Velocardiofacial syndrome
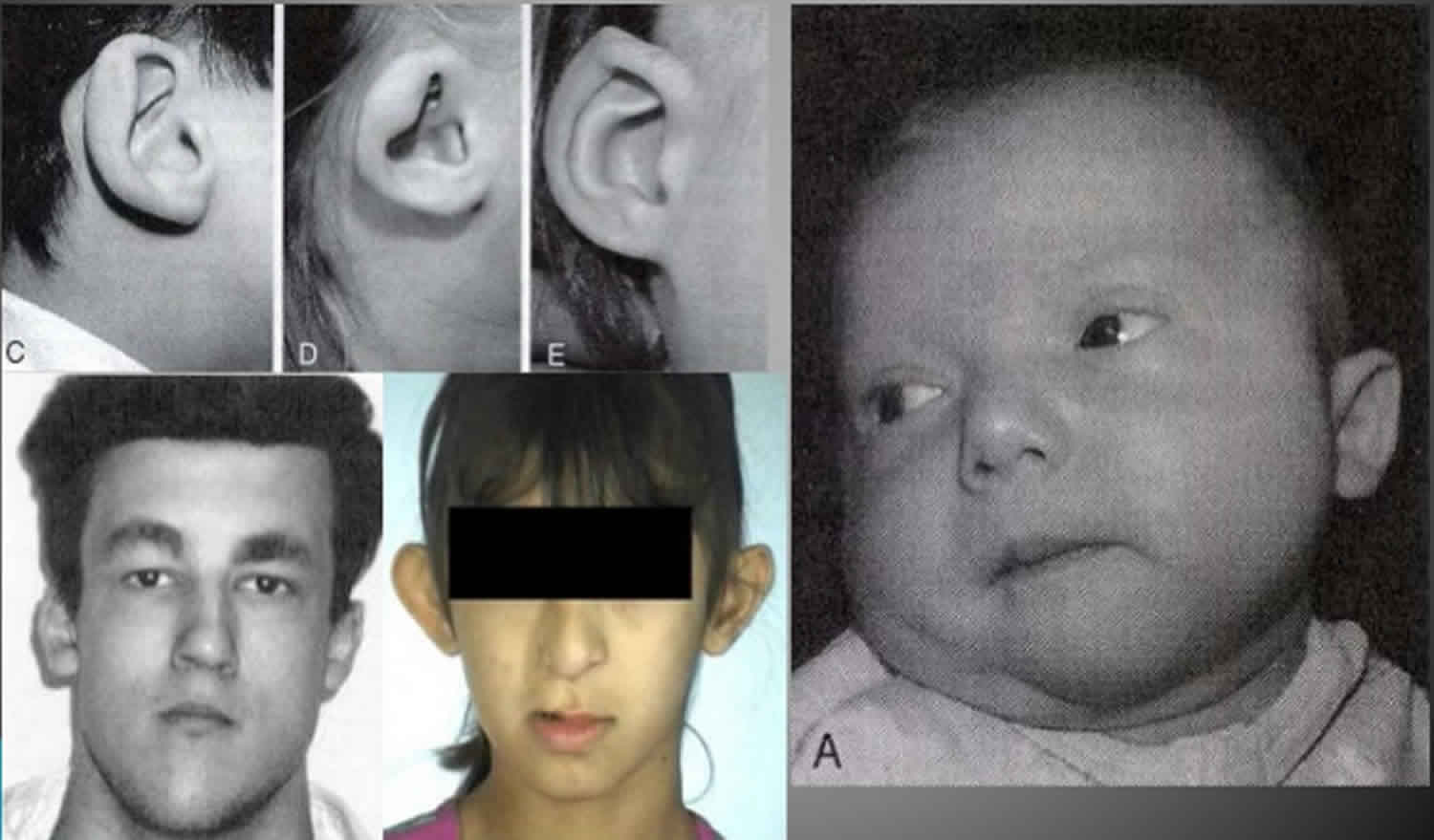
Velocardiofacial syndrome also called the 22q11.2 deletion syndrome, DiGeorge syndrome, conotruncal anomaly face syndrome, autosomal dominant Opitz G/BBB syndrome or Cayler cardiofacial syndrome, is a genetic condition that is sometimes hereditary. As a result of 22q11.2 deletion, about 30 genes are generally absent from this chromosome. Velocardiofacial syndrome is characterized by a combination of medical problems that vary from child to child. These medical problems include: cleft palate, or an opening in the roof of the mouth, and other differences in the palate; heart defects; problems fighting infection; low calcium levels (hypoparathyroidism); differences in the way the kidneys are formed or work; a characteristic facial appearance; learning disabilities; and speech and feeding problems, thrombocytopenia, scoliosis, hearing loss and developmental delay.
The name velocardiofacial syndrome comes from the Latin words “velum” meaning palate, “cardia” meaning heart and “facies” having to do with the face. Not all of these identifying features are found in each child who is born with velocardiofacial syndrome. The most common features are palatal differences (~75 percent), heart defects (75 percent), problems fighting infection (77 percent), low calcium levels (50 percent), differences in the kidney (35 percent), characteristic facial appearance (numbers vary depending on the individual’s ethnic and racial background), learning problems (~90 percent) and speech (~75 percent) and feeding problems (35 percent).
Two genes – COMT and TBX1 – are associated with velocardiofacial syndrome. However, not all of the genes that cause velocardiofacial syndrome have been identified. Most children who have been diagnosed with this syndrome are missing a small part of chromosome 22. Chromosomes are threadlike structures found in every cell of the body. Each chromosome contains hundreds of genes. A human cell normally contains 46 chromosomes (23 from each parent). The specific location or address of the missing segment in individuals with velocardiofacial syndrome is 22q11.2.
Velocardiofacial syndrome affects about 1 in 4,000 newborns. Velocardiofacial syndrome may affect more individuals, however, because some people who have the 22q11.2 deletion may not be diagnosed as they have very few signs and symptoms.
Velocardiofacial syndrome causes
Velocardiofacial syndrome is due to a 22q11.2 deletion. Most people with 22q11.2 deletion syndrome are missing a sequence of about 3 million DNA building blocks (base pairs) on one copy of chromosome 22 in each cell. This region contains 30 to 40 genes, many of which have not been well characterized. A small percentage of affected individuals have shorter deletions in the same region. This condition is described as a contiguous gene deletion syndrome because it results from the loss of many genes that are close together.
Researchers are working to identify all of the genes that contribute to the features of 22q11.2 deletion syndrome. They have determined that the loss of a particular gene on chromosome 22, TBX1, is probably responsible for many of the syndrome’s characteristic signs (such as heart defects, a cleft palate, distinctive facial features, hearing loss, and low calcium levels). Some studies suggest that a deletion of this gene may contribute to behavioral problems as well. The loss of another gene, COMT, in the same region of chromosome 22 may also help explain the increased risk of behavioral problems and mental illness. The loss of additional genes in the deleted region likely contributes to the varied features of 22q11.2 deletion syndrome.
Velocardiofacial syndrome inheritance pattern
Most cases of velocardiofacial syndrome are not inherited. Most often neither parent has the 22q11.2 deletion and so it is new in the child (93 percent) and the chance for the couple to have another child with velocardiofacial syndrome is quite low (close to zero). However, once the 22q11.2 deletion is present in a person he or she has a 50 percent chance for having children who also have the deletion (autosomal dominant inheritance). The 22q11 deletion happens as an accident when either the egg or sperm are being formed or early in fetal development. Affected people typically have no history of the disorder in their family, though they can pass the condition to their children.
In less than 10 percent of cases, a person with velocardiofacial syndrome inherits the deletion in chromosome 22 from a parent. When velocardiofacial syndrome is inherited in families, this means that other family members may be affected as well.
Since some people with the 22q11.2 deletion are very mildly affected, it is suggested that all parents of children with the deletion have testing. Furthermore, some people with the deletion have no symptoms but they have the deletion in some of their cells but not all. This is called mosaicism. Even other people have the deletion only in their egg cells or sperm cells but not in their blood cells. It is recommended that all parents of a child with a 22q11.2 deletion seek genetic counseling before or during a subsequent pregnancy to learn more about their chances of having another child with velocardiofacial syndrome.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Velocardiofacial syndrome symptoms
Despite the involvement of a very specific portion of chromosome 22, there is great variation in the symptoms of velocardiofacial syndrome. At least 30 different symptoms have been associated with the 22q11 deletion. Most of these symptoms are not present in all individuals who have velocardiofacial syndrome.
The most common symptoms include:
- cleft palate, usually of the soft palate (the roof of the mouth nearest the throat which is behind the bony palate);
- heart problems;
- similar faces (elongated face, almond-shaped eyes, wide nose, small ears);
- eye problems;
- feeding problems that include food coming through the nose (nasal regurgitation) because of the palatal differences;
- middle-ear infections (otitis media);
- low calcium due to hypoparathyroidism (low levels of the parathyroid hormone that can result in seizures);
- immune system problems which make it difficult for the body to fight infections;
- differences in the way the kidneys are formed or how they work;
- weak muscles;
- differences in the spine such as curvature of the spine (scoliosis) or bony abnormalities in the neck or upper back;
- tapered fingers.
Children who have velocardiofacial syndrome also often have learning difficulties and developmental delays. About 65 percent of individuals with the 22q11.2 deletion are found to have a non-verbal learning disability. When tested, their verbal IQ scores are greater than 10 points higher than their performance IQ scores. This combination of test scores brings down the full scale IQ scores but they won’t represent the abilities of the individual accurately. As a result of this type of learning disability, students will have relative strengths in reading and rote memorization but will struggle with math and abstract reasoning. These individuals may also have communication and social interaction problems such as autism. As adults, these individuals have an increased risk for developing mental illness such as depression, anxiety and schizophrenia.
Velocardiofacial syndrome diagnosis
Velocardiofacial syndrome is suspected as a diagnosis based on clinical examination and the presence of the signs and symptoms of the syndrome.
A special blood test called FISH (fluorescence in situ hybridization) is then done to look for the deletion in chromosome 22q11.2. More than 95 percent of individuals who have velocardiofacial syndrome have a deletion in chromosome 22q11.2.
Those individuals who do not have the 22q11.2 deletion by standard FISH testing may have a smaller deletion that may only be found using more sophisticated lab studies such as comparative genomic hybridization, MLPA, additional FISH studies performed in a research laboratory or using specific gene studies to look for mutations in the genes known to be in this region. Again, these studies may only be available through a research lab.
Velocardiofacial syndrome treatment
Treatment is based on the type of symptoms that are present. For example, heart defects are treated as they would normally be via surgical interventions in the newborn period. Individuals who have low calcium levels are given calcium supplements and frequently vitamin D to help them absorb the calcium. Palate problems are treated by a team of specialists called a cleft palate or craniofacial team and again often require surgical interventions and intensive speech therapy. Infections are generally treated aggressively with antibiotics in infants and children with immune problems.
Early intervention and speech therapies are started when possible at one year of age to assess and treat developmental delays.
Feeding disorders represent a major problem in many infants with velocardiofacial syndrome and poor outcomes for typical treatments may lead to the recommendation for gastrostomy or gavage feedings. The phenotypic factors that lead to feeding problems in velocardiofacial syndrome are hypotonia, vascular anomalies in the chest, airway compromise, slow clearing of the digestive tract with chronic constipation, chronic illness, weakness from heart disease, and temperament issues 1. It is rarely necessary to perform a gastrostomy in velocardiofacial syndrome if appropriate feeding techniques are implemented, including upright positioning during feedings, increasing the flow from the bottle by enlarging the hole in the nipple, treating constipation vigorously, and resolving the airway issues.
Hypernasal speech almost always requires surgical intervention. When cleft palate is present, it is rare for speech disorders to be successfully resolved with palate repair alone. Secondary reconstructive surgery is almost always necessary. It has been demonstrated that pharyngeal flap surgery can be highly effective 2, but an anomalous course of the internal carotid arteries presents a major risk factor 3. The use of preoperative magnetic resonance angiography to locate the internal carotids has been recommended in children with velocardiofacial syndrome 2.
The problem that most concerns parents of children with velocardiofacial syndrome is the treatment of behavioral and psychiatric disorders. The most common early psychiatric disorder in velocardiofacial syndrome is ADHD. Early data suggest that low doses of methylphenidate are effective in most cases for controlling ADHD 4. There have also been reports that psychosis in velocardiofacial syndrome may be treated effectively using antidopaminergic medications, including metyrosine 5 and L-methyldopa 6. To date, outcome studies have been limited to anecdotal reports, but having a human model for genetically caused mental illness continues to focus attention on velocardiofacial syndrome, and the possibility that COMT plays a major role may help to focus attention on the dopaminergic system.
Velocardiofacial syndrome prognosis
The prognosis for the resolution of heart, speech, and immune problems in velocardiofacial syndrome is good. The large majority of babies with velocardiofacial syndrome have successful corrections of their heart disease and will live normal life spans. Immune problems subside with time, and endocrine problems tend to be intermittent and treatable with appropriate medications. Speech problems respond well to speech therapy and surgery. Educational issues in velocardiofacial syndrome have just begun to receive some attention in the literature.
There is not yet a substantial data set on the treatment of psychiatric disorders, but the majority of patients with velocardiofacial syndrome do not require medication to treat behavioral disorders. To date, reports of significant successes with treatments designed to interrupt the abnormal dopaminergic pathway in velocardiofacial syndrome have been limited to a small number of anecdotal reports 6. However, these few reports have been exciting and positive in their outcomes and will hopefully lead to larger and better controlled studies.
There is not sufficient data as yet to determine the life span of individuals with velocardiofacial syndrome. Because the “modern era” of study of this syndrome is less than two decades old, and the delineation of the syndrome dates at its earliest to cases described by Sedlačková in 1955, there has not been an opportunity to study large samples of adults who have reached advanced age. Relying solely on anecdotal data, our clinical sample contains a number of adults with velocardiofacial syndrome in their seventh decade of life, and we have historical information on a dozen families with three generations of affected individuals with the first documented case in the family living into the eighth decade of life. Intuitively, if one were to consider some of the health related issues in velocardiofacial syndrome that might cause early death, such as severe congenital heart disease, severe immune deficiency, and possible stroke or bleeding disorders from Bernard-Soulier syndrome, an actuarial table might demonstrate that in aggregate (i.e., mean age of death), people with velocardiofacial syndrome do not have the same life span as nonaffected individuals, but it is probable that people who live into their early adult years who have not had serious health concerns up to that point will live a normal life span.
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14(1):3–10. doi:10.1002/ddrr.2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2805186[↩]
- Pharyngeal flap and the internal carotid in velocardiofacial syndrome. Tatum SA 3rd, Chang J, Havkin N, Shprintzen RJ. Arch Facial Plast Surg. 2002 Apr-Jun; 4(2):73-80.[↩][↩]
- The use of magnetic resonance angiography prior to pharyngeal flap surgery in patients with velocardiofacial syndrome. Mitnick RJ, Bello JA, Golding-Kushner KJ, Argamaso RV, Shprintzen RJ. Plast Reconstr Surg. 1996 Apr; 97(5):908-19.[↩]
- Methylphenidate treatment for attention-deficit/hyperactivity disorder in children and adolescents with velocardiofacial syndrome: an open-label study. Gothelf D, Gruber R, Presburger G, Dotan I, Brand-Gothelf A, Burg M, Inbar D, Steinberg T, Frisch A, Apter A, Weizman A. J Clin Psychiatry. 2003 Oct; 64(10):1163-9.[↩]
- Catecholamines in patients with 22q11.2 deletion syndrome and the low-activity COMT polymorphism. Graf WD, Unis AS, Yates CM, Sulzbacher S, Dinulos MB, Jack RM, Dugaw KA, Paddock MN, Parson WW. Neurology. 2001 Aug 14; 57(3):410-6.[↩]
- Replacement of antipsychotic and antiepileptic medication by L-alpha-methyldopa in a woman with velocardiofacial syndrome. O’Hanlon JF, Ritchie RC, Smith EA, Patel R. Int Clin Psychopharmacol. 2003 Mar; 18(2):117-9.[↩][↩]