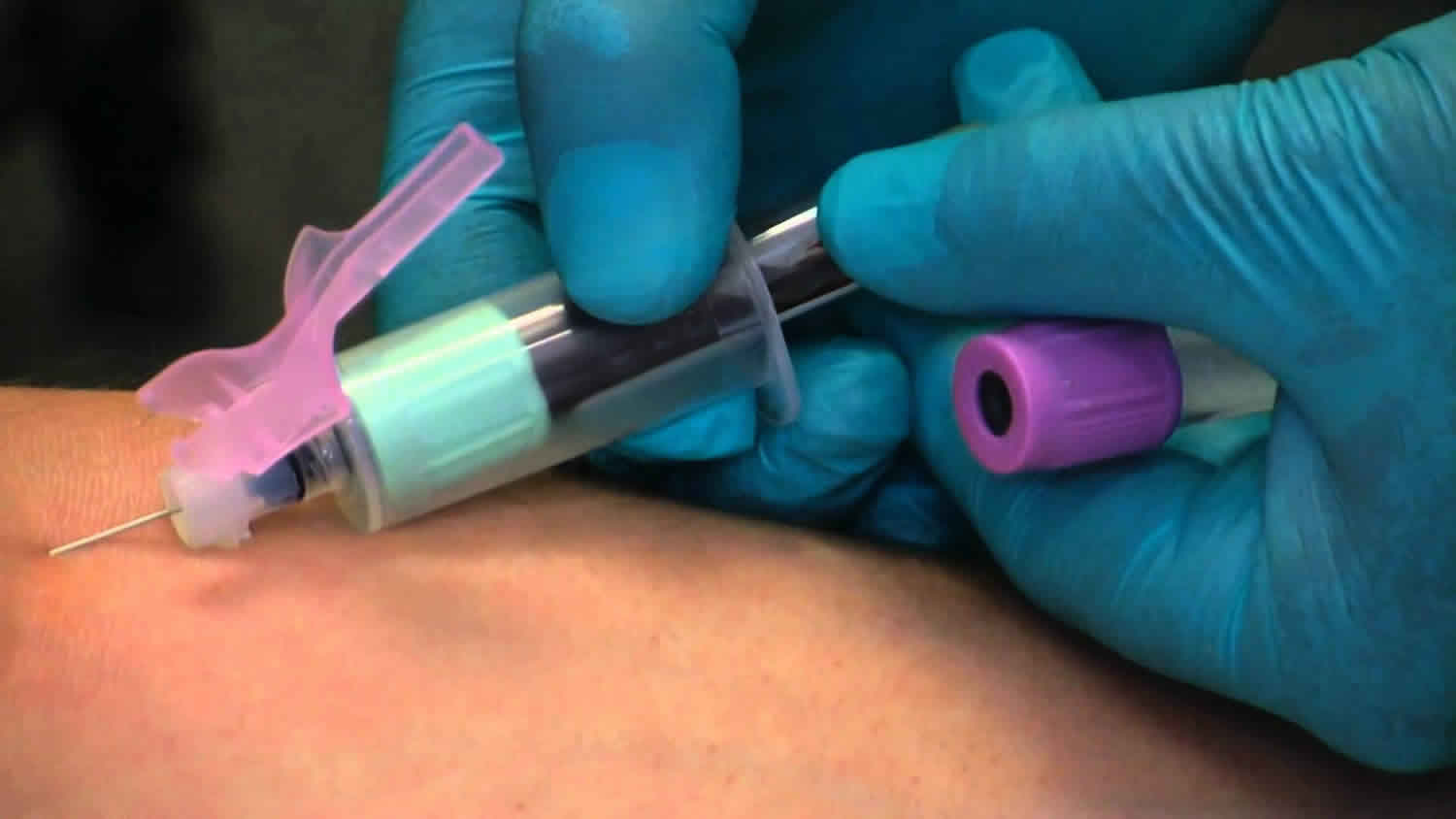
What is venipuncture
Venipuncture is also called phlebotomy, is a procedure in which a needle is used to take blood from a vein. In many patients, venous access is necessary for obtaining blood for laboratory testing and administering fluid and intravenous drugs. Venipuncture is most often done for laboratory testing. Venipuncture may also be done to remove extra red blood cells from the blood, to treat certain blood disorders.
Blood is made up of two parts:
- Fluid (plasma or serum)
- Cells
Plasma is the fluid part that contains substances such as glucose, electrolytes, proteins, and water. Serum is the fluid part that remains after the blood is allowed to clot in a test tube.
Cells in the blood include red blood cells, white blood cells, and platelets.
Blood helps move oxygen, nutrients, waste products, and other materials through the body. It helps control body temperature, fluid balance, and the body’s acid-base balance.
Tests on blood or parts of blood may give your doctor important clues about your health.
Venipuncture test
The tourniquet application causes the inner pressure of the vein to increase artificially. If prolonged or excessive, this constriction raises the hydrostatic pressure within the vessel, forcing the water to pass into the outer connective tissue. Thereafter, the collected sample can show haemoconcentration, an activated pro-coagulant response, as well as an altered platelets function 1. The prolonged venous stasis, which also favours tissue hypoxia, produces a change in pH which locally affects the electrolytes balance, especially potassium 2.
Since potassium is mainly intracellular, a false elevation in its blood level can be easily achieved through various processes. For instance, a strenuous or prolonged fist clenching or pumping leads to the increase of extracellular potassium due to the depolarization of the skeletal muscle cells 3. Nonetheless, mechanical stress on cells can similarly induce an increase of blood potassium through a membrane leakage. In this regard there are two major mechanical causes related to phlebotomy procedure: the shear stress on red blood cells flowing through a small bore needle which causes haemolysis and the needle probing of the bloodstream which damages the tissue neighbouring the venipuncture site 4. Under a clinical and laboratory standpoint, the condition in which a patient is found to have hyperkalemia with no other biochemical signs or relevant causes in the medical history is said spurious or pseudohyperkalemia (pseudohyperkalemia) 5.
A remarkable cause of pseudohyperkalemia associated with the phlebotomy procedure is the sample cross-contamination due to tube additives. As the standard haemochromocytometric analysis is carried out on anticoagulated blood sample, the sampling tubes with di- or tri-potassium salt of the ethylenediaminetetraacetic acid (K2-EDTA and K3-EDTA) are routinely used. In this regard, it is noteworthy to address two distinct ways through which a sample can be contaminated by EDTA. If a syringe is used for drawing blood, it can happen when the blood is dispended in the tube containing the anticoagulant. In this case, the syringe’s needle is thought to carry the contamination after it has accidentally touched the inner side of the tube coated with the anticoagulant. Instead, if an evacuated tubes system is used, the contamination can be due to the anticoagulated blood which stains the inner needle that perforates the tube stop. In this regard, the order in which the tubes containing different additives are drawn is relevant to prevent contamination. According to the correct order of drawing, an EDTA-containing tube should always be collected after a serum tube or a citrate-anticoagulated whole blood tube 6. Noteworthy, some authors have shown that with both Sarstedt’s and evacuated tubes the recommendation of a precise tube order does not affect the blood testing 7. However, it should be noted that drawing an EDTA tube after all the others physically prevents any possible contamination, regardless of the way the system is used as already mentioned in this paper. With respect to pseudohyperkalemia it should be finally remarked that tube mixing, necessary to achieve the proper preservation of the collected blood, should be performed only after the tube has been removed from the holder 8.
Whenever an EDTA contamination happens, an abnormal reduction of serum calcium can be shown in the factitious electrolytes imbalance 9. Such a condition, which is defined pseudohypocalcemia (PHC), is due to the strong chelating action EDTA exerts on divalent cations and ferric iron (Fe3+). Noteworthy, the strong chelation of calcium can also interfere with coagulation testing, significantly biasing both prothrombin time (PT) and activated partial thromboplastin time (APTT) 10. Therefore, the advice to draw EDTA tubes at the end should be followed whenever any other weaker anti-coagulant is used to collect blood, as in case of lithium-heparin 11.
Spurious haemolysis is another common condition associated with venipuncture, and the most frequent cause of sample unsuitability 12. The needle bore size along with the withdrawal force is the main factors of spurious hemolysis, since them both induce a mechanical stress which causes the rupture of the cell membrane. In this regard, the needle bore size, and in turn the gauge, may be regarded as a reliable predictor of haemolysis, with 21 G needles showing half the risk of producing an unsuitable sample respect to 23 G 13. However, it should be noticed that the effectiveness of larger bore needles could be mitigated by the choice of the venipuncture site, and that such a lower estimated risk of haemolysis would be reliable only when the phlebotomy is performed on large veins of the antecubital fossa 14. Moreover, a 23 G needle could be as safe as a 21 G if handled correctly, whereas smaller bore size than 23 G needle should be avoided or used in very rare and extreme circumstances 15. Actually, spurious hemolysis can affect several laboratory tests as the red blood cells contain analytes which are routinely tested in serum, like lactate dehydrogenase, aspartate aminotransferase and potassium 16. Furthermore, spurious hemolysis can cause the release of haemoglobin, whose iron-containing heme group can produce a spectrophotometric interference or, in turn, can react with some of the assay reagents. It is noteworthy to mention that swabbing the venipuncture site with alcohol-containing solutions does not cause any significant haemolysis 17. Hemolysis can be readily detected by visual inspection after sample centrifugation, and directly quantified through a spectrophotometric reading even at low concentration. However, for whole blood samples, haemolysis can be neither observed nor directly measured. Nonetheless, in recent times some authors have proposed an indirect assessment of haemolysis in whole blood samples using the automated cell blood count parameters to compute suitable and reliable indexes 18.
Although not strictly related with the procedure of phlebotomy, there are some other issues which deserve a discussion, as they are among the most frequent causes of pre-analytical errors 19. The misidentification of the patient as well as the incorrect labelling of the tubes were found to be a critical step in the quality of phlebotomy with respect to the burden of preanalytical errors 20. In this regard, the use of bar coding test tubes, along with the adoption of educational programs which helped to increase the compliance of the operators to the best practice guidelines, resulted successful in lowering the rate of unsuitable samples 21. During venipuncture, the choice of the appropriate tube to use with respect to the additive contained was another relevant factor found to significantly affect the quality of collected samples. Although the color-code scheme was devised to help the operator in recognizing the additive within the tube, the broad heterogeneity in the scheme adopted by the various manufacturers and the lack of standardization resulted in a serious issue 22. Lastly, the body positioning, which is a fundamental but often overlooked factor in laboratory preanalytics. Very recently it has been shown that changing the position from sitting to lying (and vice versa) causes a significant bias in several laboratory values, due to the gravitational adaptation which changes the hydrostatic pressure along the various body districts 23. Noteworthy, the effect that the shift in plasma volume has on hematocrit is regarded as postural pseudoanemia for the consequence it has on haematological testing 24. Although there can be several ways through which phlebotomy can affect the laboratory preanalytics, not all of them produce a significant bias as it can be seen in Table 1.
Table 1. Preanalytical bias of routine venipuncture
| Venipuncture factor | Needle gauge | Needle type | Toruniquet application | Body positioning | |
|---|---|---|---|---|---|
| Lab parameter | 23 G vs. 21 G | Winged vs. regular | 1 minute | 3 minute | Sitting vs. lying |
| Clinical chemistry | negligible | negligible | Albumin (+1.58%; ±1.3%) Calcium (+1.6%; ±0.8%) Chloride (-0.5%; ±0.5%) Potassium (-2.8%; ±1.8%) | ALT (+15.7%; ±12.0%) Albumin (+8.6%; ±1.3%) Calcium (+3.6%; ±0.8%) Chloride (-1.1%; ±0.5%) Cholesterol (+9.1%; ±4.0%) Glucose (-3.7%; ±2.2%) Iron (+8.8%; ±8.8%) Potassium (-4.8%; ±1.8%) | Albumin (+2.0%; ±1.4%) Protein (+2.9%; ±1.4%) |
| Platelets and coagulation | negligible | negligible | negligible | PT/INR (-3.1%; ±2.0%) Fibrinogen (+10.1%; ±4.8%) | n.a. |
| Haematology | n.a. | negligible | WBC (-6.7%; ±5.6%) RBC (+3.6%; ±1.7%) Haemoglobin (+3.0%; ±1.8%) Haematocrit (+3.7%; ±1.7%) Lymphocytes (-7.9%; ±7.4%) Monocytes (-14.4%; ±13.2%) | WBC (-10.1%; ±5.6%) RBC (+7.4%; ±1.7%) Haemoglobin (+6.2 ; ±1.8%) Haematocrit (+7.3%; ±1.7%) Lymphocytes (-10.7%; ±7.4%) Monocytes (-22.0%; ±13.2%) | Haemoglobin (+2.3%; ±1.8%) Haematocrit (+1.7%; ±1.7%) |
Footnote: Data in brackets are the actual and desirable bias respectively; “n.a.” indicates factors for which bias was not measured regardless to a significant effect may exist.
[Source 25 ]Veins of the arm for venipuncture
Large veins embedded i n the superficial fascia of the upper limb are often used to access a patient’s vascular system and to withdraw blood. The most significant of these veins are the cephalic vein, basilic vein and median cubital vein (Figure 1). The cephalic and basilic veins originate from the dorsal venous network on the back of the hand. The cephalic vein originates over the anatomical snuffbox at the base of the thumb, passes laterally around the distal forearm to reach the anterolateral surface of the limb, and then continues proximally. The cephalic vein crosses the elbow, then passes up the arm into a triangular depression-the clavipectoral triangle (deltopectoral triangle) between the pectoralis major muscle, deltoid muscle, and clavicle. In this depression, the vein passes into the axilla
by penetrating deep fascia just inferior to the clavicle.
The basilic vein originates from the medial side of the dorsal venous network of the hand and passes proximally up the posteromedial surface of the forearm. It passes onto the anterior surface of the limb just inferior to the elbow and then continues proximally to penetrate deep fascia about midway up the arm.
At the elbow, the cephalic and basilic veins are connected by the median cubital vein, which crosses the roof of the cubital fossa.
For straight forward blood tests the antecubital vein is usually the preferred site, and although it may not always be visible, it is easily palpated . The veins are simply distended by use of a tourniquet. A tourniquet should be applied enough to allow the veins to become prominent. The cephalic vein adjacent to the anatomical snuffboxis generally the preferred site for a short-term intravenous cannula.
Figure 1. Veins of the arm for venipuncture
Venipuncture sites
The ideal sites for venipuncture are typically in the cubital fossa of the forearm, which has the shape of an isosceles triangle with the biceps forming the base, and the lateral and medial groups of the antebrachial muscles forming the sides respectively 26. Although in the large part of individuals the running pattern of superficial veins appears well evident in this region, the path and distribution can be irregular and may require a careful inspection before attempting the needle insertion. The main veins in this area are represented by the cephalic vein, the basilic vein, the median cubital vein, the median antebrachial vein and their various tributaries and anastomosis. Their distribution and connections can be classified into four types (1 to 4), according to the dominance of cephalic vein or basilic vein with respect to the calibre of the vessel (see Figure 2) 27.
There are alternative sites for venipuncture 28. They are mainly represented by the dorsal surface of the hand, where veins lay superficially because of the poor connective tissue and muscles. It must be noted that this site has also the greater pain tolerance threshold among the upper limb sites, thus resulting in the lowest perceived pain intensity 29. Therefore, it may be eligible in those subjects with deep or small veins or with a particular issue of painful reactions. However, veins at this site have a greater mobility due to the poor surrounding connective tissue, and thus they more easily “roll” under the needle’s tip or collapse, causing a missed attempt or a rupture of the vessel.
Notably, it is important to know the spatial relationship between the path of veins and cutaneous nerves that can be accidentally pinched during needle insertion, causing intense pain and nerve damage 30. For instance, the lateral cutaneous nerve of the forearm usually descends deeply along the cephalic vein (CV) while the medial cutaneous nerve of the forearm descends superficially along the basilic vein (BV) (Figure 3) 31. The most common nerve injury related to venipuncture involves the lateral antebrachial cutaneous nerve (LACN), which can lead to the so-called “causalgia” or complex regional pain syndrome 32. The complex regional pain syndrome can range from a mild and temporary harm, which resolves within few months, up to a severe and permanent damage with chronic pain and the need of complex therapies 33. However this is a very rare consequence occurring in about 1: 25,000 individuals undergoing blood donation 34. Noteworthy, the complex regional pain syndrome may be favoured by peculiar anatomical relationships between nerves and veins, as well as by the needle probing in case of a missed attempt 35. However some authors have reported that the use of winged needles may contribute to lower the risk of nerve injury at the upper limb site 36.
Figure 2. Variations of the cubital superficial vein
Footnote: The superficial veins of the upper limb present a certain inter-individual variability in their running pattern and caliber; A) in type 1, cephalic vein (CV) and basilic vein (BV) merge into the median antebrachial vein (MABV) of the forearm; B) in type 2, the median cubital vein (MCV) forms an anastomose between CV and BV; C) in type 3, cephalic vein (CV) is threadlike and basilic vein (BV) splits in two branches of the forearm; D) in type 4, cephalic vein (CV) and basilic vein (BV) run in parallel with no evident superficial anastomoses; the dashed triangle delimits the cubital fossa area.
[Source 27 ]Due to congenital causes, extreme leanness or in children, the brachial artery can be found to run superficially, passing closely under the ulnar side of both median cubital vein and basilic vein (Figure 3) 37. In this case an excessive penetration into the vein can cause the needle to pass the vein from side to side, reaching the artery underneath. Although this happens in less than 0.01% of blood donors, it can cause sudden large hematoma with serious bleeding for the high arterial pressure, or afterwards lead to a false or true aneurism of the artery 38.
Inspection and especially palpation represent the basic approach to identify the site for venipuncture 39. Palpation allows phlebotomist to recognize the vessel elasticity, depth and consistency of the surrounding tissues. Superficial veins can be poorly evident to sight and palpation in some coloured or obese people, so the tourniquet must be applied several inches above the site of venipuncture in order to enhance their path by inducing venous stasis. To achieve satisfactory result, a moderate pressure (60 mm Hg) for a less than a minute is enough to suitably dilate the vein, avoiding the risk of inducing excessive venous stasis that can alter haematological and biochemical parameters. In terms of width of the venous vessel this condition corresponds to the achievement of the maximal venous cross-section area, as it is shown by the ultrasonography imaging 40. Interestingly, an alternative to tourniquet application can be “stimulating” the vein by tapping the vessel, although its effect may be quite small on average producing just a 4% increase 41.
Figure 3. Anatomy of the venipuncture sites (the cubital fossa of right elbow)
Footnote: Topographic anatomy of the cubital fossa (cross-section at the elbow).
Blood vessels: Cephalic vein, Radial artery and Basilic vein.
Tendons: α) biceps brachii tendon; β) triceps brachii tendon);
Nerves: a) lateral antebrachial cutaneous nerve; b) medial antebrachial cutaneous nerve; c) median nerve; d) ulnar nerve; e) posterior lateral antebrachial nerve; f) radial nerve;
Muscles and bones: 1) brachioradialis; 2) brachialis; 3) pronator tenes; 4) trochlea (humerus); 5) olecranon (ulna); 6) anconeus.
[Source 25 ]Venipuncture procedure
Most of the time, blood is drawn from a vein located on the inside of the elbow or the back of the hand.
- The site is cleaned with germ-killing medicine (antiseptic).
- An elastic band is put around the upper arm to apply pressure to the area. This makes the vein swell with blood.
- A needle is inserted into the vein.
- The blood collects into an airtight vial or tube attached to the needle.
- The elastic band is removed from your arm.
- The needle is taken out and the spot is covered with a bandage to stop bleeding.
In infants or young children, a sharp tool called a lancet may be used to puncture the skin and make it bleed. The blood collects onto a slide or test strip. A bandage may be placed over the area if there is any bleeding.
Sometimes, neither tourniquet nor tapping is effective. In all such cases, a technological help is provided by the trans-illuminating devices, which use the cold near infrared light-emitting diodes to light erythrocytes flowing inside the vessels 42. With such devices, the path of the vein appears as a colourless pattern drawn on a bright surface, as the infrared light which is absorbed by the erythrocytes is instead reflect by the neighboring tissues. In certain trans-illuminating devices the infrared image is not directly shown, but rather it is read by the device and then projected onto the skin surface to produce a guiding pattern for the operator 43. Such devices have been shown to improve the venipuncture procedure without affecting haematological parameters 44. However, they do not produce any effect over the venous cross-section area, so that small deep veins remain still hard to pierce although well visible.
Table 2. Routine venipuncture at glance
| Tools | Pain | Pre-analytics | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| > Torniquet | > Pre-procedural | > Pseudohyperkalemia | |||||||||||||||||||||||
| o elastic lace | o trypanophobia | o torniquet | |||||||||||||||||||||||
| o belt | § vasovagal reaction | § torniquet pressure (> 60 mm Hg) | |||||||||||||||||||||||
| > Needle | » distraction | § venous stasis (> 1 min) | |||||||||||||||||||||||
| o regular | » cough trick | o fist clenching | |||||||||||||||||||||||
| § 23 G | > Intra-procedural | o needle | |||||||||||||||||||||||
| § 21 G | o skin piercing | § mechanical hemolisys | |||||||||||||||||||||||
| o winged | o MACP | » bore size | |||||||||||||||||||||||
| § 23 G | § distraction | » winged needle with tubing | |||||||||||||||||||||||
| § 21 G | o nerve injury | o collecting device | |||||||||||||||||||||||
| > Collecting device | § LACN | § syringe | |||||||||||||||||||||||
| o piston syringe | § MACN | »· aspiration force | |||||||||||||||||||||||
| o evacuated tubes | > Post-procedural | » dispensing pressure | |||||||||||||||||||||||
| o Sarstedt’s hybrid system | o compression | § evacuated tube | |||||||||||||||||||||||
| > Disinfectant | § hematoma | » vacuum pressure | |||||||||||||||||||||||
| o alcoholic | § pseudo-aneurism | » mixing shake | |||||||||||||||||||||||
| § ethanol | § aneurism | » tube order | |||||||||||||||||||||||
| § isopropylic alcohol | o inflmmatory | ◊ EDTA contamination | |||||||||||||||||||||||
| § chlorhexidine 2% | § phlebitis | § pseudohypocalcemia | |||||||||||||||||||||||
| o non-alcoholic | o nerve injury | > Spurious hemolysis | |||||||||||||||||||||||
| § povidone-iodine | § causalgia | o torniquet | |||||||||||||||||||||||
| § benzalkonium chloride | § torniquet pressure (> 60 mm Hg) | ||||||||||||||||||||||||
| > Anesthetic | § venous stasis (> 1 min) | ||||||||||||||||||||||||
| o chemical | Veins | o needle | |||||||||||||||||||||||
| § topic cream | > Site | § mechanical hemolisys | |||||||||||||||||||||||
| § intradermic needless | o cubital fossa | » bore size | |||||||||||||||||||||||
| o gate control-based | § CV (type i, ii, iv) | » winged needle with tubing | |||||||||||||||||||||||
| § mechanical | § BV (type i-iv) | § traumatic venipuncture | |||||||||||||||||||||||
| » high frequency | § MABV (type i) | o collecting device | |||||||||||||||||||||||
| o thermal | § MCV (type ii) | § syringe | |||||||||||||||||||||||
| § hot pad | o hand backside | » aspiration force | |||||||||||||||||||||||
| § cold spray | > Search | » dispensing pressure | |||||||||||||||||||||||
| o torniquet | § evacuated tube | ||||||||||||||||||||||||
| o transillumination | » excessive vacuum pressure | ||||||||||||||||||||||||
| > Characteristic | » excessive mixing shake | ||||||||||||||||||||||||
| o elevation | > Haemoconcentration | ||||||||||||||||||||||||
| o depth | o torniquet pressure (> 60 mm Hg) | ||||||||||||||||||||||||
| o cross section area | o venous stasis (> 1 min) | ||||||||||||||||||||||||
Footnotes: Roots (>) are main topics, branches (o) are relevant issues and sub-items (§, », ◊) are details.
Abbreviations: MACP = motivational anticipatory cortical process; LACN = lateral antebrachial cutaneous nerve; MACN = medial antebrachial cutaneous nerve; CV = cephalic vein; BV = basilic vein; MABV = median antebrachial vein; MCV = median cubital vein.
[Source 25 ]Venipuncture steps
The venipuncture steps you need to take before the test will depend on the kind of blood test you are having. Many tests do not require special steps.
In some cases, your health care provider will tell you if you need to stop taking any medicines before you have this test or if you need to be fasting. Do not stop or change your medicines without talking to your provider first.
Venipuncture complications
Minor complications were defined as minor bruising and hematoma at the venipuncture site. Minor bruising and hematoma (blood clot under skin) were fairly common, involving 12.3% of venipunctures, with minor bruising being the most common reaction 45. Serious complications were defined as cellulitis, phlebitis (inflammation of vein), sweating (diaphoresis), hypotension, near syncope, syncope (fainting), and seizure activity. Serious complications were observed in 3.4% of patients 45. Diaphoresis with hypotension occurred in 2.6%. Syncope occurred in less than 1% of patients.
- Lima-Oliveira G, Lippi G, Salvagno GL, Gaino S, Poli G, Gelati M, et al. Venous stasis and whole blood platelet aggregometry:a question of data reliability and patient safety. Blood Coagul Fibrinolysis. 2015;26:665–8. 10.1097/MBC.0000000000000342[↩]
- Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of short-term venous stasis on clinical chemistry testing. Clin Chem Lab Med. 2005;43:869–75. 10.1515/CCLM.2005.146[↩]
- Seimiya M, Yoshida T, Sawabe Y, Sogawa K, Umemura H, Matsushita K, et al. Reducing the incidence of pseudohyperkalemia by avoiding making a fist during phlebotomy:a quality improvement report. Am J Kidney Dis. 2010;56:686–92. 10.1053/j.ajkd.2010.06.014[↩]
- Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement:a laboratory perspective for the clinician. N Am J Med Sci. 2013;5:255–9. 10.4103/1947-2714.110426[↩]
- Sevastos N, Theodossiades G, Archimandritis AJ. Pseudohyperkalemia in serum:a new insight into an old phenomenon. Clin Med Res. 2008;6:30–2. 10.3121/cmr.2008.739[↩]
- Clinical and Laboratory Standards Institute. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncutre; Approved Standard – Sixth Edition. CLSI document H3-A5. Wayne, PA, USA, 2007.[↩]
- Salvagno G, Lima-Oliveira G, Brocco G, Danese E, Guidi GC, Lippi G. The order of draw:myth or science? Clin Chem Lab Med. 2013;51:2281–5. 10.1515/cclm-2013-0412[↩]
- Lima-Oliveira G, Lippi G, Salvagno GL, Brocco G, Gaino S, Dima F, et al. Processing of diagnostic blood specimens: is it really necessary to mix primary blood tubes after collection with evacuated tube system? Biopreserv Biobank. 2014;12:53–9. 10.1089/bio.2013.0043[↩]
- Naguib MT, Evans N. Combined false hyperkalemia and hypocalcemia due to specimen contamination during routine phlebotomy. South Med J. 2002;95:1218–20. 10.1097/00007611-200295100-00025[↩]
- Lima-Oliveira G, Salvagno GL, Danese E, Favaloro EJ, Guidi GC, Lippi G. Sodium citrate blood contamination by K2 -ethylenediaminetetraacetic acid (EDTA):impact on routine coagulation testing. Int J Lab Hematol. 2015;37:403–9. 10.1111/ijlh.12301[↩]
- Lima-Oliveira G, Salvagno GL, Danese E, Brocco G, Guidi GC, Lippi G. Contamination of lithium heparin blood by K2-ethylenediaminetetraacetic acid (EDTA): an experimental evaluation. Biochem Med (Zagreb). 2014;24:359–67. 10.11613/BM.2014.038[↩]
- Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46:764–72. 10.1515/CCLM.2008.170[↩]
- Wollowitz A, Bijur PE, Esses D, John Gallagher E. Use of butterfly needles to draw blood is independently associated with marked reduction in hemolysis compared to intravenous catheter. Acad Emerg Med. 2013;20:1151–5. 10.1111/acem.12245[↩]
- Heyer NJ, Derzon JH, Winges L, Shaw C, Mass D, Snyder SR, et al. Effectiveness of practices to reduce blood sample hemolysis in EDs:a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem. 2012;45:1012–32. 10.1016/j.clinbiochem.2012.08.002[↩]
- Lippi G, Salvagno GL, Montagnana M, Brocco G, Cesare Guidi G. Influence of the needle bore size used for collecting venous blood samples on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:1009–14. 10.1515/CCLM.2006.172[↩]
- Lippi G, Pavesi F, Cattabiani C, Avanzini P, Pipitone S. Influence of in vitro hemolysis on nucleated red blood cells and reticulocyte counts. Int J Lab Hematol. 2013;35:225–8. 10.1111/ijlh.12012[↩]
- Salvagno GL, Danese E, Lima-Oliveira G, Guidi GC, Lippi G. Avoidance to wipe alcohol before venipuncture is not a source of spurious hemolysis. Biochem Med. 2013;23:201–5. 10.11613/BM.2013.023[↩]
- Lippi G, Pavesi F, Avanzini P, Chetta F, Aloe R, Pipitone S. Development of simple equations for effective screening of spurious hemolysis in whole-blood specimens. Int J Lab Hematol. 2015;37:253–8. 10.1111/ijlh.12277[↩]
- Carraro P, Plebani M. Errors in a stat laboratory:types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. 10.1373/clinchem.2007.088344[↩]
- Simundic AM, Church S, Cornes MP, Grankvist K, Lippi G, Nybo M, et al. Compliance of blood sampling procedures with the CLSI H3-A6 guidelines: An observational study by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PRE). Clin Chem Lab Med. 2015;53:1321–31. 10.1515/cclm-2014-1053[↩]
- Lillo R, Salinas M, Lopez-Garrigos M, Naranjo-Santana Y, Gutierrez M, Marin MD, et al. Reducing preanalytical laboratory sample errors through educational and technological interventions. Clin Lab. 2012;58:911–7.[↩]
- Simundic AM, Cornes MP, Grankvist K, Lippi G, Nybo M, Ceriotti F, et al. Colour coding for blood collection tube closures – a call for harmonisation. Clin Chem Lab Med. 2015;53:371–6. 10.1515/cclm-2014-0927[↩]
- Lippi G, Salvagno GL, Lima-Oliveira G, Brocco G, Danese E, Guidi GC. Postural change during venous blood collection is a major source of bias in clinical chemistry testing. Clin Chim Acta. 2015;440:164–8. 10.1016/j.cca.2014.11.024[↩]
- Jacob G, Raj SR, Ketch T, Pavlin B, Biaggioni I, Ertl AC, et al. Postural pseudoanemia:posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80:611–4. 10.4065/80.5.611[↩]
- Phlebotomy, a bridge between laboratory and patient. Biochem Med (Zagreb). 2016 Feb 15; 26(1): 17–33. doi: 10.11613/BM.2016.002 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783087/[↩][↩][↩]
- Phlebotomy tools of the trade: part 2: surveying the antecubital area. Ernst DJ, Ernst C. Home Healthc Nurse. 2002 Jun; 20(6):402-3.[↩]
- Variations of the cubital superficial vein investigated by using the intravenous illuminator. Lee H, Lee SH, Kim SJ, Choi WI, Lee JH, Choi IJ. Anat Cell Biol. 2015 Mar; 48(1):62-5.[↩][↩]
- Ernst DJ, Ernst C. Phlebotomy tools of the trade: part 3: Alternative sites for drawing blood. Home Healthc Nurse. 2003;21:156–8. 10.1097/00004045-200303000-00006[↩]
- Yoshida M, Shiiba S, Sago T, Nunomaki M, Harano N, Watanabe S. Comparison of pain tolerance thresholds of upper limb to identify the most appropriate venipuncture site. J Oral Maxillofac Surg. 2015;73:850 e1–5. 10.1016/j.joms.2015.01.027[↩]
- Berry PR, Wallis WE. Venepuncture nerve injuries. Lancet. 1977;1:1236–7. 10.1016/S0140-6736(77)92442-4[↩]
- Yamada K, Yamada K, Katsuda I, Hida T. Cubital fossa venipuncture sites based on anatomical variations and relationships of cutaneous veins and nerves. Clin Anat. 2008;21:307–13. 10.1002/ca.20622[↩]
- Ramos JA. Venipuncture-related lateral antebrachial cutaneous nerve injury:what to know? Braz J Anesthesiol. 2014;64:131–3. 10.1016/j.bjane.2013.06.003[↩]
- Elahi F, Reddy CG. Venipuncture-induced complex regional pain syndrome:a case report and review of the literature. J Med Case Rep 2014;2014:613921.[↩]
- Rayegani SM, Azadi A. Lateral antebrachial cutaneous nerve injury induced by phlebotomy. J Brachial Plex Peripher Nerve Inj. 2007;2:6.[↩]
- Fujii C. Clarification of the characteristics of needle-tip movement during vacuum venipuncture to improve safety. Vasc Health Risk Manag. 2013;9:381–90. 10.2147/VHRM.S47490[↩]
- Ohnishi H, Watanabe M, Watanabe T. Butterfly needles reduce the incidence of nerve injury during phlebotomy. Arch Pathol Lab Med. 2012;136:352. 10.5858/arpa.2011-0431-LE[↩]
- Mikuni Y, Chiba S, Tonosaki Y. Topographical anatomy of superficial veins, cutaneous nerves, and arteries at venipuncture sites in the cubital fossa. Anat Sci Int. 2013;88:46–57. 10.1007/s12565-012-0160-z[↩]
- Bhatti K, Ali S, Shamugan SK, Ward AS. True brachial artery aneurysm following blood donation:a case report of a rare complication. Eur J Vasc Endovasc Surg. 2007;13:44–6.[↩]
- Ernst DJ, Ernst C. Phlebotomy tools of the trade: part 2: surveying the antecubital area. Home Healthc Nurse. 2002;20:402–3. 10.1097/00004045-200206000-00021[↩]
- Sasaki S, Murakami N, Matsumura Y, Ichimura M, Mori M. Relationship between tourniquet pressure and a cross-section area of superficial vein of forearm. Acta Med Okayama. 2012;66:67–71.[↩]
- Ichimura M, Sasaki S, Mori M, Ogino T. Tapping but not massage enhances vasodilation and improves venous palpation of cutaneous veins. Acta Med Okayama. 2015;69:79–85.[↩]
- Lee H, Lee SH, Kim SJ, Choi WI, Lee JH, Choi IJ. Variations of the cubital superficial vein investigated by using the intravenous illuminator. Anat Cell Biol. 2015;48:62–5. 10.5115/acb.2015.48.1.62[↩]
- Miyake RK, Zeman HD, Duarte FH, Kikuchi R, Ramacciotti E, Lovhoiden G, et al. Vein imaging:a new method of near infrared imaging, where a processed image is projected onto the skin for the enhancement of vein treatment. Dermatol Surg. 2006;32:1031–8.[↩]
- Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Scartezini M, Guidi GC, et al. Transillumination:a new tool to eliminate the impact of venous stasis during the procedure for the collection of diagnostic blood specimens for routine haematological testing. Int J Lab Hematol. 2011;33:457–62.[↩]
- Complications occurring from diagnostic venipuncture. J Fam Pract. 1992 May;34(5):582-4. https://www.ncbi.nlm.nih.gov/pubmed/1578208[↩][↩]