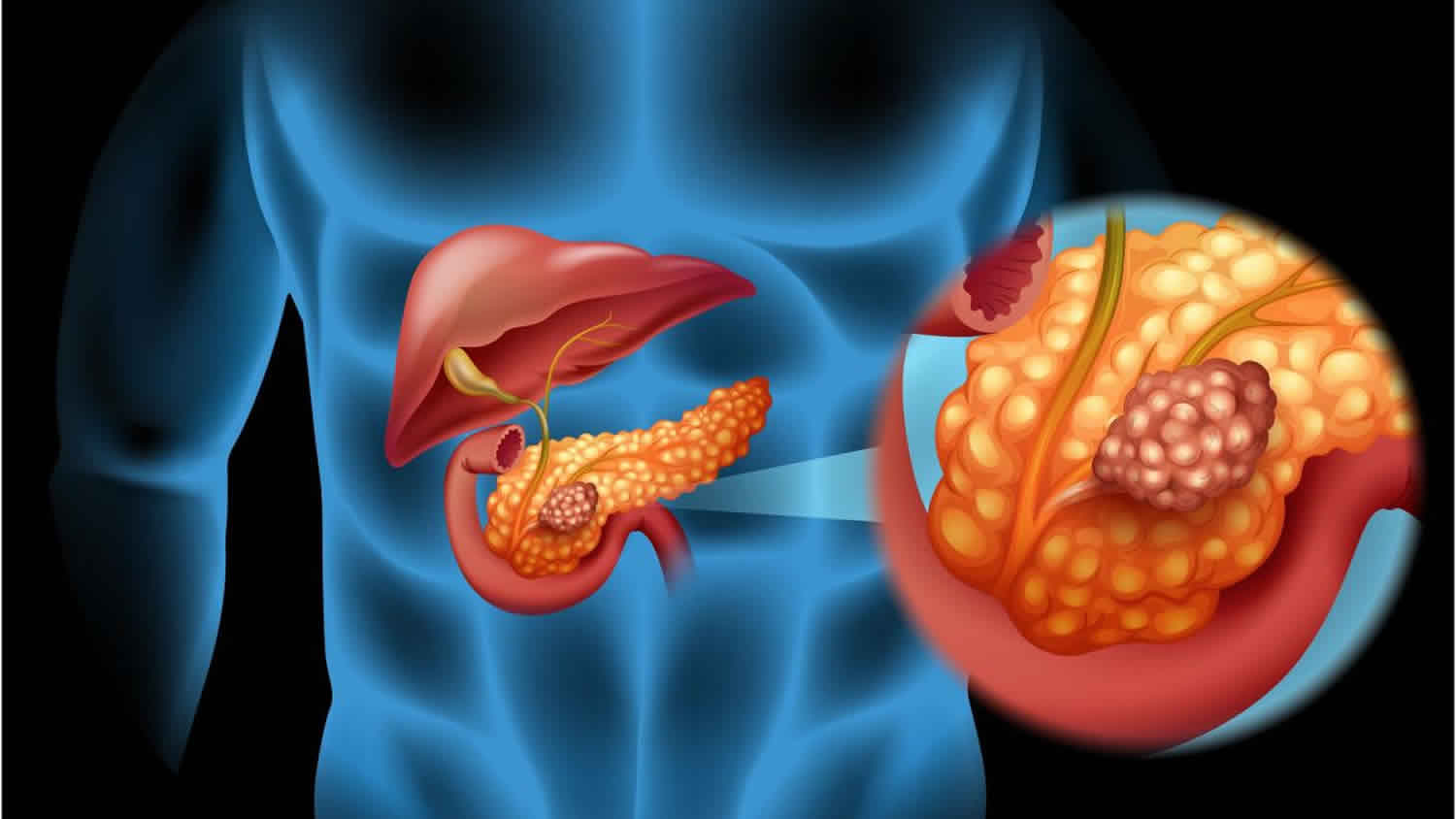
Vipoma
VIPoma is short for vasoactive intestinal peptide tumor also called Verner Morrison syndrome, is a rare type of pancreatic neuroendocrine cancer that secretes vasoactive intestinal peptide (VIP) – a hormone that stimulates the secretion and inhibits the absorption of sodium, chloride, potassium and water within the small intestine, in an unregulated manner 1. Excessive secretion can increase bowel motility and lead to signs and symptoms such as abdominal pain and cramping, severe watery diarrhea, dehydration, flushing of the face, muscle cramps due to low potassium levels (hypokalemia), and weight loss 2. VIPomas can also start in other parts of the body such as the bowel, lungs and liver 3.
All VIPomas are cancers. VIPomas often grow slowly and are diagnosed early. But some people are diagnosed when their cancer has already spread to other parts of the body (secondary tumours or metastases).
The most common places where VIPomas spread to is the liver and lymph nodes.
Around 9 out of every 10 VIPomas (90%) start in the pancreas. More rarely, VIPomas can start in the 3:
- Lungs
- Bowel
- Liver
- Adrenal glands
Werner and Morrison first described VIPoma in 1958 as a pancreatic tumor resulting in watery diarrhea and hypokalemia 4. In 1973, the team of Bloom, Polak, and Pearse confirmed that the mediator was VIP (vasoactive intestinal peptide) 5. The VIPoma syndrome is also known as Verner-Morrison syndrome or WDHA syndrome (watery diarrhea, hypokalemia, and hypochlorhydria or achlorhydria syndrome) and pancreatic cholera syndrome 6.
Verner Morrison syndrome is the name given to the collection of symptoms caused by having high levels of the hormone VIP (vasoactive intestinal peptide). It’s also called:
- VIPoma syndrome
- Pancreatic cholera
- Watery diarrhea, hypokalemia and hypochlorhydria or achlorhydria syndrome (WDHA)
The acronym WDHHA (watery diarrhea [100%], hypokalemia [100%], achlorhydria) and acidosis from bicarbonate wasting is often present for 3 or 4 years before diagnosis, with volumes usually exceeding 6 to 8 liters of stool every 24 hours 7.
VIPomas are very rare. Only around 1 person in every 10 million develop a VIPoma every year 8. Around 2 out of every 100 pancreatic neuroendocrine tumors [NETs] (2%) diagnosed every year are VIPomas.
VIPomas are usually diagnosed in middle-aged adults or in young children 9. VIPomas are often diagnosed in adults, most commonly around age 30 to 50 years and are mostly intra-pancreatic (95%). Women are more likely to be affected than men.
A small proportion of tumors secreting VIP have been reported as colorectal cancer, lung cancer, pheochromocytoma, neurofibroma, and ganglioneuroblastoma. Majority of VIPomas occur as isolated tumors, but in about 5% of patients, they are part of the multiple endocrine neoplasia type 1 (MEN1) syndromes.
In children, they are typically diagnosed between ages of 2 to 4 years. The majority of VIPomas in children are either ganglioneuromas or ganglioneuroblastomas, arising from the neural crest tissue of the sympathetic ganglia, in the mediastinum or retroperitoneum. They may also arise from the adrenal medulla 10.
Tests used to diagnose a VIPoma may include blood tests (including VIP level), imaging studies such as a CT scan or MRI, and examination of a stool sample 11. A serum vasoactive intestinal peptide (VIP) greater than 200 pg/mL is diagnostic 12. VIPomas can generally be easily localized by CT scan. Somatostatin receptor scintigraphy scanning may be a useful adjunct in detecting metastases. Unfortunately, even though these tumors are slow-growing, the majority of VIPomas are metastatic (have spread to other parts of the body) at the time of diagnosis 13.
Management typically first involves treating dehydration with fluids and correcting the imbalance of hormones and other substances (metabolic abnormalities) in the body. Medications such as octreotide and lanreotide can usually stop the diarrhea and inhibit the secretion of VIP 11. Surgery typically follows in order to remove the tumor. When there is no evidence of metastasis, complete removal of the tumor is the only possible cure 2. The majority of metastatic cases will not be cured by surgery, but symptoms may be managed and prolonged survival is often possible due to the slow-growing nature of these tumors. Targeted therapy or chemotherapy may also be used for people with progressive, advanced disease. The median survival of people with VIPomas is 96 months, but long-term survival mainly depends on the tumor grade, staging, and whether all or most of the tumor can be removed 9.
Vipoma causes
The exact cause of VIPomas is not known. VIPoma causes cells in the pancreas to produce a high level of a hormone called vasoactive intestinal peptide (VIP). VIP is a neurohormone produced in the central nervous system as well as in the neurons of the gastrointestinal (GI), respiratory, and urogenital tracts. Vasoactive intestinal peptide (VIP) hormone increases secretions from the intestines. Vasoactive intestinal peptide (VIP) functions as a vasodilator and regulator of smooth muscle activity in the gastrointestinal system, stimulator of water and electrolyte secretion from the intestinal tract, an inhibitor of gastric acid secretion, and promotor of blood flow mainly in the gastrointestinal (GI) tract.
Vipoma symptoms
VIPomas usually make large amounts of a hormone called vasoactive intestinal peptide (VIP). VIP relaxes the muscles in the stomach and bowel. It also helps to control the balance of sugar, salt and water in the gastrointestinal (GI) tract.
Symptoms usually develop slowly. In most people, the tumor has already started to spread to other parts of the body by the time it is diagnosed.
You usually have symptoms caused by the increase in the amount of VIP in your body. Symptoms include loose or water poop (diarrhea) which can be severe.
Children with VIPoma may present with failure to thrive in addition to chronic diarrhea.
Tetany has been reported and is due to hypomagnesemia. If severe, the loss of fluid and electrolytes can result in cardiac arrhythmias, myopathy, tetany and hypovolemic shock.
Symptoms of VIPoma may include any of the following:
- Abdominal pain and cramping
- Diarrhea (watery, and often in large amounts)
- Dehydration
- Flushing or redness of the face
- Muscle cramps due to low blood potassium (hypokalemia)
- Nausea
- Weight loss
Diarrhea
You might have large amounts of watery poop (stools). Some people need to go to the toilet more than 20 times a day. You can have diarrhea even when you haven’t eaten beforehand (fasting).
Between 9 and 10 out of every 10 people (90 and 100%) have diarrhea.
Typically, stool amount exceeds 700 ml per day despite fasting and can exceed over 3000 ml per day in 70% of patients 14. These stools are odorless and tea colored and result in substantial losses of fluid and electrolytes such as potassium.
Dehydration
Dehydration can cause:
- thirst
- dry skin
- a dry mouth
- tiredness
- headaches and dizziness
More than 8 out of every 10 people (more than 80%) have dehydration.
Lethargy, nausea, vomiting, muscle weakness, and cramps which occur due to dehydration and hypokalemia.
A low level of potassium in your blood (hypokalemia)
- This can cause numbness and heart problems such as an irregular heartbeat. Between 8 and 10 out of every 10 people (between 80 and 100%) have low levels of potassium.
Weigh loss
- You might lose weight even if you haven’t changed your diet.
Flushing of the skin
- The skin of your face, neck and chest may look red (flushed).
- This happens in around 2 out of every 10 people (20%).
Flushing has been reported in about 8% to 20% of the patients.
Abdominal pain
- Abdominal pain is usually mild.
Vipoma possible complications
Vipoma complications may include:
- Cancer spread (metastasis)
- Cardiac arrest from low blood potassium level
- Dehydration
Vipoma diagnosis
Your health care provider will perform a physical exam and ask about your medical history and symptoms.
Tests that may be done include:
- Stool examination for cause of diarrhea and electrolyte levels. Diagnosis of VIPoma is made in patients with secretory diarrhea usually greater than 3.0 liters per day with a serum VIP level around 250 to 500 pg/ml (reference range is less than 190 pg/ml). Secretory diarrhea has a low fecal osmotic gap of less than 50 mOsm/kg.
- Blood chemistry tests (basic or comprehensive metabolic panel).
- Electrolyte abnormalities include hypokalemia, hypochlorhydria, hypomagnesemia, hyperglycemia, and hypercalcemia. Hypokalemia and hyperchloremic metabolic acidosis occur due to a large amount of GI loss and bicarbonate wasting. Hypochlorhydria occurs secondary to the direct gastric acid inhibitory effect of VIP. Hypercalcemia may be due to dehydration, coincidental multiple endocrine neoplasia 1 (MEN1) syndrome, or secretion by the tumor of a calcitrophic peptide. Hypomagnesemia may occur from profound diarrhea. Hyperglycemia is due to the increased glycogenolytic activity of VIP.
- Blood tests can check your general health. They can also check the levels of certain substances in the blood which are sometimes raised with neuroendocrine tumors (NETs). You may also have a blood test to check for a rare inherited condition called multiple endocrine neoplasia 1 (MEN1). This test is usually only requested by specialist doctors (genetic doctors).
- VIP level in the blood. It is important to repeat levels of VIP to confirm diagnosis since levels may not be elevated between episodes of watery diarrhea. It is also imperative to determine VIP levels when the patient is symptomatic, as the VIPoma may only secrete VIP intermittently. Hence, a normal level may be a false negative. Among children suspected with VIPoma, catecholamine levels should also need to be measured. Levels of pancreatic polypeptide are elevated in tumors originating from the pancreas.
- CT scan of the abdomen. A CT scan can show up a neuroendocrine tumor (NET) and see whether it has spread anywhere else in the body.
- MRI of the abdomen. An MRI scan takes detailed pictures of the body. You might have an MRI scan to check if the VIPoma has spread to other parts of the body such as the liver and lymph nodes.
- Radioactive scans. Somatostatin receptor scintigraphy using radiolabeled somatostatin analog octreotide or lanreotide has the advantage of detecting small, occult metastases within and outside of the abdomen. These are octreotide scans (or octreoscans) or gallium PET scans. The FDA approves functional PET imaging technique with 68-Ga DOTATATE injection as the radioactive diagnostic agent for detection of somatostatin receptor positive neuroendocrine tumors in both adult and pediatric patients. You have an injection of a low dose radioactive substance, which is taken up by some vipoma cells. The vipoma cells then show up on the scan.
- Endoscopy. This test looks at the inside of your food pipe, stomach and bowel. Your doctor uses a long flexible tube which has a tiny camera and a light on the end of it. Doctors can take samples of any abnormal areas (biopsies).
- Endoscopic ultrasound scan (EUS).This test combines an ultrasound and endoscopy to look at the inside at your food pipe, stomach, pancreas and bile ducts. Your doctor uses a long flexible tube (endoscope) with a tiny camera and light on the end. It also has an ultrasound probe. The ultrasound helps the doctor find areas that might be cancer. They then can take samples (biopsies) of any abnormal areas.
Vipoma treatment
VIPoma treatment you have depends on a number of things. This includes where the tumor is, its size and whether it has spread (the stage). The best chance of a cure is surgery to remove the tumor. If the tumor has not spread to other organs, surgery can often cure the condition.
Initial management
The first treatment you’ll have is to control the diarrhea and replace the fluids and minerals you may have lost. Fluids are often given through a vein (intravenous fluids) to replace fluids lost through diarrhea. You then might have surgery to try to get rid of the tumour. But surgery isn’t always possible. Some VIPomas may have already started to spread when you are diagnosed. Or you might not be well enough to have an operation. You continue to have treatment to help your symptoms if surgery isn’t an option.
Drips to replace fluid and minerals
- You usually have a drip into your bloodstream to replace the fluid, potassium and other minerals you might have lost because of the diarrhoea.
- Exactly what you need depends on the potassium and mineral levels in your blood, and how dehydrated you are.
The next goal is to slow the diarrhea. Medicines can help control diarrhea. One such medicine is octreotide. It is a manmade form of a natural hormone that blocks the action of VIP.
Somatostatin analogues
Somatostatin is a protein made naturally in the body. It does several things including slowing down the production of hormones. Somatostatin analogues are man made versions of somatostatin.
You may have somatostatin analogues to try to slow down the tumour and help with symptoms. The most common drugs used are:
- Octreotide (Sandostatin). Octreotide is usually started at 50 to 100 mcg subcutaneous every 8 hours and titrated for symptom control. A long-acting formulation of octreotide, Sandostatin LAR is initiated at a dose of 20 mg intramuscularly (IM) monthly and titrated as needed for optimal symptom control.
- Lanreotide (Somatuline)
Octreotide helps to improve the symptoms of diarrhoea in between 8 and 9 out of every 10 people (between 80 and 90%) with VIPoma.
Glucocorticoids are used in patients refractory to somatostatin analogs 15.
Vipoma surgery
Surgery is the main treatment for VIPomas. Removing part of the tumour can reduce your symptoms. Your doctor will only suggest surgery if they think it’s possible to remove most of the tumour (at least 90%).
There are different types of operations depending on where the tumor is.
Some of these are major operations and there are risks. But if the aim is to try to cure the VIPoma, you might feel it is worth some risks. Talk to your doctor about the risks and benefits of your surgery.
You usually have open surgery. This means your surgeon makes a large cut in your tummy (abdomen) to remove the tumour. During the operation, they might also remove the nearby lymph nodes if they think your tumour is malignant (cancer).
If your VIPoma has spread to the liver, you might be able to have the liver tumour removed at the same time you have the main surgery. Your surgeon may remove just the tumor, or part of the liver too.
As per recent National Comprehensive Cancer Network (NCCN) guidelines, the post-resection follow up includes history and physical examination, multiphasic CT or MRI and serum VIP level in the initial 3 to 12 month period. After 1 year, it is recommended to follow the same measures every 6 to 12 months.
For VIPoma that started in the pancreas
You might have surgery to remove:
- just the tumour (enucleation)
- the narrowest part of the pancreas and the body of the pancreas (distal pancreatectomy)
- the whole of the pancreas (total pancreatectomy)
- the widest part of the pancreas, the duodenum, gallbladder and part of the bile duct (pylorus preserving pancreaticoduodenectomy or PPPD for short)
- the widest part of the pancreas, duodenum, gallbladder, part of the bile duct and part of the stomach (Whipple’s operation)
Radiofrequency ablation
Radiofrequency ablation uses heat made by radio waves to kill tumour cells. You might have this if the vipoma has spread to the liver.
Trans arterial embolisation
You might have this treatment if the vipoma has spread to the liver.
Trans arterial embolisation (TAE) means having a substance such as a gel or tiny beads to block the blood supply to the liver vipoma. It is also called hepatic artery embolisation.
You may also have a chemotherapy drug to the liver at the same time. This is called trans arterial chemoembolisation (TACE). But doctors don’t know for sure whether adding chemotherapy is better than having embolisation alone for vipomas that have spread to the liver.
Embolization and chemoembolisation work in two ways:
- it reduces the blood supply to the tumor and so starves it of oxygen and the nutrients it needs to grow
- it gives high doses of chemotherapy to the tumour without affecting the rest of the body
For VIPoma that started in the bowel
For a VIPoma in your bowel, your surgeon removes the tumour and checks for other tumours. You may need to have part of the bowel removed.
For VIPoma that started in the lungs
For a VIPoma in your lung, your surgeon removes the tumour and checks for other tumours. You might just have the tumour removed. Or you may need to have part of the lung removed.
Targeted drugs
Cancer cells have changes in their genes (DNA) that make them different from normal cells. These changes mean that they behave differently. Targeted drugs work by ‘targeting’ the differences that a cancer cell has and destroying them.
You may have 2 types of targeted drugs called everolimus and sunitinib if your VIPoma has started in the pancreas.
Interferon
Interferon is also called interferon alfa. You may have it if the VIPoma has spread to other parts of the body. And other treatments have stopped working.
You may have interferon alone or together with somatostatin analogues.
Radiotherapy
You may have a type of internal radiotherapy called peptide receptor radionuclide therapy (PRRT). Internal radiotherapy means having radiotherapy from inside the body (as a drip into your bloodstream).
Peptide receptor radionuclide therapy (PRRT) uses a radioactive substance called lutetium-177 or yttrium-90 attached to a somatostatin analogue.
You may have peptide receptor radionuclide therapy (PRRT) if:
- your vipoma has spread to other parts of the body
- you can’t have surgery
- your vipoma has receptors on the outside of them called somatostatin receptors (you have special scans called octreotide or gallium PET scans to check for this)
Chemotherapy
Chemotherapy uses anti cancer (cytotoxic) drugs to destroy tumour cells. You may have chemotherapy if the vipoma has spread to the liver or to other parts of your body.
The most common chemotherapy drugs used are:
- streptozotocin or temozolomide
- fluorouracil or capecitabine
- doxorubicin
Liver transplant
You may have a liver transplant if the vipoma has only spread to the liver and you’re fit and healthy. But a liver transplant might not be possible even if your doctor thinks you can have it. This is because you need a donor liver that is a close match to yours.
A liver transplant is a major operation and it has many risks. It is rarely used as a treatment for vipomas.
Vipoma survival rate
Because VIPomas are rare tumours, the survival of this disease is harder to estimate than for other, more common cancers. There are no survival statistics for VIPomas. It is difficult to collect data because VIPomas are so rare.
Survival depends on many factors. It depends on the stage and grade of the tumor when it was diagnosed. The stage describes the size of the tumor and whether it has spread. The grade means how abnormal the cells look under a microscope.
Another factor is how well you are overall.
These are general statistics based on small groups of people. Remember, they can’t tell you what will happen in your individual case. Your doctor can give you more information about your own outlook (prognosis).
An American study looked at the average length of time from either diagnosis or treatment, that people with VIPoma lived for. This is called overall survival. This study found that people with VIPoma live about 8 years on average after being diagnosed. The median survival of patients with VIPoma is 96 months 16. This means that a lot of people with VIPoma live for 8 years or more.
- Sandhu S, Jialal I. ViPoma. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507698[↩]
- Pancreatic neuroendocrine tumors: VIPoma. http://endocrinediseases.org/neuroendocrine/vipoma.shtml[↩][↩]
- VIPoma. https://about-cancer.cancerresearchuk.org/about-cancer/neuroendocrine-tumours-nets/pancreatic-nets/vipoma[↩][↩]
- VERNER JV, MORRISON AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am. J. Med. 1958 Sep;25(3):374-80[↩]
- Bloom SR, Polak JM, Pearse AG. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973 Jul 07;2(7819):14-6.[↩]
- Vinik A. Vasoactive Intestinal Peptide Tumor (VIPoma) In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. MDText.com, Inc.; South Dartmouth (MA): Jun 5, 2017[↩]
- Vinik A. Vasoactive Intestinal Peptide Tumor (VIPoma) [Updated 2017 Jun 5]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278960[↩]
- Dimitriadis GK, Weickert MO, Randeva HS, et al. Medical management of secretory syndromes related to gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2016; 23:R423.[↩]
- VIPoma: Clinical manifestations, diagnosis, and management. https://www.uptodate.com/contents/vipoma-clinical-manifestations-diagnosis-and-management[↩][↩]
- Belei OA, Heredea ER, Boeriu E, Marcovici TM, Cerbu S, Mărginean O, Iacob ER, Iacob D, Motoc AGM, Boia ES. Verner-Morrison syndrome. Literature review. Rom J Morphol Embryol. 2017;58(2):371-376.[↩]
- VIPoma. https://medlineplus.gov/ency/article/000228.htm[↩][↩]
- Davies K, Conlon KC: Neuroendocrine tumors of the pancreas. Curr Gastroenterol Rep 11 (2): 119-27, 2009.[↩]
- Perry RR, Vinik AI. Clinical review 72: diagnosis and management of functioning islet cell tumors. J. Clin. Endocrinol. Metab. 1995 Aug;80(8):2273-8[↩]
- Grier JF. WDHA (watery diarrhea, hypokalemia, achlorhydria) syndrome: clinical features, diagnosis, and treatment. South. Med. J. 1995 Jan;88(1):22-4.[↩]
- O’Dorisio TM, Mekhjian HS, Gaginella TS. Medical therapy of VIPomas. Endocrinol. Metab. Clin. North Am. 1989 Jun;18(2):545-56.[↩]
- Roland CL, Bian A, Mansour JC, Yopp AC, Balch GC, Sharma R, Xie XJ, Schwarz RE. Survival impact of malignant pancreatic neuroendocrine and islet cell neoplasm phenotypes. J Surg Oncol. 2012 May;105(6):595-600.[↩]