What is Lectin ?
Lectins are carbohydrate-binding proteins that are widely distributed in seeds and vegetative parts of edible plant species that play vital roles in many biological processes 1. Lectins have been reported in animals, plants, fungi, bacteria, and viruses, but the most well-known lectins are found in leguminous seeds 2. Lectins are responsible for innate immunity and defence mechanisms in plants 3. Because research into lectin is still in its infancy, there is some confusion with the definition of lectin. According to the National Cancer Institute, lectin is a complex molecule that has both protein and sugars 4. Lectins are able to bind to the outside of a cell and cause biochemical changes in it. The other more commonly found definition of lectins is: “Lectins are defined as proteins that preferentially recognize and bind carbohydrate complexes protruding from glycolipids and glycoproteins” 5, 6, 7, 8. In other words, lectins are carbohydrate-binding proteins, macromolecules that are highly specific for sugar moieties. Currently, lectins are defined as carbohydrate binding proteins of non-immune origin that can recognize and bind simple or complex carbohydrates in a reversible and highly specific manner 2. Lectins are a diverse group of proteins that are attracted to carbohydrates and biomolecules that contain carbohydrate groups, including varieties of sugars and sugar-modified proteins and lipids. This attraction, which is highly specific, allows lectins to influence a wide range of biological processes. For example, many bacterial and mammalian lectins control how cells move and adhere to each other 9, while some plant lectins, notably ricin and abrin, are renowned for their lethality 10.
Lectins are universally distributed in nature, being established in plants, fungi, viruses, bacteria, crustacea, insects, and animals, but legumes plants are rich source of lectins. At present, a few lectins from different fruits and vegetables have been recognized unambiguously as potential allergens by the World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee 11. For exmple, wheat agglutinin, hevein (Hev b 6.02) from the rubber tree and chitinases containing a hevein domain from different fruits and vegetables are potential food allergens 12. Moreover, other well-known lectins from legumes have been demonstrated to behave as potential food allergens taking into account their ability to specifically bind IgE from allergic patients, trigger the degranulation of sensitized basophils, and to elicit interleukin secretion in sensitized people. These allergens include members from the different families of higher plant lectins, including legume lectins, type II ribosome-inactivating proteins (RIP-II), wheat germ agglutinin (WGA), jacalin-related lectins, GNA (Galanthus nivalis agglutinin)-like lectins, and Nictaba-related lectins. Most of these potentially active lectin allergens belong to the group of seed storage proteins (legume lectins), pathogenesis-related protein family PR-3 comprising hevein and class I, II, IV, V, VI, and VII chitinases containing a hevein domain, and type II ribosome-inactivating proteins containing a ricin B-chain domain (RIP-II) 12.
Table 1. Listing of plant lectins
| Lectin icon | Lectin name | Source | |
|---|---|---|---|
| ConA | Concanavalin A | Jack bean (Canavalia ensiformis) | |
| GNA | Snowdrop lectin | Snowdrop (Galanthus nivalis) | |
| RCA | Ricinus communis Agglutinin, | Castor bean or castor oil plant (Ricinus communis) | |
| AIL | Jacalin | Jack fruit (Artocarpus integrifolia) | |
| VVL | Hairy vetch lectin | Hairy vetch (Vicia villosa) | |
| WGA | Wheat Germ agglutinin | Wheat (Triticum vulgaris) | |
| SNA | Elderberry lectin | Black elderberry (Sambucus nigra) | |
| MAL | Maackia amurensis leukoagglutinin | Amur maackia (Maackia amurensis) | |
| MAH | Maackia amurensis hemoagglutinin | Maackia amurensis | |
| UEA | Ulex europaeus agglutinin | Gorse (Ulex europaeus) | |
| PNA | Peanut agglutinin | Peanut (Arachis hypogaea) | |
| LCH | Lentil lectin | Lentil (Lens culinaris) | |
| GalPhL | Galactose specific lectin | Lima bean (Phaseolus lunatus) | |
| ManPSL | Mannose specific lectin | Peas (Pisum sativum) | |
| GalDBL | Galactose specific lectin | Horse gram (Dolichos biflorus) | |
| GalCaL | Galactose specific lectin | Siberian peashrub (Caragana arborescens) | |
In 1919, James B. Sumner at Cornell University, well known for being the first to crystallize in 1926 an enzyme, urease, isolated from jack bean (Canavalia ensiformis) a crystalline protein that he named concanavalin A and in this way obtained a pure hemagglutinin for the first time. However, nearly two decades passed before Sumner and Howell (1936) reported that concanavalin A agglutinates cells such as erythrocytes (red blood cells) and yeasts and also precipitates glycogen from solution 14. They further showed that hemagglutination by concanavalin A was inhibited by sucrose, demonstrating for the first time the sugar specificity of lectins. With much foresight, they suggested that the hemagglutination induced by concanavalin A might be a consequence of a reaction of the plant protein with carbohydrates on the surface of the red blood cells. The pioneering work of Watkins and Morgan found that the agglutination of type A red blood cells by lima bean lectin was best inhibited by α-linked N-acetyl-D-galactosamine and that of type O cells by the lectin of L. tetragonolobus was best inhibited by α-linked L-fucose. They provided the earliest evidence for the presence of sugars on cell surfaces and their potential roles as identity markers 14.
Moreover, the ability of plant agglutinins to distinguish between erythrocytes of different blood types led Boyd and Shapleigh (1954) to propose for them the name lectins, from the Latin word legere meaning “to choose” or “select”. This term was generalized to embrace all sugar-specific agglutinins of nonimmune origin, irrespective of source and blood type specificity 14. The interaction of lectins with particular carbohydrates can be as specific as the interaction between those of antigen-antibody or substrate-enzyme 8. Lectins bind not only to oligosaccharides (a carbohydrate whose molecules are composed of a relatively small number of monosaccharide units) on cells but also to free-floating glycans (compounds consisting of a large number of monosaccharides linked glycosidically) including monosaccharides. Lectin-monosaccharide interactions, however, are relatively weak with dissociation constants often on the order of micromolar to millimolar range 15, 16.
Two major discoveries made in the early 1960s were instrumental in bringing lectins into the limelight. The first of these was by Peter C. Nowell (1960), who found that the lectin of the red kidney bean (Phaseolus vulgaris), known as phytohemagglutinin, is mitogenic, that is, it possesses the ability to stimulate lymphocytes to undergo mitosis (the process by which the nucleus divides in eukaryotic organisms, producing two new nuclei that are genetically identical to the nucleus of the parent cell) 17. Within a short time, several other lectins were proven to be mitogenic. Of special significance was the finding that concanavalin A acts as a mitogen because, in contrast to phytohemagglutinin, its activity could be inhibited by low concentrations of monosaccharides, for example, mannose. This finding provided proof that mitogenic stimulation is the result of binding of lectins to sugars on the surface of the lymphocytes and was among the earliest demonstrations for a biological role of cell surface sugars. Mitogenic lectins soon became tools for the study of signal transmission into cells and for the analysis of the biochemical events that occur during lymphocyte stimulation in vitro. A most valuable outcome of such studies was the discovery in the 1970s by Robert C. Gallo and his associates at the National Institutes of Health of T cell growth factor, now known as interleukin-2, in conditioned medium of normal human lymphocytes stimulated by phytohemagglutinin 18. The second discovery was made by Joseph C. Aub at the Massachusetts General Hospital in Boston. He found that wheat germ agglutinin has the ability to preferentially agglutinate malignant cells. This was followed by the reports of Max M. Burger at Princeton University and Leo Sachs and Michael Inbar at the Weizmann Institute that concanavalin A exhibits the same ability. Together with Sachs and Ben-Ami Sela subsequently found that soybean agglutinin also possesses the same property 14.
Although some lectins are polyclonal activators both in vitro and in vivo, others may display a broad range of activities toward human lymphocytes. Indeed, the same lectin (e.g. wheat germ agglutinin or Datura lectin ) may be mitogenic, comitogenic, or antimitogenic, depending on the experimental conditions. An individual lectin may bind to several glycoproteins on the lymphocyte surface, resulting in interactions that may or may not be functionally relevant, and that may have opposing effects. Studies with lectins and with monoclonal antibodies (MAbs) have established that a surprisingly large variety of cell-surface molecules can influence the initiation and regulation of lymphocyte activation and proliferation 17.
Numerous studies concerning the biological activity of plant lectins have reported that these substances possess toxic, cytotoxic, antitumor, and anticarcinogenic properties 19.
Sources of Lectins
Lectins were initially found and described in plants, but in subsequent years multiple lectins were isolated from microorganisms and also from animals 14. Interestingly plant and animal lectins show no primary structural homology, yet they demonstrate similar preferential binding to carbohydrates. This suggests that animal and plant lectin genes may have co-evolved, thus highlighting the importance of lectin-carbohydrate interactions in living systems 6. During the past several years, however, many primary and three dimensional structures of lectins have been elucidated. It was observed that lectins from diverse sources lacked primary sequence similarity but shared similarities in their tertiary structures 14. Structural studies conducted on animal lectins suggested that the carbohydrate binding activity of most lectins was generated by limited amino acid residues designated as the carbohydrate recognition domain 14. The carbohydrate recognition domain typically recognizes the terminal non-reducing carbohydrate residues of cell membrane glycoproteins and glycolipids 5. Lectin carbohydrate recognition domains also may discriminate between anomeric isomers as a function of their specificities. For example, the lectin concanavalin A (Con A) specifically binds the α-anomer of glucose and mannose, but not the β-anomer of either 5.
Lectin families
Within the animal lectins, several highly conserved carbohydrate recognition domain amino acid sequences have been identified, thus allowing investigators to categorize the majority of these lectins into structurally related families and superfamilies 14. C-type lectins are the most abundant of all animal lectins, and the C-type lectins superfamily is grouped into three families: selectins, collectins and endocytic lectins 14, 20. A majority of C-type lectins are large, asymmetric, have one or more carbohydrate recognition domains and exist as Ca2+ (calcium) dependent proteins found in secreted or bound forms 6, 14. In contrast, the S-type lectins (galectins) in the CTL superfamily are generally small, non-glycosylated, soluble and exist as Ca2+ independent proteins found intracellularly and extracellularly (Drickamer and Taylor, 1993; Barondes et al., 1994; Drickamer, 1995; Cooper and Barondes, 1999; Minko, 2004; Sharon and Lis, 2004; Chou et al., 2009; Malik et al., 2009; Saravanan et al., 2009). Currently at least ten galectins have been identified and all bind N-acetyllactosamine (Gal-β-1-nGlcNAc-R) by recognizing the β-gal residue (Gorelik et al., 2001). The collectin family of CTLs includes collagenous lectins such as mannose binding proteins (MBPs), pulmonary surfactant SP-A and SP-D and conglutinin (Gorelik et al., 2001; Kerrigan and Brown, 2009; Ruseva et al., 2009). The number of C-type lectins has reached 50 and at least 10 galectins have been identified. The selectin family of C-type lectins includes the E-, L- and P-selectins. These selectins have a single epidermal growth like domain, an extracellular carbohydrate recognition domain, a cytoplasmic tail, a transmembrane domain, and two to nine short consensus repeat units that are homologous to complement binding proteins 21, 22. Selectins specifically bind oligosaccharides such as sLea and sLex or their sulfated equivalents 22. Another lectin family of special interest is the siglecs. The siglecs are sialic acid binding Ig-like lectins and belong to the Ig superfamily. They carry unique expression patterns in different cells, indicating that they are involved in highly specialized and specific cellular processes 23.
Table 2. Major Lectins
| Table of the major lectins | |||||
|---|---|---|---|---|---|
| Lectin Symbol | Lectin name | Source | ligand motif | ||
| Mannose binding lectins | |||||
| ConA | Concanavalin A | Canavalia ensiformis | α-D-mannosyl and α-D-glucosyl residues branched α-mannosidic structures (high α-mannose type, or hybrid type and biantennary complex type N-Glycans) | ||
| LCH | Lentil lectin | Lens culinaris | Fucosylated core region of bi- and triantennary complex type N-Glycans | ||
| GNA | Snowdrop lectin | Galanthus nivalis | α 1-3 and α 1-6 linked high mannose structures | ||
| Galactose / N-acetylgalactosamine binding lectins | |||||
| RCA | Ricin, Ricinus communis Agglutinin, RCA120 | Ricinus communis | Galβ1-4GalNAcβ1-R | ||
| PNA | Peanut agglutinin | Arachis hypogaea | Galβ1-3GalNAcα1-Ser/Thr (T-Antigen) | ||
| AIL | Jacalin | Artocarpus integrifolia | (Sia)Galβ1-3GalNAcα1-Ser/Thr (T-Antigen) | ||
| VVL | Hairy vetch lectin | Vicia villosa | GalNAcα-Ser/Thr (Tn-Antigen) | ||
| N-acetylglucosamine binding lectins | |||||
| WGA | Wheat Germ Agglutinin, WGA | Triticum vulgaris | GlcNAcβ1-4GlcNAcβ1-4GlcNAc, Neu5Ac (sialic acid) | ||
| N-acetylneuraminic acid binding lectins | |||||
| SNA | Elderberry lectin | Sambucus nigra | Neu5Acα2-6Gal(NAc)-R | ||
| MAL | Maackia amurensis leukoagglutinin | Maackia amurensis | Neu5Ac/Gcα2,3Galβ1,4Glc(NAc) | ||
| MAH | Maackia amurensis hemoagglutinin | Maackia amurensis | Neu5Ac/Gcα2,3Galβ1,3(Neu5Acα2,6)GalNac | ||
| Fucose binding lectins | |||||
| UEA | Ulex europaeus agglutinin | Ulex europaeus | Fucα1-2Gal-R | ||
| AAL | Aleuria aurantia lectin | Aleuria aurantia | Fucα1-2Galβ1-4(Fucα1-3/4)Galβ1-4GlcNAc, R2-GlcNAcβ1-4(Fucα1-6)GlcNAc-R1 | ||
What does lectin do?
Lectins perform recognition on the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins 25, 26. Lectins also mediate attachment and binding of bacteria and viruses to their intended targets.
Endogenous lectins are involved in an enormous variety of biological processes as indicated by an increasing volume of data concerning them 5, 27, 28, 29, 30. A complete and in-depth discussion of the biological significance of lectins is not within the scope of this brief review, however, a discussion of a few mammalian system examples is warranted. Endogenous lectins mediate biological processes such as cell-cell self recognition, cell-extracellular matrix interactions, gamete fertilization, embryonic development, cell growth, cell differentiation, cell signaling, cell adhesion and migration, apoptosis, immunomodulation and inflammation, host-pathogen interactions, glycoprotein folding and routing, mitogenic induction and homeostasis 31, 32, 20, 33, 34, 23.
Lectins are ubiquitous in nature and are found in many foods. Some foods such as beans and grains need to be cooked or fermented to reduce lectin content, but the lectins consumed in a typical balanced diet are not harmful. Some lectins are beneficial, such as CLEC11A which promotes bone growth, while others may be powerful toxins such as ricin 35.
Lectins may be disabled by specific mono- and oligosaccharides, which bind to ingested lectins from grains, legume, nightshade plants and dairy; binding can prevent their attachment to the carbohydrates within the cell membrane. The selectivity of lectins means that they are very useful for analyzing blood type, and they are also used in some genetically engineered crops to transfer traits, such as resistance to pests and resistance to herbicides.
Recently, direct evidence that changes in cell surface carbohydrates are important for the metastatic behavior of tumor cells 6. Cell surface carbohydrates affect tumor cell interactions with normal cells or with the extracellular matrix during metastatic spread and growth 6. These interactions can be mediated via tumor cell carbohydrates and their binding proteins known as endogenous lectins 6. The family of the discovered endogenous lectins is rapidly expanding. The biological significance of the endogenous lectins and their possible role in tumor growth and metastasis formation has started to unravel. Some lectins recognize the ‘foreign’ patterns of cell surface carbohydrates expressed by microorganisms and tumor cells, and play a role in innate and adaptive immunity 6. It was shown that lectins affect tumor cell survival, adhesion to the endothelium or extracellular matrix, as well as tumor vascularization and other processes that are crucial for metastatic spread and growth 6.
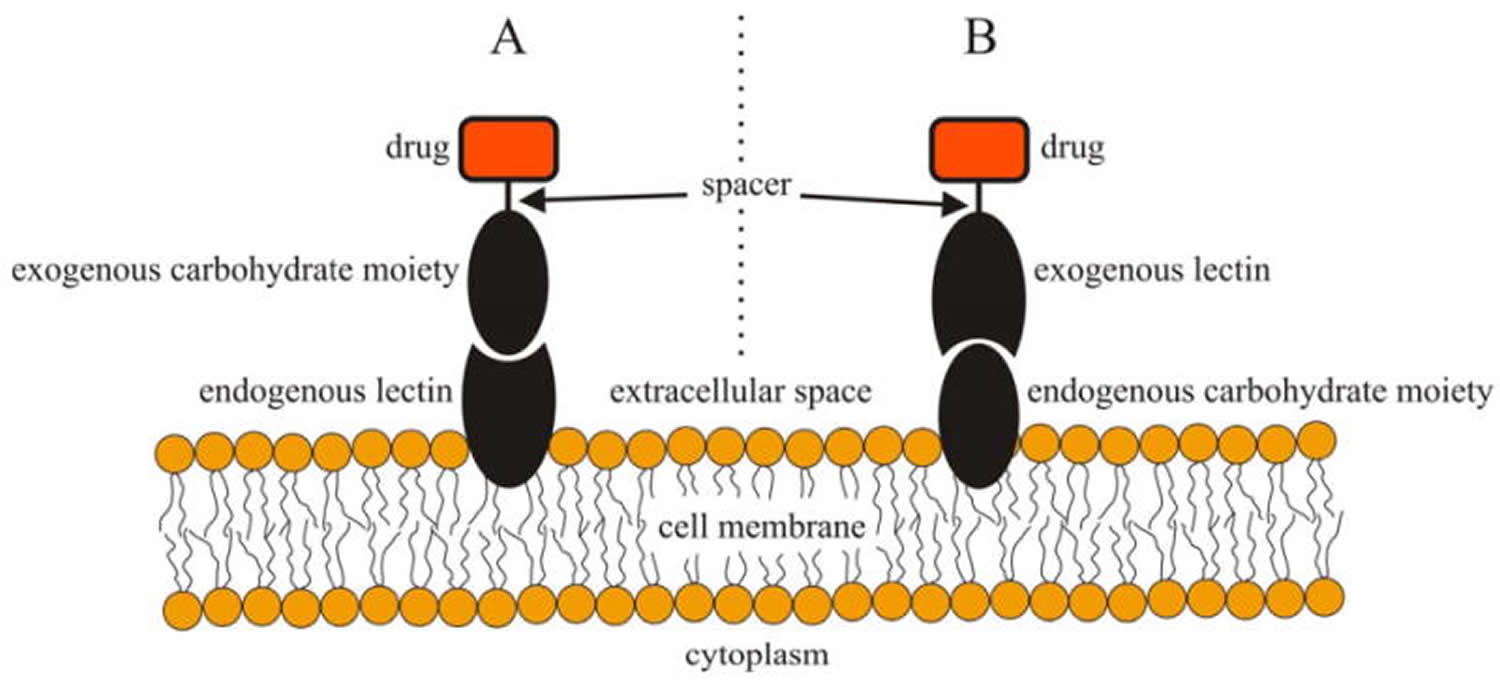
Figure 1. Schematic illustration of direct lectin targeting (A, left) and reverse lectin targeting (B, right).
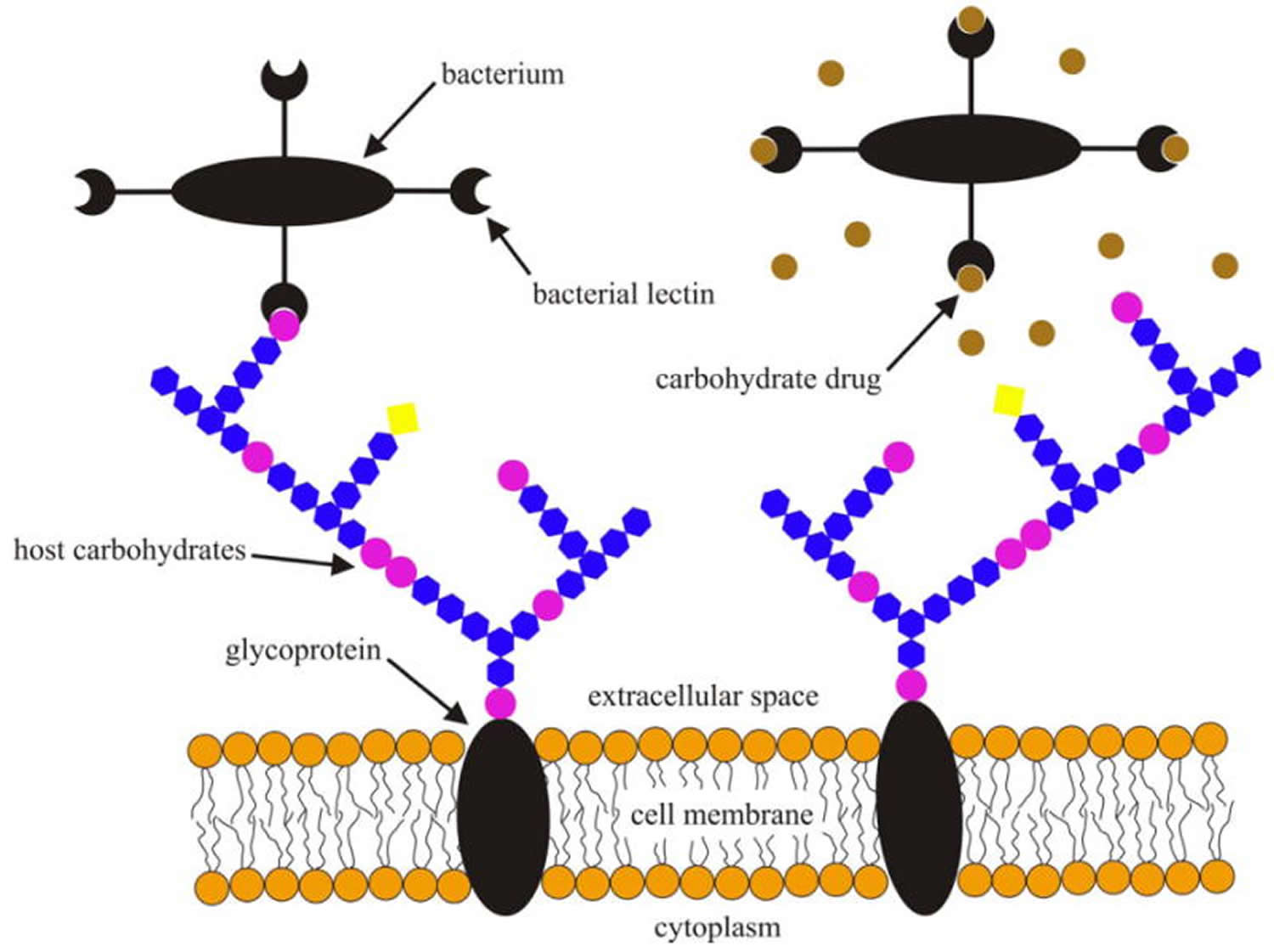
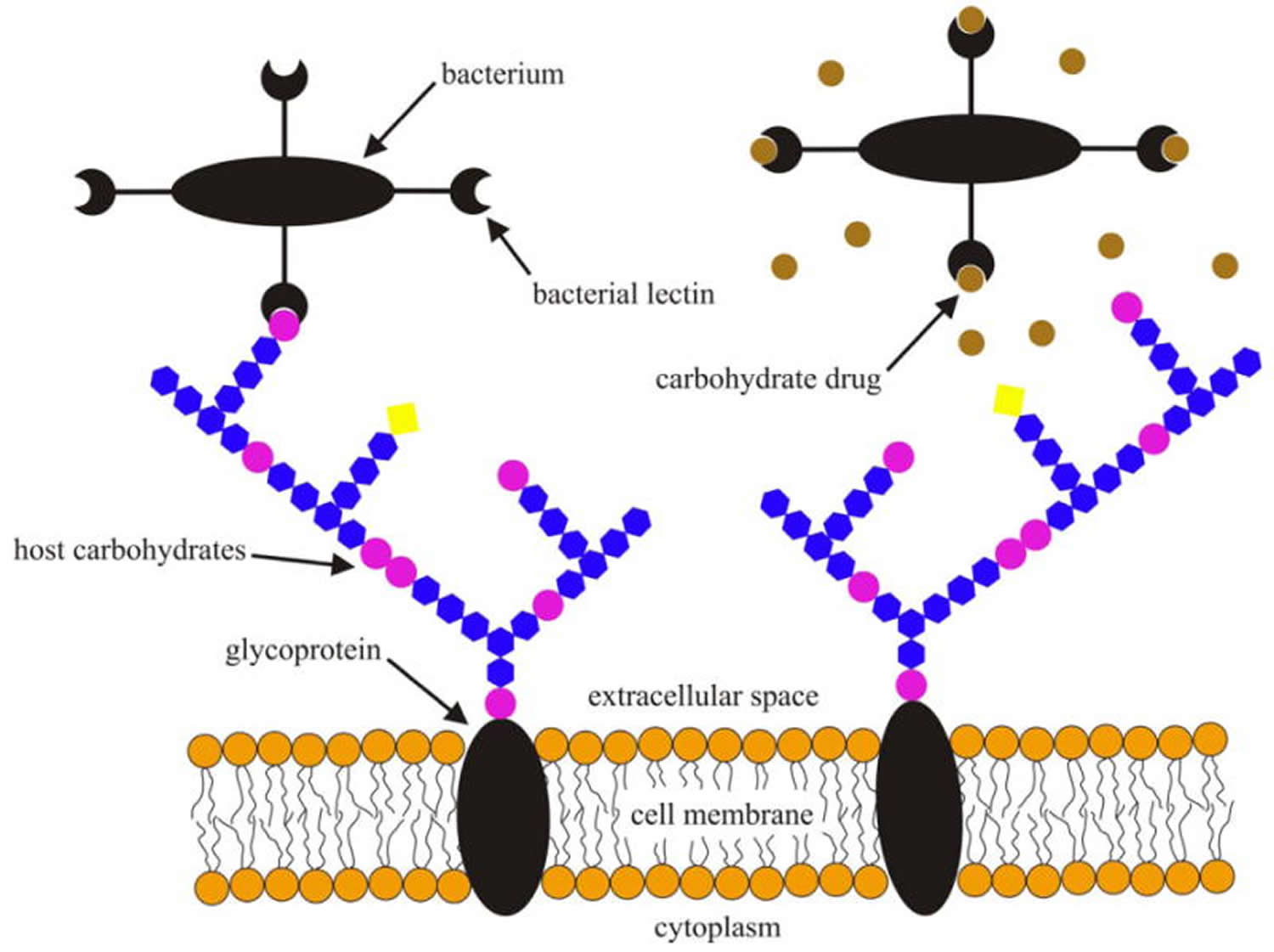
[Source 36]Figure 2. Schematic illustration of bacterial lectins binding to cell surface glycans of a host cell prior to infection (left) and specific carbohydrates or their analogs interfering with the bacterial lectin-host carbohydrate interactions (right).
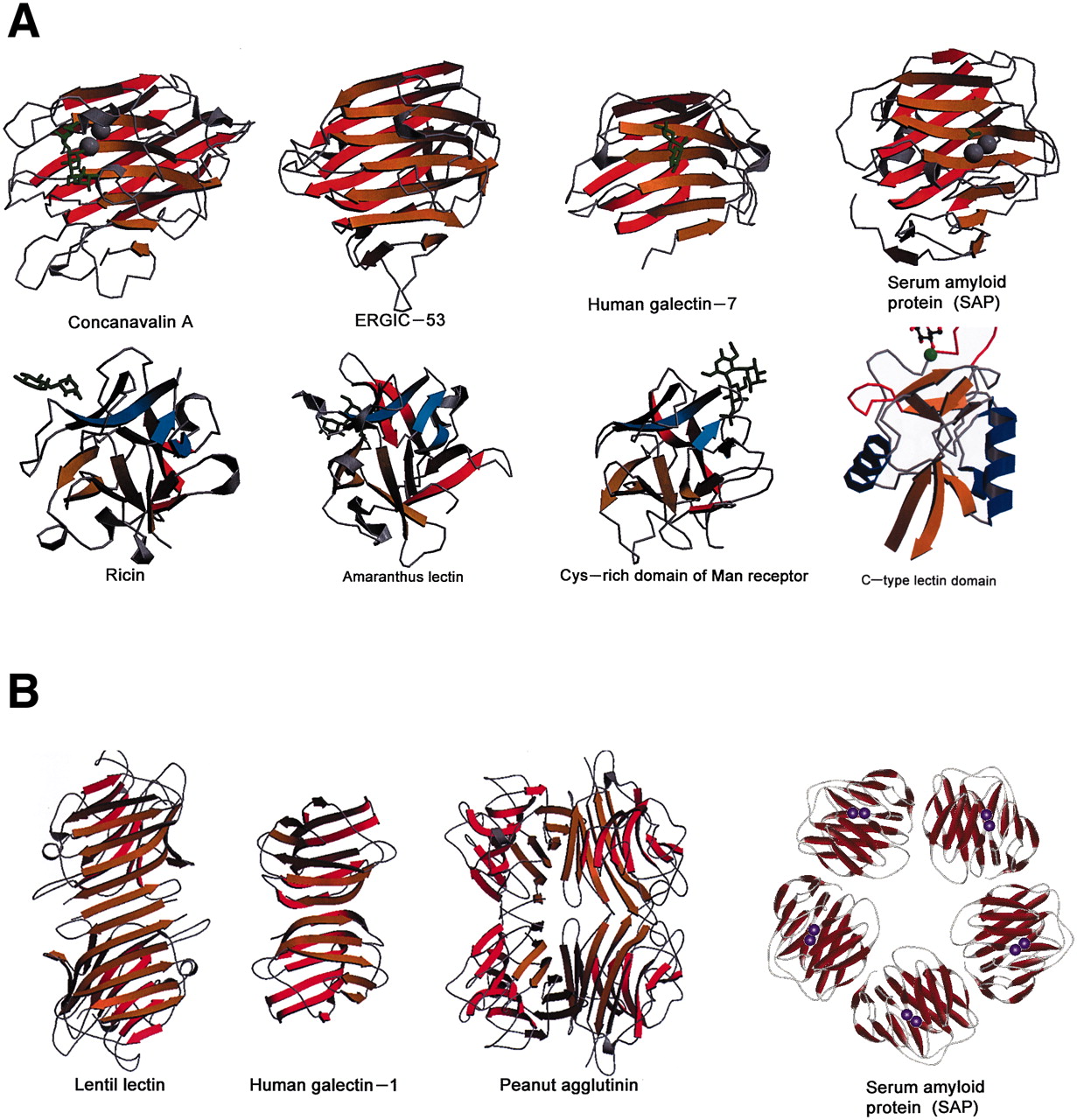
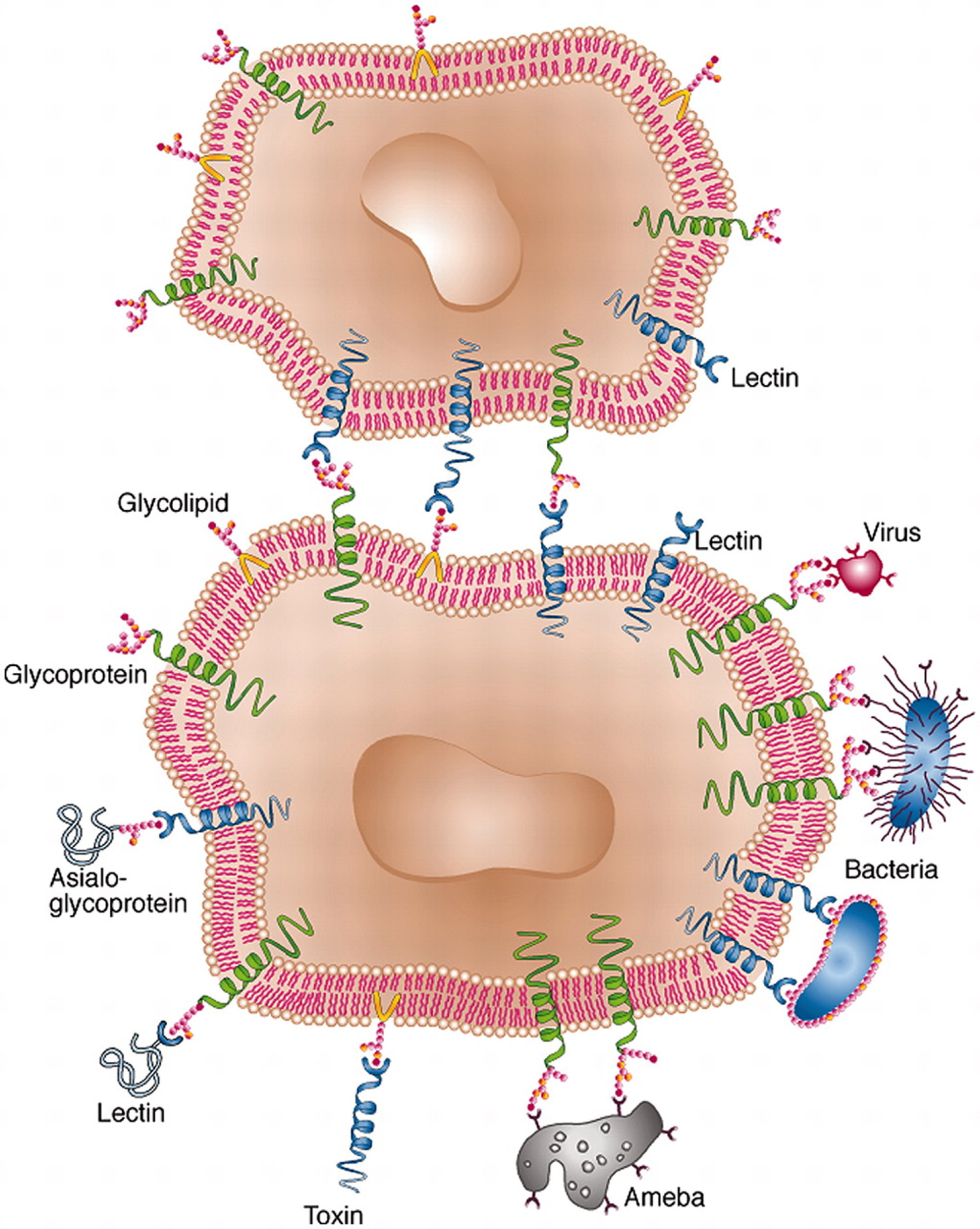
[Source 36]Figure 3. Cell surface lectin–carbohydrate interactions.
[Source 14]Note: Lectins serve as means of attachment of different kinds of cell as well as viruses to other cells via the surface carbohydrates of the latter. In some cases, cell-surface lectins bind particular glycoproteins (e.g., asialoglycoproteins), whereas in other cases the carbohydrates of cell surface glycoproteins or glycolipids serve as sites of attachment for biologically active molecules that themselves are lectins (e.g. carbohydrate-specific bacterial and plant toxins, or galectins).
Table 3. Functions of Lectins
| Lectin | Role in | |
|---|---|---|
| Microorganisms | ||
| Amoeba | Infection | |
| Bacteria | Infection | |
| Influenza virus | Infection | |
| Plants | ||
| Various | Defense | |
| Legumes | Symbiosis with nitrogen-fixing bacteria | |
| Animals | ||
| Calnexin, calreticulin, ERGIC-53 | Control of glycoprotein biosynthesis | |
| Collectins | Innate immunity | |
| Dectin-1 | Innate immunity | |
| Galectins | Regulation of cell growth and apoptosis; regulation of the cell cycle; modulation of cell–cell and cell–substratum interactions | |
| Macrophage mannose receptor | Innate immunity; clearance of sulfated glycoprotein hormones | |
| Man-6-P receptors | Targeting of lysosomal enzymes | |
| L-selectin | Lymphocyte homing | |
| E- and P-selectins | Leukocyte trafficking to sites of inflammation | |
| Siglecs | Cell-cell interactions in the immune and neural system | |
| Spermadhesin | Sperm-egg interaction | |
Table 4. Major Uses of Lectins
| Cell identification and separation |
|---|
| Detection, isolation, and structural studies of glycoproteins |
| Investigation of carbohydrates on cells and subcellular organelles; histochemistry and cytochemistry |
| Mapping of neuronal pathways |
| Mitogenic stimulation of lymphocytesb |
| Purging of bone marrow for transplantationb |
| Selection of lectin-resistant mutants |
| Studies of glycoprotein biosynthesis |
Footnotes:
a Lectins from sources other than plants are rarely in use.
b In clinical use.
Selectin functions
The discovery of the selectins and the demonstration that they play a crucial role in the control of lymphocyte homing and of leukocyte trafficking to sites of inflammation was a landmark in lectin research. Indeed, the selectins provide the best paradigm for the role of sugar–lectin interactions in biological recognition. They mediate the binding of leukocytes to endothelial cells and thereby initiate a rolling phase, in which the lectins interact transiently with glycan ligands, leading eventually to their extravasation. Prevention of adverse inflammatory reactions by inhibition of leukocyte–endothelium interactions, another application of antiadhesion therapy, has become a major aim of the biomedical and pharmacological industry. There are also indications that the selectins may function in the spread of cancer cells from the main tumor to other sites in the body and that by blocking their sugar-binding sites it may be possible to prevent the formation of metastases 14.
In the immune system, endogenous lectins are an important component of the host’s defense against invading pathogens 37, 14, 28, 16, 20, 32. In the innate immune system lectins are able to directly kill microorganisms, or they may aid in the phagocytosis of invading pathogens by dendritic cells and macrophages. Phagocytosis is an actin-dependent mechanism by which cells (phagocytes) ingest large particles that are usually greater than 0.5μm in diameter 38. Phagocytic cells are involved in a number of biological processes, including the recognition and control of invading microbes. The phagocytosed pathogens are neutralized and their proteins are processed into small peptides that are presented to T lymphocytes as a peptide-major histocompatibility complex. This antigen presentation activates specific immune responses and, therefore, lectins are also involved in the adaptive immune system 6. This is an example of indirect lectin involvement in the adaptive immune system. Lectins, however, are also directly involved in adaptive immunity. Leukocytes express L-selectins, members of the C-type Leptin superfamily, and these L-selectins aid in the homing capabilities of leukocytes 39. Interestingly, naïve T lymphocyte expression of L-selectins is high but, once activated, the L-selectin expression is low or lacking altogether. The elevated levels of L-selectin expression by naïve T lymphocytes allow them to migrate to the lymph nodes by binding to specialized high endothelial venules where they mature and become activated when presented with the proper antigens. In the latter case, the lack of L-selectin expression by activated T lymphocytes allows them to migrate and exit at the site of inflammation via high affinity interaction between integrins and their specific ligands. This L-selectin expression level-dependent behavior of T lymphocytes has been demonstrated in studies in which T lymphocytes from tumor bearing mice were restimulated in vitro and selected for their L-selectin expression levels. It was found that T lymphocytes with low L-selectin expression levels efficiently eradicated brain and pulmonary tumors while T lymphocytes with elevated levels of L-selectins demonstrated noticeably reduced tumor clearance abilities 40, 41.
Collectin functions
Collectins, also members of the C-type lectin superfamily, are thought to be involved in the pattern recognition of respiratory viruses and pathogenic bacteria 42. Mannose binding protein is an example of a protective collectin that is able to bind oligomannose residues of bacterial and fungal cell surface oligosaccharides. The structural homology between the C1q component of the complement system and the collagen-like domain of mannose binding protein allows for the initiation of complement fixation upon mannose binding protein-pathogen binding. The initiation of complement fixation is brought about by the activation of mannose binding protein associated serine proteases (MASP-1 and MASP-2). In turn, activated MASPs cleave and activate downstream complement components that eventually neutralize the invading pathogen 43, 44. Mannose binding protein is also able to activate the classical and the alternative complement pathways, thus adding additional significance to its host protection role. Furthermore, mannose binding protein activation of complement promotes the formation of C3b and C5b fragments that increase opsonization, phagocytosis and the neutralization of pathogens by macrophages 45, 44, 46. There is some evidence that suggests mannose binding protein may initiate phagocytosis by neutrophils and monocytes directly by binding to bacterial cells 45, 46. Mannose binding protein compromised individuals are more susceptible to infections, and this emphasizes the importance of mannose binding proteins in the host defense response to pathogenic invasion 46, 20. Another endogenous collectin known as mannose receptor is expressed on macrophage and dendritic cell surfaces 20. Mannose receptors recognize and bind yeasts, mycobacteria and a wide variety of Gram-positive and Gram-negative bacteria 46. Macrophages and dendritic cells, both of which are potent antigen presenting cells 47, phagocytose antigen presenting cells expressed mannose receptor bound microorganisms and process the phagocytosed proteins into short peptides that are then presented by MHC (major histocompatibility complex) class I or MHC class II molecules. Antigen presentation by MHC molecules activates T lymphocytes, and activated T lymphocytes then stimulate the adaptive immune system. Collectins such as mannose binding proteins and mannose receptors play active roles in host defense systems against invading pathogens and infection 20. It is also possible that mannose binding proteins and mannose receptors are able to target and neutralize malignant cells due to their altered glycan moieties 48, 49.
Galectin functions
Galectins are a family of soluble lectins that bind β-galactoside-containing glycans and are defined by a conserved carbohydrate recognition domain and a common structural fold 50, 51. Among the various lectin types, galectins are probably the most conserved and ubiquitous family, with members identified in most animal taxa examined so far 51. As many as fifteen galectins have been identified in mammals and proposed to mediate diverse biological processes involved in the regulation of innate and adaptive immune responses, such as cell activation, differentiation, cytokine secretion and apoptosis 52, 53.
The S-type lectins (galectins), another member of the C-type lectin superfamily, are known to be involved in a wide variety of cellular processes that include pre mRNA splicing, cell growth regulation, cell adhesion, embryogenesis, inflammation, immune function, apoptosis, angiogenesis and tumor metastasis 32. Neoplastic progression has been associated with increased galectin-3 expression in malignancies of the head, neck, gastric or anaplastic large cell lymphoma tumors, thyroid and central nervous system tumors. However, galectin-3 expression has been shown to be down regulated in carcinomas of the uterus, breast and ovary. This suggests that alterations in galectin-3 expression may affect malignant cell interactions with other normal and malignant cells via their corresponding ligands, and thus affect their local growth potential and their potential to metastasize into other anatomical locations 54, 55, 56. Galectin-3 was also shown to have anti-apoptotic effects in galectin-3 cDNA transfected human T cell leukemia Jurkat E6-1 cells 57, 58. The anti-apoptotic effects of galectin-3 are primarily associated with the C-terminus NWGR motif. This anti-apoptotic activity is abolished with a single amino acid substitution, such as glycine 182 to alanine 57, 58.
In other studies it was reported that some endogenous animal lectins, such as galectin-1 (in humans) and galectin-9 (in mice), have cytotoxic activities and are able to induce thymocyte apoptosis. Galectin induced apoptosis of thymocytes has been associated with the physiological selection processes of thymocyte maturation in the thymus . The cytotoxic activities of these galectins are not isolated to thymocytes and are also applicable to malignant cells as well. It was found that recombinant galectin-1 was able to activate apoptosis in several human B lymphoid cell lines, including Burkitt’s lymphoma, in addition to T cell Jurkat and MOLT-4 59.
Optimal signal transmission into cells and adhesion require the clustering of ligands and receptors in most systems. Lectin-carbohydrate interactions are no different and thermodynamically favorable assembly of highly ordered clustering arrays are seen. Galectins bind Gal-β-1-nGlcNAc-R by recognizing the β-gal residue, and the binding affinities to N-glycans are associated with the oligosaccharide content of N-acetyllactosamine (LacNAc) and the GlcNAc branching. GlcNAc branching-dependent affinity is important because, as described earlier, cell surface carbohydrates from human and experimental tumors showed that the most prominent alteration in glycoproteins is the presence of larger and extensively branched N-linked β-1,6-GlcNAc oligosaccharides. The β-1,6-GlcNAc branched N-glycans are tri- or tetra-antenna oligosaccharides that enable lattice formation through multivalent oligomerization by galectins and other lectins in general. Lattice formation is preceded by membrane components such as specific glycoproteins, glycolipids and receptors being rearranged into lipid raft microdomains. These lipid raft microdomains are then reorganized by galectin binding during lattice formation. Lattice formation effectively traps receptors at the cell surface and, therefore, regulates the cell surface distribution of these receptors, receptor endocytosis and their activation. For example, the T cell receptor (TCR) α/β is an N-glycan modified by the enzymatic activity of GlcNAc-TV. Glycosylation of the TCR inhibits random nonspecific clustering of TCRs by binding galectin-3. Multivalent TCR-galectin-3 lattices restrict the lateral movement of TCRs within the plasma membrane and, in turn, restrict TCR aggregation at the immune synapse. Multivalent TCR-galectin-3 lattices therefore increase the threshold for TCR activation and by doing so regulate immune response 60, 61.
Regulatory T cells over-express galectin-1 and galectin-10 that are vital for the suppressive activity of these cells. It is highly possible that the suppressive activity of regulatory T cells by over-expression of galectin-1 contributes to immune system evasion by malignant cells 62. Although, multivalent lattice formation may be one possible mechanism by which galectin-oligosaccharide interaction regulate cellular processes. The exact mechanistic nature of galectin involvement in cellular processes such as cell growth regulation, cell adhesion, embryogenesis, inflammation, immune function, apoptosis, angiogenesis and tumor metastasis remains unclear. However, a wide body of evidence strongly suggests their involvement in many of these cellular processes in normal and diseased states 63, 64, 34.
Lectin-mediated therapeutics
The concept of lectin-mediated specific drug delivery was proposed by Woodley and Naisbett in 1988 7. Delivery of targeted therapeutics via direct and reverse drug delivery systems to specific sites provides numerous advantages over traditional non-targeted therapeutics 65, 66. Targeted drug delivery increases the efficacy of treatment by enhancing drug exposure to targeted sites while limiting side effects of drugs on normal and healthy tissues. Limiting or preventing side effects in treatments is important because side effects typically lead to reduction in dosage, delay in treatment and therapy termination. Furthermore, specific drug delivery increases the uptake and internalization of therapeutics that have reduced cellular permeability. Direct or reverse targeting relies on identifying and utilizing unique moieties of the targeted site while protecting the active (drug) component during the delivery . In addition to specific moieties, other parameters such as the target environment and the path taken to reach the target must be considered in tailoring lectin-based drug delivery systems 8, 66. Drugs passing through the gastrointestinal tract are susceptible to early activation and degradation by the acidic environment and pancreatic enzymes. Alternatively, drugs administrated via the colon are vulnerable to catabolic assault by enzymes of bacterial origin (e.g. dextranase, pectinase, β-D-xylosidase, β-D-galactosidase, amylase, xylanase and β-D-glucosidase) 67. However, it is possible to develop drug delivery systems that take advantage of these bacterial enzymes. For example, a drug core in a fermentable carbohydrate coating, drug-carbohydrate conjugates (prodrugs) and drugs embedded in a biodegradable matrix are all possible designs of drugs that can utilize bacterial enzymes 68, 66.
Mistletoe Extracts and Cancer Treatment
Mistletoe is a semiparasitic plant that has been used for centuries to treat numerous human ailments. Mistletoe is used commonly in Europe, where a variety of different extracts are manufactured and marketed as injectable prescription drugs. These injectable drugs are not available commercially in the United States and are not approved as a treatment for people with cancer 69.
Mistletoe holds interest as a potential anticancer agent because extracts derived from it have been shown to kill cancer cells in vitro (taking place in a test tube) 70, 71, 72, 73, 74, 75, 76, 77, 78, 79 and to stimulate immune system cells both in vitro and in vivo (performed or taking place in a living organism) 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91.
Three components of mistletoe, namely viscotoxins, polysaccharides, and lectins, may be responsible for these effects 79, 85, 90, 92, 93. Viscotoxins are small proteins that exhibit cell-killing activity and possible immune-system-stimulating activity 94, 75, 85, 95. Lectins are complex molecules made of both protein and carbohydrates that are capable of binding to the outside of cells (e.g., immune system cells) and inducing biochemical changes in them 79, 96, 97, 19, 17. In view of mistletoe’s ability to stimulate the immune system, it has been classified as a type of biological response modifier 96. Biological response modifiers constitute a diverse group of biological molecules that have been used individually, or in combination with other agents, to treat cancer or to lessen the side effects of anticancer drugs. Mistletoe extracts have been demonstrated in preclinical settings to have other mechanisms of action, such as antiangiogenesis 98.
Mistletoe is one of the most widely studied complementary and alternative medicine therapies for cancer. In certain European countries, the preparations made from European mistletoe (Viscum album, Loranthaceae) are among the most prescribed drugs offered to cancer patients 69. Although mistletoe plants and berries are considered poisonous to humans, few serious side effects have been associated with mistletoe extract use.
The use of mistletoe as a treatment for people with cancer has been investigated in clinical studies. Reports of improved survival and/or quality of life have been common, but nearly all of the studies had major weaknesses that raise doubts about the reliability of the findings 69.
At present, the use of mistletoe cannot be recommended outside the context of well-designed clinical trials. Such trials will be valuable to determine more clearly whether mistletoe can be useful in the treatment of specific subsets of cancer patients 69.
Preparations from mistletoe extracts are most frequently used in the treatment of cancer patients in German-speaking countries 99. And the evidence from randomized clinical trials to support the application of mistletoe extracts impact on survival or improved ability to fight cancer or to withstand anticancer treatments is weak. Nevertheless, there is some evidence that mistletoe extracts may offer benefits during chemotherapy for breast cancer, but these results need replication 99. Overall, more high quality, independent clinical research is needed to truly assess the safety and effectiveness of mistletoe extracts 99. Patients receiving mistletoe therapy should be encouraged to take part in future trails.
Commercially available extracts are marketed under a variety of brand names, including Iscador, Helixor, Iscucin and Plenosol. Some extracts are marketed under more than one name. Iscador, Isorel, and Plenosol are also sold as Iscar, Vysorel, and Lektinol, respectively. All of these products are prepared from Viscum album (Loranthaceae) (Viscum album L. or European mistletoe). They are not sold as a drug in the United States 100.
In addition to European mistletoe, extracts from a type of Korean mistletoe (Viscum album var. coloratum [Kom.] Ohwi) have demonstrated in vitro and in vivo cytotoxicity in laboratory studies 101, 102, 103, 104, 105.
Mistletoe grows on several types of trees, and the chemical composition of extracts derived from it depends on the species of the host tree (e.g., apple, elm, oak, pine, poplar, and spruce), the time of year harvested, how the extracts are prepared, and the commercial producer 77, 97, 106, 107, 108, 109.
Mistletoe extracts are prepared as aqueous solutions or solutions of water and alcohol, and they can be fermented or unfermented. Some extracts are prepared according to homeopathic principles, and others are not. Accordingly, as homeopathic preparations, they are typically not chemically standardized extracts. In addition, the commercial products can be subdivided according to the species of host tree, which is typically indicated in the product name by a suffix letter. Iscador, a fermented aqueous extract of Viscum album L. that is prepared as a homeopathic drug, is marketed as IscadorM (from apple trees; Malus domestica), IscadorP (from pine trees; Pinus sylvestris), IscadorQu (from oak trees; Quercus robur), and IscadorU (from elm trees; Ulmus minor). Helixor, an unfermented aqueous extract of Viscum album L. that is standardized by its biological effect on human leukemia cells in vitro, is marketed as HelixorA (from spruce trees; Picea abies), HelixorM (from apple trees), and HelixorP (from pine trees; Pinus sylvestris).
Mistletoe extracts are usually given by subcutaneous injection, although administration by other routes (i.e., oral, intrapleural, intratumoral, and intravenous) has been described. In most reported studies, subcutaneous injections were given 2 to 3 times a week, but the overall duration of treatment varied considerably.
Viscum album is listed in the Homeopathic Pharmacopoeia of the United States, which is the officially recognized compendium for homeopathic drugs in this country 110. Although the U.S. Food and Drug Administration (FDA) has regulatory authority over homeopathic drugs, this authority is usually not exercised unless the drugs are formulated for injection or there is evidence of severe toxicity. At present, the FDA does not allow the importation or distribution of injectable preparations of mistletoe, including homeopathic formulations, except for the purpose of clinical research 100. The extracts are not available commercially in the United States and are not approved as a treatment for people with cancer 100.
Before researchers can conduct clinical drug research in the United States, they must file an Investigational New Drug (IND) application with the FDA. IND approval is also required for clinical investigation of homeopathic drugs. The FDA does not disclose information about IND applications or approvals; this information can be released only by the applicants. At least two U.S. investigators were given IND approval to study mistletoe as a treatment for people with cancer.
Lectin in Foods
Lectins are clusters of glycoproteins of nonimmune foundation that combine specifically and reversibly to carbohydrates, mainly the sugar moiety of glycoconjugates, resulting in cell agglutination and precipitation of glycoconjugates. Lectins are omnipresent proteins that are possibly there in all eukaryotic and numerous bacterial species as well as in several viruses. They play a vital function in the plants resistance against insect pests and have been found to be deadly to viruses, bacteria, fungi, insects, and prominent animals 13. Lectin are carbohydrate-binding proteins of non-immune temperament 111, 112.
Many plants contain carbohydrate-binding proteins that are commonly designated as lectins, agglutinins, or hemagglutinins. Due to the obvious differences in molecular structure, biochemical properties and carbohydrate-binding specificity, plant lectins are usually considered a complex and heterogeneous group of proteins 113. Recent advances in the structural analysis of lectins and molecular cloning of lectin genes enable subdividision of plant lectins in a limited number of subgroups of structurally and evolutionary related proteins.
Four major lectin families 113, namely:
- the legume lectins,
- the chitin-binding lectins composed of hevein domains,
- the type 2 ribosome-inactivating proteins, and
- the monocot mannose-binding lectins comprise the majority of all currently known plant lectins.
In addition to these four large families the jacalin-related lectins, the amaranthin family, and the Cucurbitaceae phloem lectins are now recognized as separate subgroups 113.
Lectins are extensively dispersed in nature and ample in plants (see Table 4). Lectins are established in diverse species of major taxonomical groups and most of as seed storage proteins 114, 115. First lectin to be purified on a large scale and accessible on a trade basis is concanavalin A. Lectins also originated in vegetative organs like roots, leaves, rhizomes, bulbs, tubers, corms, stems, bark, flowers, fruits, phloem sap, latex, and nectar 116, 117. Plant lectins possess analogous biological activities and chemical properties. The physiological, biochemical, cellular, and molecular characteristics of lectin show its involvement in plant defense mechanism, e.g., lentil, soybean, garden pea, wheat germ, Griffonia simplicifolia, Viccia graminae, Madura pomifera, Hura crepitans, Psophocarpus tetragonolobus and Abrus precatorius.
Many plant lectins are thought to play a role in the plants defence against being eaten. Accordingly, plant lectins have an obvious preference for binding to sugar structures of animal, fungal or microbial origin, and are usually at highest concentrations in plant parts essential for reproductive success such as seed germs. The intensively studied lectin wheat germ agglutinin, which protects against insects and fungi 118, is present in wheat seed in both the germ and the gluten part of endosperm 119. Peptides behaving in a lectin-like manner have also been obtained upon cleavage of gliadin in gluten 120. Sourdough lactic acid bacteria hydrolyse gliadin peptides and inhibit their lectin-like behaviour 121, which perhaps explains some of the unexplained health effects of probiotics 122. White flour consumed by humans contains a high proportion of gluten and has agglutinating activity suggestive of lectins 123, 124, 125. Thus, lectins are present in our food, they are heat-stable and resistant to breakdown in the gastrointestinal tract, they bind to the surface epithelium of the digestive tract and they can lead to anti-nutritional, mild allergic or other subclinical effects in humans and animals 126, 127. Lectins can also be transported through the gut wall into the blood circulation, where they directly influence peripheral tissues and body metabolism through the binding to glycosylated structures, such as the insulin receptor, the epidermal growth factor receptor and the interleukin 2 receptor 128, 129. Wheat germ agglutinin have effects on activation of the epidermal growth factor receptor 130, mitogenesis 131, agglutination of red blood cells 126, activation of platelets and cell adhesion molecules 132 and vascular permeability 133, 134. Wheat germ agglutinin also have several effects related to autoimmunity, allergy and inflammation 135, 136. Wheat germ agglutinin binds to several types of mammalian cells including pancreatic duct epithelial cells 137, prostatic cancer cells 138, arterial macrophages and smooth muscle cells 139, 140, glomerular capillary walls, mesangial cells and tubules of human kidney 141. Human serum contains antibodies against wheat germ agglutinin and lectins of soybean and peanut 142. Hence, lectins have sufficient properties to affect the leptin system indirectly, through effects on metabolism central to the proper function of the leptin system, and possibly also directly through interaction with leptin or the leptin receptor 143.
Legumes lectins are one of the chief lectin families out of 70 lectins were reported. The legume lectins are a family of carbohydrate binding lectins found in the seeds of plants belonging to the Fabacaea family. The accurate role of the legume lectins in vivo is unknown but they are perhaps implicated in the defense of plants against predators e.g. insects 144.
Table 5. Lectin content of some edible plants, compared to their protein content
| Edible Plant | Lectin | Lectin Content (% of Total Protein) | Reference |
| Lentil (Lens culinaris) | LcA | 2.50% | 145 |
| Pea (Pisum sativum) | PsA | 2.50% | 146 |
| Soybean (Glycine max) | SBA | 2.00% | 147 |
| Peanut (Arachis hypogaea) | PNA | 1.50% | 148 |
| Horse gram (Dolichos biflorus) | DbA | 10.00% | 149 |
| Kidney bean (Phaseolus vulgaris) | PHA | 1.00% | 150 |
| Wheat (Triticum aestivum) | WGA | 3.00% | 151 |
| Garlic (Allium sativum) | ASA | 10.00% | 152 |
| Black elderberry (Sambucus nigra) | SNA-I | 3.00% | 152 |
| Banana (Musa acuminata) | Mus a 2 | 2.30% | 153 |
Table 6. Lectins identified as potential food allergens
| Allergen | Protein | Plant Species | International Union of Immunological Societies Approved | Food Allergen | References |
| Mus a 2 | Class I chitinase | Banana (Musa acuminata) | + | + | 154 |
| Tri a 18 | Wheat germ agglutinin | Wheat (Triticum aestivum) | + | + | 155 |
| Zea m 8 | Class IV chitinase | Corn (Zea mays) | + | + | 156 |
| Bra r 2 | Chitin-binding protein | Field mustard (Brassica rapa) | + | + | 157 |
| Cas s 5 | Chitinase | Sweet chestnut (Castanea sativa) | + | + | * |
| Hev b 6 | Hevein precursor | Rubber tree (Hevea brasiliensis) | + | Contact | 158 |
| Hev b 11 | Class I chitinase | Hevea brasiliensis | + | Contact | * |
| Pers a 1 | Class I chitinase | Avocado (Persea americana) | + | + | 159 |
| Act c chitinase | Class I chitinase | Golden kiwifruit (Actinidia chinensis) | + | 160 | |
| Car p chitinase | Class I chitinase | Papaya (Carica papaya) | + | 161 | |
| Lyc es chitinase | Class I chitinase | Tomato (Lycopersicum esculentum) | + | 162 | |
| Ann ch chitinase | Class I chitinase | Custard apple (Annona cherimola) | + | 162 | |
| Pas ed chitinase | Class I chitinase | Passion fruit (Passiflora edulis) | + | 162 | |
| Vit v chitinase | Class IV chitinase | Grape (Vitis vinifera) | + | 163 | |
| AcA | GNA-like Lectin | Onion (Allium cepa) | + | 164 | |
| AsA | GNA-like lectin | Garlic (Allium sativum) | + | 165 | |
| Ara h agglutinin | Legume lectin | Peanut (Arachis hypogaea) | + | 166 | |
| DbA | Legume lectin | Horsegram (Dolichos biflorus) | + | 167 | |
| SBA | Legume lectin | Soybean (Glycine max) | + | 168 | |
| LcA | Legume lectin | Lentil (Lens culinaris) | + | 169 | |
| PHA-E | Legume lectin | Common bean (Phaseolus vulgaris) | + | 169 | |
| PHA-L | Legume lectin | Phaseolus vulgaris | + | 169 | |
| PsA | Legume lectin | Peas (Pisum sativum) | + | 169 | |
| Chia lectin | Legume lectin | Chia (Salvia hispanica) | 169 | ||
| Ricin | RIP-II | Castor oil plant (Ricinus communis) | + | 170 | |
| SNA-I | RIP-II | Black elderberry (Sambucus nigra) | + | 169 | |
| BanLec | Jacalin-related lectin | Banana (Musa acuminata) | + | 171 | |
| Cuc m 17 kDa | Nictaba-related lectin | Melon (Cucumis melo) | + | 172 |
Footnotes: List of lectins identified as potential food allergens. Lectin allergens approved by the World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee, are indicated. Lectin allergens Hev b 6 and Hev b 11 from Castor bean (Ricinus communis) latex, are responsible for contact allergies but also for IgE-binding cross-reactivity with hevein-containing food chitinase proteins.
* No references available at the WHO/IUIS Allergen Nomenclature Sub-Committee.
+ means yes.
[Source 173 ]Legume lectins
Depending on the occurrence of a single or two polypeptide chains in their constitutive protomer and their quaternary association as homotetramers or homodimers, legume lectins are classified into two groups of closely-related lectins 12:
- the homotetrameric single-chain lectins, comprise both man-specific (e.g., Con A from Jackbean, Canavalia ensiformis, and DgL from Dioclea grandiflora) and Gal/GalNAc-specific lectins (e.g., PNA from peanut, Arachis hypogaea, and SBA from soybean, Glycine max).
- the homodimeric two-chain lectins, exclusively comprise man-specific lectins (e.g., LcA from lentil, Lens culinaris, and PsA from garden pea, Pisum sativum).
Both types of lectins have been identified as potential IgE-binding allergens.
Toxicity of lectin
Lectins are present in a variety of plants, especially in seeds, where they serve as defense mechanisms against other plants and fungi. Because of their ability to bind to virtually all cell types and cause damage to several organs, lectins are widely recognized as anti-nutrients within food 174. Most lectins are resistant to heat and the effects of digestive enzymes, and are able to bind to several tissues and organs in vitro and in vivo 175.
The question of the possible physiological role of lectins has intrigued investigators from the start and focused on plant lectins, which for long time were virtually the only ones known. It was speculated, for example, that lectins may function as antibodies to protect plants against harmful soil bacteria, control seed germination, or be involved in the transport and storage of sugars, but no evidence for these speculations could be found. However, two proposals put forward in the 1970s still hold. According to first one, lectins protect plants against phytopathogenic microorganisms and insects as well as against predatory animals. The second theory assumes that they are involved in the association between leguminous plants and their symbiotic nitrogen-fixing bacteria 14.
Ingestion of the lectins present in certain improperly cooked vegetables can result in acute gastro inestinal tract distress, but the mechanism of toxicity is unknown 176. Plant lectins that are not efficiently degraded by digestive enzymes, and that have an affinity for the surface of gut epithelial cells, such as those present in the legumes, can be poisonous 177. Acute symptoms following ingestion include nausea, vomiting and diarrhea. Long-term intake in rodent models is characterized by increased cell turnover, gut hyperplasia and weight loss. Areas of epithelial cell necrosis and even zones of complete epithelial cell denudation are seen in biopsies of the stomach and intestine of rats 178 and insects 179 fed plant lectins. Indeed, the plant lectin may function as a natural insecticide. Epithelial cell microvilli particularly are affected by lectin exposure, which initiates disruption and shedding of these membrane rich surface projections. Confusingly, however, when cells are treated with lectins in vitro, even at very high doses, necrosis is not observed, though many other responses have been noted including mitogenesis, vacuole formation and inhibition of exocytosis.
The administration of the lectin wheat germ agglutinin to experimental animals caused hyperplastic and hypertrophic growth of the small intestine, hypertrophic growth of the pancreas and thymus atrophy 174. Lectin activity has been demonstrated in wheat, rye, barley, oats, corn and rice, however the best studied of the cereal grain lectins is wheat germ agglutinin 180. After ingestion, wheat germ agglutinin is capable of crossing the intestinal barrier. In animal models, WGA has been shown to reach the basolateral membrane and walls of the small blood vessels in the subepithelium of the small intestine 174.
Elevated concentration of lectin in foods, such as beans, cereal grains, seeds, and nuts may be injurious to human and animals 13. In a laboratory animal study involving mice being fed raw and irradiated diets containing kidney beans as the sole source of protein and providing 10% crude protein produced severe weight loss, followed in some cases by death 181.
Allergenic potential of plant lectins
In addition to their well-known mitogenic properties, essentially on T-lymphocytes, plant lectins are capable of inducing some allergic responses in previously sensitized people, similar to genuine allergens from plant foods and food products.
The list of lectins that have been assigned as potential food allergens by the World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee 11, comprises a restricted number of proteins including Tri a 18 (wheat germ agglutinin WGA) and a few chitinases with a hevein domain from banana (Mus a 2), turnip (Bra r 2), chestnut (Cas s 5), corn (Zea m 8), and avocado (Per a 1) (see Table 5). Two other contact allergens, Hev b 6 (hevein) and Hev b 11 (class I chitinase containing a hevein domain) from the rubber tree Hevea brasiliensis, have been included in the list because they cross-react with food chitinases of classes I, II, IV, V, VI, and VII, which also possess a hevein domain.
In addition to this official list of lectin allergens, other plant lectins have been identified as potential food allergens following their ability to bind IgE, degranulate mast cells and basophils, and trigger the interleukin responses in various allergic patients 182. These lectins have been included in Table 5 as non-IUIS approved potential food allergens (Table 6).
The non-World Health Organization/International Union of Immunological Societies (WHO/IUIS) approved lectin allergens consist of members from the different families of plant lectins, namely legume lectins, type II ribosome-inactivating proteins (RIP-II), wheat germ agglutinin, hevein and chitinases with a hevein domain, jacalin-related lectins, GNA-like lectins, and Nictaba-related lectins. All of these lectins exhibit very different structural scaffolds but share the common property to specifically recognize both simple sugars, e.g., mannose or galactose, and complex glycans, e.g., glycans of the N-acetyllactosaminic type or the high-mannose type 183.
Summary
Lectins are carbohydrate-binding proteins that preferentially recognize and bind carbohydrate complexes protruding from glycolipids and glycoproteins. Lectins play crucial roles in various biological processes such as cellular signaling, malignancy, scavenging of glycoproteins from the circulatory system, cell–cell interactions in the immune system, differentiation and protein targeting to cellular compartments and also, in host defence mechanisms, inflammation, and metastasis. Generally lectins are able to agglutinate erythrocytes and are often referred to as hemagglutinins.
Due to the property of selectivity and specificity, lectins have gained more attention from researchers in identification of cancer and degree of metastasis 184. Several lectins have been found to possess anticancer properties in vitro, in vivo, and in human case studies; they are used as therapeutic agents, preferentially binding to cancer cell membranes or their receptors, causing cytotoxicity, apoptosis, and inhibition of tumor growth 185. For example, lectin found in Mistletoe extracts have been used to treat certain cancers. In certain European countries, the preparations made from European mistletoe (Viscum album, Loranthaceae) are among the most prescribed drugs offered to cancer patients 69. Although mistletoe plants and berries are considered poisonous to humans, few serious side effects have been associated with mistletoe extract use. However, Mistletoe extracts are not available commercially in the United States and are not approved as a treatment for people with cancer 100. Although lectins seem to have great potential as anticancer agents, further research is still needed.
The galectins are believed to act as modulators of cell–substratum interactions and to be essential for the normal differentiation and growth of all multicellular animals. They are capable of inducing cell proliferation, cell arrest, or apoptosis (physiological cell death) and have been implicated in organ morphogenesis, tumor cell metastasis, leukocyte trafficking, immune response, and inflammation, as well as recognition of extracellular matrix.
Lectins are universally distributed in nature, being established in plants, fungi, viruses, bacteria, crustacea, insects, and animals, but rich source of lectins are present in most plants, especially seeds and tubers like cereals, potatoes, corn, soy and beans. Controversies arise when studies that have been conducted using lab animals are used to present cases of toxicity, leaky bowel syndrome and inflammatory diseases (e.g. rheumatoid arthritis, type 2 diabetes, wheat allergy, celiac disease) 186, 187. However, existing evidence regarding food lectins toxicity in humans is inconclusive and is likely to remain so, because until now, human epidemiological and intervention studies investigating the health effects of whole grains and legumes intake were confounded by other dietary and lifestyle factors. The science of lectinology is just beginning.
Moreover, studies involing legumes (beans, peas and lentils) and whole grains have shown legumes and whole grains are a healthy source of proteins, potassium, and complex carbohydrates, including dietary fiber and resistant starch. Legume also possess several beneficial biological activities in humans, as an antioxidant source, cholesterol- and low-density lipoprotein-lowering properties, antimutagenic and anticancer effects as well as effects on cardiovascular disease, diabetes and obesity. Bean consumption has been related to numerous health benefits, such as a decrease in cholesterol levels and cardiac diseases. Beans also offer some protection against cancer, diabetes and obesity, because of their antioxidant, antimutagenic and antiproliferative properties 188, 189. Prospective studies suggest that weight gain and increases in abdominal adiposity over time are lower in people who consume more whole grains. Analyses of the Physicians’ Health Study 190 and the Nurses’ Health Study 191 showed that those who consumed more whole grain foods consistently weighed less than those who consumed fewer whole grain foods at each follow-up period of the study. Koh-Banerjee et al. 190 estimated that for every 40-gram increase in daily whole grain intake, the 8-year weight gain was lower by 1.1 kg. It has also been shown that the intake of whole grains is associated with healthier dietary factors and a healthier lifestyle in general. In a Scandinavian cross-sectional study, the intake of whole grains was directly associated with the length of education, the intake of vegetables, fruits, dairy products, fish, shellfish, coffee, tea and margarine and inversely associated with smoking, BMI and the intake of red meat, white bread, alcohol, cakes and biscuits 192. Observational prospective and cross-sectional studies show that the intake of whole grain products is associated with reduced risks for developing type 2 diabetes, cardiovascular diseases, obesity and some types of cancer 193.
- El-Araby, M. M., El-Shatoury, E. H., Soliman, M. M., & Shaaban, H. F. (2020). Characterization and antimicrobial activity of lectins purified from three Egyptian leguminous seeds. AMB Express, 10(1), 90. https://doi.org/10.1186/s13568-020-01024-4
- Lagarda-Diaz, I., Guzman-Partida, A. M., & Vazquez-Moreno, L. (2017). Legume Lectins: Proteins with Diverse Applications. International journal of molecular sciences, 18(6), 1242. https://doi.org/10.3390/ijms18061242
- Van Damme EJ, Peumans WJ, Pusztai A, Bardocz S. Handbook of plant lectins properties and biomedical applications. Hoboken: Wiley; 1998.
- National Cancer Institute at the National Institutes of Health. Lectin. https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=44051
- Use of lectins as diagnostic and therapeutic tools for cancer. Mody R, Joshi S, Chaney W. J Pharmacol Toxicol Methods. 1995 Feb; 33(1):1-10. https://www.ncbi.nlm.nih.gov/pubmed/7727802/
- On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Gorelik E, Galili U, Raz A. Cancer Metastasis Rev. 2001; 20(3-4):245-77. https://www.ncbi.nlm.nih.gov/pubmed/12085965/
- Lectin-mediated drug targeting: history and applications. Bies C, Lehr CM, Woodley JF. Adv Drug Deliv Rev. 2004 Mar 3; 56(4):425-35. https://www.ncbi.nlm.nih.gov/pubmed/14969751/
- Drug targeting to the colon with lectins and neoglycoconjugates. Minko T. Adv Drug Deliv Rev. 2004 Mar 3; 56(4):491-509. https://www.ncbi.nlm.nih.gov/pubmed/14969755/
- Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins, and implications in cancer therapeutics. Acta histochemica. 2011;113(3):236-247. doi:10.1016/j.acthis.2010.02.004. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3027850/
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. https://www.ncbi.nlm.nih.gov/pubmed/15302520/
- Pomés A., Davies J.M., Gadermaier G., Hilger C., Holzhauser T., Lidholm J., Lopata A.L., Mueller G.A., Nandy A., Radauer C., et al. WHO/IUIS allergen nomenclature: Providing a common language. Mol. Immunol. 2018;100:3–13. doi: 10.1016/j.molimm.2018.03.003
- Barre, A., Damme, E., Simplicien, M., Benoist, H., & Rougé, P. (2020). Are Dietary Lectins Relevant Allergens in Plant Food Allergy?. Foods (Basel, Switzerland), 9(12), 1724. https://doi.org/10.3390/foods9121724
- Hivrale A, Ingale A. Plant as a plenteous reserve of lectin. Plant Signaling & Behavior. 2013;8(12):e26595. doi:10.4161/psb.26595. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4091380/
- History of lectins: from hemagglutinins to biological recognition molecules. Sharon N, Lis H. Glycobiology. 2004 Nov; 14(11):53R-62R. https://www.ncbi.nlm.nih.gov/pubmed/15229195/
- Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Bouckaert J, Berglund J, Schembri M, De Genst E, Cools L, Wuhrer M, Hung CS, Pinkner J, Slättegård R, Zavialov A, Choudhury D, Langermann S, Hultgren SJ, Wyns L, Klemm P, Oscarson S, Knight SD, De Greve H. Mol Microbiol. 2005 Jan; 55(2):441-55. https://www.ncbi.nlm.nih.gov/pubmed/15659162/
- Functions of cell surface galectin-glycoprotein lattices. Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Curr Opin Struct Biol. 2007 Oct; 17(5):513-20. https://www.ncbi.nlm.nih.gov/pubmed/17950594/
- Kilpatrick DC: Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol 11 (1): 55-65, 1999. https://www.ncbi.nlm.nih.gov/pubmed/10367282?dopt=Abstract
- Morgan, D.A., Ruscetti, F.W., and Gallo, R. (1976) Selective in vitro growth of T lymphocytes from normal human bone marrows. Science, 193, 1007–1008.
- Abdullaev FI, de Mejia EG: Antitumor effect of plant lectins. Nat Toxins 5 (4): 157-63, 1997. https://www.ncbi.nlm.nih.gov/pubmed/9407559?dopt=Abstract
- C-type lectins and phagocytosis. Kerrigan AM, Brown GD. Immunobiology. 2009; 214(7):562-75. https://www.ncbi.nlm.nih.gov/pubmed/19261355/
- Adhesion molecules controlling lymphocyte migration. Stoolman LM. Cell. 1989 Mar 24; 56(6):907-10. https://www.ncbi.nlm.nih.gov/pubmed/2647304/
- Selectin ligands. Varki A. Proc Natl Acad Sci U S A. 1994 Aug 2; 91(16):7390-7. https://www.ncbi.nlm.nih.gov/pubmed/7519775/
- Deletion polymorphism of SIGLEC14 and its functional implications. Yamanaka M, Kato Y, Angata T, Narimatsu H. Glycobiology. 2009 Aug; 19(8):841-6. https://www.ncbi.nlm.nih.gov/pubmed/19369701/
- Interchim. 2010. http://www.interchim.fr/ft/M/MS902z.pdf
- Cell-to-cell binding induced by different lectins. The Journal of Cell Biology. 1975;65(2):247-257. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2109424/pdf/jc652247.pdf
- Brudner M, Karpel M, Lear C, et al. Lectin-Dependent Enhancement of Ebola Virus Infection via Soluble and Transmembrane C-type Lectin Receptors. Schneider BS, ed. PLoS ONE. 2013;8(4):e60838. doi:10.1371/journal.pone.0060838. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614905/
- Intact cell adhesion to glycan microarrays. Nimrichter L, Gargir A, Gortler M, Altstock RT, Shtevi A, Weisshaus O, Fire E, Dotan N, Schnaar RL. Glycobiology. 2004 Feb; 14(2):197-203. https://www.ncbi.nlm.nih.gov/pubmed/14638630/
- Carbohydrates and glycoconjugates: progress in non-mammalian glycosylation, glycosyltransferases, invertebrate lectins and carbohydrate-carbohydrate interactions. Wormald MR, Sharon N. Curr Opin Struct Biol. 2004 Oct; 14(5):591-2. https://www.ncbi.nlm.nih.gov/pubmed/15465320/
- Functions of cell surface galectin-glycoprotein lattices. Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Curr Opin Struct Biol. 2007 Oct; 17(5):513-20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2100406/
- C-type lectins and phagocytosis. Kerrigan AM, Brown GD. Immunobiology. 2009; 214(7):562-75. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702671/
- Mannan-binding lectin deficiency modulates the humoral immune response dependent on the genetic environment. Ruseva M, Kolev M, Dagnaes-Hansen F, Hansen SB, Takahashi K, Ezekowitz A, Thiel S, Jensenius JC, Gadjeva M. Immunology. 2009 Jun; 127(2):279-88. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2691793/
- Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a pertussis toxin-sensitive pathway. Malik RK, Ghurye RR, Lawrence-Watt DJ, Stewart HJ. Glycobiology. 2009 Dec; 19(12):1402-7. https://www.ncbi.nlm.nih.gov/pubmed/19561030/
- Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Chou FC, Shieh SJ, Sytwu HK. Eur J Immunol. 2009 Sep; 39(9):2403-11. https://www.ncbi.nlm.nih.gov/pubmed/19670381/
- Detection of differentially expressed wound-healing-related glycogenes in galectin-3-deficient mice. Saravanan C, Cao Z, Head SR, Panjwani N. Invest Ophthalmol Vis Sci. 2009 Dec; 50(12):5690-6. https://www.ncbi.nlm.nih.gov/pubmed/19643959/
- Chan CK, Ransom RC, Longaker MT. Lectins bring benefits to bones. eLife. 2016;5:e22926. doi:10.7554/eLife.22926. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5154756/
- Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins, and implications in cancer therapeutics. Acta histochemica. 2011;113(3):236-247. doi:10.1016/j.acthis.2010.02.004. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3027850/
- The C-type lectin superfamily in the immune system. Weis WI, Taylor ME, Drickamer K. Immunol Rev. 1998 Jun; 163():19-34. https://www.ncbi.nlm.nih.gov/pubmed/9700499/
- Mechanisms of phagocytosis in macrophages. Aderem A, Underhill DM. Annu Rev Immunol. 1999; 17():593-623. https://www.ncbi.nlm.nih.gov/pubmed/10358769/
- The selectins: vascular adhesion molecules. Tedder TF, Steeber DA, Chen A, Engel P. FASEB J. 1995 Jul; 9(10):866-73. https://www.ncbi.nlm.nih.gov/pubmed/7542213/
- Purification of L-selectin(low) cells promotes the generation of highly potent CD4 antitumor effector T lymphocytes. Kagamu H, Shu S. J Immunol. 1998 Apr 1; 160(7):3444-52. https://www.ncbi.nlm.nih.gov/pubmed/9531305/
- Tumor infiltration by adoptively transferred T cells is independent of immunologic specificity but requires down-regulation of L-selectin expression. Kjaergaard J, Shu S. J Immunol. 1999 Jul 15; 163(2):751-9. https://www.ncbi.nlm.nih.gov/pubmed/10395667/
- Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. White MR, Crouch E, Chang D, Sastry K, Guo N, Engelich G, Takahashi K, Ezekowitz RA, Hartshorn KL. J Immunol. 2000 Aug 15; 165(4):2108-15. https://www.ncbi.nlm.nih.gov/pubmed/10925296/
- Serum lectin with known structure activates complement through the classical pathway. Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. J Biol Chem. 1987 Jun 5; 262(16):7451-4. https://www.ncbi.nlm.nih.gov/pubmed/3584121/
- Human mannose-binding protein activates the alternative complement pathway and enhances serum bactericidal activity on a mannose-rich isolate of Salmonella. Schweinle JE, Ezekowitz RA, Tenner AJ, Kuhlman M, Joiner KA. J Clin Invest. 1989 Dec; 84(6):1821-9. https://www.ncbi.nlm.nih.gov/pubmed/2592561/
- The human mannose-binding protein functions as an opsonin. Kuhlman M, Joiner K, Ezekowitz RA. J Exp Med. 1989 May 1; 169(5):1733-45. https://www.ncbi.nlm.nih.gov/pubmed/2469767/
- The mannose receptor is a pattern recognition receptor involved in host defense. Stahl PD, Ezekowitz RA. Curr Opin Immunol. 1998 Feb; 10(1):50-5. https://www.ncbi.nlm.nih.gov/pubmed/9523111/
- Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Stahl P, Schlesinger PH, Sigardson E, Rodman JS, Lee YC. Cell. 1980 Jan; 19(1):207-15. https://www.ncbi.nlm.nih.gov/pubmed/6766809/
- Lectin-induced apoptosis of tumour cells. Kim M, Rao MV, Tweardy DJ, Prakash M, Galili U, Gorelik E. Glycobiology. 1993 Oct; 3(5):447-53. https://www.ncbi.nlm.nih.gov/pubmed/8286857/
- Gorelik E. Mechanisms of cytotoxic activity of lectins. Trends Glycoscience and Glycobiology. 1994;6:435–45.
- Galectinomics: finding themes in complexity. Cooper DN. Biochim Biophys Acta. 2002 Sep 19; 1572(2-3):209-31. https://www.ncbi.nlm.nih.gov/pubmed/12223271/
- Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Vasta GR, Ahmed H, Odom EW. Curr Opin Struct Biol. 2004 Oct; 14(5):617-30. https://www.ncbi.nlm.nih.gov/pubmed/15465324/
- Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Brewer CF, Miceli MC, Baum LG. Curr Opin Struct Biol. 2002 Oct; 12(5):616-23. https://www.ncbi.nlm.nih.gov/pubmed/12464313/
- Dissecting the pathophysiologic role of endogenous lectins: glycan-binding proteins with cytokine-like activity? Toscano MA, Ilarregui JM, Bianco GA, Campagna L, Croci DO, Salatino M, Rabinovich GA. Cytokine Growth Factor Rev. 2007 Feb-Apr; 18(1-2):57-71. https://www.ncbi.nlm.nih.gov/pubmed/17321195/
- Differential expression of the 67-kD laminin receptor and 31-kD human laminin-binding protein in human ovarian carcinomas. van den Brûle FA, Berchuck A, Bast RC, Liu FT, Gillet C, Sobel ME, Castronovo V. Eur J Cancer. 1994; 30A(8):1096-9. https://www.ncbi.nlm.nih.gov/pubmed/7654437/
- Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Schoeppner HL, Raz A, Ho SB, Bresalier RS. Cancer. 1995 Jun 15; 75(12):2818-26. https://www.ncbi.nlm.nih.gov/pubmed/7773932/
- Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Cancer. 1997 Aug 15; 80(4):776-87. https://www.ncbi.nlm.nih.gov/pubmed/9264362/
- Expression of galectin-3 modulates T-cell growth and apoptosis. Yang RY, Hsu DK, Liu FT. Proc Natl Acad Sci U S A. 1996 Jun 25; 93(13):6737-42. https://www.ncbi.nlm.nih.gov/pubmed/8692888/
- Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Cancer Res. 1997 Dec 1; 57(23):5272-6. https://www.ncbi.nlm.nih.gov/pubmed/9393748/
- Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. J Exp Med. 1997 May 19; 185(10):1851-8. https://www.ncbi.nlm.nih.gov/pubmed/9151710/
- Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Demetriou M, Granovsky M, Quaggin S, Dennis JW. Nature. 2001 Feb 8; 409(6821):733-9. https://www.ncbi.nlm.nih.gov/pubmed/11217864/
- N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. Morgan R, Gao G, Pawling J, Dennis JW, Demetriou M, Li B. J Immunol. 2004 Dec 15; 173(12):7200-8. https://www.ncbi.nlm.nih.gov/pubmed/15585841/
- Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Cancer Cell. 2004 Mar; 5(3):241-51. https://www.ncbi.nlm.nih.gov/pubmed/15050916/
- Galectins: an evolutionarily conserved family of animal lectins with multifunctional properties; a trip from the gene to clinical therapy. Rabinovich GA. Cell Death Differ. 1999 Aug; 6(8):711-21. https://www.ncbi.nlm.nih.gov/pubmed/10467344/
- Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Am J Pathol. 2000 Mar; 156(3):899-909. https://www.ncbi.nlm.nih.gov/pubmed/10702407/
- Alteration of the glycosylation pattern of monocytic THP-1 cells upon differentiation and its impact on lectin-mediated drug delivery. Plattner VE, Ratzinger G, Engleder ET, Gallauner S, Gabor F, Wirth M. Eur J Pharm Biopharm. 2009 Nov; 73(3):324-30. https://www.ncbi.nlm.nih.gov/pubmed/19602437/
- Therapeutically targeting protein-glycan interactions. Rek A, Krenn E, Kungl AJ. Br J Pharmacol. 2009 Jul; 157(5):686-94. https://www.ncbi.nlm.nih.gov/pubmed/19371327/
- Gut flora in health and disease. Guarner F, Malagelada JR. Lancet. 2003 Feb 8; 361(9356):512-9. https://www.ncbi.nlm.nih.gov/pubmed/12583961/
- Polysaccharides in colon-specific drug delivery. Sinha VR, Kumria R. Int J Pharm. 2001 Aug 14; 224(1-2):19-38. https://www.ncbi.nlm.nih.gov/pubmed/11472812/
- National Cancer Institute at the National Institutes of Health. Mistletoe Extracts–Health Professional Version. https://www.cancer.gov/about-cancer/treatment/cam/hp/mistletoe-pdq/
- Jung ML, Baudino S, Ribéreau-Gayon G, et al.: Characterization of cytotoxic proteins from mistletoe (Viscum album L.). Cancer Lett 51 (2): 103-8, 1990. https://www.ncbi.nlm.nih.gov/pubmed/2344587?dopt=Abstract
- Kuttan G, Vasudevan DM, Kuttan R: Effect of a preparation from Viscum album on tumor development in vitro and in mice. J Ethnopharmacol 29 (1): 35-41, 1990. https://www.ncbi.nlm.nih.gov/pubmed/2345458?dopt=Abstract
- Walzel H, Jonas L, Rosin T, et al.: Relationship between internalization kinetics and cytotoxicity of mistletoe lectin I to L1210 leukaemia cells. Folia Biol (Praha) 36 (3-4): 181-8, 1990. https://www.ncbi.nlm.nih.gov/pubmed/2257937?dopt=Abstract
- Janssen O, Scheffler A, Kabelitz D: In vitro effects of mistletoe extracts and mistletoe lectins. Cytotoxicity towards tumor cells due to the induction of programmed cell death (apoptosis). Arzneimittelforschung 43 (11): 1221-7, 1993. https://www.ncbi.nlm.nih.gov/pubmed/8292069?dopt=Abstract
- Jurin M, Zarković N, Hrzenjak M, et al.: Antitumorous and immunomodulatory effects of the Viscum album L. preparation Isorel. Oncology 50 (6): 393-8, 1993 Nov-Dec. https://www.ncbi.nlm.nih.gov/pubmed/8233280?dopt=Abstract
- Schaller G, Urech K, Giannattasio M: Cytotoxicity of different viscotoxins and extracts from the European subspecies Viscum album L. Phytother Res 10 (6): 473-7, 1996.
- Gabius HJ, Darro F, Remmelink M, et al.: Evidence for stimulation of tumor proliferation in cell lines and histotypic cultures by clinically relevant low doses of the galactoside-binding mistletoe lectin, a component of proprietary extracts. Cancer Invest 19 (2): 114-26, 2001. https://www.ncbi.nlm.nih.gov/pubmed/11296616?dopt=Abstract
- Maier G, Fiebig HH: Absence of tumor growth stimulation in a panel of 16 human tumor cell lines by mistletoe extracts in vitro. Anticancer Drugs 13 (4): 373-9, 2002. https://www.ncbi.nlm.nih.gov/pubmed/11984083?dopt=Abstract
- Franz H: Mistletoe lectins and their A and B chains. Oncology 43 (Suppl 1): 23-34, 1986. https://www.ncbi.nlm.nih.gov/pubmed/3543782?dopt=Abstract
- Mengs U, Göthel D, Leng-Peschlow E: Mistletoe extracts standardized to mistletoe lectins in oncology: review on current status of preclinical research. Anticancer Res 22 (3): 1399-407, 2002 May-Jun. https://www.ncbi.nlm.nih.gov/pubmed/12168816?dopt=Abstract
- Lenartz D, Stoffel B, Menzel J, et al.: Immunoprotective activity of the galactoside-specific lectin from mistletoe after tumor destructive therapy in glioma patients. Anticancer Res 16 (6B): 3799-802, 1996 Nov-Dec. https://www.ncbi.nlm.nih.gov/pubmed/9042260?dopt=Abstract
- Fischer S, Scheffler A, Kabelitz D: Oligoclonal in vitro response of CD4 T cells to vesicles of mistletoe extracts in mistletoe-treated cancer patients. Cancer Immunol Immunother 44 (3): 150-6, 1997. https://www.ncbi.nlm.nih.gov/pubmed/9191874?dopt=Abstract
- Preisfeld A: Influence of aqueous mistletoe preparations on humoral immune parameters with emphasis on the cytotoxicity of human complement in breast cancer patients. Forsch Komplementarmed 4 (4): 224-8, 1997.
- Chernyshov VP, Omelchenko LI, Heusser P, et al.: Immunomodulatory actions of Viscum album (Iscador) in children with recurrent respiratory disease as a result of the Chernobyl nuclear accident. Complement Ther Med 5 (3): 141-6, 1997.
- Heiny BM, Albrecht V, Beuth J: Correlation of immune cell activities and beta-endorphin release in breast carcinoma patients treated with galactose-specific lectin standardized mistletoe extract. Anticancer Res 18 (1B): 583-6, 1998 Jan-Feb. https://www.ncbi.nlm.nih.gov/pubmed/9568181?dopt=Abstract
- Stein GM, Schaller G, Pfüller U, et al.: Characterisation of granulocyte stimulation by thionins from European mistletoe and from wheat. Biochim Biophys Acta 1426 (1): 80-90, 1999. https://www.ncbi.nlm.nih.gov/pubmed/9878694?dopt=Abstract
- Stein GM, Schaller G, Pfüller U, et al.: Thionins from Viscum album L: influence of the viscotoxins on the activation of granulocytes. Anticancer Res 19 (2A): 1037-42, 1999 Mar-Apr. https://www.ncbi.nlm.nih.gov/pubmed/10368652?dopt=Abstract
- Mistletoe. In: Murray MT: The Healing Power of Herbs. Roseville, Calif: Prima Publishing, 1995, pp 253-9.
- Lenartz D, Dott U, Menzel J, et al.: Survival of glioma patients after complementary treatment with galactoside-specific lectin from mistletoe. Anticancer Res 20 (3B): 2073-6, 2000 May-Jun. https://www.ncbi.nlm.nih.gov/pubmed/10928154?dopt=Abstract
- Steuer-Vogt MK, Bonkowsky V, Ambrosch P, et al.: The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: a randomised controlled clinical trial. Eur J Cancer 37 (1): 23-31, 2001. https://www.ncbi.nlm.nih.gov/pubmed/11165126?dopt=Abstract
- Goebell PJ, Otto T, Suhr J, et al.: Evaluation of an unconventional treatment modality with mistletoe lectin to prevent recurrence of superficial bladder cancer: a randomized phase II trial. J Urol 168 (1): 72-5, 2002. https://www.ncbi.nlm.nih.gov/pubmed/12050495?dopt=Abstract
- Stauder H, Kreuser ED: Mistletoe extracts standardised in terms of mistletoe lectins (ML I) in oncology: current state of clinical research. Onkologie 25 (4): 374-80, 2002. https://www.ncbi.nlm.nih.gov/pubmed/12232491?dopt=Abstract
- Pusztai A, Grant G, Gelencsér E, et al.: Effects of an orally administered mistletoe (type-2 RIP) lectin on growth, body composition, small intestinal structure, and insulin levels in young rats. J Nutr Biochem 9 (1): 31-6, 1998.
- Grossarth-Maticek R, Kiene H, Baumgartner SM, et al.: Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern Ther Health Med 7 (3): 57-66, 68-72, 74-6 passim, 2001 May-Jun. https://www.ncbi.nlm.nih.gov/pubmed/11347286?dopt=Abstract
- Jung ML, Baudino S, Ribéreau-Gayon G, et al.: Characterization of cytotoxic proteins from mistletoe (Viscum album L.). Cancer Lett 51 (2): 103-8, 1990. https://www.ncbi.nlm.nih.gov/pubmed/2344587?dopt=Abstract
- Schrader G, Apel K: Isolation and characterization of cDNAs encoding viscotoxins of mistletoe (Viscum album). Eur J Biochem 198 (3): 549-53, 1991. https://www.ncbi.nlm.nih.gov/pubmed/1710983?dopt=Abstract
- Gabius HJ, Gabius S, Joshi SS, et al.: From ill-defined extracts to the immunomodulatory lectin: will there be a reason for oncological application of mistletoe? Planta Med 60 (1): 2-7, 1994. https://www.ncbi.nlm.nih.gov/pubmed/8134410?dopt=Abstract
- Samtleben R, Hajto T, Hostanska K, et al.: Mistletoe lectins as immunostimulants (chemistry, pharmacology and clinic). In: Wagner H, ed.: Immunomodulatory Agents from Plants. Basel, Switzerland: Birkhauser Verlag, 1999, pp 223-41.
- Elluru SR, VAN Huyen JP, Delignat S, et al.: Antiangiogenic properties of viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res 29 (8): 2945-50, 2009. https://www.ncbi.nlm.nih.gov/pubmed/19661299?dopt=Abstract
- Horneber MA, Bueschel G, Huber R, et al.: Mistletoe therapy in oncology. Cochrane Database Syst Rev (2): CD003297, 2008. https://www.ncbi.nlm.nih.gov/pubmed/18425885?dopt=Abstract
- National Cancer Institute at the National Institutes of Health. Mistletoe Extracts–Health Professional Version. https://www.cancer.gov/about-cancer/treatment/cam/hp/mistletoe-pdq
- Khil LY, Kim W, Lyu S, et al.: Mechanisms involved in Korean mistletoe lectin-induced apoptosis of cancer cells. World J Gastroenterol 13 (20): 2811-8, 2007. https://www.ncbi.nlm.nih.gov/pubmed/17569116?dopt=Abstract
- Kim MS, Lee J, Lee KM, et al.: Involvement of hydrogen peroxide in mistletoe lectin-II-induced apoptosis of myeloleukemic U937 cells. Life Sci 73 (10): 1231-43, 2003. https://www.ncbi.nlm.nih.gov/pubmed/12850239?dopt=Abstract
- Choi SH, Lyu SY, Park WB: Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res 27 (1): 68-76, 2004. https://www.ncbi.nlm.nih.gov/pubmed/14969342?dopt=Abstract
- Romagnoli S, Fogolari F, Catalano M, et al.: NMR solution structure of viscotoxin C1 from Viscum album species Coloratum ohwi: toward a structure-function analysis of viscotoxins. Biochemistry 42 (43): 12503-10, 2003. https://www.ncbi.nlm.nih.gov/pubmed/14580196?dopt=Abstract
- Yoon TJ, Yoo YC, Kang TB, et al.: Antitumor activity of the Korean mistletoe lectin is attributed to activation of macrophages and NK cells. Arch Pharm Res 26 (10): 861-7, 2003. https://www.ncbi.nlm.nih.gov/pubmed/14609136?dopt=Abstract
- Ribéreau-Gayon G, Jung ML, Di Scala D, et al.: Comparison of the effects of fermented and unfermented mistletoe preparations on cultured tumor cells. Oncology 43 (Suppl 1): 35-41, 1986. https://www.ncbi.nlm.nih.gov/pubmed/3808575?dopt=Abstract
- Jäggy C, Musielski H, Urech K, et al.: Quantitative determination of lectins in mistletoe preparations. Arzneimittelforschung 45 (8): 905-9, 1995. https://www.ncbi.nlm.nih.gov/pubmed/7575759?dopt=Abstract
- Zee-Cheng RK: Anticancer research on Loranthaceae plants. Drugs Future 22 (5): 519-30, 1997.
- Kaegi E: Unconventional therapies for cancer: 3. Iscador. Task Force on Alternative Therapies of the Canadian Breast Cancer Research Initiative. CMAJ 158 (9): 1157-9, 1998. https://www.ncbi.nlm.nih.gov/pubmed/9597967?dopt=Abstract
- Viscum album. In: Homoeopathic Pharmacopoeia Convention of the United States: Homoeopathic Pharmacopoeia of the United States. Washington, DC: 2002, Monograph 9444 Visc.
- Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N. What should be called a lectin? Nature. 1980;285:66. doi: 10.1038/285066b0.
- Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S. Properties and biomedical applications. In: A handbook of plant lectins. Chichester, UK: John Wiley and Sons, 1998.
- Critical Reviews in Plant Sciences Volume 17, 1998 – Issue 6. Plant Lectins: A Composite of Several Distinct Families of Structurally and Evolutionary Related Proteins with Diverse Biological Roles. http://www.tandfonline.com/doi/abs/10.1080/07352689891304276
- EtzlerME, LienerIE, SharonN, GoldsteinIJ, eds. Distribution and function of plant lectins. In: The lectins: properties, functions and Application in Biology and Medicine. Orlando, FL USA: Academic Press, 1986: 371-435.
- Lectins and the soybean-Rhizobium symbiosis. I. Immunological investigations of soybean lines, the seeds of which have been reported to lack the 120 000 dalton soybean lectin. Su LC, Pueppke SG, Friedman HP. Biochim Biophys Acta. 1980 May 7; 629(2):292-304. https://www.ncbi.nlm.nih.gov/pubmed/7190028/
- Lectins as plant defense proteins. Peumans WJ, Van Damme EJ. Plant Physiol. 1995 Oct; 109(2):347-52. https://www.ncbi.nlm.nih.gov/pubmed/7480335/
- Purification and partial characterization of a lectin from Euphorbia neriifolia latex. Seshagirirao K, Prasad MN. Biochem Mol Biol Int. 1995 May; 35(6):1199-204. https://www.ncbi.nlm.nih.gov/pubmed/7492957/
- Sharon N, Lis H. Lectins. 2nd ed. Dordrecht ; London, Kluwer Academic Publishers; 2003. p. xviii, 454 p
- Kolberg J, Wedege E, Sollid L. Immunoblotting detection of lectins in gluten and white rice flour. Biochem Biophys Res Commun. 1987;142:717–723. https://www.ncbi.nlm.nih.gov/pubmed/3827897
- Silano M, De Vincenzi M. Bioactive antinutritional peptides derived from cereal prolamins: a review. Nahrung. 1999;43:175–184. https://www.ncbi.nlm.nih.gov/pubmed/10399351
- Di Cagno R, De Angelis M, Lavermicocca P, De Vincenzi M, Giovannini C, Faccia M, Gobbetti M. Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl Environ Microbiol. 2002;68:623–633. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC126681/
- Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr Pharm Des. 2003;9:175–191. https://www.ncbi.nlm.nih.gov/pubmed/12570667
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–958. https://www.ncbi.nlm.nih.gov/pubmed/11912237
- Minetti M, Aducci P, Teichner A. A new agglutinating activity from wheat flour inhibited by tryptophan. Biochim Biophys Acta. 1976;437:505–517. https://www.ncbi.nlm.nih.gov/pubmed/952930
- Nachbar MS, Oppenheim JD. Lectins in the United States diet: a survey of lectins in commonly consumed foods and a review of the literature. Am J Clin Nutr. 1980;33:2338–2345. https://www.ncbi.nlm.nih.gov/pubmed/7001881
- Van Damme JME. Handbook of plant lectins : properties and biomedical applications. Chichester, John Wiley; 1998. p. xiv, 452p : ill ; 26cm.
- Sharon N, Lis H. Lectins. 2nd ed. Dordrecht ; London, Kluwer Academic Publishers; 2003. p. xviii, 454 p.
- Hedo JA, Harrison LC, Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981;20:3385–3393. https://www.ncbi.nlm.nih.gov/pubmed/7260043
- Fujii M, Sugamura K, Nakamura M, Ishii T, Hinuma Y. Selective inhibition of high- but not low-affinity interleukin 2 binding by lectins and anti-interleukin 2 receptor alpha antibody. Microbiol Immunol. 1988;32:857–867. https://www.ncbi.nlm.nih.gov/pubmed/3143899
- Differential response of the epidermal growth factor receptor tyrosine kinase activity to several plant and mammalian lectins. Zeng FY, Benguría A, Kafert S, André S, Gabius HJ, Villalobo A. Mol Cell Biochem. 1995 Jan 26; 142(2):117-24. https://www.ncbi.nlm.nih.gov/pubmed/7770063/
- Mechanisms and assessment of lectin-mediated mitogenesis. Kilpatrick DC. Mol Biotechnol. 1999 Feb; 11(1):55-65. https://www.ncbi.nlm.nih.gov/pubmed/10367282/
- Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase C gamma 2 activation by Src family kinases. Ohmori T, Yatomi Y, Wu Y, Osada M, Satoh K, Ozaki Y. Biochemistry. 2001 Oct 30; 40(43):12992-3001. https://www.ncbi.nlm.nih.gov/pubmed/11669637/
- Effects of wheatgerm agglutinin and aging on the regional brain uptake of HIV-1GP120. Banks WA, Ibrahimi F, Farr SA, Flood JF, Morley JE. Life Sci. 1999; 65(1):81-9. https://www.ncbi.nlm.nih.gov/pubmed/10403496/
- Uptake and transport of lectins from the cerebrospinal fluid by cells of the immature mouse brain. Mares V, Borges LF, Sidman RL. Acta Histochem. 1984; 74(1):11-9. https://www.ncbi.nlm.nih.gov/pubmed/6428128/
- Freed DLJ. Lectins in food: Their importance in health and disease. Journal of Nutritional Medicine. 1991;2:45–65.
- Modulation of immune function by dietary lectins in rheumatoid arthritis. Cordain L, Toohey L, Smith MJ, Hickey MS. Br J Nutr. 2000 Mar; 83(3):207-17. https://www.ncbi.nlm.nih.gov/pubmed/10884708/
- Pancreatic duct glands. II. Lectin binding affinities of ductular epithelium, ductular glands, and Brunner glands. Geleff S, Böck P. Histochemistry. 1984; 80(1):31-8. https://www.ncbi.nlm.nih.gov/pubmed/6698813/
- The interaction between wheat germ agglutinin and other plant lectins with prostate cancer cells Du-145. Gabor F, Klausegger U, Wirth M. Int J Pharm. 2001 Jun 19; 221(1-2):35-47. https://www.ncbi.nlm.nih.gov/pubmed/11397565/
- Lectin binding to distinguish cell types in fixed atherosclerotic arteries. Davis HR, Glagov S. Atherosclerosis. 1986 Sep; 61(3):193-203. https://www.ncbi.nlm.nih.gov/pubmed/3533093/
- Differential lectin binding on walls of thoraco-cervical blood vessels and lymphatics in rats. Kagami H, Uryu K, Okamoto K, Sakai H, Kaneda T, Sakanaka M. Okajimas Folia Anat Jpn. 1991 Aug; 68(2-3):161-70. https://www.ncbi.nlm.nih.gov/pubmed/1758681/
- Do dietary lectins cause disease? Freed DL. BMJ. 1999 Apr 17; 318(7190):1023-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1115436/
- Natural human antibodies to dietary lectins. Tchernychev B, Wilchek M. FEBS Lett. 1996 Nov 18; 397(2-3):139-42. https://www.ncbi.nlm.nih.gov/pubmed/8955334/
- Jönsson T, Olsson S, Ahrén B, Bøg-Hansen TC, Dole A, Lindeberg S. Agrarian diet and diseases of affluence – Do evolutionary novel dietary lectins cause leptin resistance? BMC Endocrine Disorders. 2005;5:10. doi:10.1186/1472-6823-5-10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1326203/
- Legume lectin structure. Loris R, Hamelryck T, Bouckaert J, Wyns L. Biochim Biophys Acta. 1998 Mar 3; 1383(1):9-36. https://www.ncbi.nlm.nih.gov/pubmed/9546043/
- Fialová D., Tichá M., Kocourek J. Studies on phytohemagglutinins XXVI. A new type of a lentil hemagglutinin isolated from Lens esculenta Moench., subsp. Microsperma (Baumg.) barulina. Biochim. Biophys. Acta. 1975;393:170–181. doi: 10.1016/0005-2795(75)90228-7
- Rougé P. Ph.D. Thesis. Université Paul Sabatier; Toulouse, France: Jan 27, 1977. Biologie des Hémagglutinines chez le Pois (Pisum sativum L.).
- Trembley R., Feng M., Menassa R., Huner N.P.A., Jevnikar A.M., Ma S. High-yield expression of recombinant soybean agglutinin in plants using transient and stable systems. Transgenic Res. 2011;20:345–356. doi: 10.1007/s11248-010-9419-0
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea) J. Biol. Chem. 1975;250:8518–8523
- Etzler M.E. The Dolichos biflorus lectin family: A model system for studying legume lectin structure and function. In: Van Driessche E., Rougé P., Beeckmans S., Bøg-Hansen T.C., editors. Lectins, Biology-Biochemistry, Clinical Biochemistry. Volume 11. Textop; Hellerup, Denmark: 1996. pp. 3–9.
- Rigas D.A., Osgood E.E. Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J. Biol. Chem. 1955;212:607–616. doi: 10.1016/0002-9343(51)90387-7
- LeVine D., Kaplan M.J., Greenaway P.J. The purification and characterization of wheat germ agglutinin. Biochem. J. 1972;129:847–856. doi: 10.1042/bj1290847
- Peumans W.J., Van Damme E.J.M. The role of lectins in the defense of plants. In: Van Driessche E., Franz H., Beeckmans S., Pfüller U., Kallikorm A., Bøg-Hansen T.C., editors. Lectins, Biology-Biochemistry, Clinical Biochemistry. Volume 8. Textop; Hellerup, Denmark: 1993. pp. 82–91.
- Nikolic J., Mrkic I., Grozdanovic M., Popovic M., Petersen A., Jappe U., Gavrovic-Jankulovic M. Protocol for simultaneous isolation of three important banana allergens. J. Chromatogr. B. 2014;962:30–36. doi: 10.1016/j.jchromb.2014.05.020
- Sànchez-Monge R., Blanco C., Díaz-Perales A., Collada C., Carrillo T., Aragoncillo C., Salcedo G. Isolation and characterization of major banana allergens: Identification as fruit class I chitinases. Clin. Exp. Allergy. 1999;29:673–680. doi: 10.1046/j.1365-2222.1999.00526.x
- Sutton R., Skerritt J.H., Baldo B.A., Wrigley C.W. The diversity of allergens involved in baker’s asthma. Clin. Allergy. 1984;14:93–107. doi: 10.1111/j.1365-2222.1984.tb02196.x
- Volpicella M., Leoni C., Fanizza I., Distaso M., Leoni G., Farioli L., Naumann T., Pastorello E., Ceci L.R. Characterization of maize chitinase-A, a tough allergenic molecule. Allergy. 2017;72:1423–1429. doi: 10.1111/all.13164
- Hänninen A.R., Mikkola J.H., Kalkkinen N., Turjanmaa K., Ylitalo L., Reunala T., Palosuo T. Increased allergen production in turnip (Brassica rapa) by treatments activating defense mechanisms. J. Allergy Clin. Immunol. 1999;104:194–201. doi: 10.1016/S0091-6749(99)70135-1
- Bernstein D.I., Biagini R.E., Karnani R., Hamilton R., Murphy K., Bernstein C., Arif S.A., Berendts B., Yeang H.Y. In vivo sensitization to purified Hevea brasiliensis proteins in health care workers sensitized to natural rubber latex. J. Allergy Clin. Immunol. 2003;111:610–616. doi: 10.1067/mai.2003.164
- Sowka S., Hsieh L.S., Krebitz M., Akasawa A., Martin B.M., Starrett D., Peterbauer C.K., Scheiner O., Breiteneder H. Identification and cloning of Pers a 1, a 32-kDa endochitinase and major allergen of avocado, and its expression in yeast Pichia pastoris. J. Biol. Chem. 1998;273:28091–28097. doi: 10.1074/jbc.273.43.28091
- Bublin M., Mari A., Ebner C., Knulst A., Scheiner O., Hoffmann-Sommergruber K., Breiteneder H., Radauer C. IgE sensitization profiles toward green and gold kiwifruits differ among patients allergic to kiwifruit from 3 European countries. J. Allergy Clin. Immunol. 2004;114:1169–1175. doi: 10.1016/j.jaci.2004.07.016
- Chen Y.T., Hsu L.H., Huang I.P., Tsai T.C., Lee G.C., Shaw J.F. Gene cloning and characterization of a novel recombinant antifungal chitinase from papaya (Carica papaya) J. Agric. Food Chem. 2007;55:714–722. doi: 10.1021/jf062453e
- Díaz-Perales A., Collada C., Blanco C., Sanchez-Monge R., Carrillo T., Aragoncilllo C., Salcedo G. Cross-reactions in the latex-fruit syndrome: A relevant role of chitinase but not of complex asparagine-linked glycans. J. Allergy Clin. Immunol. 1999;104:681–687. doi: 10.1016/S0091-6749(99)70342-8
- Pastorello E.A., Farioli L., Pravettoni V., Ortolani C., Fortunato D., Giuffrida M.G., Perono Garoffo L., Calamari A.M., Brenna O., Conti A. Identification of grape and wine allergens as an endochitinase 4, a lipid-transfer protein, and a thaumatin. J. Allergy Allergy Clin. Immunol. 2003;111:350–359. doi: 10.1067/mai.2003.35
- Albanesi M., Pasculli C., Giliberti L., Rossi M.P., Di Bona D., Caiaffa M.F., Macchia L. Immunological characterization of onion (Allium cepa) allergy. Adv. Dermatol. Allergol. 2019;XXXVI:98–103. doi: 10.5114/ada.2019.82829
- Clement F., Pramod S.N., Venkatesh Y.P. Identity of the immunomodulatory proteins from garlic (Allium sativum) with the major garlic lectins or agglutinins. Int. Immunopharmacol. 2010;10:316–324. doi: 10.1016/j.intimp.2009.12.002
- Burks A.W., Cockrell G., Connaughton C., Guin J., Allen W., Helm R.M. Identification of peanut agglutinin and soybean trypsin inhibitor as minor legume allergens. Int. Arch. Allergy Immunol. 1994;105:143–149. doi: 10.1159/000236816
- Pramod S.N., Krishnakantha T.P., Venkatesh Y.P. Effect of horse gram lectin (Dolichos biflorus agglutinin) on degranulation of mast cells and basophils of atopic subjects: Identification as an allergen. Int. Immunopharmacol. 2006;6:1714–1722. doi: 10.1016/j.intimp.2006.07.006
- Natarajan S., Xu C., Bae H., Caperna T.J., Garrett W.M. Proteomic analysis of allergen and antinitritional proteins in wild and cultivated soybean seeds. J. Plant Biochem. Biotechnol. 2006;15:103–108. doi: 10.1007/BF03321912
- Haas H., Falcone F.H., Schramm G., Haisch K., Gibbs B.F., Klaucke J., Pöppelmann M., Becker W.-M., Gabius H.-J., Schlaak M. Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur. J. Immunol. 1999;29:918–927. doi: 10.1002/(SICI)1521-4141(199903)29:03<918::AID-IMMU918>3.0.CO;2-T
- García Jiménez S., Pastor Vargas C., de la Heras M., Sanz Matoto A., Vivanco F., Sastre J. Allergen characterization of chia seeds (Salvia hispanica), a new allergenic food. J. Investig. Allergol. Clin. Immunol. 2015;25:55–56.
- Koshte V.L., Aalbers M., Calkhoven P.G., Aalberse R.C. The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int. Arch. Allergy Immunol. 1992;97:17–24. doi: 10.1159/000236090
- González-Mancebo E., López-Torrejón G., González de Olano D., Santos S., Gandolfo-Cano M., Meléndez A., Salcedo G., Cuesta-Herranz J., Vivanco F., Pastor-Vargas C. Identification of potential allergens involved in systemic reactions to melon and watermelon. Ann. Allergy Asthma Immunol. 2010;104:271–272. doi: 10.1016/j.anai.2010.01.011
- Pomés, A., Davies, J. M., Gadermaier, G., Hilger, C., Holzhauser, T., Lidholm, J., Lopata, A. L., Mueller, G. A., Nandy, A., Radauer, C., Chan, S. K., Jappe, U., Kleine-Tebbe, J., Thomas, W. R., Chapman, M. D., van Hage, M., van Ree, R., Vieths, S., Raulf, M., Goodman, R. E., … WHO IUIS Allergen Nomenclature Sub-Committee (2018). WHO/IUIS Allergen Nomenclature: Providing a common language. Molecular immunology, 100, 3–13. https://doi.org/10.1016/j.molimm.2018.03.003
- Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins. Pusztai A, Ewen SW, Grant G, Brown DS, Stewart JC, Peumans WJ, Van Damme EJ, Bardocz S. Br J Nutr. 1993 Jul; 70(1):313-21. https://www.ncbi.nlm.nih.gov/pubmed/8399111/
- Freed D.L.J. Lectins in food: Their importance in health and disease. J. Nutr. Med. 1991;2:45–64. doi: 10.3109/13590849109084100.
- Miyake K, Tanaka T, McNeil PL. Lectin-Based Food Poisoning: A New Mechanism of Protein Toxicity. Steinhardt R, ed. PLoS ONE. 2007;2(8):e687. doi:10.1371/journal.pone.0000687. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1933252/
- Antinutritional properties of plant lectins. Vasconcelos IM, Oliveira JT. Toxicon. 2004 Sep 15; 44(4):385-403. https://www.ncbi.nlm.nih.gov/pubmed/15302522/
- In vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Lorenzsonn V, Olsen WA. Gastroenterology. 1982 May; 82(5 Pt 1):838-48. https://www.ncbi.nlm.nih.gov/pubmed/6895878/
- Binding of the insecticidal lectin Concanavalin A in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cells. Sauvion N, Nardon C, Febvay G, Gatehouse AM, Rahbé Y. J Insect Physiol. 2004 Dec; 50(12):1137-50. https://www.ncbi.nlm.nih.gov/pubmed/15670861/
- Cereal grains: humanity’s double-edged sword. Cordain L. World Rev Nutr Diet. 1999; 84():19-73. https://www.ncbi.nlm.nih.gov/pubmed/10489816/
- Rattray EAS, Palmer R, Pusztai A. Toxicity of kidney beans (Phaseolus vulgaris L). to conventional and gnotobiotic rats. J Sci Food Agric. 1974;25:1035–40. doi: 10.1002/jsfa.2740250819. https://www.ncbi.nlm.nih.gov/pubmed/4416675
- Moreno A.N., Jamur M.C., Oliver C., Roque-Barreira M.C. Mast cell degranulation induced by lectins: Effect on neutrophil recruitment. Int. Arch. Allergy Immunol. 2003;132:221–230. doi: 10.1159/000074303
- Van Damme E.J.M., Rougé P., Peumans W.J. Plant lectins. In: Kamerling J.P., Boons G.J., Lee Y.C., Suzuki A., Taniguchi N., Voragen A.G.I., editors. Carbohydrate-protein interactions: Plant Lectins. Elsevier; New York, NY, USA: 2007. pp. 564–599.
- Singh R, Nawale L, Sarkar D, Suresh CG. Two Chitotriose-Specific Lectins Show Anti-Angiogenesis, Induces Caspase-9-Mediated Apoptosis and Early Arrest of Pancreatic Tumor Cell Cycle. Facchiano A, ed. PLoS ONE. 2016;11(1):e0146110. doi:10.1371/journal.pone.0146110. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4721955/
- Lectins as bioactive plant proteins: a potential in cancer treatment. De Mejía EG, Prisecaru VI. Crit Rev Food Sci Nutr. 2005; 45(6):425-45. https://www.ncbi.nlm.nih.gov/pubmed/16183566/
- Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S. Handbook of plant lectins: properties and biomedical applications. London: Wiley; 1998. pp. 31–50.
- Freed DLJ. Do dietary lectins cause disease? : The evidence is suggestive—and raises interesting possibilities for treatment. BMJ : British Medical Journal. 1999;318(7190):1023-1024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1115436/
- Campos-Vega, R., Loarca-Piña, G., & Oomah, B. D. (2010). Minor components of pulses and their potential impact on human health. Food Research International, 43, 461–482. doi:10.1016/j.foodres.2009.09.004. http://www.sciencedirect.com/science/article/pii/S0963996909002695
- Paredes, M., Becerra, V., & Tay, J. (2009). Inorganic nutritional composition of common bean (Phaseolus vulgaris L.) genotypes race Chile. Chilean Journal of Agricultural Research, 69, 486–495. doi:10.4067/S0718-58392009000400002. http://www.inia.cl/recursosgeneticos/publicaciones/cultivadas/Paredes%20etal%202009.pdf
- Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR Jr, Spiegelman D, Willett W, Rimm E. Am J Clin Nutr. 2004 Nov; 80(5):1237-45. https://www.ncbi.nlm.nih.gov/pubmed/15531671/
- Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Am J Clin Nutr. 2003 Nov; 78(5):920-7. https://www.ncbi.nlm.nih.gov/pubmed/14594777/
- Intake of whole grain in Scandinavia: intake, sources and compliance with new national recommendations. Kyrø C, Skeie G, Dragsted LO, Christensen J, Overvad K, Hallmans G, Johansson I, Lund E, Slimani N, Johnsen NF, Halkjær J, Tjønneland A, Olsen A. Scand J Public Health. 2012 Feb; 40(1):76-84. https://www.ncbi.nlm.nih.gov/pubmed/21976053/
- Putting the whole grain puzzle together: health benefits associated with whole grains–summary of American Society for Nutrition 2010 Satellite Symposium. Jonnalagadda SS, Harnack L, Liu RH, McKeown N, Seal C, Liu S, Fahey GC. J Nutr. 2011 May; 141(5):1011S-22S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078018/