Blood brain barrier
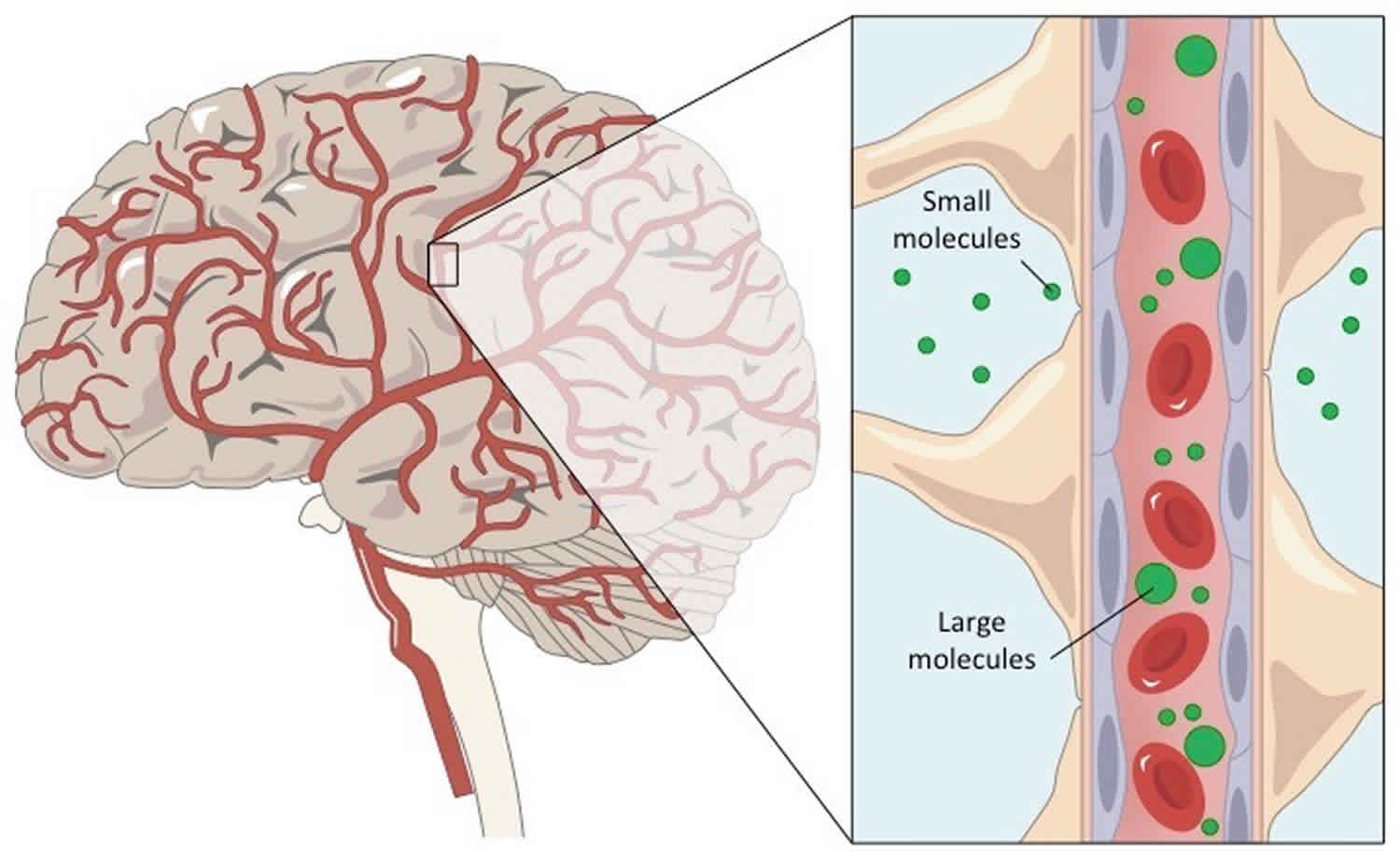
Blood-brain barrier (BBB) is a membranic structure that acts primarily to protect the brain from chemicals in the blood, while still allowing essential metabolic function. The blood-brain barrier is the barrier between the cerebral capillary blood and the interstitial fluid of the brain 1. The blood brain barrier derives from the relative impermeability of brain capillaries (complete tight junctions between epithelial cells). Blood-brain barrier (BBB) lets water, oxygen, nutrients, and fat-soluble molecules enter the neural tissue but prevents entry of most harmful substances. As the interface between the blood and the central nervous system (CNS), the blood brain barrier is critical for maintaining a steady state environment in the central nervous system (brain and spinal cord).
Blood-brain barrier is composed of endothelial cells that make up the walls of the brain capillaries, which are packed very tightly in brain capillaries. Astrocyte cells called astrocytic feet surround the endothelial cells of the blood-brain barrier (BBB), providing biochemical support to those cells. The blood-brain barrier is distinct from the similar blood-cerebrospinal fluid barrier, a function of the choroid plexus. The epithelial cells in brain capillaries are joined together around their entire perimeters by tight junctions, making these capillaries the least permeable capillaries in the body. Even so, the blood brain barrier is not an absolute barrier. All nutrients (including oxygen) and ions needed by the neurons pass through, some by special transport mechanisms in the plasma membranes of the capillary epithelial cells. Furthermore, because the barrier is ineffective against fat-soluble molecules, which easily diffuse through all cell membranes, the barrier allows
alcohol, nicotine, and anesthetic agents to reach brain neurons.
The brain has a rich supply of capillaries that provide its nervous tissue with nutrients, oxygen, and all other vital molecules. However, some bloodborne molecules that can cross other capillaries of the body cannot cross the brain capillaries. Bloodborne toxins, such as urea, mild toxins from food, and bacterial toxins, are prevented from entering brain tissue by the blood brain barrier, which protects the neurons of the central nervous system (brain and spinal cord).
Figure 1. Blood brain barrier
Blood brain barrier cells
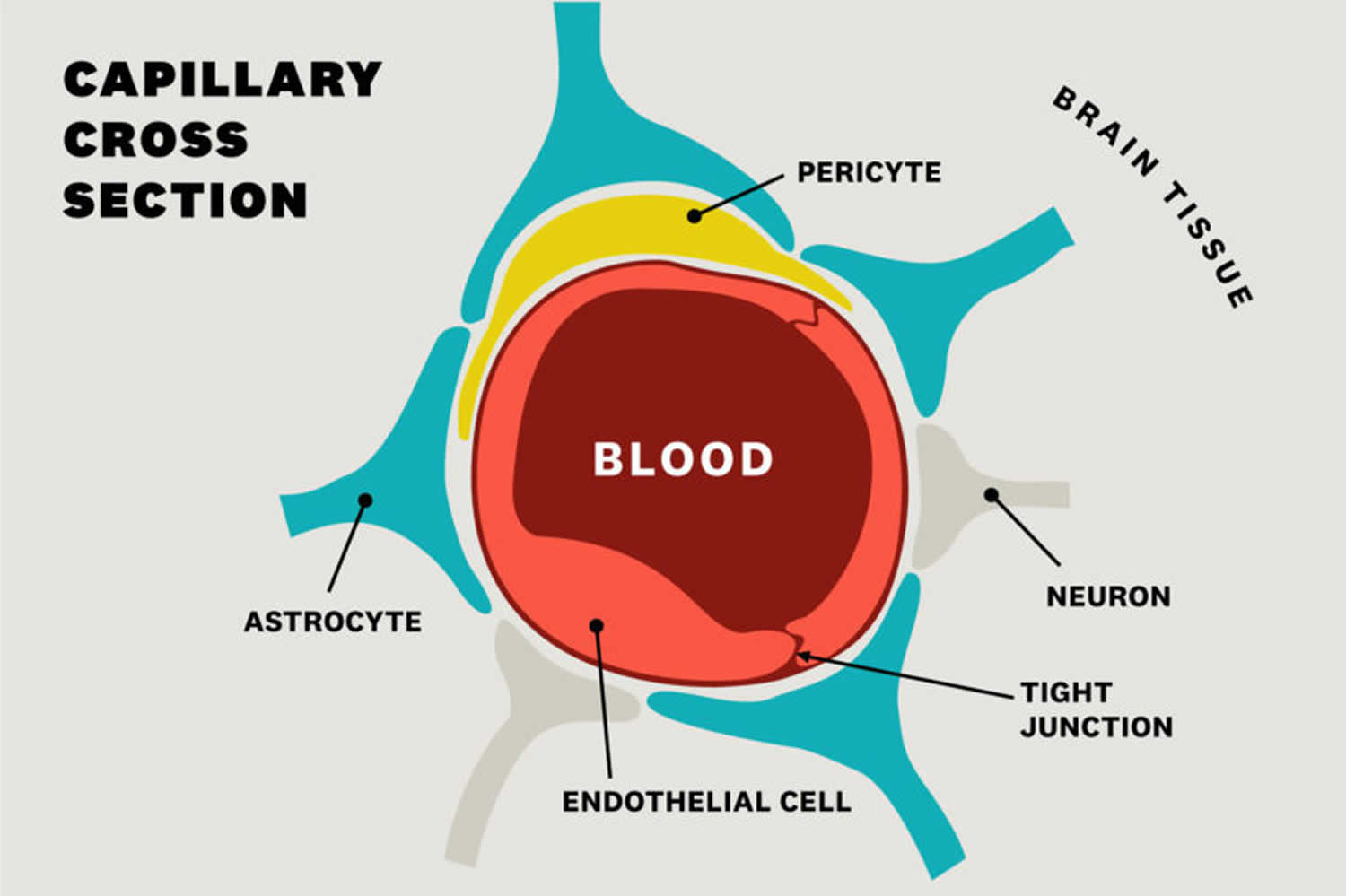
Blood brain barrier is made up of capillary endothelial cells and basement membrane, neuroglial membrane, and glial podocytes, i.e., projections of astrocytes. These 3 components work in synchronicity with one another to limit entry of various substances into the cerebral blood flow and subsequently the brain parenchyma.
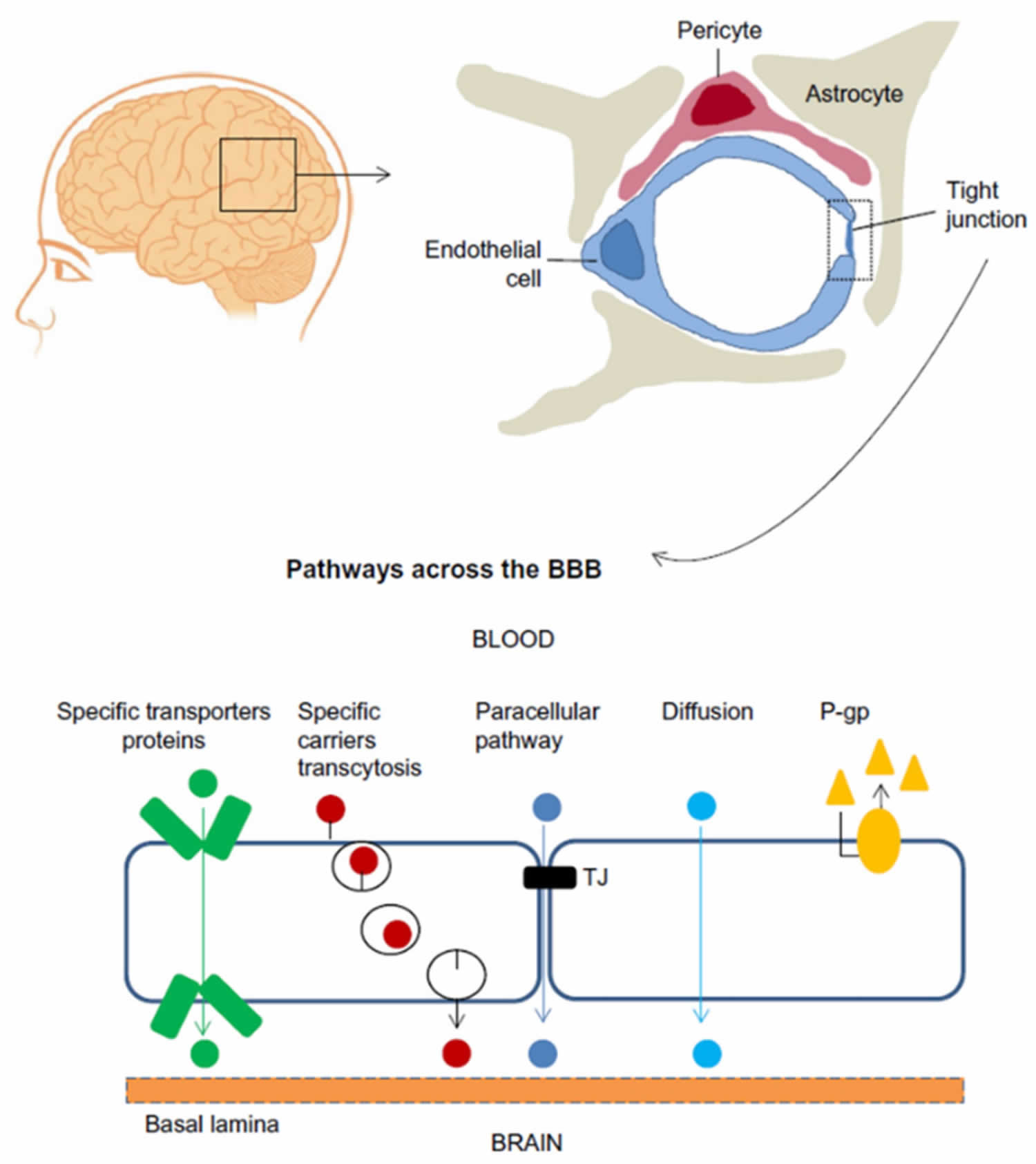
The blood-brain barrier (BBB) is a highly selective barrier that regulates passive and active transport between the brain parenchyma and peripheral blood 2. The blood-brain barrier plays a vital role in maintaining the physical and chemical homeostasis of the central nervous system (CNS), and protects the central nervous system from harmful molecules and pathogens in the blood 3. The blood-brain barrier is a complex dynamic physiological structure network, the molecular basis that underlie blood-brain barrier function depend on close interactions between adjacent brain microvascular endothelial cells 4. These cells form a layer that is tightly sealed by a junctional complex composed of tight junctions and adherens junctions 5. Tight junctions, which play an essential role in maintaining blood-brain barrier integrity, are structures formed by at least three different types of transmembrane proteins, such as occludin, claudin and junctional adhesive molecule 6. Brain microvascular endothelial cells, along with neurons, pericytes, astrocytes, microglial cells, and extracellular matrix, constitute a functional network known as the neurovascular unit 7, Figure 2 show the cellular constituents and transport pathways of the blood-brain barrier (BBB) 8. Due to the tight barrier, a number of drugs are unable to penetrate the blood-brain barrier and produce therapeutic effects in the central nervous system (CNS) 9. Disruption or dysfunction of the blood-brain barrier has been linked to a wide range of neurological disorders, including brain tumors, epilepsy, ischemic stroke, Alzheimer’s disease, and multiple sclerosis 10.
Figure 2. Blood brain barrier cells
Footnote: Cellular constituents of the blood-brain barrier (BBB). The blood-brain barrier is formed by brain microvascular endothelial cells, which are connected by tight junctions. The endothelium, together with the basal lamina, pericytes, and astrocytic end-feet forms the neurovascular unit. Some substances diffuse freely into and out of the brain parenchyma, others such as nutrients need specific transporters, while molecules such as insulin, leptin and transferrin are transported by receptor- mediated transcytosis.
[Source 4 ]Blood brain barrier function
Central nervous system (brain and spinal cord) structures are highly unique in structure and function, and therefore require a stable environment with a composition that differs from that of the peripheral circulation. For this reason, the blood-brain barrier exists to maintain a homeostatic environment in which central nervous system structures can function without disruption from other bodily functions. The blood-brain barrier functions as a semipermeable membrane that separates the peripheral blood from the cerebrospinal fluid (CSF) to maintains homeostasis within the central nervous system. It accomplishes this through several mechanisms that regulate the composition and volume of the cerebral structures.
The blood-brain barrier has several important functions that ensure a homeostatic environment within the central nervous system (brain and spinal cord). It does so primarily by regulating the composition and volume of the cerebrospinal fluid (CSF) which surrounds structures within the central nervous system (brain and spinal cord). It does so by utilizing tight junctions between endothelial cells lining the capillaries, highly specific transport proteins that are embedded into the basement membrane, and cellular enzymes that function to alter substances during passage through the cells.
Tight junctions exist between the endothelial cells of the blood-brain barrier, which permit passage of only a select few types of substances between the cells. Furthermore, only a select number of substances can pass through the endothelial cells. Such substances include lipid-soluble substances (e.g., oxygen, carbon dioxide). Hydrophilic substances, for example, hydron and bicarbonate, are not permitted to pass through cells and across the blood-brain barrier.
The capillaries in the central nervous system (brain and spinal cord) are continuous capillaries which lack fenestrations and have a continuous basal lamina. They contain only a few pinocytic vesicles, which distinguishes them from other continuous capillaries of the body and makes them well-suited to make up a selective barrier such as the blood-brain barrier.
The blood-brain barrier also prevents the entry of toxins and foreign substances from entering the central nervous system (brain and spinal cord).
Receptor-mediated transport allows glucose, ions, and other special molecules to cross the blood-brain barrier. Most large molecules and proteins are precluded from entering the barrier.
The blood-brain barrier is considered to be weaker in some areas, and thus these areas are more susceptible to changes in levels of metabolites in the blood 11. Such areas include:
- Pineal gland secretes melatonin
- Neurohypophysis: secretes neurohormones (e.g., oxytocin, vasopressin/ADH)
- Area postrema, also called the “emetic center” is triggered by toxins in the blood to cause vomiting
- Choroid plexus is specialized brain tissue that has its filtration system
Blood brain barrier clinical significance
Vasogenic edema can occur with disruption of the integrity of the blood-brain barrier. Such disruption can cause fluid shifts from the vascular compartment to the extracellular compartment. According to the Monro-Kellie doctrine, the sum of the volumes of the brain tissue, CSF, and intracranial blood is constant. Any increase in one of these 3 components will always be at the expense of the other 2. In the case of vasogenic edema, volume shifts into the extracellular compartments of the brain will cause increased intracranial pressure, resulting in compression of brain structures and vasculature 12.
Normally, ammonia enters the portal circulation and is converted by the liver to urea. However, when a hepatocellular disease is present, for example, in patients with hepatic encephalopathy, ammonia does not get converted to urea; it is instead shunted through the portosystemic collateral vessels into systemic circulation, where it traverses the blood-brain barrier and induces neuronal edema and resultant hepatic encephalopathy. ATP-binding cassette transporters prevent the brain from accumulating toxins by pumping them out of the brain. Accumulation of toxins such as ammonia during hepatic encephalopathy or other cases of severe liver disease supports the conclusion that liver diseases alter the expression and function of ATP-binding cassette transporters at the blood-brain barrier. There is growing evidence to suggest that altered ATP-binding cassette transporter expression is implicated in the development of hepatic encephalopathy 13.
The blood-brain barrier is impermeable to hydrogen ions and bicarbonate; therefore, these ions become trapped in the vascular compartment and do not enter into cerebral circulation. However, the blood-brain barrier is permeable to carbon dioxide (CO2). CO2 can pass into the brain and become converted into hydron and bicarbonate molecules. In this way, increases in arterial CO2 (PCO2) result in increases in the PCO2 of the CSF, which ultimately leads to increased H+ concentration in the CSF and consequent decrease in pH 14.
Many vasoactive substances do not affect the cerebral circulation because they cannot cross the blood-brain barrier due to their large molecular size. Leptin, a molecule secreted by adipocytes, can cross the blood-brain barrier and act on neurons of the arcuate nucleus of the hypothalamus to stimulate anorexigenic neurons and inhibit orexigenic neurons, resulting in decreased appetite and increased energy expenditure 15.
Dimethyl fumarate is a drug used to treat multiple sclerosis. It works by reducing transendothelial migration of activated leukocytes through the blood-brain barrier. It also has neuroprotective effects via activation of antioxidant pathways 16.
Use of contrast agents (e.g., gadolinium) can identify breakdown of the blood-brain barrier in patients with acute multiple sclerosis exacerbations.
Neuromyelitis optica, or “Devic’s disease,” is a disease involving the synchronous or near-synchronous development of bilateral optic neuritis and spinal cord demyelination. It results in elevated serum levels of aquaporin 4 antibodies (AQP4 antibodies). Aquaporin 4 (AQP4) is a protein found in astrocytic foot processes surrounding blood vessels that are involved in the maintenance of the blood-brain barrier 17.
L-Dopa can cross the blood-brain barrier; dopamine cannot. Carbidopa is a drug that cannot cross the blood-brain barrier; thus, it is a preferred treatment for Parkinson disease because of its exclusively peripheral action 18.
Physostigmine is an inhibitor of acetylcholinesterase (AChE) that acts at the catalytic site with the substrate and competes with substrate binding to the AChE enzyme. It can cross the blood-brain barrier 19.
Examples of drugs that can penetrate the blood-brain barrier to treat CNS infections 20:
- Isoniazid
- Pyrazinamide
- Linezolid
- Metronidazole
- Fluconazole
- Fluoroquinolones (some).
- Dotiwala AK, McCausland C, Samra NS. Anatomy, Head and Neck, Blood Brain Barrier. [Updated 2019 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519556
- Vargas-Osorio, Z.; Da Silva-Candal, A.; Piñeiro, Y.; Iglesias-Rey, R.; Sobrino, T.; Campos, F.; Castillo, J.; Rivas, J. Multifunctional Superparamagnetic Stiff Nanoreservoirs for Blood Brain Barrier Applications. Nanomaterials 2019, 9, 449.
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372.
- Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines 2019, 10(6), 375; https://doi.org/10.3390/mi10060375
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356.
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019, 317, 260–270.
- Ozaki, T.; Nakamura, H.; Kishima, H. Therapeutic strategy against ischemic stroke with the concept of neurovascular unit. Neurochem. Int. 2019, 126, 246–251.
- Guerra, M.; Blázquez, J.L.; Rodríguez, E.M. Blood-brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS 2017, 14, 19.
- Singh, A.; Kim, W.; Kim, Y.; Jeong, K.; Kang, C.S.; Kim, Y.; Koh, J.; Mahajan, S.D.; Prasad, P.N.; Kim, S. Multifunctional Photonics Nanoparticles for Crossing the Blood-Brain Barrier and Effecting Optically Trackable Brain Theranostics. Adv. Funct. Mater. 2016, 26, 7057–7066.
- Miranda, A.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Computational modeling in glioblastoma: From the prediction of blood-brain barrier permeability to the simulation of tumor behavior. Future Med. Chem. 2018, 10, 121–131.
- Kiecker C. The origins of the circumventricular organs. J. Anat. 2018 Apr;232(4):540-553.
- Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001 Jun 26;56(12):1746-8.
- Fan Y, Liu X. Alterations in Expression and Function of ABC Family Transporters at Blood-Brain Barrier under Liver Failure and Their Clinical Significances. Pharmaceutics. 2018 Jul 23;10(3).
- Nattie EE. Ionic mechanisms of cerebrospinal fluid acid-base regulation. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):3-12.
- Schuster F, Huber G, Stölting I, Wing EE, Saar K, Hübner N, Banks WA, Raasch W. Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice. Pflugers Arch. 2018 Nov;470(11):1673-1689
- Krämer T, Grob T, Menzel L, Hirnet T, Griemert E, Radyushkin K, Thal SC, Methner A, Schaefer MKE. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J. Neurochem. 2017 Dec;143(5):523-533.
- Yick LW, Ma OK, Ng RC, Kwan JS, Chan KH. Aquaporin-4 Autoantibodies From Neuromyelitis Optica Spectrum Disorder Patients Induce Complement-Independent Immunopathologies in Mice. Front Immunol. 2018;9:1438.
- Riederer P, Müller T. Monoamine oxidase-B inhibitors in the treatment of Parkinson’s disease: clinical-pharmacological aspects. J Neural Transm (Vienna). 2018 Nov;125(11):1751-1757.
- McHardy SF, Wang HL, McCowen SV, Valdez MC. Recent advances in acetylcholinesterase Inhibitors and Reactivators: an update on the patent literature (2012-2015). Expert Opin Ther Pat. 2017 Apr;27(4):455-476.
- Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013 Apr;15(2):93-117.