Children with MS
Pediatric MS also known as pediatric-onset multiple sclerosis (POMS) refers to MS occurring in people under age 18 and represents approximately 3% to 5% of all MS cases 1. MS in children is not that different from MS in adults 2, 3, 4. MS can occur at any age, but pediatric-onset multiple sclerosis (POMS) is relatively rare. Multiple studies have shown that only 3-5% of all individuals diagnosed with MS experience disease onset before 16 years of age. The majority of MS diagnoses occur between the ages of 20 and 40. Children with MS exclusively have a relapsing-remitting MS. This form of MS is characterized by recurring attacks (relapses) causing new or worsening neurologic symptoms, followed by periods without new symptoms, called remissions. During the periods of remission between attacks, there is no progression of the multiple sclerosis disease. Even though children may experience frequent relapses (possibly more than typically seen in adults), studies have shown that children also seem to have very good recovery that is often more rapid than that of adults 5.
Multiple sclerosis (MS) is thought to be an autoimmune disease that attacks the central nervous system (CNS), causing episodes of neurologic symptoms. These neurologic symptoms may include weakness, numbness, tingling, difficulty balancing, bowel or bladder problems, or changes to vision.
The central nervous system consists of the brain, spinal cord and optic nerves. MS primarily attacks the coating around nerve cells called myelin. The attacks leave spots (called plaques or lesions) that interfere with nerve conduction and produces the symptoms of MS. Symptoms of multiple sclerosis vary depending on the location of lesions.
Multiple sclerosis affects about 1 in 1,000 people. About 450,000 people in the United States and Canada are living with multiple sclerosis. Less than 5,000 children and teens are living with MS in the United States and less than 10,000 worldwide.
Multiple sclerosis is more common in women than in men. It is more common in Caucasians than in Hispanics or African Americans, and more common in temperate areas of the world away from the Equator. It is rare in Asians and other groups.
Diagnosing MS in children is more challenging than in adults because of other childhood disorders with similar symptoms and characteristics. At the time of a first attack, it is not always clear whether the diagnosis of MS is appropriate since relapsing disease has not yet occurred. New relapses or attacks will confirm your child’s diagnosis and provide insight to help their doctors better manage their care.
Although the peak age of diagnosis is between 20 to 50 years old, approximately 2.7% to 5% of people are diagnosed before the age of 16, with the majority of these cases diagnosed after the age of 10.
The treatment of MS in children and teens, as well as adults, involves several strategies:
- Modifying the disease course
- Managing relapses
- Maximizing lifestyle interventions
- Managing symptoms
There are medications that are effective at preventing relapses and disability accumulation known as disease modifying therapies (DMTs). More than a dozen DMTs are approved by the U.S. Food and Drug Administration (FDA) to treat adults with relapsing forms of MS. In May 2018, the FDA approved the use of the oral MS therapy Gilenya® (fingolimod) for the treatment of children and adolescents 10 years of age or older with relapsing forms of MS.
Many of the medications used for adults with MS have been studied in children with MS. Skilled pediatric MS healthcare providers can adapt the treatments with FDA approval in adults for their younger patients.
What does the future hold for my child with MS?
The course of MS is difficult to predict and varies from person to person, so predicting exactly how it will affect each child is not possible. Most children have relapsing remitting MS, with periods of good, if unpredictable, recovery.
Some children can be more severely affected, but rapid progression is rare. MS is not a terminal illness. Like diabetes, it’s known as a chronic or long-term condition that needs to be managed for life. Most people with MS live a normal life span, with perhaps a five to 10 year reduction in average life expectancy. Of course averages aren’t always helpful and everyone’s MS is different. With recent advances in medicine, the gap in life expectancy appears to be getting smaller.
Multiple sclerosis life expectancy
It is generally very difficult to predict the course of multiple sclerosis. The condition varies greatly in each individual but most people with multiple sclerosis can expect 95% of the normal life expectancy 6.
Some studies have shown that people who have few attacks in the first several years after diagnosis; long intervals between attacks; complete recovery from attacks and attacks that are sensory in nature (i.e. numbness or tingling) tend to have better outcomes in the long run.
People who have early symptoms of tremor, difficulty in walking, or who have frequent attacks with incomplete recoveries tend to have a more progressive disease course.
What is neuromyelitis optica (NMO)?
Children may also develop neuromyelitis optica (NMO). In NMO, immune system cells and antibodies attack and destroy myelin in the optic nerves and the spinal cord, causing optic neuritis (resulting in pain in the eye and vision loss) and transverse myelitis (causing weakness, numbness and sometimes paralysis or the arms and legs, as well as bladder and bowel problems).
The discovery of an antibody (NMO-IgG) in the blood of individuals with NMO now makes it possible to distinguish NMO from MS. NMO attacks are more severe than those seen in MS and in early disease are generally confined to optic nerves and the spinal cord.
Types of MS
MS can affect people very differently and one of the most frustrating aspects of the condition is its unpredictability – not knowing what symptoms may arise, when, or how long they will last.
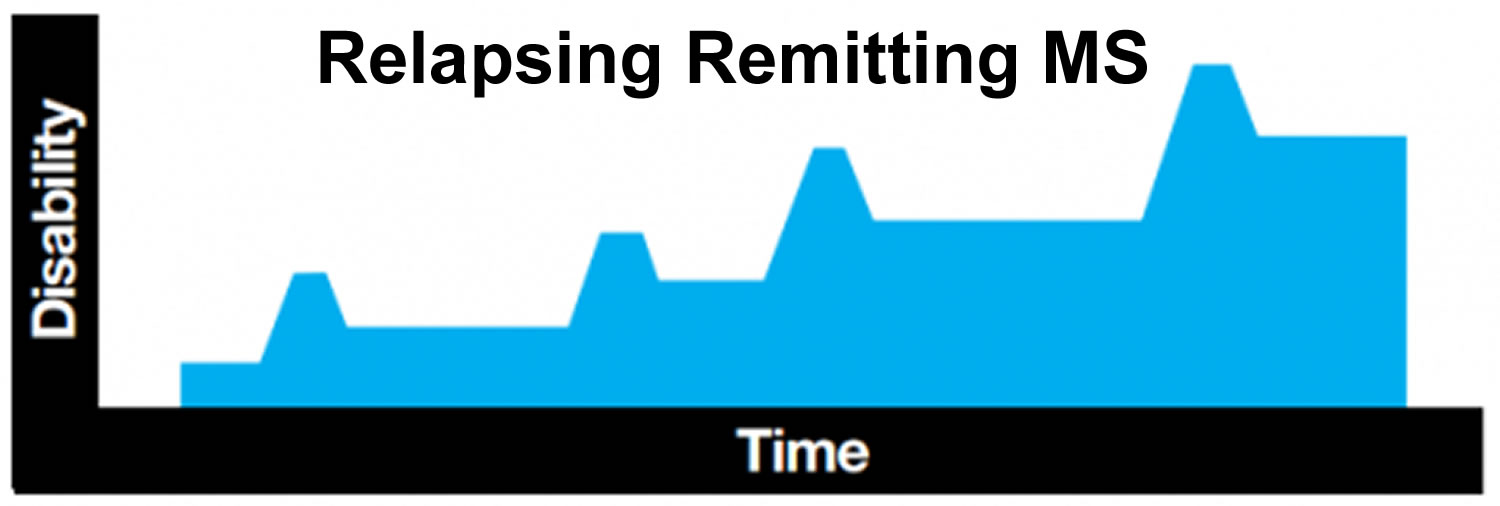
The most common form of multiple sclerosis is known as relapsing-remitting MS ~ 90 percent of cases. When people who have this kind of MS have flare-ups, the symptoms become noticeably worse. Then there is a period of recovery, when symptoms get better or disappear completely for some time. In relapsing-remitting MS, symptom flare-ups may be triggered by an infection, such as the flu. More than 50% of people who have relapsing-remitting MS develop the secondary progressive type in which there are relapses followed by a gradual worsening of the disease. About 15% to 20% of people who have MS have a form known as primary-progressive MS. In primary-progressive MS, the disease gets steadily worse, without any remissions. A fourth type—progressive relapsing MS—is rare, but the pattern follows a worsening of the disease with sudden, clear relapses. Often MS is mild, but some people lose the ability to write, speak or walk.
Relapsing remitting MS
Most people with MS, including almost all children, are first diagnosed with relapsing remitting MS. This means they experience a relapse or flare up of symptoms (also known as an attack or exacerbation) followed by a period of stability between relapses when symptoms settle down or disappear. This period of stability is known as remission. Remissions can last any length of time, even years. No one knows exactly what makes MS go into remission.
A relapse is defined as the appearance of new symptoms, or the return of old symptoms, that last for at least 24hours, and occur at least 30 days since the start of any previous relapse. If your child has a fever caused by an infection, or becomes overheated from exercise or hot weather, his or her symptoms may worsen temporarily. But this flare-up of symptoms is caused by an elevated body temperature, rather than by new MS activity, and the symptoms will fade as your child’s body temperature returns to normal. This is known as a ‘pseudo relapse’.
Relapses can take a few days to develop and can last for days, weeks or months. Symptoms can be mild or severe. Most children recover well from relapses. In the early stages of relapsing remitting MS, symptoms can disappear completely during remissions. However, after several relapses there may be some residual damage to the myelin, meaning that some symptoms may remain. These accumulating symptoms may cause your child difficulty, even if only mild.
Figure 1. Relapsing-remitting MS
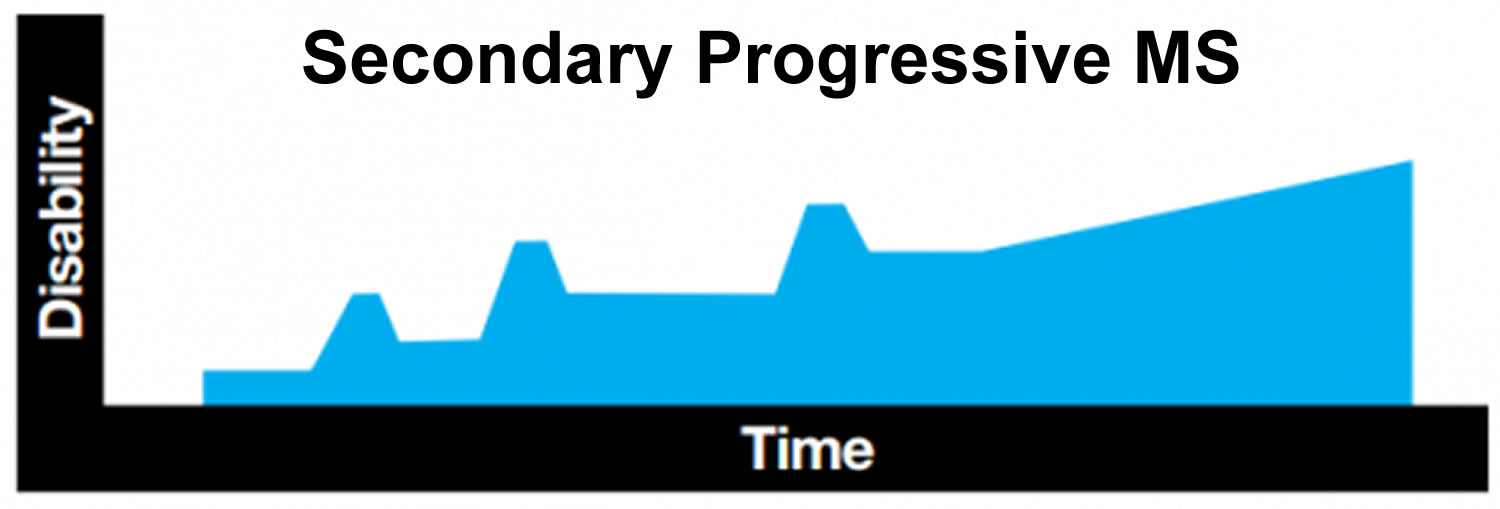
Secondary progressive MS
Many people who start out with relapsing remitting MS later develop a form that is known as secondary progressive MS. In secondary progressive MS, people experience fewer relapses, or none at all, and the disease slowly progresses over time.
In general, people who develop MS in childhood have a slower rate of progression than people who are diagnosed with MS in adulthood. It is difficult to give exact figures, and even harder to make accurate predictions for individual children. In the largest study to date looking at progression in childhood- onset MS, 50 per cent of people whose MS started before the age of 16 had developed secondary progressive MS after 28 years.
Figure 2. Secondary progressive MS
Benign MS
People with relapsing remitting MS who only have a small number of relapses, followed by a complete recovery, may be described as having benign MS. It is only possible to make a diagnosis of benign MS once a person has experienced little or no physical or cognitive disability for a period of 20 years.
However, a diagnosis of benign MS does not mean that a person will be totally free of problems; a relapse may occasionally occur after many years in which the MS has been inactive. Perhaps around 10 percent of people with MS will have a benign form of the condition.
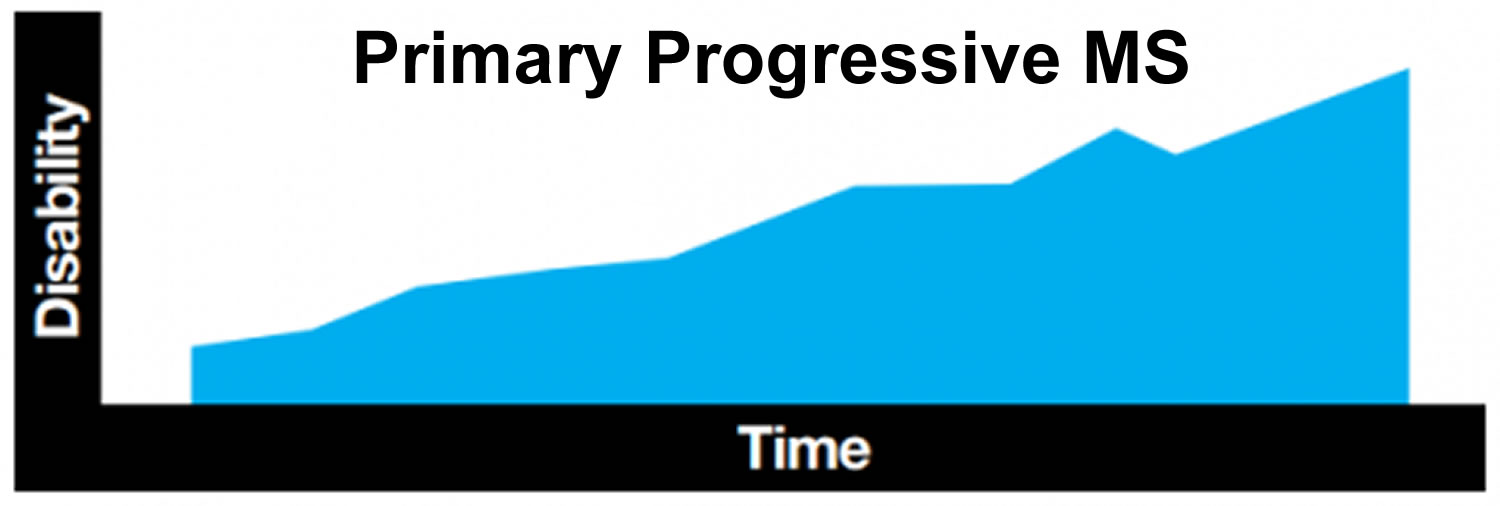
Primary progressive MS
Primary progressive MS is rare in children, with less than five percent of children with MS being diagnosed with this form. It tends to be diagnosed in older people, usually in their forties or later. From the outset, those with primary progressive MS experience steadily worsening symptoms and an increase in disability. Symptoms may level off for a time, or may continue to worsen, often with no relapses. Approximately 10 to 15 percent of adults with MS have the primary progressive form.
Figure 3. Primary progressive MS
Multiple sclerosis in children causes
The cause of multiple sclerosis is still unknown. It’s considered an autoimmune disease in which the body’s immune system attacks its own tissues. In the case of MS, this immune system malfunction destroys myelin (the fatty substance that coats and protects nerve fibers in the brain and spinal cord).
Myelin can be compared to the insulation coating on electrical wires. When the protective myelin is damaged and nerve fiber is exposed, the messages that travel along that nerve may be slowed or blocked. The nerve may also become damaged itself.
It isn’t clear why MS develops in some people and not others. A combination of genetics and environmental factors appears to be responsible.
Researchers believe there is a genetic predisposition that is triggered by some environmental factor, such as a viral infection. That means that MS is not genetically passed down from one generation to the next, like hair color or eye color, but a combination of genes can make one person more susceptible to the disease than another person. Subsequently, the average risk of developing multiple sclerosis is 1 in 750, but the risk of a child whose parent has MS is 1 in 40.
Risk factors for multiple sclerosis
These factors may increase your risk of developing multiple sclerosis:
- Age. MS can occur at any age, but most commonly affects people between the ages of 15 and 60.
- Sex. Women are about twice as likely as men are to develop MS.
- Family history. If one of your parents or siblings has had MS, you are at higher risk of developing the disease.
- Certain infections. A variety of viruses have been linked to MS, including Epstein-Barr, the virus that causes infectious mononucleosis.
- Race. White people, particularly those of Northern European descent, are at highest risk of developing MS. People of Asian, African or Native American descent have the lowest risk.
- Climate. MS is far more common in countries with temperate climates, including Canada, the northern United States, New Zealand, southeastern Australia and Europe.
- Certain autoimmune diseases. You have a slightly higher risk of developing MS if you have thyroid disease, type 1 diabetes or inflammatory bowel disease.
- Smoking. Smokers who experience an initial event of symptoms that may signal MS are more likely than nonsmokers to develop a second event that confirms relapsing-remitting MS.
Pediatric MS symptoms
Multiple sclerosis signs and symptoms in children include:
- Fatigue (an overwhelming sense of tiredness making physical or mental activity difficult)
- Changes in vision (blurred or double vision, temporary loss of sight in one eye or both)
- Weakness (loss of muscle strength and dexterity)
- Numbness or tingling: commonly in the hands or feet
- Pain sometimes mild, sometimes severe
- Balance problems and dizziness: walking difficulties, problems with coordination
- Stiffness and spasms: tightening or rigidity in particular muscle groups
- Problems with bowel/bladder
- Emotional changes (including depression, anxiety or mood swings)
- Mood changes: depression, anxiety, irritability
- Speech problems: slurring, slowing of speech, or changes in pitch or tone
- Problems with thinking and memory
Complications of multiple sclerosis
People with multiple sclerosis also may develop:
- Muscle stiffness or spasms
- Paralysis, typically in the legs
- Problems with bladder, bowel or sexual function
- Mental changes, such as forgetfulness or mood swings
- Depression
- Epilepsy
Pediatric MS diagnosis
Diagnosing MS can be difficult, both in children and adults, due to its complexity and variety of symptoms. There is no single diagnostic test for MS. In most cases, MS is diagnosed with a combination of methods. A neurologist – a doctor who specializes in conditions of the central nervous system – is the best person to evaluate your child and determine whether he or she has MS. Your child’s neurologist is likely to start with a thorough medical history and examination. Your child’s neurologist may then order tests that may include:
- Blood tests, to help rule out other diseases with symptoms similar to MS. Tests to check for specific biomarkers associated with MS are currently under development and may also aid in diagnosing the disease.
- Lumbar puncture (spinal tap), in which a small sample of the cerebral spinal fluid is removed from your spinal canal for laboratory analysis. This sample can show abnormalities in antibodies that are associated with MS. Spinal tap can also help rule out infections and other conditions with symptoms similar to MS.
- MRI, which can reveal areas of MS (lesions) on your brain and spinal cord. You may receive an intravenous injection of a contrast material to highlight lesions that indicate your disease is in an active phase.
- Evoked potential tests, which record the electrical signals produced by your nervous system in response to stimuli. An evoked potential test may use visual stimuli or electrical stimuli, in which you watch a moving visual pattern, or short electrical impulses are applied to nerves in your legs or arms. Electrodes measure how quickly the information travels down your nerve pathways.
In some patients, the first MRI scan shows multiple lesions that are so consistent with MS that an MS diagnosis may be given after only one clinical attack has happened. While not as common, early diagnosis can offer an important opportunity for early treatment.
In most people with relapsing-remitting MS, the diagnosis is fairly straightforward and based on a pattern of symptoms consistent with the disease and confirmed by brain imaging scans, such as MRI.
Diagnosing MS can be more difficult in persons with unusual symptoms or progressive disease. In these cases, further testing with spinal fluid analysis, evoked potentials and additional imaging may be needed.
To confirm a diagnosis of MS, there needs to be evidence of MS activity in two or more parts of the central nervous system (brain, spinal cord and optic nerves) that occurred at different points in time. If your child has experienced a single episode of symptoms, the diagnosis of MS cannot be confirmed.
You may have been told that your child has ‘ADEM’ (acute disseminated encephalomyelitis), ‘optic neuritis’ or has had a ‘clinically isolated syndrome’. These can all have the same symptoms as MS, but might happen only once and not return. If further episodes do occur, then MS might be diagnosed.
Table 1. Conditions potentially confused with Multiple Sclerosis
| Disease | Examples | |
|---|---|---|
| Central and peripheral nervous system disease | ||
| Degenerative disease | Amyotrophic lateral sclerosis, Huntington disease | |
| Demyelinating disease | Chronic inflammatory demyelinating polyneuropathy, progressive multifocal leukoencephalopathy | |
| Infection | Human immunodeficiency virus infection, Lyme disease, mycoplasma, syphilis | |
| Inflammatory disease | Behçet syndrome, sarcoidosis, Sjögren syndrome, systemic lupus erythematosus | |
| Structural disease | Arteriovenous malformation, herniated disk, neoplasm | |
| Vascular disease | Cerebrovascular accident, diabetes mellitus, hypertensive disease, migraine, vasculitis | |
| Genetic disorder | Leukodystrophy, mitochondrial disease | |
| Medication and illicit drug effects | Alcohol, cocaine, isoniazid, lithium, penicillin, phenytoin (Dilantin) | |
| Nutritional deficiency | Folate deficiency, vitamin B12 deficiency, vitamin E deficiency | |
| Psychiatric disease | Anxiety, conversion disorder, somatization | |
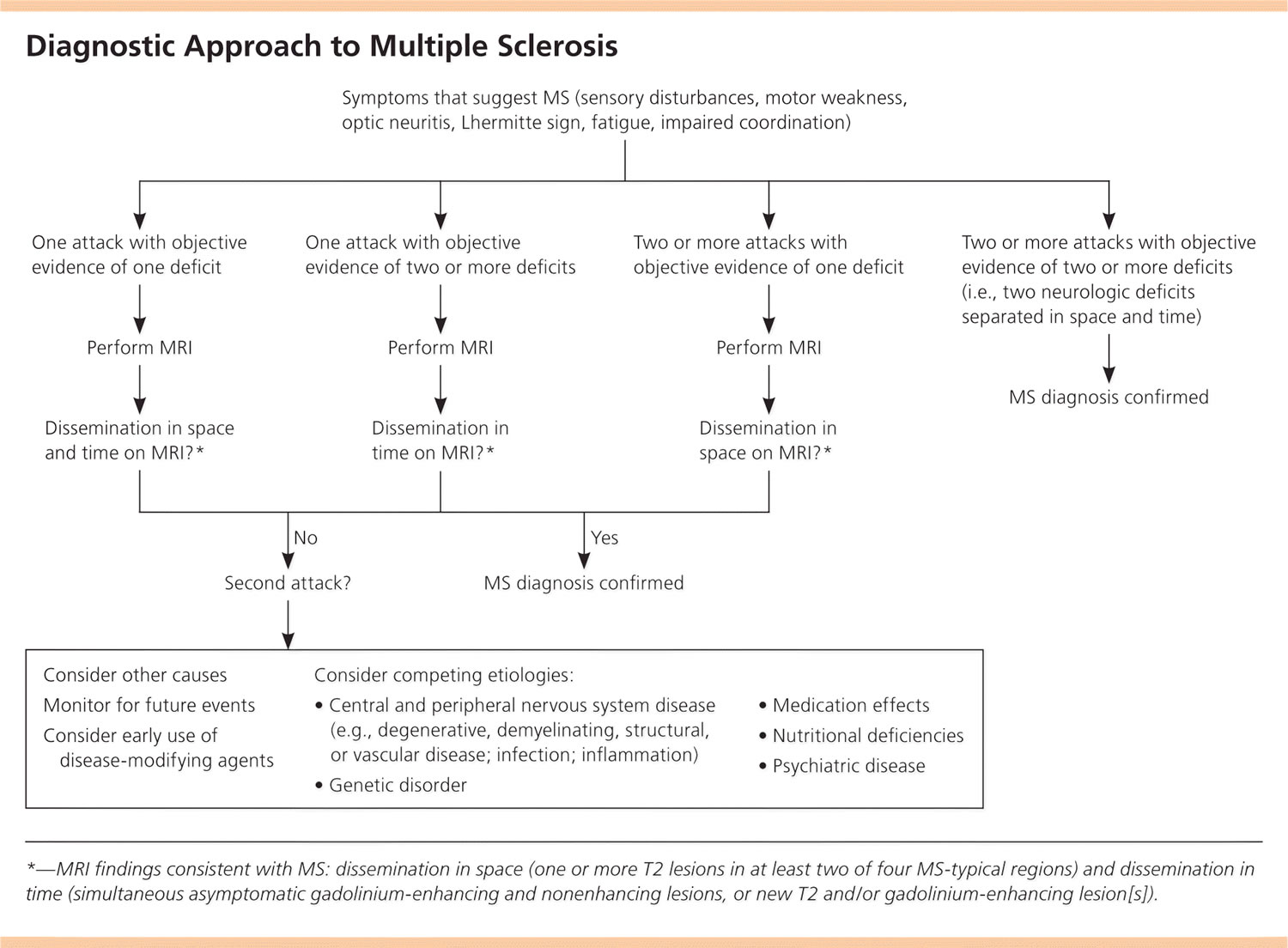
Diagnostic Criteria to Multiple Sclerosis
MS is a clinical diagnosis. Two neurologic deficits (e.g., focal weakness, sensory disturbances) separated in time and space, in the absence of fever, infection, or competing etiologies, are considered diagnostic 8, 9. Attacks may be patient-reported or objectively observed, and must last for a minimum of 24 hours. Corroborating magnetic resonance imaging (MRI) is the diagnostic standard (Figure 5) 8.
Figure 4. Diagnostic Approach to Multiple Sclerosis
Abbreviations: MRI = magnetic resonance imaging; MS = multiple sclerosis.
[Source 7]Childhood MS treatment
There is no cure for multiple sclerosis. Treatment is focused on managing symptoms during attacks, as well as slowing the disease progression to prevent relapses and limit formation of new lesions in the brain or spine.
Your child’s care team will determine the best treatment based on your child’s symptoms and condition.
Treatment of MS relapses
Corticosteroid therapy
Steroids (corticosteroids, prednisone, or methylprednisolone), most commonly IV methylprednisolone, are used to treat an attack of neurological symptoms –either the first episode or later relapses. IV methylprednisolone is given only for a few days. If needed, oral prednisone may be prescribed for a further one to two weeks. Although they do not alter the course of the condition, steroids can reduce the inflammation in the central nervous system and speed up recovery from the relapse.
Steroids do not affect the level of recovery from an attack, so if some symptoms remain several months later, steroids will have no impact on them. And although they often speed up recovery, their effectiveness can vary from one person to another and from one relapse to another for a given individual.
Neurologists specializing in MS will generally choose intravenous methylprednisolone as the steroid to treat severe MS relapses. Large doses are usually given over 3-5 days via a drip that goes into a vein (intravenous). This method may require admission to hospital, particularly for disabling relapses. Oral methylprednisolone may be prescribed instead of intravenous methylprednisolone.
All medications can have unwanted effects, and steroids are no exception. Steroids are given only for short periods of time and only for relapses that are causing a significant disruption in a person’s life.
Serious side effects are possible, but they are not common when treatment is short. Less serious side effects are common, however, and these include irritability and difficulty sleeping. Giving the steroids in the morning reduces the impact on sleep. Upset stomach is common and medications that reduce stomach acid are typically given to prevent this. High blood sugar levels may also be a side effect that resolves when the steroids are stopped.
Continuous, long-term use of steroids is known to increase a person’s risk of osteoporosis, cataracts and diabetes.
Possible short-term side effects of steroids include:
- a metallic taste in the mouth
- increased heart rate
- hot flushes or a red face
- sleeping problems
- an increased need to urinate, particularly at night
- weight gain
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) is an IV medication that is made up of antibodies from healthy blood donors. These help decrease the unwanted immune response that occurs in MS. The most common side effect is headache, but other side effects include muscle or joint pain and low-grade fever. IVIG is not usually the first treatment used for an MS relapse, but may be used in certain situations.
Plasma exchange
Plasma exchange, also called plasmapheresis or PLEX, is used when neurologic symptoms are difficult to treat. It is a process where the blood is removed from the body and washed to remove disease-causing substances, such as antibodies from the plasma (the liquid component of blood). The blood cells are then returned to the body. Multiple treatments are required, given over a couple of weeks. Plasma exchange requires the surgical placement of a special line for IV access. Risks include changes in blood pressure and fluid balance as well as infection of the access site.
Treatments to modify progression of multiple sclerosis
The goal of disease-modifying therapy (DMT) is to forestall disease, preserve function, and sustain healthy immune function while suppressing the T-cell autoimmune cascade thought to be responsible for demyelination and axonal damage. Early treatment at or before the diagnosis of clinically confirmed MS may delay damage to the central nervous system 10. The U.S. Food and Drug Administration (FDA) has approved seven agents for the treatment of MS: interferon beta, glatiramer (Copaxone), fingolimod (Gilenya), teriflunomide (Aubagio), dimethyl fumarate (Tecfidera), natalizumab (Tysabri), and mitoxantrone (Table 2). Because of the chronic nature and evolving treatment of MS, disease-modifying treatment (DMT) is typically managed by a neurologist with expertise in prescribing these potentially toxic agents.
The following US Food and Drug Administration (FDA) approved disease modifying therapies for MS have been found through clinical trials to reduce the number of relapses, delay progression of disability, and limit new disease activity (as seen on MRI) 11:
- Injectable medications
- Avonex (interferon beta-1a)
- Betaseron (interferon beta-1b)
- Copaxone (glatiramer acetate)
- Extavia (interferon beta-1b)
- Glatiramer Acetate Injection (glatiramer acetate -generic equivalent of Copaxone 20 mg and 40 mg doses)
- Glatopa (glatiramer acetate – generic equivalent of Copaxone 20mg and 40mg doses)
- Kesimpta (ofatumumab)
- Plegridy (peginterferon beta-1a)
- Rebif (interferon beta-1a)
- Oral medications
- Aubagio (teriflunomide)
- Bafiertam (monomethyl fumarate)
- Dimethyl Fumarate (dimethyl fumarate – generic equivalent of Tecfidera)
- Gilenya (fingolimod)
- Mavenclad (cladribine)
- Mayzent (siponimod)
- Ponvory (ponesimod)
- Tascenso ODT (fingolimod)
- Tecfidera (dimethyl fumarate)
- Vumerity (diroximel fumarate)
- Zeposia (ozanimod)
- Infused medications
- Briumvi (ublituximab-xiiy)
- Lemtrada (alemtuzumab)
- Novantrone (mitoxantrone)
- Ocrevus (ocrelizumab)
- Tysabri (natalizumab)
Under certain circumstances, some neurologists may use medications to treat MS that have FDA approval for other diseases called “off-label” use. For each of these medications there is some, but often limited, clinical trial evidence of efficacy. “Off-label” use medications:
- Cellcept (mycophonolate mofetil). Cellcept (mycophonolate mofetil) is an immunosuppressant taken by mouth twice daily. It works by blocking an enzyme that is needed for certain white blood cells, specifically B- and T-cells to carry out an immune system attack. CellCept has been studied as a monotherapy (use of a single drug to treat a disease or condition) and also in combination with other DMTs such as interferon-beta medications. The results suggest that Cellcept may reduce the annual number of MS relapses, limit new areas of central nervous system (CNS) damage and may slow disease worsening. Side effects and risks include increased risk of infection (including opportunistic infections such as progressive multifocal leukoencephalopathy), nausea, diarrhea, stomach pain, weakness, dizziness, difficulty sleeping, increased risk of skin cancer and lymphoma, stomach ulcers and bleeding, elevation in liver enzymes, yellowing of the skin (jaundice) and can cause fetal death or malformations.
- Cytoxan (cyclophosphamide). Cytoxan (cyclophosphamide) is an immunosuppressant medication that is given intravenously (into a vein) or orally. It works by binding to cell DNA and interfering with cell division and replication. Several clinical trials in people with MS demonstrated a reduction in relapses, fewer new areas of CNS inflammation and a variable effect on disease worsening. Side effects and risks include nausea, vomiting, hair thinning/loss, low white blood cell count, risk of infections, risk of cancers, infertility, inflammation of the bladder with bleeding, and fetal abnormalities.
- Imuran (Azathioprine). Imuran (Azathioprine) is an oral immunosuppressant drug that targets activation, proliferation, and differentiation of both T and B lymphocytes. Imuran has been used in MS for over 30 years. Several clinical trials of Imuran as a monotherapy (use of a single drug to treat a disease or condition) and in combinations with other DMTs have demonstrated an effect on relapse reduction, new CNS inflammation and disease worsening. Imuran is approved in parts of Europe for use in MS, but not in the US at this time. Side effects and risks include abdominal pain, severe nausea, vomiting, loss of appetite, mouth sores/ulcers, increased risk of infection, hair loss, change in hair color and texture, risk of malignancies and blood abnormalities, and fetal abnormalities.
- Minocycline. Minocyline is an oral tetracycline antibiotic that is FDA-approved for the treatment of a number of different types of bacterial infection. Minocycline has been studied in MS in combination with interferon beta-1a (Rebif) and in combination with glatiramer acetate (Copaxone) to determine whether adding minocycline to these disease-modifying therapy (DMT) provides added benefit. Side effects and risks include gastrointestinal problems, liver damage, mild to severe skin conditions, respiratory problems, kidney toxicity, muscle and joint pain, blood cell abnormalities, CNS disorders, and potential for fetal abnormalities.
- Rituxan (rituximab). Rituxan (rituximab) is a monoclonal antibody (a protein made in the laboratory) that targets a specific protein on the surface of B-lymphocytes. B-lymphocytes are white blood cells that are known to cause inflammation and damage in MS. Rituxan is given by intravenous (into a vein) infusion. The first dose is given in two intravenous infusions that are separated by two weeks. This dosing regimen is then repeated every six months. Several clinical trials have demonstrated that Rituxan is effective in reducing clinical relapses and limiting new inflammation in the CNS. It may also limit progression (worsening) of MS. Side effects and risks include infusion reactions (symptoms that occur during or soon after an infusion), infections (including opportunistic infections such as progressive multifocal leukoencephalopathy), allergic reactions, headache, fatigue and anemia. Rituxan should be used with caution in pregnancy.
- Statins. Statins are oral medications FDA-approved to lower cholesterol. They have been used outside of FDA approval to treat conditions such as rheumatoid arthritis and are being tested in a variety of conditions such as cancer and MS. Statins are being studied in MS because they are known to modulate the immune system and to help support the growth, health and protection of nerve cells. Side effects and risks include headache, difficulty sleeping, flushing of the skin, muscle aches, inflammation and potentially severe muscle and kidney damage, weakness, drowsiness, nausea and vomiting, abdominal cramping or pain, bloating or gas, diarrhea, constipation, rash, and fetal abnormalities.
Table 2. Disease-Modifying Agents for Relapsing Remitting Multiple Sclerosis
| Agent | Dosage | Cost (yearly)* | Evidence (95% confidence interval) | Adverse effects | |
|---|---|---|---|---|---|
| Interferon beta | $62,000 to $67,000 | Decrease in relapses: RR = 0.80 | Injection site reactions (e.g., edema, inflammation, pain); influenza-like symptoms; leukopenia; elevated liver enzyme levels; depression and suicidal thoughts (increased with preexisting depression, neutralizing antibodies) | ||
| Interferon beta-1a IM (Avonex) | Intramuscular, weekly | Decrease in progression: RR = 0.69 | |||
| Interferon beta-1a SC (Rebif) | Subcutaneous, three times per week | ||||
| Interferon beta-1b (Betaseron) | Subcutaneous, every other day | ||||
| Glatiramer (Copaxone) | Subcutaneous, daily | $70,000 | Decrease in relapses: MD = 0.51 | Injection site reactions, facial flushing, chest tightness, palpitations | |
| Decrease in disability at two years: MD = 0.33 | |||||
| Fingolimod (Gilenya) | Oral, daily | $67,000 | Annualized relapse rate vs. placebo: 0.18 vs. 0.40 | Bradycardia (contraindicated in recent heart disease or arrhythmia); elevated liver enzyme levels | |
| Decrease in progression at two years: HR = 0.70 | Melanoma, macular edema, herpes encephalitis (only occurs at higher doses) | ||||
| Teriflunomide (Aubagio) | Oral, daily | $63,000 | Annualized relapse rate vs. placebo: 0.37 vs. 0.54 | Alopecia, diarrhea, nausea, decreased white blood cell count, elevated liver enzyme levels, peripheral neuropathy | |
| Decrease in progression: HR = 0.76 | |||||
| Dimethyl fumarate (Tecfidera) | Oral, daily | $68,000 | Annualized relapse rate vs. placebo: 0.17 vs. 0.36 | Flushing, abdominal pain, lymphocytopenia, elevated liver enzyme levels | |
| Decrease in progression: HR = 0.62 | |||||
| Natalizumab (Tysabri) | Intravenous, once per month | $61,000 | Decrease in relapses: RR = 0.57 | Infusion reactions, headache, fatigue, progressive multifocal leukoencephalopathy | |
| Decrease in progression at two years: RR = 0.74 | |||||
| Mitoxantrone | Intravenous, every three months | $900 | Decrease in relapses: MD = 0.85 | Myelosuppression, elevated liver enzyme levels, decreased cardiac function, leukemia | |
| Decrease in progression: OR = 0.30 | |||||
Footnote:*—Estimated retail price of treatment for one year based on information obtained at http://www.goodrx.com. All prices are for brand name drugs except for mitoxantrone, which is available only as generic.
Abbreviations: HR = hazard ratio; MD = mean difference; OR = odds ratio; RR = relative risk
[Source 7]For primary-progressive MS, ocrelizumab (Ocrevus) is the only FDA-approved disease-modifying therapy. It slows worsening of disability in people with this type of MS.
For relapsing-remitting MS, several disease-modifying therapies are available.
Much of the immune response associated with MS occurs in the early stages of the disease. Aggressive treatment with these medications as early as possible can lower the relapse rate and slow the formation of new lesions.
Many of the disease-modifying therapies used to treat MS carry significant health risks. Selecting the right therapy for you will depend on careful consideration of many factors, including duration and severity of disease, effectiveness of previous MS treatments, other health issues, cost, and child-bearing status.
Treatment options for relapsing-remitting MS include:
Beta interferons. These medications are among the most commonly prescribed medications to treat MS. They are injected under the skin or into muscle and can reduce the frequency and severity of relapses.
Side effects of beta interferons may include flu-like symptoms and injection-site reactions.
You’ll need blood tests to monitor your liver enzymes because liver damage is a possible side effect of interferon use. People taking interferons may develop neutralizing antibodies that can reduce drug effectiveness.
Ocrelizumab (Ocrevus). This humanized immunoglobulin antibody medication is the only DMT approved by the FDA to treat both the relapse-remitting and primary progressive forms of MS. Clinical trials showed it reduced relapse rate in relapsing disease and slowed worsening of disability in both forms of the disease.
Ocrevus is given via an intravenous infusion by a medical professional. Side effects may infusion-related reactions including irritation at the injection site, low blood pressure, fever, and nausea among others. Ocrevus may also increase the risk of some types of cancer, particularly breast cancer.
Glatiramer acetate (Copaxone). This medication may help block your immune system’s attack on myelin and must be injected beneath the skin. Side effects may include skin irritation at the injection site.
Dimethyl fumarate (Tecfidera). This twice-daily oral medication can reduce relapses. Side effects may include flushing, diarrhea, nausea and lowered white blood cell count.
Fingolimod (Gilenya). This once-daily oral medication reduces relapse rate.
You’ll need to have your heart rate monitored for six hours after the first dose because your heartbeat may be slowed. Other side effects include headache, high blood pressure and blurred vision.
Teriflunomide (Aubagio). This once-daily medication can reduce relapse rate. Teriflunomide can cause liver damage, hair loss and other side effects. It is harmful to a developing fetus and should not be used by women who may become pregnant and are not using appropriate contraception, or their male partner.
Natalizumab (Tysabri). This medication is designed to block the movement of potentially damaging immune cells from your bloodstream to your brain and spinal cord. It may be considered a first line treatment for some people with severe MS or as a second line treatment in others. This medication increases the risk of a viral infection of the brain called progressive multifocal leukoencephalopathy in some people.
Alemtuzumab (Lemtrada). This drug helps reduce relapses of MS by targeting a protein on the surface of immune cells and depleting white blood cells. This effect can limit potential nerve damage caused by the white blood cells, but it also increases the risk of infections and autoimmune disorders.
Treatment with alemtuzumab involves five consecutive days of drug infusions followed by another three days of infusions a year later. Infusion reactions are common with alemtuzumab. The drug is only available from registered providers, and people treated with the drug must be registered in a special drug safety monitoring program.
Mitoxantrone. This immunosuppressant drug can be harmful to the heart and is associated with development of blood cancers. As a result, its use in treating MS is extremely limited. Mitoxantrone is usually used only to treat severe, advanced MS.
Medicines to prevent relapses and progression of MS
Treatments to prevent relapses and progression of MS may include the following categories of disease-modifying agents. Decisions about the best disease-modifying therapy can be complicated and are always made while considering other health conditions and what is best for each individual patient.
Disease modifying drugs can affect the course of MS. They are not a cure but they can reduce the number and severity of MS relapses. No controlled clinical trials of the disease modifying drugs have been completed in children and as yet, none are specifically approved for use in children under the age of 18. However pediatric clinical trials of several disease modifying drugs are currently underway.
Injectable medications
- Interferons (Avonex®, Rebif®, Betaseron®, Plegridy®): These medications mimic the effects of some proteins that your body can make to change how your immune system works. They are used to decrease the number of relapses and new lesions on MRI. Common side effects include flu-like symptoms shortly after the injection and injection site pain. Regular blood tests are required to monitor the medication. The type and frequency of injection is different with each of these medications.
- Glatiramer acetate (Copaxone®): This medicine is given by subcutaneous injection (just under the skin). This medication looks like one of the proteins that makes up myelin in the brain and spinal cord. The injection is given daily or three days a week. Common side effects include injection site reactions and, rarely, a reaction with chest pain. Regular blood tests are not required.
Oral medications
While there are several oral medications used in the treatment of MS, only fingolimod (Gilenya®) is currently under widespread use in the treatment of children with MS.
This medicine is taken daily and decreases relapses by trapping certain white blood cell in the lymph nodes to decrease immune system reactions. Blood testing is required before starting fingolimod and during treatment because it is associated with an increased risk of infection. Fingolimod also requires close monitoring with an eye doctor as it may cause an eye problem called macular edema.
The first dose of fingolimod is administered in a hospital or medical office setting because it can lower your heart rate. If you stop and then restart fingolimod, you will have to be monitored again when you restart it.
Infused medications
Infused medications such as Rituximab®, Tysabri® or Ocrevus® are given by IV infusion in a hospital setting. They are associated with increased risks of infection and require blood tests before starting as well as close monitoring and blood testing during treatment. These medications are most often used when injectable or oral medications have not been effective, but may be used in other settings as well.
Other treatments for MS
In addition to medication, certain lifestyle factors can help decrease relapses and manage MS symptoms.
- Taking vitamin D if needed. Having enough vitamin D decreases MS disease activity. Vitamin D deficiency is a risk factor for developing MS across all age groups 12. Your doctor will check your vitamin D levels and may recommend taking vitamin D3. Vitamin D may also decrease the risk of disease in other family members. A small study in adults with MS suggested that adding vitamin D to disease-modifying therapy (DMT) reduced magnetic resonance imaging (MRI) activity (fewer T1-enhancing lesions and fewer T2 burden lesions) 13, 14. Other randomized controlled trials did not find significant differences in MRI parameters or disease progression 15, 16.
- Iron and salt supplements. The potential benefit of iron and salt supplements has also been investigated, but evidence to date is insufficient for recommendation 1. Recent experiments in mice have shown that high salt intakes increase interleukin (IL)-17, causing a more severe form of experimental autoimmune encephalomyelitis 17. However, this association has not been confirmed in patients with MS. Farez 18 found that moderate salt consumption increased the MS relapse rate by 2.75 and a high intake increased it by 3.95; however, the cohort was relatively small, and confounding factors could not be excluded. Another recent randomized controlled trial in women in the Nurses’ Health Study found no association between dietary salt and risk of MS 19. In children with MS, Azary et al. 20 did not find a correlation between iron intake and relapse rate. However, other studies found significant decreased iron consumption in patients with children with MS 21 and an association between low iron intake and a diagnosis of children with MS 22. In summary, at this time, salt intake does not appear to be associated with children with MS diagnosis or time to relapse 23.
- Vitamin B7 (Biotin) supplementation. Vitamin B7 (Biotin) supplementation is thought to improve remyelination by increasing energy production and fatty acid synthesis. In an open-label pilot study of 23 patients with progressive MS, more than 90% improved clinically (motor and visual symptoms) after treatment with high-dose B7 (MD 1003) for an average of 9 months 24. This study prompted a double-blind, multicenter phase 3 trial in which 154 adults with primary or secondary progressive MS were randomized to receive either high-dose vitamin B7 (Biotin) or placebo for 12 months. High-dose vitamin B7 (Biotin) significantly improved MS-related disability in 13% compared with none in the placebo group 25. However, a trial designed to replicate these observations in a larger, international cohort (n = 642) failed to show significant improvement in disability or walking speed in patients with progressive MS. Furthermore, the side effects and laboratory changes were associated with high-dose vitamin B7 (Biotin) therapy 26. Another multicenter randomized controlled trial comparing vitamin B7 (Biotin) 300 mg versus placebo showed only modest improvements in motor scores 25. Further investigation in a larger double-blind, multicenter phase 3 randomized controlled trial in adults with progressive MS (NCT02936037) is ongoing.
- Lipoic acid. Lipoic acid inhibits lymphocyte migration into the central nervous system (CNS), increases regulatory T cells, and downregulates pro-inflammatory cytokines in experimental autoimmune encephalomyelitis 27. In patients with MS, increased lipoic acid levels may reduce matrix metalloproteinase-9 and intercellular adhesion molecules inhibiting lymphocyte migration into the central nervous system (CNS) 28. In a small randomized controlled trial, patients with MS on lipoic acid had a significant 68% reduction in brain atrophy and a nonsignificant improvement in walking speed 29. A larger, phase 2 randomized controlled trial is planned to evaluate lipoic acid in progressive MS. Lipoic acid can interact with medications, cause hypoglycemia, and have renal toxic effects.
- Cannabinoids and medical marijuana. Research on cannabinoids and medical marijuana in MS is increasing. The recent National Academies of Sciences, Engineering, and Medicine report described the evidence of cannabinoids and derivatives in adults with chronic pain 30, MS-related spasticity 31 and clinical-neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol. 2015;262(11):2520–2527. doi: 10.1007/s00415-015-7878-1)), 32 and chemotherapy-induced nausea and vomiting. No trials have been undertaken in children with MS, partly because of concerns regarding the risk of addiction to marijuana in adolescents 33. Of additional concern are the adverse effects of marijuana use on cognitive function and mood in people with MS. Furthermore, discontinuing cannabis in adults with MS may improve depression 34.
- Avoiding cigarette smoking. Researchers know that exposure to cigarette smoke increases the risk of MS. Please don’t smoke, and avoid exposure to second-hand smoke. Family members who smoke should smoke outside and change clothes when they come inside.
- Eating a healthy diet and exercising. Obesity and high body mass index (BMI) are risk factors for MS in children and adolescents 12, 35, 36. Overall, children with MS appear to have more relative obesity than their adult counterparts 37 with higher body mass index (BMI), even years before onset of symptoms 38. A multicenter prospective cohort study that analyzed the time to relapse in 219 children with MS and clinical isolated syndrome showed that each 10% of energy intake from fat increased the relapse rate by 56%, and each 10% of energy specifically from saturated fats tripled this hazard. In contrast, each equivalent of a cup of vegetables decreased the relapse rate by 50% 20. However, in adults, a diet low in saturated fat did not improve Expanded Disability Status Scale (EDSS) score 39, 40, disease progression 41 or MRI activity 40. Maintaining a healthy weight is important. It is extra important for people with MS to be sure that they are making healthy choices. A healthy diet, regular exercise, and plenty of sleep are all essential.
Home remedies for multiple sclerosis
To help relieve the signs and symptoms of MS, try to:
- Get plenty of rest.
- Exercise. If you have mild to moderate MS, regular exercise can help improve your strength, muscle tone, balance and coordination. Swimming or other water exercises are good options if you’re bothered by heat. Other types of mild to moderate exercise recommended for people with MS include walking, stretching, low-impact aerobics, stationary bicycling, yoga and tai chi.
- Cool down. MS symptoms often worsen when your body temperature rises. Avoiding exposure to heat and using devices such as cooling scarves or vests can be helpful.
- Eat a balanced diet. Results of small studies suggest that a diet low in saturated fat but high in omega-3 fatty acids, such as those found in olive and fish oils, may be beneficial. But further research is needed. Studies also suggest that vitamin D may have potential benefit for people with MS.
- Relieve stress. Stress may trigger or worsen your signs and symptoms. Yoga, tai chi, massage, meditation or deep breathing may help.
Many people with MS use a variety of alternative or complementary treatments or both to help manage their symptoms, such as fatigue and muscle pain.
Activities such as exercise, meditation, yoga, massage, eating a healthier diet, acupuncture and relaxation techniques may help boost overall mental and physical well-being, but there are few studies to back up their use in managing symptoms of MS.
Guidelines from the American Academy of Neurology recommend the use of oral cannabis extract for muscle spasticity and pain, but do not recommend cannabis in any other form for other MS symptoms due to a lack of evidence.
The guidelines also do not recommend the use of herbal supplements such as Ginkgo biloba and bee venom or magnetic therapy for MS symptoms.
Pediatric multiple sclerosis prognosis
MS is a chronic condition that needs to be managed throughout life. The course of the disease is difficult to predict and varies from person to person. Some have long periods of remission while others have more frequent attacks.
- Fernandez-Carbonell C, Charvet LE, Krupp LB. Enhancing Mood, Cognition, and Quality of Life in Pediatric Multiple Sclerosis. Paediatr Drugs. 2021 Jul;23(4):317-329. doi: 10.1007/s40272-021-00451-5[↩][↩]
- Harding KE, Liang K, Cossburn MD, Ingram G, Hirst CL, Pickersgill TP, et al. Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):141–147. doi: 10.1136/jnnp-2012-303996[↩]
- Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D, University of British Columbia MSCN Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59(7):1006–1010. doi: 10.1212/WNL.59.7.1006[↩]
- Ghezzi A, Deplano V, Faroni J, Grasso MG, Liguori M, Marrosu G, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3(1):43–46. doi: 10.1177/135245859700300105[↩]
- Pediatric MS. https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS/Pediatric-MS[↩]
- Types of MS. MS Australia. https://www.msaustralia.org.au/about-ms/types-ms[↩]
- Multiple Sclerosis: A Primary Care Perspective. Am Fam Physician. 2014 Nov 1;90(9):644-652. http://www.aafp.org/afp/2014/1101/p644.html[↩][↩][↩]
- Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.[↩][↩]
- Sicotte NL. Magnetic resonance imaging in multiple sclerosis: the role of conventional imaging. Neurol Clin. 2011;29(2):343–356.[↩]
- Bates D. Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials. Neurology. 2011;76 (1 suppl 1):S14–S25.[↩]
- Medications. https://www.nationalmssociety.org/Treating-MS/Medications[↩]
- Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88(17):1623–1629. doi: 10.1212/WNL.0000000000003849[↩][↩]
- Soilu-Hanninen M, Aivo J, Lindstrom BM, Elovaara I, Sumelahti ML, Farkkila M, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(5):565–571. doi: 10.1136/jnnp-2011-301876[↩]
- Aivo J, Lindsröm BM, Soilu-Hänninen M. A Randomised, Double-Blind, Placebo-Controlled Trial with Vitamin D3 in MS: Subgroup Analysis of Patients with Baseline Disease Activity Despite Interferon Treatment. Mult Scler Int. 2012;2012:802796. doi: 10.1155/2012/802796[↩]
- Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest. 2011;40(6):627–639. doi: 10.3109/08820139.2011.573041[↩]
- Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial. Mult Scler Int. 2012;2012:452541. doi: 10.1155/2012/452541[↩]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868[↩]
- Farez MF. Salt intake in multiple sclerosis: friend or foe? J Neurol Neurosurg Psychiatry. 2016;87(12):1276. doi: 10.1136/jnnp-2016-313768[↩]
- Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology. 2017;89(13):1322–1329. doi: 10.1212/WNL.0000000000004417[↩]
- Azary S, Schreiner T, Graves J, Waldman A, Belman A, Guttman BW, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(1):28–33. doi: 10.1136/jnnp-2017-315936[↩][↩]
- Pakpoor J, Seminatore B, Graves JS, Schreiner T, Waldman AT, Lotze TE, et al. Dietary factors and pediatric multiple sclerosis: a case-control study. Mult Scler. 2017;24:1067–1076. doi: 10.1177/1352458517713343[↩]
- McDonald J, Graves J, Waldman A, Lotze T, Schreiner T, Belman A, et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87–92. doi: 10.1016/j.msard.2016.02.011[↩]
- Nourbakhsh B, Graves J, Casper TC, Lulu S, Waldman A, Belman A, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(12):1350–1353. doi: 10.1136/jnnp-2016-313410[↩]
- Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D, et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4(2):159–169. doi: 10.1016/j.msard.2015.01.005[↩]
- Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler. 2016;22(13):1719–1731. doi: 10.1177/1352458516667568[↩][↩]
- Cree BAC, Cutter G, Wolinsky JS, Freedman MS, Comi G, Giovannoni G, et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):988–997. doi: 10.1016/S1474-4422(20)30347-1[↩]
- Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175(1–2):87–96. doi: 10.1016/j.jneuroim.2006.03.007[↩]
- Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, et al. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11(2):159–165. doi: 10.1191/1352458505ms1143oa[↩]
- Spain R, Powers K, Murchison C, Heriza E, Winges K, Yadav V, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374. doi: 10.1212/NXI.0000000000000374[↩]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358[↩]
- Leocani L, Nuara A, Houdayer E, Schiavetti I, Del Carro U, Amadio S, et al. Sativex ((R[↩]
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363[↩]
- Brook JS, Lee JY, Brook DW. Trajectories of marijuana use beginning in adolescence predict tobacco dependence in adulthood. Subst Abus. 2015;36(4):470–477. doi: 10.1080/08897077.2014.964901[↩]
- Feinstein A, Meza C, Stefan C, Staines WR. Discontinuing cannabis improves depression in people with multiple sclerosis: a short report. Mult Scler. 2021;27(4):636–639. doi: 10.1177/1352458520934070[↩]
- Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548–552. doi: 10.1212/WNL.0b013e31828154f3[↩]
- Chitnis T, Graves J, Weinstock-Guttman B, Belman A, Olsen C, Misra M, et al. Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann Clin Transl Neurol. 2016;3(12):897–907. doi: 10.1002/acn3.365[↩]
- Brenton JN, Koenig S, Goldman MD. Vitamin D status and age of onset of demyelinating disease. Mult Scler Relat Disord. 2014;3(6):684–688. doi: 10.1016/j.msard.2014.07.004[↩]
- Brenton JN, Woolbright E, Briscoe-Abath C, Qureshi A, Conaway M, Goldman MD. Body mass index trajectories in pediatric multiple sclerosis. Dev Med Child Neurol. 2019;61(11):1289–1294. doi: 10.1111/dmcn.14233[↩]
- Riccio P, Rossano R, Larocca M, Trotta V, Mennella I, Vitaglione P, et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: a pilot study. Exp Biol Med (Maywood). 2016;241(6):620–635. doi: 10.1177/1535370215618462[↩]
- Yadav V, Marracci G, Kim E, Spain R, Cameron M, Overs S, et al. Low-fat, plant-based diet in multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord. 2016;9:80–90. doi: 10.1016/j.msard.2016.07.001[↩][↩]
- Hempel S, Graham GD, Fu N, Estrada E, Chen AY, Miake-Lye I, et al. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult Scler. 2017;23(4):525–533. doi: 10.1177/1352458517690270[↩]