What is alcoholic hepatitis
Alcoholic hepatitis is a progressive inflammation of the liver caused by drinking alcohol (ethanol) 1. Alcoholic hepatitis pathogenesis is not completely understood 2. Steatosis (fatty liver) and cirrhosis (scarring of liver) frequently accompany alcoholic hepatitis. Alcoholic hepatitis is associated with high morbidity and mortality, especially when severe 3. The average 30-day mortality for severe alcoholic hepatitis patients may be as high as 17–50% 4.
Alcoholic hepatitis is most likely to occur in people who drink heavily over many years 5. However, the relationship between drinking and alcoholic hepatitis is complex. Not all heavy drinkers develop alcoholic hepatitis, and the disease can occur in people who drink only moderately.
Alcohol abuse is the most common cause of serious liver disease in Western societies. In the United States alone, alcoholic liver disease affects more than 2 million people (i.e, approximately 1% of the population). The true prevalence of alcoholic hepatitis, especially of its milder forms, is unknown, because patients may be asymptomatic and may never seek medical attention.
Globally, the prevalence of alcoholic hepatitis appears to differ widely among different countries. In the Western hemisphere, when liver biopsies were performed in people who drank moderate to heavy amounts of alcohol and were asymptomatic, the prevalence of alcoholic hepatitis was found to be approximately 25-30%.
If you’re diagnosed with alcoholic hepatitis, you must stop drinking alcohol. Mild alcoholic hepatitis generally runs a benign course and is completely reversible with the cessation of alcohol consumption. However, people who continue to drink alcohol face a high risk of serious liver damage and death.
Abstinence along with adequate nutritional support remains the cornerstone of the management of patients with alcoholic hepatitis 6. About 10% to 20% of patients with alcoholic hepatitis are likely to progress to cirrhosis annually, and 10% of the individuals with alcoholic hepatitis have regression of liver injury with abstinence.
Alcoholic hepatitis symptoms
The most common sign of alcoholic hepatitis is yellowing of the skin and whites of the eyes (jaundice).
Other signs and symptoms of alcoholic hepatitis include:
- Loss of appetite
- Nausea and vomiting
- Abdominal tenderness
- Fever, which is often low-grade
- Fatigue and weakness
- Weight loss
Just about everyone who has alcoholic hepatitis is malnourished. Drinking large amounts of alcohol suppresses the appetite, and heavy drinkers get most of their calories in the form of alcohol.
Signs and symptoms of severe alcoholic hepatitis include:
- Fluid accumulation in your abdomen (ascites)
- Confusion and behavior changes due to a buildup of toxins normally broken down and eliminated by the liver
- Kidney and liver failure.
When to see a doctor
Alcoholic hepatitis is a serious disease. Up to 30 to 40 percent of people with severe alcoholic hepatitis can die within one month.
See your doctor if:
- You have any signs or symptoms of alcoholic hepatitis
- You feel you can’t control your drinking
- You would like help to cut back on your drinking
Causes of Alcoholic hepatitis
Alcoholic hepatitis develops when the alcohol that you drink damages your liver. Just how alcohol damages the liver — and why it does so only in some heavy drinkers — isn’t clear.
It is known that:
- The body’s process for breaking down alcohol produces highly toxic chemicals
- These chemicals trigger inflammation that destroys liver cells
- Over time, scars replace healthy liver tissue, interfering with liver function
- This irreversible scarring (cirrhosis) is the final stage of alcoholic liver disease
Other factors that can contribute to alcoholic hepatitis include:
- Other types of hepatitis. If you have hepatitis C and also drink — even moderately — you’re more likely to develop cirrhosis than if you don’t drink.
- Malnutrition. Many people who drink heavily are malnourished, because they eat poorly or because alcohol and its byproducts prevent the body from properly absorbing nutrients. Lack of nutrients contributes to liver cell damage.
Risk factors for Alcoholic hepatitis
The major risk factor for alcoholic hepatitis is the amount of alcohol you consume. The amount of alcohol intake that puts a person at risk of alcoholic hepatitis isn’t known. But most people with the condition have a history of drinking more than 3.4 ounces (100 grams) — equivalent to seven glasses of wine, seven beers or seven shots of spirits — daily for at least 20 years.
Other risk factors include:
- Your sex. Women seem to have a higher risk of developing alcoholic hepatitis possibly because of differences in the way alcohol is processed in women.
- Obesity. Heavy drinkers who are overweight might be likelier to develop alcoholic hepatitis and to progress from that condition to cirrhosis.
- Genetic factors. Studies suggest there may be a genetic component in alcohol-induced liver disease although it’s difficult to separate genetic and environmental factors.
- Race and ethnicity. Although it’s difficult o separate genetic and environmental factors, African-Americans and Hispanics might be at higher risk of alcoholic hepatitis.
- Binge drinking. Consuming five or more drinks at one time might increase your risk of alcoholic hepatitis.
Alcoholic hepatitis pathophysiology
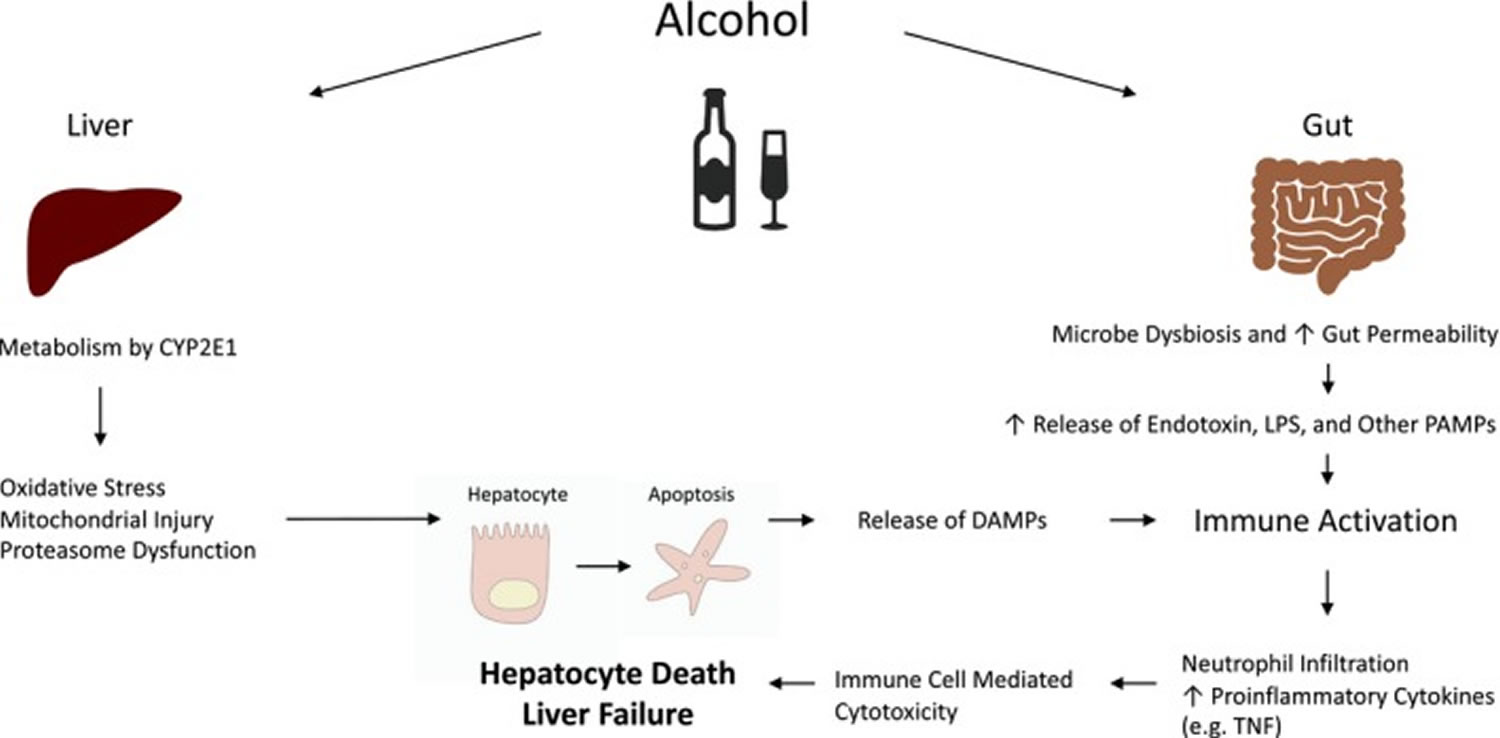
Intense research efforts focused on elucidating mechanisms of liver injury in alcoholic hepatitis have greatly advanced our understanding of alcoholic hepatitis pathophysiology (Figure 1 and 2). Ethanol-induced liver damage occurs via both direct hepatocyte injury and inflammation 7. Metabolism of ethanol also changes the redox state of hepatocytes, which interferes with carbohydrate and lipid metabolisms contributing to hepatic steatosis 8. Alcohol increases hepatocyte vulnerability to free-radicals as a result of enzyme CYP2E1 induction, mitochondrial dysfunction, depletion of anti-oxidants storage, and recruitment of inflammatory cells 7. Chronic alcohol abuse, particularly when combined with malnutrition, often compound the effect of oxidative injury by further lowering cellular resilience to oxidative stress and depleting anti-oxidant storage 7. Proteasome dysfunction also plays a role in exacerbating oxidative stress and cellular injury 7.
Secondary damage on hepatocytes mediated by inflammatory cells also plays a central role in pathophysiology of alcoholic hepatitis. Chronic exposure can lead to increased gut permeability and elevated circulating pathogen products, such as LPS (lipopolysaccharide), which are also known as PAMPs (Pathogen Associated Molecular Patterns) 9. Ethanol injured hepatocytes release aseptic inflammatory mediators, or DAMPs (Damage Associated Molecular Patterns). DAMPs and PAMPs bind to pathogen-pattern receptors such as TLRs (Toll-like Receptors) and NLRs (Nucleotide-binding Oligomerization Domain-like Receptors) on immune cells and liver parenchymal cells and potently stimulate the innate immune response. This leads to dense neutrophil infiltrations which is a hallmark of alcoholic hepatitis 9. Adaptive immune responses, mediated by B cells, T cells, and natural killer cells, also contribute to the storm of hepatic inflammation 10.
Figure 1. Alcoholic hepatitis pathophysiology
Footnote: Pathophysiology of alcohol‐induced liver injury.
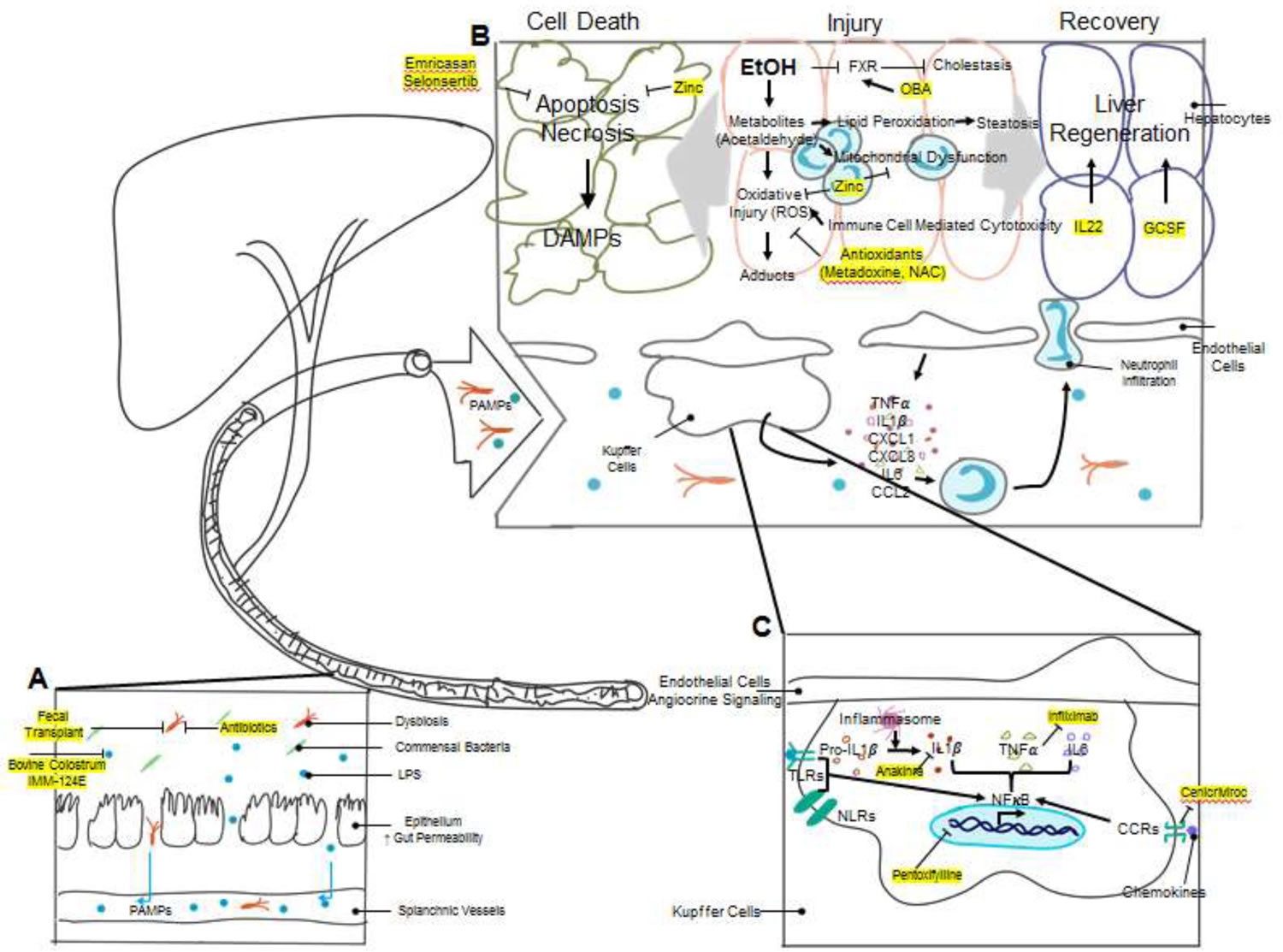
[Source 11 ]Figure 2. Alcoholic hepatitis pathophysiology and mechanisms of action of novel therapeutic agents
Footnotes: The pathogenesis of alcoholic hepatitis involves the interplay of multiple complex mechanisms. (A) Chronic alcohol use causes changes in the gut microbiome composition (dysbiosis) and breakdown of gut barrier function. Lipopolysaccharide (LPS) and other bacterial products can potently activate the innate immune system and are collectively called Pathogen-Associated Molecular Patterns (PAMPs). Increased gut permeability allows for the translocation of bacteria and PAMPs to the liver via splanchnic vasculature. Multiple therapeutics, including antibiotics, fecal transplantation, bovine colostrum, and hyperimmunized bovine colostrum IMM-124E target dysbiosis and reduce endotoxemia. (B) Excessive alcohol consumption leads to liver injury by multiple mechanisms. The toxic metabolites of ethanol, particularly acetaldehyde, cause direct hepatocyte oxidative injury as well as injury via formation of protein/DNA adducts. Ethanol metabolites also cause mitochondrial dysfunction and lipid peroxidation which leads to steatosis. Activated immune cells induce cell-mediated cytotoxicity by release of reactive oxygen species (ROS), further exacerbating oxidative injury. Antioxidants have been trialed in alcoholic hepatitis to attenuate oxidative stress. Zinc, in addition to being an antioxidant, is also protective against mitochondrial dysfunction and apoptosis. Cholestasis is another target for alcoholic hepatitis therapy. Obeticholic acid (OCA) is currently in trial as an agonist to the farnesoid X receptor (FXR), which has activity against bile synthesis. The injured liver has two different clinical outcomes, cell death and organ failure vs. liver regeneration and recovery. Hepatocyte injury activates apoptosis and necrosis pathways and releases Damage-Associated Molecular Patterns (DAMPs), which are cell derived molecules capable of activating the immune system. Emricasan and Selonsertib are two inhibitors to apoptosis signaling studied in alcoholic hepatitis. Liver injury also stimulates liver regeneration. Many cytokines, including TNFα and Interleukin-6 (IL-6), are potent activators of liver regeneration. Another cytokine, IL-22, has also been shown to stimulate liver regeneration. Growth colony stimulating factor (G-CSF) and its derivative pegylated G-CSF have shown promising results in multiple early clinical trials. (C) The accumulation of DAMPs and PAMPs in the liver activates resident liver immune cells, particularly Kupffer cells, by activation of toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs). Receptor activation enhances NFκB signaling and results in expression of pro-inflammatory molecules, including IL-1β. Pro-IL-1β undergo cleavage by caspase-1, which is activated by the inflammasome complex, to become activated IL-1β. An inhibitor to IL-1β, Anakinra, is currently studied in clinical trials. TNFα inhibitors, including Infliximab, have been tested in clinical trials. Pentoxifylline is another extensively studied compound in the treatment of alcoholic hepatitis. It suppresses NFκB signaling and reduces the production of cytokines, including TNFα. Chemokine receptors, such as C-C chemokine receptors (CCRs), promote inflammatory signaling via stimulation of the NFκB pathway, and a CCR2/CCR5 inhibitor, Cenicriviroc, has been proposed as another target for alcoholic hepatitis therapy. Inflammatory cytokines and chemokines released by Kupffer cells and other liver cells recruit circulating immune cells such as neutrophils and monocytes and promote chemotaxis and infiltration into liver parenchyma. Immune cell infiltration causes secondary cell-mediated injury to the liver parenchymal cells by oxidative injury as described above.
[Source 3 ]Prevention of alcoholic hepatitis
You might reduce your risk of alcoholic hepatitis if you:
- Drink alcohol in moderation, if at all. For healthy adults, moderate drinking means up to one drink a day for women and up to two drinks a day for men.
- The only certain way to prevent alcoholic hepatitis is to avoid all alcohol.
- Protect yourself from hepatitis C. Hepatitis C is an infectious liver disease caused by a virus. Untreated, it can lead to cirrhosis. If you have hepatitis C and drink alcohol, you’re far more likely to develop cirrhosis than is someone who doesn’t drink. Alcohol consumption may exacerbate injury caused by other pathogenic factors, including hepatitis viruses. Approximately 20% of patients presenting with alcoholic hepatitis have concomitant hepatitis C virus infection 12. Extensive epidemiologic studies suggest that the risk of cirrhosis in patients with chronic hepatitis C infection is greatly exacerbated by heavy alcohol ingestion. Possible mechanisms include the impairment of immune-mediated viral killing or enhanced virus gene expression due to the interaction of alcohol and hepatitis C virus.
- Check before mixing medications and alcohol. Ask your doctor if it’s safe to drink alcohol when taking your prescription medications. Read the warning labels on over-the-counter medications. Don’t drink alcohol when taking medications that warn of complications when combined with alcohol — especially pain relievers such as acetaminophen (Tylenol, others).
Alcoholic hepatitis complications
Complications of alcoholic hepatitis include:
- High blood pressure in the liver. Scar tissue can slow the flow of blood through your liver, causing an increase in pressure in a major blood vessel (portal vein).
- Enlarged veins (varices). Blood that can’t flow freely through the portal vein can back up into other blood vessels in the stomach and esophagus. These blood vessels have thin walls and are likely to bleed if filled with too much blood. Heavy bleeding in the upper stomach or esophagus is life-threatening and requires immediate medical care.
- Ascites. Fluid that accumulates in the abdomen might become infected and require treatment with antibiotics. Ascites aren’t life-threatening but are usually a sign of advanced alcoholic hepatitis or cirrhosis.
- Jaundice. A damaged liver can’t remove the residue of old red blood cells (bilirubin) from your blood. Bilirubin builds up and is deposited in your skin and the whites of your eyes, causing a yellow color.
- Confusion, drowsiness and slurred speech (hepatic encephalopathy). A damaged liver has trouble removing toxins from your body. The buildup of toxins can damage your brain. Severe hepatic encephalopathy can result in coma.
- Cirrhosis. This irreversible scarring of the liver frequently leads to liver failure.
- Kidney failure. A damaged liver can affect blood flow to the kidneys, resulting in damage to those organs.
Diagnosis of alcoholic hepatitis
Your doctor will conduct a physical examination and ask about your history of alcohol consumption. It is important to be honest in describing your drinking habits. Your doctor might ask to interview family members about your drinking.
To test for liver disease, your doctor might recommend:
- Liver function tests
- Blood tests
- An ultrasound, CT or MRI scan of the liver
- A liver biopsy, if other tests and imaging don’t provide a clear diagnosis or if you are at risk of other causes of hepatitis. The gold-standard for diagnosing alcoholic hepatitis remains the liver biopsy, although the diagnosis of alcoholic hepatitis in United States is often made based solely on clinical and laboratory findings 3.
In the United States, alcoholic hepatitis is often diagnosed based on clinical findings, with rapid onset of jaundice and transaminitis in the context of heavy alcohol use 13. Although liver biopsy may help to preclude alternative diagnoses of liver injury, it is not required for diagnosis of alcoholic hepatitis and is typically reserved in situations where the diagnosis is unclear. Characteristic features of alcoholic hepatitis on biopsy include ballooning degeneration of hepatocytes, alcoholic hyaline (Mallory‐Denk bodies) within damaged hepatocytes, cholestasis, and neutrophilic infiltrates 14.
Clinical manifestations of alcoholic hepatitis may include jaundice, anorexia, fever, abdominal pain, and liver decompensations due to portal hypertension 14. Typically, laboratory studies will show moderately elevated transaminases (rarely >500 IU/L), with aspartate aminotransferase‐to‐alanine aminotransferase (AST/ALT) ratio greater than 1.5. Bilirubin is elevated in acute alcoholic hepatitis as a defining feature of this disease. Findings of malnutrition may be seen in chronic alcohol abusers.
In an effort to standardize alcoholic hepatitis diagnosis for clinical research, recent clinical trials on alcoholic hepatitis has mostly adopted the alcoholic hepatitis diagnostic criteria from National Institute of Alcoholism and Alcohol Abuse (NIAAA) consensus committee 13. According to the National Institute of Alcoholism and Alcohol Abuse (NIAAA) guideline, diagnosis of alcoholic hepatitis is made based on 13:
- Alcohol use within 60 days of presentation;
- Presence of elevated liver enzymes with aspartate transaminase (AST) and alanine aminotransferase (ALT) greater than 50 IU/L but less than 400 IU/L, with AST/ALT ratio of >1.5,
- Worsening jaundice, with bilirubin greater than >3 mg/dL (>50 μmol/L), and
- Absence of any other causes of liver disease.
However, although clinical and laboratory information is usually sufficient to diagnose alcoholic hepatitis, liver biopsy is sometimes needed to discriminate from other causes of liver diseases as there can be other contributors to jaundice in alcoholic liver disease. Given presence of coagulopathy and ascites in many alcoholic hepatitis patients, transjugular approach to liver biopsy is sometimes preferred over percutaneous approach when liver biopsy is necessary. On histology, alcoholic hepatitis is characterized by neutrophil infiltration, steatosis, ballooning degeneration of hepatocyte, and Mallory-Denk bodies 15. Steatosis or fibrosis may be present in patient with concomitant nonalcoholic steatohepatitis or cirrhosis. While alcoholic hepatitis can occur with or without cirrhosis, liver biopsies of alcoholic hepatitis patients demonstrated that a majority have clinically silent liver fibrosis or cirrhosis at the time of presentation with alcoholic hepatitis. Alcoholic hepatitis patients with underlying liver cirrhosis have worse prognosis compared to those without 16. Therefore, an evaluation for the presence of liver cirrhosis should be obtained at the time of alcoholic hepatitis diagnosis to aid prognostication and to direct subsequent follow-up. Other factors such as obesity, metabolic syndrome, hepatitis C infection, and genetic traits likely also contribute to individual susceptibility to alcoholic hepatitis 17. The presence of cofactors may lower the threshold level of alcohol. Expression of certain variants of genes such as PNPLA3, HSD17β13, TM6SF2, MBOAT7 etc. have been observed in patients with alcoholic hepatitis, and research in this area has the potential to uncover novel therapeutic targets 18.
Alcoholic hepatitis prognosis
The long-term prognosis of individuals with alcoholic hepatitis depends heavily on whether patients have established cirrhosis and whether they continue to drink. With abstinence, patients with alcoholic hepatitis exhibit progressive improvement in liver function over months to years, and the histologic features of active alcoholic hepatitis resolve. If alcohol abuse continues, alcoholic hepatitis invariably persists and progresses to cirrhosis over months to years. Mild alcoholic hepatitis is a benign disorder with negligible short-term mortality. However, when alcoholic hepatitis is of sufficient severity to cause hepatic encephalopathy, jaundice, or coagulopathy, mortality can be substantial. In one study, the estimated 5-year survival after hospitalization for severe alcoholic hepatitis was 31.8%. Biopsy-documented alcoholic hepatitis has a 58% 4-year survival 19, with the worst outcomes in the first year in those with cirrhosis (35% survival) 20. A Danish survey from 1999 to 2008 21 showed increasing 28-day (12%–15%) and 84-day (14%–24%) mortality rates, and a 5-year survival of 53% and 31% in those without and with cirrhosis, respectively. Abstinence was the only independent predictor of long-term survival 22.
The overall 30-day mortality rate in patients hospitalized with alcoholic hepatitis is approximately 15%; however, in patients with severe liver disease, the rate approaches or exceeds 50%. In those lacking hepatic encephalopathy, jaundice, or coagulopathy, the 30-day mortality rate is less than 5%. Overall, the 1-year mortality rate after hospitalization for alcoholic hepatitis is approximately 40%.
In one study, the overall mortality among patients with severe alcoholic hepatitis was 66%. Age, white blood cell (WBC) count, prothrombin time (PT), and female sex were all independent risk factors for the dismal outcome 23.
To determine alcoholic hepatitis prognosis the following factors need to be considered:
- Histologically proven alcohol hepatitis
- Serum bilirubin greater than 2.5 mg/dl
- Serum albumin less than 2.5 g/dl
- Prothrombin time (PT) more than 5 seconds
Alcoholic hepatitis score
During the past several decades, various formulas and algorithms have been proposed for predicting the outcome of severe alcoholic hepatitis. The single most reliable indicator of severity is the presence of hepatic encephalopathy.
The American Association for the Study of Liver Diseases guideline recommends using prognostic scoring systems such as the Maddrey discriminant function (MDF) to stratify severity of alcoholic hepatitis and the risk of poor outcome, both initially and over the course of the illness 24.
The discriminant function (DF) of Maddrey and coworkers is based on Prothrombin time (PT) – a blood test that measures the time it takes for the liquid portion (plasma) of your blood to clot and bilirubin levels and it is calculated as follows: Maddrey’s Discriminant Function (MDF) = (4.6 × patient’s prothrombin time in seconds−control’s prothrombin time in seconds) + total serum bilirubin in milligrams per deciliter (mg/dL).
Maddrey’s discriminant function (MDF) values greater than 32 indicate severe severe alcoholic hepatitis and predict a 30-day mortality rate of approximately 20 to 30% within 1 month after presentation and 30 to 40% within 6 months after presentation 25, assuming only supportive treatment is given. However, subsequent studies have found the Maddrey’s discriminant function (MDF) to be an inexact predictor of mortality in patients with alcoholic hepatitis, especially in those who receive glucocorticoids.
Other formulas have been proposed for the assessment of prognosis of alcoholic hepatitis, but none has become popular among clinicians. For example, the model for end-stage liver disease (MELD) score, the ABIC score (age, bilirubin, international normalized ratio [INR] and the creatinine score), the Glasgow alcoholic hepatitis score (GAHS) (including the age, bilirubin, international normalized ratio [INR], blood urea nitrogen [BUN], and the peripheral white blood count) and the Lille score. The Lille score obtains data from the beginning and end of the first week of steroid therapy to assess response and subsequent need for further steroid therapy. Various combinations of scoring systems have been studied to predict outcomes accurately, and the combination of the MELD and the Lille score is one 26.
The Combined Clinical and Laboratory Index of the University of Toronto permits a linear estimate of acute mortality in persons with alcoholic hepatitis. Its major disadvantages are the large number 27 of variables that must be scored and the complexity of the calculation itself.
In contrast to the Combined Clinical and Laboratory Index, a much simpler formula for assessing mortality was proposed in a large series of 142 patients with histologically proven alcoholic hepatitis based on prothrombin time (PT), serum bilirubin level, and serum albumin level 28. According to this study, the mortality rate in patients with a serum bilirubin level greater than 2 mg/dL, a serum albumin level less than 2.5 g/dL, and a PT greater than 5 seconds was 75%. Conversely, patients who did not meet all 3 criteria had a much lower mortality rate (approximately 25%).
Recent studies indicate that CRP is a good marker of alcoholic hepatitis.
Model for end-stage liver disease (MELD) score
Several retrospective studies have shown that the MELD score is useful in predicting 30- and 90-day mortality in patients with alcoholic hepatitis (see the MELD Score calculator). Moreover, the MELD score seems to contain some practical and statistical advantages over Maddrey’s DF in predicting mortality among these patients. In a cohort of 73 patients with alcoholic hepatitis at the Mayo Clinic, the MELD score was the only independent predictor of mortality 29. Likewise, in another much larger retrospective study of 202 patients with alcoholic hepatitis, the MELD score was found superior to not only Maddrey’s DF but also to the classical Child-Turcotte-Pugh (CTP) score 30.
Glasgow alcoholic hepatitis score (GAHS)
The Glasgow alcoholic hepatitis score (GAHS) is one of the most recently described predictors of outcome in patients with alcoholic hepatitis. This scoring system uses 5 different variables, including age, bilirubin level, blood urea nitrogen (BUN) level, PT, and WBC count. The overall accuracy of GAHS, which was validated in 195 patients with alcoholic hepatitis, was 81%, when predicting 28-day outcome 27. In contrast, the modified DF had an overall accuracy of only 50% 27.
Asymmetric dimethylarginine (ADMA) score
The ADMA score is the most recently proposed predictor of adverse clinical outcome in patients with severe alcoholic hepatitis. In a small prospective study of 27 patients with alcoholic hepatitis, the ADMA score was a better predictor of outcome than the CTP score, the MDF, or the MELD score 31.
Other factors that correlate with poor prognosis include older age, impaired renal function, encephalopathy, and a rise in the WBC count in the first 2 weeks of hospitalization.
Alcoholic hepatitis treatment
Treatment for alcoholic hepatitis involves drinking cessation and therapies to ease the signs and symptoms of liver damage.
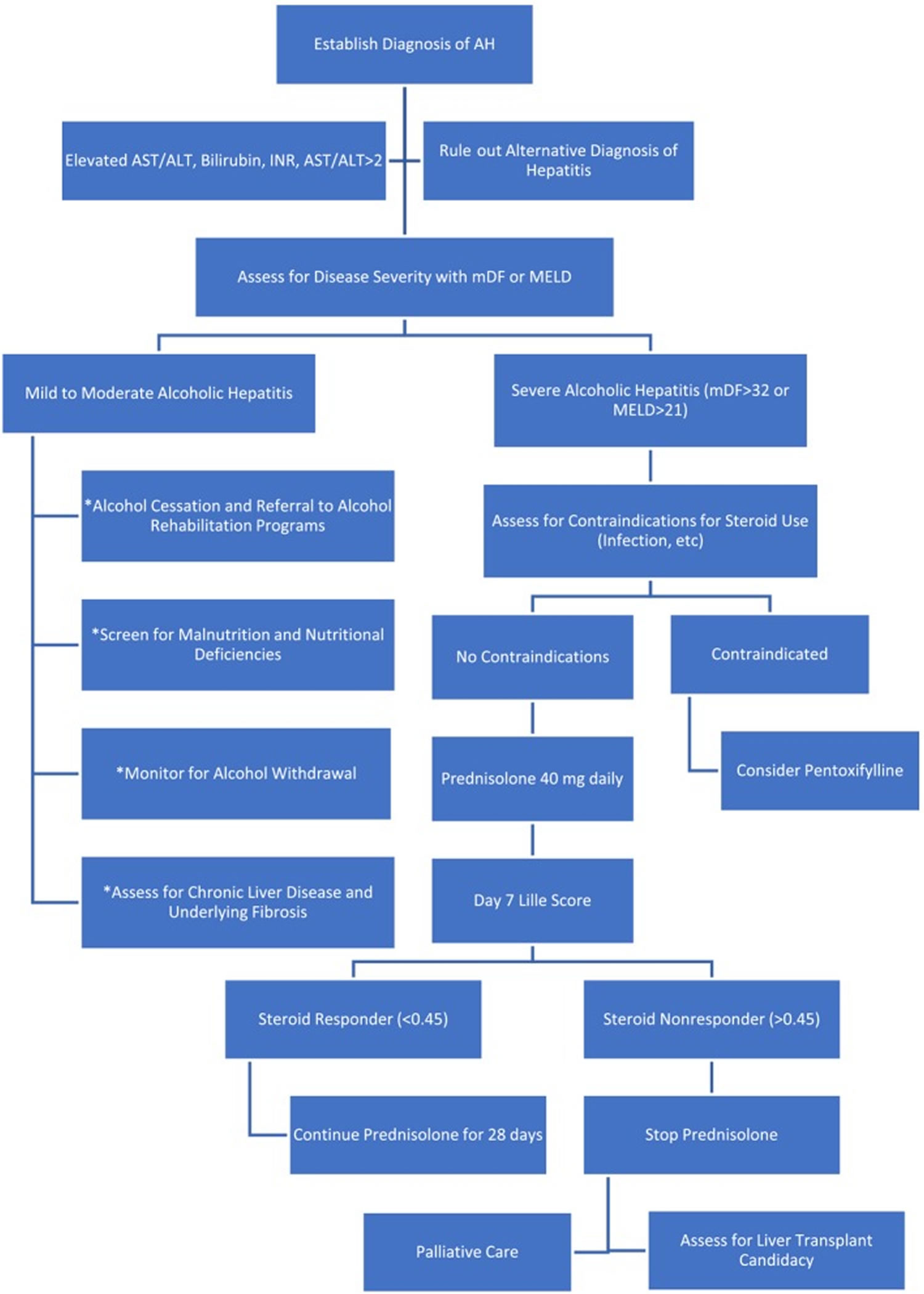
Patients with alcoholic hepatitis are subdivided into mild-moderate alcoholic hepatitis or severe alcoholic hepatitis 6. Mild to moderate alcoholic hepatitis is generally managed conservatively with alcohol cessation and monitored for development of complications 3.
Patients with an Maddrey’s Discriminant Function (MDF) greater than 32, Model for End-Stage Liver Disease (MELD) score greater than 20, ABIC (age, bilirubin, international normalized ratio, and the creatinine) score category C, or a Glasgow alcoholic hepatitis score (GAHS) of 9 predicts higher mortality with a diagnosis of severe alcoholic hepatitis. Patients with severe alcoholic hepatitis with or without hepatic encephalopathy are considered candidates for a short course of prednisolone (40 mg/day for 28 days). Prednisolone is preferred to prednisone as it does not require metabolism in the liver for its therapeutic efficacy. For patients unable to take it orally, methylprednisolone 32 mg intravenously daily, is an option. However, failure to respond to steroids within a week evident by a Lille score of greater than 0.45 indicates a lack of response to steroids which should be discontinued, thereafter. For patients with a Lille score of less than 0.45 (Lille responders), prednisolone should be continued for another three weeks. Glucocorticoids alter the expression of anti-inflammatory genes, thus promoting its anti-inflammatory role. Contraindications to steroid use include any active gastrointestinal (GI) bleeding, severe pancreatitis, uncontrolled diabetes, active infection, or renal failure. Such patients may be managed with pentoxifylline (400 mg orally, three times a day for 28 days). Hepatorenal syndrome is one of the major causes of death in patients with alcoholic hepatitis. Patients with acute kidney injury or hepatorenal syndrome respond poorly to corticosteroid therapy. Patients with bacterial infection may be treated with corticosteroids after the infection has been appropriately controlled with antibiotics. Response to prednisolone is graded as complete if Lille score is less than 0.16, partial if Lille score is between 0.16 and 0.56, or null if Lille score is greater than 0.56. A Lille score of more than 0.45 after 1 week of corticosteroid therapy is associated with 75% mortality at 6 months 32.
Many recent trials, including the STOPAH (Steroids or Pentoxifylline for Alcoholic Hepatitis) trial and meta-analysis of the use of steroids and Pentoxifylline, reveal only short-term (28-day) mortality improvements with not much difference of 6-month, or 1-year mortality 33. In the STOPAH (Steroids or Pentoxifylline for Alcoholic Hepatitis) trial, however, patients with less severe alcoholic hepatitis were included, and most patients were recruited with a clinical diagnosis of alcoholic hepatitis 33. Thus it is possible that patients with decompensated alcoholic cirrhosis may have received a diagnosis of alcoholic hepatitis, which significantly alters the result of the trial. Furthermore, the use of corticosteroids is restricted to patients in whom infection has been ruled out, which eliminates a significant proportion of alcoholic hepatitis patients 3. Finally, corticosteroids are only effective in a subset of patients and have little impact on long term survival. The Lille score is a commonly used clinical calculator to identify the patients most likely to respond to corticosteroid therapy after a 7-day trial. In patients whose Lille score is >0.45 after 7 days of corticosteroid therapy, further corticosteroids may lead to worse outcomes and thus is generally discontinued 34.
Anti-TNF (tumor necrosis factor) agents like Infliximab and Etanercept have been used with no proven survival benefits. Anti-TNF agents may even increase the incidence of infections and death.
Patients with alcoholic hepatitis are prone to infections, especially when on steroids. This is particularly important as it might lead to a poor prognosis, acute renal injury, and multi-organ dysfunction. Patients with alcoholic hepatitis are at risk of alcohol withdrawal. Lorazepam and oxazepam are the preferred benzodiazepines for prophylaxis and treatment of alcohol withdrawal. Daily caloric intake should be documented in patients with alcoholic hepatitis, and nutritional supplementation (preferably via mouth or nasogastric tube) should be considered if oral intake is less than 1200 kcal in a day.
Both pentoxifylline and prednisolone are recommended for severe alcoholic hepatitis but long-term benefits remain questionable.
Liver transplantation could be considered for patients not responsive to steroids and with a MELD of greater than 26. However, varied barriers including fear of recidivism, organ shortage, and social and ethical considerations exist. A survey of liver transplant programs conducted in 2015 revealed only 27% of the programs offering a transplant to alcoholic hepatitis patients. Of the 3,290 liver transplants performed 1.37% were on alcoholic hepatitis patients. The six months, one-year, and 5-year survival was 93%, 93%, and 87% respectively, the outcomes of which are comparable to patients with similar MELD scores. The recidivism rates are similar (17%) to patients transplanted for alcohol-related cirrhosis.
If the patient has acute renal failure, nephrology should be consulted to rule out hepatorenal syndrome.
If the patient has a change in mental status, develops seizures or focal deficits, a neurologist should be consulted. In addition, if the patient has leucocytosis and fever and there is a concern for an infection, an infectious disease consult should be obtained.
Figure 3. Alcoholic hepatitis management algorithm (treatment algorithms differ based on alcoholic hepatitis severity stratification)
Foonote: *These general management steps should be applied to all patients with alcoholic hepatitis (AH).
[Source 11 ]Drinking cessation
If you’ve been diagnosed with alcoholic hepatitis, you must stop drinking alcohol and never drink alcohol again. It’s the only way of possibly reversing liver damage or preventing the disease from becoming worse. Survival rates for people with alcoholic hepatitis who stop drinking are significantly better than survival rates for people who continue drinking.
If you are dependent on alcohol and want to stop drinking, your doctor can recommend a therapy that’s tailored for your needs. Treatment might include:
- Medications
- Counseling
- Alcoholics Anonymous or other support groups
- Outpatient or residential treatment program
Treatment for malnutrition
Your doctor might recommend a special diet to correct nutritional problems. You might be referred to a dietitian who can suggest ways to increase your consumption of the vitamins and nutrients you lack, including vitamin B1 (thiamine). A diet consisting of 100 g/day of protein should be recommended. Protein-energy malnutrition is very common in alcoholics and associated with high mortality when compared to patients with no malnutrition. Unless the patient has encephalopathy, protein should not be restricted.
If you have trouble eating, your doctor might recommend tube feeding. A tube is passed down your nose into your stomach. A special nutrient-rich liquid diet is then passed through the nasogastric tube.
Although the ideal nutrition intake goals for alcoholic hepatitis have not been studied in clinical trials, low nutritional intake, less than 21.5 kcal/kg/day, was associated with worse outcomes in severe alcoholic hepatitis 35. Current guidelines generally recommend daily protein intake of 1.2–1.5 g/kg/day and caloric intake of 30- 40 kcal/kg/day in alcoholic hepatitis patients 35. Data on the role of intensive enteric feeding has been conflicting. An early Veteran’s Affair’s (VA) hospital prospective study showed improved liver function with supplemental enteric feeding via nasogastric tube 36. However, a recent European multicenter randomized control trial failed to show mortality benefit with tube-feeding supplementation 37. This study suffered from a high rate of discontinuation of intervention in the nasogastric feeding arm, with 3 cases of aspiration pneumonia which led to 1 fatality 37. These results raised a valid concern of using nasogastric tube feeding in this patient population with a high incidence of hepatic encephalopathy. Total parenteral nutrition has also been proposed for the treatment of alcoholic hepatitis, but concerns regarding complications, particularly increased risk of infections and liver injury, limit its adaptation. Aside from protein-calorie malnutrition, deficiencies in many vitamins and minerals may impede recovery as well 35. Zinc is a trace element, and its deficiency is very common in patients with chronic alcohol use and has been shown to increase gut permeability and promote apoptosis 38. Zinc deficiency in endoplasmic reticulum and mitochondria has been shown to activate intrinsic cell death signaling in response to ethanol injury, which could not be effectively rescued by antioxidant treatment 38. A randomized control trial studying the effects of combination therapy with zinc sulfate, pentoxifylline and Anakinra showed a trend toward decreased mortality. Future trials addressing optimal strategies in treatment of macro- and micronutrient malnutrition in alcoholic hepatitis will help to direct clinical practice.
Medications to reduce liver inflammation
If you have severe alcoholic hepatitis, your doctor might recommend:
- Corticosteroids. These medications have shown some short-term benefit in increasing survival of certain people with severe alcoholic hepatitis. However, corticosteroids have serious side effects and generally aren’t prescribed if you have failing kidneys, gastrointestinal bleeding or an infection.
- Pentoxifylline. Your doctor might recommend this anti-inflammatory medication if you have severe alcoholic hepatitis and can’t take corticosteroids. The overall benefit of pentoxifylline for alcoholic hepatitis isn’t clear. Studies indicate that pentoxifylline might not be effective for people with mild alcoholic hepatitis or for people who haven’t responded to steroid treatment.
A number of therapies have been evaluated for the treatment of alcoholic hepatitis, but only two drugs have been incorporated into the treatment guidelines published by the American Association for the Study of Liver Disease and the European Association for the Study of the Liver 39. In a 2008 Cochrane meta-analysis of 15 randomized trials published since 1971 that compared glucocorticoids with placebo or no intervention for alcoholic hepatitis 40. Despite this apparent wealth of evidence, controversy persists. Advocates of the treatment cite significant reduction in short-term mortality, whereas detractors raise questions about the risks of sepsis and gastrointestinal hemorrhage with glucocorticoid therapy. In the largest placebo-controlled study to date, investigators treated 90 patients with prednisolone and found no benefit of that therapy over placebo administered in a similar group of patients 41. This study was hampered by the inclusion of patients with moderate or severe alcoholic hepatitis and those with alcohol-related cirrhosis. In the only study that required histologic confirmation of alcoholic hepatitis in all patients, prednisolone was associated with a short-term reduction in mortality, but this benefit was not apparent after 2 years 42. The systematic review by the Cochrane group revealed a trend toward a benefit with glucocorticoids that was not statistically significant 40. However a reanalysis of the five largest studies indicated a significant benefit from glucocorticoids; in this meta-analysis, 28-day mortality among patients with discriminant function scores of 32 or higher was 20% among those who were treated with prednisolone, as compared with 34% among those who received placebo 43.
Among four randomized, controlled trials in which pentoxifylline was compared with placebo for the treatment of alcoholic hepatitis, one showed a significant benefit 44. All 100 patients enrolled had a discriminant function that was greater than 32. The mortality was 24.6% in the pentoxifylline group as compared with 46.1% in the placebo group 44. The principal benefit related to pentoxifylline appeared to be a reduction in the number of deaths attributed to the hepatorenal syndrome. However, two meta-analyses have not shown any convincing benefit associated with pentoxifylline 45.
Two small studies have compared glucocorticoids with pentoxifylline, but the results were inconsistent 46. Two other studies have compared the effect of glucocorticoid monotherapy with that of combined treatment with glucocorticoids and pentoxifylline but showed no benefit from the addition of pentoxifylline 47.
Liver transplant
For many people with severe alcoholic hepatitis, a liver transplant is the only hope to avoid death. Survival rates for liver transplant for alcoholic hepatitis are similar to survival rates for transplants associated with other types of liver disease. However, most transplant centers are reluctant to perform liver transplants on people with alcoholic liver disease because of the fear they will resume drinking after surgery. For transplant to be an option, you would need:
- To find a program that will consider you
- To meet the requirements of the program, including abstaining from alcohol for six months before transplant and agreeing not to resume drinking afterward.
Clinical studies have shown improved outcomes with early liver transplantation in patients who do not respond to corticosteroids 48. However, liver transplantation for treatment of alcoholic hepatitis raises several concerns. Liver transplantation is a scarce resource and thus by default cannot be generalized to all patients with severe alcoholic hepatitis who may potentially benefit. In addition, recidivism after transplantation is a significant concern as alcoholic hepatitis patients often cannot complete alcohol rehabilitation and counseling programs generally required for others with alcoholic cirrhosis which generally requires an abstinent period of 6- months prior to liver transplant. Utilization of liver transplantation in the treatment of alcoholic hepatitis is an actively-evolving area as an increasing number of transplant centers are considering acute alcoholic hepatitis patients in their transplant programs. In patients who have undergone liver transplantation for alcoholic hepatitis, a multidisciplinary approach is needed for close monitoring. In addition to routine post-transplant care with surveillance and treatment of infections, renal injury, cardiovascular disease, osteoporosis, and de novo malignancies, patients undergoing liver transplantation for alcoholic hepatitis should receive alcohol-use disorder treatment to prevent post-transplant relapse 49.
New experimental treatments of severe alcoholic hepatitis
Many clinical trials are currently ongoing evaluating novel therapeutics in alcoholic hepatitis and have proven to be successful in pre-clinical studies (see Table 1).
Table 1. Clinical trials of Novel Therapeutic Agents for the Treatment of Alcoholic Hepatitis
| Therapy | Mechanism | Administration | Trial Design | Inclusion Criteria | Study Size | Primary Endpoint | Trial Status | Location | Clinical Trial ID# | Published Results | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||||||
| Rifaximin | Gut sterilzation | Combination with steroids | Open label, single arm | Maddrey’s Discriminant Function (MDF) ≥ 32 | 19 patients | Bacterial infections after 3-mo | Phase 2 Completed | Multicenter, Spain | NCT02116556 | No change in rate of infection | |
| Vancomycin, Gentamycin and Meropenem | Gut sterilzation | Intervention | Open label, single arm | Alcoholic hepatitis | 15 patients | Macrophage activation | Completed | Single center, Denmark | NCT03157388 | Unknown | |
| Augmentin | Gut sterilzation | Combination with steroids; vs. Placebo | RCT, double blinded | Maddrey’s Discriminant Function (MDF) ≥ 32 or MELD ≥21 | 280 patients | 2-mo survival | Phase 3 Recruiting | Multicenter, France | NCT02281929 | ||
| Ciprofloxicin | Gut sterilzation | Combination with steroids; vs. Placebo | Open label, randomized | Severe alcoholic hepatitis | 22 patients | 1-, 3-, and 6-mo survival | Phase 1 Completed | Single center, Finland | NCT02326103 | Unknown | |
| Endotoxemia | |||||||||||
| Bovine Colostrum | Decrease LPS | 1. Combination with steroids | 2. Intervention; vs. Placebo | RCT, double blinded | 1. Maddrey’s Discriminant Function (MDF) > 54 | 2. MDF>32 or MELD >=21 | 1. 10 | 2.111 patients | 1. Change in Maddrey’s Discriminant Function (MDF) after 8 weeks | 2. 3 mo survival | 1. Phase 2 Completed | 2. Phase 3. Recruiting | Single Center, India | 1.NCT02265328 | 2. NCT02473341 | 1. Improved liver function | |
| IMM 124-E | Decrease LPS | Intervention; vs. Placebo | RCT. double blinded | 20<= MELD <=28 | 56 patients | Circulating endotoxin level at 7-mo | Phase 2 Completed | Multicenter, US | NCT01968382 | Unknown | |
| Gut Microbiome | |||||||||||
| Lactobacillus subtilis/Streptoco ecus faecium | Modify gut microbiome | Intervention; vs. Placebo | RCT, double blinded | Maddrey’s Discriminant Function (MDF) ≥ 32 | 117 patients | LPS level | Phase 4 Completed | Single Center, Korea | NCT01501162 | LPS level decreased | |
| Lactobacillus Rhamnosus GG | Modify gut microbiome | Intervention; vs. Placebo | RCT, double blinded | Alcoholic hepatitis, MELD <21 | 130 patients | Change in MELD at 1-mo | Recruiting | Multicenter, US | NCT01922895 | ||
| Fecal Microbiota Transplantation | Modify gut microbiome | Intervention; vs. Steroids | RCT | Severe alcoholic hepatitis | 1.8; 2. 130 patients | Survival at 3 months | NA | Single center, India | 1. NCT03091010; 2. NCT03091010 | 1. Improved survival at 1 year | |
| IL-1 Inhibitor | |||||||||||
| Canakinumab | IL-1 Inhibition | Intervention; vs. Placebo | RCT, double blinded | Maddrey’s Discriminant Function (MDF) ≥ 32 and MELD ≤25 | 52 patients | Histological improvement at 1-month | Phase 2 Recruiting | Multiple Sites London | NCT03775109 | ||
| Anakinra, Pentoxifylline, and Zinc | IL-1 Inhibition (Anakinra) | Intervention; vs. Steroids | RCT, double blinded | Maddrey’s Discriminant Function (MDF) ≥ 32 and MELD ≥21 | 104 patients | 6-months survival | Phase 2/3 Completed | Multicenter, US | NCT01809132 | No change in survival | |
| Liver Regeneration | |||||||||||
| IL-22 (F-652) | Liver regeneration | I ntervention | Open label, single arm | 11<= MELD <=28 | 24 patients | Safety and toleratability | Phase 2a Completed, Phase 2b pending | Single center, US (Phase 2b Multicenter US) | NCT02655510 | Well tolerated, decrease in MELD | |
| GCSF | Liver regeneration | 1–2. Combination with pentoxifylline | 3. Combination with steroids; vs. Placebo 4. Intervention; vs.Placebo | 5. Combination with either steroids or pentoxifylline | 1–3. Open label, randomized, placebo controlled | 4–5. RCT, double blinded | 1–2. Inpatient severe alcoholic hepatitis | 3. Maddrey’s Discriminant Function (MDF)≥32 and Lille >0.16 | 4. Steroid Nonresponder, Lille >0.45 | 5. Maddrey’s Discriminant Function (MDF)≥32 | 1.46 | 100 | 268 | 33 nonresponders | 78 patients | 1–2. 3-months survival | 3. 2-months survival | 4.1-months survival | 5. 3-months survival | 1. Phase 4 Completed | 2–3. Phase 4 Recruiting | 5. Phase 2 Recruiting | 1–2,4. Single Center, India | 3. Multicenter, Korea, 5. Multicenter, US | 1. NCT03703674 | 3. NCT02442180 | 4. NCT01820208 | 5. NCT02776059 | 1. Increased survival 4. No change in 30 day mortality, but increased survival at 90 days | |
| GCSF+NAC | Liver regeneration | Combination with pentoxifylline; vs. GCSF alone vs. placebo | Open label, randomized, placebo controlled | Inpatient severe alcoholic hepatitis | 57 patients | 3-months survival | Phase 4 Complete | Single center, India | NCT02971306. | Increased survival in GCSF alone and GCSF+NAC | |
| Antioxidants | |||||||||||
| NAC | Antioxidant | Combination with steroids; vs. Steroid alone | RCT, double blinded | Biopsy proven alcoholic hepatitis and Maddrey’s Discriminant Function (MDF)≥32 | 180 patients | 6-months survival | Phase 3 Completed | Multicenter, France | NCT00863785 | No change in survival at 6-months, increased survival at 1-month | |
| NAC + enteric nutrition | Antioxidant | Intervention, with enteral nutrition | Open label | Biopsy proven alcoholic hepatitis and Maddrey’s Discriminant Function (MDF)≥32 | 52 patients | 6-months survival | Phase 3 Completed | Single center, Belgium | NCT00962442 | No change in survival | |
| Metacloxine | Antioxidant | 1. Combination with steroids; vs Steroids alone | 2. Combination with steroids/pentoxyfyll in | Open label, randomized | Maddrey’s Discriminant Function (MDF)≥32 | 1.70 | 2. 135 patients | 1.1- and 3-months survival | 2. 3- and 6-months survival | Phase 4 Completed | Single center, Mexico | 1. NCT02161653. | 2. NCT02161653. | Improved survival | |
| Omega 5 Fatty Acid | Antioxidant | Combination with steroids; vs. Steroids alone | RCT. double blinded | Maddrey’s Discriminant Function (MDF)≥32 | 40 patients | 30 Day Survival | Recruiting | Multiple Sites, Mexico | NCT03732586 | ||
| Supplemental Nutrition | |||||||||||
| Intensive Enteral Nutrition | Nutrition | Tube Feeding; vs. Usual meals | RCT | Maddrey’s Discriminant Function (MDF)≥32 | 136 patients | 6-months survival | Completed | Multicenter, Belgium and France | NCT01801332 | No change in survival | |
| Apoptosis | |||||||||||
| Selonsertib (GS-4997) | ASK-1 inhibitor | Combination with steroids; vs. Steroids alone | RCT, double blinded | Biopsy proven severe alcoholic hepatitis | 104 patients | Safety and toleratability | Phase 2 Completed | Multicenter, Multinational | NCT02854631 | No difference in safety, mortality, Lille response, and changes in MELD | |
| Emricasan (IDN-6556) | Pan-caspase inhibitor | Intervention; vs. Placebo | RCT, double blinded | 20< MELD <35 | 5 patients | 1-mo survival | Phase 2 Terminated | Multicenter, US | NCT01937130 | Termination due to high systemic drug levels | |
| Bile Induced Injury | |||||||||||
| ObeticholicAcid | Synthetically modified bile acid | Intervention; vs. Placebo | RCT, double blinded | 11< MELD <20 | 19 patients | Safety and toleratability; Change in MELD at 6wk | Phase 2 Completed | Multicenter, US | NCT02039219 | Unknown | |
| Lipid Metabolism | |||||||||||
| DS102 | Bioactive lipid | Intervention; vs. Placebo | RCT, double blinded | Probable or definite alcoholic hepatitis | 120 patients | Change in MELD at 1-mo | Phase 2 Not yet recruiting | NCT03452540 | |||
| DUR-928 | Small molecule epigenetic regulator | Dose escalation | Open label | 11<= MELD <=30 | 36 patients | Safety and toleratability | Phase 2 Recruiting | Multicenter, US | NCT03432260 | ||
Footnote: RCT = randomized controlled trial; LPS = lipopolysaccharide, NAC = N-acetylcysteine
Anti-inflammatory agents
Alcoholic hepatitis is a highly inflammatory condition involving complex crosstalks among various signaling pathways at the cellular level and multiple organs at the macro level. Liver Kupffer cells are believed to play an important role in instigating this inflammatory response. LPS-TLR4 binding on Kupffer cells leads to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling and expression of a slew of NFκB regulated genes 50. Among them are pro-inflammatory cytokines and chemokines, including TNFα and Interleukin-6 (IL-6). Interleukin-1 (IL-1) is also produced in precursor form downstream of NFκB signaling. Pro-IL-1β is activated and secreted upon cleavage with caspase 1, which gains function via recruitment to a multi-protein complex termed the inflammasome 51. TNFα release can be further amplified by a self-reinforcing positive feed-back loop in Kupffer cells, and TNFα signaling results in upregulation of chemokines, including C-C chemokine ligand 2 (CCL2) and C-X-C motif ligand 1 (CXCL1), leading to the recruitment of macrophages and neutrophils in propagation of liver inflammatory responses 52.
Given these observations, anti-inflammatory agents have been attractive therapeutic targets 53. Unfortunately, despite the clinical success of anti-TNFα agents in treatment of autoimmune diseases, TNFα inhibitors Infliximab and Etanercept failed in clinical trials for treatment of alcoholic hepatitis (see Figure 2). The use of TNFα inhibitors was associated with increased risk of infection and poor clinical outcomes 54. TNFα plays an important role not only in inflammation but also in hepatic regeneration. It was hypothesized that the suppression of TNFα-mediated liver regeneration combined with profound immunosuppression may have led to poor outcomes. Pentoxifylline, a nonselective phosphodiesterase (PDE) inhibitor, is another agent that has been extensively studied in treatment of alcoholic hepatitis. Pentoxifylline increases cellular cAMP, which has been shown to decrease TNFα and other pro-inflammatory cytokine production in immune cells. A multitude of clinical trials have been carried out with pentoxifylline either alone or in combination with steroids in the treatment of alcoholic hepatitis, and results have been conflicting. Initial clinical trials on pentoxifylline suggested a mortality benefit in severe alcoholic hepatitis compared to placebo, and its benefit seemed to be related to prevention of hepatorenal syndrome 44. However, pentoxifylline was shown to be inferior to steroids in a head-to-head trial of severe alcoholic hepatitis patients 46 and pentoxifylline rescue was ineffective in steroid nonresponders 55. The effects of pentoxifylline alone and in combination with prednisolone were examined in the STOPAH trial, and despite lower incidence of hepatorenal syndrome, pentoxifylline failed to show mortality benefit 33. Given these results, clinical practice has moved away from use of pentoxifylline in severe alcoholic hepatitis.
Another cytokine, IL-1β, has also been studied as a therapeutic target for alcoholic hepatitis treatment. In preclinical models, IL-1 receptor antagonists were shown to attenuate liver injury caused by alcohol 56. Anakinra, an IL-1 receptor antagonist, has been studied in clinical trials in combination with zinc sulfate and pentoxifylline compared to corticosteroids in patients with severe alcoholic hepatitis. This trial did not meet statistical significance in mortality benefit but showed a treatment effect in favor of the combination group 57. Another National Institute of Alcoholism and Alcohol Abuse (NIAAA) funded multicenter, randomized clinical trial examining the combination of Anakinra, zinc, and G-CSF is set to begin recruitment soon. More narrowly targeted and selective anti-inflammatory agents such as chemokine receptor antagonists have been used in preclinical setting with some success. Cenicriviroc, a CCR2/CCR5 receptor antagonist, was recently shown to reduce steatosis and fibrosis in a Phase II cohort with Nonalcoholic Steatohepatitis (NASH) 58. Clinical trial for treatment of alcoholic hepatitis with Cenicriviroc is needed to explore its applicability to alcoholic hepatitis.
Gut microbiome dysfunction and dysbiosis
Recent advances in our understanding of pathophysiology of alcoholic hepatitis have demonstrated that gut-liver axis dysfunction, particularly microbial dysbiosis and impaired gut barrier function, plays a significant role in development of liver diseases 59. Gut permeability increases after alcohol binge or chronic consumption and grants intestinal lumen products access to the portal circulation, leading to the propagation of inflammation and cell injury 60. Lipopolysaccharide (LPS), a component of gram-negative bacterial cell wall, and other Pathogen-Associated Molecular Patterns (PAMPs) have been well documented as perpetuating agents in this process 61. Pathogen-Associated Molecular Patterns (PAMPs) activate resident Kupffer cells in the liver through activation of TLR4 62 and preclinical studies have shown attenuation of alcohol induced liver damage by inhibiting binding of endotoxin to these Kupffer cells 63. Thus, gut-liver axis derangement is being increasingly recognized as an important driver of alcohol induced liver damage.
Recognition of gut-liver axis dysfunction in alcoholic hepatitis has led to the development of many potential therapeutics over the last two decades. A good example of gut endotoxins targeted therapy is the use of bovine colostrum, which is rich in immunoglobulins and antimicrobials thought to decrease porto-systemic endotoxemia 64. A hyperimmune bovine colostrum, IMM 124-E is further enriched for anti-LPS IgG antibodies and shown to alleviate liver injury in a clinical trial in nonalcoholic steatohepatitis (NASH) patients, possibly through its modulation of natural killer and T cells function 65. There are multiple trials underway to study the benefit of bovine colostrum in alcoholic hepatitis, including a well- designed phase II placebo-controlled RCT (Randomized Controlled Trial) currently underway studying the use of IMM-124E in alcoholic hepatitis (Table 1).
Impaired gut barrier function and dysbiosis leads not only to endotoxin but also bacterial translocation (see Figure 2). Combined with immune dysfunction present in alcoholic hepatitis, severe alcoholic hepatitis patients are at significantly elevated risk of infections, as high as 42% incidence of infections in severe alcoholic hepatitis patients within 90 days of the diagnosis 66. This risk is further exacerbated by corticosteroids treatment 66. These observations prompted many clinical trials exploring the possibility of empiric antibiotics or probiotics as treatments for alcoholic hepatitis. A small pilot study with the prophylactic use of rifaximin showed a trend toward benefit compared to control 67. Currently, multiple large trials are underway studying the prophylactic use of a variety of antibiotics including Augmentin, Ciprofloxacin, Vancomycin, Gentamycin, and Meropenem. The use of probiotics has been evaluated as well with promising preliminary data. Murine studies showed benefit with VSL3 and lactobacillus rhamnosus GG (46). A Randomized Controlled Trial is now underway at NIAAA studying the use of Lactobacillus rhamnosus in alcoholic hepatitis. Fecal transplantation, which has shown great potential in treating gut dysbiosis, was also examined in open label trials with promising results, showing a mortality benefit despite very small sample size 68. Given these early positive trends, further studies targeting gut microbiome in alcoholic hepatitis are hotly anticipated.
Antioxidants
Multiple modes of cellular injury in alcoholic hepatitis result in hepatocyte oxidative injury, including toxic ethanol metabolites, inflammatory cell-mediated cytotoxicity, as well as mitochondrial and proteasome dysfunctions. Given the central role of oxidative injury in the pathogenesis of alcoholic hepatitis, the use of anti-oxidants in alcoholic hepatitis has garnered much attention (Figure 2). Unfortunately, as a group, anti-oxidants have largely failed to demonstrate a significant mortality benefit although a few treatments showed promise. N-acetylcysteine (NAC) is an anti-oxidant widely used in the treatment of acute liver injury. A randomized control trial of N-acetylcysteine (NAC) infusion in combination with prednisolone showed mortality benefit at 30 days compared to prednisolone alone, but this effect was not seen at 3 months, which was the primary endpoint 69. Another study that combined the use of NAC and GCSF failed to show improvement of outcome beyond GCSF alone 70. Another example of antioxidant used to treat alcoholic hepatitis with promising trend is metadoxine, which is thought to increase hepatic glutathione concentrations. Metadoxine used in combination with steroid or pentoxifylline improved survival at 3 and 6 months in small open labeled trials 71. Interestingly, patients receiving metadoxine attained higher rate of sobriety in this trial, an effect that has also been observed in other retrospective analyses on alcohol dependency 72. If confirmed in larger studies, the dual antioxidative and abstinence-maintenance properties of metadoxine along with a very favorable side-effect profile, make it an attractive option for alcoholic hepatitis treatment.
Apoptosis and Liver Regeneration
The balance of signals promoting cell death and adaptive survival determines the presence of liver failure in alcoholic hepatitis. Hepatocyte death is induced by alcohol via apoptosis and necrosis processes, involving the mitochondrial pathway, caspase-dependent pathway, or ER stress 73. Caspases are a group of 11 intracellular cysteine proteases that are involved in apoptosis, profibrotic gene expression, and activation of IL-1β upon association with the inflammasome 51. Emricasan is an oral small-molecule caspase protease inhibitor currently in clinical trials. After promising preclinical studies, it was brought into trials for multiple liver diseases including alcoholic hepatitis 74. Emricasan was shown to improve liver function in patients liver fibrosis 75, but unfortunately, a recent phase II trial in severe alcoholic hepatitis patients was terminated due to concerns about liver toxicity. Another oral apoptosis inhibitor, apoptosis signal regulating kinase-1 (ASK-1) inhibitor, Selonsertib (GS-4997) was found to be safe but failed to improve mortality or liver function compared to steroids 76.
The counterbalance to cell death in alcoholic hepatitis is liver regeneration, which is the mechanism involved in recovery of liver function after toxic insults. Preclinical studies have implicated NF-kB, STAT3 and downstream pathways in the regeneration process 77. TNFα is an important player to maintain an equilibrium between proliferation, survival and apoptosis as it can induce different responses depending on the cellular context 78. Interleukin-22 (IL-22) is a promising novel therapeutic agent from the IL-10 family that has been shown in preclinical studies as having anti-apoptosis, anti-inflammation, anti-steatosis and proliferative effects 79. Preliminary data from an open-label, small cohort study with IL-22 showed efficacy signals and good safety profile in treatment of alcoholic hepatitis patients 80. A follow up phase IIb RCT on IL-22 is in preparation (Table 1). Granulocyte-colony stimulating factor (G-CSF) is another promising therapeutic agent being evaluated in clinical trials (Table 1). G-CSF was shown to mobilize hematopoietic stem cells and stimulate the proliferation of liver progenitor cells in animal models of acute liver failure 81. The activation of hematopoietic stem cells may assist with the recovery of immune dysfunction, and the proliferation of liver progenitor cells was thought to mediate liver regeneration. Several small clinical studies in India showed mortality benefit with G-CSF 82. Multiple clinical trials in Western populations are underway, and results are eagerly anticipated.
Bile metabolism has also been the target of research in alcoholic hepatitis treatment. Alcoholic hepatitis is characterized by cholestasis, and it has been hypothesized that Farnesoid X receptor (FXR) agonist obeticholic acid (OCA) protects hepatocytes against bile toxicity 81. Obeticholic acid (OCA) has shown promising results in treating chronic cholangiopathies 83. Results from a recent phase II study looking at safety and efficacy is complete but unpublished as of now.
References- Casanova J, Bataller R. Alcoholic hepatitis: Prognosis and treatment. Gastroenterol Hepatol. 2014 Apr. 37(4):262-8.
- Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009 Jul 28. 15(28):3462-71.
- Sehrawat, T. S., Liu, M., & Shah, V. H. (2020). The knowns and unknowns of treatment for alcoholic hepatitis. The lancet. Gastroenterology & hepatology, 5(5), 494–506. https://doi.org/10.1016/S2468-1253(19)30326-7
- Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O’Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015 Apr 23;372(17):1619-28. DOI: 10.1056/NEJMoa1412278
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009 Jun 25;360(26):2758-69. doi: 10.1056/NEJMra0805786
- Shah NJ, Royer A, John S. Alcoholic Hepatitis. [Updated 2021 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470217
- McClain RL CaCJ. Alcoholic Liver Disease In: Feldman M MD; Friedman Lawrence S. MD; Brandt Lawrence J. MD, editor. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. e5 ed: Saunders; 2016. p. 1409–27.
- Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004 Aug;34(1):9-19. doi: 10.1016/j.alcohol.2004.07.008
- Gao, B., Ahmad, M. F., Nagy, L. E., & Tsukamoto, H. (2019). Inflammatory pathways in alcoholic steatohepatitis. Journal of hepatology, 70(2), 249–259. https://doi.org/10.1016/j.jhep.2018.10.023
- Koskinas J, Kenna JG, Bird GL, Alexander GJ, Williams R. Immunoglobulin A antibody to a 200-kilodalton cytosolic acetaldehyde adduct in alcoholic hepatitis. Gastroenterology. 1992 Dec;103(6):1860-7. doi: 10.1016/0016-5085(92)91445-a
- Liu, M., & Shah, V. H. (2019). New Prospects for Medical Management of Acute Alcoholic Hepatitis. Clinical liver disease, 13(5), 131–135. https://doi.org/10.1002/cld.792
- Shoreibah M, Anand BS, Singal AK. Alcoholic hepatitis and concomitant hepatitis C virus infection. World J Gastroenterol. 2014 Sep 14. 20(34):11929-34.
- Crabb, D. W., Bataller, R., Chalasani, N. P., Kamath, P. S., Lucey, M., Mathurin, P., McClain, C., McCullough, A., Mitchell, M. C., Morgan, T. R., Nagy, L., Radaeva, S., Sanyal, A., Shah, V., Szabo, G., & NIAAA Alcoholic Hepatitis Consortia (2016). Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology, 150(4), 785–790. https://doi.org/10.1053/j.gastro.2016.02.042
- Carithers RL, McClain CJ. Alcoholic liver disease In: Feldman M, Friedman LS, Brandt LJ, ed. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 5th ed. St. Louis, MO: WB Saunders; 2016:1409‐1427.
- Theise N. D. (2013). Histopathology of alcoholic liver disease. Clinical liver disease, 2(2), 64–67. https://doi.org/10.1002/cld.172
- Morgan MY. The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl. 1994;2:335-43.
- Altamirano, J., & Michelena, J. (2013). Alcohol consumption as a cofactor for other liver diseases. Clinical liver disease, 2(2), 72–75. https://doi.org/10.1002/cld.197
- Atkinson SR, Way MJ, McQuillin A, Morgan MY, Thursz MR. Homozygosity for rs738409:G in PNPLA3 is associated with increased mortality following an episode of severe alcoholic hepatitis. J Hepatol. 2017 Jul;67(1):120-127. doi: 10.1016/j.jhep.2017.01.018
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993 Apr;17(4):564-76. doi: 10.1002/hep.1840170407
- Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol. 1991 Feb;86(2):210-6.
- Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011 Apr;54(4):760-4. doi: 10.1016/j.jhep.2010.07.016
- Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013 Sep. 38(6):584-95.
- Horie Y, Ishii H, Hibi T. Severe alcoholic hepatitis in Japan: prognosis and therapy. Alcohol Clin Exp Res. 2005 Dec. 29(12 Suppl):251S-8S.
- [Guideline] O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010 Jan. 51(1):307-28.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978 Aug;75(2):193-9.
- van Welzen, B. J., Mudrikova, T., El Idrissi, A., Hoepelman, A., & Arends, J. E. (2019). A Review of Non-Alcoholic Fatty Liver Disease in HIV-Infected Patients: The Next Big Thing?. Infectious diseases and therapy, 8(1), 33–50. https://doi.org/10.1007/s40121-018-0229-7
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glascow alcoholic hepatitis score. Gut. 2005 Aug. 54:14-5.
- Mihas AA, Doos WG, Spenney JG. Alcoholic hepatitis–a clinical and pathological study of 142 cases. J Chronic Dis. 1978. 31(6-7):461-72.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005 Feb. 41(2):353-8.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or discriminant function score in patients with alcoholic hepatitis. J Hepatol. 2005 May. 42:700-6.
- Mookerjee RP, Malaki M, Davies NA, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007 Jan. 45:62-71.
- Puoti C, Elmo MG, Ceccarelli D, Ditrinco M. Liver steatosis: The new epidemic of the Third Millennium. Benign liver state or silent killer? Eur J Intern Med. 2017 Dec;46:1-5. doi: 10.1016/j.ejim.2017.06.024
- Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, O’Grady JG, Akriviadis E, Sinakos E, Carithers RL Jr, Ramond MJ, Maddrey WC, Morgan TR, Duhamel A, Mathurin P. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018 Aug;155(2):458-468.e8. doi: 10.1053/j.gastro.2018.05.011
- Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, Boleslawski E, Deltenre P, Canva V, Pruvot FR, Mathurin P. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007 Jun;45(6):1348-54. doi: 10.1002/hep.21607
- Plauth, M., Bernal, W., Dasarathy, S., Merli, M., Plank, L. D., Schütz, T., & Bischoff, S. C. (2019). ESPEN guideline on clinical nutrition in liver disease. Clinical nutrition (Edinburgh, Scotland), 38(2), 485–521. https://doi.org/10.1016/j.clnu.2018.12.022
- Kearns PJ, Young H, Garcia G, Blaschke T, O’Hanlon G, Rinki M, Sucher K, Gregory P. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992 Jan;102(1):200-5. doi: 10.1016/0016-5085(92)91801-a
- Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, Hittelet A, Piquet MA, Laleman W, Orlent H, Lasser L, Sersté T, Starkel P, De Koninck X, Negrin Dastis S, Delwaide J, Colle I, de Galocsy C, Francque S, Langlet P, Putzeys V, Reynaert H, Degré D, Trépo E. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. 2016 Apr;150(4):903-10.e8. doi: 10.1053/j.gastro.2015.12.038
- Sun, Q., Zhong, W., Zhang, W., Li, Q., Sun, X., Tan, X., Sun, X., Dong, D., & Zhou, Z. (2015). Zinc deficiency mediates alcohol-induced apoptotic cell death in the liver of rats through activating ER and mitochondrial cell death pathways. American journal of physiology. Gastrointestinal and liver physiology, 308(9), G757–G766. https://doi.org/10.1152/ajpgi.00442.2014
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012 Aug;57(2):399-420. doi: 10.1016/j.jhep.2012.04.004
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis–a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008 Jun;27(12):1167-78. doi: 10.1111/j.1365-2036.2008.03685.x
- Mendenhall CL, Anderson S, Garcia-Pont P, Goldberg S, Kiernan T, Seeff LB, Sorrell M, Tamburro C, Weesner R, Zetterman R, et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med. 1984 Dec 6;311(23):1464-70. doi: 10.1056/NEJM198412063112302
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, Benhamou JP, Chaput JC, Rueff B, Poynard T. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996 Jun;110(6):1847-53. doi: 10.1053/gast.1996.v110.pm8964410
- Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011 Feb;60(2):255-60. doi: 10.1136/gut.2010.224097
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000 Dec;119(6):1637-48. doi: 10.1053/gast.2000.20189
- Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013 May;37(9):845-54. doi: 10.1111/apt.12279
- Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, Sohn JH, Yoon KT, Kim IH, Kim HS, Um SH, Baik SK, Lee JS, Suk KT, Kim SG, Suh SJ, Park SY, Kim TY, Jang JY; Korean Association for the Study of the Liver (KASL)-Alcohol Related Problems Study Group. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014 Oct;61(4):792-8. doi: 10.1016/j.jhep.2014.05.014
- Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R, Lebrec D, Moreno C, Talbodec N, Paupard T, Naveau S, Silvain C, Pageaux GP, Sobesky R, Canva-Delcambre V, Dharancy S, Salleron J, Dao T. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013 Sep 11;310(10):1033-41. doi: 10.1001/jama.2013.276300
- Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011 Nov 10;365(19):1790-800. doi: 10.1056/NEJMoa1105703
- Cuadrado A, Fábrega E, Casafont F, Pons-Romero F. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2005 Apr;11(4):420-6. doi: 10.1002/lt.20386
- Neuman MG, Maor Y, Nanau RM, Melzer E, Mell H, Opris M, Cohen L, Malnick S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules. 2015 Aug 28;5(3):2023-34. doi: 10.3390/biom5032023
- Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012 Sep;57(3):642-54. doi: 10.1016/j.jhep.2012.03.035
- Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, Kostallari E, Gao Y, Simonetto DA, Yaqoob U, Cao S, Revzin A, Beyder A, Wang RA, Kamath PS, Kubes P, Shah VH. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019 Jul;157(1):193-209.e9. doi: 10.1053/j.gastro.2019.03.013
- Greuter T, Malhi H, Gores GJ, Shah VH. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight. 2017 Sep 7;2(17):e95354. doi: 10.1172/jci.insight.95354
- Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, Kamath PS, Shah VH. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008 Dec;135(6):1953-60. doi: 10.1053/j.gastro.2008.08.057
- Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008 Mar;48(3):465-70. doi: 10.1016/j.jhep.2007.10.010
- Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012 Oct;122(10):3476-89. doi: 10.1172/JCI60777
- Szabo G, Mitchell MC, McClain CJ, Dasarathy S, McCullough AJ, Nagy L, et al. IL-1 Receptor Antagonist in Combination with Pentoxifylline and Zinc for Severe Alcoholic Hepatitis: A Multicenter Randomized Double-Bind Placebo-Controlled Clinical Trial. Hepatology. 2018;68(6):1444A–71A.
- Lefebvre E, Ratziu V, Sanyal AJ, Francque SM, Wong V, Loomba R, et al. 357 – Cenicriviroc Treatment for Adults with Non-Alcoholic Steatohepatitis: Year 2 Analysis of the Phase 2B Centaur Study. Gastroenterology. 2018;154(6, Supplement 1):S–1085.
- Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019 Apr;16(4):235-246. doi: 10.1038/s41575-018-0099-1
- Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005 Aug;289(2):G367-75. doi: 10.1152/ajpgi.00464.2004
- Bala, S., Marcos, M., Gattu, A., Catalano, D., & Szabo, G. (2014). Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PloS one, 9(5), e96864. https://doi.org/10.1371/journal.pone.0096864
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001 Jul;34(1):101-8. doi: 10.1053/jhep.2001.25350
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994 Aug;20(2):453-60.
- Döhler, J. R., & Nebermann, L. (2002). Bovine colostrum in oral treatment of enterogenic endotoxaemia in rats. Critical care (London, England), 6(6), 536–539. https://doi.org/10.1186/cc1819
- Mizrahi, M., Shabat, Y., Ben Ya’acov, A., Lalazar, G., Adar, T., Wong, V., Muller, B., Rawlin, G., & Ilan, Y. (2012). Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. Journal of inflammation research, 5, 141–150. https://doi.org/10.2147/JIR.S35227
- Vergis, N., Atkinson, S. R., Knapp, S., Maurice, J., Allison, M., Austin, A., Forrest, E. H., Masson, S., McCune, A., Patch, D., Richardson, P., Gleeson, D., Ryder, S. D., Wright, M., & Thursz, M. R. (2017). In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology, 152(5), 1068–1077.e4. https://doi.org/10.1053/j.gastro.2016.12.019
- Jimenez C, Ventura M, Sala M, Cañete N, Poca M, Simón-Talero M, et al. Use of rifaximin in alcoholic hepatitis: Pilot study. Journal of Hepatology. 2018;68:S816.
- Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, Sharma MK, Maiwall R, Jindal A, Choudhary A, Hussain MS, Sharma S, Sarin SK. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017 Apr;15(4):600-602. doi: 10.1016/j.cgh.2016.10.029
- Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011 Nov 10;365(19):1781-9. doi: 10.1056/NEJMoa1101214
- Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, Singh A. Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2018 Oct;16(10):1650-1656.e2. doi: 10.1016/j.cgh.2018.01.040
- Higuera-de la Tijera, F., Servín-Caamaño, A. I., Serralde-Zúñiga, A. E., Cruz-Herrera, J., Pérez-Torres, E., Abdo-Francis, J. M., Salas-Gordillo, F., & Pérez-Hernández, J. L. (2015). Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World journal of gastroenterology, 21(16), 4975–4985. https://doi.org/10.3748/wjg.v21.i16.4975
- Leggio L, Kenna GA, Ferrulli A, Zywiak WH, Caputo F, Swift RM, Addolorato G. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Hum Psychopharmacol. 2011 Dec;26(8):554-9. doi: 10.1002/hup.1244
- Nagy, L. E., Ding, W. X., Cresci, G., Saikia, P., & Shah, V. H. (2016). Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology, 150(8), 1756–1768. https://doi.org/10.1053/j.gastro.2016.02.035
- Mehta, G., Rousell, S., Burgess, G., Morris, M., Wright, G., McPherson, S., Frenette, C., Cave, M., Hagerty, D. T., Spada, A., & Jalan, R. (2018). A Placebo-Controlled, Multicenter, Double-Blind, Phase 2 Randomized Trial of the Pan-Caspase Inhibitor Emricasan in Patients with Acutely Decompensated Cirrhosis. Journal of clinical and experimental hepatology, 8(3), 224–234. https://doi.org/10.1016/j.jceh.2017.11.006
- Frenette CT, Morelli G, Shiffman ML, Frederick RT, Rubin RA, Fallon MB, Cheng JT, Cave M, Khaderi SA, Massoud O, Pyrsopoulos N, Park JS, Robinson JM, Yamashita M, Spada AP, Chan JL, Hagerty DT. Emricasan Improves Liver Function in Patients With Cirrhosis and High Model for End-Stage Liver Disease Scores Compared With Placebo. Clin Gastroenterol Hepatol. 2019 Mar;17(4):774-783.e4. doi: 10.1016/j.cgh.2018.06.012
- Mathurin P, Bzowej NH, Shiffman ML, Arterburn S, Nguyen T, Billin A, et al. Selonsertib in Combination with Prednisolone for the Treatment of Severe Alcoholic Hepatitis: A Phase 2 Randomized Controlled Trial. Hepatology. 2018;68(S1):1–183.
- Freimuth J, Bangen JM, Lambertz D, Hu W, Nevzorova YA, Sonntag R, Gassler N, Riethmacher D, Trautwein C, Liedtke C. Loss of caspase-8 in hepatocytes accelerates the onset of liver regeneration in mice through premature nuclear factor kappa B activation. Hepatology. 2013 Nov;58(5):1779-89. doi: 10.1002/hep.26538
- Yamada, Y., Kirillova, I., Peschon, J. J., & Fausto, N. (1997). Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1441–1446. https://doi.org/10.1073/pnas.94.4.1441
- Liu, Y., Verma, V. K., Malhi, H., Gores, G. J., Kamath, P. S., Sanyal, A., Chalasani, N., Gao, B., & Shah, V. H. (2017). Lipopolysaccharide downregulates macrophage-derived IL-22 to modulate alcohol-induced hepatocyte cell death. American journal of physiology. Cell physiology, 313(3), C305–C313. https://doi.org/10.1152/ajpcell.00005.2017
- Arab, J. P., Sehrawat, T. S., Simonetto, D. A., Verma, V. K., Feng, D., Tang, T., Dreyer, K., Yan, X., Daley, W. L., Sanyal, A., Chalasani, N., Radaeva, S., Yang, L., Vargas, H., Ibacache, M., Gao, B., Gores, G. J., Malhi, H., Kamath, P. S., & Shah, V. H. (2020). An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology (Baltimore, Md.), 72(2), 441–453. https://doi.org/10.1002/hep.31046
- Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005 Jan;33(1):108-19. doi: 10.1016/j.exphem.2004.09.005
- Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019 Sep;70(3):802-811. doi: 10.1002/hep.30516
- Trauner, M., Gulamhusein, A., Hameed, B., Caldwell, S., Shiffman, M. L., Landis, C., Eksteen, B., Agarwal, K., Muir, A., Rushbrook, S., Lu, X., Xu, J., Chuang, J. C., Billin, A. N., Li, G., Chung, C., Subramanian, G. M., Myers, R. P., Bowlus, C. L., & Kowdley, K. V. (2019). The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology (Baltimore, Md.), 70(3), 788–801. https://doi.org/10.1002/hep.30509