What is anemia
Anemia is a lack of red blood cells and hemoglobin (Hb). Red blood cells are important because they carry oxygen from the lungs around the body. Hemoglobin is contained within red blood cells and is necessary to transport and deliver oxygen from the lungs to the rest of the body. Without a sufficient supply of oxygen, many tissues and organs throughout the body can be adversely affected. People with anemia may experience fatigue and weakness and may lack energy. The World Health Organization (WHO) criteria for anemia in men is hemoglobin value less of than 13.5 g/dL, whereas it is less than 12 g/dL for women 1. The normal hemoglobin range is generally defined as 13.2 to 16.6 grams (g) of hemoglobin per deciliter (dL) of blood for men and 11.6 to 15 g/dL for women. Normal values for children vary with age. There are revised criteria for anemia in men and women with complications of chemotherapy as well as age and race. Even “special populations” such as athletes, smokers, older adults, or those living at high altitudes have suggested different ranges.
Anemia is a fairly common condition, affecting both men and women of all ages, races, and ethnic groups. However, certain people have increased risk of developing anemia. These include people with diets poor in iron and vitamins, chronic diseases such as kidney disease, diabetes, cancer, inflammatory bowel disease, a family history of inherited anemia, chronic infections such as tuberculosis or human immunodeficiency virus (HIV), and those who have had significant blood loss from injury or surgery. Anemia can be mild, moderate, or severe depending on how much the red blood cell (RBC) count and/or hemoglobin levels are decreased.
There are many forms of anemia, each with its own cause. Anemia can be temporary or long term, and it can range from mild to severe. See your doctor if you suspect you have anemia because it can be a warning sign of serious illness.
It’s important to find and treat the cause of the anemia as well as the anemia itself.
In general, the main causes of anemia include:
- Impaired or decreased production of red blood cells by the bone marrow due to nutritional deficiency (e.g., iron deficiency, B vitamin deficiencies), bone marrow failure (e.g., aplastic anemia, myelodysplastic syndrome), or diseases that involve the bone marrow (e.g., infection, lymphoma, solid tumor)
- Loss of red blood cells due to bleeding or to increased destruction of red blood cells as in hemolytic anemia
Anemia may be acute or chronic. Chronic anemia may develop slowly over a period of time with long-term illnesses such as diabetes, chronic kidney disease, or cancer. In these situations, the anemia-related symptoms may not be apparent because the underlying disease masks its symptoms and/or the body adapts to anemia when it develops over a period of time. The presence of anemia in chronic conditions may often go undetected for a period of time and sometimes may only be discovered during tests or examinations for other conditions.
Anemia may also occur in acute episodes such as with substantial blood loss (extensive injury or invasive surgery) or with certain anemias in which a significant number of red blood cells are destroyed known as hemolytic anemia. Signs and symptoms may become apparent very quickly, and the cause can be determined from a combination of physical examination, medical history, and testing.
Treatments for anemia range from taking supplements to undergoing medical procedures. You may be able to prevent some types of anemia by eating a healthy, varied diet.
Anemia in pregnancy
Anemia in pregnancy is defined by the World Health Organization as a hemoglobin (Hb) <110 g/L at any stage of pregnancy and <100 g/L postpartum. The amount of blood in your body during pregnancy increases by about 20-30 percent, which increases the supply of iron and vitamins that the body needs to make hemoglobin. During pregnancy, some women become anemic, especially iron-deficiency anemia, which means they have too few red blood cells in their body. When you don’t have enough healthy red blood cells to carry oxygen to the rest of your body, your body cannot work as well as it should, and you feel tired and run down.
The two most common causes of anemia in pregnancy are iron deficiency and acute blood loss 2.
Mild anemia is normal during pregnancy due to an increase in blood volume. More severe anemia, however, can put your baby at higher risk for anemia later in infancy. In addition, if you are significantly anemic during your first two trimesters, you are at greater risk for having a pre-term delivery (premature birth) or low birthweight baby. Premature birth is birth before 37 weeks of pregnancy. Low birthweight is when a baby weighs less than 5 pounds, 8 ounces at birth.
Being anemic also burdens the mother by increasing the risk of blood loss during labor and making it more difficult to fight infections.
Because all women are at risk for anemia during pregnancy, your doctor will do blood tests to check for the condition at different stages in your pregnancy.
What vitamins and minerals do I need during pregnancy?
During pregnancy you need folic acid, iron, calcium, vitamin D, choline, omega-3 fatty acids, B vitamins, and vitamin C. See the below table for recommended amounts.
| Key Vitamins and Minerals During Pregnancy | ||
| Nutrient (Daily Recommended Amount) | Why You and Your Fetus Need It | Best Sources |
| Calcium (1,300 milligrams for ages 14 to 18; 1,000 milligrams for ages 19 to 50) | Builds strong bones and teeth | Milk, cheese, yogurt, sardines, dark green leafy vegetables |
| Iron (27 milligrams) | Helps red blood cells deliver oxygen to your fetus | Lean red meat, poultry, fish, dried beans and peas, iron-fortified cereals, prune juice |
| Iodine (220 micrograms) | Essential for healthy brain development | Iodized table salt, dairy products, seafood, meat, some breads, eggs |
| Choline (450 milligrams) | Important for development of your fetus’s brain and spinal cord | Milk, beef liver, eggs, peanuts, soy products |
| Vitamin A (750 micrograms for ages 14 to 18; 770 micrograms for ages 19 to 50) | Forms healthy skin and eyesight Helps with bone growth | Carrots, green leafy vegetables, sweet potatoes |
| Vitamin C (80 milligrams for ages 14 to 18; 85 milligrams for ages 19 to 50) | Promotes healthy gums, teeth, and bones | Citrus fruit, broccoli, tomatoes, strawberries |
| Vitamin D (600 international units) | Builds your fetus’s bones and teeth Helps promote healthy eyesight and skin | Sunlight, fortified milk, fatty fish such as salmon and sardines |
| Vitamin B6 (1.9 milligrams) | Helps form red blood cells Helps body use protein, fat, and carbohydrates | Beef, liver, pork, ham, whole-grain cereals, bananas |
| Vitamin B12 (2.6 micrograms) | Maintains nervous system Helps form red blood cells | Meat, fish, poultry, milk (vegetarians should take a supplement) |
| Folic acid (600 micrograms) | Helps prevent birth defects of the brain and spine Supports the general growth and development of the fetus and placenta | Fortified cereal, enriched bread and pasta, peanuts, dark green leafy vegetables, orange juice, beans. Also, take a daily prenatal vitamin with 400 micrograms of folic acid. |
How can anemia in pregnancy be avoided?
There are three good ways to avoid becoming anemic while pregnant. They are:
- start your pregnancy in good health
- eat well while pregnant
- take supplements.
Starting pregnancy in good health
If you are thinking about becoming pregnant, you should see your doctor and get a check-up. At this time, you will get advice about anemia and other conditions, and particularly about taking folate supplements.
Women are advised to take a folic acid supplement for at least a month before becoming pregnant and continuing this for at least the first three months. This will help prevent anemia, and will also decrease the risk of neural tube defects such as spina bifida. The standard dose is 0.5mg of folic acid per day, but the dose may be higher for women who have diabetes, epilepsy, are overweight or have had a child with a neural tube defect. This should be discussed with a doctor.
Eating well while pregnant
Eating a healthy diet protects against anemia. Iron is found in meats, iron fortified breads and cereals, eggs, spinach and dried fruit. Vitamin B12 is found in meat, fish, shellfish, eggs and dairy products. High levels of folate are found in green leafy vegetables, beans, muesli, broccoli, beef, Brussels sprouts and asparagus Eating a diet rich in these foods will help prevent anaemia.
Women who are vegetarian can replace animal foods with lentils, beans, tofu, eggs and soy milk. Advice from a doctor or dietitian is suggested, and vitamin B12 supplements may be recommended.
Eating plenty of citrus fruit, and avoiding tea and coffee with or soon after meals, may help you absorb the iron in your food, and may help prevent anaemia.
Supplements
All women will be advised to take folate supplements, as well as eating foods rich in folate. Many women will be advised to take iron supplements. Vegetarians may be advised to take vitamin B12 supplements. If you are advised to take supplements, talk to your doctor about the best ways to take them, and how to avoid any possible side effects.
Anemia in pregnancy causes
The main reason is that the woman’s body changes during pregnancy to look after the growing child. One change is that women make more blood when they become pregnant. The average woman will have about five liters of blood when not pregnant, but will have seven to eight liters of blood in her body as she gets near term.
Making the extra blood cells requires plenty of iron, vitamin B12 and folate to make all the extra hemoglobin needed. Unfortunately, iron is hard to absorb, which makes hemoglobin hard to make. So many women become anemic during pregnancy unless they take iron supplements. Before getting pregnant, women should get about 18 milligrams (mg) of iron per day. During pregnancy, the amount of iron you need jumps to 27 mg per day. Most pregnant women get this amount from eating foods that contain iron and taking prenatal vitamins that contain iron. Some women need to take iron supplements to prevent iron deficiency.
Folate deficiency is historically regarded as the second most common cause of anemia in pregnancy after iron deficiency, although in many modern series vitamin B12 deficiency may be more prevalent, particularly in underprivileged areas 4. In studies from India, Turkey, Africa, Newfoundland, and Venezuela, 10% to 100% of pregnant women have a diagnosis of folate deficiency, whereas 30% to 100% have vitamin B12 deficiency 4.
Some women may have an illness that causes anemia. Diseases such as sickle cell anemia or thalassemia affect the quality and number of red blood cells the body produces.
Infectious cause of anemia during pregnancy are more common in nonindustrialized countries 4. Anemia during pregnancy can be caused by infections such as parvovirus B-19, cytomegalovirus (CMV), HIV, hepatitis viruses, Epstein-Barr virus (EBV), malaria, babesiosis, bartonellosis, hookworm infestation, and Clostridium toxin. If the patient’s history suggests exposure to any of these infectious agents, appropriate laboratory studies should be performed.
If you have a disease that causes anemia, talk with your health provider about how to treat anemia.
Anemia during pregnancy risk factors
You are at higher risk for becoming anemic during your pregnancy if you:
- Have two pregnancies close together
- Are pregnant with more than one child
- Are vomiting frequently due to morning sickness
- Do not consume enough iron
- Have a heavy pre-pregnancy menstrual flow
Anemia in pregnancy signs and symptoms
Anemia takes some time to develop. In the beginning, you may not have any signs or they may be mild. But as it gets worse, you may have these signs and symptoms:
- Fatigue (very common)
- Dizziness
- Headache
- Cold hands and feet
- Pale skin
- Irregular heartbeat
- Chest pain
Because your heart has to work harder to pump more oxygen-rich blood through the body, all of these signs and symptoms can occur.
Many of the symptoms of anemia during pregnancy are also symptoms you may experience even if you are not anemic; these include:
- Feeling tired or weak
- Progressive paleness of the skin
- Rapid heartbeat
- Shortness of breath
- Trouble concentrating
Doctors typically perform several tests to check the percentage of red blood cells in your plasma and the amount of hemoglobin in your blood. These are indicators of whether you are at risk for becoming anemic.
What are the risks if a pregnant woman is anemic?
If a woman becomes anemic while pregnant, it will make her even more tired than expected.
If the anemia is severe, there could be a reduced amount of amniotic fluid around baby. There is also an increased chance of miscarriage, the baby being delivered too early or having a low birth weight. Babies born from anemic mothers may also be anemic.
If a woman is anemic throughout pregnancy and loses a lot of blood during the birth, she may need to have a blood transfusion around the time of the birth.
Anemia in pregnancy complications
Serious or untreated anemia in pregnancy can cause the following complications:
- Preterm labor
- Increased blood loss during delivery
- Low birthweight
- Anemia and developmental delays in your baby
If you had significant anemia during your pregnancy, your doctor may screen your newborn for anemia.
Anemia during pregnancy prevention
Good nutrition is the best way to prevent anemia if you are pregnant or trying to become pregnant. Eating foods high in iron content (such as dark green leafy vegetables, red meat, fortified cereals, eggs, and peanuts) can help ensure that you maintain the supply of iron your body needs to function properly. Your obstetrician will also prescribe vitamins to ensure that you have enough iron and folic acid. Make sure you get at least 27 mg of iron each day. If you do become anemic during your pregnancy, it can usually be treated by taking iron supplements.
Ask your doctor about your risk for anemia and make sure you are tested at your first prenatal visit. You also may want to get tested four to six weeks after delivery. Depending on your condition, your doctor may refer you to a hematologist, a doctor who specializes in blood conditions.
You can help lower your risk of anemia by eating foods that contain iron during your entire pregnancy. Foods high in iron include:
- Poultry
- Dried fruits and beans
- Eggs
- Iron-fortified cereals, breads and pastas
- Organ meats (liver, giblets)
- Red meat
- Seafood (clams, oysters, sardines)
- Spinach and other dark leafy greens
Foods containing vitamin C can increase the amount of iron your body absorbs. So it’s a good idea to eat foods like orange juice, tomatoes, strawberries and grapefruit every day.
Calcium (in dairy products like milk) and coffee, tea, egg yolks, fiber and soybeans can block your body from absorbing iron. Try to avoid these when eating iron-rich foods.
Anemia in pregnancy diagnosis
You would usually have a blood count around the time you first see a doctor or midwife about your pregnancy. This blood count shows whether or not there is enough hemoglobin and enough blood cells. Any abnormality in these tests will guide your doctor towards other tests such as:
- iron levels
- vitamin B12 and folate levels
- genetic tests for inherited disorders such as thalassemia.
Anemia in pregnancy treatment
If you are anemic, your health care provider may prescribe an iron supplement. Treatment of iron deficiency anemia in pregnant women is similar to that in nonpregnant women and the usual dose is 60 to 120 mg of elemental iron per day 5. Intravenous iron treatment is also used during pregnancy.
Some iron supplements may cause heartburn, constipation or nausea. Here are some tips to avoid or reduce these problems:
- Take the supplement on an empty stomach. If it upsets your stomach, take the supplement with a small amount of food.
- Take the supplement with orange juice or a vitamin C supplement.
- Don’t take a supplement with dairy products (milk, cheese, yogurt), eggs, high-fiber foods (whole grain breads and cereals, raw vegetables), spinach, tea or coffee. Don’t take an iron supplement if you’re taking an antacid.
Historically, folate deficiency was the second most common cause of anemia in pregnancy, but this is being overtaken by vitamin B12 deficiencies, particularly since folate supplementation in pregnancy is advised 4 and with routine food fortification. Folic acid supplementation of 0.4 mg/day in the first 12 weeks and 2.6 micrograms/day for vitamin B12 throughout the pregnancy is recommended.
Red Blood Cells
Red blood cell (also called erythrocyte) is biconcave disc without a nucleus. This biconcave shape is an adaptation for transporting the gases oxygen and carbon dioxide. It increases the surface area through which oxygen and carbon dioxide can diffuse into and out of the cell (Figure 4). The characteristic shape of a red blood cell also places the cell membrane closer to oxygen-carrying hemoglobin (Figure 6) molecules in the cell reducing the distance for diffusion.
The average life span of red blood cells is about four months (120 days) after which it breaks down. Red blood cells are elastic and flexible, and they readily bend as they pass through small blood vessels. As the cells near the end of their four-month life span, however, they become more fragile. The cells may sustain damage simply passing through capillaries, particularly those in active muscles that must withstand strong forces. Macrophages phagocytize and destroy damaged red blood cells, primarily in the liver and spleen. Macrophages are large, phagocytic, wandering cells. During phagocytosis, the iron from the hemoglobin is retained in the liver and spleen cells and is again used in the formation of red blood cells in the body. About 2-10 million red blood cells are formed and destroyed each second in a normal person.
Each red blood cell is about one-third hemoglobin by volume. This protein imparts the color of blood. When hemoglobin binds oxygen, the resulting oxyhemoglobin is bright red, and when oxygen is released, the resulting deoxyhemoglobin is darker.
Prolonged oxygen deficiency (hypoxia) causes cyanosis, in which the skin and mucous membranes appear bluish due to an abnormally high blood concentration of deoxyhemoglobin in the superficial blood vessels. Exposure to low temperature may also result in cyanosis by constricting superficial blood vessels. This response to environmental change slows skin blood flow. As a result, more oxygen than usual is removed from the blood flowing through the vessels, increasing the concentration of deoxyhemoglobin.
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 1. Blood composition
Note: Blood consists of a liquid portion called plasma and a solid portion (the formed elements) that includes red blood cells, white blood cells, and platelets. When blood components are separated by centrifugation, the white blood cells and platelets form a thin layer, called the “buffy coat,” between the plasma and the red blood cells, which accounts for about 1% of the total blood volume. Blood cells and platelets can be seen under a light microscope when a blood sample is smeared onto a glass slide.
Red Blood Cell Counts
The number of red blood cells in a microliter (μL or mcL or 1 mm3) of blood is called the red blood cell count (RBCC or RCC). This number varies from time to time even in healthy individuals. However, the typical range for adult males is 4,700,000 to 6,100,000 cells per microliter, and that for adult females is 4,200,000 to 5,400,000 cells per microliter.
The absolute numbers for red blood cell, white blood cell, and platelet counts can vary depending on how they are measured and the instruments used to measure them. For this reason, different sources may present different, but very similar, ranges of normal values.
An increase in the number of circulating red blood cells increases the blood’s oxygen-carrying capacity, much as a decrease in the number of circulating red blood cells decreases the blood’s oxygen-carrying capacity. Changes in this number may affect health. For this reason, red blood cell counts are routinely consulted to help diagnose and evaluate the courses of certain diseases.
Blood Cell Formation
The process of blood cell formation, called hematopoiesis, begins in the yolk sac, which lies outside the human embryo. Later in the fetal development, red blood cells are manufactured (erythropoiesis) in the liver and spleen, and still later they form in bone marrow. After birth, these cells are produced in the red bone marrow.
Bone marrow is a soft, netlike mass of connective tissue within the medullary cavities of long bones, in the irregular spaces of spongy bone, and in the larger central canals of compact bone tissue. It is of two kinds: red and yellow. Red bone marrow functions in the formation of red blood cells (erythrocytes), white blood cells (leukocytes), and blood platelets. The color comes from the oxygen-carrying pigment hemoglobin in the red blood cells.
In an infant, red marrow occupies the cavities of most bones. As a person ages, yellow bone marrow, which stores fat, replaces much of the red marrow. Yellow marrow is not active in blood cell production. In an adult, red marrow is primarily found in the spongy bone of the skull, ribs, breastbone (sternum), collarbones (clavicles), backbones (vertebrae), and hip bones. If the supply of blood cells is deficient, some yellow marrow may become red marrow, which then reverts to yellow marrow when the deficiency is corrected.
Figure 2 illustrates the stages in the formation of red blood cells from hematopoietic stem cells (blood-forming cells), which are also called hemocytoblasts.
Red blood cells have nuclei during their early stages of development but lose their nuclei as the cells mature. Losing the nuclei provides more space for hemoglobin. Because mature red blood cells do not have nuclei, they cannot divide. They use none of the oxygen they carry because they do not have mitochondria. Mature red blood cells produce ATP through glycolysis only.
The average life span of a red blood cell is 120 days. Many of these cells are removed from the circulation each day, and yet the number of cells in the circulating blood remains relatively stable. This observation suggests a homeostatic control of the rate of red blood cell production.
The hormone erythropoietin controls the rate of red blood cell formation through negative feedback. The kidneys, and to a lesser extent the liver, release erythropoietin in response to prolonged oxygen deficiency (Figure 5). At high altitudes, for example, where the amount of oxygen in the air is reduced, the blood oxygen level initially decreases. This drop in the blood oxygen level triggers the release of erythropoietin, which travels via the blood to the red bone marrow and stimulates red blood cell production.
After a few days of exposure to high altitudes, many newly formed red blood cells appear in the circulating blood. The increased rate of production continues until the number of erythrocytes in the circulation is sufficient to supply tissues with oxygen. When the availability of oxygen returns to normal, erythropoietin release decreases, and the rate of red blood cell production returns to normal as well. An excessive increase in red blood cells is called polycythemia. This condition increases blood viscosity, slowing blood flow and impairing circulation.
Figure 2. Bone marrow anatomy
Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
Dietary Factors Affecting Red Blood Cell Production
Availability of B-complex vitamins—vitamin B12 and folic acid—significantly influences red blood cell production. Because these vitamins are required for DNA synthesis, they are necessary for the growth and division of cells. Cell division is frequent in blood-forming (hematopoietic) tissue, so this tissue is especially vulnerable to a deficiency of either of these vitamins.
Hemoglobin synthesis and normal red blood cell production also require iron. The small intestine absorbs iron slowly from food. The body reuses much of the iron released by the decomposition of hemoglobin from damaged red blood cells. Nonetheless, insufficient dietary iron can reduce hemoglobin synthesis.
A deficiency of red blood cells or a reduction in the amount of hemoglobin they contain results in a condition called anemia. This reduces the oxygen-carrying capacity of the blood, and the affected person may appear pale and lack energy. A pregnant woman may have a normal number of red blood cells, but she develops a relative anemia because her plasma volume increases due to fluid retention. This shows up as a decreased hematocrit.
In contrast to anemia, the inherited disorder called hemochromatosis results in the absorption of iron in the small intestine at ten times the normal rate. Iron builds up in organs, to toxic levels. Treatment is periodic blood removal, as often as every week.
Figure 3. Blood cell development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. Blood cells
Note: Blood tissue consists of red blood cells, white blood cells, and platelets suspended in plasma. (a) Idealized representation of a sample of blood. (b) Micrograph of a sample of blood (1,000x).
Figure 5. Red blood cells
Figure 6. Red blood cell formation
Note: Low blood oxygen causes the kidneys and to a lesser degree, the liver to release erythropoietin. Erythropoietin stimulates target cells in the red bone marrow to increase the production of red blood cells, which carry oxygen to tissues.
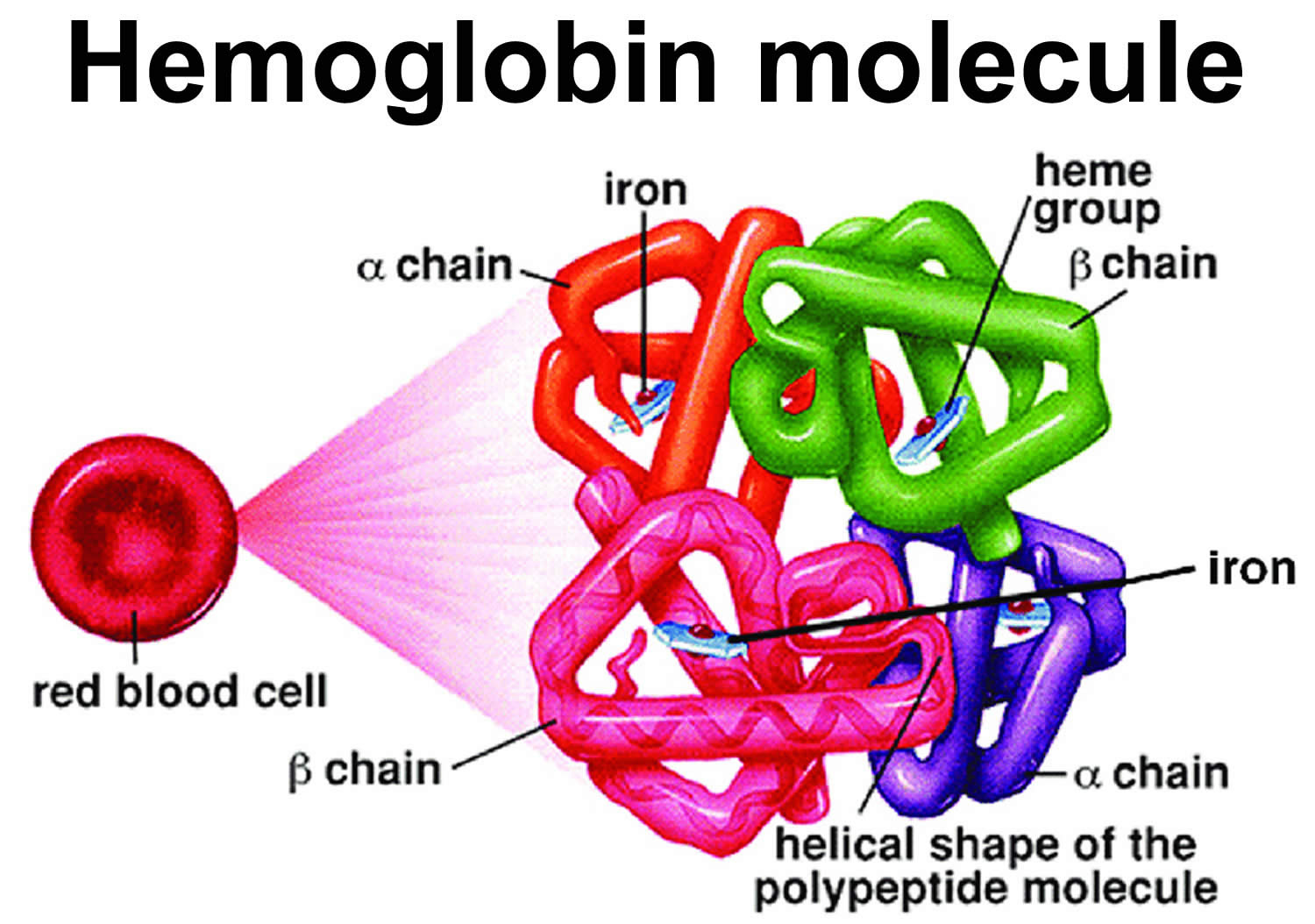
Figure 7. Red blood cell hemoglobin
Figure 8. Normal hemoglobin structure (normal hemoglobin is called hemoglobin A [HbA] and consists of 2 alpha (α) globin chains and 2 beta (β) globin chains)
Figure 9. Lifecycle of a red blood cell
 Types of anemia
Types of anemia
Anemias can also be described based on the red blood cell size and concentration of hemoglobin in them. If cell size is much smaller than normal, it is known as microcytic anemia. If it is much bigger than normal, then it is macrocytic anemia. Likewise, if the concentration of hemoglobin is much lower than normal, it is hypochromic anemia; if the concentration is much higher than normal, the red blood cells are called hyperchromic.
Within the two broad categories of general causes of anemia, there are several types with different specific causes. Some of the most common types are summarized below.
Figure 10. Anemia differential diagnosis
Different types of anemia and their causes include:
Iron deficiency anemia
Iron-deficiency anemia is a type of anemia that develops if you do not have enough iron in your body. Iron-deficiency anemia is the most common type of anemia worldwide.
Iron deficiency anemia is caused by a shortage of iron in your body. Your bone marrow needs iron to make hemoglobin to carry oxygen. Without adequate iron, your body can’t produce enough hemoglobin for red blood cells. As a result, iron deficiency anemia may leave you tired and short of breath. Low levels of hemoglobin in turn leads to production of smaller and hypochromic red blood cells.
Iron is also necessary to maintain healthy cells, skin, hair, and nails.
Examples of causes of iron deficiency anemia: Blood loss; diet low in iron; poor absorption of iron. Without iron supplementation, iron deficiency anemia occurs in many pregnant women. It is also caused by blood loss, such as from heavy menstrual bleeding, an ulcer, cancer and regular use of some over-the-counter pain relievers, especially aspirin.
If you or your child develops signs and symptoms that suggest iron deficiency anemia, see your doctor. Iron deficiency anemia isn’t something to self-diagnose or treat. So see your doctor for a diagnosis rather than taking iron supplements on your own. Overloading the body with iron can be dangerous because excess iron accumulation can damage your liver and cause other complications.
You can usually correct iron deficiency anemia with iron supplementation. Sometimes additional tests or treatments for iron deficiency anemia are necessary, especially if your doctor suspects that you’re bleeding internally.
Iron deficiency anemia symptoms
People with mild or moderate iron-deficiency anemia may not have any symptoms. But as the body becomes more deficient in iron and anemia worsens, the signs and symptoms intensify. More serious iron-deficiency anemia may cause common symptoms of anemia, such as tiredness, shortness of breath, or chest pain. Other symptoms include:
- Fatigue
- Weakness
- Headache, dizziness or lightheadedness
- Cold hands and feet
- Pale skin
- Chest pain, fast heartbeat or shortness of breath
- Inflammation or soreness of your tongue
- Brittle nails
- Unusual cravings for non-nutritive substances, such as ice, dirt or starch
- Poor appetite, especially in infants and children with iron deficiency anemia
Iron deficiency anemia complications
Mild iron deficiency anemia usually doesn’t cause complications. However, left untreated, iron deficiency anemia can become severe and lead to health problems, including the following:
- Heart problems. Iron deficiency anemia may lead to a rapid or irregular heartbeat. Your heart must pump more blood to compensate for the lack of oxygen carried in your blood when you’re anemic. This can lead to an enlarged heart or heart failure.
- Problems during pregnancy. In pregnant women, severe iron deficiency anemia has been linked to premature births and low birth weight babies. But the condition is preventable in pregnant women who receive iron supplements as part of their prenatal care.
- Growth problems. In infants and children, severe iron deficiency can lead to anemia as well as delayed growth and development. Additionally, iron deficiency anemia is associated with an increased susceptibility to infections.
Iron deficiency anemia causes
Iron deficiency anemia occurs when your body doesn’t have enough iron to produce hemoglobin. If you aren’t consuming enough iron, or if you’re losing too much iron, your body can’t produce enough hemoglobin, and iron deficiency anemia will eventually develop.
Causes of iron deficiency anemia include:
- Blood loss. Blood contains iron within red blood cells. So if you lose blood, you lose some iron. Women with heavy periods are at risk of iron deficiency anemia because they lose blood during menstruation. Slow, chronic blood loss within the body — such as from a peptic ulcer, a hiatal hernia, a colon polyp or colorectal cancer — can cause iron deficiency anemia. Gastrointestinal bleeding can result from regular use of some over-the-counter pain relievers, especially aspirin.
- Intravascular hemolysis, a condition in which red blood cells break down in the blood stream, releasing iron that is then lost in the urine. This sometimes occurs in people who engage in vigorous exercise, particularly jogging. This can cause trauma to small blood vessels in the feet, so called “march hematuria.” Intravascular hemolysis can also be seen in other conditions including damaged heart valves or rare disorders such as thrombotic thrombocytopenia purpura (TTP) or diffuse intravascular hemolysis (DIC).
- A lack of iron in your diet. Your body regularly gets iron from the foods you eat. If you consume too little iron, over time your body can become iron deficient. Examples of iron-rich foods include meat, eggs, leafy green vegetables and iron-fortified foods. For proper growth and development, infants and children need iron from their diets, too.
- An inability to absorb iron. Iron from food is absorbed into your bloodstream in your small intestine. An intestinal disorder, such as celiac disease, ulcerative colitis, Crohn’s disease, and Helicobacter pylori infection, which affects your intestine’s ability to absorb nutrients from digested food, can lead to iron deficiency anemia. If part of your small intestine has been bypassed or removed surgically, that may affect your ability to absorb iron and other nutrients. Endurance sports can make athletes lose iron through their gastrointestinal tracts and through the breakdown of red blood cells.
- Pregnancy. Without iron supplementation, iron deficiency anemia occurs in many pregnant women because their iron stores need to serve their own increased blood volume as well as be a source of hemoglobin for the growing fetus.
- Certain rare genetic conditions can block your intestines from absorbing iron or make it harder to stop bleeding.
- Other medical conditions that may cause iron-deficiency anemia include:
- Kidney disease: People who have kidney disease do not make enough of a substance called erythropoietin. Your body needs erythropoietin to make red blood cells. Your doctor may prescribe erythropoietin if you have kidney disease.
- Long-lasting conditions that lead to inflammation: These include congestive heart failure or obesity. They can make it hard for your body to regulate and use your body’s iron.
Risk factors for developing iron deficiency anemia
These groups of people may have an increased risk of iron deficiency anemia:
- Women. Because women lose blood during menstruation, women in general are at greater risk of iron deficiency anemia.
- Infants and children. Infants, especially those who were low birth weight or born prematurely, who don’t get enough iron from breast milk or formula may be at risk of iron deficiency. Children need extra iron during growth spurts. If your child isn’t eating a healthy, varied diet, he or she may be at risk of iron deficiency anemia.
- Vegetarians and vegans. People who don’t eat meat may have a greater risk of iron deficiency anemia if they don’t eat other iron-rich foods.
- Frequent blood donors. People who routinely donate blood may have an increased risk of iron deficiency anemia since blood donation can deplete iron stores. Low hemoglobin related to blood donation may be a temporary problem remedied by eating more iron-rich foods. If you’re told that you can’t donate blood because of low hemoglobin, ask your doctor whether you should be concerned.
Iron deficiency anemia prevention
You can reduce your risk of iron deficiency anemia by choosing iron-rich foods.
Choose iron-rich foods
Foods rich in iron include:
- Red meat, pork and poultry
- Seafood
- Beans
- Dark green leafy vegetables, such as spinach
- Dried fruit, such as raisins and apricots
- Iron-fortified cereals, breads and pastas
- Peas
Your body absorbs more iron from meat than it does from other sources. If you choose to not eat meat, you may need to increase your intake of iron-rich, plant-based foods to absorb the same amount of iron as does someone who eats meat.
Choose foods containing vitamin C to enhance iron absorption
You can enhance your body’s absorption of iron by drinking citrus juice or eating other foods rich in vitamin C at the same time that you eat high-iron foods. Vitamin C in citrus juices, like orange juice, helps your body to better absorb dietary iron.
Vitamin C is also found in:
- Broccoli
- Grapefruit
- Kiwi
- Leafy greens
- Melons
- Oranges
- Peppers
- Strawberries
- Tangerines
- Tomatoes
Preventing iron deficiency anemia in infants
To prevent iron deficiency anemia in infants, feed your baby breast milk or iron-fortified formula for the first year. Cow’s milk isn’t a good source of iron for babies and isn’t recommended for infants under 1 year. After age 6 months, start feeding your baby iron-fortified cereals or pureed meats at least twice a day to boost iron intake. After one year, be sure children don’t drink more than 20 ounces (591 milliliters) of milk a day. Too much milk often takes the place of other foods, including those that are rich in iron.
How much iron do you need?
The amount of iron you need each day depends on your age, your sex, and whether you consume a mostly plant-based diet. Average daily recommended amounts are listed below in milligrams (mg). Vegetarians who do not eat meat, poultry, or seafood need almost twice as much iron as listed in the table because the body doesn’t absorb nonheme iron in plant foods as well as heme iron in animal foods.
Iron recommended intake
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 0.27 mg |
| Infants 7–12 months | 11 mg |
| Children 1–3 years | 7 mg |
| Children 4–8 years | 10 mg |
| Children 9–13 years | 8 mg |
| Teens boys 14–18 years | 11 mg |
| Teens girls 14–18 years | 15 mg |
| Adult men 19–50 years | 8 mg |
| Adult women 19–50 years | 18 mg |
| Adults 51 years and older | 8 mg |
| Pregnant teens | 27 mg |
| Pregnant women | 27 mg |
| Breastfeeding teens | 10 mg |
| Breastfeeding women | 9 mg |
Iron deficiency anemia diagnosis
To diagnose iron deficiency anemia, your doctor may run tests to look for:
- Red blood cell size and color. With iron deficiency anemia, red blood cells are smaller and paler in color than normal.
- Hematocrit. This is the percentage of your blood volume made up by red blood cells. Normal levels are generally between 35.5 and 44.9 percent for adult women and 38.3 to 48.6 percent for adult men. These values may change depending on your age.
- Hemoglobin. Lower than normal hemoglobin levels indicate anemia. The normal hemoglobin range is generally defined as 13.2 to 16.6 grams (g) of hemoglobin per deciliter (dL) of blood for men and 11.6 to 15 g/dL for women.
- Ferritin. This protein helps store iron in your body, and a low level of ferritin usually indicates a low level of stored iron.
Additional diagnostic tests
If your bloodwork indicates iron deficiency anemia, your doctor may order additional tests to identify an underlying cause, such as:
- Endoscopy. Doctors often check for bleeding from a hiatal hernia, an ulcer or the stomach with the aid of endoscopy. In this procedure, a thin, lighted tube equipped with a video camera is passed down your throat to your stomach. This allows your doctor to view the tube that runs from your mouth to your stomach (esophagus) and your stomach to look for sources of bleeding.
- Colonoscopy. To rule out lower intestinal sources of bleeding, your doctor may recommend a procedure called a colonoscopy. A thin, flexible tube equipped with a video camera is inserted into the rectum and guided to your colon. You’re usually sedated during this test. A colonoscopy allows your doctor to view inside some or all of your colon and rectum to look for internal bleeding.
- Ultrasound. Women may also have a pelvic ultrasound to look for the cause of excess menstrual bleeding, such as uterine fibroids.
Your doctor may order these or other tests after a trial period of treatment with iron supplementation.
Sometimes it is difficult to diagnose the cause of iron deficiency, or your doctor may be concerned that there is a problem other than iron deficiency causing the anemia. These may include inherited blood disorders called thalassemiasin which red blood cells also appear small and pale, hemoglobinopathies such as sickle cell disease (but not sickle cell trait alone), or other blood disorders. People with chronic infections or conditions such as kidney failure, autoimmune diseases, and inflammatory disorders may also have small red blood cells. When the cause of the anemia is not clear, your doctor may refer you to a hematologist, a medical specialist in blood disorders, for consultation and further evaluation.
Iron deficiency anemia treatment
To treat iron deficiency anemia, your doctor may recommend that you take iron supplements. Your doctor will also treat the underlying cause of your iron deficiency, if necessary.
Medicinal iron
The amount of iron needed to treat patients with iron deficiency is higher than the amount found in most daily multivitamin supplements. The amount of iron prescribed by your doctor will be in milligrams (mg) of elemental iron. Your doctor may recommend over-the-counter iron tablets to replenish the iron stores in your body. Your doctor will let you know the correct dose for you. Most people with iron deficiency need 150-200 mg per day of elemental iron (2 to 5 mg of iron per kilogram of body weight per day). Iron is also available in liquid form for infants and children.
There is no evidence that any one type of iron salt, liquid, or pill is better than the others, and the amount of elemental iron varies with different preparations. To be sure of the amount of iron in a product, check the packaging. In addition to elemental iron, the iron salt content (ferrous sulfate, fumarate, or gluconate) may also be listed on the package, which can make it confusing for consumers to know how many tablets or how much liquid to take to get the proper dosage of iron.
Iron is absorbed in the small intestine (duodenum and first part of the jejunum). This means that enteric-coated iron tablets may not work as well. If you take antacids, you should take iron tablets two hours before or four hours after the antacid.
To improve the chances that your body will absorb the iron in the tablets, you may be instructed to:
- Take iron tablets on an empty stomach. If possible, take your iron tablets when your stomach is empty. However, because iron tablets can upset your stomach, you may need to take your iron tablets with meals.
- Don’t take iron with antacids. Medications that immediately relieve heartburn symptoms can interfere with the absorption of iron. Take iron two hours before or four hours after you take antacids.
- Take iron tablets with vitamin C (ascorbic acid). Vitamin C improves the absorption of iron. Your doctor might recommend taking your iron tablets with a glass of orange juice or with a 250 mg of vitamin C supplement.
Iron supplements can cause constipation, so your doctor may also recommend a stool softener. Iron may turn your stools black, which is a harmless side effect.
Iron deficiency can’t be corrected overnight. You may need to take iron supplements for several months or longer to replenish your iron reserves. Generally, you’ll start to feel better after a week or so of treatment. Ask your doctor when to have your blood rechecked to measure your iron levels. To be sure that your iron reserves are replenished, you may need to take iron supplements for a year or more.
Intravenous iron
In some cases your doctor may recommend intravenous (IV) iron. Intravenous (IV) iron may be necessary to treat iron deficiency in patients who do not absorb iron well in the gastrointestinal tract, patients with severe iron deficiency or chronic blood loss, patients who are receiving supplemental erythropoietin, a hormone that stimulates blood production, or patients who cannot tolerate oral iron. If you need intravenous iron, your doctor may refer you to a hematologist to supervise the iron infusions. Intravenous iron comes in different preparations:
- Iron dextran
- Iron sucrose
- Ferric gluconate
Large doses of iron can be given at one time when using iron dextran. Iron sucrose and ferric gluconate require more frequent doses spread over several weeks. Some patients may have an allergic reaction to intravenous iron, so a test dose may be administered before the first infusion. Allergic reactions are more common with iron dextran and may necessitate switching to a different preparation. Severe side effects other than allergic reactions are rare and include urticaria (hives), pruritus (itching), and muscle and joint pain.
Dietary iron
- Meat: beef, pork, or lamb, especially organ meats such as liver
- Poultry: chicken, turkey, and duck, especially liver and dark meat
- Fish, especially shellfish, sardines, and anchovies
- Leafy green members of the cabbage family including broccoli, kale, turnip greens, and collard greens
- Legumes, including lima beans, peas, pinto beans, and black-eyed peas
- Iron-enriched pastas, grains, rice, and cereals.
Blood transfusions
Red blood cell transfusions may be given to patients with severe iron-deficiency anemia who are actively bleeding or have significant symptoms such as chest pain, shortness of breath, or weakness. Transfusions are given to replace deficient red blood cells and will not completely correct the iron deficiency. Red blood cell transfusions will only provide temporary improvement. It is important to find out why you are anemic and treat the cause as well as the symptoms.
Treating underlying causes of iron deficiency
If iron supplements don’t increase your blood-iron levels, it’s likely the anemia is due to a source of bleeding or an iron-absorption problem that your doctor will need to investigate and treat. Depending on the cause, iron deficiency anemia treatment may involve:
- Medications, such as oral contraceptives to lighten heavy menstrual flow
- Antibiotics and other medications to treat peptic ulcers
- Surgery to remove a bleeding polyp, a tumor or a fibroid
If iron deficiency anemia is severe, you may need iron given intravenously or you may need blood transfusions to help replace iron and hemoglobin quickly.
Vitamin deficiency anemia
In addition to iron, your body needs folate and vitamin B-12 to produce enough healthy red blood cells. A diet lacking in these and other key nutrients can cause decreased red blood cell production.
Examples of causes of vitamin deficiency anemia: Lack of intrinsic factor (needed for vitamin B12 absorption); diet low in B vitamins; decreased absorption of B vitamins
Additionally, some people may consume enough vitamin B-12, but their bodies aren’t able to process vitamin B-12. This can lead to vitamin B-12 deficiency anemia, also known as pernicious anemia.
Pernicious anemia
Pernicious anemia is megaloblastic anemia resulting from a deficiency of vitamin B12 (cobalamin), which in turn is caused by a lack of intrinsic factor (IF) 6, 7. Intrinsic factor is a glycoprotein that binds cobalamin and thereby enables its absorption in the terminal ileum. Pernicious anemia is often described as an autoimmune disorder due to the findings of gastric autoantibodies directed against both intrinsic factor (IF) and parietal cells and the increased frequency of other autoimmune diseases seen in patients with pernicious anemia 8.
Pernicious anemia symptoms
If you have vitamin B12–deficiency anemia, you may have the typical symptoms of anemia at first, such as fatigue, paleness, shortness of breath, headaches, or dizziness. If left untreated, you may start to notice brain and nervous system symptoms. This is because vitamin B12 is also needed for your brain and your nerves to work properly.
Your pernicious anemia symptoms may include:
- Diarrhea or constipation
- Nausea
- Vomiting
- Fatigue, lack of energy, or lightheadedness when standing up or with exertion
- Loss of appetite
- Pale skin (mild jaundice)
- Shortness of breath, mostly during exercise
- Heartburn
- Swollen, red tongue or bleeding gums
- Glossitis, which is a painful, smooth, red tongue
If you have a low vitamin B12 level for a long time, you can have nervous system damage. Symptoms can include:
- Confusion
- Short-term memory loss
- Forgetfulness
- Depression
- Loss of balance
- Trouble walking
- Uncontrollable muscle movements
- Numbness and tingling in the hands and feet
- Problems concentrating
- Irritability
- Hallucinations
- Delusions
- Optic nerve atrophy
- Vision problems
- Problems with smell or taste
Pernicious anemia possible complications
People with pernicious anemia may have gastric polyps. They are also more likely to develop gastric cancer and gastric carcinoid tumors.
People with pernicious anemia are more likely to have fractures of the back, upper leg, and upper forearm.
Brain and nervous system problems may continue or be permanent if treatment is delayed.
A woman with a low B12 level may have a false positive Pap smear. This is because vitamin B12 deficiency affects the way certain cells (epithelial cells) in the cervix look.
Pernicious anemia causes
Pernicious anemia is a condition that develops when your body does not have enough vitamin B12. Vitamin B12 deficiency also is called cobalamin deficiency and combined systems disease. The body needs vitamin B12 to make healthy red blood cells and to keep its nervous system working properly. Pernicious anemia is caused by a lack of a protein called intrinsic factor (IF) or other issues, such as infections, surgery, medicines, or diet. You may develop vitamin B12–deficiency anemia if your body is not able to absorb enough vitamin B12 from the foods you eat. Older adults are more likely to have digestive problems that make it harder to absorb vitamin B12. You can develop vitamin B12–deficiency if you do not eat enough food with vitamin B12, such as if you follow a strict vegetarian or vegan diet. But this is rare. In the United States, vitamin B12–deficiency anemia is most often due to other risk factors.
Pernicious anemia is more common in people with northern European or African ancestry.
In rare cases, children are born with an inherited disorder that prevents their bodies from making intrinsic factor (IF). This disorder is called congenital pernicious anemia.
You can develop vitamin B12 deficiency or pernicious anemia for the following reasons:
- Lack of intrinsic factor (IF): Intrinsic factor is a protein made in the stomach, which helps the body absorb vitamin B12. People who have pernicious anemia do not produce intrinsic factor. In some people, an autoimmune response causes a lack of intrinsic factor (IF). An autoimmune response occurs if the body’s immune system makes antibodies (proteins) that mistakenly attack and damage the body’s tissues or cells. In pernicious anemia, the body makes antibodies that attack and destroy the parietal cells. The parietal cells line the stomach and make intrinsic factor (IF). Why this autoimmune response occurs isn’t known, but as a result of this attack, the stomach stops making intrinsic factor (IF), and this leads to vitamin B12 deficiency. A lack of intrinsic factor also can occur if you’ve had part or all of your stomach surgically removed. This type of surgery reduces the number of parietal cells available to make intrinsic factor.
- Malabsorption in the small intestine. Sometimes pernicious anemia occurs because the body’s small intestine can’t properly absorb vitamin B12. This may be the result of:
- Too many of the wrong kind of bacteria in the small intestine. This is a common cause of pernicious anemia in older adults. The bacteria use up the available vitamin B12 before the
small intestine can absorb it. - Diseases that interfere with vitamin B12 absorption.
- One example is celiac disease. This is a genetic disorder that makes your body unable to tolerate a protein called gluten.
- Another example is Crohn’s disease, an inflammatory bowel disease.
- HIV also may interfere with vitamin B12 absorption.
- Certain medicines that alter bacterial growth or prevent the small intestine from properly absorbing vitamin B12. Examples include antibiotics, some heartburn medicines and metformin to treat diabetes and seizure medicines.
- Surgical removal of part or all of the small intestine.
- A tapeworm infection. The tapeworm feeds off of the vitamin B12. Eating undercooked, infected fish may cause this type of infection.
- Too many of the wrong kind of bacteria in the small intestine. This is a common cause of pernicious anemia in older adults. The bacteria use up the available vitamin B12 before the
- Lifestyle habits: Drinking too much alcohol can make it harder for your body to absorb vitamin B12. For men this is more than two drinks in a day. For women, it’s more than one drink in a day.
- Medicines: Taking certain medicines can make it harder for your body to absorb vitamin B12 over time. These include some heartburn medicines, antibiotics, and metformin to treat diabetes and seizure medicines.
- Medical conditions: Some medical conditions can raise your risk of vitamin B12–deficiency anemia. These include:
- Autoimmune diseases, such as celiac disease, type 1 diabetes, and thyroid disease
- Chronic pancreatic disease
- Genetic conditions, such as Imerslund-Gräsbeck syndrome, inherited intrinsic factor deficiency, and inherited transcobalamin deficiency
- Intestinal and digestive conditions, such as ulcerative colitis, Crohn’s disease, and Helicobacter pylori infection
- Vitiligo
- Stomach surgery: Surgery on your stomach or intestines, such as weight-loss surgery or gastrectomy, can make it harder for your body to absorb vitamin B12.
- Diet lacking vitamin B12. Some people get pernicious anemia because they don’t have enough vitamin B12 in their diets. This cause of pernicious anemia is less common than other causes. Good food sources of vitamin B12 include:
- Breakfast cereals with added vitamin B12
- Meats such as beef, liver, poultry, and fish
- Eggs and dairy products such as milk, yogurt, and cheese
- Foods fortified with vitamin B12, such as soy-based beverages and vegetarian burgers
Pernicious anemia prevention
If you are otherwise healthy, maintaining a normal diet enriched in vitamin B12 is important.
Vitamin B12 is found naturally in a wide variety of animal foods and is added to some fortified foods. Plant foods have no vitamin B12 unless they are fortified. You can get recommended amounts of vitamin B12 by eating a variety of foods including the following foods that are good sources of vitamin B12:
- Beef liver and clams are the best sources of vitamin B12.
- Fish such as catfish and salmon, meat, chicken, eggs, milk, yogurt, cheese, and other dairy products also contain vitamin B12.
- Some breakfast cereals, nutritional yeasts and other food products that are fortified with vitamin B12. To find out if vitamin B12 has been added to a food product, check the product labels.
Several food sources of vitamin B12 are listed in Table 5 below.
Pernicious anemia diagnosis
Your doctor will perform a physical exam. Tests that may be done include:
- Bone marrow examination (only needed if diagnosis is unclear)
- Complete blood count (CBC)
- Reticulocyte count
- Lactate dehydrogenase (LDH) level
- Serum bilirubin
- Methylmalonic acid (MMA) level
- Homocysteine level (amino acid found in blood)
- Vitamin B12 level
- Levels of antibodies against intrinsic factor (IF) or the parietal cells that make intrinsic factor (IF)
Pernicious anemia treatment
The goal of pernicious anemia treatment is to increase your vitamin B12 level 6:
- Treatment involves an intramuscular (IM) injection of 1000 mcg vitamin B12 every day or every other day during the first week of treatment. The next month, you’ll receive injections every week, subsequently followed by monthly injections.
- The alternative to intramuscular injection of vitamin B12 is high-dose oral vitamin B12. A 1000 to 2000 mcg/day has been demonstrated to be effective, although recommendations are to always use the parenteral route in severe neurological manifestations. Approved sublingual and intranasal formulations of vitamin B12 are also available 9. Oral dosage is recommended for patients unable to take intramuscular (IM) injections, but cobalamin levels must be measured frequently to ensure absorption. Finally, oral therapy is not recommended for patients with central nervous system (CNS) symptoms.
Monitoring
The earliest sign of treatment response is an increase in reticulocyte count, usually within three days of treatment 6. Following changes in the decrease of biochemical markers such as methylmalonic acid (MMA) and plasma homocysteine levels have been observed in the first five days of treatment. Sustained normalization of serum cobalamin usually occurs following two weeks of therapy 10. The macrocytosis correction takes place during the first month of treatment. A clinical interview should be considered every year to monitor for new symptoms. These may include epigastric pain, dysphagia, iron deficiency, and/or others that can require gastroscopic investigation.
The key management principle is the importance of routine follow-up. Patients with underlying causes like chronic pancreatitis, bacterial overgrowth, or tapeworm will require additional treatments. Blood transfusions are not required in most patients. With treatment, the symptoms of heart failure resolve, but some patients may require concomitant diuretic therapy.
Anemia of Chronic Disease
Certain diseases — such as cancer, HIV/AIDS, diabetes, tuberculosis, rheumatoid arthritis, kidney disease, Crohn’s disease, cancer and other chronic inflammatory diseases — can interfere with the production of red blood cells.
Aplastic anemia
Aplastic anemia is a rare but serious blood condition that occurs when your bone marrow cannot make enough new blood cells for your body to work normally. Aplastic anemia can develop quickly or slowly, and it can be mild or serious. Causes of aplastic anemia include infections, certain medicines, autoimmune diseases, viral infections and exposure to toxic chemicals. At this time, there is no way to prevent aplastic anemia.
People of all ages can develop aplastic anemia. Those at increased risk of aplastic anemia may include:
- People undergoing high-dose radiation or chemotherapy for cancer
- People exposed to certain environmental toxins, such as pesticides, arsenic, and benzene
- People taking certain medicines, such as those used to treat rheumatoid arthritis and some types of antibiotics
- People with certain infectious diseases, autoimmune disorders, or inherited conditions that can damage the bone marrow
Treatment for aplastic anemia might include medications, blood transfusions or a stem cell transplant, also known as a bone marrow transplant.
Aplastic anemia causes
Aplastic anemia is caused by damage to stem cells inside your bone marrow, which is the sponge-like tissue within your bones. Many diseases and conditions can damage the stem cells in bone marrow. As a result, the bone marrow makes fewer red blood cells, white blood cells, and platelets.
The most common cause of bone marrow damage is from your immune system attacking and destroying the stem cells in your bone marrow. This is a type of autoimmune illness, a disease that makes your body attack itself.
Other causes of aplastic anemia include some medicines, such as those used in chemotherapy, and exposure to toxins or chemicals in the environment.
You can also inherit aplastic anemia, in rare cases.
Other factors that can injure bone marrow and affect blood cell production include:
- Radiation and chemotherapy treatments. While these cancer-fighting therapies kill cancer cells, they can also damage healthy cells, including stem cells in bone marrow. Aplastic anemia can be a temporary side effect of these treatments.
- Exposure to toxic chemicals. Toxic chemicals, such as some used in pesticides and insecticides, and benzene, an ingredient in gasoline, have been linked to aplastic anemia. This type of anemia might improve if you avoid repeated exposure to the chemicals that caused your illness.
- Use of certain drugs. Some medications, such as those used to treat rheumatoid arthritis and some antibiotics, can cause aplastic anemia.
- Autoimmune disorders. An autoimmune disorder, in which your immune system attacks healthy cells, might involve stem cells in your bone marrow.
- A viral infection. Viral infections that affect bone marrow can play a role in the development of aplastic anemia. Viruses that have been linked to aplastic anemia include hepatitis, Epstein-Barr, cytomegalovirus, parvovirus B19 and HIV.
- Pregnancy. Your immune system might attack your bone marrow during pregnancy.
- Unknown factors. In many cases, doctors aren’t able to identify the cause of aplastic anemia (idiopathic aplastic anemia).
Some people with aplastic anemia also have a rare disorder known as paroxysmal nocturnal hemoglobinuria (PNH), which causes red blood cells to break down too soon. This condition can lead to aplastic anemia, or aplastic anemia can evolve into paroxysmal nocturnal hemoglobinuria.
Fanconi’s anemia is a rare, inherited disease that leads to aplastic anemia. Children born with it tend to be smaller than average and have birth defects, such as underdeveloped limbs. The disease is diagnosed with the help of blood tests.
Risk factors for developing aplastic anemia
Aplastic anemia is rare. Factors that can increase your risk of aplastic anemia include:
- Treatment with high-dose radiation or chemotherapy for cancer
- Exposure to toxic chemicals
- The use of some prescription drugs — such as chloramphenicol, which is used to treat bacterial infections, and gold compounds used to treat rheumatoid arthritis
- Certain blood diseases, autoimmune disorders and serious infections
- Pregnancy, rarely
Aplastic anemia prevention
There’s no prevention for most cases of aplastic anemia. Avoiding exposure to insecticides, herbicides, organic solvents, paint removers and other toxic chemicals might lower your risk of the disease.
Aplastic anemia symptoms
Aplastic anemia symptoms include:
- Fatigue
- Frequent infections or infections that last a long time
- Easy bruising or bleeding
- Prolonged bleeding from cuts
- Nosebleeds and bleeding gums
- Shortness of breath
- Rapid or irregular heart rate
- Pale skin
- Skin rash
- Dizziness
- Nausea
- Headache
- Fever
Lower-than-normal numbers of red blood cells (anemia), white blood cells (leukopenia), and platelets (thrombocytopenia) cause the signs and symptoms of aplastic anemia.
- A lower-than-normal number of red blood cells (anemia) can cause fatigue; weakness; shortness of breath; pale skin, gums, and nail beds; dizziness; headaches; cold hands and feet; and chest pain.
- A lower-than-normal number of white blood cells (leukopenia) can cause fever, frequent or severe infections, and lingering flu-like symptoms.
- A lower-than-normal number of platelets (thrombocytopenia) can cause easy bleeding or bruising, petechiae (pinpoint red spots on the skin), nosebleeds, bleeding gums, blood in the stool, and heavy menstrual periods.
While aplastic anemia signs and symptoms can be mild, moderate, or severe; severe aplastic anemia can be life-threatening.
People who have another blood disorder called paroxysmal nocturnal hemoglobinuria (PNH) and aplastic anemia may have other signs and symptoms, including blood in the urine, swelling or pain in the abdomen, swelling in the legs, headaches, and jaundice (a medical condition marked by a yellowing of the skin or the whites of the eyes).
Aplastic anemia complications
Aplastic anemia can raise your risk of complications such as bleeding, leukemia, or other serious blood conditions. Without treatment, aplastic anemia can lead to serious medical conditions such as an irregular heartbeat and heart failure.
Aplastic anemia diagnosis
To diagnose aplastic anemia, your doctor will order tests to find out whether you have low numbers of cells in your bone marrow and blood.
- Blood tests. Normally, red blood cell, white blood cell and platelet levels stay within certain ranges. In aplastic anemia all three of these blood cell levels are low.
- Bone marrow biopsy. A doctor uses a needle to remove a small sample of bone marrow from a large bone in your body, such as your hipbone. The sample is examined under a microscope to rule out other blood-related diseases. In aplastic anemia, bone marrow contains fewer blood cells than normal. Confirming a diagnosis of aplastic anemia requires a bone marrow biopsy.
Once you’ve received a diagnosis of aplastic anemia, you might need other tests to determine the cause.
- Other tests. In addition to the bone marrow test, your doctor may recommend a chest x-ray, a computed tomography (CT) scan, ultrasound imaging, liver tests, tests for viral infections, tests for vitamin B12 and folate levels in your blood, and/or a specialized test for paroxysmal nocturnal hemoglobinuria (PNH). These tests can help your doctor determine the severity of your anemia, what is causing it, and whether you have paroxysmal nocturnal hemoglobinuria (PNH).
Aplastic anemia treatment
Treatments for aplastic anemia, which will depend on the severity of your condition and your age, may include the following:
- Blood and bone marrow transplants, which may cure aplastic anemia in some people
- Blood transfusions
- Medicines to stop your immune system from destroying the stem cells in your bone marrow
- Medicines to help your body make new blood cells
- Removing or staying away from toxins in your environment
Your doctor will monitor your condition and screen you for blood conditions regularly. If you take medicine that affects your immune system, you will also need to take steps to prevent infection and get annual flu shots.
Severe aplastic anemia, in which your blood cell counts are extremely low, is life-threatening and requires immediate hospitalization.
Blood transfusions
Although not a cure for aplastic anemia, blood transfusions can control bleeding and relieve symptoms by providing blood cells your bone marrow isn’t producing. You might receive:
- Red blood cells. These raise red blood cell counts and help relieve anemia and fatigue.
- Platelets. These help prevent excessive bleeding.
While there’s generally no limit to the number of blood transfusions you can have, complications can sometimes arise with multiple transfusions. Transfused red blood cells contain iron that can accumulate in your body and can damage vital organs if an iron overload isn’t treated. Medications can help rid your body of excess iron.
Over time your body can develop antibodies to transfused blood cells, making them less effective at relieving symptoms. The use of immunosuppressant medication makes this complication less likely.
Stem cell transplant
A stem cell transplant to rebuild the bone marrow with stem cells from a donor might be the only successful treatment option for people with severe aplastic anemia. A stem cell transplant, also called a bone marrow transplant, is generally the treatment of choice for people who are younger and have a matching donor — most often a sibling.
If a donor is found, your diseased bone marrow is first depleted with radiation or chemotherapy. Healthy stem cells from the donor are filtered from the blood. The healthy stem cells are injected intravenously into your bloodstream, where they migrate to the bone marrow cavities and begin creating new blood cells.
The procedure requires a lengthy hospital stay. After the transplant, you’ll receive drugs to help prevent rejection of the donated stem cells.
A stem cell transplant carries risks. Your body may reject the transplant, leading to life-threatening complications. In addition, not everyone is a candidate for transplantation or can find a suitable donor.
Immunosuppressants
For people who can’t undergo a bone marrow transplant or for those whose aplastic anemia is due to an autoimmune disorder, treatment can involve drugs that alter or suppress the immune system (immunosuppressants).
Drugs such as cyclosporine (Gengraf, Neoral, Sandimmune) and anti-thymocyte globulin suppress the activity of immune cells that are damaging your bone marrow. This helps your bone marrow recover and generate new blood cells. Cyclosporine and anti-thymocyte globulin are often used together.
Corticosteroids, such as methylprednisolone (Medrol, Solu-Medrol), are often used with these drugs.
Although effective, these drugs further weaken your immune system. It’s also possible for anemia to return after you stop these drugs.
Bone marrow stimulants
Certain drugs — including colony-stimulating factors, such as sargramostim (Leukine), filgrastim (Neupogen) and pegfilgrastim (Neulasta), epoetin alfa (Epogen/Procrit), and eltrombopag (Promacta) — help stimulate the bone marrow to produce new blood cells. Growth factors are often used with immune-suppressing drugs.
Having aplastic anemia weakens your immune system, which leaves you more prone to infections.
If you have aplastic anemia, see your doctor at the first sign of infection, such as a fever. You don’t want the infection to get worse, because it could prove life-threatening. If you have severe aplastic anemia, your doctor might prescribe antibiotics or antiviral medications to help prevent infections.
Other treatments
Aplastic anemia caused by radiation and chemotherapy treatments for cancer usually improves after those treatments stop. The same is true for most other drugs that induce aplastic anemia.
Pregnant women with aplastic anemia are treated with blood transfusions. For many women, pregnancy-related aplastic anemia improves once the pregnancy ends. If that doesn’t happen, treatment is still necessary.
Anemias associated with bone marrow disease
A variety of diseases, such as leukemia and myelofibrosis, can cause anemia by affecting blood production in your bone marrow. The effects of these types of cancer and cancer-like disorders vary from mild to life-threatening.
Hemolytic anemia
Hemolytic anemia develops when red blood cells are destroyed faster in the bloodstream or in the spleen than bone marrow can replace them. Certain blood diseases increase red blood cell destruction. You can inherit a hemolytic anemia, or you can develop it later in life. Hemolytic anemia can be further subdivided as to where the hemolysis is taking place – intravascularly or extravascularly. Hemolytic anemia can develop quickly or slowly, and it can be mild or serious.
Doctors diagnose hemolytic anemia based on the underlying cause of your anemia. Certain conditions can cause hemolysis to happen too fast or too often. Conditions that may lead to hemolytic anemia include inherited blood disorders such as sickle cell disease or thalassemia, autoimmune disorders, bone marrow failure, or infections. Some medicines or side effects from blood transfusions may cause hemolytic anemia. Sometimes, the cause is not known.
Hemolytic anemia affects people of all ages and races and both sexes. The different types of hemolytic anemia affect various populations. Some types of acquired hemolytic anemia also affect certain populations. For example, alloimmune hemolytic anemia can occur in pregnant women and their fetuses. Mechanical hemolytic anemia may occur in people who have artificial heart valves
or who use a heart-lung bypass machine during open-heart surgery.
Hemolytic anemia treatment will depend on the cause. People who have mild hemolytic anemia may not need treatment. Serious hemolytic anemia that is not treated or managed can cause irregular heart rhythms, a heart that is larger than normal, and heart failure if anemia gets severe.
Hemolytic anemia symptoms
Hemolytic anemia signs and symptoms vary widely and depend on the type and severity of the hemolytic anemia. Hemolytic anemia symptoms may include tiredness, dizziness, weakness, and a spleen or liver that is larger than normal.
You may not have symptoms if the anemia is mild. If the problem develops slowly, the first symptoms may be:
- Feeling weak or tired more often than usual, or with exercise
- Feelings that your heart is pounding or racing
- Headaches
- Problems concentrating or thinking
If the anemia gets worse, symptoms may include:
- Lightheadedness when you stand up
- Pale skin
- Shortness of breath
- Chest pain
- Pain in the upper abdomen or back
- Sore tongue
- Enlarged spleen
- Jaundice (a medical condition marked by a yellow color of the skin or the whites of the eyes). This sign often is very severe in hemolytic anemia.
Hemolytic anemia causes
Hemolytic anemia develops when your bone marrow cannot make enough new red blood cells to replace the ones that have been destroyed too soon. There are many types of hemolytic anemia and many causes. Hemolytic anemia can be acquired or inherited. Hemolytic anemia may be due to mechanical causes (leaky heart valves or aneurysms), infections, autoimmune disorders, or congenital abnormalities in the red blood cell. Inherited abnormalities may affect the hemoglobin or the red blood cell structure or function. Examples of inherited hemolytic anemias include some types of thalassemia and low levels of enzymes such as glucose-6 phosphate dehydrogenase deficiency. Some medicines or side effects from blood transfusions may cause hemolytic anemia. Sometimes, the cause is not known.
There are several possible causes of hemolytic anemia. Red blood cells may be destroyed due to:
- An autoimmune problem in which the immune system mistakenly sees your own red blood cells as foreign substances and destroys them. These are the 3 types of immune hemolytic anemia:
- Autoimmune hemolytic anemia (AIHA). Autoimmune hemolytic anemia is the main cause of hemolytic anemia. The immune system makes antibodies (proteins) that attack the red blood cells. Autoimmune hemolytic anemia can develop very suddenly. Certain diseases or infections can raise the risk for autoimmune hemolytic anemia (for example, lupus, chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, other blood cancers, Epstein-Barr virus, cytomegalovirus, mycoplasma pneumonia, hepatitis, and HIV). Some autoimmune hemolytic anemia antibodies become active only in warm temperatures, others only in cold temperatures.
- Alloimmune hemolytic anemia. With alloimmune hemolytic anemia, a person’s immune system makes antibodies against blood that is a different type from his or her own blood. This may occur in a blood transfusion from a donor who has a different blood type. Alloimmune hemolytic anemia also can occur during pregnancy if the fetus has a different blood type than the mother (this condition is called Rh incompatibility).
- Drug-induced hemolytic anemia. Some medicines (for example, penicillin, acetaminophen, antimalaria medicines, and levodopa) may cause an immune reaction that destroys red blood cells.
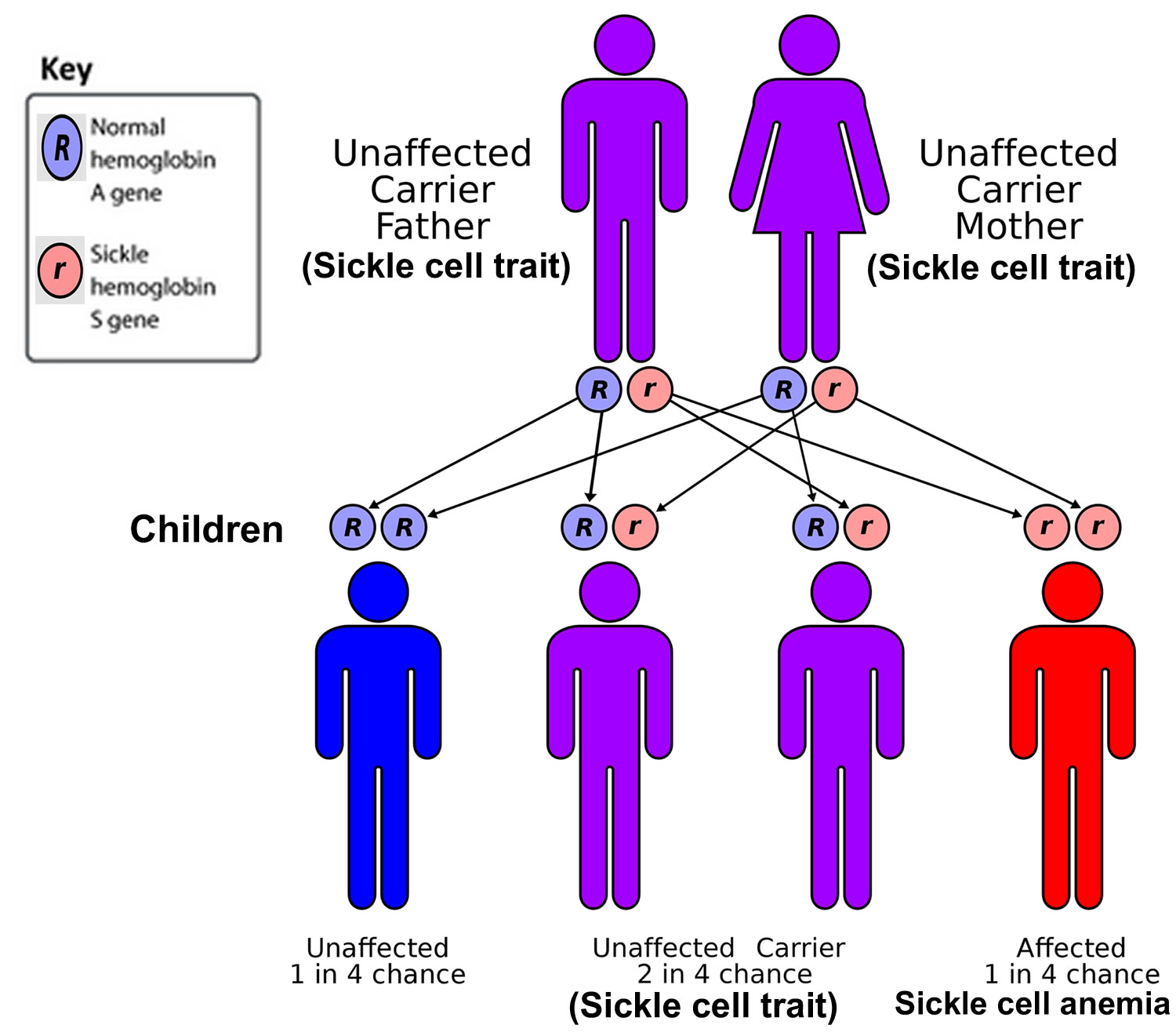
- Genetic defects within the red cells (such as sickle cell anemia, thalassemia, and G6PD deficiency)
- Bone marrow failure
- Exposure to certain chemicals, medicines, and toxins
- Anti-malaria drugs (quinine compounds)
- Arsenic
- Dapsone
- Intravenous water infusion (not half-normal saline or normal saline)
- Metals (chromium/chromates, platinum salts, nickel compounds, copper, lead, cis-platinum)
- Nitrites
- Nitrofurantoin
- Penicillin
- Phenazopyridine (Pyridium)
- Rho immune globulin (WinRho)
- Ribavirin
- Snake bites (some snake venom contains hemolytic toxins)
- Sulfonamides
- Sulfones
- Infections
- Blood clots in small blood vessels
- Complications from blood transfusions from a donor with a blood type that does not match yours
- Inherited blood conditions such as sickle cell disease, thalassemia, hereditary spherocytosis, hereditary elliptocytosis (ovalocytosis), G6PD deficiency (glucose-6-phosphate dehydrogenase deficiency), pyruvate kinase deficiency
- Mechanical hemolytic anemia. Mechanical hemolytic anemia develops because red blood cells are physically damaged. This damage may result from a heart-lung bypass machine (used during open-heart surgery), an artificial heart valve that is not working well, an increase in body temperature due to exposure to extreme heat or extensive burns, or preeclampsia (very high blood pressure during pregnancy).
- Paroxysmal nocturnal hemoglobinuria (PNH). Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired (not inherited) disorder that leads to the premature death and impaired production of blood cells. In paroxysmal nocturnal hemoglobinuria (PNH) abnormal stem cells in the bone marrow make blood cells with a faulty outer membrane. This causes the body to destroy its red blood cells and make too few white blood cells and platelets.
Hemolytic anemia diagnosis
To diagnose hemolytic anemia, your doctor will do a physical exam and order blood tests. Additional tests may include a urine test, a bone marrow test, or genetic tests.
These tests can identify the type of hemolytic anemia:
- Absolute reticulocyte count
- Coombs test, direct and indirect
- Donath-Landsteiner test
- Cold agglutinins
- Free hemoglobin in the serum or urine
- Hemosiderin in the urine
- Platelet count
- Protein electrophoresis – serum
- Pyruvate kinase
- Serum haptoglobin levels
- Serum LDH
- Carboxyhemoglobin level
Hemolytic anemia treatment
Hemolytic anemia treatment depends on the type and cause. If you have mild hemolytic anemia, you may not have any symptoms or need treatment. For others, hemolytic anemia can often be treated or managed. Treatments may include the following:
- Blood transfusions
- Medicines. For immune causes, medicines that suppress the immune system may be used.
- When blood cells are being destroyed at a fast pace, the body may need extra folic acid and iron supplements to replace what is being lost.
- Surgery to remove your spleen called splenectomy. This is because the spleen acts as a filter that removes abnormal cells from the blood. If large numbers of red blood cells are destroyed during a short period, they will become trapped in the spleen. Over time, this can cause the spleen to become larger than normal.
- Blood and bone marrow transplants
If your hemolytic anemia is caused by medicines or another health condition, your doctor may change your treatment to manage or stop hemolytic anemia.
Treatments for acquired hemolytic anemia
- Immune hemolytic anemia
- Corticosteroids and other medicines to suppress the immune system
- Removal of the spleen
- Plasmapheresis (a procedure to remove antibodies from the blood)
- Avoidance of cold temperatures (for example, wear gloves, a hat, and a scarf; dress warmly in air conditioning; keep your car warm when driving in cold weather)
- Intravenous gamma globulin, a medicine that may increase the lifespan of red blood cells and possibly reduce the number of antibodies produced
- Mechanical hemolytic anemia
- Folic acid supplements
- Blood transfusions
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Iron and folic acid supplements
- Eculizumab, an antibody that blocks the destruction of red blood cells in paroxysmal nocturnal hemoglobinuria
Treatments for inherited hemolytic anemia
- Hemoglobin disorders (sickle cell disease and thalassemias)
- For sickle cell disease:
- Folic acid supplements (made from the synthetic form of folate), antibiotics to prevent infection, medicine to reduce the number of faulty red blood cells in the blood, and a medicine called hydroxyurea, which may help the body make more healthy hemoglobin and reduce the amount of faulty hemoglobin that leads to sickle cells
- For thalassemias:
- Blood transfusions to replace destroyed red blood cells, and blood and marrow stem cell transplants
- Folic acid supplements
- For sickle cell disease:
- Disorders of the red blood cell outer membrane (hereditary spherocytosis and hereditary elliptocytosis)
- Blood transfusions
- Removal of the spleen (rarely)
- Enzyme deficiencies
- For G6PD deficiency:
- Avoidance of substances that trigger the condition, such as fava beans, naphthalene (a substance in mothballs), and certain medicines
- For pyruvate kinase deficiency:
- Folic acid supplements and blood transfusions.
- For G6PD deficiency:
Sickle cell disease
Sickle cell disease is a group of inherited red blood cell disorders that affects hemoglobin (Hb), the protein that carries oxygen to cells throughout the body. Within the umbrella of sickle cell disease, many subgroups exist, namely sickle cell anemia (HbSS), hemoglobin SC disease (HbSC), and hemoglobin sickle-beta-thalassemia [HbSB or HbSBetaThal]. Sickle cell beta-thalassemia (HbSB) can present in 2 forms. The first form is one in which there is a small amount of normal hemoglobin present called sickle cell beta-thalassemia+ (Sickle Beta-Plus Thalassemia or HbSB+). The sickle cell disease combined with beta-thalassemia (Sickle Beta-Plus Thalassemia) is generally a “mild” form of sickle cell disease 11. The second form is the absence of normal hemoglobin called sickle cell beta-thalassemia zero (Sickle Beta-Zero Thalassemia or HbSB0), and these patients present similarly to those with sickle cell disease 12. Several other minor variants within the group of sickle cell diseases also, albeit not as common as the aforementioned varieties. Lastly, it is important to mention the sickle cell trait (HbAS), which carries a heterozygous mutation and seldom presents with any clinical signs or symptoms. Normal hemoglobin is called hemoglobin A (α2β2) and consists of four protein subunits: two subunits of alpha (α) globin chains, which is produced by hemoglobin alpha (HBA1 or HBA2) gene and two subunits of beta (β) globin chains, which is produced by HBB gene (hemoglobin beta gene). Each of these protein subunits is attached (bound) to an iron-containing molecule called heme; each heme contains an iron molecule in its center that can bind to one oxygen molecule. Hemoglobin within red blood cells binds to oxygen molecules in the lungs. A complete hemoglobin protein is capable of carrying four oxygen molecules at a time (one attached to each heme molecule). Oxygen attached to hemoglobin gives blood its bright red color. These red blood cells then travel through the bloodstream and deliver oxygen to tissues throughout the body (see Figure 4). Adult red blood cells normally contain the following hemoglobin chain combinations: hemoglobin A (α2β2 or alpha2-beta 2) >95%; hemoglobin A2 (α2δ2 or alpha2-delta2) 2% to 3.4%; fetal hemoglobin F (α2γ2 or alpha2-gamma2) <1%.
Sickle cell disease is the most common genetic disease in the United States, affecting 1 in 500 African Americans 13. About 1 in 12 African Americans carry the autosomal recessive mutation, and approximately 300,000 infants are born with sickle cell anemia annually. Sickle cell disease affects more than 100,000 people in the United States and 20 million people worldwide.
Normally, red blood cells are disc shaped and flexible to move easily through the blood vessels. If you have sickle cell disease, your red blood cells are crescent or “sickle” shaped. The sickle-shaped red blood cells are not flexible and cannot change shape easily. Many of them burst apart as they move through your blood vessels. The sickle red blood cells usually only last 10 to 20 days, instead of the normal 90 to 120 days. Your body may have trouble making enough new red blood cells to replace the ones that you lost. Because of this, you may not have enough red blood cells. This is a condition called anemia, and it can make you feel tired.
The sickle-shaped red blood cells can also stick to blood vessel walls, causing a blockage that slows or stops the flow of blood. When this happens, oxygen can’t reach nearby tissues. The lack of oxygen can cause attacks of sudden, severe pain, called pain crises. These attacks can occur without warning. If you get one, you might need to go to the hospital for treatment.
The blocked blood flow through the body can also lead to serious problems, including stroke, eye problems and infections.
Sickle cell disease is inherited, meaning that it runs in families. People who have sickle cell disease inherit two abnormal hemoglobin genes, called hemoglobin-Beta gene (HBB gene), one from each parent. The HBB gene provides instructions for making beta-globin, which is one part of hemoglobin (see Figure 4 below). Sickle cell disease is caused by a point mutation in the hemoglobin-Beta gene (HBB gene) found on chromosome 11. Various versions of beta-globin result from different mutations in the HBB gene. A point mutation of HBB gene replaces A with T at codon 6 of beta hemoglobin chain 14. This causes the switch from glutamic acid to valine amino acid at position 6 in beta-globin, written as Glu6Val or E6V. Replacing glutamic acid with valine causes the abnormal version of beta-globin known as hemoglobin S (HbS) subunits to stick together and form long, rigid molecules that bend red blood cells into a sickle (crescent) shape when exposed to a low oxygen threshold. Other mutations in the HBB gene lead to additional abnormal versions of beta-globin such as hemoglobin C (HbC) and hemoglobin E (HbE). HBB gene mutations can also result in an unusually low level of beta-globin; this abnormality is called beta thalassemia.
If you’re a carrier of sickle cell or have sickle cell trait, it means you carry one of the hemoglobin-Beta (HBB) gene that causes sickle cell disease, but you do not have the condition yourself.
A person has sickle cell disease trait (HbAS) also called sickle cell carrier when the hemoglobin S (Hb S) gene is inherited from only one parent, and a normal hemoglobin gene — hemoglobin A (Hb A) — is inherited from the other parent 15. People who have sickle cell trait (sickle cell carrier) are generally healthy. However, there have been reports of adverse conditions such as anaesthetics can cause problems due to the patient’s sickle cell trait status. If you have sickle cell trait Always notify your dentist or doctor before treatment commences to be on the safe side. There is also a small chance that you may experience pain at high altitudes (generally above 10,000 feet), including long-haul flying in unpressurized planes and mountain climbing. It is important you say you have sickle cell trait before undertaking such activities as you may need to breathe oxygen. Extreme exercise may also precipitate problems and if you are a professional athlete you should have a training programme that takes account of this. Therefore, sickle cell trait may not be completely benign and these patients should be managed aggressively whenever they develop some of these complications 15.
If you have sickle cell trait, you are a carrier of the hemoglobin S (Hb S) gene, so you can pass it on when you have a child. If the child’s other parent also has sickle cell trait or another abnormal hemoglobin gene, such as beta-thalassemia, hemoglobin C, hemoglobin D, or hemoglobin E, that child has a chance of having sickle cell disease.
If both parents are sickle cell carriers (also known as having the sickle cell trait), there’s a:
- 1 in 4 chance each child they have will not inherit any sickle cell genes and will not have sickle cell disease or be able to pass it on
- 1 in 2 chance each child they have will just inherit a copy of the sickle cell gene from 1 parent and be a carrier
- 1 in 4 chance each child they have will inherit copies of the sickle cell gene from both parents and will be born with sickle cell disease
Worldwide, it is estimated that there are 300 million people with sickle cell trait and one-third of this number are in sub-Saharan Africa 16. The prevalence of sickle cell trait is higher in areas where malaria is endemic. Gibson and colleague mentions that the prevalence is as high as 25% in some part of Africa and 60% in Saudi Arabia 17. Because of the high migration of people from areas of high prevalence like Africa, Middle East, the prevalence of both sickle cell trait and disease will increase in the western part of the world.
Many states routinely screen newborns for sickle cell so that treatment can begin as soon as possible. Early diagnosis and treatment can reduce the risk of complications.
Hemoglobin electrophoresis is a blood test that can determine if a person is a carrier of sickle cell, or has any of the diseases associated with the sickle cell gene.
Sickle cell disease is a lifelong illness. A blood and bone marrow transplant is currently the only cure for sickle cell disease, but there are effective treatments that can reduce symptoms and prolong life. Treatments for sickle cell include antibiotics, pain management and blood transfusions. A new drug treatment, hydroxyurea, which is an anti-tumor drug, appears to stimulate the production of fetal hemoglobin F (α2γ2), a type of hemoglobin usually found only in newborns. Fetal hemoglobin (α2γ2) helps prevent the “sickling” of red blood cells. Patients treated with hydroxyurea also have fewer attacks of acute chest syndrome and need fewer blood transfusions.
Figure 11. Sickle cell anemia genetics
Sickle cell disease symptoms
If a person has sickle cell disease, it is present at birth. The severity of the symptoms of sickle cell disease can vary greatly depending on the specific genetic type and even within those of the same type. Each child may experience symptoms differently, and symptoms can be very difficult to predict. But most newborns do not have any problems from the disease until they are about 5 or 6 months of age.
Sickle cell disease can cause a wide range of symptoms and can change over time. These can start from a few months of age, although many children have few or no symptoms if treatment is started early on. Over time, you may experience symptoms depending on how sickle cell disease affects your health.
Sickle cell disease main symptoms are:
- painful episodes (sickle cell pain crises or vaso-occlusive crisis)
- getting infections often
- anemia
Anemia
Nearly all people with sickle cell disease have anemia, where the hemoglobin in the blood is low. Hemoglobin is the substance found in red blood cells that’s used to transport oxygen around the body. Anemia does not usually cause many symptoms or it may delay normal growth and development and decrease energy and endurance. Sometimes anemia can get worse if you become infected with the virus that causes slapped cheek syndrome (parvovirus). This can lead to a sudden drop in the number of red blood cells and symptoms of severe anemia include extreme tiredness (fatigue), shortness of breath, dizziness and fainting, headaches, rapid heartbeat or irregular heartbeat.
Splenic sequestration crisis or an aplastic crisis can also cause severe anemia symptoms. These conditions can be life-threatening.
Anemia is usually treated with a blood transfusion.
Vaso-occlusive crisis or painful episodes
Episodes of pain known as sickle cell crises or vaso-occlusive crisis, are one of the most common and distressing symptoms of sickle cell disease. They happen when blood vessels to part of the body become blocked. The pain can be severe and lasts for up to 7 days on average.
A sickle cell crisis often affects a particular part of the body, such as the:
- hands or feet (particularly in young children)
- ribs and breastbone
- spine
- pelvis
- tummy
- legs and arms
Pain can occur anywhere but most often occurs in the bones of the arms, legs, chest, and spine.
Painful swelling of the small bones of the hands and feet, also known as dactylitis, occurs mostly in infants and toddlers with sickle cell disease. This condition occurs when blood flow is blocked in the small bones of the hands and feet.
Priapism (a persistent and painful erection of the penis) results from sickling that occurs in the penis. This results in a painful and unwanted erection. Priapism (a persistent and painful erection of the penis) if not promptly treated, it can result in impotence. If you experience an erection that lasts for 4 hours or more, go to the hospital to see a hematologist and urologist.
Any interruption in blood flow to the body can result in pain, swelling, dysfunction, and possible death of the surrounding tissue not receiving adequate blood and oxygen.
How often someone with sickle cell disease gets episodes of pain varies a lot. Some people may have one every few weeks, while others may have less than 1 a year. The average is 1 bad episode a year.
It’s not always clear what triggers bad pain, but sometimes painful episodes can be caused by the weather (such as wind, rain or cold), dehydration, stress or strenuous exercise.
Acute chest syndrome
Acute chest syndrome occurs when sickle-shaped cells stick together and block the flow of oxygen in the vessels in the lungs. Acute chest syndrome can be life-threatening and is the leading cause of death in children and adults with sickle cell disease. Acute chest syndrome can be triggered by asthma crisis, infection (viral or bacterial), or pain (particularly in the chest) and can progress rapidly to respiratory failure. Acute chest syndrome resembles pneumonia and includes fever and breathing symptoms such as cough or difficulty catching breath. Acute chest syndrome often occurs suddenly, when the body is under stress from infection, fever, or dehydration, and multiple episodes can cause permanent lung damage.
Infections
People with sickle cell disease are more vulnerable to infections and sepsis, particularly when they’re young. Infections can range from mild, such as colds, to much more serious and potentially life threatening, such as meningitis.
In most children with sickle cell disease, by toddlerhood, the spleen becomes scarred and permanently damaged and no longer has full function. The spleen is important in the body’s defense against serious bacterial infections; therefore, children with sickle cell disease are at risk for life-threatening bacterial infections. Fever (more than 38.5⁰C or 101.5⁰F) is a symptom that must be evaluated immediately by a doctor to rule out a life-threatening bacterial infection and treated with antibiotics right away if required. Some people will need to be hospitalized.
Vaccinations and daily doses of antibiotics can help reduce the risk of many infections.
Splenic sequestration
Sickle cells can block the exit of blood from the spleen resulting in pooling of sickle-shaped cells in the spleen, causing a sudden worsening of the anemia. The spleen becomes enlarged and painful from the increase in trapped blood volume. It can be life threatening if not treated promptly. A severe episode of splenic sequestration requires surgical removal of the spleen.
Stroke
Stroke is a serious life-threatening medical emergency that happens when the blood supply to part of the brain is cut off. Stroke is a sudden and severe complication that can occur in children with sickle cell disease. Sickle-shaped cells can block the major blood vessels that supply the brain with oxygen. Interruption in the flow of blood and oxygen to the brain can result in devastating damage to the brain. Symptoms of a stroke can include weakness, particularly on one side of the body; slurred speech; seizure; confusion; dizziness or loss of coordination; or a severe headache.
The sooner a person receives treatment for a stroke, the less damage is likely to happen. If you suspect that you or someone else is having a stroke, phone your local emergency services number immediately and ask for an ambulance. Immediate treatment may save someone’s life and increase the chances for successful rehabilitation and recovery.
Having had one stroke, a child is much more likely to have more strokes and requires preventative therapy with chronic repeated transfusion for life.
Jaundice
Jaundice is when your skin or the whites of your eyes turn yellow. Jaundice is a common sign and symptom of sickle cell disease. Sickle red blood cells are destroyed prematurely in the spleen. The recycling of sickle hemoglobin from these cells produces increased levels of the yellow bilirubin protein that discolors your skin and eyes. Chronic high bilirubin levels can lead to gallstone formation.
Other problems
Sickle cell disease can also sometimes cause a wide range of other problems which may include:
- delayed growth during childhood and delayed puberty
- gallstones, which can cause tummy (abdominal) pain and yellow skin and eyes (jaundice)
- painful open sores on the lower legs (leg ulcers)
- strokes or transient ischemic attacks, where the flow of blood to the brain is blocked or interrupted
- a serious lung condition called acute chest syndrome, which can cause a fever, cough, chest pain and breathing difficulties. You will need to be admitted to the hospital, where you may receive antibiotics, oxygen therapy, or a blood transfusion.
- swelling of the spleen, which can cause shortness of breath, a rapid heartbeat, tummy pain, a swollen tummy and anemia
- eyesight problems, such as floaters, blurred or patchy vision, reduced night vision and occasionally sudden vision loss
- high blood pressure in the blood vessels that carry blood from the heart to the lungs (pulmonary hypertension)
- kidney or urinary problems, including blood in the urine and bedwetting
Sickle cell disease causes
Sickle cell disease is inherited, meaning that it runs in families. People who have sickle cell disease inherit two abnormal hemoglobin genes, called hemoglobin S (Hb S), one from each parent. Sickle cell disease is caused by a point mutation in the hemoglobin-Beta gene (HBB) found on chromosome 11. The hemoglobin beta (HBB) gene provides instructions for making a protein called beta-globin. Beta-globin is a component (subunit) of a larger protein called hemoglobin, which is located inside red blood cells (see Figure 8 above). This point mutation replaces A with T at codon 6 of hemoglobin-Beta chain. This causes the switch from glutamic acid to valine amino acid. The valine-type hemoglobin causes red cells to sickle when exposed to a low oxygen threshold.
If both parents are sickle cell carriers (also known as having the sickle cell trait), there’s a:
- 1 in 4 chance each child they have will not inherit any sickle cell genes and will not have sickle cell disease or be able to pass it on
- 1 in 2 chance each child they have will just inherit a copy of the sickle cell gene from 1 parent and be a carrier
- 1 in 4 chance each child they have will inherit copies of the sickle cell gene from both parents and will be born with sickle cell disease
If you’re a carrier of sickle cell or have sickle cell trait, it means you carry one of the hemoglobin S (Hb S) gene that causes sickle cell disease, but you do not have the condition yourself.
About 100,000 Americans have sickle cell disease. In the United States, most people who have sickle cell disease are of African ancestry or identify themselves as Black.
- About 1 in 13 Black or African American babies is born with sickle cell trait.
- About 1 in every 365 Black or African American babies is born with sickle cell disease.
There are also many people who have sickle cell disease who come from Hispanic, southern European, Middle Eastern, or Asian Indian backgrounds.
People who do not know whether they carry an abnormal hemoglobin S (Hb S) gene can ask their doctor to have their blood tested.
Couples who are planning to have children and know that they are at risk of having a child with sickle cell disease may want to meet with a genetic counselor. A genetic counselor can answer questions about the risk and explain the choices that are available.
Sickle cell disease diagnosis
Sickle cell disease is diagnosed with a simple blood test that checks for hemoglobin S (HbS) – the defective form of hemoglobin. To confirm the diagnosis, a sample of blood is examined under a microscope to check for large numbers of sickled red blood cells – the hallmark trait of the disease.
In more than 40 states, testing for the defective sickle cell gene is routinely performed on newborns. It often is included in routine newborn screening tests at birth in the hospital. It’s important to test babies for sickle cell disease. Early diagnosis and treatment can help prevent and manage symptoms of sickle cell disease.
Talk to your doctor if you or your partner has sickle cell disease or if it runs in your family. If you’re trying to get pregnant, your doctor can tell you options for genetic counseling. If you have sickle cell disease and are pregnant, you should receive extra care to monitor your sickle cell disease and treat problems early. Your baby can be tested for sickle cell disease during pregnancy.
Newborn screening
When a child has sickle cell disease, early diagnosis is important to better prevent complications. Every state in the United States, the District of Columbia, and the U.S. territories require that every baby be tested for sickle cell disease as part of a newborn screening program. In newborn screening programs, blood from a heel prick is collected in “spots” on a special paper. The hemoglobin from this blood is then analyzed in special labs. Newborn screening results are sent to the doctor who ordered the test and to the child’s primary doctor.
If a baby is found to have sickle cell disease, health providers from a special follow-up newborn screening group contact the family directly to make sure that the parents know the results. The child is always retested to be sure that the diagnosis is correct.
Newborn screening programs also find out whether the baby has an abnormal hemoglobin trait. If so, the parents are informed, and counseling is offered. Remember that when a child has sickle cell trait or sickle cell disease, a future sibling or the child’s own future child may be at risk.
Prenatal screening
Doctors can also diagnose sickle cell disease before a baby is born. This is done using a sample of amniotic fluid, the liquid in the sac surrounding a growing embryo, or of tissue taken from the placenta, the organ that attaches the umbilical cord to the mother’s womb.
Testing before birth can be done as early as 8 to 10 weeks into the pregnancy. This testing looks for the sickle hemoglobin gene rather than the abnormal hemoglobin.
Sickle cell carriers testing
Screening for sickle cell disease is offered to all pregnant women, although most women will be at low risk and will not need to have a blood test to check if they’re a carrier.
A new technique used in conjunction with in vitro fertilization (IVF), called pre-implantation genetic diagnosis (PGD), enables parents who carry the sickle cell trait to test embryos for the defective gene before implantation, and to choose to implant only those embryos free of the sickle cell gene.
Sickle cell disease treatment
Sickle cell disease usually requires lifelong treatment. Children and adults with sickle cell disease are supported by a team of different healthcare professionals working together at a specialist sickle cell center. Babies who have sickle cell disease may see a hematologist, a doctor who specializes in blood diseases such as sickle cell disease. For newborns, the first sickle cell disease visit should take place before 8 weeks of age.
A blood and bone marrow transplant is currently the only cure for some patients who have sickle cell disease 18. After early diagnosis, your doctor may recommend medicines or blood transfusions to manage complications, including chronic pain.
Medicine to prevent the sickling of red blood cells
The U.S. Food and Drug Administration (FDA) approved Voxelotor in 2019 to treat sickle cell disease in adults and children 12 years and older. The oral medicine prevents red blood cells from forming the sickle shape and binding together. This may decrease the destruction of some red blood cells, which in turn lowers the risk for anemia and improves blood flow to your organs.
Possible side effects include headache, diarrhea, abdominal pain, nausea, fatigue, and fever. Rarely, allergic reactions may occur, causing rashes, hives, or mild shortness of breath. Talk to your doctor about other medicines you take.
Medicine to reduce vaso-occlusive and pain crises
In 2019, the FDA approved crizanlizumab-tmca (ADAKVEO®) to reduce the number of pain crises experienced by adults and children 16 years and older who have sickle cell disease. The medicine, which is given through an IV in the vein, helps prevent blood cells from sticking to blood vessel walls and causing blood flow blockage, inflammation, and pain crises.
Possible side effects include nausea, joint pain, back pain, and fever.
Hydroxyurea
Hydroxyurea is an oral medicine that has been shown to reduce or prevent several sickle cell disease complications.
- Use in adults: Many studies of adults with hemoglobin SS (also known as sickle cell anemia, which is the most common and most severe type of sickle cell disease) or hemoglobin Sβ thalassemia (Sickle Beta Thalassemia) showed that hydroxyurea reduced the number of episodes of pain crises and acute chest syndrome. It also improved anemia and decreased the need for transfusions and hospital admissions.
- Use in children: Studies in children with severe hemoglobin SS or Sβ thalassemia (Sickle Beta Thalassemia) showed that hydroxyurea reduced the number of vaso-occlusive crises and hospitalizations. A study of children between the ages of 9 and 18 months with hemoglobin SS or Sβ thalassemia also showed that hydroxyurea reduced the number of pain episodes and dactylitis. There is no information about how safe or effective hydroxyurea is in children under 9 months of age
- Pregnancy: Pregnant people should not use hydroxyurea.
Since hydroxyurea can decrease several complications of sickle cell disease, most experts recommend that children and adults with hemoglobin SS or Sβ0 thalassemia (Sickle Beta Zero Thalassemia) who have frequent painful episodes, recurrent chest crises, or severe anemia take hydroxyurea daily.
Possible side effects include decreased white cell count or platelet count. Rarely, it can worsen anemia. These side effects usually go away quickly if a patient stops taking the medicine. When the patient restarts it, the doctor usually prescribes a lower dose.
It is still unclear whether hydroxyurea can cause problems later in life in people who have sickle cell disease and take the medicine for many years. Studies so far suggest that it does not put people at a higher risk of cancer and does not affect growth in children, but further studies are needed.
Medicine to reduce risk of infection
In children who have sickle cell disease, taking penicillin two times a day has been shown to reduce the chance of having a severe infection in the bloodstream. Newborns need to take liquid penicillin. Older children can take tablets.
Many doctors will stop prescribing penicillin after a child has reached the age of 5. Some prefer to continue this antibiotic throughout life, particularly if a person has hemoglobin SS or hemoglobin Sβ0 thalassemia (Sickle Beta Zero Thalassemia), since people who have sickle cell disease are still at risk. All people who have had surgical removal of the spleen, called a splenectomy, or a past infection with pneumococcus should keep taking penicillin throughout life.
Blood transfusions
Your doctor may recommend transfusion to treat and prevent certain sickle cell disease complications.
These transfusions may include:
- Acute transfusions treat complications that cause severe anemia. Doctors may also use transfusions when a patient has an acute stroke, in many cases of acute chest crises, and in multi-organ failure. A patient who has sickle cell disease usually receives blood transfusions before surgery, to prevent complications.
- Red blood cell transfusions increase the number of red blood cells and provide normal red blood cells that are more flexible than red blood cells with sickle hemoglobin.
- Regular or ongoing blood transfusions may help lower the chances of another stroke in people who have had an acute stroke.
Doctors also recommend blood transfusions for children who have abnormal transcranial Doppler ultrasound results, because transfusions can reduce the chance of having a first stroke. Some doctors use this approach to treat complications that do not improve with hydroxyurea. Doctors may also use transfusions in people who have too many side effects from hydroxyurea. Possible complications of blood transfusions include alloimmunization, which can make it hard to find a matching unit of blood for a future transfusion; infection; and iron overload.
Blood and bone marrow transplant
A blood and bone marrow transplant is currently the only cure for sickle cell disease, but it is not for everyone. Most patients who have sickle cell disease either are too old for a transplant or do not have a relative who is a good enough genetic match to be a donor. A well-matched donor is needed for a patient to have the best chance for a successful transplant.
Most sickle cell disease transplants are currently performed in children who have had complications such as strokes, acute chest crises, and recurring pain crises. These transplants usually use a matched donor. Blood and bone marrow transplants are riskier in adults.
Several medical centers are looking into new ways to help more people who have sickle cell disease get a transplant. These include blood and bone marrow transplant techniques in children and adults who do not have a matched donor in the family or who are older than most recipients.
Blood and bone marrow transplants are successful in about 85% of children when the donor is related and HLA (human leukocyte antigen)-matched. Even with this high success rate, transplants still have risks. Complications can include severe infections, seizures, and other clinical problems. About 5% of people who have received such transplants have died. Sometimes transplanted cells attack the recipient’s organs. This is called graft-versus-host disease. You will get medicine to prevent many of the complications, but they still can happen.
Sickle cell anemia
Sickle cell anemia also called homozygous sickle cell disease or HbSS disease, is the most common and most severe type of sickle cell disease. Sickle cell anemia is caused by a defective form of hemoglobin that forces red blood cells to assume an abnormal crescent or sickle shape. These irregular blood cells die prematurely, resulting in a chronic shortage of red blood cells or anemia (low number of red blood cells).
Red blood cells are usually round and flexible, so they move easily through blood vessels. In sickle cell anemia, some red blood cells are shaped like sickles or crescent moons. These sickle cells also become rigid and sticky, which can slow or block blood flow. Signs and symptoms of sickle cell disease usually begin in early childhood and may include anemia, repeated infections, and periodic episodes of pain (called crises). Children with sickle cell anemia are prone to infections, which often start with a fever and can be life-threatening, seek prompt medical attention for a fever greater than 101.5 °F (38.5 °C).
Sickle cell anemia is caused by genetic changes (mutations) in the HBB gene and is inherited in an autosomal recessive pattern. For a baby to be born with sickle cell anemia, both parents must carry a sickle cell gene. In the United States, sickle cell anemia most commonly affects people of African, Mediterranean and Middle Eastern descent.
There’s no cure for most people with sickle cell anemia. Treatments can relieve pain and help prevent complications associated with the disease.
Sickle cell anemia symptoms
Sickle cell anemia signs and symptoms usually appear around 6 months of age. They vary from person to person and may change over time. Signs and symptoms can include:
- Anemia. Sickle cells break apart easily and die. Red blood cells usually live for about 120 days before they need to be replaced. But sickle cells typically die in 10 to 20 days, leaving a shortage of red blood cells (anemia). Without enough red blood cells, the body can’t get enough oxygen and this causes fatigue.
- Episodes of pain. Periodic episodes of extreme pain, called pain crises, are a major symptom of sickle cell anemia. Pain develops when sickle-shaped red blood cells block blood flow through tiny blood vessels to your chest, abdomen and joints. The pain varies in intensity and can last for a few hours to a few days. Some people have only a few pain crises a year. Others have a dozen or more a year. A severe pain crisis requires a hospital stay. Some adolescents and adults with sickle cell anemia also have chronic pain, which can result from bone and joint damage, ulcers, and other causes.
- Swelling of hands and feet. The swelling is caused by sickle-shaped red blood cells blocking blood circulation in the hands and feet.
- Frequent infections. Sickle cells can damage the spleen, increasing vulnerability to infections. Infants and children with sickle cell anemia commonly receive vaccinations and antibiotics to prevent potentially life-threatening infections, such as pneumonia.
- Delayed growth or puberty. Red blood cells provide the body with the oxygen and nutrients needed for growth. A shortage of healthy red blood cells can slow growth in infants and children and delay puberty in teenagers.
- Vision problems. Tiny blood vessels that supply the eyes can become plugged with sickle cells. This can damage the retina — the portion of the eye that processes visual images — and lead to vision problems.
Sickle cell anemia causes
Sickle cell anemia is caused by genetic changes (mutations) in the HBB gene and is inherited in an autosomal recessive pattern.
The HBB gene provides instructions for making a protein called beta-globin. Beta-globin is a component (subunit) of a larger protein called hemoglobin (Hb), which is located inside red blood cells. In adults, hemoglobin normally consists of four protein subunits: two subunits of beta-globin and two subunits of a protein called alpha-globin, which is produced from another gene called HBA. Each of these protein subunits is attached (bound) to an iron-containing molecule called heme; each heme contains an iron molecule in its center that can bind to one oxygen molecule. Hemoglobin within red blood cells binds to oxygen molecules in the lungs. These cells then travel through the bloodstream and deliver oxygen to tissues throughout the body.
Mutation in the HBB gene results in the production of an abnormal version of beta-globin called hemoglobin S or HbS. In sickle cell anemia, hemoglobin S replaces both beta-globin subunits in hemoglobin. The mutation that causes hemoglobin S changes a single protein building block (amino acid) in beta-globin. Specifically, the amino acid glutamic acid is replaced with the amino acid valine at position 6 in beta-globin, written as Glu6Val or E6V. Replacing glutamic acid with valine causes the abnormal hemoglobin S subunits to stick together and form long, rigid molecules that bend red blood cells into a sickle (crescent) shape. The sickle-shaped cells die prematurely, which can lead to a shortage of red blood cells (anemia). The sickle-shaped cells are rigid and can block small blood vessels, causing severe pain and organ damage.
Autosomal means the gene is located on any chromosome except the X or Y chromosomes (sex chromosomes). Genes, like chromosomes, usually come in pairs. Recessive means that both copies of the responsible gene must have a disease-causing change (pathogenic variant) in order for a person to have the disease. Mutation is an older term that is still sometimes used to mean pathogenic variant. A person who has an autosomal recessive disease receives a gene with a pathogenic variant from each of their parents. Each parent is a carrier which means they have a pathogenic variant in only one copy of the gene. Carriers of an autosomal recessive disease usually do not have any symptoms of the disease. When two carriers of an autosomal recessive disease have children, there is a 25% (1 in 4) chance to have a child who has the disease.
For a baby to be born with sickle cell anemia, both parents must carry a sickle cell gene. In the United States, sickle cell anemia most commonly affects people of African, Mediterranean and Middle Eastern descent.
Sickle cell anemia prevention
If you carry the sickle cell trait, seeing a genetic counselor before trying to conceive can help you understand your risk of having a child with sickle cell anemia. A genetic counselor can also explain possible treatments, preventive measures and reproductive options.
Sickle cell anemia complications
Sickle cell anemia can lead to a host of complications, including:
- Stroke. Sickle cells can block blood flow to an area of the brain. Signs of stroke include seizures, weakness or numbness of the arms and legs, sudden speech difficulties, and loss of consciousness. If your child has any of these signs and symptoms, seek medical treatment immediately. A stroke can be fatal.
- Acute chest syndrome. A lung infection or sickle cells blocking blood vessels in the lungs can cause this life-threatening complication, resulting in chest pain, fever and difficulty breathing. It might require emergency medical treatment.
- Pulmonary hypertension. People with sickle cell anemia can develop high blood pressure in their lungs. This complication usually affects adults. Shortness of breath and fatigue are common symptoms of this condition, which can be fatal.
- Organ damage. Sickle cells that block blood flow to organs deprive the affected organs of blood and oxygen. In sickle cell anemia, blood is also chronically low in oxygen. This lack of oxygen-rich blood can damage nerves and organs, including kidneys, liver and spleen, and can be fatal.
- Splenic sequestration. A large number of sickle cells can get trapped in the spleen, causing it to enlarge and possibly causing belly pain on the left side of the body. This can be life-threatening. Parents of children with sickle cell anemia should learn to regularly feel their child’s spleen for enlargement.
- Blindness. Sickle cells can block tiny blood vessels that supply the eyes. Over time, this can lead to blindness.
- Leg ulcers. Sickle cell anemia can cause painful open sores on the legs.
- Gallstones. The breakdown of red blood cells produces a substance called bilirubin. A high level of bilirubin in the body can lead to gallstones.
- Priapism. In this condition, men with sickle cell anemia can have painful, long-lasting erections. Sickle cells can block the blood vessels in the penis, which can lead to impotence over time.
- Deep vein thrombosis (DVT). Sickling of red cells can cause blood clots, increasing the risk of a clot lodging in a deep vein (deep vein thrombosis) or a lung (pulmonary embolism). Either can cause serious illness or even death.
- Pregnancy complications. Sickle cell anemia can increase the risk of high blood pressure and blood clots during pregnancy. It can also increase the risk of miscarriage, premature birth and having low birth weight babies.
Sickle cell anemia diagnosis
A blood test can check for the form of hemoglobin that underlies sickle cell anemia. In the United States, this blood test is part of routine newborn screening. But older children and adults can be tested, too.
In adults, a blood sample is drawn from a vein in the arm. In young children and babies, the blood sample is usually collected from a finger or heel. The sample is then sent to a laboratory, where it’s screened for the sickle cell form of hemoglobin.
If you or your child has sickle cell anemia, your doctor might suggest other tests to check for possible complications of the disease.
If you or your child carries the sickle cell gene, you’ll likely be referred to a genetic counselor.
Sickle cell anemia assessing stroke risk
A special ultrasound machine can reveal which children have a higher risk of stroke. This painless test, which uses sound waves to measure blood flow in the brain, can be used in children as young as 2 years. Regular blood transfusions can decrease stroke risk.
Tests to detect sickle cell genes before birth
Sickle cell disease can be diagnosed in an unborn baby by sampling some of the fluid surrounding the baby in the mother’s womb (amniotic fluid). If you or your partner has sickle cell anemia or the sickle cell trait, ask your doctor about this screening.
Sickle cell anemia treatment
Management of sickle cell anemia is usually aimed at avoiding pain episodes, relieving symptoms and preventing complications. Treatments might include medications and blood transfusions. For some children and teenagers, a stem cell transplant might cure the disease.
Sickle cell anemia medications
- Hydroxyurea (Droxia, Hydrea, Siklos). Daily hydroxyurea reduces the frequency of painful crises and might reduce the need for blood transfusions and hospitalizations. But it can increase the risk of infections. Don’t take the drug if you’re pregnant.
- L-glutamine oral powder (Endari). The FDA recently approved this drug for treatment of sickle cell anemia. It helps in reducing the frequency of pain crises.
- Crizanlizumab (Adakveo). This drug, given by injection, can help reduce the frequency of pain crises in adults and children older than 16. Side effects can include nausea, joint pain, back pain and fever.
- Voxelotor (Oxbryta). This drug is used to treat sickle cell disease in adults and children older than 12. Taken orally, this drug can lower the risk of anemia and improve blood flow throughout the body. Side effects can include headache, nausea, diarrhea, fatigue, rash and fever.
- Pain-relieving medications. Your doctor might prescribe narcotics to help relieve pain during sickle cell pain crises.
Sickle cell anemia preventing infections
Children with sickle cell anemia might receive penicillin between the ages of about 2 months old until at least age 5 years. Doing so helps prevent infections, such as pneumonia, which can be life-threatening to children with sickle cell anemia.
Adults who have sickle cell anemia might need to take penicillin throughout their lives if they’ve had pneumonia or surgery to remove the spleen.
Childhood vaccinations are important for preventing disease in all children. They’re even more important for children with sickle cell anemia because their infections can be severe.
Your child’s doctor should ensure that your child receives all the recommended childhood vaccinations, as well as vaccines against pneumonia, meningitis, hepatitis B and an annual flu shot. Vaccines are also important for adults with sickle cell anemia.
Sickle cell anemia home remedies
Taking the following steps to stay healthy might help you avoid complications of sickle cell anemia:
- Take folic acid supplements daily and choose a healthy diet. Bone marrow needs folic acid and other vitamins to make new red blood cells. Ask your doctor about a folic acid supplement and other vitamins. Eat a variety of colorful fruits and vegetables, as well as whole grains.
- Drink plenty of water. Dehydration can increase your risk of a sickle cell crisis. Drink water throughout your day, aiming for about eight glasses a day. Increase the amount of water you drink if you exercise or spend time in a hot, dry climate.
- Avoid temperature extremes. Exposure to extreme heat or cold can increase your risk of a sickle cell crisis.
- Exercise regularly, but don’t overdo it. Talk with your doctor about how much exercise is right for you.
- Use nonprescription medications with caution. Use pain medications, such as ibuprofen (Advil, Motrin IB, Children’s Motrin, others) or naproxen sodium (Aleve), sparingly, if at all, because of the possible effect on your kidneys. Ask your doctor before taking nonprescription drugs.
- Don’t smoke. Smoking increases your risk of pain crises.
Sickle cell anemia during surgical and other procedures
- Blood transfusions. These are used to treat and prevent complications, such as stroke, in people with sickle cell disease. In a red blood cell transfusion, red blood cells are removed from a supply of donated blood, then given through a vein to a person with sickle cell anemia. This increases the number of normal red blood cells, which helps reduce symptoms and complications. Risks include an immune response to the donor blood, which can make it hard to find future donors; infection; and excess iron buildup in your body. Because excess iron can damage your heart, liver and other organs, you might need treatment to reduce iron levels if you undergo regular transfusions.
- Stem cell transplant also known as bone marrow transplant, this procedure involves replacing bone marrow affected by sickle cell anemia with healthy bone marrow from a donor. The procedure usually uses a matched donor, such as a sibling, who doesn’t have sickle cell anemia. Because of the risks associated with a bone marrow transplant, including death, the procedure is recommended only for people, usually children, who have significant symptoms and complications of sickle cell anemia. A stem cell transplant is the only known cure for sickle cell anemia. Clinical trials are ongoing to address stem cell transplantation in adults and gene therapies.
Other anemias
There are many other conditions that can, for various reasons, result in some level of anemia, such as:
- Bleeding—significant bleeding resulting from, for example, trauma or surgery (acute) or from gastrointestinal bleeding (ulcers) occurring over time (chronic)
- Leukemia (acute or chronic)
- Lymphoma
- Myelodysplastic syndrome
- Multiple myeloma
- Myeloproliferative neoplasms (e.g., myelofibrosis)
- Infections (e.g., HIV)
- Thalassemia
- Malarial anemia
Thalassemia
Thalassemia is an inherited blood disorder, which means that thalassemia is passed to children by parents who carry the mutated thalassemia gene. If you have thalassemia, your body may not make enough hemoglobin, which can lead to fewer healthy red blood cells. This can lead to a condition called anemia.
There are two main types of thalassemia: alpha thalassemia and beta thalassemia, depending on which part of an oxygen-carrying protein in the red blood cells is lacking. Alpha thalassemia is caused by reduced or absent production of alpha-globin subunits, while beta thalassemia is caused by reduced or absent production of beta-globin subunits. Each of these types can be mild, moderate, or serious, depending on how much hemoglobin your body makes.
Alpha thalassemia minor and beta thalassemia minor, also known as alpha thalassemia trait or beta thalassemia trait, are common conditions in many demographics.
The more severe form of thalassemia is thalassemia major also called Cooley’s anemia 19. It is a serious disease that requires regular blood transfusions and extensive medical care.
Those with thalassemia major or Cooley’s anemia usually show symptoms within the first two years of life. They become pale and listless and have poor appetites. They grow slowly and often develop jaundice. Without treatment, the spleen, liver and heart soon become greatly enlarged. Bones become thin and brittle. Heart failure and infection are the leading causes of death among children with untreated thalassemia major (Cooley’s anemia).
The use of frequent blood transfusions and antibiotics has improved the outlook for children with thalassemia major (Cooley’s anemia). Frequent transfusions keep their hemoglobin levels near normal and prevent many of the complications of the disease. But repeated blood transfusions lead to iron overload – a buildup of iron in the body – that can damage the heart, liver and other organs. Drugs known as “iron chelators” can help rid the body of excess iron, preventing or delaying problems related to iron overload.
Thalassemia has been cured using bone marrow transplants. However, this treatment is possible only for a small minority of patients who have a suitable bone marrow donor. The transplant procedure itself is still risky and can result in death.
Scientists are working to develop a gene therapy that may offer a cure for thalassemia. Such a treatment might involve inserting a normal beta globin gene (the gene that is abnormal in this disease) into the patient’s stem cells, the immature bone marrow cells that are the precursors of all other cells in the blood.
Another form of gene therapy could involve using drugs or other methods to reactivate the patient’s genes that produce fetal hemoglobin – the form of hemoglobin found in fetuses and newborns. Scientists hope that spurring production of fetal hemoglobin will compensate for the patient’s deficiency of adult hemoglobin.
Thalassemia symptoms
The symptoms of thalassemia are caused by anemia. Anemia is a condition that develops when your blood produces a lower-than-normal amount of healthy red blood cells. Anemia often develops in people with thalassemia because the body cannot make enough hemoglobin. Without enough hemoglobin, red blood cells in your blood stream cannot work properly to effectively deliver oxygen to cells throughout your body.
Symptoms of anemia include:
- Feeling tired or weak
- Shortness of breath
- Paleness
- Dizziness and fainting
- Headaches
Depending on the type of thalassemia you have, you may experience mild anemia or no symptoms at all.
Symptoms in young children
Children who have more serious types of thalassemia often have symptoms by the time they are 2 years old. These can include:
- Pale skin or yellowing of the skin and eyes (jaundice)
- A large abdomen from a spleen or liver that is larger than normal
- Changes or problems with bones in the face
- Dark urine
- Poor appetite
- Intellectual or developmental disabilities
More serious forms of thalassemia are often diagnosed during the newborn screening.
Thalassemia causes
Thalassemia is an inherited blood disorder that causes your body to produce less hemoglobin than normal. Hemoglobin is a protein in red blood cells that helps them carry oxygen to all parts of the body. Hemoglobin is made of two kinds of protein chains called alpha globin and beta globin. Thalassemia develops when faulty genes prevent your body from making the right amount of alpha globin or beta globin chains. When this happens, red blood cells cannot carry enough oxygen to your body’s organs and tissues.
There are two main types of thalassemia, alpha thalassemia and beta thalassemia. Beta thalassemia is caused by changes (mutations) in the HBB gene while alpha thalassemia is caused by mutations in the HBA1 and/or HBA2 genes are located on the short arm (p) of chromosome 16 (16p13.3). Both types of thalassemia are inherited in an autosomal recessive manner.
A child who inherits one mutated gene from one parent but normal genes from the other is called a “carrier”, which is sometimes also called “thalassemia trait.” Most carriers often have no signs of illness and they lead completely normal, healthy lives or they may experience mild anemia. However, carriers can pass the faulty genes on to their children. If you inherit faulty genes from both parents, your disease may be moderate to serious.
A child who inherits two thalassemia trait genes – one from each parent – will have the disease. A child of two carriers has a 25 percent chance of receiving two trait genes and developing the disease, and a 50 percent chance of being a thalassemia trait carrier.
Most individuals with alpha thalassemia have milder forms of the disease, with varying degrees of anemia. The most severe form of alpha thalassemia, which affects mainly individuals of Southeast Asian, Chinese and Filipino ancestry, results in fetal or newborn death.
A child who inherits two copies of the mutated gene for beta thalassemia will have beta thalassemia disease. The child can have a mild form of the disease, known as thalassemia intermedia, which causes milder anemia that rarely requires transfusions.
Alpha thalassemia
You need four genes (two from each parent) to make enough alpha globin protein chains. If one or more of the genes is missing, you will have alpha thalassemia, which means your body does not make enough alpha globin protein.
- If you’re only missing one gene, you’re a “silent” carrier. This means you won’t have any signs of illness.
- If you’re missing two genes, you have alpha thalassemia trait (also called alpha thalassemia minor). This means you may have mild symptoms of anemia.
- If you’re missing three genes, you likely have hemoglobin H disease (which a blood test can detect). This type of thalassemia causes moderate to severe anemia.
- Very rarely, a baby is missing all four genes. This condition is called alpha thalassemia major or hydrops fetalis. Babies who have hydrops fetalis usually die before or shortly after birth.
Beta thalassemia
You need two genes (one from each parent) to make enough beta globin protein chains. If one or both of these genes are altered, you’ll have beta thalassemia. This means that your body won’t make enough beta globin protein.
- If you have one altered gene, you’re a carrier. This condition is called beta thalassemia trait or beta thalassemia minor. It causes mild anemia symptoms.
- If both genes are altered, you’ll have beta thalassemia intermedia or beta thalassemia major (also called Cooley’s anemia). The intermedia form of the disorder causes moderate anemia. The major form causes serious anemia symptoms.
Thalassemia prevention
Since thalassemia is caused by changes (mutations) in genes, there is no way to prevent it. People who do not know whether they carry a faulty gene that can cause thalassemia can ask their healthcare provider for a blood test.
Couples who are planning to have children and know that they are at risk of having a child with thalassemia may want to meet with a genetic counselor. A genetic counselor can answer questions about the risk and explain the choices that are available.
If you are pregnant and you or your partner has a family history of thalassemia, your provider may also recommend prenatal testing. Prenatal testing is done using a sample of amniotic fluid, the liquid in the sac surrounding a growing embryo, or of tissue taken from the placenta, the organ that attaches the umbilical cord to the mother’s womb. Testing before birth is safe and can be done as early as 8 to 10 weeks into the pregnancy.
Thalassemia diagnosis
Doctors diagnose thalassemias using blood tests.
Your doctor may order the following tests to determine whether you or your child have thalassemia:
- Complete blood count (CBC) measures the amount of hemoglobin and different types of blood cells (such as red blood cells) in your blood. People who have thalassemia have fewer healthy red blood cells and less hemoglobin than normal. Depending on the type of thalassemia, your red blood cells may look smaller and show signs of disease under a microscope.
- Special hemoglobin tests measure the types of hemoglobin in a sample of blood. This test can help to distinguish between different medical conditions caused by problems with hemoglobin.
- Genetic testing can help determine what specific type of thalassemia you have.
Thalassemia treatment
Treatments for thalassemia depend on the type and how serious it is. If you are a carrier or have alpha or beta thalassemia trait, you likely have mild or no symptoms and may not need treatment.
If you have a more serious thalassemia type like hemoglobin H disease, beta thalassemia intermedia, or beta thalassemia major you may experience moderate to serious anemia symptoms. You may need treatments such as blood transfusions, medicine, a splenectomy, or a blood and bone marrow transplant.
- Medicines called luspatercept (Reblozyl) and hydroxyurea may be prescribed by your doctor to treat thalassemia. Luspatercept can lessen the number of blood transfusions needed for people with moderate to severe anemia as a result of thalassemia. Hydroxyurea is usually used to treat sickle cell disease and can help lower the risk of health problems from thalassemia.
- Splenectomy is surgery to remove the spleen. Your doctor may recommend splenectomy to improve your symptoms if you have mild to moderate thalassemia. However, removing the spleen lowers the body’s ability to fight infections.
Blood transfusions
Blood transfusions are the main way to treat moderate or severe thalassemia. This treatment gives you red blood cells with healthy hemoglobin.
During a blood transfusion, a needle is used to insert an intravenous (IV) line into one of your blood vessels. You receive healthy blood through this line. The procedure usually takes 1 to 4 hours. How often blood transfusions are needed depends on how serious your condition and symptoms are.
- Occasional blood transfusions may be needed for people who have hemoglobin H disease or beta thalassemia intermedia. Specifically, a transfusion may be needed when your body is under stress, such as during an infection, pregnancy, or surgery.
- Regular blood transfusions (every 3 to 4 weeks) may be needed for people who have beta thalassemia major. These transfusions help maintain healthy hemoglobin and red blood cell levels.
Iron chelation therapy
The hemoglobin in red blood cells is an iron-rich protein. Regular blood transfusions can cause iron buildup, or iron overload, which can lead to potentially life-threatening complications.
To prevent this, doctors use iron chelation therapy in people who receive regular blood transfusions to remove excess iron from the body. Three medicines are used for iron chelation therapy:
- Deferasirox is a pill taken once daily. Side effects can include skin rash, nausea, and diarrhea.
- Deferiprone is a pill that may be used if other treatments do not work. It can lower your white blood cell numbers, which can put you at risk for infections.
- Deferoxamine is a liquid medicine that is given slowly under the skin, usually with a small portable pump used overnight. This therapy takes time and can be mildly painful. Side effects can include problems with vision and hearing.
Talk to your doctor if you’re pregnant or thinking about becoming pregnant. You may need to switch to a different iron chelation therapy medicine.
Blood and bone marrow transplant
A blood or bone marrow transplant, also called a hematopoietic stem cells transplant, replaces blood-forming stem cells that aren’t working properly with healthy donor cells. A stem cell transplant is the only treatment that can cure thalassemia. However, only a small number of people who have severe thalassemia are able to find a good donor match and are a good fit for the procedure.
Complications of anemia
Left untreated, anemia can cause many health problems, such as:
- Severe fatigue. When anemia is severe enough, you may be so tired that you can’t complete everyday tasks.
- Pregnancy complications. Pregnant women with folate deficiency anemia may be more likely to experience complications, such as premature birth.
- Heart problems. Anemia can lead to a rapid or irregular heartbeat (arrhythmia). When you’re anemic your heart must pump more blood to compensate for the lack of oxygen in the blood. This can lead to an enlarged heart or heart failure.
- Death. Some inherited anemias, such as sickle cell anemia, can be serious and lead to life-threatening complications. Losing a lot of blood quickly results in acute, severe anemia and can be fatal.
Anemia causes
Anemia occurs when your blood doesn’t have enough red blood cells. This can happen if:
- Your body doesn’t make enough healthy red blood cells in your bone marrow – this can be due to an inherited disease, a lack of iron or vitamins in the diet, or bone marrow disease
- Bleeding causes you to lose red blood cells more quickly than they can be replaced, whether that be heavy periods, a fast bleed or one so slow you didn’t even notice it
- You have a disease that destroys red blood cells.
Risk factors for anemia
These factors place you at increased risk of anemia:
- A diet lacking in certain vitamins. Having a diet that is consistently low in iron, vitamin B-12 and folate increases your risk of anemia.
- Intestinal disorders. Having an intestinal disorder that affects the absorption of nutrients in your small intestine — such as Crohn’s disease and celiac disease — puts you at risk of anemia.
- Menstruation. In general, women who haven’t experienced menopause have a greater risk of iron deficiency anemia than do men and postmenopausal women. That’s because menstruation causes the loss of red blood cells.
- Pregnancy. If you’re pregnant and aren’t taking a multivitamin with folic acid, you’re at an increased risk of anemia.
- Chronic conditions. If you have cancer, kidney failure or another chronic condition, you may be at risk of anemia of chronic disease. These conditions can lead to a shortage of red blood cells. Slow, chronic blood loss from an ulcer or other source within your body can deplete your body’s store of iron, leading to iron deficiency anemia.
- Family history. If your family has a history of an inherited anemia, such as sickle cell anemia, you also may be at increased risk of the condition.
- Other factors. A history of certain infections, blood diseases and autoimmune disorders, alcoholism, exposure to toxic chemicals, and the use of some medications can affect red blood cell production and lead to anemia.
- Age. People over age 65 are at increased risk of anemia.
Prevention of anemia
Many types of anemia can be mild, short term, or even prevented. Other types may last a lifetime but are easily treated. Still other anemias are severe, life-threatening conditions that need prompt and intense treatment.
You can take action to prevent, treat, and control anemia. These actions can give you greater energy, improve your quality of life, and help you live a long time.
You can reduce your chances of getting anemia by:
- Having a healthy diet. Eating healthy means following a healthy eating pattern that includes a variety of nutritious foods and drinks. It also means getting the number of calories that’s
right for you (not eating too much or too little). To eat healthy, try to follow these tips:- Choose nutrient-dense foods and beverages.
- Eat vegetables, fruits, whole grains, fat-free or low-fat dairy products, and a variety of foods with protein, such as seafood, lean meats and poultry, eggs, beans, peas, nuts, seeds, and
soy products. - Limit certain nutrients and ingredients, such as salt, added sugars, saturated fats, and refined grains.
- Stay at a healthy weight by balancing the calories you eat and drink with the calories you burn.
- Clean, handle, cook, and chill food properly to prevent foodborne illnesses.
- Avoiding substances that can cause or trigger anemia. For example, exposure to chemicals or toxins in the environment can cause some types of anemia. Other types of anemia are triggered by certain foods or cold temperatures. If you have one of these types of anemia, avoid these triggers if you can. If you have hemolytic anemia, reduce your chances of getting an infection by washing your hands often, avoiding people who have colds, and staying away from crowds.
- Seeing your doctor if you are feeling unusually tired.
- Visit your doctor regularly for checkups and ongoing care, and tell him or her about any new or changing symptoms.
- If you are diagnosed with anemia, follow your doctor’s advice about diet, supplements, medicines, and other treatment methods.
- Older children and teens who have severe anemia may have an increased risk of injury or infection. Talk with your doctor about ways to keep them as healthy as possible and discuss whether they need to avoid certain activities.
- Girls and women who have heavy menstrual periods may need regular screenings and follow-up care with their doctors to prevent or control iron-deficiency anemia.
Eat a vitamin-rich diet
Many types of anemia can’t be prevented. But iron deficiency anemia and vitamin deficiency anemias can be avoided by having a diet that includes a variety of vitamins and nutrients, including:
- Iron. Iron-rich foods include beef and other meats, beans, lentils, iron-fortified cereals, dark green leafy vegetables, and dried fruit.
- Folate. This nutrient, and its synthetic form folic acid, can be found in fruits and fruit juices, dark green leafy vegetables, green peas, kidney beans, peanuts, and enriched grain products, such as bread, cereal, pasta and rice.
- Vitamin B-12. Foods rich in vitamin B-12 include meat, dairy products, and fortified cereal and soy products.
- Vitamin C. Foods rich in vitamin C include citrus fruits and juices, peppers, broccoli, tomatoes, melons and strawberries. These items help increase iron absorption.
Consider a multivitamin
If you’re concerned about getting enough vitamins from the food you eat, ask your doctor whether a multivitamin may be right for you.
Consider genetic counseling
If you have a family history of an inherited anemia, such as sickle cell anemia or thalassemia, talk to your doctor and possibly a genetic counselor about your risk and what risks you may pass on to your children.
Prevent malaria
Anemia can be a complication of malaria. If you plan on traveling to a place where malaria is common, talk with your doctor beforehand about taking preventive drugs. In areas where malaria is common, prevention involves reducing exposure to mosquitoes, for example, by using bed nets treated with insecticide.
Anemia signs and symptoms
If you have anemia, you will feel tired and short of breath, even when doing things you could usually do easily.
You may also have a fast or irregular heartbeat, look pale, have cold feet or hands, feel dizzy or have problems thinking.
It’s important to see your doctor if you have any of these symptoms.
Signs and symptoms of anemia
- Fatigue
- Weakness
- Pale or yellowish skin
- Irregular heartbeats
- Shortness of breath
- Dizziness or lightheadedness
- Chest pain
- Cold hands and feet
- Headache
At first anemia can be so mild that it goes unnoticed. But symptoms worsen as anemia worsens.
Anemia diagnosis
If you have anemia, your doctor will talk to you and examine you to work out how severe the anaemia is, and what the cause could be. You might be asked to have more tests, depending on what your doctor has learned from talking to you and examining you.
To diagnose anemia, your doctor may ask you about your medical and family history, perform a physical exam, and run the following tests
Anemia tests
Several routine laboratory tests may be used to help diagnose anemia as well as help to determine the underlying cause. These are listed below. Depending on the results of these, the medical history of the person, and signs and symptoms, other tests may be done as follow up to help diagnose the cause of anemia and to help guide treatment. (Click on the links for the different types of anemia at the top of this page to read about these specific tests.)
Complete Blood Count (CBC)
Anemia may first be detected when a routine test that counts the number and relative proportion of each of the different types of cells in the blood stream, called a complete blood count (CBC), is done during a health exam or as part of testing for other conditions. A CBC is often ordered as part of a yearly physical exam and helps to evaluate overall health and to screen for a wide variety of disorders.
A complete blood count (CBC) is used to count the number of blood cells in a sample of your blood. For anemia your doctor will be interested in the levels of the red blood cells contained in the blood (hematocrit) and the hemoglobin in your blood.
- Normal adult hematocrit values vary from one medical practice to another but are generally between 40 and 52 percent for men and 35 and 47 percent for women. Normal adult hemoglobin values are generally 14 to 18 grams per deciliter for men and 12 to 16 grams per deciliter for women.
- A test to determine the size and shape of your red blood cells. Some of your red blood cells may also be examined for unusual size, shape and color.
With anemia, some of the components of the CBC that may show abnormal results include:
- RBC count—typically low
- Hemoglobin—low
- Hematocrit—low
- RBC indices—these include mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). They give a healthcare practitioner information about the size of the red blood cells and the amount and concentration of hemoglobin in RBCs present in someone’s blood at that moment. For example, the size and hemoglobin concentration of RBCs can help with diagnosing anemia because those characteristics can vary for different kinds of anemia.
Blood Smear and Differential
If results of the CBC indicate anemia, it may be followed up with an examination of a blood smear or a differential, which counts white blood cells. The smear review can provide additional information, such as the shape of red blood cells and the presence of abnormal cells, which can help diagnose and classify anemia.
Reticulocyte Count
This test provides information on the number of relatively immature red blood cells in a person’s blood sample. When someone has anemia (low RBC count, hemoglobin, and hematocrit), the results of this test can help determine the cause and/or help classify the type of anemia. For example, for a person with anemia, an inappropriately low reticulocyte count often indicates decrease in red blood cell production in the bone marrow.
Results from these tests may give clues as to the cause. Several other tests may be run to help determine the cause of the anemia and to guide treatment. See the individual discussions of the different types of anemia for more on these.
Additional diagnostic tests
If you receive a diagnosis of anemia, your doctor may order additional tests to determine the underlying cause. For example, iron deficiency anemia can result from chronic bleeding of ulcers, benign polyps in the colon, colon cancer, tumors or kidney problems.
Occasionally, it may be necessary to study a sample of your bone marrow to diagnose anemia.
Anemia treatment
The treatment for anemia depends on what causes it and how serious it is. But there are usually two parts:
- treating the anemia itself
- treating the cause of the anemia.
People who have mild anemia may not need treatment. If your anemia is caused by medicines or another health condition, your doctor may change your treatment to manage or stop your anemia.
How to treat anemia
- Iron deficiency anemia. Treatment for iron-deficiency anemia usually involves taking iron supplements and making changes to your diet. If the underlying cause of iron deficiency is loss of blood — other than from menstruation — the source of the bleeding must be located and stopped. This may involve surgery.
- Vitamin deficiency anemias. Treatment for folic acid and vitamin B-12 deficiency involves dietary supplements and increasing these nutrients in your diet. If your digestive system has trouble absorbing vitamin B-12 from the food you eat, you may need vitamin B-12 shots. At first, you may receive the shots every other day. Eventually, you’ll need shots just once a month, which may continue for life, depending on your situation.
- Anemia of chronic disease. There’s no specific treatment for this type of anemia. Doctors focus on treating the underlying disease. If symptoms become severe, a blood transfusion or injections of synthetic erythropoietin, a hormone normally produced by your kidneys, may help stimulate red blood cell production and ease fatigue.
- Aplastic anemia. Treatment for aplastic anemia may include blood transfusions to boost levels of red blood cells. You may need a bone marrow transplant if your bone marrow is diseased and can’t make healthy blood cells.
- Anemias associated with bone marrow disease. Treatment of these various diseases can include medication, chemotherapy or bone marrow transplantation.
- Hemolytic anemias. Managing hemolytic anemias includes avoiding suspect medications, treating related infections and taking drugs that suppress your immune system, which may be attacking your red blood cells. Depending on the severity of your anemia, a blood transfusion or plasmapheresis may be necessary. Plasmapheresis is a type of blood-filtering procedure. In certain cases, removal of the spleen can be helpful.
- Sickle cell anemia. Treatment for sickle cell anemia may include the administration of oxygen, pain-relieving drugs, and oral and intravenous fluids to reduce pain and prevent complications. Doctors also may recommend blood transfusions, folic acid supplements and antibiotics. A bone marrow transplant may be an effective treatment in some circumstances. A cancer drug called hydroxyurea (Droxia, Hydrea) also is used to treat sickle cell anemia.
- Thalassemia. This anemia may be treated with blood transfusions, folic acid supplements, medication, removal of the spleen (splenectomy), or a blood and bone marrow stem cell transplant.
Anemia diet
Iron
Iron is a mineral that our bodies need for many functions. In the human body, iron is present in all cells and has several vital functions — as a carrier of oxygen to the tissues from the lungs in the form of hemoglobin (Hb), as a facilitator of oxygen use and storage in the muscles as myoglobin, as a transport medium for electrons within the cells in the form of cytochromes, and as an integral part of enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and mortality 20, 21.
Dietary iron has two main forms: heme and nonheme 22. Plants and iron-fortified foods contain nonheme iron only, whereas meat, seafood, and poultry contain both heme and nonheme iron 23. Heme iron, which is formed when iron combines with protoporphyrin IX, contributes about 10% to 15% of total iron intakes in western populations 24.
In adults, the recommended dietary allowance of iron is 8 to 11 mg per day for men and 8 to 18 mg for women in whom higher levels are recommended during pregnancy (27 mg per day) 25. Iron is poorly absorbed and body and tissue iron stores are controlled largely by modifying rates of absorption. Adequate amounts of iron are found in most Western diets, with highest levels found in red meats and moderate levels in fish, poultry, green vegetables, cereals and grains (some of which are fortified with iron).
How much iron do you need?
The amount of iron you need each day depends on your age, your sex, and whether you consume a mostly plant-based diet. Average daily recommended amounts are listed below in milligrams (mg). Vegetarians who do not eat meat, poultry, or seafood need almost twice as much iron as listed in the table because the body doesn’t absorb nonheme iron in plant foods as well as heme iron in animal foods.
Table 1. Iron recommended intake
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 0.27 mg |
| Infants 7–12 months | 11 mg |
| Children 1–3 years | 7 mg |
| Children 4–8 years | 10 mg |
| Children 9–13 years | 8 mg |
| Teens boys 14–18 years | 11 mg |
| Teens girls 14–18 years | 15 mg |
| Adult men 19–50 years | 8 mg |
| Adult women 19–50 years | 18 mg |
| Adults 51 years and older | 8 mg |
| Pregnant teens | 27 mg |
| Pregnant women | 27 mg |
| Breastfeeding teens | 10 mg |
| Breastfeeding women | 9 mg |
What foods provide iron?
Iron is found naturally in many foods and is added to some fortified food products. You can get recommended amounts of iron by eating a variety of foods, including the following:
- Lean meat, seafood, and poultry.
- Iron-fortified breakfast cereals and breads.
- White beans, lentils, spinach, kidney beans, and peas.
- Nuts and some dried fruits, such as raisins.
Iron in food comes in two forms: heme iron and nonheme iron. Nonheme iron is found in plant foods and iron-fortified food products. Meat, seafood, and poultry have both heme and nonheme iron.
Heme iron has higher bioavailability than nonheme iron, and other dietary components have less effect on the bioavailability of heme than nonheme iron 24. The bioavailability of iron is approximately 14% to 18% from mixed diets that include substantial amounts of meat, seafood, and vitamin C (ascorbic acid, which enhances the bioavailability of nonheme iron) and 5% to 12% from vegetarian diets 23. In addition to ascorbic acid, meat, poultry, and seafood can enhance nonheme iron absorption, whereas phytate (present in grains and beans) and certain polyphenols in some non-animal foods (such as cereals and legumes) have the opposite effect 26. Unlike other inhibitors of iron absorption, calcium might reduce the bioavailability of both nonheme and heme iron. However, the effects of enhancers and inhibitors of iron absorption are attenuated by a typical mixed western diet, so they have little effect on most people’s iron status.
Several food sources of iron are listed in Table 2. Some plant-based foods that are good sources of iron, such as spinach, have low iron bioavailability because they contain iron-absorption inhibitors, such as polyphenols 27.
Your body absorbs iron from plant sources better when you eat it with meat, poultry, seafood, and foods that contain vitamin C, like citrus fruits, strawberries, sweet peppers, tomatoes, and broccoli.
Table 2: Selected Food Sources of Iron
| Food | Milligrams per serving | Percent DV* |
|---|---|---|
| Breakfast cereals, fortified with 100% of the DV for iron, 1 serving | 18 | 100 |
| Oysters, eastern, cooked with moist heat, 3 ounces | 8 | 44 |
| White beans, canned, 1 cup | 8 | 44 |
| Chocolate, dark, 45%–69% cacao solids, 3 ounces | 7 | 39 |
| Beef liver, pan fried, 3 ounces | 5 | 28 |
| Lentils, boiled and drained, ½ cup | 3 | 17 |
| Spinach, boiled and drained, ½ cup | 3 | 17 |
| Tofu, firm, ½ cup | 3 | 17 |
| Kidney beans, canned, ½ cup | 2 | 11 |
| Sardines, Atlantic, canned in oil, drained solids with bone, 3 ounces | 2 | 11 |
| Chickpeas, boiled and drained, ½ cup | 2 | 11 |
| Tomatoes, canned, stewed, ½ cup | 2 | 11 |
| Beef, braised bottom round, trimmed to 1/8” fat, 3 ounces | 2 | 11 |
| Potato, baked, flesh and skin, 1 medium potato | 2 | 11 |
| Cashew nuts, oil roasted, 1 ounce (18 nuts) | 2 | 11 |
| Green peas, boiled, ½ cup | 1 | 6 |
| Chicken, roasted, meat and skin, 3 ounces | 1 | 6 |
| Rice, white, long grain, enriched, parboiled, drained, ½ cup | 1 | 6 |
| Bread, whole wheat, 1 slice | 1 | 6 |
| Bread, white, 1 slice | 1 | 6 |
| Raisins, seedless, ¼ cup | 1 | 6 |
| Spaghetti, whole wheat, cooked, 1 cup | 1 | 6 |
| Tuna, light, canned in water, 3 ounces | 1 | 6 |
| Turkey, roasted, breast meat and skin, 3 ounces | 1 | 6 |
| Nuts, pistachio, dry roasted, 1 ounce (49 nuts) | 1 | 6 |
| Broccoli, boiled and drained, ½ cup | 1 | 6 |
| Egg, hard boiled, 1 large | 1 | 6 |
| Rice, brown, long or medium grain, cooked, 1 cup | 1 | 6 |
| Cheese, cheddar, 1.5 ounces | 0 | 0 |
| Cantaloupe, diced, ½ cup | 0 | 0 |
| Mushrooms, white, sliced and stir-fried, ½ cup | 0 | 0 |
| Cheese, cottage, 2% milk fat, ½ cup | 0 | 0 |
| Milk, 1 cup | 0 | 0 |
Footnotes: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for iron is 18 mg for adults and children age 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 28 ]Folate
Folate is also known vitamin B9 (Folacin, Folic Acid, Pteroylglutamic acid) that is naturally present in many foods. Folic Acid is a form of folate that is manufactured and used in dietary supplements and fortified foods 29. Your body need folate to make DNA and other genetic material. Folate is also needed for the body’s cells to divide.
In women and pregnant mothers, folic acid is very important because it can help prevent some major birth defects of the baby’s brain and spine (anencephaly and spina bifida) 30.
Every woman needs folic acid every day, whether she’s planning to get pregnant or not, for the healthy new cells the body makes daily. Think about the skin, hair, and nails. These – and other parts of the body – make new cells each day.
Centers for Disease Control and Prevention urges women to take 400 mcg of folic acid every day, starting at least one month before getting pregnant and while she is pregnant, to help prevent major birth defects of the baby’s brain and spine.
Folic acid and folate also help your body make healthy new red blood cells. Red blood cells carry oxygen to all the parts of your body. If your body does not make enough red blood cells, you can develop anemia. Anemia happens when your blood cannot carry enough oxygen to your body, which makes you pale, tired, or weak. Also, if you do not get enough folic acid, you could develop a type of anemia called folate-deficiency anemia 31.
Folate functions as a coenzyme or cosubstrate in single-carbon transfers in the synthesis of nucleic acids (DNA and RNA) and metabolism of amino acids 32. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of S-adenosyl-methionine, an important methyl donor 32. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 33.
When consumed, food folates are hydrolyzed to the monoglutamate form in the gut prior to absorption by active transport across the intestinal mucosa 34. Passive diffusion also occurs when pharmacological doses of folic acid are consumed 34. Before entering the bloodstream, the monoglutamate form is reduced to tetrahydrofolate (THF) and converted to either methyl or formyl forms. The main form of folate in plasma is 5-methyl-THF. Folic acid can also be found in the blood unaltered (known as unmetabolized folic acid), but whether this form has any biological activity or can be used as a biomarker of status is not known 35.
The total body content of folate is estimated to be 10 to 30 mg; about half of this amount is stored in the liver and the remainder in blood and body tissues. A serum folate concentration is commonly used to assess folate status, with a value above 3 nanograms (ng)/mL indicating adequacy 33. This indicator, however, is sensitive to recent dietary intake, so it might not reflect long-term status. Erythrocyte folate concentration provides a longer-term measure of folate intakes, so when day-to-day folate intakes are variable—such as in people who are ill and whose folate intake has recently declined—it might be a better indicator of tissue folate stores than serum folate concentration 34. An erythrocyte folate concentration above 140 ng/mL indicates adequate folate status 34, although some researchers have suggested that higher values are optimal for preventing neural tube defects 36.
Why do women need folic acid?
Everyone needs folic acid to be healthy. But it is especially important for women:
- Before and during pregnancy. Folic acid protects unborn children against serious birth defects called neural tube defects. These birth defects happen in the first few weeks of pregnancy, often before a woman knows she is pregnant. Folic acid might also help prevent other types of birth defects and early pregnancy loss (miscarriage). Since about half of all pregnancies in the United States are unplanned 37, experts recommend all women get enough folic acid even if you are not trying to get pregnant.
- To keep the blood healthy by helping red blood cells form and grow. Not getting enough folic acid can lead to a type of anemia called folate-deficiency anemia. Folate-deficiency anemia is more common in women of childbearing age than in men.
Do Women need to take Folic Acid every day even if you’re not planning to get pregnant?
Yes. All women who can get pregnant need to take 400 to 800 micrograms of folic acid every day, even if you’re not planning to get pregnant 38. There are several reasons why:
- Your birth control may not work or you may not use birth control correctly every time you have sex. In a survey by the Centers for Disease Control and Prevention, almost 40% of women with unplanned pregnancies were using birth control 39.
- Birth defects of the brain and spine can happen in the first few weeks of pregnancy, often before you know you are pregnant. By the time you find out you are pregnant, it might be too late to prevent the birth defects.
- You need to take folic acid every day because it is a water soluble B-vitamin. Water soluble means that it does not stay in the body for a long time. Your body metabolizes (uses) folic acid quickly, so your body needs folic acid each day to work properly.
What can happen if Women do Not get enough Folic Acid during Pregnancy?
If you do not get enough folic acid before and during pregnancy, your baby is at higher risk for neural tube defects.
Neural tube defects are serious birth defects that affect the spine, spinal cord, or brain and may cause death. These include:
- Spina bifida 40. This condition happens when an unborn baby’s spinal column does not fully close during development in the womb, leaving the spinal cord exposed. As a result, the nerves that control the legs and other organs do not work. Children with spina bifida often have lifelong disabilities. They may also need many surgeries.
- Anencephaly 41. This means that most or all of the brain and skull does not develop in the womb. Almost all babies with this condition die before or soon after birth.
How much Folic Acid do Women need?
All women need 400 micrograms of folic acid every day. Women who can get pregnant should get 400 to 800 micrograms of folic acid from a vitamin or from food that has added folic acid, such as breakfast cereal 42. This is in addition to the folate you get naturally from food.
Some women may need more folic acid each day. See the chart to find out how much folic acid you need.
| If you: | Amount of folic acid you may need daily 42 |
|---|---|
| Could get pregnant or are pregnant | 400–800 micrograms 42 Your doctor may prescribe a prenatal vitamin with more. |
| Had a baby with a neural tube defect (such as spina bifida) and want to get pregnant again | 4,000 micrograms. Your doctor may prescribe this amount. Research shows taking this amount may lower the risk of having another baby with spina bifida 43 |
| Have a family member with spina bifida and could get pregnant | 4,000 micrograms. Your doctor may prescribe this amount. |
| Have spina bifida and want to get pregnant | 4,000 micrograms. Your doctor may prescribe this amount. Women with spina bifida have a higher risk of having children with the condition. |
| Take medicines to treat epilepsy, type 2 diabetes, rheumatoid arthritis, or lupus | Talk to your doctor or nurse. Folic acid supplements can interact with these medicines. |
| Are on dialysis for kidney disease | Talk to your doctor or nurse. |
| Have a health condition, such as inflammatory bowel disease or celiac disease, that affects how your body absorbs folic acid | Talk to your doctor or nurse. |
How can you be sure you get enough folic acid?
You can get enough folic acid from food alone. Many breakfast cereals have 100% of your recommended daily value (400 micrograms) of folic acid.
If you are at risk for not getting enough folic acid, your doctor may recommend that you take a vitamin with folic acid every day. Most U.S. multivitamins have at least 400 micrograms of folic acid. Check the label on the bottle to be sure. You can also take a pill that contains only folic acid.
If swallowing pills is hard for you, try a chewable or liquid product with folic acid.
Are some women at risk for not getting enough folic acid?
Yes, certain groups of women do not get enough folic acid each day 44.
- Women who can get pregnant need more folic acid (400 to 800 micrograms). 43
- Nearly one in three African-American women does not get enough folic acid each day.
- Spanish-speaking Mexican-American women often do not get enough folic acid. However, Mexican-Americans who speak English usually get enough folic acid. 45
Not getting enough folic acid can cause health problems, including folate-deficiency anemia, and problems during pregnancy for you and your unborn baby.
How a Woman can get enough Folic Acid
There are two easy ways to be sure to get enough folic acid each day:
- Take a vitamin that has folic acid in it every day. Most multivitamins sold in the United States have the amount of folic acid women need each day. Women can also choose to take a small pill (supplement) that has only folic acid in it each day.
- Multivitamins and folic acid pills can be found at most local pharmacy, grocery, or discount stores.
- Check the label to be sure it contains 100% of the daily value (DV) of folic acid, which is 400 micrograms (mcg).
- Eat a bowl of breakfast cereal that has 100% of the daily value of folic acid every day.
Not every cereal has this amount. Make sure you check the label on the side of the box, and look for one that has “100%” next to folic acid or 400 micrograms (mcg).
How much folate do you need?
The amount of folate you need depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) of dietary folate equivalents (DFEs).
All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get naturally from foods.
Table 3: Recommended Dietary Allowances (RDAs) for Folate
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 65 mcg DFE* |
| Infants 7–12 months* | 80 mcg DFE* |
| Children 1–3 years | 150 mcg DFE |
| Children 4–8 years | 200 mcg DFE |
| Children 9–13 years | 300 mcg DFE |
| Teens 14–18 years | 400 mcg DFE |
| Adults 19–50 years | 400 mcg DFE |
| Adults 51–70 years | 400 mcg DFE |
| Adults 71+ years | 400 mcg DFE |
| Pregnant teens and women | 600 mcg DFE |
| Breastfeeding teens and women | 500 mcg DFE |
Footnote: * Adequate Intake (AI)
[Source 34 ]Do you need folic acid after menopause?
Yes. Women who have gone through menopause still need 400 micrograms of folic acid every day for good health. Talk to your doctor or nurse about how much folic acid you need.
What foods provide folate?
Folate is naturally present in many foods and food companies add folic acid to other foods, including bread, cereal, and pasta. You can get recommended amounts by eating a variety of foods, including the following:
- Leafy Green Vegetables (especially asparagus, Brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens).
- Fruits and fruit juices (especially oranges and orange juice).
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans).
- Grains (including whole grains; fortified cold cereals; enriched flour products such as bread, bagels, cornmeal, and pasta; and rice).
- Folic acid is added to many grain-based products, enriched breads, cereals and corn masa flour (used to make corn tortillas and tamales, for example). To find out whether folic acid has been added to a food, check the product label.
Beef liver is high in folate but is also high in cholesterol, so limit the amount you eat. Only small amounts of folate are found in other animal foods like meats, poultry, seafood, eggs, and dairy products.
In January 1998, the U.S. Food and Drug Administration (FDA) began requiring manufacturers to add folic acid to enriched breads, cereals, flours, cornmeals, pastas, rice, and other grain products 46. Because cereals and grains are widely consumed in the United States, these products have become very important contributors of folic acid to the American diet. The fortification program was projected to increase folic acid intakes by approximately 100 mcg/day 47, but the program actually increased mean folic acid intakes in the United States by about 190 mcg/day 48. In April 2016, FDA approved the voluntary addition of folic acid to corn masa flour at levels consistent with other enriched grain products 49.
The Canadian government has also required the addition of folic acid to many grains, including white flour, enriched pasta, and cornmeal, since November 1, 1998 50. Other countries, including Costa Rica, Chile, and South Africa, have also established mandatory folic acid fortification programs 51.
Table 4. Selected Food Sources of Folate and Folic Acid
| Food | mcg DFE per serving | Percent DV* |
|---|---|---|
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 23 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Spaghetti, cooked, enriched, ½ cup† | 83 | 21 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Bread, white, 1 slice† | 43 | 11 |
| Peanuts, dry roasted, 1 ounce | 41 | 10 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Cantaloupe, raw, 1 wedge | 14 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, ½ breast | 3 | 1 |
Footnotes: * DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of products within the context of a total diet. The DV for folate is 400 mcg for adults and children aged 4 and older. However, the FDA does not require food labels to list folate content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
† Fortified with folic acid as part of the folate fortification program.
[Source 28 ]Vitamin B12
Vitamin B12 is also known as Cyanocobalamin is a nutrient that helps keep the body’s nerve and blood cells healthy and helps make DNA, the genetic material in all cells. Vitamin B12 also helps prevent a type of anemia called megaloblastic anemia that makes people tired and weak.
Vitamin B12 is a water-soluble vitamin that is naturally present in some foods, added to others, and available as a dietary supplement and a prescription medication. Vitamin B12 exists in several forms and contains the mineral cobalt 52, 53, 54, 55, so compounds with vitamin B12 activity are collectively called “cobalamins”. Methylcobalamin and 5-deoxyadenosylcobalamin are the forms of vitamin B12 that are active in human metabolism 56.
Two steps are required for the body to absorb vitamin B12 from food.
- First, food-bound vitamin B12 is released in the stomach’s acid environment (hydrochloric acid and and gastric protease in the stomach separate vitamin B12 from the protein to which vitamin B12 is attached in food) and is bound to R protein (haptocorrin) 56. When synthetic vitamin B12 is added to fortified foods and dietary supplements, it is already in free form and thus, does not require this separation step.
- Second, pancreatic enzymes cleave this B12 complex (B12-R protein) in the small intestine. After cleavage, intrinsic factor (a protein made by the stomach), secreted by parietal cells in the gastric mucosa, binds with the free vitamin B12. Intrinsic factor is required for absorption of vitamin B12, which takes place in the terminal ileum 56, 57. Approximately 56% of a 1 mcg oral dose of vitamin B12 is absorbed, but absorption decreases drastically when the capacity of intrinsic factor is exceeded (at 1–2 mcg of vitamin B12) 58. Some people have pernicious anemia, a condition where they cannot make intrinsic factor. As a result, they have trouble absorbing vitamin B12 from all foods and dietary supplements.
Pernicious anemia is an autoimmune disease that affects the gastric mucosa and results in gastric atrophy. This leads to the destruction of parietal cells, achlorhydria, and failure to produce intrinsic factor, resulting in vitamin B12 malabsorption 54, 56, 59, 60, 61. If pernicious anemia is left untreated, it causes vitamin B12 deficiency, leading to megaloblastic anemia and neurological disorders, even in the presence of adequate dietary intake of vitamin B12. For more details see below – Groups at Risk of Vitamin B12 Deficiency.
In the blood plasma, vitamin B12 is bound to transcobalamins I and II. Transcobalamin II is responsible for delivering vitamin B12 to tissues. The liver stores large amounts of vitamin B12. Enterohepatic reabsorption helps retain vitamin B12. Liver vitamin B12 stores can normally sustain physiologic needs for 3 to 5 yr if B12 intake stops (eg, in people who become vegans) and for months to 1 yr if enterohepatic reabsorption capacity is absent.
Vitamin B12 is required for proper red blood cell formation, neurological function, and DNA synthesis 52, 53, 54, 55, 56. Vitamin B12 functions as a cofactor for methionine synthase and L-methylmalonyl-CoA mutase. Methionine synthase catalyzes the conversion of homocysteine to methionine 56, 62. Methionine is required for the formation of S-adenosylmethionine, a universal methyl donor for almost 100 different substrates, including DNA, RNA, hormones, proteins, and lipids. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the degradation of propionate 54, 56, 62, an essential biochemical reaction in fat and protein metabolism. Succinyl-CoA is also required for hemoglobin synthesis.
Large amounts of vitamin B12 seem to be nontoxic but are not recommended for regular use (ie, as a general tonic).
How much vitamin B12 do you need?
The amount of vitamin B12 you need each day depends on your age. Average daily recommended amounts for different ages are listed below in micrograms (mcg).
The table below lists the current Recommended Dietary Allowance (RDA) for vitamin B12 in micrograms (mcg). For infants aged 0 to 12 months, the Food and Nutrition Board established an adequate intake (AI) for vitamin B12 that is equivalent to the mean intake of vitamin B12 in healthy, breastfed infants.
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 0.4 mcg |
| Infants 7–12 months | 0.5 mcg |
| Children 1–3 years | 0.9 mcg |
| Children 4–8 years | 1.2 mcg |
| Children 9–13 years | 1.8 mcg |
| Teens 14–18 years | 2.4 mcg |
| Adults | 2.4 mcg |
| Pregnant teens and women | 2.6 mcg |
| Breastfeeding teens and women | 2.8 mcg |
Footnotes:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
What foods provide vitamin B12?
Vitamin B12 is naturally found in animal products, including fish, meat, poultry, eggs, milk, and milk products. Vitamin B12 is generally not present in plant foods, but fortified breakfast cereals are a readily available source of vitamin B12 with high bioavailability for vegetarians 56, 64, 65, 66. Some nutritional yeast products also contain vitamin B12. Fortified foods vary in formulation, so it is important to read product labels to determine which added nutrients they contain.
The U.S. Department of Agriculture’s (USDA’s) Nutrient Database Web site 64 lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin B12 arranged by nutrient content 67 and by food name 68.
Dietary vitamin B12 deficiency usually results from inadequate absorption, but deficiency can develop in vegans who do not take vitamin supplements. Infants of vegan mothers should receive supplemental vitamin B12 from birth. Deficiency causes megaloblastic anemia, damage to the white matter of the spinal cord and brain, and peripheral neuropathy. Diagnosis is usually made by measuring serum vitamin B12 levels. The Schilling test helps determine etiology 69.
Vitamin B12 is found naturally in a wide variety of animal foods and is added to some fortified foods. Plant foods have no vitamin B12 unless they are fortified. You can get recommended amounts of vitamin B12 by eating a variety of foods including the following foods that are good sources of vitamin B12:
- Beef liver and clams are the best sources of vitamin B12.
- Fish such as catfish and salmon, meat, chicken, eggs, milk, yogurt, cheese, and other dairy products also contain vitamin B12.
- Some breakfast cereals, nutritional yeasts and other food products that are fortified with vitamin B12. To find out if vitamin B12 has been added to a food product, check the product labels.
Several food sources of vitamin B12 are listed in Table 5 below.
Table 5: Vitamin B12 Food Sources
| Food | Micrograms (mcg) per serving | Percent DV* |
|---|---|---|
| Clams, cooked, 3 ounces | 84.1 | 1402 |
| Liver, beef, cooked, 3 ounces | 70.7 | 1178 |
| Breakfast cereals, fortified with 100% of the DV for vitamin B12, 1 serving | 6 | 100 |
| Trout, rainbow, wild, cooked, 3 ounces | 5.4 | 90 |
| Salmon, sockeye, cooked, 3 ounces | 4.8 | 80 |
| Trout, rainbow, farmed, cooked, 3 ounces | 3.5 | 58 |
| Tuna fish, light, canned in water, 3 ounces | 2.5 | 42 |
| Cheeseburger, double patty and bun, 1 sandwich | 2.1 | 35 |
| Haddock, cooked, 3 ounces | 1.8 | 30 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B12, 1 serving | 1.5 | 25 |
| Beef, top sirloin, broiled, 3 ounces | 1.4 | 23 |
| Milk, low-fat, 1 cup | 1.2 | 18 |
| Yogurt, fruit, low-fat, 8 ounces | 1.1 | 18 |
| Cheese, Swiss, 1 ounce | 0.9 | 15 |
| Beef taco, 1 soft taco | 0.9 | 15 |
| Ham, cured, roasted, 3 ounces | 0.6 | 10 |
| Egg, whole, hard boiled, 1 large | 0.6 | 10 |
| Chicken, breast meat, roasted, 3 ounces | 0.3 | 5 |
Footnotes: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers determine the level of various nutrients in a standard serving of food in relation to their approximate requirement for it. The DV for vitamin B12 is 6.0 mcg. However, the FDA does not require food labels to list vitamin B12 content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 28 ]Vitamin B12 Supplements
In dietary supplements, vitamin B12 is usually present as cyanocobalamin 56, a form that the body readily converts to the active forms methylcobalamin and 5-deoxyadenosylcobalamin. Dietary supplements can also contain methylcobalamin and other forms of vitamin B12.
Existing evidence does not suggest any differences among forms with respect to absorption or bioavailability. However the body’s ability to absorb vitamin B12 from dietary supplements is largely limited by the capacity of intrinsic factor. For example, only about 10 mcg of a 500 mcg oral supplement is actually absorbed in healthy people 58.
In addition to oral dietary supplements, vitamin B12 is available in sublingual preparations as tablets or lozenges. These preparations are frequently marketed as having superior bioavailability, although evidence suggests no difference in efficacy between oral and sublingual forms 70, 71.
Vitamin B12 Prescription Medications
Vitamin B12, in the form of cyanocobalamin and occasionally hydroxocobalamin, can be administered parenterally as a prescription medication, usually by intramuscular injection 72. Parenteral administration is typically used to treat vitamin B12 deficiency caused by pernicious anemia and other conditions that result in vitamin B12 malabsorption and severe vitamin B12 deficiency 72.
Vitamin B12 is also available as a prescription medication in a gel formulation applied intranasally, a product marketed as an alternative to vitamin B12 injections that some patients might prefer 73. This formulation appears to be effective in raising vitamin B12 blood levels 74, although it has not been thoroughly studied in clinical settings.
- Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf[↩]
- Anaemia in pregnancy. Best Practice & Research Clinical Obstetrics & Gynaecology Volume 26, Issue 1, February 2012, Pages 3-24. https://doi.org/10.1016/j.bpobgyn.2011.10.010[↩]
- Nutrition During Pregnancy. https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy[↩]
- Lee AI, Okam MM. Anemia in pregnancy. Hematology/oncology Clinics of North America. 2011 Apr;25(2):241-59, vii. DOI: 10.1016/j.hoc.2011.02.001[↩][↩][↩][↩]
- McDonagh M, Cantor A, Bougatsos C, Dana T, Blazina I. Routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Evidence synthesis no. 123. AHRQ publication no. 13-05187-EF-2. Rockville, Md.: Agency for Healthcare Research and Quality; 2015.[↩]
- Rodriguez NM, Shackelford K. Pernicious Anemia. [Updated 2021 Jul 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540989[↩][↩][↩]
- Htut, TW, Thein, KZ, Oo, TH. Pernicious anemia: pathophysiology and diagnostic difficulties. J Evid Based Med. 2021; 14: 161- 169. https://doi.org/10.1111/jebm.12435[↩]
- Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol. 2009 Nov 7;15(41):5121-8. doi: 10.3748/wjg.15.5121[↩]
- Andres E, Serraj K. Optimal management of pernicious anemia. J Blood Med. 2012;3:97-103. doi: 10.2147/JBM.S25620[↩]
- Nazarenko GI, Kolenkin SM, Lugovskaia SA, Mikolauskas VP. Znachenie pokazateleĭ avtomatizirovannogo analiza retikulotsitov dlia diagnostiki i otsenki éffektivnosti lecheniia B12-defitsitnoĭ anemii [Significance of parameters of automated reticulocyte analysis in diagnosis and evaluation of treatment outcome in B12-deficient anemia]. Klin Lab Diagn. 2004 May;(5):42-5. Russian.[↩]
- Sickle Beta Plus Thalassemia. https://dshs.texas.gov/newborn/pdf/FactSBPlusThal.doc[↩]
- Sangani V, Pokal M, Balla M, Merugu GP, Khokher W, Gayam V, Konala VM. Fat Embolism Syndrome in Sickle Cell β-Thalassemia Patient With Osteonecrosis: An Uncommon Presentation in a Young Adult. J Investig Med High Impact Case Rep. 2021 Jan-Dec;9:23247096211012266. doi: 10.1177/23247096211012266[↩]
- Sedrak A, Kondamudi NP. Sickle Cell Disease. [Updated 2021 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482384[↩]
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. doi: 10.1371/journal.pmed.1001484[↩]
- Ashorobi D, Ramsey A, Yarrarapu SNS, et al. Sickle Cell Trait. [Updated 2022 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537130[↩][↩]
- El Ariss AB, Younes M, Matar J, Berjaoui Z. Prevalence of Sickle Cell Trait in the Southern Suburb of Beirut, Lebanon. Mediterr J Hematol Infect Dis. 2016 Feb 20;8(1):e2016015. doi: 10.4084/MJHID.2016.015[↩]
- Gibson JS, Rees DC. How benign is sickle cell trait? EBioMedicine. 2016 Sep;11:21-22. doi: 10.1016/j.ebiom.2016.08.023[↩]
- Sickle Cell Disease. https://www.nhlbi.nih.gov/health/sickle-cell-disease/treatment[↩]
- Khan I, Shaikh H. Cooley Anemia. [Updated 2022 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557522[↩]
- U.S. National Library of Medicine. Medline Plus. Iron. https://medlineplus.gov/iron.html#cat_51[↩]
- Centers for Disease Control and Prevention. Recommendations to Prevent and Control Iron Deficiency in the United States. https://www.cdc.gov/mmwr/preview/mmwrhtml/00051880.htm[↩]
- Wessling-Resnick M. Iron. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler RG, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:176-88.[↩]
- Aggett PJ. Iron. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:506-20.[↩][↩]
- Murray-Kolbe LE, Beard J. Iron. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:432-8.[↩][↩]
- U.S. National Library of Medicine. Iron. https://livertox.nlm.nih.gov/Iron.htm[↩]
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr 2010;91:1461S-7S. http://ajcn.nutrition.org/content/91/5/1461S.long[↩]
- Rutzke CJ, Glahn RP, Rutzke MA, Welch RM, Langhans RW, Albright LD, et al. Bioavailability of iron from spinach using an in vitro/human Caco-2 cell bioassay model. Habitation 2004;10:7-14. https://www.ncbi.nlm.nih.gov/pubmed/15880905[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/[↩][↩][↩]
- U.S. National Library of Medicine, MedlinePlus – Folic Acid – https://medlineplus.gov/folicacid.html[↩]
- Centers for Disease Control and Prevention – Facts About Folic Acid – https://www.cdc.gov/ncbddd/folicacid/about.html[↩]
- Office on Women’s Health, U.S. Department of Health and Human Services – Folic acid – https://www.womenshealth.gov/a-z-topics/folic-acid[↩]
- Bailey LB, Gregory JFr (2006). Folate. Present Knowledge in Nutrition. B. Bowman and R. Russell. Washington, DC, International Life Sciences Institute. I: 278-301.[↩][↩]
- Carmel R (2005). Folic Acid. Modern Nutrition in Health and Disease. M. Shils, M. Shike, A. Ross, B. Caballero and R. Cousins. Baltimore, MD, Lippincott Williams & Wilkins: 470-481.[↩][↩]
- Institute of Medicine. Food and Nutrition Board (1998). Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC, National Academy Press. https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline[↩][↩][↩][↩][↩]
- Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, et al. (2011). Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 94(1): 303S-312S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3127517/[↩]
- Colapinto CK, O’Connor DL, Dubois L, Tremblay MS (2012). Folic acid supplement use is the most significant predictor of folate concentrations in Canadian women of childbearing age. Appl Physiol Nutr Metab 37(2): 284-292. http://www.nrcresearchpress.com/doi/full/10.1139/h11-161[↩]
- Finer, L.B., Zolna, M.R. (2016). Declines in unintended pregnancy in the United States, 2008-2011. The New England Journal of Medicine; 374(9):843–52.[↩]
- U.S. Preventive Services Task Force. (2016). Final Recommendation Statement: Folic Acid for the Prevention of Neural Tube Defects: Preventive Medication – https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication[↩]
- Mosher, W.D., Jones, J., Abma, J.C. (2012). Intended and Unintended Births in the United States: 1982–2010 (PDF, 404 KB). National Health Statistics Reports; no. 55.[↩]
- CDC. (2016). Spina Bifida. – https://www.cdc.gov/ncbddd/spinabifida/facts.html[↩]
- CDC. (2015). Facts about Anencephaly. – https://www.cdc.gov/ncbddd/birthdefects/anencephaly.html[↩]
- U.S. Preventive Services Task Force. (2016). Final Recommendation Statement: Folic Acid for the Prevention of Neural Tube Defects: Preventive Medication[↩][↩][↩]
- CDC. (2016). Folic Acid Recommendations.- https://www.cdc.gov/ncbddd/folicacid/recommendations.html[↩][↩]
- Bailey, R.L., Dodd, K.W., Gahche, J.J., Dwyer, J.T., McDowell, M.A., Yetley, E.A., et al. (2010). Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. American Journal of Clinical Nutrition; 91(1): 231–237.[↩]
- Hamner, H.C., Cogswell, M.E., Johnson, M.A. (2011). Acculturation factors are associated with folate intakes among Mexican American women. The Journal of Nutrition; 141(10): 1889–97.[↩]
- U.S. Food and Drug Administration (1996). Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register 61(44). https://www.gpo.gov/fdsys/pkg/FR-1996-03-05/pdf/96-5014.pdf[↩]
- Whittaker P, Tufaro PR, Rader JI (2001). Iron and folate in fortified cereals. J Am Coll Nutr 20(3): 247-254. https://www.ncbi.nlm.nih.gov/pubmed/11444421[↩]
- Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH Jacques PF (2002). Folic acid intake from fortification in United States exceeds predictions. J Nutr 132(9): 2792-2798. http://jn.nutrition.org/content/132/9/2792.long[↩]
- Food and Drug Administration. Final rule: food additives permitted for direct addition to food for human consumption; folic acid. Federal Register 81(73):22176-22183, April 15, 2016. https://www.federalregister.gov/documents/2016/04/15/2016-08792/food-additives-permitted-for-direct-addition-to-food-for-human-consumption-folic-acid[↩]
- Government of Canada (1998). Regulations Amending the Food and Drug Regulations (1066). Canada Gazette 132(24).[↩]
- Centers for Disease Control and Prevention (2010). CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep 59(31): 980-984. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5931a2.htm[↩]
- Herbert V. Vitamin B12 in Present Knowledge in Nutrition. 17th ed. Washington, DC: International Life Sciences Institute Press, 1996.[↩][↩]
- Herbert V, Das K. Vitamin B12 in Modern Nutrition in Health and Disease. 8th ed. Baltimore, MD: Williams & Wilkins, 1994.[↩][↩]
- Combs G. Vitamin B12 in The Vitamins. New York: Academic Press, Inc., 1992.[↩][↩][↩][↩]
- Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and folate deficiency. Sem Hematol 1999;36:35-46. https://www.ncbi.nlm.nih.gov/pubmed/9930567?dopt=Abstract[↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem 2000;46:1277-83. https://www.ncbi.nlm.nih.gov/pubmed/10926922?dopt=Abstract[↩]
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood.2008;112:2214-21. https://www.ncbi.nlm.nih.gov/pubmed/18606874?dopt=Abstract[↩][↩]
- Gueant JL, Safi A, Aimone-Gastin I, Rabesona H, Bronowicki J P, Plenat F, et al. Autoantibodies in pernicious anemia type I patients recognize sequence 251-256 in human intrinsic factor. Proc Assoc Am Physicians 1997;109:462-9. https://www.ncbi.nlm.nih.gov/pubmed/9285945?dopt=Abstract[↩]
- Kapadia CR. Vitamin B12 in health and disease: part I—inherited disorders of function, absorption, and transport. Gastroenterologist 1995;3:329-44. https://www.ncbi.nlm.nih.gov/pubmed/8775094?dopt=Abstract[↩]
- Johnson MA. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr Rev 2007;65:451-8. https://www.ncbi.nlm.nih.gov/pubmed/17972439?dopt=Abstract[↩]
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc 2008;67:75-81. https://www.ncbi.nlm.nih.gov/pubmed/18234134?dopt=Abstract[↩][↩]
- National Institute of Health. Vitamin B12. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/[↩]
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page, https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/[↩][↩]
- Subar AF, Krebs-Smith SM, Cook A, Kahle LL. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc 1998;98:537-47. https://www.ncbi.nlm.nih.gov/pubmed/9597026?dopt=Abstract[↩]
- Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson WF, et al. Plasma vitamin B12 concentrations relate to intake source in the Framingham Offspring Study. Am J Clin Nutr 2000;71:514-22. https://www.ncbi.nlm.nih.gov/pubmed/10648266?dopt=Abstract[↩]
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page, https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Content.pdf[↩]
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page, https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Food.pdf[↩]
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin B 12. https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-b-12[↩]
- Yazaki Y, Chow G, Mattie M. A single-center, double-blinded, randomized controlled study to evaluate the relative efficacy of sublingual and oral vitamin B-complex administration in reducing total serum homocysteine levels. J Altern Complement Med 2006;12:881-5. https://www.ncbi.nlm.nih.gov/pubmed/17109579?dopt=Abstract[↩]
- Sharabi A, Cohen E, Sulkes J, Garty M. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol 2003;56:635-8. https://www.ncbi.nlm.nih.gov/pubmed/14616423?dopt=Abstract[↩]
- Andrès E, Federici L, Affenberger S, Vidal-Alaball J, Loukili NH, Zimmer J, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract 2007;56:537-42. https://www.ncbi.nlm.nih.gov/pubmed/17605945?dopt=Abstract[↩][↩]
- Suzuki DM, Alagiakrishnan K, Masaki KH, Okada A, Carethers M. Patient acceptance of intranasal cobalamin gel for vitamin B12 replacement therapy. Hawaii Med J 2006;65:311-4. https://www.ncbi.nlm.nih.gov/pubmed/17265990?dopt=Abstract[↩]
- Slot WB, Merkus FW, Van Deventer SJ, Tytgat GN. Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology.1997;113:430-3. https://www.ncbi.nlm.nih.gov/pubmed/9247460?dopt=Abstract[↩]