What is anion gap
The serum anion gap is a helpful parameter in the clinical diagnosis of various conditions. The anion gap is the difference between the measured cations (positively charged ions) and the measured anions (negatively charged ions) in serum, plasma, or urine. The magnitude of this difference (i.e., “gap”) in the serum is often calculated in medicine when attempting to identify the cause of metabolic acidosis, a lower than normal pH in the blood. If the gap is greater than normal, then high anion gap metabolic acidosis is diagnosed.
An anion gap blood test is a way to help clinicians check the levels of acid in your blood in order to determine the cause of a metabolic acidosis 1. An anion gap blood test is based on the results of another blood test called an electrolyte panel. The electrolyte panel measures the blood levels of the main electrolytes in the body: sodium (Na+), potassium (K+), chloride (Cl–), and bicarbonate (HCO3– sometimes reported as total CO2). Electrolytes are electrically charged minerals that help control the balance of chemicals in your body called acids and bases. Some of these minerals have a positive electric charge. Others have a negative electric charge.
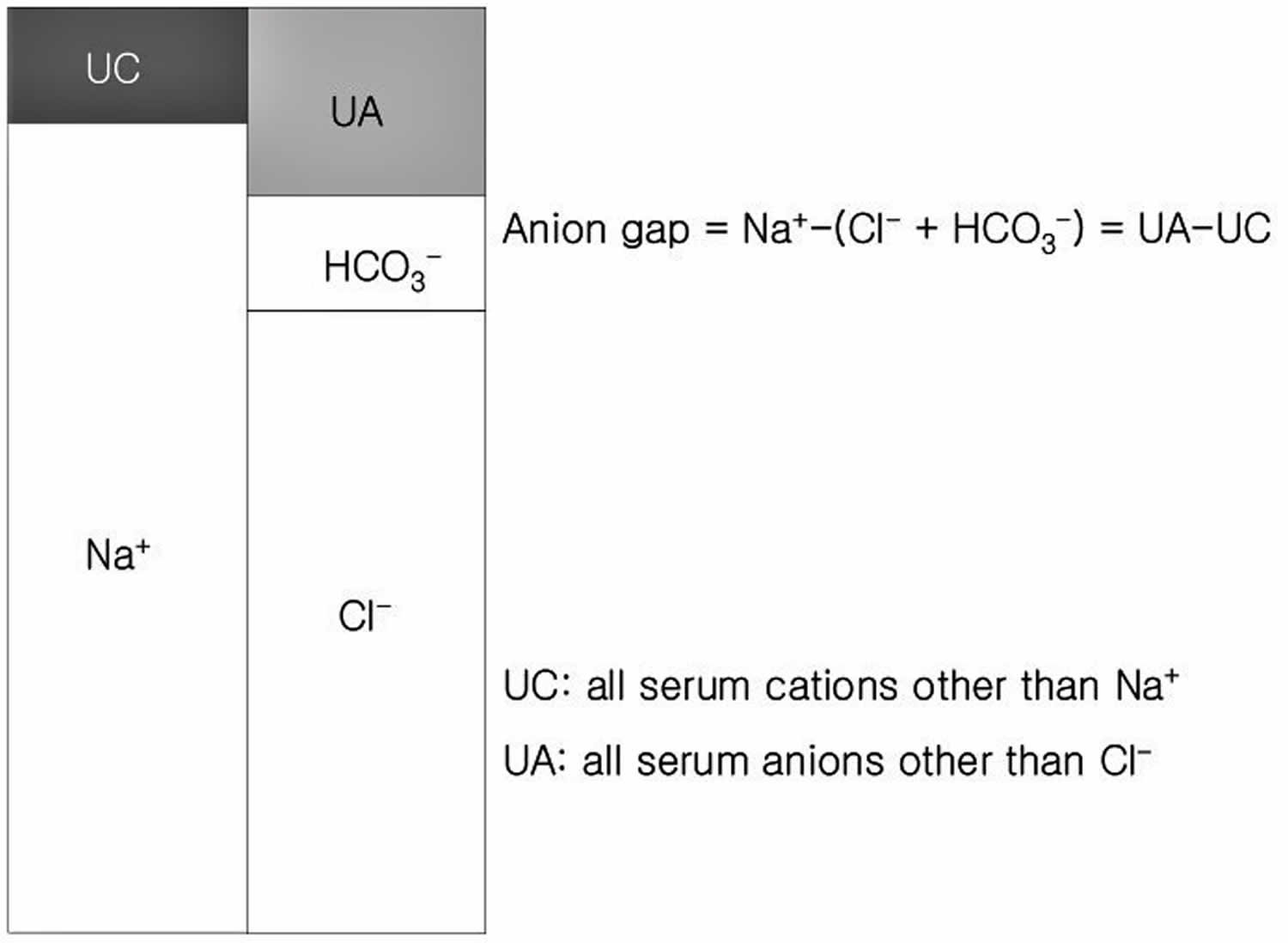
Clinically, anion gap is equal to the difference between the plasma concentrations of the major cation [Na+] and the major measured anions ([Cl–]+[HCO3–]) 2. It is important to understand that this entity actually equals [anionic proteins+inorganic phosphate+sulphate+organic anions]-[potassium+calcium+magnesium+cationic proteins] (see Figure 1). Since there are more unmeasured anions (negatively charged ion) than unmeasured cations (positively charged ion), the value of anion gap is usually positive.
The classical value of a normal anion gap is considered 12±4 mEq/L, when sodium was determined by flame photometry (based on the principle of flame atomic emission spectrometry) and chloride by a colorimetric assay (mercuric-nitrate-thiocyanate colorimetric assay). Since the 1980s, ion-selective electrodes for specific ionic species were used for the measurement of serum electrolytes. The difference between the ionic concentration in the electrode (known) and the sample creates an electrical potential (measured) and the sample ionic concentration can be calculated. The measurement by ion-selective electrodes has caused a shift of the anion gap from 12±4 mEq/L down 6±3 mEq/L 3. It is worthy for clinicians to understand the range of normal anion gap and the measuring methods for serum sodium and chloride in the laboratories that support their practice.
The formula for anion gap is:
[Na+] – ([Cl–]+[HCO3–]) = Sodium – (Chloride + Bicarbonate) = Sodium + Unmeasured cations = Chloride + Bicarbonate + Unmeasured anions
Another important formula to use with metabolic acidosis is the Winter formula. This equation provides the clinician with the expected PCO2 value. This is important because there could be another acid-base disorder present.
The Winter formula is:
Expected pCO2 = (Bicarbonate x 1.5) + 8 +/- 2
If the patient’s pCO2 (amount of carbon dioxide gas dissolved in the blood) is within the the expected pCO2, there is no mixed disorder, just respiratory compensation. When the value is lower or higher than expected, there is a mixed disorder. If the pCO2 is greater than expected pCO2, this indicates an additional respiratory acidosis. If the pCO2 is less than expected pCO2, there is an additional respiratory alkalosis occurring.
A shortcut for the Winter formula is that the last two digits of the pH +/- 2 is about equal to the expected pCO2. The pH of the human body ranges between 7.35 to 7.45, with the average at 7.40. A pH below 7.35 is an acidemia or acidosis, and a pH above 7.45 is an alkalemia or alkalosis.
Figure 1. Anion gap calculation
[Source 2 ]Humans are electrically neutral, but all cations (positively charged ions) and anions (negatively charged ions) are not being measured. The normal anion gap is equal to 8 +/- 4 1. Most of this number is due to albumin; this anion is not accounted for in the formula which is a large reason why the anion gap is not closer to zero. Albumin is normally 4 mg/dL. Because of the large effect of albumin on anion gap, if a patient’s albumin level is abnormal, their expected anion gap will not be accurate. This can be corrected using simple math. The normal anion gap and albumin level differ by a factor of three (normal anion gap of 12, normal albumin of 4 mg/dL). If a patient has an anion gap of 24, that means there are 12 units of the conjugate base present that normally would not be due to the combination of hydrogen ions with bicarbonate. If this same patient has an albumin level of 3mg/dL, their expected anion gap should actually be about 9. This means that, rather than 12 units of the conjugate base present, there are really 15 units.
Urine anion gap formula
The following is the equation for urine anion gap where Na is sodium, K is potassium, and Cl is chloride:
Urine anion gap = Urine Na (sodium) + Urine K (potassium) – Urine Cl (chloride)
A positive urine anion gap value is indicative of renal bicarbonate loss, such as renal tubular acidosis. Negative values are found with non-renal bicarbonate losses, such as diarrhea 4.
The urine anion gap provides an estimate of urinary ammonium (NH4) excretion 5. The normal renal response to metabolic acidosis is to increase acidic ammonium (NH4) excretion renally. Therefore, a positive urine anion gap between 20 and 90 mEq/L is indicative of low or normal NH4 excretion, such as is seen in distal renal tubular acidosis. A negative urine anion gap between -20 and -50 mEq/L is indicative of increased ammonium (NH4) excretion. This occurs in patients with metabolic acidosis generated by profuse watery diarrhea. A urine anion gap approaching 0 is indeterminate.
Anion gap blood test
The anion gap blood test is used to show whether your blood has an imbalance of electrolytes or too much or not enough acid.
When is an anion gap blood test ordered?
Your health care provider may have ordered an anion gap blood test if you have signs of an imbalance in your blood acid levels. These signs may include:
- Shortness of breath
- Vomiting
- Abnormal heartbeat
- Confusion
Will I need to do anything to prepare for the test?
You don’t need any special preparations for an anion gap blood test. If your health care provider has also ordered other blood tests, you may need to fast (not eat or drink) for several hours before the test. Your health care provider will let you know if there are any special instructions to follow.
Anion gap normal range
Depends on measurement methods used, anion gap can range between 6±3 mEq/L 3 or 8 +/- 4 1.
What do abnormal anion gap test results mean?
If your results show a high anion gap, you may have acidosis, which means higher than normal levels of acid in the blood. Acidosis may be a sign of dehydration, diarrhea, or too much exercise. It may also indicate a more serious condition such as kidney disease or diabetes.
If your results show a low anion gap, it may mean you have a low level of albumin, a protein in the blood. Low albumin may indicate kidney problems, heart disease, or some types of cancer. Since low anion gap results are uncommon, retesting is often done to ensure the results are accurate. Talk to your health care provider to learn what your results mean.
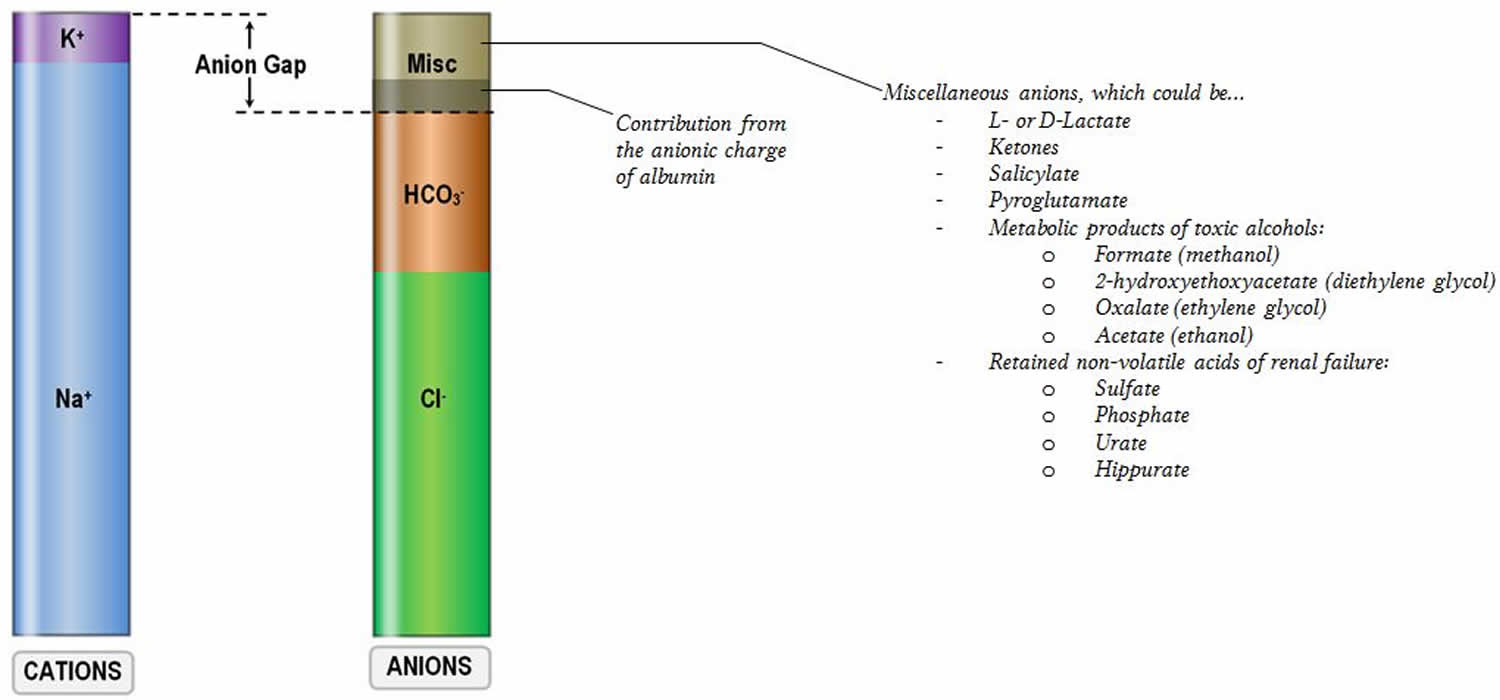
Figure 2. Anion gap in major causes of metabolic acidosis
[Source 2 ]Anion gap high
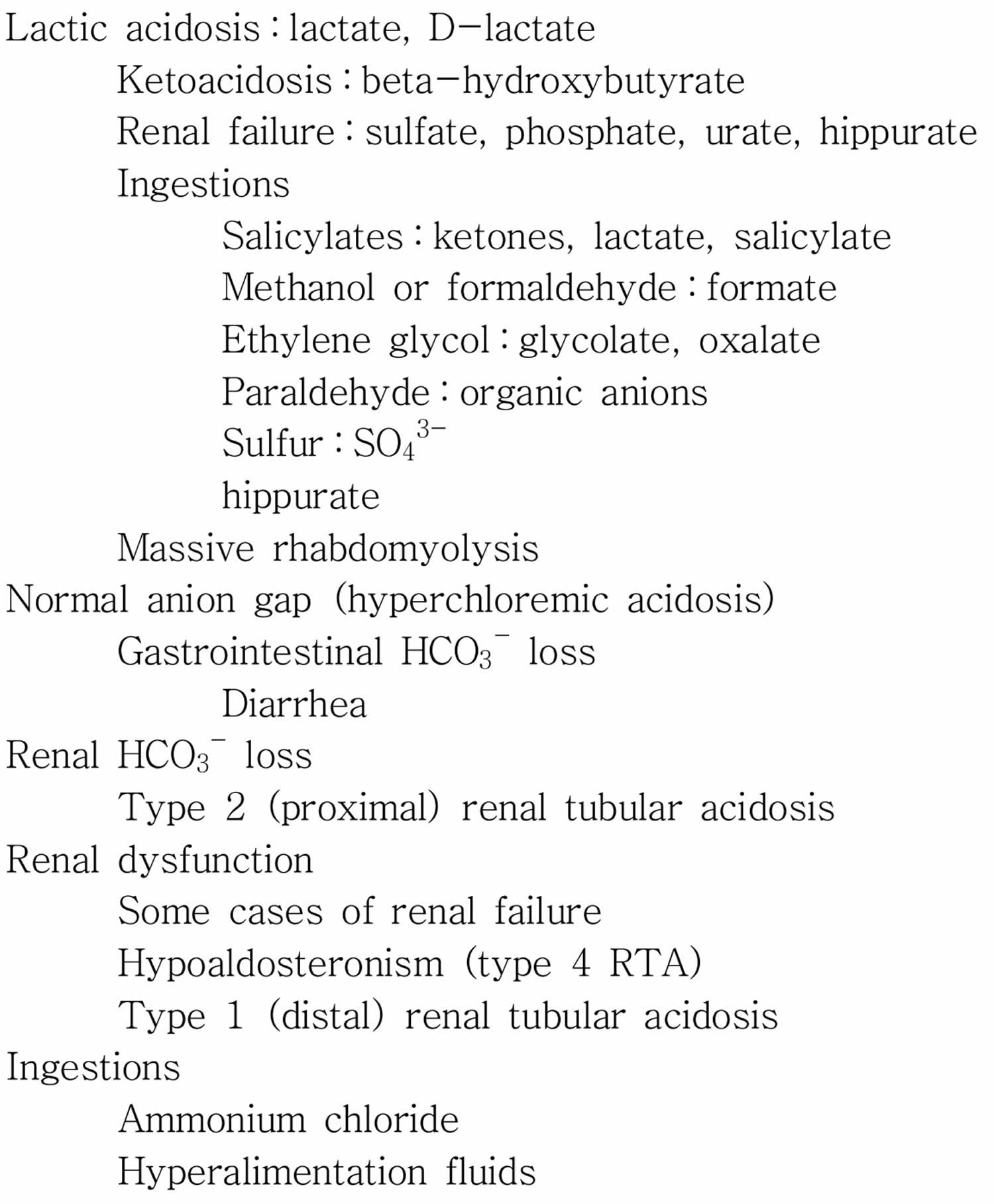
An increased anion gap usually is caused by an increase in unmeasured anions (negatively charged ions), and that most commonly occurs when there is an increase in unmeasured organic acids, that is, an acidosis 6. Acids (e.g., lactate and pyruvate) are protons donors and must be buffered by bicarbonate [HCO3–]. The consumption of bicarbonate [HCO3–] by the unmeasured anions (negatively charged ions) will increase the anion gap by lowering the serum bicarbonate level. The total numbers of anions (negatively charged ions) and cations (positively charged ions) are still equal, but the gap is increased because of a lowering of a measured anion (negatively charged ion), the serum bicarbonate [HCO3–]. The causes of an increase in organic acids have been well outlined. The most common ones can be remembered by the mnemonic MUDPILES : methanol, uremia, diabetic ketoacidosis, paraldehyde, infection, lactic acidosis, ethylene glycol, and salicylates 7. A new mnemonic, GOLDMARK, has been suggested to be an improvement. GOLDMARK is an anagram for glycols (ethylene and propylene), oxoproline, lactate, methanol, aspirin, renal failure, and ketones 1. If a patient has an anion gap over 12, these mnemonics are helpful to remember the possible causes of the disorder. These conditions produce an acid load that consumes bicarbonate, increases the anion gap, and lowers serum pH. If the patient is acidotic and has an elevated anion gap, it is almost certainly caused by one of these conditions, each one with us characteristic signs, symptoms, and laboratory values.
Narrow anion gap metabolic acidosis
If the acidosis involves a normal anion gap, there is a loss of bicarbonate [HCO3–] rather than an increased amount of hydrogen ions, with a concomitant increase in chloride [Cl–] ions. To keep a physiological neutral state, chloride [Cl–] ions migrate out of the cells and into the extracellular space. This causes the patient’s serum chloride to increase and keeps the anion gap at a normal level 1. This means that a metabolic acidosis without an abnormal anion gap is also a hyperchloremic metabolic acidosis 1. A metabolic acidosis without an increased anion gap results from many processes including severe diarrhea, type 1 renal tubular acidosis, long-term use of carbonic anhydrase inhibitors, and suctioning of gastric contents. When a patient has a narrow ion gap hyperchloremic acidosis, the doctor can calculate the urine anion gap to help determine etiology.
Anion gap low
A low anion gap often does not elicit the same warning to clinicians as a high anion gap and hence often remains either undiscovered or neglected 2. The classical differential diagnosis of a low anion gap has changed since the ion-selective electrode has been introduced. A low anion gap has several utilities. First, it can be an early and sometimes only sign of an underlying disease process such as paraproteinemia. In addition to displacement of sodium-containing water from serum by large amounts of non-sodium-containing paraproteins, some paraproteins (e.g., IgG in multiple myeloma) can have a net positive charge at physiological pH. This leads to an increase in unmeasured cations and a low anion gap 8. Concomitant severe hypercalcemia and hypoalbuminemia are often contributing factors to a low anion gap in multiple myeloma9). Since the only cation included in the anion gap calculation is sodium, severe hyperkalemia (high blood potassium), hypercalcemia (high blood calcium), hypermagnesemia (high blood magnesium) or lithium intoxication theoretically can also lead to a significantly decreased anion gap. Second, at normal serum pH of 7.4, the majority of plasma proteins are anionic. Albumin with an average negative charge of 18 per mole at physiological pH has been shown to be responsible for approximately 75% of the unmeasured anions of the normal anion gap. A drop in albumin by 10 g/L therefore will cause the anion gap to fall by approximately 2.5 mEq/L at constant pH 9. Hypoalbuminemia is probably the commonest cause of a clinically relevant lowered anion gap 2. Third, a low anion gap can mask an underlying high anion gap acidosis and potentially delay intervention. While an increase in the anion gap is almost always caused by retained unmeasured anions, a decrease in the anion gap can be generated by multiple mechanisms.
References- Hopkins E, Sharma S. Physiology, Acid Base Balance. [Updated 2018 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507807
- Lee S, Kang KP, Kang SK. Clinical usefulness of the serum anion gap. Electrolyte Blood Press. 2006;4(1):44-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3894543/
- The fall of the serum anion gap. Winter SD, Pearson JR, Gabow PA, Schultz AL, Lepoff RB. Arch Intern Med. 1990 Feb; 150(2):311-3. https://www.ncbi.nlm.nih.gov/pubmed/2302006/
- Burger MK, Schaller DJ. Physiology, Acidosis, Metabolic. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482146
- Sharma S, Aggarwal S. Hyperchloremic Acidosis. [Updated 2018 Feb 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482340
- Adrogue HJ, Madias NE. Management of life-threatening acid-base disorders : First of two parts. N Engl J Med. 1998;338:26–34.
- Ishihara K, Szerlip HM. Anion gap acidosis. Semin Nephrol. 1998;18:83–97.
- Keshgegian AA. Anion gap and immunoglobulin concentration. Am J Clin Pathol. 1980;74:282–284.
- Durward A, Mayer A, Skellet S, Taylor D, Hanna S, Tibby SM, Murdoch IA. Hypoalbuminemia in critically ill children : incidence, prognosis, and influence on the anion gap. Arch Dis Child. 2003;88:419–422