Anomalous coronary artery
Coronary artery anomalies covers a very wide spectrum of anatomical entities with diverse clinical manifestations and varying degrees of severity and their clinical significance varies from benign without ischemic consequences to ischemia-related symptoms and arrhythmias leading to an increased risk of sudden cardiac death 1. Anomalous origin of a coronary ostium from the contralateral sinus is the anomaly most frequently associated with sudden cardiac death, in particular in the case of anomalous left coronary artery from the right sinus or if the anomalous coronary artery has a course between the aorta and the pulmonary artery or other high-risk features. Other coronary artery anomalies, like anomalous origin of the left coronary artery from the pulmonary artery or atresia of the left coronary artery, have also been associated with extensive ischemia and high risk of sudden cardiac death early in infancy or in early adulthood. Accurate diagnosis, risk-assessment and appropriate choice of treatment are of utmost importance, since coronary artery anomalies constitute the second most common cause of sudden cardiac death in young competitive athletes 1. The rapid advancement of imaging techniques, including intravascular ultrasound, optical coherence, computed tomography and magnetic resonance imaging, have provided doctors with a wealth of new information on the subject as well as more accurate diagnostic criteria regarding the severity of these conditions that allow risk-stratification for sudden cardiac death and provide guidelines for optimal treatment. Consultation by cardiologists in cases of suspected or diagnosed coronary artery anomalies and systematic referral of diagnosed patients to specialized centers play a significant role in timely diagnosis, appropriate management and successful prevention of sudden cardiac death.
Anomalous course of a coronary artery is an uncommon type of congenital (present at birth) disorders of the coronary artery anatomy and constitute the second most common cause of sudden cardiac death in young competitive athletes 1. Anomalous coronary artery though relatively uncommon and usually discovered incidentally during coronary angiography, they have attracted interest as they constitute the second most frequent cause of sudden death in young adults participating in competitive sports 2. Anomalous coronary artery may represent a benign (80.6%) and incidental finding, but can also be potentially serious anomalies (19.4%) predisposing patients to life-threatening myocardial ischemia or arrhythmias, depending on where the anomalous coronary artery runs. Potentially serious anomalies include ectopic origin from the pulmonary artery, ectopic origin from the opposite aortic sinus, single coronary artery, and large coronary fistulae 3. These anomalies may be associated with sudden death.
Normal coronary artery anatomy is characterized by two ostia centrally placed in the right and left sinus of Valsalva. The main left coronary artery originates from the left ostium, branching into the left anterior descending artery and circumflex artery, which courses around the left atrioventricular groove; the right coronary artery arises from the right ostium, providing an infundibular branch to the anterior side of the heart, and then courses backward in the atrioventricular groove 4. The three main coronary arteries branch superiorly to the atria and inferiorly to the ventricles; they end in broom-like arborisations, which penetrate the myocardium 5. Because of ventricular contraction myocardial perfusion of the left ventricle occurs mainly in diastole, while the myocardium of the right ventricle is perfused during both heart cycles 6.
Anomalous origin of a coronary artery from the contralateral sinus is the anomaly most frequently associated with sudden cardiac death, in particular if the anomalous coronary artery has a course between the aorta and the pulmonary artery. However, other coronary anomalies, like anomalous origin of the left coronary artery from the pulmonary artery, atresia of the left main stem and coronary fistulae, have also been implicated in cases of sudden cardiac death. Patients are usually asymptomatic, and in most of the cases, coronary anomalies are discovered incidentally during coronary angiography or on autopsy following sudden cardiac death. However, in some cases, symptoms like angina, syncope, heart failure and myocardial infarction may occur.
The incidence of coronary artery anomalies is approximately 1% among patients undergoing cardiac catheterization 7, 0.29% among autopsy specimens 8 and less than 0.1% among prospective echocardiographic screenings 9. Most anomalies are incidentally detected and do not create clinical problems 7. However, 19-33% of sudden cardiac deaths in the young population are attributable to coronary artery anomalies 10. An anomalous origin of the right coronary artery from the left sinus is a very rare anomaly, and its incidence is 0.019% to 0.49% on coronary angiography 7. However, recent angiographic studies have reported a relatively high incidence (5.6%) of coronary artery anomalies and anomalous right coronary artery origins from the left sinus (0.92%) 11. Anomalous origin of the right coronary artery (0.17%) is more common than anomalous origin of left coronary artery (left coronary artery, 0.047%) 3.
Normal coronary artery anatomy
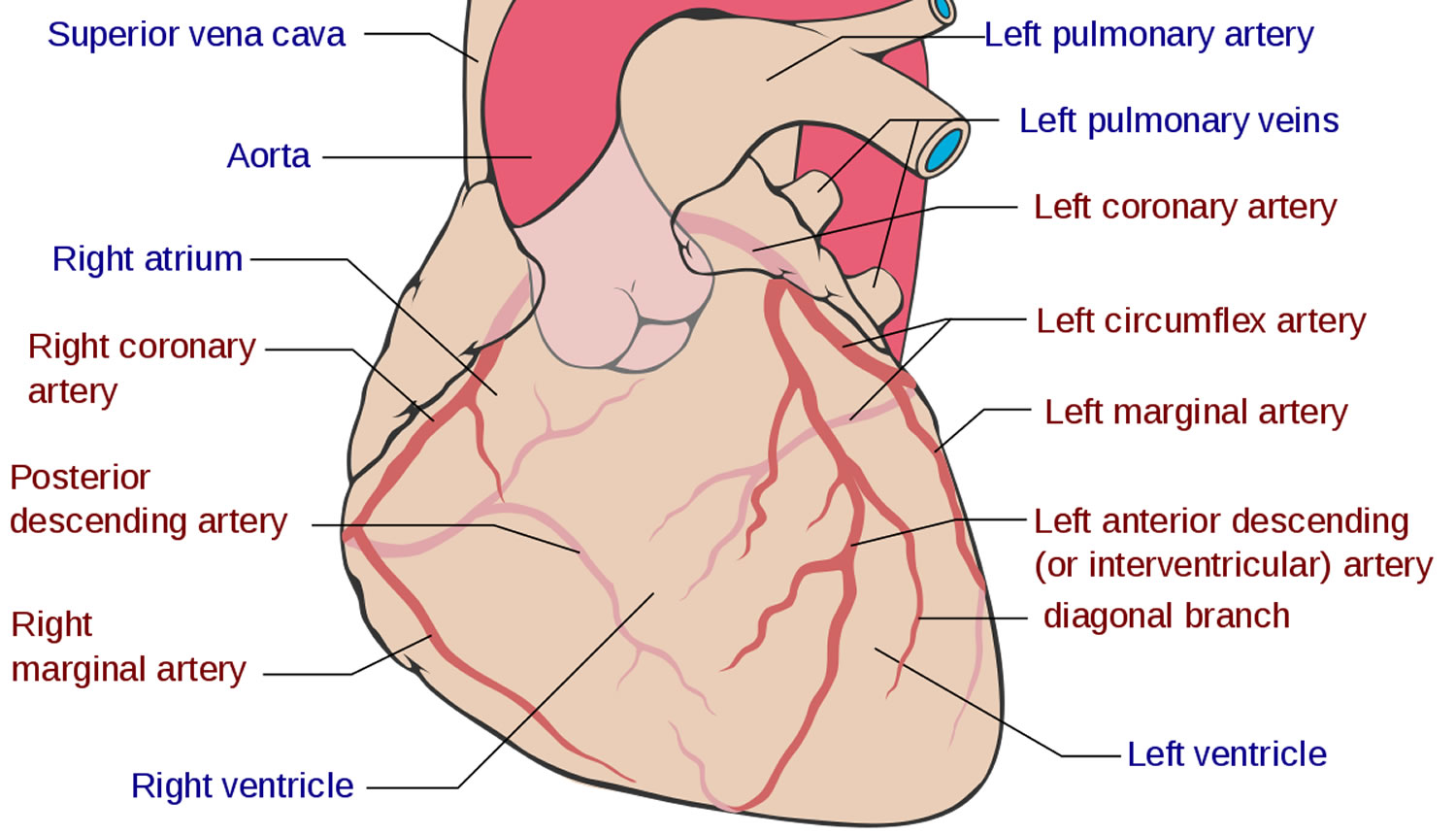
Before venturing to explore the various coronary anatomy variations and anomalies, it is worth going through a brief overview of normal coronary anatomy (Figure 1). Normally there are two main coronary arteries, which stem from the sinuses of Valsalva and descend towards the cardiac apex. The left main coronary artery originates from the left sinus of Valsalva and crosses between the main pulmonary artery and the left atrial appendage. The left main coronary artery has an average length of 2-4 cm and normally bifurcates into the left anterior descending artery and the left circumflex artery. The right coronary artery stems from the right sinus of Valsalva. The left anterior descending artery descends towards the apex of the heart in the epicardial fat across the anterior interventricular sulcus. Its length varies between 10 and 13 cm and gives rise to diagonal and septal branches. It supplies the anterior wall, the apex and a significant portion of the interventricular septum. The left circumflex artery has a length of 5-8 cm and crosses the coronary sulcus on the diaphragmatic cardiac surface. It gives rise to the obtuse marginal branches and supplies mostly the lateral wall of the left ventricle. The right coronary artery passes to the right between the pulmonary artery and the right auricle, descends across the right atrioventricular sulcus and continues posteriorly after the acute margin of the heart. Normal length is 12-14 cm. During its course, it may give rise to several branches, like the conus branch, sinoatrial branch, right ventricular branch, atrioventricular nodal branch, posterior descending branch and posterolateral branch. It can broadly be considered as the artery that supplies the right side of the heart 2. According to Angelini 12, the coronary anatomy falls within the spectrum of normality when: (1) there are two to four coronary ostia located at the upper midsection of the anterior aspect of the right and left sinuses of Valsalva. This is because sometimes the left anterior descending artery and left circumflex artery may have separate origins from the left sinus, whereas the right coronary artery and the conus branch may have separate origins from the right sinus; (2) a proximal stem bifurcating into major arteries is present only in the left coronary system (left main coronary artery bifurcating into left anterior descending artery and left circumflex artery); (3) coronary arteries originate from the aortic wall with an angle of 45°-90°, follow an extramural (subepicardial) course, provide adequate branches for the perfusion of myocardium and terminate at the capillary bed distally; and (4) right coronary artery perfuses the right ventricular free wall, left anterior descending artery perfuses the anteroseptal wall and the obtuse marginal branch of left circumflex artery perfuses the free wall of the left ventricle (essential perfusion territories).
Figure 1. Normal coronary artery
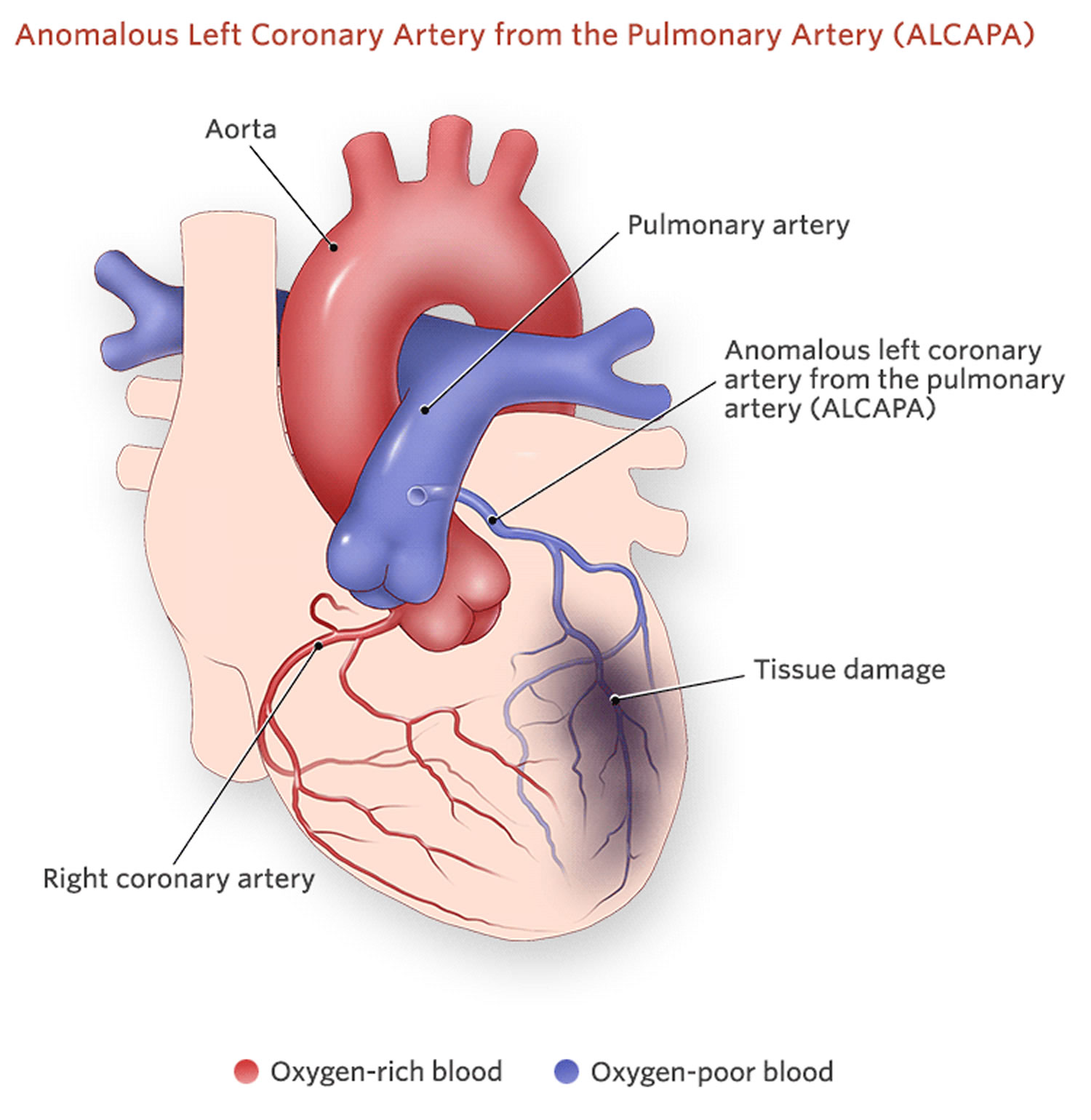
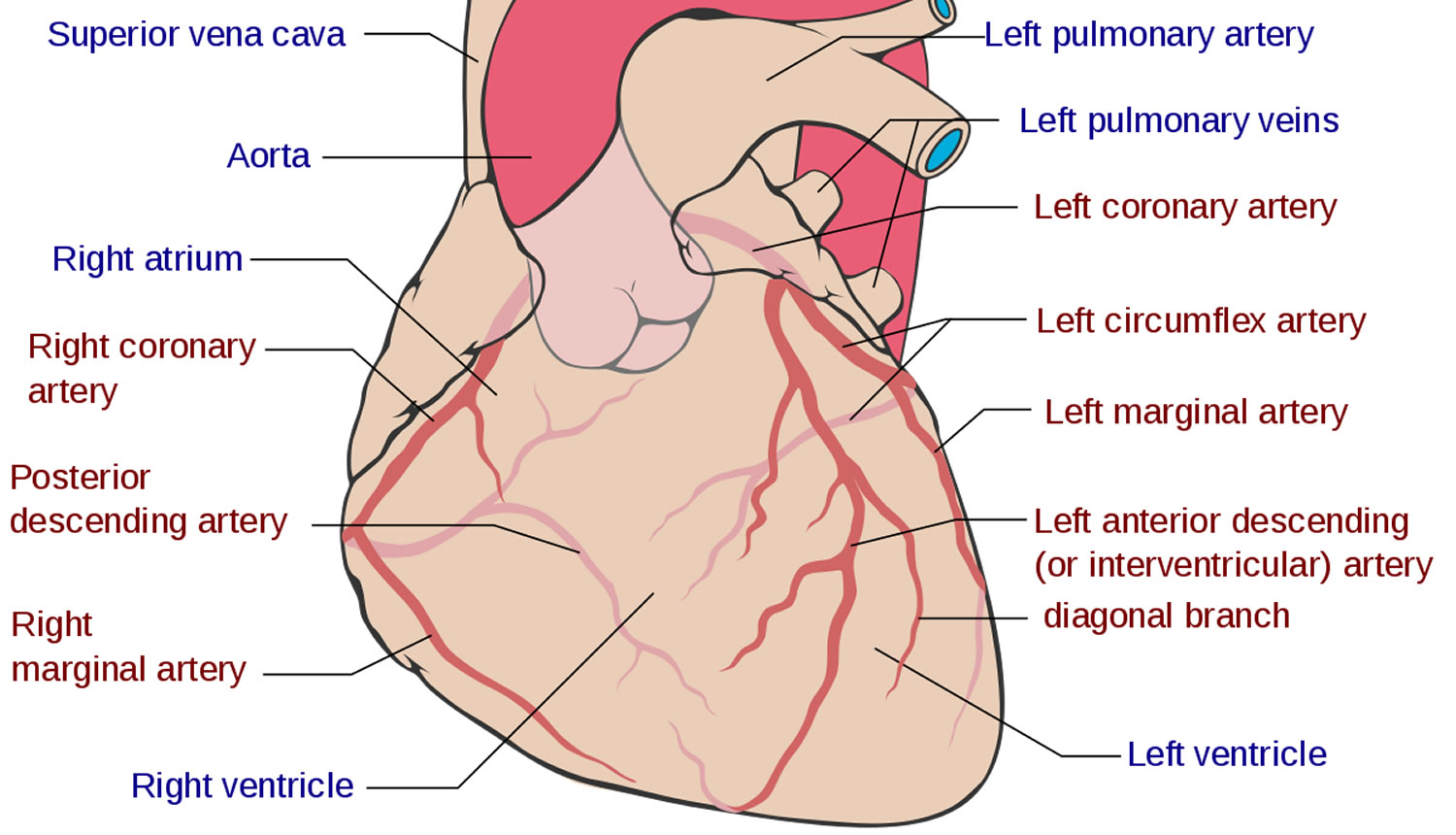 Figure 2. Anomalous left coronary from pulmonary artery (ALCAPA)
Figure 2. Anomalous left coronary from pulmonary artery (ALCAPA)
Coronary artery anomalies
Several classification schemes have been proposed for coronary artery anomalies 1. Based on clinical significance some authors categorize them as major and minor. Based on functional relevance they can be classified as: (1) anomalies that are associated with definite ischemia (for example, anomalous origin of the left coronary artery from the pulmonary artery or atresia of a coronary artery); (2) anomalies that are not associated with ischemia (for example, separate ostia of left anterior descending artery and right coronary artery with absence of left coronary artery, split left anterior descending artery or split right coronary artery); and (3) anomalies with exceptional ischemia (like anomalous origin of coronary artery from the contralateral sinus and coronary fistulae). However, the most detailed and accurate classification has been proposed by Angelini 12 and is based on anatomical features. According to this classification, coronary anomalies can be characterized as: (1) anomalies of origination and course of coronaries (separate ostia of left anterior descending artery and right coronary artery, anomalous location of coronary ostia within the aortic root, outside the sinuses of Valsalva or at the contralateral sinus, single coronary artery); (2) anomalies of intrinsic coronary anatomy (split RCA/left anterior descending artery, ostial stenosis/atresia, ectasia, hypoplasia, intramural course/bridging, absent coronary artery, ectopic origin of first septal branch, ectopic origin of posterior descending branch from the left anterior descending artery or a septal branch); (3) anomalies of coronary termination (mainly fistulas); and (4) anomalous collateral vessels.
Table 1 summarizes the most common variations, aberrations and anomalies for coronary anatomy, based on anomalies of origin, course, intrinsic coronary anatomy and termination.
Table 1. Classification of coronary artery variants, aberrations and anomalies
| Variations/anomalies of origin and course |
|
|
|
| Within sinuses of Valsalva |
|
|
| Outside sinuses of Valsalva |
|
|
|
|
| Anomalous origin from contralateral sinus |
|
| Variations/anomalies of intrinsic coronary anatomy |
|
|
|
|
|
|
|
|
|
| Anomalies of coronary termination |
|
|
Anomalous coronary artery origination and course
Absence of left coronary artery
Absence of the left coronary artery (separate ostia of left anterior descending artery and left circumflex artery) is the most common coronary anomaly, with an incidence ranging between 0.41%-0.67%. It is a benign anomaly that causes no hemodynamic impairment or ischemic consequences 3.
Anomalous location of coronary ostia
A coronary ostium may have an anomalous origin but may be still located within the proper coronary sinus; in those cases, the coronary ostium may originate from a higher position or a lower position compared to the normal site of origin, or may stem from the commissural level. On the other hand, when the anomalous coronary ostium is located outside the proper coronary sinuses, it can present in a wide variety of locations, like the non-coronary sinus of Valsalva, the ventricles and ectopic sites in the aorta or the large arteries (ascending or descending aorta, anonymous artery, carotid arteries), or even smaller arteries (bronchial arteries, internal thoracic artery, etc). Anomalous origin of a coronary artery from the contralateral sinus of Valsalva is particularly interesting from a clinical point of view, because these anomalies can be associated with sudden cardiac death, especially when the anomalous coronary artery crosses interarterially between the aorta and the pulmonary artery 13.
Anomalous origin of coronary ostium from contralateral sinus
A coronary artery that originates from the contralateral sinus of Valsalva can follow five potential paths towards its perfusion territory: (1) pre-pulmonic, anterior to the right ventricular outflow tract (usually benign, though rarely associated with angina); (2) retro-aortic, posterior to the aortic root (no hemodynamic consequences); (3) trans-septal, with a subpulmonic intramyocardial course; (4) retro-cardiac, behind the mitral and tricuspid valves, in the posterior atrioventricular groove; and (5) inter-arterial, between the aorta and the pulmonary artery 2.
The inter-arterial path has clinical significance, as it has been associated with an increased risk of sudden cardiac death, especially in young athletes 2. The causes are not clear and several explanations have been proposed. In some cases, autopsy findings have shown an acute angle in the take-off of the anomalous coronary artery with a slit-like lumen and a proximal course between the aorta and the pulmonary trunk 14. In other cases, histological examination has also shown an intramural course of the anomalous coronary within the aortic wall. It has been proposed that the acute angulation and slit-like ostium of the anomalous coronary vessel predispose to myocardial ischemia. Exercise-induced expansion of the aortic root and the pulmonary trunk compresses and exacerbates the slit-like ostium, resulting in further ischemia 15. A scissors-like mechanism that compresses the anomalous coronary between the aorta and the pulmonary artery has also been suggested 12. Intravascular ultrasound studies have demonstrated an intramural proximal intussusception of the ectopic artery at the aortic wall for a variable distance 16. The intussuscepted segment of the vessel is smaller in circumference compared to its more distal segment (circumferential hypoplasia), and its cross-section is ovoid instead of circular. These parameters lead to a lateral luminal compression that is present throughout the whole of the cardiac cycle but even more pronounced during systole 12. Furthermore, the intramural segment has thin inner and outer aortic wall layers. Therefore, the section of the aorta that is penetrated by the ectopic artery is a localized weak spot, prone to more extensive distensibility, which can further exacerbate stenosis and ischemia 17.
Ectopic right coronary artery originating from the left sinus of Valsalva has a frequency of 0.03%-0.92% and ectopic left anterior descending artery arising from the right sinus of Valsalva has a frequency of 0.03% 2. Both of these anomalies may be associated with an intramural inter-arterial course, in which case there is an increased risk of sudden cardiac death. Ectopic origin of the left circumflex artery from the right sinus of Valsalva (Figure (Figure8)8) or from the proximal right coronary artery is the second most common coronary artery anomaly, with a frequency of 0.37% 2, but it is considered benign and the course of the ectopic left circumflex artery is usually retroaortic or retrocardiac 12.
It must be noted that anomalous origin of a coronary artery from an opposite sinus of Valsalva is related with sudden death mostly in young athletes < 35 years old but less frequently in older patients. Symptoms like angina, syncope, heart failure and myocardial infarction may appear in both age groups but are seen more frequently in older patients. It has been proposed that stiffening of the aortic wall in older adults is the reason why sudden death is less frequent in this age group 18.
Diagnosis of anomalous origin of a coronary artery from the contralateral sinus is often difficult, since most of the times patients are asymptomatic prior to sudden cardiac death. However, there have been some reports of premonitory symptoms like syncope or chest pain prior to sudden death 19. Diagnostic workup in patients with the above symptoms (especially young athletes) must include electrocardiography, Holter monitoring and focused expert echocardiography. If at least two normally located coronary ostia can be identified with echocardiography, no further investigations are needed 12. However, if echocardiographic findings are inconclusive, further imaging with computed tomography or magnetic resonance imaging is recommended 12. Treadmill evaluation is unfortunately hampered by a high incidence of false positive and false negative results 17. If the anomaly is confirmed, patients should undergo nuclear stress testing to evaluate exercise-induced ischemia and to establish a baseline for follow-up. Coronary angiography may reveal additional obstructive coronary disease, and intracoronary imaging establishes the severity of the condition 12. Intravascular ultrasound and/or optical coherence tomography findings that determine the severity of an anomalous origin of a coronary artery from an opposite sinus include the length of the intramural segment, the hypoplasia index that quantifies the severity of the circumferential hypoplasia (ratio of the circumference of the intramural segment vs the circumference of the more distal epicardial segment of the vessel), the vessel asymmetry score (ratio of transverse to longitudinal diameter in a cross-sectional image from intravascular ultrasound) and the systolic vs diastolic cross-sectional area of stenosis during a cardiac cycle at rest and during simulated exercise with infusion of saline, atropine and dobutamine (SAD test) 15. When an anomalous left coronary artery with origin from the right sinus of Valsalva is diagnosed incidentally during coronary angiography, specific angiographic anatomical findings help differentiate between the inter-arterial and the more benign trans-septal varieties of this anomaly, since in the trans-septal origin the left coronary artery gives rise to the first septal branch, presents a mild concentric myocardial bridge effect at the distal segment and connects with mid-left anterior descending artery 15. However, computed tomography remains the most accurate method to characterize the course of ectopic coronary arteries.
Asymptomatic patients with anomalous right coronary artery arising from the left sinus of Valsalva with a negative nuclear stress test need regular follow-up only. Symptomatic patients and asymptomatic patients with a positive nuclear stress test must be further assessed with intracoronary imaging with intravascular ultrasound or optical coherence tomography, and if a significant stenosis or high risk features are found, percutaneous coronary intervention with stent 12 or surgical repair should be offered. Patients with anomalous left coronary artery arising from the right sinus of Valsalva should undergo surgical repair regardless of symptoms, if younger than 35 years old. Older patients should undergo surgery if they develop symptoms or if they have a positive nuclear stress test 15. Surgical options include osteoplasty (creation of a new ostium at the end of the ectopic artery’s intramural segment), direct reimplantation of the ectopic artery at the aortic root (a challenging procedure) and unroofing of the intramural segment (excising of the common wall located between the aorta and the anomalous coronary) 15. Currently, unroofing is the most favored of these procedures. Bypass surgery with an internal mammary artery is not preferred, because of the high risk of regression of the graft lumen due to competitive flow 20.
Anomalous origin of coronary arteries outside the aortic root
The most dramatic clinical appearance in this group of congenital coronary anomalies occurs when the ectopic coronary arises from the pulmonary artery. The right coronary artery originates from the pulmonary artery in 0.002% of the general population. In this anomaly, blood flows from the left coronary artery to the right coronary artery via collaterals and further back into the pulmonary artery. Most patients with this anomaly are asymptomatic; however, sudden cardiac death, heart failure and syncope have been occasionally reported 2.
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is called Bland-White-Garland syndrome and has a low prevalence of 0.008%. The right coronary artery is dilated and provides extensive collaterals to left coronary artery. This condition may coexist with aortic coarctation and a patent ductus arteriosus. Most patients (about 85%) develop symptoms of myocardial ischemia and heart failure in infancy and die within the first year of life. However, a minority of patients may remain asymptomatic and survive into adulthood, probably because of adequate blood flow to the left coronary artery territory from the right coronary artery collaterals. Treatment is surgical and reimplantation of the left coronary artery onto the aorta is the preferred method. However, the procedure is more technically challenging in adults due to the frailty of the coronary artery. Ligation of the left coronary artery ostium and coronary artery bypass grafting with venous or arterial grafts is a viable alternative in these cases 21. The left anterior descending artery arises from the pulmonary artery extremely rarely in 0.0008% of the general population and is associated with myocardial ischemia and sudden cardiac death 2. There have also been reports of all coronary arteries originating from the pulmonary artery. Patients die within the first month of life, and the condition frequently coexists with patent ductus arteriosus and other major congenital cardiac anomalies 22. Finally, cases of accessory coronary arteries arising from the pulmonary artery have been documented. The most frequent artery involved is the conus branch, and this anomaly has no clinical significance 2.
Single coronary artery
There have been several reports of presence of a single coronary artery with a variety of anatomies. A single coronary artery may originate either from the left or the right Valsalva sinus and may sometimes coexist with other congenital anomalies 2. Lipton et al 23 proposed an anatomical classification of single coronary arteries based on location of the ostium (R: right Valsalva sinus, L: left Valsalva sinus), anatomical distribution (I: single coronary artery following course of normal left or right coronary artery, II: a coronary artery with abnormal origin from the proximal segment of the other coronary artery, III: single coronary artery with a origin from the right Valsalva sinus, with the left anterior descending artery and left circumflex artery originating separately from the common trunk) and course of the transverse trunk (A: anterior course to the great vessels, B: between the aorta and the pulmonary artery, P: posterior course, S: septal course, C: combined type).
When the single coronary artery has an origin from the left sinus, the right coronary artery may originate from the proximal or mid segment of the left anterior descending artery and follows a course anterior to the pulmonary artery or between the aorta and the pulmonary artery towards the right atrioventricular groove. The prevalence of this anomaly is around 0.024%-0.066% in the general population 24. This anomaly is usually benign, provided that the course of the anomalous right coronary artery is not inter-arterial. If, however, the anomalous right coronary artery crosses between the aorta and the pulmonary artery, myocardial ischemia and sudden cardiac death may occur 25. Rarely, the right coronary artery may be supplied by the distal left circumflex artery.
When the single coronary artery originates from the right sinus, the left coronary artery follows an anterior or inter-arterial path towards its perfusion territory. Prevalence is around 0.02%-0.05%, and this condition is associated with sudden cardiac death more frequently than an anomalous single coronary artery originating from the left sinus. The anterior variant is usually – but not always – benign, whereas the inter-arterial variant is the potentially most threatening one 26.
Anomalies of intrinsic coronary artery anatomy
Split right coronary artery
A split right coronary artery is the most common type of coronary artery anomaly, with a frequency around 1% in the general population. It is a benign anomaly in which the right coronary artery has a split posterior descending branch. The split right coronary artery is divided early into an anterior and posterior branch. The anterior branch runs through the free wall of the right ventricle and supplies a posterior descending branch that follows the distal posterior interventricular groove, whereas the posterior branch follows the right atrioventricular groove and leads to a posterior descending branch that runs through the proximal segment of the posterior interventricular groove 27.
Split left anterior descending artery
There have been reported four types of split left anterior descending artery. In types 1-3, the left anterior descending artery branches into two subdivisions, a short one that terminates high in the anterior interventricular groove and a longer one that branches off the proper left anterior descending artery, descends on the left (type 1) or the right (type 2) ventricular side of the anterior interventricular groove or intramyocardially (type 3) and finally reenters the anterior interventricular groove distally. Sometimes in type 3 the intramyocardial left anterior descending artery never emerges but instead provides septal branches to the apical septum. Type 4 is an entirely different entity, in which a short left anterior descending artery originates from the left coronary artery and terminates high in the anterior interventricular groove, while a duplicated left anterior descending artery arises from the right coronary artery and follows a pre-pulmonic, septal or inter-arterial course towards the distal anterior interventricular groove. This anomaly is relatively benign; however, it may complicate significantly surgical intervention 28.
Atresia of left coronary artery
True atresia of the left coronary artery is an extremely rare congenital disorder in which there is neither left coronary ostium nor left coronary artery. The left anterior descending artery and the left circumflex artery connect but end blindly proximally. The left system receives blood retrogradely via collaterals from the right coronary artery, but in most cases blood flow is inadequate for the needs of the perfusion territory, resulting in symptoms of myocardial ischemia and an increased risk of sudden cardiac death. This condition often coexists with supravalvular aortic stenosis and other congenital cardiac defects. Patients usually become symptomatic during infancy or early childhood, but there have been reports of patients who remained asymptomatic during childhood and young adulthood and developed symptoms later in life. Due to poor prognosis, atresia of the left coronary artery requires surgical intervention with coronary artery bypass grafting and an internal mammary artery graft to the left anterior descending artery in adults, whereas in children surgical reconstruction of the left coronary artery with a baffle of ascending aorta may be preferable 2.
Hypoplasia of coronary arteries
Congenital hypoplasia of coronary arteries presents as a narrowed luminal diameter (less than 1.5 mm) in one or two of the three main coronary arteries with no compensatory branches. Limitations to blood flow caused by the narrow lumen lead to symptoms of myocardial ischemia and sudden cardiac death. Most frequent variants in reported cases are hypoplasia of both left circumflex artery and right coronary artery and hypoplasia of the left anterior descending artery. Treatment options are extremely limited. Transmyocardial revascularization and implantable cardioverter-defibrillator have been suggested in the literature 29.
Anomalies of coronary artery termination
Coronary fistulae are defined as abnormal connections between the termination of a coronary artery or its branches and a low-pressure vascular space, like a cardiac chamber or a great vessel. They may present as small discrete fistulae or more complex arteriovenous malformations. Reported prevalence is 0.3%-0.87% in the general population. Most patients are asymptomatic; however, there have been reports of symptoms of myocardial ischemia, heart failure, arrhythmia and sudden cardiac death, pulmonary hypertension, rupture and endocarditis, usually after the age of 50.
Several mechanisms for the causes of these symptoms have been proposed. Fistulae draining into the right heart chambers (60% of cases) function as left to right shunts and may cause right ventricular volume overload. Termination in a low pressure space causes enlargement and tortuosity of the fistulous coronary artery that leads to vascular wall degeneration, aneurysmatic dilatation and predisposition to rupture. Furthermore, the dilatation of the involved coronary artery may cause distortion of the aortic root and aortic valve disruption and regurgitation. Myocardial ischemia may result from two separate mechanisms: (1) a persistent or episodic steal of blood flow from the normal coronary branches to the competing fistulous low-pressure tract; and (2) stenosis and obstruction of side branches secondary to thrombus formation related to ulceration and atherosclerosis in the aneurysmal coronary artery. Indications for closing a coronary fistula are not well established. Symptomatic patients should definitely be treated as well as patients with a pulmonary to systemic flow ratio that exceeds 1.5 and patients with severe aneurysmal degeneration. Available options are surgical closure at the drainage site or catheter-based repair with catheter occlusion devices. It is generally recommended to intervene before adulthood, since negative postoperative remodeling of the fistulous artery is much more common in children compared to adults 15.
Furthermore, scientists have recently described a fistulous tract from the left ventricle to the Thebesian venous network of the myocardium 30. Thebesian veins are small valveless veins in the walls of all four heart chambers that act as an alternative channel of nutrition to the myocardium or as venous drainage conduits. They are more prevalent in the right heart chambers, but they can also appear in the left ventricle. Aznaouridis et al 30 described the case of inadvertent angiography of a large posterior interventricular cardiac vein during a left ventriculogram. This happened because the end-hole of the angiographic catheter inadvertently engaged the endocardial opening of a small Thebesian vein, leading to retrograde opacification of the cardiac vein through the Thebesian network 30.
Anomalous coronary artery symptoms
Although the spectrum of symptoms and associated syndromes is broad, the coronary artery anomalies that give rise to symptoms are limited to those that cause significant alteration in myocardial perfusion or result in pronounced left-to-right shunting (steal). The majority (approximately 80%) of anomalous coronary artery (anomalous origin of the right or left coronary artery) are benign and asymptomatic. However, on rare occasions, particularly young patients, it can result in myocardial ischemia, myocardial infarction, arrhythmias or sudden death 31. Most symptomatic patients present at a young age. The coronary anomalies most likely to cause myocardial infarction, ischaemia, or ventricular tachycardia are anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), large coronary arteriovenous fistulas, and those anomalies associated with a coronary artery coursing between the great vessels. The other coronary artery anomalies are rarely associated with symptoms or sudden death 4.
When the right coronary artery originates from the pulmonary trunk (ARCAPA), an anomaly rarer than ALCAPA (left coronary artery from the pulmonary artery), there is no ischaemia because the right ventricle is under low pressure; without ischaemia there is no stimulus to form collateral anastomoses with branches of the left coronary artery and thus there is no great tendency to establish fistulous flow.
Both coronary arteries arising from the pulmonary trunk constitute an extremely rare situation. In such cases symptoms appear within a few days of birth, and death follows within two weeks; it is compatible with life only if associated with pulmonary hypertension.
Anomalous coronary artery diagnosis
Cardiac catheterization remains the gold standard in the diagnosis of coronary artery anomalies. The objective of angiocardiography is to establish the integrity and source of the coronary arteries. An aortic root injection may be sufficient to show the early filling of the right coronary artery and the delayed passage via collateral vessels into the left coronary artery and finally the pulmonary trunk.
The finding of coronary artery filling from the pulmonary trunk should rule out ligation of the left main coronary artery at its origin as a form of treatment for the patient.
The diagnosis of ALCAPA in an infant, usually seriously ill, is an indication for urgent surgery; diagnosis in older patients should also be an indication for surgery. Excellent surgical results have been reported following re-establishment of a dual coronary artery system, with direct reimplantation of the coronary artery into the aorta 32, left subclavian artery to left coronary artery anastomosis, or transpulmonary baffling or Takeuchi procedure 33, as it restores oxygenated blood flow to the left ventricular myocardium immediately and more completely. Although clinical improvement with simple ligature of the left coronary artery is possible in individual cases, perioperative mortality associated with this operation seems higher and late results of left ventricular function are less satisfactory 34.
Generally, in infants and young children, dramatic improvement of left ventricular function occurs within the first year after establishment of a dual coronary artery system. The younger the patient the quicker the recovery of ventricular function; this probably reflects the enormous potential for tissue growth in infants which is an argument for operating early. Global left ventricular function was found to be reduced only when major structural anomalies such as left ventricular aneurysm, obstruction of the anastomosis of the left coronary artery with the aorta, and mitral valve replacement were present.
Children with myocardial scars on postoperative nuclear imaging generally have undergone surgery at an older age. This indicates that myocardial ischaemia in ALCAPA may result in more pronounced scarring when surgery is done later in life, emphasising the importance of early surgical intervention as soon as the diagnosis is established 35.
Positron emission tomography, as a non-invasive method for quantitative assessment of myocardial perfusion, can show that myocardial blood flow and coronary flow reserve are greatly reduced in the left coronary artery territories of long term survivors of ALCAPA repair, irrespective of the angiographic status of the graft. This may result in myocardial ischaemia during periods of increased oxygen demand such as exercise, contributing to impaired exercise performance.
Although the extent and severity of stress perfusion defects in adults with coronary artery disease have been shown to have prognostic significance and relate to adverse cardiovascular events, it is unknown whether these prognostic indicators are applicable to long term survivors of ALCAPA repair.
Myocardial perfusion imaging or stress echocardiography should be used to identify patients with severely reduced flow reserve and exercise induced ischaemia. Advice on physical activity should be based on the result of these imaging studies. Anti-ischaemic medications (β blockers) should be considered in patients with evidence of exercise induced myocardial ischaemia or severely reduced myocardial flow reserve. In addition, patient education about known risk factors for coronary artery disease is important.
Anomalous coronary artery treatment
An anomalous left main coronary artery with its origin from the pulmonary artery (ALCAPA) is one of the few clinically significant coronary anomalies. Without surgical correction, ALCAPA is fatal. Simple ligation of the anomalous left coronary artery was the first successful operation described for ALCAPA. Ligation of the left main coronary artery from the pulmonary artery excluded the left-to-right shunt, thus allowing the collaterals from the right coronary artery to perfuse the ventricle. This procedure has since fallen out of favor due to the significant risk of sudden death. Additional surgical corrections include bypass, reimplantation, and in situ conduit procedures, with the ultimate goal of developing a dual coronary artery system. Bypass grafting has used the left subclavian artery, internal mammary artery, and saphenous vein graft, but the results have been disappointing. Ultimately, reimplantation, if possible, is the ideal technique. This approach depends on the left coronary artery to be sufficient length for mobilization to the left sinus of Valsalva. If arterial mobilization is an issue, an ostium can be created in another part of the aorta for reimplantation. Takeuchi fashioned a conduit to direct flow from the aorta to the anomalous artery by creating an aortopulmonary window and intrapulmonary baffle with a flap of the pulmonary artery 36. Dehiscence of the baffle–a cited complication–results in the redevelopment of the left-to-right shunt. The outcome depends on the degree of irreversible ventricular dysfunction. However, neither severe ventricular dysfunction nor mitral insufficiency is a contraindication to revascularization, as significant recovery is the norm. Management of the mitral insufficiency remains controversial. While the regurgitation resolves in most cases with revascularization, the extremely rare case of substantial myocardial ischemia with infarcted papillary muscles exists. In this case, valvular repair has shown good results 37.
Debate on treatment policy
Sudden death without symptoms occurs frequently in patients with anomalous left coronary arteries, so surgical repair is recommended 38. However, sudden death is extremely rare in asymptomatic patient with anomalous right coronary arterys, and there is no sudden death in children under 10 years of age or adults over 30 years of age 38. Eckart et al. 10 reported 21 coronary artery anomalies related to sudden death among 6,300,000 military recruits, and all cases were anomalous left coronary arterys from the right coronary sinus. According to the multi-detector CT imaging-based study of Lee et al., significant stenosis (>50%) of an anomalous right coronary artery occurred in only 1 of 24 patients, and this patient, whose symptoms disappeared after an unroofing procedure, now has an outstanding acute takeoff angle and a small orifice. Other patients with more obtuse angles and mild or absent narrowing of the orifice and artery exhibit no anomaly-related problems in the absence of treatment. One report has suggested that subclinical ischemic changes in the myocardium are relatively frequent (8 of 16 patients) in anomalous right coronary artery patients in the postoperative period 39. Treatment of anomalous right coronary artery with an interarterial course from the left coronary sinus is still debated because most anomalous right coronary arterys are benign, with a small risk of sudden death and late myocardial ischemia after surgery is undertaken. Pelliccia 40 insists on treatment as follows. Young patients (<35 years) with symptoms or ischemia should undergo surgery. In young patients (<35 years) without symptoms or ischemia, the best therapy is uncertain. Older patients without symptoms or ischemia do not need surgical therapy. Strenuous exercise should be limited. Gersony 38 suggests that anomalous right coronary arterys should be followed without intervention and believes that the benefit of excessive exercise limitation is doubtful. If a young, symptomatic patient has significant luminal narrowing on imaging studies, surgical intervention should be considered.
References- Kastellanos S, Aznaouridis K, Vlachopoulos C, Tsiamis E, Oikonomou E, Tousoulis D. Overview of coronary artery variants, aberrations and anomalies. World J Cardiol. 2018;10(10):127–140. doi:10.4330/wjc.v10.i10.127 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6205847
- Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 2016;8:537–555.
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40.
- Hauser M. Congenital anomalies of the coronary arteries. Heart. 2005;91(9):1240–1245. doi:10.1136/hrt.2004.057299 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769093
- Gregg DE, Fisher LC. Blood supply to the heart. In: Handbook of physiology. Washington DC: American Physiological Society, 1963.
- Epstein SE, Cannon RO, Talbot TL. Hemodynamic principles in the control of coronary blood flow. Am J Cardiol 1985;56:4–10.
- Garg N, Tewari S, Kapoor A, Gupta DK, Sinha N. Primary congenital anomalies of the coronary arteries: a coronary: arteriographic study. Int J Cardiol. 2000;74:39–46.
- Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956;14:800–805.
- Pelliccia A, Spataro A, Maron BJ. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am J Cardiol. 1993;72:978–979.
- Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834.
- Angelini P. Coronary artery anomalies–current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29:271–278.
- Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–1305.
- Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369.
- Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501.
- Moscucci M. 2005. Grossman and Baim’s Cardiac Catheterization, Angiography, and Intervention. 8th Edition. Lippincott Williams Wilkins (LWW) pp. 335–353.
- Angelini P, Velasco JA, Ott D, Khoshnevis GR. Anomalous coronary artery arising from the opposite sinus: descriptive features and pathophysiologic mechanisms, as documented by intravascular ultrasonography. J Invasive Cardiol. 2003;15:507–514.
- Angelini P. Coronary artery anomalies–current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29:271–278.
- Cheitlin MD. Coronary anomalies as a cause of sudden death in the athlete. In: Estes NAM, Salem DN, Wang JJP, eds, editors. Sudden Cardiac Death in the Athlete. Armonk NY: Futura Publishing Co; 1998. pp. 379–391.
- Ghosh PK, Agarwal SK, Kumar R, Chandra N, Puri VK. Anomalous origin of right coronary artery from left aortic sinus. J Cardiovasc Surg (Torino) 1994;35:65–70.
- Angelini P. Letter by Angelini regarding article, “long-term outcome and impact of surgery on adults with coronary arteries originating from the opposite coronary cusp” Circulation. 2011;124:e383.
- Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, Schmitt B, Weng YG, Böttcher W, Hübler M, Berger F, Hetzer R. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg. 2011;142:868–874.
- Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986;111:941–963.
- Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: diagnosis, angiographic classification, and clinical significance. Radiology. 1979;130:39–47.
- Yurtdaş M, Gülen O. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Cardiol J. 2012;19:122–129.
- Wann S, Schuchard G. Images in clinical medicine. Anomalous origin of the right coronary artery. N Engl J Med. 2006;355:e8.
- Flessas D, Mamarelis I, Maniatis V, Souretis G, Laschos N, Kotoulas C, Lazaridis K. An unusual pattern of three major components of the cardiovascular system: multimodality imaging and review of the literature. J Cardiothorac Surg. 2013;8:61.
- Gulel O, Yazici M, Durna K, Demircan S. A rare coronary anomaly: double right coronary artery. Clin Cardiol. 2007;30:309.
- Agarwal PP, Kazerooni EA. Dual left anterior descending coronary artery: CT findings. AJR Am J Roentgenol. 2008;191:1698–1701.
- McFarland CA, Svamy RSA. Hypoplastic coronary artery disease: a rare cause of sudden cardiac death and its treatment with an implantable defibrillator. J Cardiol Cases. 2011;4:e148–e151.
- Aznaouridis K, Masoura C, Kastellanos S, Alahmar A. Inadvertent cardiac phlebography. World J Cardiol. 2017;9:558–561.
- Lee BY. Anomalous right coronary artery from the left coronary sinus with an interarterial course: is it really dangerous?. Korean Circ J. 2009;39(5):175–179. doi:10.4070/kcj.2009.39.5.175 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2771782
- Kirklin JW, Barrat-Boyes BG. Cardiac surgery. volume II, 3 ed. Churchill Livingstone 2003 1251.
- Takeuchi S, Imamura H, Katsumoto J, et al. New surgical method for repair of anomalous left coronary artery from the pulmonary artery. J Thorac Cardiovasc Surg 1979;78:7.
- Kreutzer C, Schlichter AJ, Roman MI, et al. Emergency ligation of anomalous left coronary artery arising from the pulmonary artery. Ann Thorac Surg 2000;69:1591.
- Alexanderson E, Attie F, Zabal C, et al. Surgical treatment of anomalous origin of the left coronary artery from the pulmonary artery: late results evaluated with dobutamine stress gated single-photon emission computed tomography sestamibi study. J Nucl Cardiol 1997;4:341–2.
- Takeuchi S, Imamura H, Katsumoto K, Hayashi I, Katohgi T, Yozu R, Ohkura M, Inoue T. New surgical method for repair of anomalous left coronary artery from pulmonary artery. J. Thorac. Cardiovasc. Surg. 1979 Jul;78(1):7-11.
- Yamanaka S, Uchimuro T, Amagaya S, Yun R, Onga Y, Itou C, Saito D, Shimizu M, Wada K, Yoshio T, Takanashi S. [Aorta-left Main Trunk Interposition for Adult Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery Using a Prosthetic Graft;Report of a Case]. Kyobu Geka. 2018 Nov;71(12):1045-1047.
- Gersony WM. Management of anomalous coronary artery from the contralateral coronary sinus. J Am Coll Cardiol. 2007;50:2083–2084.
- Brothers JA, McBride MG, Seliem MA, et al. Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol. 2007;50:2078–2082.
- Pelliccia A. Congenital coronary artery anomalies in young patients: new perspectives for timely identification. J Am Coll Cardiol. 2001;37:598–600.