What is Burkitt lymphoma
Burkitt lymphoma (small noncleaved cell lymphoma) is a rare form of a highly aggressive and very fast-growing type of B-cell non-Hodgkin lymphoma predominantly affecting young children and young adults in Central Africa, but Burkitt lymphoma has also been reported in other areas 1, 2, 3. Burkitt-like lymphoma is considered to be a morphologic variant of Burkitt lymphoma 4. In the United States, Burkitt and Burkitt-like lymphoma/leukemia account for about 40% of childhood non-Hodgkin lymphoma 5. The Burkitt lymphoma seen in Africa seems to be associated with infection by the Epstein-Barr virus (EBV), although the pathogenic mechanism is unclear.
There are 3 recognized forms of Burkitt lymphoma:
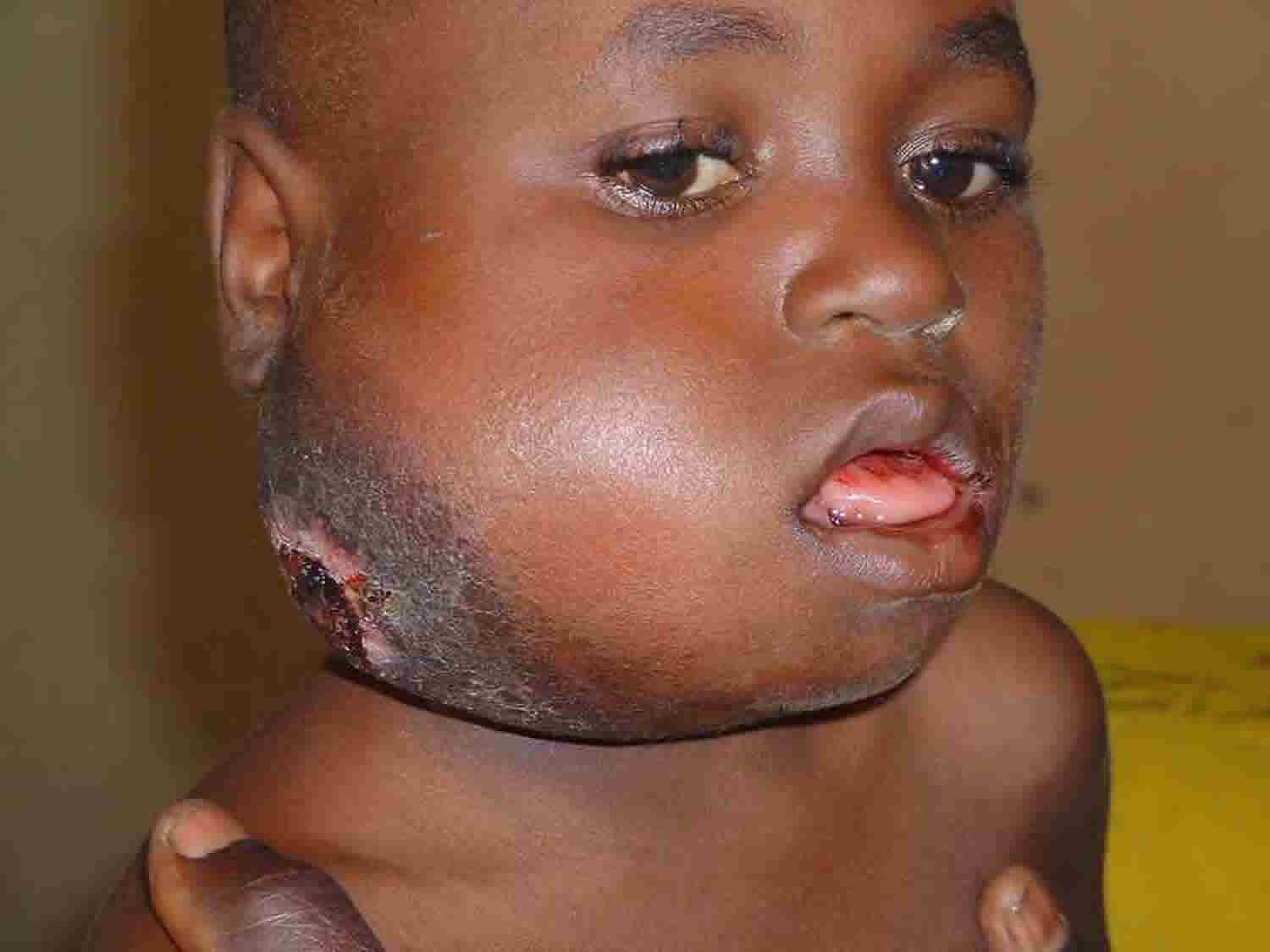
- Endemic (African) Burkitt lymphoma – the most common form, found mainly in central Africa, where it is associated with the Epstein Barr virus (EBV) 6. Endemic (African) Burkitt lymphoma is most common in children. Endemic (African) Burkitt lymphoma often manifests as enlargement of the jaw or facial bones 7.
- Sporadic Burkitt lymphoma – a rarer form, seen in all parts of the world, that often develops in the abdomen with bone marrow involvement. The kidneys, ovaries, breasts or other organs may also be involved 6. This form commonly affects children and young adults 8.
- Immunodeficiency-associated Burkitt lymphoma – occurs primarily in people with HIV infection, and less commonly in people with other immunodeficiency disorders or recipients of organ transplants 9.
These Burkitt lymphoma variants are generally distinguishable by viral association, tumor location, and geographic distribution, although there are exceptions.
Burkitt lymphoma signs and symptoms may differ depending on the form of Burkitt lymphoma and the organs or body systems involved 6. When Burkitt lymphoma spreads, weakness and fatigue often develop. Burkitt lymphoma cells may build up in the lymph nodes and other organs, causing swelling 7. Central nervous system involvement is possible with all forms of Burkitt lymphoma, particularly when there is advanced-stage disease 8.
The exact cause of Burkitt lymphoma is not known. Epstein Barr virus (EBV) infection appears to play a role in virtually all cases of endemic (African) Burkitt lymphoma, and a minority of sporadic and immunodeficiency-associated Burkitt lymphoma 9. While acquired (not inherited) genetic changes involving the chromosome translocations that involve the Myc gene and other genes are present within Burkitt lymphoma cancer cells, it is unclear what causes these genetic changes to occur 3. A chromosome translocation means that a chromosome is broken, which allows it to associate with parts of other chromosomes. The classic chromosome translocation in Burkitt lymophoma involves chromosome 8, the site of the Myc gene. This changes the pattern of Myc’s expression, thereby disrupting its usual function in controlling cell growth and proliferation. Scientists are still not sure what causes chromosome translocation. However, research in model organisms such as mice is leading us toward a better understanding of how translocations occur and, hopefully, how this process contributes to Burkitt lymphoma and other cancers such as leukemia.
Without timely treatment, Burkitt lymphoma is rapidly fatal. Treatment involves intensive chemotherapy, which includes chemotherapy to the fluid surrounding the brain and spinal cord 3. Current chemotherapy regimens are effective in approximately 40-90% of patients. Treatment success depends on age, stage of the disease, treatment regimen, and site of the treatment facility. The variability of tumor response demonstrates the need for new treatments to improve patient outcomes and quality of life. The majority of people treated with aggressive therapy achieve long-term remission 9. If Burkitt lymphoma cancer does not respond to chemotherapy alone, a bone marrow transplant may be done.
What is Burkitt-like lymphoma?
The distinction between Burkitt and Burkitt-like lymphoma/leukemia is controversial 5. Burkitt lymphoma consists of uniform, small, noncleaved cells, whereas the diagnosis of Burkitt-like lymphoma is highly disputed among pathologists because of features that are consistent with diffuse large B-cell lymphoma 10. Studies have demonstrated that the vast majority of Burkitt-like or atypical Burkitt lymphoma/leukemia has a gene expression signature similar to Burkitt lymphoma/leukemia 11. Additionally, as many as 30% of pediatric diffuse large B-cell lymphoma cases will have a gene signature similar to Burkitt lymphoma/leukemia 12.
Is Burkitt lymphoma inherited?
Burkitt lymphoma is not an inherited condition. Burkitt lymphoma almost always occurs in people with no family history of Burkitt lymphoma. There has been one report (in 1986) describing Burkitt lymphoma in more than one family member (two sisters). However, this occurrence was thought to be due to an inherited lymphocyte disorder that may have predisposed the sisters to developing Burkitt lymphoma 13.
While Burkitt lymphoma is associated with genetic changes involving the MYC gene and immunoglobulin genes (genes that provide instructions for antibodies), these genetic changes are acquired (not inherited), and are limited to the cancer cells 13. They are not passed on to offspring.
Is there a known association between Burkitt lymphoma and CHARGE syndrome when they occur in the same family?
A thorough search of available resources does not yield any information about an association between Burkitt lymphoma and CHARGE syndrome.
Burkitt lymphoma causes
Burkitt lymphoma was first discovered in children in certain parts of Africa. It also occurs in the United States.
The African type of Burkitt lymphoma is closely associated with the Epstein-Barr virus (EBV), the main cause of infectious mononucleosis. The North American form of Burkitt lymphoma is not linked to Epstein-Barr virus (EBV).
People with HIV/AIDS have an increased risk for this condition. Burkitt lymphoma is most often seen in males.
Haluska et al. 14 suggested the following scenario for African Burkitt lymphoma: Epstein-Barr virus (EBV) is a polyclonal activator of B lymphocytes, and infection of normal B cells in vitro by Epstein-Barr virus (EBV) is associated with immortalization. In regions of equatorial Africa where Burkitt lymphoma is endemic, 80% of children demonstrate evidence of Epstein-Barr virus (EBV) infection. Malaria is also hyperendemic in the area and causes immunosuppression. Polyclonal B-lymphocyte proliferation therefore proceeds unchecked in the absence of T-cell suppression, probably enlarging the population of cells susceptible to translocation. Translocation involving the immunoglobulin-H (IgH) locus leads to deregulation of the MYC oncogene. In Europe and North America, childhood Epstein-Barr virus (EBV) infection is less frequent, as is malaria. Burkitt lymphoma appears to occur in mature B cells following antigenic stimulation and during isotype switching.
Epstein-Barr virus (EBV) is associated with nearly all Burkitt lymphoma in Africa, but is only associated with 20% or fewer cases of sporadic Burkitt lymphoma worldwide. All Burkitt lymphoma tumors share the translocation of immunoglobulin and MYC genes. Following Epstein-Barr virus (EBV) infection of primary B lymphocytes, Epstein-Barr virus (EBV)-determined nuclear antigens (EBNA) appear, first EBNA2, a transcriptional activator of specific viral and cellular genes, particularly in the NOTCH pathway, then EBNA-leader protein and the other EBNAs. Latent membrane proteins are then expressed, including LMP1, which interacts with TRAFs, and the abundant EBERs (Epstein-Barr virus (EBV)-encoded small nonpolyadenylated RNAs), which are transcribed by RNA polymerase III.
Komano et al. 15 showed that Epstein-Barr virus (EBV)-negative Burkitt lymphoma clones infected with recombinant virus regained the ability of the Epstein-Barr virus (EBV)-positive parent clone to grow on soft agar and to be tumorigenic in immunodeficient SCID mice. In addition, the Epstein-Barr virus (EBV)-positive lines expressed higher levels of BCL2 and were more resistant to apoptosis than Epstein-Barr virus (EBV)-negative cells. Transfection of EBNA1, which is required for replication of the viral episome, into Epstein-Barr virus (EBV)-negative Burkitt lymphoma lines did not restore the malignant phenotype or apoptosis resistance. Komano et al. 15 concluded that persistence of Epstein-Barr virus (EBV) is required for Burkitt lymphoma malignancy and apoptosis resistance.
Komano et al. 16 showed that transfection of EBER1 and EBER2 into Epstein-Barr virus (EBV)-negative Burkitt lymphoma lines restored the capacity for malignancy and apoptosis resistance. They suggested that Epstein-Barr virus (EBV) infection upregulates BCL2 expression, protects cells from MYC-induced apoptosis, and permits MYC to exert its oncogenic functions.
Kitagawa et al. 17 found that the EBERs of Epstein-Barr virus (EBV)-positive Akata and Mutu Burkitt lymphoma cell lines activated higher levels of IL10 expression than Epstein-Barr virus (EBV)-negative cells and enabled growth of Burkitt lymphoma cells. RT-PCR analysis revealed that Epstein-Barr virus (EBV)-positive but not Epstein-Barr virus (EBV)-negative Burkitt lymphoma tumors expressed both EBERs and IL10, suggesting that Burkitt lymphoma cells use IL10 as an autocrine growth factor. IL10 enhanced the growth of Epstein-Barr virus (EBV)-negative cells in culture, but transfection of IL10 into such cells did not confer tumorigenicity in SCID mice. Kitagawa et al. 17 proposed that RNA molecules can regulate cell growth.
The Epstein-Barr virus (EBV) growth-transforming (Latency III) program of gene expression is extinguished in tumor cells, and only a single viral protein, EBNA1, is expressed via the alternative Latency I program. It was not known if Burkitt lymphoma arises from a B-cell subset in which Epstein-Barr virus (EBV) naturally adopts a Latency I infection or if selection of a clone with limited antigen expression from an Epstein-Barr virus (EBV)-transformed Latency III progenitor pool occurs. Kelly et al. 18 identified a subset of Burkitt lymphoma tumors in which the Latency III-associated EBNA promoter Wp is active and most EBNAs are expressed, but where a gene deletion has specifically abrogated the expression of EBNA2. Kelly et al. 18 concluded that Burkitt lymphoma can be selected from a Latency III progenitor and that the principal selection pressure is for downregulation of the c-Myc antagonist EBNA2.
Schmitz et al. 19 used high-throughput RNA sequencing and RNA interference screening to discover essential regulatory pathways in Burkitt lymphoma that cooperate with MYC, the defining oncogene of this cancer. In 70% of sporadic Burkitt lymphoma cases, mutations affecting the transcription factor TCF3 (E2A) or its negative regulator ID3 fostered TCF3 dependency. TCF3 activated the prosurvival phosphatidylinositol-3OH kinase pathway in Burkitt lymphoma, in part by augmenting tonic B-cell receptor signaling. In 38% of sporadic Burkitt lymphoma cases, oncogenic CCND3 mutations produced highly stable cyclin D3 isoforms that drive cell cycle progression.
Varano et al. 20 studied the effects of BCR ablation on MYC-driven mouse B-cell lymphomas and compared them with observations in human Burkitt lymphoma. Whereas BCR ablation does not, per se, significantly affect lymphoma growth, BCR-negative (BCR-) tumor cells rapidly disappear in the presence of their BCR-expressing (BCR+) counterparts in vitro and in vivo. This requires neither cellular contact nor factors released by BCR+ tumor cells. Instead, BCR loss induces the rewiring of central carbon metabolism, increasing the sensitivity of receptor-less lymphoma cells to nutrient restriction. The BCR attenuates glycogen synthase kinase-3-beta (GSK3-beta) activity to support MYC-controlled gene expression. BCR- tumor cells exhibit increased GSK3-beta activity and are rescued from their competitive growth disadvantage by GSK3-beta inhibition. BCR- lymphoma variants that restore competitive fitness normalize GSK3-beta activity after constitutive activation of the MAPK pathway, commonly through Ras mutations. Similarly, in Burkitt lymphoma, activating RAS mutations may propagate immunoglobulin-crippled tumor cells, which usually represent a minority of the tumor bulk. Thus, while BCR expression enhances lymphoma cell fitness, BCR-targeted therapies may profit from combinations with drugs targeting BCR- tumor cells.
Burkitt lymphoma tumor biology
The malignant cells show a mature B-cell phenotype and are negative for the enzyme terminal deoxynucleotidyl transferase. These malignant cells usually express surface immunoglobulin, most bearing a clonal surface immunoglobulin M with either kappa or lambda light chains. A variety of additional B-cell markers (e.g., CD19, CD20, CD22) are usually present, and most childhood Burkitt and Burkitt-like lymphomas/leukemias express CALLA (CD10) 21.
Burkitt lymphoma expresses a characteristic chromosomal translocation, usually t(8;14) and more rarely t(8;22) or t(2;8). Each of these translocations juxtaposes the MYC oncogene and immunoglobulin locus regulatory elements, resulting in the inappropriate expression of MYC, a gene involved in cellular proliferation 22. The presence of one of the variant translocations t(2;8) or t(8;22) does not appear to affect response or outcome 23.
While MYC translocations are present in all Burkitt lymphoma, cooperating genomic alterations appear to be required for lymphoma development. Recurring mutations that have been identified in Burkitt lymphoma in pediatric and adult cases are listed below. The clinical significance of these mutations for pediatric Burkitt lymphoma remains to be elucidated.
- Activating mutations in the transcription factor TCF3 and inactivating mutations in its negative regulator ID3 are observed in approximately 70% of Burkitt lymphoma cases 24.
- Mutations in TP53 are observed in one-third to one-half of cases 25.
- Mutations in cyclin D3 (CCND3) are commonly observed in sporadic Burkitt lymphoma (approximately 40% of cases) but are rare in endemic Burkitt lymphoma 25.
- Mutations in MYC itself are observed in approximately one-half of Burkitt lymphoma cases and appear to increase MYC stability 26.
Cytogenetic evidence of MYC rearrangement is the gold standard for diagnosis of Burkitt lymphoma/leukemia. For cases in which cytogenetic analysis is not available, the World Health Organization (WHO) has recommended that the Burkitt-like diagnosis be reserved for lymphoma resembling Burkitt lymphoma/leukemia or with more pleomorphism, large cells, and a proliferation fraction (i.e., MIB-1 or Ki-67 immunostaining) of 99% or greater 21. BCL2 staining by immunohistochemistry is variable. The absence of a translocation involving the BCL2 gene does not preclude the diagnosis of Burkitt lymphoma/leukemia and has no clinical implications 27.
Burkitt lymphoma signs and symptoms
Childhood Burkitt lymphoma (non-Hodgkin lymphoma) can cause many different signs and symptoms, depending on where it is in the body.
Burkitt lymphoma may first be noticed as a swelling of the lymph nodes (glands) in the head and neck. These swollen lymph nodes are often painless, but can grow very rapidly.
In the types commonly seen in the United States, the cancer often starts in the belly area (abdomen). The disease can also start in the ovaries, testes, brain, kidneys, liver, and spinal fluid.
Common symptoms include:
- Enlarged lymph nodes (seen or felt as lumps under the skin)
- Swollen abdomen (belly)
- Feeling full after only a small amount of food
- Shortness of breath or cough
- Fever
- Unexplained weight loss
- Night sweats
- Fatigue (feeling very tired)
Enlarged lymph nodes
Non-Hodgkin lymphoma may grow in lymph nodes under the skin (on the sides of the neck, in the underarm area, above the collar bone, or in the groin area). The enlarged nodes are often seen or felt as lumps under the skin and are not usually painful. They are often first noticed by the child, parent, or a health care provider.
Enlarged lymph nodes in children are more often caused by infections than by non-Hodgkin lymphoma. Lymph nodes that grow in reaction to infection are called reactive nodes or hyperplastic nodes and are often tender to the touch.
Lymphoma in the abdomen (belly)
Lymphoma growing inside the abdomen can make it swollen and painful. There may also be a buildup of fluid that causes even more swelling.
Lymphoma can sometimes enlarge the spleen and make it press on the stomach. This can make a child feel full after eating only a small amount of food.
When lymphoma causes swelling near the intestines, bowel movements may be blocked, which may lead to belly pain, nausea, and vomiting.
Lymphoma can also block urine from leaving the kidneys. This can lead to low urine output, tiredness, loss of appetite, nausea, or swelling in the hands or feet.
Lymphoma in the chest
When lymphoma starts in the thymus (a small organ in the middle of the chest) or lymph nodes in the chest, it can press on the nearby trachea (windpipe). This can lead to coughing, shortness of breath, and trouble breathing.
The superior vena cava (SVC) is a large vein that carries blood from the head and arms back to the heart. It passes next to the thymus and lymph nodes inside the chest. Lymphomas in this area may press on the superior vena cava, which can make the blood back up in the veins. This is can lead to swelling in the face, neck, arms, and upper chest (sometimes with a bluish-red skin color). It can also cause trouble breathing, as well as headaches, dizziness, and a change in consciousness if it affects the brain. This condition, known as superior vena cava syndrome, can be life-threatening, so it needs to be treated right away.
Lymphoma in the brain and spinal cord
Some types of lymphoma can spread to the area around the brain and spinal cord. This can cause problems such as headache, nausea, vision changes, facial numbness, and trouble talking.
Lymphoma in the skin
Some lymphomas can affect the skin itself. They can cause itchy, red or purple lumps or nodules under the skin.
General lymphoma symptoms (B symptoms)
Along with causing symptoms in the part of the body where it starts, non-Hodgkin lymphoma can also cause general symptoms such as:
- Fever and chills
- Sweating (particularly at night)
- Unexplained weight loss
When talking about lymphoma, doctors sometimes call these B symptoms. B symptoms are often found in more rapidly growing lymphomas.
Other symptoms can be caused by low blood cell counts. Blood counts can become low if lymphoma spreads to the bone marrow and crowds out the normal, healthy cells that make new blood cells. This can lead to problems like:
- Severe or frequent infections (from low white blood cell counts)
- Easy bruising or bleeding (from low blood platelet counts)
- Fatigue and pale skin (from low red blood cell counts; anemia)
Many of the signs and symptoms above are more likely to be caused by something other than a lymphoma, such as an infection. Still, if your child has any of these symptoms, check with the doctor so that the cause can be found and treated, if needed.
Burkitt lymphoma possible complications
Possible complications of Burkitt lymphoma include:
- Complications of treatment
- Spread of the cancer
Burkitt lymphoma diagnosis
Burkitt lymphoma is usually found when a child is brought to a doctor because of signs or symptoms he or she is having. This might lead the doctor to suspect the child could have a lymphoma, but tests are needed to confirm this. The exams and tests below are used to diagnose lymphoma, to find out what type it is, and to learn how advanced it is.
Medical history and physical exam
If any signs and symptoms suggest a child might have lymphoma, the doctor will ask about the symptoms and how long they have been present. The doctor might also ask if there is any history of possible risk factors, such as immune system problems.
During the physical exam, the doctor will probably focus on any enlarged lymph nodes or other areas of concern. For example, the abdomen (belly) may be felt for signs of an enlarged spleen or liver.
The most common cause of enlarged lymph nodes in a child is an infection, so this is often what doctors think of first. Because of this, the diagnosis of Burkitt lymphoma in a child can sometimes be delayed. There is usually little cause for concern in children with swollen lymph nodes unless they are very large (more than 1 inch across). Even in these instances, the child is usually watched closely for a time or given a course of antibiotics first to see if the nodes will shrink. If they don’t, more tests are done, such as a biopsy to remove part or all of a swollen node (see next section). But if the lymph nodes seem to be growing quickly or the child’s health seems to be getting worse, a biopsy may be needed right away.
Tests for Burkitt lymphoma (Non-Hodgkin Lymphoma) in Children
Biopsy
A doctor can’t diagnose Burkitt lymphoma in a child based only on symptoms or a physical exam. Most of the symptoms non-Hodgkin lymphoma can cause are more often caused by other problems, like infections. They may also be caused by other kinds of cancers. If a child does have non-Hodgkin lymphoma, it’s important to know which type it is, because each type is treated slightly differently.
The only way to diagnose these things for sure is to remove some or all of an abnormal lymph node (or tumor) for viewing under a microscope and other lab tests. This is called a biopsy.
Types of biopsies used to diagnose non-Hodgkin lymphoma
There are several types of biopsies. Doctors choose which one to use based on the situation. The goal is to get a sample large enough to make an accurate diagnosis as quickly as possible, with as few side effects as possible.
Surgical (excisional or incisional) biopsy: These are the most common types of biopsies done if lymphoma is suspected. An exception might be large chest tumors, for which a needle biopsy (described below) might be used instead.
In these procedures, a surgeon cuts through the skin to remove either an entire lymph node (excisional biopsy) or a small part of a large tumor (incisional biopsy).
If the node is near the skin surface, this is an operation that might be done with either local anesthesia (numbing medicine at the biopsy site) and sedation, or with general anesthesia (where the child is in a deep sleep). If the node is inside the chest or abdomen, then general anesthesia is typically needed.
This method almost always provides enough of a sample to diagnose the exact type of non-Hodgkin lymphoma.
Needle biopsy: These biopsies use hollow needles to remove small pieces of tissue. There are 2 main types:
- In an fine needle aspiration (FNA) biopsy, the doctor uses a very thin, hollow needle attached to a syringe to withdraw (aspirate) a small amount of tissue from an enlarged lymph node or a tumor mass.
- For a core needle biopsy, the doctor uses a larger needle to remove a slightly larger piece of tissue.
If an enlarged lymph node is just below the skin, the doctor can aim the needle while feeling the node. If the enlarged node or tumor is deep in the body (such as in the chest or abdomen), the doctor can guide the needle while watching it on a CT scan or ultrasound (see discussion of imaging tests later in this section).
The main advantage of a needle biopsy is that it does not require surgery. This can be especially important for children with tumors in the chest, because general anesthesia (where the child is in a deep sleep) can sometimes be dangerous for these children. A needle biopsy is also useful when the lymphoma is in places other than the lymph nodes, such as the bones.
In children, needle biopsies can often be done using local anesthesia to numb the area, along with sedation to make the child sleepy. General anesthesia is needed less often.
The main drawback of needle biopsies (especially fine needle aspiration [FNA]) is that sometimes the needle might not remove enough of a sample to make a definite diagnosis. Most doctors don’t use needle biopsies if they strongly suspect lymphoma (unless other types of biopsies can’t be done for some reason). But if the doctor suspects that lymph node swelling is caused by an infection (even after antibiotics), a needle biopsy may be the first type of biopsy done. If a biopsy is needed, doctors typically prefer to do a core biopsy instead of fine needle aspiration (FNA). An excisional biopsy might still be needed to diagnose and classify lymphoma, even after a needle biopsy has been done.
Once lymphoma has been diagnosed, needle biopsies are sometimes used to check areas in other parts of the body that might be lymphoma spreading or coming back after treatment.
Other types of biopsies
These other types of biopsies are not normally used to diagnose lymphoma, but they might be done if a lymphoma has already been diagnosed to help show how far it has spread.
Bone marrow aspiration and biopsy: These tests can show if a lymphoma has reached the bone marrow. The 2 tests are usually done at the same time. The biopsy samples are usually taken from the back of the pelvic (hip) bones, although sometimes they may be taken from the front of the hip bones or from other bones.
For a bone marrow aspiration, the skin over the hip and the surface of the bone is numbed with local anesthetic. In most cases, children will be given other medicines to make them drowsy or asleep during the biopsy. A thin, hollow needle is then inserted into the bone, and a syringe is used to suck out a small amount of liquid bone marrow.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is put into the bone. Once the biopsy is done, pressure will be applied to the site to help stop any bleeding.
Lumbar puncture (spinal tap): This test is used to look for lymphoma cells in the cerebrospinal fluid (CSF), which is the fluid that surrounds the brain and spinal cord.
For this test, the doctor first numbs an area in the lower part of the back near the spine. The doctor usually also gives the child medicine to make him or her sleep during the procedure. A small, hollow needle is then placed between the bones of the spine to withdraw some of the fluid.
In children already diagnosed with lymphoma, a lumbar puncture can also be used to put chemotherapy drugs into the CSF to try to prevent or treat the spread of lymphoma to the spinal cord and brain.
Pleural or peritoneal fluid sampling: If lymphoma spreads to the thin membranes that line the inside of the chest and abdomen it can cause fluid to build up. Pleural fluid (inside the chest) or peritoneal fluid (inside the belly) can be removed by putting a hollow needle t through the skin into the chest or abdomen.
- When this procedure is used to remove fluid from the chest, it’s called a thoracentesis.
- When it’s used to collect fluid from inside the belly, it’s known as a paracentesis.
Before the procedure, the doctor uses a local anesthetic to numb the skin and may give the child other medicines so they are drowsy or asleep during the procedure. The fluid is then drawn out and looked at with a microscope to check for lymphoma cells
Lab tests on biopsy samples
All biopsy samples and fluids are looked at by a pathologist (a doctor with special training in using lab tests to identify cancer cells). The doctor uses a microscope to look at the size and shape of the cells and how they are arranged. This can show if a child has lymphoma, and sometimes what type of lymphoma it is, as well. But usually other types of lab tests will be needed, too.
Flow cytometry and immunohistochemistry
For flow cytometry and immunohistochemistry, samples of cells are treated with antibodies, which are proteins that stick only to certain other proteins on cells. For immunohistochemistry, the cells are then looked at with a microscope to see if the antibodies stuck to them (meaning they have these proteins), while for flow cytometry a special machine is used.
These tests can help determine if a lymph node is swollen because of lymphoma, some other cancer, or a non-cancerous disease. The tests can also be used for immunophenotyping – determining which type of lymphoma a child has, based on certain proteins in or on the cells. Different types of lymphocytes have different proteins on their surface, which correspond to the type of lymphocyte and how mature it is.
Chromosome tests
Doctors use these tests to evaluate the chromosomes (long strands of DNA) in the lymphoma cells. In some types of lymphoma, the cells have changes in their chromosomes, such as having too many, too few, or abnormal chromosomes. These changes can often help identify the type of lymphoma.
Cytogenetics: In this type of lab test, the cells are looked at under a microscope to see if the chromosomes have any abnormalities. A drawback of this test is that it usually takes about 2 to 3 weeks because the lymphoma cells must grow in lab dishes for a couple of weeks before their chromosomes are ready to be seen with a microscope.
Fluorescent in situ hybridization (FISH): This test looks more closely at lymphoma cell DNA using fluorescent dyes that only attach to specific gene or chromosome changes. Fluorescent in situ hybridization (FISH): can find most chromosome changes (such as translocations) that can be seen under a microscope on standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing. Fluorescent in situ hybridization (FISH): is very accurate and results are usually ready within a couple of days, which is why this test is now used in many medical centers.
Polymerase chain reaction (PCR): This is a very sensitive DNA test that can also find some chromosome changes too small to be seen with a microscope, even if there are very few lymphoma cells in a sample.
Blood tests
Blood tests measure the amounts of certain types of cells and chemicals in the blood. They are not used to diagnose lymphoma, but they might be one of the first types of tests done in children with symptoms to help the doctor determine what is going on. If a child has been diagnosed with lymphoma, these tests can also sometimes help tell how advanced the lymphoma is.
- The complete blood count (CBC) is a test that measures the levels of different cells in the blood, such as the red blood cells, the white blood cells, and the platelets. In children already known to have lymphoma, low blood cell counts might mean that the lymphoma is growing in the bone marrow and damaging new blood cell production.
- Blood levels of lactate dehydrogenase (LDH) may be checked. Lactate dehydrogenase (LDH) will often be abnormally high in patients with fast-growing lymphomas.
- Blood chemistry tests can help detect liver or kidney problems caused by the spread of lymphoma cells or certain chemotherapy drugs. These tests can also help determine if treatment is needed to correct low or high blood levels of certain minerals.
- Tests may also be done to make sure the blood is clotting properly.
- For some types of lymphoma, the doctor might also want to order other blood tests to see if the child has been infected with certain viruses, such as the Epstein-Barr virus (EBV), hepatitis B virus (HBV), or human immunodeficiency virus (HIV). Infections with some of these viruses can affect your child’s treatment.
Imaging tests
Imaging tests use x-rays, sound waves, magnetic fields, or radioactive substances to create pictures of the inside of the body. These tests might be done for a number of reasons, including:
- To look for possible causes of certain symptoms (such as trouble breathing)
- To help determine the stage (extent) of the lymphoma
- To help show if treatment is working
- To look for possible signs of lymphoma coming back after treatment
A child with a known or suspected lymphoma might need one or more of these tests.
Chest x-ray
A chest x-ray may be done to look for enlarged lymph nodes inside the chest.
Computed tomography (CT or CAT) scan
The CT scan combines many x-rays to make detailed, cross-sectional images of the body. CT scans can be used to look for enlarged lymph nodes or other masses in the chest, abdomen, pelvis, head, and neck.
During the test, your child will need to lie still on a table that slides in and out of the ring-shaped scanner. Some younger children may be given medicine to help keep them calm or even asleep during the test to help make sure the pictures come out well.
CT-guided needle biopsy: A CT scan can also be used to guide a biopsy needle precisely into a suspected tumor or enlarged lymph node. For this procedure, the child remains asleep on the CT scanning table, while the doctor advances a biopsy needle through the skin and toward the area. CT scans are repeated until the needle is in the right place. A biopsy sample is then removed and looked at under a microscope.
Ultrasound (sonogram)
Ultrasound uses sound waves and their echoes to create pictures of internal organs or masses.
Ultrasound can be used to look at lymph nodes near the surface of the body or to look inside the abdomen (belly) for enlarged lymph nodes or organs such as the liver, spleen, and kidneys. (It can’t be used to look inside the chest because the ribs block the sound waves.) It is also sometimes used to help guide a biopsy needle into an enlarged lymph node.
Magnetic resonance imaging (MRI) scan
An MRI scan, like a CT scan, shows detailed images of soft tissues in the body. This test is not used as often as CT scans for lymphoma, but MRI is very useful for looking at the brain and spinal cord if a child has symptoms that might be caused by problems in the nervous system.
MRI scans take longer than CT scans, often up to an hour. Your child may have to lie inside a narrow tube, which can be distressing, so sedation is sometimes needed. Newer, more open MRI machines may be another option, although your child will still have to lie still.
Positron emission tomography (PET) scan
For a PET scan, a slightly radioactive sugar is injected into the blood. (The amount of radioactivity used is very low and will pass out of the body within a day or so.) Because lymphoma cells grow quickly, they absorb more of the sugar. After about an hour, your child will be moved onto a table in the PET scanner. He or she will lie on the table for about 30 minutes while a special camera creates a picture of areas of radioactivity in the body. Younger children may be given medicine to help keep them calm or even asleep during the test.
The picture from a PET scan is not detailed like a CT or MRI scan, but it provides helpful information about the whole body.
PET scans can be used for many reasons in a child with lymphoma:
- They can help tell if an enlarged lymph node contains lymphoma.
- They can help spot small areas in the body that might be lymphoma, even if the area looks normal on a CT scan.
- They can help tell if a lymphoma is responding to treatment. Some doctors will repeat the PET scan after 1 or 2 courses of chemotherapy. If the chemotherapy is working, the lymphoma will no longer show up as well on the scan.
- They can be used after treatment to help decide if an enlarged lymph node still contains lymphoma or is just scar tissue.
PET/CT or PET/MRI scan: Some newer machines can do both a PET as well as a CT or MRI scan at the same time. This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT or MRI scan.
Bone scan
A bone scan is not usually needed unless a child is having bone pain or has lab test results that suggest the lymphoma might have reached the bones.
For this test, a radioactive substance called technetium is injected into the blood. (The amount of radioactivity used is very low and will pass out of the body within a day or so. Technetium travels to damaged areas of the bone over a couple of hours. Your child then lies on a table for about 30 minutes while a special camera detects the radioactivity and creates a picture of the skeleton. Younger children may be given medicine to help keep them calm or even asleep during the test.
A bone scan can detect bone damage from lymphoma. But a bone scan may also show other things that are not cancer, so other tests might be needed to be sure.
Burkitt lymphoma staging
A lymphoma’s stage s based on the results of physical exams, biopsies, and imaging tests (CT scan, PET scan, etc.), which are described in Tests for Burkitt lymphoma (non-Hodgkin Lymphoma) in Children.
A staging system is a standard way for the cancer care team to describe how far a cancer has spread. The staging system most often used to describe the spread of non-Hodgkin Lymphoma in children is called the St. Jude staging system. This is different from the staging system used for lymphomas in adults.
St. Jude staging system
The St. Jude system divides non-Hodgkin Lymphoma in children into 4 stages:
- Stage I and II lymphomas are usually considered limited-stage disease and are treated the same way.
- Stage III and IV lymphomas are usually thought of as advanced-stage disease and are also treated alike.
Stage 1
The lymphoma is in only one place, either as a single tumor not in lymph nodes, or in lymph nodes in one part of the body (the neck, groin, underarm, etc.). The lymphoma is not in the chest or abdomen (belly).
Stage 2
Stage II lymphomas are not in the chest, and one of the following applies:
- The lymphoma is a single tumor and is also in nearby lymph nodes in only one part of the body (the neck, groin, underarm, etc.).
- The lymphoma is more than one tumor and/or in more than one set of lymph nodes, all of which are either above or below the diaphragm (the thin breathing muscle that separates the chest and abdomen). For example, this might mean nodes in the underarm and neck area are affected but not the combination of underarm and groin nodes.
- The lymphoma started in the digestive tract (usually at the end of the small intestine) and can be removed by surgery. It might or might not have reached nearby lymph nodes.
Stage 3
For stage III lymphomas, one of the following applies:
- The lymphoma started in the chest (usually in the thymus or lymph nodes in the center of the chest or the lining of the lung).
- The lymphoma started in the abdomen and has spread too widely within the abdomen to be removed completely by surgery.
- The lymphoma is located next to the spine (and may be elsewhere as well).
- The lymphoma is more than one tumor or in more than one set of lymph nodes that are both above and below the diaphragm. For example, the lymphoma is in both underarm and groin lymph nodes.
Stage 4
The lymphoma is in the central nervous system (brain or spinal cord) and/or the bone marrow when it is first found. (If more than 25% of the bone marrow is made up of cancer cells, called blasts, the cancer is classified as acute lymphoblastic leukemia instead of lymphoma.)
Burkitt lymphoma prognosis
Without timely treatment, Burkitt lymphoma is rapidly fatal 28. More than one half of people with Burkitt lymphoma can be cured with intensive chemotherapy. The cure rate may be lower if the Burkitt lymphoma cancer spreads to the bone marrow or spinal fluid. The outlook is poor for both children and adults if Burkitt lymphoma returns after improvement (relapses) or does not go into remission as a result of the first cycle of chemotherapy 29. However, the majority (more than 80% of children and adolescents) of people treated with aggressive therapy achieve long-term remission (survive at least 5 years) 30, 9.
In general, children with Burkitt lymphoma have better survival rates than adults with Burkitt lymphoma. The prognosis in children correlates with the extent of disease at the time of diagnosis. Those with limited disease when diagnosed and treated have a survival rate greater than 90%. Children with more extensive disease, especially involving the bone marrow and central nervous system, have long-term survival rates of 50-90% 6.
While the majority of adults with Burkitt lymphoma also achieve long-term remission with aggressive therapy, adults (particularly those with advanced stage disease) do more poorly than children 6. In addition to the extent of disease at the time of diagnosis, prognosis depends on the age of the adult. In general, survival rates decrease with age and are lowest for elderly patients 6. Unfortunately, specific statistics regarding the prognosis for adults with Burkitt lymphoma appear to be more scarce 31.
How long can you live with Burkitt lymphoma?
In high-income countries and with current treatments, more than 80% of children and adolescents with non-Hodgkin lymphoma will survive at least 5 years, although outcome depends on a number of factors, including clinical stage and histology 27.
Cancer survival rates don’t tell the whole story. Survival rates are often based on previous outcomes of large numbers of children who had the disease, but they can’t predict for sure what will happen in any particular child’s case. There are some limitations to keep in mind:
- The outlook for children with non-Hodgkin lymphoma varies by the type and stage (extent) of the lymphoma. But other factors can also affect a child’s outlook, such as the location and size of the tumor(s), and how well the lymphoma responds to treatment. The outlook for each child is specific to their circumstances.
- The numbers below are among the most current available. But to get these survival rates, doctors have to look at children were treated at least several years ago. As treatments are improving over time, children who are now being diagnosed with non-Hodgkin lymphoma may have a better outlook than these statistics show.
Your child’s doctor can tell you how these numbers might apply to your child’s particular situation.
Survival rates tell you what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell exactly what will happen with any person, but they may help give you a better understanding about how likely it is that treatment will be successful.
- Treatment of limited stage (stage 1 and 2) Burkitt lymphomas is usually very successful, with a long-term survival rate of over 90% 32.
- The long-term survival rate for children with more advanced (stage 3 or 4) Burkitt lymphoma ranges from about 80% to 90% 32.
Prognostic factors for childhood non-Hodgkin lymphoma include the following:
- Response to therapy.
- Stage at diagnosis/presence of minimal disseminated disease (MDD).
- Sites of disease at diagnosis.
- Tumor biology.
- Age.
- Immune response to tumor.
Response to therapy
Response to therapy in pediatric lymphoma is one of the most important prognostic markers. Regardless of histology, pediatric non-Hodgkin lymphoma that is refractory to first-line therapy has a very poor prognosis 12.
One of the most important predictive factors is response to the initial prophase treatment; poor responders (i.e., <20% resolution of disease) have an event-free survival of 30% 33.
International pediatric non-Hodgkin lymphoma response criteria have been proposed but require prospective evaluation. The clinical utility of these new criteria are under investigation 34.
Unlike in acute leukemia, in pediatric non-Hodgkin lymphoma, the prognostic value of minimal residual disease after therapy is initiated remains uncertain and requires further investigation.
One study suggests an inferior outcome for patients with Burkitt lymphoma/leukemia who had detectable minimal residual disease after induction chemotherapy 35. However, other studies found that detectable minimal residual disease at the end of induction was not prognostic, possibly because of the low number of relapses in patients with disease detected in blood or bone marrow at diagnosis 36.
Stage at diagnosis
In general, patients with low-stage disease (i.e., single extra-abdominal/extrathoracic tumor or totally resected intra-abdominal tumor) have an excellent prognosis (a 5-year survival rate of approximately 90%), regardless of histology 37. Apart from this finding, the outcome by clinical stage, if the correct therapy is given, does not differ significantly.
A surrogate for tumor burden (i.e., elevated levels of LDH) has been shown to be prognostic in many studies 38.
Minimal disseminated disease is generally defined as submicroscopic bone marrow involvement that is present at diagnosis. minimal disseminated disease is generally detected by sensitive methods such as flow cytometry or reverse transcription–polymerase chain reaction (RT-PCR). Patients with morphologically involved bone marrow with more than 5% lymphoma cells are considered to have stage 4 disease.
The role of minimal disseminated disease for Burkitt lymphoma remains to be defined. One study suggests minimal disseminated disease is predictive of outcome 39, while another study does not 36.
Sites of disease at diagnosis
In pediatric non-Hodgkin lymphoma, some sites of disease appear to have prognostic value, including the following:
- Bone marrow and CNS: Bone marrow and CNS (central nervous system = brain and spinal cord) involvement at diagnosis usually requires more intensive therapy 37. Although these intensive therapies produce improved outcomes, patients who present with CNS disease continue to have the worst outcomes 40. Patients with mature B-cell lymphoma/leukemia with CNS disease at presentation have a 3-year event-free survival of around 70%, while those with bone marrow involvement alone have a 3-year event-free survival of 90% 35. The combination of CNS involvement and bone marrow disease appears to impact outcome the most 41.
- Mediastinum: Mediastinal involvement in children and adolescents with nonlymphoblastic non-Hodgkin lymphoma results in an inferior outcome 35. In children and young adults with primary mediastinal B-cell lymphoma, series have reported a 3-year event-free survival of 50% to 70% 42. However, studies using the dose-adjusted (DA)–EPOCH protocol (etoposide, prednisone, vincristine, and doxorubicin) with rituximab have reported an event-free survival higher than 80% 43.
- Viscera: For anaplastic large cell lymphoma, a retrospective study by the European Intergroup for Childhood non-Hodgkin lymphoma found a high-risk group of patients defined by involvement of mediastinum, skin, or viscera 44. In a subsequent study analysis from EICnon-Hodgkin lymphoma utilizing biologic risk factors, the clinical risk features were not found to be significant 45. In the CCG-5941 (https://clinicaltrials.gov/ct2/show/NCT00002590) study for anaplastic large cell lymphoma patients, these clinical risk factors could not be confirmed; only bone marrow involvement predicted inferior progression-free survival 46.
- Bone: Although previously thought to be a poor prognostic site, patients with non-Hodgkin lymphoma arising in bone have an excellent prognosis, regardless of histology 47.
- Testicle: Testicular involvement does not affect prognosis 48.
- Head and Neck: For mature B-cell non-Hodgkin lymphoma, overall survival is comparable to that observed for patients with primary tumors at other sites. Head and neck primary tumors are associated with higher rates of disseminated and CNS disease and lower rates of LDH levels that were more than twofold higher than the upper limit of normal. Childhood non-Hodgkin lymphoma of the head and neck site was not associated with inferior overall survival 49.
- Skin: The prognostic implication of skin involvement is limited to anaplastic large cell lymphoma and depends on whether the disease is localized to skin. ALK-negative, skin-limited anaplastic large cell lymphoma appears to have an excellent prognosis. However, studies from European Intergroup for Childhood non-Hodgkin lymphoma and the Children’s Oncology Group have demonstrated that skin involvement in systemic anaplastic large cell lymphoma does not appear to have positive prognostic value 45.
Tumor biology
Mature B-cell lymphoma: Compared with treatments for adults, aggressive Burkitt regimens in pediatrics have been used to treat both Burkitt lymphoma/leukemia and large B-cell histologies, resulting in no difference in outcome based on histology 50. The exception is primary mediastinal B-cell lymphoma, which has had an inferior outcome with these regimens 42. For pediatric Burkitt lymphoma/leukemia patients, secondary cytogenetic abnormalities, other than MYC rearrangement, are associated with an inferior outcome 51 and cytogenetic abnormalities involving gain of 7q or deletion of 13q appeared to have an inferior outcome on the FAB/LMB-96 chemotherapy protocol 52. For pediatric patients with diffuse large B-cell lymphoma and chromosomal rearrangement at MYC (8q24), outcome appeared to be worse 52. A subset of pediatric diffuse large B-cell lymphoma cases were found to have a translocation that juxtaposes the IRF4 oncogene next to one of the immunoglobulin loci and has been associated with favorable prognosis compared with diffuse large B-cell lymphoma cases lacking this finding 53.
Age
Non-Hodgkin lymphoma in infants is rare (1% in Berlin-Frankfurt-Münster trials from 1986 to 2002) 54. In this retrospective review, the outcome for infants was inferior compared with the outcome for older patients with non-Hodgkin lymphoma 54.
Adolescents have been reported to have outcomes inferior to those of younger children 55. This adverse effect of age appears to be most pronounced for adolescents with diffuse large B-cell lymphoma, and to a lesser degree T-cell lymphoblastic lymphoma, compared with younger children with these diagnoses 55. On the other hand, for patients with Burkitt and Burkitt-like lymphoma/leukemia who were treated on the FAB/LMB-96 (https://clinicaltrials.gov/ct2/show/NCT00002757) clinical trial, adolescent age (≥15 years) was not an independent risk factor for inferior outcome 35.
Immune response to tumor
An immune response against the ALK protein (i.e., anti-ALK antibody titer) appeared to correlate with lower clinical stage and predicted relapse risk but not overall survival 56. A study by the European Intergroup for Childhood non-Hodgkin lymphoma, which combined the level of anti-ALK antibody with minimal disseminated disease, demonstrated that patients with newly diagnosed anaplastic large cell lymphoma could be stratified into three risk groups, with a progression-free survival of 28% (low risk), 68% (intermediate risk), and 93% (all remaining patients) 45.
Burkitt lymphoma relapse rate
Experts are not aware of statistics regarding the recurrence rate (or risk of relapse) of Burkitt lymphoma in general. There have been studies that have “lumped together” both relapsing and refractory cases (those that are resistant to treatment). Depending on the study, the combined incidence of these cases has ranged from about 1 in 7 people (15.2%) to 1 in 16 people (6.4%) 30.
The majority of Burkitt lymphoma relapses occur during the first year of treatment for Burkitt lymphoma. Therefore, people who remain disease-free at 10-12 months are considered cured, although reports of delayed relapses (even several or more years later) have been described 57.
Due to the risk of recurrence, it is typically recommended that individuals be monitored at least every 2 months during the first year after chemotherapy is completed. They should additionally be monitored every 3 months the following year, and every 6 months thereafter 6.
Burkitt lymphoma treatment
Chemotherapy (sometimes along with other drugs) is the main treatment for all children with non-Hodgkin lymphoma, because it can reach all parts of the body and kill lymphoma cells wherever they may be. Even if the lymphoma appears to be limited to a single swollen lymph node, non-Hodgkin lymphoma in a child has often spread by the time it is diagnosed. Lymphoma cells are probably in other organs, but these are too small to be felt by the doctor or seen on imaging tests.
Sometimes high-dose chemotherapy followed by a stem cell transplant might be needed if the lymphoma comes back after treatment.
Unlike mature B-lineage non-Hodgkin lymphoma seen in adults, there is no difference in outcome based on histology (Burkitt or Burkitt-like lymphoma/leukemia or diffuse large B-cell lymphoma). Pediatric Burkitt and Burkitt-like lymphoma/leukemia and diffuse large B-cell lymphoma are clinically very aggressive and are treated with very intensive regimens 34.
Tumor lysis syndrome is often present at diagnosis or after initiation of treatment. This emergent clinical situation should be anticipated and addressed before treatment is started.
Which chemo drugs are used to treat non-Hodgkin lymphoma in children?
Children with non-Hodgkin lymphoma get a combination of several chemo drugs over a period of time. The number of drugs, their doses, and the length of treatment depend on the type and stage of the lymphoma. Some of the chemo drugs commonly used to treat childhood lymphoma include:
- Cyclophosphamide (Cytoxan)
- Vincristine (Oncovin)
- Doxorubicin (Adriamycin)
- Prednisone
- Dexamethasone
- Cytarabine, also known as ara-C (Cytosar)
- Methotrexate
- L-asparaginase (Elspar), PEG-L-asparaginase (pegaspargase, Oncaspar)
- Etoposide (VePesid, others)
- 6-mercaptopurine (Purinethol)
- Ifosfamide (Ifex)
Doctors give chemo in cycles, in which a period of treatment is followed by a rest period to allow the body time to recover. Each chemo cycle generally lasts for several weeks.
Most chemo treatments are given in an outpatient setting (in the doctor’s office or clinic or hospital outpatient department), but some – especially at the start of treatment – may need to be given while the child stays in the hospital.
- Stages 1 and 2: While chemo is the main treatment of these lymphomas, surgery may be done before chemo if the tumor is in only one area, such as a large abdominal (belly) tumor. Several different chemo drugs are used. The length of treatment ranges from about 9 weeks to 6 months. Most pediatric oncologists feel that the 9-week treatment is adequate if all of the tumor is removed with surgery first. Chemotherapy into the spinal fluid is needed only if the lymphoma is growing around the head or neck.
- Stages 3 and 4: Children with more advanced lymphomas need more intensive chemotherapy. Because these lymphomas tend to grow quickly, the chemo cycles are short, with little rest between courses of treatment.
For example, a treatment plan known as the French LMB protocol regimen alternates between different combinations of drugs every 3 to 4 weeks for a total of about 6 to 8 months. Other similar treatment regimens are the German BFM protocol and the St. Jude Total B regimen.
Chemotherapy must also be given into the spinal fluid.
Possible risks and side effects
Chemo drugs can cause side effects. These depend on the type and dose of drugs given and how long treatment lasts. Common side effects can include:
- Hair loss
- Mouth sores
- Loss of appetite
- Nausea and vomiting
- Diarrhea
- Increased chance of infections (due to low white blood cell counts)
- Easy bruising or bleeding (due to low blood platelet counts)
- Fatigue (due to low red blood cell counts)
These side effects usually go away after treatment is finished. If serious side effects occur, the dose of chemo may be reduced or treatment may be delayed.
There are often ways to lessen these side effects. For example:
- Drugs can be given to help prevent or reduce nausea and vomiting.
- Infections can be very serious in people getting chemo. Drugs known as growth factors can be given to keep the blood cell counts higher.
- Tumor lysis syndrome is a possible side effect of chemo in children who have large numbers of lymphoma cells in the body before treatment. It occurs most often with the first cycle of chemo. Killing the lymphoma cells releases their contents into the bloodstream. This can overwhelm the kidneys, which can’t get rid of all of these substances at once. Excess amounts of certain minerals may also lead to heart and nervous system problems. This can be prevented by making sure the child gets lots of fluids during treatment and by giving drugs such as bicarbonate, allopurinol, and rasburicase, which help the body get rid of these substances.
Some possible side effects occur only with certain drugs. For example, drugs such as doxorubicin can damage the heart. Your child’s doctor may order heart function tests (like a MUGA scan or echocardiogram) if your child is getting one of these drugs.
Be sure to ask your child’s doctor or nurse about any specific side effects you should watch for and about what you can do about them.
Along with the side effects listed above, there are possible long-term effects of chemo in children, such as possible effects on fertility later in life.
References- National Center for Biotechnology Information (US). Genes and Disease [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1998-. Burkitt lymphoma. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22257
- Burkitt lymphoma. https://ocg.cancer.gov/programs/cgci/projects/burkitt-lymphoma
- Burkitt Lymphoma. https://www.merckmanuals.com/home/blood-disorders/lymphomas/burkitt-lymphoma
- Boerma EG, van Imhoff GW, Appel IM, Veeger NJ, Kluin PM, Kluin-Nelemans JC. Gender and age-related differences in Burkitt lymphoma–epidemiological and clinical data from The Netherlands. Eur J Cancer. 2004 Dec. 40(18):2781-7.
- PDQ Pediatric Treatment Editorial Board. Childhood Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version. 2018 Aug 22. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65738
- Burkitt Lymphoma and Burkitt-like Lymphoma. https://emedicine.medscape.com/article/1447602-overview
- Burkitt Lymphoma. https://www.merckmanuals.com/professional/hematology-and-oncology/lymphomas/burkitt-lymphoma
- Update on Burkitt Lymphoma. Hematol Oncol Clin North Am. 2016 Dec;30(6):1333-1343. doi: 10.1016/j.hoc.2016.07.009. https://www.ncbi.nlm.nih.gov/pubmed/27888884
- Epidemiology, clinical manifestations, pathologic features, and diagnosis of Burkitt lymphoma. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-pathologic-features-and-diagnosis-of-burkitt-lymphoma
- Kluin PM, Harris NL, Stein H: B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer, 2008, pp 265-6.
- Klapper W, Szczepanowski M, Burkhardt B, et al.: Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood 112 (4): 1374-81, 2008.
- Deffenbacher KE, Iqbal J, Sanger W, et al.: Molecular distinctions between pediatric and adult mature B-cell non-Hodgkin lymphomas identified through genomic profiling. Blood 119 (16): 3757-66, 2012.
- BURKITT LYMPHOMA. http://www.omim.org/entry/113970
- Haluska, F. G., Tsujimoto, Y., Croce, C. M. Mechanisms of chromosome translocation in B- and T-cell neoplasia. Trends Genet. 3: 11-15, 1987.
- Komano, J., Sugiura, M., Takada, K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt’s lymphoma cell line Akata. J. Virol. 72: 9150-9156, 1998.
- Komano, J., Maruo, S., Kurozumi, K., Oda, T., Takada, K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt’s lymphoma cell line Akata. J. Virol. 73: 9827-9831, 1999.
- Kitagawa, N., Goto, M., Kurozumi, K., Maruo, S., Fukayama, M., Naoe, T., Yasukawa, M., Hino, K., Suzuki, T., Todo, S., Takada, K. Epstein-Barr virus-encoded poly(A)- RNA supports Burkitt’s lymphoma growth through interleukin-10 induction. EMBO J. 19: 6742-6750, 2000
- Kelly, G., Bell, A., Rickinson, A. Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nature Med. 8: 1098-1104, 2002.
- Schmitz, R., Young, R. M., Ceribelli, M., Jhavar, S., Xiao, W., Zhang, M., Wright, G., Shaffer, A. L., Hodson, D. J., Buras, E., Liu, X., Powell, J., and 30 others. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490: 116-120, 2012.
- Varano, G., Raffel, S., Sormani, M., Zanardi, F., Lonardi, S., Zasada, C., Perucho, L., Patrocelli, V., Haake, A., Lee, A. K., Bugatti, M., Paul, U., and 9 others. The B-cell receptor controls fitness of MYC-driven lymphoma cells via GSK3-beta inhibition. Nature 546: 302-306, 2017.
- Leoncini L, Raphael M, Stein H: Burkitt lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer, 2008, pp 262-4.
- Miles RR, Cairo MS, Satwani P, et al.: Immunophenotypic identification of possible therapeutic targets in paediatric non-Hodgkin lymphomas: a children’s oncology group report. Br J Haematol 138 (4): 506-12, 2007.
- Gualco G, Weiss LM, Harrington WJ Jr, et al.: Nodal diffuse large B-cell lymphomas in children and adolescents: immunohistochemical expression patterns and c-MYC translocation in relation to clinical outcome. Am J Surg Pathol 33 (12): 1815-22, 2009.
- Rohde M, Bonn BR, Zimmermann M, et al.: Relevance of ID3-TCF3-CCND3 pathway mutations in pediatric aggressive B-cell lymphoma treated according to the non-Hodgkin Lymphoma Berlin-Frankfurt-Münster protocols. Haematologica 102 (6): 1091-1098, 2017.
- Havelange V, Pepermans X, Ameye G, et al.: Genetic differences between paediatric and adult Burkitt lymphomas. Br J Haematol 173 (1): 137-44, 2016.
- Chakraborty AA, Scuoppo C, Dey S, et al.: A common functional consequence of tumor-derived mutations within c-MYC. Oncogene 34 (18): 2406-9, 2015.
- Masqué-Soler N, Szczepanowski M, Kohler CW, et al.: Clinical and pathological features of Burkitt lymphoma showing expression of BCL2–an analysis including gene expression in formalin-fixed paraffin-embedded tissue. Br J Haematol 171 (4): 501-8, 2015.
- Burkitt lymphoma. https://www.merckmanuals.com/home/blood-disorders/lymphomas/burkitt-lymphoma
- Burkitt lymphoma. https://medlineplus.gov/ency/article/001308.htm
- Kim H, Park ES, Lee SH, et al. Clinical outcome of relapsed or refractory burkitt lymphoma and mature B-cell lymphoblastic leukemia in children and adolescents. Cancer Res Treat. 2014;46(4):358-65. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4206068
- Casulo C, Friedberg J. Treating Burkitt Lymphoma in Adults. Curr Hematol Malig Rep. September, 2015; 10(3):266-271.
- Survival Rates for Childhood Non-Hodgkin Lymphoma. https://www.cancer.org/cancer/childhood-non-hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html
- Patte C, Auperin A, Gerrard M, et al.: Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 109 (7): 2773-80, 2007.
- Goldman S, Smith L, Anderson JR, et al.: Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia 27 (5): 1174-7, 2013.
- Cairo MS, Sposto R, Gerrard M, et al.: Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol 30 (4): 387-93, 2012.
- Gerrard M, Cairo MS, Weston C, et al.: Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol 141 (6): 840-7, 2008.
- Cairo MS, Gerrard M, Sposto R, et al.: Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 109 (7): 2736-43, 2007.
- Minard-Colin V, Auperin A, Pillon M, et al.: Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. [Abstract] J Clin Oncol 34 (Suppl 15): A-10507, 2016.
- Jourdain A, Auperin A, Minard-Colin V, et al.: Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Société Française des Cancers de l’Enfant study. Haematologica 100 (6): 810-7, 2015.
- Williams D, Mori T, Reiter A, et al.: Central nervous system involvement in anaplastic large cell lymphoma in childhood: results from a multicentre European and Japanese study. Pediatr Blood Cancer 60 (10): E118-21, 2013.
- Cairo MS, Gerrard M, Sposto R, et al.: Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 109 (7): 2736-43, 2007
- Gerrard M, Waxman IM, Sposto R, et al.: Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood 121 (2): 278-85, 2013.
- Giulino-Roth L, O’Donohue T, Chen Z, et al.: Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol 179 (5): 739-747, 2017.
- Le Deley MC, Reiter A, Williams D, et al.: Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood 111 (3): 1560-6, 2008.
- Mussolin L, Damm-Welk C, Pillon M, et al.: Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia 27 (2): 416-22, 2013.
- Lowe EJ, Sposto R, Perkins SL, et al.: Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Children’s Cancer Group Study 5941. Pediatr Blood Cancer 52 (3): 335-9, 2009.
- Zhao XF, Young KH, Frank D, et al.: Pediatric primary bone lymphoma-diffuse large B-cell lymphoma: morphologic and immunohistochemical characteristics of 10 cases. Am J Clin Pathol 127 (1): 47-54, 2007.
- Dalle JH, Mechinaud F, Michon J, et al.: Testicular disease in childhood B-cell non-Hodgkin’s lymphoma: the French Society of Pediatric Oncology experience. J Clin Oncol 19 (9): 2397-403, 2001.
- Lervat C, Auperin A, Patte C, et al.: Head and neck presentations of B-NHL and B-AL in children/adolescents: experience of the LMB89 study. Pediatr Blood Cancer 61 (3): 473-8, 2014.
- Patte C, Auperin A, Gerrard M, et al.: Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 109 (7): 2773-80, 2007
- Poirel HA, Cairo MS, Heerema NA, et al.: Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Leukemia 23 (2): 323-31, 2009.
- Nelson M, Perkins SL, Dave BJ, et al.: An increased frequency of 13q deletions detected by fluorescence in situ hybridization and its impact on survival in children and adolescents with Burkitt lymphoma: results from the Children’s Oncology Group study CCG-5961. Br J Haematol 148 (4): 600-10, 2010.
- Salaverria I, Philipp C, Oschlies I, et al.: Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood 118 (1): 139-47, 2011.
- Mann G, Attarbaschi A, Burkhardt B, et al.: Clinical characteristics and treatment outcome of infants with non-Hodgkin lymphoma. Br J Haematol 139 (3): 443-9, 2007.
- Burkhardt B, Oschlies I, Klapper W, et al.: Non-Hodgkin’s lymphoma in adolescents: experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM protocols. Leukemia 25 (1): 153-60, 2011.
- Ait-Tahar K, Damm-Welk C, Burkhardt B, et al.: Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 115 (16): 3314-9, 2010.
- A case of clonally distinct relapse of Burkitt lymphoma 9 years after complete remission. Int J Hematol. 2015 May;101(5):520-4. doi: 10.1007/s12185-014-1729-1. Epub 2015 Jan 3. https://www.ncbi.nlm.nih.gov/pubmed/25555480