Familial pancreatic cancer
Familial pancreatic cancer is a term to describe families with a high rate of pancreatic cancer or families with a clustering of pancreatic cancer diagnoses. Families are considered to have familial pancreatic cancer if there are at least 2 members of the family with pancreatic cancer who are first-degree relatives, such as a parent, child, or siblings of one another, or if there are at least 3 members of the family who have pancreatic cancer. Healthy individuals who come from a family with familial pancreatic cancer are likely to have an increased risk of developing pancreatic cancer in their lifetime.
At this time, there is no specific test for familial pancreatic cancer. Families are considered to have familial pancreatic cancer if there are:
- 2 or more members of a family who are first-degree relatives, such as parents, children, or siblings, who have been diagnosed with pancreatic cancer, or
- 3 or more close relatives from the same side of the family who have been diagnosed with pancreatic cancer.
If you have symptoms of pancreatic cancer, talk with your doctor. They will perform a physical exam and ask you about your medical history. Your doctor will also recommend specific tests to help find pancreatic cancer.
An estimated 57,600 adults (30,400 men and 27,200 women) in the United States will be diagnosed with pancreatic cancer. About 10% of those cases are thought to be caused by familial pancreatic cancer.
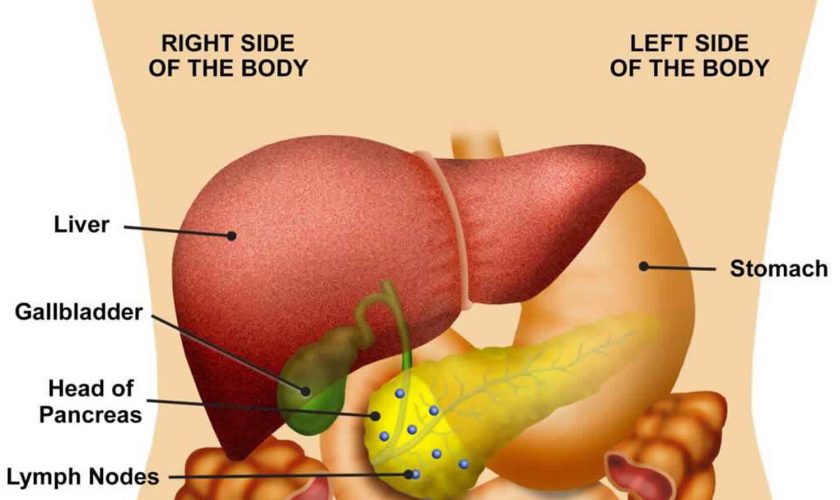
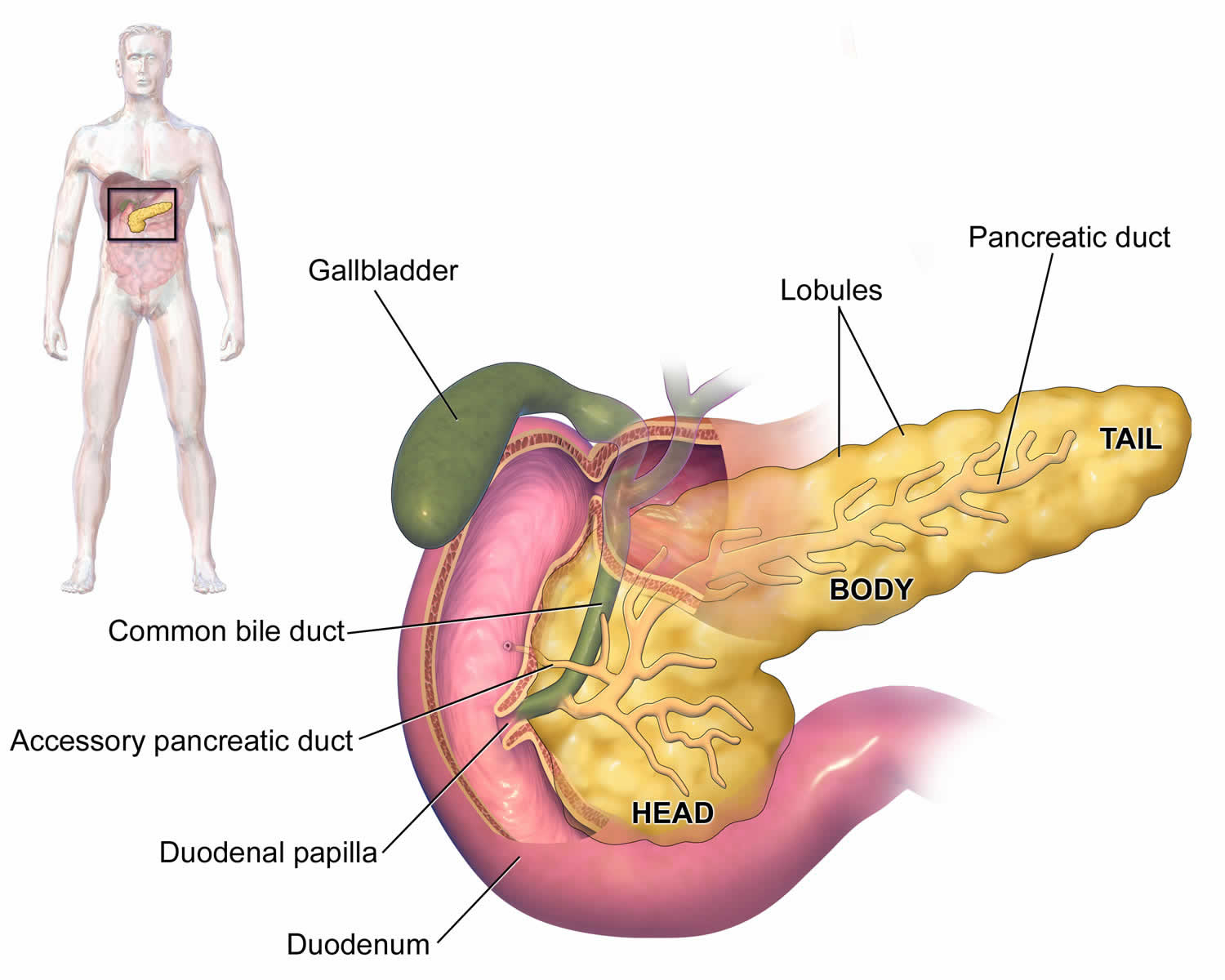
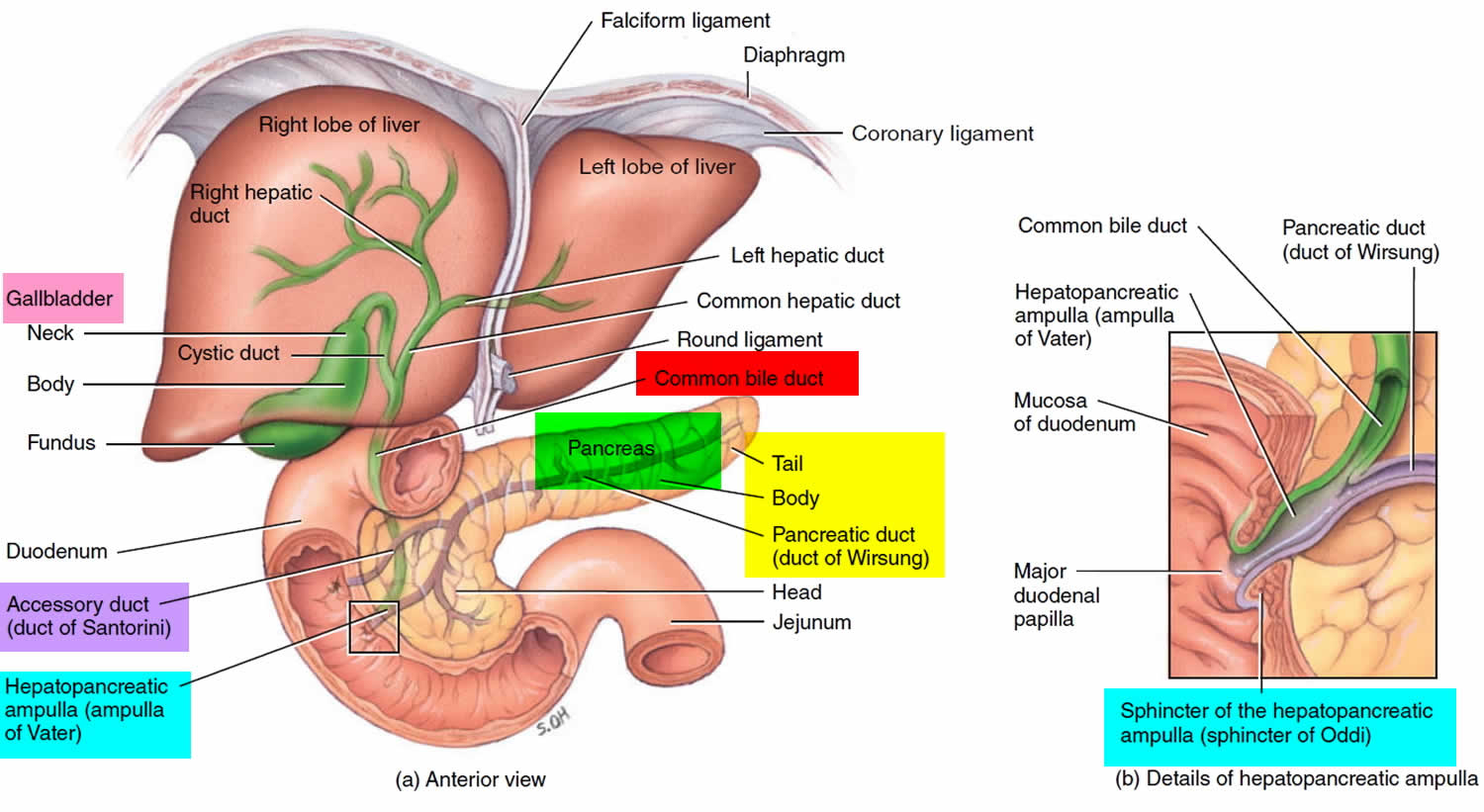
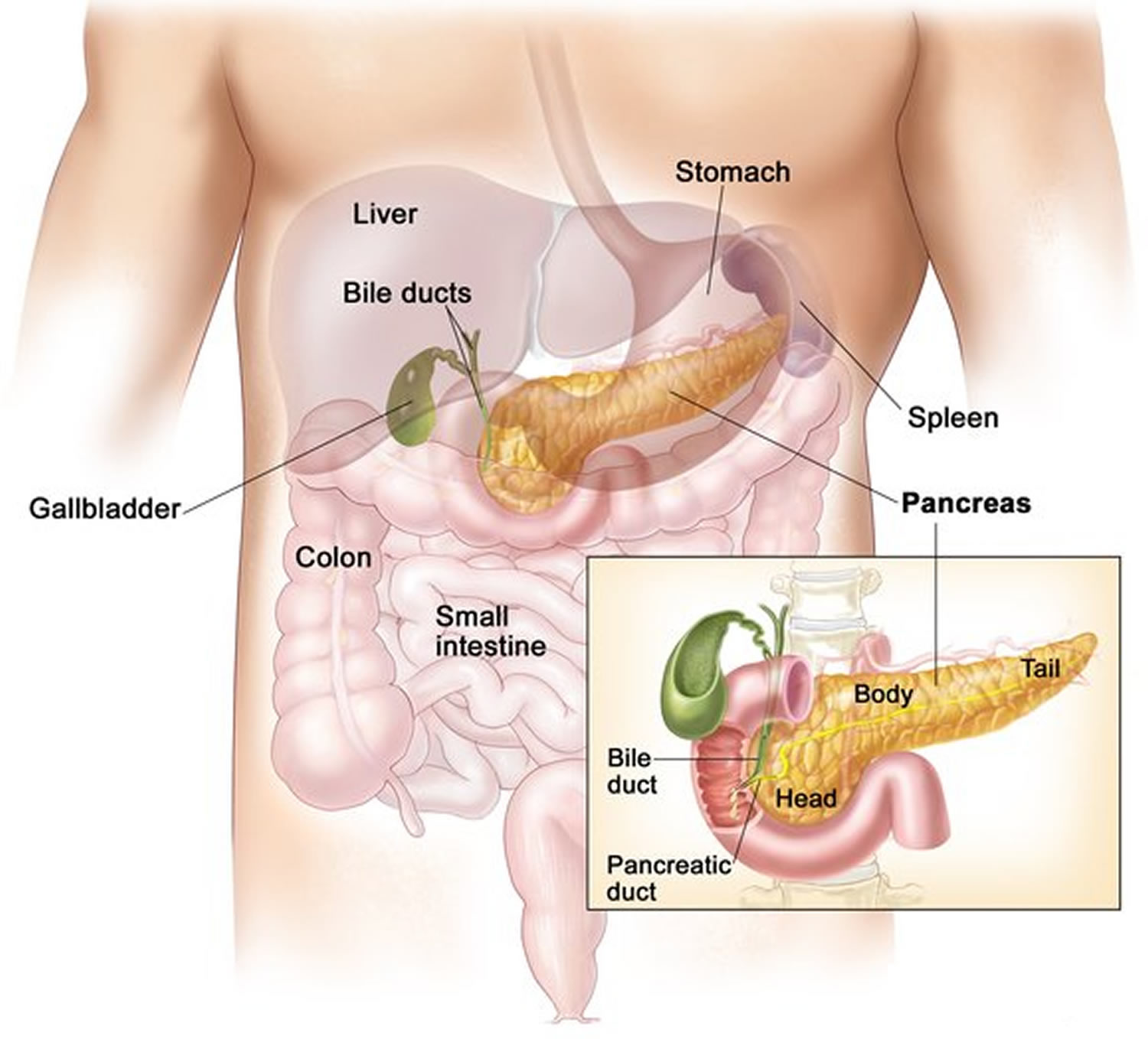
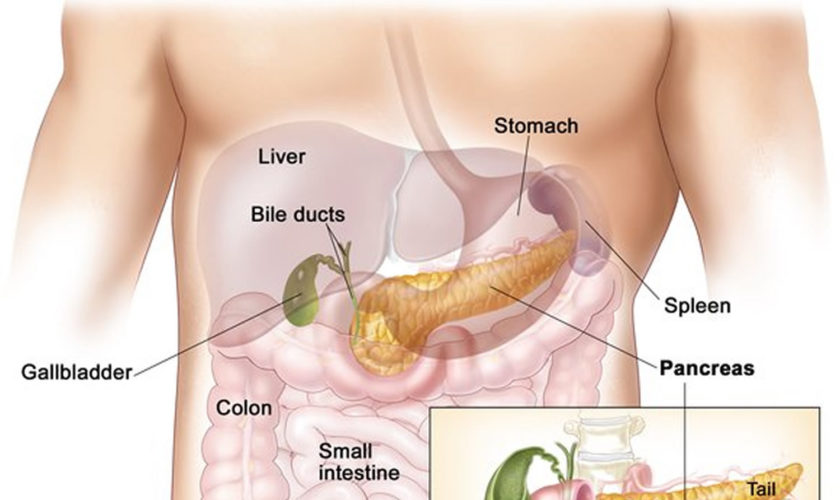
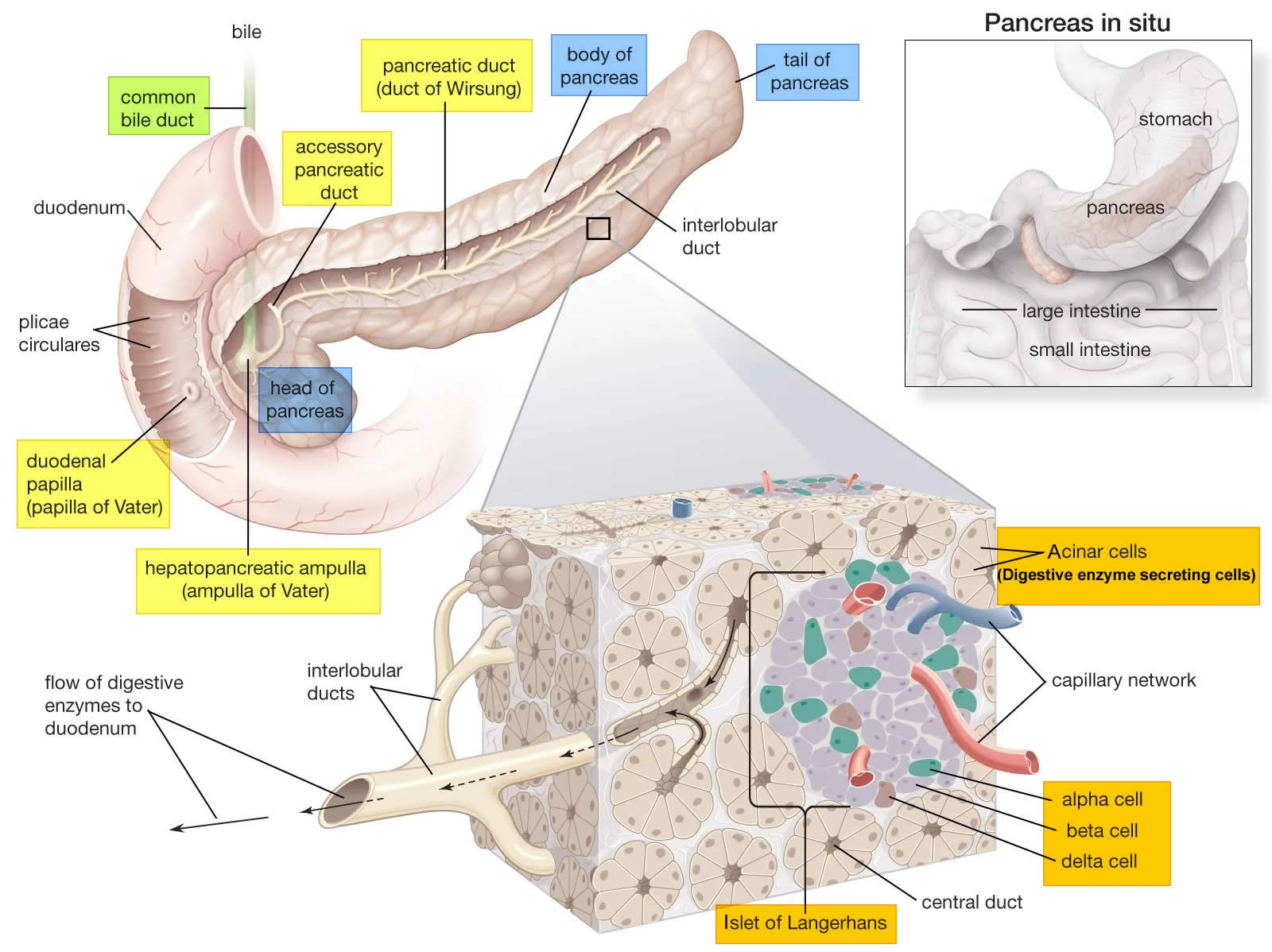
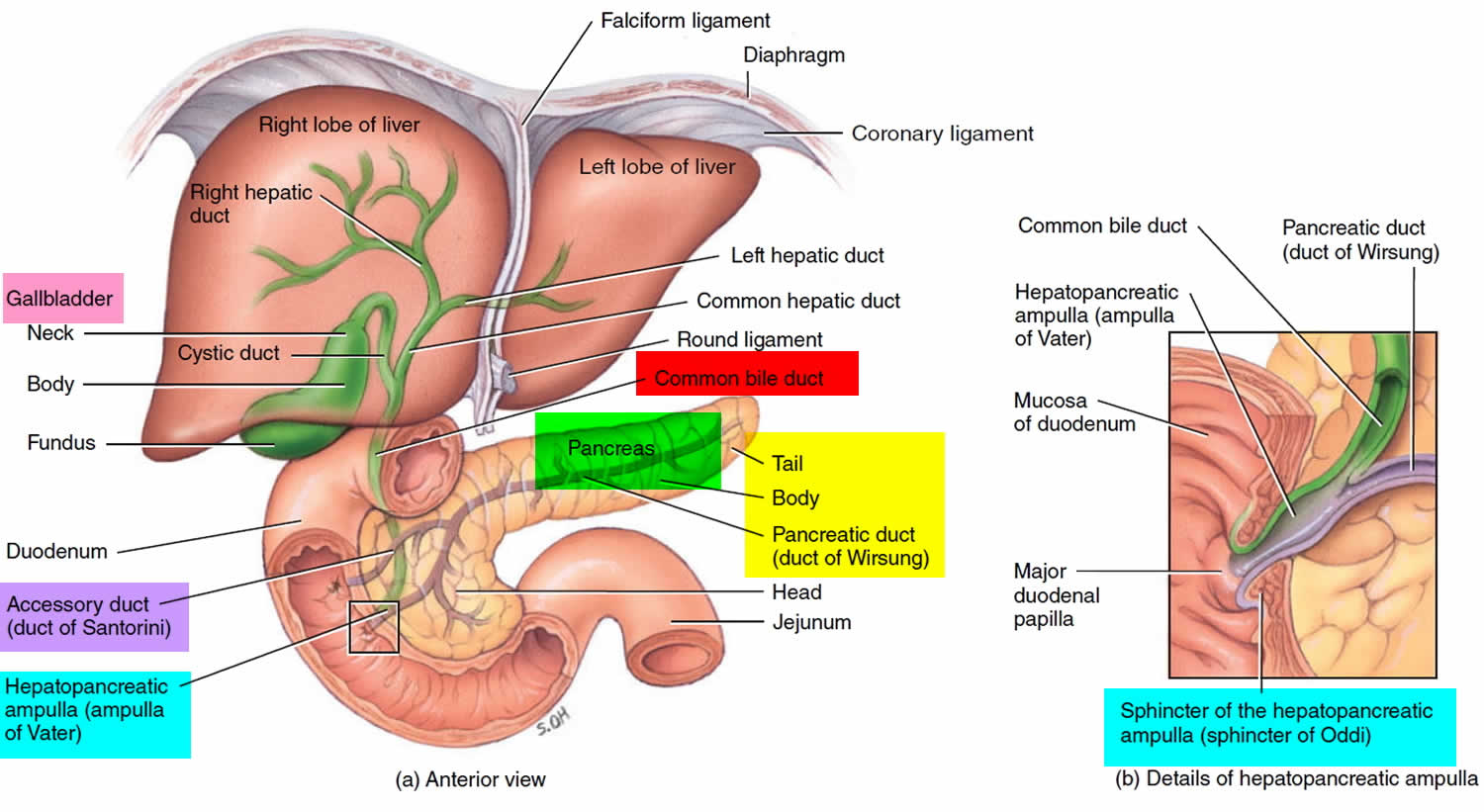
Figure 1. Pancreas anatomy
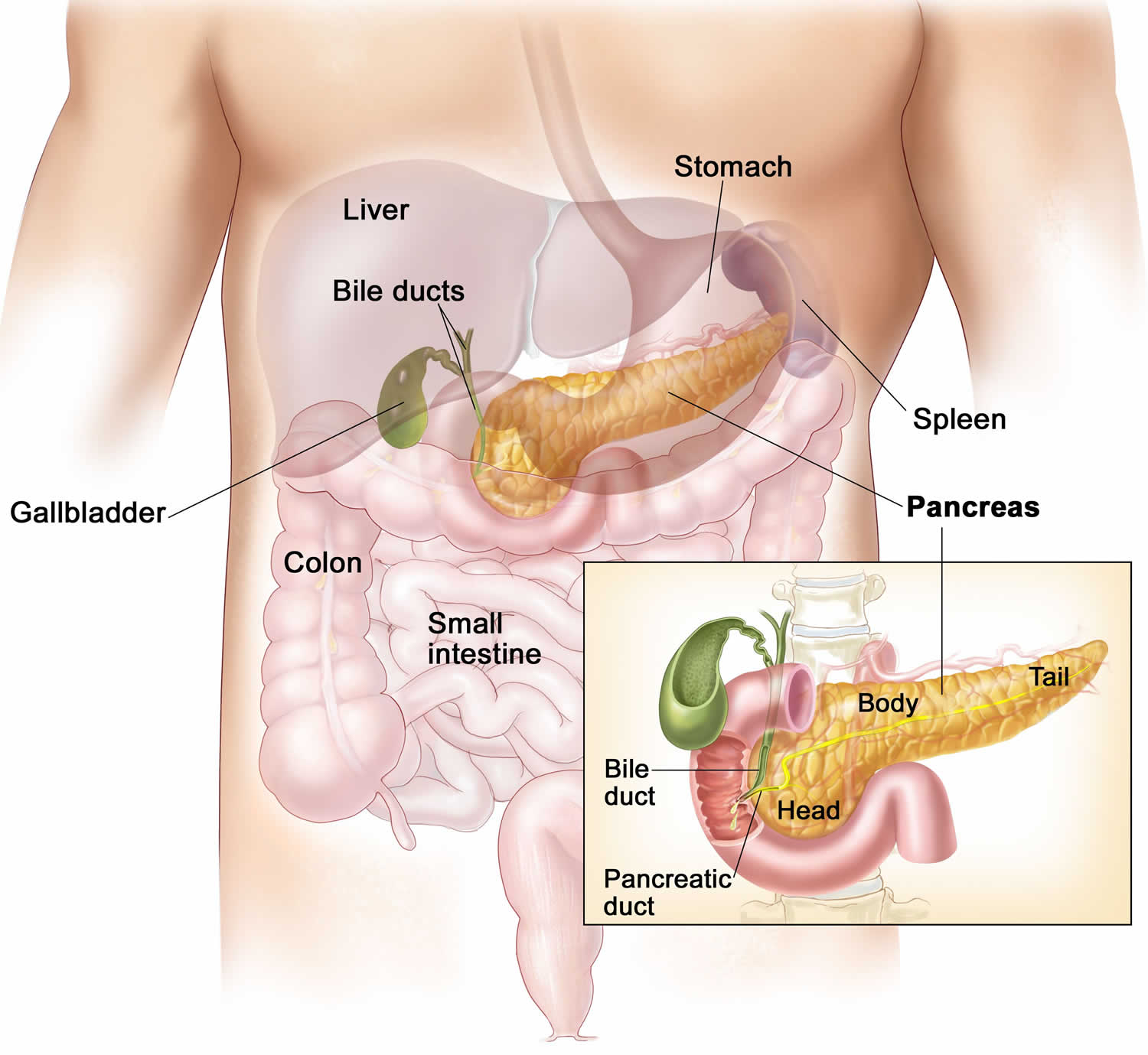
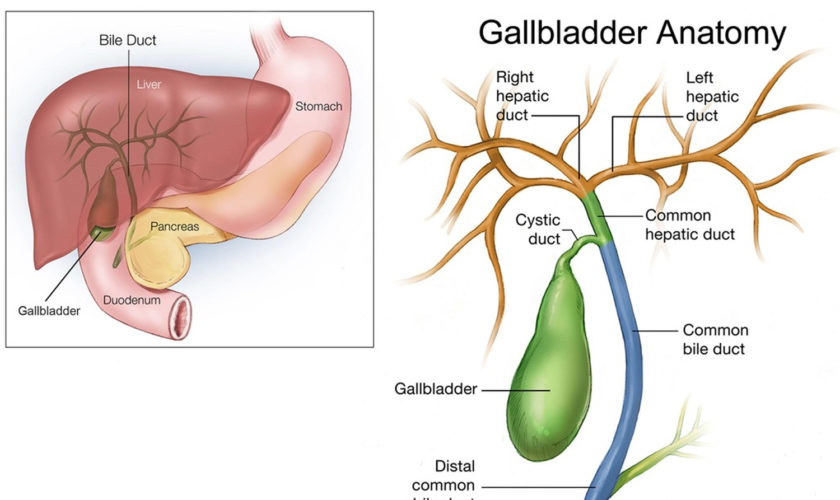
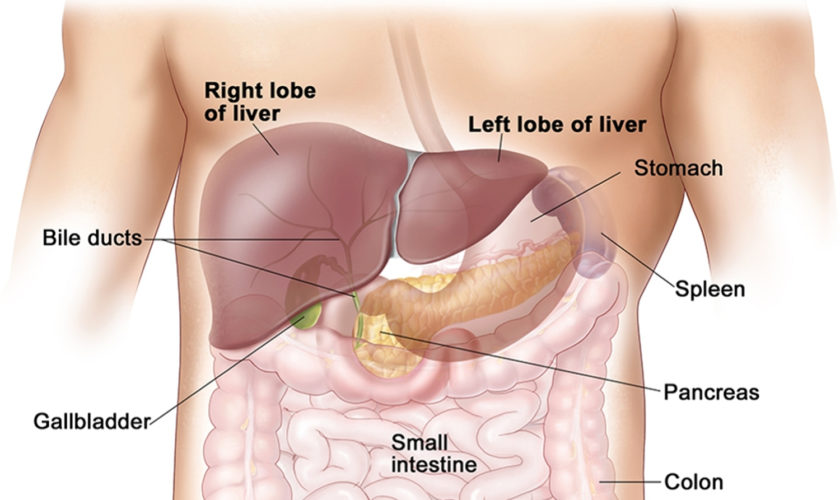
Figure 2. Pancreas location
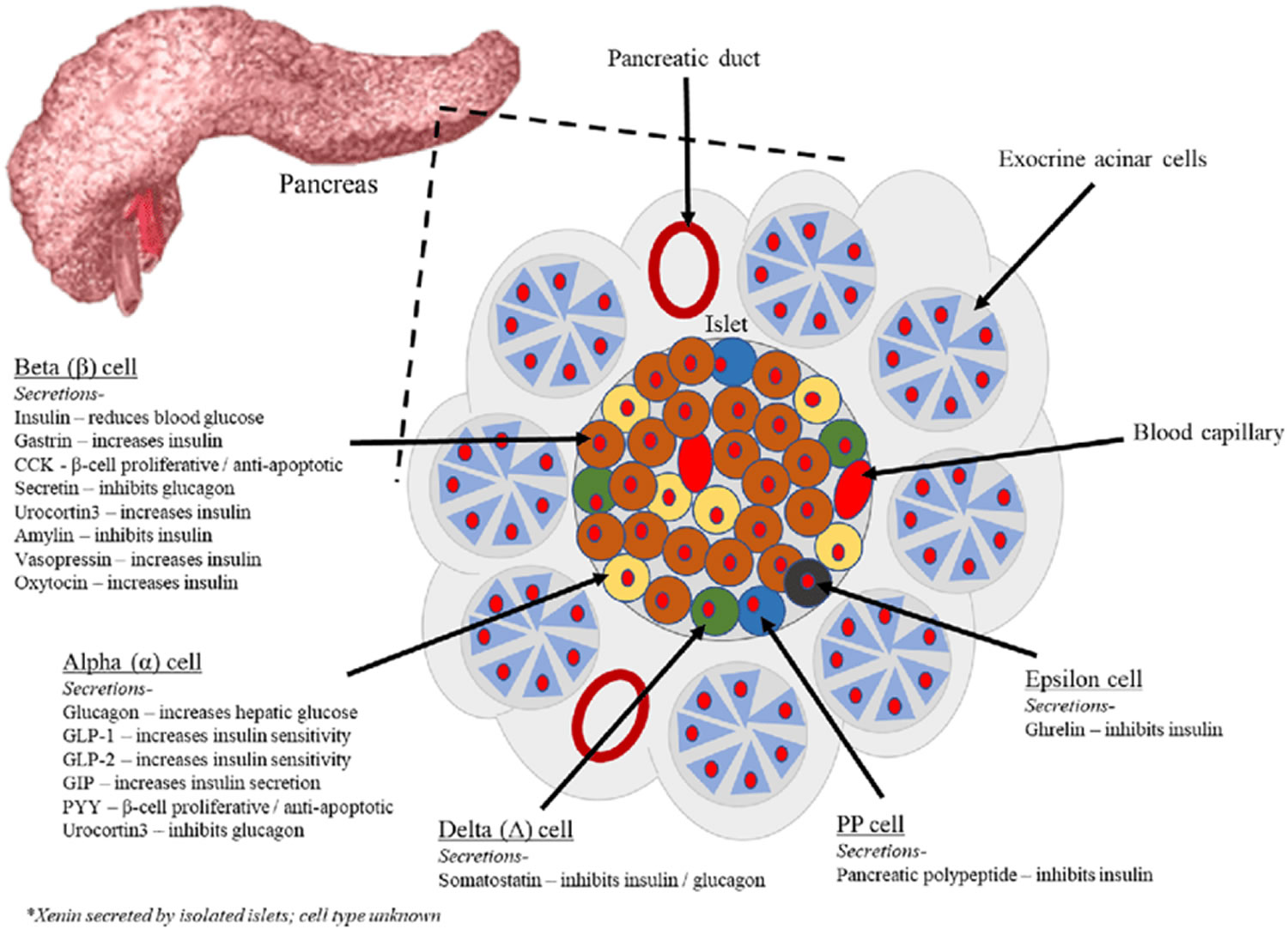
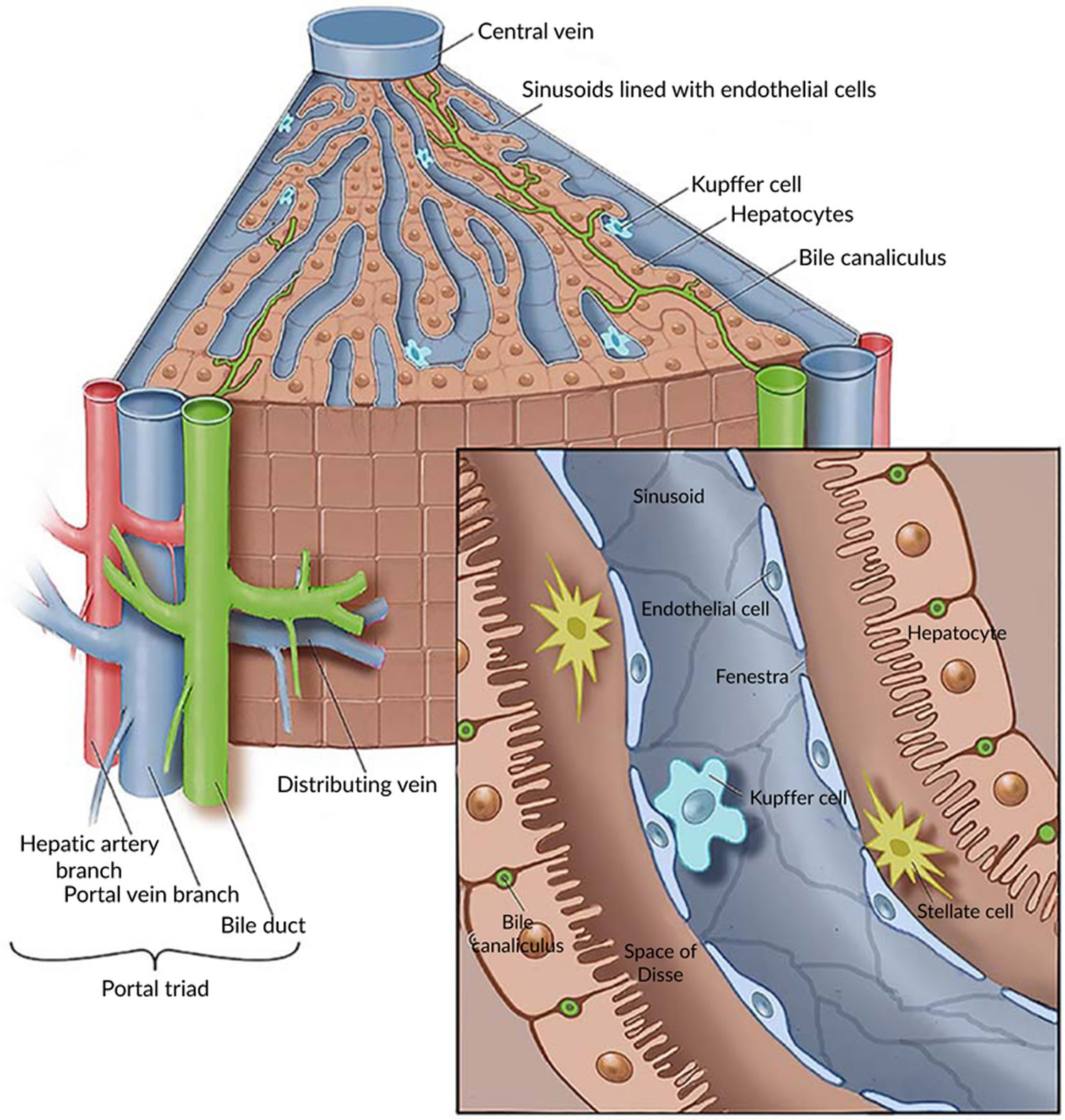
Figure 3. Pancreas cell types
Familial pancreatic cancer causes
Individuals from familial pancreatic cancer families should consider genetic testing to see if there is a specific germline genetic mutation that may have caused the pancreatic cancers in their family. A germline mutation is a genetic mutation found in every cell of a person’s body from birth. Some genes linked to familial pancreatic cancer families include BRCA1, BRCA2, PALB2, CDKN2A, and ATM, and the genes linked to Lynch syndrome (MLH1, MSH2, MSH6, PMS2, and EPCAM). Germline mutations in genes that cause other rare inherited cancer syndromes (TP53 mutations in Li-Fraumeni syndrome and STK11 mutations for Peutz-Jeghers syndrome) can also increase the risk of pancreatic cancer. Individuals who carry germline genetic mutations in these genes are at an increased risk of pancreatic cancer as well as other types of cancers. Genetic testing for these genes is available, but your decision to have genetic testing should be discussed carefully with a medical professional with expertise in this area.
It is important to note that genetic testing is still evolving, and only 10% to 20% of families with familial pancreatic cancer will have a mutation identified by genetic testing. Currently, most families with familial pancreatic cancer will have normal genetic testing results, suggesting that the genes responsible for most familial pancreatic cancer families have not yet been discovered. Researchers continue to search for other specific genes that may be linked to familial pancreatic cancer. Since most familial pancreatic cancer families will have normal genetic testing results, it is important to realize that individuals from familial pancreatic cancer families are still at an increased risk of pancreatic cancer, even when genetic testing results are normal. Talk with a genetic counselor before you have any genetic testing.
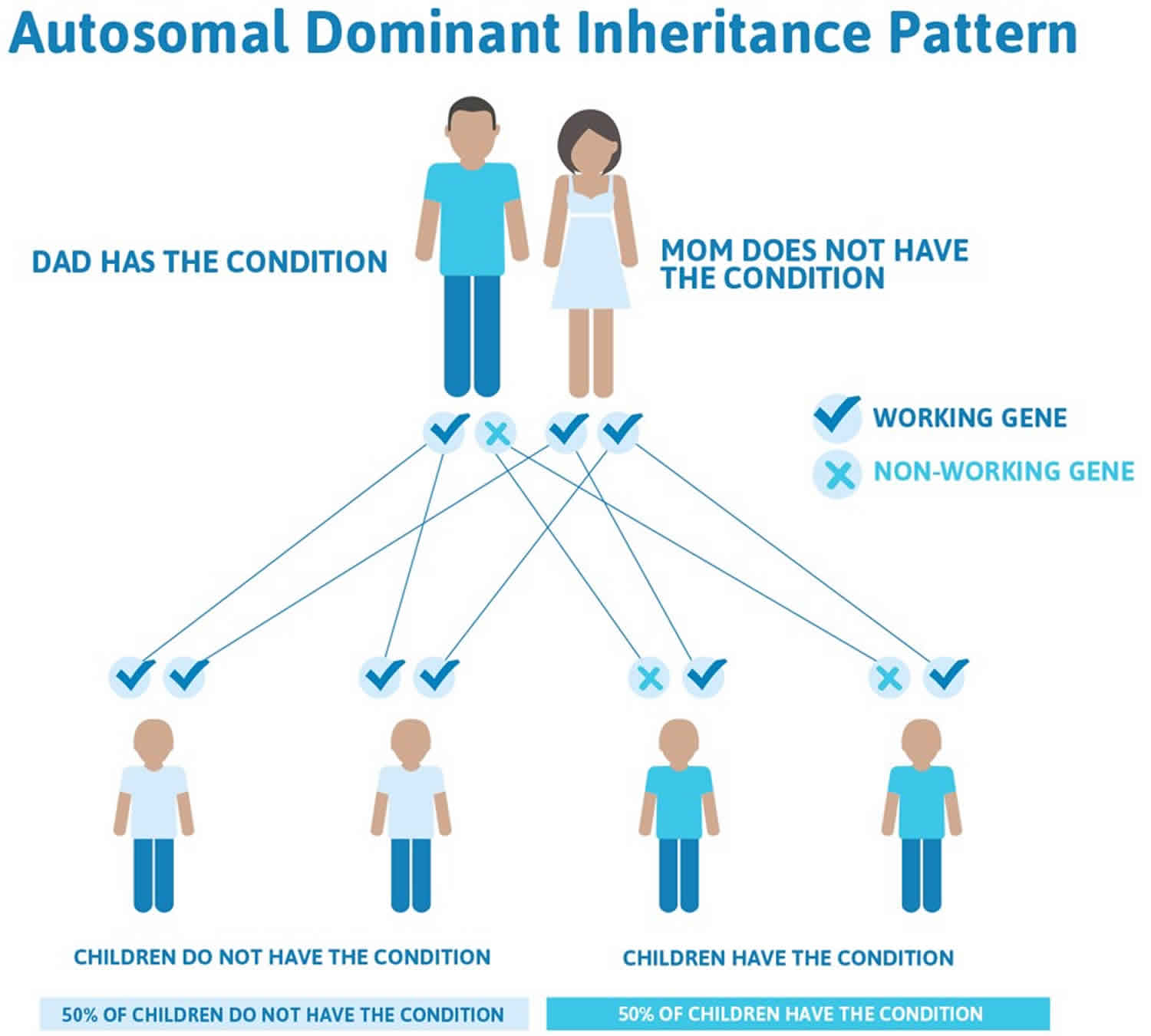
Familial pancreatic cancer inheritance pattern
Normally, every cell has 2 copies of each gene: 1 inherited from the mother and 1 inherited from the father. Researchers think that familial pancreatic cancer typically follows an autosomal dominant inheritance pattern, even though the specific genes that cause familial pancreatic cancer are mostly unknown. In autosomal dominant inheritance, a mutation happens in only 1 copy of the gene. This means that a parent with a gene mutation may pass along a copy of their normal gene. Or, that parent may pass along a copy of the gene with the mutation. Therefore, a child who has a parent with a mutation has a 50% chance of inheriting that mutation. A brother, sister, or parent of a person who has a mutation also has a 50% chance of having the same mutation. However, if the parents test negative for the mutation (meaning each person’s test results found no mutation), the risk to the siblings significantly decreases but their risk may still be higher than an average risk.
Options exist for people interested in having a child when a prospective parent carries a gene mutation that increases the risk for this hereditary cancer syndrome. Preimplantation genetic diagnosis (PGD) is a medical procedure done in conjunction with in-vitro fertilization (IVF). It allows people who carry a specific known genetic mutation to reduce the likelihood that their children will inherit the condition. A woman’s eggs are removed and fertilized in a laboratory. When the embryos reach a certain size, 1 cell is removed and is tested for the hereditary condition in question. The parents can then choose to transfer embryos which do not have the mutation. Preimplantation genetic diagnosis has been in use for over 2 decades and has been used for several hereditary cancer predisposition syndromes. However, this is a complex procedure with financial, physical, and emotional factors to consider before starting. For more information, talk with an assisted reproduction specialist at a fertility clinic.
Figure 4. Familial pancreatic cancer autosomal dominant inheritance pattern
Genetic counseling and testing
If you’ve been diagnosed with pancreatic cancer, your doctor might suggest speaking with a genetic counselor to determine if you could benefit from genetic testing.
Some people with pancreatic cancer have gene mutations (such as BRCA mutations) in all the cells of their body, which put them at increased risk for pancreatic cancer (and possibly other cancers). Testing for these gene mutations can sometimes affect which treatments might be helpful. It might also affect whether other family members should consider genetic counseling and testing as well.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
What are the estimated cancer risks associated with familial pancreatic cancer?
The lifetime risk of pancreatic cancer for the average individual without a family history of pancreatic cancer is approximately 1%. Individuals with a family history of pancreatic cancer are at an increased lifetime risk for developing pancreatic cancer. This risk is likely higher for individuals from a family with familial pancreatic cancer. The following cancer risk estimates are generalized and should be interpreted with caution since the actual risk for each individual may be different:
- Individuals from familial pancreatic cancer families who have 1 first-degree relative, meaning a parent, sibling, or child, with pancreatic cancer are estimated to have an increased lifetime risk of pancreatic cancer that is 3 to 5 times higher than the general population.
- Individuals from familial pancreatic cancer families who have 2 first-degree relatives with pancreatic cancer are estimated to have an increased lifetime risk of pancreatic cancer that is 5 to 7 times higher than the general population.
- Individuals from familial pancreatic cancer families who have 3 or more first-degree relatives with pancreatic cancer are estimated to have an increased lifetime risk of pancreatic cancer that may be up to 30 times higher than the general population.
Individuals who carry germline mutations in known genes linked to pancreatic cancer risk (BRCA1, BRCA2, PALB2, CDKN2A, ATM, TP53, STK11, MLH1, MSH2, MSH6, PMS2, and EPCAM) are also at an increased risk of various cancers, including pancreatic cancer. For individuals with a mutation in 1 of these genes, the risk of pancreatic cancer may be particularly higher if there is also a history of pancreatic cancer in the family. Recent studies have found that 4% to 10% of individuals with pancreatic cancer will have a mutation in 1 of these genes. Individuals with pancreatic cancer who are of Ashkenazi Jewish ancestry are even more likely to carry 1 of these genetic mutations. Some national guidelines now recommend genetic testing for any person diagnosed with pancreatic cancer, regardless of their family history of cancer or age at diagnosis.
Tobacco use increases an individual’s lifetime risk of pancreatic cancer, regardless of their family history. Tobacco use may significantly increase the risk of pancreatic cancer for individuals from familial pancreatic cancer families.
Familial pancreatic cancer signs and symptoms
Early pancreatic cancers often do not cause any signs or symptoms. By the time they do cause symptoms, they have often grown very large or already spread outside the pancreas.
Having one or more of the symptoms below does not mean you have pancreatic cancer. In fact, many of these symptoms are more likely to be caused by other conditions. Still, if you have any of these symptoms, it’s important to have them checked by a doctor so that the cause can be found and treated, if needed.
Jaundice and related symptoms
Jaundice is yellowing of the eyes and skin. Most people with pancreatic cancer and nearly all people with ampullary cancer will have jaundice as one of their first symptoms.
Jaundice is caused by the buildup of bilirubin, a dark yellow-brown substance made in the liver. Normally, the liver releases a liquid called bile that contains bilirubin. Bile goes through the common bile duct into the intestines, where it helps break down fats. It eventually leaves the body in the stool. When the common bile duct becomes blocked, bile can’t reach the intestines, and the amount of bilirubin in the body builds up.
Cancers that start in the head of the pancreas are near the common bile duct. These cancers can press on the duct and cause jaundice while they are still fairly small, which can sometimes lead to these tumors being found at an early stage. But cancers that start in the body or tail of the pancreas don’t press on the duct until they have spread through the pancreas. By this time, the cancer has often spread beyond the pancreas.
When pancreatic cancer spreads, it often goes to the liver. This can also cause jaundice.
There are other signs of jaundice as well as the yellowing of the eyes and skin:
- Dark urine: Sometimes, the first sign of jaundice is darker urine. As bilirubin levels in the blood increase, the urine becomes brown in color.
- Light-colored or greasy stools: Bilirubin normally helps give stools their brown color. If the bile duct is blocked, stools might be light-colored or gray. Also, if bile and pancreatic enzymes can’t get through to the intestines to help break down fats, the stools can become greasy and might float in the toilet.
- Itchy skin: When bilirubin builds up in the skin, it can start to itch as well as turn yellow.
Pancreatic cancer is not the most common cause of jaundice. Other causes, such as gallstones, hepatitis, and other liver and bile duct diseases, are much more common.
Belly or back pain
Pain in the abdomen (belly) or back is common in pancreatic cancer. Cancers that start in the body or tail of the pancreas can grow fairly large and start to press on other nearby organs, causing pain. The cancer may also spread to the nerves surrounding the pancreas, which often causes back pain. Pain in the abdomen or back is fairly common and is most often caused by something other than pancreatic cancer.
Weight loss and poor appetite
Unintended weight loss is very common in people with pancreatic cancer. These people often have little or no appetite.
Nausea and vomiting
If the cancer presses on the far end of the stomach it can partly block it, making it hard for food to get through. This can cause nausea, vomiting, and pain that tend to be worse after eating.
Gallbladder or liver enlargement
If the cancer blocks the bile duct, bile can build up in the gallbladder, making it larger. Sometimes a doctor can feel this (as a large lump under the right side of the ribcage) during a physical exam. It can also be seen on imaging tests.
Pancreatic cancer can also sometimes enlarge the liver, especially if the cancer has spread there. The doctor might be able to feel the edge of the liver below the right ribcage on an exam, or the large liver might be seen on imaging tests.
Blood clots
Sometimes, the first clue that someone has pancreatic cancer is a blood clot in a large vein, often in the leg. This is called a deep vein thrombosis or DVT. Symptoms can include pain, swelling, redness, and warmth in the affected leg. Sometimes a piece of the clot can break off and travel to the lungs, which might make it hard to breathe or cause chest pain. A blood clot in the lungs is called a pulmonary embolism or PE.
Still, having a blood clot does not usually mean that you have cancer. Most blood clots are caused by other things.
Diabetes
Rarely, pancreatic cancers cause diabetes (high blood sugar) because they destroy the insulin-making cells. Symptoms can include feeling thirsty and hungry, and having to urinate often. More often, cancer can lead to small changes in blood sugar levels that don’t cause symptoms of diabetes but can still be detected with blood tests.
Pancreatic cancer complications
As pancreatic cancer progresses, it can cause complications such as:
- Weight loss. A number of factors may cause weight loss in people with pancreatic cancer. The cancer itself may cause weight loss. Nausea and vomiting caused by cancer treatments or a tumor pressing on your stomach may make it difficult to eat. Or your body may have difficulty processing nutrients from food because your pancreas isn’t making enough digestive juices. Your doctor may recommend pancreatic enzyme supplements to aid in digestion. Try to maintain your weight by adding extra calories where you can and making mealtime as pleasant and relaxed as possible.
- Jaundice. Pancreatic cancer that blocks the liver’s bile duct can cause jaundice. Signs include yellow skin and eyes, dark-colored urine, and pale-colored stools. Jaundice usually occurs without abdominal pain. Your doctor may recommend that a plastic or metal tube (stent) be placed inside the bile duct to hold it open. This is done with the help of a procedure called endoscopic retrograde cholangiopancreatography (ERCP) (see Figure 5). During ERCP an endoscope is passed down your throat, through your stomach and into the upper part of your small intestine. A dye is then injected into the pancreatic and bile ducts through a small hollow tube (catheter) that’s passed through the endoscope. Finally, images are taken of the ducts.
- Pain. A growing tumor may press on nerves in your abdomen, causing pain that can become severe. Pain medications can help you feel more comfortable. Radiation therapy might help stop tumor growth temporarily to give you some relief. In severe cases, your doctor might recommend a procedure to inject alcohol into the nerves that control pain in your abdomen (celiac plexus block). This procedure stops the nerves from sending pain signals to your brain.
- Bowel obstruction. Pancreatic cancer that grows into or presses on the first part of the small intestine (duodenum) can block the flow of digested food from your stomach into your intestines. Your doctor may recommend a tube (stent) be placed in your small intestine to hold it open. Or surgery may be necessary to attach your stomach to a lower point in your intestines that isn’t blocked by cancer.
Figure 5. Endoscopic retrograde cholangiopancreatography (ERCP)
Footnote: Endoscopic retrograde cholangiopancreatography (ERCP) uses a dye to highlight the bile ducts on X-ray images. A thin, flexible tube (endoscope) with a camera on the end is passed down your throat and into your small intestine. The dye enters the ducts through a small hollow tube (catheter) passed through the endoscope.
Familial pancreatic cancer diagnosis
If a person has signs and symptoms that might be caused by pancreatic cancer, certain exams and tests will be done to find the cause. If cancer is found, more tests will be done to help determine the extent (stage) of the cancer.
Medical history and physical exam
Your doctor will ask about your medical history to learn more about your symptoms. The doctor might also ask about possible risk factors, including smoking and your family history.
Your doctor will also examine you to look for signs of pancreatic cancer or other health problems. Pancreatic cancers can sometimes cause the liver or gallbladder to swell, which the doctor might be able to feel during the exam. Your skin and the whites of your eyes will also be checked for jaundice (yellowing).
If the results of the exam are abnormal, your doctor will probably order tests to help find the problem. You might also be referred to a gastroenterologist (a doctor who treats digestive system diseases) for further tests and treatment.
Imaging tests
Imaging tests use x-rays, magnetic fields, sound waves, or radioactive substances to create pictures of the inside of your body. Imaging tests might be done for a number of reasons both before and after a diagnosis of pancreatic cancer, including:
- To look for suspicious areas that might be cancer
- To learn how far cancer may have spread
- To help determine if treatment is working
- To look for signs of cancer coming back after treatment
Computed tomography (CT) scan
The CT scan makes detailed cross-sectional images of your body. CT scans are often used to diagnose pancreatic cancer because they can show the pancreas fairly clearly. They can also help show if cancer has spread to organs near the pancreas, as well as to lymph nodes and distant organs. A CT scan can help determine if surgery might be a good treatment option.
If your doctor thinks you might have pancreatic cancer, you might get a special type of CT known as a multiphase CT scan or a pancreatic protocol CT scan. During this test, different sets of CT scans are taken over several minutes after you get an injection of an intravenous (IV) contrast.
CT-guided needle biopsy: CT scans can also be used to guide a biopsy needle into a suspected pancreatic tumor. But if a needle biopsy is needed, most doctors prefer to use endoscopic ultrasound (described below) to guide the needle into place.
Magnetic resonance imaging (MRI)
MRI scans use radio waves and strong magnets instead of x-rays to make detailed images of parts of your body. Most doctors prefer to look at the pancreas with CT scans, but an MRI might also be done.
Special types of MRI scans can also be used in people who might have pancreatic cancer or are at high risk:
- MR cholangiopancreatography (MRCP), which can be used to look at the pancreatic and bile ducts, is described below in the section on cholangiopancreatography.
- MR angiography (MRA), which looks at blood vessels, is mentioned below in the section on angiography.
Ultrasound
Ultrasound (US) tests use sound waves to create images of organs such as the pancreas. The two most commonly used types for pancreatic cancer are:
- Abdominal ultrasound: If it’s not clear what might be causing a person’s abdominal symptoms, this might be the first test done because it is easy to do and it doesn’t expose a person to radiation. But if signs and symptoms are more likely to be caused by pancreatic cancer, a CT scan is often more useful.
- Endoscopic ultrasound (EUS): This test is more accurate than abdominal US and can be very helpful in diagnosing pancreatic cancer. This test is done with a small US probe on the tip of an endoscope, which is a thin, flexible tube that doctors use to look inside the digestive tract and to get biopsy samples of a tumor.
Cholangiopancreatography
This is an imaging test that looks at the pancreatic ducts and bile ducts to see if they are blocked, narrowed, or dilated. These tests can help show if someone might have a pancreatic tumor that is blocking a duct. They can also be used to help plan surgery. The test can be done in different ways, each of which has pros and cons.
Endoscopic retrograde cholangiopancreatography (ERCP): For this test, an endoscope (a thin, flexible tube with a tiny video camera on the end) is passed down the throat, through the esophagus and stomach, and into the first part of the small intestine. The doctor can see through the endoscope to find the ampulla of Vater (where the common bile duct empties into the small intestine).
X-rays taken at this time can show narrowing or blockage in these ducts that might be due to pancreatic cancer. The doctor doing this test can put a small brush through the tube to remove cells for a biopsy or place a stent (small tube) into a bile or pancreatic duct to keep it open if a nearby tumor is pressing on it.
Magnetic resonance cholangiopancreatography (MRCP): This is a non-invasive way to look at the pancreatic and bile ducts using the same type of machine used for standard MRI scans. Unlike ERCP, it does not require an infusion of a contrast dye. Because this test is non-invasive, doctors often use MRCP if the purpose is just to look at the pancreatic and bile ducts. But this test can’t be used to get biopsy samples of tumors or to place stents in ducts.
Percutaneous transhepatic cholangiography (PTC): In this procedure, the doctor puts a thin, hollow needle through the skin of the belly and into a bile duct within the liver. A contrast dye is then injected through the needle, and x-rays are taken as it passes through the bile and pancreatic ducts. As with ERCP, this approach can also be used to take fluid or tissue samples or to place a stent into a duct to help keep it open. Because it is more invasive (and might cause more pain), PTC is not usually used unless ERCP has already been tried or can’t be done for some reason.
Positron emission tomography (PET) scan
For a PET scan, you are injected with a slightly radioactive form of sugar, which collects mainly in cancer cells. A special camera is then used to create a picture of areas of radioactivity in the body.
This test is sometimes used to look for spread from exocrine pancreatic cancers.
PET/CT scan: Special machines can do both a PET and CT scan at the same time. This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan. This test can help determine the stage (extent) of the cancer. It might be especially useful for spotting cancer that has spread beyond the pancreas and wouldn’t be treatable by surgery.
Angiography
This is an x-ray test that looks at blood vessels. A small amount of contrast dye is injected into an artery to outline the blood vessels, and then x-rays are taken.
An angiogram can show if blood flow in a particular area is blocked by a tumor. It can also show abnormal blood vessels (feeding the cancer) in the area. This test can be useful in finding out if a pancreatic cancer has grown through the walls of certain blood vessels. Mainly, it helps surgeons decide if the cancer can be removed completely without damaging vital blood vessels, and it can also help them plan the operation.
X-ray angiography can be uncomfortable because the doctor has to put a small catheter into the artery leading to the pancreas. Usually the catheter is put into an artery in your inner thigh and threaded up to the pancreas. A local anesthetic is often used to numb the area before inserting the catheter. Once the catheter is in place, the dye is injected to outline all the vessels while the x-rays are being taken.
Angiography can also be done with a CT scanner (CT angiography) or an MRI scanner (MR angiography). These techniques are now used more often because they can give the same information without the need for a catheter in the artery. You might still need an IV line so that a contrast dye can be injected into the bloodstream during the imaging.
Blood tests
Several types of blood tests can be used to help diagnose pancreatic cancer or to help determine treatment options if it is found.
Liver function tests: Jaundice (yellowing of the skin and eyes) is often one of the first signs of pancreatic cancer. Doctors often get blood tests to assess liver function in people with jaundice to help determine its cause. Certain blood tests can look at levels of different kinds of bilirubin (a chemical made by the liver) and can help tell whether a patient’s jaundice is caused by disease in the liver itself or by a blockage of bile flow (from a gallstone, a tumor, or other disease).
Tumor markers: Tumor markers are substances that can sometimes be found in the blood when a person has cancer. Tumor markers that may be helpful in pancreatic cancer are:
- CA 19-9. CA 19-9 is a tumor marker that may be helpful in pancreatic cancer. A drop in the CA 19-9 level after surgery (compared to the level before surgery) and low levels of CA 19-9 after pancreas surgery tend to predict a better prognosis (outlook).
- Carcinoembryonic antigen (CEA), which is not used as often as CA 19-9
Neither of these tumor marker tests is accurate enough to tell for sure if someone has pancreatic cancer. Levels of these tumor markers are not high in all people with pancreatic cancer, and some people who don’t have pancreatic cancer might have high levels of these markers for other reasons. Still, these tests can sometimes be helpful, along with other tests, in figuring out if someone has cancer.
In people already known to have pancreatic cancer and who have high CA19-9 or CEA levels, these levels can be measured over time to help tell how well treatment is working. If all of the cancer has been removed, these tests can also be done to look for signs the cancer may be coming back.
Other blood tests: Other tests, like a complete blood count (CBC) or chemistry panel, can help evaluate a person’s general health (such as kidney and bone marrow function). These tests can help determine if they’ll be able to withstand the stress of a major operation.
Biopsy
A person’s medical history, physical exam, and imaging test results may strongly suggest pancreatic cancer, but usually the only way to be sure is to remove a small sample of tumor and look at it under the microscope. This procedure is called a biopsy. Biopsies can be done in different ways.
Percutaneous (through the skin) biopsy: For this test, a doctor inserts a thin, hollow needle through the skin over the abdomen and into the pancreas to remove a small piece of a tumor. This is known as a fine needle aspiration (FNA). The doctor guides the needle into place using images from ultrasound or CT scans.
Endoscopic biopsy: Doctors can also biopsy a tumor during an endoscopy. The doctor passes an endoscope (a thin, flexible, tube with a small video camera on the end) down the throat and into the small intestine near the pancreas. At this point, the doctor can either use endoscopic ultrasound (EUS) to pass a needle into the tumor or endoscopic retrograde cholangiopancreatography (ERCP) to place a brush to remove cells from the bile or pancreatic ducts.
Surgical biopsy: Surgical biopsies are now done less often than in the past. They can be useful if the surgeon is concerned the cancer has spread beyond the pancreas and wants to look at (and possibly biopsy) other organs in the abdomen. The most common way to do a surgical biopsy is to use laparoscopy (sometimes called keyhole surgery). The surgeon can look at the pancreas and other organs for tumors and take biopsy samples of abnormal areas.
Some people might not need a biopsy
Rarely, the doctor might not do a biopsy on someone who has a tumor in the pancreas if imaging tests show the tumor is very likely to be cancer and if it looks like surgery can remove all of it. Instead, the doctor will proceed with surgery, at which time the tumor cells can be looked at in the lab to confirm the diagnosis. During surgery, if the doctor finds that the cancer has spread too far to be removed completely, only a sample of the cancer may be removed to confirm the diagnosis, and the rest of the planned operation will be stopped.
If treatment (such as chemotherapy or radiation) is planned before surgery, a biopsy is needed first to be sure of the diagnosis.
Lab tests of biopsy samples
The samples obtained during a biopsy (or during surgery) are sent to a lab, where they are looked at under a microscope to see if they contain cancer cells.
If cancer is found, other tests might be done as well. For example, tests might be done to see if the cancer cells have mutations (changes) in certain genes, such as the BRCA genes (BRCA1 or BRCA2) or NTRK genes. This might affect whether certain targeted therapy drugs might be helpful as part of treatment.
Genetic testing for pancreatic cancer risk
It is unknown if screening for pancreatic cancer is effective, and there is no routine screening for pancreatic cancer that is currently recommended for the general population. The medical community continues to research who to screen, which tests to use, and how often to use them.
Given that individuals from familial pancreatic cancer families, or individuals with germline genetic mutations in BRCA1, BRCA2, PALB2, CDKN2A, ATM, MLH1, MSH2, MSH6, PMS2, STK11, and EPCAM, are at increased risk for pancreatic cancer, there is special interest in researching pancreatic cancer screening for these high-risk individuals. It’s important to talk with your doctor about the screening options below, as each person is different.
Current guidelines recommend that healthy individuals from familial pancreatic cancer families should consider pancreatic cancer screening beginning at age 50, or 10 years younger than the earliest pancreatic cancer diagnosis in the family, if at least 1 of the pancreatic cancers in their family was in a first-degree relative. Guidelines also recommend that individuals with germline mutations in the genes listed above should consider screening beginning at age 50, or 10 years younger than the earliest pancreatic cancer diagnosis in the family, if they have a family history of pancreatic cancer. Some experts have recommended that all individuals with germline mutations in STK11 (which causes Peutz-Jeghers syndrome) or CDKN2A (which causes familial atypical multiple mole melanoma [FAMMM] syndrome), have screening regardless of their family history, with Peutz-Jeghers syndrome patients being recommended to begin screening at age 30 to 35 and FAMMM syndrome patients being recommended to begin by age 40. The screening tests that are most commonly used include:
- Magnetic resonance imaging (MRI) – An MRI uses magnetic fields to produce detailed images of the pancreas.
- Endoscopic ultrasound (EUS) – A thin, lighted tube is passed through the patient’s mouth and stomach. The tube goes down into the small intestine to take a picture of the pancreas.
Screening options are likely to change over time as new technologies are developed and more is learned about familial pancreatic cancer. It’s important to talk with your doctor about screening tests that are right for you.
Pancreatic cancer staging
After someone is diagnosed with pancreatic cancer, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics and to help them decide on treatment.
The earliest stage pancreas cancers are stage 0 (carcinoma in situ), and then range from stages I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means a more advanced cancer. Cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
The staging system used most often for pancreatic cancer is the American Joint Committee on Cancer (AJCC) TNM system, which is based on 3 key pieces of information:
- The extent of the tumor (T): How large is the tumor and has it grown outside the pancreas into nearby blood vessels?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes? If so, how many of the lymph nodes have cancer?
- The spread (metastasized) to distant sites (M): Has the cancer spread to distant lymph nodes or distant organs such as the liver, peritoneum (the lining of the abdominal cavity), lungs or bones?
The system described below is the most recent American Joint Committee on Cancer TNM system, effective January 2018. It is used to stage most pancreatic cancers except for well-differentiated pancreatic neuroendocrine tumors (NETs), which have their own staging system.
The staging system in the table uses the pathologic stage. It is determined by examining tissue removed during an operation. This is also known as the surgical stage. Sometimes, if the doctor’s physical exam, imaging, or other tests show the tumor is too large or has spread to nearby organs and cannot be removed by surgery right away or at all, radiation or chemotherapy might be given first. In this case, the cancer will have a clinical stage. It is based on the results of physical exam, biopsy, and imaging tests. The clinical stage can be used to help plan treatment. Sometimes, though, the cancer has spread further than the clinical stage estimates, and may not predict the patient’s outlook as accurately as a pathologic stage. For more information, see Cancer Staging.
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
Cancer staging can be complex. If you have any questions about your stage, please ask your doctor to explain it to you in a way you understand. (Additional information of the TNM system also follows the stage table below.)
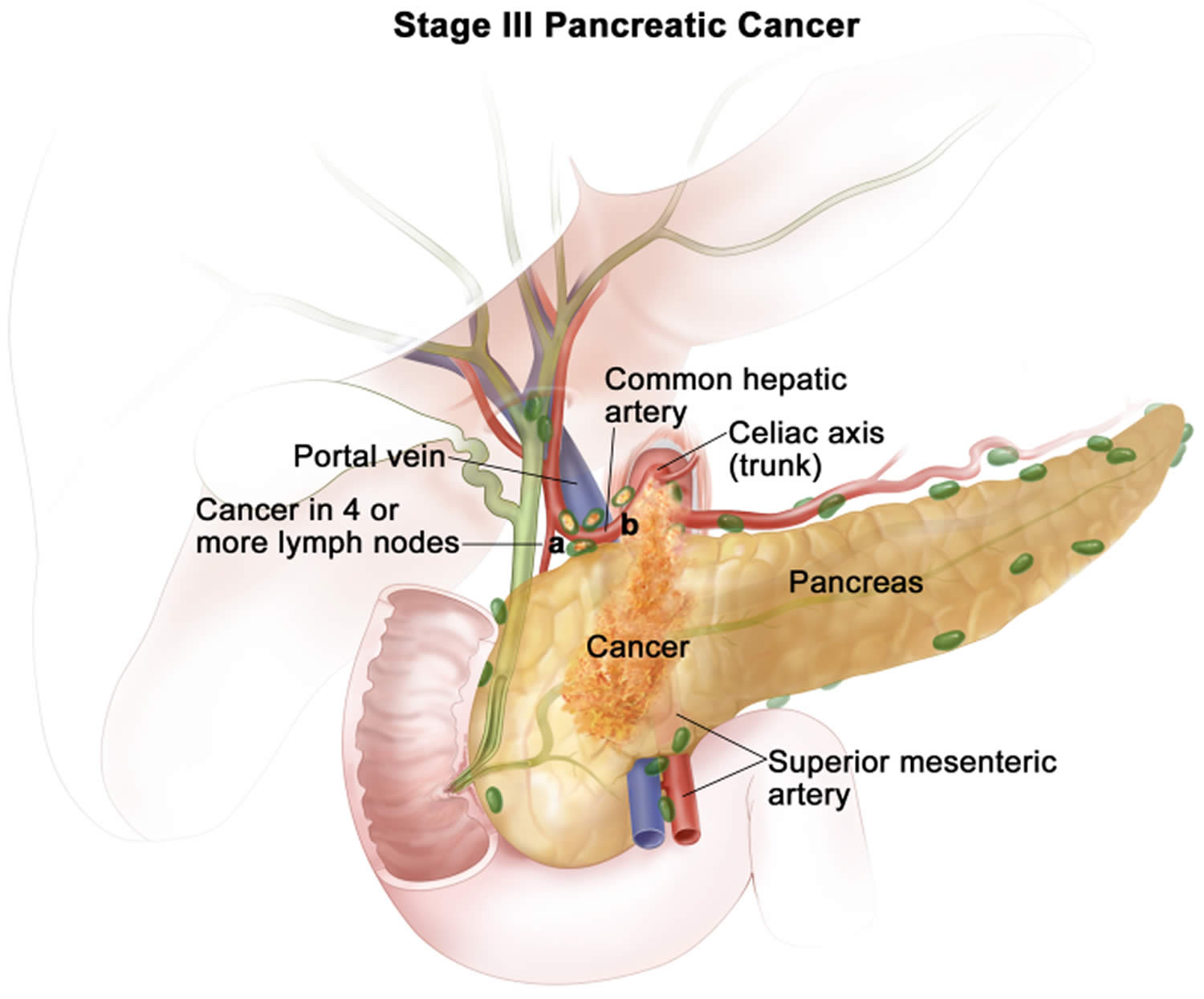
Table 1. Stages of pancreatic cancer
| American Joint Committee on Cancer (AJCC) Stage | Stage grouping | Stage description* |
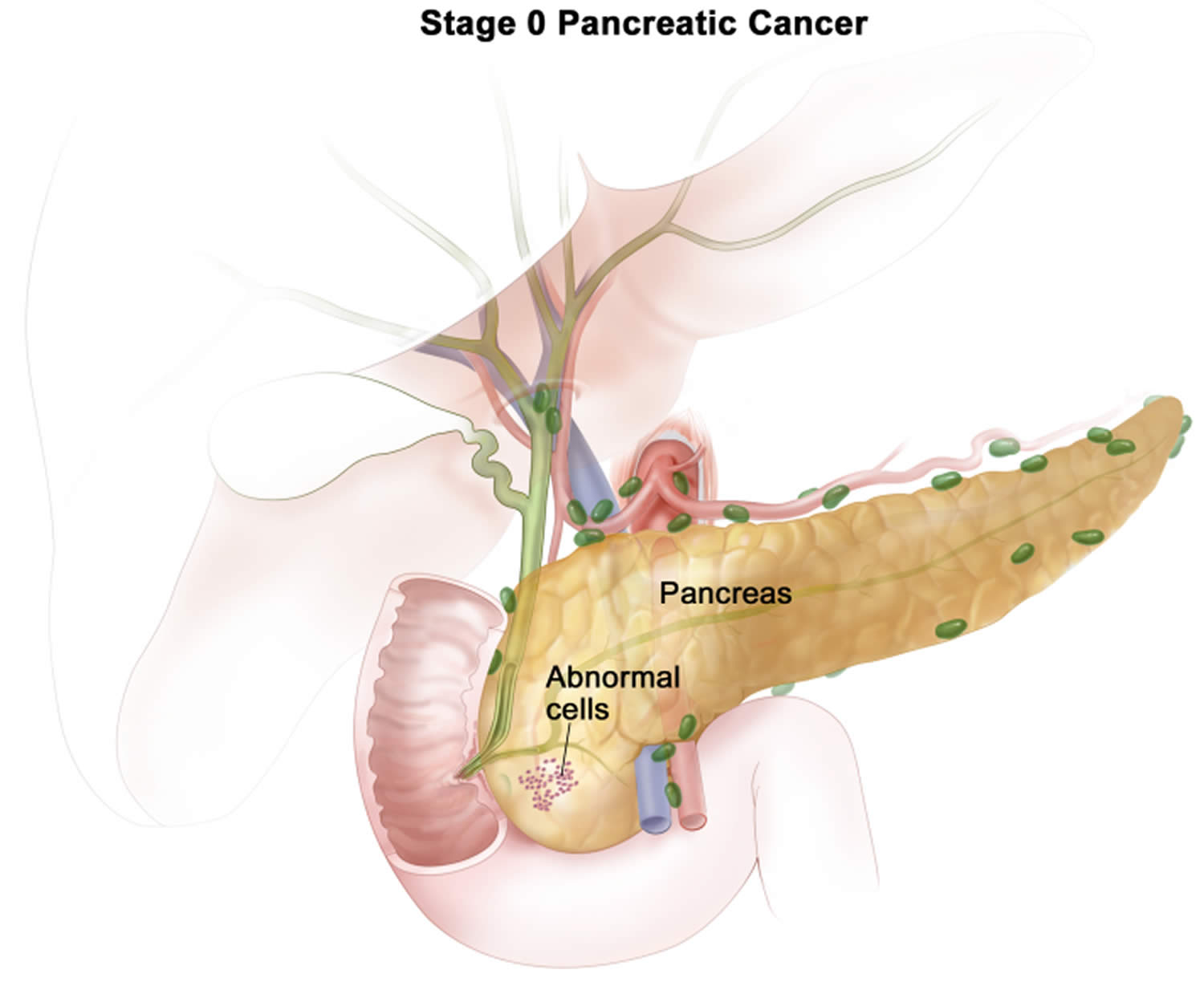
| 0 | Tis N0 M0 | The cancer is confined to the top layers of pancreatic duct cells and has not invaded deeper tissues. It has not spread outside of the pancreas. These tumors are sometimes referred to as carcinoma in situ (Tis). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
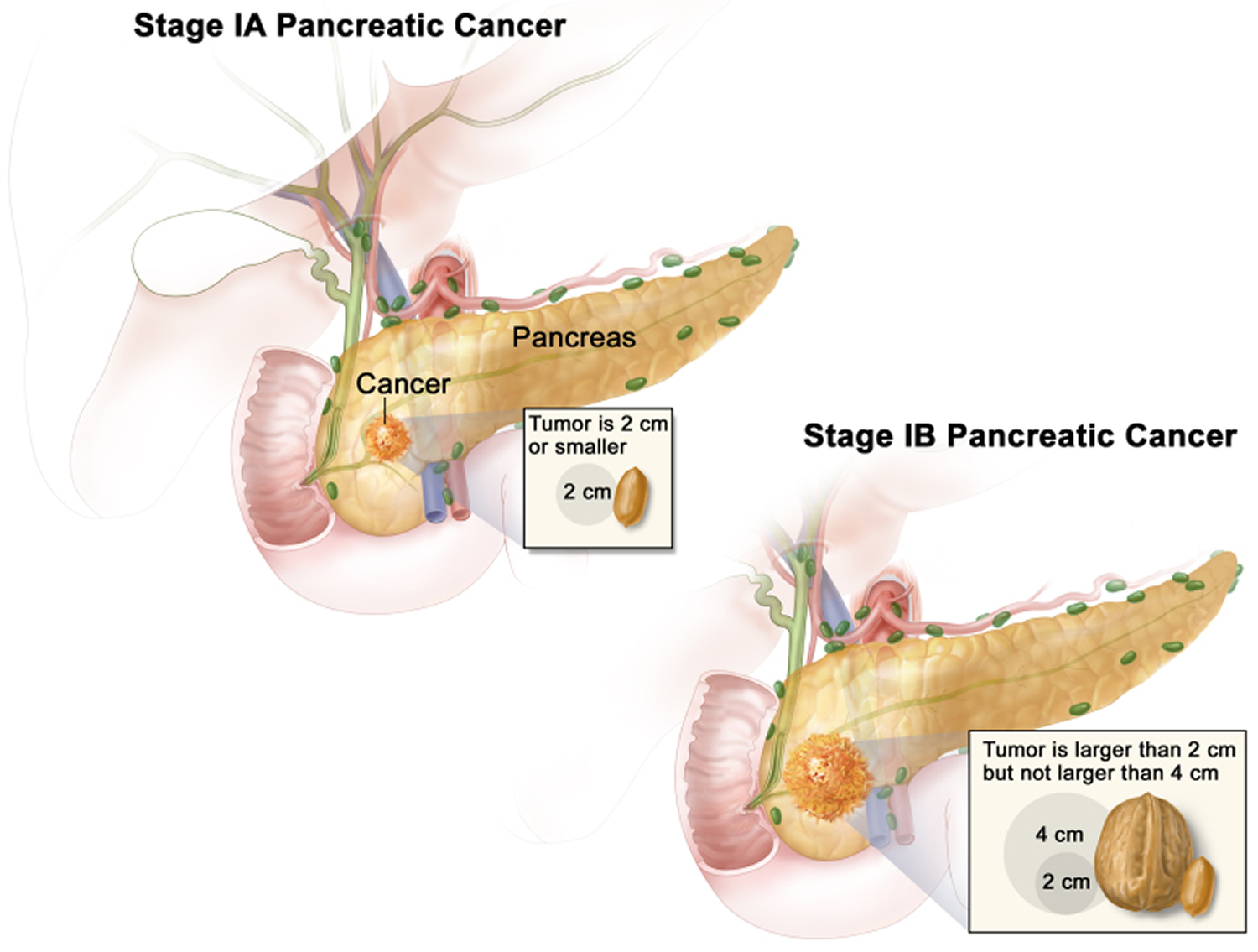
| 1A | T1 N0 M0 | The cancer is confined to the pancreas and is no bigger than 2 cm (0.8 inch) across (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1B | T2 N0 M0 | The cancer is confined to the pancreas and is larger than 2 cm (0.8 inch) but no more than 4cm (1.6 inches) across (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2A | T3 N0 M0 | The cancer is confined to the pancreas and is bigger than 4 cm (1.6 inches) across (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
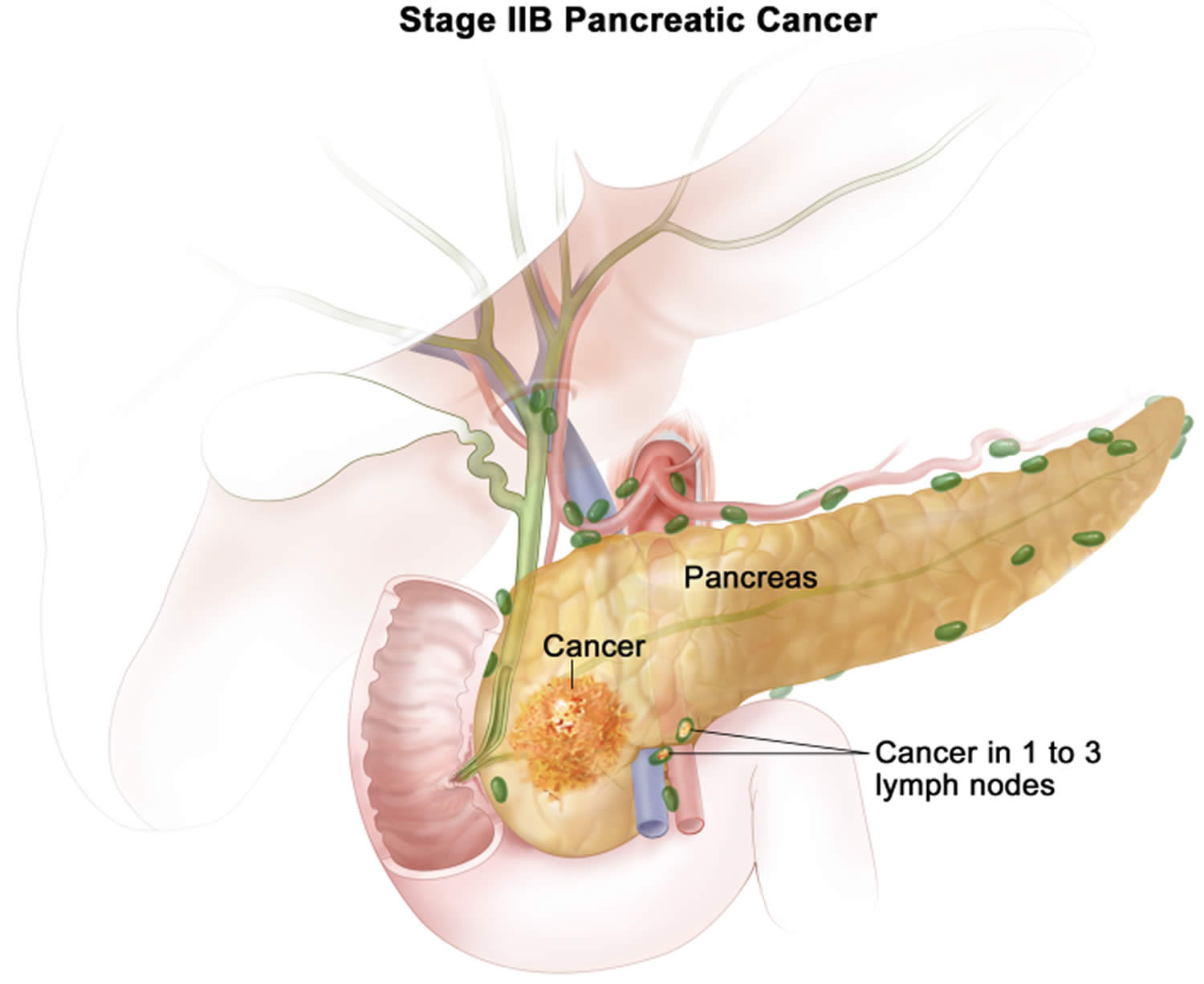
| 2B
| T1 N1 M0 | The cancer is confined to the pancreas and is no bigger than 2 cm (0.8 inch) across (T1) AND it has spread to no more than 3 nearby lymph nodes (N1). It has not spread to distant sites (M0). |
| T2 N1 M0 | The cancer is confined to the pancreas and is larger than 2 cm (0.8 inch) but no more than 4cm (1.6 inches) across (T2) AND it has spread to no more than 3 nearby lymph nodes (N1). It has not spread to distant sites (M0). | |
| T3 N1 M0 | The cancer is confined to the pancreas and is bigger than 4 cm (1.6 inches) across (T3) AND it has spread to no more than 3 nearby lymph nodes (N1). It has not spread to distant sites (M0). | |
| 3 | T1 N2 M0 | The cancer is confined to the pancreas and is no bigger than 2 cm (0.8 inch) across (T1) AND it has spread to 4 or more nearby lymph nodes (N2). It has not spread to distant sites (M0). |
| OR | ||
| T2 N2 M0 | The cancer is confined to the pancreas and is larger than 2 cm (0.8 inch) but no more than 4cm (1.6 inches) across (T2) AND it has spread to 4 or more nearby lymph nodes (N2). It has not spread to distant sites (M0). | |
| OR | ||
| T3 N2 M0 | The cancer is confined to the pancreas and is bigger than 4 cm (1.6 inches) across (T3) AND it has spread to 4 or more nearby lymph nodes (N2). It has not spread to distant sites (M0). | |
| OR | ||
| T4 Any N M0 | The cancer is growing outside the pancreas and into nearby major blood vessels (T4). The cancer may or may not have spread to nearby lymph nodes (Any N). It has not spread to distant sites (M0). | |
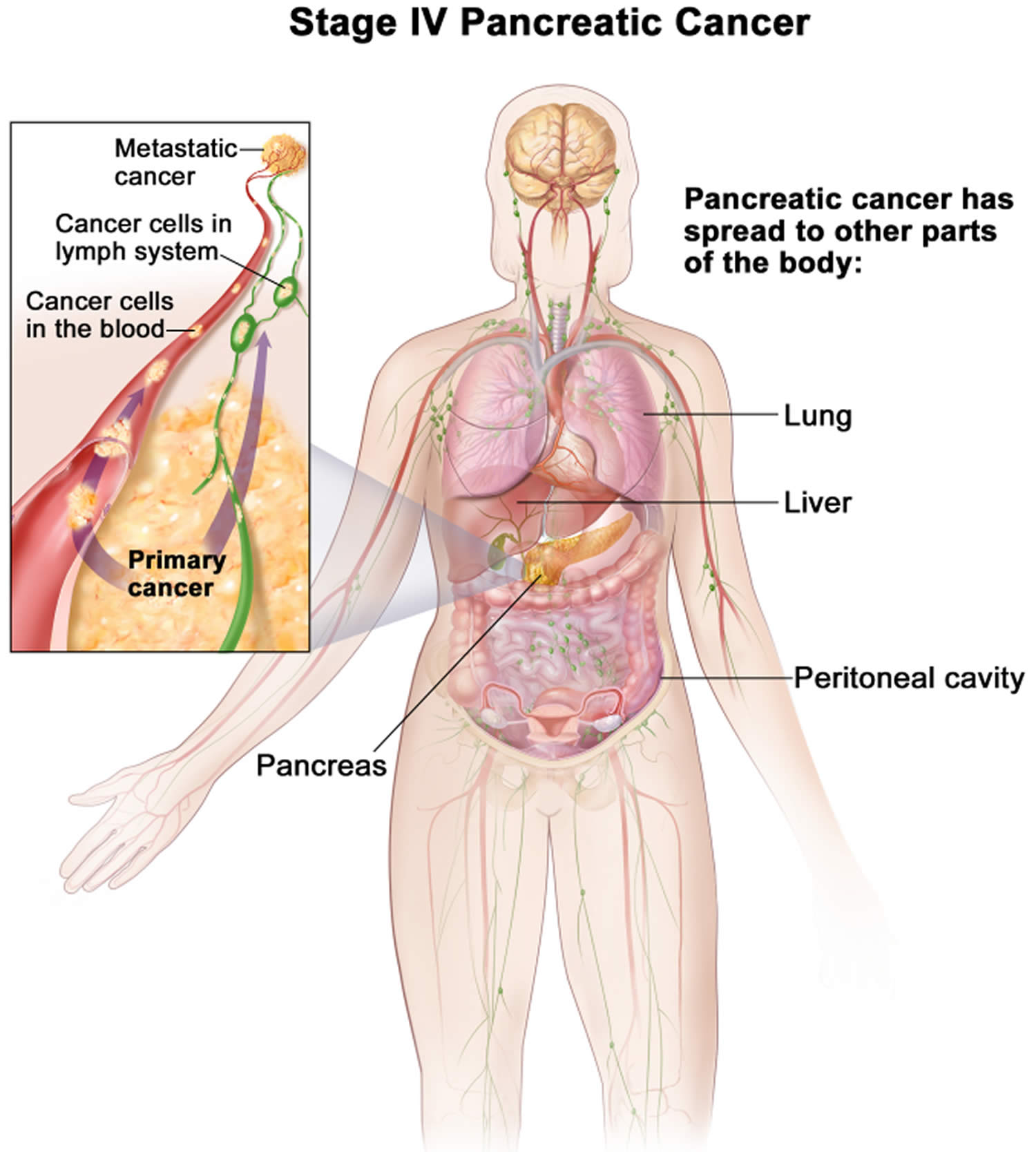
| 4 | Any T Any N M1 | The cancer has spread to distant sites such as the liver, peritoneum (the lining of the abdominal cavity), lungs or bones (M1). It can be any size (Any T) and might or might not have spread to nearby lymph nodes (Any N). |
Footnotes: * The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Stage 0 pancreatic cancer
Stage 0 pancreatic cancer is also called carcinoma in situ, this means the cancer is confined to the top layers of pancreatic duct cells and has not invaded deeper tissues. The cancer has not spread outside of the pancreas to nearby lymph nodes (N0) or to distant sites (M0).
Stage 1 pancreatic cancer
Stage 1 pancreatic cancer means the cancer is not more than 4cm in size and it hasn’t spread outside the pancreas. Stage 1 pancreatic cancer is divided into 1A and 1B.
- Stage 1A pancreatic cancer means the cancer is completely inside the pancreas and is 2cm or less. There is no cancer in the lymph nodes Open a glossary item or other areas of the body. In TNM staging, this is the same as T1, N0, M0.
- Stage 1B pancreatic cancer means the cancer is completely inside the pancreas and is larger than 2cm but no bigger than 4cm. There is no cancer in the lymph nodes or other areas of the body. In TNM staging, this is the same as T2, N0, M0.
Stage 2 pancreatic cancer
Stage 2 pancreatic cancer is divided into into 2A and 2B:
- Stage 2A pancreatic cancer means the cancer is bigger than 4 cm but is still within the pancreas. It has not spread to the lymph nodes Open a glossary item or other areas of the body. In TNM staging, this is the same as T3, N0, M0.
- Stage 2B pancreatic cancer means the cancer is any size within the pancreas and has spread to no more than 3 nearby lymph nodes. It has not spread anywhere else in the body. In TNM staging, this is the same as T1, 2 or 3, N1, M0.
Stage 3 pancreatic cancer
Stage 3 pancreatic cancer is also called locally advanced cancer.
- Stage 3 pancreatic cancer can mean that the cancer is any size within the pancreas and has spread to 4 or more nearby lymph nodes. In TNM staging, this is the same as T1, 2 or 3, N2, M0.
- OR
- Stage 3 pancreatic cancer can also mean the cancer has started to grow outside the pancreas into the major blood vessels nearby. It may or may not have spread into the lymph nodes. It hasn’t spread to any other areas of the body. In TNM staging, this is the same as T4, Any N, M0.
Stage 4 pancreatic cancer
Stage 4 pancreatic cancer is also called advanced (metastatic) cancer, this means that the cancer has spread to other areas of the body, such as the liver or lungs. In TNM staging, this is the same as Any T, Any N, M1.
Other prognostic factors
Although not formally part of the TNM system, other factors are also important in determining a person’s prognosis (outlook).
Tumor grade
The grade describes how closely the cancer looks like normal tissue under a microscope.
- Grade 1 (G1) means the cancer looks much like normal pancreas tissue.
- Grade 3 (G3) means the cancer looks very abnormal.
- Grade 2 (G2) falls somewhere in between.
Low-grade cancers (G1) tend to grow and spread more slowly than high-grade (G3) cancers. Most of the time, Grade 3 pancreas cancers tend to have a poor prognosis (outlook) compared to Grade 1 or 2 cancers.
Extent of resection
For patients who have surgery, another important factor is the extent of the resection — whether or not all of the tumor is removed:
- R0: All of the cancer is thought to have been removed. (There are no visible or microscopic signs suggesting that cancer was left behind.)
- R1: All visible tumor was removed, but lab tests of the removed tissue show that some small areas of cancer were probably left behind.
- R2: Some visible tumor could not be removed.
Resectable versus unresectable pancreatic cancer
The AJCC staging system gives a detailed summary of how far the cancer has spread. But for treatment purposes, doctors use a simpler staging system, which divides cancers into groups based on whether or not they can be removed (resected) with surgery:
- Resectable
- Borderline resectable
- Unresectable (either locally advanced or metastatic)
Resectable
If the cancer is only in the pancreas (or has spread just beyond it) and the surgeon believes the entire tumor can be removed, it is called resectable. In general, this would include most stage IA, IB, and IIA cancers in the TNM system.
It’s important to note that some cancers might appear to be resectable based on imaging tests, but once surgery is started it might become clear that not all of the cancer can be removed. If this happens, only some of the cancer may be removed to confirm the diagnosis (if a biopsy hasn’t been done already), and the rest of the planned operation will be stopped to help avoid the risk of major side effects.
Borderline resectable
This term is used to describe some cancers that might have just reached nearby blood vessels, but which the doctors feel might still be removed completely with surgery.
Unresectable
These cancers can’t be removed entirely by surgery.
Locally advanced: If the cancer has not yet spread to distant organs but it still can’t be removed completely with surgery, it is called locally advanced. Often the reason the cancer can’t be removed is because it has grown into or surrounded nearby major blood vessels. (This would include some stage III cancers in the TNM system.)
Surgery to try to remove these tumors would be very unlikely to be helpful and could still have major side effects. Some type of surgery might still be done, but it would be a less extensive operation with the goal of preventing or relieving symptoms or problems like a blocked bile duct or intestinal tract, instead of trying to cure the cancer.
Metastatic: If the cancer has spread to distant organs, it is called metastatic (Stage IV). These cancers can’t be removed completely. Surgery might still be done, but the goal would be to prevent or relieve symptoms, not to try to cure the cancer.
Familial pancreatic cancer treatment
Treatment for pancreatic cancer depends on the stage and location of the cancer as well as on your overall health and personal preferences. For most people, the first goal of pancreatic cancer treatment is to eliminate the cancer, when possible. When that isn’t an option, the focus may be on improving your quality of life and preventing the cancer from growing or causing more harm.
Depending on the type and stage of the cancer and other factors, treatment options for people with pancreatic cancer can include:
- Surgery
- Ablation or Embolization
- Radiation Therapy
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Palliative care
Treatment may include surgery, radiation, chemotherapy or a combination of these. When pancreatic cancer is advanced and these treatments aren’t likely to offer a benefit, your doctor will offer symptom relief (palliative care) that makes you as comfortable as possible.
Deciding which treatment you need
To decide about what treatment you need, your healthcare team looks at your test and scan results to see if they can remove (resect) the cancer. Your cancer may be:
- Resectable, which means pancreatic cancer can be removed with surgery. Generally, stage 1 and 2 pancreatic cancers are resectable.
- Borderline resectable, which means the cancer is right next to a main blood vessel and so it is less clear if surgery is possible. You may have chemotherapy first to shrink the cancer.
- Unresectable, which means that surgery to remove the cancer is not possible. The cancer may have grown into nearby organs (locally advanced cancer) or to more distant areas of the body (metastatic cancer).
Pancreatic cancer surgery
The type of surgery you need depends on where the cancer is in your pancreas.
To find out if it might be possible to remove the cancer, your surgeon will look at:
- the size of the tumor
- where it is in the pancreas
- whether the cancer has grown into the tissues around the pancreas
- whether the cancer is in any of the lymph nodes around the pancreas
- whether the cancer has grown into the major blood vessels in or around the pancreas
- whether the cancer has spread to any other parts of the body
To determine which type of surgery might be best, it’s important to know the stage (extent) of the cancer. But it can be hard to stage pancreatic cancer accurately just using imaging tests. Sometimes laparoscopy is done first to help determine the extent of the cancer and if it can be resected.
For this procedure, the surgeon makes a few small incisions (cuts) in the abdomen (belly) and inserts long, thin instruments. One of these has a small video camera on the end so the surgeon can see inside the abdomen and look at the pancreas and other organs. Biopsy samples of tumors and other abnormal areas can show how far the cancer has spread.
Two general types of surgery can be used for pancreatic cancer:
- Potentially curative surgery is used when the results of exams and tests suggest that it’s possible to remove (resect) all the cancer.
- Palliative surgery may be done if tests show that the cancer is too widespread to be removed completely. This surgery is done to relieve symptoms or to prevent certain complications like a blocked bile duct or intestine, but the goal is not to cure the cancer.
If it is possible to remove your cancer your surgeon might suggest a:
- Total pancreatectomy. Your surgeon removes all your pancreas. You can live relatively normally without a pancreas but do need lifelong insulin and enzyme replacement.
- Distal pancreatectomy. Your surgeon removes the body and tail of your pancreas. Your surgeon may also remove your spleen.
- Pylorus preserving pancreaticduodenectomy (PPPD). A PPPD operation means removing:
- the head of your pancreas
- the duodenum – the first part of the small bowel (intestine)
- gallbladder
- part of the bile duct
- Kausch Whipple operation also called Whipple procedure is the same as a pylorus preserving pancreaticduodenectomy (PPPD) operation but you also have part of your stomach removed.
- Surgery for tumors affecting nearby blood vessels. Many people with advanced pancreatic cancer are not considered eligible for the Whipple procedure or other pancreatic surgeries if their tumors involve nearby blood vessels. At a very few medical centers in the United States, highly specialized and experienced surgeons will safely perform these operations with removal and reconstruction of parts of blood vessels in select patients.
Each of these surgeries carries the risk of bleeding and infection. After surgery some people experience nausea and vomiting if the stomach has difficulty emptying (delayed gastric emptying). Expect a long recovery after any of these procedures. You’ll spend several days in the hospital and then recover for several weeks at home.
Extensive research shows pancreatic cancer surgery tends to cause fewer complications when done by highly experienced surgeons at centers that do many of these operations. Don’t hesitate to ask about your surgeon’s and hospital’s experience with pancreatic cancer surgery. If you have any doubts, get a second opinion.
Whipple procedure (pancreaticoduodenectomy)
Whipple procedure (pancreaticoduodenectomy) is the most common operation to remove a cancer in the head of the pancreas.
During this operation, the surgeon removes the head of the pancreas and sometimes the body of the pancreas as well. Nearby structures such as part of the small intestine, part of the bile duct, the gallbladder, lymph nodes near the pancreas, and sometimes part of the stomach are also removed. The remaining bile duct and pancreas are then attached to the small intestine so that bile and digestive enzymes can still go into the small intestine. The end pieces of the small intestine (or the stomach and small intestine) are then reattached so that food can pass through the digestive tract (gut).
Most often, this operation is done through a large incision (cut) down the middle of the belly. Some doctors at major cancer centers also do the operation laparoscopically, which is sometimes known as keyhole surgery.
A Whipple procedure is a very complex operation that requires a surgeon with a lot of skill and experience. It carries a relatively high risk of complications that can be life threatening. When the operation is done in small hospitals or by doctors with less experience, as many as 15% of patients may die as a result of surgical complications. In contrast, when the operation is done in cancer centers by surgeons experienced in the procedure, fewer than 5% of patients die as a direct result of surgery.
To have the best outcome, it’s important to be treated by a surgeon who does many of these operations and to have the surgery at a hospital where many of them are done. In general, people having this type of surgery do better when it’s done at a hospital that does at least 15 to 20 Whipple procedures per year.
Still, even under the best circumstances, many patients have complications from the surgery. These can include:
- Leaking from the various connections between organs that the surgeon has to join
- Infections
- Bleeding
- Trouble with the stomach emptying after eating
- Trouble digesting some foods (which might require taking some pills to help with digestion)
- Weight loss
- Changes in bowel habits
- Diabetes
Distal pancreatectomy
In this operation, the surgeon removes only the tail of the pancreas or the tail and a portion of the body of the pancreas. The spleen is usually removed as well. The spleen helps the body fight infections, so if it’s removed you’ll be at increased risk of infection with certain bacteria. To help with this, doctors recommend that patients get certain vaccines before this surgery.
This surgery is used to treat cancers found in the tail and body of the pancreas. Unfortunately, many of these tumors have usually already spread by the time they are found and surgery is not always an option.
Total pancreatectomy
This operation removes the entire pancreas, as well as the gallbladder, part of the stomach and small intestine, and the spleen. This surgery might be an option if the cancer has spread throughout the pancreas but can still be removed. But this type of surgery is used less often than the other operations because there doesn’t seem to be a major advantage in removing the whole pancreas, and it can have major side effects.
It’s possible to live without a pancreas. But when the entire pancreas is removed, people are left without the cells that make insulin and other hormones that help maintain safe blood sugar levels. These people develop diabetes, which can be hard to manage because they are totally dependent on insulin shots. People who have had this surgery also need to take pancreatic enzyme pills to help them digest certain foods.
Before you have this operation, your doctor will recommend that you get certain vaccines because the spleen will be removed.
Potentially curative surgery
Studies have shown that removing only part of a pancreatic cancer doesn’t help patients live longer, so potentially curative surgery is only done if the surgeon thinks all of the cancer can be removed.
This is a very complex surgery and it can be very hard for patients. It can cause complications and might take weeks or months to recover from fully. If you’re thinking about having this type of surgery, it’s important to weigh the potential benefits and risks carefully.
Fewer than 1 in 5 pancreatic cancers appear to be confined to the pancreas at the time they are found. Even then, not all of these cancers turn out to be truly resectable (able to be completely removed). Sometimes after the surgeon starts the operation it becomes clear that the cancer has grown too far to be completely taken out. If this happens, the operation may be stopped, or the surgeon might continue with a smaller operation with a goal of relieving or preventing symptoms. This is because the planned operation would be very unlikely to cure the cancer and could still lead to major side effects. It would also lengthen the recovery time, which could delay other treatments.
Surgery offers the only realistic chance to cure pancreatic cancer, but it doesn’t always lead to a cure. Even if all visible cancer is removed, often some cancer cells have already spread to other parts of the body. These cells can grow into new tumors over time, which can be hard to treat.
Curative surgery is done mainly to treat cancers in the head of the pancreas. Because these cancers are near the bile duct, they often cause jaundice, which sometimes allows them to be found early enough to be removed completely. Surgeries for other parts of the pancreas are described below, and are done if it’s possible to remove all of the cancer.
Palliative surgery
If the cancer has spread too far to be removed completely, any surgery being considered would be palliative (intended to relieve symptoms). Because pancreatic cancer can spread quickly, most doctors don’t advise major surgery for palliation, especially for people who are in poor health.
Sometimes surgery might be started with the hope it will cure the patient, but once it begins the surgeon discovers this is not possible. In this case, the surgeon might do a less extensive, palliative operation known as bypass surgery to help relieve symptoms.
Cancers growing in the head of the pancreas can block the common bile duct as it passes through this part of the pancreas. This can cause pain and digestive problems because bile can’t get into the intestine. The bile chemicals will also build up in the body, which can cause jaundice, nausea, vomiting, and other problems. There are two main options to relieve bile duct blockage in this situation:
- Stent placement. The most common approach to relieving a blocked bile duct does not involve actual surgery. Instead, a stent (small tube, usually made of metal) is put inside the duct to keep it open. This is usually done through an endoscope (a long, flexible tube) while you are sedated. Often this is part of an endoscopic retrograde cholangiopancreatography (ERCP). The doctor passes the endoscope down the throat and all the way into the small intestine. Through the endoscope, the doctor can then put the stent into the bile duct. The stent can also be put in place through the skin during a percutaneous transhepatic cholangiography (PTC). The stent helps keep the bile duct open even if the surrounding cancer presses on it. But after several months, the stent may become clogged and may need to be cleared or replaced. Larger stents can also be used to keep parts of the small intestine open if they are in danger of being blocked by the cancer. A bile duct stent can also be put in to help relieve jaundice before curative surgery is done (which would typically be a couple of weeks later). This can help lower the risk of complications from surgery.
- Bypass surgery. In people who are healthy enough, another option for relieving a blocked bile duct is surgery to reroute the flow of bile from the common bile duct directly into the small intestine, bypassing the pancreas. This typically requires a large incision (cut) in the abdomen, and it can take weeks to recover from this. Sometimes surgery can be done through several small cuts in the abdomen using special long surgical tools. This is known as laparoscopic or keyhole surgery. Having a stent placed is often easier and the recovery is much shorter, which is why this is done more often than bypass surgery. But surgery can have some advantages, such as:
- It can often give longer-lasting relief than a stent, which might need to be cleaned out or replaced.
- It might be an option if a stent can’t be placed for some reason.
- During surgery, the surgeon may be able to cut some of the nerves around the pancreas or inject them with alcohol. Because pancreatic cancer often causes pain if it reaches these nerves, this procedure may reduce or get rid of any pain caused by the cancer.
- Sometimes, the end of the stomach is disconnected from the duodenum (the first part of the small intestine) and attached farther down the small intestine during this surgery as well. This is known as a gastric bypass. This is done because over time the cancer might grow large enough to block the duodenum, which can cause pain and vomiting and often requires urgent surgery. Bypassing the duodenum before this happens can sometimes help avoid this.
- Bypass surgery can still be a major operation, so it’s important that you are healthy enough to tolerate it and that you talk with your doctor about the possible benefits and risks before you have the surgery.
Ablation or embolization treatments for pancreatic cancer
Ablation and embolization treatments are different ways of destroying tumors, rather than removing them with surgery. They are used much less often for pancreatic cancers but can sometimes be used to help treat pancreatic cancer that has spread to other organs, especially the liver.
These treatments are very unlikely to cure cancers on their own. They are more likely to be used to help prevent or relieve symptoms, when there are only a few areas of spread, and are often used along with other types of treatment.
Ablative treatments
Ablation refers to treatments that destroy tumors, usually with extreme heat or cold. They are generally best for tumors no more than about 2 cm (a little less than an inch) across. Typically, with this type of treatment you will not need to stay in the hospital. There are different kinds of ablative treatments:
- Radiofrequency ablation (RFA) uses high-energy radio waves for treatment. A thin, needle-like probe is put through the skin and into the tumor. Placement of the probe is guided by ultrasound or CT scans. The tip of the probe releases a high-frequency electric current which heats the tumor and destroys the cancer cells.
- Microwave thermotherapy is similar to RFA, except it uses microwaves to heat and destroy the cancer cells.
- Ethanol (alcohol) ablation also known as percutaneous ethanol injection kills the cancer cells by injecting concentrated alcohol directly into the tumor. This is usually done through the skin using a needle guided by ultrasound or CT scans.
- Cryosurgery also known as cryotherapy or cryoablation destroys a tumor by freezing it with a thin metal probe. The probe is guided through the skin and into the tumor, using ultrasound. Then very cold gasses are passed through the probe to freeze the tumor, killing the cancer cells. This method may be used to treat larger tumors than the other ablation techniques, but it sometimes requires general anesthesia (where you are put into a deep sleep).
Side effects of ablation treatments
Possible side effects after ablation therapy include abdominal pain, infection, and bleeding inside the body. Serious complications are uncommon, but they are possible.
Embolization treatments
During embolization, substances are injected into an artery to try to block the blood flow to cancer cells, causing them to die. This may be used for larger tumors (up to about 5 cm or 2 inches across) in the liver.
There are 3 main types of embolization:
- Arterial embolization also known as trans-arterial embolization (TAE) involves putting a catheter (a thin, flexible tube) into an artery through a small cut in the inner thigh and threaded up into the hepatic artery feeding the tumor. Blood flow is blocked (or reduced) by injecting materials that plug up that artery. Most of the healthy liver cells will not be affected because they get their blood supply from a different blood vessel, the portal vein.
- Chemoembolization also known as trans-arterial chemoembolization (TACE) combines embolization with chemotherapy. Most often, this is done by using tiny beads that give off a chemotherapy drug during the embolization. Trans-arterial chemoembolization (TACE) can also be done by giving chemotherapy through the catheter directly into the artery, then plugging up the artery.
- Radioembolization combines embolization with radiation therapy. In the United States, this is done by injecting small radioactive beads (called microspheres) into the hepatic artery. The beads lodge in the blood vessels near the tumor, where they give off small amounts of radiation to the tumor site. Since the radiation travels a very short distance, its effects are limited mainly to the tumor.
Possible side effects after embolization include abdominal pain, fever, nausea, infection, and blood clots in nearby blood vessels. Serious complications are not common, but they can happen.
Chemotherapy for pancreatic cancer
Chemotherapy uses drugs to help kill cancer cells. These drugs can be injected into a vein or taken orally. You may receive one chemotherapy drug or a combination of them. Chemo drugs enter the bloodstream and reach almost all areas of the body, making this treatment potentially useful for cancers whether or not they have spread.
Chemo is often part of the treatment for pancreatic cancer and may be used at any stage:
- Before surgery (neoadjuvant chemotherapy): Chemo can be given before surgery (sometimes along with radiation) to try to shrink the tumor so it can be removed with less extensive surgery. Neoadjuvant chemo is often used to treat cancers that are too big to be removed by surgery at the time of diagnosis (called locally advanced cancers).
- After surgery (adjuvant chemotherapy): Chemo can be used after surgery (sometimes along with radiation) to try to kill any cancer cells that have been left behind or have spread but can’t be seen, even on imaging tests. If these cells were allowed to grow, they could form new tumors in other places in the body. This type of treatment might lower the chance that the cancer will come back later.
- For advanced pancreatic cancer: Chemo can be used when the cancer is advanced and can’t be removed completely with surgery, or if surgery isn’t an option, or if the cancer has spread to other organs.
When chemo is given along with radiation, it is known as chemoradiation. It helps the radiation work better, but can also have more side effects.
In most cases (especially as adjuvant or neoadjuvant treatment), chemo is most effective when combinations of drugs are used. For people who are healthy enough, 2 or more drugs are usually given together. For people who are not healthy enough for combined treatments, a single drug (usually gemcitabine, 5-FU, or capecitabine) can be used.
The most common drugs used for both adjuvant and neoadjuvant chemo include:
- Gemcitabine (Gemzar)
- 5-fluorouracil (5-FU)
- Oxaliplatin (Eloxatin)
- Albumin-bound paclitaxel (Abraxane)
- Capecitabine (Xeloda)
- Cisplatin
- Irinotecan (Camptosar)
Chemotherapy for advanced pancreatic cancer:
- Gemcitabine (Gemzar)
- 5-fluorouracil (5-FU) or Capecitabine (Xeloda) (an oral 5FU drug)
- Irinotecan (Camptosar) or Liposomal Irinotecan (Onivyde)
- Platinum agents : Cisplatin and Oxaliplatin (Eloxatin)
- Taxanes: Paclitaxel (Taxol), Docetaxel (Taxotere), and Albumin-bound paclitaxel (Abraxane)
Chemo drugs for pancreatic cancer can be given into a vein (IV) or by mouth as a pill. The infusion can be done in a doctor’s office, chemotherapy clinic, or in a hospital setting.
Often, a slightly larger and sturdier IV is required in the vein system to give chemo. They are known as central venous catheters (CVCs), central venous access devices (CVADs), or central lines. They are used to put medicines, blood products, nutrients, or fluids right into your blood. They can also be used to take out blood for testing.
Doctors give chemo in cycles, with each period of treatment followed by a rest period to give you time to recover from the effects of the drugs. Cycles are most often 2 or 3 weeks long. The schedule varies depending on the drugs used. For example, with some drugs, the chemo is given only on the first day of the cycle. With others, it is given for a few days in a row, or once a week. Then, at the end of the cycle, the chemo schedule repeats to start the next cycle.
Adjuvant and neoadjuvant chemo is often given for a total of 3 to 6 months, depending on the drugs used. The length of treatment for advanced pancreatic cancer is based on how well it is working and what side effects you may have.
Chemo drugs can cause side effects. These depend on the type and dose of drugs given and how long treatment lasts. Common possible side effects include:
- Nausea and vomiting
- Loss of appetite
- Hair loss
- Mouth sores
- Diarrhea or constipation
Chemo can also affect the blood-forming cells of the bone marrow, which can lead to:
- Increased chance of infection (from low white blood cells)
- Bleeding or bruising (from low platelet counts)
- Fatigue or shortness of breath (from low red blood cells)
These side effects usually go away after treatment is finished. There are often ways to lessen these side effects. For example, drugs can be given to help prevent or reduce nausea and vomiting.
Some chemo drugs can cause other side effects. For example:
- Drugs such as cisplatin, oxaliplatin, and paclitaxel can damage nerves, which can lead to symptoms of numbness, tingling, or even pain in the hands and feet (called peripheral neuropathy). For a day or so after treatment, oxaliplatin can cause nerve pain that gets worse with exposure to cold, including when swallowing cold foods or liquids.
- Cisplatin can damage the kidneys. Doctors try to prevent this by giving the patient lots of intravenous (IV) fluid before and after the drug is given.
- Cisplatin can affect hearing. Your doctor may ask if you have any ringing in the ears or hearing loss during treatment.
Targeted therapy for pancreatic cancer
As researchers have learned more about the changes in pancreatic cancer cells that help them grow, they have developed newer drugs to specifically target these changes. These targeted drugs work differently from standard chemo drugs. Sometimes they work when standard chemo drugs don’t, and they often have different (and less severe) side effects.
EGFR inhibitor
Erlotinib (Tarceva) is a drug that targets a protein on cancer cells called EGFR, which normally helps the cells grow. In people with advanced pancreatic cancer, this drug can be given along with the chemo drug gemcitabine. Some people may benefit more from this combination than others.
Erlotinib (Tarceva) is taken as a pill, once a day.
Common side effects of erlotinib include an acne-like rash on the face and neck, diarrhea, loss of appetite, and feeling tired. Less common but more serious side effects can include serious lung, liver, or kidney damage; holes (perforations) forming in the stomach or intestines; serious skin conditions; and bleeding or blood clotting problems.
PARP inhibitor
In a small number of pancreatic cancers, the cells have changes in one of the BRCA genes (BRCA1 or BRCA2). Changes in one of these genes can sometimes lead to cancer.
Olaparib (Lynparza) is a type of drug known as a PARP inhibitor. PARP enzymes are normally involved in a pathway that helps repair damaged DNA inside cells. The BRCA genes are normally involved in a different pathway of DNA repair, and mutations in one of these genes can block that pathway. By blocking the PARP pathway as well, this drug makes it very hard for tumor cells with a mutated BRCA gene to repair damaged DNA, which often leads to their death.
Olaparib can be used to treat advanced pancreatic cancer in people with a known or suspected BRCA gene mutation, whose cancer has not gotten worse after at least 4 months of chemo that included a platinum drug (such as oxaliplatin or cisplatin).
This drug has been shown to help shrink or slow the growth of some advanced pancreatic cancers, although so far it’s not clear if it can help people live longer.
Olaparib (Lynparza) is taken by mouth as pills, typically twice a day.
Side effects of this drug can include nausea, vomiting, diarrhea or constipation, fatigue, feeling dizzy, loss of appetite, taste changes, low red blood cell counts (anemia), low white blood cell counts (with an increased risk of infection), belly pain, and muscle and joint pain. Less common but more serious side effects can include inflammation in the lungs and the development of certain blood cancers, such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).
NTRK inhibitors
A small number of pancreatic cancers have changes in one of the NTRK genes. These gene changes can sometimes lead to abnormal cell growth and cancer.
Larotrectinib (Vitrakvi) and entrectinib (Rozlytrek) target the proteins made by the NTRK genes. These drugs can be used in people with advanced pancreatic cancer that has been found to have an NTRK gene change, typically when the cancer is still growing despite other treatments.
These drugs are taken as pills, once or twice daily.
Common side effects of these drugs can include dizziness, fatigue, nausea, vomiting, constipation, weight gain, and diarrhea. Less common but more serious side effects can include abnormal liver tests, heart problems, and confusion.
Immunotherapy for pancreatic cancer
Immunotherapy is the use of medicines to stimulate a person’s own immune system to recognize and destroy cancer cells more effectively. Certain types of immunotherapy can be used to treat pancreatic cancer.
Immune checkpoint inhibitors
An important part of the immune system is its ability to keep itself from attacking the body’s normal cells. To do this, it uses “checkpoint” proteins on immune cells, which act like switches that need to be turned on (or off) to start an immune response. Cancer cells sometimes use these checkpoints to keep the immune system from attacking them. But drugs that target these checkpoints hold a lot of promise as cancer treatments.
Drugs called checkpoint inhibitors can be used for people whose pancreatic cancer cells have tested positive for specific gene changes, such as a high level of microsatellite instability (MSI-H), or changes in one of the mismatch repair (MMR) genes. Changes in MSI or in MMR genes (or both) are often seen in people with Lynch syndrome.
The drugs are used for people whose cancer starts growing again after chemotherapy. They might also be used to treat people whose cancer can’t be removed with surgery, has come back (recurred) after treatment, or has spread to other parts of the body (metastasized).
PD-1 inhibitor
Pembrolizumab (Keytruda) is a drug that targets PD-1, a checkpoint protein on immune system cells called T cells, that normally helps keep these cells from attacking normal cells in the body. By blocking PD-1, this drug boosts the immune response against pancreatic cancer cells and can often shrink tumors.
This drug is given as an intravenous (IV) infusion every 2 or 3 weeks.
Side effects can include fatigue, cough, nausea, itching, skin rash, decreased appetite, constipation, joint pain, and diarrhea.
Other, more serious side effects occur less often. This drug works by basically removing the brakes from the body’s immune system. Sometimes the immune system starts attacking other parts of the body, which can cause serious or even life-threatening problems in the lungs, intestines, liver, hormone-making glands, kidneys, or other organs.
It’s very important to report any new side effects to your health care team promptly. If serious side effects do occur, treatment may need to be stopped and you may get high doses of corticosteroids to suppress your immune system.
Radiation therapy for pancreatic cancer
Radiation therapy uses high-energy beams, such as those made from X-rays and protons, to destroy cancer cells. You may receive radiation treatments before or after cancer surgery, often in combination with chemotherapy. Or your doctor may recommend a combination of radiation and chemotherapy treatments when your cancer can’t be treated surgically.
Radiation therapy usually comes from a machine that moves around you, directing radiation to specific points on your body (external beam radiation). In specialized medical centers, radiation therapy may be delivered during surgery (intraoperative radiation).
Radiation therapy traditionally uses X-rays to treat cancer. Some medical centers offer proton beam radiation therapy, which may be a treatment option for some people with advanced pancreatic cancer.
The type of radiation most often used to treat pancreatic cancer is known as external beam radiation therapy, which focuses radiation from a source outside of the body on the cancer.
Getting radiation therapy is much like getting an x-ray, but the radiation is stronger. The procedure itself is painless. Each treatment lasts only a few minutes, although the setup time – getting you into place for treatment – usually takes longer. Most often, radiation treatments are given 5 days a week for several weeks.
When might radiation therapy be used?
- Radiation might be given after surgery (known as adjuvant treatment) to try to lower the chance of the cancer coming back. The radiation is typically given along with chemotherapy, which is together known as chemoradiation or chemoradiotherapy.
- For borderline resectable tumors, radiation might be given along with chemotherapy before surgery (neoadjuvant treatment) to try to shrink the tumor and make it easier to remove completely.
- Radiation therapy combined with chemotherapy may be used as part of the main treatment in people whose cancers have grown beyond the pancreas and can’t be removed by surgery (locally advanced/unresectable cancers).
- Radiation is sometimes used to help relieve symptoms (such as pain) in people with advanced cancers or in people who aren’t healthy enough for other treatments like surgery.
Some of the more common side effects of radiation therapy include:
- Skin changes in areas getting radiation, ranging from redness to blistering and peeling
- Nausea and vomiting
- Diarrhea
- Fatigue
- Loss of appetite
- Weight loss
Radiation can also lower blood counts, which can increase the risk of serious infection.
Usually these effects go away within a few weeks after the treatment is complete. Ask your doctor what side effects to expect and how to prevent or relieve them.
Resectable pancreatic cancer treatment
Surgeons usually consider pancreatic cancer to be resectable if it looks like it is still just in the pancreas or doesn’t extend far beyond the pancreas, and has not grown into nearby large blood vessels. A person must also be healthy enough to withstand surgery to remove the cancer, which is a major operation.
If imaging tests show a reasonable chance of removing the cancer completely, surgery is the preferred treatment if possible, as it offers the only realistic chance for cure. Based on where the cancer started, usually either a Whipple procedure (pancreaticoduodenectomy) or a distal pancreatectomy is used.
Sometimes even when a cancer is thought to be resectable, it becomes clear during the surgery that not all of it can be removed. If this happens, continuing the operation might do more harm than good. The surgery might be stopped, or the surgeon might continue with a smaller operation with a goal of relieving or preventing problems such as bile duct blockage.
- Neoadjuvant treatment (treatment before surgery). Sometimes, if the tumor is thought to be resectable but is very large, has many nearby large lymph nodes, or is causing significant pain, chemotherapy or chemoradiation may be given before surgery to shrink the tumor (known as neoadjuvant treatment). This may make it easier to remove all the cancer at the time of surgery. Additional chemo may still be recommended after surgery.
- Adjuvant treatment (treatment after surgery). Even when the surgeon thinks all of the cancer has been removed, the cancer might still come back. Giving chemotherapy (chemo), either alone or with radiation therapy (chemoradiation), after surgery (known as adjuvant treatment) might help some patients live longer. The chemo drugs most often used are gemcitabine (Gemzar) or 5-FU.
Borderline resectable pancreatic cancer treatment
A small number of pancreatic cancers have reached nearby blood vessels but have not grown deeply into them or surrounded them. These cancers might still be removable by surgery, but the odds of removing all of the cancer are lower, so they are considered borderline resectable.
These cancers are often treated first with neoadjuvant chemotherapy (sometimes along with radiation therapy) to try to shrink the cancer and make it easier to remove. Imaging tests (and sometimes laparoscopy) are then done to make sure the cancer hasn’t grown too much to be removed. As long as it hasn’t, surgery is then done to remove it. This might be followed by more chemotherapy.
Another option might be to have surgery first, followed by adjuvant chemotherapy (and possibly radiation). If, during the surgery, it becomes clear that not all of the cancer can be removed, continuing the operation might do more harm than good. The surgery might be stopped, or the surgeon might continue with a smaller operation with a goal of relieving or preventing problems such as bile duct blockage.
Locally advanced pancreatic cancer treatment
Locally advanced cancers have grown too far into nearby blood vessels or other tissues to be removed completely by surgery, but have not spread to the liver or distant organs and tissues. Surgery to try to remove these cancers does not help people live longer. Therefore, if surgery is done, it is to relieve bile duct blockage or to bypass a blocked intestine caused by the cancer pressing on other organs.
Chemotherapy, sometimes followed by chemoradiation, is the standard treatment option for locally advanced cancers. This may help some people live longer even if the cancer doesn’t shrink. Giving chemo and radiation therapy together may work better to shrink the cancer, but this combination has more side effects and can be harder on patients than either treatment alone. Sometimes, targeted therapy may be added to chemotherapy before chemoradiation is given.
Other times, immunotherapy given alone may also be an option.
Surgery might be done after chemo or chemoradiation, if imaging shows the cancer has become smaller and can be removed completely by surgery.
Stage 4 pancreatic cancer treatment
Stage 4 pancreatic cancer also called advanced (metastatic) pancreatic cancer, means that the cancer has spread to other areas of the body, such as the liver or lungs. Stage 4 pancreatic cancers have spread too much to be removed by surgery. Even when imaging tests show that the spread is only to one other part of the body, it is often assumed that small groups of cancer cells (too small to be seen on imaging tests) have already reached other organs of the body.
Chemotherapy is typically the main treatment for these cancers. It can sometimes shrink or slow the growth of these cancers for a time and might help people live longer, but it is not expected to cure the cancer.
Gemcitabine is one of the drugs used most often. It can be used alone (especially for people in poor health), or it can be combined with other drugs like albumin-bound paclitaxel (Abraxane), capecitabine (Xeloda), or the targeted drug erlotinib (Tarceva).
Another option, especially for people who are otherwise in good health, is a combination of chemo drugs called FOLFIRINOX. This consists of 4 drugs: 5-FU, leucovorin, irinotecan (Camptosar), and oxaliplatin (Eloxatin). This treatment might help people live longer than getting gemcitabine alone, but it can also have more severe side effects.
In certain cases, immunotherapy or targeted therapy may be options for people whose cancer cells have certain gene changes.
Other treatments might also be used to help prevent or relieve symptoms from these cancers. For example, radiation therapy or some type of nerve block might be used to help relieve cancer pain, or a stent might be placed during an endoscopy to help keep the bile duct open.
Because the treatments now available don’t work well for many people, you may want to think about taking part in a clinical trial of new drugs or combinations of drugs.
Pancreatic cancer that progresses or recurs treatment
If cancer continues to grow during treatment (progresses) or comes back (recurs), your treatment options will depend on:
- Where and how much the cancer has spread
- What treatments you have already had
- Your health and desire for more treatment
It’s important that you understand the goal of any further treatment, as well as the likelihood of benefits and risks.
When pancreatic cancer recurs, it most often shows up first in the liver, but it may also spread to the lungs, bone, or other organs. This is usually treated with chemotherapy if you are healthy enough to get it. If you have had chemo before and it kept the cancer away for some time, the same chemo might be helpful again. Otherwise, different chemo drugs might be tried, sometimes along with targeted therapy. Immunotherapy may also be helpful in some cases of recurrent pancreatic cancer. Other treatments such as radiation therapy or stent placement might be used to help prevent or relieve symptoms from the cancer.
If the cancer progresses while you are getting chemotherapy, another type of chemotherapy might be tried if you are healthy enough.
At some point, it might become clear that standard treatments are no longer controlling the cancer. If you want to continue getting treatment, you might think about taking part in a clinical trial of a newer pancreatic cancer treatment. While these are not always the best option for every person, they may benefit you, as well as future patients.
Ampulla of Vater cancer treatment
The ampulla of Vater is the area where the pancreatic duct and the common bile duct empty into the duodenum (the first part of the small intestine). Cancer at this site (known as ampullary cancer) can start in the pancreatic duct, the duodenum, or the common bile duct. In many patients, ampullary cancer can’t be distinguished from pancreatic cancer until surgery has been done.
These cancers often cause early symptoms such as jaundice, so they are often found while they are still resectable. Surgery with the Whipple procedure is often successful in treating these early stage cancers. Adjuvant chemoradiotherapy is often recommended after surgery.
More advanced ampullary cancers are treated like pancreatic cancer.
Clinical trials
Clinical trials are studies to test new treatments, such as systemic therapy, and new approaches to surgery or radiation therapy. If the treatment being studied proves to be safer and more effective than are current treatments, it can become the new standard of care.
Clinical trials for pancreatic cancer might give you a chance to try new targeted therapy, chemotherapy drugs, immunotherapy treatments or vaccines.
Clinical trials can’t guarantee a cure, and they might have serious or unexpected side effects. On the other hand, cancer clinical trials are closely monitored to ensure they’re conducted as safely as possible. And they offer access to treatments that wouldn’t otherwise be available to you.
Talk to your doctor about what clinical trials might be appropriate for you.
Pancreatic cancer diet
Having cancer of the pancreas will affect your eating and drinking habits, whatever your stage of cancer or treatment. Many people with pancreatic cancer lose weight. The pancreas is not only close to the stomach and bowel, it produces insulin and enzymes which help to digest food.
If you’ve had all or part of your pancreas removed, you may need to take insulin or tablets to regulate your blood sugar. You may also need to take enzyme supplements when you eat to help your digestion.
Blood sugar
If you are on insulin or tablets to regulate your blood sugar, your doctor will ask you to check your urine for sugar. Too much sugar in the urine indicates that the sugar balance of your body is not yet right.
If you are on insulin, you will probably also have to test your blood sugar levels. You will have to prick your finger and squeeze a drop of blood onto a test strip. This will show how much sugar is in your blood. You will then know how much insulin to take.
It takes time to get used to doing these tests. You will be shown how to do it before you leave hospital.
You may also have a nurse to visit you at home to help you and answer your questions.
Enzyme supplements
Digestive enzymes help your body to break down and absorb fats and proteins. Without enough enzymes, you may have diarrhoea or your poo (stools) may float, look pale and smell offensive. This is due to the undigested fat in the stool.
It might be difficult to put on weight as you are unable to absorb the nutrients from your food. If your pancreas is not working properly due to the cancer or you’ve had all or part of your pancreas removed, you may need to take enzyme supplements to reduce these effects.
Types of enzyme supplements
There are several different types of enzyme supplement. Creon is the most commonly used. The dose depends on:
- how well the remaining part of your pancreas is working
- your diet
You might need to take more enzymes if you are about to eat a large or fatty meal.
How to take them
You swallow the enzyme capsules whole, immediately before your meal. If you find it difficult to swallow capsules, you can open them and mix the granules in soft acidic foods that are at room temperature and easy to swallow. This can include apple sauce or mashed banana.
You must not chew or crush the granules. Have a drink of water afterwards to make sure none of the granules stay in your mouth as they can irritate the lining and cause mouth ulcers.
Your dietician will give you a diet plan to suit you and advise you on taking the supplements. It can take a bit of time to get the right dose of enzymes for you.
Snacks and small meals
You may find it easier to have lots of small meals through the day, rather than sticking to the traditional three meals a day.
It is a good idea to have plenty of nutritious snacks to hand that you can have whenever you feel like eating. If you can manage it, it’s best to choose full fat versions of yoghurts and puddings, so that you get the most calories.
You could try:
- yoghurts or fromage frais
- other soft puddings such as trifle or chocolate mousse
- dried fruit
- stewed or fresh fruit (bananas are high in calories)
- nuts
- cheese
- instant soups (make up with milk to boost calories)
- cereal
- milky drinks
- flapjacks
Some of these ideas may not suit your digestion but they might be worth a try. If in doubt, check with your dietitian.
Try to think of quick ways of having the things you like to eat. If possible, get someone to prepare your favourite foods in advance and freeze them in small portions. A microwave makes defrosting and heating easier and quicker.
Managing diarrhea
If you have diarrhea after pancreatic surgery, it is probably related to difficulty digesting fat. Avoid very high fibre foods (such as cereal and dried fruit) for a time as these may make things worse. Tell your doctor, nurse or dietitian.
You may need some medicines to control your symptoms. If you’re taking enzyme supplements, your dietitian may need to alter the dose. They can also suggest some changes to your diet that may help.
Nutritional supplements
If you are finding it hard to eat, there are plenty of nutritional supplements available on prescription. Some are powders you sprinkle on your food and some are drinks that are complete meals in themselves.
Sipping a supplement between meals throughout the day can really boost your calorie intake. Again, ask your doctor or dietitian.
Supportive (palliative) care
Advanced pancreatic cancer means that a cancer that began in the pancreas has spread to another part of the body or has come back after previous treatment. Advanced cancer can cause symptoms. Tell your doctor or nurse about any symptoms that you have so they can help you.
Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care. Palliative care can be used while undergoing aggressive treatments, such as surgery, chemotherapy and radiation therapy.
When palliative care is used along with other appropriate treatments — even soon after the diagnosis — people with cancer may feel better and live longer.
Palliative care is provided by teams of doctors, nurses and other specially trained professionals. These teams aim to improve the quality of life for people with cancer and their families. Palliative care is not the same as hospice care or end-of-life care.
Treatments such as chemotherapy or radiotherapy can sometimes help to shrink the cancer, reduce symptoms and help you feel better. Other treatments such as stents can treat specific symptoms such as a blockage in the stomach.
Tiredness and feeling unwell
Tiredness is a common symptom of advanced cancer. It can feel a bit overwhelming and as though you don’t have any energy.
Let your doctor or nurse know if you’re very tired as they might be able to prescribe medicine to help or other treatments. For example, a blood transfusion can give you more energy if you’re tired due to anaemia (low red blood cell levels).
- Resting. It’s important to rest a few times throughout the day. Resting regularly can help you feel less tired and more able to cope. You don’t have to sleep during these times. Just sitting or lying down will help.
- Exercise. Exercising can be hard when you feel very tired. Research has shown that daily light to moderate exercise can give you more energy. Going for a gentle walk is enough. Gentle exercises in bed or standing up can help if you can’t move around easily. Your hospital physiotherapist or community palliative care team might be able to help you plan an exercise programme that suits your needs.
- Sleeping. You might feel more tired if you have trouble sleeping at night. It can help to change a few things about when and where you sleep.
A blockage in the stomach
The cancer might block the entrance to the stomach or the entrance to the bowel. Then food can’t pass through. This causes pain, sickness and makes you feel very unwell. You may need to go to the hospital if this happens. They might put a tube called a stent into your stomach to allow food to pass through. Surgery to bypass or remove part of your stomach can help if you’re well enough to cope with it.
Loss of appetite
You might not feel like eating and may lose weight. It is important to eat as much as you can. Most hospital pancreatic cancer specialist teams include a dietitian. Talk to the dietitian about help with eating and getting high calorie drinks to boost your calorie intake if you need them.
Tips:
- Eating several small meals and snacks throughout the day can be easier to manage.
- Ask your doctor or dietitian to recommend high calorie drinks to sip if you are worried about losing weight.
- Eat whatever you feel like eating rather than what you think you should eat.
- Eat plenty of calories when you can to make up for times when you don’t feel like eating.
- Drink plenty of fluids even if you can’t eat.
- Don’t fill your stomach with a large amount of liquid before eating.
- Try to eat high calorie foods to keep your weight up.
A swollen abdomen (ascites)
You might have a swollen tummy (abdomen) if your cancer has spread to the liver. The swelling is due to a build up of fluid called ascites. It can make your clothes feel tighter. Your tummy might feel bloated. You might also find it difficult to sit comfortably or to move around.
Your doctor might drain off the fluid by putting a small flexible tube into the tummy (abdomen). They might use an ultrasound scan to make sure the tube is in the right place. Draining off the fluid helps you to feel more comfortable.
Sometimes the fluid builds up again over a few weeks. If this happens, you might have another tube into your abdomen called an indwelling peritoneal catheter (IPC). This means you can drain the fluid off when you need to at home. This means you won’t need to go to hospital each time.
Bowel problems
You might pass pale, offensive smelling stools (poo) that float. This is called steatorrhea (fatty poop).
Bowel problems such as diarrhea or constipation can be caused by the cancer. They can also be caused by cancer treatments or medicines. For example, painkillers commonly cause constipation.
Talk to your doctor or nurse if you have bowel problems. They can help by giving you medicine. And they can refer you to a dietitian for advice on what to eat or drink.
Pain control for pancreatic cancer
Pain can be a major problem for people with pancreatic cancer. These cancers can invade and press on nerves near the pancreas, which can cause pain in the abdomen or back. Treatment is available to help relieve this pain. If you are having any pain, please be sure to tell your doctor or nurse. Pain is easier to control if the treatment is started when you first have it. You and your doctor or nurse can talk about the best ways to treat your pain. A pain specialist can also help develop a treatment plan.
Some proven ways to relieve pain from pancreatic cancer include:
- Pain medicines. For most patients, morphine or similar drugs (opioids) can help control the pain. Many people are worried about these drugs because they fear becoming addicted, but studies have shown that the risk of this is low if the patient takes the drug for pain as directed by the doctor. Pain medicines work best when they are taken on a regular schedule. They do not work as well if they are only used when the pain becomes severe. Several long-acting forms of morphine and other opioids are in pill form and only need be taken once or twice a day. There is even a long-acting form of the drug fentanyl that is applied as a patch every 3 days. Common side effects of these drugs are nausea and feeling sleepy, which often get better over time. Constipation is a common side effect that does not get better on its own, so it needs to be treated. Most people need to take stool softeners and/or laxatives daily.
- Other treatments for pain. Sometimes certain procedures might be needed to treat pain. For example, cutting or injecting alcohol into some of the nerves (that carry pain sensations) near the pancreas can often improve pain and may allow you to use lower doses of pain medicines. If you are having surgery for some reason (such as to remove the cancer or relieve bile duct blockage), this can usually be done as part of the same operation. This can also be done as a separate procedure. The doctor might do a nerve block by injecting the nerves near the pancreas with either an anesthetic or a medicine that destroys the nerves. This can be done with the help of an ultrasound or CT scan either by:
- passing a needle through the skin or
- by using an endoscope (a long, flexible tube that is passed down the throat and past the stomach) that guides a needle to the nerves.
Treating the cancer with chemotherapy and/or radiation therapy can also sometimes relieve pain by shrinking the size of the cancer.
Treatments to help you cope with distress
People with cancer frequently experience distress. Some research suggests distress is more common in people with pancreatic cancer than it is in people with other types of cancer.
If you’re distressed, you may have difficulty sleeping and find yourself constantly thinking about your cancer. You may feel angry or sad.
Discuss your feelings with your doctor. Specialists can help you sort through your feelings and help you devise strategies for coping. In some cases, medications may help.
Integrative medicine and alternative therapies may also help you cope with distress. Examples include:
- Art therapy
- Exercise
- Meditation
- Music therapy
- Relaxation exercises
- Spirituality
Talk with your doctor if you’re interested in these treatment options.
Coping and support
Learning you have a life-threatening illness can be devastating. Some of the following suggestions may help:
- Learn what you need to know about your cancer. Learn enough about your cancer to help you make decisions about your care. Ask your doctor about the details of your cancer and your treatment options. Ask about trusted sources of further information. If you’re doing your own research, good places to start include the National Cancer Institute and the Pancreatic Cancer Action Network.
- Assemble a support system. Ask your friends and family to form a support network for you. They may feel helpless and uncertain after your diagnosis. Helping you with simple tasks might give them comfort. And you might find relief in not having to worry about certain tasks. Think of things you want help with, such as meal preparation or getting to appointments.
- Find someone to talk with. Although friends and family can be your best allies, in some cases they have difficulty coping with the shock of your diagnosis. In these cases, talking with a counselor, medical social worker, or a pastoral or religious counselor can be helpful. Ask your doctor for a referral.
- Connect with other cancer survivors. You may find comfort in talking with other cancer survivors. Contact your local chapter of the American Cancer Society to find cancer support groups in your area. The Pancreatic Cancer Action Network can connect you with a pancreatic cancer survivor who can provide support by phone or email.
- Consider hospice. Hospice care provides comfort and support to terminally ill people and their loved ones. It allows family and friends — with the aid of nurses, social workers and trained volunteers — to care for and comfort a loved one at home or in a hospice residence. Hospice care also provides emotional, social and spiritual support for people who are ill and those closest to them.
Pancreatic neuroendocrine tumor treatment
For pancreatic neuroendocrine tumors (PNETs), treatment options might include surgery, ablation or embolization treatments, radiation therapy, or different types of medicines.
Treatment of pancreatic neuroendocrine tumors (PNETs) depends to a large extent on whether they can be resected (removed) completely or not. But other factors, such as your overall health, can also affect treatment options. Talk to your doctor if you have any questions about the treatment plan they recommend.
Pancreatic NETs are more likely to be resectable than exocrine pancreas cancers (the most common type of pancreatic cancer). Most NETs that have not spread to distant parts of the body are resectable. Even some NETs that have spread might be resectable if they have not spread too far (such as only to a few spots in the liver).
Treating resectable tumors
Pancreatic NETs that have not spread outside the pancreas should be completely removed, if possible, because these tumors are more likely to be cured with surgery. The procedure used depends on the type of tumor, its size, and its location in the pancreas. Types of potentially curative surgery include enucleation (removing only the tumor), central pancreatectomy, distal pancreatectomy, the Whipple procedure (pancreaticoduodenectomy), and total pancreatectomy. The type of surgery needed depends on several factors, including the location, size, and specific kind of pancreatic NET (functioning or nonfunctioning). Lymph nodes are often removed to check for tumor spread.
Before any surgery, medicines are often given to control any symptoms caused by the tumor. For example, drugs to block stomach acid (like proton pump inhibitors) are used for gastrinomas. Often, people with insulinomas are treated with diazoxide to keep blood sugar from getting too low. If the tumor was visible on somatostatin receptor scintigraphy, a somatostatin analog such as octreotide may be used to control any symptoms.
Surgery alone is all that is needed for many pancreatic NETs, but after surgery, close monitoring is important to look for signs that the cancer may have come back or spread.
Enucleation (removing just the tumor)
Sometimes if a pancreatic NET is small, just the tumor itself is removed. This is called enucleation. This operation may be done using a laparoscope, so that only a few small cuts on the belly are needed. This operation may be all that is needed to treat an insulinoma. Small gastrinomas and some other pancreatic NETs may also be treated with enucleation, but sometimes the duodenum (the first part of the small intestine) is removed as well. The lymph nodes around the pancreas might also be removed so that they can be checked for cancer cells.
Central pancreatectomy
A central pancreatectomy is used to treat small, low grade tumors. For this operation, the surgeon removes only the neck and part of the body of the pancreas keeping the head and tail intact. This helps maintain most of the function of the pancreas.
Distal pancreatectomy
A distal pancreatectomy is used to treat pancreatic NETs found in the tail and body of the pancreas. In this operation, the surgeon removes only the tail of the pancreas or the tail and a portion of the body of the pancreas. The spleen is usually removed as well. The spleen helps the body fight infections, so if it’s removed you’ll be at increased risk of infection with certain bacteria. To help with this, doctors recommend that patients get certain vaccines before this surgery.
Whipple procedure (pancreaticoduodenectomy)
A Whipple procedure is used to treat pancreatic NETs found in the head of the pancreas. During this operation, the surgeon removes the head of the pancreas and sometimes the body of the pancreas as well. Nearby structures such as part of the small intestine, part of the bile duct, the gallbladder, lymph nodes near the pancreas, and sometimes part of the stomach are also removed. The remaining bile duct and pancreas are then attached to the small intestine so that bile and digestive enzymes can still go into the small intestine. The pieces of the small intestine (or the stomach and small intestine) are then reattached so that food can pass through the digestive tract.
Most often, this operation is done through a large incision (cut) down the middle of the belly. Some doctors at major cancer centers also do the operation laparoscopically, which is sometimes known as keyhole surgery.
This is a very complex operation that requires a surgeon with a lot of skill and experience. It carries a relatively high risk of complications that can be life threatening. When the operation is done in small hospitals or by doctors with less experience, as many as 15% of patients may die as a result of surgical complications. In contrast, when the operation is done in cancer centers by surgeons experienced in the procedure, less than 5% of patients die as a direct result of surgery.
To have the best outcome, it’s important to be treated by a surgeon who does many of these operations and to have the surgery at a hospital where many of them are done. In general, people having this type of surgery do better when it’s done at a hospital where at least 15 to 20 Whipple procedures are done per year.
Still, even under the best circumstances, many patients have complications from the surgery. These can include:
- Leaking from the various connections between organs that the surgeon has joined
- Infections
- Bleeding
- Trouble with the stomach emptying after eating
- Trouble digesting some foods (which might require taking pancreatic enzymes in pill form to help with digestion)
- Weight loss
- Changes in bowel habits
- Diabetes
Total pancreatectomy
Total pancreatectomy might be an option if the cancer has spread throughout the pancreas but can still be removed. This operation removes the entire pancreas, as well as the gallbladder, part of the stomach and small intestine, and the spleen. But this type of surgery is used less often than the other operations because there doesn’t seem to be a major advantage in removing the whole pancreas, and it can have major side effects.
It’s possible to live without a pancreas. But when the entire pancreas is removed, people are left without the cells that make insulin and other hormones that help maintain safe blood sugar levels. These people develop diabetes, which can be hard to manage because they are totally dependent on insulin shots. People who have had this surgery also need to take pancreatic enzyme pills to help them digest certain foods.
Before you have this operation, your doctor will recommend that you get certain vaccines because the spleen will be removed.
Palliative surgery
If the cancer has spread too far to be removed completely, any surgery being considered would be palliative (intended to relieve symptoms). This type of surgery may be considered in some people with pancreatic NETs whose tumor has recurred and is causing local problems or is making too many hormones that are causing symptoms.
Sometimes surgery might be started with the hope it will cure the patient, but once it begins the surgeon discovers this is not possible. In this case, the surgeon might do a less extensive, palliative operation known as bypass surgery instead to help prevent or relieve symptoms.
Cancers growing in the head of the pancreas can block the common bile duct as it passes through this part of the pancreas. This can cause pain and digestive problems because bile can’t get into the intestine. The bile chemicals will also build up in the body, which can cause jaundice, nausea, vomiting, and other problems.
There are 2 main options for relieving bile duct blockage: stent placement, and bypass surgery.
- Stent placement. The most common approach to relieving a blocked bile duct does not involve actual surgery. Instead, a stent (small tube, usually made of metal) is put inside the duct to keep it open. This is usually done through an endoscope (a long, flexible tube) while you are sedated. Often this is part of an endoscopic retrograde cholangiopancreatography (ERCP). The doctor passes the endoscope down the throat and all the way into the small intestine. The doctor can then insert the stent into the bile duct through the endoscope. The stent can also be put in place through the skin during a percutaneous transhepatic cholangiography (PTC). The stent helps keep the bile duct open even if the surrounding cancer presses on it. But after several months, the stent may become clogged and may need to be cleared or replaced. Larger stents can also be used to keep parts of the small intestine open if they are in danger of being blocked by the cancer. A bile duct stent can also be put in to help relieve jaundice before curative surgery is done (which would typically be a couple of weeks later). This can help lower the risk of complications from surgery.
- Bypass surgery. In people who are healthy enough, another option for relieving a blocked bile duct is surgery to reroute the flow of bile from the common bile duct directly into the small intestine, bypassing the pancreas. This typically requires a large incision (cut) in the abdomen, and it can take weeks to recover from this. Sometimes surgery can be done through several small cuts in the abdomen using special long surgical tools. This is known as laparoscopic or keyhole surgery. Having a stent placed is often easier and the recovery is much shorter, which is why this is done more often than bypass surgery. But this surgery can have some advantages:
- It can often give longer-lasting relief than a stent, which might need to be cleaned out or replaced.
- It might be an option if a stent can’t be placed for some reason.
- During surgery, the surgeon may be able to cut some of the nerves around the pancreas or inject them with alcohol. This may reduce or get rid of any pain caused by the cancer.
- Sometimes, the end of the stomach is disconnected from the duodenum (the first part of the small intestine) and attached farther down the small intestine during this surgery as well. This is known as a gastric bypass. This is done because over time the cancer might grow large enough to block the duodenum, which can cause pain and vomiting and often requires urgent surgery. Bypassing the duodenum before this happens can sometimes help avoid this.
- Bypass surgery can still be a major operation, so it’s important that you are healthy enough to withstand it and that you talk with your doctor about the possible benefits and risks before you have the surgery.
Surgery for cancer that has spread
Surgery may be used to remove metastases if a pancreatic NET has spread to the liver (the most common site of spread) or the lungs. Surgically removing metastases can improve symptoms and help patients with pancreatic NETs live longer. In rare cases, a liver transplant might be used to treat pancreatic NETs that have spread to the liver.
Treating unresectable tumors
Unresectable tumors can’t be removed completely with surgery. Pancreatic NETs are often slow growing, so lab and imaging tests are used to monitor the tumor(s) and look for signs of growth.
People with NETs that have spread outside the pancreas often have symptoms like diarrhea or hormone problems. These can often be helped with medicines like octreotide, lanreotide, diazoxide, and proton pump inhibitors. Some of these might also slow the growth of the tumor.
If further treatment is needed, chemotherapy or targeted drugs (such as sunitinib or everolimus) might be used, but this is usually delayed until a person is having symptoms that can’t be controlled with other drugs or has signs of tumor growth on scans. Surgery or ablative techniques might also be used to treat cancer spread to the liver.
For people with poorly differentiated tumors (neuroendocrine carcinomas), chemotherapy is typically the first treatment.
For adults with somatostatin (a type of hormone) receptor-positive pancreatic neuroendocrine tumors, a radiopharmaceutical drug, called Lutathera (lutetium Lu 177 dotatate), is also an option for treatment.
If treatment is no longer working at some point, you might want to think about taking part in a clinical trial testing a newer treatment. While these are not always the best option for every person, they may benefit you as well as future patients.
Ablation or embolization for pancreatic cancer
Ablation and embolization treatments are different ways of destroying tumors, rather than removing them with surgery. Ablation or embolization can sometimes be used to help treat pancreatic neuroendocrine tumor (NET) that has spread to other organs, especially the liver. When pancreatic NETs have spread to other sites, these treatments can often reduce tumor size and improve symptoms. But these treatments are very unlikely to cure cancers on their own. They are more likely to be used to help prevent or relieve symptoms, and are often used along with other types of treatment.
Ablative treatments (ablation)
Ablation refers to treatments that destroy tumors, usually with extreme heat or cold. They are generally best for tumors no more than about 2 cm (a little less than an inch) across. There are different kinds of ablative treatments:
- Radiofrequency ablation (RFA) uses high-energy radio waves. A thin, needle-like probe is put through the skin and into the tumor. Placement of the probe is guided by an ultrasound or CT scan. The tip of the probe releases a high-frequency electric current which heats the tumor and destroys the cancer cells.
- Microwave thermotherapy is similar to RFA, except it uses microwaves to heat and destroy the cancer cells.
- Ethanol (alcohol) ablation also known as percutaneous ethanol injection kills the cancer cells with concentrated alcohol injected directly into the tumor. This is usually done using a needle through the skin, guided by ultrasound or CT scans.
- Cryosurgery also known as cryotherapy or cryoablation destroys a tumor by freezing it with a thin metal probe. The probe is guided through the skin and into the tumor using an ultrasound. Then very cold gasses are passed through the probe to freeze the tumor, killing the cancer cells. This method may be used to treat larger tumors than the other ablation techniques, but it sometimes requires general anesthesia (where you are asleep).
Possible side effects after ablation therapy include abdominal pain, infection, and bleeding inside the body. Serious complications are uncommon, but they are possible.
Embolization
During embolization, substances are injected into an artery to try to block the blood flow to cancer cells, causing them to die. This may be used for larger tumors (up to 5cm or 2 inches across) in the liver.
There are 3 main types of embolization:
- Arterial embolization also known as trans-arterial embolization (TAE) involves putting a catheter (a thin, flexible tube) into an artery through a small cut in the inner thigh and threading it up into the hepatic artery feeding the tumor. Blood flow is blocked (or reduced) by injecting materials to plug up that artery. Most of the healthy liver cells will not be affected because they get their blood supply from a different blood vessel, the portal vein.
- Chemoembolization also known as trans-arterial chemoembolization (TACE) combines embolization with chemotherapy. Most often, this is done by using tiny beads that give off a chemotherapy drug during the embolization. TACE can also be done by giving chemotherapy through the catheter directly into the artery, then plugging up the artery.
- Radioembolization combines embolization with radiation therapy. In the United States, this is done by injecting small radioactive beads (called microspheres) into the hepatic artery. The beads lodge in the blood vessels near the tumor, where they give off small amounts of radiation to the tumor site for several days. Since the radiation travels a very short distance, its effects are limited mainly to the tumor.
Possible complications after embolization include abdominal pain, fever, nausea, infection, and blood clots in nearby blood vessels. Serious complications are not common, but they can happen.
Radiation therapy for pancreatic neuroendocrine tumor
Radiation therapy uses high-energy rays (such as x-rays) or radioactive particles to kill cancer cells. Surgery is the main treatment for most pancreatic neuroendocrine tumors (NETs), but radiation therapy may be an option for those who can’t have surgery for some reason. It may also be given after surgery in some cases if there’s a chance some of the tumor was not removed and is causing problems. Radiation is sometimes used to treat pancreatic NETs that have spread to the bone and are causing pain. It may also be used in the form of radioembolization to treat NETs that have spread to the liver.
External beam radiation therapy
External beam radiation therapy uses a machine that delivers a beam of radiation to a specific part of the body. Before your treatment starts, the radiation team will determine the correct angles for aiming the radiation beams and the proper dose of radiation. The treatment is much like getting an x-ray, but the radiation is stronger. The procedure itself is painless. Each treatment lasts only a few minutes, although the setup time – getting you into place for treatment – usually takes longer. Most often, radiation treatments are given 5 days a week for several weeks, but this can vary based on the reason it’s being given.
Some common side effects of radiation therapy include:
- Skin changes in areas getting radiation, ranging from redness to blistering and peeling
- Nausea and vomiting
- Diarrhea
- Fatigue
- Loss of appetite
- Weight loss
- Low blood counts, which can increase the risk of serious infection.
Usually these side effects go away within a few weeks after the treatment is complete. Ask your doctor what side effects to expect and how to prevent or relieve them.
Radioembolization
Radioembolization combines embolization with radiation therapy and can be used to treat liver metastases. Small beads called microspheres are attached to a radioactive element called yttrium-90 (or 90Y) and then injected into an artery close to the liver. The beads travel in the liver blood vessels until they get stuck in small blood vessels near the tumor. There they give off radioactivity for a short while, killing nearby tumor cells. The radiation travels a very short distance, so its effects are limited mainly to the tumor.
Peptide receptor radionuclide therapy (PRRT)
People with somatostatin receptor-positive neuroendocrine tumors may be candidates for peptide receptor radionuclide therapy (PRRT). In PRRT, a radioactive element is linked to a small part (peptide) of a somatostatin analog, and injected into a vein in the arm. The drug travels throughout the body, attaches to the somatostatin receptor (a protein) on the cancer cell, and gives off radiation to kill it. The radiation is delivered directly to the tumor, so there is less effect on healthy tissue. There are several drugs that might be used:
- The radioactive element Yttrium-90
- The radioactive element Lutathera (lutetium or Lu-177 dotatate)
If you are already taking octreotide or lanreotide, you will most likely need to stop taking these medicines for a certain time before you can be treated with PRRT.
Common side effects of peptide receptor radionuclide therapy (PRRT) include low levels of white blood cells, abnormal liver tests, nausea and vomiting, high levels of blood sugar, and pain.
Serious side effects include low levels of blood cells, development of certain blood or bone marrow cancers, kidney damage, liver damage, abnormal levels of hormones in the body, and infertility. Tell your cancer care team if you are pregnant or might become pregnant, because Lu-177 dotatate can harm the baby. There is not enough information regarding Yttrium-90 in pregnant women so you should discuss this with your doctor.
Since these drugs expose you to radiation, people who might come into contact with you need to follow certain radiation safety practices to limit their exposure.
Chemotherapy for pancreatic neuroendocrine tumor
Chemotherapy (chemo) uses anti-cancer drugs injected into a vein or taken by mouth to kill cancer cells. These drugs enter the bloodstream and reach almost all areas of the body, making this treatment useful for some types of cancers that have spread.
Chemo is most often used to treat pancreatic neuroendocrine tumors (PNETs) if they:
- Have not responded to other medicines (such as somatostatin drugs or targeted therapy),
- Have spread to other organs,
- Are large or growing quickly,
- Are causing severe symptoms, or
- Are high grade (grade 3)
The most commonly used drugs for pancreatic NETs include:
- Doxorubicin (Adriamycin)
- Streptozocin
- Fluorouracil (5-FU)
- Dacarbazine (DTIC)
- Temozolomide (Temodar)
- Capecitabine (Xeloda)
- Oxaliplatin (Eloxatin)
Some tumors might be treated with more than one drug. Possible combinations include:
- Doxorubicin plus streptozocin
- 5-FU plus doxorubicin plus streptozocin
- Temozolomide plus capecitabine
- 5-FU plus streptozocin
Chemo drugs are typically given into a vein (IV), either as an injection over a few minutes or as an infusion over a longer period of time. This can be done in a doctor’s office, chemotherapy clinic, or in a hospital setting.
Doctors give chemo in cycles, with each period of treatment followed by a rest period to give you time to recover from the effects of the drugs. Cycles are most often 2 or 3 weeks long. The schedule varies depending on the drugs used. For example, with some drugs, the chemo is given only on the first day of the cycle. With others, it is given for a few days in a row, or once a week. Then, at the end of the cycle, the chemo schedule repeats to start the next cycle.
The length of treatment for advanced pancreatic NETs is based on how well it is working and what side effects you have.
Chemo drugs attack cells that are dividing quickly, which is why they work against cancer cells. But other cells in the body, such as those in the bone marrow (where new blood cells are made), the lining of the mouth and intestines, and the hair follicles, also divide quickly. These cells are also likely to be affected by chemo, which can lead to side effects.
The side effects of chemo depend on the type and dose of drugs given and the length of time they are taken. Common side effects can include:
- Nausea and vomiting
- Loss of appetite
- Hair loss
- Mouth sores
- Diarrhea or constipation
- Increased chance of infections (from having too few white blood cells)
- Easy bruising or bleeding (from having too few blood platelets)
- Fatigue (from having too few red blood cells)
Most side effects go away after treatment is finished. Tell your cancer care team about any side effects or changes you notice while getting chemotherapy, so that they can be treated promptly. Often medicines can help prevent or minimize many of the side effects. For example, your doctor can prescribe drugs to help prevent or reduce nausea and vomiting. In some cases, the doses of the chemo drugs might need to be lowered or treatment might need to be delayed or stopped to keep the effects from getting worse.
Targeted drug therapy for pancreatic neuroendocrine tumor
Targeted drugs used to treat pancreatic neuroendocrine tumors (NETs) work by blocking angiogenesis (the growth of new blood vessels that nourish cancers) or important proteins (called tyrosine kinases) in cancer cells that help them grow and survive.
Sunitinib (Sutent)
Sunitinib blocks several tyrosine kinases and attacks new blood vessel growth. It has been shown to help slow tumor growth. This drug is taken as a pill once a day.
The most common side effects are nausea, diarrhea, changes in skin or hair color, mouth sores, weakness, and low blood cell counts. Other possible effects include tiredness, high blood pressure, heart problems, bleeding, hand-foot syndrome (redness, pain, and skin peeling of the palms of the hands and the soles of the feet), and low thyroid hormone levels.
Everolimus (Afinitor)
Everolimus blocks a protein known as mTOR, which normally helps cells grow and divide. It has been shown to help treat advanced pancreatic NETs. Everolimus is a pill taken once a day.
Common side effects of this drug include mouth sores, infections, nausea, loss of appetite, diarrhea, skin rash, feeling tired or weak, fluid buildup (usually in the legs), and increases in blood sugar and cholesterol levels. A less common but serious side effect is damage to the lungs, which can cause shortness of breath or other problems.
Belzutifan (Welireg)
Belzutifan is a type of drug known as a HIF inhibitor. It blocks a protein called hypoxia-inducible factor 2 alpha (HIF-2a), which is involved in both cancer cell growth and the formation of new blood vessels in tumors. This drug is taken as pills, typically once a day.
Belzutifan can be used in people with von Hippel-Lindau (VHL) disease who have a pancreatic NET and don’t need surgery right away.
Common side effects of this drug include low red blood cell counts (anemia), feeling tired and/or dizzy, nausea, headache, increased blood sugar levels, and changes in lab tests showing the drug might be affecting the kidneys. Less common but more serious side effects can include very low red blood cell counts (severe anemia), which might require blood transfusions, and low oxygen levels in the body, for which you might need oxygen therapy or even be admitted to the hospital.
Advanced pancreatic neuroendocrine tumors treatment
For people with advanced pancreatic neuroendocrine tumors (NETs), several medicines can help control symptoms and tumor growth. These drugs are used mainly when the tumor can’t be removed with surgery.
Somatostatin analogs
Somatostatin analogs are related to somatostatin, a natural hormone in the body. They can help slow the growth of neuroendocrine tumor cells. They can be very helpful for some patients with pancreatic NETs because these drugs stop tumors from releasing hormones into the bloodstream, which can often relieve symptoms and help patients feel better. They also seem to help slow the growth of some tumors, but cannot cure them.
These drugs can help reduce diarrhea in patients with VIPomas, glucagonomas, and somatostatinomas, help the rash of glucagonomas, and lower the levels of insulin in insulinomas. They are very useful in people who have carcinoid syndrome (facial flushing, diarrhea, wheezing, rapid heart rate), although this syndrome is not as common with NETs in the pancreas as it is with NETs found in other places. The drugs are also helpful for people whose tumors show up on a somatostatin receptor scintigraphy (SRS) scan or gallium-68 Dotatate scans.
- Octreotide (Sandostatin): One version of octreotide is short-acting and is injected 2 to 4 times a day under the skin. There is also a long-acting form of the drug (called Sandostatin LAR Depot) that only needs to be given once a month, by injection into a muscle. Depending on the severity of symptoms, some people are given injections every day when first starting treatment. Once symptoms are controlled, the longer-acting monthly injection may then be used. Other times, the long acting drug may be started from the beginning.
- Lanreotide (Somatuline Depot): This somatostatin analog is injected under the skin about once a month.
Either drug may be given by your doctor or nurse, or you may learn how to give the injection at home.
The main side effects of these drugs are pain at the site of the injection, and rarely, stomach cramps, nausea, vomiting, headaches, dizziness, and fatigue. These drugs can also cause sludge to build up in the gallbladder, which can lead to gallstones. They can also make the body resistant to the action of insulin, which can raise blood sugar levels and make pre-existing diabetes harder to control. As a result, these drugs are only used to treat insulinomas if the tumor has somatostatin receptors as seen by a positive somatostatin receptor scintigraphy (SRS) or gallium-68 Dotatate scan.
Other drugs used for specific pancreatic neuroendocrine tumors
Gastrinomas make too make gastrin, which increases stomach acid levels, and can lead to stomach ulcers. Proton pump inhibitors, for example omeprazole (Prilosec), esomeprazole (Nexium), or lansoprazole (Prevacid), block stomach acid production and may be given to decrease the chance of ulcers forming.
Insulinomas make too much insulin which causes very low blood glucose (sugar) levels. If the somatostatin receptor scintigraphy (SRS) or gallium-68 Dotatate scans are negative, showing the cancer does not have the somatostatin protein, then other treatments besides somatostatin analogs are considered to even out glucose levels. Diazoxide, a drug that keeps insulin from being released into the bloodstream, or diet changes (higher carbohydrate intake or more frequent meals) may be started to raise glucose levels.
Glucagonomas make too much glucagon, a hormone that increases blood glucose (sugar) levels. It works opposite of insulin. These cancers may be treated with medicines for diabetes if somatostatin analogs alone are not enough to control the high glucose levels.
VIPomas make too much vasoactive intestinal peptide (VIP), a hormone that regulates water and mineral (such as potassium and magnesium) levels in the gut. Treatment may involve giving intravenous (IV) fluids to treat the dehydration from diarrhea as well as certain minerals that are low.
Pancreatic cancer life expectancy and survival
Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful. Some people will want to know the survival rates for their cancer, and some people won’t. If you don’t want to know, you don’t have to.
Pancreatic cancer survival rate
Statistics on the outlook for a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer. For example, a 5-year survival rate of 70% means that an estimated 70 out of 100 people who have that cancer are still alive 5 years after being diagnosed. Keep in mind, however, that many of these people live much longer than 5 years after diagnosis. In general, people who can be treated with surgery tend to live longer than those not treated with surgery.
But remember, the 5-year relative survival rates are estimates – your outlook can vary based on a number of factors specific to you.
Cancer survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with pancreatic cancer may have a better outlook than these statistics show.
- These statistics are based on the stage of the cancer when it was first diagnosed. They do not apply to cancers that later come back or spread, for example.
- The outlook for people with pancreatic cancer varies by the stage (extent) of the cancer – in general, the survival rates are higher for people with earlier stage cancers. But many other factors can affect a person’s outlook, such as age and overall health, and how well the cancer responds to treatment. The outlook for each person is specific to their circumstances.
- Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful. Some people will want to know the survival rates for their cancer, and some people won’t.
Your doctor can tell you how these numbers may apply to you, as he or she is familiar with your particular situation.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for pancreatic cancer in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by American Joint Committee on Cancer (AJCC) TNM stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: There is no sign that the cancer has spread outside of the pancreas. This usually includes Stage 0 and 1 cancers.
- Regional: The cancer has spread from the pancreas to nearby structures or lymph nodes. This typically includes Stage 2 and 3 cancers.
- Distant: The cancer has spread to distant parts of the body such as the lungs, liver or bones. This usually includes all stage 4 cancers.
Table 3. 5-year relative survival rates for pancreatic cancer
| SEER Stage | 5-year Relative Survival Rate |
| Localized | 42% |
| Regional | 14% |
| Distant | 3% |
| All SEER stages combined | 11% |
Footnote: SEER= Surveillance, Epidemiology, and End Results
Understanding the numbers
- Based on people diagnosed with pancreatic cancer between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, tumor grade, extent of resection, level of tumor marker (CA 19-9) and other factors will also affect your outlook.
- People now being diagnosed with pancreatic cancer may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Survival rates for exocrine pancreatic cancer
The numbers below come from the National Cancer Data Base and are based on people diagnosed with exocrine pancreatic cancer between 1992 and 1998 3. In general, people who can be treated with surgery tend to live longer than those not treated with surgery.
- The 5-year survival rate for people with stage 1A pancreatic cancer is about 14%. For stage 1B cancer, the 5-year survival rate is about 12%.
- For stage 2A pancreatic cancer, the 5-year survival rate is about 7%. For stage 2B cancer, the 5-year survival rate is about 5%.
- The 5-year survival rate for stage 3 pancreatic cancer is about 3%.
- Stage 4 pancreatic cancer has a 5-year survival rate of about 1%. Still, there are often treatment options available for people with this stage of cancer.
According to Surveillance, Epidemiology, and End Results Program (SEER 18) 2007-2013, All Races, Both Sexes ~ 8.2 Percent Surviving 5 Years 4.
Survival by Stage
Cancer stage at diagnosis, which refers to extent of a cancer in the body, determines treatment options and has a strong influence on the length of survival 4. In general, if the cancer is found only in the part of the body where it started it is localized (sometimes referred to as stage 1). If it has spread to a different part of the body, the stage is regional or distant. The earlier pancreatic cancer is caught, the better chance a person has of surviving five years after being diagnosed. For pancreatic cancer, 9.7% are diagnosed at the local stage. The 5-year survival for localized pancreatic cancer is 31.5%.
Remember, these survival rates are only estimates – they can’t predict what will happen to any individual person. We understand that these statistics can be confusing and may lead you to have more questions. Talk to your doctor to better understand your specific situation.
Survival rates for neuroendocrine pancreatic tumors (treated with surgery)
For pancreatic neuroendocrine tumors (PNETs), survival statistics by stage are only available for patients treated with surgery. These numbers come from the National Cancer Data Base and are based on patients diagnosed between 1985 and 2004.
- The 5-year survival rate for people with stage 1 pancreatic NETs is about 61%.
- For stage 2 pancreatic NETs, the 5-year survival rate is about 52%.
- The 5-year relative survival rate for stage 3 pancreatic NETs is about 41%.
- Stage 4 pancreatic NETs have a 5-year survival rate of about 16%. Still, there are often treatment options available for people with these cancers.
In this database, the overall 5-year survival rate for people who did not have their tumors removed by surgery was 16%.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for pancreatic NET in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by AJCC TNM stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: There is no sign the cancer has grown outside of the pancreas.
- Regional: The cancer has grown outside the pancreas into nearby tissues or has spread to nearby lymph nodes.
- Distant: The cancer has spread to distant parts of the body such as the lungs, liver or bones.
Table 4. 5-year relative survival rates for pancreatic neuroendocrine tumors (PNETs)
| SEER Stage | 5-year Relative Survival Rate |
|---|---|
| Localized | 93% |
| Regional | 74% |
| Distant | 24% |
| All SEER stages combined | 53% |
Footnotes:
- These numbers are based on people diagnosed with pancreatic NET between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, tumor grade, tumor function, and other factors can also affect your outlook.
- People now being diagnosed with pancreatic NET may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
- Pancreatic Cancer Stages. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/staging.html
- Survival Rates for Pancreatic Cancer. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html
- Pancreatic Cancer Survival Rates, by Stage. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html
- Cancer Stat Facts: Pancreatic Cancer. https://seer.cancer.gov/statfacts/html/pancreas.html
- Survival Rates for Pancreatic Neuroendocrine Tumor. https://www.cancer.org/cancer/pancreatic-neuroendocrine-tumor/detection-diagnosis-staging/survival-rates.html