What is the Pancreas
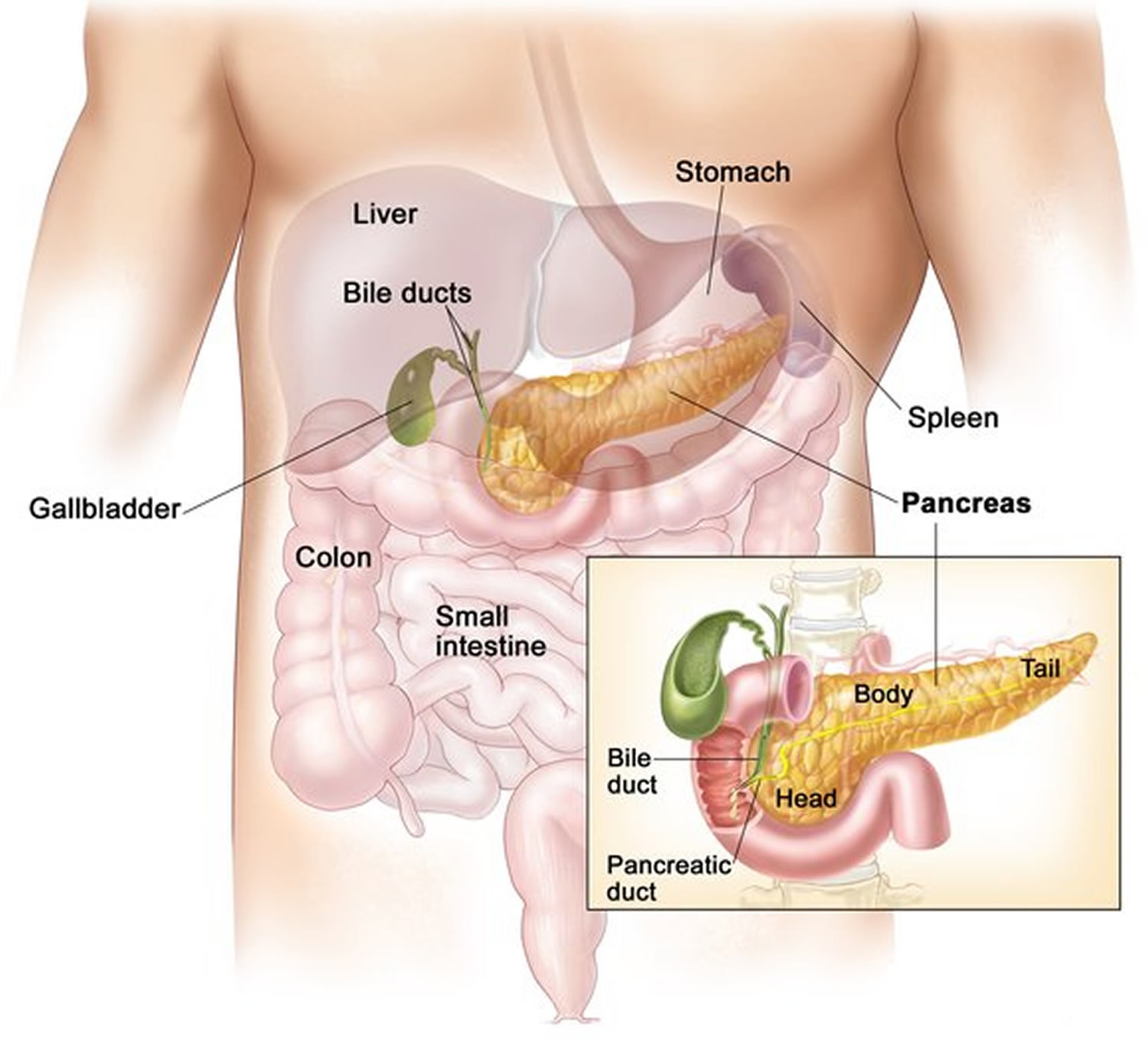
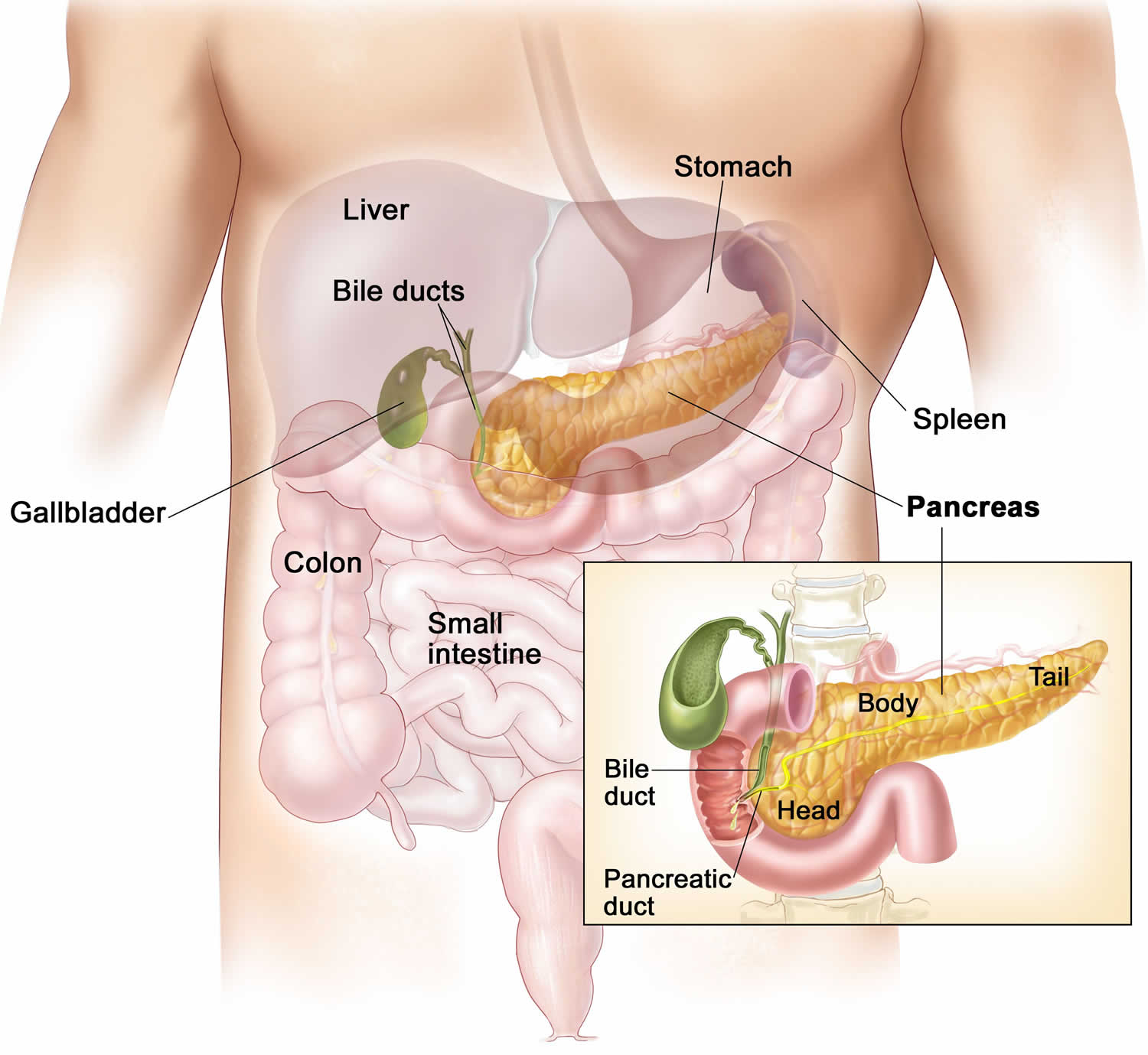
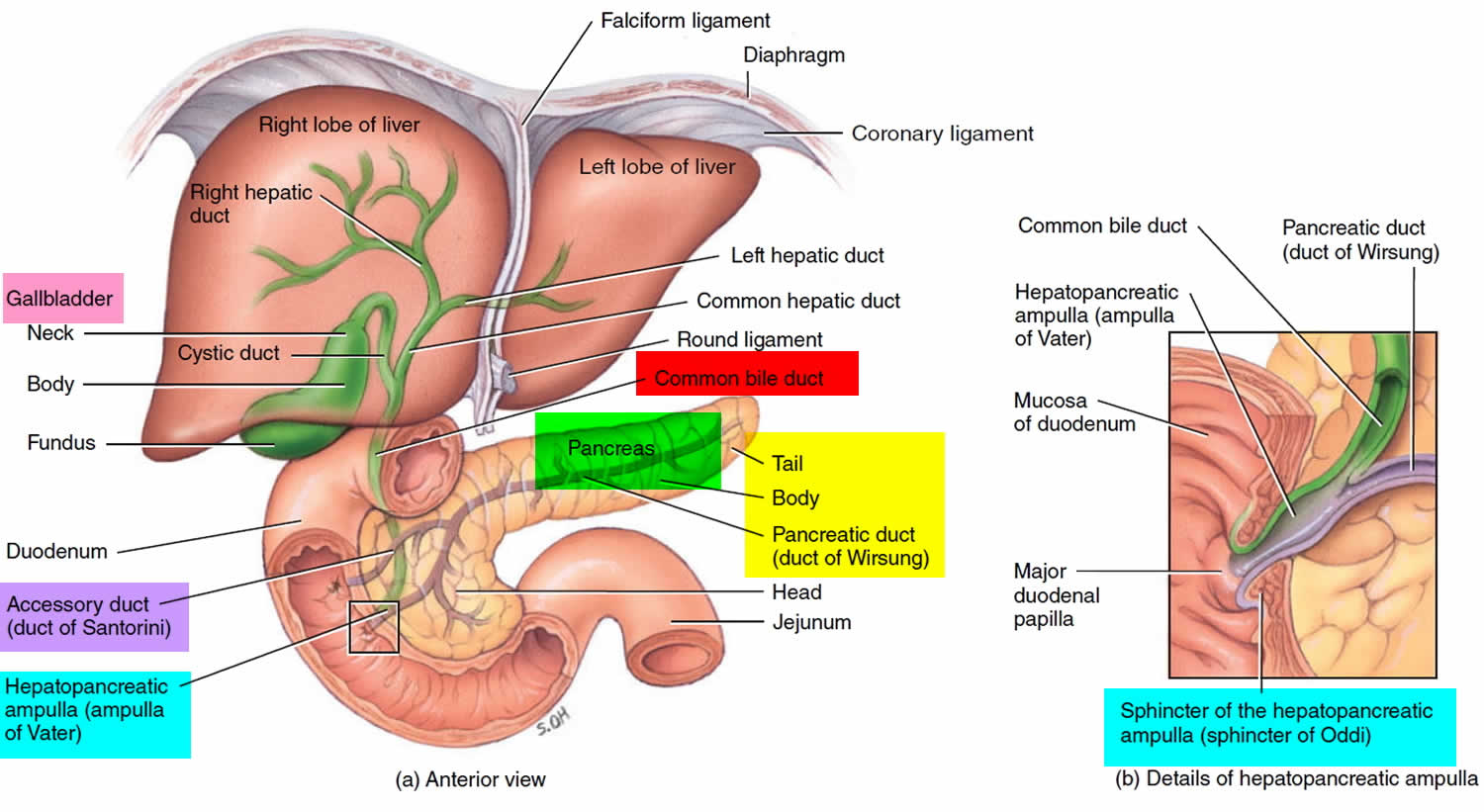
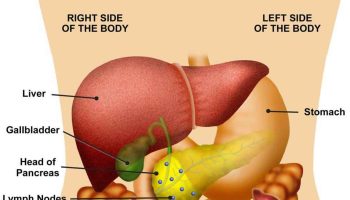
The pancreas is a large gland that sits behind the greater curvature of the stomach and close to the first part of the small intestine (the duodenum). The pancreas is shaped a bit like a fish with a wide head, a tapering body, and a narrow, pointed tail. In adults it’s about 12–15 cm (5–6 inches) long and 2.5 cm (1 in.) thick but less than 2 inches (5 centimeters) wide. The pancreas is both an endocrine and exocrine gland (see Figures 1 and 2).
The pancreas has 3 parts, the head, body, and tail.
- the wide end is called the head. The head of the pancreas is on the right side of the abdomen (belly), behind where the stomach meets the duodenum (the first part of the small intestine).
- the bit in the middle is called the body. The body of the pancreas is behind the stomach.
- the thin end is called the tail. The tail of the pancreas is on the left side of the abdomen next to the spleen.
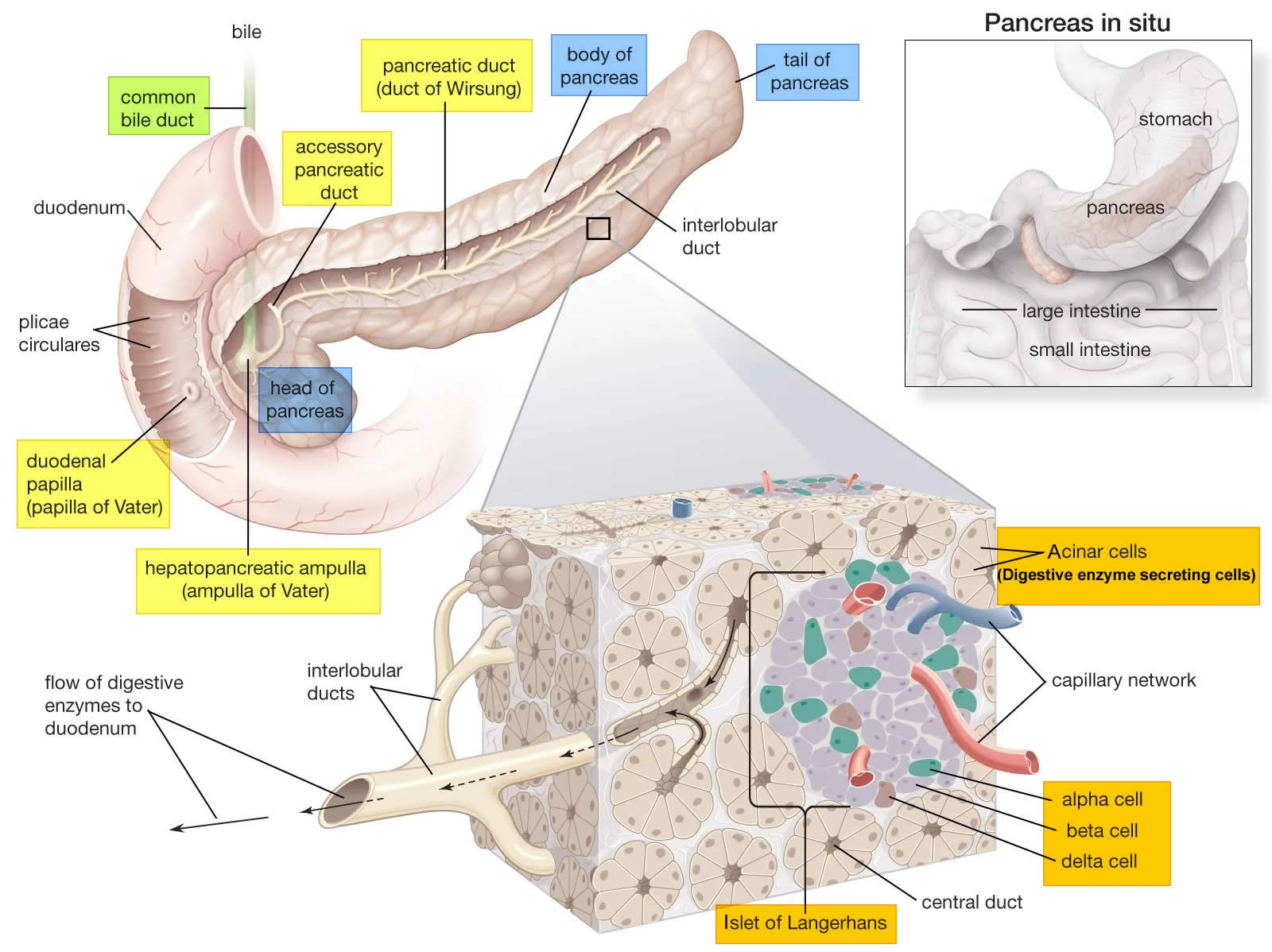
About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The digestive enzymes are first released into tiny tubes called central ducts. These merge to form larger ducts, which empty into the pancreatic duct (duct of Wirsung). The pancreatic duct merges with the common bile duct (the duct that carries bile from the liver), and empties into the duodenum (the first part of the small intestine) at the ampulla of Vater (also known as the hepatopancreatic ampulla). The ampulla of Vater (hepatopancreatic ampulla) is where the pancreatic duct and bile duct join together to drain into the duodenum, which is the first part of the small intestine. The passage of pancreatic juice and bile through the hepatopancreatic ampulla (ampulla of Vater) into the duodenum of the small intestine is regulated by a mass of smooth muscle surrounding the ampulla known as the sphincter of the hepatopancreatic ampulla, or sphincter of Oddi. The other major duct of the pancreas, the accessory duct (duct of Santorini), that branches from the main pancreatic duct and opens independently into the duodenum about 2.5 cm (1 in.) superior to the hepatopancreatic ampulla (ampulla of Vater) at the minor duodenal papilla. The accessory duct (duct of Santorini) bypasses the sphincter and allows pancreatic juice to be released into the duodenum even when bile is held back.
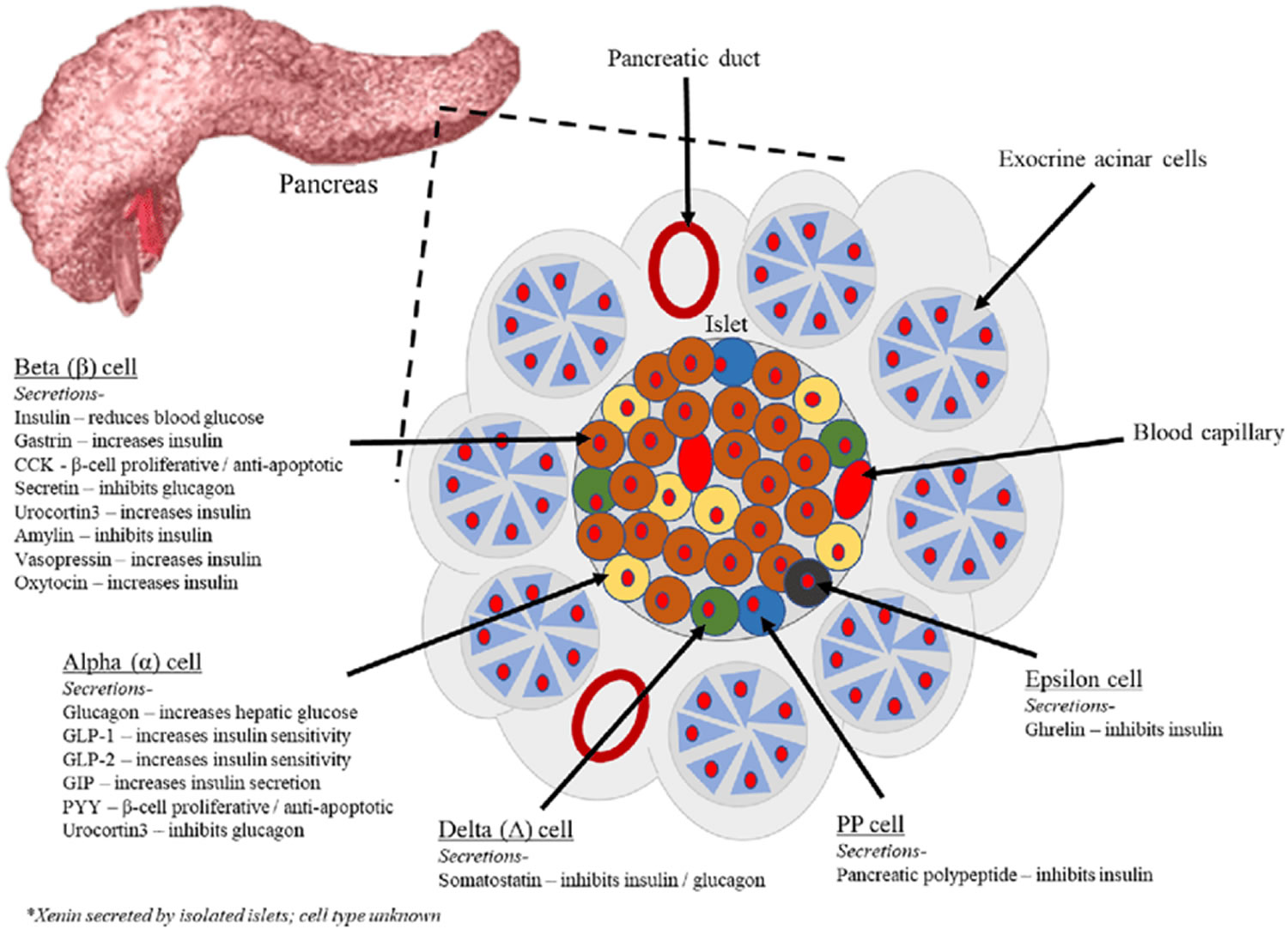
The endocrine part of the pancreas consists of groups of cells that are closely associated with blood vessels. These remaining 1% of the cell clusters form “islands” of cells called pancreatic islets (Islets of Langerhans). The Islets of Langerhans cells secrete the hormones glucagon, insulin, somatostatin, and pancreatic polypeptide. The pancreatic islets include two distinct types of cells—alpha cells, which secrete the hormone glucagon, and beta cells, which secrete the hormone insulin (Figure 2). Both insulin and glucagon are important hormones which help control blood sugar levels and are released directly into the bloodstream.
Pancreatic islets (Islets of Langerhans) are relatively concentrated in the tail of the pancreas, whereas the head is more exocrine. Over 90% of pancreatic cancers arise from the ducts of the exocrine portion (ductal carcinomas), so cancer is most common in the head of the pancreas.
Figure 1. The pancreas
Figure 2. Pancreas cell types
Footnotes: Exocrine pancreatic acinar cells constitute most of the pancreatic tissue, these cells produce digestive enzymes which are transported via the pancreatic ducts. The endocrine pancreas is illustrated with all cell types; alpha, beta, delta, pancreatic polypeptide (PP) and epsilon. The endocrine pancreas cells are arranged in compact Islets of Langerhans and secrete a number of classical and ‘nonclassical’ peptides, as depicted.
Figure 3. Pancreas location
Figure 4. Relationship of the pancreas to the liver, gallbladder, and duodenum
What does the pancreas do?
About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The cells of the secretory acini exhibit a high density of rough ER (endoplasmic reticulum) and secretory vesicles (zymogen granules). The acini open into a system of branched ducts that eventually converge on the main pancreatic duct. This duct runs lengthwise through the middle of the gland and joins the bile duct at the hepatopancreatic ampulla (ampulla of Vater). The hepatopancreatic sphincter (sphincter of Oddi) thus controls the release of both bile and pancreatic juice into the duodenum. Usually, however, there is a smaller accessory pancreatic duct (duct of Santorini) that branches from the main pancreatic duct and opens independently into the duodenum at the minor duodenal papilla, proximal to the major papilla. The accessory duct (duct of Santorini) bypasses the hepatopancreatic sphincter (sphincter of Oddi) and allows pancreatic juice to be released into the duodenum even when bile is held back.
Pancreatic juice is an alkaline mixture of water, enzymes, zymogens, sodium bicarbonate, and other electrolytes. The acini secrete the enzymes and zymogens, whereas the ducts secrete the sodium bicarbonate. The bicarbonate buffers HCl (hydrochloric acid) arriving from the stomach.
Sodium bicarbonate buffers the hydrochloric acid arriving from the stomach, with the reaction:
- HCl + NaHCO3 ⟶ NaCl + H2CO3 (carbonic acid).
The carbonic acid then breaks down to carbon dioxide (CO2) and water. CO2 is absorbed into the blood and ultimately exhaled. What is left in the small intestine, therefore, is salt water—sodium chloride (NaCl) and H2O. Sodium bicarbonate is therefore important in protecting the intestinal mucosa from hydrochloric acid (HCl) as well as raising the intestinal pH to the level needed for activity of the pancreatic and intestinal digestive enzymes.
The pancreatic zymogens are trypsinogen, chymotrypsinogen and procarboxypeptidase. When trypsinogen is secreted into the intestinal lumen, it is converted to trypsin by enteropeptidase, an enzyme on the brush border of the duodenum. Trypsin is autocatalytic—it converts trypsinogen into still more trypsin. Trypsin also converts the other two zymogens into chymotrypsin and carboxypeptidase, in addition to its primary role of digesting dietary protein.
Pancreatic acinar cells also secrete a protein called trypsin inhibitor that combines with any trypsin formed accidentally in the pancreas or in pancreatic juice and blocks its enzymatic activity.
Other pancreatic enzymes include pancreatic amylase, which digests starch; pancreatic lipase, which digests fat; and ribonuclease and deoxyribonuclease, which digest RNA and DNA, respectively. Unlike the zymogens, these enzymes are not altered after secretion. They become fully active, however, only upon exposure to bile or ions in the intestinal lumen.
Regulation of Pancreatic Secretion
Three stimuli are chiefly responsible for the release of pancreatic juice and bile.
- Acetylcholine (ACh), coming from the vagus nerves and enteric neurons. ACh stimulates the pancreatic acini to secrete their enzymes even during the cephalic phase of gastric control, before food is swallowed. The enzymes remain stored in the pancreatic acini and ducts, however, in preparation for release later when chyme enters the duodenum.
- Cholecystokinin (CCK), secreted by the mucosa of the duodenum and proximal jejunum (the next segment of the small intestine), primarily in response to fats in the small intestine. CCK also stimulates the pancreatic acini to secrete enzymes, but it is named for its strongly stimulatory effect on the gallbladder. It induces contractions of the gallbladder and relaxation of the hepatopancreatic sphincter, discharging bile into the duodenum.
- Secretin, produced by the same regions of the small intestine, mainly in response to the acidity of chyme from the stomach. Secretin stimulates the ducts of both the liver and pancreas to secrete an abundant sodium bicarbonate solution. In the pancreas, this flushes the enzymes into the duodenum.
Hormones of the Pancreatic Islets
The pancreas is primarily an exocrine digestive gland. Scattered throughout the exocrine tissue, are 1 to 2 million endocrine groups of cells that are closely associated with blood vessels called pancreatic islets (islets of Langerhans). Although they are less than 2% of the pancreatic tissue, the islets of Langerhans secrete the hormone glucagon and the hormone insulin of vital importance, especially in the regulation of glycemia, the blood glucose concentration. The pancreatic islets of Langerhans include two distinct types of cells—alpha cells, which secrete the hormone glucagon, and beta cells, which secrete insulin hormone. A typical islet measures about 75 × 175 μm and contains from a few to 3,000 cells. Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). Islets of Langerhans respond directly to blood nutrient levels associated with the cycle of eating and fasting. Their functions are as follows:
- Alpha (α) cells, or A cells, secrete glucagon between meals when the blood glucose concentration falls below 100 mg/dL (5.6 mmol/L). Glucagon exerts two primary actions on the liver: (1) glycogenolysis, the breakdown of glycogen into glucose; and (2) gluconeogenesis, the synthesis of glucose from fats and proteins. These effects lead to the release of glucose into circulation, thus raising the blood glucose level. In adipose tissue, glucagon stimulates fat catabolism and the release of free fatty acids. Glucagon is also secreted in response to rising amino acid levels in the blood after a high-protein meal. It promotes amino acid absorption and thereby provides cells with the raw material for gluconeogenesis.
- Beta (β) cells, or B cells, secrete two hormones, insulin and amylin. Insulin, “the hormone of nutrient abundance,” is secreted during and immediately following a meal when blood nutrient levels are rising. Osteocalcin, a hormone from the osteoblasts of bone, also stimulates multiplication of beta cells, insulin secretion, and insulin sensitivity of other body tissues. The principal targets of insulin are the liver, skeletal muscles, and adipose tissue. In times of plenty, insulin stimulates cells to absorb glucose, fatty acids, and amino acids and to store or metabolize them; therefore, it lowers the level of blood glucose and other nutrients. It promotes the synthesis of glycogen, fat, and protein, thereby promoting the storage of excess nutrients for later use and enhancing cellular growth and differentiation. It also antagonizes glucagon, thus suppressing the use of already-stored fuels. The brain, liver, kidneys, and red blood cells absorb and use glucose without need of insulin, but insulin does promote glycogen synthesis in the liver. Insulin insufficiency or inaction is well known as the cause of diabetes. The beta cells also secrete another hormone, amylin, simultaneously with insulin. Amylin helps to reduce spikes in blood glucose by slowing the emptying of the stomach; modulating the secretion of gastric enzymes, acid, and bile; inhibiting glucagon secretion; and stimulating the sense of satiety (having had enough to eat).
- Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin a peptide hormone that inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells. Somatostatin also work with amylin to limit the secretion of stomach acid.
- Other, minor types of pancreatic cells, about 5% of the total, are called pancreatic polypeptide (PP) and G cells. Pancreatic polypeptide (PP) cells secrete pancreatic polypeptide, a hormone that may inhibit the exocrine activity of the pancreas.
Any hormone that raises blood glucose concentration is called a hyperglycemic hormone. You may have noticed that glucagon is not the only hormone that does so; so do growth hormone, epinephrine, norepinephrine, cortisol, and corticosterone. Insulin is called a hypoglycemic hormone because it lowers blood glucose levels.
Glucagon raises the blood sugar concentration by stimulating the liver to break down glycogen and convert certain noncarbohydrates, such as amino acids, into glucose. These actions raise the blood glucose concentration. Glucagon much more effectively elevates blood glucose than does epinephrine (adrenaline).
A negative feedback system regulates glucagon secretion. A low blood glucose concentration stimulates alpha cells to release glucagon. When the blood glucose concentration rises, glucagon secretion falls. This control prevents hypoglycemia when the blood glucose concentration is relatively low, such as between meals, or when glucose is used rapidly, such as during exercise.
The main effect of insulin is to lower the blood glucose level, exactly opposite that of glucagon. Insulin does this in part by promoting facilitated diffusion of glucose into cells that have insulin receptors, for use in cellular respiration. Such cells include those of adipose tissue, liver, and skeletal muscle. (Glucose uptake by active skeletal muscle does not require insulin.) Insulin also stimulates the liver to form glycogen from glucose and inhibits conversion of noncarbohydrates into glucose. In addition, insulin promotes transport of amino acids into cells, increases the rate of protein synthesis, and stimulates adipose cells to synthesize and store fat.
A negative feedback system sensitive to the blood glucose concentration regulates insulin secretion. When the blood glucose concentration is high, such as after a meal, beta cells release insulin. Insulin helps prevent too high a blood glucose concentration by promoting glycogen formation in the liver and entrance of glucose into adipose and muscle cells.
When glucose concentration falls, such as between meals or during the night, insulin secretion decreases. As insulin secretion decreases, less glucose enters adipose and resting muscle cells. Cells that lack insulin receptors and are therefore not dependent on insulin, such as nerve cells, can still take up glucose from the blood. At the same time that insulin is decreasing, glucagon secretion is increasing. Nerve cells, including those of the brain, obtain glucose by a facilitated diffusion mechanism that does not require insulin, but rather depends only on the blood glucose concentration. For this reason, nerve cells are particularly sensitive to changes in blood glucose concentration. Conditions that cause such changes—for example, oversecretion of insulin leading to decreased blood glucose—are likely to affect brain functions.
Insulin and glucagon are coordinated to maintain a relatively stable blood glucose concentration, despite great variation in the amount of carbohydrates a person eats. About 85% to 90% of people with diabetes mellitus have type 2 diabetes, in which the beta cells produce insulin but body cells lose the ability to recognize it. On the other hand, type 1 diabetes mellitus usually appears before age twenty and it is an autoimmune disease: the immune system destroys the beta cells of the pancreas.