Lateral hypothalamus
Lateral hypothalamus also called the lateral hypothalamic area 1 is considered to be a small (6 × 6 × 3.5 mm) target within the hypothalamus located in the midbrain, lying inferior to the fornix and superoposterior to the optic nerve and chiasm 2, 3. Lateral hypothalamus is divided into three rostro-caudal zones – the anterior lateral hypothalamus, the tuberal lateral hypothalamus, and the posterior lateral hypothalamus 4. The anterior lateral hypothalamus is continuous rostrally with the lateral preoptic area, and extends caudally to the level of the rostral pole of the venteromedial nucleus (VMN). While the tuberal lateral hypothalamus is coextensive with the venteromedial nucleus (VMN), the posterior lateral hypothalamus follows the tuberal division at the level of the mamillary complex 5. The lateral hypothalamus is home to a heterogeneous population of neurons that include both gamma-aminobutyric acid (GABA)-ergic and glutamatergic neurons as well as subpopulations of neurons expressing neuropeptides that have been linked to the modulation of motivated behaviors such as orexin (hypocretin) 6, 7, melanin-concentrating hormone (MCH) 8, cocaine- and amphetamine-regulated transcript (CART)9, neurotensin (NT) 10, leptin receptor 11 and galanin 12, 13. These neurons connect to other brain structures via efferent projections from the lateral hypothalamus to multiple structures including the amygdala, hippocampal formation, thalamus, the pons, brainstem and spinal cord, as well as intra-structural projections within the lateral hypothalamus to other hypothalamic subnuclei 14, 15, 16, 17. The lateral hypothalamus also projects densely onto the ventral tegmental area (VTA) 18, 19.
The large number of inputs, outputs, neuron types and functions present within the lateral hypothalamus suggest that this structure is very complex and hosts an extremely diverse population of neurons, with many neurons co-expressing multiple neuropeptides and projecting to numerous target neural structures 20, 21, moving forward it will be important to gain a more precise understanding of these neurons, projections and functions to better disentangle the many roles of the lateral hypothalamus 20. Interestingly, neurons in the lateral hypothalamus are the largest in the hypothalamus and are topographically well organized 5. Chief among them are the orexin neurons that project widely to the neuraxis and undertake many important functions. A growing body of evidence suggests that orexin neurons play a key role in regulating wakefulness 22, sleep 23, food intake 24, autonomic and endocrine functions 25, reward-related behaviors 26 and pain-related behaviors 27. However, despite significant progress in research, a more refined understanding of the detailed functions of orexin neurons of the lateral hypothalamus in the regulation of inflammatory pain is needed 28.
The lateral hypothalamus has been implicated in numerous functions including sleep-wake transitions 29, 30, 31, feeding 32, energy balance 33, stress 34 and reward 35, 36 and plays a critical role in maintaining physiological and behavioral homeostasis. As well as being dubbed a “feeding center” or “hunger center” by Anand and Brobeck 32, the lateral hypothalamus has also been labeled as a “pleasure center” 37 after it was shown that electrode implantation into the medial forebrain bundle in the lateral hypothalamus resulted in persistent intracranial self-stimulation 38. It has been suggested that this behavior is a result of stimulation of the descending fibers in the medial forebrain bundle that feed into the ventral tegmental area (VTA), likely triggering a reward response 39.
The lateral hypothalamus was discovered to be the source of extensive projections of neurons containing 2 newly discovered neuropeptides: melanin-concentrating hormone and orexin 40, 41. Both neuronal populations have broad projections throughout the central nervous system (CNS), and direct injection of either melanin-concentrating hormone or orexin into the ventricles will cause rats to feed, and these levels increase in rats during periods of starvation 42, 40. Multiple early animal studies demonstrated that low-frequency stimulation of the lateral hypothalamus resulted in an excitatory stimulation of these fibers, with animals demonstrating food-seeking and food-hoarding behavior, increased gastrointestinal blood flow, and activation of vagal pathways 43, 44, 45. In 2007, Sani et al. 46 reported on the use of high-frequency deep brain stimulation (DBS) of the bilateral lateral hypothalamus in 16 rats that resulted in a 2.3% weight loss in stimulated rats and a 13.8% weight gain in unstimulated controls. After lateral hypothalamic damage in rats only simple automatisms (such as grooming, chewing, licking) are present, but intense stimuli can activate more complex actions (walking, orientation, swimming) 47. In the anorexic stage, tactile stimuli dominate in steering locomotion and “spontaneous” locomotion depends on activation from the empty stomach 47.
Anatomical and electrophysiological studies have shown strong neuronal connections between the olfactory system and the hypothalamus 48. Anterograde and retrograde axonal tracing studies have revealed that projections from the olfactory system are more prominent to the lateral hypothalamus than to the thalamus 48. Furthermore, four primary areas in the posterior lateral hypothalamus (anterior olfactory nucleus, olfactory tubercle, piriform cortex, and anterior cortical nucleus of amygdala) were shown to receive this input from the olfactory bulb 48.
The lateral hypothalamus became classically known as the “feeding center” or “hunger center” of the brain after multiple early animal studies demonstrated that bilateral lateral hypothalamus lesions in rats resulted in decreased food intake, weight loss, and decreased food-seeking behavior leading to anorexia, somnolence, akinesia, and sensory neglect combine to produce complete aphagia, indicating the vast importance of lateral hypothalamus circuitry in appetite and/or eating or drinking properties of feeding behavior 49, 50, 51, 52, 53, 54, 55, 56.
Targeted photostimulation of projections from the lateral hypothalamus to the paraventricular hypothalamus in mice elicited voracious feeding and repetitive self-grooming behavior 57. Compared with sham-operated controls, rats with bilateral lateral hypothalamus lesions gained significantly less weight with equal quantities of digested food, and they exhibited increased core body temperature 58, 59.
The first pilot study of bilateral lateral hypothalamus deep brain stimulation (DBS) for obesity in humans was performed in 2013 by Whiting et al. 60. Three patients who met the criteria for morbid obesity, who had had multiple unsuccessful attempts at lifestyle modification, and in whom weight loss surgery (bariatric surgery) had failed underwent stereotactic deep brain stimulation (DBS) electrode placement bilaterally in the lateral hypothalamus 60. The study was primarily focused on safety outcomes, and no serious adverse effects were observed during the mean follow-up of 35 months 60. Transient nausea, anxiety, and temperature change sensations were noted during programming changes but lasted less than 5 minutes. Throughout most of this pilot study, stimulation parameters were set at a frequency of 185 Hz and a pulse width of 90 µsec. The resting metabolic rate was tested systematically using monopolar stimulation at different voltages and electrode contacts to find the optimized stimulation parameters and contacts. Although the study was primarily focused on safety, early data on weight change showed a trend toward weight loss in 2 of the 3 patients at optimized parameters 60. Other studies are currently being completed that examine the effect of different frequencies and pulse widths on resting metabolic rate and sleep energy expenditure, and long-term studies are examining weight changes, effects on comorbidities, and the durability of resting metabolic rate changes 3.
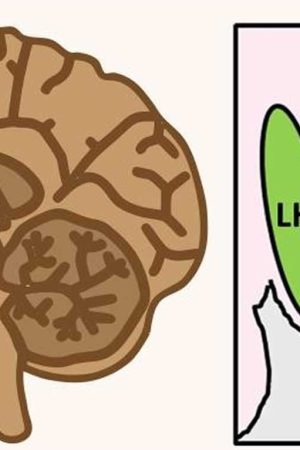
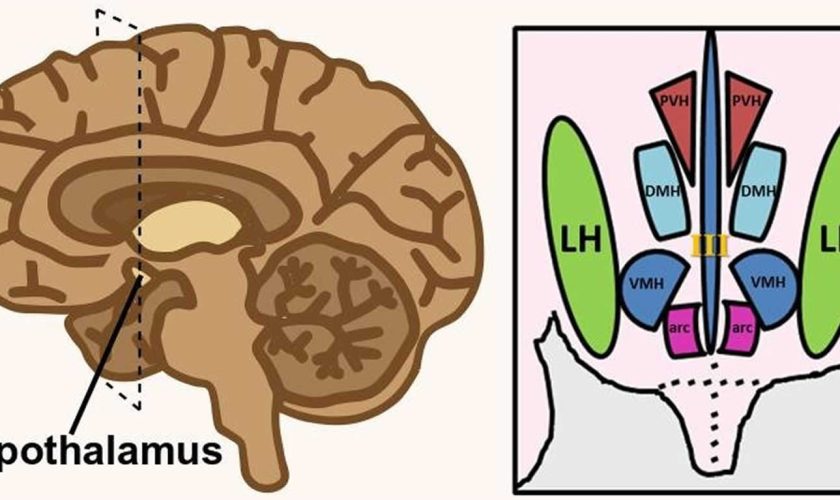
Figure 1. Hypothalamus
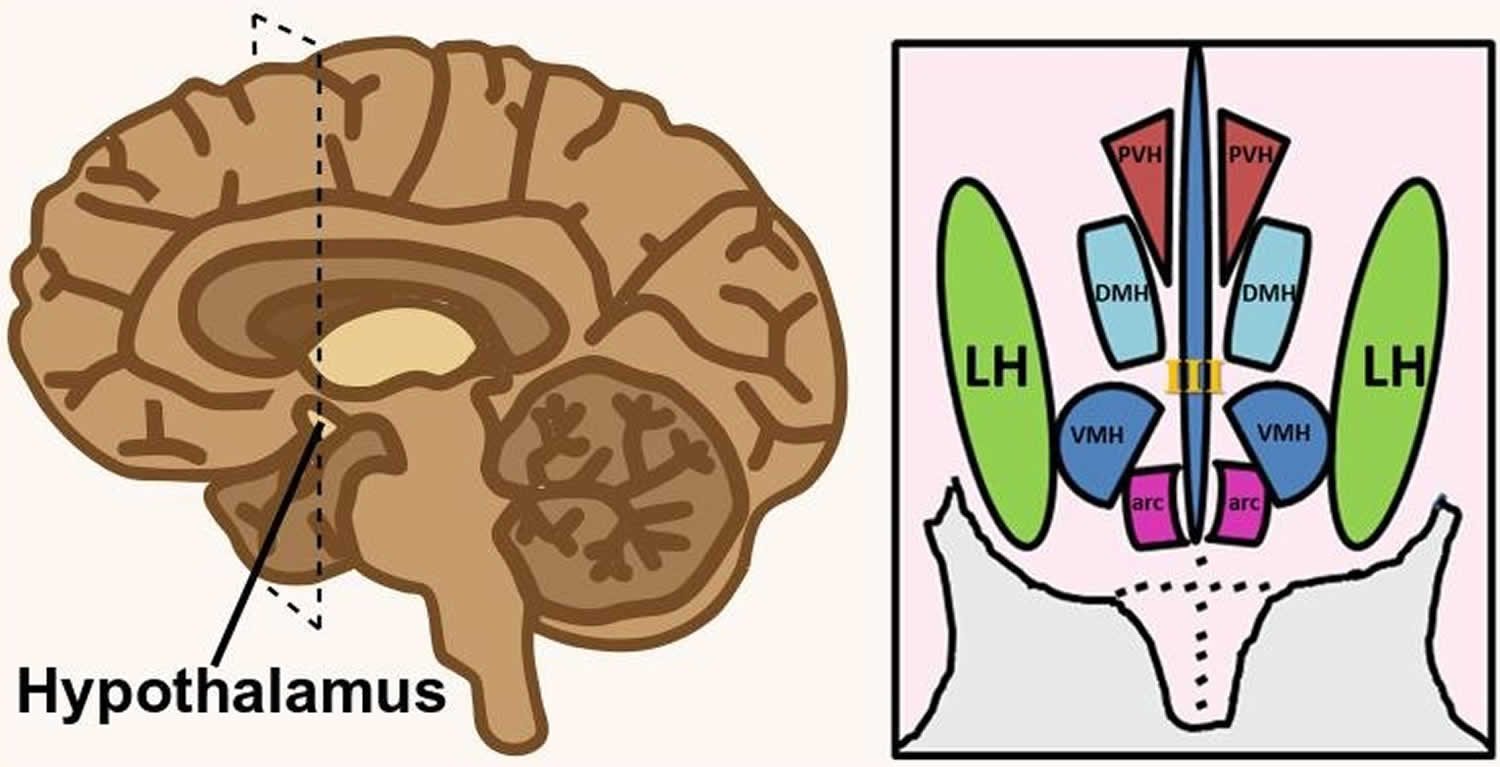
Figure 2. Lateral hypothalamus
Footnote: Hypothalamus is composed of several spatially clustered neural populations. (Left) Location of the hypothalamus at the base of the forebrain. Dashed lines indicate a coronally-cut section of the hypothalamus shown on the right. (Right) Simplified diagram of hypothalamic nuclei involved in feeding behavior and energy metabolism.
Abbreviations: III = third ventricle; arc = arcuate nucleus; DMH = dorsomedial hypothalamus; LH = lateral hypothalamus; PVH = paraventricular hypothalamus; VMH = ventromedial hypothalamus.
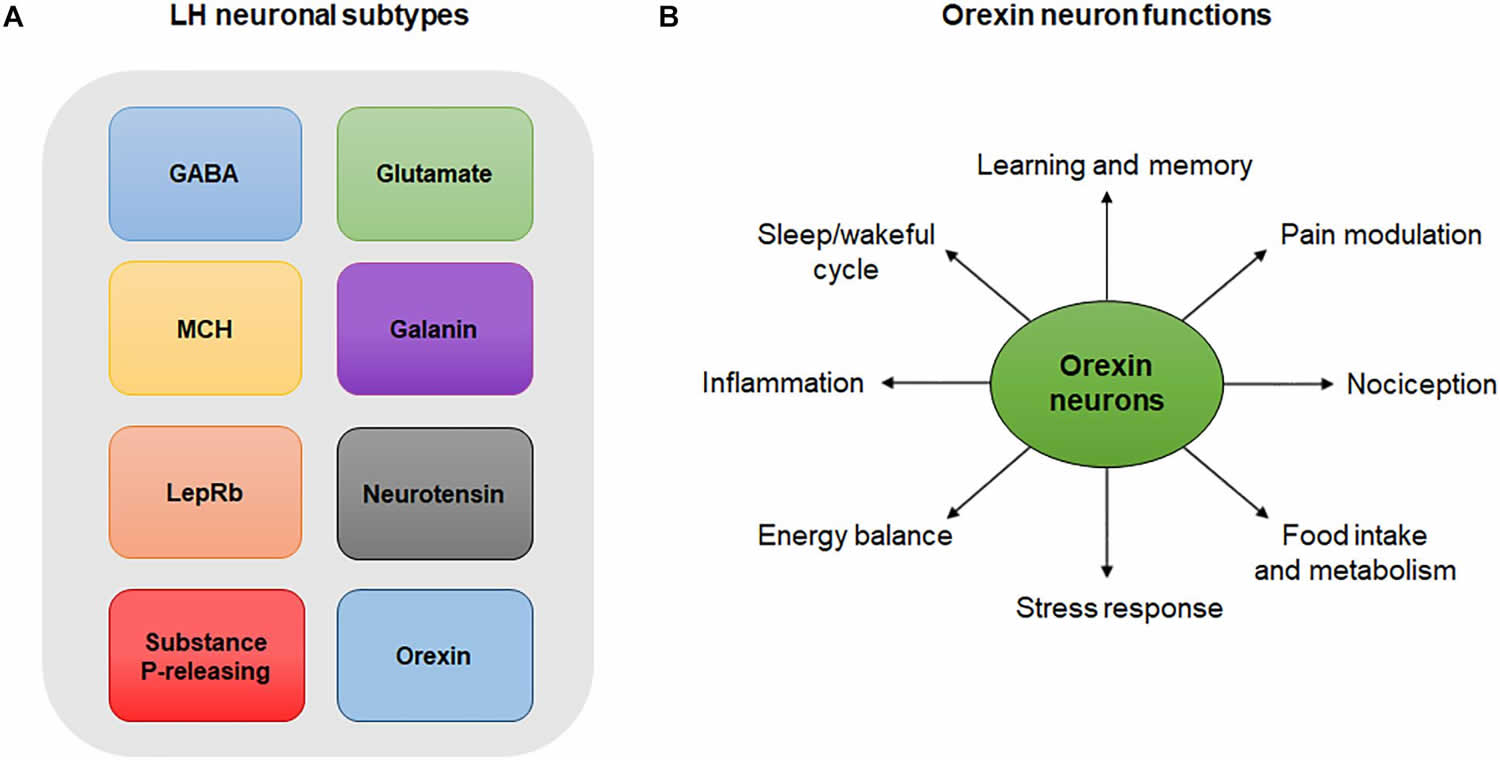
[Source 57 ]Figure 3. Lateral hypothalamus neurons and functions
Footnotes: (A) Lateral hypothalamus neuronal subtypes. Simplistic diagram showing existing neuronal populations in the lateral hypothalamus. Neuronal populations in the lateral hypothalamus include, but are not limited to, GABA neurons, glutamate neurons, melanin-concentrating hormone (MCH)-expressing neurons, galanin-expressing neurons, leptin-receptor (LepRb)-expressing neurons, neurotensin-releasing neurons, substance P-releasing neurons and orexin neurons. The degree to which these neuronal populations overlap is not represented in this diagram. (B) Orexin neuron functions. Orexin neurons are involved in numerous physiological and behavioral processes including sleep/wakeful cycles, learning, memory, pain, nociception, food intake, metabolism, stress, energy balance and inflammation.
[Source 28 ]Lateral hypothalamus function
The lateral hypothalamus is generally known as the hunger center or feeding center, and two of its main functions are the stimulation of feeding behavior and arousal 61, 3, 62. Electrical stimulation of the lateral hypothalamus results in ravenous eating behavior, and animals are extremely motivated to work for a food reward 56. The neurons of the lateral hypothalamus are mainly orexin expressing neurons and they respond to both melanocortins and neuropeptide Y 63, 64. Orexin neurons stimulate wakefulness, and a loss of orexin neurons causes narcolepsy 65, which in turn is associated with increased risk on the development of type 2 diabetes 66. Together, these properties of orexin promote alertness in a fasted state, which is crucial for food-seeking behavior 67. Recent animal studies indicated that the lateral hypothalamus plays a key role in eliciting violent forms of aggression 68, 69, 70, 71, 72
The lateral hypothalamus is also a site for integration of autonomic and endocrine responses, and a crucial regulator of pituitary function and homeostatic balance 73, 74. The lateral hypothalamus plays a key role in regulating autonomic functions and relays information to all major parts of the brain including the major hypothalamic nuclei 75, 56, 76, 77, 78. Converging evidence from functional, structural, and behavioral studies confirmed the importance of this region not only in regulating metabolism and feeding behavior, but also in serving as a motivation-cognition interface 56, 62.
Lateral hypothalamus neurons control feeding, blood pressure, heart rate, water intake and sodium excretion largely through the activation of adrenergic receptors 79, 80, 81. In addition, they receive inhibitory noradrenergic input from the locus coeruleus, which helps prevent excessive activity in the arousal pathway during the waking cycle 82. Also, beta (β)-adrenoceptors activation by noradrenaline in the lateral hypothalamus appears to be involved in the suppression of feeding behavior 83. On the other hand, activation of alpha 1 (α1)-adrenoceptors of the lateral hypothalamus has been linked to behavioral activation and exploration, despite the insignificant number of these receptors in the lateral hypothalamus 84.
The lateral hypothalamus also plays an important role in the brain reward system. This was demonstrated by studies using intracranial self-stimulation in rodents showing that animals will willingly perform an operant response to receive rewarding pulses of electrical stimulation within the lateral hypothalamus 85, 86. The rewarding effect of lateral hypothalamus self-simulation is largely influenced by the dopamine and opioid systems as alterations in these systems were shown to either suppress or disrupt the self-stimulation behavior 87, 86. The lateral hypothalamus, through GABAergic neurons, also plays an important role in learning to respond to cues that predict the delivery of a reward 88. In addition, GABAergic neurons of the lateral hypothalamus highly project to the ventral tegmental area (VTA) 88, a center rich in dopaminergic neurons that is known to be crucial for learning, reward processes and feeding behavior 89, 90.
Recently, many reports have implicated the lateral hypothalamus in the regulation of inflammatory pain 91, 92, 93. Studies have shown that stimulation of the lateral hypothalamus produces analgesic and anti-nociceptive effects in an animal model of inflammatory pain 92 and that this effect is largely due to the activation of α-adrenoceptors in the dorsal horn of the spinal cord 94, 92, and to the involvement of lateral hypothalamic orexin neurons 95.
Ventral tegmental area structure and reward function
The ventral tegmental area (VTA) is a semi-circular nucleus which lies along the midline in the midbrain, it is home to a heterogeneous population of neurons containing multiple neurotransmitters including neurotensin (NT) 96, cholecystokinin (CCK) 97 and dopamine 98. The ventral tegmental area (VTA) dopaminergic system has been implicated in brain-stimulation reward and food reward, psychomotor stimulation, learning and memory formation 99, 100, 101, 102, 103 and it has been shown that goal-directed behavior is promoted by dopamine release from ventral tegmental area (VTA) dopaminergic neurons 104, 105, 106. Studies have shown that both the synaptic connections and intrinsic excitability of dopaminergic neurons are highly plastic dependent on the experiences of the animals 107, 108, 109, 102, 110. This suggests the possibility for experience/outcome-based modulation of behavioral motivation to be mediated via ventral tegmental area (VTA) dopaminergic neurons and a “directing” role for dopamine in goal-oriented behaviors.
Ventral tegmental area (VTA) dopaminergic neuron involvement in reward processing has been studied extensively in an attempt to understand how these neurons code for rewards and the mechanisms through which they are able to modulate animal behaviors. However the complexity of the ventral tegmental area (VTA), as well as the lateral hypothalamus inputs into the ventral tegmental area (VTA), require equally complex methods to investigate specific neuron populations within such heterogeneous neuron populations. Eshel et al. 111 carried out a complex set of experiments using a multi-method approach combining computational modeling, extra-cellular recordings, optogenetics and viral injections to investigate the computational mechanisms by which ventral tegmental area (VTA) dopaminergic neurons calculate reward prediction error. Performing extra-cellular recordings of dopaminergic neurons while delivering expected and unexpected rewards, and using subsequent optogenetic manipulations to investigate the importance of ventral tegmental area (VTA) GABAergic neurons to normal ventral tegmental area (VTA) dopaminergic function. They found that as the size of the reward the animal receives increases, so does the dopaminergic neuron response, which was consistent with previous results 112, 113, they also found that expectation of a reward resulted in a suppression of the dopaminergic neuron response, and that this response fit to a subtractive computational model better than an alternative divisive model 111. Then, Eshel et al. 111 investigated the role of ventral tegmental area (VTA) GABAergic neurons in their subtraction model of ventral tegmental area (VTA) dopaminergic neuron suppression in expected rewards by optogenetically mimicking normal ventral tegmental area (VTA) GABAergic neuron firing patterns and observing ventral tegmental area (VTA) dopaminergic activity. They found that ventral tegmental area (VTA) GABA stimulation resulted in the suppression of dopamine responses to unexpected rewards in a similar pattern to that seen in animals receiving expected rewards. This ventral tegmental area (VTA) GABA-induced suppression of dopaminergic responses also fit with a subtractive computational model. Additionally, they showed that inhibition of ventral tegmental area (VTA) GABAergic neurons partially reversed the expectation-dependent suppression of ventral tegmental area (VTA) dopamine reward responses. Taken together this suggests that ventral tegmental area (VTA) dopaminergic neurons calculate reward-error using a subtractive model, and that ventral tegmental area (VTA) GABAergic neurons play a role in the temporal expectation modulation of dopamine responses in a manner that is consistent with the ramping expectation function in some models of prediction error computational models 114, 115. This modulation of reward response in the ventral tegmental area (VTA) may play an important role in directing motivated behaviors to rewards that are less predictable over rewards that are more regularly available. This also suggests a mechanism by which ventral tegmental area (VTA) dopaminergic neurons can rationalize between multiple rewards within an environment by modulating the reward value of more reliable rewards to be less rewarding than unpredictable rewards to shift the animals drive to focus on less readily-available rewards. This series of experiments shows the multitude of benefits that can be gained by using a multi-method approach to investigate neural circuits, by using behavioral protocols, optogenetics, electrophysiology and computational modeling (as well as investigating the role of both dopamine and GABA activity for comparison) these researchers were able to gain a deeper understanding of ventral tegmental area (VTA) dopaminergic activity by observing it from multiple angles.
The development of new techniques and biomarkers has allowed a closer look at the roles of different populations of lateral hypothalamus neurons in ventral tegmental area (VTA) function. For example, optogenetic stimulation of lateral hypothalamus GABA inputs to the ventral tegmental area (VTA) results in conditioned place preference 116, reduces ventral tegmental area (VTA) GABA activity, and drives nucleus accumbens dopamine release 117. Nieh et al. 117 additionally showed that optogenetic stimulation of glutamatergic lateral hypothalamus inputs to the ventral tegmental area (VTA) results in conditioned place aversion. Interestingly, the behavioral response to optogenetic stimulation of ventral tegmental area (VTA)-innervating lateral hypothalamus GABAergic neurons differed depending on the stimulation frequency, with low frequency stimulation (5–10 Hz) resulting in increased feeding, and high frequency stimulation (40 Hz) appeared to trigger reward, resulting in a place preference 116. It is possible that these two functions may be being mediated by two different neuropeptides co-expressed within lateral hypothalamus GABAergic neurons, or that this stimulation triggers a general “drive” and the stimulation frequencies result in the release of different neuropeptides in the ventral tegmental area (VTA), resulting in a target for the “drive” response. This could suggest that the lateral hypothalamus and the ventral tegmental area (VTA) are the “drive” and “focus” sources for motivated behaviors, respectively, with lateral hypothalamus activation producing a general energizing of the animal to perform a behavior, and the ventral tegmental area (VTA) then directing that energy to a specific goal-oriented behavior—depending on which neurotransmitters are released, or the stimulation frequency, or some other determining factor. Additionally, the development of this gene-targeting methodology also opens up the possibility to investigate the multiple other neuron types in the lateral hypothalamus that are known to project to the ventral tegmental area (VTA) to better understand how these neuron types differentiate between multiple input signals and determine which environmental goals to pursue 20.
GABA Neurons
Lateral hypothalamus neurons are composed of many overlapping neuronal populations that play distinct functions in the central nervous system (CNS) (Figure 3) 28. Studies over the past few decades have largely focused on the functions of GABAergic neurons of the lateral hypothalamus and their projections in reward and feeding behavior 118. Evidence suggests that these neurons encode information necessary for associating specific cues with reward delivery. In experiments employing optogenetics, a highly specific technique that involves the use of light to activate or inhibit neurons, inhibition of lateral hypothalamus GABAergic neurons was shown to reduce responding to a cue predicting a food reward, indicating that these neurons encode information pertaining to reward prediction 88. On the other hand, optogenetic inhibition of lateral hypothalamus GABA neurons that project to the ventral tegmental area (VTA) increased responding to the food reward-paired cue, suggesting that these neurons may play a role in relaying reward-predictive information to other neuronal structures 88. Interestingly, a study evaluating the role of lateral hypothalamus GABA neurons projecting to the ventral tegmental area (VTA) showed that optogenetic activation of this pathway can either induce a feeding or rewarding effect depending on the frequency of the stimulation used 119. Last but not least, a recent study by Giardino et al. 120 found that the bed nucleus of the stria terminalis sends two non-overlapping GABAergic projections to the lateral hypothalamus that express several neuropeptides including corticotropin-releasing factor (CRF) and cholecystokinin (CCK).
Glutamate Neurons
Glutamate neurons of the lateral hypothalamus mediate important physiological processes in the central nervous system (CNS). Studies have shown that lateral hypothalamus glutamate neurons produce behavioral functions opposite to those of lateral hypothalamus GABAergic neurons. In mice, optogenetic activation of putative glutamate neurons of the lateral hypothalamus suppressed feeding and produced aversion-related phenotypes 121, while the opposite effect was observed following optogenetic or chemogenetic activation of lateral hypothalamus GABA neurons 122. The opposite functions of lateral hypothalamus glutamate and GABA neurons in feeding and reward-related processes are mainly explained by differences in their projection pattern. Glutamate neurons of the lateral hypothalamus send dense projections to the lateral habenula (LHb), a region involved in processing aversive stimuli 123, in contrast to lateral hypothalamus GABA neurons, whose projections mainly target the ventral tegmental area (VTA) 119. Besides its role in the regulation of feeding and aversion-related behaviors, lateral hypothalamus glutamate neurons have been implicated in compulsive 124 and hyperkinetic behaviors 125.
Melanin-Concentrating Hormone (MCH)-Expressing Neurons
Neurons expressing melanin-concentrating hormone (MCH) are also widely present in the lateral hypothalamus. Functional and anatomical studies showed that melanin-concentrating hormone (MCH) neurons co-express the vesicular glutamate transporter 2 (VGLUT2), indicating a glutamatergic identity 125 and project to several regions of the central nervous system (CNS) ranging from the cortex to the spinal cord 126. Melanin-concentrating hormone (MCH) is an appetite stimulant (orexigenic) hypothalamic peptide that exerts inhibitory effects on lateral hypothalamic neurons 127. Although the mechanism of action of melanin-concentrating hormone (MCH) is yet to be fully determined, its inhibitory effect on lateral hypothalamus neurons is largely mediated by the attenuation of excitatory glutamate transmission presynaptically 127. Melanin-concentrating hormone (MCH) was also shown to depress synaptic activity of lateral hypothalamus GABA neurons, suggesting a substantial level of complexity in its modulation of lateral hypothalamus neuron activity 128. Mounting evidence suggests that MCH neurons directly regulate feeding behavior. Both administration of melanin-concentrating hormone (MCH) 129, 130 and activation of MCH receptors 131 increases food intake and facilitates body weight gain in rodents. Conversely, genetic knockout of the MCH gene 132 or pharmacological blockade of MCH receptors 131 leads to substantial decreases in food intake in mice. In addition, through their direct projections to gonadotropin-releasing hormone (GnRH) synthesizing neurons, melanin-concentrating hormone (MCH) neurons convey critical homeostatic signals to the reproductive axis, and contribute considerably to the functional connection between the regulation of food intake and reproduction 128, 133.
Galanin and Leptin-Receptor Expressing Neurons
Another type of neuron found in the lateral hypothalamus is the galanin-containing neuron 134. These neurons represent a GABAergic subpopulation of lateral hypothalamus neurons with a distinct molecular phenotype and projection pattern. Unlike GABAergic neurons of the lateral hypothalamus, which project to the ventral tegmental area (VTA), galanin neurons of the lateral hypothalamus lack direct ventral tegmental area (VTA) innervation 134. Instead, galanin neurons of the lateral hypothalamus strongly innervate the locus coeruleus 135, a site involved in the control of arousal 136, 137 and reward processing 138, 139. Galanin is a 29 amino acid neuropeptide widely distributed in the brain 140, 141, 142 that acts as an inhibitor of synaptic transmission in the hypothalamus 143. Galanin also acts in the hypothalamus to produce behavioral hyperalgesia through activation of two descending pronociceptive pathways; one that involves the medullary dorsal reticular nucleus, and another one that involves serotonin neurons acting on the spinal cord 144. Galanin has also been reported to promote feeding behavior. Chemogenetic activation of lateral hypothalamus galanin neurons 134 or central injection of galanin 145 enhances food-seeking behavior, while targeted knockout of the galanin gene 146 or the galanin receptor 147 reduces dietary fat intake.
Another neuronal population of the lateral hypothalamus involved in the regulation of feeding behaviors is the leptin-receptor (LepRb) expressing neuron. LepRb-expressing neurons are widely expressed in the brain, but are particular enriched within the hypothalamus and the brainstem 148. In mice, leptin acts on leptin-receptor (LepRb)-expressing neurons of the lateral hypothalamus to decrease feeding and body weight 149. Leptin-receptor (LepRb)-expressing neurons of the lateral hypothalamus also innervate the ventral tegmental area (VTA), and leptin action on these neurons increases ventral tegmental area (VTA) dopaminergic neuron activity, suggesting a link between the anorexic effect of leptin and the mesolimbic dopaminergic system 149. Interestingly, leptin-receptor (LepRb)-expressing neurons in the lateral hypothalamus are thought to be GABAergic 149, and a subpopulation of these neurons in the lateral hypothalamus was shown to co-express the inhibitory acting neuropeptide galanin 150, suggesting that the anorexic effect of leptin is likely due to its interaction with other neuropeptidergic receptors in the lateral hypothalamus.
Substance P and Neurotensin-Releasing Neurons
Substance P and neurotensin-releasing neurons are also found in the lateral hypothalamus 151, 152. Substance P is a member of the tachykinin neuropeptide family and is associated with multiple physiological processes including wound healing, neurogenic inflammation and tissue homeostasis 153. In the lateral hypothalamus, substance P-containing neurons have been proposed to exert antinociceptive functions by activating spinally projecting serotonin neurons in the rostral ventromedial medulla (RVM) 91. These cells activate spinally projecting serotonin neurons either through direct contact, or indirectly through the innervation of interneurons in the rostral ventromedial medulla (RVM), thereby altering nociceptive responses in the dorsal horn of the spinal cord 91.
On the other hand, neurotensin neurons of the lateral hypothalamus are involved in the regulation of the sleep/wake cycle 154 and are implicated in feeding behavior 155 and reward processes 156. Studies exploring the role of neurotensin in reward and feeding have indicated that this neuropeptide promotes reward by enhancing glutamate transmission in the mesolimbic dopaminergic system 156 and promotes weight loss by suppressing the increased appetitive drive through activation of the G-protein-coupled neurotensin receptor-1 155. Finally, neurotensin neurons of the lateral hypothalamus have been implicated in a number of other physiological processes, including hyperthermia and energy balance, though the central mechanisms by which these processes are mediated remain to be fully elucidated 157, 158.
Orexin Neurons and Other Neuronal Populations of the lateral hypothalamus
By being an extensively researched population of cells in the past recent years, orexinergic neurons, and especially those of the lateral hypothalamus, have been shown to have different roles in inflammatory pain and in the balance of psychological functions (Figure 3B). Orexinergic neurons synthesize two neuropeptides (Orexin A and B) from the precursor prepro-orexin 159. Furthermore, inhibition of orexin neurons by local GABAergic neurons of the lateral hypothalamus is thought to disrupt the sleep cycle (Ferrari et al., 2018). On the other hand, inhibition of orexin neurons through activation of acetylcholine and dynorphin promotes wakefulness 160.
Other neuronal populations in the lateral hypothalamus not mentioned above include neurons that express cocaine- and amphetamine-regulated transcript, thyrotropin-releasing hormone, encephalin, urocortin-3 and corticotropin-releasing hormone 161, 162.
Orexin Neurons
Orexin also known as hypocretin, is a neuropeptide secreted by orexin neurons in the lateral hypothalamic area. They are two types of orexin; orexin A (hypocretin-1) and orexin B (hypocretin-2). These neuropeptides originate from the same precursor known as prepro-orexin 159. Orexin-A is a 33-amino-acid peptide while orexin-B is a 28-amino-acid peptide 159. Both orexin A and orexin B bind to the G-protein coupled receptors orexin receptor 1 (OX-1) and 2 (OX-2) (also known as hypocretin receptors type 1 and 2) 163. Orexin A binds to both OX-1 and OX-2 with the same affinity while orexin B has a higher affinity for OX-2 over OX-1 receptors 164.
In the central nervous system (CNS), orexin (hypocretin) is co-localized with other transmitters, some of which include dynorphin 165, glutamate 166, 167, galanin 168 and prolactin 169. Experiments employing in situ hybridization and immunohistochemical techniques indicate that orexin neurons in the lateral hypothalamus mostly express the vesicular glutamate transporters, VGLUT1 and VGLUT2, suggesting that they are glutamatergic 170.
Orexin neurons project their axons to most parts in the brain and spinal cord, especially to areas that are involved in the modulation of pain 171. In addition, orexin neurons in the lateral hypothalamus send projections to multiple sites related to arousal including the serotonergic dorsal raphe 172. Orexin neurons also project to the tuberomammillary nucleus (TMN) 167, a center involved in the control of arousal, learning and memory 173, 174. Pre-synaptically, orexin increases the release of glutamate and GABA in the hypothalamus, while post-synaptically, it increases Ca2+ levels, thus leading to the depolarization, hence activation, of tuberomammillary nucleus (TMN neurons by glutamatergic orexin terminals 167.
Last but not least, orexin neurons have been shown to directly interact with neuropeptide Y (NPY), a peptide that plays a role in the regulation of feeding behavior, metabolism and energy balance 175. This neuropeptide is primarily synthesized by neurons in the arcuate nucleus (ARC) and is present in different areas of the brain including the cortex, hippocampus, hindbrain and hypothalamus 175. Through its heavy projections to the arcuate nucleus (ARC) 176, orexin neurons interact with neuropeptide Y (NPY) to regulate numerous physiological processes and behaviors including food intake and calcium signaling 177, 178.
The distribution of OX-1 and OX-2 receptors has been established in different species including rats and mice. Studies employing in situ hybridization, immunohistochemistry and quantitative reverse transcription–polymerase chain reaction in rodents found that these receptors are widely distributed throughout the brain and spinal cord 179, 180. Although some overlap exist in the distribution pattern of OX-1 and OX-2 receptors, these receptors are differentially expressed in the central nervous system (CNS) 179, 181.
OX-1 receptors are primarily expressed in the ventromedial hypothalamic nucleus, prefrontal and infralimbic cortex, hippocampus, paraventricular thalamic nucleus, dorsal raphe, and locus coeruleus 179, 181, 180, and to a lesser extent in the medial preoptic area, lateroanterior and dorsomedial hypothalamic nuclei, lateral mammillary nucleus and posterior hypothalamic area 179. They are also found in the periaqueductal gray and dorsal root ganglia, which suggests a role in the regulation of pain 180, 182, and in the spinal cord, which suggests a role in the regulation of the parasympathetic and sympathetic system 180. On the other hand, OX-2 receptors are predominantly expressed in the tuberomammillary nucleus (TMN), paraventricular nucleus (PVN), cerebral cortex, nucleus accumbens (NAc), subthalamic and paraventricular thalamic nuclei, septal nuclei, raphe nuclei, and anterior pretectal nucleus, and to a lesser extent in the ventromedial/dorsomedial hypothalamic nuclei and the posterior and lateral hypothalamic areas 179, 181.
Interestingly, a study examining the expression of OX-1 and OX-2 receptors mRNA with in situ hybridization in rats and mice found some species-specific differences 183. For instance, OX-1 receptors are expressed in the caudate putamen and ventral tuberomammillary nucleus (TMN) in rats only, while they are detected in the bed nucleus of the stria terminalis, medial division, posteromedial part in mice only 183. On the other hand, OX-2 receptors show similar pattern of expression between the two species, though they are more widely expressed in the ventral tuberomammillary nucleus (TMN) of rats compared to mice 183. This differential distribution of orexin receptors is consistent with the proposed multifaceted roles of orexin in regulating homeostasis and other functions in the central nervous system (CNS).
Orexin A and B neuropeptides, as demonstrated in the literature, are also widely expressed in different regions of the brain and spinal cord. Findings from immunohistochemical and radioimmunoassay techniques indicate that orexin A fibers are found throughout the hypothalamus, septum, thalamus, locus coeruleus and spinal cord, and in the paraventricular and supraoptic nucleus 184, 185, 186, 187. In addition, orexin A fibers colocalize with substance P positive afferents of dorsal root ganglia neurons, which further strengthens its confirmed role in the regulation of pain 188. On the other hand, orexin B fibers are distributed sparsely in the hypothalamus and the spinal cord 184, 185, but are absent in the paraventricular and supraoptic nucleus 187. Interestingly, a study investigating the distribution of orexin A and orexin B in the brain of nocturnal and diurnal rodents found striking differences among species, in particular in the lateral mammillary nucleus, ventromedial hypothalamic nucleus and flocculus 187.
- Lateral hypothalamic area. http://braininfo.rprc.washington.edu/centraldirectory.aspx?type=h&ID=409[
]
- Schaltenbrand G, Wahren W, Hassler R: Atlas for stereotaxy of the human brain, in: Atlas for Stereotaxy of the Human Brain, ed 2. Stuttgart: Thieme, 1977, p 84[
]
- Whiting AC, Oh MY, Whiting DM. Deep brain stimulation for appetite disorders: a review. Neurosurg Focus. 2018 Aug;45(2):E9. https://doi.org/10.3171/2018.4.FOCUS18141[
][
][
]
- Berthoud, H. R., and Munzberg, H. (2011). The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 104, 29–39. doi: 10.1016/j.physbeh.2011.04.051[
]
- Bernardis, L. L., and Bellinger, L. L. (1993). The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Biobehav. Rev. 17, 141–193. doi: 10.1016/s0149-7634(05)80149-6[
][
]
- de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., et al. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U S A 95, 322–327. doi: 10.1073/pnas.95.1.322[
]
- Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. doi: 10.1016/s0092-8674(00)80949-6[
]
- Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247. doi: 10.1038/380243a0[
]
- Kristensen, P., Judge, M. E., Thim, L., Ribel, U., Christjansen, K. N., Wulff, B. S., et al. (1998). Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76. doi: 10.1038/29993[
]
- Luttinger, D., King, R. A., Sheppard, D., Strupp, J., Nemeroff, C. B., and Prange, A. J. (1982). The effect of neurotensin on food consumption in the rat. Eur. J. Pharmacol. 81, 499–503. doi: 10.1016/0014-2999(82)90116-9[
]
- Leinninger, G. M., Opland, D. M., Jo, Y.-H., Faouzi, M., Christensen, L., Cappellucci, L. A., et al. (2011). Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323. doi: 10.1016/j.cmet.2011.06.016[
]
- Skofitsch, G., Jacobowitz, D. M., and Zamir, N. (1985). Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res. Bull. 15, 635–649. doi: 10.1016/0361-9230(85)90213-8[
]
- Melander, T., Hökfelt, T., and Rökaeus, A. (1986). Distribution of galaninlike immunoreactivity in the rat central nervous system. J. Comp. Neurol. 248, 475–517. doi: 10.1002/cne.902480404[
]
- Ricardo, J. A., and Koh, E. T. (1978). Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 153, 1–26. doi: 10.1016/0006-8993(78)91125-3[
]
- Berk, M. L., and Finkelstein, J. A. (1982). Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res. Bull. 8, 511–526. doi: 10.1016/0361-9230(82)90009-0[
]
- Ter Horst, G. J., and Luiten, P. G. M. (1987). Phaseolus vulgaris leuco-agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res. Bull. 18, 191–203. doi: 10.1016/0361-9230(87)90190-0[
]
- Ter Horst, G. J., De Boer, P., Luiten, P. G. M., and Van Willigen, J. D. (1989). Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 31, 785–797. doi: 10.1016/0306-4522(89)90441-7[
]
- Phillipson, O. T. (1979). Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J. Comp. Neurol. 187, 117–143. doi: 10.1002/cne.901870108[
]
- Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A., and Uchida, N. (2012). Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873. doi: 10.1016/j.neuron.2012.03.017[
]
- Tyree SM, de Lecea L. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front Syst Neurosci. 2017 Jul 6;11:50. doi: 10.3389/fnsys.2017.00050[
][
][
]
- Bonnavion, P., Mickelsen, L. E., Fujita, A., de Lecea, L., and Jackson, A. C. (2016). Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 594, 6443–6462. doi: 10.1113/jp271946[
]
- Eggermann, E., Bayer, L., Serafin, M., Saint-Mleux, B., Bernheim, L., Machard, D., et al. (2003). The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J. Neurosci. 23, 1557–1562. doi: 10.1523/jneurosci.23-05-01557.2003[
]
- Inutsuka, A., and Yamanaka, A. (2013). The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. 4:18. doi: 10.3389/fendo.2013.00018[
]
- Barson, J. R., and Leibowitz, S. F. (2017). Orexin/hypocretin system: role in food and drug overconsumption. Int. Rev. Neurobiol. 136, 199–237. doi: 10.1016/bs.irn.2017.06.006[
]
- Grimaldi, D., Silvani, A., Benarroch, E. E., and Cortelli, P. (2014). Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology 82, 271–278. doi: 10.1212/WNL.0000000000000045[
]
- Espana, R. A. (2012). Hypocretin/orexin involvement in reward and reinforcement. Vitam. Horm. 89, 185–208. doi: 10.1016/B978-0-12-394623-2.00010-X[
]
- Inutsuka, A., Yamashita, A., Chowdhury, S., Nakai, J., Ohkura, M., Taguchi, T., et al. (2016). The integrative role of orexin/hypocretin neurons in nociceptive perception and analgesic regulation. Sci. Rep. 6:29480. doi: 10.1038/srep29480[
]
- Fakhoury M, Salman I, Najjar W, Merhej G, Lawand N. The Lateral Hypothalamus: An Uncharted Territory for Processing Peripheral Neurogenic Inflammation. Front Neurosci. 2020 Feb 12;14:101. https://doi.org/10.3389/fnins.2020.00101[
][
][
]
- Adamantidis A. R., Zhang F., Aravanis A. M., Deisseroth K., de Lecea L. (2007). Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424. 10.1038/nature06310[
]
- Adamantidis A. R., Carter M. C., de Lecea L. (2010). Optogenetic deconstruction of sleep-wake circuitry in the brain. Front. Mol. Neurosci. 2:31. 10.3389/neuro.02.031.2009[
]
- Carter M. E., Adamantidis A., Ohtsu H., Deisseroth K., de Lecea L. (2009). Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 29, 10939–10949. 10.1523/JNEUROSCI.1205-09.2009[
]
- Anand B. K., Brobeck J. R. (1951). Localization of a “feeding center” in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 77, 323–324. 10.3181/00379727-77-18766[
][
]
- BROBECK JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946 Oct;26(4):541-59. doi: 10.1152/physrev.1946.26.4.541[
]
- Bonnavion P., Jackson A. C., Carter M. E., de Lecea L. (2015). Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun. 6:6266. 10.1038/ncomms7266[
]
- Olds J., Milner P. (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 47, 419–427. 10.1037/h0058775[
]
- Hoebel B. G., Teitelbaum P. (1962). Hypothalamic control of feeding and self-stimulation. Science 135, 375–377. 10.1126/science.135.3501.375[
]
- Olds J. (1970). Pleasure centers in the brain. Eng. Sci. 33, 22–31.[
]
- Olds J., Olds M. E. (1965). Drives, rewards, and the brain. New Direct. Psychol. 2, 327–410.[
]
- Bielajew C, Shizgal P. Evidence implicating descending fibers in self-stimulation of the medial forebrain bundle. J Neurosci. 1986 Apr;6(4):919-29. doi: 10.1523/JNEUROSCI.06-04-00919.1986[
]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998 Feb 20;92(4):573-85. doi: 10.1016/s0092-8674(00)80949-6[
][
]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992 May 8;319(2):218-45. doi: 10.1002/cne.903190204[
]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996 Mar 21;380(6571):243-7. doi: 10.1038/380243a0[
]
- Folkow B, Rubinstein EH. Behavioural and autonomic patterns evoked by stimulation of the lateral hypothalamic area in the cat. Acta Physiol Scand. 1965 Dec;65(4):292-9. doi: 10.1111/j.1748-1716.1965.tb04276.x[
]
- Herberg LJ, Blundell JE. Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science. 1967 Jan 20;155(3760):349-50. doi: 10.1126/science.155.3760.349[
]
- MENDELSON J, CHOROVER SL. LATERAL HYPOTHALAMIC STIMULATION IN SATIATED RATS: T-MAZE LEARNING FOR FOOD. Science. 1965 Jul 30;149(3683):559-61. doi: 10.1126/science.149.3683.559[
]
- Sani S, Jobe K, Smith A, Kordower JH, Bakay RA. Deep brain stimulation for treatment of obesity in rats. J Neurosurg. 2007 Oct;107(4):809-13. doi: 10.3171/JNS-07/10/0809[
]
- Levitt DR, Teitelbaum P. Somnolence, akinesia, and sensory activation of motivated behavior in the lateral hypothalamic syndrome. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2819-23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC432863/pdf/pnas00050-0353.pdf[
][
]
- Price J.L., Slotnick B.M., Revial M.F. Olfactory projections to the hypothalamus. J. Comp. Neurol. 1991;306:447–461. doi: 10.1002/cne.903060309[
][
][
]
- Taghva A, Corrigan JD, Rezai AR. Obesity and brain addiction circuitry: implications for deep brain stimulation. Neurosurgery. 2012 Aug;71(2):224-38. doi: 10.1227/NEU.0b013e31825972ab[
]
- ANAND BK, BROBECK JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951 Jun;77(2):323-4. doi: 10.3181/00379727-77-18766[
]
- Hetherington AM, Ranson SW: Hypothalamic lesions and adiposity in the rat. Anat Rec 78:149–172, 1940[
]
- MONTEMURRO DG, STEVENSON JA. The localization of hypothalamic structures in the rat influencing water consumption. Yale J Biol Med. 1955 Dec-1956 Feb;28(3-4):396-403. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2603397/pdf/yjbm00310-0233.pdf[
]
- Grossman SP, Dacey D, Halaris AE, Collier T, Routtenberg A. Aphagia and adipsia after preferential destruction of nerve cell bodies in hypothalamus. Science. 1978 Nov 3;202(4367):537-9. doi: 10.1126/science.705344[
]
- Grossman SP, Grossman L. Iontophoretic injections of kainic acid into the rat lateral hypothalamus: effects on ingestive behavior. Physiol Behav. 1982 Sep;29(3):553-9. doi: 10.1016/0031-9384(82)90281-5[
]
- Stricker EM, Swerdloff AF, Zigmond MJ. Intrahypothalamic injections of kainic acid produce feeding and drinking deficits in rats. Brain Res. 1978 Dec 15;158(2):470-3. doi: 10.1016/0006-8993(78)90692-3[
]
- Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016 Feb;19(2):198-205. doi: 10.1038/nn.4220[
][
][
][
]
- Mangieri, Leandra. (2018). HYPOTHALAMIC CIRCUITS IN THE CONTROL OF FEEDING AND EMOTIONAL BEHAVIORS. https://www.researchgate.net/publication/329341331_HYPOTHALAMIC_CIRCUITS_IN_THE_CONTROL_OF_FEEDING_AND_EMOTIONAL_BEHAVIORS[
][
]
- Harrell LE, Decastro JM, Balagura S. A critical evaluation of body weight loss following lateral hypothalamic lesions. Physiol Behav. 1975 Jul;15(1):133-6. doi: 10.1016/0031-9384(75)90292-9[
]
- Keesey RE, Powley TL. Self-stimulation and body weight in rats with lateral hypothalamic lesions. Am J Physiol. 1973 Apr;224(4):970-8. doi: 10.1152/ajplegacy.1973.224.4.970[
]
- Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, Alcindor D, Prostko ER, Cheng BC, Angle C, Cantella D, Whiting BB, Mizes JS, Finnis KW, Ravussin E, Oh MY. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013 Jul;119(1):56-63. doi: 10.3171/2013.2.JNS12903[
][
][
][
]
- Kalsbeek MJT, Yi CX. The infundibular peptidergic neurons and glia cells in overeating, obesity, and diabetes. Handb Clin Neurol. 2021;180:315-325. https://doi.org/10.1016/B978-0-12-820107-7.00019-7[
]
- Petrovich, G. D. (2018). Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front. Syst. Neurosci. 12:14. doi: 10.3389/fnsys.2018.00014[
][
]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003 Feb 15;23(4):1487-97. doi: 10.1523/JNEUROSCI.23-04-01487.2003[
]
- Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LL, Norris AW, Lutter M, Rahmouni K, Cui H. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes. 2015 Jun;64(6):1976-87. doi: 10.2337/db14-1257[
]
- Shan L, Dauvilliers Y, Siegel JM. Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol. 2015 Jul;11(7):401-13. doi: 10.1038/nrneurol.2015.99[
]
- Mohammadi S, Dolatshahi M, Zare-Shahabadi A, Rahmani F. Untangling narcolepsy and diabetes: Pathomechanisms with eyes on therapeutic options. Brain Res. 2019 Sep 1;1718:212-222. doi: 10.1016/j.brainres.2019.04.013[
]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013 Apr 18;7:28. doi: 10.3389/fnbeh.2013.00028[
]
- Woodworth C. H. (1971). Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol. Behav. 6, 345–353. 10.1016/0031-9384(71)90166-1[
]
- Koolhaas J. M. (1978). Hypothalamically induced intraspecific aggressive behaviour in the rat. Exper. Brain Res. 32, 365–375. 10.1007/BF00238708[
]
- Tulogdi A., Biro L., Barsvari B., Stankovic M., Haller J., Toth M. (2015). Neural mechanisms of predatory aggression in rats-implications for abnormal intraspecific aggression. Behav. Brain Res. 283, 108–115. 10.1016/j.bbr.2015.01.030[
]
- Biro L., Sipos E., Bruzsik B., Farkas I., Zelena D., Balazsfi D., et al.. (2018). Task division within the prefrontal cortex: distinct neuron populations selectively control different aspects of aggressive behavior via the hypothalamus. J. Nerosci. 38, 3234–17. 10.1523/JNEUROSCI.3234-17.2018[
]
- Haller J. The Role of the Lateral Hypothalamus in Violent Intraspecific Aggression-The Glucocorticoid Deficit Hypothesis. Front Syst Neurosci. 2018 Jun 8;12:26. doi: 10.3389/fnsys.2018.00026[
]
- Ferguson, A. V., and Samson, W. K. (2003). The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front. Neuroendocrinol. 24, 141–150. doi: 10.1016/S0091-3022(03)00028-1[
]
- Timper, K., and Bruning, J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model. Mech. 10, 679–689. doi: 10.1242/dmm.026609[
]
- Seoane-Collazo, P., Ferno, J., Gonzalez, F., Dieguez, C., Leis, R., Nogueiras, R., et al. (2015). Hypothalamic-autonomic control of energy homeostasis. Endocrine 50, 276–291. doi: 10.1007/s12020-015-0658-y[
]
- Szymusiak R., McGinty D. (2008). Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 1129 275–286. 10.1196/annals.1417.027[
]
- Seoane-Collazo P., Ferno J., Gonzalez F., Dieguez C., Leis R., Nogueiras R., et al. (2015). Hypothalamic-autonomic control of energy homeostasis. Endocrine 50 276–291. 10.1007/s12020-015-0658-y[
]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004 Feb;29(1):41-59. doi: 10.1385/MN:29:1:41[
]
- Shiraishi T. (1991). Noradrenergic neurons modulate lateral hypothalamic chemical and electrical stimulation-induced feeding by sated rats. Brain Res. Bull. 27 347–351. 10.1016/0361-9230(91)90123-2[
]
- Saad W. A., Guarda I. F., Ferreira A. C., de Arruda Camargo L. A., Neto A. F., dos Santos T. A. (2000). Participation of alpha-1 and alpha-2 adrenoceptors of the lateral hypothalamic area in water intake, and renal sodium, potassium and urinary volume excretion induced by central administration of angiotensin II. Brain Res. Bull. 52 491–497. 10.1016/s0361-9230(00)00285-289[
]
- Mendonca M. M., Santana J. S., da Cruz K. R., Ianzer D., Ghedini P. C., Nalivaiko E., et al. (2018). Involvement of GABAergic and adrenergic neurotransmissions on paraventricular nucleus of hypothalamus in the control of cardiac function. Front. Physiol. 9:670 10.3389/fphys.2018.00670[
]
- Breton-Provencher V., Sur M. (2019). Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22 218–228. 10.1038/s41593-018-0305-z[
]
- Leibowitz S. F. (1970). Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 67 1063–1070. 10.1073/pnas.67.2.1063[
]
- Lin Y., Quartermain D., Dunn A. J., Weinshenker D., Stone E. A. (2008). Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse 62 516–523. 10.1002/syn.20517[
]
- Fakhoury M., Rompre P. P., Boye S. M. (2016). Role of the dorsal diencephalic conduction system in the brain reward circuitry. Behav. Brain Res. 296 431–441. 10.1016/j.bbr.2015.10.038[
]
- Ide S., Takahashi T., Takamatsu Y., Uhl G. R., Niki H., Sora I., et al. (2017). Distinct roles of opioid and dopamine systems in lateral hypothalamic intracranial self-stimulation. Int. J. Neuropsychopharmacol. 20 403–409. 10.1093/ijnp/pyw113[
][
]
- Koob G. F., Fray P. J., Iversen S. D. (1978). Self-stimulation at the lateral hypothalamus and locus coeruleus after specific unilateral lesions of the dopamine system. Brain Res. 146 123–140. 10.1016/0006-8993(78)90222-6[
]
- Sharpe M. J., Marchant N. J., Whitaker L. R., Richie C. T., Zhang Y. J., Campbell E. J., et al. (2017). Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Curr. Biol. 27 2089–2100.e5. 10.1016/j.cub.2017.06.024[
][
][
][
]
- Hommel J. D., Trinko R., Sears R. M., Georgescu D., Liu Z. W., Gao X. B., et al. (2006). Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51 801–810. 10.1016/j.neuron.2006.08.023[
]
- Ranaldi R. (2014). Dopamine and reward seeking: the role of ventral tegmental area. Rev. Neurosci. 25 621–630. 10.1515/revneuro-2014-0091[
]
- Holden J. E., Pizzi J. A. (2008). Lateral hypothalamic-induced antinociception may be mediated by a substance P connection with the rostral ventromedial medulla. Brain Res. 1214 40–49. 10.1016/j.brainres.2008.03.051[
][
][
]
- Jeong Y., Holden J. E. (2009a). Lateral hypothalamic-induced alpha-adrenoceptor modulation occurs in a model of inflammatory pain in rats. Biol. Res. Nurs. 10 331–339. 10.1177/1099800408325053[
][
][
]
- Wardach J., Wagner M., Jeong Y., Holden J. E. (2016). Lateral hypothalamic stimulation reduces hyperalgesia through spinally descending orexin-a neurons in neuropathic pain. West J. Nurs. Res. 38 292–307. 10.1177/0193945915610083[
]
- Aimone L. D., Gebhart G. F. (1987). Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Res. 403 290–300. 10.1016/0006-8993(87)90066-7[
]
- Inutsuka A., Yamashita A., Chowdhury S., Nakai J., Ohkura M., Taguchi T., et al. (2016). The integrative role of orexin/hypocretin neurons in nociceptive perception and analgesic regulation. Sci. Rep. 6:29480. 10.1038/srep29480[
]
- Kalivas P. W., Miller J. S. (1984). Neurotensin neurons in the ventral tegmental area project to the medial nucleus accumbens. Brain Res. 300, 157–160. 10.1016/0006-8993(84)91351-9[
]
- Studler J. M., Simon H., Cesselin F., Legrand J. C., Glowinski J., Tassin J. P. (1981). Biochemical investigation on the localization of the cholecystokinin octapeptide in dopaminergic neurons originating from the ventral tegmental area of the rat. Neuropeptides 2, 131–139. 10.1016/0143-4179(81)90062-7[
]
- Oades R. D., Halliday G. M. (1987). Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. Rev. 12, 117–165. 10.1016/0165-0173(87)90011-7[
]
- Yokel R. A., Wise R. A. (1975). Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science 187, 547–549. 10.1126/science.1114313[
]
- De Wit H., Wise R. A. (1977). Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can. J. Psychol. 31, 195–203. 10.1037/h0081662[
]
- Berridge K. C. (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191, 391–431. 10.1007/s00213-006-0578-x[
]
- Friedman A. K., Walsh J. J., Juarez B., Ku S. M., Chaudhury D., Wang J., et al.. (2014). Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319. 10.1126/science.1249240[
][
]
- Popescu A. T., Zhou M. R., Poo M.-M. (2016). Phasic dopamine release in the medial prefrontal cortex enhances stimulus discrimination. Proc. Natl. Acad. Sci. U S A 113, E3169–E3176. 10.1073/pnas.1606098113[
]
- Phillips P. E. M., Stuber G. D., Heien M. L. A. V., Wightman R. M., Carelli R. M. (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618. 10.1038/nature01476[
]
- Grace A. A., Floresco S. B., Goto Y., Lodge D. J. (2007). Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227. 10.1016/j.tins.2007.03.003[
]
- Gallistel CR, Gomita Y, Yadin E, Campbell KA. Forebrain origins and terminations of the medial forebrain bundle metabolically activated by rewarding stimulation or by reward-blocking doses of pimozide. J Neurosci. 1985 May;5(5):1246-61. doi: 10.1523/JNEUROSCI.05-05-01246.1985[
]
- Stuber G. D., Klanker M., De Ridder B., Bowers M. S., Joosten R. N., Feenstra M. G., et al.. (2008). Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321, 1690–1692. 10.1126/science.1160873[
]
- Mao D., Gallagher K., McGehee D. S. (2011). Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J. Neurosci. 31, 6710–6720. 10.1523/JNEUROSCI.5671-10.2011[
]
- Collo G., Cavalleri L., Spano P. (2014). Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: critical role of BDNF and dopamine. Front. Pharmacol. 5:259. 10.3389/fphar.2014.00259[
]
- Gore B. B., Soden M. E., Zweifel L. S. (2014). Visualization of plasticity in fear-evoked calcium signals in midbrain dopamine neurons. Learn. Mem. 21, 575–579. 10.1101/lm.036079.114[
]
- Eshel N., Bukwich M., Rao V., Hemmelder V., Tian J., Uchida N. (2015). Arithmetic and local circuitry underlying dopamine prediction errors. Nature 525, 243–246. 10.1038/nature14855[
][
][
]
- Tobler P. N., Fiorillo C. D., Schultz W. (2005). Adaptive coding of reward value by dopamine neurons. Science 307, 1642–1645. 10.1126/science.1105370[
]
- Cohen J. Y., Haesler S., Vong L., Lowell B. B., Uchida N. (2012). Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. 10.1038/nature10754[
]
- Hazy T. E., Frank M. J., O’Reilly R. C. (2010). Neural mechanisms of acquired phasic dopamine responses in learning. Neurosci. Biobehav. Rev. 34, 701–720. 10.1016/j.neubiorev.2009.11.019[
]
- Rivest F., Kalaska J. F., Bengio Y. (2014). Conditioning and time representation in long short-term memory networks. Biol. Cybern. 108, 23–48. 10.1007/s00422-013-0575-1[
]
- Barbano M. F., Wang H.-L., Morales M., Wise R. A. (2016). Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J. Neurosci. 36, 2975–2985. 10.1523/JNEUROSCI.3799-15.2016[
][
]
- Nieh E. H., Vander Weele C. M., Matthews G. A., Presbrey K. N., Wichmann R., Leppla C. A., et al.. (2016). Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298. 10.1016/j.neuron.2016.04.035[
][
]
- Suyama S., Yada T. (2018). New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 68 717–722. 10.1007/s12576-018-0622-8[
]
- Barbano M. F., Wang H. L., Morales M., Wise R. A. (2016). Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J. Neurosci. 36 2975–2985. 10.1523/JNEUROSCI.3799-15.2016[
][
]
- Giardino W., Eban-Rothschild A., Christoffel D., Li S. B., Malenka R., Lecea L. (2018). Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21 1084–1095. 10.1038/s41593-018-0198-x[
]
- Jennings J. H., Rizzi G., Stamatakis A. M., Ung R. L., Stuber G. D. (2013). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341 1517–1521. 10.1126/science.1241812[
]
- Jennings J. H., Ung R. L., Resendez S. L., Stamatakis A. M., Taylor J. G., Huang J., et al. (2015). Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160 516–527. 10.1016/j.cell.2014.12.026[
]
- Stamatakis A. M., Van Swieten M., Basiri M. L., Blair G. A., Kantak P., Stuber G. D. (2016). Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. 36 302–311. 10.1523/JNEUROSCI.1202-15.2016[
]
- Mangieri L. R., Lu Y., Xu Y., Cassidy R. M., Xu Y., Arenkiel B. R., et al. (2018). A neural basis for antagonistic control of feeding and compulsive behaviors. Nat. Commun. 9:52. 10.1038/s41467-017-02534-9[
]
- Schneeberger M., Tan K., Nectow A. R., Parolari L., Caglar C., Azevedo E., et al. (2018). Functional analysis reveals differential effects of glutamate and MCH neuropeptide in MCH neurons. Mol. Metab. 13 83–89. 10.1016/j.molmet.2018.05.001[
][
]
- Bittencourt J. C., Elias C. F. (1998). Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 805 1–19. 10.1016/s0006-8993(98)00598-8[
]
- Gao X. B. (2009). Electrophysiological effects of MCH on neurons in the hypothalamus. Peptides 30 2025–2030. 10.1016/j.peptides.2009.05.006[
][
]
- Gao X. B., van den Pol A. N. (2001). Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J. Physiol. 533(Pt 1), 237–252. 10.1111/j.1469-7793.2001.0237b.x[
][
]
- Qu D., Ludwig D. S., Gammeltoft S., Piper M., Pelleymounter M. A., Cullen M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380 243–247. 10.1038/380243a0[
]
- Clegg D. J., Air E. L., Benoit S. C., Sakai R. S., Seeley R. J., Woods S. C. (2003). Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284 R494–R499. 10.1152/ajpregu.00399.2002[
]
- Shearman L. P., Camacho R. E., Sloan Stribling D., Zhou D., Bednarek M. A., Hreniuk D. L., et al. (2003). Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur. J. Pharmacol. 475 37–47. 10.1016/s0014-2999(03)02146-2140[
][
]
- Shimada M., Tritos N. A., Lowell B. B., Flier J. S., Maratos-Flier E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396 670–674. 10.1038/25341[
]
- Skrapits K., Kanti V., Savanyu Z., Maurnyi C., Szenci O., Horvath A., et al. (2015). Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front. Cell Neurosci. 9:348. 10.3389/fncel.2015.00348[
]
- Qualls-Creekmore E., Yu S., Francois M., Hoang J., Huesing C., Bruce-Keller A., et al. (2017). Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J. Neurosci. 37 6053–6065. 10.1523/JNEUROSCI.0155-17.2017[
][
][
]
- Laque A., Yu S., Qualls-Creekmore E., Gettys S., Schwartzenburg C., Bui K., et al. (2015). Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol. Metab. 4 706–717. 10.1016/j.molmet.2015.07.002[
]
- Gonzalez M. M., Aston-Jones G. (2006). Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep 29 1327–1336. 10.1093/sleep/29.10.1327[
]
- Sara S. J., Bouret S. (2012). Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76 130–141. 10.1016/j.neuron.2012.09.011[
]
- Bouret S., Richmond B. J. (2015). Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci. 35 4005–4014. 10.1523/JNEUROSCI.4553-14.2015[
]
- Hofmeister J., Sterpenich V. (2015). A role for the locus ceruleus in reward processing: encoding behavioral energy required for goal-directed actions. J. Neurosci. 35 10387–10389. 10.1523/JNEUROSCI.1734-15.2015[
]
- Skofitsch G., Jacobowitz D. M. (1986). Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides 7 609–613. 10.1016/0196-9781(86)90035-5[
]
- Gentleman S. M., Falkai P., Bogerts B., Herrero M. T., Polak J. M., Roberts G. W. (1989). Distribution of galanin-like immunoreactivity in the human brain. Brain Res. 505 311–315. 10.1016/0006-8993(89)91458-3[
]
- Perez S. E., Wynick D., Steiner R. A., Mufson E. J. (2001). Distribution of galaninergic immunoreactivity in the brain of the mouse. J. Comp. Neurol. 434 158–185. 10.1002/cne.1171[
]
- Kinney G. A., Emmerson P. J., Miller R. J. (1998). Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J. Neurosci. 18 3489–3500. 10.1523/jneurosci.18-10-03489.1998[
]
- Amorim D., Viisanen H., Wei H., Almeida A., Pertovaara A., Pinto-Ribeiro F. (2015). Galanin-mediated behavioural hyperalgesia from the dorsomedial nucleus of the hypothalamus involves two independent descending pronociceptive pathways. PLoS One 10:e0142919. 10.1371/journal.pone.0142919[
]
- Kyrkouli S. E., Stanley B. G., Seirafi R. D., Leibowitz S. F. (1990). Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides 11 995–1001. 10.1016/0196-9781(90)90023-x[
]
- Adams A. C., Clapham J. C., Wynick D., Speakman J. R. (2008). Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J. Neuroendocrinol. 20 199–206. 10.1111/j.1365-2826.2007.01638.x[
]
- Zorrilla E. P., Brennan M., Sabino V., Lu X., Bartfai T. (2007). Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol. Behav. 91 479–485. 10.1016/j.physbeh.2006.11.011[
]
- Elmquist J. K., Bjorbaek C., Ahima R. S., Flier J. S., Saper C. B. (1998). Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 395 535–547. 10.1002/(sici)1096-9861(19980615)395:4<535::aid-cne9>3.0.co;2-2[
]
- Leinninger G. M., Jo Y. H., Leshan R. L., Louis G. W., Yang H., Barrera J. G., et al. (2009). Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10 89–98. 10.1016/j.cmet.2009.06.011[
][
][
]
- Laque A., Zhang Y., Gettys S., Nguyen T. A., Bui K., Morrison C. D., et al. (2013). Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab. 304 E999–E1011. 10.1152/ajpendo.00643.2012[
]
- Ljungdahl A., Hokfelt T., Nilsson G. (1978). Distribution of substance P-like immunoreactivity in the central nervous system of the rat–I. Cell bodies and nerve terminals. Neuroscience 3 861–943. 10.1016/0306-4522(78)90116-90111[
]
- Yamano M., Inagaki S., Kito S., Tohyama M. (1986). A substance P-containing pathway from the hypothalamic ventromedial nucleus to the medial preoptic area of the rat: an immunohistochemical analysis. Neuroscience 18 395–402. 10.1016/0306-4522(86)90161-2[
]
- Suvas S. (2017). Role of substance P Neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 199 1543–1552. 10.4049/jimmunol.1601751[
]
- Gerashchenko D., Shiromani P. J. (2004). Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol. Neurobiol. 29 41–59. 10.1385/MN:29:1,41[
]
- Woodworth H. L., Beekly B. G., Batchelor H. M., Bugescu R., Perez-Bonilla P., Schroeder L. E., et al. (2017). Lateral hypothalamic neurotensin neurons orchestrate dual weight loss behaviors via distinct mechanisms. Cell Rep. 21 3116–3128. 10.1016/j.celrep.2017.11.068[
][
]
- Kempadoo K. A., Tourino C., Cho S. L., Magnani F., Leinninger G. M., Stuber G. D., et al. (2013). Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci. 33 7618–7626. 10.1523/JNEUROSCI.2588-12.2013[
][
]
- Brown J., Sagante A., Mayer T., Wright A., Bugescu R., Fuller P. M., et al. (2018). Lateral hypothalamic area neurotensin neurons are required for control of orexin neurons and energy balance. Endocrinology 159 3158–3176. 10.1210/en.2018-0031[
]
- Naganuma F., Kroeger D., Bandaru S. S., Absi G., Madara J. C., Vetrivelan R. (2019). Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 17:e3000172. 10.1371/journal.pbio.3000172[
]
- Chieffi S., Carotenuto M., Monda V., Valenzano A., Villano I., Precenzano F., et al. (2017). Orexin System: the key for a healthy life. Front. Physiol. 8:357 10.3389/fphys.2017.00357[
][
][
]
- Ferrari L. L., Park D., Zhu L., Palmer M. R., Broadhurst R. Y., Arrigoni E. (2018). Regulation of lateral hypothalamic orexin activity by local GABAergic neurons. J. Neurosci. 38 1588–1599. 10.1523/JNEUROSCI.1925-17.2017[
]
- Bonnavion P., Mickelsen L. E., Fujita A., de Lecea L., Jackson A. C. (2016). Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 594 6443–6462. 10.1113/JP271946[
]
- Tyree S. M., de Lecea L. (2017). Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front. Syst. Neurosci. 11:50 10.3389/fnsys.2017.00050[
]
- Smart D., Jerman J. (2002). The physiology and pharmacology of the orexins. Pharmacol. Ther. 94 51–61. 10.1016/s0163-7258(02)00171-7[
]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:696 10.1016/s0092-8674(02)09256-5[
]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001 Oct 1;21(19):RC168. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6762880/pdf/ns2119rc168.pdf[
]
- Abrahamson E. E., Leak R. K., Moore R. Y. (2001). The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12 435–440. 10.1097/00001756-200102120-00048[
]
- Torrealba F., Yanagisawa M., Saper C. B. (2003). Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119 1033–1044. 10.1016/s0306-4522(03)00238-0[
][
][
]
- Barson J. R., Chang G. Q., Poon K., Morganstern I., Leibowitz S. F. (2011). Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience 193 10–20. 10.1016/j.neuroscience.2011.07.057[
]
- Risold P. Y., Griffond B., Kilduff T. S., Sutcliffe J. G., Fellmann D. (1999). Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci. Lett. 259 153–156. 10.1016/s0304-3940(98)00906-909[
]
- Rosin D. L., Weston M. C., Sevigny C. P., Stornetta R. L., Guyenet P. G. (2003). Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol. 465 593–603. 10.1002/cne.10860[
]
- Watanabe S., Kuwaki T., Yanagisawa M., Fukuda Y., Shimoyama M. (2005). Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport 16 5–8. 10.1097/00001756-200501190-00002[
]
- Brown R. E., Sergeeva O. A., Eriksson K. S., Haas H. L. (2002). Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J. Neurosci. 22 8850–8859. 10.1523/jneurosci.22-20-08850.2002[
]
- Huston J. P., Wagner U., Hasenohrl R. U. (1997). The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: evidence for an inhibitory role. Behav. Brain Res. 83 97–105. 10.1016/s0166-4328(97)86052-4[
]
- Sakai K., Takahashi K., Anaclet C., Lin J. S. (2010). Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decarboxylase knock-out mice. Front. Behav. Neurosci. 4:53. 10.3389/fnbeh.2010.00053[
]
- Beck B. (2006). Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361 1159–1185. 10.1098/rstb.2006.1855[
][
]
- Guan J. L., Saotome T., Wang Q. P., Funahashi H., Hori T., Tanaka S., et al. (2001). Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. Neuroreport 12 547–551. 10.1097/00001756-200103050-00023[
]
- Yamanaka A., Kunii K., Nambu T., Tsujino N., Sakai A., Matsuzaki I., et al. (2000). Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 859 404–409. 10.1016/s0006-8993(00)02043-6[
]
- Muroya S., Funahashi H., Yamanaka A., Kohno D., Uramura K., Nambu T., et al. (2004). Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 19 1524–1534. 10.1111/j.1460-9568.2004.03255.x[
]
- Trivedi P., Yu H., MacNeil D. J., Van der Ploeg L. H., Guan X. M. (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438 71–75. 10.1016/s0014-5793(98)01266-6[
][
][
][
][
]
- Hervieu G. J., Cluderay J. E., Harrison D. C., Roberts J. C., Leslie R. A. (2001). Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103 777–797. 10.1016/s0306-4522(01)00033-31[
][
][
][
]
- Marcus J. N., Aschkenasi C. J., Lee C. E., Chemelli R. M., Saper C. B., Yanagisawa M., et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435 6–25. 10.1002/cne.1190[
][
][
]
- Ho Y. C., Lee H. J., Tung L. W., Liao Y. Y., Fu S. Y., Teng S. F., et al. (2011). Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J. Neurosci. 31 14600–14610. 10.1523/JNEUROSCI.2671-11.2011[
]
- Ikeno T., Yan L. (2018). A comparison of the orexin receptor distribution in the brain between diurnal Nile grass rats (Arvicanthis niloticus) and nocturnal mice (Mus musculus). Brain Res. 1690 89–95. 10.1016/j.brainres.2018.04.002[
][
][
]
- Cutler D. J., Morris R., Sheridhar V., Wattam T. A., Holmes S., Patel S., et al. (1999). Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides 20 1455–1470. 10.1016/s0196-9781(99)00157-6[
][
]
- Date Y., Mondal M. S., Matsukura S., Nakazato M. (2000). Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci. Lett. 288 87–90. 10.1016/s0304-3940(00)01195-1192[
][
]
- Bingham S., Davey P. T., Babbs A. J., Irving E. A., Sammons M. J., Wyles M., et al. (2001). Orexin-A, an hypothalamic peptide with analgesic properties. Pain 92 81–90. 10.1016/s0304-3959(00)00470-x[
]
- Nixon J. P., Smale L. (2007). A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav. Brain Funct. 3:28. 10.1186/1744-9081-3-28[
][
][
]
- Colas D., Manca A., Delcroix J. D., Mourrain P. (2014). Orexin A and orexin receptor 1 axonal traffic in dorsal roots at the CNS/PNS interface. Front. Neurosci. 8:20 10.3389/fnins.2014.00020[
]