Cholinergic crisis
Cholinergic crisis is a clinical condition that develops as a result of over-stimulation of nicotinic and muscarinic receptors at the neuromuscular junctions and synapses due to an excess of neurotransmitter acetylcholine (ACh), as of a result of the inactivation or inhibition of the acetylcholine esterase (AChE) enzyme, the enzyme responsible for the degradation of acetylcholine (ACh) 1. Acetylcholine (ACh) is found in at the synapses of ganglia, the neuromuscular junction, and the muscular system of the visceral organs. Acetylcholine (ACh) is synthesized at the nerve terminal from acetyl coenzyme A (acetyl CoA). Excessive accumulation of acetylcholine (ACh) at the neuromuscular junctions and synapses causes symptoms of both muscarinic and nicotinic toxicity. The action of acetylcholine at the postsynaptic membrane is not terminated by reuptake of the neurotransmitter but rather by the action of a powerful hydrolytic enzyme acetylcholine esterase (AChE). Acetylcholine esterase (AChE) enzyme is found in the synaptic cleft. The action of acetylcholine esterase (AChE) enzyme breaks up acetylcholine into choline and acetate. The cholinergic nerve terminal has a sodium choline transporter that takes up choline produced from hydrolysis of acetylcholine 2. Excessive acetylcholine esterase (AChE) inhibition can cause cholinergic crisis, defined by symptoms including pupils ≤ 2 mm in diameter, muscular fasciculation, cramps, muscular weakness, paralysis, heart rate ≤ 60 beats per minute (bradycardia), systolic blood pressure ≤ 80 mmHg, decreased consciousness (Glasgow Coma Scale < 15), excessive sweating, increased salivation, diarrhea, blurry vision, poor air entry due to bronchorrhea and/or bronchospasm 3.
Normally acetylcholinesterase terminates synaptic transmission through hydrolyzation of the neurotransmitter acetylcholine in brain synapses and neuromuscular junctions 4. Irreversible inhibition of acetylcholinesterase with organophosphorus compounds 4 leads to overstimulation of cholinergic receptors via excessive accumulation of acetylcholine, resulting in respiratory failure or even death 5. Although reversible cholinesterase inhibitors are beneficial for the treatment of Alzheimer’s disease 6, myasthenia gravis 7, and neurogenic bladder 8, they can still cause adverse cholinergic reactions.
Patients receiving pharmaceutical cholinesterase inhibitors rarely develop cholinergic crisis in clinical practice. However, relatively higher doses of distigmine bromide were prescribed for the treatment of neurogenic bladder in Japan compared with in other countries until 2010, and several case reports 9 and case series 10 of cholinergic crisis associated with therapeutic doses of cholinesterase inhibitors have therefore been reported by Japanese doctors.
In clinical practice, cholinergic crisis is most commonly seen in 11:
- Patients with myasthenia gravis on treatment with high dose acetylcholinesterase inhibitors (AChEI).
- Patients after general anesthesia who received high doses acetylcholinesterase inhibitors to reverse the effects of neuromuscular blocking agents, for example, neostigmine.
- Exposure to a chemical substance that causes inactivation of acetylcholinesterase. Examples of such substances are nerve gas like sarin, tabun, soman and other organophosphates like pesticides and insecticides.
Cholinergic crisis is commonly seen in the pediatric population and patients with myasthenia gravis. In the pediatric age group, cholinergic crisis is usually as a result of accidental contact or ingestion of organophosphates. Children living in rural areas are at a very high risk. Since 2013, there are stricter federal regulations in the United States for the sale of organophosphates.
Globally, about three million people have been exposed to organophosphate poisoning annually with approximately 300,000 deaths. Poisoning is from either accidental or intentional ingestion of agricultural insecticides or pesticides. In cholinergic crisis related to organophosphates, poisoning can be sourced to food products like wheat, flour, cooking oil, fruits, and vegetables.
Since World War II, production of nerve gases like sarin and tabun has been limited. Manufacturing nerve gasses has been considered a war crime since the Geneva Convention in 1925 12. Decreased production has significantly reduced the incidence of nerve gas poisoning in modern times.
Cholinergic crisis causes
Several clinical conditions can trigger a cholinergic crisis. Outlined below are the most commonly encountered disorders:
Overmedication with acetylcholine esterase inhibitors in the treatment of myasthenia gravis
Myasthenia gravis is an autoimmune condition that affects the neuromuscular junction by producing autoantibodies against the acetylcholine (ACh) receptors at the postsynaptic membrane 13. Myasthenia gravis is characterized by generalized weakness or easy fatigability that can rapidly progress to respiratory failure. Another form of myasthenia gravis commonly seen in women is associated with the production of antibodies against muscle-specific tyrosine kinase (MuSK) 14.
One of the treatment modalities for myasthenia gravis is the use of acetylcholinesterase inhibitors (AChEI) such as pyridostigmine. acetylcholine esterase inhibitors prevents the breakdown of ACh by inactivating acetylcholine esterase. This stops the breakdown of acetylcholine (ACh) and increases its level and duration of action at the postsynaptic membrane. Excessive use of acetylcholine esterase inhibitors in the treatment of a patient with myasthenia gravis may precipitate a cholinergic crisis which is characterized by both muscarinic and nicotinic toxicity 12.
Myasthenic crisis is a complication of myasthenia gravis. Precipitants of the myasthenic crisis include infection, surgery, menstruation, and certain medications like quinidine, calcium channel blockers (verapamil, nifedipine, felodipine) and antibiotics (gentamicin, ampicillin, streptomycin, erythromycin, ciprofloxacin) 15. The clinical symptomatology of myasthenic crisis and cholinergic crisis are very similar. The cholinergic crisis should always be considered in myasthenic crisis although the cholinergic crisis is not that common in myasthenia crisis 16.
It is important to identify which of the two conditions is causing muscular weakness. A simple test that can be done involves giving a dose of edrophonium, 2 mg intravenously. The edrophonium medication produces clinical improvement in myasthenic crisis but worsening of symptoms in cholinergic crisis
Exposure to organophosphates
Cholinergic crisis can be precipitated by exposure to drugs that inhibit acetylcholine esterase, for example, nerve gas and organophosphate compounds used in pesticides, insecticides, and herbicides. Exposure might be via inhalation of vapors, ingestion, or direct contact of the chemical with the skin or mucous membrane 17..
Organophosphates are chemical compounds used extensively as agents of chemical warfare. Nerve gases are one of the deadliest agents in chemical warfare. Examples of such chemicals are sarin, tabun, soman, GF, and VX. Organophosphates work by inhibiting the action of acetylcholine esterase. This causes excessive stimulation of muscarinic and nicotinic receptors at the postsynaptic membrane. Acetylcholine (ACh) binds to the endplates of smooth muscles and secretory glands causing nausea, vomiting, bronchospasm, miosis, blurry vision, bronchorrea, and sialohorrea. Nicotinic effect on skeletal muscle can cause fasciculation and flaccid paralysis. Nerve gas poisoning can vary in severity from mild to moderate or severe.
Acute or chronic exposure to pesticides and insecticides containing organophosphates also can trigger a cholinergic crisis. Commonly used insecticides are malathion, parathion, diazinon, fenthion, and trichlorfon. Typically, patients are encountered in rural settings where pesticides and herbicides are used extensively. Examples of pesticides apart from organophosphates are carbamate, organochlorine, and pyrethroid insecticides. Apart from the muscarinic and nicotinic effects seen in cholinergic crisis, patients exposed to organophosphates might also exhibit neurological symptoms like a headache, dizziness, tremor, and paresthesia 18.
Reversal of neuromuscular blockage
Lastly, use of a reversal agent like neostigmine or pyridostigmine for neuromuscular blockages can also trigger a cholinergic crisis 19. Neostigmine is a compound that inhibits acetylcholine esterase and is commonly used to reverse the effects of non-depolarizing paralytic agents like vecuronium, rocuronium, mivacurium, and pancuronium. Inhibiting acetylcholine esterase allows for the accumulation of acetylcholine (ACh) at the neuromuscular junction thus overcoming the competitive inhibition of non-depolarizing blocking agents. Neostigmine, like other acetylcholine esterase inhibitors, can stimulate the muscarinic receptors and cause the cholinergic crisis. Bronchospasm, miosis, increased peristalsis, and secretions are usually seen after administration of neostigmine. To minimize these effects on muscarinic receptors, an anticholinergic agent like glycopyrrolate is concomitantly administered during reversal of neuromuscular blocking agents.
Cholinergic crisis pathophysiology
Acetylcholine acts on both muscarinic and nicotinic receptors.
Muscarinic receptors are located throughout the body. They are activated by the action of muscarine and acetylcholine. These receptors are part of the G protein-coupled receptors. On activation, there is an increase in intracellular cyclic adenosine monophosphate (AMP). Activation of cyclic AMP triggers the action of protein kinase. The muscarinic receptors form part of the parasympathetic that helps with the regulation of secretions (both in the bronchial tree and the gastrointestinal tract), heart rate, pupillary response, and urination.
Muscarinic effects of acetylcholine includes:
- Eye miosis and blurry vision,
- Gastrointestinal system: nausea, vomiting, and diarrhea
- Respiratory system: low lung compliance, bronchoconstriction, and bronchorrhea
- Secretory system: increased secretions in the tracheobronchial and gastrointestinal system
- Cardiovascular system: bradycardia
- Genitourinary: urinary frequency and urgency
Nicotinic receptors belong to the ligand-gated ion family of receptors. They are stimulated by acetylcholine and nicotine. They are located in muscle fiber at the neuromuscular junctions and autonomic ganglia for both sympathetic and parasympathetic nervous system 12.
Nicotinic effects of acetylcholine
Acetylcholine binds to the endplate of skeletal muscle and synaptic ganglia causing the following effects:
- Voluntary muscle fasciculation: flaccid paralysis
- Cardiovascular effects: tachycardia that may progress to bradycardia from the opposing effects of the stimulation of muscarinic and nicotinic receptors.
Cholinergic crisis signs and symptoms
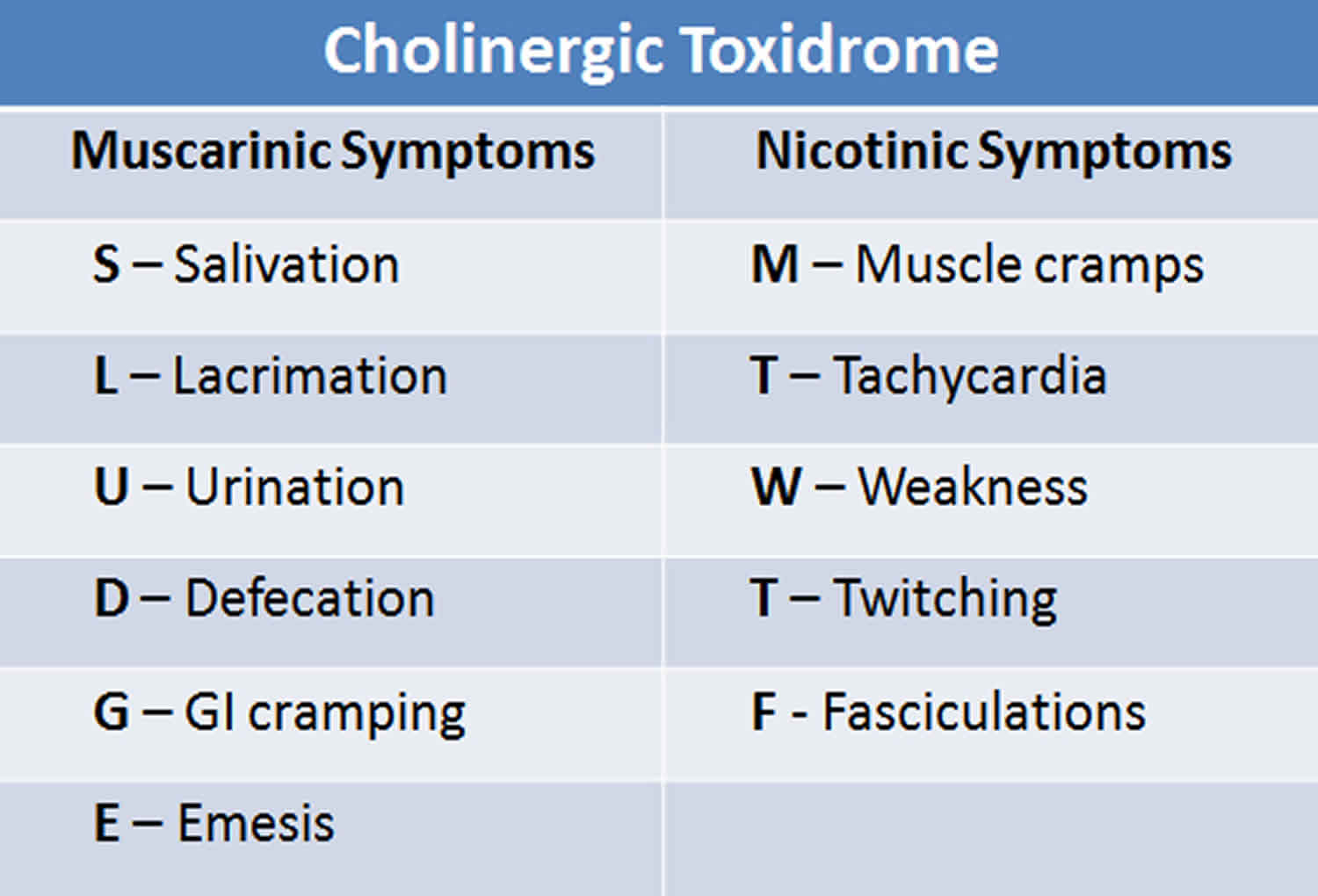
The diagnostic work of cholinergic crisis can pose a clinical challenge, especially for those unfamiliar with the clinical signs and symptoms. A very detailed history taking with a thorough physical examination is necessary. In the physical examination, particular attention should be paid to the nervous, respiratory, cardiovascular, and gastrointestinal system as this is where the clinical manifestation is most profound. A good mnemonic to remember is SLUDGEM and DUMBELS for the muscarinic effect of acetylcholine.
Clinical findings related to stimulation of muscarinic receptors
- S- Salivation
- L- Lacrimation
- U -Urinary frequency
- D-Diarrhea
- G- Gastrointestinal cramping and pain
- E- Emesis
- M- Miosis
Another mnemonic that is commonly used for symptoms is “DUMBELS.”
- D- Diaphoresis and Diarrhea
- U -Urinary frequency
- M-Miosis
- B-Bronchospasm and Bronchorrhea
- E – Emesis
- L – Lacrimation
- S – Salivation
Clinical findings related to stimulation of nicotinic receptors
- Muscular weakness
- Muscular fatigue and fasciculation
- Respiratory muscle weakness
- Tachycardia
- Hypertension
Clinical findings related to stimulation of the central nervous system
- Seizures
- Coma
- Ataxia
- Slurred speech
- Agitation and restlessness.
Cholinergic crisis complications
The following complications can develop in cholinergic crisis. These problems are related to the overstimulation of the muscarinic and nicotinic receptors.
Outlined below are the complications as it affects each system.
Respiratory system
- Respiratory failure from profound weakness of respiratory muscles
- Aspiration pneumonia from hypersalivation and bronchorrhea
- Severe bronchospasm
Cardiovascular system
- Bradycardia
- Hypotension
- Hypertension
- Arrhythmia
Central nervous system
- Hallucination
- Psychosis
- Seizure
- Altered mental status
Gastrointestinal system
- Electrolytes abnormalities related to gastrointestinal losses from vomiting and diarrhea
Cholinergic crisis diagnosis
Evaluation of patients with cholinergic crisis involves a detailed history taking and physical examination for the toxidromes associated with the crisis. Clinical diagnosis of cholinergic crisis can be established based on the toxidromes listed above.
In the history taking, it is very pertinent to determine the cause of the cholinergic crises:
- Medications for the treatment of myasthenia gravis or glaucoma, including pyridostigmine
- Ingestion or exposure to insecticides, pesticides, or herbicides
- Exposure to nerve gas
- Reversal of neuromuscular blockage
Time is paramount in the initial assessment. When, how, and where the ingestion or exposure took place is necessary to elicit from the history. This is because there is a 48-hour window during which to administer pralidoxime as an antidote. Pralidoxime will react with the enzyme that breaks down acetylcholine after contact with the inhibitor of acetylcholine esterase, in this instance the nerve gas or insecticide. The reactivated enzyme acetylcholine esterase will expedite the molecular degradation of acetylcholine. The degradation of acetylcholine will terminate the overstimulation of the postsynaptic membrane by acetylcholine.
Included in the evaluation are ancillary studies:
- Complete Blood Count (CBC) to check if there is an elevation of white blood cell count to rule out an infectious process.
- Comprehensive Metabolic Panel (CMP) to rule out electrolytes abnormalities related to organophosphate poisoning
- Red Blood Cell Cholinesterase activity is usually decreased, and this can help in confirming the diagnosis. Plasma pseudocholinesterase can also be used but is less accurate than red blood cell cholinesterase activity 20.
- Electrocardiography (ECG) to check for the presence of arrhythmia associated with organophosphate poisoning.
- Chest X-ray to evaluate for the presence of pulmonary edema or aspiration.
- Head CT scan is indicated if the patient’s mental status is altered or there are significant changes in the Glasgow Coma Scale.
Cholinergic crisis treatment
The management of cholinergic crisis encompasses three stages:
- prehospital care,
- emergency department management, and
- inpatient care.
Prehospital care
Prehospital care includes the initial stabilization of the patient and removal of the offending toxic agent. Decontamination should be initiated as soon as possible if poisoning with organophosphate or nerve gas is the primary culprit of cholinergic crisis. All clothing should be removed from the patient’s body to prevent continued contamination and to prevent cross contamination of first responders.
Emergency room management
Regardless of the etiology of cholinergic crises, the core principle in stabilization is ABC: Airway, Breathing, and Circulation.
Airway and Breathing
Care should be taken to ensure that the airway is patent and the patient is breathing spontaneously. Airway should be secured if there is concern for airway compromise.
Indications for advanced airway management and intubation in cholinergic crisis are:
- Copious oral and nasal secretions compromising the patency of the airway
- Altered mental status with a Glasgow Coma Score less than 8
- Evidence of hemodynamic instability
- Profound weakness of the respiratory muscles
Circulation
Vascular access should be established immediately with two large bore peripheral intravenous access. Fluid should be started to maintain adequate circulation with continuous pulse oximetry and monitoring of vital signs. In case of hemodynamic instability a central venous access should be established for infusion of vasoactive medications.
In the emergency department, the primary focus on initial management is the maintenance of airway and hemodynamic stability. If the patient is already intubated, ventilatory support should be continued .
Inpatient management
Inpatient care includes continued cardiopulmonary support and monitoring. Patients with cholinergic crisis should be admitted to the intensive care unit (ICU).
Cholinergic crisis antidote
Two types of antidotes are used for a cholinergic crisis: atropine and oximes.
Atropine
The first antidote is atropine. It is an effective agent for the muscarinic effect of acetylcholine. It competitively binds to the post synaptic muscarinic receptor thereby preventing further action of acetylcholine. Atropine dose is about 0.03- 0.05 mg/kg for pediatric and about 2 mg for adult patients. It is recommended to give atropine until signs of atropinization is present:
Signs of atropinization
- Tachycardia
- Warm, dry and flushed skin
- Mydriasis
Atropine does not have any effect on the nicotinic receptors.
Oximes
For the nicotinic effect in cholinergic crisis, the antidote is a class of drugs called the “oximes.” Examples of oximes are pralidoxime and obidoxime 21.
In the United States, pralidoxime chloride is the most commonly used antidote. Its mechanism of action is like that of a “molecular crowbar” that separates the bonded nerve gas or organophosphate from acetylcholinesterase. The separated acetylcholine esterase can then continue the process of chemical breakdown of acetylcholine. There is a window period during which oximes can be given before there is an irreversible bonding of nerve gas to acetylcholine esterase. This phenomenon is known as “aging” 22. The aging half life ranges from two minutes for soman to several hours for sarin
Pralidoxime should be given to patients with signs of respiratory muscle weakness or generalized muscular weakness. It should be administered until there is an improvement in muscle weakness. It does not cross the blood-brain barrier hence the central nervous system effect of organophosphate poisoning is not neutralized. This is achieved by using atropine.
Other medications in cholinergic crisis
Seizure and agitation in cholinergic crisis can be treated with benzodiazepine like midazolam or lorazepam. Care should also be taken to avoid drugs like loop diuretics, theophylline, and caffeine and succinylcholine in organophosphate poisoning as this can make the symptoms of toxicity worse.
In the management of cholinergic crisis secondary to reversal of neuromuscular blockage with neostigmine, atropine or glycopyrrolate can be administered to lessen the cholinergic effects of the neuromuscular blockage reversal.
Consultation with a clinical toxicologist and intensivist is recommended in the treatment of cholinergic crisis.
Cholinergic crisis prognosis
The mortality rate in cholinergic crisis ranges from 3% to 25%. The most common cause of death is progressive respiratory failure.
References- Ohbe H, Jo T, Matsui H, Fushimi K, Yasunaga H. Cholinergic Crisis Caused by Cholinesterase Inhibitors: a Retrospective Nationwide Database Study. J Med Toxicol. 2018;14(3):237–241. doi:10.1007/s13181-018-0669-1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097965
- LOEWI O. On the intraneural state of acetylcholine. Experientia. 1956 Sep 15;12(9):331-3.
- Abedin MJ, Sayeed AA, Basher A, Maude RJ, Hoque G, Faiz MA. Open-label randomized clinical trial of atropine bolus injection versus incremental boluses plus infusion for organophosphate poisoning in Bangladesh. J Med Toxicol. 2012;8(2):108–117. doi: 10.1007/s13181-012-0214-6.
- Gorecki L, Korabecny J, Musilek K, Nepovimova E, Malinak D, Kucera T, et al. Progress in acetylcholinesterase reactivators and in the treatment of organophosphorus intoxication: a patent review (2006-2016). Expert Opin Ther Pat. 2017;27(9):971–85.
- Marrs TC. Organophosphate poisoning. Pharmacol Ther. 1993;58(1):51–66. doi: 10.1016/0163-7258(93)90066-M
- Korabecny J, Andrs M, Nepovimova E, Dolezal R, Babkova K, Horova A, et al. 7-Methoxytacrine-p-anisidine hybrids as novel dual binding site acetylcholinesterase inhibitors for Alzheimer’s disease treatment. Molecules. 2015;20(12):22084–101.
- Komloova M, Musilek K, Dolezal M, Gunn-Moore F, Kuca K. Structure-activity relationship of quaternary acetylcholinesterase inhibitors—outlook for early myasthenia gravis treatment. Curr Med Chem. 2010;17(17):1810–1824. doi: 10.2174/092986710791111198
- Sugimoto K, Akiyama T, Shimizu N, Matsumura N, Hashimoto M, Minami T, et al. Acotiamide hydrochloride hydrate added to combination treatment with an alpha-blocker and a cholinergic drug improved the QOL of women with acute urinary retention: case series. Res Rep Urol. 2017;9:141–3.
- Yamanaka S, Fujita I, Murota T, Kawakita M, Matsuda T. Cholinergic crisis following administration of distigmine bromide: a case report. Hinyokika Kiyo. 2002;48(1):21–23.
- Huang X, Liu WB, Men LN, Feng HY, Li Y, Luo CM, et al. Clinical features of myasthenia gravis in southern China: a retrospective review of 2,154 cases over 22 years. Neurol Sci. 2013;34(6):911–7.
- Adeyinka A, Kondamudi NP. Cholinergic Crisis. [Updated 2019 Mar 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482433
- Lacomis D. Myasthenic crisis. Neurocrit Care. 2005;3(3):189-94.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM. 2009 Feb;102(2):97-107.
- Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr. Opin. Neurol. 2005 Oct;18(5):519-25.
- Francis JK, Higgins E. Permanent Peripheral Neuropathy: A Case Report on a Rare but Serious Debilitating Side-Effect of Fluoroquinolone Administration. J Investig Med High Impact Case Rep. 2014 Jul-Sep;2(3):2324709614545225
- Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011 Jan;1(1):16-22.
- Newmark J. Nerve agents. Neurologist. 2007 Jan;13(1):20-32.
- Rastogi SK, Tripathi S, Ravishanker D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J Occup Environ Med. 2010 Aug;14(2):54-7.
- Neely GA, Kohli A. Neostigmine. [Updated 2019 Oct 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470596
- Eckert S, Eyer P, Herkert N, Bumm R, Weber G, Thiermann H, Worek F. Comparison of the oxime-induced reactivation of erythrocyte and muscle acetylcholinesterase following inhibition by sarin or paraoxon, using a perfusion model for the real-time determination of membrane-bound acetylcholinesterase activity. Biochem. Pharmacol. 2008 Feb 01;75(3):698-703.
- Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22(3):165-90.
- Burillo-Putze G, Hoffman RS, Howland MA, Duenas-Laita A. Late administration of pralidoxime in organophosphate (fenitrothion) poisoning. Am J Emerg Med. 2004 Jul;22(4):327-8.