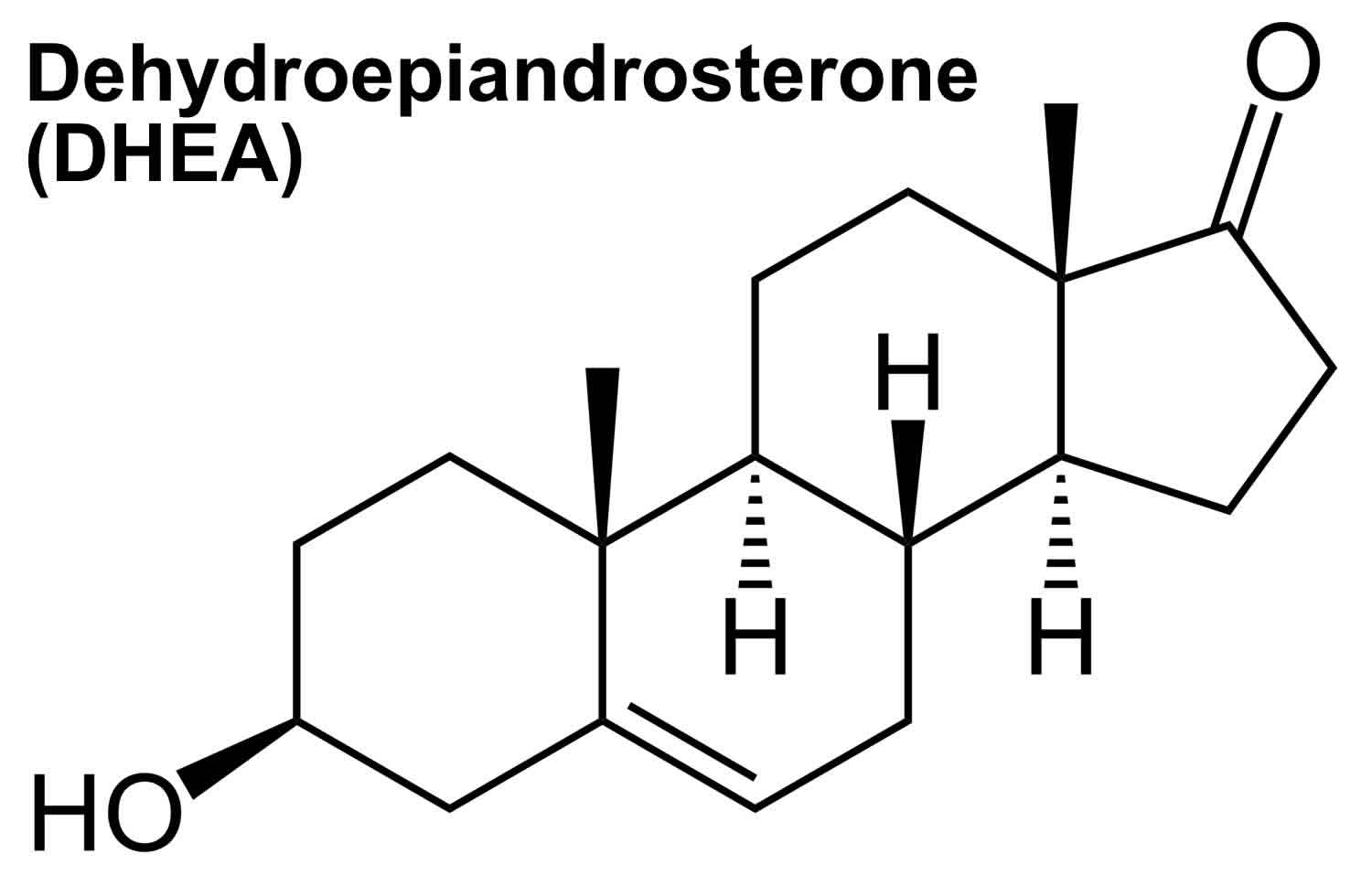
Dehydroepiandrosterone
DHEA also called Dehydroepiandrosterone, Androstenolone, Prasterone, 3b-hydroxyandrost-5-en-17-one, 3Beta-hydroxy-5-androsten-17-one, are adrenal steroid precursor hormones (prohormones) with weak androgenic effects that is converted into male sex hormones (androgens) and/or female sex hormones (estrogens) in peripheral tissues in your body to exert their effects 1, 2. Dehydroepiandrosterone sulphate (DHEAS) has a long half-life and provides a stable pool of circulating DHEA 2. DHEA and DHEAS are involved in the development of male sexual characteristics at puberty. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) are made and secreted primarily by the zona reticularis of the adrenal glands (adrenal cortex) in response to adrenocorticotropic hormone (ACTH) 2. The ovaries also synthesize DHEA; however, the ovaries lack the enzyme DHEA-sulphotransferase so that dehydroepiandrosterone sulphate (DHEAS) is almost exclusively synthesized and secreted by the adrenal glands. Furthermore, dehydroepiandrosterone (DHEA) is claimed to be synthesized in the human brain as well 3. In fact, DHEA concentrations in the human brain have been shown to be higher than that in circulation, while DHEAS concentrations are lower 4, 5, which not only supports the theory of local synthesis, but also testifies to the importance of this hormone in the central nervous system (brain and spinal cord) 3. DHEA can also be made in a laboratory; this man-made synthetic form is known as prasterone 6. Although DHEA and DHEAS are secreted in greater quantities, androstenedione is qualitatively more important since it is more readily converted to testosterone in peripheral tissues 2. Roughly, the relative androgenic potency of DHEA, androstenedione, testosterone, and dihydrotestosterone (DHT) are 5:10:100:300, respectively 2. As ACTH is the main regulator of adrenal androgen production in adults, both DHEA and androstenedione exhibit circadian periodicity in concert with ACTH and cortisol and their plasma concentrations increase rapidly following ACTH administration; also, they are suppressed by glucocorticoid administration. Because of its slow metabolic clearance, DHEAS does not exhibit diurnal rhythm variation.
The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 7, 8. DHEA and DHEAS levels rise steadily and peak in the second to third decade of life (serum DHEAS concentration can be as high as 10 μM in young adults) 9, 10, 11, 12. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life 13. In a healthy aged 65- to 81-yr-old population, plasma DHEAS concentration is 2.2 µM in women and 3.3 µM in men 14. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 12. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 12. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 15. Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 16. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 15. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 15. To compensate for the decrease in aged people, over-the-counter DHEA sulfate supplements are available and are sometimes promoted as an anti-aging therapy. But there is no reliable evidence to support these anti-aging claims of DHEA supplements. In fact, DHEA supplements may cause serious side effects. If you have questions about DHEA supplements, talk to your health care provider.
The potential clinical roles of dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulfate (DHEAS) have been studied extensively, as previous epidemiologic and prospective studies found DHEA or DHEAS levels seem to go down as people get older and indicate an inverse relation between low circulating levels of dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulphate (DHEAS) with a host of aging-associated pathologies such as sexual dysfunction, degenerative disorders and increased frailty, mood defects, poor sense of well-being and mortality from all causes in the elderly, attributing to DHEA or DHEAS anti-aging properties 17, 3, 18, 15, as well as higher risk of hospital admission 19, poor muscle strength 20 and mobility 19, 21, and higher prevalence of frailty 22, insulin resistance, obesity, cardiovascular disease 23 and mortality from cardiovascular disease 24. At the same time, a positive relation between higher levels of DHEAS and better health and well-being was documented 25. Furthermore, animal (primarily rats) studies have suggested many beneficial effects of DHEA treatment, including improved immune function and prevention of atherosclerosis, cancer, diabetes, and obesity. Therefore, the therapeutic role of DHEA replacement as an anti-aging factor for the prevention and/or treatment of the above conditions was studied; however, recent systematic reviews of the reports do not seem promising 26, 27, 28, 29, 30, 31, 32, 2.
- Thinning of vaginal tissue (vaginal atrophy). A prescription DHEA product is available for vaginal atrophy. Using vaginal inserts containing DHEA can reduce pain during sex by up to 15% after menopause.
- Aging. In theory, taking DHEA supplements to maintain DHEA levels could slow the aging process, possibly improving well-being, cognitive function and body composition. But so far research hasn’t proved this to be true. More studies are needed to better understand whether DHEA supplementation can counteract some of the effects of aging.
- Aging skin. Taking DHEA by mouth or applying it to the skin might improve skin appearance after menopause and in people over the age of 60 years. A small study suggested that taking DHEA supplements might improve skin hydration and firmness, and decrease aging spots in elderly adults.
- Depression. Changes in levels of DHEA have been linked to depression. Several preliminary studies show improvement in depression symptoms when taking DHEA as a dietary supplement, but more research is needed. Taking 30-500 mg of DHEA by mouth daily seems to improve symptoms of depression. Lower doses don’t seem to help.
- Inability to become pregnant within a year of trying to conceive (infertility). Taking DHEA by mouth before in-vitro fertilization (IVF) might improve the chances of pregnancy and having a baby. But it’s not clear if taking DHEA helps prevent miscarriage after IVF.
- Osteoporosis. Study findings on the effects of DHEA supplementation in the treatment of osteoporosis are mixed. More research is needed to determine whether taking DHEA supplements improves bone density in older adults with low DHEA.
DHEA levels seem to be lower in people with depression, Alzheimer’s disease and after menopause 33, 34, 35, 36, 37, 38, 39, 40, 5.
There is interest in using DHEA for a number of health purposes (e.g., for aging and aging related problems such as body shape, bone strength, muscle strength, physical performance or quality of life in people older than 60 or memory and thinking skills [cognitive function] in healthy older people, people with HIV, or in healthy young adults), but there isn’t enough reliable information to say whether it might be helpful. The authors of a Cochrane Systematic Review regarding the supplementation of DHEA in peri- and post-menopausal women, questioned the effectiveness of DHEA in women, and concluded that the overall quality of the studies analyzed was moderate to low and that the study outcomes were inconsistent and could not be pooled to obtain an overall effect due to the diversity of the measurement methods employed 41. Without exception, all recent reviews of the available data regarding DHEA replacement for the management of aging-related disorders do not support its use in clinical practice 26, 27, 28, 29, 30, 31, 32, 2.
There’s no scientific evidence to support taking DHEA to improve exercise or athletic performance. DHEA is banned by the National Collegiate Athletic Association (NCAA), the International Olympic Committee (IOC) and the World Anti-Doping Agency (WADA) 42, 43. Don’t confuse DHEA with 7-alpha-hydroxy-DHEA, 7-beta-hydroxy-DHEA, and 7-keto-DHEA. These are all different forms of DHEA but are not the same as DHEA.
DHEA supplementation has historically been used in the treatment of reproductive-related diseases, particularly in women, where it has been shown to alleviate menopause-related pathologies such as vaginal tissue thinning (vaginal atrophy) 44. DHEA supplements are also used for aging skin, depression, infertility, muscle strength, heart disease, erectile dysfunction (ED), and many other conditions, but there is no good scientific evidence to support many of these other uses 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56. Studies have shown quality control of DHEA supplement to often be low.
As an ingredient in dietary supplement products, DHEA is mostly marketed for bodybuilding as a “testosterone booster” (which it is not) or for nootropic (“cognitive enhancer”) actions. Research on the effects of DHEA on muscle strength and physical performance had mixed results, but most studies indicate DHEA supplementation has no effect on muscle strength in younger or older adults. DHEA might eventually prove to have benefits in treating people diagnosed with certain conditions, such as adrenal insufficiency and lupus. However, further studies are needed.
DHEA is considered as a hormone in Europe and available only by prescription, while in the United States DHEA is considered as a nutritional supplement and is sold over the counter without a prescription. This difference has no scientific foundation and is mostly a matter of declaration 2. Most DHEA supplements are made in laboratories from a substance called diosgenin, a plant sterol found in soy and wild yams. DHEA supplements were taken off the U.S. market in 1985 because of their unproven safety and effectiveness, but were reintroduced as a dietary supplement after the Dietary Supplement Health and Education Act was passed in 1994 2. At present, questionable over-the-counter DHEA preparations lacking pharmacokinetic and pharmacodynamic data are widely used in the United States 57, 58. There is no standard dosage of DHEA replacement; some studies have used between 25 and 200 milligrams a day, or sometimes even higher amounts. DHEA in current preparations has a long half-life 23, which allows a single intake a day. Target levels of DHEA are around the middle of normal range for healthy young subjects, measured in a blood sample 24 hour after the last intake 59.
Previous studies have shown that the end products of DHEA supplementation depend on the patient’s gender, with a non-symmetrical transformation of DHEA favoring androgens in women and estrogens in men 26, 60, 61. The above refer to oral administration of DHEA supplements; percutaneous administration of DHEA seems to provoke similar increases in both estrogens and androgens in the two genders 62.
DHEA supplements are generally well tolerated in studies using oral or percutaneous administration, with daily doses ranging from 25 mg to 1,600 mg. In women DHEA when administered orally is mainly converted to androgen metabolites. As a result, some minimal androgenic adverse effects have been reported, including mild acne, seborrhea (a red, itchy rash and white scales skin), facial hair growth, and ankle swelling 23, 29, 63.
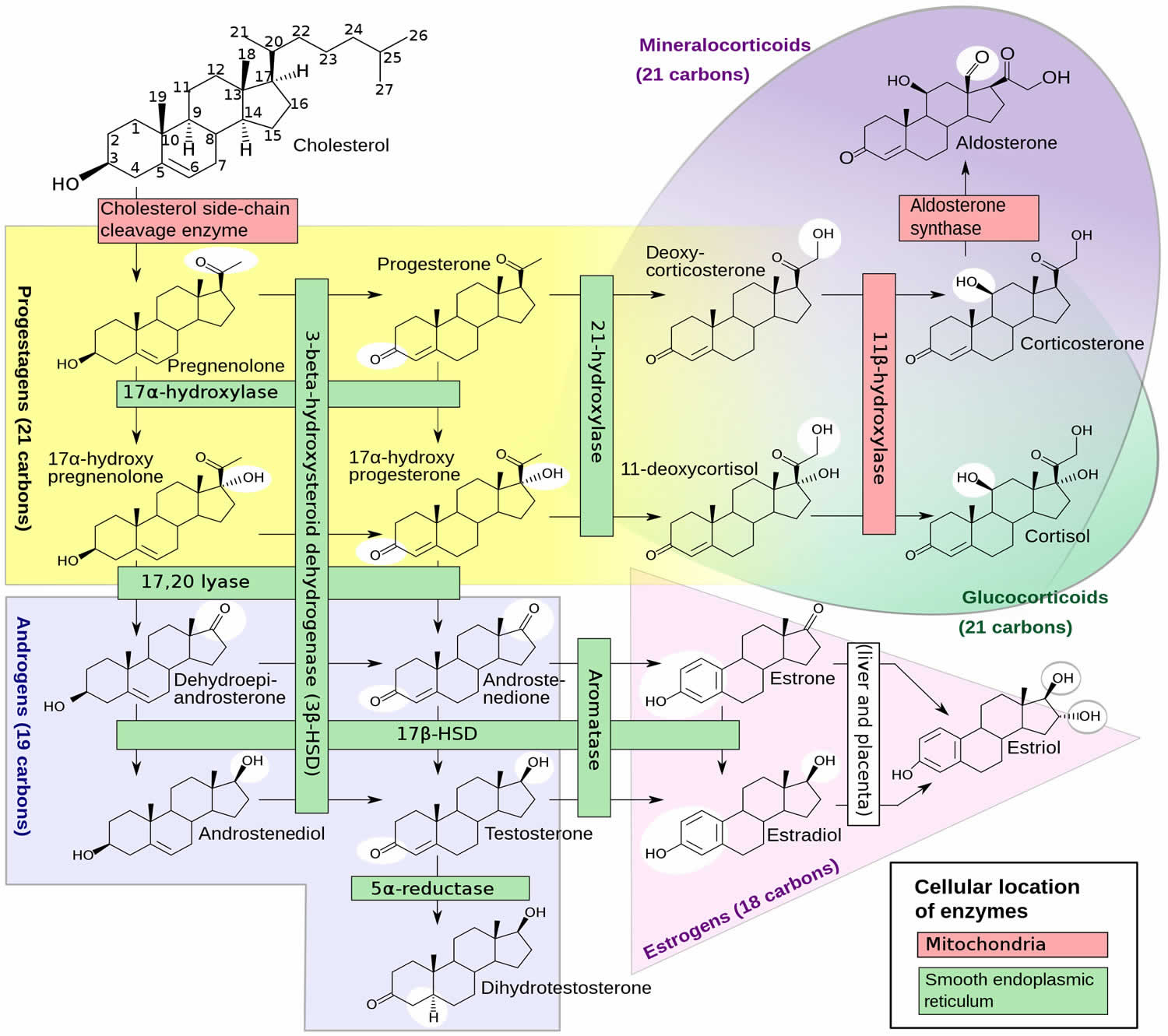
Figure 1. DHEA Biosynthesis
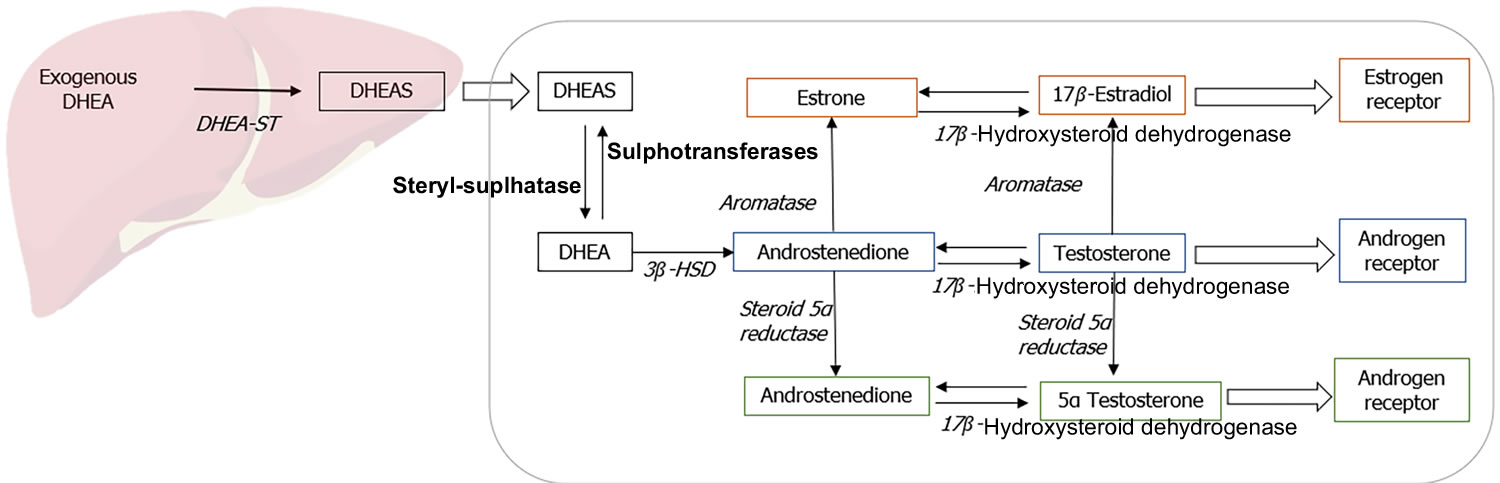
Figure 2. Metabolism of DHEA
Footnote: Metabolism of exogenously administered dehydroepiandrosterone (DHEA).
Abbreviations: DHEA = Dehydroepiandrosterone; DHEAS = Dehydroepiandrosterone sulfate.
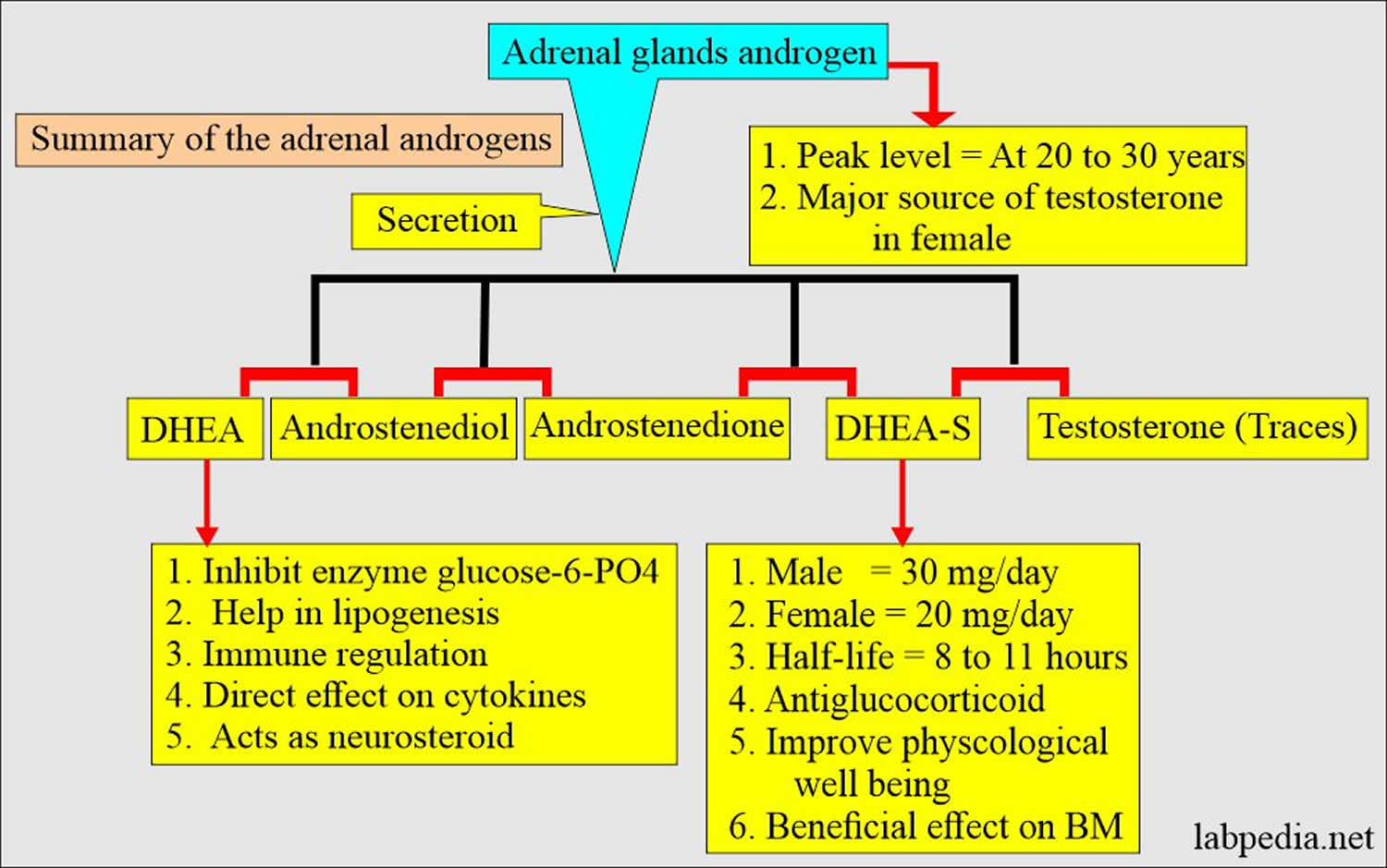
[Spurce 8 ]Figure 3. Adrenal gland androgens
[Source 64 ]Figure 4. Adrenal androgens summary
[Source 64 ]How does DHEA work?
The exact mechanism of action and clinical role of DHEA and DHEAS remain unclear 2, 8. As DHEA has minor steroidogenic activity, it acts predominantly by conversion to androgens and estrogens in peripheral target tissues (Figure 1 and 2). It also functions as a neurosteroid and acts via receptors for N methyl-D aspartate receptors (NMDA) and gamma amino butyric acid alpha (GABAα), peroxisome proliferator-activated receptor α (PPARα), or receptors for pregnane X, androstanol, and estrogen receptor β 65.
About 75%-90% of DHEA is produced by the zona reticularis of the adrenal gland while the rest is produced in the ovaries and the brain 8. DHEA sulfated form, dehydroepiandrosterone sulfate (DHEAS), is exclusively synthesized by the adrenals. DHEA has a shorter half-life and is secreted in a pulsatile manner, mirroring the circadian rhythm of corticotrophin (ACTH). In contrast, dehydroepiandrosterone sulfate (DHEAS) has a longer half-life and relatively more stable levels across the day, providing a continuous reservoir of DHEA.
The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 7, 8. DHEA and DHEAS levels rise steadily and peak in the second to third decade of life 66, 11, 12. Serum DHEA concentration is around 20 nM in young adults and decreases with age to 7 nM 9. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life 13. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 12. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 12. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 15. Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 16. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 15. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 15.
In adult women, dehydroepiandrosterone sulfate (DHEAS) is the most abundant steroid hormone, with daily production rates being approximately 8 to 16 mg/day, which are almost exclusively adrenal glands 67. DHEAS is formed from DHEA in the highly specialized zona reticularis of the adrenals, which has high sulfuryl transferase activity. The hydrophilic DHEAS is the major circulating form of DHEA and is interconverted in various tissues with DHEA by DHEA sulfotransferases and hydroxysteroid sulfatases 67. In premenopausal women, the production rate of DHEA is approximately 6–8 mg/day. Approximately 50% of DHEA is secreted by the adrenal glands, with the ovaries producing approximately 1 to 2 mg of DHEA per day; the remaining DHEA production occurs in peripheral tissues 67. In contrast, the production of testosterone (T) in premenopausal women is approximately 0.2 to 0.25 mg/day, which is about 25–40 times less than that of DHEA and even much lower than that of DHEAS 68. The ovaries of some postmenopausal women continue to produce some testosterone (T), but not DHEA 68, 69. In the circulation, DHEAS and DHEA are found in low micromolar and low nanomolar concentrations, respectively. Rosenfeld et al. 70 reported a circulating half-life of 1 to 3 hours for DHEA and 10 to 20 hours for DHEAS. Sex hormone-binding globulin (SHBG) weakly binds DHEA but not DHEAS 71.
Regular exercise is known to increase DHEA production in the body 72, 73. Calorie restriction has also been shown to increase DHEA in rhesus monkeys 74. Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction 75.
Peripherally, DHEA appears to exert its primary effects through its estrogenic and androgenic metabolites because a unique DHEA receptor has not been characterized. However, DHEA has been shown to exhibit weak agonist effects on the estrogen receptors (ER) α and β and be a weak antagonist of the androgen receptor (AR) 76. There is evidence that DHEA is synthesized within the brain and may act locally as an excitatory neurosteroid by antagonizing the actions of the gamma-aminobutyric acid type A receptor (GABAα) and stimulating the N-methyl-d-aspartate receptor (NMDA), peroxisome proliferator-activated receptor α (PPARα), or receptors for pregnane X, androstanol, and estrogen receptor β 8, 77, 78. A putative plasma membrane-bound G coupled receptor activated by DHEA has been identified on bovine aortic endothelial cells 79, 80. Liu et al. 81 subsequently have shown that physiological concentrations of DHEA activate this plasma membrane receptor on vascular endothelial cells and stimulate endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms.
DHEA and DHEAS can be converted to many different metabolites; Figure 2 depicts some of the more important ones. DHEA and DHEAS are metabolized in extragonadal target tissues such as the brain, bone, and body fat either by aromatization to estrone or by 5α-reduction to testosterone (T), with the testosterone (T) being converted to either estradiol or 5 alpha-dihydrotestosterone (DHT) in the same cells 82. The transformation of DHEA into active androgens and estrogens depends upon the level of expression of the various steroidogenic and metabolizing enzymes in each cell type. With the increase in aromatase gene expression in body fat with age 83, 84, older women potentially have greater capacity to synthesize estrone from DHEA and DHEAS. Furthermore, the ultimate effects of the complex metabolism of DHEA and DHEAS will involve the absolute levels of each metabolite, their receptor content within the target cell, the presence and levels of specific coactivator and corepressor proteins that modify the transcriptional response, and the up- or down-regulation of receptor levels by other hormones 16.
DHEA uses
DHEA has most often been used by adults at a dose of 50 mg by mouth daily for up to 1 year. DHEA is also available in topical creams and vaginal products. Speak with a healthcare provider to find out what type of product and dose might be best for a specific condition.
Data from epidemiologic and prospective studies indicate an inverse relation between low circulating levels of dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulphate (DHEAS) and a host of aging-associated pathologies such as sexual dysfunction, mood defects, and poor sense of well-being 18, 15, as well as higher risk of hospital admission 19, poor muscle strength 20 and mobility 19, 21, and higher prevalence of frailty 22, insulin resistance, obesity, cardiovascular disease 23 and mortality from cardiovascular disease 24. At the same time, a positive relation between higher levels of DHEAS and better health and well-being was documented 25. Furthermore, animal (primarily rodent) studies have suggested many beneficial effects of DHEA treatment, including improved immune function and prevention of atherosclerosis, cancer, diabetes, and obesity. Therefore, the therapeutic role of DHEA replacement as an anti-aging factor for the prevention and/or treatment of the above conditions was studied; however, recent systematic reviews of the reports do not seem promising 26, 27, 28, 29, 30, 31, 32, 2.
- Thinning of vaginal tissue (vaginal atrophy). A prescription DHEA product is available for vaginal atrophy. Using vaginal inserts containing DHEA can reduce pain during sex by up to 15% after menopause.
- Aging. In theory, taking DHEA supplements to maintain DHEA levels could slow the aging process, possibly improving well-being, cognitive function and body composition. But so far research hasn’t proved this to be true. More studies are needed to better understand whether DHEA supplementation can counteract some of the effects of aging.
- Aging skin. Taking DHEA by mouth or applying it to the skin might improve skin appearance after menopause and in people over the age of 60 years. A small study suggested that taking DHEA supplements might improve skin hydration and firmness, and decrease aging spots in elderly adults.
- Depression. Changes in levels of DHEA have been linked to depression. Several preliminary studies show improvement in depression symptoms when taking DHEA as a dietary supplement, but more research is needed. Taking 30-500 mg of DHEA by mouth daily seems to improve symptoms of depression. Lower doses don’t seem to help.
- Inability to become pregnant within a year of trying to conceive (infertility). Taking DHEA by mouth before in-vitro fertilization (IVF) might improve the chances of pregnancy and having a baby. But it’s not clear if taking DHEA helps prevent miscarriage after IVF.
- Osteoporosis. Study findings on the effects of DHEA supplementation in the treatment of osteoporosis are mixed. More research is needed to determine whether taking DHEA supplements improves bone density in older adults with low DHEA.
There is interest in using DHEA for a number of other purposes (e.g., for aging and aging related problems such as body shape, bone strength, muscle strength, physical performance or quality of life in people older than 60 or memory and thinking skills [cognitive function] in healthy older people, people with HIV, or in healthy young adults), but there isn’t enough reliable information to say whether it might be helpful. The authors of a Cochrane Systematic Review regarding the supplementation of DHEA in peri- and post-menopausal women, questioned the effectiveness of DHEA in women, and concluded that the overall quality of the studies analyzed was moderate to low and that the study outcomes were inconsistent and could not be pooled to obtain an overall effect due to the diversity of the measurement methods employed 41. Without exception, all recent reviews of the available data regarding DHEA replacement for the management of aging-related disorders do not support its use in clinical practice 26, 27, 28, 29, 30, 31, 32, 2.
There’s no scientific evidence to support taking DHEA to improve exercise or athletic performance. The National Collegiate Athletic Association (NCAA), the International Olympic Committee (IOC) and the World Anti-Doping Agency (WADA) prohibit the use of DHEA in athletic competitions.
DHEA and infertility
DHEA has been found to improve ovarian steroidogenesis and also leads to an increase in insulin-like growth factor 1 (IGF-1) which is speculated to have a favorable effect on oocyte quality and follicular development 85, 86. In a randomized prospective study by Wiser et al 87, they enrolled 33 women with poor ovarian reserve (17 in the DHEA group and 16 in the control group) and observed the effects of DHEA supplementation on in vitro fertilization (IVF). Patients in the DHEA group had a significantly higher live birth rate as compared to controls 87. In a meta-analysis by Qin et al 88, they included nine studies and observed that clinical pregnancy rates were significantly increased in women with diminished ovarian reserve supplemented with DHEA. However, when the analysis was restricted to randomized control trials, there was no significant difference in pregnancy rates. In a recent meta-analysis by Schwarze et al 89, they included five studies with a total of 910 individuals with diminished ovarian reserve, of which 413 received DHEA. They observed that DHEA supplementation was associated with significantly improved pregnancy rates and decreased abortion frequency 89. There was no effect on the number of oocytes retrieved. Hence, the results have been largely conflicting and it is difficult to draw any conclusions as the definitions for diminished ovarian reserve, stimulation protocols used, and dosing and duration of DHEA varied notably between studies 8. Taken together, it implies that DHEA may have a role in the peri-implantation period but have less impact on ovulation induction/oocytes retrieval 8. Future randomized controlled trials planned with primary endpoints of implantation success in such group of subjects, may yield a favorable role for DHEA supplementation.
Genitourinary syndrome of menopause
Genitourinary syndrome of menopause, a term first introduced in 2014, is a relatively common entity with a prevalence ranging from 27% to 82%. Genitourinary syndrome of menopause encompasses symptoms ranging from vulvovaginal dryness and painful intercourse (dyspareunia) to urinary urgency and pain or a burning sensation when urinating (dysuria), and leads to significant impairment in quality of life and sexual function 8. In a randomized prospective double-blind placebo-controlled trial by Labrie et al 90, the efficacy of 0.5% intravaginal DHEA in women with genitourinary syndrome of menopause was assessed. They observed that vaginal DHEA (Prasterone) significantly relieved painful intercourse (dyspareunia) with improvement in vaginal secretions and epithelial integrity. Prasterone was first approved by the FDA in 2016 for the treatment of dyspareunia due to genitourinary syndrome of menopause 91. Recent North American Menopause Society guidelines suggest vaginal DHEA as an alternative agent in individuals with genitourinary syndrome of menopause symptoms after the initial use of non-hormonal agents 92.
DHEA and Sexual dysfunction
Serum levels of DHEA and DHEAS start declining from the third decade, leading to decreased androgen levels. From epidemiologic studies sexual function problems are common among women and increase with increasing age 93, 94, 95. The sex steroid hormones estrogens and androgens seem to play an important role in the sexual life of women; androgens affect the reusability, pleasure, and intensity of orgasm in women and are particularly implicated in the neurovascular smooth muscle response of swelling and lubrication, whereas estrogens contribute to vulval and vaginal congestive response and affect mood and sexual responses 96. Conditions such as menopausal symptoms, loss of libido, vulvovaginal atrophy-related sexual dysfunction, and poor sense of well-being seen in menopausal and peri-menopausal women were related to the age-associated decline in sex steroids 97. Furthermore, interventional studies in postmenopausal women with estrogens have shown much improvement on vaginal atrophy and vasomotor symptoms 97, 98, 99, 100; there is also much clinical evidence for the efficacy of transdermal testosterone treatment for low sexual function in women 95, 101, 102, 103, 104, 105.
Given that a) the adrenal steroids are the most abundant sex steroids in post-menopausal women and provide a large reservoir of precursors for the intracellular production of androgens and estrogens in non-reproductive tissues, b) DHEA levels decline with age, c) pre- and post-menopausal women with lower sexual responsiveness have lower levels of serum DHEAS 106 and d) treatment of postmenopausal women with estrogen and testosterone have shown some improvement in sexual function, it was proposed that restoring the circulating levels of DHEA to those found in young women may improve sexual function and well-being in postmenopausal women 107. In a randomized, double-blind, placebo-controlled trial by Panjari et al 29, 93 post-menopausal women with low libido were included and the effect of DHEA on sexual function was assessed. They observed that there was no significant improvement in sexual function with regard to the primary outcome measures which included the change in total satisfying sexual events and the Sabbatsberg Sexual Self-Rating Scale total score. There was no significant change in secondary outcome measures as well, which included measures of well-being and quality of life 29.
A recent review of the available data concluded that current evidence does not support the routine use of DHEA in women with adrenal insufficiency 30. Furthermore, the more recent placebo-controlled randomized trials that are of superior design compared to the early trials, as they use validated measures of sexual function, have larger sample sizes, and are of longer duration, failed to document any significant benefit of oral DHEA therapy on well-being or sexual function in women 31, 26, 27, 28, 29. It has been hypothesized that the efficacy of DHEA to improve sexual function might be dependent on the route of its administration. In women, androgens and estrogens are produced from DHEA in the vagina tissue. As vaginal atrophy and dryness are common symptoms of estrogen deficiency during menopause, causing dyspareunia and sexual dysfunction 108, a possible benefit that emerged is that vaginally administered DHEA may improve the postmenopausal vaginal atrophy-related sexual dysfunction 109 without increasing the circulating levels of estrogen above the postmenopausal range 61, 109, 110, 111, 112. Despite initial promising, beneficial effects on sexual function, again, even with intravaginally administered DHEA, a study failed to show significant benefits 31.
In a systematic review and meta-analysis of 28 studies of DHEA therapy in 1273 post-menopausal women, DHEA therapy did not improve sexual function, quality of life, or menopausal symptoms and was associated with androgenic side effects 113. It is to be noted that these studies had a duration less than 3 months. Also, oral DHEA was used in all of these, and there are no studies on percutaneous DHEA. Currently, the Endocrine Society guidelines recommend against the use of DHEA for sexual dysfunction and other related indications because of a lack of long-term safety efficacy data 114.
DHEA and Erectile dysfunction
In men lower circulating levels of DHEA was related to erectile dysfunction. A double-blind, placebo-controlled study that enrolled men with erectile dysfunction treated with oral DHEA 50 mg daily has shown some promise for improving sexual performance in men who had low DHEA blood levels 112. However, high-quality studies have demonstrated inconsistent results regarding DHEA supplementation for improving sexual function, libido, and erectile dysfunction. Although research in this area is promising, additional well-designed studies are required 2.
DHEA and Adrenal insufficiency
Adrenal insufficiency is associated with reduced androgen levels which have been suggested to have multiple effects including loss of libido, reduced energy, and consequently decreased quality of life despite optimal glucocorticoid replacement when compared to a healthy population 59, 115, 116. DHEA supplementation has been suggested as a potential add-on therapy to conventional adrenal replacement therapy with glucocorticoids and mineralocorticoids to mitigate these effects 8. However, the exact physiological roles of DHEA still remain unclear and the routine therapy of individuals with adrenal insufficiency is still controversial 2.
Some authors reported significant improvements of mood, well-being, sexual thoughts, libido, interest, and satisfaction following DHEA replacement, particularly in females 59, 115, 116, 117. In a randomized double-blind placebo-controlled study by Binder et al 118, they included 23 young women with secondary hypoadrenalism and observed significant improvements in pubic hair growth and psychological well-being. In a subsequent meta-analysis by Alkatib et al 30 which included 10 studies in women with either primary or secondary adrenal insufficiency, DHEA supplementation lead to minor improvements in quality of life. However, there was no effect on sexual function or anxiety 30.
Other analyses of DHEA administration in women with primary and secondary adrenal insufficiency have resulted in inconsistent and unreproducible results 117. Currently, the Endocrine Society Clinical Practice Guidelines suggest that DHEA replacement (25–50 mg as a single oral dose in the morning) may be considered in women with adrenal insufficiency with low energy levels, low libido and depressive symptoms despite optimal glucocorticoid and mineralocorticoid replacement 119. Monitoring is done by clinical and biochemical markers such as measurement of DHEAS, testosterone, androstenedione, and sex hormone-binding globulin (SHBG) 24 hour after the last DHEA dose. If the patient fails to report a sustained, beneficial effect of replacement after 6 months, the treatment should be discontinued 8.
DHEA and Anorexia nervosa
DHEA levels have also been implicated to play a role in low bone mass in anorexia nervosa. In a randomized control trial by Gordon et al 120, which compared the effects of DHEA vs conventional hormone replacement therapy in young women with anorexia nervosa, they observed that while hip bone mineral density (BMD) increased significantly with both therapies, DHEA therapy was associated with increased bone formation markers. DHEA therapy in addition was associated with significant improvement in psychological parameters. However, in a recent systematic review and meta-analysis 121, DHEA treatment was not found to be associated with improvement in bone mineral density (BMD) compared with placebo after adjustment for weight gain. Therefore, while DHEA does play a role in the bone pathology in anorexia nervosa, evidence with treatment remains sparse and further randomized trials are needed.
DHEA and Inflammatory bowel disease (IBD)
DHEA has anti-inflammatory properties 122, 123. DHEA levels appear to be low in people with ulcerative colitis and Crohn’s disease, irrespective of the patient’s age 124, 125. A phase 2 small pilot trial in patients with active inflammatory bowel disease refractory to other drugs, treated with 200 mg dehydroepiandrosterone per day orally for 56 days showed that DHEA may decrease the clinical activity of the disease and may even cause a remission 126. More studies are needed before saying for sure whether DHEA helps inflammatory bowel disease (IBD) or not.
DHEA and Autoimmune diseases
DHEA has been found to modulate inflammatory responses by blunting the production of pro-inflammatory cytokines, downregulating complement activation via the generation of C1 inhibitor, and enhancing T-cell and NK cell cytotoxicity 127. In accordance, DHEAS levels have also been found to be decreased in multiple autoimmune diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), autoimmune hypothyroidism, fibromyalgia, and polymyalgia rheumatica 128, 129, 130.
In a randomized double-blind placebo-controlled study by Nordmark et al 131, they included 41 women with systemic lupus erythematosus (SLE) on steroids and assessed the efficacy of DHEA supplementation. They observed significant improvement in some domains of health-related quality of outcome measures which included an improvement in mental health. There was also an improvement in sexual well-being while there was no improvement in other domains such as physical function, general health, or vitality.
Similarly, DHEA levels have also been found to be low in Sjogren’s syndrome, which has been hypothesized as a potential cause of fatigue in these individuals. In a multicenter randomized controlled trial by Virkki et al 132, they included 107 individuals with primary Sjogren’s syndrome and assessed the efficacy of DHEA administration on several measures of fatigue. They observed that DHEA supplementation at a dose of 50 mg significantly improved measures of fatigue but a similar improvement was observed with placebo as well 132. Their results were similar to an earlier study by Hartkamp et al 133, and hence the authors suggested cognitive behavioral interventions in these individuals. In autoimmune hypothyroidism, Shukla et al 134 investigated the relationship between DHEAS levels and arthralgias in individuals with primary hypothyroidism. They assessed 73 individuals with subclinical hypothyroidism and observed that DHEAS levels < 43.6 mcg/dL significantly predicted early rheumatoid changes in individuals with primary hypothyroidism 134. They postulated the inhibition of 11-β HSD1, a possible bystander effect due to hypothalamic-pituitary-axis suppression and its immunosuppressive effects as some of the mechanisms to explain the effects. Therefore, there is some evidence to suggest a potential role of DHEA in multiple immunological diseases, and clinical interventions targeting this area merit further investigation.
DHEA and Systemic Lupus Erythematosus (SLE)
Several randomized controlled clinical studies have reported that regardless of the patient’s age, taking DHEA (50-200mg/day) along with other medications improves quality of life for people with mild to moderate SLE, decreases corticosteroid requirements, and reduces the frequency of flare-ups, though it probably does not change the overall course of their disease 116, 135, 136. A study had shown DHEA replacement may increase bone mass in women with lupus 137. A 2007 report in the Cochrane Database of Systematic Reviews 32 suggests a “modest but clinically significant impact” of DHEA replacement on health-related quality of life in the short-term for people with SLE; the impact on disease activity was inconsistent. Long- term outcomes and safety remain unstudied.
DHEA and Rheumatoid arthritis (RA)
DHEA levels have been found to be low in people with rheumatoid arthritis 138, 139 and decrease further with glucocorticoid therapy 140. Considering the well-demonstrated immune-suppressive activities exerted by the adrenal androgens and their derivatives 141, 142, the use of DHEA as potential therapy for management of male and female rheumatoid arthritis patients was studied. Preliminary data from animal studies showed benefits of DHEA treatment in collagen-induced arthritis 143, 144. However, in carefully controlled human clinical trials, DHEA treatment produced only modest benefits 145, probably with the exception of female-treated RA patients who benefit the most by DHEA replacement 146. The noted discrepancy in benefits from DHEA treatment between animals and humans may be related to the low endogenous DHEA in rodents relative to humans because of low levels of cytochrome P450 17α-hydroxylase 147, but also because of different DHEA metabolism between species; remarkably, in rodents DHEA has many highly oxygenated metabolites and a surprisingly complex metabolism that results in production of a multitude highly oxygenated species that may exert the beneficial effects on arthritis 63.
DHEA as Anti-ageing agent
Concomitant to its potential multiple actions on well-being, sexual function, and cognition, early interest in DHEA came about with it being promoted as the “fountain of youth hormone.” In the DHEAge study by Baulieu and colleagues 148, they observed the effects of DHEA supplementation at 50 mg daily for a year in 280 older men and women (age 60-79, 140 each). While there was some improvement in some parameters such as sexual function in women over 70 years of age, BMD at the femoral neck, and skin indices, there was no difference in libido, BMD, or sexual function in men 148. Moreover, there was no difference in body composition or muscle strength in women. Thereafter, in a double-blind randomized placebo-controlled study by Nair et al 26, they investigated the effects of DHEA administration in 87 elderly men with low levels of DHEAS and bioavailable testosterone and 57 elderly women with low DHEAS levels. There was no improvement in body composition, quality of life, physical performance, or insulin supplementation with DHEA supplementation 26. Similarly, there have been studies that have found no effects of DHEA supplementation on sexual function or well-being parameters 115, 149. Taken together, studies have argued against the use of DHEA as a cure-all elixir and the lack of long-term safety data does not justify the use of DHEA in healthy elderly individuals.
DHEA in Muscle strength, physical function and performance
The increasing incidence of fractures with advancing age has been related, among other factors, with the aging-related reduced muscle mass and strength, that increase the tendency for falling 150. A body of evidence exists on the effect of circulating DHEA or DHEAS on various markers of strength and physical function in older individuals. Studies in elderly individuals support a positive relation between DHEA blood levels and muscle mass 20, muscle strength 20, 151, and mobility 21, as well as better self-reported 152 and objectively assessed physical function 153, and measured peak volume of oxygen consumed per minute 154 in elderly with higher DHEA or DHEAS concentrations. In this direction, higher DHEAS levels were associated with increased bone mass density (BMD) in both men 155 and post-menopausal women and inversely related to risk for falls 156. Finally, low DHEAS levels have been associated with a higher prevalence of frailty, a geriatric syndrome of loss of reserve characterized by weight loss, fatigue, weakness, and vulnerability to adverse events 22, 157, and low back pain in both genders and slow rehabilitation of low-back pain in women 25, 158, 159.
Reports from interventional studies support a therapeutic role of DHEA replacement in aging-associated musculoskeletal defects. For example, DHEA exerted positive effects on muscle strength, body composition 160, 161, 162, and physical performance 163, as well as on bone mass density (BMD) in both lumbar spine and the hip 164, 165, 166, 167, 168, 148, 26, 162 when administered to post-menopausal women and elderly people over a period of 52 weeks. The above positive effects on musculoskeletal system were attributed to the DHEA-related increase of insulin-like growth factor-1 (IGF-1) levels 161, 169 and bioavailability (decrease of insulin growth factor binding protein-1 [IGFBP-1]) 169 in both men and women and/or to the increase of androgen levels mostly in women 161, 169, 170. Some other data also suggest aromatase activity of primary human osteoblasts converting DHEA to estrone 171, while it was shown in vitro that DHEA inhibits apoptosis and promotes proliferation of rat osteoblasts through MAPK signaling pathways, independently from androgen and estrogen effects 172. The above support a positive effect of DHEA on bone through conversion to estrogens, but also independently from its hormonal end-products. Other studies, however, failed to show a beneficial effect of DHEA supplementation on muscle function 173, 174, 149 or on BMD 160, 175, 163; of note all these studies were conducted over a shorter period (26 weeks only). Whether these conflicting data result from DHEA’s mild/moderate effect or from great differences between study designs, such as short duration of treatment and small number of participants, is difficult to say 27. Overall, the effect of DHEA supplementation on bone mass density (BMD) is small in relation to other treatments for bone loss, and no fracture data are available. Therefore, its therapeutic utility in rehabilitation and/or fracture/frailty prevention and treatment protocols for older patients remains unclear.
A recent systematic review 27 of the literature concluded that overall, the benefit of DHEA on muscle strength and physical function in older adults remains inconclusive 176, 177. Some measures of muscle strength may improve, although DHEA does not appear to routinely benefit measures of physical function or performance. Therefore, consensus has not been reached. Further large clinical trials are necessary to better identify the clinical role of DHEA supplementation in this population.
DHEA and Age-Related Cognitive Decline
The incidence of dementia increases exponentially with increasing age in both men and women 178. The number of elderly people nowadays is the fastest growing segment of the population, which means the related personal, social, and economic burdens of dementia are extremely high and could increase dramatically over the next few decades. It has been proposed that DHEA and DHEAS may exert neuroprotective effects in the brain mainly through DHEA-dependent neural stem cell stimulation, genomic activity modulation, and upregulation of androgen receptor levels 179, 180, as well as via the DHEA-induced inhibition of pro-inflammatory factor production, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) 181 that are involved in the pathogenesis of the amyloid plaques of Alzheimer disease 147. Higher serum levels of DHEAS have been related to more favorable cognitive function in older people, such as better concentration and working memory 182, 183 and higher scores on the Mini Mental State Examination (MMSE) 184. In this direction, low DHEA or DHEAS levels in particular brain regions were thought to play a role in the development of Parkinson disease, which is the second most common neurodegenerative disorder, just behind Alzheimer’s disease 185, while DHEA administration showed some beneficial effect in a primate model of Parkinson disease 186, 187. Inverse relations between DHEAS levels in saliva 187 and circulation 183 and some domains of memory impairment were also documented, supporting the hypothesis that DHEA supplementation may improve cognition in the elderly; yet solid evidence of associations between the endogenous levels of DHEA or DHEAS and measures of cognitive function is lacking.
No studies with DHEA replacement, either acute administration or chronic (up to 12 months) supplementation, have shown a benefit in cognitive function in healthy elderly populations 28, 188, 187, 189, 190, 191, 192. Furthermore, DHEA supplementation failed to show any benefit in patients with Alzheimer’s disease 193 and had only minimal beneficial effect on specific cognitive domains such as the verbal fluency in older women with mild to moderate cognitive impairment 194. Remarkably, some other studies have shown a negative effect of DHEAS replacement on cognitive performance 191, 195, 196. It should be noted however, that most studies included only small groups of patients and were up to a yearlong, which is probably not enough time to address the potential role of DHEA or DHEAS in neurodegenerative disorders.
DHEA supplementation in Depression
The prevalence of depression increases in cohorts of the elderly and has been independently related to high morbidity and mortality 197. In the central nervous system, DHEA is considered a neurosteroid with a wide range of functions. Animal studies demonstrated several DHEA-modulated neurotransmitters, including dopamine, glutamate, and c-amino butyric acid 181, as well as DHEA-induced increased activity of serotonin (5-hydroxytryptamine) neurons 198, providing the cellular basis for a potential antidepressant effect of DHEA. Furthermore, typical neuroleptic-like effects of DHEA were displayed amphetamine-induced schizophrenia models in mice suggesting potential role of DHEA replacement in the treatment of schizophrenia 199.
Previous studies suggested a strong relation between low levels of DHEA or DHEAS and major depression in children and adolescents 200, as well as adults and the elderly 201, 202. On the contrary, higher DHEAS levels were positively associated with depressive symptoms during the menopausal transition 203 and depression in patients with major depression 204, 205; whether the elevated DHEAS levels in the above studies represent increased adrenal activity that could explain the depressive symptoms is not clear, as cortisol was not measured. Moreover, successful treatment of depression was followed by reductions in both DHEAS 204, 205, 206 and DHEA levels 206, making the relation between DHEA or DHEAS and depression even more confusing.
Several interventional studies have shown that DHEA replacement may improve negative and depressive symptoms 107, 207, 208, 209, 210. In women with adrenal insufficiency, oral DHEA replacement significantly improved the overall well-being, as well as scores for depression and anxiety 174; similar results were found in the management of the negative symptoms of schizophrenia 210. Recent placebo-controlled randomized trials, however, failed to demonstrate a beneficial effect of DHEA therapy on mood, quality of life, perceptions of physical and emotional health, and life satisfaction in postmenopausal women 26, 28, 29. However, recent data have suggested that increased circulating DHEA and DHEAS levels may predict SSRI-associated remission in major depression 211. Therefore, the therapeutic role of DHEA on mood disorders remains unclear.
DHEA supplementation in Schizophrenia
DHEA has also been found to play a role in the pathophysiology of schizophrenia. It has been suggested to modulate neuronal differentiation and synaptogenesis. Additionally, it also interacts with multiple hormone receptor systems including gamma-aminobutyric acid, glutamate, and dopamine 212. In a systematic review and meta-analysis by Misiak et al 213, which included 19 studies, DHEAS levels were found to be significantly elevated in individuals with schizophrenia.
It has long been suggested that long-term psychosocial stress may cause or contribute to different diseases and symptoms, including atherosclerosis 214, coronary heart disease 215 and acute coronary events 216, as well as accelerated aging 217, 218. Whether DHEA or DHEAS levels are related to psychological stress or not is still debatable. Exposure to prolonged psychosocial stress has been related to reduced 219, 220, 221 or elevated levels of DHEA or DHEAS 222, while some other studies failed to show any clear association in any direction 223, 224. A recent study by Lennartsson et al. 225 demonstrated that DHEA and DHEAS levels are markedly lower in individuals that report perceived stress at work than in individuals who report no perceived stress at work. Whether this is of clinical importance is not clear.
DHEA and ADHD
Some research has shown that DHEA levels are too low in people with attention-deficit/hyperactivity disorder (ADHD), and treatment with methylphenidate or bupropion (stimulant type of medications) normalizes DHEA levels 226.
DHEA and Cardiovascular disease (CVD)
Cardiovascular disease (CVD) is the broad term for problems with the heart and blood vessels and its prevalence increases with advancing age 227. Cardiovascular disease (CVD) is often due to atherosclerosis. Cardiovascular disease (CVD) occurs when fat and cholesterol build up in blood vessel (artery) walls. This buildup is called plaque. Over time, plaque can narrow blood vessels and cause problems throughout the body. If an artery becomes blocked, it can lead to heart attack or stroke. Low androgen levels have been related to atherogenic profile in men 228, 229, while data from acute coronary units have shown a transient fall of the testosterone levels in the first 24 hours after heart attack (myocardial infarction) 230, 231, which probably deprives these patients of testosterone’s pro-fibrinolytic activity 232, 233 and may actually result in increased 30-day mortality rate following acute heart attack 234; the above findings suggest a strong relation between sex steroid hormones and cardiovascular disease morbidity and mortality. Many studies have previously documented a significant inverse relation between low DHEA or DHEAS levels and key elements involved in the development of atherosclerosis and cardiovascular disease, including carotid artery intima-media thickness 235, 236, oxidative stress 237, 238 and endothelial dysfunction 239, independent of other coronary risk factors. Low DHEAS levels 240, 241, 242 were also predictive of severe coronary atherosclerosis on coronary angiography 243, but also of earlier cardiac allograft vasculopathy development in heart transplant patients 244.
These findings are suggestive of anti-atherogenic and cardioprotective effect of DHEA or DHEAS. Numerous epidemiological studies have, therefore, looked at the specific relation between plasma levels of adrenal androgens and cardiovascular disease. Most have shown that low plasma levels of DHEA or DHEAS were clearly associated with increased incidence of atherosclerotic vascular diseases 154, 243, 245, 246 and cardiovascular morbidity 243, 247, 248, independently from classic cardiovascular risk factors, as well as with increased cardiovascular disease-related mortality in elderly men but not in postmenopausal women, unless they had pre-existing coronary disease 249, 13, 24, 250, 251, 252.
The plasma levels of DHEA were also inversely associated with the progression 253 and prognosis of heart failure 254, at least in men. The exact pathophysiologic background is still more or less unclear. Some preliminary data in patients with type 2 diabetes mellitus suggest that the adrenal androgens may increase the generation of activated protein C, an important anticoagulant protein that protects from acute coronary events 236. Furthermore, DHEA may directly stimulate eNOS phosphorylation/activation in endothelial cells and nitric oxide (NO) production 80, 181, 255, which in turn induces vasodilation, and preserves myocardial perfusion 256. DHEA may also exert anti-inflammatory actions 181, 257, through which it may alleviate endothelial dysfunction, atherogenesis 258, and the acute thrombotic complications of atheroma 181, 257, 259, 260 enhanced by systemic inflammation. The protective effects of DHEA on endothelium were also shown in several in vitro studies in which DHEA increased endothelial proliferation 261 and protected endothelial cells against apoptosis 238, 262. Finally, DHEA can alleviate oxidative stress and inflammation in vascular smooth muscle cells via ERK1/2 and NF-κB signaling pathways, although it has no effect on their phenotype transition 263.
However, other studies have failed to show a significant relation between DHEA or DHEAS and cardiovascular disease. In men for example, myocardial infarction occurrence was not altered by DHEAS levels, and acute myocardial infarctions were seen in patients with either low or high DHEAS levels 264, 265. Similarly in women, lower DHEAS levels in ischemic heart disease patients versus control were observed in some studies, but not in others 246, 266, 267. The reasons that account for the discrepancies among the above studies are not clear. It can be argued that smoking could be a possible confounding variable for both DHEAS levels and cardiovascular disease, as smoking increases DHEA-S levels but also increases the incidence of adverse cardiovascular events 268, 269. The discrepancies among the above studies may also be attributed to population variability; for example, in the study by Mazat et al. 13 the relative risk of an 8-year mortality associated with low DHEAS was 3.4 times higher in males under 70 years compared to older men (odds ratio of 6.5 versus 1.9). Finally, DHEAS was checked just once in some retrospective studies, often several years before the adverse cardiovascular events 270.
Whether exogenously administered DHEA could reduce key elements involved in the generation and progression of the atherosclerotic process was addressed in humans with atherosclerosis and experimental animal models. The human studies have shown a beneficial effect of DHEA on angiographic evidence of atherosclerosis and improvement of vascular endothelial function 261, 243, 271. Several animal studies have also clearly demonstrated the inhibitory effect of orally administered DHEA on atherosclerosis and plaque progression 272, 273 as well as beneficial effects on ischemia–reperfusion injury in the microcirculation 274, 275 and cardiac dysfunction 276, 277. Arterial stiffness, which is also considered a risk factor for cardiovascular disease, significantly improved after DHEA replacement in both elderly men and women 278, 279. Whether the above findings could be translated into DHEA administration in clinical practice for the reduction of cardiovascular disease morbidity and/or mortality is not well documented and supported by current reports. However, since DHEA is a well-tolerated molecule and an inexpensive drug, additional large multi-centric clinical studies could address its role in the prevention and/or management of cardiovascular disease.
DHEA and Cerebrovascular disease
Stroke is the third-leading cause for disability worldwide 280; therefore, early risk stratification for an optimized allocation of health care resources is imperative. The ischemic strokes that account for the great majority of all stroke cases (87 percent) occur as a result of acute obstruction of atherosclerotic blood vessels supplying blood to the brain 281. Considering DHEAS has neuroprotective and antiatherosclerotic properties 282, 283, 284 and its synthesis has been documented in the brain 147, 285, 286, the role of DHEA or DHEAS in acute stroke incidence and outcome was investigated. Interestingly, in women from Nurses’ Health Study, lower DHEAS levels were associated with a greater risk of ischemic stroke 287. In addition, it was suggested that DHEAS levels in the blood may predict the severity and functional outcome of acute strokes 288, 289. Whether the above findings suggest baseline DHEAS levels could alter stroke management in clinical practice or whether DHEA replacement has a therapeutic potential role in stroke management need to be addressed.
DHEA and Pulmonary hypertension
The previously described vasorelaxant properties of DHEA in systemic circulation were also investigated in pulmonary hypertension in animal models and in humans. Several studies have shown that DHEA replacement could effectively prevent and reverse hypoxic pulmonary hypertension, pulmonary arterial remodeling, and right ventricular hypertrophy in rats 290, 291 in a dose-dependent manner 292 and also prevent the age-related frailty induced by hypoxic pulmonary hypertension in older mice 291. The effect of DHEA is selective to the pulmonary circulation since the systemic blood pressure was not altered. It was shown that the beneficial effects of DHEA on pulmonary hypertension were at least partly independent of its conversion to estrogen/testosterone and eNOS activation. Some of the potential molecular mechanism by which DHEA promotes pulmonary artery relaxation appears to involve K+ channel activation, upregulation of soluble guanylate cyclase 293, 294, 295, downregulation of hypoxia inducible factor 1a (HIF-1a) 296, and by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α 297.
DHEA may inhibit and reverse chronic hypoxia-induced pulmonary hypertension in rats. Little is known, however, about the effects of DHEA on the pulmonary circulation in humans. The levels of DHEA or DHEAS in patients with pulmonary hypertension over time have not been determined, but the recent Multi-Ethnic Study of Atherosclerosis (MESA) – Right Ventricle (RV) Study found that higher DHEA levels were associated with increased right ventricle mass and stroke volume in women 298. Another prospective study suggested a strong inverse correlation between natural DHEA/DHEA-S blood levels and the ten-year mortality in old male smokers and ex-smokers 13. Prompted by the experimental findings in the pulmonary circulation, a recent study investigated whether DHEA can improve the clinical and hemodynamic status of patients with pulmonary hypertension associated to chronic obstructive pulmonary disease; eight patients with the disease were treated with DHEA (200mg daily orally) for 3 months. The results were very promising as DHEA treatment significantly improved the pulmonary hemodynamics and the physical performance of the patients, without worsening gas exchange, as do other pharmacological treatments of pulmonary hypertension 299.
Putting together the above evidence, there are experimental data to support the beneficial role of DHEA treatment in models with pulmonary hypertension, but only a few studies supporting its beneficial effect in patients with pulmonary hypertension associated with chronic obstructive pulmonary disease. Further clinical studies would probably clarify its therapeutic role in the management of pulmonary hypertension in clinical practice.
DHEA and Obesity
Animal studies support a beneficial effect of DHEA administration on obesity 300, 301, 302. In humans, two sets of longitudinal analyses of studies with women in menopausal transition showed that elevated DHEAS level is negatively related to body mass index (BMI) 18, 15. On the other hand, baseline analyses by Santoro et al 303 did not find much association between DHEAS and body mass index (BMI), waist-hip ratio, or waist. Childhood obesity is associated with higher levels of DHEAS 304. Similarly, a 2-year, placebo-controlled, randomized, double-blind study involving elderly men and women with low levels of DHEAS, showed no significant effect of DHEA replacement (75 mg per day orally) on body composition measurements 26. Interestingly, a, recent meta-analysis of intervention studies showed that DHEA supplementation in elderly men can induce only a small positive effect on body composition which is strictly dependent on DHEA conversion into its bioactive metabolites such as androgens or estrogens 305. Putting together these results, current data regarding DHEA effect on BMI contradict each other, and its usage in clinical practice for body weight management is not suggested or recommended at the present.
DHEA and Cholesterol levels
In women, the effects of sex steroids on lipid profile differ according to the steroid treatment (estrogen or androgen) and to the route of administration. Thus, oral methyltestosterone lowers high-density lipoprotein (HDL)-cholesterol 306, and oral estrogen increases HDL-cholesterol and triglycerides and lowers low-density lipoprotein (LDL)-cholesterol and total cholesterol 307, 308, while transdermal estradiol and transdermal testosterone have little or no effect on lipids 309. Combined oral estrogen and methyltestosterone is associated with lowering of HDL-cholesterol 310, 311. Considering that DHEA can be converted intracellularly to estrogens and androgens, the effect on the lipid profile could be mixed and may vary between individuals. Most of the recent well-designed studies, addressing this issue report no association or even an adverse association (at least in women) 312, 313 between plasma levels of DHEA 314, 250 or DHEA administration 173, 161, 308, 315, 240 and the lipid profile.
DHEA and Insulin resistance
DHEA may at least theoretically improve endothelial function 261, and ameliorate local/systemic inflammation 122, 123 and oxidative stress 237, 316, 317. These effects in association with DHEAS’s inverse relation with body mass index (BMI) 318, 18, 15, 319 would most probably suggest beneficial effect of DHEA or DHEAS supplement on insulin sensitivity 319. This hypothesis was confirmed by reports from animal studies in which DHEA replacement had a beneficial effect on insulin sensitivity 300, 301. In human studies, however, the results are rather inconsistent. In some studies, the lower levels of DHEA seen with aging have been associated with impaired glucose tolerance, insulin resistance, and diabetes 320, 321, while in another 322 exactly the opposite relation was shown as higher levels of DHEA were associated with impaired glucose tolerance and diabetes mellitus in post-menopausal women. The truth regarding DHEA or DHEAS and insulin resistance and its associated conditions gets even more complicated considering conflicting results from interventional studies with DHEA replacement. Therefore, an ameliorating effect of long-term treatment with DHEA on insulin resistance was described in a group of middle-aged hypo-adrenal women treated with DHEA 323, but also in groups of elderly men 324 and postmenopausal women 323, 324, 325, 326, 327 replaced with DHEA. The DHEA dose used ranged between 25 and 100 mg/day oally and the duration of treatment varied between 3 and 12 months; in one study transdermal DHEA was used 327. Most other interventional studies addressing this issue, failed to demonstrate any significant effect of DHEA on insulin resistance/sensitivity 328, 174, 175, 176, 26, 161 and so did a recent review of the available data regarding use of DHEA in women with adrenal insufficiency 30. Mortola and Yen 116 reported worsening insulin resistance with DHEA replacement in postmenopausal women; in this study however, the number of participants was small (n=6), the duration of treatment short (28 days), and the DHEA dosage supraphysiological (1600 mg/day orally). Putting together the above, the relation between DHEA and carbohydrate metabolism is still uncertain.
Is DHEA safe?
DHEA hasn’t been studied enough to know whether it’s safe to take. DHEA has been studied for a number of potential medical uses, but most research studies have been relatively small and for less than 24 months. In women, taking DHEA supplements for months can increase testosterone levels, which can cause acne and facial hair growth, but a variety of other side effects have been noted. Women might develop masculine features, and men might experience breast tenderness or enlargement, aggression, or testicular wasting, but it depends on the amount being taken.
There isn’t enough information about its long-term effects, but DHEA is being explored for possible uses to treat other medical conditions. In general, extensive research is ongoing and, so far, mostly inconclusive. DHEA can be converted into estrogen, so doctors should advise patients with any hormone-sensitive cancer (such as prostate, breast, or ovarian cancer) not to take DHEA.
Don’t use DHEA if you’re pregnant or breastfeeding.
Consider avoiding use of DHEA if you have high cholesterol or a condition that affects the supply of blood to the heart (ischemic heart disease). DHEA might reduce high-density lipoprotein (HDL), or “good,” cholesterol levels.
Use of DHEA also might worsen psychiatric disorders and increase the risk of mania in people who have mood disorders.
DHEA also might cause oily skin, acne and unwanted, male-pattern hair growth in women (hirsutism).
- When taken by mouth, DHEA is possibly safe when used short-term 329. DHEA has been used safely for up to 2 years, usually in doses of 50 mg daily 329. DHEA is possibly unsafe when used in high doses or long-term 329. Do not use DHEA in doses higher than 50-100 mg daily or for a long period of time. Using higher doses or using it long-term can increase the risk of serious side effects including cancer 329. DHEA is a hormone that can affect how estrogen works in the body. If you have any condition that might be made worse by estrogen, do not use DHEA. Hormone-sensitive conditions such as breast cancer, uterine cancer, ovarian cancer, endometriosis, or uterine fibroids.
- When applied to the skin, DHEA is possibly safe when used appropriately. DHEA cream has been used safely for up to 1 year.
- When applied into the vagina, DHEA is possibly safe when used appropriately. DHEA vaginal inserts have been safely used for up to 3 months.
Pregnancy and breast-feeding
DHEA is possibly unsafe when taken during pregnancy or breast-feeding. It can cause higher than normal levels of a male hormone called androgen. This might be harmful to the baby. Do not use DHEA if you are pregnant or breast-feeding.
Diabetes
DHEA can affect how insulin works in the body. If you have diabetes, monitor your blood sugar carefully if you are taking DHEA.
High cholesterol
DHEA might lower high-density lipoprotein (HDL or “good”) cholesterol, especially in females. If you have high cholesterol or heart disease, talk with your doctor before taking DHEA.
Liver problems
DHEA might make liver problems worse. Do not use DHEA if you have liver problems.
Depression and mood disorders
DHEA might cause excitability, impulsiveness, and irritability in people with mood disorders. If you have a mood disorder, talk to your healthcare provider before taking DHEA.
Polycystic ovary syndrome (PCOS)
Don’t use DHEA if you have PCOS. Taking DHEA might make polycystic ovary syndrome (PCOS) worse.
Interactions with other medications
- Estrogens. DHEA might increase estrogen levels in your body. Taking DHEA along with estrogen might cause too much estrogen in your body and cause symptoms of excess estrogen, such as nausea, headache and insomnia.
- Testosterone. Taking DHEA with testosterone might cause too much testosterone in your body. This might increase the effects and side effects of testosterone. Combining DHEA and testosterone might cause symptoms such as low sperm count and enlarged breasts in men (gynecomastia) and the development of typically male characteristics in women.
- Fulvestrant (Faslodex). Estrogen-sensitive cancers are cancers that are affected by estrogen levels in the body. Fulvestrant is used for estrogen-sensitive cancers. DHEA might increase estrogen in your body and decrease the effects of fulvestrant for treating cancer. Do not take DHEA if you are taking fulvestrant.
- Medications changed by the liver (Cytochrome P450 3A4 (CYP3A4) substrates). Some medications are changed and broken down by the liver. DHEA might change how quickly the liver breaks down these medications. This could change the effects and side effects of these medications.
- Medications for depression (Antidepressant drugs). There is some concern that taking DHEA along with antidepressant drugs might increase the risk for serious side effects. Speak with a healthcare provider before taking DHEA if you are taking an antidepressant.
- Selective serotonin reuptake inhibitors (SSRIs). Use of DHEA with this type of antidepressant might cause manic symptoms.
- Antipsychotics. Use of DHEA with antipsychotics such as clozapine (Clozaril, Versacloz, others) might reduce the drug’s effectiveness.
- Carbamazepine (Tegretol, Carbatrol, others). Use of DHEA with this drug used to treat seizures, nerve pain and bipolar disorder might reduce the drug’s effectiveness.
- Valproic acid. Use of DHEA with this medication used to treat seizures and bipolar disorder might reduce the drug’s effectiveness.
- Medications for estrogen sensitive cancers (Aromatase inhibitors). The body changes DHEA to estrogen in the body. Aromatase inhibitors are used to help lower estrogen levels in the body. Taking DHEA might decrease the effects of aromatase inhibitors.
- Medications that slow blood clotting (Anticoagulant / Antiplatelet drugs). DHEA might slow blood clotting. Taking DHEA along with medications that also slow blood clotting might increase the risk of bruising and bleeding.
- Tamoxifen (Nolvadex). Estrogen-sensitive cancers are cancers that are affected by estrogen levels in the body. Tamoxifen is used to help treat and prevent these types of cancer. DHEA increases estrogen levels in the body and might decrease the effects of tamoxifen. Do not take DHEA if you are taking tamoxifen.
- Triazolam (Halcion). DHEA might decrease how quickly the body breaks down triazolam. Taking DHEA with triazolam might increase the effects and side effects of triazolam, causing excessive sedation and affecting your breathing and heart rate.
- Lithium. Use of DHEA with lithium might reduce the drug’s effectiveness.
- Tuberculosis vaccine. Taking DHEA might reduce the effects of the tuberculosis vaccine. Do not take DHEA if you are receiving a tuberculosis vaccine.
- Herbs and supplements that might slow blood clotting. DHEA might slow blood clotting and increase the risk of bleeding. Taking it with other supplements with similar effects might increase the risk of bleeding in some people. Examples of supplements with this effect include garlic, ginger, ginkgo, nattokinase, and Panax ginseng.
- Licorice. Taking licorice increases levels of DHEA in the body. Taking licorice with DHEA might increase the side effects of DHEA.
DHEA side effects
The major concerns with DHEA revolve around its ability to convert to androgen and estrogen metabolites. Reported androgenic side effects of DHEA are usually mild and might include mild acne, facial hair growth, upset stomach and seborrhea 330. DHEA also might cause oily skin, acne and unwanted, male-pattern hair growth in women (hirsutism). DHEA has also been associated with a pro-atherogenic state with decreased high-density lipoprotein (HDL) or “good,” cholesterol levels 331, 312. It has also been seen that DHEA leads to proliferation of breast cancer cells via stimulation of estrogen receptors 332 Hence, there have been concerns with the use of DHEA in hormone dependent cancers including breast cancer, endometrial cancer, and prostate cancer 333, 334. Currently, there is limited evidence on the long-term safety data and caution needs to exercised.
As long as long-term safety data for DHEA supplementation are lacking, the American Cancer Society advices caution in its use in people who have cancer, especially types of cancer that respond to hormones, such as certain types of breast cancer, prostate cancer, ovarian cancer and endometrial cancer 335. If you have any form of cancer or are at risk of cancer, don’t use DHEA.
Don’t use DHEA if you’re pregnant or breastfeeding.
Consider avoiding use of DHEA if you have high cholesterol or a condition that affects the supply of blood to the heart (ischemic heart disease). DHEA might reduce high-density lipoprotein (HDL), or “good,” cholesterol levels.
Use of DHEA also might worsen psychiatric disorders and increase the risk of mania in people who have mood disorders.
DHEA-sulfate test
Dehydroepiandrosterone sulfate test or DHEAS blood test checks how much DHEA-sulfate (DHEAS) is in your blood. DHEA-sulfate (DHEAS) is a male sex hormone (androgen) that is found in both men and women. Dehydroepiandrosterone Sulfate (DHEAS) is a steroid precursor hormone (prohormone) made by your adrenal glands with weak androgenic effects that is converted into male sex hormones (androgens) and/or female sex hormones (estrogens) in peripheral tissues in your body to exert their effects 1, 2. The DHEA-sulfate test is generally done to check how well your adrenal glands are working and to look into conditions that might cause hormone production to be off.
Although DHEA-sulfate is the most abundant hormone in the body, its exact function is still not known. DHEA-sulfate (DHEAS) plays an important role in making the male sex hormone testosterone and androstenedione and the female sex hormone estrogen. DHEA-sulfate (DHEAS) is also responsible for the onset of sexual differentiation in males and females and the development of secondary male physical characteristics at puberty such as a deep voice and facial hair.
- In men, the DHEA-sulfate (DHEAS) male hormone effect may not be important if testosterone level is normal.
- In women, DHEA contributes to normal libido and sexual satisfaction.
- DHEA may also have effects on the immune system.
DHEA-sulfate (DHEAS) is mostly made in the adrenal glands, two small glands located above your kidneys (Figure 1). They help control heart rate, blood pressure, and other body functions. Smaller amounts of DHEAS are made in a man’s testicles and in a woman’s ovaries. If your DHEAS levels are not normal, it may mean there is a problem with your adrenal glands or sex organs (testicles or ovaries).
The DHEA-sulfate (DHEAS) test is used to determine whether or not your adrenal glands are functioning properly and to detect adrenal tumors or cancers 336. DHEAS blood test also helps determine the cause of masculine (male) physical characteristics in girls and women (virilization) or early puberty in boys (precocious puberty). DHEA-sulfate test can help figure out what’s causing your irregular periods, excessive hair growth (hirsutism), infertility, and a decreased sex drive (low libido) 64. DHEAS test is also used to find out if someone has a growth in their adrenal gland, congenital adrenal hyperplasia, diagnosis of hyperandrogenism (in conjunction with measurements of other sex steroids) or polycystic ovary syndrome (PCOS) or to assess delayed puberty or premature adrenarche. Babies may also need testing if they have genitals that are not clearly male or female in appearance (ambiguous genitalia). Boys may need this test if they have signs of early puberty (precocious puberty). People on long-term glucocorticoid medicine can also use the DHEAS test to check how well their adrenal glands are working or to rule out Cushing’s syndrome. It is an important test for people who think their male sex hormones (androgens) or female sex hormones (estrogens) might be out of order.
A DHEA-sulfate (DHEAS) test is most often used to 337:
- Find out if your adrenal glands are working right
- Diagnose adrenal glands tumors or cancers
- Diagnose disorders of the testicles or ovaries
- Find out the cause of early puberty in boys
- Find out the cause of excess body hair growth (hirsutism) and development of masculine features in women and girls (virilization)
A DHEAS test is often done along with other sex hormone tests. These include testosterone tests for men and estrogen tests for women.
The DHEAS test is ordered along with tests for testosterone and several other male hormones (androgens) to 336:
- Evaluate whether the adrenal glands are working properly
- Distinguish between DHEAS-secreting conditions caused by the adrenal glands and those that originate in the testicles — or rarely, in the ovaries (ovarian tumors)
- Help diagnose tumors in the outer layer (cortex) of the adrenal gland (adrenocortical tumors) and adrenal cancers
- Help diagnose congenital adrenal hyperplasia and enlargement of the adrenal glands (hyperplasia) in adults
In women, DHEAS levels are often measured, along with other hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estrogen, and testosterone, to help diagnose polycystic ovary syndrome (PCOS) and rule out other causes of infertility, lack of menstrual period (amenorrhea), and excess hair on the face and body (hirsutism).
DHEAS levels may be ordered with other hormones to investigate and diagnose the cause of the development of masculine physical characteristics (virilization) in young girls and early (precocious) puberty in young boys.
An initial workup in adults might also include total testosterone and bioavailable testosterone. Depending on results, this may be supplemented with measurements of sex hormone-binding globulin (SHBG) and occasionally other androgenic steroids (eg, 17-hydroxyprogesterone).
What do the DHEA-sulfate test results mean?
A DHEA-sulfate (DHEAS) test is most often used to 337:
- Find out if your adrenal glands are working right
- Diagnose adrenal glands tumors or cancers
- Diagnose disorders of the testicles or ovaries
- Find out the cause of early puberty in boys
- Find out the cause of excess body hair growth (hirsutism) and development of masculine features in women and girls (virilization)
A DHEAS test is often done along with other sex hormone tests. These include testosterone tests for men and estrogen tests for women.
The DHEAS test is ordered along with tests for testosterone and several other male hormones (androgens) to 336:
- Evaluate whether the adrenal glands are working properly
- Distinguish between DHEAS-secreting conditions caused by the adrenal glands and those that originate in the testicles — or rarely, in the ovaries (ovarian tumors)
- Help diagnose tumors in the outer layer (cortex) of the adrenal gland (adrenocortical tumors) and adrenal cancers
- Help diagnose congenital adrenal hyperplasia and enlargement of the adrenal glands (hyperplasia) in adults
In women, DHEAS levels are often measured, along with other hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estrogen, and testosterone, to help diagnose polycystic ovary syndrome (PCOS) and rule out other causes of infertility, lack of menstrual period (amenorrhea), and excess hair on the face and body (hirsutism).
DHEAS levels may be ordered with other hormones to investigate and diagnose the cause of the development of masculine physical characteristics (virilization) in young girls and early (precocious) puberty in young boys.
DHEAS normal range
Normal blood levels of DHEA-sulfate can differ by sex and age. Normal value ranges may vary slightly among different laboratories. Some labs use different measurements or test different specimens. Talk to your health care provider about the meaning of your specific test results.
Normal DHEA-sulfate levels in females
Typical normal DHEA-sulfate ranges for females are 338:
- Ages 18 to 19: 145 to 395 micrograms per deciliter (µg/dL) or 3.92 to 10.66 micromoles per liter (µmol/L)
- Ages 20 to 29: 65 to 380 µg/dL or 1.75 to 10.26 µmol/L
- Ages 30 to 39: 45 to 270 µg/dL or 1.22 to 7.29 µmol/L
- Ages 40 to 49: 32 to 240 µg/dL or 0.86 to 6.48 µmol/L
- Ages 50 to 59: 26 to 200 µg/dL or 0.70 to 5.40 µmol/L
- Ages 60 to 69: 13 to 130 µg/dL or 0.35 to 3.51 µmol/L
- Ages 69 and older: 17 to 90 µg/dL or 0.46 to 2.43 µmol/L
Normal DHEA-sulfate levels in males
Typical normal DHEA-sulfate ranges for males are 338:
- Ages 18 to 19: 108 to 441 µg/dL or 2.92 to 11.91 µmol/L
- Ages 20 to 29: 280 to 640 µg/dL or 7.56 to 17.28 µmol/L
- Ages 30 to 39: 120 to 520 µg/dL or 3.24 to 14.04 µmol/L
- Ages 40 to 49: 95 to 530 µg/dL or 2.56 to 14.31 µmol/L
- Ages 50 to 59: 70 to 310 µg/dL or 1.89 to 8.37 µmol/L
- Ages 60 to 69: 42 to 290 µg/dL or 1.13 to 7.83 µmol/L
- Ages 69 and older: 28 to 175 µg/dL or 0.76 to 4.72 µmol/L
High DHEA-sulfate levels
If your DHEAS blood test results show high levels of DHEA sulfate (DHEAS), it may mean you have one of the following conditions:
- Congenital adrenal hyperplasia, an inherited disorder of the adrenal glands
- A tumor of the adrenal gland. It may be benign (noncancerous) or cancerous.
- Polycystic ovary syndrome (PCOS). PCOS is a common hormone disorder affecting childbearing women. It is one of the leading causes of female infertility. About 25% to 50% of women with PCOS have elevated DHEAS.
- Body changes of a girl in puberty happening earlier than normal.
- Rarely, an ovarian tumor that produces DHEA-sulfate.
A high dehydroepiandrosterone sulfate (DHEAS) blood level may indicate that excess DHEA sulfate (DHEAS) production is causing or contributing to your symptoms. Elevated DHEA-sulfate levels can cause symptoms or signs of hyperandrogenism in women. However, an increased level of DHEAS is not diagnostic of a specific condition. Most mild to moderate elevations in DHEAS levels are idiopathic (unknown cause). It usually indicates the need for further testing to pinpoint the cause of the hormone imbalance. However, pronounced elevations of DHEA-sulfate with levels of 600 micrograms per deciliter (µg/dL) or more may be indicative of androgen-producing adrenal tumors. DHEA sulfate (DHEAS) levels are elevated in more than 90% of patients with adrenal tumors, usually well above 600 mcg/dL. This is particularly true for androgen-secreting adrenal carcinomas (adrenal gland cancer), as they have typically lost the ability to produce down-stream androgens, such as testosterone 339. By contrast, androgen-secreting adrenal adenomas (adrenal benign tumors) may also produce excess testosterone and secrete lesser amounts of DHEAS 339.
In small children, congenital adrenal hyperplasia (CAH) due to 3 beta-hydroxysteroid deficiency is associated with very high levels of DHEA sulfate (DHEAS) production, often 5- to 10-fold elevations. Lesser elevations may be observed in 21-hydroxylase deficiency (the most common form of congenital adrenal hyperplasia) and 11 beta-hydroxylase deficiency. By contrast, steroidogenic acute regulatory protein or 17 alpha-hydroxylase deficiencies are characterized by low DHEA-sulfate levels. Consequently, DHEAS testing should not be used as the primary
tool for congenital adrenal hyperplasia (CAH) diagnosis. Similarly, discovering a high DHEA sulfate (DHEAS) level in an infant or child with symptoms or signs of possible congenital adrenal hyperplasia (CAH) should prompt additional testing, as should the discovery of very high DHEAS levels in an adult. In the latter case, adrenal tumors need to be excluded and additional adrenal steroid profile testing may assist in diagnosing congenital adrenal hyperplasia (CAH). If you have questions about your results, talk to your doctor.
Girls below the age of 7 to 8 and boys before age 8 to 9, who present with early development of pubic hair, or, in boys, penile enlargement, may be suffering from either premature adrenarche or premature puberty or both 339. Measurement of DHEAS and androstenedione, alongside determination of sensitive estradiol,total testosterone and bioavailable testosterone or free testosterone, sex hormone-binding globulin (SHBG), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels will allow correct diagnosis in most cases 339. In premature adrenarche, only the adrenal androgens, chiefly DHEA sulfate (DHEAS), will be above prepubertal levels, whereas early puberty will also show a fall in sex hormone-binding globulin (SHBG) levels and variable elevations of gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and gonadal sex-steroids above the prepuberty reference range.
Many drugs and hormones can result in changes in DHEA sulfate (DHEAS) levels. Whether any of these secondary changes in DHEAS levels are of clinical significance and how they should be related to the established normal reference ranges is unknown 339. In most cases, the drug-induced changes are not large enough to cause diagnostic confusion, but when interpreting mild abnormalities in DHEAS levels, drug and hormone interactions should be taken into account. Examples of drugs that may increase DHEA sulfate (DHEAS) levels include metformin, troglitazone, prolactin, many neuroleptic drugs (by indirect implication)), danazol, calcium channel blockers (eg, diltiazem, amlodipine), and nicotine 339.
Low DHEA-sulfate levels
If your DHEAS blood test results show low levels of DHEA sulfate (DHEAS), it may mean you have one of the following conditions:
- Adrenal gland disorders that produce lower than normal amounts of adrenal hormones, including adrenal insufficiency and Addison disease
- Hypopituitarism, a condition in which the pituitary gland does not make enough pituitary hormones
- Taking glucocorticoid medicines (Cushing’s syndrome)
If you have questions about your results, talk to your doctor.
Many drugs and hormones can result in changes in DHEA sulfate (DHEAS) levels. Whether any of these secondary changes in DHEAS levels are of clinical significance and how they should be related to the established normal reference ranges is unknown 339. In most cases, the drug-induced changes are not large enough to cause diagnostic confusion, but when interpreting mild abnormalities in DHEAS levels, drug and hormone interactions should be taken into account. Examples of drugs and hormones that can reduce DHEA sulfate (DHEAS) levels include: insulin, oral contraceptive drugs,
corticosteroids, central nervous system agents that induce hepatic enzymes (eg, carbamazepine, clomipramine, imipramine, phenytoin), many antilipemic drugs (eg, statins, cholestyramine), dopaminergic drugs (eg, levodopa/dopamine, bromocriptine), fish oil, and vitamin E 339.
DHEA sulfate levels normally decline with age in both men and women. The production of DHEA and DHEAS start increasing in boys and girls around the age of 6 to 8 years as a consequence of the maturation of the zona reticularis of the adrenal cortex and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche 7, 8. DHEA and DHEAS levels rise steadily and peak in the second to third decade of life (serum DHEAS concentration can be as high as 10 μM in young adults) 9, 10, 11, 12. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life 13. In a healthy aged 65- to 81-yr-old population, plasma DHEAS concentration is 2.2 µM in women and 3.3 µM in men 14. However, the interindividual variability across adulthood is substantial, and the normal range of serum DHEA and DHEAS is therefore very wide at each decade of life 12. DHEAS levels in women in their mid 70s are about 77% lower than women in their third decade of life, with age alone explaining about 30% of the variation in DHEAS levels 12. Despite the overall decline in DHEAS with age, levels across the menopausal transition vary according to the transitional phase 15. Between the early and late perimenopause, DHEAS appears to increase on average by 3.95% in most women and then decline to levels seen in the early perimenopause by the late postmenopause 16. Women who do not exhibit this rise in DHEAS across the menopause have lower levels as they enter menopause 15. Overall Chinese women tend to have higher DHEAS levels and African-American women have lower DHEAS levels than Caucasian women 15. To compensate for the decrease in aged people, over-the-counter DHEA sulfate supplements are available and are sometimes promoted as an anti-aging therapy. But there is no reliable evidence to support these anti-aging claims of DHEA supplements. In fact, DHEA supplements may cause serious side effects. If you have questions about DHEA supplements, talk to your health care provider.
References- Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005 Nov;187(2):169-96. https://joe.bioscientifica.com/view/journals/joe/187/2/1870169.xml
- Papadopoulou-Marketou N, Kassi E, Chrousos GP. Adrenal Androgens and Aging. [Updated 2023 Jan 18]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279006
- Powrie YSL, Smith C. Central intracrine DHEA synthesis in ageing-related neuroinflammation and neurodegeneration: therapeutic potential? J Neuroinflammation. 2018 Oct 16;15(1):289. doi: 10.1186/s12974-018-1324-0
- Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol. 2015;145:254–260. doi: 10.1016/j.jsbmb.2014.03.008
- Arbo BD, Bennetti F, Ribeiro MF. Astrocytes as a target for neuroprotection: modulation by progesterone and dehydroepiandrosterone. Prog Neurobiol. 2016;144:27–47. doi: 10.1016/j.pneurobio.2016.03.010
- DHEA: CAN I USE IT? https://www.opss.org/article/dhea-can-i-use-it
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004 Nov;22(4):337-47. doi: 10.1055/s-2004-861550
- Jethwani P, Rastogi A, Shukla R. Dehydroepiandrosterone sulfate supplementation in health and diseases. World J Meta-Anal 2023; 11(4): 102-111 https://www.wjgnet.com/2308-3840/full/v11/i4/102.htm
- Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169–196, 2005. doi: 10.1677/joe.1.06264
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551–555, 1984. doi: 10.1210/jcem-59-3-551
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997 Aug;82(8):2396-402. doi: 10.1210/jcem.82.8.4160
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005 Jul;90(7):3847-53. doi: 10.1210/jc.2005-0212
- Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, Baulieu EE. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):8145-50. doi: 10.1073/pnas.121177998
- Geisler C, Schweitzer L, Müller MJ. Functional correlates of detailed body composition in healthy elderly subjects. J Appl Physiol 124: 182–189, 2018. doi: 10.1152/japplphysiol.00162.2017
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009 Aug;94(8):2945-51. doi: 10.1210/jc.2009-0386
- Susan R. Davis and others, DHEA Replacement for Postmenopausal Women, The Journal of Clinical Endocrinology & Metabolism, Volume 96, Issue 6, 1 June 2011, Pages 1642–1653, https://doi.org/10.1210/jc.2010-2888
- Burns BR. Hormone Therapy: Aging-Related Hormone Replacement Therapy and Supplementation. FP Essent. 2023 Aug;531:22-26.
- Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011 May;18(5):494-8. doi: 10.1097/gme.0b013e3181fb53fc
- Forti P, Maltoni B, Olivelli V, Pirazzoli GL, Ravaglia G, Zoli M. Serum dehydroepiandrosterone sulfate and adverse health outcomes in older men and women. Rejuvenation Res. 2012 Aug;15(4):349-58. doi: 10.1089/rej.2011.1248
- Valenti G, Denti L, Maggio M, Ceda G, Volpato S, Bandinelli S, Ceresini G, Cappola A, Guralnik JM, Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004 May;59(5):466-72. doi: 10.1093/gerona/59.5.m466
- Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Bernardi M, Pratelli L, Pizzoferrato A, Porcu S, Gasbarrini G. Determinants of functional status in healthy Italian nonagenarians and centenarians: a comprehensive functional assessment by the instruments of geriatric practice. J Am Geriatr Soc. 1997 Oct;45(10):1196-202. doi: 10.1111/j.1532-5415.1997.tb03769.x
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004 Apr;16(2):153-7. doi: 10.1007/BF03324545
- Legrain S, Massien C, Lahlou N, Roger M, Debuire B, Diquet B, Chatellier G, Azizi M, Faucounau V, Porchet H, Forette F, Baulieu EE. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000 Sep;85(9):3208-17. doi: 10.1210/jcem.85.9.6805
- Ohlsson C, Labrie F, Barrett-Connor E, Karlsson MK, Ljunggren O, Vandenput L, Mellström D, Tivesten A. Low serum levels of dehydroepiandrosterone sulfate predict all-cause and cardiovascular mortality in elderly Swedish men. J Clin Endocrinol Metab. 2010 Sep;95(9):4406-14. doi: 10.1210/jc.2010-0760
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999 Apr;39(4):327-48. doi: 10.1177/00912709922007903
- Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006 Oct 19;355(16):1647-59. doi: 10.1056/NEJMoa054629
- Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011 Jun;59(6):997-1002. doi: 10.1111/j.1532-5415.2011.03410.x
- Kritz-Silverstein D, von Mühlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008 Jul;56(7):1292-8. doi: 10.1111/j.1532-5415.2008.01768.x
- Panjari M, Bell RJ, Jane F, Wolfe R, Adams J, Morrow C, Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009 Sep;6(9):2579-90. doi: 10.1111/j.1743-6109.2009.01381.x
- Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009 Oct;94(10):3676-81. doi: 10.1210/jc.2009-0672
- Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dubé R, Côté I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009 Sep-Oct;16(5):923-31. doi: 10.1097/gme.0b013e31819e85c6
- van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000 Sep 15;152(6):514-27. doi: 10.1093/aje/152.6.514
- Maninger Nicole, Wolkowitz Owen M., Reus Victor I., Epel Elissa S., Mellon Synthia H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002
- Sunderland Trey, Merril CarlR., Harrington MichaelG., Lawlor BrianA., Molchan SusanE., Martinez Rick, Murphy DennisL. REDUCED PLASMA DEHYDROEPIANDROSTERONE CONCENTRATIONS IN ALZHEIMER’S DISEASE. The Lancet. 1989;334(8662):570. doi: 10.1016/S0140-6736(89)90700-9
- Yanase T, Fukahori M, Taniguchi S, Nishi Y, Sakai Y, Takayanagi R, et al. Serum dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S) in Alzheimer’s disease and in cerebrovascular dementia. Endocr J. 1996;43:119–123. doi: 10.1507/endocrj.43.119
- Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, et al. Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer’s disease and vascular dementia. Eur J Endocrinol. 2000;142:466–471. doi: 10.1530/eje.0.1420466
- Hillen T, Lun A, Reischies FM, Borchelt M, Steinhagen-Thiessen E, Schaub RT. DHEA-S plasma levels and incidence of Alzheimer’s disease. Biol Psychiatry. 2000;47:161–163. doi: 10.1016/S0006-3223(99)00217-6
- Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, et al. Relationships between cortisol, dehydroepiandrosterone sulphate and insulin-like growth factor-I system in dementia. J Endocrinol Invest. 2001;24:139–146. doi: 10.1007/BF03343833
- Genedani S, Rasio G, Cortelli P, Antonelli F, Guidolin D, Galantucci M, et al. Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer’s disease patients and in Parkinson’s disease patients. Neurotox Res. 2004;6:327–332. doi: 10.1007/BF03033443
- Aldred S, Mecocci P. Decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) concentrations in plasma of Alzheimer’s disease (AD) patients. Arch Gerontol Geriatr. 2010;51:e16–e18. doi: 10.1016/j.archger.2009.07.001
- Scheffers CS, Armstrong S, Cantineau AEP, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri‐ or postmenopausal phase. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No.: CD011066. DOI: 10.1002/14651858.CD011066.pub
- NCAA Banned Substances. https://www.ncaa.org/sports/2015/6/10/ncaa-banned-substances.aspx
- The Prohibited List. https://www.wada-ama.org/en/prohibited-list?item-id=5027
- Labrie Fernand, Archer David, Bouchard Céline, Fortier Michel, Cusan Leonello, Gomez José-Luis, Girard Ginette, Baron Mira, Ayotte Normand, Moreau Michèle, Dubé Robert, Côté Isabelle, Labrie Claude, Lavoie Lyne, Berger Louise, Gilbert Lucy, Martel Céline, Balser John. Intravaginal dehydroepiandrosterone (Prasterone), the physiological and a highly efficient treatment of vaginal atrophy. Menopause. 2009;16(5):907–922. doi: 10.1097/gme.0b013e31819e8e2d
- Chen H, Jin Z, Sun C, Santos HO, Kord Varkaneh H. Effects of dehydroepiandrosterone (DHEA) supplementation on cortisol, leptin, adiponectin, and liver enzyme levels: A systematic review and meta-analysis of randomised clinical trials. Int J Clin Pract. 2021 Nov;75(11):e14698. doi: 10.1111/ijcp.14698
- Zhu Y, Qiu L, Jiang F, Găman MA, Abudoraehem OS, Okunade KS, Zhang M. The effect of dehydroepiandrosterone (DHEA) supplementation on estradiol levels in women: A dose-response and meta-analysis of randomized clinical trials. Steroids. 2021 Sep;173:108889. doi: 10.1016/j.steroids.2021.108889
- Krysiak R, Szkróbka W, Okopień B. Impact of dehydroepiandrosterone on thyroid autoimmunity and function in men with autoimmune hypothyroidism. Int J Clin Pharm. 2021 Aug;43(4):998-1005. doi: 10.1007/s11096-020-01207-w
- Wang X, Feng H, Fan D, Zou G, Han Y, Liu L. The influence of dehydroepiandrosterone (DHEA) on fasting plasma glucose, insulin levels and insulin resistance (HOMA-IR) index: A systematic review and dose response meta-analysis of randomized controlled trials. Complement Ther Med. 2020 Dec;55:102583. doi: 10.1016/j.ctim.2020.102583
- Li Y, Ren J, Li N, Liu J, Tan SC, Low TY, Ma Z. A dose-response and meta-analysis of dehydroepiandrosterone (DHEA) supplementation on testosterone levels: perinatal prediction of randomized clinical trials. Exp Gerontol. 2020 Nov;141:111110. doi: 10.1016/j.exger.2020.111110
- Peixoto C, José Grande A, Gomes Carrilho C, Nardi AE, Cardoso A, Barciela Veras A. Dehydroepiandrosterone for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. J Neurosci Res. 2020 Dec;98(12):2510-2528. doi: 10.1002/jnr.24721
- Qin Y, O Santos H, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020 Aug 28;30(9):1465-1475. doi: 10.1016/j.numecd.2020.05.015
- Sandoughi M, Kaykhaei MA, Langarizadeh E, Dashipour A. Effects of dehydroepiandrosterone on quality of life in premenopausal women with rheumatoid arthritis: A preliminary randomized clinical trial. Int J Rheum Dis. 2020 Dec;23(12):1692-1697. doi: 10.1111/1756-185X.13975
- Lin H, Li L, Wang Q, Wang Y, Wang J, Long X. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA supplementation of bone mineral density in healthy adults. Gynecol Endocrinol. 2019 Nov;35(11):924-931. doi: 10.1080/09513590.2019.1616175
- Chen SN, Tsui KH, Wang PH, Chern CU, Wen ZH, Lin LT. Dehydroepiandrosterone Supplementation Improves the Outcomes of in vitro Fertilization Cycles in Older Patients With Diminished Ovarian Reserve. Front Endocrinol (Lausanne). 2019 Nov 15;10:800. doi: 10.3389/fendo.2019.00800
- Gravisse N, Vibarel-Rebot N, Labsy Z, Do MC, Gagey O, Dubourg C, Audran M, Collomp K. Short-term Dehydroepiandrosterone Intake and Supramaximal Exercise in Young Recreationally-trained Women. Int J Sports Med. 2018 Sep;39(9):712-719. doi: 10.1055/a-0631-3008
- Chern CU, Tsui KH, Vitale SG, Chen SN, Wang PH, Cianci A, Tsai HW, Wen ZH, Lin LT. Dehydroepiandrosterone (DHEA) supplementation improves in vitro fertilization outcomes of poor ovarian responders, especially in women with low serum concentration of DHEA-S: a retrospective cohort study. Reprod Biol Endocrinol. 2018 Sep 17;16(1):90. doi: 10.1186/s12958-018-0409-z
- HEALTH PRODUCTS FOR SENIORS. “Anti-Aging” Products Pose Potential for Physical and Economic Harm. September 2001. https://ods.od.nih.gov/pubs/gao-01-1129.pdf
- Dietary Supplements for Exercise and Athletic Performance. https://ods.od.nih.gov/factsheets/ExerciseAndAthleticPerformance-HealthProfessional
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009 Apr;94(4):1059-67. doi: 10.1210/jc.2009-0032
- Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010 Jun;66(2):172-9. doi: 10.1016/j.maturitas.2009.12.017
- Labrie F, Bélanger A, Bélanger P, Bérubé R, Martel C, Cusan L, Gomez J, Candas B, Chaussade V, Castiel I, Deloche C, Leclaire J. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol. 2007 Feb;103(2):178-88. doi: 10.1016/j.jsbmb.2006.09.034
- Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008 May;110(1-2):1-9. doi: 10.1016/j.jsbmb.2008.02.003
- Marwah A, Marwah P, Lardy H. Ergosteroids. VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: a liquid chromatographic-mass spectrometric study. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Feb 15;767(2):285-99. doi: 10.1016/s1570-0232(01)00570-0
- Androgens: Androstenedione (AD), DHEA- S (Dehydroepiandrosterone sulphate), DHEA (dehydroepiandrosterone). https://labpedia.net/androgens-androstenedione-ad-dhea-s-dehydroepiandrosterone-sulphate-dhea-dehydroepiandrosterone
- Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. J Mol Endocrinol. 2016 Apr;56(3):R139-55. doi: 10.1530/JME-16-0013
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551-5. doi: 10.1210/jcem-59-3-551
- Kalimi M, Regelson M 1990 The biological role of dehydroepiandrosterone (DHEA). New York: Walter de Gryter
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986 May;15(2):213-28. doi: 10.1016/s0300-595x(86)80021-4
- Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007 Aug;92(8):3040-3. doi: 10.1210/jc.2007-0581
- Rosenfeld RS, Rosenberg BJ, Hellman L. Direct analysis of dehydroisoandrosterone in plasma. Steroids. 1975 Jun;25(6):799-805. doi: 10.1016/0039-128x(75)90044-6
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981 Jul;53(1):58-68. doi: 10.1210/jcem-53-1-58
- Filaire E, Duché P, Lac G. Effects of amount of training on the saliva concentrations of cortisol, dehydroepiandrosterone and on the dehydroepiandrosterone: cortisol concentration ratio in women over 16 weeks of training. Eur J Appl Physiol Occup Physiol. 1998 Oct;78(5):466-71. doi: 10.1007/s004210050447
- Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19-69 years. J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):B158-65. doi: 10.1093/gerona/57.4.b158
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003 Jan-Feb;38(1-2):35-46. doi: 10.1016/s0531-5565(02)00146-8
- Roberts E. The importance of being dehydroepiandrosterone sulfate (in the blood of primates): a longer and healthier life? Biochem Pharmacol. 1999 Feb 15;57(4):329-46. doi: 10.1016/s0006-2952(98)00246-9
- Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005 Nov;146(11):4568-76. doi: 10.1210/en.2005-0368
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998 Nov;23(8):963-87. doi: 10.1016/s0306-4530(98)00071-7
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009 Jan;30(1):65-91. doi: 10.1016/j.yfrne.2008.11.002
- Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem. 2002 Jun 14;277(24):21379-88. doi: 10.1074/jbc.M200491200
- Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids. 2004 Apr;69(4):279-89. doi: 10.1016/j.steroids.2004.02.004
- Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology. 2008 Mar;149(3):889-98. doi: 10.1210/en.2007-1125
- Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003 Apr;24(2):152-82. doi: 10.1210/er.2001-0031
- Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994 Feb;78(2):428-32. doi: 10.1210/jcem.78.2.8106632
- Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005 Mar;12(2):210-5. doi: 10.1097/00042192-200512020-00016
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994 Apr;100(1-2):51-4. doi: 10.1016/0303-7207(94)90278-x
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000 Oct;15(10):2129-32. doi: 10.1093/humrep/15.10.2129
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010 Oct;25(10):2496-500. doi: 10.1093/humrep/deq220
- Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. J Gynecol Obstet Hum Reprod. 2017 Jan;46(1):1-7. doi: 10.1016/j.jgyn.2016.01.002
- Schwarze JE, Canales J, Crosby J, Ortega-Hrepich C, Villa S, Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: A systematic review and meta-analysis. JBRA Assist Reprod. 2018 Nov 1;22(4):369-374. doi: 10.5935/1518-0557.20180046
- Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016 Mar;23(3):243-56. doi: 10.1097/GME.0000000000000571
- FDA approves Intrarosa for postmenopausal women experiencing pain during sex. https://www.fda.gov/news-events/press-announcements/fda-approves-intrarosa-postmenopausal-women-experiencing-pain-during-sex
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020 Sep;27(9):976-992. doi: 10.1097/GME.0000000000001609
- Nijland E, Davis S, Laan E, Schultz WW. Female sexual satisfaction and pharmaceutical intervention: a critical review of the drug intervention studies in female sexual dysfunction. J Sex Med. 2006 Sep;3(5):763-777. doi: 10.1111/j.1743-6109.2006.00285.x
- Hayes RD, Dennerstein L, Bennett CM, Koochaki PE, Leiblum SR, Graziottin A. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007 Jan;87(1):107-12. doi: 10.1016/j.fertnstert.2006.05.071
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000 Sep 7;343(10):682-8. doi: 10.1056/NEJM200009073431002
- Genazzani AD, Lanzoni C, Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging. 2007;24(3):173-85. doi: 10.2165/00002512-200724030-00001
- Nappi RE, Polatti F. The use of estrogen therapy in women’s sexual functioning (CME). J Sex Med. 2009 Mar;6(3):603-16; quiz 618-9. doi: 10.1111/j.1743-6109.2008.01198.x
- Bachmann GA, Leiblum SR. The impact of hormones on menopausal sexuality: a literature review. Menopause. 2004 Jan-Feb;11(1):120-30. doi: 10.1097/01.GME.0000075502.60230.28
- Alexander JL, Kotz K, Dennerstein L, Kutner SJ, Wallen K, Notelovitz M. The effects of postmenopausal hormone therapies on female sexual functioning: a review of double-blind, randomized controlled trials. Menopause. 2004 Nov-Dec;11(6 Pt 2):749-65. doi: 10.1097/01.gme.0000142887.31811.97
- Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003 Jan;188(1):286-93. doi: 10.1067/mob.2003.117
- Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003 Sep-Oct;10(5):390-8. doi: 10.1097/01.GME.0000060256.03945.20
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005 Jul 25;165(14):1582-9. doi: 10.1001/archinte.165.14.1582
- Davis SR, van der Mooren MJ, van Lunsen RH, Lopes P, Ribot C, Rees M, Moufarege A, Rodenberg C, Buch A, Purdie DW. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006 May-Jun;13(3):387-96. doi: 10.1097/01.gme.0000179049.08371.c7. Erratum in: Menopause. 2006 Sep-Oct;13(5):850. Ribot, Jean [corrected to Ribot, Claude].
- Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005 May;105(5 Pt 1):944-52. doi: 10.1097/01.AOG.0000158103.27672.0d
- Davis S, Papalia MA, Norman RJ, O’Neill S, Redelman M, Williamson M, Stuckey BG, Wlodarczyk J, Gard’ner K, Humberstone A. Safety and efficacy of a testosterone metered-dose transdermal spray for treating decreased sexual satisfaction in premenopausal women: a randomized trial. Ann Intern Med. 2008 Apr 15;148(8):569-77. doi: 10.7326/0003-4819-148-8-200804150-00001
- Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005 Jul 6;294(1):91-6. doi: 10.1001/jama.294.1.91
- Panjari M, Davis SR. DHEA therapy for women: effect on sexual function and wellbeing. Hum Reprod Update. 2007 May-Jun;13(3):239-48. doi: 10.1093/humupd/dml055
- Avis NE, Brockwell S, Randolph JF Jr, Shen S, Cain VS, Ory M, Greendale GA. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009 May-Jun;16(3):442-52. doi: 10.1097/gme.0b013e3181948dd0
- Labrie F, Cusan L, Gomez JL, Côté I, Bérubé R, Bélanger P, Martel C, Labrie C. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008 Sep;111(3-5):178-94. doi: 10.1016/j.jsbmb.2008.06.003. Erratum in: J Steroid Biochem Mol Biol. 2008 Nov;112(1-3):169.
- Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dubé R, Côté I, Labrie C, Lavoie L, Berger L, Martel C, Balser J. High internal consistency and efficacy of intravaginal DHEA for vaginal atrophy. Gynecol Endocrinol. 2010 Jul;26(7):524-32. doi: 10.3109/09513590903511547
- Ibe C, Simon JA. Vulvovaginal atrophy: current and future therapies (CME). J Sex Med. 2010 Mar;7(3):1042-50; quiz 1051. doi: 10.1111/j.1743-6109.2009.01692.x
- Reiter WJ, Pycha A, Schatzl G, Pokorny A, Gruber DM, Huber JC, Marberger M. Dehydroepiandrosterone in the treatment of erectile dysfunction: a prospective, double-blind, randomized, placebo-controlled study. Urology. 1999 Mar;53(3):590-4; discussion 594-5. doi: 10.1016/s0090-4295(98)00571-8
- Scheffers CS, Armstrong S, Cantineau AE, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015 Jan 22;1:CD011066. doi: 10.1002/14651858.CD011066.pub2
- Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014 Oct;99(10):3489-510. doi: 10.1210/jc.2014-2260
- Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001 Oct;86(10):4686-92. doi: 10.1210/jcem.86.10.7974
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999 Sep 30;341(14):1013-20. doi: 10.1056/NEJM199909303411401
- Løvås K, Gebre-Medhin G, Trovik TS, Fougner KJ, Uhlving S, Nedrebø BG, Myking OL, Kämpe O, Husebye ES. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003 Mar;88(3):1112-8. doi: 10.1210/jc.2002-020769
- Binder G, Weber S, Ehrismann M, Zaiser N, Meisner C, Ranke MB, Maier L, Wudy SA, Hartmann MF, Heinrich U, Bettendorf M, Doerr HG, Pfaeffle RW, Keller E; South German Working Group for Pediatric Endocrinology. Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab. 2009 Apr;94(4):1182-90. doi: 10.1210/jc.2008-1982
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89. doi: 10.1210/jc.2015-1710
- Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002 Nov;87(11):4935-41. doi: 10.1210/jc.2002-020545
- Lin J, Kao TW, Cheng YC, Fan KC, Huang YC, Liu CW. Dehydroepiandrosterone status and efficacy of dehydroepiandrosterone supplementation for bone health in anorexia nervosa: A systematic review and meta-analysis. Int J Eat Disord. 2022 Jun;55(6):733-746. doi: 10.1002/eat.23714
- Straub RH, Konecna L, Hrach S, Rothe G, Kreutz M, Schölmerich J, Falk W, Lang B. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab. 1998 Jun;83(6):2012-7. doi: 10.1210/jcem.83.6.4876
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998 Dec 4;273(49):32833-41. doi: 10.1074/jbc.273.49.32833
- Straub RH, Vogl D, Gross V, Lang B, Schölmerich J, Andus T. Association of humoral markers of inflammation and dehydroepiandrosterone sulfate or cortisol serum levels in patients with chronic inflammatory bowel disease. Am J Gastroenterol. 1998 Nov;93(11):2197-202. doi: 10.1111/j.1572-0241.1998.00535.x
- de la Torre B, Hedman M, Befrits R. Blood and tissue dehydroepiandrosterone sulphate levels and their relationship to chronic inflammatory bowel disease. Clin Exp Rheumatol. 1998 Sep-Oct;16(5):579-82.
- Andus T, Klebl F, Rogler G, Bregenzer N, Schölmerich J, Straub RH. Patients with refractory Crohn’s disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14. doi: 10.1046/j.1365-2036.2003.01433.x
- Prall SP, Muehlenbein MP. DHEA Modulates Immune Function: A Review of Evidence. Vitam Horm. 2018;108:125-144. doi: 10.1016/bs.vh.2018.01.023
- Nilsson E, de la Torre B, Hedman M, Goobar J, Thörner A. Blood dehydroepiandrosterone sulphate (DHEAS) levels in polymyalgia rheumatica/giant cell arteritis and primary fibromyalgia. Clin Exp Rheumatol. 1994 Jul-Aug;12(4):415-7.
- Durcan L, Petri M. Immunomodulators in SLE: Clinical evidence and immunologic actions. J Autoimmun. 2016 Nov;74:73-84. doi: 10.1016/j.jaut.2016.06.010
- Vernerova L, Mravcova M, Paulikova L, Vlcek M, Marko A, Meskova M, Penesova A, Rovensky J, Wendl J, Raslova K, Vohnout B, Jochmanova I, Lazurova I, Killinger Z, Steiner G, Smolen J, Imrich R. Contribution of Genetic Factors to Lower DHEAS in Patients with Rheumatoid Arthritis. Cell Mol Neurobiol. 2018 Jan;38(1):379-383. doi: 10.1007/s10571-017-0522-0
- Nordmark G, Bengtsson C, Larsson A, Karlsson FA, Sturfelt G, Rönnblom L. Effects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosus. Autoimmunity. 2005 Nov;38(7):531-40. doi: 10.1080/08916930500285550
- Virkki LM, Porola P, Forsblad-d’Elia H, Valtysdottir S, Solovieva SA, Konttinen YT. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren’s syndrome. Arthritis Care Res (Hoboken). 2010 Jan 15;62(1):118-24. doi: 10.1002/acr.20022
- Hartkamp A, Geenen R, Godaert GL, Bootsma H, Kruize AA, Bijlsma JW, Derksen RH. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: a randomised controlled trial. Ann Rheum Dis. 2008 Jan;67(1):91-7. doi: 10.1136/ard.2007.071563
- Shukla R, Ganeshani M, Agarwal M, Jangir R, Kandel G, Sankanagoudar S, Srivastava S. Dehydroepiandrostenedione sulphate (DHEAS) levels predict high risk of rheumatoid arthritis (RA) in subclinical hypothyroidism. PLoS One. 2021 Feb 16;16(2):e0246195. doi: 10.1371/journal.pone.0246195
- van Vollenhoven RF, Engleman EG, McGuire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1994 Sep;37(9):1305-10. doi: 10.1002/art.1780370906
- Chang DM, Lan JL, Lin HY, Luo SF. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7. doi: 10.1002/art.10615
- van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. 1999;8(3):181-7. doi: 10.1191/096120399678847588
- Wilder RL. Adrenal and gonadal steroid hormone deficiency in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1996 Mar;44:10-2.
- Kanik KS, Chrousos GP, Schumacher HR, Crane ML, Yarboro CH, Wilder RL. Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab. 2000 Apr;85(4):1461-6. doi: 10.1210/jcem.85.4.6534
- Yukioka M, Komatsubara Y, Yukioka K, Toyosaki-Maeda T, Yonenobu K, Ochi T. Adrenocorticotropic hormone and dehydroepiandrosterone sulfate levels of rheumatoid arthritis patients treated with glucocorticoids. Mod Rheumatol. 2006;16(1):30-5. doi: 10.1007/s10165-005-0453-3
- Danenberg HD, Alpert G, Lustig S, Ben-Nathan D. Dehydroepiandrosterone protects mice from endotoxin toxicity and reduces tumor necrosis factor production. Antimicrob Agents Chemother. 1992 Oct;36(10):2275-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC245489/pdf/aac00044-0231.pdf
- Keller ET, Chang C, Ershler WB. Inhibition of NFkappaB activity through maintenance of IkappaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996 Oct 18;271(42):26267-75. doi: 10.1074/jbc.271.42.26267
- Williams PJ, Jones RH, Rademacher TW. Reduction in the incidence and severity of collagen-induced arthritis in DBA/1 mice, using exogenous dehydroepiandrosterone. Arthritis Rheum. 1997 May;40(5):907-11. doi: 10.1002/art.1780400519
- Röntzsch A, Thoss K, Petrow PK, Henzgen S, Bräuer R. Amelioration of murine antigen-induced arthritis by dehydroepiandrosterone (DHEA). Inflamm Res. 2004 May;53(5):189-98. doi: 10.1007/s00011-003-1244-y
- Giltay EJ, van Schaardenburg D, Gooren LJ, Dijkmans BA. Dehydroepiandrosterone sulfate in patients with rheumatoid arthritis. Ann N Y Acad Sci. 1999 Jun 22;876:152-4. doi: 10.1111/j.1749-6632.1999.tb07633.x
- Cutolo M. Sex hormone adjuvant therapy in rheumatoid arthritis. Rheum Dis Clin North Am. 2000 Nov;26(4):881-95. doi: 10.1016/s0889-857x(05)70174-5
- Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R. Immunological pathogenesis of main age-related diseases and frailty: Role of immunosenescence. European Geriatric Medicine. 2010;1(2):112–121.
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharrière O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4279-84. doi: 10.1073/pnas.97.8.4279
- Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, Allen S, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999 May;84(5):1527-33. doi: 10.1210/jcem.84.5.5672
- Society, A.G., Society, G., Of, A.A. and On Falls Prevention, O.S.P. (2001), Guideline for the Prevention of Falls in Older Persons. Journal of the American Geriatrics Society, 49: 664-672. https://doi.org/10.1046/j.1532-5415.2001.49115.x
- Kostka T, Arsac LM, Patricot MC, Berthouze SE, Lacour JR, Bonnefoy M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol. 2000 May;82(1-2):83-90. doi: 10.1007/s004210050655
- Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, Finch C, Schneider E, Cotman C, McClearn G, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993 Oct;46(10):1129-40. doi: 10.1016/0895-4356(93)90112-e
- Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998 Fall;6(4):277-84. https://doi.org/10.1097/00019442-199800640-00002
- Abbasi A, Duthie EH Jr, Sheldahl L, Wilson C, Sasse E, Rudman I, Mattson DE. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc. 1998 Mar;46(3):263-73. doi: 10.1111/j.1532-5415.1998.tb01036.x
- Clarke BL, Ebeling PR, Jones JD, Wahner HW, O’Fallon WM, Riggs BL, Fitzpatrick LA. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif Tissue Int. 2002 Mar;70(3):137-45. doi: 10.1007/s00223-001-1072-4
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporos Int. 2008 Sep;19(9):1307-14. doi: 10.1007/s00198-008-0573-7
- Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009 Jul;38(4):401-6. doi: 10.1093/ageing/afp015
- Hasselhorn HM, Theorell T, Vingård E; Musculoskeletal Intervention Center (MUSIC)-Norrtälje Study Group. Endocrine and immunologic parameters indicative of 6-month prognosis after the onset of low back pain or neck/shoulder pain. Spine (Phila Pa 1976). 2001 Feb 1;26(3):E24-9. doi: 10.1097/00007632-200102010-00005
- Schell E, Theorell T, Hasson D, Arnetz B, Saraste H. Stress biomarkers’ associations to pain in the neck, shoulder and back in healthy media workers: 12-month prospective follow-up. Eur Spine J. 2008 Mar;17(3):393-405. doi: 10.1007/s00586-007-0554-0
- Kaiman DS, Colker CM, Swain MA, Torina GC, Shi Q. A randomized, double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy overweight adults. Current Therapeutic Research. 2000;61(7):435–442.
- Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998 Oct;49(4):421-32. doi: 10.1046/j.1365-2265.1998.00507.x
- Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf). 2000 Nov;53(5):561-8. doi: 10.1046/j.1365-2265.2000.01131.x
- Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010 Sep;58(9):1707-14. doi: 10.1111/j.1532-5415.2010.03019.x
- Weiss EP, Shah K, Fontana L, Lambert CP, Holloszy JO, Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. 2009 May;89(5):1459-67. doi: 10.3945/ajcn.2008.27265
- von Mühlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008 May;19(5):699-707. doi: 10.1007/s00198-007-0520-z
- Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008 Dec;93(12):4767-73. doi: 10.1210/jc.2007-2614
- Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006 Aug;91(8):2986-93. doi: 10.1210/jc.2005-2484
- Labrie F, Diamond P, Cusan L, Gomez JL, Bélanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997 Oct;82(10):3498-505. doi: 10.1210/jcem.82.10.4306
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994 Jun;78(6):1360-7. doi: 10.1210/jcem.78.6.7515387. Erratum in: J Clin Endocrinol Metab 1995 Sep;80(9):2799.
- Yen SS, Morales AJ, Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects. Ann N Y Acad Sci. 1995 Dec 29;774:128-42. doi: 10.1111/j.1749-6632.1995.tb17377.x
- Nawata H, Tanaka S, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M. Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):165-74. doi: 10.1016/0960-0760(95)00031-t
- Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol. 2007 Apr;38(4):467-79. doi: 10.1677/jme.1.02173
- Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003 Sep;6(3):151-6.
- Callies F, Fassnacht M, van Vlijmen JC, Koehler I, Huebler D, Seibel MJ, Arlt W, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab. 2001 May;86(5):1968-72. doi: 10.1210/jcem.86.5.7483
- Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998 Jul;70(1):107-10. doi: 10.1016/s0015-0282(98)00121-6
- Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008 Feb;93(2):534-8. doi: 10.1210/jc.2007-1027
- Percheron G, Hogrel JY, Denot-Ledunois S, Fayet G, Forette F, Baulieu EE, Fardeau M, Marini JF; Double-blind placebo-controlled trial. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003 Mar 24;163(6):720-7. doi: 10.1001/archinte.163.6.720
- Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998 Sep;51(3):728-33. doi: 10.1212/wnl.51.3.728
- Lu SF, Mo Q, Hu S, Garippa C, Simon NG. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J Neurobiol. 2003 Nov;57(2):163-71. doi: 10.1002/neu.10260
- Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3202-7. doi: 10.1073/pnas.0307325101
- Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)–a precursor steroid or an active hormone in human physiology. J Sex Med. 2011 Nov;8(11):2960-82; quiz 2983. doi: 10.1111/j.1743-6109.2011.02523.x
- Hildreth KL, Gozansky WS, Jankowski CM, Grigsby J, Wolfe P, Kohrt WM. Association of serum dehydroepiandrosterone sulfate and cognition in older adults: sex steroid, inflammatory, and metabolic mechanisms. Neuropsychology. 2013 May;27(3):356-363. doi: 10.1037/a0032230
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008 Mar;93(3):801-8. doi: 10.1210/jc.2007-2128
- Valenti G, Ferrucci L, Lauretani F, Ceresini G, Bandinelli S, Luci M, Ceda G, Maggio M, Schwartz RS. Dehydroepiandrosterone sulfate and cognitive function in the elderly: The InCHIANTI Study. J Endocrinol Invest. 2009 Oct;32(9):766-72. doi: 10.1007/BF03346534
- Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab. 2002 Nov;87(11):5138-43. doi: 10.1210/jc.2002-020878
- Bélanger N, Grégoire L, Bédard PJ, Di Paolo T. DHEA improves symptomatic treatment of moderately and severely impaired MPTP monkeys. Neurobiol Aging. 2006 Nov;27(11):1684-93. doi: 10.1016/j.neurobiolaging.2005.09.028
- van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001 Aug;26(6):591-612. doi: 10.1016/s0306-4530(01)00014-2
- Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990 Sep;71(3):696-704. doi: 10.1210/jcem-71-3-696
- Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006 Oct 18;2006(4):CD006221. doi: 10.1002/14651858.CD006221
- Bielohuby M, Roemmler J, Manolopoulou J, Johnsen I, Sawitzky M, Schopohl J, Reincke M, Wolf E, Hoeflich A, Bidlingmaier M. Chronic growth hormone excess is associated with increased aldosterone: a study in patients with acromegaly and in growth hormone transgenic mice. Exp Biol Med (Maywood). 2009 Aug;234(8):1002-9. doi: 10.3181/0901-RM-34
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998 Aug;23(6):617-29. doi: 10.1016/s0306-4530(98)00032-8
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999 Nov;30(3):264-88. doi: 10.1016/s0165-0173(99)00021-1
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, De Souza E, Sadowsky C, Roberts E; DHEA-Alzheimer’s Disease Collaborative Research. DHEA treatment of Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Neurology. 2003 Apr 8;60(7):1071-6. doi: 10.1212/01.wnl.0000052994.54660.58
- Yamada S, Akishita M, Fukai S, Ogawa S, Yamaguchi K, Matsuyama J, Kozaki K, Toba K, Ouchi Y. Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatr Gerontol Int. 2010 Oct;10(4):280-7. doi: 10.1111/j.1447-0594.2010.00625.x
- Parsons TD, Kratz KM, Thompson E, Stanczyk FZ, Buckwalter JG. Dhea supplementation and cognition in postmenopausal women. Int J Neurosci. 2006 Feb;116(2):141-55. doi: 10.1080/00207450500341506
- Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000 Jan 15;47(2):144-50. doi: 10.1016/s0006-3223(99)00099-2
- Steffens DC. A multiplicity of approaches to characterize geriatric depression and its outcomes. Curr Opin Psychiatry. 2009 Nov;22(6):522-6. doi: 10.1097/YCO.0b013e32832fcd93
- Robichaud M, Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol. 2004 Jul;182(1):11-21. doi: 10.1677/joe.0.1820011
- Kilic FS, Kulluk D, Musmul A. Effects of dehydroepiandrosterone in amphetamine-induced schizophrenia models in mice. Neurosciences (Riyadh). 2014 Apr;19(2):100-5.
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996 Mar;26(2):245-56. doi: 10.1017/s0033291700034644
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000 Nov 15;48(10):989-95. doi: 10.1016/s0006-3223(00)00955-0
- Barrett-Connor E, von Mühlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999 Jun;47(6):685-91. doi: 10.1111/j.1532-5415.1999.tb01590.x
- Morrison MF, Freeman EW, Lin H, Sammel MD. Higher DHEA-S (dehydroepiandrosterone sulfate) levels are associated with depressive symptoms during the menopausal transition: results from the PENN Ovarian Aging Study. Arch Womens Ment Health. 2011 Oct;14(5):375-82. doi: 10.1007/s00737-011-0231-5
- Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm (Vienna). 1998;105(4-5):537-42. doi: 10.1007/s007020050077
- Assies J, Visser I, Nicolson NA, Eggelte TA, Wekking EM, Huyser J, Lieverse R, Schene AH. Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findings. Psychiatry Res. 2004 Sep 30;128(2):117-22. doi: 10.1016/j.psychres.2004.05.016
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF 3rd, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry. 2001 Nov 15;50(10):767-74. doi: 10.1016/s0006-3223(01)01198-2
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005 Feb;62(2):154-62. doi: 10.1001/archpsyc.62.2.154
- Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997 Feb 1;41(3):311-8. doi: 10.1016/s0006-3223(96)00043-1
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999 Apr;156(4):646-9. doi: 10.1176/ajp.156.4.646
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003 Feb;60(2):133-41. doi: 10.1001/archpsyc.60.2.133
- Hough CM, Lindqvist D, Epel ES, Denis MS, Reus VI, Bersani FS, Rosser R, Mahan L, Burke HM, Wolkowitz OM, Mellon SH. Higher serum DHEA concentrations before and after SSRI treatment are associated with remission of major depression. Psychoneuroendocrinology. 2017 Mar;77:122-130. doi: 10.1016/j.psyneuen.2016.11.035
- Vuksan-Ćusa B, Šagud M, Radoš I. The role of dehydroepiandrosterone (DHEA) in schizophrenia. Psychiatr Danub. 2016 Mar;28(1):30-3.
- Misiak B, Piotrowski P, Chęć M, Samochowiec J. Cortisol and dehydroepiandrosterone sulfate in patients with schizophrenia spectrum disorders with respect to cognitive performance. Compr Psychoneuroendocrinol. 2021 Mar 3;6:100041. doi: 10.1016/j.cpnec.2021.100041
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982 Sep-Oct;2(5):359-68. doi: 10.1161/01.atv.2.5.359
- Kivimäki M, Nyberg ST, Batty GD, et al. IPD-Work Consortium. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012 Oct 27;380(9852):1491-7. doi: 10.1016/S0140-6736(12)60994-5
- Marmot M. Psychosocial factors and cardiovascular disease: epidemiological approaches. Eur Heart J. 1988 Jun;9(6):690-7. doi: 10.1093/oxfordjournals.eurheartj.a062569
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17312-5. doi: 10.1073/pnas.0407162101
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010 Apr;27(4):327-38. doi: 10.1002/da.20686. Erratum in: Depress Anxiety. 2010 Jul;27(7):693.
- Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. Neuroendocrine and immunological correlates of chronic stress in ‘strictly healthy’ populations. Neuroimmunomodulation. 2010;17(1):9-18. doi: 10.1159/000243080
- Izawa S, Saito K, Shirotsuki K, Sugaya N, Nomura S. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: a study of a two-week teaching practice. Psychoneuroendocrinology. 2012 Jun;37(6):852-8. doi: 10.1016/j.psyneuen.2011.10.001
- Brzoza Z, Kasperska-Zajac A, Badura-Brzoza K, Matysiakiewicz J, Hese RT, Rogala B. Decline in dehydroepiandrosterone sulfate observed in chronic urticaria is associated with psychological distress. Psychosom Med. 2008 Jul;70(6):723-8. doi: 10.1097/PSY.0b013e31817bcc8d
- Lac G, Dutheil F, Brousse G, Triboulet-Kelly C, Chamoux A. Saliva DHEAS changes in patients suffering from psychopathological disorders arising from bullying at work. Brain Cogn. 2012 Nov;80(2):277-81. doi: 10.1016/j.bandc.2012.07.007
- Du CL, Lin MC, Lu L, Tai JJ. Correlation of Occupational Stress Index with 24-hour Urine Cortisol and Serum DHEA Sulfate among City Bus Drivers: A Cross-sectional Study. Saf Health Work. 2011 Jun;2(2):169-75. doi: 10.5491/SHAW.2011.2.2.169
- Kim MS, Lee YJ, Ahn RS. Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Med J. 2010 Mar;51(2):212-8. doi: 10.3349/ymj.2010.51.2.212
- Lennartsson AK, Theorell T, Rockwood AL, Kushnir MM, Jonsdottir IH. Perceived stress at work is associated with lower levels of DHEA-S. PLoS One. 2013 Aug 28;8(8):e72460. doi: 10.1371/journal.pone.0072460
- Lee MS, Yang JW, Ko YH, Han C, Kim SH, Lee MS, Joe SH, Jung IK. Effects of methylphenidate and bupropion on DHEA-S and cortisol plasma levels in attention-deficit hyperactivity disorder. Child Psychiatry Hum Dev. 2008 Jun;39(2):201-9. doi: 10.1007/s10578-007-0081-6
- Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, Shepherd MD, Seibel JA; AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012 Mar-Apr;18 Suppl 1:1-78. doi: 10.4158/ep.18.s1.1
- Phillips GB, Pinkernell BH, Jing TY. Are major risk factors for myocardial infarction the major predictors of degree of coronary artery disease in men? Metabolism. 2004 Mar;53(3):324-9. doi: 10.1016/j.metabol.2003.11.008
- Freedman DS, O’Brien TR, Flanders WD, DeStefano F, Barboriak JJ. Relation of serum testosterone levels to high density lipoprotein cholesterol and other characteristics in men. Arterioscler Thromb. 1991 Mar-Apr;11(2):307-15. doi: 10.1161/01.atv.11.2.307
- Tripathi Y, Hegde BM. Serum estradiol and testosterone levels following acute myocardial infarction in men. Indian J Physiol Pharmacol. 1998 Apr;42(2):291-4.
- Pugh PJ, Channer KS, Parry H, Downes T, Jone TH. Bio-available testosterone levels fall acutely following myocardial infarction in men: association with fibrinolytic factors. Endocr Res. 2002 Aug;28(3):161-73. doi: 10.1081/erc-120015055
- Glueck CJ, Glueck HI, Stroop D, Speirs J, Hamer T, Tracy T. Endogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic men. J Lab Clin Med. 1993 Oct;122(4):412-20.
- De Pergola G, De Mitrio V, Sciaraffia M, Pannacciulli N, Minenna A, Giorgino F, Petronelli M, Laudadio E, Giorgino R. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism. 1997 Nov;46(11):1287-93. doi: 10.1016/s0026-0495(97)90232-8
- Militaru C, Donoiu I, Dracea O, Ionescu DD. Serum testosterone and short-term mortality in men with acute myocardial infarction. Cardiol J. 2010;17(3):249-53. https://journals.viamedica.pl/cardiology_journal/article/view/21373/16977
- Fukui M, Kitagawa Y, Nakamura N, Kadono M, Yoshida M, Hirata C, Wada K, Hasegawa G, Yoshikawa T. Serum dehydroepiandrosterone sulfate concentration and carotid atherosclerosis in men with type 2 diabetes. Atherosclerosis. 2005 Aug;181(2):339-44. doi: 10.1016/j.atherosclerosis.2005.01.014
- Suzuki T, Yano Y, Sakamoto M, Uemura M, Yasuma T, Onishi Y, Sasaki R, Matsumoto K, Hayashi T, Maruyama-Furuta N, Akatsuka H, Gabazza EC, Sumida Y, Takei Y. Correlation of circulating dehydroepiandrosterone with activated protein C generation and carotid intima-media thickness in male patients with type 2 diabetes. Diabet Med. 2012 Jul;29(7):e41-6. doi: 10.1111/j.1464-5491.2012.03573.x
- Camporez JP, Akamine EH, Davel AP, Franci CR, Rossoni LV, Carvalho CR. Dehydroepiandrosterone protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats. J Physiol. 2011 May 15;589(Pt 10):2585-96. doi: 10.1113/jphysiol.2011.206078
- Nheu L, Nazareth L, Xu GY, Xiao FY, Luo RZ, Komesaroff P, Ling S. Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids. 2011 Dec 20;76(14):1590-6. doi: 10.1016/j.steroids.2011.09.015
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Association of plasma dehydroepiandrosterone-sulfate levels with endothelial function in postmenopausal women with coronary risk factors. Hypertens Res. 2008 Jan;31(1):69-74. doi: 10.1291/hypres.31.69
- Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009 Jul 20;63(3):240-5. doi: 10.1016/j.maturitas.2009.03.020
- Gebre-Medhin G, Husebye ES, Mallmin H, Helström L, Berne C, Karlsson FA, Kämpe O. Oral dehydroepiandrosterone (DHEA) replacement therapy in women with Addison’s disease. Clin Endocrinol (Oxf). 2000 Jun;52(6):775-80. doi: 10.1046/j.1365-2265.2000.01017.x
- Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007 Mar;56(3):753-66. doi: 10.2337/db06-1504. Erratum in: Diabetes. 2007 May;56(5):1486.
- Herrington DM. Dehydroepiandrosterone and coronary atherosclerosis. Ann N Y Acad Sci. 1995 Dec 29;774:271-80. doi: 10.1111/j.1749-6632.1995.tb17387.x-i1
- Herrington DM, Nanjee N, Achuff SC, Cameron DE, Dobbs B, Baughman KL. Dehydroepiandrosterone and cardiac allograft vasculopathy. J Heart Lung Transplant. 1996 Jan;15(1 Pt 1):88-93.
- Shono N, Kumagai S, Higaki Y, Nishizumi M, Sasaki H. The relationships of testosterone, estradiol, dehydroepiandrosterone-sulfate and sex hormone-binding globulin to lipid and glucose metabolism in healthy men. J Atheroscler Thromb. 1996;3(1):45-51. doi: 10.5551/jat1994.3.45.
- Bernini GP, Sgro’ M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab. 1999 Jun;84(6):2008-12. doi: 10.1210/jcem.84.6.5824
- Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996 Aug 23;125(1):1-13. doi: 10.1016/0021-9150(96)05864-9
- Mitchell LE, Sprecher DL, Borecki IB, Rice T, Laskarzewski PM, Rao DC. Evidence for an association between dehydroepiandrosterone sulfate and nonfatal, premature myocardial infarction in males. Circulation. 1994 Jan;89(1):89-93. doi: 10.1161/01.cir.89.1.89
- Barrett-Connor E, Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation. 1995 Mar 15;91(6):1757-60. doi: 10.1161/01.cir.91.6.1757
- Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA, Stanczyk FZ, Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab. 2010 Nov;95(11):4985-92. doi: 10.1210/jc.2010-0143
- Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001 Sep;86(9):4171-7. doi: 10.1210/jcem.86.9.7838
- Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996 Nov 12;93(23):13410-5. doi: 10.1073/pnas.93.23.13410
- Nakamura S, Yoshimura M, Nakayama M, Ito T, Mizuno Y, Harada E, Sakamoto T, Saito Y, Nakao K, Yasue H, Ogawa H. Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation. 2004 Sep 28;110(13):1787-93. doi: 10.1161/01.CIR.0000143072.36782.51
- Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006 Oct 24;114(17):1829-37. doi: 10.1161/CIRCULATIONAHA.106.649426
- Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006 May;20(5):1153-63. doi: 10.1210/me.2005-0266
- Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res. 1994 Apr;74(4):629-40. doi: 10.1161/01.res.74.4.629
- Wang L, Hao Q, Wang YD, Wang WJ, Li DJ. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells. Atherosclerosis. 2011 Jan;214(1):47-57. doi: 10.1016/j.atherosclerosis.2010.07.043
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115-26. doi: 10.1056/NEJM199901143400207
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996 Oct;7(5):330-5. doi: 10.1097/00041433-199610000-00012
- Belosjorow S, Bolle I, Duschin A, Heusch G, Schulz R. TNF-alpha antibodies are as effective as ischemic preconditioning in reducing infarct size in rabbits. Am J Physiol Heart Circ Physiol. 2003 Mar;284(3):H927-30. doi: 10.1152/ajpheart.00374.2002
- Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, Sudhir K, Komesaroff PA. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab. 2004 Sep;89(9):4708-15. doi: 10.1210/jc.2003-031560
- Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007 Jul;148(7):3068-76. doi: 10.1210/en.2006-1378
- Chen J, Xu L, Huang C. DHEA inhibits vascular remodeling following arterial injury: a possible role in suppression of inflammation and oxidative stress derived from vascular smooth muscle cells. Mol Cell Biochem. 2014 Mar;388(1-2):75-84. doi: 10.1007/s11010-013-1900-7
- Hautanen A, Mänttäri M, Manninen V, Tenkanen L, Huttunen JK, Frick MH, Adlercreutz H. Adrenal androgens and testosterone as coronary risk factors in the Helsinki Heart Study. Atherosclerosis. 1994 Feb;105(2):191-200. doi: 10.1016/0021-9150(94)90049-3
- Słowínska-Srzednicka J, Zgliczyński S, Ciświcka-Sznajderman M, Srzednicki M, Soszyński P, Biernacka M, Woroszyłska M, Ruzyłło W, Sadowski Z. Decreased plasma dehydroepiandrosterone sulfate and dihydrotestosterone concentrations in young men after myocardial infarction. Atherosclerosis. 1989 Oct;79(2-3):197-203. doi: 10.1016/0021-9150(89)90124-x
- Haffner SM, Moss SE, Klein BE, Klein R. Sex hormones and DHEA-SO4 in relation to ischemic heart disease mortality in diabetic subjects. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1996 Oct;19(10):1045-50. doi: 10.2337/diacare.19.10.1045
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002 Mar 1;155(5):437-45. doi: 10.1093/aje/155.5.437
- Khaw KT, Tazuke S, Barrett-Connor E. Cigarette smoking and levels of adrenal androgens in postmenopausal women. N Engl J Med. 1988 Jun 30;318(26):1705-9. doi: 10.1056/NEJM198806303182601
- Salvini S, Stampfer MJ, Barbieri RL, Hennekens CH. Effects of age, smoking and vitamins on plasma DHEAS levels: a cross-sectional study in men. J Clin Endocrinol Metab. 1992 Jan;74(1):139-43. doi: 10.1210/jcem.74.1.1530789
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003 Apr;24(2):183-217. doi: 10.1210/er.2001-0025
- Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5. doi: 10.1210/jc.2002-021603
- Eich DM, Nestler JE, Johnson DE, Dworkin GH, Ko D, Wechsler AS, Hess ML. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation. 1993 Jan;87(1):261-9. doi: 10.1161/01.cir.87.1.261
- Gordon GB, Bush DE, Weisman HF. Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest. 1988 Aug;82(2):712-20. doi: 10.1172/JCI113652
- Aragno M, Parola S, Brignardello E, Mauro A, Tamagno E, Manti R, Danni O, Boccuzzi G. Dehydroepiandrosterone prevents oxidative injury induced by transient ischemia/reperfusion in the brain of diabetic rats. Diabetes. 2000 Nov;49(11):1924-31. doi: 10.2337/diabetes.49.11.1924
- Ayhan S, Tugay C, Norton S, Araneo B, Siemionow M. Dehydroepiandrosterone protects the microcirculation of muscle flaps from ischemia-reperfusion injury by reducing the expression of adhesion molecules. Plast Reconstr Surg. 2003 Jun;111(7):2286-94. doi: 10.1097/01.PRS.0000060242.85268.8F
- Tagashira H, Bhuiyan S, Shioda N, Fukunaga K. Distinct cardioprotective effects of 17β-estradiol and dehydroepiandrosterone on pressure overload-induced hypertrophy in ovariectomized female rats. Menopause. 2011 Dec;18(12):1317-26. doi: 10.1097/gme.0b013e31821f915b
- Bhuiyan MS, Tagashira H, Fukunaga K. Dehydroepiandrosterone-mediated stimulation of sigma-1 receptor activates Akt-eNOS signaling in the thoracic aorta of ovariectomized rats with abdominal aortic banding. Cardiovasc Ther. 2011 Aug;29(4):219-30. doi: 10.1111/j.1755-5922.2010.00196.x
- Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001 Nov;38(5):1049-53. doi: 10.1161/hy1101.095329
- Weiss EP, Villareal DT, Ehsani AA, Fontana L, Holloszy JO. Dehydroepiandrosterone replacement therapy in older adults improves indices of arterial stiffness. Aging Cell. 2012 Oct;11(5):876-84. doi: 10.1111/j.1474-9726.2012.00852.x
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2197-223. doi: 10.1016/S0140-6736(12)61689-4. Erratum in: Lancet. 2013 Feb 23;381(9867):628. AlMazroa, Mohammad A [added]; Memish, Ziad A [added].
- Donkor ES. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res Treat. 2018 Nov 27;2018:3238165. doi: 10.1155/2018/3238165
- Herrington DM, Gordon GB, Achuff SC, Trejo JF, Weisman HF, Kwiterovich PO Jr, Pearson TA. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol. 1990 Nov;16(6):862-70. doi: 10.1016/s0735-1097(10)80334-1
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4089-91. doi: 10.1073/pnas.95.8.4089
- Lapchak PA, Chapman DF, Nunez SY, Zivin JA. Dehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model: possible involvement of GABA(A) receptors. Stroke. 2000 Aug;31(8):1953-6; discussion 1957. doi: 10.1161/01.str.31.8.1953
- Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4704-7. doi: 10.1073/pnas.78.8.4704
- Baulieu EE. Neuroactive neurosteroids: dehydroepiandrosterone (DHEA) and DHEA sulphate. Acta Paediatr Suppl. 1999 Dec;88(433):78-80. doi: 10.1111/j.1651-2227.1999.tb14408.x
- Jiménez MC, Sun Q, Schürks M, Chiuve S, Hu FB, Manson JE, Rexrode KM. Low dehydroepiandrosterone sulfate is associated with increased risk of ischemic stroke among women. Stroke. 2013 Jul;44(7):1784-9. doi: 10.1161/STROKEAHA.111.000485
- Pappa T, Vemmos K, Saltiki K, Mantzou E, Stamatelopoulos K, Alevizaki M. Severity and outcome of acute stroke in women: relation to adrenal sex steroid levels. Metabolism. 2012 Jan;61(1):84-91. doi: 10.1016/j.metabol.2011.06.003
- Blum CA, Mueller C, Schuetz P, Fluri F, Trummler M, Mueller B, Katan M, Christ-Crain M. Prognostic value of dehydroepiandrosterone-sulfate and other parameters of adrenal function in acute ischemic stroke. PLoS One. 2013 May 1;8(5):e63224. doi: 10.1371/journal.pone.0063224
- Hampl V, Bíbová J, Povýsilová V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J. 2003 May;21(5):862-5. doi: 10.1183/09031936.03.00084503
- Debonneuil EH, Quillard J, Baulieu EE. Hypoxia and dehydroepiandrosterone in old age: a mouse survival study. Respir Res. 2006 Dec 18;7(1):144. doi: 10.1186/1465-9921-7-144
- Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007 Jun 1;74(3):377-87. doi: 10.1016/j.cardiores.2007.01.021
- Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9488-93. doi: 10.1073/pnas.1633724100
- Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca(2+)-activated K(+)-channel opener. Am J Physiol. 1998 Feb;274(2):L186-95. doi: 10.1152/ajplung.1998.274.2.L186
- Gupte SA, Li KX, Okada T, Sato K, Oka M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther. 2002 Apr;301(1):299-305. doi: 10.1124/jpet.301.1.299
- Dessouroux A, Akwa Y, Baulieu EE. DHEA decreases HIF-1alpha accumulation under hypoxia in human pulmonary artery cells: potential role in the treatment of pulmonary arterial hypertension. J Steroid Biochem Mol Biol. 2008 Mar;109(1-2):81-9. doi: 10.1016/j.jsbmb.2007.12.001
- Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol. 2014 Feb 15;306(4):L383-91. doi: 10.1152/ajplung.00301.2013
- Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med. 2011 Mar 1;183(5):659-67. doi: 10.1164/rccm.201007-1027OC
- Dumas de La Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, Jougon J, Marthan R, Moore N, Fayon M, Baulieu EÉ, Dromer C. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol (Paris). 2012 Feb;73(1):20-5. doi: 10.1016/j.ando.2011.12.005
- Mohan PF, Ihnen JS, Levin BE, Cleary MP. Effects of dehydroepiandrosterone treatment in rats with diet-induced obesity. J Nutr. 1990 Sep;120(9):1103-14. doi: 10.1093/jn/120.9.1103
- Cleary MP, Zisk JF. Anti-obesity effect of two different levels of dehydroepiandrosterone in lean and obese middle-aged female Zucker rats. Int J Obes. 1986;10(3):193-204.
- Hansen PA, Han DH, Nolte LA, Chen M, Holloszy JO. DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol. 1997 Nov;273(5):R1704-8. doi: 10.1152/ajpregu.1997.273.5.R1704
- Wellman M, Shane-McWhorter L, Orlando, Jennings JP. The role of dehydroepiandrosterone in diabetes mellitus. Pharmacotherapy. 1999 May;19(5):582-91. doi: 10.1592/phco.19.8.582.31533
- Corvalán C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr. 2013 Feb;97(2):318-25. doi: 10.3945/ajcn.112.037325
- Corona G, Rastrelli G, Giagulli VA, Sila A, Sforza A, Forti G, Mannucci E, Maggi M. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab. 2013 Sep;98(9):3615-26. doi: 10.1210/jc.2013-1358
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD004509. doi: 10.1002/14651858.CD004509.pub2
- Darling GM, Johns JA, McCloud PI, Davis SR. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N Engl J Med. 1997 Aug 28;337(9):595-601. doi: 10.1056/NEJM199708283370903
- Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999 Nov;84(11):3896-902. doi: 10.1210/jcem.84.11.6153
- Davis SR, Goldstat R, Newman A, Berry K, Burger HG, Meredith I, Koch K. Differing effects of low-dose estrogen-progestin therapy and pravastatin in postmenopausal hypercholesterolemic women. Climacteric. 2002 Dec;5(4):341-50.
- Flöter A, Nathorst-Böös J, Carlström K, von Schoultz B. Serum lipids in oophorectomized women during estrogen and testosterone replacement therapy. Maturitas. 2004 Feb 20;47(2):123-9. doi: 10.1016/s0378-5122(03)00246-9
- Leão LM, Duarte MP, Silva DM, Bahia PR, Coeli CM, de Farias ML. Influence of methyltestosterone postmenopausal therapy on plasma lipids, inflammatory factors, glucose metabolism and visceral fat: a randomized study. Eur J Endocrinol. 2006 Jan;154(1):131-9. doi: 10.1530/eje.1.02065
- Srinivasan M, Irving BA, Dhatariya K, Klaus KA, Hartman SJ, McConnell JP, Nair KS. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J Clin Endocrinol Metab. 2009 Mar;94(3):761-4. doi: 10.1210/jc.2008-1774
- Srinivasan M, Irving BA, Frye RL, O’Brien P, Hartman SJ, McConnell JP, Nair KS. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab. 2010 Apr;95(4):1617-25. doi: 10.1210/jc.2009-2000
- Bell RJ, Davison SL, Papalia MA, McKenzie DP, Davis SR. Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause. 2007 Jul-Aug;14(4):630-8. doi: 10.1097/GME.0b013e31802b6cb1
- Christiansen JJ, Andersen NH, Sørensen KE, Pedersen EM, Bennett P, Andersen M, Christiansen JS, Jørgensen JO, Gravholt CH. Dehydroepiandrosterone substitution in female adrenal failure: no impact on endothelial function and cardiovascular parameters despite normalization of androgen status. Clin Endocrinol (Oxf). 2007 Mar;66(3):426-33. doi: 10.1111/j.1365-2265.2007.02750.x
- Jacob MH, Janner Dda R, Belló-Klein A, Llesuy SF, Ribeiro MF. Dehydroepiandrosterone modulates antioxidant enzymes and Akt signaling in healthy Wistar rat hearts. J Steroid Biochem Mol Biol. 2008 Nov;112(1-3):138-44. doi: 10.1016/j.jsbmb.2008.09.008
- Jia C, Chen X, Li X, Li M, Miao C, Sun B, Fan Z, Ren L. The effect of DHEA treatment on the oxidative stress and myocardial fibrosis induced by Keshan disease pathogenic factors. J Trace Elem Med Biol. 2011 Jul;25(3):154-9. doi: 10.1016/j.jtemb.2011.04.001
- Liu CH, Laughlin GA, Fischer UG, Yen SS. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: evidence for a reduced 17,20-desmolase enzymatic activity. J Clin Endocrinol Metab. 1990 Oct;71(4):900-6. doi: 10.1210/jcem-71-4-900
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996 Jun 1;97(11):2601-10. doi: 10.1172/JCI118709
- Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005 Nov;187(2):169-96. doi: 10.1677/joe.1.06264
- Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007 Apr;92(4):1289-95. doi: 10.1210/jc.2006-1895
- Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1996 Jan;81(1):59-64. doi: 10.1210/jcem.81.1.8550794
- Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005 Mar;54(3):765-9. doi: 10.2337/diabetes.54.3.765
- Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004 Nov 10;292(18):2243-8. doi: 10.1001/jama.292.18.2243
- Lasco A, Frisina N, Morabito N, Gaudio A, Morini E, Trifiletti A, Basile G, Nicita-Mauro V, Cucinotta D. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001 Oct;145(4):457-61. doi: 10.1530/eje.0.1450457
- Gómez-Santos C, Larqué E, Granero E, Hernández-Morante JJ, Garaulet M. Dehydroepiandrosterone-sulphate replacement improves the human plasma fatty acid profile in plasma of obese women. Steroids. 2011 Dec 11;76(13):1425-32. doi: 10.1016/j.steroids.2011.07.011
- Diamond P, Cusan L, Gomez JL, Bélanger A, Labrie F. Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol. 1996 Sep;150 Suppl:S43-50.
- Johannsson G, Burman P, Wirén L, Engström BE, Nilsson AG, Ottosson M, Jonsson B, Bengtsson BA, Karlsson FA. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002 May;87(5):2046-52. doi: 10.1210/jcem.87.5.8494
- DHEA. https://medlineplus.gov/druginfo/natural/331.html
- Wierman ME, Kiseljak-Vassiliades K. Should Dehydroepiandrosterone Be Administered to Women? J Clin Endocrinol Metab. 2022 May 17;107(6):1679-1685. doi: 10.1210/clinem/dgac130
- Ng MK, Nakhla S, Baoutina A, Jessup W, Handelsman DJ, Celermajer DS. Dehydroepiandrosterone, an adrenal androgen, increases human foam cell formation: a potentially pro-atherogenic effect. J Am Coll Cardiol. 2003 Dec 3;42(11):1967-74. doi: 10.1016/j.jacc.2003.07.024
- Maggiolini M, Donzé O, Jeannin E, Andò S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999 Oct 1;59(19):4864-9.
- Stoll BA. Dietary supplements of dehydroepiandrosterone in relation to breast cancer risk. Eur J Clin Nutr. 1999 Oct;53(10):771-5. doi: 10.1038/sj.ejcn.1600889
- Arnold JT. DHEA metabolism in prostate: For better or worse? Mol Cell Endocrinol. 2009 Mar 25;301(1-2):83-8. doi: 10.1016/j.mce.2008.10.019
- Schwartz AG. Dehydroepiandrosterone, Cancer, and Aging. Aging Dis. 2022 Apr 1;13(2):423-432. doi: 10.14336/AD.2021.0913
- DHEA-S Test. https://www.testing.com/tests/dheas
- DHEA Sulfate Test. https://medlineplus.gov/lab-tests/dhea-sulfate-test
- DHEA-sulfate test. https://medlineplus.gov/ency/article/003717.htm
- Dehydroepiandrosterone Sulfate. https://www.mayocliniclabs.com/api/sitecore/TestCatalog/DownloadTestCatalog?testId=113595