Natamycin in food
Natamycin also known as pimaricin (E235), is a natural antifungal (a fungicide of the polyene macrolide group) that can be used as an antifungal to treat most fungus infections and has been used globally as a food preservative in a variety of foods and beverages to increase their shelf life without any effect on flavor or appearance 1, 2, 3, 4, 5, 6. The application of natamycin as an anti-fungal preservative and its effect on shelf life is detailed in Table 1 below. Natamycin is most commonly preferred over the other preservatives as it is free from odor and color. Natamycin acts as an antifungal preservative on various food products like yogurt, fruits, khoa, sausages, juices and wines 5, 7. Natamycin is authorized for food preservation by European Union Directive 95/2/EC 8, 9. Natamycin is permitted for the surface treatment on cheese and on dried cured sausages. World Health Organization (WHO), European Food Safety Authority (EFSA), and Food and drug administration (FDA) has listed natamycin or pimaricin as generally recognized as safe (GRAS) status after thorough evaluation, and being considered as a natural preservative by the European Union and labelled as E235 10. Natamycin is allowed in many countries for application on the surface of cheese, skin, and specific meat products. In South Africa and China, natamycin is allowed to be used in fruit juices. China also uses natamycin for the surface treatment of baked products. Natamycin’s application in fish products, wine, yogurt, and tinned food is only allowed in South Africa. The Food and Drug Administration (FDA) has authorized the use of natamycin in yogurt. In a recent study by Chen et al. 11 demonstrated that combination of natamycin-fludioxonil or a natamycin-propiconazole were shown to have a synergistic effect resulting in a > 85.0% reduction of green mold and sour rot thereby managing post-harvest fruit decay of citrus.
Natamycin was reported to be effective against nearly all mold and yeasts (such as Candida spp., Aspergillus spp., Cephalosporium spp., Fusarium spp. and Penicillium spp.) but proved to be ineffective against bacteria and viruses 12, 13. Natamycin is not only certified for safe use in foods but also used for the treatment of fungal infections in humans. Natamycin is the only antifungal medication approved by U.S. Food and Drug Administration (FDA) 14. Natamycin is also used to treat some types of fungus infections of the eye 15, 16, 17, 18. Natamycin is especially effective against Aspergillus and Fusarium corneal infections 19.
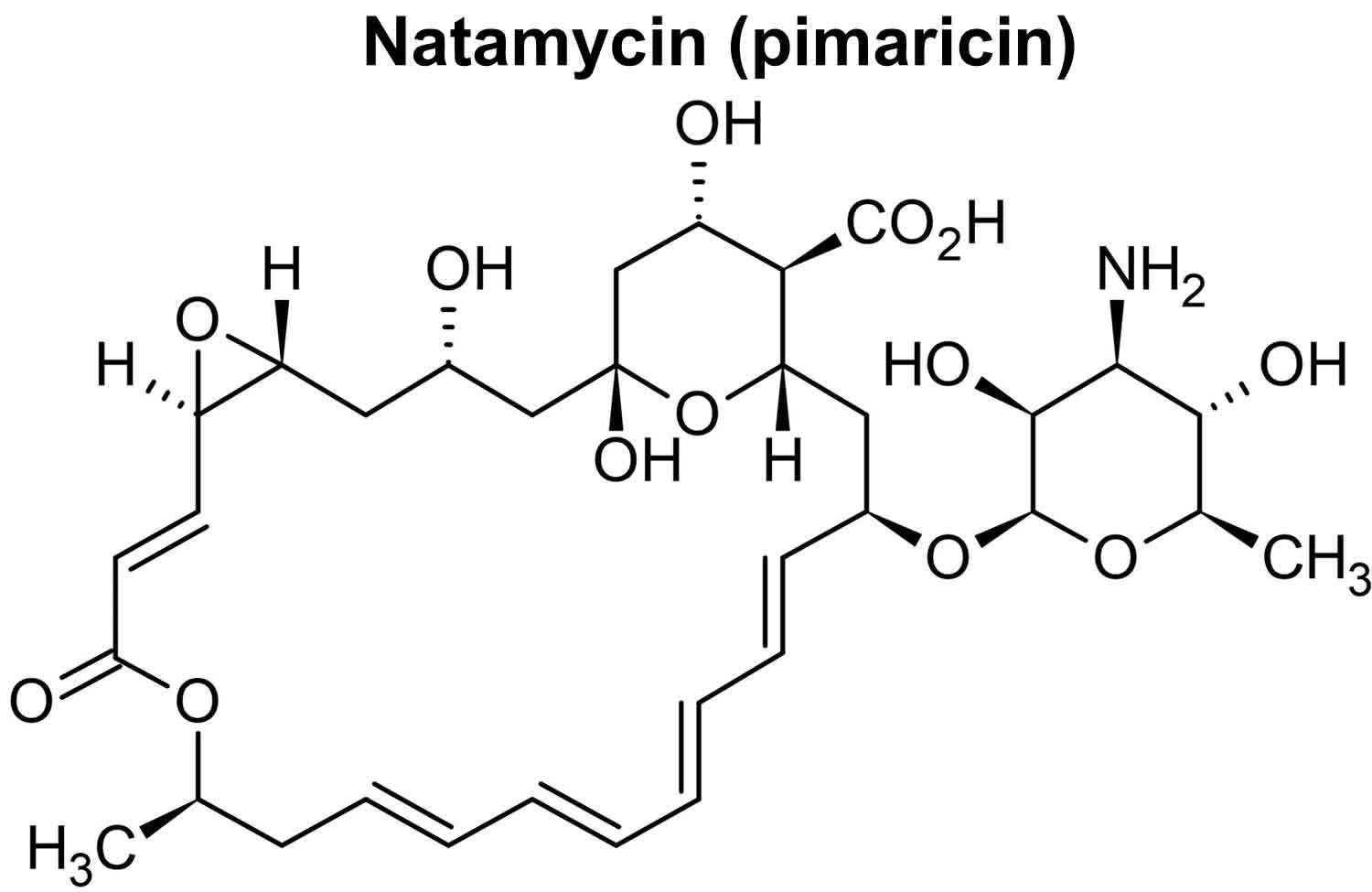
Natamycin (pimaricin) is an antibiotic formed by submerged fermentation of certain Streptomyces bacteria strains, such as Streptomyces natalensis, Streptomyces chattanoogensis, and Streptomyces gilvosporeu 1, 20, 21, 22, 23, 24, 25, 26. Structurally, natamycin is characterized by a series of conjugated double bonds enclosed in a macrocyclic lactone ring with the number of hydroxyl bonds (Figure 1). The lactone ring consists of 25 carbon atoms. So natamycin is classified as a macrolide antibiotic joined to a mycosamine moiety (3-amino-3,6-dideoxy-d-mannose) by an ether linkage 12. The mycosamine moiety is a six-membered pyranose ring present at the C15 position. Natamycin is also classified as a tetraene antibiotic because it is made up of 4 conjugated double bonds. The combination of mycosamine group and carboxyl moiety is present in the structure of natamycin makes it amphoteric 27. Chemically, it is 22-(3-amino-3,6-dideoxy-b-d-manno-pyranosol)oxy-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxiatri[22.3.1.05.7]ocatosa-8,14,16,18,20-pantanene-25-carboxylic acid 28.
Figure 1. Natamycin (pimaricin)
Table 1. Application of natamycin in various food products
| Food product | Natamycin concentration | Target micro-organisms | Load reduction/microbial population | Shelf life achieved days/storage temp (°C) | Effect on nutritional/sensory properties | References |
|---|---|---|---|---|---|---|
| Vanilla flavor stirred yoghurt | 8 ppm | Yeast | Reduces yeast count by 69.36% | 35/5.5 °C | Minimal changes in physicochemical properties (pH, total soluble solids, titratable acidity) | Bakar 29 |
| Plain yoghurt | 10 ppm | Yeast and molds | Yeast and mold counts were reduced to minimum level (3.36 ± 0.66 log10 cfu/g) | 40 | Sensory properties were remained good upto 40 days | Sara et al. 30 |
| Doogh | 10 ppm | Coliform, Escherichia coli, lactic acid bacteria, coagulase positive Staphylococci, yeast and molds | The microbial count was reduced to zero and fungus count was significantly reduced | 60/25 | NA | Mohamadi et al. 31 |
| Khoa | 0.50% | Yeast and mold | Reduce the yeast and mold count | 40/5 | Lower extent of lipolysis and proteolysis was observed | Rajarajan et al. 32 |
| Orange Lemon | 4.5 mg/mL 5 mg/mL | Penicillium italicum Peniillium digitatum | Reduce the chances of disease from 90 to 100% Reduce the chances of disease from 90 to 100% | 14–21 14–21 | Reduced the incidence of decay by 40% Reduced the chances of spoilage | Yİğİter et al. 33 |
| Muffins Bread Loaves Pan Bread Pan Bread | 4 to 5 µg/cm² 2.5 µg/cm² Natamycin (14 ppm) + vinegar Natamycin (14 ppm) + vinegar + cultured wheat flour (2%) | Yeast and mold | Zero mold growth was observed | 60 30 15 30 | Inhibits the mold growth efficiently | Delves-Broughton et al. 34 |
Footnote: ppm = parts per million. One ppm is equivalent to 1 milligram of something per liter of water (mg/L) or 1 milligram of something per kilogram soil (mg/kg).
[Source 5 ]Natamycin in dairy products
Dairy products are highly susceptible to microbial spoilage due to favorable composition like high water activity, moderate pH, proteins, salts, etc. Several fungi like Debaryomyces hansenii, Kluyveromyces lactis, Kluyveromyces maxianus, Penicillium breviocompactum, Rhodotorula mucilaginosa, and Yarrowia lipolytica present in the environment can spoil the dairy products 35. Therefore, preservatives and food additives play a very crucial role in maintaining the quality and enhancing the shelf life of dairy products. Natamycin has been commonly used in dairy products worldwide. Due to its low solubility, it is generally applied on the food products surfaces to enhance their shelf life. An advantage of nataycin over use of sorbates in the limited migration characteristics into the food matrix 36. Cheese is the major dairy product where the application of natamycin plays a role for several years. Cheese is susceptible to mold growth due to the large surface area on exposure to the external environment. There are other factors like extra handling while cutting the cheese, contaminated starter culture, and dirty processing machines, making the cheese favorable for microbial contamination or mold growth 37. Mold contamination results in the formation of metabolites that could induce undesirable flavors and aroma to it. Production of carcinogenic mycotoxin by molds is the biggest threat for cheesemakers 38. The most concern in soft cheese is fungal growth during their storage. Ombarak and Shelaby 39 studied the inhibitory action of natamycin at different concentrations (5–20 ppm) on mold growth in Egyptian fresh soft cheese (Tallaga cheese). Natamycin showed the best inhibitory action at 20 ppm with an increased shelf life of cheese up to 4 weeks. The combination of preservatives is an excellent approach to enhance the shelf life as well as the consumer acceptance of the food products. Natamycin and nisin acted synergistically and increased the shelf of Galoytri cheese (traditional Greek cheese) for more than 28 days by inhibiting the yeast and molds 40. The application of natamycin on cheese can be done by spraying, dipping, coating emulsions, and direct addition 36. The coating application of natamycin with the carrier as alginate and zein films were studied on shelf life of Kashar cheese by Küçük et al. 41. They reported that increasing concentrations of natamycin (100, 200, 500, 1000, 2000, 400 ppm) on films showed higher antifungal activity. For the strains of Aspergillus niger and Penicillium camemberti alginate films showed greater inhibitory activity than zein fims in terms of compatibility with natamycin. In adddition, comparison with Aspergillus niger greater inhibition zones formed against Penicillium camemberti indicating its high sensitivity towards natamycin. However, codex standards define the maximum permitted levels of Natamycin in processed cheese, ripened cheese and cheese analogues as 40 mg/kg in US and 20 mg/kg in Germany 42.
Probiotics are gaining huge popularity in global markets due to their beneficial health effects for their role in colonizing good bacteria in the gut. Although probiotics are reported to be microbiologically safe, they get contaminated with acid-tolerant fungi. Studies showed that plain yogurt treated with natamycin (10 ppm) was reported to have a shelf life up to 40 days as the yeast and mold growth was reduced (3.36 ± 0.66 log 10 cfu/g) 30. Natamycin at the concentration of 8–10 ppm was shown to reduce the yeast counts up to 65.99 % in vanilla-flavored yogurt stored at 5 ± 1 °C without altering their native sensory attributes 43. Growth of Mucor circinelloides (involved in bloating the container) in yogurt was inhibited by applying 8 ppm of natamycin to yogurt (refrigerated at 15 °C) resulted in shelf-life extension of up to 30 days without compromising the sensory aspects.
Natamycin in meat and meat products
The primary application of natamycin are intended for surface treatment of fermented sausages. The codex commission has set maximum permitted levels of natamycin in cured meat, game meats, dried and processed meat as 6 mg/kg with penetration depth of not more 5 mm 42. Meat Sausages have a larger surface area, thereby acting as a medium for undesirable mold growth. In case of fermented sausages where ripenining causes decrease in various volatile compounds like carbonyl, carboxylic acids, alcohols, phenols, etc. are formed during smoking. These volatiles not only imparts aroma and flavor to sausage but also acts as an antifungal agent 44. Sausages are also treated with different preservatives like sorbic acid, potassium sorbate, natamycin, etc. to extend the shelf life and minimize the losses by fungal growth. However, Natamycin showed a better antimycotic effect on sausages that were heat-treated and fermented than sorbates 45. A combination of natamycin, sodium lactate, and nisin was used to suppress mold growth on emulsion-type sausages and extend their shelf life 46. A dosage of 300 ppm of natamycin reduced the chances of Aspergillus niger contamination by 44.80% on minced beef meat and kept the overall acceptability intact for 8 days 47. The combination of several preservatives like nisin, natamycin and polylysine at the concentration of 0.1, 0.05, and 0.1 shows inhibition ration of 95.1% against the spoilage microbes in ham 48. Matary et al. 49 studied the effect of irradiation and natamycin treatment on yeast and mould counts in the 60 samples of fresh minced meat. They found that samples treated with 0.1% of natamycin showed significant decrease in yeast and mould count at 0, 5, 10 and 15 days of storage at 4 °C. Nevertheless, application of natamycin is well known in the poultry feed industry to control the disease caused by Aspergillus without interfering in the growth performance of broiler.
Natamycin in fruits and vegetables
Postharvest loss due to fungal infection is a severe threat to various agricultural commodities causing significant financial loss. There are two major postharvest fungi namely Botrytis cinerea and Penicillium expansum, which are involved in grey mold disease that infects strawberries, apples, and grapes, etc., and blue mold disease. Blue mold disease was caused by the production of mycotoxin (patulin) by Penicillium expansum 50. The concentration of 100 mg/L and 200 mg/L of natamycin prevented grey mold disease in grapefruit and blue mold disease in jujube fruit. Natamycin prevents these two diseases by inhibiting spore germination through permeabilization 3. Dipping strawberries in 20 mg/L of natamycin for 5 minute can inhibit mold growth, respiration rate, and fruit rot. The postharvest quality of button mushroom (stored at 4 ± 1 °C) was improved by a combined effect of natamycin (0.5 mM) and 100% oxygen (100 mL/min flow rate) in cold storage. This combination effect was shown to inhibit yeast and mold growth besides maintained firmness, delayed browning, cap opening, and reduced respiration rate. This combination also enhanced the mushrooms’ shelf life by inhibiting the spoilage enzymes like polyphenol oxidase, peroxidase, and phenylalanine ammonia-lyase 51.
Fermented olives are highly prone to yeast and mold growth during fermentation if the conditions (temperature, pH, starter culture, time, etc.) are not appropriately maintained. Due to uncontrolled conditions (i.e. traditional fermentation), a fungal layer may be formed on the exposed area of brine in which olives were preserved and might lead to undesirable olive softening 52. Fermentation of black olives in brine (8% w/v) and natamycin (0.01% w/v) medium inhibits yeast and mold without affecting the other desirable bacteria, helps in more vigorous fermentation with delivered a product with high titratable acidity 53. The chances of Aspergillus ochraceus growth in the olive paste were inhibited by using a dose of 350 µg/g natamycin. This also reduced the production of penicillic acid to 96% 54.
Soaking of mulberry in natamycin solution (0.3 g/L) reduced the decay rate (23.3% on the 10th day), malondialdehyde content, phenylalanine ammonia-lyase, and polyphenol oxidase activity throughout storage 55. It also reduced the total phenolic, glucose, fructose, and anthocyanin content. Natamycin was also proved to be beneficial in maintaining the total soluble solids, total acids, sucrose levels, color, and firmness of mulberries during storage. This soaking treatment of natamycin enhanced the catalase, superoxide dismutase, and peroxidase in mulberries.
Natamycin in bakery products
Baked goods are susceptible to spoilage by mold, the pattern, and incidence of which depends on the food’s moisture content. Most of the baked items like cake, pastries, bread, and muffins have high water activity (aw), due to which they get spoil rapidly. The molds that are associated with the spoilage are Penicillium (P. chrysogenum, P. brevicompactum, and P. roqueferti), Mucor, Aspergillus, Rhizopus, Wallemia, Chrysonilia sitophila, and Eurotium respectively 56.
Although the baking temperatures are sufficient for the inhibition of fungi, their vegetative cells, and mold spores, nevertheless mold spores can recontaminate the baked product during post-processing 57. Several chemical preservatives (sorbate and propionate) are utilized for the inhibition of molds in baked goods. These preservatives are proved to be ineffective at pH 6 (normal pH of baked goods) and give an inappropriate taste to the baked commodities 58. In addition, several strains of yeast and mold like Monascus ruber and pencillium roqueforti has degrade sorbate or propionic acid preservatives. Treatment of baked products with natamycin can enhance the shelf life without destroying the taste and other sensory attributes. Moreover, addition of natamycin in dough would inhibit yeast fermentation. Studies on vacuum packed Psyllo (pastry product) by treatment of chitosan and natamycin on shelf life extension by inhibition of spoilage microflora was studied by Tsiraki et al. 59. They found that Chitosan at 1.5%, w/v and natamycin at 10 mg/L, w/v showed have significant effect on yeast and mould count by extending the shelf life upto 11 days without affecting the sensorial characteristics 59. The detailed study on application of natamycin on surface of various baked goods were extensively reviewed by Delves-Broughton et al. 60. The FDA has set permissible levels for addition of natamycin for various bakery products as 14 mg/kg for bread, 20 mg/kg for tortillas, 7 mg/kg for US style muffins. Similarly in China, natamycin residue in moon cakes should not exceed 10 mg/kg.
Natamycin in packaging material
Natamycin has the antimycotic effect that could be utilized as a coating material or incorporated in food packaging material to increase food products shelf life. Various researches have conducted studies on natamycin as an antifungal agent to make edible coatings or composite films for food application are listed in Table 2. The major concern while making the composite films (or coating) is the low solubility of the natamycin which reduces the antimycotic efficiency of the films 61. The inclusion of natamycin in methyl-β-cyclodextrin could increase the solubility of natamycin by inserting the hydrophobic part of natamycin (C16-C26) into the rim formed by the natamycin/methyl-β-cyclodextrin (N/ME-β-CD) complex 62. This also inhibits the Botrytis cinerea in cherry tomatoes.
Table 2. Natamycin in food packaging
| Composition of coating/composite film | Product | Shelf life (days) | Discussion | References |
|---|---|---|---|---|
| Natamycin (0.05 mg), 0.2 g Tween 80 (Surfactant), 2.75% (Chitosan), Glycerol (25% w/w to chitosan) | Halloumi cheese | 35 | Coating inhibits yeast and moulds growth No effects on sensory attributes of the product The brine requirement for the cheese reduced from 15 to 10% | Mehyar et al. 63 |
| Starch:glycerol:water (2.5:1:46.5), Natamycin (0.027 g), 0.0068 g Nisin (pH 2) | Port salut cheese | 8 | The film controlled and inhibited the growth of Saccharomyces cerevisiae and Listeria innocua respectively | Resa et al. 64 |
| Cinnamaldehyde (5%) cross linked with gliadin films, Natamycin (0.5) | Soft sliced cheese | NA | The cross-linking networks increased the migration of natamycin thereby increased the preservative effect Films showed good barrier against oxygen permeation Film provided antimycotic activity against Penicillium species, Alternaria solani, Colletotrichum acutatum | Balaguer et al. 65 |
| Tritical flour (4.0 g/mL), Glycerol (30 g/100 g flour), Natamycin (0.08 g/100 mL of film solution) | Soft cheese | 14 | Filmcontrolled the growth of Aspergillus niger and Candida albicans | Romero et al. 66 |
| Glycerol (0.5 g), Chitosan (0.5 g), Lactic acid (1% v/v), Natamycin (0.5 mg/mL) | Saloio cheese | 27 | Coating decreased the growth of yeast and mold to 1.1 log (CFU g−1) Increased the O2 and CO2 permeability without altering the water vapour permeability | Fajardo et al. 67 |
| Starch, glycerol, water (1.8:1:32.5), natamycin (9.25 mg/dm2) | NA | NA | Film showed fungicidal activity against Saccharomyces cerevisiae Contamination was not observed up to 10 days | Resa et al. 68 |
| Whey protein isolate (7%), malic acid (3%), sorbitol (1.5%), nisin (50 IU/mL), natamycin (0.002 g/mL), EDTA (0.1% w/v), Tween 80 (0.15% w/v), sucrose monostearate and sucrose distearate (0.075% w/v) | NA | NA | Film showed the inhibitory effect against L. monocytogenes, Ps. aeruginosa, Y. lipolytica and P. commune and P. chrysogenum | Pintado et al. 69 |
| Whey protein isolate (10%), glycerol (5% wt/wt), guar gum (0.7% wt/wt), sunflower oil (10% wt/wt), tween 20 (0.2% wt/wt), lactic acid (6 g/L) and natamycin (0.25 g/L) | Semi hard cheese | 60 | The edible coating decreased the hardness, water loss and color changes The coating did not altered the fat and salt content Edible coating did not allow the growth of pathogens but allowed lactic acid bacteria to grow throughout the storage period | Ramos et al. 70 |
| Chitosan (1.5 g), natamycin (5 g), acetic acid (1% w/w), tween 80 (2% v/v) | Ground cherry | 50 | The activity of superoxide dismutase and ascorbate peroxidase (reactive oxygen species scavenging enzymes) was enhanced which delayed the senescence period The coating reduces the chances of accumulation of malondialdehyde | Hao et al. 71 |
Is natamycin in food safe?
In 1957, the first toxicological study of natamycin for rats, mice, and guinea pigs was reported by Struyk et al 72. Lethal dose (LD50) is the amount of an ingested substance that kills 50 percent of a test sample. It is expressed in mg/kg, or milligrams of substance per kilogram of body weight. The study by Struyk et al 72 reported the LD50 of natamycin for guinea pigs as 450 mg/kg. Another acute oral toxicity study was performed on rats (both male and female) and male rabbits. After oral administration, the LD50 values of natamycin were reported to be 2.73 g/kg, 4.67 g/kg, and 1.42 g/kg for male rats, female rats and male rabbits, respectively 73.
Three subchronic toxicity studies with natamycin were performed on animals (two studies were performed on rat and one study on dogs). The study performed on rats (fed on 500 mg/kg of natamycin supplemented diet) showed no deviation in hematological, biological factors and organs weight 74. In another study performed on rats, reduction in mean body weight and mean food intake have been reported 73. The No Observed Adverse Effect level (NOAEL) of the study was 45 mg/kg body weight per day for Beagle dogs exposed to natamycin for 3 months. The natamycin at the levels of 0, 12, and 25 mg/kg body weight per day for 3 months exposure resulted in transient diarrhea and slight reduction in body weight of the dogs 75. A two year chronic toxicity study was performed on rats and dogs. Rats were exposed to the different levels of natamycin (0, 125, 250, 500 or 1000 mg/kg diet). Reduction in growth rate and food intake was seen only in the highest dose group (1000 mg/kg of natamycin in diet). The No Observed Adverse Effect level (NOAEL) was reported to be 22.4 mg/kg body weight per day. This study also concluded that there was no significant difference between the natamycin treated groups and their respective control groups in terms of numbers and types of tumors 76, 73. In the dog, obesity was observed in the group that was fed with highest dose of natamycin (500 mg/kg of natamycin in diet), whereas 6.25 mg/kg body weight per day or less dietary levels of natamycin diet did not affect the body weight and hence considered as its NOAEL 73.
A reproductive study with natamycin was performed on three generations of rat 77. An increased number of fetus born dead and decreased number of fetus born alive (surviving at 21 days at F1 generation) was noticed at highest dose group (100 mg/kg body weight per day). Natamycin dietary dose of 50 mg/kg body weight per day or less did not affect the growth, reproduction, and pathology. The NOAEL of this study was considered to be 50 mg/kg body weight per day 77.
Rasgele and Kaymak 78 presented a study on cytotoxic (toxic to cells) and genotoxic (damage to DNA which may lead to cancer) effects of natamycin in mice bone marrow. In this study, different doses of natamycin (20, 400 and 800 mg/kg) were intraperitoneally given to the mice (both male and female) for different time periods (6, 12, 24, 48 and 72 hours). Chromosomal assay confirmed that natamycin was not clastogenic (a mutagenic agent) and did not increase chromosome aberrations 78. Natamycin (400 and 800 mg/kg) treatment induced the micronucleus formation (in 24 and 48 h) in both male and female mice due to which natamycin might be aneugenic. Decrease in mitotic index (MI) and Polychromatic erythrocyte/normochromatic erythrocyte (at all the concentrations of natamycin) ratio were reported, reflecting the cytotoxic (toxic to cells) effect of natamycin on mice bone marrow 78.
Natamycin (400 and 800 mg/kg) can alter the serum levels of liver enzymes (alanine transaminase [ALT], lactate dehydrogenase [LDH] and alkaline phosphatase [ALP]) and cause degenerative disorders in liver of mice 78. The effect of different concentrations of natamycin (13, 18, 23 and 28 µg/mL for 24–48 h) on human lymphocytes was studied. The study concluded that natamycin showed cytotoxicty by reducing the replication index (RI), mitotic index (MI) and nuclear division index (NDI) in lymphocytes of humans 79. In contrast to the previous study, the natamycin was reported to show low toxicity when ingested. In this study, a Langmuir monolayer (contains 25% sterol and mimics a natural cell membrane in mammals) was prepared to examine the effect of natamycin and reported a negligible or low toxic effect of natamycin 80.
To assess the long term toxicity effect of natamycin, groups of 35–40 male and female rats were fed with diets containing natamycin at a concentration of 0, 125, 250, 500, or 1000 mg/kg for 2 years 81. The animals remained in good health, and their survival was unaffected by treatment. Nausea, vomiting, and diarrhea have been observed occasionally after an oral dose of 300–400 mg of natamycin daily; no changes in peripheral blood cells were observed 81. A group of 10 patients with systemic mycoses received oral doses of 50–1000 mg/day for 13–180 days. Nausea, vomiting, and diarrhea occurred in those receiving 600–1000 mg/day 82. No allergic sensitization occurred among 111 patients being treated with natamycin for a variety of conditions 83. No history of allergic reactions was found in 73 workers engaged for an average of 5 years in the manufacture of natamycin.
Natamycin was extensively reviewed in 2003 by Joint Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) Expert Committee on Food Additives (JECFA), who concluded that the previously established Acceptable Daily Intake (ADI = as an estimate of the amount of a chemical that can be ingested daily over a lifetime without appreciable risk to health) of 0–0.3 mg/kg body weight 84 was satisfactory and the consumption of treated cheese and meats would not exceed this Acceptable Daily Intake of 0–0.3 mg/kg body weight 85. European Union had permitted the use of clarified natamycin in Ripened cheeses and uncut cheese products at the level of 1 mg/dm² for external use only 86. Several potential metabolites of natamycin like aponatamycin, mycosamine hydrochloride, and dinatamycinolidediole were reported to have Lethal dose (LD50 = is the amount of an ingested substance that kills 50 percent of a test sample) values of 3200, 3700, and > 4000 mg/kg, respectively.
Natamycin allergy
Allergy occurs when your immune system reacts to a foreign substance such as natamycin or a food that doesn’t cause a reaction in most people. Your immune system produces substances known as antibodies. When you have allergies, your immune system makes antibodies that identify a particular allergen (e.g., natamycin) as harmful, even though it isn’t. When you come into contact with the allergen, your immune system’s reaction can inflame your skin, sinuses, airways or digestive system.
The severity of allergies varies from person to person and can range from minor irritation to anaphylaxis — a potentially life-threatening emergency. Anaphylaxis is the most severe type of allergic reaction and should always be treated as a medical emergency, call your local emergency services number or seek emergency medical help. Anaphylaxis requires immediate treatment with adrenaline (epinephrine) auto-injector (Auvi-Q, EpiPen, others), injected into the outer mid-thigh. Delayed treatment can result in fatal anaphylaxis. Anaphylaxis to drugs can affect breathing, the heart and blood pressure. Even if your symptoms improve after an epinephrine injection, you should go to the emergency department to make sure symptoms don’t return when the effects of the injection wear off.
If you’ve had a severe allergy attack or any signs and symptoms of anaphylaxis in the past, make an appointment to see your health care provider. Evaluation, diagnosis and long-term management of anaphylaxis are complicated, so you’ll probably need to see a provider who specializes in allergies and immunology.
While most allergies can’t be cured, treatments can help relieve your allergy symptoms.
When drug allergy is uncertain, skin testing or a medically supervised test called a ‘drug challenge’ can be conducted by clinical immunology/allergy specialists in hospital clinics.
If a true drug allergy is diagnosed after specialist assessment, the drug must be avoided. Documentation of a diagnosed drug allergy should be recorded in My Health Record, as well as in GP, hospital and pharmacy records. People with a diagnosed drug allergy should always carry or wear medical alert identification.
An allergic reaction to a drug (e.g., natamycin) is called ‘immediate’ when it occurs within one to six hours after taking a medication. An allergic reaction to a drug is called ‘non-immediate’ when the reaction occurs after 24 hours of starting a medication.
Signs of mild to moderate allergic reactions to a drug (e.g., natamycin) can include itchy rashes (hives or welts), swelling (angioedema) of lips, face or eyes, tingling mouth, abdominal (stomach) pain and vomiting. Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Severe non-immediate rashes due to drugs are associated with fever, flu-like and other systemic symptoms, and can be life-threatening. These are called severe cutaneous adverse reactions (SCAR) and require urgent specialist care.
Natamycin eye drops
Natamycin has long been considered the standard of care for fungal keratitis and is the only topical ocular antifungal approved by the US Food and Drug Administration 15, 16, 17, 18. Natamycin eye drops is used to treat fungal infections of the eyes as topical treatment of blepharitis, conjunctivitis, and keratitis (corneal infection) caused by susceptible fungi. Natamycin is especially effective against Aspergillus and Fusarium corneal infections 19.
Use natamycin as ordered by your doctor. Follow all directions on your prescription label. Do not use natamycin eye drops in larger or smaller amounts or for longer than recommended.
- For the eye only.
- Shake this medicine well just before each use.
- Use as you have been told, even if your signs get better.
- Wash your hands before and after using the eye drops.
- Do not use if the solution is leaking or has particles.
- Do not touch the container tip to the eye, lid, or other skin.
- Tilt your head back slightly and pull down your lower eyelid to create a small pocket. Hold the dropper above the eye with the tip down. Look up and away from the dropper and squeeze out a drop.
- After use, keep your eyes closed. Put pressure on the inside corner of the eye. Do this for 1 to 2 minutes. This keeps the drug in your eye.
- Wait at least 10 minutes before using any other eye drops your doctor has prescribed.
- Do not use other eye medications unless your doctor tells you to.
- Do not wear contact lenses if any signs or symptoms of fungal blepharitis, conjunctivitis, or keratitis are present.
- Do not use this medication while wearing contact lenses. Natamycin ophthalmic may contain a preservative that can discolor soft contact lenses.
Use this medication for the full prescribed length of time. Your symptoms may improve before the infection is completely cleared. Skipping doses may also increase your risk of further infection that is resistant to antibiotics.
Your doctor should check your progress at regular visits. For some eye infections, these visits may be as often as several times a week.
If your symptoms do not improve within 7 to 10 days of treatment, or if they become worse, see your doctor.
Natamycin eye drops dosage
Natamycin eye drops dose will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
- For fungus infection of the eye
- Natamycin eye drops dosage form:
- Adults—Use one drop in the eye every four to six hours. For more serious infections, your doctor may tell you to use one drop in the eye every one or two hours for three or four days, then one drop six to eight times a day thereafter.
- Children—Use and dose must be determined by your doctor.
- Natamycin eye drops dosage form:
If you miss a dose of this medicine, apply the missed dose as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not use extra medicine to make up the missed dose.
Fungal Blepharitis or Conjunctivitis
- Instill 1 drop of 5% Natamycin eye drops into conjunctival sac of affected eye(s) 4–6 times daily.
Fungal Keratitis
- Instill 1 drop of 5% Natamycin eye drops into conjunctival sac of affected eye(s) every 1–2 hours. After 3–4 days, may decrease frequency to 1 drop 6–8 times daily; in many cases, frequency may be gradually decreased every 4–7 days.
If no signs of improvement after 7–10 days of topical therapy, infection may be caused by pathogens not susceptible to natamycin; base continued therapy on clinical reevaluation and additional laboratory studies.
If evidence of improvement, continue topical therapy for 14–21 days or until there are no signs or symptoms of active fungal keratitis.
Natamycin eye drops side effects
Check with your doctor as soon as possible if any of the following side effects occur while using natamycin eye drops:
- allergic reaction,
- change in vision,
- chest pain,
- corneal opacity,
- shortness of breath (dyspnea),
- eye discomfort,
- eye edema,
- eye redness (eye hyperemia),
- eye irritation or pain,
- foreign body sensation,
- paresthesia,
- tearing.
- Davidson PM, Doan C. Natamycin. In Antimicrobials in Food (pp 339–356). CRC Press 2020
- Davidson PM, Doan CH (1993). Natamycin. In, Davidson DP, Branen AL eds. Antimicrobials in Foods (pp. 395–407). New York, Marcel Dekker.
- He C, Zhang Z, Li B, Xu Y, Tian S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biology and Technology. 2019;151:134–141. doi: 10.1016/j.postharvbio.2019.02.009
- Delves-Broughton J, Thomas LV, Williams G (2006). Natamycin as an antimycotic preservative on cheese and fermented sausages. Food Aust. 58, 19–21.
- Meena M, Prajapati P, Ravichandran C, Sehrawat R. Natamycin: a natural preservative for food applications-a review. Food Sci Biotechnol. 2021 Oct 5;30(12):1481-1496. doi: 10.1007/s10068-021-00981-1
- Rencüzoğullari E, Azirak S, Canimoglu S, Parlak S, Buyukleyla M. Effects of natamycin on sister chromatid exchanges, chromosome aberrations and micronucleus in human lymphocytes. Drug Chem Toxicol. 2009;32(1):47-52. https://doi.org/10.1080/01480540802431371
- Galus S., Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015;45:273–283. doi: 10.1016/j.tifs.2015.07.011
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Scientific Opinion on the use of natamycin (E 235) as a food additive. EFSA Journal 2009; 7(12):1412 [25 pp.]. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.1412
- Commission staff working document – Annex to: Report from the Commission to the European Parliament and the Council on the progress of the re-evaluation of food additives {COM(2007) 418 final} https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52007SC0998&qid=1693977229375
- EFSA. Scientific opinion on the use of natamycin (E 235) as a food additive EFSA panel on food additives and nutrient sources added to food (ANS) EFSA J. 2009;1412:1–25.
- Chen D, Förster H, Adaskaveg JE. Natamycin, a Biofungicide for Managing Major Postharvest Fruit Decays of Citrus. Plant Dis. 2021 May;105(5):1408-1414. https://apsjournals.apsnet.org/doi/epdf/10.1094/PDIS-08-20-1650-RE
- Raab W. Natamycin (Pimaricin): Its Properties and Possibilities in Medicine 1972. 10.1111/j.1439-0507.1974.tb04240.x
- Hsiao CH, Yeh LK, Chen HC, Lin HC, Chen PY, Ma DH, Tan HY. Clinical characteristics of Alternaria keratitis. Journal of Ophthalmology. 2014;2014:1–7. doi: 10.1155/2014/536985
- Arora R, Gupta D, Goyal J, Kaur R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clinical & Experimental Ophthalmology. 2011;39(5):434–440. doi: 10.1111/j.1442-9071.2010.02473.x
- Malecha MA (2004). Fungal keratitis caused by Scopulariopsis brevicaulis treated successfully with natamycin. Cornea 23, 201–203.
- Farrell S, McElnea E, Moran S, Knowles S, Murphy CC. Fungal keratitis in the Republic of Ireland. Eye (Lond). 2017 Oct;31(10):1427-1434. doi: 10.1038/eye.2017.82
- Sun CQ, Lalitha P, Prajna NV, Karpagam R, Geetha M, O’Brien KS, Oldenburg CE, Ray KJ, McLeod SD, Acharya NR, Lietman TM; Mycotic Ulcer Treatment Trial Group. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology. 2014 Aug;121(8):1495-500.e1. doi: 10.1016/j.ophtha.2014.03.004
- Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006 Apr;113(4):526-30. doi: 10.1016/j.ophtha.2005.10.063
- Prajna NV, Rao RA, Mathen MM, Prajna L, George C, Srinivasan M (2002). Simultaneous bilateral fungal keratitis caused by different fungi, Ind. J. Ophthalmol. 50(3), 213–214.
- Delves-Broughton, J. 2014. Permitted Preservatives – Natamycin. Pages 87-91 in: Encyclopedia of Food Microbiology, 2nd ed. C. A. Batt and M. L. Tortorello, eds. Elsevier, London, U.K. https://doi.org/10.1016/B978-0-12-384730-0.00269-X
- Stark, J., and Tan, H. S. 2003. Natamycin. Pages 179-195 in: Food Preservatives. N. J. Russell and G. W. Gould, eds. Springer, Boston, MA, U.S.A. https://doi.org/10.1007/978-0-387-30042-9_9
- Thomas LV and Delves-Broughton J, 2003. Natamycin. In: Encyclopedia of Food Sciences and Nutrition. Eds. B Caballero, L Trugo and P Finglas, pp. 4109–4115. Elsevier Science Ltd.
- Aparicio JF, Fouces R, Mendes MV, Olivera N, Martín JF (2000). A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem. Biol. 7(11), 895–905.
- Aparicio JF, Caffrey P, Gil JA, Zotchev SB (2003). Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 61, 179–188.
- WHO. (2002). Safety evaluation of certain food additives and contaminants, natamycin (pimaricin). WHO Food Additives Series No. 48, 1–26.
- Liang J, Lin J, Xu Z, Su W, Cen P (2007). Space-flight mutation of Streptomyces gilvosporeus for enhancing natamycin production. Chin. J. Chern. Eng. 15, 720–724.
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes. 864(3–4): 257–304. 10.1016/0304-4157(86)90002-X
- Delves-Broughton J, Thomas LV, Doan CH, Davidson PM. Natamycin. In Davidson PM, Sofos JN, Branen AL (3rd ed.). Antimicrobials in food (pp 237–274). Taylor & Francis 2005
- Bakar D. Comparative studies on the qualities of commercialized yoghurt in Kumasi and the effect of Natamycin on yoghurt during storage (Doctoral dissertation) 2011
- Sara AE, Ekbal MA, Adham MA, Hamdi AM. The role of natamycin fortification to extend shelf life of plain yoghurt. Benha Veterinary Medical Journal. 2014;27:140–149.
- Mohamadi S, Mofid V, Zeinali T, Rahmani A, Sadighara P, Peivasteh-Roudsari L. Microbial and Chemical Characteristics of Doogh (Iranian Fermented Milk Drink). Int J Food Sci. 2021 Dec 17;2021:3009795. doi: 10.1155/2021/3009795
- Rajarajan G, Kumaresan G, Annal R, Pandiyan C. Extending the shelf life of Khoa using antifungal agents. International Journal Chemistry Science. 2010;8(5):560–563.
- Yİğİter B, Onay F, Akgul NB, Akocak PB. Natamycin treatment to control postharvest mold development and improve storability of citrus fruits. Journal of Food, Agriculture and Environment. 12: 188–192, 2014
- Delves-Broughton J, Steenson L, Dorko C, Erdmann J, Mallory S, Norbury F, Thompson B. Use of natamycin as a preservative on the surface of baked goods: a case study. In Doona CJ, Kustin K, Feeherry FE, Case Studies in Novel Food Processing Technologies: Innovations in Processing, Packaging and Predictive Modelling (303–320). Woodhead Publishing 2010
- Delavenne E, Ismail R, Pawtowski A, Mounier J, Barbier G, Le Blay G. Assessment of lactobacilli strains as yogurt bioprotective cultures. Food Control. 2013;30(1):206–213. doi: 10.1016/j.foodcont.2012.06.043
- Elsser-Gravesen D, Elsser-Gravesen A. Biopreservatives. Adv Biochem Eng Biotechnol. 2014;143:29-49. doi: 10.1007/10_2013_234
- Kure CF, Skaar I, Brendehaug J. Mould contamination in production of semi-hard cheese. International Journal of Food Microbiology. 2004;93(1):41–49. doi: 10.1016/j.ijfoodmicro.2003.10.005
- Dalie DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria–Potential for control of mould growth and mycotoxins: A review. Food Control. 2010;21(4):370–380. doi: 10.1016/j.foodcont.2009.07.011
- Ombarak RA, Shelaby HH. The inhibitory effect of natamycin and potassium sorbate on mold growth in egyptian fresh soft cheese (Tallaga Cheese) Alexandria Journal for Veterinary Sciences. 2017;53(2):33–37. doi: 10.5455/ajvs.264557
- Kallinteri LD, Kostoula OK, Savvaidis IN. Efficacy of nisin and/or natamycin to improve the shelf-life of Galotyri cheese. Food Microbiology. 2013;36(2):176–181. doi: 10.1016/j.fm.2013.05.006
- Küçük GS, Çelik ÖF, Mazi BG, Türe H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packaging Technology and Science. 2020;33(1):39–48. doi: 10.1002/pts.2483
- Lee NK, Paik HD. Status, antimicrobial mechanism, and regulation of natural preservatives in livestock food systems. Korean Journal for Food Science of Animal Resources. 2016;36(4):547. doi: 10.5851/kosfa.2016.36.4.547
- Dzigbordi B, Adubofuor J, Dufie WF. The effects of different concentrations of natamycin and the point of addition on some physicochemical and microbial properties of vanilla-flavoured yoghurt under refrigerated condition. International Food Research Journal. 20: 3287–3292.
- Ledesma E, Rendueles M, Díaz M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control. 2016;60:64–87. doi: 10.1016/j.foodcont.2015.07.016
- Pipek P, Rohlík BA, Lojkova A, Staruch L. Suppression of mould growth on dry sausages. Czech Journal of Food Sciences 28: 258–263. 2010 doi:10.17221/121/2010-CJFS
- Jingwei GXZKW, Yunxia R. Studies on the Effect of compound natural preservatives used in emulsion-type sausage. Journal of Chinese Institute of Food Science and Technology. 2009 doi: 10.1016/j.tifs.2015.05.003
- Salem, A. M., Amin, R. A., Khater, D. F. & Shokr, L. A. (2016) Antifungal effect of some chemical preservatives on aspergillus niger in minced beef meat. Benha Veterinary Medical Journal 30: 295–301. 10.21608/bvmj.2016.31399
- Zhang X, Chi YL, Miao T, Jia DY, Yao K. Antibacterial effects of different preservatives on the major spoilage microbes in traditional fermented ham. China Condiment. 01, 2013
- El-Matary DA, Baher WM, Zaki NM. Effect of irradiation and natamycin on decontamination of fungi from laboratory inoculated minced meat
- Williamson B, Tudzynski B, Tudzynski P, Van Kan JA. Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology. 2007;8(5):561–580. doi: 10.1111/j.1364-3703.2007.00417.x
- Jiang T. Effect of natamycin in combination with pure oxygen treatment on postharvest quality and selected enzyme activities of button mushroom (Agaricus bisporus) Journal of Agricultural and Food Chemistry. 2012;60(10):2562–2568. doi: 10.1021/jf205160c
- Arroyo-Lopez FN, Querol A, Bautista-Gallego J, Garrido-Fernandez A. Role of yeasts in table olive production. International Journal of Food Microbiology. 2008;128(2):189–196. doi: 10.1016/j.ijfoodmicro.2008.08.018
- Hondrodimou O, Kourkoutas Y, Panagou EZ. Efficacy of natamycin to control fungal growth in natural black olive fermentation. Food Microbiology. 2011;28(3):621–627. doi: 10.1016/j.fm.2010.11.015
- Gourama H, Bullerman LB. Effects of potassium sorbate and natamycin on growth and penicillic acid production by Aspergillus ochraceus. Journal of Food Protection. 1988;51(2):139–144. doi: 10.4315/0362-028x-51.2.139
- Wen M, Lin X, Yu Y, Wu J, Xu Y, Xiao G. Natamycin treatment reduces the quality changes of postharvest mulberry fruit during storage. Journal of Food Biochemistry. 2019;43(8):12934. doi: 10.1111/jfbc.12934
- Saranraj P, Geetha M. Microbial spoilage of bakery products and its control by preservatives. International Journal of Pharmaceutical & Biological Archives. 2012;3(1):38–48.
- Ponte JG, Tsen CC. Bakery products. In Beuchat, L.R. (2nd ed.) Food and beverage mycology pp 233–264.1987 Van Nostrand Reinhold
- Seiler DAL. Factors affecting the use of mould inhibitors in bread and cake. In Microbial Inhibitors in Food: Proceedings of the Fourth International Symposium on Food Microbiology (pp 211–220). 1964 Almqvist & Wiksell
- Tsiraki MI, El-Obeid T, Yehia HM, Karam L, Savvaidis IN. Effects of chitosan and natamycin on vacuum-packaged phyllo: A pastry product. Journal of Food Protection. 2018;81(12):1982–1987. doi: 10.4315/0362-028X.JFP-18-236
- Delves-Broughton J, Steenson L, Dorko C, Erdmann J, Mallory S, Norbury F, Thompson B. Use of natamycin as a preservative on the surface of baked goods: a case study. In Doona CJ, Kustin K, Feeherry FE, Case Studies in Novel Food Processing Technologies: Innovations in Processing, Packaging and Predictive Modelling (303–320). 2010 Woodhead Publishing
- Medina E, Caro N, Abugoch L, Gamboa A, Diaz-Dosque M, Tapia C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. Journal of Food Engineering. 2019;240:191–198. doi: 10.1016/j.jfoodeng.2018.07.023
- Yang Y, Huan C, Liang X, Fang S, Wang J, Chen J. Development of starch-based antifungal coatings by incorporation of natamycin/methyl-β-cyclodextrin inclusion complex for postharvest treatments on cherry tomato against Botrytis cinerea. Molecules. 2019;24(21):3962. doi: 10.3390/molecules24213962
- Mehyar GF, Al Nabulsi AA, Saleh M, Olaimat AN, Holley RA. Effects of chitosan coating containing lysozyme or natamycin on shelf-life, microbial quality, and sensory properties of Halloumi cheese brined in normal and reduced salt solutions. Journal of Food Processing and Preservation. 2018;42(1):1–9. doi: 10.1111/jfpp.13324
- Resa CPO, Gerschenson LN, Jagus RJ. Starch edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian Port Salut cheese. Food Control. 2016;59:737–742. doi: 10.1007/s11947-012-0960-0
- Balaguer MP, Fajardo P, Gartner H, Gomez-Estaca J, Gavara R, Almenar E, Hernandez-Munoz P. Functional properties and antifungal activity of films based on gliadins containing cinnamaldehyde and natamycin. International Journal of Food Microbiology. 2014;173:62–71. doi: 10.1016/j.ijfoodmicro.2013.12.013
- Romero V, Borneo R, Passalacqua N, Aguirre A. Biodegradable films obtained from triticale (x TriticosecaleWittmack) flour activated with natamycin for cheese packaging. Food Packaging and Shelf Life. 2016;10:54–59. doi: 10.1016/j.fpsl.2016.09.003
- Fajardo P, Martins JT, Fuciños C, Pastrana L, Teixeira JA, Vicente AA. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. Journal of Food Engineering. 2010;101(4):349–356. doi: 10.1016/j.jfoodeng.2010.06.029
- Resa CPO, Gerschenson LN, Jagus RJ. Effect of natamycin on physical properties of starch edible films and their effect on Saccharomyces cerevisiae activity. Food and Bioprocess Technology. 2013;6(11):3124–3133. doi: 10.1007/s11947-012-0960-0
- Pintado CM, Ferreira MA, Sousa I. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin and natamycin. Food Control. 2010;21(3):240–246. doi: 10.1016/j.foodcont.2009.05.017
- Ramos ÓL, Pereira JO, Silva SI, Fernandes JC, Franco MI, Lopes-da-Silva JA, Malcata FX. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. Journal of Dairy Science. 2012;95(11):6282–6292. doi: 10.3168/jds.2012-5478
- Hao XL, Zhang JJ, Li XH, Wang W. Application of a chitosan coating as a carrier for natamycin to maintain the storage quality of ground cherry (Physalis pubescens L.). Journal of Zhejiang University-Science B. 18: 807–815. 2017 doi:10.1631/jzus.B1600295
- STRUYK AP, HOETTE I, DROST G, WAISVISZ JM, VAN EEK T, HOOGERHEIDE JC. Pimaricin, a new antifungal antibiotic. Antibiot Annu. 1957-1958;5:878-85.
- Levinskas GJ, Ribelin WE, Shaffer CB. Acute and chronic toxicity of pimaricin. Toxicology and Applied Pharmacology. 1966;8(1):97–109. doi: 10.1016/0041-008X(66)90105-0
- Hutchison EB, Ribelin WE, Levinskas GJ. Report on acid-degraded pimaricin: Ninety-eight day repeated feeding to rats. Central Medical Department: Unpublished report submitted to WHO by American Cyanamid Co.; 1966.
- Van Eeken CJ, Birtwhistle RDR, Aboulwafa-wan Velthoven MJE. Three months study in dogs of the toxicity of natamycin by addition to the food. Unpublished report No.12.401, 24 October 1984. Submitted to WHO by Gist-Brocades Research and Development 1984
- Levinskas GJ, Shaffer CB, Bushey C, Kunde ML, Stackhouse DW, Vidone LB, Javier B, Monell E. Two-year feeding to rats. Submitted to WHO by American Cyanamid Co: Unpublished report from the Central Medical Department; 1963.
- Cox GE, Bailey DE, Morgareidge K. Multigeneration reproduction studies in rats with delvocid brand of pimaricin. Unpublished report No.1-1052 submitted to WHO by Food and Drug Research Laboratories Inc 1973
- Rasgele P, KaymaK F. Effects of food preservative natamycin on liver enzymes and total protein in Mus Musculus. Bulgarian Journal of Agricultural Science. 2013;19(2):298–302.
- Rencüzoğullari E, Azirak S, Canimoglu S, Parlak S, Buyukleyla M. Effects of natamycin on sister chromatid exchanges, chromosome aberrations and micronucleus in human lymphocytes. Drug Chem Toxicol. 2009;32(1):47-52. doi: 10.1080/01480540802431371
- Arima AA, Pavinatto FJ, Oliveira ON Jr, Gonzales ERP. The negligible effects of the antifungal natamycin on cholesterol-dipalmitoyl phosphatidylcholine monolayers may explain its low oral and topical toxicity for mammals. Colloids Surf B Biointerfaces. 2014 Oct 1;122:202-208. doi: 10.1016/j.colsurfb.2014.06.058
- Anonymous. Absorption of pimaricin following oral administration. Unpublished report submitted to WHO by the Royal Netherlands Fermentation Industries Ltd., Delft 1968
- NEWCOMER VD, STERNBERG TH, WRIGHT ET, REISNER RM, McNALL EG, SORENSEN LJ. The treatment of systemic mycoses with orally administered pimaricin: preliminary report. Ann N Y Acad Sci. 1960 Aug 27;89:240-6. doi: 10.1111/j.1749-6632.1960.tb20145.x
- EDWARDS G, LATOUCHE CJ. THE TREATMENT OF BRONCHOPULMONARY MYCOSES WITH A NEW ANTIBIOTIC–PIMARICIN. Lancet. 1964 Jun 20;1(7347):1349-53. doi: 10.1016/s0140-6736(64)92038-0
- European Commission (1992). Food-science and techniques. Report of the Scientific Committee for Food (Twenty sixth series). CEC, Luxembourg.
- NATAMYCIN (PIMARICIN). WHO FOOD ADDITIVES SERIES: 48. SAFETY EVALUATION OF CERTAIN FOOD ADDITIVES AND CONTAMINANTS. https://www.inchem.org/documents/jecfa/jecmono/v48je06.htm
- European Union Commission Regulation (EU) 2015/647. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R0647