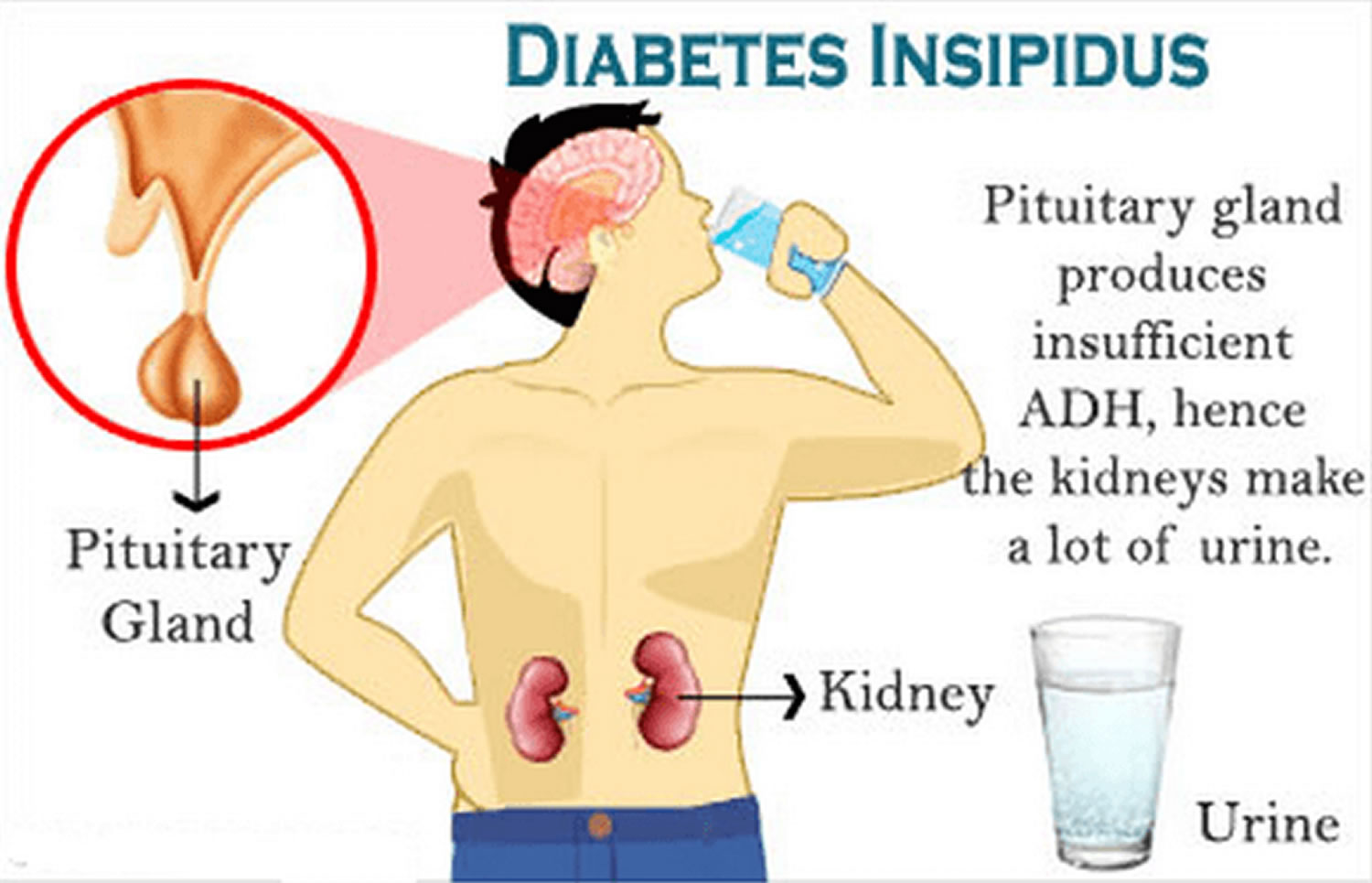
Diabetes insipidus
Diabetes insipidus also called “water diabetes”, arginine vasopressin deficiency and arginine vasopressin resistance, is a rare disorder that occurs when a person’s kidneys pass an abnormally large volume of urine that is insipid (dilute and odorless urine) 1. The two main symptoms of diabetes insipidus are 2, 3:
- Extreme thirst (polydipsia). It’s likely that you’ll feel thirsty all the time and have a ‘dry’ feeling that’s always present, no matter how much water you drink. Maintaining proper water balance by drinking enough fluids is critical for poeple with diabetes insipidus, as they tend to lose a lot of water with frequent urination, which can lead to life-threatening dehydration. However, drinking too much water is also dangerous, as it may lead to a rare condition called water intoxication.
- Passing large amounts of urine (polyuria), even at night (nocturia). You may pass pale, watery urine every 15-20 minutes. The amount of urine passed can range from 3 liters in mild cases to up to 20 liters in severe cases.
- You may also feel generally unwell and ‘run down’ much of the time for no apparent reason.
In most people, the kidneys pass about 1 to 2 quarts (0.946 to 1.89 liter) of urine a day. In people with diabetes insipidus, the kidneys can pass 3 to 21 quarts (2.83 to 20 liters) of urine a day. As a result, a person with diabetes insipidus may feel the need to drink large amounts of liquids. You become extremely thirsty, so you drink. Then you urinate. This cycle can keep you from sleeping or even make you wet the bed. Your body produces lots of urine that is almost all water.
Four underlying conditions can lead to diabetes insipidus:
- Central diabetes insipidus is the most common type and is caused by damage to a person’s hypothalamus or pituitary gland causing disruptions in the normal production, storage, and release of vasopressin hormone [arginine vasopressin (AVP)] also called antidiuretic hormone (ADH). The disruption of vasopressin or antidiuretic hormone (ADH) causes the kidneys to remove too much fluid from the body, leading to an increase in urination. In infants and children, central diabetes insipidus is often an inherited condition. Other causes include tumors, infections, inflammation, surgery and head injury.
- Nephrogenic diabetes insipidus occurs when the pituitary produces enough vasopressin hormone or antidiuretic hormone (ADH) but the kidneys fail to recognize it because of an inherited or acquired kidney disease. Nephrogenic diabetes insipidus may be due to:
- An inherited disorder 4:
- X-linked nephrogenic diabetes insipidus accounts for 90% of cases of congenital nephrogenic diabetes insipidus and occurs with a frequency of 4 to 8 per 1 million male live births.
- Autosomal nephrogenic diabetes insipidus accounts for approximately 10% of the remaining cases.
- Certain medicines, including lithium and antiviral medicines such as foscarnet (Foscavir).
- Low levels of potassium in the blood.
- High levels of calcium in the blood.
- A blocked urinary tract or a urinary tract infection.
- A chronic kidney condition.
- An inherited disorder 4:
- Dipsogenic diabetes insipidus also known as primary polydipsia occurs due to a defect in the thirst mechanism located in a person’s hypothalamus. This defect results in an abnormal increase in thirst and liquid intake that suppresses vasopressin or antidiuretic hormone (ADH) secretion and increases urine output. The same events and conditions that damage the hypothalamus or pituitary—surgery, infection, inflammation, a tumor, head injury—can also damage the thirst mechanism. Certain medications or mental health problems such as schizophrenia may predispose a person to dipsogenic diabetes insipidus.
- Gestational diabetes insipidus occurs only during pregnancy. In some cases, an enzyme made by the placenta—a temporary organ joining mother and baby—breaks down the mother’s vasopressin or antidiuretic hormone (ADH). In other cases, pregnant women produce more prostaglandin, a hormone-like chemical that reduces kidney sensitivity to vasopressin. Most pregnant women who develop gestational diabetes insipidus have a mild case that does not cause noticeable symptoms. Gestational diabetes insipidus usually goes away after the mother delivers the baby; however, it may return if the mother becomes pregnant again.
Normal vasopressin hormone or antidiuretic hormone (ADH) stimulates the kidneys to produce more concentrated urine, thus reducing water loss and retaining water in the body. Vasopressin or antidiuretic hormone (ADH) plays an important role in controlling the fluid balance in your body. Without the antidiuretic hormone (ADH), the kidneys do not work properly to keep enough water in your body. The result is a rapid loss of water from your body in the form of dilute urine. This results in the need to drink large amounts of water due to extreme thirst and to make up for excessive water loss in the urine (10 to 20 liters a day).
The hallmarks of diabetes insipidus include polyuria (> 50 mL/kg), dilute urine (osmolality < 300 mOsm/L), and increased thirst with the intake of up to 20 L/day fluid intake 2. The kidneys pass large amounts of urine (water) irrespective of the body’s hydration state 5. Untreated diabetes insipidus can cause hypovolemia, dehydration, and electrolyte imbalances 6, 7.
Diabetes insipidus is a rare disease, affecting about 1 in 25,000 people worldwide 8, 4. Diabetes insipidus can present at any age, and the prevalence is equal among males and females 7. The age of presentation depends on the cause 9. Less than 10% of diabetes insipidus is hereditary 7.
To check for diabetes insipidus, your doctor may order one of several tests:
- A urine test. This will show how much water is in your urine. It can rule out type 1 or type 2 diabetes. (If you have type 1 or type 2 diabetes, there will be excess sugar in your urine.)
- A blood test. This will check for high sodium levels – another indication of diabetes insipidus.
- A water deprivation test. Water deprivation test can take several hours. You aren’t allowed to drink any liquids during this time. Your weight, urine and blood will be checked every hour.
- An MRI (magnetic resonance image) scan. The scan can show problems in the brain that could be causing your diabetes insipidus.
There’s no cure for diabetes insipidus. But you can work with your doctor to manage the symptoms. Usually, diabetes insipidus is caused by a problem with your pituitary gland (central diabetes insipidus) or your kidneys (nephrogenic diabetes insipidus). Treatment depends on the cause of the problem. Treatment includes relieving thirst, lowering the amount of urine your body makes and preventing dehydration. Treating these symptoms will add a great deal to your quality of life.
For central diabetes insipidus (the most common type of diabetes insipidus) in which the hypothalamus does not produce enough anti-diuretic hormone (ADH), medicines that deliver synthetic vasopressin called desmopressin (DDAVP) is given either as a nasal spray, tablets, or injections to controls urine output and fluid balance and prevents dehydration. Drinking too much while taking desmopressin (DDAVP) can overload your body with fluids. This can make you feel sick, weak, or dizzy.
For nephrogenic diabetes insipidus in which the kidneys fail to respond to anti-diuretic hormone (ADH), water pills (diuretics) are used to balance your body salt and water. Your doctor may also recommend that you reduce salt in your diet. You’ll also need to drink enough water to avoid dehydration.
Other treatments will depend on the cause of diabetes insipidus:
- If it is caused by tumors or abnormal growths on your hypothalamus or pituitary gland: Your doctor may suggest surgery to remove the growths.
- If a medicine is causing diabetes insipidus: Your doctor may prescribe another type. He or she will look for a medicine that won’t cause excessive thirst and urination.
Talk to your doctor about which option is right for you.
Figure 1. The hypothalamus and pituitary gland location
Figure 2. The hypothalamus and pituitary gland (anterior and posterior) endocrine pathways and target organs
Diabetes insipidus vs Diabetes mellitus
Diabetes insipidus and diabetes mellitus which includes both type 1 and type 2 diabetes—are unrelated, although both conditions cause frequent urination and constant thirst. Diabetes mellitus also known as diabetes which involves insulin problems and high blood sugar or high blood glucose, resulting from the body’s inability to use blood glucose for energy. People with diabetes insipidus have normal blood glucose levels; however, their kidneys cannot balance fluid in the body. The cause of the fluid imbalance depends on the type of diabetes insipidus.
- Central diabetes insipidus. Damage to the pituitary gland or hypothalamus from surgery, a tumor, a head injury or an illness can cause central diabetes insipidus. That damage affects the production, storage and release of antidiuretic hormone (ADH). An inherited disorder may cause this condition too. It also can be the result of an autoimmune reaction that causes the body’s immune system to damage the cells that make antidiuretic hormone (ADH).
- Nephrogenic diabetes insipidus. This happens when there’s a problem with the kidneys that makes them unable to properly respond to antidiuretic hormone (ADH). That problem may be due to:
- An inherited disorder.
- Certain medicines, including lithium and antiviral medicines such as foscarnet (Foscavir).
- Low levels of potassium in the blood.
- High levels of calcium in the blood.
- A blocked urinary tract or a urinary tract infection.
- A chronic kidney condition.
- Gestational diabetes insipidus. This rare form of diabetes insipidus only happens during pregnancy. It develops when an enzyme made by the placenta destroys antidiuretic hormone (ADH) in a pregnant person.
- Primary polydipsia also called dipsogenic diabetes insipidus. People who have this disorder constantly feel thirsty and drink lots of fluids. It can be caused by damage to the thirst-regulating mechanism in the hypothalamus. It also has been linked to mental illness, such as schizophrenia.
Can diabetes insipidus be prevented or avoided?
Most of the time, diabetes insipidus is a permanent condition. You likely won’t be able to prevent it. It is often associated with another health problem, such as abnormal kidney function or tumors. Even though you can’t prevent it in these cases, you can often manage the symptoms.
The kidneys and what do they do
You have two kidneys that are reddish, kidney bean–shaped organs located just above the waist between the peritoneum and the posterior wall of the abdomen. The kidneys are located between the levels of the last thoracic vertebrae T12 and third lumbar (L3) vertebrae, a position where they are partially protected by ribs 11 and 12. A typical adult kidney is 10–12 cm (4–5 in.) long, 5–7 cm (2–3 in.) wide, and 3 cm (1 in.) thick—about the size of a bar of bath soap—and weighs about 135–150 g (4.5–5 oz).
Every day, the kidneys normally filter about 120 to 150 quarts (113.5 to 141.9 liters) of blood to produce about 1 to 2 quarts (0.946 to 1.89 liter) of urine, composed of wastes and extra fluid. The urine flows from the kidneys to the bladder through tubes called ureters. The bladder stores urine. When the bladder empties, urine flows out of the body through a tube called the urethra, located at the bottom of the bladder.
Figure 3. Kidney location
How is fluid regulated in the body?
A person’s body regulates fluid by balancing liquid intake and removing extra fluid. Thirst usually controls a person’s rate of liquid intake, while urination removes most fluid, although people also lose fluid through sweating, breathing, or diarrhea. The hormone vasopressin, also called antidiuretic hormone (ADH), controls the fluid removal rate through urination. The hypothalamus, a small gland located at the base of the brain, produces vasopressin or antidiuretic hormone (ADH). The nearby pituitary gland stores the vasopressin and releases it into the bloodstream when the body has a low fluid level. Vasopressin signals the kidneys to absorb less fluid from the bloodstream, resulting in less urine. When the body has extra fluid, the pituitary gland releases smaller amounts of vasopressin, and sometimes none, so the kidneys remove more fluid from the bloodstream and produce more urine.
Types of diabetes insipidus
The types of diabetes insipidus include:
- central
- nephrogenic
- dipsogenic
- gestational
Each type of diabetes insipidus has a different cause.
Central Diabetes Insipidus
Central diabetes insipidus also called neurogenic diabetes insipidus happens when damage to a person’s hypothalamus or pituitary gland causes disruptions in the normal production, storage, and release of vasopressin (AVP) or antidiuretic hormone (ADH). The disruption of vasopressin (AVP) or antidiuretic hormone (ADH) causes the kidneys to remove too much fluid from the body, leading to an increase in urination.
Central diabetes insipidus is the most common form of diabetes insipidus, occurring in both the sexes equally and at any age.
Damage to the hypothalamus or pituitary gland can result from the following:
- surgery
- infection
- inflammation
- a tumor
- head injury
These subtypes of central diabetes insipidus is known as acquired central diabetes insipidus.
Central diabetes insipidus can also result from an inherited defect in the gene that produces vasopressin (AVP) or antidiuretic hormone (ADH) also known as hereditary central diabetes insipidus, although this cause is rare.
In some cases, the cause of central diabetes insipidus is unknown (idiopathic diabetes insipidus). Based on a literature review, the most common cause of central diabetes insipidus is idiopathic diabetes insipidus 10, 11. In a report of 79 participants, central diabetes insipidus was idiopathic in 52 percent of cases 11. Other cases were from a tumor or infiltrative disease in 38 percent of cases 11.
Idiopathic central diabetes insipidus
Approximately 30 to 50 percent of cases of central diabetes insipidus are idiopathic 3. Idiopathic diabetes insipidus cases are suggested to be associated with an autoimmune process in most patients 12, 13, 14. The autoimmune process is characterized by lymphocytic inflammation of the pituitary gland, specifically the pituitary stalk and the posterior pituitary gland. Early in its course, imaging of the gland (MRI pituitary gland sequence) reveals thickening or enlargement of these structures. A longitudinal study demonstrated the presence of cytoplasmic antibodies directed against vasopressin cells (Ab-positive) in patients with endocrine abnormalities 13.
Another study of 150 patients with central diabetes insipidus evaluated their association with other autoimmune diseases and their correlation with imaging findings. The study reported cause of central diabetes insipidus was idiopathic in 43 percent, familial in 4 percent, granulomatous in 8 percent, and an acquired cause like cranial trauma, tumor, or surgery in 45 percent of cases 14.
Antibodies to vasopressin cells were found in approximately one-third of the patients with idiopathic disease and about one-quarter with non-idiopathic disease 14. Antibody positivity was independently associated with age less than 30 years at disease onset in those with the idiopathic illness, a history of autoimmune disease, or pituitary stalk thickening. Autoimmune central diabetes insipidus was highly probable in young patients with autoimmune disease and pituitary stalk thickening history.
The autoantigens involved in idiopathic central diabetes insipidus are not entirely identified. In patients with lymphocytic infundibuloneurohypophysitis (LINH), autoantibodies to rabphilin-3A, a regulator of secretory vesicle trafficking, are found in a large majority of patients 15. Other autoimmune conditions associated with central diabetes insipidus include Immunoglobulin (Ig) G4-related systemic syndrome, granulomatosis with polyangiitis (PGA), and autoimmune polyglandular syndrome type 1. 16.
Hereditary central diabetes insipidus and congenital disease
Many familial and congenital diseases have been associated with central diabetes insipidus. These include familial central diabetes insipidus, Wolfram syndrome, proprotein convertase subtilisin/kexin type 1 (PCSK1) gene deficiency, and congenital diseases such as congenital hypopituitarism and septo-optic dysplasia.
- Familial central diabetes insipidus also called familial neurohypophyseal diabetes insipidus (FNDI), is an autosomal dominant disease caused by mutations in the gene encoding antidiuretic hormone (ADH) 17. Antidiuretic hormone (ADH) and its corresponding carrier, neurophysin 2, are synthesized as a composite precursor by the magnocellular neurons of the supraoptic and paraventricular nuclei of the hypothalamus 18.
- Wolfram syndrome or DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness) syndrome is characterized by central diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, with cognitive and psychiatric issues that may appear later in life 19; it is inherited as an autosomal recessive trait with incomplete penetrance.
- Proprotein convertase subtilisin/kexin-type 1 (PCSK1) gene deficiency — The proprotein convertase subtilisin/kexin type 1 (PCSK1) gene encodes a 753-amino acid precursor, preproPC1/3, which is processed in the ER into its proenzyme form, proPC1/3. ProPC1/3 is then modified by cleavage of its prodomain into active PC1/3 20. PC1/3 is involved in processing numerous digestive and hypothalamic prohormones, including antidiuretic hormone (ADH). A deficiency of antidiuretic hormone (ADH) leads to central diabetes insipidus.
- Congenital hypopituitarism — central diabetes insipidus has been described in patients with congenital hypopituitarism with or without ectopia of the posterior pituitary lobe 21, 22. The defects in posterior pituitary function in these disorders include symptomatic central diabetes insipidus, nocturia, reduced antidiuretic hormone (ADH) release after osmotic challenge, and hypodipsia or polydipsia.
Neurosurgery or trauma
Traumatic injury to the hypothalamus and posterior pituitary or neurosurgery with a transsphenoidal approach usually induces diabetes insipidus 23, 24, 25. The incidence of central diabetes insipidus in such instances varies with the extent of injury (10-20 percent for surgical removal of adenoma limited to the sella to 60-80 percent after removal of large tumors). Minimally invasive endoscopic pituitary surgery has seen a lower incidence of central diabetes insipidus than the traditional approach 26. Craniopharyngioma (a benign tumour of the suprasellar region) is associated with central diabetes insipidus both before and particularly after surgery 27.
Despite the relatively high frequency of central diabetes insipidus in patients undergoing neurosurgery, most cases of polyuria in this setting are not due to central diabetes insipidus 28. More common causes are the excretion of excess fluid administered during surgery and an osmotic diuresis induced by mannitol or glucocorticoids (which cause hyperglycemia and glucosuria) to reduce cerebral edema. These conditions are generally differentiated from central diabetes insipidus by measuring the urine osmolality and the response to water restriction and the administration of antidiuretic hormone (ADH).
Cancer
Primary or secondary (most often due to lung cancer, leukemia, or lymphoma) tumors in the brain can involve the hypothalamic-pituitary region and lead to central diabetes insipidus 10. Central diabetes insipidus may also be observed in myelodysplastic syndrome
Hypoxic encephalopathy
Hypoxic encephalopathy or severe ischemia (as seen with cardiopulmonary arrest or shock) can lead to diminished ADH release 29. The severity of this defect varies, ranging from mild and asymptomatic to marked polyuria.
Infiltrative disorders
Infiltrative disorders like Langerhan’s cell histiocytosis, granulomatosis with polyangiitis, autoimmune lymphocytic hypophysitis, and sarcoidosis are associated with central diabetes insipidus 30, 31, 32, 33, 34.
Nephrogenic Diabetes Insipidus
Nephrogenic diabetes insipidus occurs when your kidneys do not respond normally to vasopressin or antidiuretic hormone (ADH) and continue to remove too much fluid from your bloodstream. This water, which is meant for and needed by the body, is instead passed out in large quantities as dilute urine, leaving nephrogenic diabetes insipidus patients chronically thirsty and in danger of dehydration. The urine is called dilute urine because it has a high ratio of water to particles – such as minerals and salts – due to the unabsorbed water. Nephrogenic diabetes insipidus patients require a steady supply of water to alleviate their thirst and prevent dehydration.
Nephrogenic diabetes insipidus pathology can be due to resistance at the antidiuretic hormone (ADH) site of activity in the collecting tubules or interference with the countercurrent mechanism, for example, due to medullary injury or decreased sodium chloride reabsorption in the medullary aspect of the thick ascending limb of the loop of Henle 3.
Nephrogenic diabetes insipidus may be either acquired or hereditary from inherited gene changes, or gene mutations, that prevent the kidneys from responding to vasopressin or antidiuretic hormone (ADH).
Other causes of acquired nephrogenic diabetes insipidus include:
- chronic kidney disease
- certain medications, particularly lithium
- low potassium levels in the blood
- high calcium levels in the blood
- blockage of the urinary tract
The most common causes of nephrogenic diabetes insipidus are hereditary nephrogenic diabetes insipidus in children; chronic lithium ingestion and hypercalcemia in adults 3. Acquired causes are often partially reversible with cessation of the offending drug or correction of hypercalcemia.
The causes of nephrogenic diabetes insipidus can also be unknown.
Acquired nephrogenic diabetes insipidus
Acquired nephrogenic diabetes insipidus is the more common form of nephrogenic diabetes insipidus and can occur at any time of life. Acquired nephrogenic diabetes insipidus occurs more frequently than inherited nephrogenic diabetes insipidus, but is still a rare disorder. It is usually less severe than inherited nephrogenic diabetes insipidus and usually occurs in adults. This is because acquired nephrogenic diabetes insipidus is generally due to a treatment or pathology that develops later in life.
Nephrogenic diabetes insipidus can be acquired at any time 3, 35, 36, 37:
- through the use of certain prescription drugs,
- as a result of a physical condition,
- chronic kidney failure,
- other kidney diseases,
- abnormally low levels of potassium (hypokalemia). Persistent severe hypokalemia can impair urinary concentrating ability. The mechanisms by which this occurs are incompletely understood. Downregulation of urea transporters may also contribute to the impairment of urinary concentrating ability induced by potassium depletion 38
- abnormally high levels of calcium (hypercalcemia). A plasma calcium concentration persistently above 11 mg/dl (2.75 mmol/L) can impair renal concentrating ability 39. The mechanisms with which these occur are not entirely understood. This defect may be associated with reductions in sodium chloride reabsorption in the thick ascending loop of Henle, thereby interfering with the countercurrent mechanism and ADH’s ability to increase collecting tubule water permeability 39. The concentrating defect induced by hypercalcemia is generally reversible with a normal serum calcium concentration restoration. However, the defect may persist in patients with permanent medullary damage.
- sickle cell disease or trait,
- autosomal dominant polycystic kidney disease and medullary cystic kidney disease,
- renal amyloidosis,
- one or more of the ureters (the fibromuscular tubes shunting urine from the kidney to the bladder) being blocked,
- Sjögren syndrome 40,
- as the result of protein starvation,
- on a transient basis during pregnancy (a transient nephrogenic diabetes insipidus is noted in a few patients during the second half of their pregnancy) 41,
- because of an underlying systemic disease or disorder.
Most often, acquired nephrogenic diabetes insipidus is a result of the use of the drug, lithium 3, 42. The adverse effect of lithium is mediated by the entry into the principal cells in the collecting tubule via the epithelial sodium channel (ENaC) 43. Lithium inhibits the signaling pathway at cytotoxic concentrations, leading to dysfunction of the aquaporin-2 (AQP2) water channel 43.
Acquired nephrogenic diabetes insipidus can also result from the use of other drugs, such as cidofovir, foscarnet, ofloxacin, ifosfamide, colchicine, methoxyflurane, orlistat, amphotericin B, gentamicin, loop diuretics and demeclocycline 3, 42. Drug-induced nephrogenic diabetes insipidus is typically reversible, at least in part.
Inherited nephrogenic diabetes insipidus
There are three types of inherited nephrogenic diabetes insipidus 35:
- X-linked nephrogenic diabetes insipidus is the most common type of inherited nephrogenic diabetes insipidus, affecting around 90 percent of the cases of inherited nephrogenic diabetes insipidus 4. X-linked nephrogenic diabetes insipidus are due to mutations in the AVPR2 gene located on the X chromosome (Xq-28), which encodes for a dysfunctional vasopressin V2 receptor (V2R) 44. X-linked nephrogenic diabetes insipidus affects males more often than females. X-linked nephrogenic diabetes insipidus occurs with a frequency of 4 to 8 per 1 million male live births 7. Males are certain to be seriously affected by X-linked nephrogenic diabetes insipidus if they inherit the gene, whereas females are usually affected mildly or not at all. Rarely, girls may be affected as severely as boys. Females who carry this gene, whether or not they show symptoms, will pass it on to their daughters and their sons 50% of the time.
- Autosomal recessive nephrogenic diabetes insipidus is a much rarer type of inherited nephrogenic diabetes insipidus. It affects males and females equally. For a child to have the disease, both parents must carry this gene. Parents who are both carriers of this form of nephrogenic diabetes insipidus have a 25% chance with each pregnancy of having another affected child.
- Autosomal dominant nephrogenic diabetes insipidus is an extremely rare type of nephrogenic diabetes insipidus. It affects both males and females. For a child to inherit this type of nephrogenic diabetes insipidus, only one parent need carry the gene.
Inherited nephrogenic diabetes insipidus is very rare. Those who inherit it may begin showing symptoms in the first few days of life.
For a hormone to transmit its hormonal message, it must first link with a hormone receptor specific to it. Normally, the hormone arginine vasopressin (AVP) links up to a hormone receptor called the vasopressin-2 receptor (V2R) that is located in the principal cells of a part of the kidney called the collecting duct. This linking of arginine vasopressin (AVP) and vasopressin-2 receptor (V2R) initiates a molecular sequence that makes the cells of the kidney collecting duct more water permeable. This enables the kidneys to reabsorb the water flowing through the collecting duct. It is this sequence that breaks down when a person has nephrogenic diabetes insipidus 42.
The vasopressin-2 receptor (V2R) receptor, like all receptors, is a protein. Cells synthesize proteins using instructions encoded in genes. When a gene is mutated, it is likely to produce defective proteins incapable of carrying out their function in the body. The transmission of mutated genes from parents to child forms the basis of inherited disorders. To date, researchers have identified the genetic basis of three forms of inherited nephrogenic diabetes insipidus:
X-linked nephrogenic diabetes insipidus is caused by mutations of the AVPR2 gene (V2R gene) that result in defective vasopressin V2 receptors (V2Rs).
Both autosomal recessive nephrogenic diabetes insipidus and autosomal dominant nephrogenic diabetes insipidus are caused by mutations of the aquaporin-2 (AQP2) gene that encodes the ADH-sensitive water channels in the collecting tubule cells resulting in defective AQP2s 45, 46.
Up to 90% of the cases of inherited nephrogenic diabetes insipidus are the result of mutations of the AVPR2 gene (V2R gene). The remaining cases are the result of mutations of the AQP2 gene. Though each type of inherited nephrogenic diabetes insipidus has a different genetic cause, the symptoms of each are identical.
What is different about the two forms of inherited nephrogenic diabetes insipidus is that males are the most likely to display symptoms of X-linked nephrogenic diabetes insipidus, whereas males and females are equally likely to display symptoms of the autosomal recessive form of nephrogenic diabetes insipidus. This is because the V2R gene is carried on the X-chromosome. Males have one X-chromosome, and females have two X-chromosomes. Therefore, if a male inherits a mutated V2R gene from his mother, he will most likely have nephrogenic diabetes insipidus, whereas the female, should she carry a mutated V2R gene on one of her X-chromosomes, has a good chance of having a normal V2R gene on her other X-chromosome. Her normal V2R gene will mask the effects of her mutated V2R gene. Autosomal recessive nephrogenic diabetes insipidus is carried on a non-sex chromosome, so males and females are equally likely to inherit it, though a mutated AQP2 gene must be inherited from both parents for nephrogenic diabetes insipidus to express itself in their child.
In 1969 Bode and Crawford hypothesized in “Nephrogenic diabetes insipidus in North America–The Hopewell hypothesis” that all inherited nephrogenic diabetes insipidus originated from a common carrier (which implied a common genetic mutation the same for all nephrogenic diabetes insipidus patients) 47. Since then, researchers have disproved this hypothesisby discovering over 70 different mutations of the V2R gene 42. Many of these mutations are located at different points on the V2R gene and disable the V2Rsproduced by these genes in different ways. Researchers have also discovered more than 13 different AQP2 gene mutations, located at different points on the AQP2 gene, that disrupt AQP2s in different ways. Inherited nephrogenic diabetes insipidus has been identified in different ethnic groups worldwide 42.
Dipsogenic Diabetes Insipidus (primary polydipsia)
A defect in the thirst mechanism, located in a person’s hypothalamus, causes dipsogenic diabetes insipidus or primary polydipsia. This defect results in an abnormal increase in thirst and liquid intake that suppresses vasopressin or antidiuretic hormone (ADH) secretion and increases urine output. The same events and conditions that damage the hypothalamus or pituitary—surgery, infection (e.g., tuberculous meningitis), inflammation (e.g., sarcoidosis), a tumor, head injury, multiple sclerosis —can also damage the thirst mechanism. Certain medications (e.g., phenothiazine) or mental health problems such as schizophrenia, compulsive behavior, or stress reduction may predispose a person to dipsogenic diabetes insipidus (primary polydipsia) 6, 48, 49.
Gestational Diabetes Insipidus
Gestational diabetes insipidus occurs only during pregnancy and in about 1 in 30,000 pregnancies 3. In some cases, an enzyme made by the placenta called placental cysteine aminopeptidase breaks down the mother’s vasopressin or antidiuretic hormone (ADH) 50. In other cases, pregnant women produce more prostaglandin, a hormone-like chemical that reduces kidney sensitivity to vasopressin. Most pregnant women who develop gestational diabetes insipidus have a mild case that does not cause noticeable symptoms 41. Gestational diabetes insipidus typically presents in the third trimester and usually goes away after the mother delivers the baby (about 2 or 3 weeks postpartum); however, it may return if the mother becomes pregnant again, therefore diagnosis to reveal any underlying pathology is necessary 51, 1.
Diabetes insipidus complications
The two main complications of diabetes insipidus are dehydration and an electrolyte imbalance. Complications are more likely if the condition goes undiagnosed or is poorly controlled.
Dehydration
If you have diabetes insipidus, your body will find it difficult to retain enough water, even if you drink fluid constantly. This can lead to dehydration (a severe lack of water in the body).
If you or someone you know has diabetes insipidus, it’s important to look out for the signs and symptoms of dehydration.
Signs of dehydration include:
- thirst
- dry skin
- fatigue
- sluggishness
- dizziness
- confusion
- nausea
- dizziness or light-headedness
- headache
- dry mouth and lips
- sunken features (particularly the eyes)
- confusion and irritability
Severe dehydration can lead to seizures, permanent brain damage, and even death.
Dehydration can be treated by rebalancing the level of water in your body.
If you’re severely dehydrated, you may need intravenous fluid replacement in hospital. This is where fluids are given directly through a drip into your vein.
Electrolyte imbalance
Diabetes insipidus can also cause an electrolyte imbalance. Electrolytes are minerals in your blood that have a tiny electric charge, such as sodium, calcium, potassium, chlorine, magnesium and bicarbonate.
If the body loses too much water, the concentration of these electrolytes can go up simply because the amount of water they’re contained in has gone down.
This dehydration disrupts other functions of the body, such as the way muscles work. It can also lead to:
- headache
- fatigue (feeling tired all the time)
- irritability
- muscle pain.
Diabetes insipidus causes
Diabetes insipidus is usually caused by problems with a hormone called vasopressin, which is also known as antidiuretic hormone (ADH), that helps your kidneys balance the amount of fluid in your body. Vasopressin or antidiuretic hormone (ADH) is produced by the hypothalamus and stored in the pituitary gland until needed. The hypothalamus is an area of your brain that controls mood and appetite. The pituitary gland is located below your brain, behind the bridge of your nose.
Vasopressin or antidiuretic hormone (ADH) regulates the level of water in your body by controlling the amount of urine your kidneys produce. When the level of water in your body decreases, your pituitary gland releases vasopressin or antidiuretic hormone (ADH) to conserve water and stop the production of urine.
In diabetes insipidus, vasopressin or antidiuretic hormone (ADH) fails to properly regulate your body’s level of water, and allows too much urine to be produced and passed from your body.
Specific causes vary among the four types of diabetes insipidus: central, nephrogenic, , and .
There are 4 main types of diabetes insipidus 52:
- Central diabetes insipidus – where the body doesn’t produce enough vasopressin, so excessive amounts of water are lost in large amounts of urine
- Nephrogenic diabetes insipidus – where vasopressin is produced at the right levels but, for a variety of reasons, the kidneys don’t respond to it in the normal way.
- Dipsogenic diabetes insipidus also known as primary polydipsia occurs due to a defect in the thirst mechanism located in a person’s hypothalamus. This defect results in an abnormal increase in thirst and liquid intake that suppresses vasopressin or antidiuretic hormone (ADH) secretion and increases urine output. The same events and conditions that damage the hypothalamus or pituitary—surgery, infection, inflammation, a tumor, head injury—can also damage the thirst mechanism. Certain medications or mental health problems such as schizophrenia may predispose a person to dipsogenic diabetes insipidus.
- Gestational diabetes insipidus occurs only during pregnancy. In some cases, an enzyme made by the placenta—a temporary organ joining mother and baby—breaks down the mother’s vasopressin or antidiuretic hormone (ADH). In other cases, pregnant women produce more prostaglandin, a hormone-like chemical that reduces kidney sensitivity to vasopressin. Most pregnant women who develop gestational diabetes insipidus have a mild case that does not cause noticeable symptoms. Gestational diabetes insipidus usually goes away after the mother delivers the baby; however, it may return if the mother becomes pregnant again.
Possible underlying causes for 4 types of diabetes insipidus are described below.
Central diabetes insipidus
The three most common causes of central diabetes insipidus are:
- a brain tumor that damages the hypothalamus or pituitary gland
- a severe head injury that damages the hypothalamus or pituitary gland
- complications that occur during brain or pituitary surgery
No cause can be found for about a third of all cases of central diabetes insipidus. These cases, known as idiopathic, appear to be related to the immune system attacking the normal, healthy cells producing vasopressin. It’s unclear what causes the immune system to do this.
Less common causes of central diabetic insipidus include:
- cancers that spread from another part of the body to the brain
- Wolfram syndrome, which is a rare genetic disorder that also causes vision loss
- brain damage caused by a sudden loss of oxygen, which can occur during a stroke or drowning
- infections, such as meningitis and encephalitis, that can damage the brain
Nephrogenic diabetes insipidus
Your kidneys contain nephrons, which are tiny intricate structures that filter waste products from the blood and help produce urine. They also control how much water is reabsorbed into your body and how much is passed in the urine.
In a healthy person, vasopressin acts as a signal to the nephrons to reabsorb water into the body. In nephrogenic diabetes insipidus, the nephrons in the kidney aren’t able to respond to this signal, leading to excessive water loss in large amounts of urine. Your thirst increases to try to balance this loss from the body.
Nephrogenic diabetes insipidus can be congenital (present at birth) or acquired (where it develops later in life as a result of an external factor). These are described in more detail below.
Congenital nephrogenic diabetes insipidus
Two genetic mutations (abnormal changes in genes that leads to them not working properly) have been identified that cause congenital nephrogenic diabetes insipidus.
The first, known as the vasopressinR2 gene mutation, is responsible for 90% of all cases of congenital diabetes insipidus. However, it’s still rare, occurring in an estimated 1 in 250,000 births.
The vasopressinR2 gene mutation can only be passed down by mothers (who may appear to not be affected) to their sons (who are affected).
The remaining 10% of cases of congenital nephrogenic diabetes insipidus are caused by the AQP2 gene mutation, which can affect both males and females.
Acquired nephrogenic diabetes insipidus
Lithium is the most common cause of acquired nephrogenic diabetes insipidus. It’s a medication that’s often used to treat bipolar disorder. Long-term lithium use can damage the cells of the kidneys so they no longer respond to vasopressin.
Just over half of all people on long-term lithium therapy develop some degree of nephrogenic diabetes insipidus. Stopping lithium treatment often restores normal kidney function, although in many cases the damage to the kidneys is permanent.
Due to these risks, it’s recommended that you have kidney function tests every three months if you’re taking lithium.
Other causes of acquired nephrogenic diabetes insipidus include:
- hypercalcaemia – a condition where there’s too much calcium in the blood (high calcium levels can damage the kidneys)
- hypokalemia – a condition where there isn’t enough potassium in the blood (all the cells in the body, including kidney cells, require potassium to function properly)
- pyelonephritis (kidney infection) – where the kidneys are damaged by an infection
- ureteral obstruction – where one or both tubes (ureters) that connect the kidneys to the bladder become blocked by an object, such as a kidney stone, which damages the kidneys.
Dipsogenic diabetes insipidus (primary polydipsia)
A defect in the thirst mechanism, located in a person’s hypothalamus, causes dipsogenic diabetes insipidus or primary polydipsia. This defect results in an abnormal increase in thirst and liquid intake that suppresses vasopressin or antidiuretic hormone (ADH) secretion and increases urine output. The same events and conditions that damage the hypothalamus or pituitary—surgery, infection (e.g., tuberculous meningitis), inflammation (e.g., sarcoidosis), a tumor, head injury, multiple sclerosis —can also damage the thirst mechanism. Certain medications (e.g., phenothiazine) or mental health problems such as schizophrenia, compulsive behavior, or stress reduction may predispose a person to dipsogenic diabetes insipidus (primary polydipsia) 6, 48, 49.
Gestational diabetes insipidus
Gestational diabetes insipidus occurs only during pregnancy and in about 1 in 30,000 pregnancies 3. In some cases, an enzyme made by the placenta called placental cysteine aminopeptidase breaks down the mother’s vasopressin or antidiuretic hormone (ADH) 50. In other cases, pregnant women produce more prostaglandin, a hormone-like chemical that reduces kidney sensitivity to vasopressin. Most pregnant women who develop gestational diabetes insipidus have a mild case that does not cause noticeable symptoms 41. Gestational diabetes insipidus typically presents in the third trimester and usually goes away after the mother delivers the baby (about 2 or 3 weeks postpartum); however, it may return if the mother becomes pregnant again, therefore diagnosis to reveal any underlying pathology is necessary 51, 1.
Diabetes insipidus signs and symptoms
The most common signs and symptoms of diabetes insipidus are:
- Extreme thirst or polydipsia (water intake of up to 20 liters per day)
- Frequent urination or polyuria
- Excretion of an excessive amount of diluted urine (producing 8–16 liters of pale or colorless, watery urine).
If you have diabetes insipidus, you may pass pale, watery urine every 15-20 minutes. The amount of urine passed can range from 3 liters in mild cases to up to 20 liters in severe cases.
Other signs may include needing to get up at night to urinate (nocturia) and bed-wetting.
It’s also likely that you’ll feel thirsty all the time and have a ‘dry’ feeling that’s always present, no matter how much water you drink.
If you need to pass urine regularly and always feel thirsty, your sleeping patterns and daily activities may be disrupted. This can cause tiredness, irritability and difficulty concentrating, which can affect your daily life further.
You may also feel generally unwell and ‘run down’ much of the time for no apparent reason.
Other symptoms are dizziness, weakness, fatigue, and signs of dehydration (fever, dry skin and mucus membranes, weight loss, poor skin turgor). Low blood pressure (hypotension) and rapid heart rate (tachycardia) with decreased right atrial and pulmonary artery occlusion pressures and an altered level of consciousness may occur 7. Young children may present with severe dehydration, vomiting, constipation, fever, irritability, sleep disturbances, retardation of growth, and failure to thrive. Mental retardation can be caused by repeated and unrecognized dehydration 6, 4..
Symptoms in children
Excessive thirst can be difficult to recognize in children who are too young to speak.
Signs and symptoms that could suggest diabetes insipidus include:
- excessive crying
- irritability
- slower than expected growth
- hyperthermia (high body temperature)
- unexplained weight loss
In older children, symptoms of diabetes insipidus include:
- wetting the bed (enuresis) – although most children who wet the bed don’t have diabetes insipidus
- loss of appetite
- feeling tired all the time (fatigue)
Diabetes insipidus diagnosis
As the symptoms of diabetes insipidus are similar to those of other conditions, including type 1 diabetes and type 2 diabetes, tests will be needed to confirm which condition you have.
If diabetes insipidus is diagnosed, the tests will also be able to identify the type you have – central or nephrogenic diabetes insipidus.
A health care provider can diagnose a person with diabetes insipidus based on the following:
- medical and family history
- physical exam
- urinalysis
- blood tests
- water deprivation test
- desmopressin challenge test
- magnetic resonance imaging (MRI)
The diagnostic algorithm of diabetes insipidus is described in Figure 4. The ability of the brain to produce ADH and of the kidney to respond to it is measured by the water deprivation test (Hare-Hickey test) and desmopressin challenge test. A 24-hour urine volume is used to confirm polyuria. During water deprivation, hourly measurements of body weight and urine osmolality are made, until 2–3 samples vary by <30 mOsm/kg (or <10%), or until the patient loses 5% of his/her body weight. Serum ADH is then measured and desmopressin (5 units) is injected. Urine osmolality is measured 30–60 minutes after this 49, 5, 4. The test is discontinued if the patient loses >5% of his/her body weight and/or plasma sodium (Na+) exceeds 143 mEq/L and/or urine osmolality increases to normal. Response to the administration of desmopressin distinguishes between central diabetes insipidus and nephrogenic diabetes insipidus. The interpretation of the water deprivation test is described in Table 1 below.
Findings suggestive of primary polydipsia (Dipsogenic Diabetes Insipidus) include the history of psychiatric disease, fluctuating symptoms and gradual onset of polydipsia, posterior pituitary bright spot and normal thickness of the pituitary stalk, and hyponatremia after a therapeutic trial with desmopressin 1.
Table 1. Interpretation of the water deprivation test and the desmopressin challenge test in the diagnosis of diabetes insipidus
[Source 7 ]Figure 4. Diabetes insipidus diagnostic algorithm
Footnote: Diagnostic flowchart for central and nephrogenic diabetes insipidus.
Abbreviations: AVP = Arginine vasopressin; Posm = Plasma osmolality; Uosm = Urine osmolality; DI = diabetes insipidus
[Source 7 ]Medical and Family History
Taking a medical and family history can help a health care provider diagnose diabetes insipidus. A health care provider will ask the patient to review his or her symptoms and ask whether the patient’s family has a history of diabetes insipidus or its symptoms.
Physical Exam
A physical exam can help diagnose diabetes insipidus. During a physical exam, a health care provider usually examines the patient’s skin and appearance, checking for signs of dehydration.
Urinalysis
Urinalysis tests a urine sample. A patient collects the urine sample in a special container at home, in a health care provider’s office, or at a commercial facility. A health care provider tests the sample in the same location or sends it to a lab for analysis. The test can show whether the urine is dilute or concentrated. The test can also show the presence of glucose, which can distinguish between diabetes insipidus and diabetes mellitus. The health care provider may also have the patient collect urine in a special container over a 24-hour period to measure the total amount of urine produced by the kidneys.
Blood Tests
A blood test involves drawing a patient’s blood at a health care provider’s office or a commercial facility and sending the sample to a lab for analysis. The blood test measures sodium levels, the levels of antidiuretic hormone (ADH) in your blood, which can help diagnose diabetes insipidus and in some cases determine the type.
Your blood and urine may also be tested for substances such as glucose (blood sugar), calcium and potassium. If you have diabetes insipidus, your urine will be very dilute, with low levels of other substances. A large amount of sugar in your urine may be a sign of type 1 or type 2 diabetes rather than diabetes insipidus.
Copeptin (the C-terminal glycoprotein of the vasopressin prohormone)
Endogenous vasopressin measurement is an important test to differentiate between central diabetes insipidus and nephrogenic diabetes insipidus. Copeptin (the C-terminal glycoprotein of the vasopressin prohormone) can be considered a stable surrogate for endogenous plasma vasopressin. Vasopressin (AVP) is unstable, largely attached to platelets and is rapidly cleared. The small size of vasopressin makes estimation difficult 1, 53. In one study, copeptin levels were found to be <2.5 pmol/L in all patients with central diabetes insipidus, suggesting that it could be used to distinguish central diabetes insipidus from primary polydipsia (dipsogenic diabetes insipidus) and nephrogenic diabetes insipidus 54.
Fluid Deprivation Test
A fluid deprivation test measures changes in a patient’s body weight and urine concentration after restricting liquid intake. If you have diabetes insipidus, you’ll continue to pass large amounts of dilute urine, when normally you’d only pass a small amount of concentrated urine.
A health care provider can perform two types of fluid deprivation tests:
- A short form of the deprivation test. A health care provider instructs the patient to stop drinking all liquids for a specific period of time, usually during dinner. The next morning, the patient will collect a urine sample at home. The patient then returns the urine sample to his or her health care provider or takes it to a lab where a technician measures the concentration of the urine sample.
- A formal fluid deprivation test. A health care provider performs this test in a hospital to continuously monitor the patient for signs of dehydration. Patients do not need anesthesia. A health care provider weighs the patient and analyzes a urine sample. The health care provider repeats the tests and measures the patient’s blood pressure every 1 to 2 hours until one of the following happens:
- The patient’s blood pressure drops too low or the patient has a rapid heartbeat when standing.
- The patient loses 5 percent or more of his or her initial body weight.
- Urine concentration increases only slightly in two to three consecutive measurements.
At the end of the test, a health care provider will compare the patient’s blood sodium, vasopressin levels, and urine concentration to determine whether the patient has diabetes insipidus. Sometimes, the health care provider may administer medications during the test to see if they increase a patient’s urine concentration. In other cases, the health care provider may give the patient a concentrated sodium solution intravenously at the end of the test to increase the patient’s blood sodium level and determine if he or she has diabetes insipidus.
Vasopressin test
After the water deprivation test, you may be given a small dose of vasopressin, usually as an injection. This will show how your body reacts to the hormone, which helps to identify the type of diabetes insipidus you have.
If the dose of vasopressin stops you producing urine, it’s likely your condition is due to a shortage of vasopressin. If this is the case, you may be diagnosed with central or cranial diabetes insipidus.
If you continue to produce urine despite the dose of vasopressin, this suggests there’s already enough vasopressin in your body, but your kidneys aren’t responding to it. In this case, you may be diagnosed with nephrogenic diabetes insipidus.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a test that takes pictures of the body’s internal organs and soft tissues without using x-rays. A specially trained technician performs the procedure in an outpatient center or a hospital, and a radiologist—a doctor who specializes in medical imaging—interprets the images. A patient does not need anesthesia, although people with a fear of confined spaces may receive light sedation. An MRI may include an injection of a special dye, called contrast medium. With most MRI machines, the person lies on a table that slides into a tunnel-shaped device that may be open ended or closed at one end. Some MRI machines allow the patient to lie in a more open space. MRIs cannot diagnose diabetes insipidus. Instead, an MRI can show if the patient has problems with his or her hypothalamus or pituitary gland or help the health care provider determine if diabetes insipidus is the possible cause of the patient’s symptoms.
Genetic screening
If your doctor suspects an inherited form of diabetes insipidus, he or she will look at your family history of polyuria and may suggest genetic screening.
Diabetes insipidus treatment
The primary treatment for diabetes insipidus involves drinking enough liquid to prevent dehydration. A health care provider may refer a person with diabetes insipidus to a nephrologist—a doctor who specializes in treating kidney problems—or to an endocrinologist—a doctor who specializes in treating disorders of the hormone-producing glands. Treatment for frequent urination or constant thirst depends on the patient’s type of diabetes insipidus:
Central diabetes insipidus
Mild central diabetes insipidus may not require any medical treatment.
Central diabetes insipidus is considered mild if you produce approximately 3-4 liters of urine over 24 hours.
If this is the case, you may be able to ease your symptoms by increasing the amount of water you drink, to avoid dehydration. Your doctor or endocrinologist (specialist in hormone conditions) may advise you to drink a certain amount of water every day, usually at least 2.5 liters.
However, if you have more severe central diabetes insipidus, drinking water may not be enough to control your symptoms. As your condition is due to a shortage of vasopressin, your doctor or endocrinologist may prescribe a treatment that takes the place of vasopressin, known as desmopressin (DDAVP).
Other treatment options for central diabetes insipidus include a low-solute diet (low salt, low protein), thiazide diuretics, chlorpropamide, carbamazepine, and non-steroidal anti-inflammatory drugs (NSAID) 55, 56, 57.
Desmopressin (DDAVP)
A synthetic, or man-made, hormone called desmopressin (DDAVP) treats central diabetes insipidus 58. Desmopressin is more powerful and more resistant to being broken down than the vasopressin naturally produced by your body. It works just like natural vasopressin, stopping your kidneys producing urine when the level of water in your body is low. The medication comes as an injection, a nasal spray, or a pill. The medication works by replacing the vasopressin that a patient’s body normally produces. This treatment helps a patient manage symptoms of central diabetes insipidus; however, it does not cure the disease.
If you’re prescribed desmopressin as a nasal spray, you’ll need to spray it inside your nose once or twice a day, where it’s quickly absorbed into your bloodstream.
If you’re prescribed desmopressin tablets, you may need to take them more than twice a day. This is because desmopressin is absorbed into your blood less effectively through your stomach than through your nasal passages, so you need to take more to have the same effect.
Your doctor or endocrinologist may suggest switching your treatment to tablets if you develop a cold that prevents you from using the nasal spray.
Desmopressin is very safe to use and has few side effects.
Desmopressin is considered safe during pregnancy.
However, possible side effects can include:
- headache
- stomach pain
- feeling sick
- blocked or runny nose
- nosebleeds
If you take too much desmopressin or drink too much fluid while taking it, it can cause your body to retain too much water.
This can result in:
- headaches
- dizziness
- feeling bloated
- hyponatremia – a low level of sodium (salt) in your blood
Symptoms of hyponatremia include:
- a severe or prolonged headache
- confusion
- nausea and vomiting
If you think you may have hyponatremia, stop taking desmopressin immediately and call your doctor for advice.
Carbamazepine
Carbamazepine is an anticonvulsant and psychotropic drug used to treat epilepsy or intellectual disabilities. It may stimulate the release of vasopressin from the pituitary gland and act directly on renal tubules. Carbamazepine increased water absorption in the absence of vasopressin in vitro. The effect was cyclic adenosine monophosphate (cAMP)-dependent. In rats, carbamazepine increased water permeability and absorption in the inner medullary collecting duct, acted directly on the V2R-protein G complex and increased AQP2 expression 59. Meinders et al. 60, studied six central diabetes insipidus patients treated with 200–800 mg carbamazepine daily. All patients had a reduction in urinary water output (from 8–16 L/day to 1.9–6 L/day) and an increase in urine osmolality (from 60–120 mOsm/kg to 150–532 mOsm/kg). ADH (vasopressin) levels were undetectable in diabetes insipidus patients and healthy subjects, suggesting that carbamazepine did not stimulate the release of, or inhibit the breakdown of vasopressin or ADH.
Hyponatremia has been detected in 4.8–40% of patients receiving carbamazepine for neurological disorders 61, 59, 62 and among patients receiving it for back pain 63. Prolonged carbamazepine treatment has been linked to reduction in antidiuretic activity possibly due to auto-induction of carbamazepine 64.
Chlorpropamide
The antidiuretic drug, chlorpropamide, decreases the clearance of solute-free water if the neurohypophysis has the residual secretory capacity and normalizes plasma osmolality 65. Chlorpropamide administered to children and adults (125–1000 mg daily) reduced urine output from 5.4–10.7 L/day to <2 L/day. Maximal diuresis was achieved in 3–4 days, and the effect was dose-dependent 66.
Chlorpropamide may enhance the neurohypophyseal secretion of vasopressin, have a vasopressin-like effect or potentiate the effect of minute amounts of vasopressin which persist in diabetes insipidus 66, 67. Chlorpropamide may potentiate vasopressin-induced water transport through activation of renal tubular adenylyl cyclase which generates cAMP rather than affecting water movement 67.
Clofibrate
Clofibrate is a hypolipidemic agent which stimulates ADH production in patients with partial central diabetes insipidus 68, 5. Clofibrate treatment (500 mg every 6 hours) significantly reduced the mean urine clearance from 280 mL/h to 141 mL/h, and the mean free water clearance from 158 mL/h to 10 mL/h in 6 cases of diabetes insipidus. Urine osmolality increased from 152 mOsm/kg to 317 mOsm/kg, with a concomitant decrease in urinary ADH excretion in 4/6 patients. There was a significant antidiuretic activity even in patients who were water-loaded 68.
Chlorpropamide treatment causes a greater reduction in mean urine volume (59% vs. 47%), and higher urine osmolality compared to clofibrate treatment. However, clofibrate significantly reduces free water clearance. Furthermore, chlorpropamide can cause serious hypoglycemia, unlike clofibrate 69. Clofibrate is generally safe, but there have been reports of clofibrate-induced myopathy 70. It is likely that hypothyroidism coexisting in patients with secondary diabetes insipidus may precipitate the development of clofibrate-induced myopathy 70.
Indapamide
Indapamide is an antihypertensive, diuretic drug which could be considered as an alternative drug for mild central diabetes insipidus 9. The molecular structure is similar to hydrochlorothiazide and chlorpropamide. As with thiazides, the activity of indapamide may be due to proximal tubular water reabsorption 71. The first report on the use of indapamide (at 2.5 mg/day) for diabetes insipidus demonstrated a reduction in the 24-h urinary volume (from 5–16 L to 2.3–9.2 L) in 3 patients with central diabetes insipidus 72.
Among patients with treatment-naive central diabetes insipidus, there was a smaller reduction in mean urine output volume (48.49% ± 10.69% vs. 40.56% ± 9.7%) in patients receiving indapamide compared to those receiving chlorpropamide. Urine osmolality increased in all nine patients receiving indapamide and in 7/11 patients receiving chlorpropamide, while serum osmolality decreased in 4/9 and 7/11 patients, respectively. Chlorpropamide was discontinued due to hypoglycemia in 2 patients while there were no adverse effects among patients receiving indapamide 71.
Nephrogenic diabetes insipidus
In some cases, nephrogenic diabetes insipidus goes away after treatment of the cause. For example, switching medications or taking steps to balance the amount of calcium or potassium in the patient’s body may resolve the problem.
As nephrogenic diabetes insipidus is caused by your kidneys not responding to vasopressin, rather than a shortage of vasopressin, it usually can’t be treated with desmopressin. However, it’s still important to drink plenty of water to avoid dehydration.
If your condition is mild, your doctor or endocrinologist may suggest reducing the amount of salt and protein in your diet, which will help your kidneys produce less urine. This may mean eating less salt and protein-rich food, such as processed foods, meat, eggs and nuts. Don’t alter your diet without first seeking medical advice. Your doctor or endocrinologist will be able to advise you about which foods to cut down on.
If you have more severe nephrogenic diabetes insipidus, you may be prescribed a combination of thiazide diuretics and an NSAID to help reduce the amount of urine your kidneys produce.
Medications for nephrogenic diabetes insipidus include diuretics, either alone or combined with aspirin or ibuprofen. Health care providers commonly prescribe diuretics to help patients’ kidneys remove fluid from the body. Paradoxically, in people with nephrogenic diabetes insipidus, a class of diuretics called thiazides reduces urine production and helps patients’ kidneys concentrate urine. Aspirin or ibuprofen also helps reduce urine volume.
Thiazide diuretics
Thiazide diuretics can reduce the rate the kidney filters blood, which reduces the amount of urine passed from the body over time. Thiazide diuretics can be used to treat both nephrogenic diabetes insipidus and central diabetes insipidus 5, 73.
Thiazide diuretics act at the distal convoluted tubule and inhibit cotransport of sodium and chloride. Prolonged administration reduces extracellular fluid volume, allowing water and sodium reabsorption at the proximal tubules. Ultimately, there is reduction of urine output 73. An animal model of lithium-induced nephrogenic diabetes insipidus demonstrated that chronic hydrochlorothiazide treatment up-regulates NCC and ENaC, which enhances sodium reabsorption along the distal segments of the nephron 74.
Treatment with chlorothiazide (5–10 mg/kg/day) or hydrochlorothiazide (1–2 mg/kg/day) was effective and safe, with hospitalization for hypernatremia required in only 1/13 patients 75.
Side effects are uncommon but include:
- dizziness when standing
- indigestion
- very sensitive skin
- erectile dysfunction (impotence) in men
This last side effect is usually temporary and should resolve itself if you stop taking the medication.
Treatment with chlorothiazide or hydrochlorothiazide reduced water intake by 23–40% in children with nephrogenic diabetes insipidus 76. The combination of amiloride and hydrochlorothiazide was superior to hydrochlorothiazide alone and prevented urinary potassium loss, hypokalemia, and alkalosis 77. Though hydrochlorothiazide decreases urine volume by up to 50% in pregnancy, the risks of hypokalemia and hypovolemia cause it to be used sparingly in gestational diabetes insipidus 51.
Amiloride
Amiloride is a diuretic that is useful in the treatment of lithium-induced nephrogenic diabetes insipidus. It reduces transcellular lithium transport, intracellular lithium concentration and lithium-induced inactivation of GSK-3-xβ. Blocking of ENaC with amiloride reduces the lithium-induced down-regulation of AQP2 expression and protects the cellular composition of the collecting duct 78. In a study of the effect of amiloride versus placebo on lithium-induced nephrogenic diabetes insipidus, amiloride administered for 6 weeks increased maximal urine osmolality and AQP2 excretion 79.
Non-steroidal anti-inflammatory drugs (NSAIDs)
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and indomethacin, help reduce urine volume further when they’re used in combination with thiazide diuretics.
Weinstock and Moses 80 reported that indomethacin effectively treated nephrogenic diabetes insipidus that persisted after lithium therapy was discontinued. When used along with desmopressin, there was a marked decrease in polyuria 80. A patient who did not respond to desmopressin, thiazides, and amiloride for the treatment of lithium-induced nephrogenic diabetes insipidus, responded immediately to indomethacin treatment with a reduction in urine volume from 24 L/day to 12 L/day, and ultimately to 2 L/day 81. Indomethacin successfully treated streptozocin-induced nephrogenic diabetes insipidus, with a rapid correction in polyuria that was independent of the glomerular filtration rate 82. Indomethacin may impair kidney function 80 and have severe adverse effects such as gastrointestinal bleeding, hyperkalemia, hypernatremia, and elevated creatinine 6.
Furthermore, long-term use of NSAIDs increases your risk of developing a stomach ulcer. To counter this increased risk, an additional medication called a proton pump inhibitor (PPI) may be prescribed. Proton pump inhibitors (PPIs) help protect your stomach lining against the harmful effects of NSAIDs, reducing the risk of ulcers forming.
Dipsogenic diabetes insipidus
Researchers have not yet found an effective treatment for dipsogenic diabetes insipidus. People can try sucking on ice chips or sour candies to moisten their mouths and increase saliva flow, which may reduce the desire to drink. For a person who wakes multiple times at night to urinate because of dipsogenic diabetes insipidus, taking a small dose of desmopressin at bedtime may help. Initially, your health care provider will monitor your blood sodium levels to prevent hyponatremia, or low sodium levels in the blood.
Gestational diabetes insipidus
Doctors treat gestational diabetes insipidus with desmopressin, which is safe for both mother and baby. An expectant mother’s placenta does not destroy desmopressin (man made vasopressin) as it does vasopressin. Gestational diabetes insipidus usually goes away after the baby is born, but may return if the mother becomes pregnant again.
Most people with diabetes insipidus can prevent serious problems and live a normal life if they follow their health care professional’s recommendations and keep their symptoms under control.
Lifestyle and home remedies
If you have diabetes insipidus:
- Prevent dehydration. As long as you take your medication and have access to water when the medication’s effects wear off, you’ll prevent serious problems. Plan ahead by carrying water with you wherever you go, and keep a supply of medication in your travel bag, at work or at school.
- Wear a medical alert bracelet or carry a medical alert card in your wallet. If you have a medical emergency, a health care professional will recognize immediately your need for special treatment.
Eating, Diet, and Nutrition
Researchers have not found that eating, diet, and nutrition play a role in causing or preventing diabetes insipidus.
Diabetes insipidus prognosis
The prognosis for most patients with diabetes insipidus is excellent as long as the underlying primary cause can be treated 3. Lithium discontinuation can restore normal kidney function, but the nephrogenic diabetes insipidus may be permanent in some patients.
As long as the individual has access to water, mortality can be avoided 3. However, diabetes insipidus can lead to cardiovascular collapse, fever, and hypernatremia in children and older patients.
Without medical treatment, the potential diabetes insipidus complications include 3:
- Chronic dehydration
- Tachycardia (rapid heart rate)
- Decreased temperature
- Hypotension
- Weight loss
- Fatigue
- Headaches
- Kidney damage
- Brain damage.
- Fenske W, Allolio B. Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab. 2012 Oct;97(10):3426-37. doi: 10.1210/jc.2012-1981[↩][↩][↩][↩][↩]
- Grace M, Balachandran V; Preethy; Menon S. Idiopathic central diabetes Insipidus. Indian J Med Sci. 2011 Oct;65(10):452-5[↩][↩]
- Hui C, Khan M, Khan Suheb MZ, et al. Diabetes Insipidus. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470458[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, Rossi A, Maghnie M. Diabetes insipidus–diagnosis and management. Horm Res Paediatr. 2012;77(2):69-84. doi: 10.1159/000336333[↩][↩][↩][↩][↩]
- Makaryus AN, McFarlane SI. Diabetes insipidus: diagnosis and treatment of a complex disease. Cleve Clin J Med. 2006 Jan;73(1):65-71. doi: 10.3949/ccjm.73.1.65[↩][↩][↩][↩]
- Crawford A, Harris H. Water world, part 2: Understanding diabetes insipidus in adults. Nurs Crit Care. 2012;7:12–6.[↩][↩][↩][↩][↩]
- Kalra S, Zargar AH, Jain SM, Sethi B, Chowdhury S, Singh AK, Thomas N, Unnikrishnan AG, Thakkar PB, Malve H. Diabetes insipidus: The other diabetes. Indian J Endocrinol Metab. 2016 Jan-Feb;20(1):9-21. doi: 10.4103/2230-8210.172273[↩][↩][↩][↩][↩][↩][↩]
- Levy M, Prentice M, Wass J. Diabetes insipidus. British Medical Journal. 2019;364. doi: 10.1136/bmj.l321[↩]
- Saifan C, Nasr R, Mehta S, Sharma Acharya P, Perrera I, Faddoul G, Nalluri N, Kesavan M, Azzi Y, El-Sayegh S. Diabetes insipidus: a challenging diagnosis with new drug therapies. ISRN Nephrol. 2013 Mar 24;2013:797620. doi: 10.5402/2013/797620[↩][↩]
- Kimmel DW, O’Neill BP. Systemic cancer presenting as diabetes insipidus. Clinical and radiographic features of 11 patients with a review of metastatic-induced diabetes insipidus. Cancer. 1983 Dec 15;52(12):2355-8. doi: 10.1002/1097-0142(19831215)52:12<2355::aid-cncr2820521232>3.0.co;2-j[↩][↩]
- Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, Zecca S, Tinelli C, Gallucci M, Bernasconi S, Boscherini B, Severi F, Aricò M. Central diabetes insipidus in children and young adults. N Engl J Med. 2000 Oct 5;343(14):998-1007. doi: 10.1056/NEJM200010053431403[↩][↩][↩]
- Imura H, Nakao K, Shimatsu A, Ogawa Y, Sando T, Fujisawa I, Yamabe H. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993 Sep 2;329(10):683-9. doi: 10.1056/NEJM199309023291002[↩]
- De Bellis A, Colao A, Di Salle F, Muccitelli VI, Iorio S, Perrino S, Pivonello R, Coronella C, Bizzarro A, Lombardi G, Bellastella A. A longitudinal study of vasopressin cell antibodies, posterior pituitary function, and magnetic resonance imaging evaluations in subclinical autoimmune central diabetes insipidus. J Clin Endocrinol Metab. 1999 Sep;84(9):3047-51. doi: 10.1210/jcem.84.9.5945[↩][↩]
- Pivonello R, De Bellis A, Faggiano A, Di Salle F, Petretta M, Di Somma C, Perrino S, Altucci P, Bizzarro A, Bellastella A, Lombardi G, Colao A. Central diabetes insipidus and autoimmunity: relationship between the occurrence of antibodies to arginine vasopressin-secreting cells and clinical, immunological, and radiological features in a large cohort of patients with central diabetes insipidus of known and unknown etiology. J Clin Endocrinol Metab. 2003 Apr;88(4):1629-36. doi: 10.1210/jc.2002-020791[↩][↩][↩]
- Iwama S, Sugimura Y, Kiyota A, Kato T, Enomoto A, Suzuki H, Iwata N, Takeuchi S, Nakashima K, Takagi H, Izumida H, Ochiai H, Fujisawa H, Suga H, Arima H, Shimoyama Y, Takahashi M, Nishioka H, Ishikawa SE, Shimatsu A, Caturegli P, Oiso Y. Rabphilin-3A as a Targeted Autoantigen in Lymphocytic Infundibulo-neurohypophysitis. J Clin Endocrinol Metab. 2015 Jul;100(7):E946-54. doi: 10.1210/jc.2014-4209[↩]
- Johnston PC, Chew LS, Hamrahian AH, Kennedy L. Lymphocytic infundibulo-neurohypophysitis: a clinical overview. Endocrine. 2015 Dec;50(3):531-6. doi: 10.1007/s12020-015-0707-6[↩]
- Christensen JH, Rittig S. Familial neurohypophyseal diabetes insipidus–an update. Semin Nephrol. 2006 May;26(3):209-23. doi: 10.1016/j.semnephrol.2006.03.003[↩]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001 Jul;81(3):1197-267. doi: 10.1152/physrev.2001.81.3.1197[↩]
- Bischoff AN, Reiersen AM, Buttlaire A, Al-Lozi A, Doty T, Marshall BA, Hershey T; Washington University Wolfram Syndrome Research Group. Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet J Rare Dis. 2015 May 30;10:66. doi: 10.1186/s13023-015-0282-1[↩]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012 May;11(5):367-83. doi: 10.1038/nrd3699[↩]
- Yagi H, Nagashima K, Miyake H, Tamai S, Onigata K, Yutani S, Kuroume T. Familial congenital hypopituitarism with central diabetes insipidus. J Clin Endocrinol Metab. 1994 Apr;78(4):884-9. doi: 10.1210/jcem.78.4.8157716[↩]
- Lukezic, M., Righini, V., Di Natale, B., De Angelis, R., Norbiato, G., Bevilacqua, M. and Chiumello, G. (2000), Vasopressin and thirst in patients with posterior pituitary ectopia and hypopituitarism. Clinical Endocrinology, 53: 77-83. https://doi.org/10.1046/j.1365-2265.2000.01030.x[↩]
- Nemergut EC, Zuo Z, Jane JA Jr, Laws ER Jr. Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J Neurosurg. 2005 Sep;103(3):448-54. doi: 10.3171/jns.2005.103.3.0448[↩]
- Ghirardello S, Hopper N, Albanese A, Maghnie M. Diabetes insipidus in craniopharyngioma: postoperative management of water and electrolyte disorders. J Pediatr Endocrinol Metab. 2006 Apr;19 Suppl 1:413-21.[↩]
- Hadjizacharia P, Beale EO, Inaba K, Chan LS, Demetriades D. Acute diabetes insipidus in severe head injury: a prospective study. J Am Coll Surg. 2008 Oct;207(4):477-84. doi: 10.1016/j.jamcollsurg.2008.04.017[↩]
- Sigounas DG, Sharpless JL, Cheng DM, Johnson TG, Senior BA, Ewend MG. Predictors and incidence of central diabetes insipidus after endoscopic pituitary surgery. Neurosurgery. 2008 Jan;62(1):71-8; discussion 78-9. doi: 10.1227/01.NEU.0000311063.10745.D8[↩]
- Crowley, R.K., Hamnvik, O.P., O’Sullivan, E.P., Behan, L.A., Smith, D., Agha, A. and Thompson, C.J. (2010), Morbidity and mortality in patients with craniopharyngioma after surgery. Clinical Endocrinology, 73: 516-521. https://doi.org/10.1111/j.1365-2265.2010.03838.x[↩]
- Seckl J, Dunger D. Postoperative diabetes insipidus. BMJ. 1989 Jan 7;298(6665):2-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1835326/pdf/bmj00213-0006.pdf[↩]
- Wickramasinghe LS, Chazan BI, Mandal AR, Baylis PH, Russell I. Cranial diabetes insipidus after upper gastrointestinal hemorrhage. Br Med J (Clin Res Ed). 1988 Apr 2;296(6627):969. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2545439/pdf/bmj00279-0025a.pdf[↩]
- Grois N, Fahrner B, Arceci RJ, Henter JI, McClain K, Lassmann H, Nanduri V, Prosch H, Prayer D; Histiocyte Society CNS LCH Study Group. Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010 Jun;156(6):873-881.e1. doi: 10.1016/j.jpeds.2010.03.001[↩]
- Dunger DB, Broadbent V, Yeoman E, Seckl JR, Lightman SL, Grant DB, Pritchard J. The frequency and natural history of diabetes insipidus in children with Langerhans-cell histiocytosis. N Engl J Med. 1989 Oct 26;321(17):1157-62. doi: 10.1056/NEJM198910263211704[↩]
- Garovic VD, Clarke BL, Chilson TS, Specks U. Diabetes insipidus and anterior pituitary insufficiency as presenting features of Wegener’s granulomatosis. Am J Kidney Dis. 2001 Jan;37(1):E5. doi: 10.1016/s0272-6386(01)90002-2[↩]
- Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab. 1995 Aug;80(8):2302-11. doi: 10.1210/jcem.80.8.7629223[↩]
- Stuart CA, Neelon FA, Lebovitz HE. Disordered control of thirst in hypothalamic-pituitary sarcoidosis. N Engl J Med. 1980 Nov 6;303(19):1078-82. doi: 10.1056/NEJM198011063031902[↩]
- NDI Facts. https://ndif.org/ndi-facts[↩][↩]
- Miller M, Dalakos T, Moses AM, Fellerman H, Streeten DH. Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med. 1970 Nov;73(5):721-9. doi: 10.7326/0003-4819-73-5-721[↩]
- Davis J, Desmond M, Berk M. Lithium and nephrotoxicity: a literature review of approaches to clinical management and risk stratification. BMC Nephrol. 2018 Nov 3;19(1):305. doi: 10.1186/s12882-018-1101-4[↩]
- Jung JY, Madsen KM, Han KH, Yang CW, Knepper MA, Sands JM, Kim J. Expression of urea transporters in potassium-depleted mouse kidney. Am J Physiol Renal Physiol. 2003 Dec;285(6):F1210-24. doi: 10.1152/ajprenal.00111.2003[↩]
- Christ-Crain M, Bichet DG, Fenske WK, Goldman MB, Rittig S, Verbalis JG, Verkman AS. Diabetes insipidus. Nat Rev Dis Primers. 2019 Aug 8;5(1):54. doi: 10.1038/s41572-019-0103-2[↩][↩]
- Kidney disease in primary Sjögren syndrome. https://www.uptodate.com/contents/kidney-disease-in-primary-sjogren-syndrome?search=nephrogenic%20diabetes%20insipidus&source=search_result&selectedTitle=7~81&usage_type=default&display_rank=7[↩]
- Aleksandrov N, Audibert F, Bedard MJ, Mahone M, Goffinet F, Kadoch IJ. Gestational diabetes insipidus: a review of an underdiagnosed condition. J Obstet Gynaecol Can. 2010 Mar;32(3):225-31. doi: 10.1016/s1701-2163(16)34448-6[↩][↩][↩]
- Nephrogenic Diabetes Insipidus Description. https://ndif.org/ndi-facts/description[↩][↩][↩][↩][↩]
- Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009 May;5(5):270-6. doi: 10.1038/nrneph.2009.43[↩][↩]
- Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol. 2008 May;28(3):245-51. doi: 10.1016/j.semnephrol.2008.03.005[↩]
- Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015 Oct;11(10):576-88. doi: 10.1038/nrneph.2015.89[↩]
- Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005 Oct;16(10):2836-46. doi: 10.1681/ASN.2005040371[↩]
- Bode HH, Crawford JD. Nephrogenic diabetes insipidus in North America. The Hopewell hypothesis. N Engl J Med. 1969 Apr 3;280(14):750-4. doi: 10.1056/NEJM196904032801404[↩]
- Kohli A, Verma S Jr, Sharma A Jr. Psychogenic polydipsia. Indian J Psychiatry. 2011 Apr;53(2):166-7. doi: 10.4103/0019-5545.82554[↩][↩]
- Sarma KV. Algorithmic approach for the diagnosis of polyuria. In: Muruganathan A, editor. Medicine Update. Vol. 23. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2013. pp. 311–3.[↩][↩][↩]
- Hime MC, Richardson JA. Diabetes insipidus and pregnancy. Case report, incidence and review of literature. Obstet Gynecol Surv. 1978 Jun;33(6):375-9. doi: 10.1097/00006254-197806000-00001[↩][↩]
- Ananthakrishnan S. Diabetes insipidus in pregnancy: etiology, evaluation, and management. Endocr Pract. 2009 May-Jun;15(4):377-82. doi: 10.4158/EP09090.RA[↩][↩][↩]
- Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nature Reviews. Disease Primers. 2019;5(1):54. doi: 10.1038/s41572-019-0103-2[↩]
- Nils G Morgenthaler, Joachim Struck, Christine Alonso, Andreas Bergmann, Assay for the Measurement of Copeptin, a Stable Peptide Derived from the Precursor of Vasopressin, Clinical Chemistry, Volume 52, Issue 1, 1 January 2006, Pages 112–119, https://doi.org/10.1373/clinchem.2005.060038[↩]
- de Fost M, Oussaada SM, Endert E, Linthorst GE, Serlie MJ, Soeters MR, DeVries JH, Bisschop PH, Fliers E. The water deprivation test and a potential role for the arginine vasopressin precursor copeptin to differentiate diabetes insipidus from primary polydipsia. Endocr Connect. 2015 Jun;4(2):86-91. doi: 10.1530/EC-14-0113[↩]
- Webster B, Bain J. Antidiuretic effect and complications of chlorpropamide therapy in diabetes insipidus. J Clin Endocrinol Metab. 1970 Feb;30(2):215-27. doi: 10.1210/jcem-30-2-215[↩]
- Radó JP. Combination of carbamazepine and chlorpropamide in the treatment of “hyporesponder” pituitary diabetes insipidus. J Clin Endocrinol Metab. 1974 Jan;38(1):1-7. doi: 10.1210/jcem-38-1-1[↩]
- Welch WJ, Ott CE, Lorenz JN, Kotchen TA. Effects of chlorpropamide on loop of Henle function and plasma renin. Kidney Int. 1986 Nov;30(5):712-6. doi: 10.1038/ki.1986.245[↩]
- Vokes TJ, Gaskill MB, Robertson GL. Antibodies to vasopressin in patients with diabetes insipidus. Implications for diagnosis and therapy. Ann Intern Med. 1988 Feb;108(2):190-5. doi: 10.7326/0003-4819-108-2-190[↩]
- Ana C. de Bragança, Zenaide P. Moyses, Antonio J. Magaldi, Carbamazepine can induce kidney water absorption by increasing aquaporin 2 expression, Nephrology Dialysis Transplantation, Volume 25, Issue 12, December 2010, Pages 3840–3845, https://doi.org/10.1093/ndt/gfq317[↩][↩]
- Meinders AE, Cejka V, Robertson GL. The antidiuretic action of carbamazepine in man. Clin Sci Mol Med. 1974 Oct;47(4):289-99. doi: 10.1042/cs0470289[↩]
- Henry DA, Lawson DH, Reavey P, Renfrew S. Hyponatraemia during carbamazepine treatment. Br Med J. 1977 Jan 8;1(6053):83-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1604051/pdf/brmedj00444-0025b.pdf[↩]
- Van Amelsvoort, T., Bakshi, R., Devaux, C.B. and Schwabe, S. (1994), Hyponatremia Associated with Carbamazepine and Oxcarbazepine Therapy: A Review. Epilepsia, 35: 181-188. https://doi.org/10.1111/j.1528-1157.1994.tb02930.x[↩]
- Kamiyama T, Iseki K, Kawazoe N, Takishita S, Fukiyama K. Carbamazepine-induced hyponatremia in a patient with partial central diabetes insipidus. Nephron. 1993;64(1):142-5. doi: 10.1159/000187295[↩]
- Radó JP. Decreased antidiuretic response in diabetes insipidus during prolonged treatment with carbamazepine presumably due to enzym-induction. Endokrinologie. 1976 Jul;67(3):322-30.[↩]
- Hocken AG, Longson D. Reduction of free water clearance by chlorporpamide. Br Med J. 1968 Feb 10;1(5588):355-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1984885/pdf/brmedj02070-0043.pdf[↩]
- Cushard WG Jr, Beauchamp CJ, Martin ND. Oral therapy of diabetes insipidus with chlorpropamide. Calif Med. 1971 Aug;115(2):1-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1517999/pdf/califmed00128-0027.pdf[↩][↩]
- Wales JK. Mode of action of chlorpropamide in the treatment of diabetes insipidus. Proc R Soc Med. 1971 Oct;64(10):1070-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1812776/pdf/procrsmed00277-0082.pdf[↩][↩]
- Moses AM, Howanitz J, van Gemert M, Miller M. Clofibrate-induced antidiuresis. J Clin Invest. 1973 Mar;52(3):535-42. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC302290/pdf/jcinvest00179-0007.pdf[↩][↩]
- Thompson P Jr, Earll JM, Schaaf M. Comparison of clofibrate and chlorpropamide in vasopressin-responsive diabetes insipidus. Metabolism. 1977 Jul;26(7):749-62. doi: 10.1016/0026-0495(77)90062-2[↩]
- Matsukura S, Matsumoto J, Chihara K, Yoshimoto Y, Fujita T. Clofibrate-induced myopathy in patients with diabetes insipidus. Endocrinol Jpn. 1980 Jun;27(3):401-3. doi: 10.1507/endocrj1954.27.401[↩][↩]
- Tetiker T, Sert M, Koçak M. Efficacy of indapamide in central diabetes insipidus. Arch Intern Med. 1999 Sep 27;159(17):2085-7. doi: 10.1001/archinte.159.17.2085[↩][↩]
- Koçak M, Karademir BM, Tetiker T. Antidiuretic effect of indapamide in central diabetes insipidus. Acta Endocrinol (Copenh). 1990 Dec;123(6):657-60. doi: 10.1530/acta.0.1230657[↩]
- Abraham MB, Rao S, Price G, Choong CS. Efficacy of Hydrochlorothiazide and low renal solute feed in Neonatal Central Diabetes Insipidus with transition to Oral Desmopressin in early infancy. Int J Pediatr Endocrinol. 2014;2014(1):11. doi: 10.1186/1687-9856-2014-11[↩][↩]
- Kim GH, Lee JW, Oh YK, Chang HR, Joo KW, Na KY, Earm JH, Knepper MA, Han JS. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004 Nov;15(11):2836-43. doi: 10.1097/01.ASN.0000143476.93376.04[↩]
- Al Nofal A, Lteif A. Thiazide Diuretics in the Management of Young Children with Central Diabetes Insipidus. J Pediatr. 2015 Sep;167(3):658-61. doi: 10.1016/j.jpeds.2015.06.002[↩]
- O’DOHERTY NJ, ROSSER J, SLATER RJ. Diabetes insipidus: the influence of chlorothiazide therapy in affected children. Can Med Assoc J. 1962 Mar 31;86(13):559-67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1849045/pdf/canmedaj00937-0002.pdf[↩]
- Alon U, Chan JC. Hydrochlorothiazide-amiloride in the treatment of congenital nephrogenic diabetes insipidus. Am J Nephrol. 1985;5(1):9-13. doi: 10.1159/000166896[↩]
- Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009 Jul;76(1):44-53. doi: 10.1038/ki.2009.91[↩]
- Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, Leader JP, Walker RJ. Lithium-induced nephrogenic diabetes insipidus: renal effects of amiloride. Clin J Am Soc Nephrol. 2008 Sep;3(5):1324-31. doi: 10.2215/CJN.01640408[↩]
- Weinstock RS, Moses AM. Desmopressin and indomethacin therapy for nephrogenic diabetes insipidus in patients receiving lithium carbonate. South Med J. 1990 Dec;83(12):1475-7. doi: 10.1097/00007611-199012000-00026[↩][↩][↩]
- Lam SS, Kjellstrand C. Emergency treatment of lithium-induced diabetes insipidus with nonsteroidal anti-inflammatory drugs. Ren Fail. 1997 Jan;19(1):183-8. doi: 10.3109/08860229709026274[↩]
- Delaney V, de Pertuz Y, Nixon D, Bourke E. Indomethacin in streptozocin-induced nephrogenic diabetes insipidus. Am J Kidney Dis. 1987 Jan;9(1):79-83. doi: 10.1016/s0272-6386(87)80166-x[↩]