What is empyema
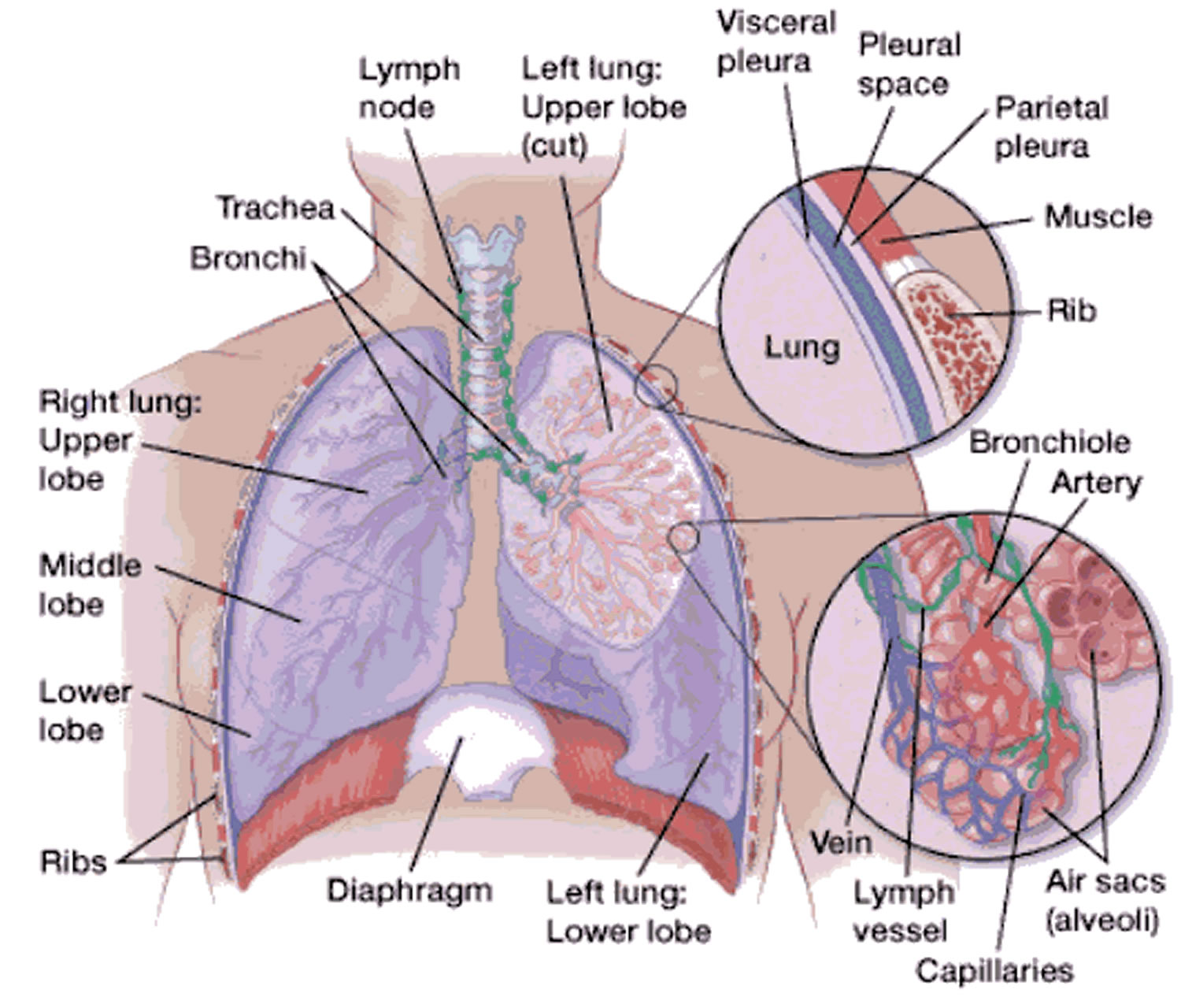
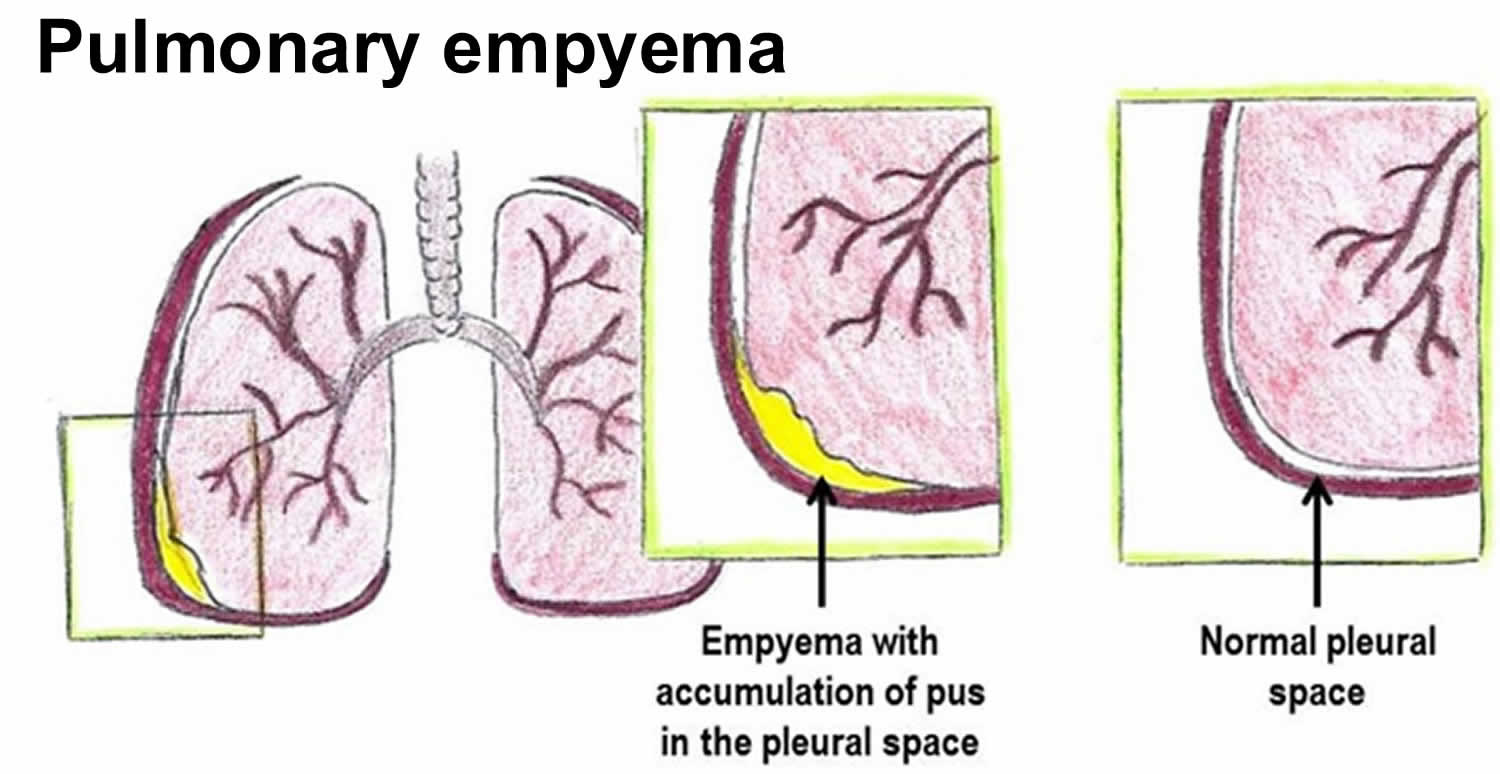
Empyema is the medical term for pockets of pus that have collected inside a body cavity. The term empyema is most commonly used to refer to pus-filled pockets that develop in the pleural space also called empyema thoracis. The pleural space is the slim space between the outside of the lungs (visceral pleura) and the inside of the chest cavity (parietal pleura). Empyema can form if a bacterial infection is left untreated, or if it fails to fully respond to treatment.
Although empyema can occasionally be life threatening, lung empyema is not a common condition, as most bacterial infections are effectively treated with antibiotics before they get to this stage.
In the United States, there are approximately 32,000 pulmonary empyema cases per year. Empyema is associated with elevated morbidity and mortality, around 20% to 30% of patients affected will either die or required further surgery in the first year after developing empyema. Early treatment is crucial in the management of empyema 1.
Empyema is usually caused by an infection that spreads from the lung. It leads to a buildup of pus in the pleural space.
There can be a pint (1/2 liter) or more of infected fluid. This fluid puts pressure on the lungs.
Risk factors for lung empyema include:
- Bacterial pneumonia
- Tuberculosis
- Chest surgery
- Lung abscess
- Trauma or injury to the chest
- In rare cases, empyema can occur after thoracentesis. This is a procedure in which a needle is inserted through the chest wall to remove fluid in the pleural space for medical diagnosis or treatment.
Empyema is usually associated with pneumonia but may also develop after thoracic surgery or thoracic trauma 2. Empyema most commonly occurs as a complication in pneumonia due to Staphylococcus aureus. Around 20% of patients with pneumonia will develop a parapneumonic effusion that may lead to empyema. Seventy percent of patients with empyema have parapneumonic effusion, the other 30% of cases are related to trauma, post-thoracic surgery, esophageal ruptures, or cervical infections, and a small number are not related to previous pneumonia or intervention, this is known as primary empyema 3.
Empyema can cause fever, chest pains, breathlessness and coughing up mucus.
Figure 1. Lungs pleural space
Lung abscess vs Empyema
A lung abscess is a pus-filled cavity in the lung surrounded by inflamed tissue and caused by an infection. A lung abscess usually occurs as a complication of inadequately treated pneumonia, when the bacteria are able to replicate to large numbers and cause damage to the lung tissue. The damaged tissue is walled off by the immune system and becomes an abscess.
A lung abscess is usually caused by bacteria that normally live in your mouth or throat and that are inhaled (aspirated) into the lungs, resulting in an infection. Often, gum disease (periodontal disease) is the source of the bacteria that cause a lung abscess.
Your body has many defenses (such as a cough) to help prevent bacteria from getting into the lungs. Infection occurs primarily when a person is unconscious or very drowsy because of sedation, anesthesia, alcohol or drug use, or a disease of the nervous system and is thus less able to cough to clear the aspirated bacteria.
In people whose immune system functions poorly, a lung abscess may be caused by organisms that are not typically found in the mouth or throat, such as fungi (pulmonary aspergillosis) or Mycobacterium tuberculosis (the organism that causes tuberculosis). Other bacteria that can cause lung abscesses are streptococci and staphylococci, including methicillin-resistant Staphylococcus aureus (MRSA), which is a serious infection.
Blockage (obstruction) of the airways also can lead to abscess formation. If the branches of the windpipe (bronchi) are blocked by a tumor or a foreign object, an abscess can form because secretions (mucus) can accumulate behind the obstruction. Bacteria sometimes enter these secretions. The obstruction prevents the bacteria-laden secretions from being coughed back up through the airway.
Less commonly, abscesses result when bacteria or infected blood clots travel through the bloodstream to the lung from another infected site in the body (septic pulmonary emboli).
Usually, people develop only one lung abscess as a result of aspiration or airway obstruction. If several abscesses develop, they are usually in the same lung. When an infection reaches the lungs through the bloodstream, however, many scattered abscesses may develop in both lungs. This problem is most common among people who inject drugs using dirty needles or unsterile methods.
Eventually, most abscesses rupture into an airway, producing a lot of sputum that gets coughed up. A ruptured abscess leaves a cavity in the lung that is filled with fluid and air. Sometimes an abscess ruptures into the space between the lungs and the chest wall (pleural space), filling the space with pus, a condition called empyema. Very rarely, if an abscess destroys a blood vessel wall, it may lead to serious bleeding.
What is brain empyema
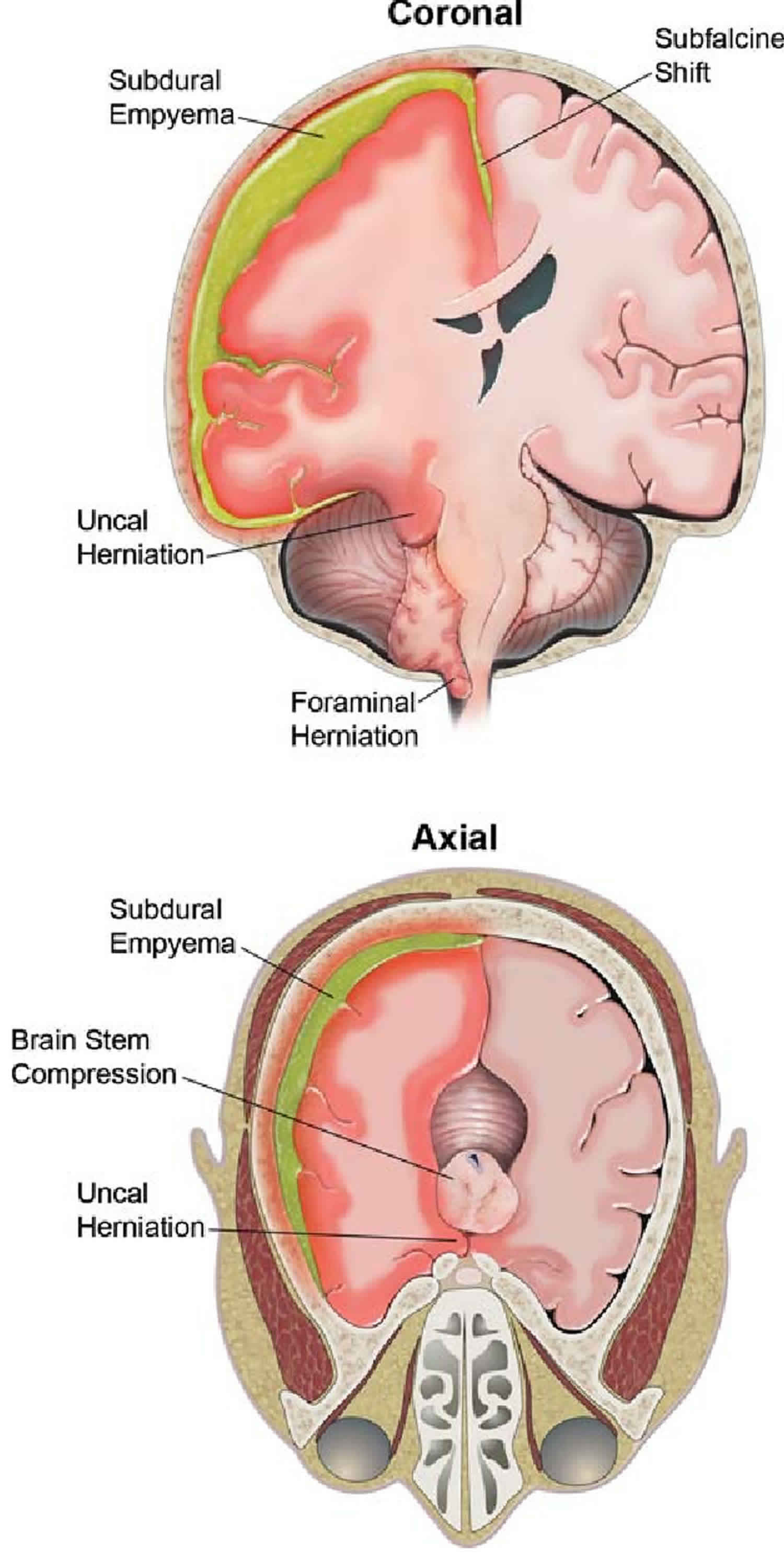
Subdural empyema, an accumulation of purulent material between the dura and arachoid mater, can be a rare complication of sinusitis, mastoiditis, meningitis, dental abscess, influenza, or a number of other infections 4. As a complication of sinusitis, pus may erode the bony walls of the sinuses directly invading the leptomeninges, or bacteria may infect the bridging veins that run through the bony calvarium and leptomeninges, carrying bacteria along the veins into the leptomeninges. Empyemas form in sequestered potential spaces and may rapidly grow to a large size with significant mass effect on surrounding structures, as large collections of purulent material trigger a cascade of severe inflammatory reaction within the surrounding tissues. The subdural space may be opened up diffusely around a cerebral hemisphere, creating a large cavity for pus to accumulate around the brain, particularly over the cerebral convexities 5. As pus accumulates in the subdural space around the brain, a space-occupying mass is formed and there is a significant, rapid increase in intracranial pressure and mechanical mass effect on the adjacent cerebral hemisphere. The result is midline or downward herniation of the brain.
However, empyema is also a collection of bacterial purulent material that inflames the adjacent brain tissue (cerebritis) and thromboses the cortical veins that traverse the leptomeninges. Cerebritis may create significant edema and swelling in the brain. Cortical vein thrombosis results in stasis of arterial cerebral blood flow due to the back pressure from the thrombosed vein with hypoperfusion and ischemia/infarction in the drainage territory of the vein. If a large dural venous sinsus thromboses, the resulting venous hypertension impairs cerebrospinal fluid (CSF) reabsorption and results in hydrocephalus. The severe inflammatory response in the leptomeninges may also cause a vasopspasm of the arteries in the subarachnoid space resulting in direct arterial ischemia of the brain. The synergistic cascade of rapidly accumulating pus surrounding the brain with raised intracranial pressure and mass effect, severe inflammatory edema, hydrocephalus, and infarction results in death if the patient is not diagnosed and treated emergently 6.
Figure 3. Brain empyema
Footnote: Right-sided subdural empyema causing extensive, life-threatening herniation. The patient is a 15-year-old, right-handed male who presented to the Emergency Department with sinusitis, worsening headache, low-grade fever, dizziness, neck pain, right-sided facial droop, and unequal pupil dilation. His mother noted that the patient had been sick for ∼4 days prior to his hospitalization. He reportedly began experiencing headache 4 days prior to his admission, followed by increasing motor slowing, fatigue, and one episode of vomiting. The day he presented to the Emergency Department, the child reportedly had become unable to dress himself or walk without assistance. An intravenous contrast-enhanced CT scan at his admission detected a right-sided pneumonoccal subdural empyema, originating from the right frontal sinus. The patient immediately underwent a frontal craniotomy, evacuation of the empyema, and cranialization of the right frontal sinus. There were no complications of encephalitis or meningitis, according to analysis of the CSF. A peripherally inserted central catheter (PICC) line was inserted that same day for administration of ampicillin and was removed on Day 45 upon completion of that antibiotic course.
[Source 6 ]The prevalence of subdural empyema is difficult to determine, with the most recent formal study suggesting an incidence rate of one case per 193,000 people per year 7. However, as that study was conducted in a region of South Africa with a very low socioeconomic index, the incidence rate of subdural empyema in a more developed area of the world, such as the United States, is likely even lower 8. It is estimated that 3% of all patients hospitalized for sinusitis develop subdural empyema 9. Approximately 70% of cases occur in individuals aged 10–30 10. Numerous studies suggest that subdural empyema is more commonly seen in males 11.
The onset of symptoms in cases of subdural empyema is often abrupt 12, and most patients present at about 7–12 days 13. Any earlier detection is extremely difficult due to the nonspecific nature of initial symptoms, which typically include fever, focal cranial pain, and headache 8. Lethargy and altered mental status are commonly observed upon initial presentation for medical evaluation 14. Over 70% of cases present with hemiparesis due to mass effect and up to 48% experience seizures prior to undergoing treatment 15. In severe or prolonged cases, brain abscesses may develop 13, and as the brain herniates, seizures, stupor, hemiparesis, and cranial nerve palsies progress. If untreated, patients will progress to coma and eventual death 14. Even with appropriate treatment, mortality rates are reported from 14% to 28% 16.
Contrast-enhanced computed tomography (CT) imaging is commonly used and “very reliable” 8 in diagnosing subdural empyema 9. However, as CT sometimes fails to detect early-stage subdural empyema 17, some healthcare professionals prefer the greater sensitivity of magnetic resonance imaging (MRI). MRI is also more sensitive in detecting the complications of subdural empyema, including cerebritis, abscess, and infarction. After diagnosis, standard of care is immediate craniotomy or burr hole surgery to remove purulent material. Although burr hole drainage is more common, craniotomy may be more effective 11. There is much debate over which technique is optimal, and the severity of the empyema is often the determining factor. Following surgery, patients are treated with an antibiotic regimen determined by the pathogens identified as the causal source of the empyema 8. Due to the rarity of subdural empyema, the duration of antibiotic therapy is not well established, although 4–6 weeks has been suggested 8. In addition to antibiotics, corticosteroids are occasionally used due to their established role in the treatment of meningitis, although the rationale and success of using corticosteroids to treat subdural empyema has not been well documented 18.
Prior to the development of CT, overall mortality rates for subdural empyema were consistently 25%–40%, whereas current mortality rates typically are in the range of 8%–22% 19. Mortality rates are as high as 57%–80% for patients who are comatose on initial presentation, in contrast to 0%–7% mortality for patients who present while still conscious 20. Any lasting neurological disability as a result of subdural empyema is rare 8. However, survivors commonly experience lingering hemiparesis, and epilepsy develops in 17%–42% of survivors 19. Thus, anti-seizure medications and occupational, physical, or speech therapy may be prescribed for survivors.
Due to the rarity of this condition, there is no known neuropsychological profile of patients in either acute or recovery stages of subdural empyema 6.
Empyema causes
The lungs and inside of the chest cavity are lined with a smooth layer called the pleura. These layers are almost in contact, but separated by a thin space (the pleural space) filled with a small amount of lubricant called pleural fluid. The pleural fluid can sometimes build up and become infected, and a collection of pus forms. This can thicken and cause areas of the pleura to stick together, creating pockets of pus.
Empyema can worsen to become many more pockets of pus, with thick deposits coating the outer layer of the lungs.
These deposits prevent the lungs expanding properly.
Pneumonia and other possible causes
The most common cause of empyema is pneumonia caused by a bacterial infection of the lungs. An empyema can form when pneumonia fails to fully respond to treatment in a straightforward way.
The development of empyema can be described in a sequence of events. During an inflammatory process such as pneumonia, there is an increase in fluid production in the pleural cavity known as the exudate stage. As the disease progresses microorganisms, usually bacteria, can colonize the fluid and generated an empyema. This fluid is characterized by elevated lactate dehydrogenase, proteins, neutrophils, and dead cells. Macroscopically is a thick opaque fluid found in the fibrinopurulent stage. After the resolution of the infection and as a consequence of the inflammation, there is a process of fibrosis that can lead to restriction of the lung parenchyma. Appropriate and early intervention is vital to decrease complications and mortality 21.
Other possible causes are:
- Bronchiectasis – a long-term condition where the airways of the lungs become abnormally widened, leading to a build-up of mucus that can make the lungs more vulnerable to infection
- A blood clot or another blockage – this can prevent blood flow to the lungs, causing some of the lung tissue to die (known as a pulmonary infarction)
- Surgery to the chest – empyema is a rare complication
- An endoscopy – empyema is a rare complication
- Serious injury to the chest
- An infection elsewhere in the body that’s spread through the bloodstream
- An infection caused by inhaled food if you have swallowing problems – this is rare
- Tuberculosis – this is rare in the US
You’re more at risk of developing an empyema if you:
- have diabetes
- have a weakened immune system
- have acid reflux
- drink too much alcohol or take a lot of recreational drugs
Both adults and children can be affected.
Risk factors for empyema
A number of conditions are risk factors for the development of an empyema.
Unresolved pneumonia, usually due to Staphylococcus aureus (and particularly a lung abscess) can lead to infection that spreads to the pleural space (space between the lining of the chest cavity and the lung).
Other conditions such as bronchiectasis, airway-obstructing cancer, thoracic surgery, penetrating wounds, and secondary spread from a distant focus (particularly a subphrenic abscess) are all possible mechanisms of disease development.
Occasionally an empyema may arise as primary pathology (especially if due to Mycobacteria or Nocardia infection).
Empyema symptoms
Symptoms of bacterial pneumonia include:
- Fever
- Weak cough
- No interest in activity
- Poor appetite
If empyema develops, symptoms become worse, and your may have the following:
- Very tired or lack of energy
- Fever over 102°F (38.9 °C) and night sweats
- Shallow and rapid breathing
- Difficulty breathing
- A cough and coughing up mucus containing pus
- Weight loss
- Chest pain
Sometimes the pus pushes on the healthy lung and it becomes hard to breathe. This can cause:
- Shortness of breath with gasping
- Pale or grey color
- Difficulty walking
- Chest pain with anxiety
Lung empyema possible complications
Having empyema may lead to the following:
- Pleural thickening
- Reduced lung function
- Bronchopleural fistula (which is a permanent communication between a bronchus and the pleural space) – allowing for air to enter the pleural space as well as the pus – leading to a pyopneumothorax (air or pus in the plural cavity).
How is pulmonary empyema diagnosed?
An empyema is usually suspected when a person with severe pneumonia doesn’t improve with treatment and they start to show some of the symptoms listed above.
Tests to diagnose pulmonary empyema include:
- Chest x-ray to confirm that an empyema is present and estimate the amount of pus in the chest
- Ultrasound of the chest to determine the thickness and amount of pus
- A computed tomography study (CT scan) may be done if there is any question about the diagnosis, but is usually not necessary
- Blood tests to check for changes due to the empyema. A blood sample will also be taken to count the number of white blood cells and other markers of infection.
- A culture of the pus from the chest is the best way to identify the bacteria causing empyema. The type of bacteria causing the infection is identified so the most effective antibiotics can be given.
- If the patient is coughing up mucus, a sample of this should be taken to be inspected under a microscope.
Empyema treatment
Treatment of empyema usually involves medical and surgical treatment.
- Pain medicine is used to keep you comfortable
- Antibiotics are given for 14 days or more depending on the bacteria causing the infection
- A chest tube (a small tube inserted between the ribs through the chest wall) is placed to drain the pus from the inside of the chest
- The chest tube stays in place for 3 to 5 days
- Oftentimes, medicine is given through the chest tube to help dissolve and drain the thick pus
What else you need to know
- You will be eligible for discharge after the chest tube is removed and you are able to drink fluids and take oral medications.
- You may easily lose 1 – 5 pounds during a complicated infection and nutrition is important to help with wound healing.
- Remember, the lung is temporarily injured during pneumonia, but will heal with antibiotics and nutrition.
- When empyema complicates pneumonia, the risk for permanent lung damage and death goes up. Long-term treatment with antibiotics and drainage are needed.
- In general, most people fully recover from empyema.
- Lung function studies performed months or years after pulmonary empyema reveal that children have remarkable healing ability.
- Pulmonary empyema generally does not limit a normal person’s ability to participate in normal activity within one month of the event.
- Additional antibiotics for 1 or more weeks may be required.
Antibiotics
Some patients will just need antibiotics given directly into a vein through a drip (intravenously). But they may need to stay in hospital for a long period.
In community-acquired empyema, the use of a third or fourth-generation cephalosporin plus metronidazole or ampicillin with a beta-lactamase inhibitor will provided good coverage 2. In hospital-acquired or trauma-related, and surgery-related empyema, coverage of Pseudomonas and MRSA by adding vancomycin, cefepime, and metronidazole or piperacillin-tazobactam is essential . Due to the difficulty isolating anaerobes, the coverage for this organism should continue regardless of negative cultures. There is not a proven benefit of intrapleural antibiotics. Antibiotic should be given for 2 to 6 weeks, depending on patient response, source control, and organism 22.
Chest drain
Some patients may need both antibiotics and a chest drain. A chest drain is a flexible plastic tube that’s inserted through the chest wall and into the affected area to drain it of fluid. The area where the tube is inserted is numbed, and the patient may also be given a light sedative before having the drain inserted.
Painkillers are given to ease any pain while the chest drain is in. The chest tube usually stays in place until an X-ray or ultrasound scan shows all the fluid has drained from the chest and the lungs are fully expanded.
Sometimes injections may be given through the chest drain to help clear the infected pockets of pus.
The patient may need to stay in hospital until the tube is removed.
Some patients may be able to go home with the chest tube still in place, in which case a specialist nurse will offer support and advice on how to manage this at home.
The nurse will demonstrate how to position, empty and change the bag until the family or patient feels confident they can do this themselves.
Tube thoracostomy is the most common type of drainage, bore tube versus smaller tubes have not shown any difference regarding mortality and prognosis, but bigger tubes are associated with more pain. For this reason, small tubes are frequently placed, generally smaller than 14F 2. The position of the chest tube should be confirming with an x-ray or CT scan. Lack of clinical improvement in the first 24 hours is usually related to tube malposition or blockage. Blockage of the chest tube can be prevented with frequent flushing, but the necessary amount and frequency of this process is unclear. Any indication of a persistent fluid or other locations should be addressed with more aggressive therapy including a larger tube, more tubes, or surgery. The chest tube can usually be removed when the daily production of pleural fluid is proximal 350 ml/day or less.
The use of intrapleural medication has been around for at least 60 years. Fibrinolytics such as urokinase, streptokinase, and tissue plasminogen activator have been tried, as well as, mucolytics such as DNase. The isolated use of fibrinolytics, so far, has not shown differences in mortality or surgical intervention. On the other hand, some studies have shown that the combination of fibrinolytic and mucolytic, specifically TPA and DNase increases the amount of fluid drainage and the need for surgery, unfortunately, no changes in mortality have been shown. Currently, the use of intrapleural agents is not a standard of care 2.
Empyema surgery
Surgery may be needed if the pleural empyema doesn’t improve. This is only carried out if other treatments haven’t worked. In 10-20% of patients, surgery may be needed to help drain the pus. This surgery is called a VATs procedure (Video-Assisted Thoracoscopic surgery). Video-Assisted Thoracoscopic surgery (VATs) is a less invasive procedure, there is less blood loss, less pain for the patient, better respiratory outcome, decrease length of stay, and a decreased 30 days mortality 2. Several small incisions are made in the chest wall: one for a small video camera and others for instruments. Pus and blood are removed from the chest. After the procedure you return to the hospital room with a chest tube in place to collect any remaining pus. This chest tube stays in place for 3 to 5 days. Pain medications are given to keep you comfortable.
There is always a dilemma of when to convert to open-thoracotomy, and some of the clear indications for this are uncontrolled bleeding, damage to a structure that cannot be repaired with laparoscopy, and a patient who is not able to tolerate one-lung ventilation. Also, when the evacuation of the cavity or lung expansion are not achieved with Video-Assisted Thoracoscopic surgery (VATs), an open-thoracotomy is indicated.
After the acute phase, some patients develop fibrosis and lung restriction that can cause dyspnea and exercise-intolerance as symptoms. Decortication may help to alleviate these symptoms and is considered when pulmonary restriction is present 6 months after the resolution of the infection but there are still issues in a patient’s quality of life.
Stoma
A chest drain isn’t suitable for all patients. Some will instead opt to have an opening made in their chest, known as a stoma. A special bag is placed over the stoma to collect the fluid that leaks from the empyema.
This is worn on the body, and may be more discreet and interfere less with your lifestyle than a chest drain. But with modern treatments, getting a stoma is uncommon.
References- Bostock IC, Sheikh F, Millington TM, Finley DJ, Phillips JD. Contemporary outcomes of surgical management of complex thoracic infections. J Thorac Dis. 2018 Sep;10(9):5421-5427.
- Garvia V, Paul M. Empyema. [Updated 2018 Nov 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459237
- Madhi F, Levy C, Morin L, Minodier P, Dubos F, Zenkhri F, Dommergues MA, Mezgueldi E, Levieux K, Pneumonia Study Group. Béchet S, Varon E, Cohen R., GPIP (Pediatric Infectious Disease Group). Change in Bacterial Causes of Community-Acquired Parapneumonic Effusion and Pleural Empyema in Children 6 Years After 13-Valent Pneumococcal Conjugate Vaccine Implementation. J Pediatric Infect Dis Soc. 2018 Nov 08
- Wu T. J., Chiu N. C., Huang F. Y.. Subdural empyema in children: 20-Year experience in a medical center, Journal of Microbiology, Immunology, and Infection , 2008, vol. 411, pg. 62-67
- Bowen B. C., Donovan-Post M. J.. Atlas S. W.. Intracranial infection, Magnetic resonance imaging of the brain and spine , 1991New YorkRavepg. 517
- Alice Ann Holland, Michael Morriss, Paul C. Glasier, Peter L. Stavinoha; Complicated Subdural Empyema in an Adolescent, Archives of Clinical Neuropsychology, Volume 28, Issue 1, 1 February 2013, Pages 81–91, https://doi.org/10.1093/arclin/acs104
- Nathoo N., Nadvi S. S., van Dellen J. R., Gouws E.. Intracranial subdural empyemas in the era of computed tomography: A review of 699 cases, Neurosurgery , 1999, vol. 44 3(pg. 529-535) https://doi.org/10.1097/00006123-199903000-00055
- Bartt R. E.. Cranial epidural abscess and subdural empyema. In M. J. Aminoff, F. Boller, & D. F. Swaab (Series Eds.), Handbook of Clinical Neurology: Vol. 96. Bacterial infections of the central nervous system , 2010, pp. 75–89
- Hicks C. W., Weber J. G., Reid J. R., Moodley M.. Identifying and managing intracranial complications of sinusitis in children: A retrospective series, The Pediatric Infectious Disease Journal , 2011, vol. 30 3(pg. 222-226) https://doi.org/10.1097/INF.0b013e3181f86398
- Bayonne E., Kania R., Tran P., Huy B., Herman P.. Intracranial complications of rhinosinusitis: A review, typical imaging data, and algorithm of management, Rhinology , 2009, vol. 471, pg. 59-65
- Banerjee A. D., Pandey P., Devi B. I., Sampath S., Chandramouli B. A.. Pediatric supratentorial subdural empyemas: A retrospective analysis of 65 cases, Pediatric Neurosurgery , 2009, vol. 45 1(pg. 11-18) https://doi.org/10.1159/000202619
- Bleck T. P., Greenlee J. E.. Mandell G. L., Bennett J. E., Dolin R.. Epidural abscess, Mandell, Douglas, and Bennett’s principles and practice of infectious diseases , 2000, vol. Vol. 1 5th ed.PhiladelphiaChurchill Livingstone, pg. 1031-1034
- Adame N., Hedlund G., Byington C. L.. Sinogenic intracranial empyema in children, Pediatrics , 2005, vol. 116 3(pg. e461-e467) https://doi.org/10.1542/peds.2004-2501
- Osman Farah J., Kandasamy J., May P., Buxton N., Mallucci C.. Subdural empyema secondary to sinus infection in children, Child’s Nervous System , 2009, vol. 25 2(pg. 199-205) https://doi.org/10.1007/s00381-008-0665-x
- Hartman B. J., Helfgott D. C., Weingarten K.. Scheld W. M., Whitley R. J., Marra C. M.. Subdural empyema and suppurative intracranial phlebitis, Infections of the central nervous system , 2004 PhiladelphiaLippincott Williams & Wilkins, pg. 523-535
- Agrawal A., Timothy J., Pandit L., Shetty L., Shetty J. P.. A review of subdural empyema and its management, Infectious Diseases in Clinical Practice , 2007, vol. 15 3(pg. 149-153) https://doi.org/10.1097/01.idc.0000269905.67284.c7
- van de Beek D., Campeau N. G., Wijdicks E. M. F.. The clinical challenge of recognizing infratentorial empyema, Neurology , 2007, vol. 69 (pg. 477-481) https://doi.org/10.1212/01.wnl.0000266631.19745.32
- de Gans J., van de Beek D.. European Dexamethasone in Adulthood Bacterial Meningitis Study InvestigatorsDexamethasone in adults with bacterial meningitis, New England Journal of Medicine , 2002, vol. 347 (pg. 1549-1556) https://doi.org/10.1056/NEJMoa021334
- Hartman B. J., Helfgott D. C., Weingarten K.. Scheld W. M., Whitley R. J., Marra C. M.. Subdural empyema and suppurative intracranial phlebitis, Infections of the central nervous system , 2004PhiladelphiaLippincott Williams & Wilkins, pg. 523-535
- Dill S. R., Cobbs C. G., McDonald C. K.. Subdural empyema: Analysis of 32 cases and review, Clinical Infectious Diseases , 1995, vol. 20 (pg. 372-386) https://doi.org/10.1093/clinids/20.2.372
- Feller-Kopman D, Light R. Pleural Disease. N. Engl. J. Med. 2018 Feb 22;378(8):740-751.
- Kelly MM, Coller RJ, Kohler JE, Zhao Q, Sklansky DJ, Shadman KA, Thurber A, Barreda CB, Edmonson MB. Trends in Hospital Treatment of Empyema in Children in the United States. J. Pediatr. 2018 Nov;202:245-251.e1.