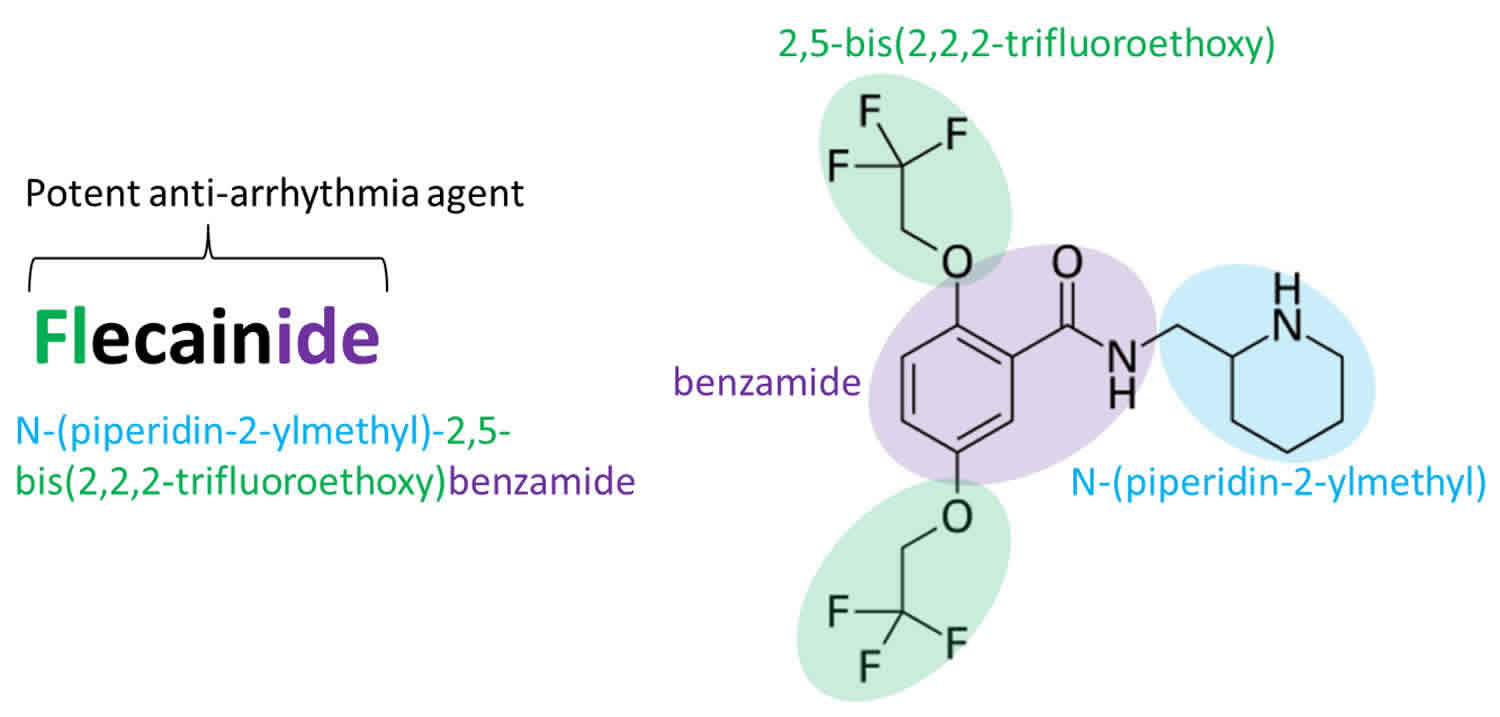
Flecainide
Flecainide is an oral antiarrhythmic agent (antiarrhythmic Class 1C) that is used in certain situations to prevent or treat certain types of life-threatening irregular heartbeats (arrhythmias) that include paroxysmal supraventricular tachycardias (PSVTs), atrioventricular nodal re-entrant tachycardia (AVNRT), AV re-entrant tachycardia (AVRT), and paroxysmal atrial fibrillation or atrial flutter in patients who do not have structural heart disease and arrhythmic long QT syndromes (LQTS), as well as the Ca2+-mediated, catecholaminergic polymorphic ventricular tachycardia (CPVT) 1, 2. However, flecainide can also exert pro‐arrhythmic effects most notably following myocardial infarction and when used to diagnose Brugada syndrome 1. Flecainide works by slowing electrical signals in the heart to stabilize the heart rhythm. Flecainide appears to act by blocking open sodium channels and outward potassium channels 3, 4, 5, 6, 7. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction. Flecainide was approved for use in the United States in 1985. Flecainide current indications include prevention and treating life-threatening ventricular arrhythmias and conversion of paroxysmal supraventricular tachycardia (PSVTs), Wolf-Parkinson-White syndrome, atrioventricular nodal re-entrant tachycardia (AVNRT), AV re-entrant tachycardia (AVRT), and atrial fibrillation/atrial flutter in patients who do not have structural heart disease, in whom other agents were unsuccessful.
Flecainide is available only with your doctor’s prescription.
Flecainide is a benzamide derivative analogue of the local anesthetic procaine and has electrophysiological effects that resemble quinidine. Flecainide appears to act by blocking open sodium channels and outward potassium channels 8. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction.
Oral bioavailability of Flecainide is nearly 100% but decreases when administered with milk 2. Flecainide is 40% protein-bound, and half-life elimination varies across age groups. In newborns: less than 28 hours, 3 months: 11 to 12 hours, 12 months: 6 hours. In children: 8 hours, adolescents 12 to 15 years: approximately 11 hours, adults: 12 to 24 hours. It takes 1 to 6 hours to peak in serum after administration. Flecainide is metabolized by your liver via the CYP450 system; specifically, it is a CYP2D6 substrate. Flecainide is excreted in the urine (30%) and, to a lesser extent, the feces (5%) 9, 10, 11. Its half-life is approximately 20 hours 12.
Flecainide is available in tablets of 50, 100 and 150 mg generically to be taken by mouth and under the brand name Tambocor. The usual maintenance dose in adults is 50 to 200 mg twice daily (once every 12 hours). Some people may take flecainide once every 8 hours if they experience side effects or if their condition cannot be controlled by taking flecainide every 12 hours. Take flecainide at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take flecainide exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
You may be hospitalized when you begin your treatment with flecainide. Your doctor will monitor you carefully during this time and for as long as you continue to take flecainide. Your doctor will probably start you on a low dose of flecainide and gradually increase your dose, not more than once every 4 days. Your doctor may also decrease your dose once your condition is controlled.
Flecainide may control your condition, but will not cure it. Continue to take flecainide even if you feel well. Do not stop taking flecainide without talking to your doctor. If you suddenly stop taking flecainide, your condition may become worse.
Flecainide most common side effects include dizziness, visual blurring, headache, fatigue, anxiety, gastrointestinal upset and nausea.
In a study of people who had experienced heart attacks within the past 2 years, people who took flecainide were more likely to have another heart attack or to die than people who did not take flecainide. There is not enough information to tell whether taking flecainide also increases the risk of heart attack or death in people who have not had heart attacks within the past 2 years. Because of this serious risk and because flecainide has not been shown to help people with irregular heartbeats to live longer, flecainide should be used only to treat people with life-threatening irregular heartbeats.
Tell you doctor if you have atrial fibrillation or atrial flutter (conditions in which the upper chambers of the heart do not beat effectively). People with atrial fibrillation or atrial flutter who take flecainide may have a higher risk of developing certain types of irregular heartbeats.
Talk to your doctor about the risks of taking flecainide.
How long does it take for flecainide to work?
Time to Peak
- Serum: ~3 hours (range: 1 to 6 hours)
Half-Life Elimination
- Newborns: Up to ≤29 hours; 3 months: 11 to 12 hours; 12 months: 6 hours
- Children: ~8 hours
- Adolescents 12 to 15 years: ~11 to 12 hours
- Adults: ~20 hours (range: 12 to 27 hours); increased in patients with heart failure (NYHA Class III) or renal dysfunction
After administration of one XL capsule, plasma flecainide concentrations gradually increase after a lag time of 2 to 3 hours to reach a peak between the 21st and 25th hour and remain at plateau levels until after the 30th hour. After reaching the steady state concentration (3-5days), it exerts maximum action.
Flecainide mechanism of action
Flecainide acts on the fast-inward sodium (Na+) ion channel and has a high affinity to activated or open sodium (Na+) channels 2. Flecainide prolongs depolarization and increases refractoriness due to slow release from its binding site. Flecainide potently acts on the His-Purkinje system. It also works by inhibiting rapid delayed rectifier (IKr) channels, delaying potassium rectifier current resulting in prolongation of action potential duration in both ventricular and atrial muscle fibers 13. Flecainide is shown to block ryanodine receptor opening, which reduces calcium release from sarcoplasmic reticulum resulting after depolarization and triggered activity 14. Hence, indications for flecainide include catecholaminergic polymorphic ventricular tachycardia (CPVT).
Flecainide contraindications
According to the American College of Cardiology/American Heart Association/European Society of Cardiology, the use of flecainide is considered contraindicated in patients with structural heart disease. Other contraindications include hypersensitivity, documented second or third-degree AV block, sick sinus syndrome, bundle branch block, cardiogenic shock, and acquired/congenital QT prolongation with a history of Torsades de Pointes.
Caution is also advised in myocardial dysfunction, congestive heart failure, QT prolongation, electrolyte abnormalities, and pacemaker use 15.
Significant drug interactions include ritonovir, cisapride, despiramine, dronedarone, quinidine, saquinavir, and tipranavir. Concurrent use with these agents is contraindicated. It interacts with many other drugs where therapy modification may be necessary, so thorough medication reconciliation is necessary with flecainide, as with all drugs.
Flecainide special precautions
Before taking flecainide:
- tell your doctor and pharmacist if you are allergic to flecainide or any other medications.
- tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: acetazolamide (Diamox); amiodarone (Cordarone, Pacerone); ammonium chloride; antacids; beta blockers such as atenolol (Tenormin), labetalol (Trandate), metoprolol (Lopressor, Toprol XL), nadolol (Corgard), and propranolol (Inderal); carbamazepine (Carbatrol, Tegretol); cimetidine (Tagamet); clozapine (Clozaril); dichlorphenamide; digoxin (Lanoxin); diltiazem (Cardizem, Tiazac); disopyramide (Norpace); methazolamide; nifedipine (Adalat, Procardia); phenytoin (Dilantin); phenobarbital; quinidine; sodium bicarbonate (baking soda, Citrocarbonate, Soda Mint); and verapamil (Calan, Verelan). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have heart block (condition in which electrical signals are not passed normally from the upper chambers of the heart to the lower chambers). Your doctor may tell you not to take flecainide.
- tell your doctor if you have a pacemaker (device that is surgically placed under the skin to control irregular heartbeats) and if you have or have ever had a heart attack, heart failure, or any type of heart disease; low or high levels of potassium in the blood; or liver or kidney disease. Also tell your doctor if you follow a strict vegetarian diet.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking flecainide, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking flecainide.
- if you are giving this medication to an infant, be sure to talk to the doctor if there will be any major changes in the amount of milk the infant drinks. Milk can affect how the medication is absorbed in the body.
It is important that your doctor check your progress at regular visits to make sure the medicine is working properly. This will allow for changes to be made in the amount of medicine you are taking, if necessary.
Check with your doctor right away if you develop any of the following:
- chest pain;
- shortness of breath;
- swelling of your hands, ankles, or feet; or weight gain.
These may be symptoms of heart failure.
This medicine can cause changes in your heart rhythm, such as conditions called PR, QRS, or QT prolongation. It may cause fainting or serious side effects in some patients. Contact your doctor right away if your symptoms do not improve or if they become worse.
Your doctor may want you to carry a medical identification card or bracelet stating that you are using flecainide.
Before having any kind of surgery (including dental surgery) or emergency treatment, tell the medical doctor or dentist in charge that you are taking flecainide.
Flecainide may cause some people to become dizzy, lightheaded, or less alert than they are normally. Make sure you know how you react to flecainide before you drive, use machines, or do anything else that could be dangerous if you are dizzy or are not alert.
If you have been using flecainide regularly for several weeks, do not suddenly stop using it. Check with your doctor for the best way to gradually reduce the amount you are taking before stopping completely.
Do not take other medicines unless they have been discussed with your doctor. This includes prescription or nonprescription (over-the-counter [OTC]) medicines and herbal or vitamin supplements.
Pregnancy
Pregnancy Category C: Animal studies have shown an adverse effect and there are no adequate studies in pregnant women OR no animal studies have been conducted and there are no adequate studies in pregnant women.
Using flecainide during pregnancy may pose some risk to the fetus, so clinicians must perform a risk/benefit analysis. Fetal risks include fetal heart rate variability, acceleration impairment, and QT interval abnormalities 16. Fetuses may also experience neonatal hyperbilirubinemia, although this data is unclear.
Breastfeeding
Flecainide is present in breast milk; the relative infant dose is 8% when the maternal dosing is 200mg/day. The relative infant dosing is calculated using the highest average breast milk concentration compared to the maternal dosage. Breastfeeding is acceptable as long as relative infant dosing is under 10% 16.
Flecainide interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking flecainide, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using flecainide with any of the following medicines is not recommended. Your doctor may decide not to treat you with this medication or change some of the other medicines you take.
- Amisulpride
- Bepridil
- Cisapride
- Dronedarone
- Levomethadyl
- Mesoridazine
- Pimozide
- Piperaquine
- Ritonavir
- Saquinavir
- Sparfloxacin
- Terfenadine
- Thioridazine
- Tipranavir
- Vernakalant
- Ziprasidone
Using flecainide with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Acecainide
- Ajmaline
- Alfuzosin
- Amiodarone
- Amitriptyline
- Amoxapine
- Anagrelide
- Apomorphine
- Aprindine
- Arbutamine
- Aripiprazole
- Aripiprazole Lauroxil
- Arsenic Trioxide
- Artemether
- Asenapine
- Astemizole
- Azimilide
- Azithromycin
- Bedaquiline
- Bendroflumethiazide
- Boceprevir
- Bretylium
- Buprenorphine
- Bupropion
- Buserelin
- Ceritinib
- Chloral Hydrate
- Chloroquine
- Chlorothiazide
- Chlorpromazine
- Chlorthalidone
- Ciprofloxacin
- Citalopram
- Clarithromycin
- Clofazimine
- Clomipramine
- Clozapine
- Cobicistat
- Crizotinib
- Cyclobenzaprine
- Dabrafenib
- Darifenacin
- Dasabuvir
- Dasatinib
- Degarelix
- Delamanid
- Delavirdine
- Desipramine
- Deslorelin
- Deutetrabenazine
- Dibenzepin
- Disopyramide
- Dofetilide
- Dolasetron
- Domperidone
- Donepezil
- Droperidol
- Duloxetine
- Efavirenz
- Encorafenib
- Enflurane
- Erythromycin
- Escitalopram
- Etravirine
- Fingolimod
- Fluconazole
- Fluoxetine
- Foscarnet
- Gatifloxacin
- Gemifloxacin
- Glasdegib
- Gonadorelin
- Goserelin
- Granisetron
- Halofantrine
- Haloperidol
- Halothane
- Histrelin
- Hydrochlorothiazide
- Hydroflumethiazide
- Hydroquinidine
- Hydroxychloroquine
- Hydroxyzine
- Ibutilide
- Iloperidone
- Imipramine
- Inotuzumab Ozogamicin
- Isoflurane
- Isradipine
- Ivabradine
- Ivosidenib
- Ketoconazole
- Lacosamide
- Lapatinib
- Leuprolide
- Levofloxacin
- Lidocaine
- Lidoflazine
- Lofexidine
- Lorcainide
- Lumefantrine
- Macimorelin
- Mefloquine
- Methadone
- Metolazone
- Metronidazole
- Mifepristone
- Moxifloxacin
- Nafarelin
- Nilotinib
- Norfloxacin
- Nortriptyline
- Octreotide
- Ofloxacin
- Ombitasvir
- Ondansetron
- Osimertinib
- Paliperidone
- Panobinostat
- Paritaprevir
- Pasireotide
- Pazopanib
- Peginterferon Alfa-2b
- Pentamidine
- Pimavanserin
- Pirmenol
- Pitolisant
- Polythiazide
- Posaconazole
- Prajmaline
- Prilocaine
- Probucol
- Procainamide
- Prochlorperazine
- Promethazine
- Propafenone
- Protriptyline
- Quetiapine
- Quinidine
- Ranolazine
- Ribociclib
- Risperidone
- Salmeterol
- Sematilide
- Sertindole
- Sertraline
- Sevoflurane
- Simeprevir
- Siponimod
- Sodium Phosphate
- Sodium Phosphate, Dibasic
- Sodium Phosphate, Monobasic
- Solifenacin
- Sorafenib
- Sotalol
- Spiramycin
- Sulfamethoxazole
- Sulpiride
- Sultopride
- Sunitinib
- Tacrolimus
- Tedisamil
- Telaprevir
- Telavancin
- Telithromycin
- Tetrabenazine
- Tizanidine
- Toremifene
- Trazodone
- Trichlormethiazide
- Triclabendazole
- Trifluoperazine
- Trimethoprim
- Trimipramine
- Triptorelin
- Vandetanib
- Vardenafil
- Vasopressin
- Vemurafenib
- Vinflunine
- Voriconazole
- Zolmitriptan
- Zotepine
- Zuclopenthixol
Using flecainide with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Cimetidine
- Digoxin
- Paroxetine
- Propranolol
- Verapamil
Other Interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using flecainide with any of the following is usually not recommended, but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use flecainide, or give you special instructions about the use of food, alcohol, or tobacco.
- Milk
Using flecainide with any of the following may cause an increased risk of certain side effects but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use flecainide, or give you special instructions about the use of food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of flecainide. Make sure you tell your doctor if you have any other medical problems, especially:
- AV block (type of abnormal heart rhythm), with no pacemaker or
- Bundle branch block (heart rhythm problem), with no pacemaker or
- Cardiogenic shock (shock caused by heart attack) or
- Chronic atrial fibrillation or
- Heart attack, recent—Should not be used in patients with these conditions.
- Congestive heart failure (severe) or
- Heart disease (e.g., cardiomyopathy) or
- Sick sinus syndrome (type of abnormal heart rhythm)—Use with caution. May make these conditions worse.
- Electrolyte imbalance (e.g., high or low potassium in the blood)—Should be corrected first before using flecainide.
- Kidney disease or
- Liver disease—Use with caution. The effects may be increased because of slower removal of the medicine from the body.
- If you have a permanent pacemaker—Use with caution. Flecainide may interfere with the pacemaker and require more careful follow-up by the doctor.
What is flecainide used for?
Flecainide is used to prevent or treat certain types of life-threatening irregular heartbeats (arrhythmias) such as paroxysmal supraventricular tachycardia (PSVT) and paroxysmal atrial fibrillation/flutter (PAF). Flecainide is also used to prevent life-threatening sustained ventricular tachycardia (sustained VT). Flecainide is in a class of medications called antiarrhythmics. It works by slowing electrical signals in the heart to stabilize the heart rhythm.
There is a chance that flecainide may cause new or make worse existing heart rhythm problems when it is used. Since it has been shown to cause severe problems in some patients, it is only used to treat serious heart rhythm problems. Discuss this possible effect with your doctor.
Flecainide dose
The dose of flecainide will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of flecainide. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
For oral dosage form (tablets) per American College of Cardiology, American Heart Association and Heart Rhythm Society guidelines:
- For paroxysmal supraventricular tachycardia (PSVT) and paroxysmal atrial fibrillation/atrial flutter:
- Adults: 50 to 300 milligrams (mg) daily by mouth divided into 8 to 12-hour intervals. Start with 50 mg orally every 12 hours, then increase 100 mg per day every four days. The maximum dose is 300 mg daily. Dose adjustment is made based on serum levels. Your doctor may increase your dose as needed.
- Children: Use and dose must be determined by your doctor. Dose is based on body size and must be determined by your child’s doctor. The starting dose is 100 milligrams (mg) per square meter (m²) per day for infants 6 months and older and 50 mg/m² per day in infants younger than 6 months. Doses are divided into two or three equal doses per day.
- For sustained ventricular tachycardia (sustained VT) prophylaxis:
- Adults: 100 to 400 milligrams (mg) daily by mouth, divided into 8 or 12-hour intervals. Start 100 mg daily every 12 hours, and increase dosing by 100 mg per day every four days. The maximum dose is 400 mg daily. Dose adjustment is made based on serum levels. Your doctor may increase your dose as needed. However, the dose is usually not more than 400 mg per day.
- Children: Use and dose must be determined by your doctor. Dose is based on body size and must be determined by your child’s doctor. The starting dose is 100 milligrams (mg) per square meter (m²) per day for infants 6 months and older and 50 mg/m² per day in infants younger than 6 months. Doses are divided into two or three equal doses per day.
No dosage changes are necessary for liver disease, as per the manufacturer. With kidney disease and CrCl less than 35ml/min/1.73m², caution is necessary when increasing the dose at 4-day intervals.
There are an increased response and a steep relationship between dose and concentration. Plasma levels require monitoring in patients with severe kidney failure or hepatic disease. Drug overdose could be fatal with flecainide. The prescriber should adjust dosing based on clinical response 12.
In patients with renal failure or hepatic impairment, it is prudent to monitor EKG and blood pressure and to obtain periodic serum trough concentration. Therapeutic trough concentration is between 0.2 to 1 mcg/ml. Lower trough concentration is sufficient in pediatric patients.
Consult facility guidelines or manufacturer prescribing data for pediatric dose regimens.
Adult dose for Ventricular Tachycardia
- Initial dose: 100 mg orally every 12 hours.
- Maintenance dose: May be increased in increments of 50 mg bid every 4 days until efficacy is achieved. Most patients with SUSTAINED VT do not require more than 150 mg every 12 hours (300 mg/day), and the maximum dose recommended is 400 mg/day.
Adult dose for Atrial Fibrillation
- Initial dose: 50 mg orally every 12 hours.
- Maintenance dose: May be increased in increments of 50 mg bid every 4 days until efficacy is achieved.
Adult dose for Atrial Flutter
- Initial dose: 50 mg orally every 12 hours.
- Maintenance dose: May be increased in increments of 50 mg bid every 4 days until efficacy is achieved.
Adult dose for Wolff-Parkinson-White Syndrome
- Initial dose: 50 mg orally every 12 hours.
- Maintenance dose: May be increased in increments of 50 mg bid every 4 days until efficacy is achieved.
Adult dose for Paroxysmal Supraventricular Tachycardia
- Initial dose: 50 mg orally every 12 hours.
- Maintenance dose: May be increased in increments of 50 mg bid every 4 days until efficacy is achieved.
Pediatric dose for Supraventricular Tachycardia
Less than 1 month:
- Supraventricular tachycardia: Limited data available: Initial: 2 mg/kg/day orally divided every 12 hours; titrate to clinical response, monitor serum concentration; mean dose required to suppress SVT: 3.35 ± 1.35 mg/kg/day in 17 neonates (n=20 treated neonates; mean PNA: 11.5 days; mean GA: 36.8 weeks; mean birthweight: 2.8 kg); study did not report resultant serum concentrations.
1 month or older:
- Initial: 1 to 3 mg/kg/day orally or 50 to 100 mg/m²/day orally in 3 divided doses; usual: 3 to 6 mg/kg/day or 100 to 150 mg/m²/day in 3 divided doses; up to 8 mg/kg/day or 200 mg/m²/day for uncontrolled patients with subtherapeutic levels; higher doses have been reported, however they may be associated with an increased risk of proarrhythmias; a review of world literature reports the average effective dose to be 4 mg/kg/day or 140 mg/m²/day.
Renal Dose Adjustments
- CrCl=35 mL/min or less: Initial dose: 100 mg orally once a day or 50 mg twice a day. It may take longer than 4 days before a new steady-state plasma level is reached following a dosage change.
- In patients with less severe renal dysfunction: Initial dose: 100 mg every 12 hours.
Liver Dose Adjustments
Flecainide should not be used in patients with significant liver dysfunction unless the potential benefits clearly outweigh the risks, as elimination from plasma can be markedly slower in patients with significant hepatic impairment. If it is deemed necessary, frequent and early plasma level monitoring is required to guide dosage and dosage increases should be made very cautiously when plasma levels have plateaued (after more than four days).
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Flecainide side effects
Flecainide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- dizziness
- changes in vision
- headache
- weakness
- uncontrollable shaking of a part of your body
- constipation
- stomach pain
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately:
- fast, pounding, or irregular heartbeat
- chest pain
- shortness of breath
- extreme tiredness
- nausea
- loss of appetite
- persistent cough with blood-tinged mucus
- swelling of the hands, feet, ankles, or lower legs
- confusion
- unusual bleeding or bruising
- pain in the upper right part of the stomach
- yellowing of the skin or eyes
- flu-like symptoms
More common
- difficult or labored breathing
- dizziness, fainting, or lightheadedness
- fast, irregular, pounding, or racing heartbeat or pulse
- shortness of breath
- tightness in the chest
- wheezing
Less common
- burning, crawling, itching, numbness, prickling, “pins and needles”, or tingling feelings
- chest pain
- fainting
- feeling of warmth
- fever
- increased sweating
- partial or slight paralysis
- redness of the face, neck, arms, and occasionally, upper chest
- shakiness and unsteady walk
- shakiness in the legs, arms, hands, or feet
- swelling of the feet or lower legs
- trembling or shaking of the hands or feet
- unsteadiness, trembling, or other problems with muscle control or coordination
Rare
- arm, back, or jaw pain
- black, tarry stools
- bleeding gums
- blood in the urine or stools
- blurred vision
- chest discomfort
- chest tightness or heaviness
- chills
- confusion
- convulsions
- cough
- decrease in the frequency of urination
- decrease in urine volume
- difficulty in passing urine (dribbling)
- difficulty with breathing
- dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position
- frequent urination
- general feeling of discomfort or illness
- headache
- increased volume of pale, dilute urine
- nausea
- nervousness
- noisy breathing
- painful or difficult urination
- pinpoint red spots on the skin
- pounding in the ears
- sensation of pins and needles
- slow or fast heartbeat
- sore throat
- sores, ulcers, or white spots on the lips or in the mouth
- stabbing pain
- sweating
- swollen glands
- thickening of bronchial secretions
- troubled breathing
- unusual bleeding or bruising
- unusual tiredness or weakness
- yellow eyes or skin
Flecainide may cause other side effects. Call your doctor if you have any unusual problems while taking flecainide.
Flecainide toxicity
Broadly speaking, flecainide like other sodium channel blockers cause metabolic, cardiac, and neurologic symptoms. This leads to hemodynamic compromise and metabolic acidosis, potentiating the effects of the medications and causing further sodium blockade 17. Patients with potential flecainide toxicity require an immediate electrocardiogram (ECG). Flecainide toxicity leads to a widening of the QRS complex, lengthening of the QT interval, a new right axis deviation, bradydysrhythmias, ventricular tachycardia, ventricular fibrillation or torsades des pointes 18. Brugada phenocopy, a sodium channelopathy disorder, can also be seen during acute toxicity 19.
Symptoms of flecainide toxicity may include:
- nausea
- vomiting
- seizures
- slow, fast, or irregular heartbeat
- loss of consciousness
- sudden death
Sodium channel blockers cross the blood-brain barrier and act through multiple mechanisms. They inhibit the gamma-aminobutyric acid (GABA) system (primarily lidocaine), activate the sodium ouabain-sensitive current, stimulate 5-TH2C receptors, antagonize H1 receptors and block all noradrenaline activating effect. It is through these actions that adrenergic stimulation occurs. These medications in large doses are also pro-convulsant through the above mechanisms 20.
Co-ingestion of other drugs can alter the elimination kinetics. A recent case report describes how propafenone delayed the metabolism of metoprolol by inhibition of CYP2D. This interaction of the two medications led to a more profound toxicity, and in this case cardiovascular collapse 21.
It is vital to consider and evaluate for any other co-ingestion.
Obtain electrolyte, renal and hepatic profiles, acetaminophen level, salicylate level, arterial or venous blood gas, drug screen, and a complete blood count. Evaluate for an anion gap, and osmolal gap as this could indicate coingestants not detectable on standard testing.
Flecainide toxicity management
Immediate initial management must begin with an assessment of airway, breathing, and circulation. Many patients present with hypotension, bradycardia or tachycardia and altered mental status. An endotracheal tube or other advanced airways should be placed in patients who are unable to protect their airway.
The cornerstone of treatment is the administration of sodium bicarbonate. It is indicated for patients with an ECG demonstrating a QRS duration >100 ms or any suspicious QT prolongation or dysrhythmia 22. Sodium bicarbonate is beneficial in raising the serum pH and increasing the extracellular sodium. Alkalinization leads to an increase of the electrochemical gradient across cell membranes which helps to offload sodium channels. It might also increase the protein binding of the offending agent. Patients should be given 1-2mEq/kg as a bolus dose 17. Bolus doses can be administered until the QRS duration is less than 100 ms. This can be followed with a continuous infusion of sodium bicarbonate of 2-3 50mEq ampules in one liter of D5W (5% dextrose in water) 22. Hypertonic saline has been used but is not routinely recommended; it remains an option in dire circumstances as reported in a case of flecainide overdose 23.
Management of hypotension requires a combination of volume resuscitation and vasopressor and inotropic support. Use of inotropic agents such as dobutamine helps increase cardiac output while the effects of the toxicity dissipate. Addition of vasopressors such as norepinephrine, vasopressin, or epinephrine might be considered for hemodynamic support. These agents lead to vasoconstriction and an increase in the systemic vascular resistance, resulting in increased systemic blood pressure.
Patients with sodium channel blocker toxicity from lidocaine have benefitted from the administration of 20% lipid emulsion if they are hemodynamically unstable 24. The mechanism of action of lipid emulsion is unclear; however, it is hypothesized that it acts as a lipid sink, with an electrochemical gradient drawing the lidocaine into the lipid. Patients should be given a 1.5mL/kg bolus followed by a 0.25mL/kg infusion 25. Few studies exist on lipid emulsion for other sodium channel toxicities.
Extracorporeal membrane oxygenation (ECMO) has been used in a refractory case with reported survival 23. In general, the drugs which cause sodium channel blockade toxicity are highly lipophilic, have a wide volume of distribution, and are not dialyzable.
Seizure management is accomplished with benzodiazepine medications, such as lorazepam and midazolam. For refractory seizures, loading of antiepileptic medications such as levetiracetam is recommended. Phenytoin and its derivatives should be avoided in this situation as phenytoin is itself a sodium channel blocker and will likely lead to clinical deterioration. Intubation and sedation with propofol should be considered for seizures refractory to other management.
Flecainide toxicity prognosis
Complications of sodium channel blocker toxicity include cardiogenic shock, hypotension, bradycardia or tachycardia, cardiovascular collapse, respiratory depression, encephalopathy, status epilepticus, and death.
Class I antiarrhythmic toxicity is associated with a significantly higher mortality rate (22.5%) compared with other drugs (1%) 19. Prompt recognition and treatment are vital to minimize morbidity and mortality.
References- Salvage SC, Chandrasekharan KH, Jeevaratnam K, Dulhunty AF, Thompson AJ, Jackson AP, Huang CL. Multiple targets for flecainide action: implications for cardiac arrhythmogenesis. Br J Pharmacol. 2018 Apr;175(8):1260-1278. doi: 10.1111/bph.13807
- Arunachalam K, Alzahrani T. Flecainide. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542291
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Flecainide. [Updated 2018 Jan 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548023
- Paul AA, Witchel HJ, Hancox JC. Inhibition of the current of heterologously expressed HERG potassium channels by flecainide and comparison with quinidine, propafenone and lignocaine. Br J Pharmacol. 2002 Jul;136(5):717-29. doi: 10.1038/sj.bjp.0704784
- Rolf S, Haverkamp W, Borggrefe M, Musshoff U, Eckardt L, Mergenthaler J, Snyders DJ, Pongs O, Speckmann EJ, Breithardt G, Madeja M. Effects of antiarrhythmic drugs on cloned cardiac voltage-gated potassium channels expressed in Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000 Jul;362(1):22-31. doi: 10.1007/s002100000257
- Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, Napolitano C, Coetzee WA, Boyden PA, Priori SG. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011 Jul 22;109(3):291-5. doi: 10.1161/CIRCRESAHA.111.247338
- Nagatomo T, January CT, Makielski JC. Preferential block of late sodium current in the LQT3 DeltaKPQ mutant by the class I(C) antiarrhythmic flecainide. Mol Pharmacol. 2000 Jan;57(1):101-7.
- Dokken K, Fairley P. Sodium Channel Blocker Toxicity. [Updated 2018 Nov 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534844
- Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985 Jan;29(1):1-33. doi: 10.2165/00003495-198529010-00001
- Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986 Jul 3;315(1):36-41. doi: 10.1056/NEJM198607033150106
- Conard GJ, Ober RE. Metabolism of flecainide. Am J Cardiol. 1984 Feb 27;53(5):41B-51B. doi: 10.1016/0002-9149(84)90501-0
- Tamargo J, Le Heuzey JY, Mabo P. Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol. 2015 May;71(5):549-67. doi: 10.1007/s00228-015-1832-0
- Salvage, S.C., Chandrasekharan K.H., Jeevaratnam K., Dulhunty A.F., Thompson A.J., Jackson A.P., and Huang C.L.H.. 2018. Multiple targets for flecainide action: Implications for cardiac arrhythmogenesis. Br. J. Pharmacol. 175:1260–1278. 10.1111/bph.13807
- Bannister ML, MacLeod KT, George CH. Moving in the right direction: elucidating the mechanisms of interaction between flecainide and the cardiac ryanodine receptor. Br J Pharmacol. 2022 Jun;179(11):2558-2563. doi: 10.1111/bph.15718
- Rivner H, Lambrakos LK. Flecainide Toxicity Leading to Loss of Pacemaker Capture and Cardiac Arrest. JACC Case Rep. 2021 Jan 27;3(4):586-590. doi: 10.1016/j.jaccas.2020.11.030
- Tamirisa KP, Elkayam U, Briller JE, Mason PK, Pillarisetti J, Merchant FM, Patel H, Lakkireddy DR, Russo AM, Volgman AS, Vaseghi M. Arrhythmias in Pregnancy. JACC Clin Electrophysiol. 2022 Jan;8(1):120-135. doi: 10.1016/j.jacep.2021.10.004
- Mirrakhimov AE, Ayach T, Barbaryan A, Talari G, Chadha R, Gray A. The Role of Sodium Bicarbonate in the Management of Some Toxic Ingestions. Int J Nephrol. 2017;2017:7831358
- Di Grande A, Giuffrida C, Narbone G, Le Moli C, Nigro F, Di Mauro A, Pirrone G, Tabita V, Alongi B. Management of sodium-channel blocker poisoning: the role of hypertonic sodium salts. Eur Rev Med Pharmacol Sci. 2010 Jan;14(1):25-30.
- Arı ME, Ekici F. Brugada-Phenocopy Induced by Propafenone Overdose and Successful Treatment: A Case Report. Balkan Med J. 2017 Sep 29;34(5):473-475
- Grant AO. On the mechanism of action of antiarrhythmic agents. Am. Heart J. 1992 Apr;123(4 Pt 2):1130-6
- Kacirova I, Grundmann M, Kolek M, Vyskocilova-Hrudikova E, Urinovska R, Handlos P. Lethal suicide attempt with a mixed-drug intoxication of metoprolol and propafenone – A first pediatric case report. Forensic Sci. Int. 2017 Sep;278:e34-e40
- Kacirova I, Grundmann M, Kolek M, Vyskocilova-Hrudikova E, Urinovska R, Handlos P. Lethal suicide attempt with a mixed-drug intoxication of metoprolol and propafenone – A first pediatric case report. Forensic Sci. Int. 2017 Sep;278:e34-e40.
- Szadkowski M, Drapkin Z, Hewes H, Caravati EM, Plumb J. A Teenager With Seizures and Cardiac Arrest After Drug Overdose: Are We Numb to the Danger? Pediatr Emerg Care. 2017 Sep;33(9):657-659
- Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med. 2010 Oct 05;18:51
- Vu NM, Hill TE, Summers MR, Vranian MN, Faulx MD. Management of life-threatening flecainide overdose: A case report and review of the literature. HeartRhythm Case Rep. 2016 May;2(3):228-231