Fontan procedure
Fontan procedure is the the third-stage and final surgery to treat hypoplastic left heart syndrome (in children whose heart has only one main pumping chamber or ventricle), and usually happens between 18 months to 5 years of age. Fontan procedure requires open heart surgery in the operating room and your child connected to the heart-lung machine the same as Stage 2 (Hemi-Fontan or Glenn operation). During the Fontan procedure the inferior vena cava (IVC), a large vein that carries deoxygenated blood from the lower body into the heart, is disconnected from the heart and attached to the pulmonary artery directly. Blood will reach the lungs from the lower body by the inferior vena cava connection. After this operation, all of the deoxygenated blood from the body goes to the lungs without passing through the heart. After the final surgery is completed, the blood flow from the upper body will return to the lungs by the superior vena cava (SVC) connection. After the all the staged operations for single ventricle defects, the heart functions like a one-sided pump with two chambers. The heart no longer receives deoxygenated blood from the veins. Instead, this blood flows directly to the lungs. The heart receives oxygenated blood from the lungs and pumps it to the body. This is called Fontan circulation.
The Fontan procedure is done so that almost all the deoxygenated blood coming back from the body goes to the lungs. After this stage, most children are much “pinker” because now nearly all of the blood pumped out to the body goes to the lungs first. The Fontan procedure has allowed children with the most complex forms of heart disease to enjoy a mostly normal life.
The name of the procedure comes from the name of the doctor who was the first to describe this operation in 1971, Dr Francis Fontan 1. Fontan procedure improves blood flow from the lower body to the lungs, which further decreases the workload of the single ventricle and improves oxygen levels. Until now, blood low in oxygen from the lower part of the body has mixed with blood high in oxygen. During the Fontan operation, surgeons connect the veins from the body directly to the arteries to the lungs.
Frequent surveillance in infancy and early childhood is important to minimize risk factors for the eventual Fontan operation.
Your child will also need a customized series of diagnostic tests between the planned stages of surgery, and throughout childhood. Additional surgical or catheter therapies, or in rare cases heart transplantation, may also be recommended.
Figure 1. Hypoplastic left heart syndrome
Stage 1. Norwood procedure
The Stage 1 Norwood procedure, first described by Norwood et al. in 1979, has become the standard of care for neonates with hypoplastic left heart syndrome 2. The Norwood procedure allows the right ventricle to become the systemic ventricle while the pulmonary flow is provided through a Gortex tube graft called a modified Blalock Taussig shunt (BT shunt). This stage usually requires the use of deep hypothermic circulatory arrest due to the abnormal aortic arch anatomy. This operation will occur within several days of birth. The purpose of Norwood procedure is to ensure that blood-flow is controlled enough to prevent damage to the heart and lungs, and that enough blood is reaching the lungs to keep the child alive until the second operation.
- The atrial septum is removed to allow free flow of oxygenated blood entering the left atrium from the pulmonary veins to reach the right ventricle
- A neoaorta is created by sewing the hypoplastic ascending aorta to the main pulmonary artery to provide a common outflow tract to the systemic circulation from the right ventricle.
- A systemic-to-pulmonary shunt (Blalock Taussig shunt) is created by connecting the right subclavian or innominate artery to the right pulmonary artery. This replaces the ductus arteriosus as the source of pulmonary blood. Consequently, prostaglandin E1 is no longer needed to maintain ductal patency 2. The size of the shunt provides partial restriction to pulmonary blood flow and may reduce the incidence of pulmonary over circulation.
Depending on the type of heart defect, different surgical procedures may be used, including the Norwood procedure.
Figure 2. Norwood procedure
Stage 2. Hemi-Fontan or Glenn operation
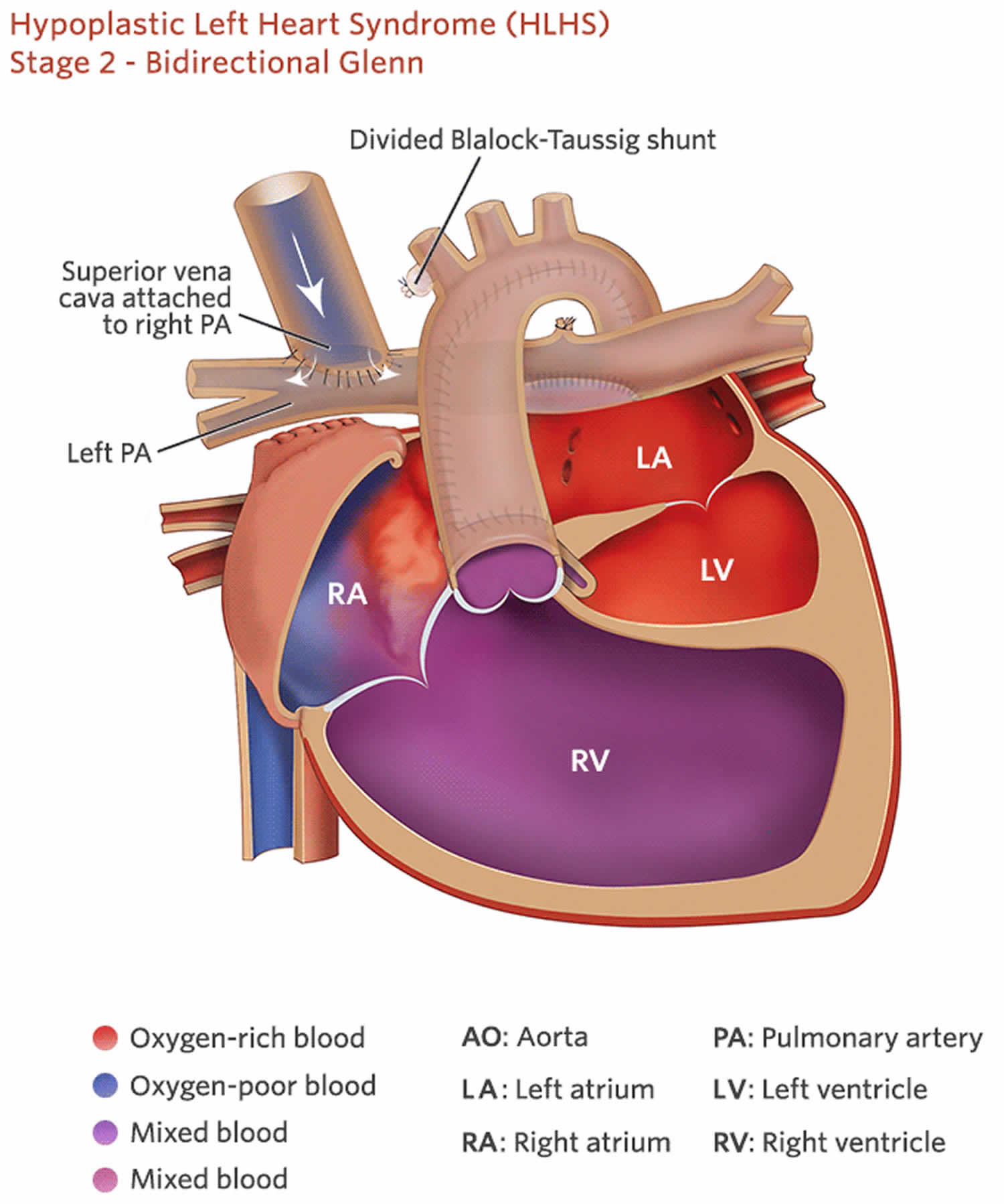
The Hemi-Fontan or Glenn operation (Bidirectional Glenn), the second procedure usually occurs within 4-6 months months of birth. During this surgery the superior vena cava — a large vein that carries deoxygenated blood from the upper body into the heart — is disconnected from the heart and attached to the pulmonary artery. After this operation, deoxygenated blood from the upper body goes to the lungs without passing through the heart.
Both of these surgical procedures, the Hemi-Fontan or Bidirectional Glenn procedures, result in a decreased volume load on the right ventricle, thereby preventing right ventricular hypertrophy/increased wall thickness and potentially improving diastolic function 3. The systemic venous return via the inferior vena cava (IVC) empties into the common atrium where there is a mixing of oxygenated and deoxygenated blood. Before this procedure, cardiac catheterization will be performed to document normal pulmonary artery pressures. Elevated pulmonary pressures would need to be corrected before proceeding with the second stage repair. After the second stage repair, the pulmonary and systemic systems are changed from a parallel circulation to a partial in series circulation 4. The following are steps involved with each procedure:
Hemi-Fontan
- Systemic-to-pulmonary artery shunt removal (Blalock-Taussig shunt)
- Anastomosis between the superior vena cava and right atrial (RA) confluence and the central and branch pulmonary arteries to direct the superior vena cava blood to the pulmonary circulation
- Homograft patch augmentation of the central and branch pulmonary arteries
- Interruption of the superior vena cava blood from reaching right atrial using the homograft stopper 3.
Bidirectional Glenn
- Systemic-to-pulmonary artery shunt removal (Blalock-Taussig shunt)
- End-to-side anastomosis of the superior vena cava to a branch pulmonary artery, typically right pulmonary artery 3.
Hybrid procedure 2nd stage repair
- Superior cavopulmonary anastomosis (superior vena cava to pulmonary artery anastomosis) via Hemi-Fontan or Bidirectional Glenn
- Creation of a neoaorta, as described in the Stage I palliation, with aortic arch reconstruction
- Atrial septectomy
- Removal of the pulmonary artery bands and pulmonary arterioplasty if needed
- Removal or resection of the ductus/stent complex 5.
Figure 3. Hemi-Fontan procedure
Figure 4. Fontan procedure
Summary
The Fontan procedure often is the only definitive palliative surgical option for patients with a variety of complex congenital heart conditions involving a single, dominant ventricle. Improved patient selection, patient preparation, and surgical techniques have led to improved outcomes, and many patients with Fontan circulation enjoy a high quality of life; however, there are many complications of the procedure such as exercise intolerance, ventricle failure, right atrium dilatation and arrhythmia, systemic and hepatic venous hypertension, portal hypertension, coagulopathy, pulmonary arteriovenous malformations, venovenous shunts, and lymphatic dysfunction. MR imaging is best for postoperative evaluation of patients with Fontan circulation, and cardiac transplantation remains the only definitive treatment for those with failing Fontan circulation.
Fenestrated fontan or no fenestration?
A fenestration is a communication between the channel carrying the deoxygenated blood to the lungs and the heart. It allows the passage of blood from the veins to the heart when the lungs are not functioning well. If the lungs are swollen such as during pneumonia, or soon after surgery, the blood will not flow passively very well through them, and there may not be enough blood going back to the heart. The heart could then not pump enough blood to the body and the patient may feel sicker than if they were just limited by their sick lungs. This fenestration is like a pop-up valve acting as a safety mechanism. It is known to be particularly useful soon after surgery.
It is not yet known whether it is good or not to keep this fenestration open for a long time or not. Some believe that it improves the exercise capacity, and that because on peak exercise, some more blue blood will go to the heart, and the heart will then be able to pump more blood through the body. Others think that the heart will not function as well if the blood that the heart ejects is more blue (with less oxygen), and believe that the people will be able to exercise better with the fenestration closed. We hope that future research will be able to show whether Fontan patients would be better with, or without, fenestration late after surgery.
How does a normal heart function?
It is important to look at a normal heart and how it usually functions. Oxygen-poor (blue) blood returns to the right atrium from the body and travels to the right ventricle. It is then pumped through the pulmonary artery into the lungs where it receives oxygen. Oxygen-rich blood (red) returns to the left atrium from the lungs and passes into the left ventricle. The blood is then pumped out to the body through the aorta.
What is hypoplastic left heart syndrome?
Hypoplastic left heart syndrome is a congenital (present at birth) complex heart defect. Sometime around the first 8 weeks of fetal (unborn child) development, the heart did not develop properly. The term hypoplastic left heart syndrome means that most of the structures on the left side of the heart are small and underdeveloped.
With hypoplastic left heart syndrome these parts of the heart may be underdeveloped to some degree:
- Aortic arch
- Mitral and aortic valves
- Left ventricle
The exact cause of hypoplastic left heart syndrome is unknown. In the United States about 1,000 – 2,000 babies are born with hypoplastic left heart syndrome each year. Hypoplastic left heart syndrome occurs more often in males (67%). Without surgery the child with hypoplastic left heart syndrome will not survive.
Who needs a Fontan procedure?
Today, heart surgeons try to offer a Fontan procedure to all children who are born with abnormal hearts that cannot be repaired with two pumping chambers (ventricles).
One pumping chamber, the left ventricle, pushes the blood to the body, and the other, the right ventricle, pushes the blood to the lungs. Sometimes, these children are completely missing a ventricle and have what is called a single ventricle. Often, there are two ventricles, but one of them is too small to be really useful. At times, there are two good ventricles with some holes, but the connections between the ventricles and the collecting chambers and/or the vessels going to and out of the heart is so abnormal that it is impossible to close these holes and use the two ventricles separately.
After the Fontan procedure, all these children are said to have the heart functioning with a single pumping chamber, and we say that they have a “functional single ventricle”.
There are many different conditions of the heart that necessitates a Fontan procedure. The most frequent ones are called:
- Tricuspid atresia,
- Hypoplastic left heart syndrome
- Unbalanced atrio-ventricular septal defect
- Double outlet right ventricle
- Double inlet left ventricle
- Pulmonary atresia with intact ventricular septum
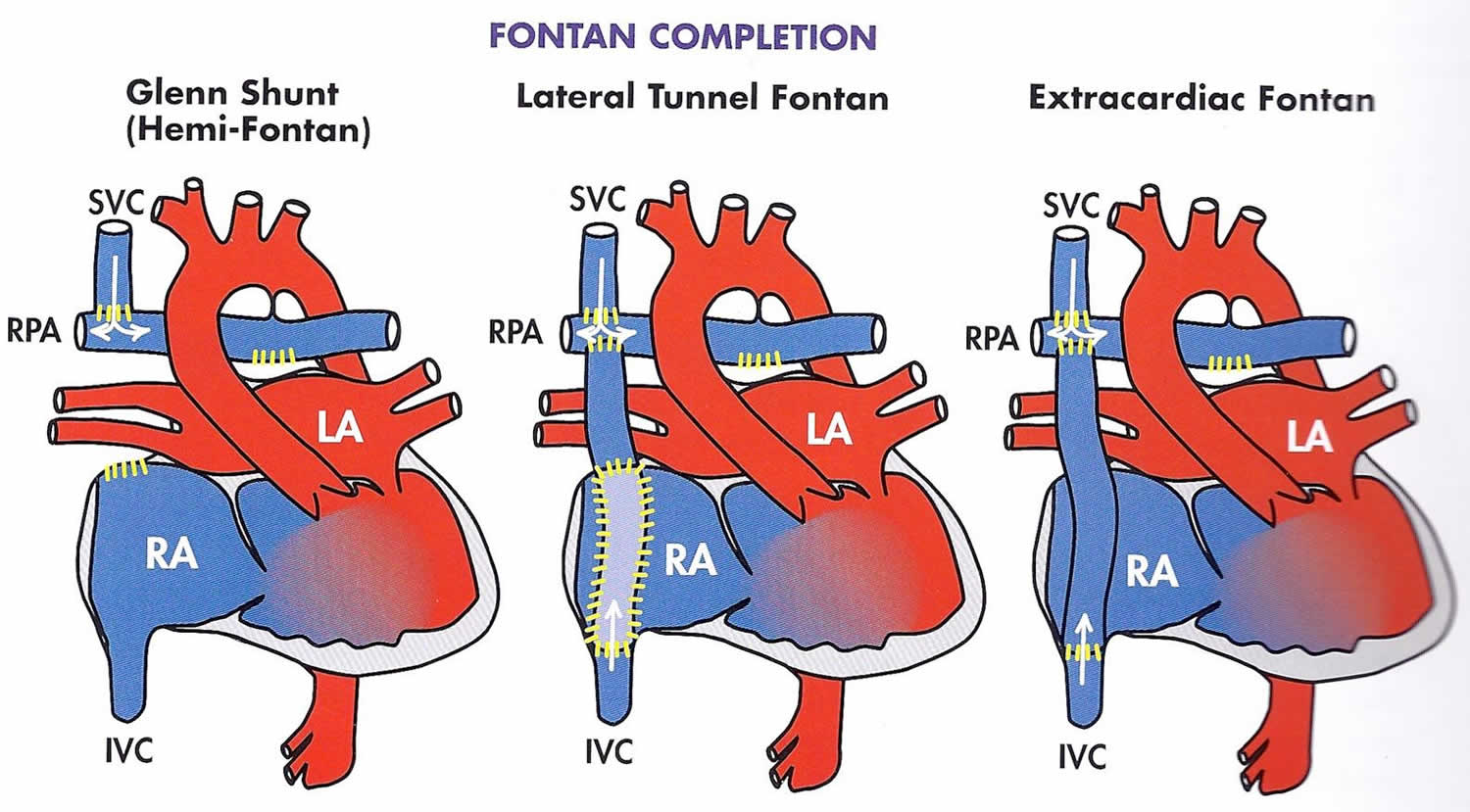
Fontan procedure types
There are 3 different variations for the Fontan procedure that has evolved over time.
Figure 5. Fontan procedure types
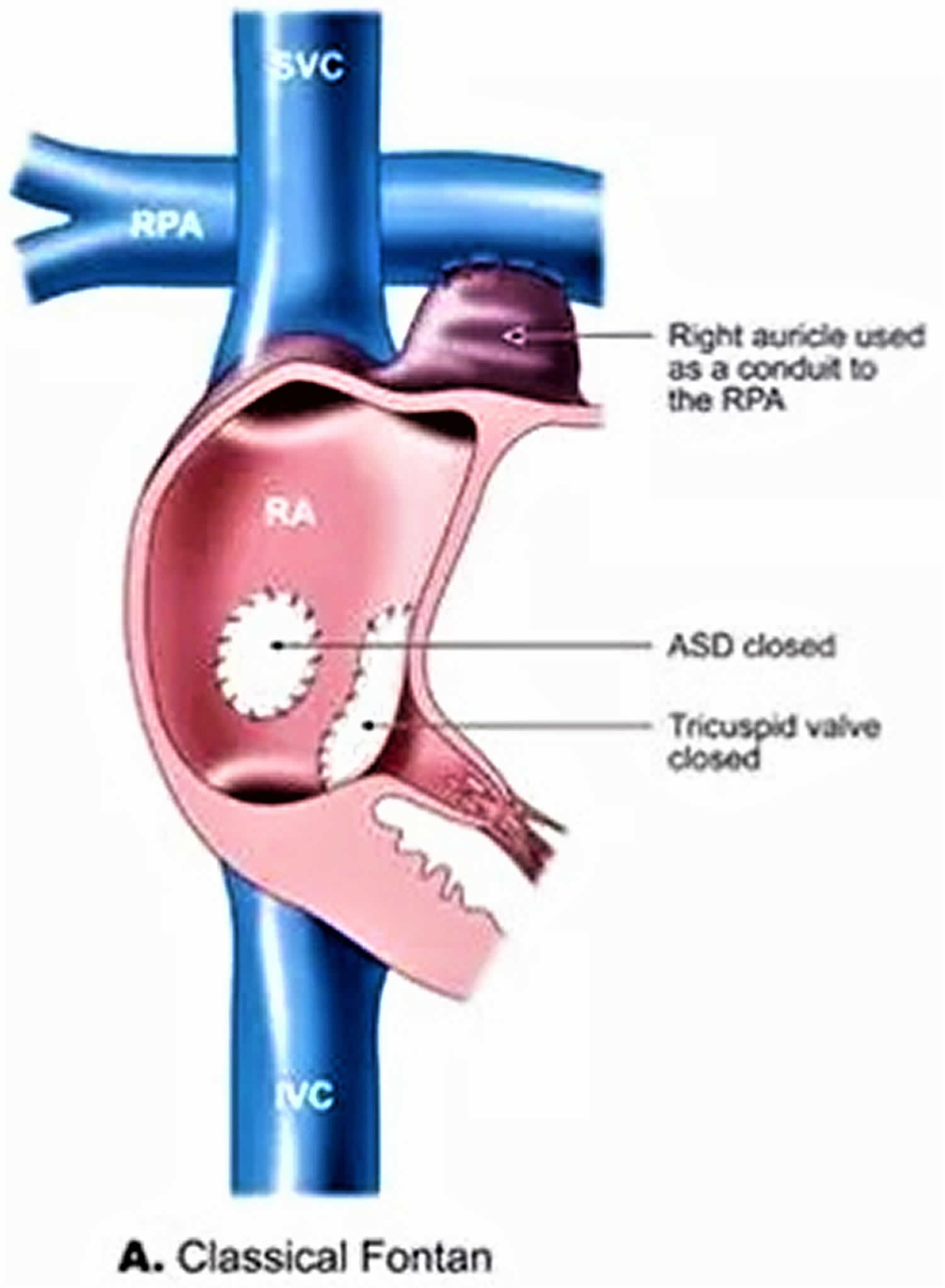
Atrio-Pulmonary connection (AP Fontan)
The AP Fontan (AtrioPulmonary connection) also known as the “classical” Fontan procedure originally described by Dr Fontan, is the way the operation was performed initially. In this operation, the collecting chamber of the heart taking the blue blood without oxygen coming from the body (the right atrium) was isolated from the rest of the heart. An expansion of this cavity (the right atrial appendage) was then connected to the right pulmonary artery.
Figure 6. Atriopulmonary Fontan
Footnote: Pictures showing the anatomy of the original Fontan procedure, in which the SVC was connected to the right pulmonary artery (RPA) and the right atrium to the pulmonary artery. Abbreviations: LPA = left pulmonary artery.
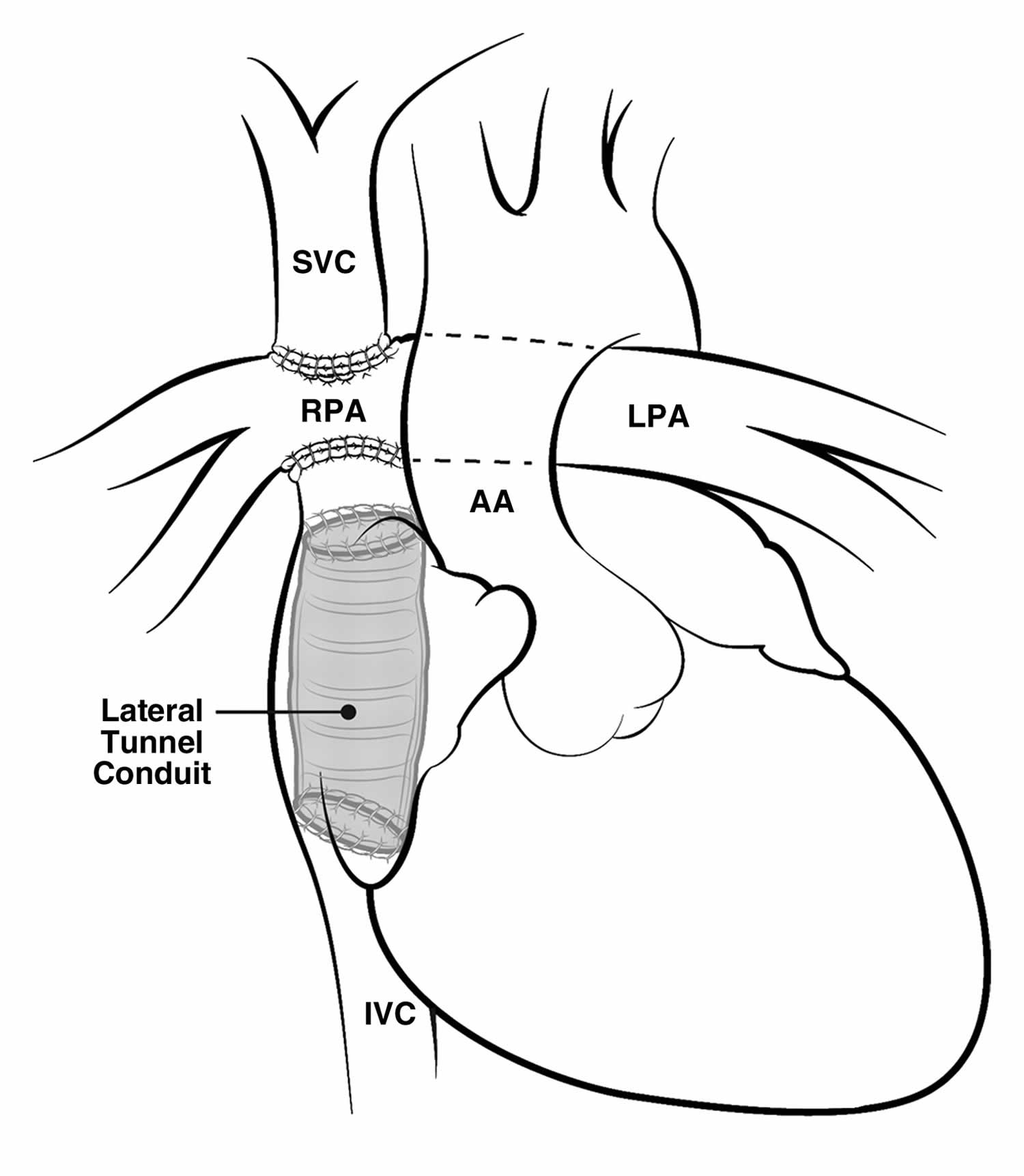
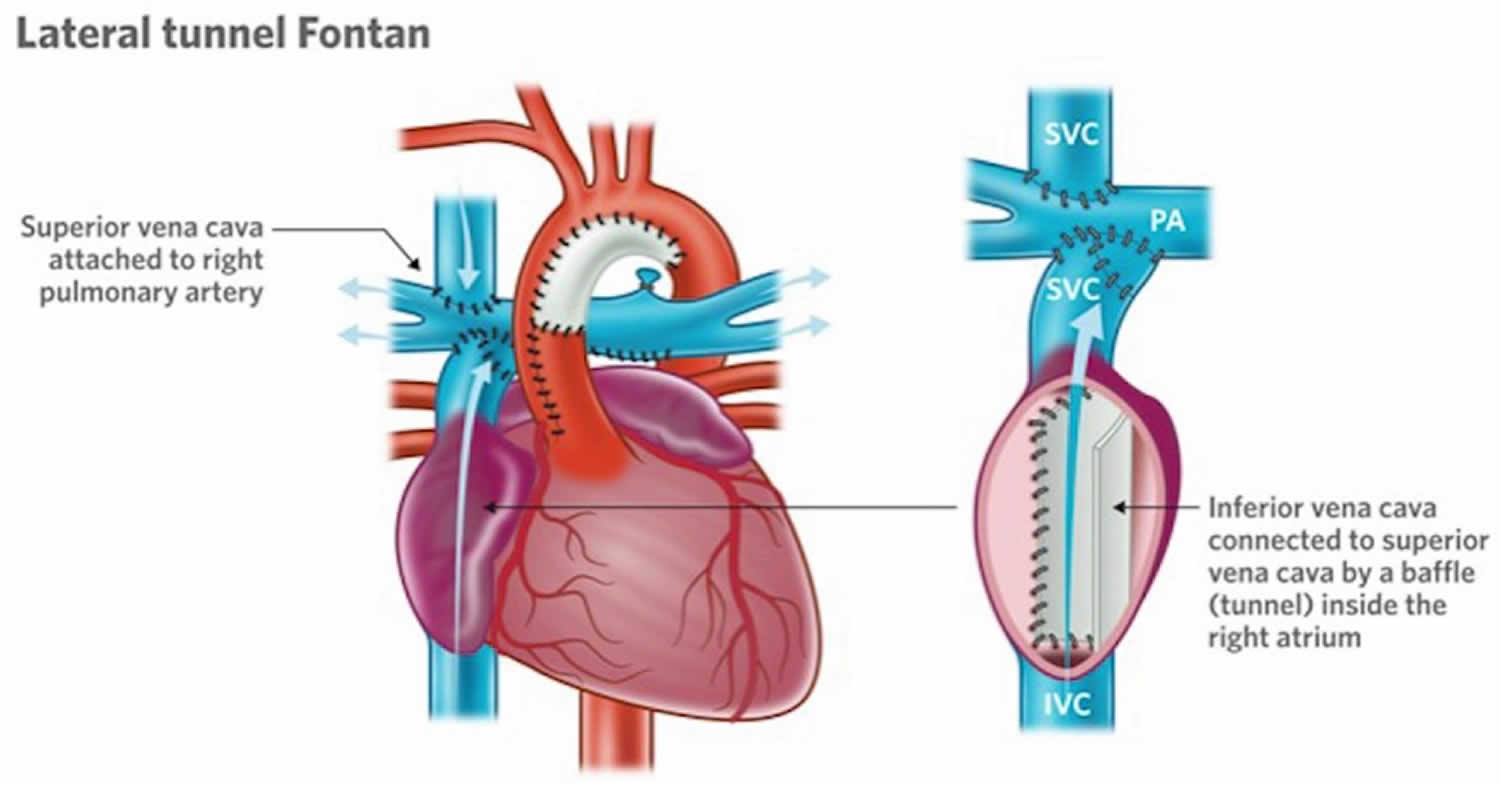
Lateral Tunnel Fontan
After many years, it was observed that the right atrium of some of the children who had a Fontan operation was progressively dilating. This dilatation seems to be occurring because of the turbulences of the blood as it was arriving from the veins coming from the body into this collecting chamber. This dilatation was annoying, because it was responsible for the formation of clots in the heart, and some of these patients had fast heart rates that made them sick.
A new design of the operation was then performed. In this operation commonly known as the “lateral tunnel fontan” or also “total cavo-pulmonary connection”, the vein draining the blood from the upper part of the body (the superior vena cava) was attached to the upper part of the right lung artery and the collecting chamber was attached to the underside of this same right lung artery. A half tube of artificial material was then sutured inside the collecting chamber to direct the blood coming from the lower part of the body in what looked much more like the inside of a cylinder inside the heart. The lateral tunnel fontan operation was designed so that this tube inside the heart would still have some potential for growth.
Figure 7. Lateral tunnel fontan
Footnote: Images showing the intraatrial method of creating cavopulmonary Fontan circulation, in which an intraatrial conduit connects the IVC to the right pulmonary artery (RPA).
Abbreviations: AA = ascending aorta, LPA = left pulmonary artery.
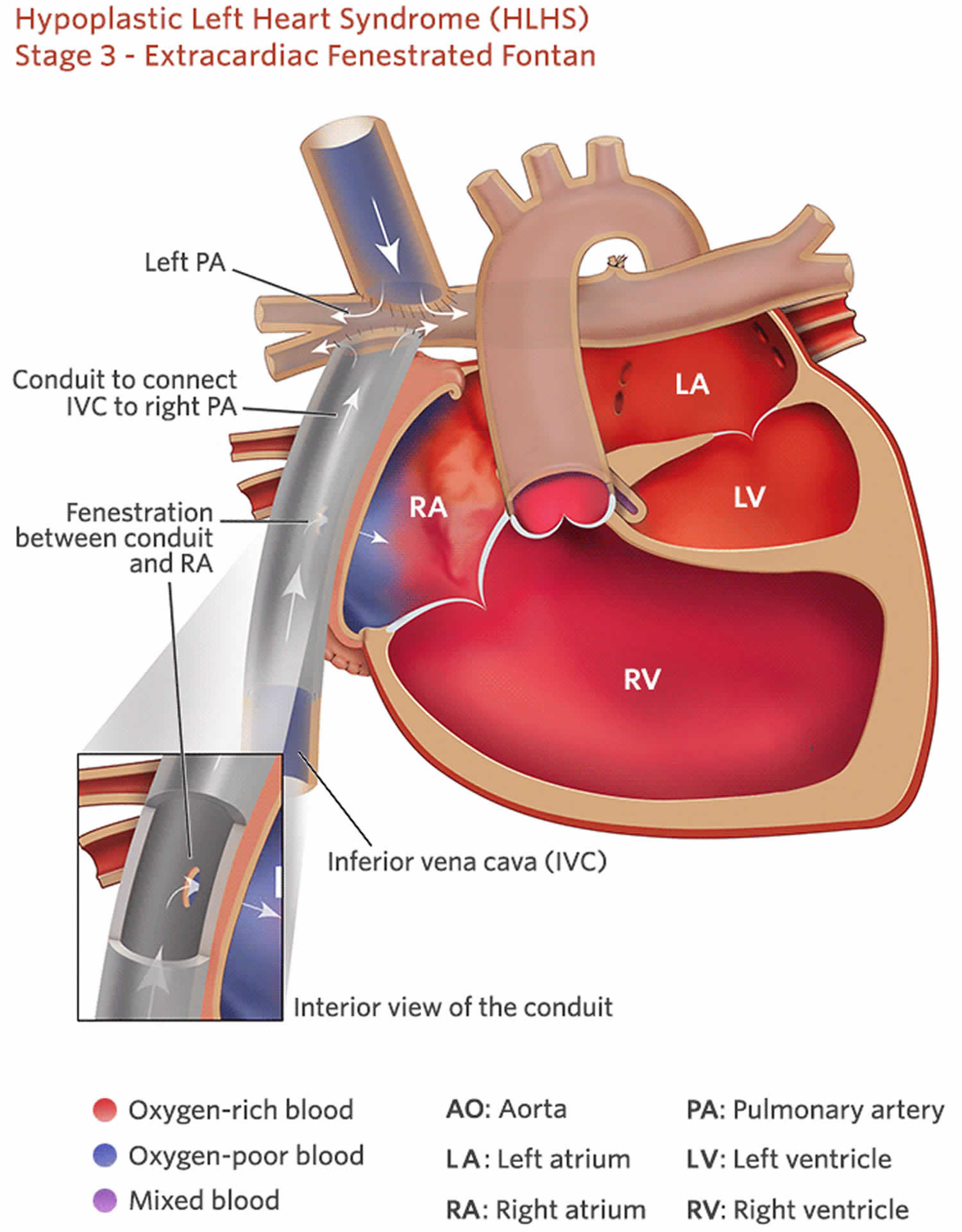
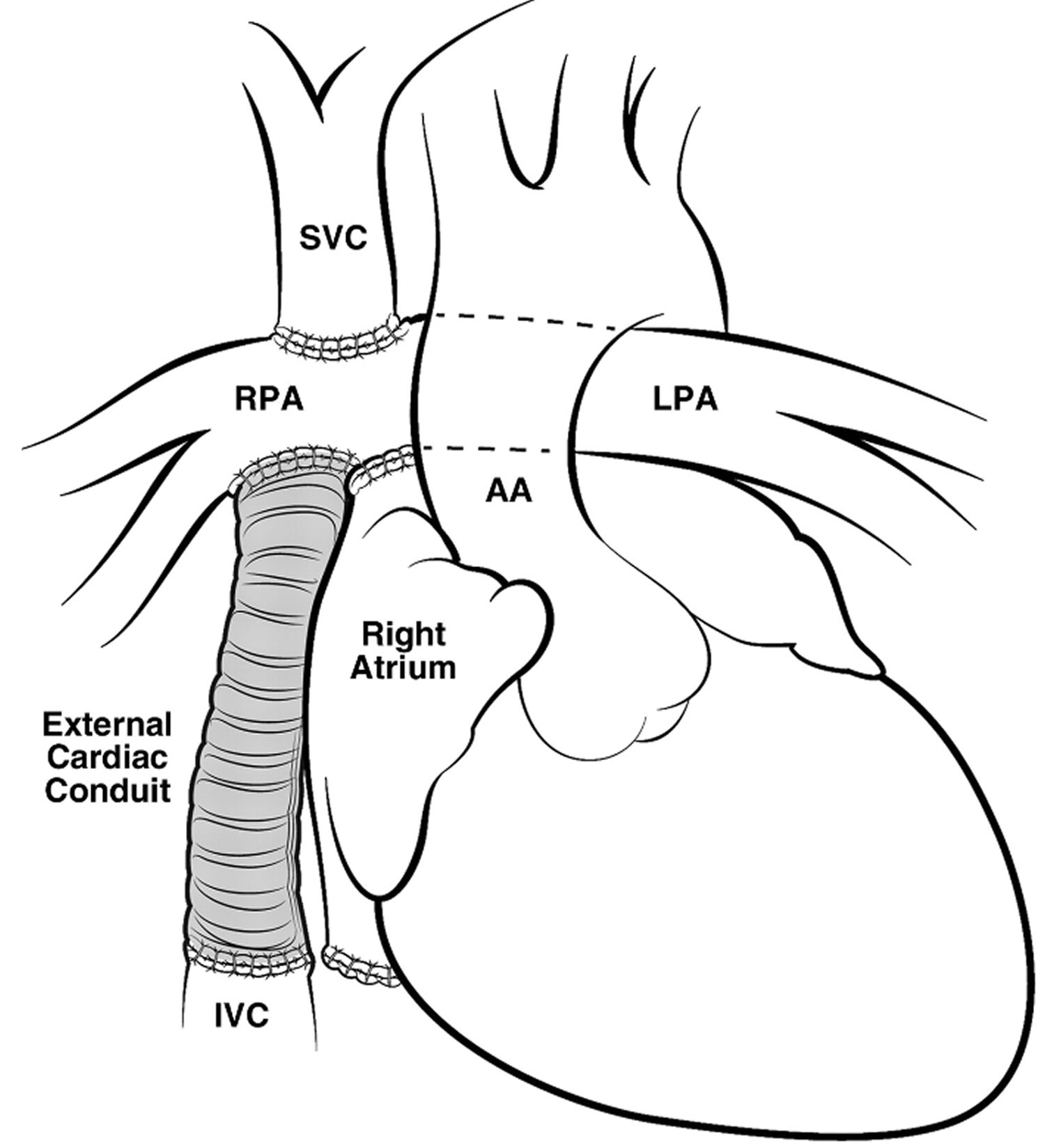
Extra-Cardiac Conduit Fontan
In Extra-Cardiac Conduit Fontan operation the superior vena cava is also, like in the Lateral Tunnel Fontan operation, connected to the right lung artery, but the vein coming from the lower part of the body (the inferior vena cava) is connected to an artificial (e.g. gore-tex) tube. The other end of this tube is then connected to the underside of the right lung artery. Extra-Cardiac Conduit Fontan operation was designed to try to have the best possible flow from the veins into the lung.
Figure 8. Extra-Cardiac Conduit Fontan
Footnote: Pictures showing the extraatrial method of creating cavopulmonary Fontan circulation, in which an extraatrial conduit connects the IVC to the right pulmonary artery (RPA).
Abbreviations: AA = ascending aorta, LPA = left pulmonary artery.
How does the Fontan procedure work?
After the Fontan procedure, the blood without oxygen comes back from the body directly in the lungs, without being pushed by the heart. There are two main driving forces allowing this flow into the lungs.
The first one is an increased pressure in the veins. Instead of a pressure of 0 to 5 millimeter of mercury (the equivalent of the weight of a column of water of 1 square centimetre over a height of 5 centimeter, very little), the pressure in the veins after a Fontan procedure is around 15 to 20 milimeter of mercury.
The second driving force is the breathing. As you breathe in, the size of the inside of the chest is increased, and the air is sucked in the airways. At the same time, the blood is sucked into the lungs. When you breathe out, the opposite occurs. The size of what is inside of the chest is reduced and the air is pushed out of the lungs. At the same time, the blood is pushed out of the lungs. The breathing acts like a pump for the blood flowing passively in and out of the lungs. That is why it is important for patients who had a Fontan operation to have good lungs. It has been shown that after Fontan, the blood circulates better in the body of those who keep exercising.
For the Fontan operation to work well, it is also important to have good lung arteries, without restriction, and to have a well a functioning heart, even if the heart is working as a single pump instead of two.
How is the Fontan procedure done?
The patch that was placed in the right upper chamber is removed (1). A wall, called a baffle (2), is built in the right upper chamber. The baffle guides the blue blood coming from the lower body into the blood vessels that go to the lungs (the pulmonary arteries). A small hole, called a fenestration (3), is made in the baffle. This allows a small amount of blue blood to go across the baffle into the right upper chamber. This hole works like a pop-off valve in case the pressure in the lungs gets too high. The size of the hole may vary. For most children, a small hole is made that will close by itself over time. In some children, a larger hole is needed. Closure of large holes is usually done six to twelve months later during a heart catheterization.
Figure 9. How the Fontan procedure is done
Fontan procedure recovery
Recovery after Fontan heart surgery will be similar to the Stage 2 hemi-fontan procedure where the length of stay varies from one to three weeks. After surgery, the baby will go to the Cardiac Intensive Care Unit (CICU). As long as the baby is on the breathing machine or needs to be watched closely the baby will stay in the CICU. After the baby fully recovers from surgery, the baby will be transferred to the regular cardiac floor. One important difference is that the child may have more than one chest tube to drain fluid from around the lung. Now all of the blood from the body is returning to the lungs through a “passive route” that requires a higher pressure. Therefore, fluid can leak out around the lungs and needs to be drained. Sometimes these chest tubes need to stay in for a longer time to drain this fluid. The child can be recovering on the regular cardiac floor and is encouraged to be out of bed and playing at this time.
Fontan and exercise
Increasingly the importance of regular physical activity is being recognised for people with a Fontan circulation.
“Cardio” type exercise programs with a component of moderate-intensity weight training has been shown to be of benefit for the cardiovascular system in other forms of heart disease but research investigating such physical activity in people with Fontan-hearts is limited. Some small studies have shown cardio exercise (such as brisk walking, swimming, dancing and jogging) improves exercise capacity in Fontan-hearts and recent Australian research has suggested that healthier limb muscles after a doctor-supervised, strength-training program helped the Fontan circulation to work more effectively both at rest and during exercise, probably by helping to pump blood back through the lungs and into the heart.
More research is needed to clarify the optimal exercise regimen that might include aspects of “cardio” exercise and some carefully supervised strength-training to help build muscle bulk, but what we do know already is that maintaining an active lifestyle with regular physical activity several days a week is beneficial.
A good target during exercise is to reach a level of exertion at which you are breathing hard, but can still have a conversation without having to stop to catch your breath. Lifting really heavy weights that are so difficult that you have to hold your breath to strain or grunt should be avoided as this can have negative effects on your cardiovascular system.
Before undertaking any vigorous exercise training program it is important that you discuss your plans with your cardiologist. They may also be able to give you some helpful targets for your heart rate and level of exertion.
Fontan procedure complications
Creation of Fontan circulation is palliative by nature, with proved good results in patients with ideal hemodynamics and substantial morbidity and mortality in those with unfavorable hemodynamics and those who underwent older surgical techniques. Risk factors for complications include elevated pulmonary artery pressure, anatomic abnormalities of the right and left pulmonary arteries, atrial-ventricular valve regurgitation, and poor ventricular function.
Many patients with Fontan circulation lead almost normal lives; however, some experience progressive exercise intolerance, and in patients with surgically constructed conduits, conduits may develop stenosis or become dilated.
Potential complications of Fontan circulation include 6:
- Left ventricle – ventricular failure causing exercise intolerance, ischemia and infacrti0n
- Pulmonary circulation – stenosis, dilatation or leakage of anastomosis, pulmonary stenosis, pulmonary hypertension
- Inferior vena cava – increased pressure causing liver cirrhosis, liver failure and portal hypertension; increased risk for liver carcinoma
- Right atrium with classic Fontan circulation – dilatation (can be severe); poor, turbulent flow; blood clot formation
- Collateral vessels and shunts – pulmonary arteriovenous malformation, aortopulmonary collateral vessels
- Lymphatic system – protein losing enteropathy, plastic bronchitis, pericardial and pleural effusions, chylothorax
- Blood vessels – blood clots and emboli including pulmonary embolism
Right atrium with classic Fontan circulation
Patients with atriopulmonary Fontan circulation are predisposed to development of complications. The right atrium is exposed to elevated systemic and right atrial pressure, which leads to right atrial dilatation and hypertrophy 6. Dilatation may be severe, and it may lead to complications such as arrhythmia and swirling of blood in the enlarged atrium, which causes stasis and results in poor blood flow to the lungs. Dilatation also may be a predisposing factor for clot formation. Patients with Fontan circulation also may have undergone atriotomy, a procedure that may injure the sinus node or conducting fibers and cause atrial arrhythmia. Many patients who undergo the Fontan procedure also require conversion of the old circuit to an extracardiac cavopulmonary circuit. These patients also may require a right atrial maze procedure (to reduce arrhythmia), right atrial reduction plasty, and possibly a pacemaker 7.
Inferior Vena Cava
Systemic venous hypertension of Fontan circulation has detrimental effects on infradiaphragmatic venous and splanchnic circulation 8. Chronic congestive heart failure leads to increased hepatic sinusoidal pressure and loss of venous pressure gradient, which leads to cirrhosis, portal venous hypertension, and hepatic dysfunction. Portal hypertension may manifest as splenomegaly and portosystemic shunts. Hepatic encephalopathy has been reported in patients who underwent Fontan procedures, and hepatic dysfunction may progress to cardiac cirrhosis or hepatocellular carcinoma 9.
Left ventricle
Because of its original hemodynamic state of volume overload, the ventricle of a functionally univentricular heart may dilate, hypertrophy, and become hypocontractile 6. Total bypass of the right side of the heart and completion of the total cavopulmonary circuit result in a marked reduction of preload to the systemic ventricle. Chronic preload depletion perpetuates systolic and diastolic dysfunction of the ventricle, resulting in impaired compliance, poor ventricular filling, and eventually low cardiac output. The congenital malformation itself also may be a predisposing factor for ventricular dysfunction, and the systemic ventricle may be a morphologic right ventricle or an indeterminate primitive ventricle, both of which may fail after years of systemic loading; failure manifests as exercise intolerance. High right atrial pressure may lead to disadvantageous coronary blood flow, which may affect myocardial perfusion and function. Coronary sinus blood may be surgically redirected to drain into the left atrium 7.
Pulmonary circulation
In the absence of the hydraulic force of the right ventricle, Fontan circulation results in a paradox of systemic venous hypertension (mean pressure, >10 mm Hg) and pulmonary artery hypotension (mean pressure, <15 mm Hg). The absence of pulsatile blood flow and low mean pressure in the pulmonary artery underfill the pulmonary vascular bed and increase pulmonary vascular resistance. The pulmonary arteries may be morphologically abnormal (ie, small, discontinuous, or stenosed) 6. Pulmonary vascular resistance is an important determinant of cardiac output in patients with Fontan circulation, and it is important to identify restricted blood flow to and from the lungs. Stenosis or leakage of surgical anastomoses between the venae cavae and pulmonary arteries may adversely affect pulmonary blood flow 10.
Collateral vessels and shunts
Collateral vessels and shunts may lead to substantial right-to-left shunts and cyanosis, which may be caused by incomplete closure or a residual atrial septal defect 7. Fenestration may be surgically created between the surgical conduits and the right atrium at the expense of cyanosis from the right-to-left shunt 11. Surgical redirection of coronary sinus blood flow to the left atrium usually results in modest arterial desaturation and a right-to-left shunt 7. Due to the absence of pulsatile blood flow and underfilling of the pulmonary vascular bed, patients with Fontan circulation are at increased risk for formation of pulmonary arteriovenous malformations 6. Patent collateral vessels between systemic veins and pulmonary veins or patent systemic veins that extend directly into the left atrium—a result of their pressure difference—are other potential causes of desaturation in patients with Fontan circulation 12.
Left-to-right shunts also may occur. Aortopulmonary collateral vessels are common in patients with Fontan circuits and have been identified as a risk factor for Fontan operations and morbidity. These collateral vessels also may lead to hemodynamic shunting, which results in volume overload of the systemic ventricle and increased pulmonary blood flow and pulmonary pressure. Aortopulmonary collateral vessels may arise from the thoracic aorta, internal mammary arteries, or brachiocephalic arteries 13.
Blood vessels
In addition to a dilated atrium and low cardiac output, many patients with Fontan circulation also have coagulation abnormalities associated with hepatic congestion and chronic cyanosis–induced polycythemia, which results in increased frequency of pulmonary thromboembolic events 10. Massive pulmonary embolism is the most common cause of sudden out-of-hospital death in patients with Fontan circulation 7. The reported incidences of venous thromboembolism and stroke are 3%–16% and 3%–19%, respectively 14.
Lymphatic system
Fontan circulation operates at or sometimes beyond the functional limits of the lymphatic system. Lymphatic circulation may be affected by high venous pressure and impaired thoracic duct drainage. Increased pulmonary lymphatic pressure may result in interstitial pulmonary edema or lymphedema. Leakage into the thorax or pericardium may lead to pericardial and pleural effusions (often right-sided) and chylothorax, usually in the postoperative period 7.
Protein-losing enteropathy is a relatively uncommon manifestation of failing Fontan circulation. Its cause is unclear; however, loss of enteric protein may be due to elevated systemic venous pressure that is transmitted to the hepatic circulation (hepatic and portal veins). Protein-losing enteropathy may lead to hypoproteinemia, immunodeficiency, hypocalcemia, and coagulopathy, and it may occur in the long term. Patients with protein-losing enteropathy usually have a poor prognosis 10.
Plastic bronchitis is a rare but serious complication of the Fontan procedure, occurring in less than 1%–2% of patients. It is characterized by noninflammatory mucinous casts that form in the tracheobronchial tree and obstruct the airway6. Clinical manifestations include dyspnea, cough, wheezing, and expectoration of casts, which may cause severe respiratory distress with asphyxia, cardiac arrest, or death. The exact cause of plastic bronchitis is unknown; however, it has been suggested that high intrathoracic lymphatic pressure or obstruction of lymphatic flow may lead to the development of lymphoalveolar fistula and bronchial casts 15. Medical management is difficult; success rates vary, and patients often require repeat bronchoscopy to remove the thick casts. Surgical ligation of the thoracic duct may cure plastic bronchitis by decreasing intrathoracic lymphatic pressure and flow 16.
References- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971 May. 26(3):240-8.
- Yabrodi M, Mastropietro CW. Hypoplastic left heart syndrome: from comfort care to long-term survival. Pediatr. Res. 2017 Jan;81(1-2):142-149.
- Roeleveld PP, Axelrod DM, Klugman D, Jones MB, Chanani NK, Rossano JW, Costello JM. Hypoplastic left heart syndrome: from fetus to fontan. Cardiol Young. 2018 Nov;28(11):1275-1288.
- Gobergs R, Salputra E, Lubaua I. Hypoplastic left heart syndrome: a review. Acta Med Litu. 2016;23(2):86-98.
- Pizarro C, Derby CD, Baffa JM, Murdison KA, Radtke WA. Improving the outcome of high-risk neonates with hypoplastic left heart syndrome: hybrid procedure or conventional surgical palliation? Eur J Cardiothorac Surg. 2008 Apr;33(4):613-8.
- The Fontan Procedure: Anatomy, Complications, and Manifestations of Failure. Tyler B. Fredenburg, Tiffanie R. Johnson, and Mervyn D. Cohen RadioGraphics 2011 31:2, 453-463 https://doi.org/10.1148/rg.312105027
- Gewillig M. The Fontan circulation. Heart 2005;91(6):839–846.
- Greenberg SB, Morrow WR, Imamura M, Drummond-Webb J. Magnetic resonance flow analysis of classic and extracardiac Fontan procedures: the seesaw sign. Int J Cardiovasc Imaging 2004;20(5):397–405; discussion 407–408.
- Kiesewetter CH, Sheron N, Vettukattill JJ et al.. Hepatic changes in the failing Fontan circulation. Heart 2007;93(5):579–584.
- Khambadkone S. The Fontan pathway: what’s down the road? Ann Pediatr Cardiol 2008;1(2):83–92.
- Lemler MS, Scott WA, Leonard SR, Stromberg D, Ramaciotti C. Fenestration improves clinical outcome of the Fontan procedure: a prospective, randomized study. Circulation 2002;105(2):207–212.
- de Leval MR. The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med 2005;2(4):202–208.
- Ichikawa H, Yagihara T, Kishimoto H et al.. Extent of aortopulmonary collateral blood flow as a risk factor for Fontan operations. Ann Thorac Surg 1995;59(2):433–437.
- Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after Fontan procedures: the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg 1998;115(3):493–498.
- Hug MI, Ersch J, Moenkhoff M, Burger R, Fanconi S, Bauersfeld U. Chylous bronchial casts after Fontan operation. Circulation 2001;103(7):1031–1033.
- Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: is thoracic duct ligation a real surgical option? Ann Thorac Surg 2006;81(6):2281–2283.