What is Alkaline Diet
Much has been written in the lay literature as well as many online sites expounding on the benefits of the alkaline diet. This post is an attempt to balance the evidence that is found in the scientific literature.
Life on earth depends on appropriate pH levels in and around living organisms and cells. Human life requires a tightly controlled pH level in the serum of about 7.4 (a slightly alkaline range of 7.35 to 7.45) to survive 1. The ‘acidity’ of human blood is highly stable (pH = 7.35–7.45) in healthy individuals and cancer patients 2. The pH is a number that shows how acidic or alkaline a substance is. A pH of less than 7 is acidic, and greater than 7 is alkaline. The pH of blood is about 7.4. Acidosis also called acidemia is a condition in which there is a shift in the acid-base balance of your body to have more acid than normal, often causing the pH of your blood and body tissues to fall below pH 7.35 (the healthy normal range of pH is between 7.35-7.45, with the average at 7.40). Acidosis occurs when acid builds up or when bicarbonate [HCO3–] (a base) is lost.
Acidosis can be due to increased acid or decreased base:
- Increased acid production within the body
- Consumption of substances that are metabolized to acids
- Decreased acid excretion
- Increased excretion of base
Acidosis may also be caused by decreased carbon dioxide (CO2) elimination in respiratory disorders such as emphysema, by metabolic problems such as kidney disease and diabetes, or as the result of ingesting poisons (ethlylene glycol, methanol) or overdosing on certain medication (salicylates); it can also be caused by losing bicarbonate (HCO3–), as in diarrhea.
Alkaline diet refers to the idea that as foods are digested and metabolized, they create a more alkaline environment in the body 3, 4. For example, animal protein is a major source of sulphur-containing amino acids that create a higher acid load in the body. The amount of acids released is related to the type of amino acids they contain. In fact, amino acids can be divided into neutral (alanine, phenylalanine, glycine, isoleucine, leucine, methionine, proline, tryptophan, valine, asparagine, glutamine, serine, threonine, cysteine, and tyrosine), acidic (aspartate and glutamate), and alkaline (arginine, histidine, and lysine). Specifically, lysine, arginine, and histidine form hydrochloric acid, while cysteine and methionine are converted to sulfuric acid 5. Moreover, foods containing phosphorus, both of natural origin or from food additives, further increase the acid load introduced with the diet 6. On the other hand, vegetables and fruits are major sources of certain minerals, especially potassium, after being metabolized, produce alkali species that are able to neutralize acids 7.
The proponents of alkaline diet believe that an acidic environment promotes ill health whereas an alkaline environment is beneficial and promotes good health, based on the “acid–ash” hypothesis developed over 100 years ago 8, 9. The acid–ash hypothesis suggests that to achieve a more alkaline load, you must consume more fruit and vegetables with only a moderate intake of protein. The diet also calls for avoiding added sugars. They claimed that the food you eat can affect your body’s pH and that as your blood that is naturally alkaline (pH 7.4) is being upset by eating acid foods. By trying to change your blood pH to be more alkaline through eating mostly alkaline foods, followers of alkaline diet believe that they can cure disease, including cancer. Proponents of the Alkaline Diet say that when you load up on excess amounts of protein, sugar, caffeine and other highly processed foods, your pH levels are thrown off track, your bodies become more acidic, and that can lead to weight gain and disease. The remedy, they say? Eat fresh vegetables, fresh fruit and other “alkaline-promoting foods” such as soy products, legumes, and certain nuts and grains. “Acid-promoting foods”, which include meat, fish, poultry, dairy products, processed foods, white sugar, white flour, and caffeine, are discouraged. Because alkaline promoting foods has a higher pH level than does acid promoting foods, proponents of the alkaline diet say that it can neutralize acid in your bloodstream, boost your metabolism and help your body absorb nutrients more effectively. Some even say that alkaline promoting foods can help prevent disease and slow the aging process. Researchers haven’t verified these claims. Despite this, many of those following an alkaline diet insist on testing the pH of their urine multiple times per day to make sure that their bodies aren’t too acidic. Moreover, marketers of alkaline diet imply that the diet changes will raise your blood pH, but a well-conducted randomized trial of alkaline diet changes altered only your blood pH by 0.014 units, while the urine pH increased by 1.02 units 10. Another study reported that high fruit and vegetable consumption and low meat intake could significantly alkalinize the urine pH in healthy men and women of ages up to 79 years 11. While diet and other metabolic processes can affect the pH level of your urine, what you eat does not determine your blood’s pH level. And the pH of your urine has no effect on weight loss and no correlation with the risk of cancer or inflammation-related medical conditions.
The alkaline diet proponent suggests that eating an alkaline diet can create a hostile alkaline environment and therefore kill cancer. Interest in an alkaline diet mostly stems from laboratory studies suggesting that cancerous tumor cells have an acidic environment (pH ~6.5) surrounding them, which promotes the invasiveness of the cancer cells (e.g., by activating proteolytic enzymes and digesting their surroundings) whilst inhibiting the growth of the normal host cells 12, 13, 14. However, neither cancer cells nor healthy cells can survive in an alkaline environment. Scientists are investigating whether this acidic environment promotes cancer development or enhances metastasis 15.
Your blood pH (7.4) is tightly regulated by your kidneys and respiratory system. Any excess acid is excreted in the urine. Your blood pH is not altered by your dietary intake. The only situation in which blood pH is altered is during metabolic acidosis, when an individual is critically ill. Furthermore, there is no scientific literature establishing the benefit of an alkaline diet for the prevention of cancer at this time 16. A 2016 systematic review of studies on alkaline diet and cancer found that an alkaline diet could change the pH of urine to be more alkaline, but not that of the whole body 16. The body regulates the pH of blood through several internal processes. Food does not affect it. This systematic review of the literature revealed a lack of evidence for or against diet acid load and/or alkaline water for the initiation or treatment of cancer 16. Promotion of alkaline diet and alkaline water to the public for cancer prevention or treatment is not justified 16. Additionally, a review of the body of evidence regarding the acid–ash or alkaline hypothesis for bone health found that the hypothesis is not supported and there is no evidence that altering the diet acid load improves bone health 17.
Since the 2016 review 16, a 2019 observational study 18 found that diets categorized as more acid-producing were associated with increased risk, and alkaline diets were associated with decreased risk, of estrogen receptor-negative (ER-) and triple-negative breast cancers. Another observational study 19 found that scores suggesting a more acidic diet were linked with increased markers of inflammation and poorer outcomes among some early-stage breast cancer survivors. Diet scores associated with blood and urine pH are derived from calculations based on total animal protein consumption and levels of one or a few minerals.
Current scientific evidence points to the benefits of alkaline diet for people with chronic kidney disease 20, 21, 22, 5. Many studies have shown that a diet high in acid load is associated with an increased incidence of chronic kidney disease (CKD), CKD progression 23, 24, 25 and diabetes 26. Chronic kidney disease (CKD) means that your kidneys are damaged and can’t filter blood as they should. This damage can cause toxin and wastes to build up in your body 27. Chronic kidney disease (CKD) can also cause other problems that can harm your health 5. Chronic kidney disease (CKD) common complications include hyperkalemia, metabolic acidosis, calcium-phosphorus metabolism impairment, water and sodium metabolism alterations, changes in the composition of the gut microbiota, oxidative stress, hyperhomocysteinemia, chronic low-grade inflammation, and normocytic normochromic anemia 28, 29, 30, 31, 32. Diabetes and high blood pressure are the most common causes of chronic kidney disease (CKD). If left unchecked, chronic kidney disease (CKD) can evolve into end-stage renal disease (ESRD). At this stage, the kidneys are no longer able to remove enough wastes and excess fluids from the body. At this point, you would need dialysis or a kidney transplant.
In summary, eating a diet with plenty of fruits and vegetables – which is what some people mean when referring to an alkaline diet – gives many health benefits and can help maintain a healthy weight. A study from more than 100,000 people starting in the mid-1980s until 2014 found that eating an average of five servings of fruits and vegetables a day is linked to a reduced risk of death from heart and respiratory diseases. Eating more fruit is also associated with a lower risk of cancer. Most types of fruits and vegetables led to these results, except fruit juices and starchy vegetables such as peas, corn, and potatoes. Try to eat about 3 to 5 servings every day. Fruit is also a good source of fiber, vitamins, and minerals. You should try to eat about 2 to 3 servings of fruit each day. However, the average American adult only eats about one serving of fruit and 1.5 servings of vegetables a day.
The Role of pH in Various Cells, Organs, and Membranes
The pH in our body may vary considerably from one area to another with the highest acidity in the stomach (pH of 1.35 to 3.5) to aid in digestion and protect against opportunistic microbial organisms. But even in the stomach, the layer just outside the epithelium is quite basic to prevent mucosal injury.
The skin is quite acidic (pH 4–6.5) to provide an acid mantle as a protective barrier to the environment against microbial overgrowth. There is a gradient from the outer horny layer (pH 4) to the basal layer (pH 6.9). This is also seen in the vagina where a pH of less than 4.7 protects against microbial overgrowth.
The urine may have a variable pH from acid to alkaline depending on the need for balancing the internal environment. Foods can be categorized by the potential renal acid loads.
- Fruits, vegetables, fruit juices, potatoes, and alkali-rich and low phosphorus beverages (red and white wine, mineral soda waters) having a negative acid load.
- Whereas, grain products, meats, dairy products, fish, and alkali poor and low phosphorus beverages (e.g., pale beers, cocoa) have relatively high acid loads.
How your body maintains a healthy and stable blood pH
The pH of blood is about 7.4 (a slightly alkaline range of 7.35 to 7.45). Your blood pH is highly stable within a normal range of pH 7.35 to 7.45 and it’s tightly regulated by your kidneys and respiratory system. The primary pH buffering system in the human body is the bicarbonate (HCO3–) and carbon dioxide (CO2). Bicarbonate (HCO3–) functions as an alkalotic substance. Carbon dioxide (CO2) functions as an acidic substance. Therefore, an increase in serum bicarbonate (HCO3–) or a decrease in CO2 (carbon dioxide) will make blood more alkaline. The opposite is also true where decreases in bicarbonate (HCO3–) or an increase in carbon dioxide (CO2) will make blood more acidic. Acidosis also called acidemia is a condition in which there is a shift in the acid-base balance of your body to have more acid than normal, often causing the pH of your blood and body tissues to fall below pH 7.35 (the healthy normal range of pH is between 7.35-7.45, with the average at 7.40). Acidosis occurs when acid builds up or when bicarbonate [HCO3–] (a base) is lost. The carbon dioxide (CO2) levels are physiologically regulated by the pulmonary system through respiration, whereas the serum bicarbonate (HCO3–) levels are regulated through your kidneys by two mechanisms: bicarbonate [HCO3–] (a base) reclamation mainly in the proximal tubule and bicarbonate [HCO3–] (a base) generation predominantly in the distal nephron. Elevated pH above 7.45 and elevated plasma bicarbonate (HCO3–) level above 30 meq/L characterize metabolic alkalosis. When bicarbonate (HCO3–) is elevated the arterial partial pressure of carbon dioxide (PaCO2) must also be elevated to maintain pH to its normal range. Therefore with metabolic alkalosis, the compensation is to decrease alveolar ventilation (hypoventilation) in order to increase the arterial partial pressure of carbon dioxide (PaCO2).
To understand acid-base buffering system, it is important to recall that pH is governed by the ratio bicarbonate [HCO3–] (a base)/arterial partial pressure of carbon dioxide (PaCO2) (an acid). So long as the ratio is normal, pH will be normal.
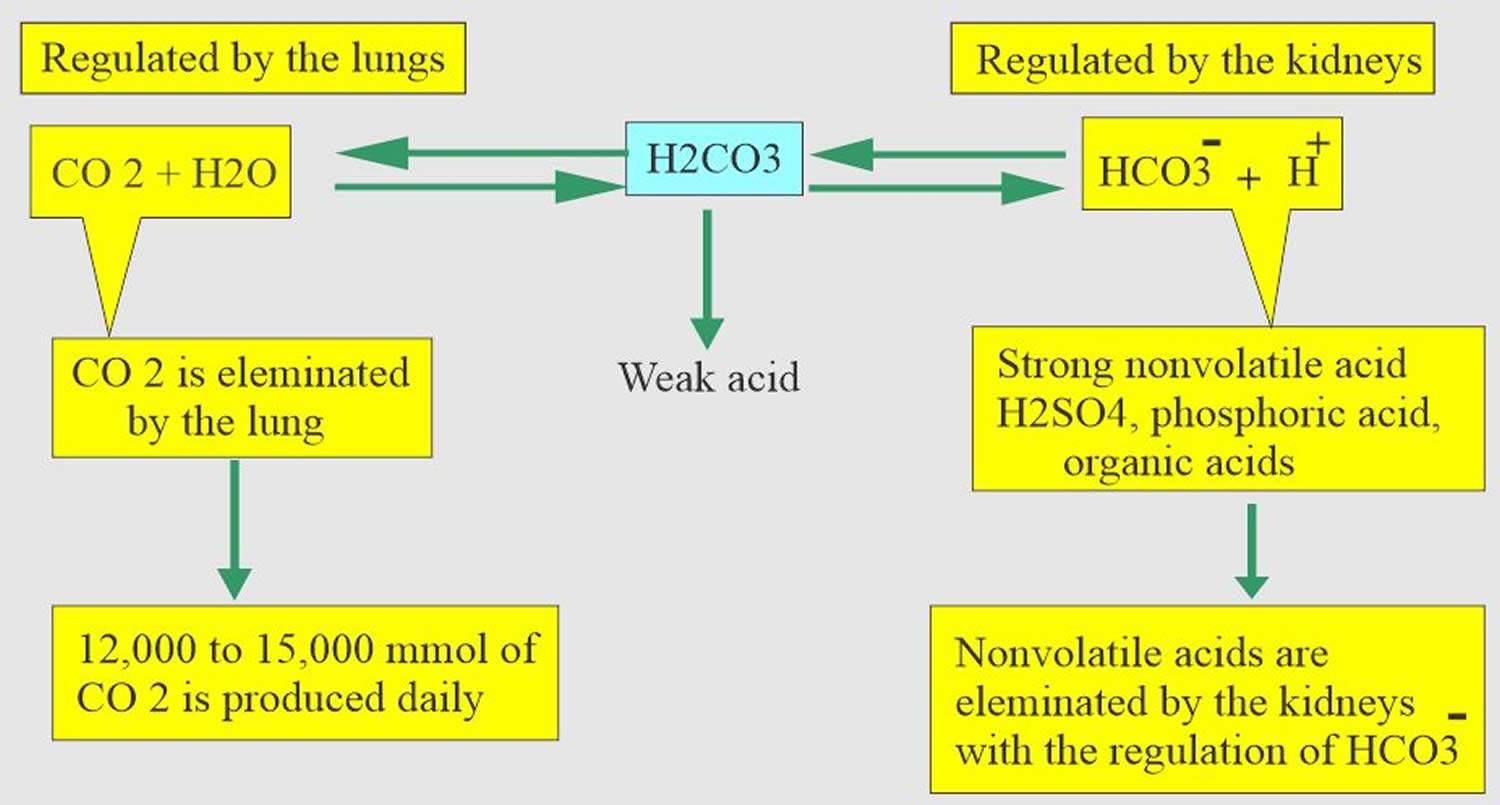
Normal body functions and metabolism generate large quantities of acids that must be neutralized and/or eliminated to maintain blood pH balance. Most of the acid is carbonic acid (H2CO3), which is created from carbon dioxide (CO2) and water (H2O). Carbon dioxide (CO2) is produced as the body uses glucose (sugar) or fat for energy. In its normal state, the body maintains carbon dioxide (arterial partial pressure of carbon dioxide [PaCO2]) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. Lesser quantities of lactic acid, ketoacids, and other organic acids are also produced.
- Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
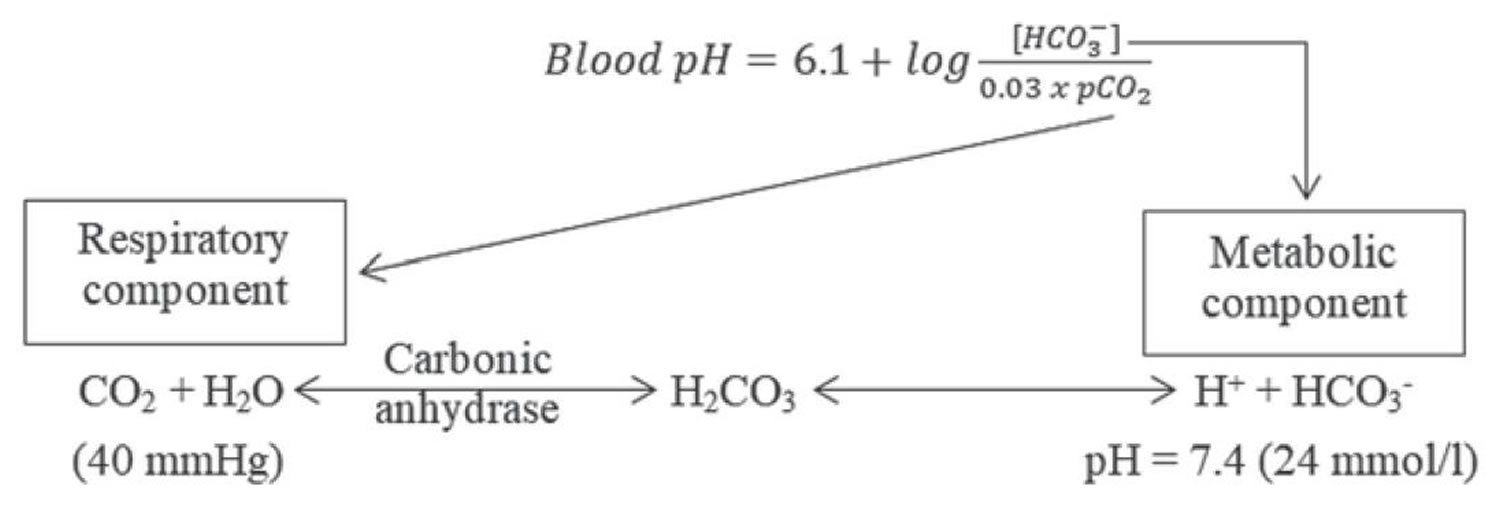
According to the Henderson-Hasselbalch equation (Figure 1), maintaining physiological pH depends on arterial partial pressure of carbon dioxide (PaCO2), which in turn depends on alveolar ventilation (hypoventilation causes acidosis and hyperventilation causes alkalosis). The kidneys participate in maintaining the stable pH by reabsorption of bicarbonate (3,600 mmol of bicarbonate is filtrated in glomeruli during 24 hour) and excretion of hydrogen ions from nonvolatile acids (including sulfur and phosphate) as titratable acidity (0.3 mmol hydrogen ions/kg/day) and in the form of ammonium ion (0.7 mmol hydrogen ions/kg/day) 33, 34.
Serum bicarbonate (HCO3–) concentration can be calculated from a blood gas sample using the Henderson-Hasselbalch equation, as follows (see Figure 1 below):
- pH = 6.10 + log (HCO3– ÷ 0.03 × PaCO2)
- Alternatively, bicarbonate (HCO3–) = 24 × PaCO2 ÷ [H+]
Because pH and arterial partial pressure of carbon dioxide (PaCO2) are directly measured, bicarbonate (HCO3–) can be calculated.
Another means of assessing serum bicarbonate (HCO3–) concentration is with the total carbon dioxide content in serum, which is routinely measured with serum electrolytes obtained from venous blood. In this method, a strong acid is added to serum, which interacts with bicarbonate in the serum sample, forming carbonic acid. Carbonic acid dissociates to carbon dioxide and water; then, carbon dioxide is measured.
Note that the carbon dioxide measured includes bicarbonate and dissolved carbon dioxide. The contribution of dissolved carbon dioxide is quite small (0.03 × PaCO2) and is usually ignored, although it accounts for a difference of 1-3 mEq/L between the measured total carbon dioxide content in venous blood and the calculated bicarbonate in arterial blood. Thus, at an arterial partial pressure of carbon dioxide (PaCO2) of 40, a total carbon dioxide (CO2) content of 25 means a true bicarbonate concentration of 23.8 (ie, 25 – 0.03 × 40).
Your lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the metabolic acidosis, you increase your breathing rate (hyperventilation) to increase carbon dioxide (CO2) elimination 35, 36.
- The lungs flush acid out of your body by exhaling carbon dioxide (CO2). Raising and lowering the respiratory rate alters the amount of carbon dioxide (CO2) that is breathed out, and this can affect blood pH within minutes 37.
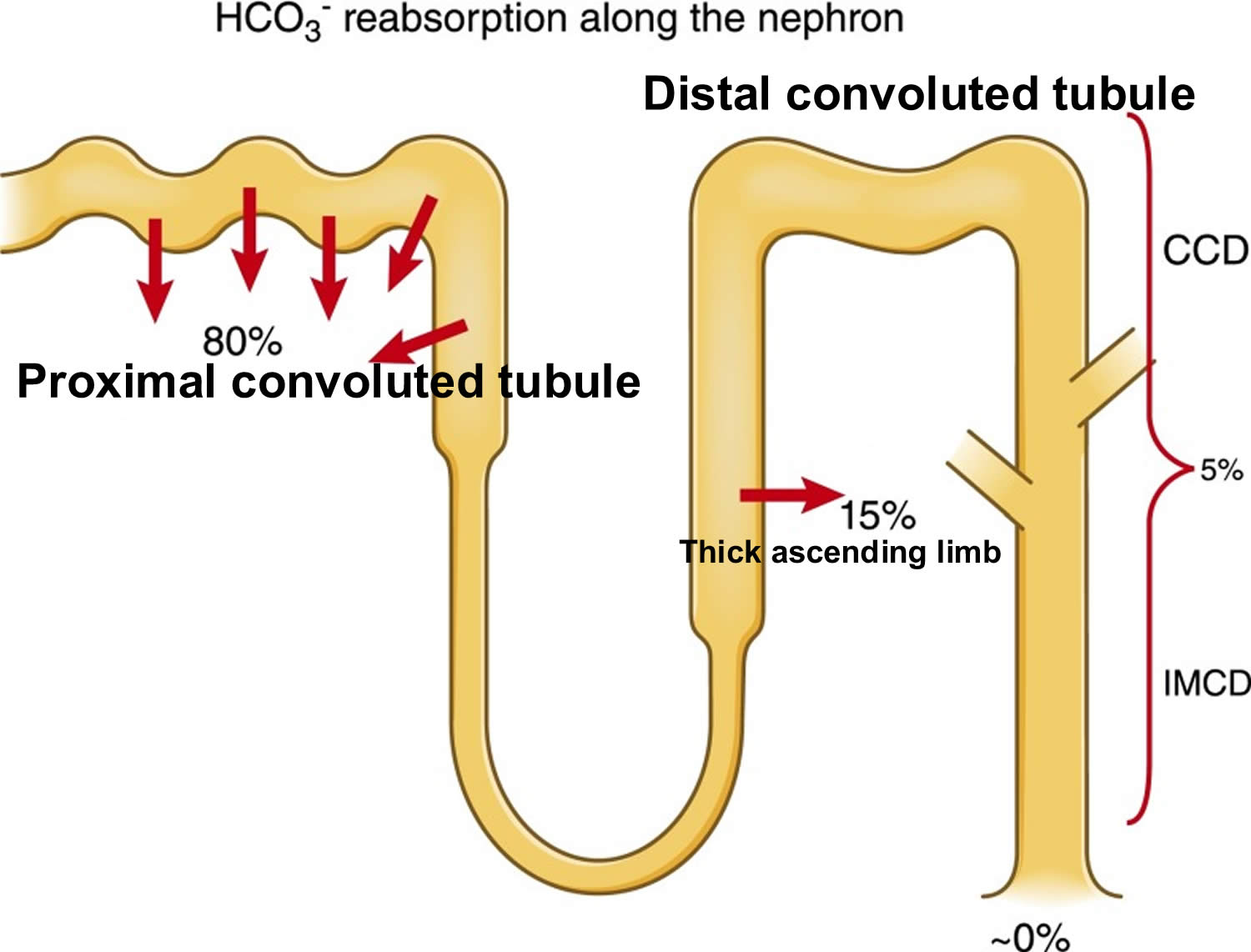
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in carbon dioxide (CO2), taking hours or days. Bicarbonate (HCO3–) reabsorption occurs in the kidneys in every part of the tubules. About 85–90% of the filtered bicarbonate is reabsorbed in the proximal tubules, 10% in the ascending arms of the Henle loop, 6% in the distal tubules, and 4% in the collecting tubules 33, 34.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled. The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, carbon dioxide (CO2), bicarbonate (HCO3–) and phosphates.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains carbon dioxide (CO2) in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination. In a state of hypoventilation (breathing that is too shallow or too slow to meet the needs of the body), the body produces more carbon dioxide (CO2) than it can eliminate, causing a net retention of carbon dioxide (CO2). The increased carbon dioxide (CO2) is what leads to an increase in hydrogen ions (H+) and a slight increase in bicarbonate (HCO3–), as seen by a right shift in the following equilibrium reaction of carbon dioxide:
- Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: carbon dioxide (CO2), H2CO3 (carbonic acid), and bicarbonate (HCO3–). When hydrogen ions (H+) is high, bicarbonate (HCO3–) buffers the low pH. When hydroxide (OH–) is high, H2CO3 (carbonic acid) buffers the high pH. In respiratory acidosis, the slight increase in bicarbonate (HCO3–) serves as a buffer for the increase in hydrogen ions (H+), which helps minimize the drop in pH. The increase in hydrogen ions inevitably causes the decrease in pH, which is the mechanism behind metabolic acidosis.
Figure 1. Henderson-Hasselbalch equation
[Source 38 ]Figure 2. Acid-base buffering system
Figure 3. Kidneys control of plasma bicarbonate (HCO3–)
Abbreviations: CCD = cortical collecting duct; IMCD = inner medullary collecting duct
[Source 34 ]Respiration
The pulmonary system adjusts pH using carbon dioxide (CO2); upon expiration, carbon dioxide (CO2) is projected into the environment. Due to carbon dioxide (CO2) forming carbon dioxide (CO2) in the body when combining with water (H2O), the amount of carbon dioxide (CO2) expired can cause pH to increase or decrease. When the respiratory system is utilized to compensate for metabolic pH disturbances, the effect occurs in minutes to hours 39.
Renal adaptation
The renal system affects pH by reabsorbing bicarbonate (HCO3–) and excreting fixed acids 39, 40. Whether due to pathology or necessary compensation, the kidney excretes or reabsorbs these substances which affect pH. The nephron is the functional unit of the kidney. Blood vessels called glomeruli transport substances found in the blood to the renal tubules so that some can be filtered out while others are reabsorbed into the blood and recycled. This is true for hydrogen ions and bicarbonate. If bicarbonate (HCO3–) is reabsorbed and/or acid is secreted into the urine, the pH becomes more alkaline (pH increases). When bicarbonate (HCO3–) is not reabsorbed or acid is not excreted into the urine, pH becomes more acidic (pH decreases). The metabolic compensation from the renal system takes longer to occur, days rather than minutes or hours.
The renal adaptations are extensive 41:
- Increased urinary excretion of sulfate, phosphate, urate, and chloride;
- Increased urinary excretion of calcium;
- Decreased urinary excretion of citrate;
- Increased urinary excretion of ammonium ions; and
- Kidney vasodilatation and increased glomerular filtration rate.
The kidneys mitigate but do not eliminate all the excess acidity. As the kidneys lose function with aging (when GFR is lower than 30 mL/min/1.73 m²), their ability to excrete acid becomes impaired, which may be another explanation for the loss of bone with aging 42. In fact, counteracting metabolic acidosis helps to preserve muscle mass and to improve bone metabolism 43, 44, 45.
Bone for acid buffering
The major reservoir of base is the skeleton (in the form of alkaline salts of calcium), which provides the buffer needed to maintain blood pH and plasma bicarbonate concentrations when renal and respiratory adaptations are inadequate. Acid-promoting diets are associated with increased urinary excretion of both calcium and bone matrix protein and decreased bone density 46. Neutralizing acid intake with diet or alkalinizing supplements decreases urine Calcium and bone matrix protein excretion. Also, to a much smaller degree, skeletal muscle can act as a buffer.
Other buffer systems
Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases which accept hydrogen ions which keep the pH from plummeting. The phosphate buffer system, while present globally, is important for the regulation of urine pH. Proteins assist with intracellular pH regulation. Red blood cells use the reaction above to help hemoglobin buffer; carbon dioxide can diffuse across red blood cells and combine with water. This alone would cause an increase in hydrogen ions; however, hemoglobin can bind hydrogen ions. Hemoglobin also can bind carbon dioxide without this reaction. This depends on the amount of oxygen that is bound to hemoglobin. This is called the Haldane effect and the Bohr effect. When hemoglobin is saturated with oxygen, it has a lower affinity for carbon dioxide (CO2) and hydrogen ions and is able to release i
The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health?
There is some good news for fans of alkaline eating. The Alkaline Diet is a plant-based and discourages added sugar, so it may help your weight and health, although not because of the pH. People who eat balanced, plant-based diets tend to have lower risks of chronic diseases like high blood pressure and diabetes. There is also research indicating that vegetarian diets may lead to lower risk for some types of cancer. However it is not clear yet if these lower cancer rates are due to diet alone, or other lifestyle factors that often go in tandem with plant-based diets (like not smoking).
As for weight, a diet rich in whole grains and fruits and veggies, and low in processed foods may be a good way to get or stay slim, however you’ll still need to pay attention to portion sizes, total calorie intake and exercise regularly.
- Chronic Acidosis and Bone Disease
There is online information promoting an alkaline diet for bone health as well as a number of books. However, a recent systematic review of the literature looking for evidence supporting the alkaline diet for bone health found no protective role of dietary acid load in osteoporosis 47.
There is evidence that in healthy humans the increased sodium in the diet can predict the degree of hyperchloremic metabolic acidosis when consuming a net acid producing diet 48. As well, there is evidence that there are adverse effects of sodium chloride in the aging population. A high sodium diet will exacerbate disuse-induced bone and muscle loss during immobilization by increasing bone resorption and protein wasting 49. Excess dietary sodium has been shown to result in hypertension and osteoporosis in women 50, 51. As well, dietary potassium which is lacking in the modern diet would modulate pressor and hypercalciuric effects of excess of sodium chloride 52.
Excess dietary protein with high acid renal load may decrease bone density if not buffered by ingestion of supplements or foods that are alkali rich 53. However, adequate protein is necessary for prevention of osteoporosis and sarcopenia; therefore, increasing the amount of fruit and vegetables may be necessary rather than reducing protein 54.
- Alkaline Diets and Muscle
As we age, there is a loss of muscle mass, which may predispose to falls and fractures. A three-year study looking at a diet rich in potassium, such as fruits and vegetables, as well as a reduced acid load, resulted in preservation of muscle mass in older men and women 55. Conditions such as chronic renal failure that result in chronic metabolic acidosis result in accelerated breakdown in skeletal muscle 56. Correction of acidosis may preserve muscle mass in conditions where muscle wasting is common such as diabetic ketosis, trauma, sepsis, chronic obstructive lung disease, and renal failure 57. In situations that result in acute acidosis, supplementing younger patients with sodium bicarbonate prior to exhaustive exercise resulted in significantly less acidosis in the blood than those that were not supplemented with sodium bicarbonate 58.
- Alkaline Supplementation and Growth Hormone
It has long been known that severe forms of metabolic acidosis in children, such as renal tubular acidosis, are associated with low levels of growth hormone with resultant short stature. Correction of the acidosis with bicarbonate 59 or potassium citrate 60 increases growth hormone significantly and improved growth. The use of enough potassium bicarbonate in the diet to neutralize the daily net acid load in postmenopausal women resulted in a significant increase in growth hormone and resultant osteocalcin 61. Improving growth hormone levels may improve quality of life, reduce cardiovascular risk factors, improve body composition, and even improve memory and cognition 62. As well this results in a reduction of urinary calcium loss equivalent to 5% of bone calcium content over a period of 3 years 63.
- Alkaline Diet and Back Pain
There is some evidence that chronic low back pain improves with the supplementation of alkaline minerals 64. With supplementation there was a slight but significant increase in blood pH and intracellular magnesium. Ensuring that there is enough intracellular magnesium allows for the proper function of enzyme systems and also allows for activation of vitamin D 65. This in turn has been shown to improve back pain 66.
- Alkalinity and Chemotherapy
The effectiveness of chemotherapeutic agents is markedly influenced by pH. Numerous agents such as epirubicin and adriamycin require an alkaline media to be more effective. Others, such as cisplatin, mitomycin C, and thiotepa, are more cytotoxic in an acid media 67. Cell death correlates with acidosis and intracellular pH shifts higher (more alkaline) after chemotherapy may reflect response to chemotherapy 68. It has been suggested that inducing metabolic alkalosis may be useful in enhancing some treatment regimes by using sodium bicarbonate, carbicab, and furosemide 69. Extracellular alkalinization by using bicarbonate may result in improvements in therapeutic effectiveness 70. There is no scientific literature establishing the benefit of an alkaline diet for the prevention of cancer at this time.
Conclusion
Alkaline diets result in a more alkaline urine pH and may result in reduced calcium in the urine, however, as seen in some recent reports, this may not reflect total calcium balance because of other buffers such as phosphate. There is no substantial evidence that this improves bone health or protects from osteoporosis.
Alkaline diets may result in a number of health benefits as outlined below 71:
- Increased fruits and vegetables in an alkaline diet would improve the K/Na ratio and may benefit bone health, reduce muscle wasting, as well as mitigate other chronic diseases such as hypertension and strokes. There is some evidence that the K/Na ratio does matter and that the significant amount of salt in our diet is detrimental.
- The resultant increase in growth hormone with an alkaline diet may improve many outcomes from cardiovascular health to memory and cognition.
- An increase in intracellular magnesium, which is required for the function of many enzyme systems, is another added benefit of the alkaline diet. Available magnesium, which is required to activate vitamin D, would result in numerous added benefits in the vitamin D apocrine/exocrine systems.
- Alkalinity may result in added benefit for some chemotherapeutic agents that require a higher pH.
From the evidence outlined above, it would be prudent to consider an alkaline diet to reduce morbidity and mortality of chronic disease that are plaguing our aging population. One of the first considerations in an alkaline diet, which includes more fruits and vegetables, is to know what type of soil they were grown in since this may significantly influence the mineral content. At this time, there are limited scientific studies in this area, and many more studies are indicated in regards to muscle effects, growth hormone, and interaction with vitamin D.
- Schwalfenberg GK. The alkaline diet: is there evidence that an alkaline pH diet benefits health? J Environ Public Health. 2012;2012:727630. doi: 10.1155/2012/727630[↩]
- Ali M., Alam S.P., Kumar S., Anupam Kumar R., Kumar A. Does blood pH change in cancer patients. Int. J. Curr. Res. 2016;8:29543–29544.[↩]
- Remer T., Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994;59:1356–1361. doi: 10.1093/ajcn/59.6.1356[↩]
- Anthony Sebastian, Lynda A Frassetto, Deborah E Sellmeyer, Renée L Merriam, R Curtis Morris, Jr, Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors, The American Journal of Clinical Nutrition, Volume 76, Issue 6, December 2002, Pages 1308–1316, https://doi.org/10.1093/ajcn/76.6.1308[↩]
- Noce A, Marrone G, Wilson Jones G, Di Lauro M, Pietroboni Zaitseva A, Ramadori L, Celotto R, Mitterhofer AP, Di Daniele N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients. 2021 Jul 24;13(8):2534. doi: 10.3390/nu13082534[↩][↩][↩]
- D’Alessandro C., Piccoli G.B., Cupisti A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015;16:9. doi: 10.1186/1471-2369-16-9[↩]
- Wesson D.E., Nathan T., Rose T., Simoni J., Tran R.M. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007;71:210–217. doi: 10.1038/sj.ki.5002036[↩]
- Sherman H, Gettler A. The balance of acid-forming and base-forming elements in foods, and its relation to ammonia metabolism. J Biol Chem 1912;11:323–38.[↩]
- Dwyer J, Foulkes E, Evans M, Ausman L. Acid/alkaline ash diets: time for assessment and change. J Am Diet Assoc. 1985 Jul;85(7):841-5.[↩]
- Buclin T, Cosma M, Appenzeller M et al.. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int 2001;12:493–9. 10.1007/s001980170095[↩]
- Welch A.A., Mulligan A., Bingham S.A., Khaw K.-T. Urine pH is an indicator of dietary acid–base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008;99:1335–1343. doi: 10.1017/S0007114507862350[↩]
- Kato Y., Ozawa S., Miyamoto C., Maehata Y., Suzuki A., Maeda T., Baba Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89. doi: 10.1186/1475-2867-13-89[↩]
- Lee S.-H., McIntyre D., Honess D., Hulikova A., Pacheco-Torres J., Cerdán S., Swietach P., Harris A.L., Griffiths J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer. 2018;119:622–630. doi: 10.1038/s41416-018-0216-5[↩]
- Pilon-Thomas S., Kodumudi K.N., El-Kenawi A.E., Russell S., Weber A.M., Luddy K., Damaghi M., Wojtkowiak J.W., Mulé J.J., Ibrahim-Hashim A., et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2015;76:1381–1390. doi: 10.1158/0008-5472.CAN-15-1743[↩]
- Fais S, Marunaka Y. The Acidic Microenvironment: Is It a Phenotype of All Cancers? A Focus on Multiple Myeloma and Some Analogies with Diabetes Mellitus. Cancers (Basel). 2020 Nov 2;12(11):3226. doi: 10.3390/cancers12113226[↩]
- Fenton TR, Huang T. Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ Open. 2016 Jun 13;6(6):e010438. doi: 10.1136/bmjopen-2015-010438[↩][↩][↩][↩][↩]
- Fenton TR, Tough SC, Lyon AW et al.. Causal assessment of dietary acid load and bone disease: a systematic review & meta-analysis applying Hill’s epidemiologic criteria for causality. Nutr J 2011;10:41 10.1186/1475-2891-10-41[↩]
- Park YM, Steck SE, Fung TT, Merchant AT, Elizabeth Hodgson M, Keller JA, Sandler DP. Higher diet-dependent acid load is associated with risk of breast cancer: Findings from the sister study. Int J Cancer. 2019 Apr 15;144(8):1834-1843. doi: 10.1002/ijc.31889[↩]
- Wu T, Seaver P, Lemus H, Hollenbach K, Wang E, Pierce JP. Associations between Dietary Acid Load and Biomarkers of Inflammation and Hyperglycemia in Breast Cancer Survivors. Nutrients. 2019 Aug 15;11(8):1913. doi: 10.3390/nu11081913[↩]
- Passey C. Reducing the dietary acid load: How a more alkaline diet benefits patients with chronic kidney disease. J. Ren. Nutr. 2017;27:151–160. doi: 10.1053/j.jrn.2016.11.006[↩]
- Yari Z, Mirmiran P. Alkaline Diet: a Novel Nutritional Strategy in Chronic Kidney Disease? Iran J Kidney Dis. 2018 Jul;12(4):204-208. http://www.ijkd.org/index.php/ijkd/article/view/3576/1012[↩]
- Rodrigues Neto Angéloco L, Arces de Souza GC, Almeida Romão E, Garcia Chiarello P. Alkaline Diet and Metabolic Acidosis: Practical Approaches to the Nutritional Management of Chronic Kidney Disease. J Ren Nutr. 2018 May;28(3):215-220. doi: 10.1053/j.jrn.2017.10.006[↩]
- Ko B.J., Chang Y., Ryu S., Kim E.M., Lee M.Y., Hyun Y.Y., Lee K.B. Dietary acid load and chronic kidney disease in elderly adults: Protein and potassium intake. PLoS ONE. 2017;12:e0185069. doi: 10.1371/journal.pone.0185069[↩]
- Rebholz C.M., Coresh J., Grams M.E., Steffen L.M., Anderson C.A., Appel L.J., Crews D.C. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am. J. Nephrol. 2015;42:427–435. doi: 10.1159/000443746[↩]
- Banerjee T., Crews D.C., Wesson D.E., Tilea A., Saran R., Rios Burrows N., Williams D.E., Powe N.R. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15:137. doi: 10.1186/1471-2369-15-137[↩]
- Fagherazzi G., Vilier A., Bonnet F., Lajous M., Balkau B., Boutron-Rualt M.C., Clavel-Chapelon F. Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia. 2014;57:313–320. doi: 10.1007/s00125-013-3100-0[↩]
- Cupisti A., Gallieni M., Avesani C.M., D’Alessandro C., Carrero J.J., Piccoli G.B. Medical nutritional therapy for patients with chronic kidney disease not on dialysis: The low protein diet as a medication. J. Clin. Med. 2020;9:3644. doi: 10.3390/jcm9113644[↩]
- Chen T.K., Knicely D.H., Grams M.E. Chronic kidney disease diagnosis and management: A review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745[↩]
- Noce A., Canale M.P., Capria A., Rovella V., Tesauro M., Splendiani G., Annicchiarico-Petruzzelli M., Manzuoli M., Simonetti G., Di Daniele N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging. 2015;7:269–279. doi: 10.18632/aging.100740[↩]
- Noce A., Marrone G., Di Lauro M., Urciuoli S., Pietroboni Zaitseva A., Wilson Jones G., Di Daniele N., Romani A. Cardiovascular protection of nephropathic male patients by oral food supplements. Cardiovasc. Ther. 2020;2020:1807941. doi: 10.1155/2020/1807941[↩]
- Annalisa N., Alessio T., Claudette T.D., Erald V., Antonino de L., Nicola D.D. Gut microbioma population: An indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediat. Inflamm. 2014;2014:901308. doi: 10.1155/2014/901308[↩]
- Dessi M., Noce A., Dawood K.F., Galli F., Taccone-Gallucci M., Fabrini R., Bocedi A., Massoud R., Fucci G., Pastore A., et al. Erythrocyte glutathione transferase: A potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids. 2012;43:347–354. doi: 10.1007/s00726-011-1085-x[↩]
- Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009 Dec;33(4):275-81. doi: 10.1152/advan.00054.2009[↩][↩]
- Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2232-42. doi: 10.2215/CJN.07400715[↩][↩][↩]
- Kisaka T, Cox TA, Dumitrescu D, Wasserman K. CO2 pulse and acid-base status during increasing work rate exercise in health and disease. Respir Physiol Neurobiol. 2015 Nov;218:46-56. doi: 10.1016/j.resp.2015.07.005[↩]
- Brinkman JE, Toro F, Sharma S. Physiology, Respiratory Drive. [Updated 2022 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482414[↩]
- Brinkman JE, Sharma S. Respiratory Alkalosis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482117[↩]
- Adamczak M, Surma S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis (Basel). 2021 Jun 4;7(6):452-467. doi: 10.1159/000516371[↩]
- Hopkins E, Sharma S. Physiology, Acid Base Balance. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507807[↩][↩]
- Wesson DE, Nathan T, Rose T, Simoni J, Tran RM. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007 Feb;71(3):210-7. doi: 10.1038/sj.ki.5002036[↩]
- Acidosis: An Old Idea Validated by New Research. Integr Med (Encinitas). 2015;14(1):8-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566456/[↩]
- Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271(6 Pt 2):F1114–F1122[↩]
- Kopple J.D., Kalantar-Zadeh K., Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005;67:S21–S27. doi: 10.1111/j.1523-1755.2005.09503.x[↩]
- Dubey A.K., Sahoo J., Vairappan B., Haridasan S., Parameswaran S., Priyamvada P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transplant. 2020;35:121–129. doi: 10.1093/ndt/gfy214[↩]
- Noce A., Marrone G., Ottaviani E., Guerriero C., Di Daniele F., Pietroboni Zaitseva A., Di Daniele N. Uremic sarcopenia and its possible nutritional approach. Nutrients. 2021;13:147. doi: 10.3390/nu13010147[↩]
- Diet acids and alkalis influence calcium retention in bone. Buclin T, Cosma M, Appenzeller M, Jacquet AF, Décosterd LA, Biollaz J, Burckhardt P. Osteoporos Int. 2001; 12(6):493-9.[↩]
- Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: a systematic review & meta-analysis applying Hill’s epidemiologic criteria for causality. Nutrition Journal. 2011;10(1, article 41). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114717/[↩]
- Frassetto LA, Morris RC, Jr., Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. American Journal of Physiology—Renal Physiology. 2007;293(2):F521–F525. https://www.ncbi.nlm.nih.gov/pubmed/17522265[↩]
- Frings-Meuthen P, Buehlmeier J, Baecker N, et al. High sodium chloride intake exacerbates immobilization-induced bone resorption and protein losses. Journal of Applied Physiology. 2011;111(2):537–542. https://www.ncbi.nlm.nih.gov/pubmed/21596917[↩]
- Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet. 1999;354(9183):971–975. https://www.ncbi.nlm.nih.gov/pubmed/10501357[↩]
- Devine A, Criddle RA, Dick IM, Kerr DA, Prince RL. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. American Journal of Clinical Nutrition. 1995;62(4):740–745. https://www.ncbi.nlm.nih.gov/pubmed/7572702[↩]
- Morris RC, Jr., Schmidlin O, Frassetto LA, Sebastian A. Relationship and interaction between sodium and potassium. Journal of the American College of Nutrition. 2006;25(3):262S–270S. https://www.ncbi.nlm.nih.gov/pubmed/16772638[↩]
- Barzel US, Massey LK. Excess dietary protein may can adversely affect bone. Journal of Nutrition. 1998;128(6):1051–1053. https://www.ncbi.nlm.nih.gov/pubmed/9614169[↩]
- Heaney RP, Layman DK. Amount and type of protein influences bone health. American Journal of Clinical Nutrition. 2008;87(5):156S–157S. https://www.ncbi.nlm.nih.gov/pubmed/18469289[↩]
- Dawson-Hughes B, Harris SS, Ceglia L. Alkaline diets favor lean tissue mass in older adults. American Journal of Clinical Nutrition. 2008;87(3):662–665. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597402/[↩]
- Garibotto G, Russo R, Sofia A, et al. Muscle protein turnover in chronic renal failure patients with metabolic acidosis or normal acid-base balance. Mineral and Electrolyte Metabolism. 1996;22(1–3):58–61. https://www.ncbi.nlm.nih.gov/pubmed/8676826[↩]
- Caso G, Garlick PJ. Control of muscle protein kinetics by acid-base balance. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8(1):73–76. https://www.ncbi.nlm.nih.gov/pubmed/15586003[↩]
- Webster MJ, Webster MN, Crawford RE, Gladden LB. Effect of sodium bicarbonate ingestion on exhaustive resistance exercise performance. Medicine and Science in Sports and Exercise. 1993;25(8):960–965. https://www.ncbi.nlm.nih.gov/pubmed/8396707[↩]
- Frassetto L, Morris, Jr. R.C. RC, Jr., Sellmeyer DE, Todd K, Sebastian A. Diet, evolution and aging—the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. European Journal of Nutrition. 2001;40(5):200–213. https://www.ncbi.nlm.nih.gov/pubmed/11842945[↩]
- McSherry E, Morris RC., Jr. Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. Journal of Clinical Investigation. 1978;61(2):509–527. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC372562/[↩]
- Frassetto L, Morris RC, Jr., Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 1997;82(1):254–259. https://www.ncbi.nlm.nih.gov/pubmed/8989270[↩]
- Wass JAH, Reddy R. Growth hormone and memory. Journal of Endocrinology. 2010;207(2):125–126. https://www.ncbi.nlm.nih.gov/pubmed/20696696[↩]
- Frassetto L, Morris RC, Jr., Sebastian A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):831–834. https://www.ncbi.nlm.nih.gov/pubmed/15572425[↩]
- Vormann J, Worlitschek M, Goedecke T, Silver B. Supplementation with alkaline minerals reduces symptoms in patients with chronic low back pain. Journal of Trace Elements in Medicine and Biology. 2001;15(2-3):179–183. https://www.ncbi.nlm.nih.gov/pubmed/11787986[↩]
- Zofková I, Kancheva RL. The relationship between magnesium and calciotropic hormones. Magnesium Research. 1995;8(1):77–84. https://www.ncbi.nlm.nih.gov/pubmed/7669510[↩]
- Schwalfenberg G. Improvement of chronic back pain or failed back surgery with vitamin D repletion: a case series. Journal of the American Board of Family Medicine. 2009;22(1):69–74. https://www.ncbi.nlm.nih.gov/pubmed/19124636[↩]
- Groos E, Walker L, Masters JR. Intravesical chemotherapy. Studies on the relationship between pH and cytotoxicity. Cancer. 1986;58(6):1199–1203. https://www.ncbi.nlm.nih.gov/pubmed/3091241[↩]
- Smith SR, Martin PA, Edwards RHT. Tumour pH and response to chemotherapy: an in vivo 31P magnetic resonance spectroscopy study in non-Hodgkin’s lymphoma. British Journal of Radiology. 1991;64(766):923–928. https://www.ncbi.nlm.nih.gov/pubmed/1954534[↩]
- Raghunand N, Gillies RJ. pH and chemotherapy. Novartis Foundation Symposium. 2001;240:199–211. https://www.ncbi.nlm.nih.gov/pubmed/11727930[↩]
- Raghunand N, He X, Van Sluis R, et al. Enhancement of chemotherapy by manipulation of tumour pH. British Journal of Cancer. 1999;80(7):1005–1011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363059/[↩]
- J Environ Public Health. 2012; 2012: 727630. Published online 2011 Oct 12. doi: 10.1155/2012/727630. The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health ? – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195546/[↩]