Magnesium
Magnesium (Mg or Mg2+) is the fourth most common mineral in your body (after calcium, potassium, and sodium) and the second richest intracellular cation (a positively charged ion) after potassium that your body needs to stay healthy and is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines such as antacids and laxatives 1, 2. Magnesium is an essential mineral which acts as a cofactor (a compound that is essential for the activity of an enzyme) in more than 300 enzyme systems that regulate diverse biochemical reactions in your body, including making protein, bone, and DNA, energy production, oxidative phosphorylation, glycolysis (the metabolic pathway in which glucose is broken down to produce energy), regulating muscle and nerve function, blood sugar control, blood pressure regulation, ion transport and cell signaling 3, 4, 5, 6, 7, 8, 9, 10. According to the United States Food and Nutrition Board, recommended daily allowance for magnesium is 420 mg for adult males and 320 mg for adult females, respectively 11, 6. Approximately 10% of the daily magnesium requirement is derived from water 12. Green vegetables, nuts, seeds, and unprocessed cereals are rich sources of magnesium. Also, some magnesium is available in fruits, fish, meat, and milk products 13.
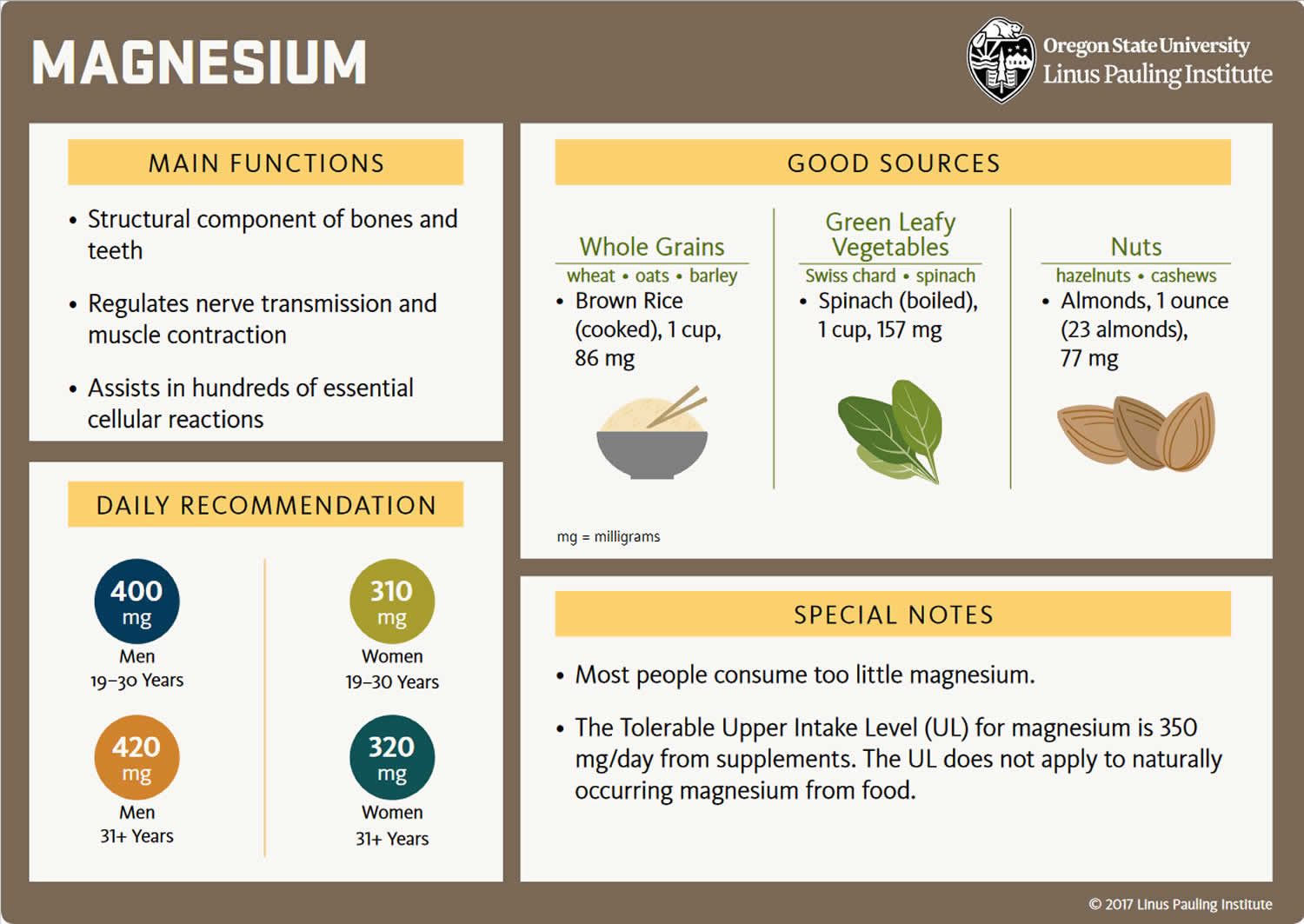
You can get recommended amounts of magnesium by eating a variety of foods, including the following 14:
- Legumes, nuts, seeds, whole grains, and green leafy vegetables (such as spinach)
- Fortified breakfast cereals and other fortified foods
- Milk, yogurt, and some other milk products
Magnesium contributes to the structural development of bone and is required for the synthesis of DNA, RNA, and the antioxidant glutathione. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm 8. Magnesium deficiency in healthy individuals who are consuming a balanced diet is quite rare because magnesium is abundant in both plant and animal foods and because the kidneys are able to limit urinary excretion of magnesium when intake is low 5. However, before you reach for a magnesium supplement, though, you should know that just a few servings of magnesium-rich foods a day can meet your need for this important nutrient.
The majority of the population in the Western countries consume less than the recommended amount of magnesium, contributed by the consumption of processed foods, demineralized water, and agricultural practices using soil deficient in magnesium for growing food 11, 15, 16. Approximately half (48%) of the US population has been shown to consume less than the daily requirement of magnesium from foods 17, partly because of the processing of food, a lower consumption of whole grains and fruits and vegetables than recommended, and a greater consumption of fast food that has a low magnesium content 18. The 2015 Dietary Guidelines Advisory Committee found magnesium to be underconsumed relative to the Estimated Average Requirement (EAR) and characterized it as a shortfall nutrient of public health concern 19. The European Food Safety Authority (EFSA) recently published a scientific opinion on dietary reference values for magnesium and found that “although the role of Mg in bone structure and physiology is well established, there are few quantitative data for using this relation for setting dietary reference values for magnesium” 20. Nevertheless, the impact of chronically low magnesium intake on serum magnesium concentrations and long-term health remains poorly studied; most trials have been of short duration, and most observational studies have lacked repeated serum measures.
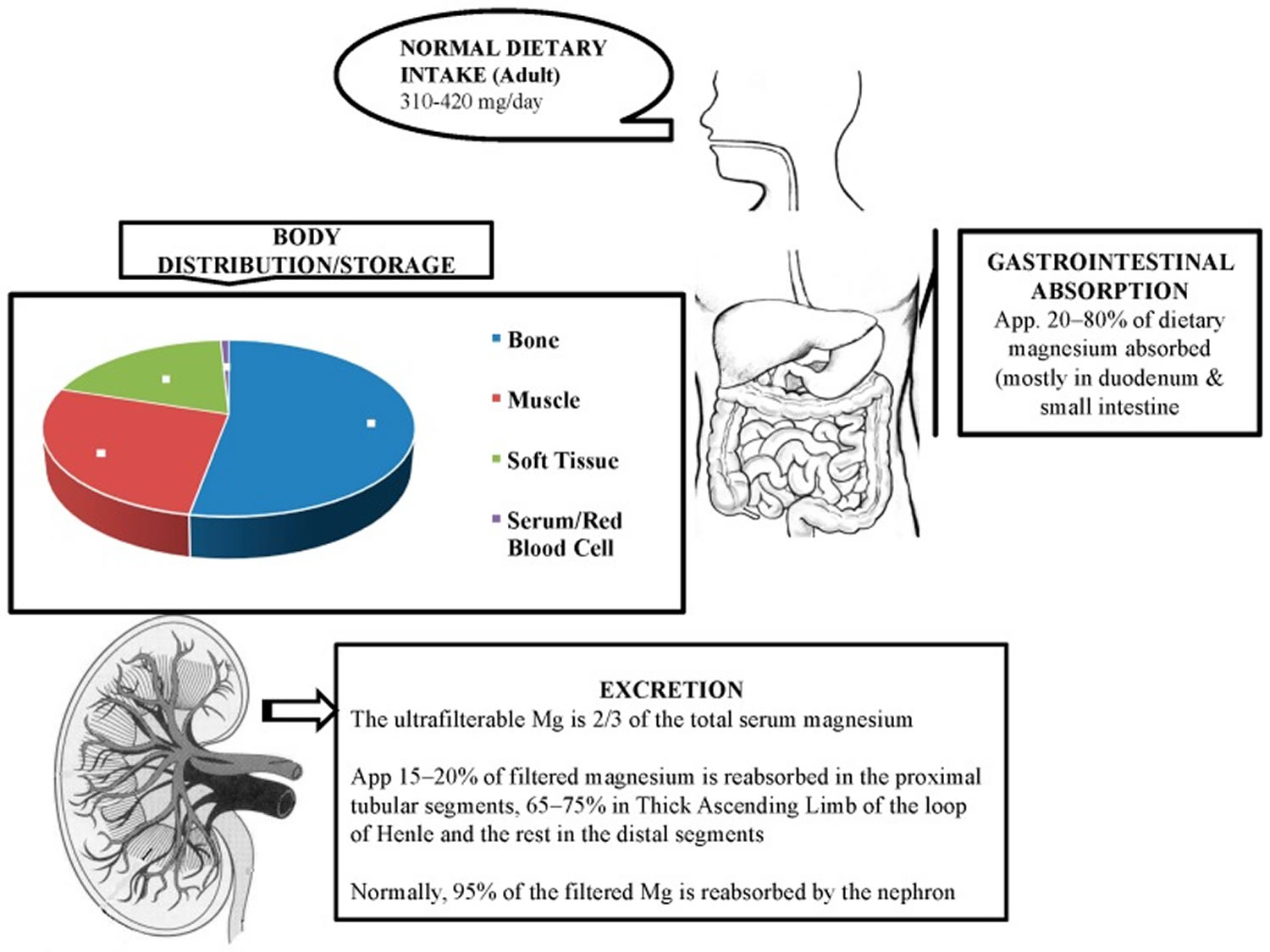
Magnesium enters your body through your diet and is absorbed by your small intestine and large intestine (colon). The total body magnesium in the average adult is approximately 1000 mmol or 25 g magnesium, that is, 20 mmol/kg of lean body mass, with 50% to 60% present in your bones and most of the rest in your muscles and other soft tissues (34% to 50%) 15, 11, 21, 22. Around one-third of the bone magnesium content is available for exchange to maintain the levels of extracellular magnesium 15.
Less than 1% of total magnesium in the body is available in serum and red blood cells, accounting for the extracellular magnesium in your body 11. The magnesium levels in blood serum and extracellular fluids are kept under tight control through its absorption, reservoir, and excretion process by various organs such as the gut, bone, and kidney 23. Besides these organs, several hormones, namely vitamin D, parathyroid hormone (PTH), and estrogen, are involved in the regulation of normal level of magnesium 13, 24. The relationship between parathyroid hormone (PTH) and magnesium is complex and similar to calcium; high serum magnesium levels suppress the secretion of parathyroid hormone (PTH) via activation of calcium-sensing receptor (CaSR) present on the chief cells of the parathyroid glands 12. In contrast, low serum magnesium stimulates parathyroid hormone (PTH) secretion 12.
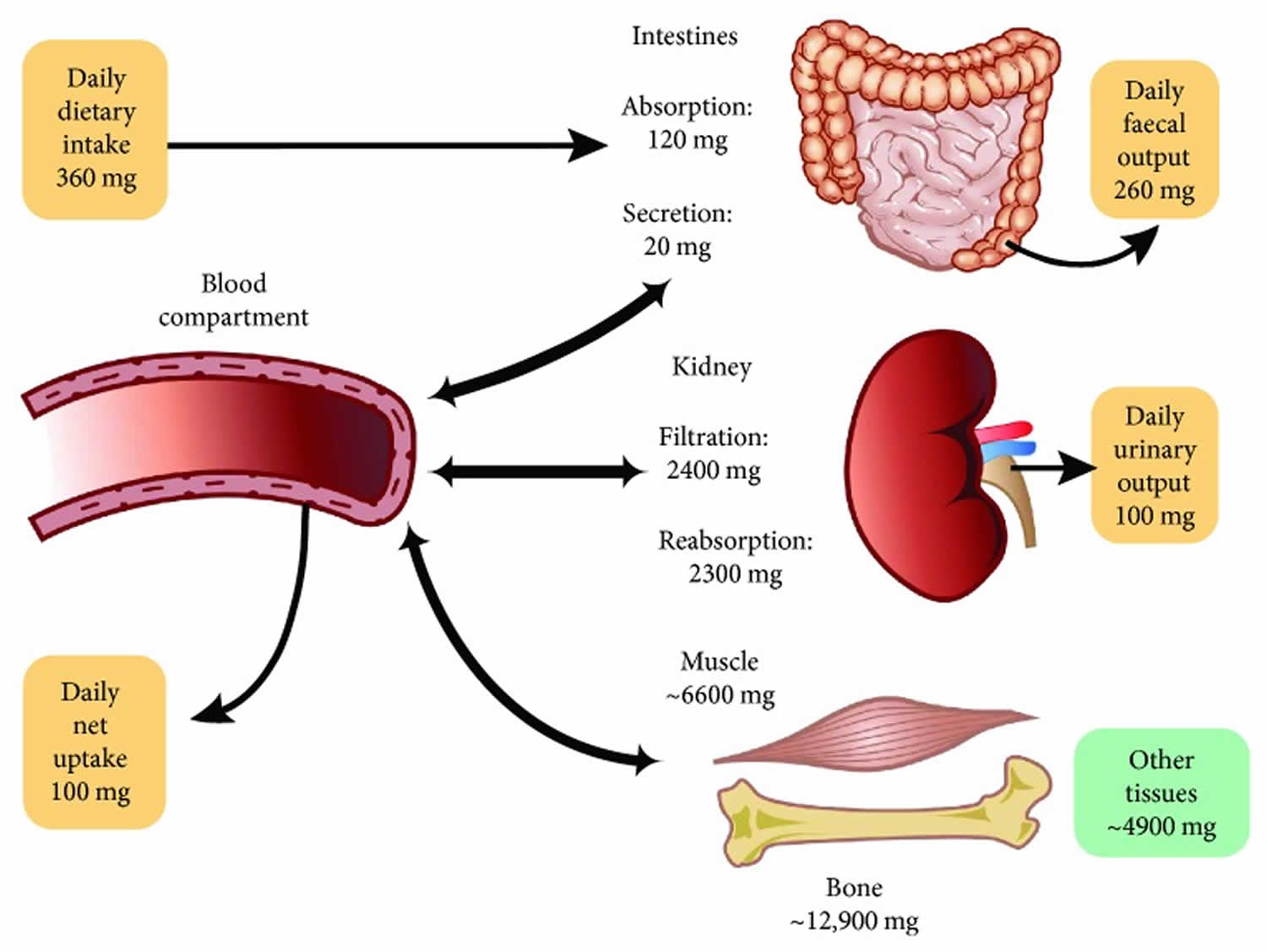
The magnesium homeostasis is primarily regulated by your kidneys 15. The glomeruli filter around 2400 mg of magnesium per day 25. Normally, only 3% of filtered magnesium appears in urine. About 97% of excreted magnesium is passively reabsorbed, mainly by the thick ascending limb of the loop of Henle (65%) and to a lesser extent in the distal tubules (30%) 26, 27, 28, 15, 29. Only around 100 mg to 120 mg of magnesium is excreted in the urine each day, and the kidneys can regulate the amount excreted, depending on the serum level of magnesium 15, 7, 28. Urinary excretion is reduced when magnesium status is low 6. Figure 2 below illustrates the magnesium balance in the human body.
Relatively little is known about cellular magnesium transport mechanisms 28. This factor is essential for the pathophysiology of kidney-related hypermagnesemia as along the loop of Henle, not only the volume of the filtrate gets reduced, but also the osmolarity decreases significantly (-66%), and consequently the solutes become less concentrated. Furthermore, this explains the high resorbent capacity of the kidney, which generally maintains magnesium equilibrium until the creatinine clearance falls below 20 ml/minute 26. Thus, an increase in plasma magnesium levels is practically impossible to achieve with diet alone in conditions of perfect kidney health. However, the odds of hypermagnesemia can increase by taking mega-doses of magnesium. The pathophysiology of hypermagnesemia related to excess laxative use is different. In this case, the huge amount of magnesium given through the digestive tract can lead to overwhelming the excretory mechanism, especially in cases with underlying subclinical renal failure 26.
Magnesium concentration within red blood cells is three times higher than in plasma 30. In mammalian cells, Mg2+ is an abundant cation present at concentrations ranging from 5 to 20 millimoles (mmol)/L (12.15 to 48.6 mg/dL) 31. In the plasma, the magnesium concentration is a little lower at around 1 mmol/L (2.43 mg/dL) 25. Many different reference values for serum magnesium have been proposed, which collectively suggest that, in healthy adults plasma magnesium concentration ranges somewhere between 0.6 and 1.2 millimoles (mmol)/L (1.46 to 2.92 mg/dL) 32, 6, 33. The Canadian Health Measure Survey Cycle 3 conducted in 2012–2013, measured serum magnesium in subjects aged 3–79 years 34. They reported that 9.5% to 16.6% of adults and 15.8% to 21.8% of adolescents (12–19 years) had serum magnesium concentrations < 0.75 mmol/L (< 1.82 mg/dL), which is a level currently accepted as an indication of hypomagnesemia or magnesium deficiency 34, 35, 36, 37. However, it has recently been suggested that serum magnesium concentrations < 0.75 mmol/L (< 1.82 mg/dL) value is likely to be too low and should be raised to <0.85 mmol/L (< 2.07 mg/dL), as values in this range are associated with increased health risks 32, 38. Razzaque 39 suggests that individuals with serum magnesium levels between 0.75 to 0.85 mmol/L (1.82 to 2.07 mg/dL) to undergo further investigation to confirm body magnesium status.
Approximately 30% of total plasma magnesium is protein-bound and approximately 70% is filterable through artificial membranes (15% complexed, 55% free Mg2+ ions) 40. With a glomerular filtration rate (GFR) of approximately 150 L per day and an ultrafiltrable magnesium concentration of 14 mg/L, the filtered magnesium load is approximately 2,100 mg per day.
Assessing magnesium status is difficult because most magnesium is inside cells or in bone 8. The most commonly used and readily available method for assessing magnesium status is measurement of serum magnesium concentration, even though serum levels have little correlation with total body magnesium levels or concentrations in specific tissues 37. Other methods for assessing magnesium status include measuring magnesium concentrations in red blood cells, saliva, and urine; measuring ionized magnesium concentrations in blood, plasma, or serum; and conducting a magnesium-loading (or “tolerance”) test. No single method is considered satisfactory 41. Some experts 21 but not others 8 consider the tolerance test (in which urinary magnesium is measured after parenteral infusion of a dose of magnesium) to be the best method to assess magnesium status in adults. To comprehensively evaluate magnesium status, both laboratory tests and a clinical assessment might be required 37.
Clinical and preclinical studies revealed that the magnesium level is found to be low in various pathological conditions such as migraine, diabetes, osteoporosis, asthma, preeclampsia, cardiovascular diseases and its correction is an important treatment strategy for these conditions 42, 43, 44, 45, 46, 47.
Low magnesium levels, also called hypomagnesemia, may have no or few nonspecific symptoms in the short term. Early signs of magnesium deficiency (hypomagnesemia) include weakness, loss of appetite, fatigue, nausea, and vomiting 3. Afterwards, muscle contractions and cramps, numbness, tingling, personality changes, coronary spasms, abnormal heart rhythms (cardiac arrhythmias) and seizures can occur when magnesium deficiency worsens 48, 49. Finally, severe magnesium deficiency can result in low blood calcium (hypocalcemia) or low blood potassium (hypokalemia) because mineral homeostasis is disrupted 50. Long term (chronic) or severe low magnesium levels can increase your risk of high blood pressure, heart disease, type 2 diabetes and osteoporosis 51. Persistent or severe magnesium deficiencies can cause nausea, loss of appetite, fatigue, confusion, muscle cramps, seizures, changes in heart rate, and numbness or tingling. Severe magnesium deficiency can impede vitamin D and also affect calcium metabolism and exacerbate calcium deficiencies. Magnesium deficiencies or hypomagnesemia may be seen in those with gastrointestinal or kidney disorders, those suffering from chronic alcoholism, and older people 5. Poor dietary intake, gastrointestinal problems, and increased urinary loss of magnesium may all contribute to magnesium depletion in people suffering from alcoholism 5. Older adults have relatively low dietary magnesium intakes 52, 53. Intestinal magnesium absorption tends to decrease with age, and urinary magnesium excretion tends to increase with age; thus, suboptimal dietary magnesium intake may increase the risk of magnesium depletion in the elderly 54.
The following conditions increase the risk of magnesium deficiency 55:
- Gastrointestinal disorders: Prolonged diarrhea, Crohn’s disease, malabsorption syndromes, celiac disease, surgical removal of a portion of the small intestine, and intestinal inflammation due to radiation may all lead to magnesium depletion.
- Kidney disorders (magnesium wasting): Diabetes mellitus and long-term use of certain diuretics (see Drug interactions) may result in increased urinary loss of magnesium. Multiple other medications can also result in renal magnesium wasting 56.
- Endocrine and metabolic disorders: Several conditions, such as diabetes mellitus, parathyroid gland disorders, phosphate depletion, primary aldosteronism, and even excessive lactation, can lead to magnesium depletion.
An excess of magnesium also called hypermagnesemia may happen with the ingestion of antacids and laxatives that contain magnesium and with a decreased ability of the kidneys to excrete the excess magnesium (Mg). But clinically significant hypermagnesemia is rare due to the kidney’s ability to increase excretion when needed. Symptoms of excess magnesium (hypermagnesemia) can be similar to those of magnesium deficiency and include nausea and vomiting, muscle weakness, fatigue, loss of appetite, trouble breathing, an irregular heart rate and in severe cases, cardiac arrest (the sudden stopping of the heart) 57. Severe hypermagnesemia (levels greater than 12 mg/dL) can lead to cardiovascular complications (hypotension, and arrhythmias) and neurological disorder (confusion and lethargy). Higher values of serum magnesium (exceeding 15 mg/dL) can induce cardiorespiratory arrest and coma 26.
According to European Register on Nutrition and Health Claims 58, 3, the following claims about magnesium have been authorized. The claim may be used only for food which is at least a source of magnesium as referred to in the claim “a source of” magnesium as listed in the Annex to Regulation (EC) No 1924/2006.
- “Magnesium contributes to a reduction of tiredness and fatigue”;
- “Magnesium contributes to electrolyte balance”;
- “Magnesium contributes to normal energy-yielding metabolism”;
- “Magnesium contributes to normal functioning of the nervous system”;
- “Magnesium contributes to normal muscle function”;
- “Magnesium contributes to normal protein synthesis”;
- “Magnesium contributes to normal psychological function”;
- “Magnesium contributes to the maintenance of normal bones”;
- “Magnesium contributes to the maintenance of normal teeth”;
- “Magnesium has a role in the process of cell division”.
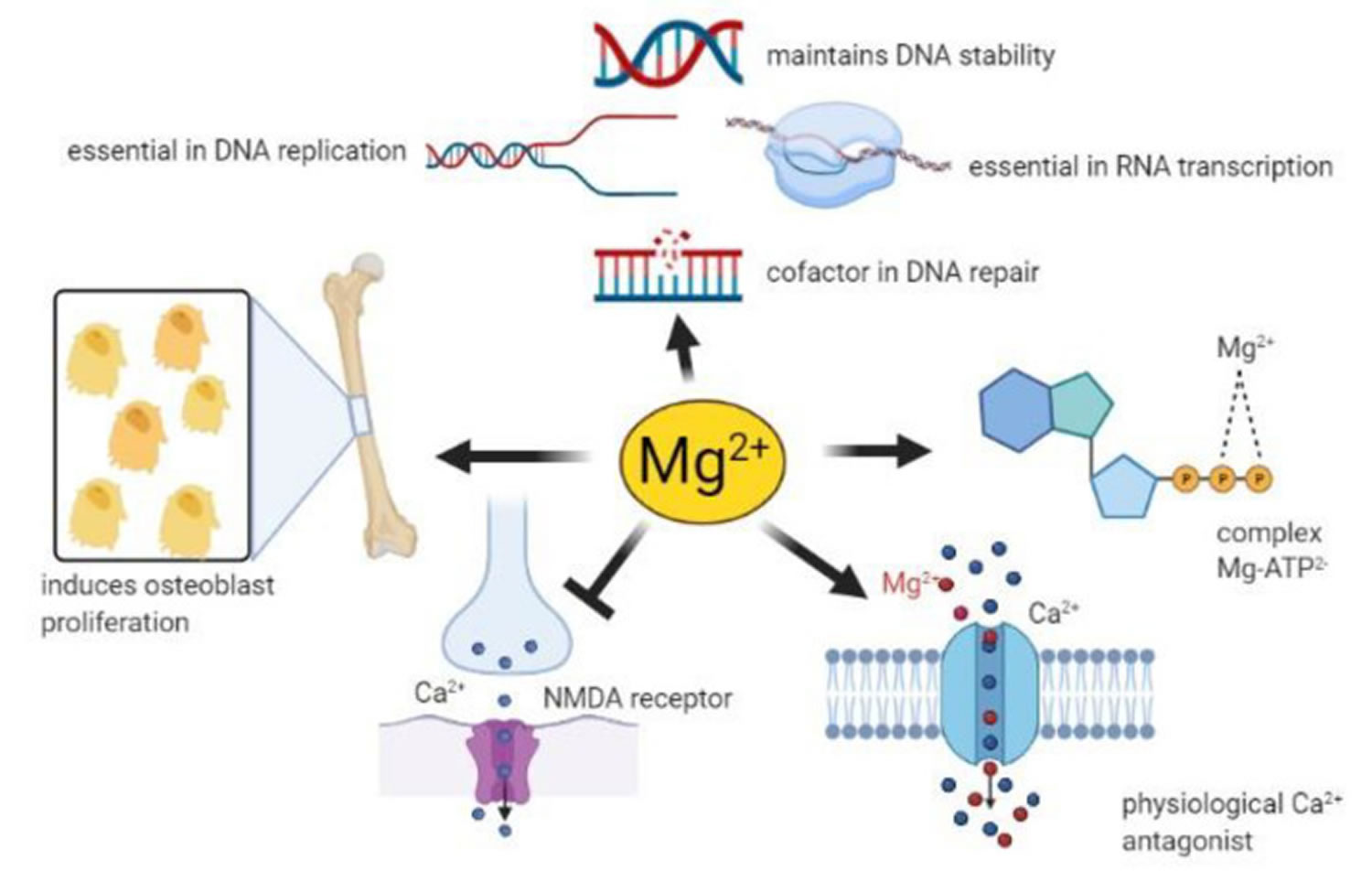
Figure 1. Physiology of magnesium
Footnote: Magnesium homeostasis is facilitated by intestinal absorption, bone which acts as a reservoir/store, and kidneys which are responsible for magnesium excretion.
[Source 59 ]Figure 2. Magnesium homeostasis
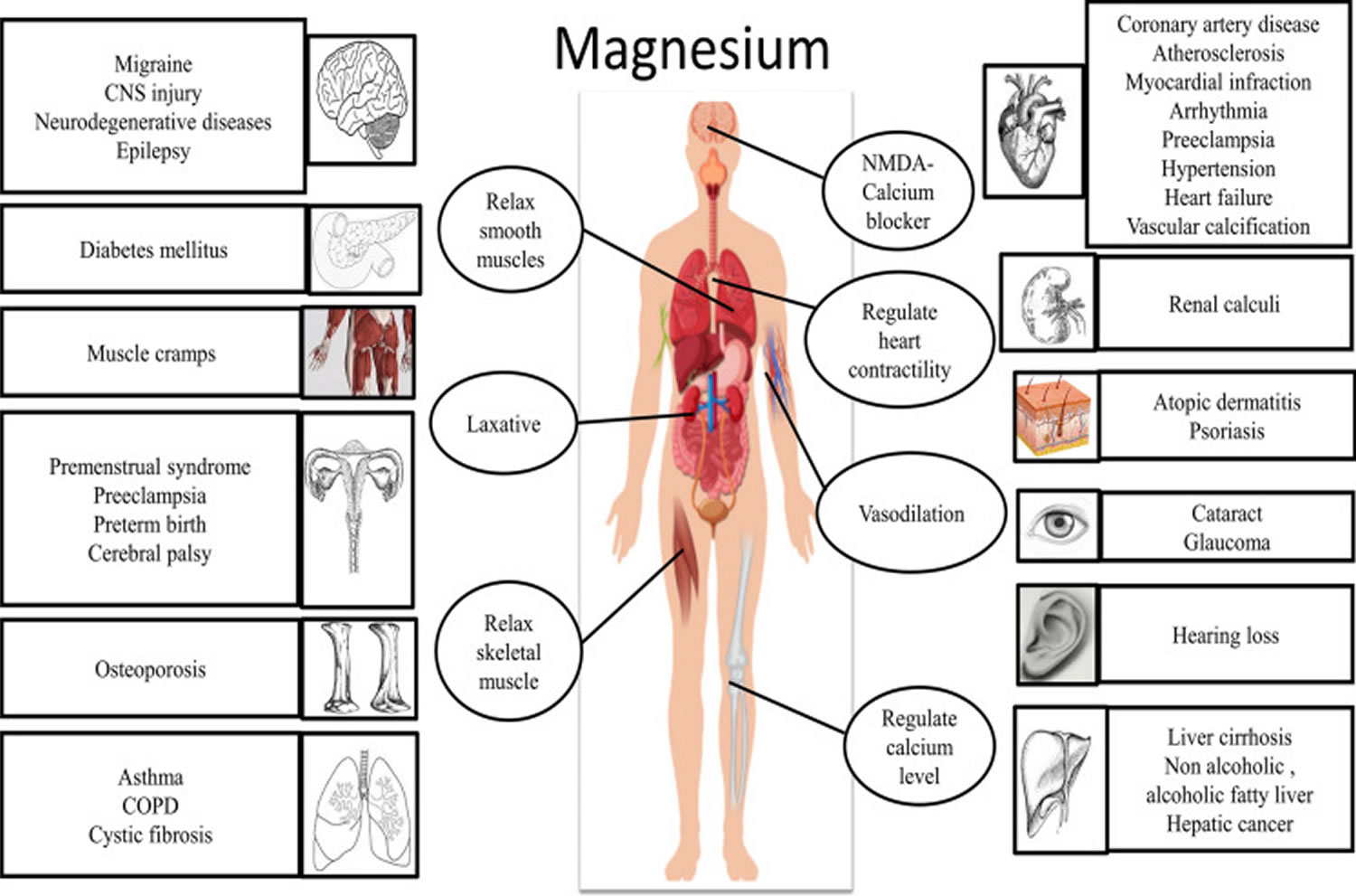
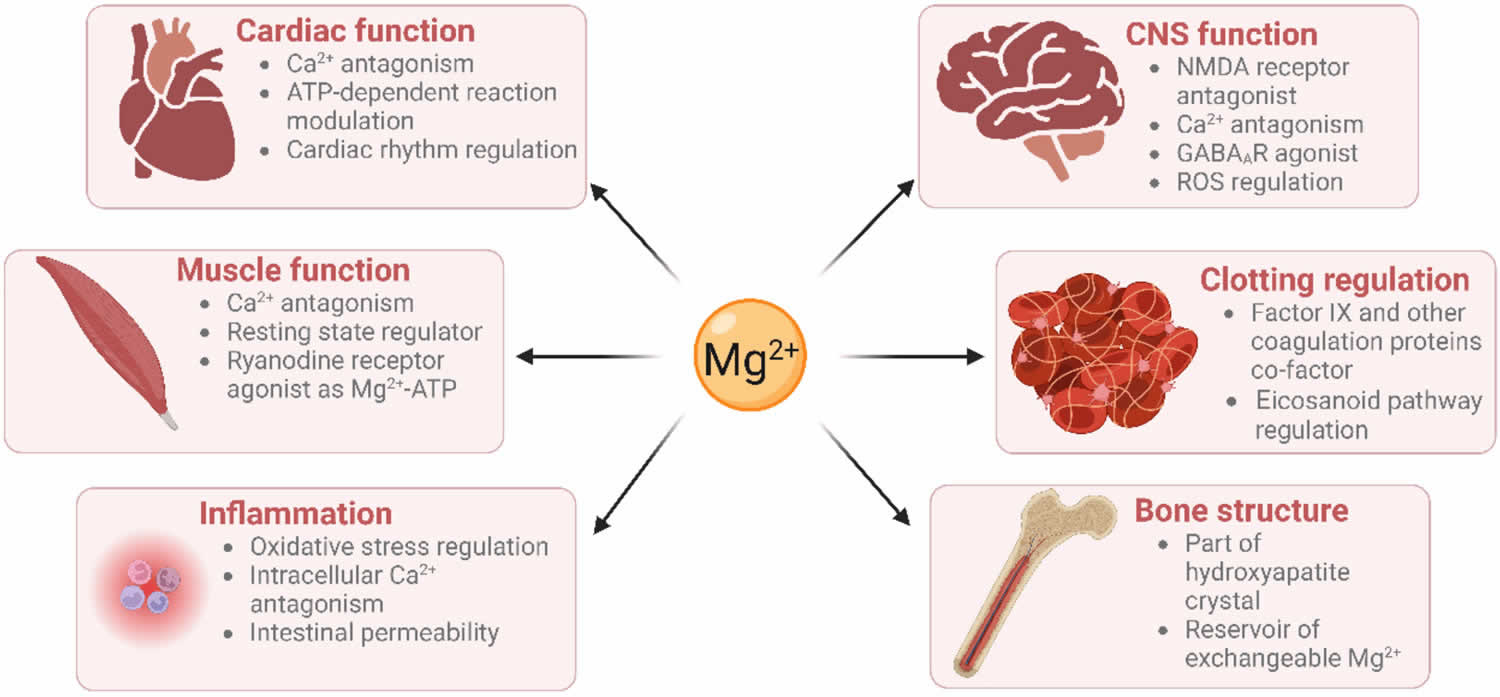
[Source 12 ]Figure 3. Physiological roles of magnesium
Footnote: Physiological roles of magnesium on vital systems. Text in the circle represents the physiological role of magnesium (Mg) in various vital organs. Text in the rectangle indicate the diseases or disorders associated with magnesium deficiency.
[Source 23 ]Figure 4. Magnesium role in many cellular processes
Footnote: Neuronal magnesium concentrations downregulate the excitability of the N-methyl-D-aspartate (NMDA) receptor, which is essential for excitatory synaptic transmission and neuronal plasticity in learning and memory 60. Magnesium blocks the calcium channel in the NMDA receptor and must be removed for glutamatergic excitatory signaling. Low serum magnesium levels increase NMDA receptor activity thus enhancing Ca2+ and Na+ influx and neuronal excitability. For these reasons, a deficiency of magnesium has been hypothesized in many neurological disorders, such as migraine, chronic pain, epilepsy, Alzheimer’s disease, Parkinson’s disease and stroke, as well as anxiety and depression 61.
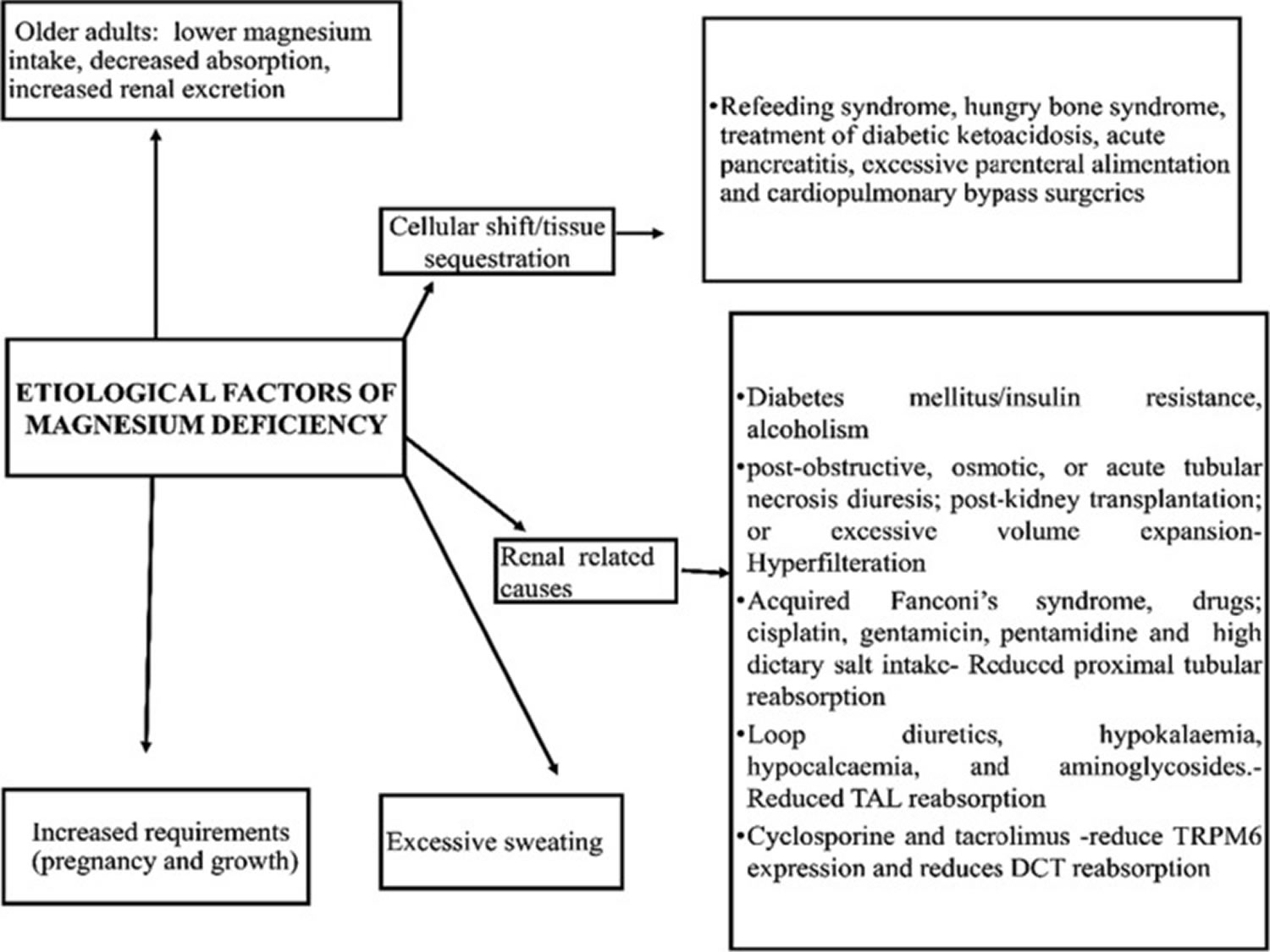
[Source 3 ]Figure 5. Factors associated with magnesium deficiency
[Source 23 ]What does magnesium do?
Every organ in your body, especially your heart, muscles, and kidneys, needs the mineral magnesium. Magnesium also contributes to the makeup of teeth and bones. Magnesium is important for physiological functions in your body, including regulating muscle and nerve function, blood sugar levels, and blood pressure; making protein and bone; regulating inflammation; maintaining hemostasis (a process that prevent and stop bleeding from a blood vessel), deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) stability; as well as making the antioxidant glutathione 62, 63, 64, 65, 25, 3, 5, 6, 7, 8, 9, 10. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm 8.
Role of magnesium in the human body 66, 67, 68, 13, 66, 25, 69:
- Cofactor in more than 300 enzymes involved in:
- Protein synthesis, muscle and nerve transmission, neuromuscular conduction, and blood glucose and blood pressure regulation.
- Platelet function (clotting and/or thrombus formation).
- Muscle contraction/relaxation.
- Insulin regulation.
- Bone formation
- Role in active transport
- Facilitates active transport of calcium and potassium ions across cell membranes, which is essential for the conduction of nerve impulses (neurotransmission), muscle contraction, maintaining vascular tone and normal heart rhythm.
- Structural roles
- Important for the structure of bones, proteins, many enzymes, mitochondria, DNA, and RNA.
- Role in immunological functions
- Involved in macrophage activation, adherence, and bactericidal activity of granulocyte oxidative burst, lymphocyte proliferation, and endotoxin binding to monocytes.
Magnesium is a cofactor (a compound that is essential for the activity of an enzyme) in more than 300 enzyme 67, 65. These include protein kinases which are commonly utilized to regulate gene transcription in response to extracellular stimuli 70. Magnesium is also required for the structure and functioning of DNA and RNA polymerases 71, 72. These DNA and RNA polymerases are not only involved in nucleic acid synthesis, but some are also involved in DNA repair and genome maintenance 25. Virtually all enzymes taking part in mismatch repair, nucleotide repair, and base excision repair use magnesium as a cofactor 25. Given that defects in genome maintenance pathways are considered a hallmark of many cancers, magnesium deficiency might contribute to oncogenesis (the process through which healthy cells become transformed into cancer cells) 73. Furthermore, magnesium deficiency has been shown to be associated with diverse pathologies including prediabetes, platelet hyper-reactivity, pre-eclampsia, heart attack (acute myocardial infarction) and even some therapies 74, 75, 76, 42.
In fact, magnesium is used as a primary ingredient in some laxatives 77. Phillips’ Milk of Magnesia®, for example, provides 500 mg elemental magnesium (as magnesium hydroxide) per tablespoon; the directions advise taking up to 4 tablespoons/day for adolescents and adults 78. Although such a dose of magnesium is well above the safe upper level, some of the magnesium is not absorbed because of the medication’s laxative effect. Magnesium is also included in some remedies for heartburn and upset stomach due to acid indigestion 77. Extra-strength Rolaids®, for example, provides 55 mg elemental magnesium (as magnesium hydroxide) per tablet, although Tums® is magnesium free.
Magnesium is also used for the treatment of pre-eclampsia and eclampsia 42, 59. Pre-eclampsia is a disorder of pregnancy characterized by hypertension and proteinuria. Eclampsia is the occurrence of one or more convulsions associated with pre-eclampsia. Magnesium sulfate is now the drug of choice for women with eclampsia and is better than antiepileptic drugs. The Magpie trial 79 was a randomized controlled trial comparing magnesium sulfate with a placebo for pre-eclampsia. The results demonstrated a reduction by about 50% in the risk of eclampsia in the pre-eclamptic women 79.
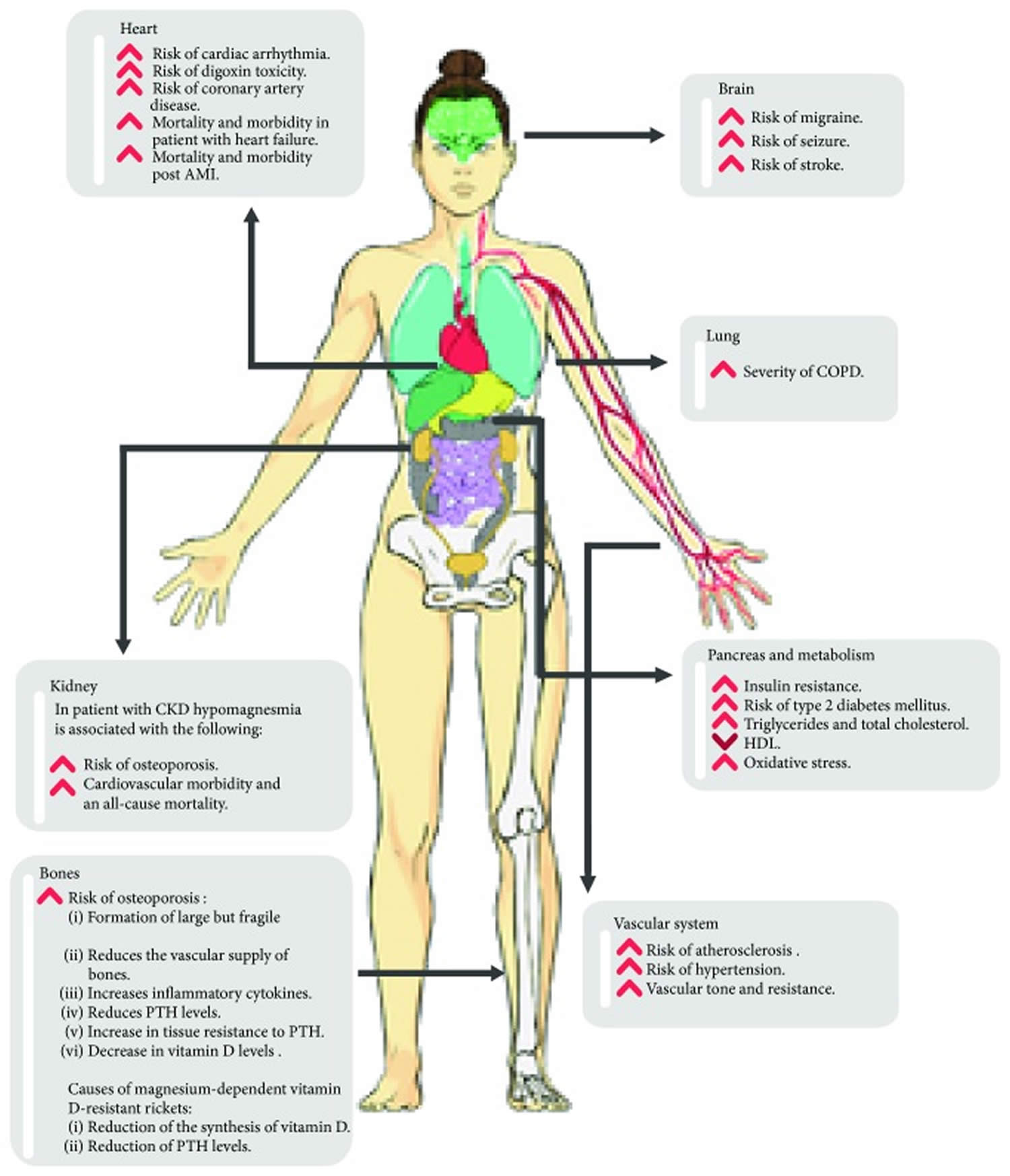
Figure 6. Magnesium function
Footnote: Magnesium has roles in many physiological processes
[Source 25 ]Energy production
The metabolism of carbohydrates (sugars) and fats to produce energy requires numerous magnesium-dependent chemical reactions. Magnesium is required by the adenosine triphosphate (ATP)-synthesizing protein in mitochondria. ATP, the molecule that provides energy for almost all metabolic processes, exists primarily as a complex with magnesium (MgATP) 56.
Synthesis of essential molecules
Magnesium is required for a number of steps during synthesis of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and proteins. Several enzymes participating in the synthesis of carbohydrates and lipids require magnesium for their activity. Glutathione, an important antioxidant, requires magnesium for its synthesis 56.
Structural roles in bone, cell membranes, and chromosomes
Magnesium plays a structural role in bone, cell membranes, and chromosomes 56.
Ion transport across cell membranes
Magnesium is required for the active transport of ions like potassium and calcium across cell membranes. Through its role in ion transport systems, magnesium affects the conduction of nerve impulses, muscle contraction, and normal heart rhythm 56.
Cell signaling
Cell signaling requires Mg-ATP for the phosphorylation of proteins and the formation of the cell-signaling molecule, cyclic adenosine monophosphate (cAMP). cAMP is involved in many processes, including the secretion of parathyroid hormone (PTH) from the parathyroid glands 56.
Cell migration
Calcium and magnesium concentrations in the fluid surrounding cells affect the migration of a number of different cell types. Such effects on cell migration may be important in wound healing 56.
Therapeutic use of Magnesium
Magnesium is a primary ingredient in some laxatives 77. Phillips’ Milk of Magnesia®, for example, provides 500 mg elemental magnesium (as magnesium hydroxide) per tablespoon; the directions advise taking up to 4 tablespoons/day for adolescents and adults 78. Although such a dose of magnesium is well above the safe upper level, some of the magnesium is not absorbed because of the medication’s laxative effect. Magnesium is also included in some remedies for heartburn and upset stomach due to acid indigestion 77. Extra-strength Rolaids®, for example, provides 55 mg elemental magnesium (as magnesium hydroxide) per tablet, although Tums® is magnesium free.
Magnesium is also used for the treatment of pre-eclampsia and eclampsia 59. Pre-eclampsia is a disorder of pregnancy characterized by hypertension and proteinuria. Eclampsia is the occurrence of one or more convulsions associated with pre-eclampsia. Magnesium sulfate is now the drug of choice for women with eclampsia and is better than antiepileptic drugs. The Magpie trial 79 was a randomized controlled trial comparing magnesium sulfate with a placebo for pre-eclampsia. The results demonstrated a reduction by about 50% in the risk of eclampsia in the pre-eclamptic women 79.
How much magnesium do I need?
The amount of magnesium you need depends on your age and sex. Average daily recommended magnesium amounts are listed below in milligrams (mg). Intake recommendations for magnesium and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies 6. Dietary Reference Intake (DRI) is the general term for a set of reference values used to plan and assess nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Estimated Average Requirement (EAR): average daily level of intake estimated to meet the requirements of 50% of healthy individuals. It is usually used to assess the adequacy of nutrient intakes in population groups but not individuals.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects.
Table 1 lists the current Recommended Dietary Allowances (RDAs) for magnesium 6. For infants from birth to 12 months, the Food and Nutrition Board established an Adequate Intake (AI) for magnesium that is equivalent to the mean intake of magnesium in healthy, breastfed infants, with added solid foods for ages 7–12 months.
As per the United States Food and Nutrition, Recommended Daily Allowance (RDA) of magnesium is 420 mg for males and 320 mg for females 6. Around 10% of magnesium is obtained through drinking water. Other sources of magnesium include green vegetables, unprocessed cereals, nuts, seeds, fish, meat, and milk products 49.
Too much magnesium from foods isn’t a concern for healthy adults. However, the same can’t be said for supplements. High doses of magnesium from supplements or medications can cause nausea, abdominal cramping and diarrhea. In addition, the magnesium in supplements can interact with some types of antibiotics and other medicines. Check with your doctor or pharmacist if you’re considering magnesium supplements, especially if you routinely use magnesium-containing antacids or laxatives.
Table 1. Recommended Dietary Allowances (RDAs) for Magnesium
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 30 mg |
| Infants 7–12 months | 75 mg |
| Children 1–3 years | 80 mg |
| Children 4–8 years | 130 mg |
| Children 9–13 years | 240 mg |
| Teen boys 14–18 years | 410 mg |
| Teen girls 14–18 years | 360 mg |
| Men | 400–420 mg |
| Women | 310–320 mg |
| Pregnant teens | 400 mg |
| Pregnant women | 350–360 mg |
| Breastfeeding teens | 360 mg |
| Breastfeeding women | 310–320 mg |
Footnote: *Adequate Intake (AI) is the intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 80 ]Are you getting enough magnesium?
Dietary surveys of people in the United States consistently show that many people consume less than recommended amounts of magnesium 81. An analysis of data from the National Health and Nutrition Examination Survey (NHANES) of 2013-2016 found that 48% of Americans of all ages ingest less magnesium from food and beverages than their respective Estimated Average Requirements (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals); adult men aged 71 years and older and adolescent males and females are most likely to have low intakes 82. In a study using data from National Health and Nutrition Examination Survey 2003–2006 to assess mineral intakes among adults, average intakes of magnesium from food alone were higher among users of dietary supplements (350 mg for men and 267 mg for women, equal to or slightly exceeding their respective Estimated Average Requirements) than among nonusers (268 mg for men and 234 for women) 83. When supplements were included, average total intakes of magnesium were 449 mg for men and 387 mg for women, well above Estimated Average Requirement (EAR) levels.
No current data on magnesium status in the United States are available. Determining dietary intake of magnesium is the usual proxy for assessing magnesium status. The National Health and Nutrition Examination Survey has not determined serum magnesium levels in its participants since 1974 84 and magnesium is not evaluated in routine electrolyte testing in hospitals and clinics 7.
What foods provide Magnesium?
Magnesium is widely distributed in plant and animal foods and in beverages 81. Green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains, are good sources of magnesium 6, 8. In general, foods containing dietary fiber provide magnesium. Magnesium is also added to some breakfast cereals and other fortified foods. Some types of food processing, such as refining grains in ways that remove the nutrient-rich germ and bran, lower magnesium content substantially 6. Selected food sources of magnesium are listed in Table 2.
You can get recommended amounts of magnesium by eating a variety of foods, including the following 14:
- Legumes, nuts, seeds, whole grains, and green leafy vegetables (such as spinach)
- Fortified breakfast cereals and other fortified foods
- Milk, yogurt, and some other milk products
Tap, mineral, and bottled waters can also be sources of magnesium, but the amount of magnesium in water varies by source and brand (ranging from 1 mg/L to more than 120 mg/L) 85.
Approximately 30% to 40% of the dietary magnesium consumed is typically absorbed by the body 7, 86.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides comprehensive list of foods containing magnesium arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Magnesium-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Magnesium-Food.pdf).
Table 2. Magnesium Rich Foods
| Food | Milligrams (mg) per serving | Percent Daily Value (DV)* |
|---|---|---|
| Pumpkin seeds, roasted, 1 ounce | 156 | 37 |
| Chia seeds, 1 ounce | 111 | 26 |
| Almonds, dry roasted, 1 ounce | 80 | 19 |
| Spinach, boiled, ½ cup | 78 | 19 |
| Cashews, dry roasted, 1 ounce | 74 | 18 |
| Peanuts, oil roasted, ¼ cup | 63 | 15 |
| Cereal, shredded wheat, 2 large biscuits | 61 | 15 |
| Soymilk, plain or vanilla, 1 cup | 61 | 15 |
| Black beans, cooked, ½ cup | 60 | 14 |
| Edamame, shelled, cooked, ½ cup | 50 | 12 |
| Peanut butter, smooth, 2 tablespoons | 49 | 12 |
| Potato, baked with skin, 3.5 ounces | 43 | 10 |
| Rice, brown, cooked, ½ cup | 42 | 10 |
| Yogurt, plain, low fat, 8 ounces | 42 | 10 |
| Breakfast cereals, fortified with 10% of the DV for magnesium, 1 serving | 42 | 10 |
| Oatmeal, instant, 1 packet | 36 | 9 |
| Kidney beans, canned, ½ cup | 35 | 8 |
| Banana, 1 medium | 32 | 8 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 26 | 6 |
| Milk, 1 cup | 24–27 | 6 |
| Halibut, cooked, 3 ounces | 24 | 6 |
| Raisins, ½ cup | 23 | 5 |
| Bread, whole wheat, 1 slice | 23 | 5 |
| Avocado, cubed, ½ cup | 22 | 5 |
| Chicken breast, roasted, 3 ounces | 22 | 5 |
| Beef, ground, 90% lean, pan broiled, 3 ounces | 20 | 5 |
| Broccoli, chopped and cooked, ½ cup | 12 | 3 |
| Rice, white, cooked, ½ cup | 10 | 2 |
| Apple, 1 medium | 9 | 2 |
| Carrot, raw, 1 medium | 7 | 2 |
Footnote: *DV = Daily Value. Daily Values (DVs) were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The Daily Value (DV) for magnesium is 400 mg for adults and children aged 4 and older. However, the FDA does not require food labels to list magnesium content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 87]Magnesium Supplements
Magnesium supplements are available in a variety of forms, including magnesium oxide, magnesium hydroxide, magnesium gluconate, magnesium chloride, and magnesium citrate salts, as well as a number of amino acid chelates like magnesium aspartate (Table 3) 7, 8. Magnesium hydroxide, oxide, or trisilicate salts are used as antacids to mitigate gastric hyperacidity and symptoms of gastroesophageal reflux disease. The Supplement Facts panel on a dietary supplement label declares the amount of elemental magnesium in the product, not the weight of the entire magnesium-containing compound.
Absorption of magnesium from different kinds of magnesium supplements varies. Forms of magnesium that dissolve well in liquid are more completely absorbed in the gut than less soluble forms 7, 88. Small studies have found that magnesium in the aspartate, citrate, lactate, and chloride forms is absorbed more completely and is more bioavailable than magnesium oxide and magnesium sulfate 88, 89, 90, 91, 92. One study found that very high doses of zinc from supplements (142 mg/day) can interfere with magnesium absorption and disrupt the magnesium balance in the body 93.
Table 3. Clinically available magnesium supplements with their uses
| Magnesium supplement | Elemental content (%) | Bioavailability (%) | Particular uses |
|---|---|---|---|
| Magnesium oxide | 60 | 4 | Effervescent magnesium oxide is better absorbed (8%) than tablets |
| Magnesium carbonate | 45 | 15–40 | Treatment of hypomagnesemia, heart burn, stomach upset and acid indigestion |
| Magnesium sulphate | 10 | 4 | Most commonly used clinical supplement |
| Magnesium hydroxide | 42 | 4 | Antacid and a cathartic |
| Magnesium citrate | 16 | 12 | Nephrolithiasis (kidney stones) |
| Magnesium lactate | 12 | 12 | Treatment of hypomagnesemia vomiting or diarrhea or in gastrointestinal diseases |
| Magnesium chloride | 12 | 12 | Treatment or prevention of hypomagnesemia |
| Magnesium aspartate | 10 | 41–45% (for 5 mg) | To treat fatigue and muscle hyper excitability |
Interactions with Medications
Several types of medications have the potential to interact with magnesium supplements or affect magnesium status. Magnesium interferes with the absorption of digoxin (a heart medication), nitrofurantoin (an antibiotic), and certain anti-malarial drugs, which could potentially reduce drug efficacy 5. Bisphosphonates (e.g., alendronate, etidronate), which are drugs used to treat osteoporosis, and magnesium should be taken two hours apart so that the absorption of the bisphosphonates is not inhibited 94, 95. Magnesium has also been found to reduce the efficacy of chlorpromazine (a tranquilizer), penicillamine, oral anticoagulants, and the quinolone and tetracycline classes of antibiotics 94, 95. Intravenous magnesium might inhibit calcium entry into smooth muscle cells and lead to hypotension and muscular weakness if administered with calcium channel blockers (e.g., nifedipin, nicardipin) 95. Because intravenous magnesium has increased the effects of certain muscle-relaxing medications used during anesthesia, it is advisable to let medical staff know if you are taking oral magnesium supplements, laxatives, or antacids prior to surgical procedures. Moreover, long-term use (three months or longer) of proton-pump inhibitors (drugs used to reduce the amount of stomach acid) may increase the risk of hypomagnesemia 96, 97. High doses of furosemide (Lasix) and some thiazide diuretics (e.g., hydrochlorothiazide), if taken for extended periods, may interfere with magnesium reabsorption in the kidneys and result in magnesium depletion 95. Many other medications may also result in renal magnesium loss 56.
People taking these and other medications on a regular basis should discuss their magnesium intakes with their healthcare providers. Tell your doctor, pharmacist, and other health care providers about any dietary supplements and prescription or over-the-counter medicines you take. They can tell you if the dietary supplements might interact with your medicines or if the medicines might interfere with how your body absorbs, uses, or breaks down nutrients.
Bisphosphonates
Magnesium-rich supplements or medications can decrease the absorption of oral bisphosphonates, such as alendronate (Fosamax®), used to treat osteoporosis 98. Use of magnesium-rich supplements or medications and oral bisphosphonates should be separated by at least 2 hours 81.
Antibiotics
Magnesium can form insoluble complexes with tetracyclines, such as demeclocycline (Declomycin®) and doxycycline (Vibramycin®), as well as quinolone antibiotics, such as ciprofloxacin (Cipro®) and levofloxacin (Levaquin®) 81. These antibiotics should be taken at least 2 hours before or 4–6 hours after a magnesium-containing supplement 99.
Diuretics
Chronic treatment with loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®), and thiazide diuretics, such as hydrochlorothiazide (Aquazide H®) and ethacrynic acid (Edecrin®), can increase the loss of magnesium in urine and lead to magnesium depletion 100. In contrast, potassium-sparing diuretics, such as amiloride (Midamor®) and spironolactone (Aldactone®), reduce magnesium excretion 100.
Proton pump inhibitors
Prescription proton pump inhibitor (PPI) drugs, such as esomeprazole magnesium (Nexium®) and lansoprazole (Prevacid®), when taken for prolonged periods (typically more than a year) can cause hypomagnesemia 97. In cases that FDA reviewed, magnesium supplements often raised the low serum magnesium levels caused by PPIs. However, in 25% of the cases, supplements did not raise magnesium levels and the patients had to discontinue the PPI. FDA advises healthcare professionals to consider measuring patients’ serum magnesium levels prior to initiating long-term PPI treatment and to check magnesium levels in these patients periodically 97.
Can too much magnesium be harmful?
Too much magnesium from food does not pose a health risk in healthy individuals because your kidneys eliminate the excess magnesium in the urine 28. However, high doses of magnesium from dietary supplements or medications often result in diarrhea that can be accompanied by nausea and abdominal cramping 101, 102. The initial symptom of excess magnesium supplementation is diarrhea — a well-known side effect of magnesium that is used therapeutically as a laxative. The diarrhea and laxative effects of magnesium salts are due to the osmotic activity of unabsorbed salts in the intestine and colon and the stimulation of gastric motility 81. Forms of magnesium most commonly reported to cause diarrhea include magnesium carbonate, chloride, gluconate, and oxide 81.
The most common cause of hypermagnesemia is kidney failure. Other causes include the following 103, 104:
- Excessive intake
- Lithium therapy
- Hypothyroidism (underactive thyroid)
- Addison disease
- Familial hypocalciuric hypercalcemia
- Milk alkali syndrome
- Depression
The risk of magnesium toxicity increases with impaired renal function or kidney failure because the ability to remove excess magnesium is reduced or lost 28, 101. Individuals with impaired kidney function are at higher risk for adverse effects of magnesium supplementation, and symptoms of magnesium toxicity have occurred in people with impaired kidney function taking moderate doses of magnesium-containing laxatives or antacids 105, 5. Very large doses of magnesium-containing laxatives and antacids (typically providing more than 5,000 mg/day magnesium) have been associated with magnesium toxicity 106, including fatal hypermagnesemia in a 28-month-old boy 107 and an elderly man 108. Patients with symptomatic hypermagnesemia can present different clinical signs and symptoms depending on the level and the time in which the electrolytic disturbance has occurred. Patients with serum magnesium concentrations of under 4 mg/dL may be asymptomatic or paucisymptomatic 26. Patients with serum magnesium concentrations of less than 7.0 mg/dL may experience weakness, nausea, vomiting, dizziness, and confusion 26. Patients with serum magnesium concentrations of 7 to 12 mg/dL may have decreased reflexes, worsening confusional state, drowsiness, bladder paralysis, retention of urine, facial flushing, headache, and constipation 26. A slight reduction in blood pressure (hypotension) and blurred vision caused by diminished accommodation and convergence can manifest. For higher magnesium concentrations values over 12.0 mg/dL, you may have paralytic ileus, decreased breathing rate, difficulty breathing, depression and lethargy before progressing to muscle weakness, muscle paralysis, extreme hypotension, electrocardiogram (ECG) changes including an increase in PR and QRS interval with sinus bradycardia, and atrioventricular block, irregular heartbeat, coma and cardiac arrest (exceeding 15.0 mg/dL) may occur 28, 26. When associated with hypocalcemia (low blood calcium), hypermagnesemia may induce choreiform movements and seizures. The clinical picture becomes particularly severe, and there are few case reports of patients who survived to higher hypermagnesaemia levels 109.
In summary hypermagnesemia signs and symptoms depend on the serum magnesium concentrations 26:
- Mild hypermagnesemia (less than 7 mg/dL) – Asymptomatic or paucisymptomatic: weakness, nausea, dizziness, and confusion
- Moderate hypermagnesemia (7 to 12 mg/dL) – Decreased reflexes, worsening of the confusional state and sleepiness, bladder paralysis, flushing, headache, and constipation. A slight reduction in blood pressure, bradycardia, and blurred vision caused by diminished accommodation and convergence are usually present.
- Severe hypermagnesemia (greater than 12 mg/dL) – Muscle flaccid paralysis, decreased breathing rate, more evident hypotension and bradycardia, prolongation of the P-R interval, atrioventricular block, and lethargy are common. Coma and cardiorespiratory arrest can occur for higher values (over 15 mg/dL).
Elevated serum concentrations of magnesium (hypermagnesemia) may result in a fall in blood pressure (hypotension). Some of the later effects of magnesium toxicity, such as lethargy, confusion, disturbances in normal cardiac rhythm, and deterioration of kidney function, are related to severe hypotension. As hypermagnesemia progresses, muscle weakness and difficulty breathing may occur. Severe hypermagnesemia may result in cardiac arrest 56, 101. The Food and Nutrition Board of the US Institute of Medicine set the tolerable upper intake level (UL) for magnesium at 350 mg/day; this tolerable upper intake level (UL) represents the highest level of daily supplemental magnesium intake likely to pose no risk of diarrhea or gastrointestinal disturbance in almost all individuals (see Table 4) 101. The Food and Nutrition Board cautions that individuals with kidney disease or impairment are at higher risk for adverse effects from excess supplemental magnesium intake. However, the Food and Nutrition Board also notes that there are some conditions that may warrant higher doses of magnesium under medical supervision 101.
Table 4. Tolerable Upper Intake Levels (ULs) for Supplemental Magnesium
| Age | Male | Female | Pregnant | Lactating |
|---|---|---|---|---|
| Birth to 12 months | None established | None established | ||
| 1–3 years | 65 mg | 65 mg | ||
| 4–8 years | 110 mg | 110 mg | ||
| 9–18 years | 350 mg | 350 mg | 350 mg | 350 mg |
| 19+ years | 350 mg | 350 mg | 350 mg | 350 mg |
Magnesium toxicity and overdose treatment
Patients with normal kidney function (GFR over 60 ml/min) and mild asymptomatic hypermagnesemia require no treatment except the removal of all sources of exogenous magnesium 26. The half-time of elimination of magnesium is approximately 28 hours 26.
In more severe cases, close monitoring of the ECG, blood pressure, and neuromuscular function and early treatment are necessary 26, 110:
- In patients with symptomatic hypermagnesemia that is causing cardiac effects or respiratory distress, antagonize the effects by infusing calcium gluconate or chloride [Dosage: 1 g in 2 to 5 min (repeatable over 5 minutes)]. Calcium antagonizes the toxic effect of magnesium, and these ions electrically oppose each other at their sites of action. This treatment usually leads to immediate symptomatic improvement. In subjects with frankly impaired ability to excrete magnesium (eg, end-stage renal disease), renal replacement therapy may also be necessary.
- Intravenous normal saline (e.g., at 150 ml/hour)
Severe clinical conditions require increasing renal magnesium excretion through 26, 110:
- Intravenous loop diuretics (e.g., furosemide 1 mg/kg). Furosemide (Lasix) may promote excretion of magnesium. It increases excretion of water by interfering with the chloride-binding cotransport system, which in turn inhibits sodium and chloride reabsorption in the ascending loop of Henle and distal renal tubule.
- OR
- Hemodialysis, when kidney function is impaired, or the patient is symptomatic from severe hypermagnesemia. This approach usually removes magnesium efficiently (up to 50% reduction after a 3- to 4-hour treatment). Dialysis can, however, increase the excretion of calcium by developing hypocalcemia, thus possibly worsening the symptoms and signs of hypermagnesaemia.
The use of diuretics must be associated with infusions of saline solutions to avoid further electrolyte disturbances (e.g., hypokalemia) and metabolic alkalosis. Your healthcare provider must perform serial measurements of calcium and magnesium. In association with electrolytic correction, it is often necessary to support cardiorespiratory activity. As a consequence, the treatment of this electrolyte disorder can frequently require intensive care unit (ICU) admission 111.
Particular clinical conditions require a specific approach. For instance, during the management of eclampsia, the magnesium infusion is stopped if urine output drops to less than 80 mL (in 4 hours), deep tendon reflexes are absent, or the respiratory rate is below 12 breaths/minute. A 10% calcium gluconate or chloride solution (10 mL intravenously repeatable over 5 minutes) can serve as an antidote 26.
Magnesium toxicity and overdose prognosis
The prognosis of hypermagnesemia depends on magnesium values and on the clinical condition that induced hypermagnesemia 26. Values that are not excessively high (mild hypermagnesemia) and in the absence of triggering and aggravating conditions (e.g., renal insufficiency) are benign conditions. On the contrary, high values (severe hypermagnesemia) expose the patient to high risks and high chance of death 26.
What happens if you don’t get enough magnesium?
In the short term, getting too little magnesium does not produce obvious symptoms. When healthy people have low intakes, the kidneys help retain magnesium by limiting the amount lost in urine. However, habitually low magnesium intakes for a long period of time or excessive losses of magnesium due to certain health conditions and chronic alcoholism can lead to magnesium deficiency. In addition, some medical conditions and medications interfere with the body’s ability to absorb magnesium or increase the amount of magnesium that the body excretes, which can also lead to magnesium deficiency.
Early signs of magnesium deficiency include loss of appetite, nausea, vomiting, fatigue, and weakness. As magnesium deficiency worsens, numbness, tingling, muscle contractions and cramps, seizures, personality changes, abnormal heart rhythms, and coronary spasms can occur. Severe magnesium deficiency can result in hypocalcemia or hypokalemia (low serum calcium or potassium levels, respectively) because mineral homeostasis is disrupted 7.
Groups at Risk of Magnesium Inadequacy
The following groups of people are more likely than others to get too little magnesium:
- People with gastrointestinal diseases (such as Crohn’s disease and celiac disease)
- People with type 2 diabetes
- People with long-term alcoholism
- Older people
Magnesium inadequacy can occur when intakes fall below the RDA but are above the amount required to prevent overt deficiency. The following groups are more likely than others to be at risk of magnesium inadequacy because they typically consume insufficient amounts or they have medical conditions (or take medications) that reduce magnesium absorption from the gut or increase losses from the body.
People with gastrointestinal diseases
The chronic diarrhea and fat malabsorption resulting from Crohn’s disease, gluten-sensitive enteropathy (celiac disease), and regional enteritis can lead to magnesium depletion over time 7. Resection or bypass of the small intestine, especially the ileum, typically leads to malabsorption and magnesium loss 7.
People with type 2 diabetes
Magnesium deficits and increased urinary magnesium excretion can occur in people with insulin resistance and/or type 2 diabetes 112. The magnesium loss appears to be secondary to higher concentrations of glucose in the kidney that increase urine output 7.
People with alcohol dependence
Magnesium deficiency is common in people with chronic alcoholism 7. In these individuals, poor dietary intake and nutritional status; gastrointestinal problems, including vomiting, diarrhea, and steatorrhea (fatty stools) resulting from pancreatitis; renal dysfunction with excess excretion of magnesium into the urine; phosphate depletion; vitamin D deficiency; acute alcoholic ketoacidosis; and hyperaldosteronism secondary to liver disease can all contribute to decreased magnesium status 7.
Older adults
Older adults have lower dietary intakes of magnesium than younger adults 113. In addition, magnesium absorption from the gut decreases and renal magnesium excretion increases with age 114. Older adults are also more likely to have chronic diseases or take medications that alter magnesium status, which can increase their risk of magnesium depletion 115.
Magnesium and health
Scientists are studying magnesium to understand how it affects health. Here are some examples of what this research has shown.
High blood pressure
High blood pressure is a major risk factor for heart disease and stroke. Magnesium supplements might decrease blood pressure, but only by a small amount. Some studies show that people who have more magnesium in their diets have a lower risk of some types of heart disease and stroke.
While results from intervention studies have not been entirely consistent 54, the latest review of the data highlighted a therapeutic benefit of magnesium supplements in treating hypertension. A 2006 meta-analysis of 12 clinical trials found that magnesium supplementation for 8–26 weeks in 545 hypertensive participants resulted in only a small reduction (2.2 mmHg) in diastolic blood pressure 116. The dose of magnesium ranged from approximately 243 to 973 mg/day. A 2012 meta-analysis of 22 randomized, placebo-controlled trials of magnesium supplementation in 1,173 adults with either normal blood pressure (normotensive) or hypertension (treated with medication or untreated) concluded that oral supplementation with magnesium (mean dose of 410 mg/day; range of 120 to 973 mg/day) for a median period of 11.3 months significantly reduced systolic blood pressure by 2 to 3 mm Hg and diastolic blood pressure by 3 to 4 mm Hg; the effects were somewhat larger when supplemental magnesium intakes of the participants in the nine crossover-design trials exceeded 370 mg/day 117. A diet containing more magnesium because of added fruits and vegetables, more low-fat or non-fat dairy products, and less fat overall was shown to lower systolic and diastolic blood pressure by an average of 5.5 and 3.0 mmHg, respectively 118. While oral magnesium supplementation may be helpful in hypertensive individuals who are depleted of magnesium due to chronic diuretic use and/or inadequate dietary intake 55, this Dietary Approaches to Stop Hypertension (DASH) diet — a diet rich in fruit, vegetables, and low-fat dairy and low in saturated and total fats — has been linked to significant reductions in systolic and diastolic blood pressures 119, so any independent contribution of magnesium cannot be determined.
Magnesium doses required to achieve a decrease in blood pressure appeared to depend on whether participants with high blood pressure were treated with antihypertensive medications, including diuretics. Intervention trials on treated participants showed a reduction in hypertension with magnesium doses from 243 mg/day to 486 mg/day, whereas untreated patients required doses above 486 mg/day to achieve a significant decrease in blood pressure. A 2016 meta-analysis of randomized controlled studies with 2,028 participants found that supplemental magnesium at a median dose of 368 mg/day (range: 238-960 mg/day) for a median duration of three months (range: 3 weeks-6 months) increased serum magnesium concentration by 0.05 mmol/L (27 trials) and reduced systolic blood pressure by 2 mm Hg and diastolic blood pressure by 1.78 mm Hg (37 trials) 120. A 2017 meta-analysis restricted to trials in participants with underlying preclinical (insulin resistance or prediabetes) or clinical conditions (type 2 diabetes mellitus or coronary heart disease) found a 4.18 mm Hg reduction in systolic blood pressure and a 2.27 mm Hg reduction in diastolic blood pressure with supplemental doses of magnesium ranging between 365 mg/day and 450 mg/day for one to six months 121.
In 2022, U.S. Food and Drug Administration (FDA) approved a qualified health claim for conventional foods and dietary supplements that contain magnesium 122. One example of this claim states, “Consuming diets with adequate magnesium may reduce the risk of high blood pressure (hypertension). However, FDA has concluded that the evidence is inconsistent and inconclusive.” FDA also specifies that foods and dietary supplements carrying this claim on their labels must provide at least 84 mg of magnesium per serving and, for dietary supplements, no more than 350 mg 122.
Heart disease
Several prospective studies have examined associations between magnesium intakes and heart disease. The Atherosclerosis Risk in Communities study assessed heart disease risk factors and levels of serum magnesium in a cohort of 14,232 white and African-American men and women aged 45 to 64 years at baseline 123. Over an average of 12 years of follow-up, individuals in the highest quartile of the normal physiologic range of serum magnesium (at least 0.88 mmol/L) had a 38% reduced risk of sudden cardiac death compared with individuals in the lowest quartile (0.75 mmol/L or less). However, dietary magnesium intakes had no association with risk of sudden cardiac death. Another prospective study tracked 88,375 female nurses in the United States to determine whether serum magnesium levels measured early in the study and magnesium intakes from food and supplements assessed every 2 to 4 years were associated with sudden cardiac death over 26 years of follow-up 124. Women in the highest compared with the lowest quartile of ingested and plasma magnesium concentrations had a 34% and 77% lower risk of sudden cardiac death, respectively. Another prospective population study of 7,664 adults aged 20 to 75 years in the Netherlands who did not have cardiovascular disease found that low urinary magnesium excretion levels (a marker for low dietary magnesium intake) were associated with a higher risk of ischemic heart disease over a median follow-up period of 10.5 years. Plasma magnesium concentrations were not associated with risk of ischemic heart disease 125. A systematic review and meta-analysis of prospective studies found that higher serum levels of magnesium were significantly associated with a lower risk of cardiovascular disease, and higher dietary magnesium intakes (up to approximately 250 mg/day) were associated with a significantly lower risk of ischemic heart disease caused by a reduced blood supply to the heart muscle 126.
Higher magnesium intakes might reduce the risk of stroke. In a meta-analysis of 7 prospective trials with a total of 241,378 participants, an additional 100 mg/day magnesium in the diet was associated with an 8% decreased risk of total stroke, especially ischemic rather than hemorrhagic stroke 127. One limitation of such observational studies, however, is the possibility of confounding with other nutrients or dietary components that could also affect the risk of stroke. But in many of these studies, it’s hard to know how much of the effect was due to magnesium as opposed to other nutrients.
A large, well-designed clinical trial is needed to better understand the contributions of magnesium from food and dietary supplements to heart health and the primary prevention of cardiovascular disease.
Cardiovascular disease
Several large prospective cohort studies, including the Health Professionals Follow-up Study (HPFS) and the Nurses’ Health Study (NHS), have examined magnesium intakes in relation to cardiovascular outcomes. In the most recent analysis of the Nurses’ Health Study, which followed nearly 90,000 female nurses for 28 years, those in the highest quintile of magnesium intake had a 39% lower risk of fatal myocardial infarction (heart attack) but not nonfatal coronary heart disease (coronary artery disease) compared to those in the lowest quintile (>342 mg/day versus <246 mg/day) 128. A meta-analysis of nine prospective cohort studies, mostly conducted in participants without cardiovascular disease at baseline, reported a 22% lower risk of coronary heart disease per 200 mg/day incremental intake in dietary magnesium 129. A more recent meta-analysis by Fang et al. 130 included six studies and reported a 10% lower risk of coronary artery disease with higher versus lower dietary magnesium intakes.
Higher magnesium intakes were associated with an 8 to 11% reduction in stroke risk in two meta-analyses of prospective studies, each including over 240,000 participants 131, 132. The most recent pooled analysis of 14 studies found a 12% lower risk of stroke with higher versus lower magnesium intakes and estimated a 7% risk reduction of stroke associated with each 100-mg increment in daily magnesium intake 130.
Only two prospective studies have examined the risk of heart failure in relation to magnesium intakes. The pooled analysis suggested a 31% lower risk of heart failure with higher dietary magnesium intakes 130.
Finally, a meta-analysis of 13 prospective studies in over 475,000 participants reported that the risk of total cardiovascular events, including stroke, nonfatal myocardial infarction, and coronary heart disease, was 15% lower in individuals with higher intakes of magnesium 133. However, in the recent meta-analysis of prospective studies by Fang et al. 130 and Xu et al. 134, found no associations between dietary magnesium intake and risk of total cardiovascular disease and all-cause mortality.
In a prospective analysis of NHANES data from 14,353 participants, followed for a median period of 28.6 years, the risk of all-cause and stroke mortality was significantly increased in those with low rather than normal serum concentrations of magnesium (<0.7 mmol/L versus 0.8-0.89 mmol/L) 135. In contrast, hypermagnesemia (serum magnesium concentration >0.89 mmol/L) — rather than hypomagnesemia — in people with heart failure was associated with an increased risk of cardiovascular and all-cause mortality 136.
One large prospective study (almost 14,000 men and women) associated higher serum magnesium concentrations with a lower risk of coronary artery disease in women but not in men 137. This study was included in a meta-analysis of four studies that showed no evidence of a reduced risk of coronary artery disease with increasing serum magnesium concentrations 129. In contrast, a 0.2 mmol/L increment in serum magnesium concentration was associated with a 30% lower risk of total cardiovascular disease in a pooled analysis of eight prospective cohort studies 129. In the recently published British Regional Heart Study that followed 3,523 men for a mean 15 years, there was no association between serum magnesium concentration and incidental coronary artery disease events, yet serum magnesium concentration was inversely associated with the risk of heart failure 138.
It is important to note that while these prospective cohort studies assessed the association between dietary magnesium and cardiovascular disease, they did not account for the use of supplemental magnesium by a significant fraction of participants.
Aneurysmal subarachnoid hemorrhage
Occurrence of hypomagnesemia has been reported in patients who suffered from a subarachnoid hemorrhage (a type of stroke) caused by the rupture of a cerebral aneurysm 139. Poor neurologic outcomes following an aneurysmal subarachnoid hemorrhage have been linked to inappropriate calcium-dependent contraction of arteries known as cerebral arterial vasospasm, leading to delayed cerebral ischemia 140. Because magnesium is a calcium antagonist and potent vasodilator, several randomized controlled trials have examined whether intravenous magnesium sulfate infusions could reduce the incidence of vasospasm after an aneurysmal subarachnoid hemorrhage. A meta-analysis of nine randomized controlled trials found that magnesium therapy after an aneurysmal subarachnoid hemorrhage significantly reduced vasospasm but failed to prevent neurologic deterioration or decrease the risk of death 141. Another meta-analysis of 13 trials in 2,413 an aneurysmal subarachnoid hemorrhage sufferers concluded that the infusion of magnesium sulfate had no benefit regarding neurologic outcome and mortality, despite a reduction in the incidence of delayed cerebral ischemia 142. The post-hoc analysis of a small randomized controlled trial suggested that maintaining magnesium sulfate infusion for 10 days post-an aneurysmal subarachnoid hemorrhage or until signs of vasospasm disappear might protect against secondary cerebral infarction when markers of vasoconstriction and reduced brain perfusion are present 143, 144. Current evidence does not support the use of magnesium supplementation in clinical practice for an aneurysmal subarachnoid hemorrhage patients beyond magnesium status normalization.
Complications of heart surgery
Atrial fibrillation also called atrial arrhythmia is a condition defined as the occurrence of persistent heart rate abnormalities; such arrhythmias often complicate the recovery of patients after cardiac surgery. The use of magnesium in the prophylaxis of postoperative atrial arrhythmia after coronary artery bypass grafting has been evaluated as a sole or adjunctive agent to classical antiarrhythmic molecules (namely, β-blockers and amiodarone) in several prospective, randomized controlled trials. A meta-analysis of 21 intervention studies showed that intravenous magnesium infusions could significantly reduce postoperative atrial fibrillation in treated compared to untreated patients 145. The results of a more recent meta-analysis of 22 placebo-controlled trials suggested that magnesium may effectively reduce atrial fibrillation when administered post-operatively, as a bolus, and for more than 24 hours 146. However, another meta-analysis of four trials found that magnesium was no more effective than other antiarrhythmic agents 146. Moreover, the meta-analysis of five randomized controlled trials also suggested that intravenous magnesium added to β-blocker treatment did not decrease the risk of atrial arrhythmia compared to β-blocker alone and was associated with more adverse effects (bradycardia and hypotension) 147. Presently, high-quality evidence is still lacking to support the use of magnesium in the prophylaxis of post-operative atrial fibrillation and other arrhythmias in patients with contraindications to first-line antiarrhythmic agents 146.
Hemostasis
Magnesium is known to have antiplatelet and antithrombotic effects and is involved in hemostasis (a process that prevent and stop bleeding from a blood vessel) as a co-factor for factor IX and membrane-bound coagulation proteins and as a regulator of the eicosanoid synthesis pathway, which produces inflammatory mediators including prostaglandins and thromboxane 148, 25. Factor IX is part of the intrinsic pathway of the coagulation cascade, it activates factor X and is activated by activated factor VIII. The activation of factor IX is calcium dependent 149. Mutation of the factor IX gene is a hallmark of Hemophilia B, a blood clotting disorder which is life threatening and shortens life expectancy 150. Magnesium has been shown to stabilize the native conformation of factor IX, and consequently to increase its activity 151. Moreover, magnesium appears to be important for the early key stages of coagulation by enhancing the activity of the tissue factor-factor VIIa complex, which activates factor X 152.
Furthermore, during the initial stages of the coagulation process, when endothelial cell membranes are exposed to the blood stream, blood coagulation proteins reversibly interact with these membranes to trigger the coagulation cascade. Seven coagulation enzymes are bound to the cell surface through their gamma-carboxyglutamate-rich (GLA) domains. GLA domain folding is dependent on both calcium and magnesium. The binding of these metal ions leads to the exposure of hydrophobic residues that ultimately help integration into the membrane bilayer. Under physiological conditions, the metal ions binding sites of GLA domains are occupied concurrently by magnesium and calcium, with two to three of the nine binding sites occupied by magnesium 153, 154.
Finally, magnesium has been shown to inhibit the eicosanoid synthesis pathway in platelets. This pathway produces thromboxane which, once released, amplifies platelet aggregation. Magnesium sulfate is thought to modify platelet membrane fluidity, which in turn interferes with fibrinogen binding to the GPIIb/Iia complex and inhibits phosphoinositide breakdown and the formation of thromboxane 155. Moreover, more recent research has shown that a similar inhibition occurs in macrophages using another magnesium salt, magnesium isoglycyrrhizinate. They showed that magnesium inhibits key enzymes involved in eicosanoid synthesis, which suggests that magnesium might have a direct inhibitory role on this pathway as well as through action on membrane fluidity 156.
Metabolic syndrome
Metabolic syndrome refers to the concomitant presentation of several metabolic disorders in an individual, including dyslipidemia, hypertension, insulin resistance, and obesity 157. People with metabolic syndrome are at greater risk of developing type 2 diabetes mellitus, cardiovascular disease, and some types of cancer 158, 159, 160. A 2015 analysis of data from the US National Health and Nutrition Examination Survey (NHANES 2001-2010) in 9,148 adults (mean age, 50 years) found a 32% lower risk of metabolic syndrome in those in the highest versus lowest quantile of magnesium intake (≥355 mg/day versus <197 mg/day) 161. Several meta-analyses of primarily cross-sectional studies have also reported an inverse association between dietary magnesium intake and risk of metabolic syndrome 162, 163, 164. Moreover, lower serum magnesium concentrations have been reported in individuals with metabolic syndrome compared to controls 164, 165. However, circulating magnesium represents only 1% of total body stores and is tightly regulated; therefore, serum magnesium concentrations do not best reflect magnesium status 166. At present, additional evidence is needed from prospectively designed studies to clarify the potential relationship between dietary and circulating magnesium and the risk of metabolic syndrome.
Systemic inflammation, which contributes to the development of metabolic disorders, has been inversely correlated with magnesium intakes in a cross-sectional study of 11,686 women (≥45 years). In this study, the lowest prevalence of metabolic syndrome was found in the group of women in the highest quintile of magnesium intakes (median intake, 422 mg/day) 167. Several randomized controlled trials also reported a reduction in circulating C-reactive protein (CRP) — a marker of inflammation — following oral magnesium supplementation 168. This might constitute a potential mechanism through which magnesium could play a role in the prevention of metabolic disorders.
Anti-inflammatory
Experimental studies performed in animals and in test tubes have demonstrated that magnesium modulates the inflammatory response. The decrease in extracellular magnesium activated the transcription of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) in the endothelium cells 169. Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) has a role in triggering the global immune and inflammatory responses and controlling the gene expressions of cytokines, chemokines, growth factors, and adhesion molecules 170. In conjugation with the inhibitory protein IkB, NFkB remains inactive in the cytoplasm. Through exposure to bacteria, viruses, cytokines, or oxidative stress, NFkB activation is initiated. In this stage, through the proteolysis of IkB, a nuclear recognition site is revealed, and NFkB translocates into the nucleus. It attaches to DNA and, thus, determines mRNA expression 171.
The consequence of NFkB activation is endothelial dysfunction, which triggers a proinflammatory and proatherogenic phenotype 170. It is certain that oxidative stress and chronic inflammation are inseparable phenomena. Nitric oxide produced by endothelial cells stabilizes IkB by inhibiting the expression of the adhesion molecule, clearly reducing the inflammatory response. Magnesium supplementation significantly attenuates the translocation of NFkB from the cytoplasm to the nucleus, inhibits the degradation of IkB in endothelial cells, and implicitly reduces the inflammatory response 172.
The central role in mediating the inflammatory response induced by magnesium deficiency is assigned to IL-1α, which produces chemokines and adhesion molecules by activating NFkB. These principally increase IL-8 and RANTES (regulated upon activation, normal T-cell expressed and secreted), which are chemokines that are overexpressed in patients with atherosclerotic lesions. Moreover, the inhibition of IL-1α prevents low-magnesium-induced adhesion of monocytoid cells to the endothelium 169.
Another important factor involved in inflammation seems to be substance P (SP), a peptide of the tachykinin family found in both the central and peripheral nervous systems 173. Magnesium deficiency is associated with neurogenic inflammation mediated by the release of substance P, a physiopathological event preceded by significant increases in inflammation parameters (circulating IL-1, IL-6, tumor necrosis factor TNFα, histamine, PGE2, white blood cells, and cardiac tissue inflammation) and in oxidative stress factors. By restricting magnesium, the inhibition of neutral endopeptidase (NEP), a specific SP-degrading enzyme, maintains a high level of neurogenic inflammation, leading to increased intestinal and cardiac dysfunction 174.
The mechanism by which magnesium deficiency produces inflammatory stress is closely related to the role of magnesium as an antagonist of calcium. Hypomagnesemia causes an increase in intracellular calcium by activating L-type calcium channels or by releasing it from intracellular stores, such as the sarcoplasmic reticulum. The release of TNFα is induced by the increase in intracellular calcium and, thus, the inflammatory response is initiated, resulting in the production of cytokines 175.
Decreases in extracellular magnesium concentration in experimental animals induced an inflammatory response that determined the stimulation of phagocytic cells, with increases in polymorphonuclear leukocytes, mainly neutrophils and eosinophils, and macrophages also noted. Moreover, an increase in proinflammatory cytokines, especially IL-6 and TNFα, was observed 176. The production of these cytokines was significantly reduced by the increase in intracellular magnesium following a magnesium treatment in vivo 177. The acute phase proteins of alpha2-macroglobulin and alpha1-acid glycoprotein, increased in parallel with the mRNA level that encoded them 178. Magnesium is involved in the prevention of cardiovascular disease, diabetes, and metabolic syndrome by reducing systemic inflammation and improving endothelial dysfunction. In chronic diseases associated with magnesium deficiency, the most commonly used inflammatory marker is C-reactive protein (CRP). In the majority of cases, magnesium deficiency is associated with a low degree of inflammation or pathological conditions for which inflammatory stress is considered a risk factor. At normal serum magnesium concentrations, there is no significant improvement in inflammatory markers, probably due to other nutritional and metabolic factors affecting inflammatory and oxidative stress 179, 175.
Magnesium deficiency has been shown to be accompanied by high C-reactive protein (CRP) in individuals whose magnesium dietary intakes are below the Recommended Dietary Allowance (average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals). Magnesium supplementation has significantly improved serum CRP levels 180, 181. Similar studies showed that the serum CRP level was elevated 1.94 times in children consuming less than 75% of their magnesium Recommended Dietary Allowance (average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals) 180. The results from various meta-analyses, systematic reviews, and studies have shown that dietary magnesium intake is significantly and inversely associated with serum CRP levels 175, 177, 182, 183, 184, 185. An inverse association between magnesium intake and metabolic syndrome has also been reported 186, 187. The favorable impact of magnesium on systemic inflammation is also reflected in patients with diabetes. Regarding the relationship between magnesium intake and serum inflammatory marker levels and HOMA-IR, it seems that magnesium intake is significantly inversely related to high-sensitivity C-reactive protein (hs-CRP), IL-6, fibrinogen, and HOMA-IR, and serum magnesium level is inversely related to hs-CRP and HOMA-IR 188. Another meta-analysis concluded that there is an inverse relationship between dietary magnesium intake and serum magnesium concentrations with the risk of total cardiovascular events 189. It is abundantly clear that magnesium deficiency maintains both hyperinflammation in acute inflammatory processes and low-grade inflammation in chronic diseases 190, 191.
Immune system
Magnesium is important in acquired immunity via regulating lymphocyte growth 192. An in vitro study (test tube study) carried out in chicken B cell line DT40 revealed that the removal of magnesium channel, TRPM7, results in cell death and can be partially corrected by magnesium supplementation 193. Mutation in a magnesium transporter, MagT1, is reported in patients with X-linked immunodeficiency diseases, Epstein–Barr virus infection, and neoplasia 194. Low CD4+ T cells and defective activation of T-lymphocytes are due to the decreased magnesium influx, which fails to activate PLCγ1 195. The importance of magnesium for CD4+ activation is also evident from reported studies conducted in asthma patients 196. However, further studies are essential to conclude the effect of magnesium on T cell signaling.
Magnesium has an important role in synthesizing and releasing immune cells and other associated processes like cell adhesion and phagocytosis 197. Magnesium acts as a natural calcium antagonist, the molecular basis for inflammatory response could be the result of modulation of intracellular calcium concentration 198. Besides, magnesium acts as a cofactor for the synthesis of immunoglobulin, CI 3 convertase, antibody-dependent cytolysis, macrophage responses to lymphocyte, IgM lymphocyte binding, T helper B cell adherence, substance P binding with lymphoblast, and binding of antigen to macrophage 199, 200. Magnesium deficiency affects various immune functions like the decline in NK cell level, monocytes and T cell ratio, increased oxidative stress after strenuous exercise, and elevated cytokine IL-6 level and inflammatory events. Deficiency of magnesium may be prone to recurrent bacterial and fungal infection 201. Many studies have demonstrated that in humans, a moderate or subclinical magnesium deficiency can induce chronic low-grade inflammation or exacerbate inflammatory stress caused by other factors 202. This low-grade inflammation increases the secretion of pro-inflammatory cytokines, which stimulate the resorption of bone by the induction of the differentiation of osteoclasts from their precursors 203. The ability of magnesium to decrease the inflammatory response and oxidative stress, as well as improving lung inflammation, possibly by inhibiting IL-6 pathway, NF-κB pathway, and L-type calcium channels 204, has raised the hypothesis of a possible magnesium supplementation in the prevention and treatment of COVID-19 patients, as suggested in the recent papers by Tang et al 205 and Iotti 206. Based Tang et al 207 basic and clinical research study, it is evident that magnesium effectively treats respiratory diseases like asthma and pneumonia because of its anti-inflammatory, antioxidant, and smooth muscle relaxant properties. A substantial decrease in the need for oxygen or intensive treatment assistance is reported in elderly COVID-19 patients upon the intake of vitamin D, magnesium, and vitamin B12 in combination 208. Iran’s clinical trial registry 209 confirmed that magnesium sulphate inhalation is effective in improving respiratory symptoms such as shortness of breath, cough and oxygen saturation in COVID-19 patients.
Magnesium deficient animal model exhibits inflammation as the first noticeable change with elevated levels of pro-inflammatory mediators like TNFα with declined anti-inflammatory cytokine levels 210, 200. The activation of immune cells like monocyte, macrophages, and polymorphonuclear cells are involved in the release of inflammatory mediators like cytokine, free radical and eicosanoids 200. Administration of magnesium reduces leukocyte activation and oxidative damage to peripheral blood lymphocyte DNA in athletes and sedentary young men 211. Thus, magnesium is an important factor for optimum immune cell functioning by regulating the proliferation and function of lymphocytes 197. In vitro studies also prove the role of magnesium in reducing leukocyte activation through its calcium antagonistic action 199. Magnesium deficiency results in the stress condition that activate the sympathetic system and hypothalamic-pituitary axis causes fat accumulation and release of neuropeptides; results in the immune response followed by inflammatory cascades 212.
Magnesium can inhibit cytokine storm and hyper oxidative stress in COVID-19 patients through various mechanisms such as its antioxidant, immune-modulatory, and anti-inflammatory activities 213.
Type 2 diabetes
People with higher amounts of magnesium in their diets tend to have a lower risk of developing type 2 diabetes. Magnesium helps the body break down sugars and might help reduce the risk of insulin resistance (a condition that leads to diabetes) 214, 215. Scientists are studying whether magnesium supplements might help people who already have type 2 diabetes control their disease. Low magnesium (hypomagnesaemia) might worsen insulin resistance, a condition that often precedes diabetes, or it might be a consequence of insulin resistance 216. Diabetes leads to increased urinary losses of magnesium, and the subsequent magnesium inadequacy might impair insulin secretion and action, thereby worsening diabetes control 8.
It has been suggested that low magnesium (hypomagnesaemia) is caused by diabetes rather than contributing to type 2 diabetes onset, which is based on the findings of a cohort study reporting hypomagnesaemia (<0.7 mmol/L) being more common in patients with type 2 diabetes but not pre-diabetes 217. However, other cohort studies challenge this. The 2015 dose–response meta-analysis of prospective cohort studies published by Fang and colleagues 218 found an inverse correlation between magnesium intake and type 2 diabetes. The number of pooled participants totalled about 26,300 cases of type 2 diabetes with follow-ups ranging from 4 to 30 years, and the dietary magnesium intake was self-reported using a validated food frequency questionnaire 218. Moreover, in a 2017 meta analysis, which included 11 studies, Wu and colleagues 219 found an inverse correlation between circulating magnesium concentration and type 2 diabetes as well as chronic heart disease and hypertension. Finally, other cohort studies in both Western and non-Western populations have reported associations between magnesium and type 2 diabetes development 220, 221.
A prospective cohort study that followed more than 25,000 individuals, 35 to 65 years of age, for seven years found no difference in incidence of type 2 diabetes mellitus when comparing the highest (377 mg/day) quintile of magnesium intake to the lowest quintile (268 mg/day) 222. However, inclusion of this study in a meta-analysis of eight cohort studies showed that the risk of type 2 diabetes was inversely correlated with magnesium intake 222. A meta-analysis of 7 of these studies, which included 286,668 patients and 10,912 cases of diabetes over 6 to 17 years of follow-up, found that a 100 mg/day increase in total magnesium intake decreased the risk of diabetes by a statistically significant 15% 214. Another meta-analysis of 8 prospective cohort studies that followed 271,869 men and women over 4 to 18 years found a significant inverse association between magnesium intake from food and risk of type 2 diabetes; the relative risk reduction was 23% when the highest to lowest intakes were compared 222.
The most recent meta-analysis of 25 prospective cohort studies, including 637,922 individuals and 26,828 new cases of type 2 diabetes mellitus, found that higher magnesium intakes were associated with a 17% lower risk of type 2 diabetes mellitus 223. Several meta-analyses conducted to date reported an 8 to 15% decrease in the risk of developing type 2 diabetes mellitus with each 100 mg-increment in dietary magnesium intake 223, 224, 225, 214.
A 2011 meta-analysis of prospective cohort studies of the association between magnesium intake and risk of type 2 diabetes included 13 studies with a total of 536,318 participants and 24,516 cases of diabetes 224. The mean length of follow-up ranged from 4 to 20 years. Investigators found an inverse association between magnesium intake and risk of type 2 diabetes in a dose-responsive fashion, but this association achieved statistical significance only in overweight (body mass index [BMI] 25 or higher) but not normal-weight individuals (BMI less than 25) 224. Again, a limitation of these observational studies is the possibility of confounding with other dietary components or lifestyle or environmental variables that are correlated with magnesium intake.
Insulin resistance, characterized by alterations in both insulin secretion by the pancreas and insulin action on target tissues, has been linked to inadequate magnesium status. A cross-sectional analysis of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium, which included 15 cohorts with a total of 52,684 diabetes-free participants, showed that magnesium intakes were inversely associated with fasting insulin concentrations after multiple adjustments, including various lifestyle factors, body mass index (BMI), caffeine intake, and fiber intake 225. It is thought that pancreatic beta-cells, which secrete insulin, could become less responsive to changes in insulin sensitivity in magnesium-deficient subjects 226. A randomized, double-blind, placebo-controlled trial that enrolled 97 healthy adults with significant hypomagnesemia (serum magnesium concentration ≤0.70 mmol/L) showed that daily consumption of 638 mg of magnesium (from a solution of magnesium chloride) for three months improved the function of pancreatic beta-cells, resulting in lower fasting glucose and insulin concentrations 227. In a follow-up randomized controlled trial, the administration of 382 mg/day of magnesium for four months to participants (mean age, 42 years) with both hypomagnesemia (serum magnesium concentration <0.74 mmoles/L) and impaired fasting glucose improved serum magnesium concentrations, as well as fasting and post-load glucose concentrations 228. Other metabolic markers, including serum triglycerides, HDL-cholesterol, and a measure of insulin resistance, also improved in magnesium- versus placebo-treated individuals 228. Additionally, similar metabolic improvements have been reported following the supplementation of magnesium (382 mg/day for four months) to participants who were both hypomagnesemic and lean yet metabolically obese (i.e., with metabolic disorders usually associated with obesity) 229. In another study, supplementation with 365 mg/day of magnesium (from magnesium aspartate hydrochloride) for six months reduced insulin resistance in 27 overweight individuals with normal values of serum and intracellular magnesium 230. This latter study suggests that magnesium might have additional effects on glucose tolerance and insulin sensitivity that go beyond the normalization of serum magnesium concentrations in hypomagnesemic individuals.
Only a few small, short-term clinical trials have examined the potential effects of supplemental magnesium on control of type 2 diabetes and the results are conflicting 231, 215, 232, 233. For example, 128 patients with poorly controlled diabetes in a Brazilian clinical trial received a placebo or a supplement containing either 500 mg/day or 1,000 mg/day magnesium oxide (providing 300 or 600 mg elemental magnesium, respectively) 233. After 30 days of supplementation, plasma, cellular, and urine magnesium levels increased in participants receiving the larger dose of the supplement, and their glycemic control improved 233. In another small trial in Mexico, participants with type 2 diabetes and hypomagnesemia who received a liquid supplement of magnesium chloride (providing 300 mg/day elemental magnesium) for 16 weeks showed significant reductions in fasting glucose and glycosylated hemoglobin concentrations compared with participants receiving a placebo, and their serum magnesium levels became normal 234. In contrast, neither a supplement of magnesium aspartate (providing 369 mg/day elemental magnesium) nor a placebo taken for 3 months had any effect on glycemic control in 50 patients with type 2 diabetes who were taking insulin 235. A recent cost–benefit analysis study showed that 22.3% fewer men with pre-diabetes taking a magnesium supplement develop type 2 diabetes compared to placebo and supported such supplementation as a cost-effective preventative measure 231. In addition, in a study of obese patients, serum magnesium levels increased by 13.2% and HbA1c decreased nine months post-weight loss surgery. This is likely to be due to a combination of weight loss, lifestyle changes and recommendation to take over the counter multi-vitamin tablets for four weeks 236.
More research is needed to better understand whether magnesium can help treat diabetes. The American Diabetes Association states that there is insufficient evidence to support the routine use of magnesium to improve glycemic control in people with diabetes 232. It further notes that there is no clear scientific evidence that vitamin and mineral supplementation benefits people with diabetes who do not have underlying nutritional deficiencies.
Osteoporosis
Magnesium is important for healthy bones. Magnesium is involved in bone formation and influences the activities of osteoblasts and osteoclasts 237. Around 60% of total body magnesium is stored in the bone and is known to influence both bone matrix and bone mineral metabolism. Magnesium at the surface of bones is also available for dynamic exchange with blood 238. As the magnesium content of bone mineral decreases, hydroxyapatite crystals of bone may become larger and more brittle. Some studies have found lower magnesium content and larger hydroxyapatite crystals in bones of women with osteoporosis compared to disease-free women 239. Magnesium also affects the concentrations of both parathyroid hormone (PTH) and the active form of vitamin D (calcitriol or 1,25-dihydroxyvitamin D), which are major regulators of bone homeostasis. Inadequate serum magnesium concentrations are known to result in low serum calcium concentrations, resistance to parathyroid hormone (PTH) action, and resistance to some of the effects of vitamin D (calcitriol), all of which can lead to increased bone loss 5. Lower serum magnesium concentrations may not be unusual in postmenopausal women with osteoporosis 240, and hypomagnesemia has been reported as an adverse effect of using the prescription drug teriparatide (Forteo) in the treatment of osteoporosis 241.
Several population-based studies have found positive associations between magnesium intake and bone mineral density in both men and women 242. Other research has found that women with osteoporosis have lower serum magnesium levels than women with osteopenia and those who do not have osteoporosis or osteopenia 243. These and other findings indicate that magnesium deficiency might be a risk factor for osteoporosis 237.
Higher dietary magnesium intakes have been associated with increased site-specific 244 and total-body bone mineral density (BMD) 245 in observational studies, including studies of older adults. More recently, a large cohort study conducted in almost two-thirds of the Norwegian population found the level of magnesium in drinking water to be inversely associated with the risk of hip fracture 246. In the Women’s Health Initiative study, data analysis from 4,778 participants (mean age, 63 years) followed for about seven years showed that higher magnesium intakes were associated with higher hip and whole-body bone mineral density (BMD) but not with reduced hip or total fractures 247. Moreover, the highest versus lowest quintile of total magnesium intakes was associated with a 23% increased risk of lower arm and wrist fractures 247. In a case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk study, which included 5,319 individuals, total magnesium and potassium intakes were found to be inversely associated with heel bone (calcaneus) broadband ultrasound attenuation measurements — which are predictive of the risk of incidental fracture — and with the risk of hip fractures 248.
Few studies have addressed the effect of magnesium supplementation on bone mineral density (BMD) or osteoporosis in humans. In a small group of postmenopausal women with osteoporosis, magnesium supplementation of 750 mg/day for six months followed by 250 mg/day for 18 more months resulted in increased bone mineral density (BMD) at the wrist after one year, with no further increase after two years of supplementation 249. A study in postmenopausal women who were taking estrogen replacement therapy and a multivitamin supplement found that supplementation with an additional 400 mg/day of magnesium and 600 mg/day of calcium resulted in increased bone mineral density (BMD) at the heel compared to postmenopausal women receiving estrogen replacement therapy only 250. A more recent randomized controlled study conducted in 20 postmenopausal women with osteoporosis suggested that high-dose supplementation with magnesium citrate (1,830 mg/day) for one month could reduce the rapid rate of bone loss that characterizes osteoporosis 251. Evidence is not yet sufficient to suggest that supplemental magnesium in excess of the Recommended Dietary Allowance (average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals) could be effective in the prevention of osteoporosis unless normalization of serum magnesium concentration is required 252.
Although limited in number, studies suggest that increasing magnesium intakes from food or supplements might increase bone mineral density in postmenopausal and elderly women 80. For example, one short-term study found that 290 mg/day elemental magnesium (as magnesium citrate) for 30 days in 20 postmenopausal women with osteoporosis suppressed bone turnover compared with placebo, suggesting that bone loss decreased 251.
Diets that provide recommended levels of magnesium enhance bone health, but further research is needed to elucidate the role of magnesium in the prevention and management of osteoporosis.
Magnesium-dependent vitamin D-resistant Rickets
Magnesium acts as a cofactor for binding of vitamin D to its transport protein (vitamin D-binding protein), and it is required for conversion of vitamin D into the active form in the liver and the kidneys 13. Calcitriol or 1,25-Dihydroxyvitamin D has been shown to stimulate the absorption of magnesium from the intestines. Magnesium deficiency can cause magnesium-dependent vitamin D-resistant rickets by reducing the synthesis of vitamin D and impairing PTH function 13. Adequate replacement of magnesium is essential for the treatment of magnesium-dependent rickets 253.
Neuropathic pain
The effect of magnesium on neuropathic pain has been examined in some clinical studies. The intravenous administration of magnesium sulfate was found to partially or completely alleviate pain in patients with postherpetic neuralgia, a type of neuropathic pain caused by herpes zoster infection (shingles) 254, 255. In a more recent randomized controlled trial in 45 patients with either postherpetic neuralgia or neuropathic pain of traumatic or surgical origin, oral supplementation of magnesium failed to improve measures of pain and quality of life compared to a placebo 256. Another trial is underway to examine the impact of intravenous magnesium with ketamine on neuropathic pain 257.
Post-operative pain
Several intervention studies have examined the role of magnesium on pain control and analgesic requirement in patients during the immediate post-surgery period.
After cesarean section
Pain management strategies after cesarean section usually involve the injection of an analgesic into either the epidural space (for epidural analgesia) or the subarachnoid space (for spinal [intrathecal] analgesia). A recent meta-analysis of nine randomized controlled trials summarized the evidence regarding the potential use of magnesium sulfate to control or relieve postoperative pain in 827 women who underwent cesarean section 258. All the trials evaluated the effect of a first-line analgesic regimen (i.e., bupivacaine or lidocaine, with or without opioids) with and without the addition of magnesium sulfate. The results suggested that the anesthesia (8 studies) and sensory blockade (6 studies) lasted longer in women who received the additional magnesium sulfate. The use of magnesium sulfate also resulted in lower pain score (3 studies) and in lower postoperative consumption of analgesics (4 studies). Additionally, there was no difference in occurrence of side effects between regimens 258. A recent randomized controlled trial in 60 healthy women undergoing elective cesarean section confirmed that the addition of magnesium sulfate to a bupivacaine/opioid regimen increased the duration of spinal anesthesia and lowered the pain level, yet did not improve the potency of bupivacaine 259. In another study in women with mild preeclampsia who received an epidural injection of ropivacaine after cesarean section, spinal infusion of magnesium sulfate increased the duration of sensory and motor blockade, as well as the time before patients requested an analgesic, compared to midazolam 260.
After a variety of other surgeries
The efficacy of intravenous magnesium has also been examined for local, regional, or systemic pain control following a range of different surgeries. A review of four small randomized controlled studies suggested that, when added to local analgesics, magnesium infusion to patients undergoing tonsillectomy might decrease pain and incidence of laryngospasm, extend the time to first post-operative analgesic requirement, and reduce the number of post-operative analgesic requests 261. Similar observations were reported in two additional meta-analyses, yet there was discrepancy regarding the ability of magnesium to alleviate pain 262, 263. The review of eight trials by Xie et al. 263, of which only two scored pain using the same scale, showed no pain reduction with magnesium compared to control. Finally, both meta-analyses reported no reduction in risk of post-operative nausea and vomiting with intravenous magnesium administration 261, 263. A 2018 meta-analysis of four randomized controlled trials in 263 patients also suggested that magnesium sulfate infusion may help reduce pain scores at 2 and 8 hours (but not 24 hours) after laparoscopic cholecystectomy 264. Recent studies have examined the use of magnesium sulfate for pain control after other surgeries, including hysterectomy 265, 266, spinal surgery 267, 268, or during endoscopic sinus 269 or cochlear implantation 270 surgery. Despite conflicting results or reports of limited benefits of magnesium, further research is needed before any conclusions can be drawn.
Asthma
The occurrence of hypomagnesemia may be greater in patients with asthma than in individuals without asthma 271. Several clinical trials have examined the effect of intravenous magnesium infusions on acute asthmatic attacks in children or adults who did not respond to initial treatment in the emergency room. Indeed, magnesium can promote bronchodilation in subjects with asthma by interfering with mechanisms like the activation of N-methyl D-aspartate (NMDA) receptors that trigger bronchoconstriction through facilitating calcium influx in airway smooth muscle cells 272. In a meta-analysis of six (quasi) randomized controlled trials in 325 children with acute asthma treated with a short-acting β2-adrenergic receptor agonist (e.g., salbutamol) and systemic steroids, intravenous magnesium sulfate treatment improved measurements of the respiratory function and reduced the risk of hospital admission by 30% compared to control 273. Another meta-analysis of randomized controlled trials primarily conducted in adults with asthma exacerbations indicated that single infusions of 1.2 to 2 g of magnesium sulfate over 15 to 30 minutes could reduce the risk of hospital admission and improve lung function after initial treatments failed (i.e., oxygen, short-acting β2 agonist, and steroids) 274.
The use of nebulized, inhaled magnesium for treating asthma has also been investigated. A recent systematic review of 25 randomized controlled trials, including adults, children, or both, found little evidence that inhaled magnesium sulfate alone or along with a β2-adrenergic receptor agonist and/or a muscarinic anticholinergic (e.g., ipratropium) could improve pulmonary function in patients with acute asthma 275. In addition, oral magnesium supplementation is of no known value in the management of chronic asthma 276, 277.
Migraine headaches
People who have migraine headaches sometimes have low levels of magnesium in their blood and other tissues 278. Several small studies found that magnesium supplements can modestly reduce the frequency of migraines.
However, research on the use of magnesium supplements to prevent or reduce symptoms of migraine headaches is limited. Three of four small, short-term, placebo-controlled trials found modest reductions in the frequency of migraines in patients given up to 600 mg/day magnesium 278. The authors of a review on migraine prophylaxis suggested that taking 300 mg magnesium twice a day, either alone or in combination with medication, can prevent migraines 279.
In their evidence-based guideline update, the American Academy of Neurology and the American Headache Society concluded that magnesium therapy is “probably effective” for migraine prevention 280. Because the typical dose of magnesium used for migraine prevention exceeds the Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects), this treatment should be used only under the direction and supervision of a healthcare provider.
More research is needed to determine whether magnesium supplements can help reduce the risk of migraines or ease migraine symptoms.
Preeclampsia and eclampsia
Preeclampsia and eclampsia are hypertensive disorders of pregnancy that may occur at any time after 20 weeks’ gestation and persist up to six weeks following birth. Preeclampsia (sometimes called toxemia of pregnancy) affects approximately 4% of pregnant women in the US 281. Preeclampsia is defined as the presence of elevated blood pressure (hypertension), protein in the urine (proteinuria), and severe swelling (edema) during pregnancy 282. Eclampsia occurs with the addition of seizures to the triad of preeclamptic symptoms (hypertension, proteinuria & edema) and is a significant cause of perinatal and maternal mortality 282, 283. Although cases of preeclampsia are at high risk of developing eclampsia, one-quarter of eclamptic women do not initially exhibit preeclamptic symptoms 284.
Although lower magnesium concentrations have been reported in the blood and brain of women with preeclampsia than in healthy pregnant women, there is no evidence that magnesium imbalance may cause adverse pregnancy events. A 2014 meta-analysis of 10 randomized controlled trials found no effect of oral magnesium salt administration during normal and at-risk pregnancies on the risk of preeclampsia, perinatal mortality, and small-for-gestational age infants 285.
For many years, high-dose intravenous magnesium sulfate has been the treatment of choice for preventing eclamptic seizures that may occur in association with severe preeclampsia in pregnancy or during labor 286, 287. A systematic review of seven randomized trials in 1,396 women with eclampsia compared the effect of magnesium sulfate administration with diazepam (a known anticonvulsant) treatment on perinatal outcomes. Risks of recurrent seizures and maternal death were significantly reduced by the magnesium regimen compared to diazepam 284. Moreover, the use of magnesium for the care of eclamptic women resulted in newborns with higher Apgar scores; there was no significant difference in the risk of preterm birth and perinatal mortality 284. Additional research has confirmed that infusion of magnesium sulfate should always be considered in the management of severe preeclampsia and eclampsia to prevent initial and recurrent seizures 288. Moreover, the World Health Organization (WHO) recommends the use of magnesium sulfate — administered either intramuscularly or intravenously — as first-line treatment for the prevention of eclampsia in women with severe preeclampsia, in preference to other anticonvulsants 289. Further research is needed to evaluate the efficacy of magnesium salt infusion in eclampsia prophylaxis in women with mild preeclampsia 290. In addition, it is unclear whether prolonging magnesium use post-partum in women who presented with severe preeclampsia during pregnancy is necessary to lower the risk of eclampsia after delivery 291.
Perinatal neuroprotection
While intravenous magnesium sulfate is included in the medical care of preeclampsia and eclampsia, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine support its use in two additional situations: specific conditions of short-term prolongation of pregnancy and neuroprotection of the fetus in anticipated premature delivery 292.
Preterm birth, which is defined by the premature delivery of an infant between the 20th and 37th weeks of estimated gestation, is associated with an increased risk of perinatal mortality and both short- and long-term morbidity. The American College of Obstetricians and Gynecologists approves the use of different classes of drugs — known as tocolytics — that are meant to delay delivery for long enough so that antenatal corticoids can be used to accelerate lung maturation in the fetus of women at imminent risk of preterm labor 293. A 2014 meta-analysis of 37 trials found that intravenous infusion of magnesium sulfate was no more efficacious than commonly used tocolytics (e.g., β-adrenergic receptor agonists, calcium channel blockers, prostaglandin inhibitors) in delaying delivery or preventing serious infant outcomes 294. Very limited evidence also suggested that high- versus low-dose magnesium infusion may reduce the length of hospital stays in neonates admitted to intensive care units 295.
The relationship between magnesium sulfate and risk of cerebral damage in premature infants has been assessed in observational studies. A meta-analysis of six case-control and five prospective cohort studies showed that the use of magnesium significantly reduced the risk of cerebral palsy, as well as mortality 296. However, the high degree of heterogeneity among the cohort studies and the fact that corticosteroid exposure (which is known to decrease antenatal mortality) was higher in the cases of children exposed to magnesium compared to controls imply a cautious interpretation of the results. Nonetheless, a meta-analysis of five randomized controlled trials, which included 5,493 women at risk of preterm birth and 6,135 babies, found that magnesium therapy given to mothers delivering before term decreased the risk of cerebral palsy by 32% without causing severe adverse maternal events, but this treatment did not reduce the risk of other neurologic impairments or mortality in early childhood 297. Another meta-analysis conducted on five randomized controlled trials found that intravenous magnesium administration to newborns who suffered from perinatal asphyxia could be beneficial in terms of short-term neurologic outcomes, although there was no effect on mortality 298. Additional trials are needed to evaluate the long-term benefits of magnesium in pediatric care.
Magnesium deficiency
Magnesium deficiency also called hypomagnesemia or low magnesium is a condition in which the amount of magnesium in your blood (plasma) is lower than normal. Normal serum magnesium levels are between 0.6 millimoles/liter (mmol/L) and 1.1 mmol/L (1.46 mg/dL to 2.68 mg/dL) 299. Magnesium deficiency or hypomagnesemia is an electrolyte disturbance caused by a low serum magnesium level less than 0.6 mmol/L (less than 1.46 mg/dL) in the blood 299. When the level of magnesium in your body drops below normal, symptoms develop due to low magnesium. However, it is typically asymptomatic until serum magnesium concentration is less than 0.5 mmol/L (1.22 mg/dL) 300. While mild magnesium deficiency may not elicit clinical symptoms, it may be associated with an increased risk of developing chronic diseases (see Figure 7 below) 166.
Magnesium deficiency in healthy individuals who are consuming a balanced diet is quite rare because magnesium is abundant in both plant and animal foods and because the kidneys are able to limit urinary excretion of magnesium when intake is low. During low intakes of magnesium, the magnesium absorbed from the diet is increased, the amount in urine is decreased, and body magnesium reserves (bone is the major reserve) are used 301. When the dietary intake of magnesium is adequate, the opposite occurs. Magnesium homeostasis involves the kidney (primarily through the proximal tubule, the thick ascending loop of Henle, and the distal tubule), small bowel (primarily through the jejunum and ileum), and bone. Hypomagnesemia occurs when something, whether a drug or a disease condition, alters the homeostasis of magnesium 299.
Low magnesium levels or hypomagnesemia can occur secondary to kidney and gastrointestinal losses 302, 303.
Common causes of magnesium deficiency or hypomagnesemia include 304:
- Alcohol use disorder
- Burns that affect a large area of the body
- Chronic diarrhea
- Excessive urination (polyuria), such as in uncontrolled diabetes and during recovery from acute kidney failure
- Hyperaldosteronism (disorder in which the adrenal gland releases too much of the hormone aldosterone into the blood)
- Kidney tubule disorders
- Malabsorption syndromes, such as celiac disease and inflammatory bowel disease
- Malnutrition
- Medicines
- Diuretics (furosemide, thiazide)
- Epidermal growth factor (EGF) receptor inhibitors (cetuximab)
- Proton pump inhibitors (all, such as omeprazole)
- Calcineurin inhibitors (cyclosporin A, tacrolimus)
- Platinum derivatives (cisplatin, carboplatin)
- Antimicrobials (aminoglycosides, pentimidine, rapamycin, amphotericin B, foscarnet)
- Pancreatitis (swelling and inflammation of the pancreas)
- Excessive sweating
- Genetic causes 305
- Hypercalciuric hypomagnesemias
- Bartter syndrome
- Gitelman syndrome
- EAST (epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome
- Mitochondrial hypomagnesemias
- Autosomal dominant tubulointerstitial kidney disease
- Autosomal dominant hypocalcemia with hypocalciuria
- Episodic ataxia type 1
- Familial hypomagnesemia with hypocalcemia and nephrocalcinosis
- Hypertension, hypercholesterolemia and hypomagnesemia
- Hyperphenylalaninemia BH4-deficient
- Hypomagnesemia with secondary hypocalcemia
- Hypomagnesemia with seizures and mental retardation
- Hyperuricemia, pulmonary hypertension, renal failure and alkalotic syndrome (HUPRAS)
- Kenny−Chaffey syndrome type 2
- Kearns-Sayre syndrome
- Neonatal inflammatory skin and bowel disease type 2 (NISBD2)
- Renal cysts and diabetes
Signs and symptoms of of magnesium deficiency can be nonspecific and usually overlap with symptoms of other electrolyte imbalances 12. The severity of symptoms and signs depends on the degree of magnesium depletion, rate, extent and duration of magnesium decline 12. Low magnesium symptoms usually occur when serum magnesium levels fall below 0.5 mmol/L (1.2 mg/dL) 306. The signs and symptoms of hypomagnesemia may affect every system including neuromuscular, cardiovascular, renal, and gastrointestinal systems 15, 66. The signs and symptoms of hypomagnesemia may include 307, 308, 309:
- Neuromuscular
- Tremors, muscle fasciculation, muscle spasms and cramps, muscle contractions, numbness, tingling, generalized weakness and tiredness.
- Central nervous system
- Agitation, depression, sudden change in behavior, encephalopathy, and seizures.
- Cardiovascular
- Cardiac arrhythmia, ECG changes and cardiac ischemia.
- Gastrointestinal
- Loss of appetite, nausea, and vomiting.
- Metabolic
- Low serum potassium concentrations (hypokalemia) and low serum calcium concentrations (hypocalcemia). Magnesium deficiency also can cause hypocalcemia (low blood calcium), as the two are interrelated. Decreased magnesium causes impaired magnesium-dependent adenyl cyclase generation of cyclic adenosine monophosphate (cAMP), decreasing parathyroid hormone (PTH) release. Hypocalcemia persisted despite increased secretion of parathyroid hormone (PTH), which regulates calcium homeostasis. Usually, increased parathyroid hormone (PTH) secretion quickly results in the mobilization of calcium from bone and normalization of blood calcium concentration. As the magnesium depletion progressed, parathyroid hormone (PTH) secretion diminished to low concentrations. In addition to hypomagnesemia, signs of severe magnesium deficiency included hypocalcemia, low serum potassium concentrations (hypokalemia), retention of sodium, low circulating parathyroid hormone (PTH) concentrations, neurological and muscular symptoms (tremor, muscle spasms, tetany), loss of appetite, nausea, vomiting, and personality changes 56.
- Untreated, magnesium deficiency or hypomagnesemia can lead to:
- Cardiac arrest
- Respiratory arrest
- Coma
- Death
In addition, (severe) hypomagnesemia may have further consequences during pregnancy, as suggested by findings that a magnesium deficient diet in pregnant mice was able to induce fetal malformations 310. Conversely, supplementation with magnesium sulfate (MgSO4) during pregnancy is a treatment for pre-eclampsia 311, 312, suggesting a role for a relative shortage of magnesium in this disease too. Furthermore, some diseases, such as Parkinson’s disease and diabetes, have merely been associated with low serum magnesium concentrations 313. However, it is not yet clear whether hypomagnesemia is the cause, a consequence or simply an epiphenomenon in these diseases.
Magnesium also affects the electrical activity of the heart muscle (myocardium) and vascular tone, which is why patients with hypomagnesemia are at risk for cardiac arrhythmias. In addition, when magnesium is low, there is inhibition of renal outer medullary potassium channels, leading to increased urinary excretion and depletion of intracellular potassium levels. This reduces the threshold required for generating an action potential in the heart muscle cell (cardiac myocyte) 299. Moreover, reduced intracellular potassium levels also prolong the time to repolarize the cell membrane, increasing the risk of arrhythmias 299. In a systematic review and meta-analysis of experimental studies, authors showed that subjects with prediabetes had significantly lower serum Mg concentration than healthy controls (about 0.07 mmol/L) 74.
The risk of magnesium deficiency or hypomagnesemia depends on multiple characteristics in various healthcare settings. The reported prevalence in the general population is 2.5% to 15% 299. In hospitalized patients, magnesium deficiency or hypomagnesemia ranges from 12 to 20% 314, 315. The prevalence is even higher in critically ill patients, estimated to be 65% in a study 300. In a study of 100 critically ill children (mean age 4.9 years) admitted to a pediatric intensive care unit in India, the prevalence of magnesium deficiency or hypomagnesemia was about 55%. A study revealed a 30% prevalence of magnesium deficiency or hypomagnesemia in patients with chronic alcohol use disorder 316.
The diagnosis of magnesium deficiency or low magnesium is challenging 69, because magnesium serum concentration does not reflect the total content in the human body 317. You may have a “normal” serum magnesium concentration (0.7–1 mmol/L or 1.7–2.4 mg/dL) 318, but have relatively low levels of skeletal or cellular magnesium 319. Up till now, no single reliable indicator of magnesium deficiency is considered satisfactory 69. The difficulties of accessing total body magnesium concentration concerns its main two compartments, namely bone and muscle, while in blood it is present only in very small amounts (less than 1%) 320, 12, 321. It is possible that an individual can be in a magnesium-depleted state but have plasma or serum values within the “normal” range 25. Consequently, the clinical impact of magnesium deficiency may be underestimated. In plasma, the concentration of free magnesium is reported to be ~0.6 mmol/L (~14 mg/L) 322, with about 30% complexed by proteins 323. The major magnesium binding protein in plasma is serum albumin 324, 322.
In clinical practice, the total serum or plasma magnesium concentration is the most commonly used test to assess the magnesium status, and the normal reference range is usually 0.7–1 mmol/L (1.7–2.43 mg/dL) 318, 325. Plasma magnesium concentrations are closely related to bone metabolism, as there is continuous exchange between the skeleton and blood 326. However, the normal value varies from laboratory to laboratory, and different studies have used slightly different ranges. This may partially explain the differences in the prevalence of magnesium imbalances reported in different groups of patients with similar characteristics 15.
The treatment of patients with hypomagnesemia is based on a patient’s kidney function, the severity of their symptoms, and hemodynamic stability 299. If a patient is hemodynamically unstable in an acute hospital setting, 1 to 2 grams of magnesium sulfate can be given in about 15 minutes Gragossian A, Bashir K, Bhutta BS, et al. Hypomagnesemia. [Updated 2022 Nov 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500003. For symptomatic, severe hypomagnesemia in a stable patient, 1 to 2 grams of magnesium sulfate can be given over one hour Gragossian A, Bashir K, Bhutta BS, et al. Hypomagnesemia. [Updated 2022 Nov 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500003. Non-emergent repletion of magnesium in adult patient is generally 4 to 8 grams of magnesium sulfate given slowly over 12 to 24 hours 299. In children patients, the magnesium sulfate dose is 25 to 50 mg/kg (with a maximum of 2 grams) 327, 328.
For an asymptomatic patient with hypomagnesemia who is not hospitalized and can tolerate medications by mouth, sustained-release oral replacement should be tried first. Asymptomatic patients with hypomagnesemia can benefit from oral sustained-release magnesium preparations (magnesium chloride containing 64-71.5 mg or magnesium L-lactate containing 84 mg elemental magnesium) 299. After repletion, serum electrolyte levels must be rechecked (whether in an inpatient or outpatient setting) to ensure effective treatment. Although serum magnesium levels rise quickly with treatment, intracellular magnesium takes longer to replete. Therefore, patients with normal kidney function should try to continue magnesium repletion for two days after the level normalizes 299.
Magnesium deficiency causes
Magnesium deficiency causes include insufficient dietary supply, malabsorption, endocrine causes, renal disease, redistribution and intracellular shift of magnesium, medication use, or other factors such as chronic alcoholism or stress 320, 329:i3–i14. doi: 10.1093/ndtplus/sfr163)), 330.
Magnesium deficiency or hypomagnesemia can be secondary to decreased intake, as seen in the following 15, 331:
- Decreased intake. Several dietary surveys have shown that people in North America and Europe consume less than recommended daily allowance (RDA) for magnesium as a result of food processing and the use of poor soil for agriculture 13, 16, 332. Hypomagnesemia can also occur in times of prolonged fasting, total parenteral nutrition, or
- Starvation
- Alcohol use disorder (with a reported prevalence of 30%) 333
- Anorexia nervosa
- Terminal cancer
- Critically ill patients who are receiving total parenteral nutrition
- Prolonged nasogastric suctioning.
Magnesium deficiency or hypomagnesemia can also be secondary to the following medications 300, 334, 329:i3–i14. doi: 10.1093/ndtplus/sfr163)):
- Loop and thiazide diuretics
- Proton pump inhibitors
- Aminoglycoside antibiotics
- Amphotericin B
- Pentamidine
- Digitalis
- Tacrolimus
- Chemotherapeutic drugs, such as cisplatin, cyclosporine
- Antibodies that bind to epidermal growth factor (EGF) receptors (cetuximab, matuzumab, panitumumab)
- Laxative abuse
Magnesium deficiency or hypomagnesemia can also result from redistribution from the extracellular to the intracellular compartment 335, 330:
- Treatment of diabetic ketoacidosis by insulin
- Refeeding syndrome
- Correction of metabolic acidosis
- Acute pancreatitis
- Ethanol withdrawal syndrome
- Pregnancy
- Lactation
- Cardiopulmonary surgeries
Magnesium deficiency or hypomagnesemia can be induced by malabsorption or gastrointestinal disorders 320, 306:
- Crohn’s disease,
- Ulcerative colitis,
- Inflammatory bowel diseases,
- Malabsorption syndromes,
- Celiac disease,
- Short bowel syndrome,
- Whipple’s disease,
- Acute diarrhea
- Chronic diarrhea,
- Pancreatic insufficiency
- Acute pancreatitis
- Gastric bypass surgery
- Surgical removal of a portion of the small intestine
- Intestinal inflammation due to radiation.
Magnesium deficiency or hypomagnesemia can be induced by kidney losses 300, 306:
- Hungry bone syndrome (an increased magnesium uptake by renewing bone following parathyroidectomy or thyroidectomy, causing a decrease in serum magnesium)
- Genetic disorders 305
- Gitelman syndrome. Gitelman syndrome is caused by recessive mutations in the gene that codes for the thiazide-sensitive sodium chloride cotransporter (SLC12A3) in the distal convoluted tubule. Transcellular reabsorption of magnesium in the distal convoluted tubule is impaired, leading to increased calcium reabsorption and the subsequent hypocalciuria (low urine calcium) and fluid loss have the tendency to lower blood pressure. In addition, fluid loss activates the renin-angiotensin-aldosterone system, and increased aldosterone causes increased potassium secretion in exchange for sodium and subsequent hypokalemia (low blood potassium).
- Bartter syndrome
- Familial hypomagnesemia with hypercalciuria and nephrocalcinosis
- Hypercalciuric hypomagnesemias: Mutations affect the reabsorption of magnesium and calcium ions in the thick ascending limb of Henle, leading to hypercalciuric hypomagnesemia that ultimately results in nephrocalcinosis or chronic kidney disease.
- Renal malformations and early-onset diabetes mellitus caused by HNF1-beta mutation
- Autosomal recessive isolated hypomagnesemia caused by EGF mutation
- Autosomal dominant isolated hypomagnesemia caused by Na-K-ATPase gamma subunit, Kv1.1, and cyclin M2 mutations.
- Intestinal hypomagnesemia with secondary hypocalcemia
- EAST (SeSAME) syndrome. EAST (SeSAME) syndrome is caused by loss-of-function mutations in the gene encoding the potassium channel, KCNJ10 (Kir4.1), on the basolateral membrane of the distal convoluted tubule. It results in hypomagnesemia, salt wasting, metabolic alkalosis, and hypokalemia. The mechanism, however, is poorly understood 336.
- Acquired renal tubular dysfunction may result in increased urinary loss of magnesium 329:i3–i14. doi: 10.1093/ndtplus/sfr163)), 330:
- Post-kidney transplantation
- Recovery from acute tubular necrosis
- Postobstructive diuresis
- Chronic renal failure
- Dialysis
- Excessive volume expansion
- Chronic metabolic acidosis
- Diabetes mellitus
- Long-term use of certain diuretics
Magnesium deficiency or hypomagnesemia due to endocrine disorders 329:i3–i14. doi: 10.1093/ndtplus/sfr163)), 320:
- Aldosteronism,
- Hyperparathyroidism,
- Hyperthyroidism,
- Poorly-controlled diabetes
Other causes
Poor dietary intake, gastrointestinal problems, and increased urinary loss of magnesium may all contribute to magnesium depletion in people suffering from alcoholism. Older adults have relatively low dietary intakes of magnesium 53, 52. Intestinal magnesium absorption tends to decrease with age, and urinary magnesium excretion tends to increase with age; therefore, suboptimal dietary magnesium intake may increase the risk of magnesium depletion in the elderly 54.
Groups at risk of magnesium deficiency
The following groups of people are more likely than others to get too little magnesium:
- People with gastrointestinal diseases (such as Crohn’s disease and celiac disease)
- People with type 2 diabetes
- People with long-term alcoholism
- Older people
Magnesium inadequacy can occur when intakes fall below the RDA but are above the amount required to prevent overt deficiency. The following groups are more likely than others to be at risk of magnesium inadequacy because they typically consume insufficient amounts or they have medical conditions (or take medications) that reduce magnesium absorption from the gut or increase losses from the body.
People with gastrointestinal diseases
The chronic diarrhea and fat malabsorption resulting from Crohn’s disease, gluten-sensitive enteropathy (celiac disease), and regional enteritis can lead to magnesium depletion over time 7. Resection or bypass of the small intestine, especially the ileum, typically leads to malabsorption and magnesium loss 7.
People with type 2 diabetes
Magnesium deficits and increased urinary magnesium excretion can occur in people with insulin resistance and/or type 2 diabetes 112. The magnesium loss appears to be secondary to higher concentrations of glucose in the kidney that increase urine output 7.
People with alcohol dependence
Magnesium deficiency is common in people with chronic alcoholism 7. In these individuals, poor dietary intake and nutritional status; gastrointestinal problems, including vomiting, diarrhea, and steatorrhea (fatty stools) resulting from pancreatitis; renal dysfunction with excess excretion of magnesium into the urine; phosphate depletion; vitamin D deficiency; acute alcoholic ketoacidosis; and hyperaldosteronism secondary to liver disease can all contribute to decreased magnesium status 7.
Older adults
Older adults have lower dietary intakes of magnesium than younger adults 113. In addition, magnesium absorption from the gut decreases and renal magnesium excretion increases with age 114. Older adults are also more likely to have chronic diseases or take medications that alter magnesium status, which can increase their risk of magnesium depletion 115.
Magnesium deficiency prevention
It is important to consume an adequate amount of magnesium through diet. The good sources of magnesium include green vegetables, nuts, seeds, unprocessed cereals, and dark chocolate (Table 2). A lower concentration of magnesium is present in fruits, meat, fish, and dairy products. Drinking water supplies about 10% of daily magnesium intake 329:i3–i14. doi: 10.1093/ndtplus/sfr163)). It is worth adding that dietary factors such as lactose, fructose, or glucose can enhance magnesium absorption, while a high intake of zinc, fiber, free fatty acids, oxalate, or phytate can cause its decrease 337.
Magnesium deficiency signs and symptoms
The clinical signs and symptoms of magnesium deficiency or hypomagnesemia are often non-specific 12. The major signs and symptoms of low magnesium or magnesium deficiency include neuromuscular and cardiovascular manifestations and other electrolyte abnormalities. Early signs of magnesium deficiency may be fatigue, weakness, loss of appetite, nausea, or vomiting. As magnesium deficiency worsens, the next symptoms may include tremors, agitation and muscle fasciculation, cramps, seizures, cardiac arrhythmia, ventricular tachycardia, personality changes, or depression 338. In addition, clinical symptoms of hypomagnesemia are often correlated with a rapid decrease in magnesium levels compared to a gradual change. Hypomagnesemia is frequently accompanied by other electrolyte abnormalities, especially hypokalemia and hypocalcemia. Thus, the diagnosis of magnesium deficiency should be supported by laboratory determinations of other macroelements, i.a., calcium, potassium, or phosphorus 339:i15–i24. doi: 10.1093/ndtplus/sfr164)). The impact of chronic magnesium deficiency is shown is Figure 7.
Magnesium deficiency or low magnesium common symptoms include 304:
- Abnormal eye movements (nystagmus)
- Convulsions
- Fatigue
- Muscle spasms or cramps
- Muscle weakness
- Numbness
Early presentation of low magnesium or hypomagnesemia includes nausea, vomiting, loss of appetite, fatigue, and weakness 15. Patients may complain of dysphagia, muscular weakness, and other symptoms as described below 335. A case report describes symptoms of cerebellar ataxia, generalized convulsions, intermittent downbeat nystagmus, and supraventricular tachycardia in a 59-year-old man with severe hypomagnesemia 340.
Figure 7. Chronic magnesium depletion consequences
[Source 12 ]Neuromuscular signs and symptoms
- Neuromuscular hyperexcitability (often the first clinical manifestation) 327
- Tremors
- Tetany, including positive Trousseau and Chvostek signs, muscle spasms, and muscle cramps. It may occur in the absence of hypocalcemia and alkalosis and is thought to be due to the lowering of the threshold for nerve stimulation 341.
- Choreoathetosis 342
- Seizures
- Vertical nystagmus
- Apathy
- Delirium
- Depression
- Agitation
- Psychosis
- Delirium
- Coma
Cardiovascular signs and symptoms
- Electrocardiogram (ECG) changes, including widening of the QRS complex, peaked T waves (with mild to moderate deficiency), prolongation of the PR interval, and diminution of the T wave (with severe deficiency) 343
- Atrial and ventricular premature systoles
- Atrial fibrillation (AF)
- Ventricular arrhythmias, including torsades de pointes
- Cardiac ischemia
- Increased risk of digoxin toxicity by inhibiting Na-K-ATPase and depleting intracellular potassium 344
- Hypertension. Low dietary magnesium and hypomagnesemia might be a contributing factor in the pathophysiology of hypertension. Magnesium reduces vascular tone and resistance by enhancing vasodilator effect of nitric oxide, antagonizing the vasoconstrictor effect of calcium, bradykinin, angiotensin 2, serotonin, and prostaglandin in F2α, and protecting the vascular endothelium through its antioxidant effect 345. Several clinical trials have been conducted to study the effect of magnesium supplementation on the blood pressure, and at present, there is no strong evidence to support the use of magnesium supplementation in the routine management of hypertension 11, 346.
Other Electrolyte and Hormone Abnormalities
- Hypocalcemia
- Symptoms typically occur at magnesium levels below 0.5 mmol/L or 1.22 mg/dL.
- Milder hypomagnesemia (between 0.55 mmol/L and 0.65 mmol/L) lowers the plasma calcium concentration only slightly (0.2 mg/dL or 0.05 mmol/L) 347
- Hypoparathyroidism
- Hypokalemia (about 60% of cases).
Magnesium deficiency complications
Dangerously low levels of magnesium have the potential to cause fatal cardiac arrhythmias, such as torsades de pointes (polymorphous ventricular tachycardia with marked QT prolongation) 299. Moreover, hypomagnesemia in patients with heart attack (acute myocardial infarction) puts them at a higher risk of ventricular arrhythmias within the first 24 hours. Moreover, it may also cause chondrocalcinosis 305.
Magnesium deficiency diagnosis
Your health care provider will do a physical exam and ask about your symptoms.
History may include the causes adn symptoms mentioned above.
On examination, vertical nystagmus and tetany may be observed. The following signs can be checked:
- Chvostek sign: Tapping on facial nerve leads to twitching of facial muscles
- Trousseau sign: Carpopedal spasm induced by inflated blood pressure cuff
Tests that may be ordered include blood tests, urine tests and an electrocardiogram (ECG) to rule out arrhythmias.
Blood and urine tests that may be done include:
- Calcium blood test
- Comprehensive metabolic panel
- Potassium blood test
- Urine magnesium test
- Genetic testing may be considered if there is positive family history, unexplained hypomagnesemia, or if discovered early in infancy.
The diagnosis of magnesium deficiency or low magnesium is challenging 69, because magnesium serum concentration does not reflect the total content in the human body 317. You may have a “normal” serum magnesium concentration (0.7–1 mmol/L or 1.7–2.4 mg/dL) 318, but have relatively low levels of skeletal or cellular magnesium 319. Up till now, no single reliable indicator of magnesium deficiency is considered satisfactory 69. The difficulties of accessing total body magnesium concentration concerns its main two compartments, namely bone and muscle, while in blood it is present only in very small amounts (less than 1%) 320, 12, 321. It is possible that an individual can be in a magnesium-depleted state but have plasma or serum values within the “normal” range 25. Consequently, the clinical impact of magnesium deficiency may be underestimated. In plasma, the concentration of free magnesium is reported to be ~0.6 mmol/L (~14 mg/L) 322, with about 30% complexed by proteins 323. The major magnesium binding protein in plasma is serum albumin 324, 322.
In clinical practice, the total serum or plasma magnesium concentration is the most commonly used test to assess the magnesium status, and the normal reference range is usually 0.7–1 mmol/L (1.7–2.43 mg/dL) 318, 325. Plasma magnesium concentrations are closely related to bone metabolism, as there is continuous exchange between the skeleton and blood 326. However, the normal value varies from laboratory to laboratory, and different studies have used slightly different ranges. This may partially explain the differences in the prevalence of magnesium imbalances reported in different groups of patients with similar characteristics 15.
Normal serum magnesium does not necessarily mean adequate content of total body magnesium because only less than 0.3% of total body magnesium is found in serum 15. Serum magnesium is in most places not part of routine blood tests, and it should be assessed in the relevant clinical conditions such as arrhythmia, low blood potassium (hypokalemia), low blood calcium (hypocalcemia), diarrhea, and chronic alcoholism that tend to be associated with magnesium derangement 68. Serum magnesium test is also recommended if the patient is critically ill or when being administered certain medications known to cause hypomagnesemia. Table 3 lists other more accurate but lesser used measures of assessing magnesium status 13, 348.
The magnesium loading test also known as the magnesium retention test is considered to be the gold standard for measuring magnesium status, which basically determines magnesium retention using 24-hour urine collection following the intravenous administration of magnesium 166. A magnesium deficiency is indicated if a patient has <80% excretion (over 24 hours) of an infused magnesium load (2.4 mg/kg of lean body weight given over the initial 4 hours) 349, 350. Despite the magnesium loading test or magnesium retention test is a good indicator of magnesium deficiency or hypomagnesemia in adults, it appears to be poorly sensitive to changes in magnesium status in healthy people. Moreover, the magnesium loading test or magnesium retention test is invasive and cumbersome, and thus difficult to use routinely 351.
Additional tests for magnesium deficiency involve measuring the magnesium/creatinine ratio in spot urine or in 24 hour urine collections 319. It is also possible to directly measure magnesium in the urine; this can be used to gain insight into kidney functioning and magnesium wasting. A 24 hour urinary magnesium level of > 24 mg is indicative of magnesium wasting 352. Another method to assess magnesium status is through measurements of plasma ionized magnesium, which represents the physiologically active form of magnesium. However, it is unknown whether plasma ionized magnesium reflect body stores 351.
In practice, magnesium status is usually determined through assessments of dietary magnesium intake, serum magnesium concentration, and/or urinary magnesium concentration 351. However, each of these indicators has limitations. Although predominantly used in epidemiological studies and the sole indicator available to clinicians, serum magnesium concentration has been found to poorly respond to magnesium supplementation 5. Regarding dietary intakes of magnesium, about 30 to 40% of ingested magnesium is absorbed, yet absorption varies with the amount of magnesium ingested and with the food matrix composition 5. Finally, a state of magnesium deficiency has not been associated to a clear cutoff concentration of magnesium in the urine 5. Urinary magnesium concentration fluctuates rapidly with dietary intakes, but measurements of 24-hour urinary magnesium can be used in addition to other indicators to assess population status. Presently, a combination of all three markers — dietary, serum, and urinary magnesium — may be used to get a valid assessment of magnesium status 351.
For differentiating hypomagnesemia of renal origin from intestinal hypomagnesemia is determination of the fractional excretion of magnesium (FEMg) 353, which can be calculated with the following formula 305:
- Fractional excretion of magnesium (FEMg) = [([Mg2+]urine × [creatinine]plasma)/ (0.7 [Mg2+]plasma × [creatinine]urine)] × 100 %
Note: The factor of 0.7 is included to adjust the total plasma magnesium concentration to the freely filtered fraction.
A fractional excretion of magnesium (FEMg) of >4 % in a hypomagnesemic patient is consistent with renal magnesium wasting, while a patient with a FEMg of <2 % will likely have an extra-renal origin of their hypomagnesemia 353. However, a FEMg <4 % does not rule out renal magnesium wasting. First, a low glomerular filtration rate (GFR) may result in a lower filtered load of magnesium. If the absorptive capacity of the kidney for magnesium is sufficient to cope with this lower load, the result may be a normal or even low FEMg. By the same mechanism, severe (renal) hypomagnesemia may result in a lower filtered load of magnesium and thus a normal or low FEMg. To account for these confounding factors, the serum magnesium levels of hypomagnesemic patients should be increased by means of intravenous magnesium supplementation before the FEMg is measured 354.
Table 5. Assessment of magnesium status
| Test | Comments |
|---|---|
| Serum magnesium | Sometimes not adequate since less than 0.3% of total body magnesium is found in serum. However, it is easy, accessible, and cheap. |
| 24 hours excretion in urine or the fractional excretion of magnesium | Helps in differentiating renal wasting of magnesium from inadequate intake or poor absorption as an etiology for hypomagnesemia. |
| Magnesium loading test | Identifies patients with normomagnesic magnesium deficiency. |
| Assesses intestinal absorption of magnesium. | |
| Indirectly assesses bone status of magnesium. | |
| Magnesium concentration in red blood cell counts | Can give early indication of magnesium deficiency. |
| Isotopic analysis of magnesium | Assesses the absorption of magnesium from the gastrointestinal tract in research setting. |
| Ionized magnesium | More accurate, especially in critically ill patients with rapid change in hemodynamics. |
| Not effected by low albumin. | |
Magnesium deficiency treatment
Treatment depends on the type of low magnesium problem you have and may include:
- Fluids given through a vein (IV)
- Magnesium by mouth or through a vein
- Medicines to relieve symptoms.
The treatment of patients with hypomagnesemia is based on a patient’s kidney function, the severity of their symptoms, and hemodynamic stability 299. If a patient is hemodynamically unstable in an acute hospital setting, 1 to 2 grams of magnesium sulfate can be given in about 15 minutes 299. For symptomatic, severe hypomagnesemia in a stable patient, 1 to 2 grams of magnesium sulfate can be given over one hour 299. Non-emergent repletion of magnesium in adult patient is generally 4 to 8 grams of magnesium sulfate given slowly over 12 to 24 hours 299. In children patients, the magnesium sulfate dose is 25 to 50 mg/kg (with a maximum of 2 grams) 327, 328.
For an asymptomatic patient with hypomagnesemia who is not hospitalized and can tolerate medications by mouth, sustained-release oral replacement should be tried first. Asymptomatic patients with hypomagnesemia can benefit from oral sustained-release magnesium preparations (magnesium chloride containing 64-71.5 mg or magnesium L-lactate containing 84 mg elemental magnesium) 299. After repletion, serum electrolyte levels must be rechecked (whether in an inpatient or outpatient setting) to ensure effective treatment. Although serum magnesium levels rise quickly with treatment, intracellular magnesium takes longer to replete. Therefore, patients with normal kidney function should try to continue magnesium repletion for two days after the level normalizes 299.
Use caution in repleting magnesium in patients with abnormal kidney function (defined as creatinine clearance less than 30 mL/min/1.73 m²) 299. These patients are at risk of hypermagnesemia (too much magnesium). Studies recommend reducing the magnesium dose by 50% and closely monitoring magnesium levels in these patients 299.
Magnesium deficiency prognosis
Magnesium deficiency prognosis or outcome depends on the condition that is causing the low magnesium. Patients with low magnesium or hypomagnesemia from an identifiable cause have a good prognosis for complete recovery 299. However, in critically ill patients, low magnesium or hypomagnesemia is associated with higher mortality, the need of mechanical ventilation and increased length of ICU stay in patients admitted to ICU 355.
- Tangvoraphonkchai K., Davenport A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018;25:251–260. doi: 10.1053/j.ackd.2018.02.010[↩]
- National Institutes of Health. Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/[↩]
- Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients. 2021 Mar 30;13(4):1136. doi: 10.3390/nu13041136[↩][↩][↩][↩][↩]
- Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010 Jun;23(2):60-72. doi: 10.1684/mrh.2010.0202[↩]
- Magnesium. https://lpi.oregonstate.edu/mic/minerals/magnesium[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare; 2010:527-37.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins; 2012:159-75.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Pham PCT, Pham PAT, Pham SV, Pham PTT, Pham PMT, Pham PTT. Hypomagnesemia: a clinical perspective. Int J Nephrol Renovasc Dis. 2014 doi: 10.2147/IJNRD.S42054[↩][↩]
- Bharadwaj J, Darbari A, Naithani M. Magnesium: the fifth electrolyte. J Med Nutr Nutraceuticals. 2014;3:186. doi: 10.4103/2278-019x.131955[↩][↩]
- de Baaij J. H. F., Hoenderop J. G. J., Bindels R. J. M. Magnesium in man: implications for health and disease. Physiological Reviews. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014[↩][↩][↩][↩][↩]
- Al Alawi AM, Majoni SW, Falhammar H. Magnesium and Human Health: Perspectives and Research Directions. Int J Endocrinol. 2018 Apr 16;2018:9041694. doi: 10.1155/2018/9041694[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Grober U., Schmidt J., Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199–8226. doi: 10.3390/nu7095388[↩][↩][↩][↩][↩][↩][↩]
- Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-Consumer[↩][↩]
- Jahnen-Dechent W., Ketteler M. Magnesium basics. Clinical Kidney Journal. 2012;5(Supplement 1):i3–i14. doi: 10.1093/ndtplus/sfr163[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Olza J., Aranceta-Bartrina J., Gonzalez-Gross M., et al. Reported dietary intake, disparity between the reported consumption and the level needed for adequacy and food sources of calcium, phosphorus, magnesium and vitamin D in the Spanish population: findings from the ANIBES study † Nutrients. 2017;9(2) doi: 10.3390/nu9020168[↩][↩]
- Moshfegh A, Goldman JD, Ahuja J, Rhodes D, LaComb R. What we eat in America, NHANES 2005–2006: usual nutrient intakes from food and water compared to 1997 Dietary Reference Intakes for vitamin D, calcium, phosphorus, and magnesium. Washington (DC): USDA; 2009.[↩]
- Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y, Van Horn LV. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv Nutr. 2016 Nov 15;7(6):977-993. doi: 10.3945/an.116.012765[↩]
- US Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015/advisory-report[↩]
- European Food Safety Authority. Scientific opinion on dietary reference values for magnesium. EFSA J 2015;13:4186.[↩]
- Volpe SL. Magnesium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames, Iowa; John Wiley & Sons, 2012:459-74.[↩][↩]
- Schuchardt J.P., Hahn A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017;13:260–278. doi: 10.2174/1573401313666170427162740[↩]
- Mathew AA, Panonnummal R. ‘Magnesium’-the master cation-as a drug-possibilities and evidences. Biometals. 2021 Oct;34(5):955-986. doi: 10.1007/s10534-021-00328-7[↩][↩][↩][↩]
- Groenestege W. M., Hoenderop J. G., van den Heuvel L., Knoers N., Bindels R. J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. Journal of the American Society of Nephrology. 2006;17(4):1035–1043. doi: 10.1681/ASN.2005070700[↩]
- Fritzen R, Davies A, Veenhuizen M, Campbell M, Pitt SJ, Ajjan RA, Stewart AJ. Magnesium Deficiency and Cardiometabolic Disease. Nutrients. 2023 May 17;15(10):2355. doi: 10.3390/nu15102355[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Cascella M, Vaqar S. Hypermagnesemia. [Updated 2023 May 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549811[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Quamme GA. Control of magnesium transport in the thick ascending limb. Am J Physiol. 1989 Feb;256(2 Pt 2):F197-210. doi: 10.1152/ajprenal.1989.256.2.F197[↩]
- Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41(2):357-62. doi: 10.1007/s11255-009-9548-7[↩][↩][↩][↩][↩][↩]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US); 1997. Available from: https://www.ncbi.nlm.nih.gov/books/NBK109825/ doi: 10.17226/5776[↩]
- Ismail A.A.A., Ismail Y., Ismail A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM. 2018;111:759–763. doi: 10.1093/qjmed/hcx186[↩]
- Rude R. Magnesium disorders. In: Kokko J., Tannen R., editors. Fluids and Electrolytes. W.B. Saunders Company; Philadelphia, PA, USA: 1996. pp. 421–445.[↩]
- Rosanoff A., West C., Elin R., Micke O., Baniasadi S., Barbagallo M., Campbell E., Cheng F.C., Costello R.B., Gamboa-Gomez C., et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022;61:3697–3706. doi: 10.1007/s00394-022-02916-w[↩][↩]
- Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010 Dec;23(4):S194-8. doi: 10.1684/mrh.2010.0213[↩]
- Bertinato J., Wang K.C., Hayward S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients. 2017;9:296. doi: 10.3390/nu9030296[↩][↩]
- Witkowski M., Hubert J., Mazur A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011;24:163–180. doi: 10.1684/mrh.2011.0292[↩]
- Gröber U., Schmidt J., Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388[↩]
- Gibson, RS. Principles of Nutritional Assessment, 2nd ed. New York, NY: Oxford University Press, 2005.[↩][↩][↩]
- Micke O, Vormann J, Kraus A, Kisters K. Serum magnesium: time for a standardized and evidence-based reference range. Magnes Res. 2021 May 1;34(2):84-89. doi: 10.1684/mrh.2021.0486[↩]
- Razzaque M.S. Magnesium: Are we consuming enough? Nutrients. 2018;10:1863. doi: 10.3390/nu10121863[↩]
- Hypermagnesemia. https://emedicine.medscape.com/article/246489-overview[↩]
- Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnes Res. 2011 Dec;24(4):163-80. doi: 10.1684/mrh.2011.0292[↩]
- Abad C., Vargas F. R., Zoltan T., et al. Magnesium sulfate affords protection against oxidative damage during severe preeclampsia. Placenta. 2015;36(2):179–185. doi: 10.1016/j.placenta.2014.11.008[↩][↩][↩]
- Dolati S, Rikhtegar R, Mehdizadeh A, Yousefi M. The role of magnesium in pathophysiology and migraine treatment. Biol Trace Elem Res. 2019 doi: 10.1007/s12011-019-01931-z[↩]
- Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388[↩]
- DiNicolantonio JJ, O’Keefe JH, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Hear. 2018 doi: 10.1136/openhrt-2017-000668[↩]
- Li X, Han X, Yang J, Bao J, Di X, Zhang G, Liu H. Magnesium sulfate provides neuroprotection in eclampsia-like seizure model by ameliorating neuroinflammation and brain edema. Mol Neurobiol. 2017;54:7938–7948. doi: 10.1007/s12035-016-0278-4[↩]
- Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169–1175. doi: 10.1161/STROKEAHA.108.527788[↩]
- Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5:i3–i14. doi: 10.1093/ndtplus/sfr163[↩]
- Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol. 2008;19:1451–1458. doi: 10.1681/ASN.2008010098[↩][↩]
- Gröber U. Magnesium and Drugs. Int. J. Mol. Sci. 2019;20:2094. doi: 10.3390/ijms20092094[↩]
- Ahmed F, Mohammed A. Magnesium: the forgotten electrolyte—a review on hypomagnesemia. Med Sci. 2019;7:56. doi: 10.3390/medsci7040056[↩]
- Moshfegh A, Goldman J, Ahuja J, Rhodes D, LaComb R. What We Eat in America, NHANES 2005-2006: Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium. 2009. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0506/usual_nutrient_intake_vitD_ca_phos_mg_2005-06.pdf[↩][↩]
- Sebastian RS, Cleveland LE, Goldman JD, Moshfegh AJ. Older adults who use vitamin/mineral supplements differ from nonusers in nutrient intake adequacy and dietary attitudes. J Am Diet Assoc. 2007 Aug;107(8):1322-32. doi: 10.1016/j.jada.2007.05.010[↩][↩]
- Food and Nutrition Board, Institute of Medicine. Magnesium. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, D.C.: National Academy Press; 1997:190-249. https://nap.nationalacademies.org/read/5776/chapter/8[↩][↩][↩]
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. China: Williams & Wilkins; 2014:159-175.[↩][↩]
- Rude RK, Shils ME. Magnesium. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore: Lippincott Williams & Wilkins; 2006:223-247.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Magnesium Blood Test. https://medlineplus.gov/lab-tests/magnesium-blood-test[↩]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for magnesium. EFSA Journal 2015; 13( 7):4186, 63 pp. doi:10.2903/j.efsa.2015.4186[↩]
- Ahmed F, Mohammed A. Magnesium: The Forgotten Electrolyte-A Review on Hypomagnesemia. Med Sci (Basel). 2019 Apr 4;7(4):56. doi: 10.3390/medsci7040056[↩][↩][↩]
- Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504[↩]
- Kirkland A.E., Sarlo G.L., Holton K.F. The Role of Magnesium in Neurological Disorders. Nutrients. 2018;10:730. doi: 10.3390/nu10060730[↩]
- Maier J.A.M., Locatelli L., Fedele G., Cazzaniga A., Mazur A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022;24:223. doi: 10.3390/ijms24010223[↩]
- Kirkland A.E., Sarlo G.L., Holton K.F. The role of magnesium in neurological disorders. Nutrients. 2018;10:730. doi: 10.3390/nu10060730[↩]
- Maier J.A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021;115:37–44. doi: 10.1016/j.semcdb.2020.11.002[↩]
- Andreini C., Bertini I., Cavallaro G., Holliday G.L., Thornton J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5[↩][↩]
- Martin K. J., Gonzalez E. A., Slatopolsky E. Clinical consequences and management of hypomagnesemia. Journal of American Society of Nephrology. 2009;20(11):2291–2295. doi: 10.1681/ASN.2007111194[↩][↩][↩]
- Viering D. H. H. M., de Baaij J. H. F., Walsh S. B., Kleta R., Bockenhauer D. Genetic causes of hypomagnesemia, a clinical overview. Pediatric Nephrology. 2017;32(7):1123–1135. doi: 10.1007/s00467-016-3416-3[↩][↩]
- Ryan M. F. The role of magnesium in clinical biochemistry: an overview. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine. 1991;28(1):19–26. doi: 10.1177/000456329102800103[↩][↩]
- Pelczyńska M, Moszak M, Bogdański P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients. 2022 Apr 20;14(9):1714. doi: 10.3390/nu14091714[↩][↩][↩][↩][↩]
- Buelens F.P., Leonov H., de Groot B.L., Grubmüller H. ATP-magnesium coordination: Protein structure-based force field evaluation and corrections. J. Chem. Theory Comput. 2021;17:1922–1930. doi: 10.1021/acs.jctc.0c01205[↩]
- Brautigam C.A., Steitz T.A. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998;8:54–63. doi: 10.1016/S0959-440X(98)80010-9[↩]
- Suh W.C., Leirmo S., Record M.T., Jr. Roles of Mg2+ in the mechanism of formation and dissociation of open complexes between Escherichia coli RNA polymerase and the lambda PR promoter: Kinetic evidence for a second open complex requiring Mg2+ Biochemistry. 1992;31:7815–7825. doi: 10.1021/bi00149a011[↩]
- de Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014[↩]
- Mousavi E., Ghoreishy S., Hemmati S.M., Mohammadi A. Association between Magnesium Concentrations and Prediabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2021;11:24388. doi: 10.1038/s41598-021-03915-3[↩][↩]
- Salehidoost R., Taghipour Boroujeni G., Feizi A., Aminorroaya A., Amini M. Effect of oral magnesium supplement on cardiometabolic markers in people with prediabetes: A double blind randomized controlled clinical trial. Sci. Rep. 2022;12:18209. doi: 10.1038/s41598-022-20277-6[↩]
- Al Alawi A.M., Majoni S.W., Falhammar H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018;2018:9041694. doi: 10.1155/2018/9041694[↩]
- Guerrera MP, Volpe SL, Mao JJ. Therapeutic uses of magnesium. Am Fam Physician 2009;80:157-62. https://www.aafp.org/afp/2009/0715/p157.html[↩][↩][↩][↩]
- https://www.phillipsdigestive.com/products/milk-magnesia/[↩][↩]
- Altman D., Carroli G., Duley L., Farrell B., Moodley J., Neilson J., Smith D., Magpie Trial Collaborative Group Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The magpie trial: A randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0[↩][↩][↩][↩]
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997. https://www.nap.edu/read/5776/chapter/1[↩][↩][↩]
- Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional[↩][↩][↩][↩][↩][↩]
- U.S. Department of Agriculture, Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America, NHANES 2013-2016. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/usual/Usual_Intake_gender_WWEIA_2013_2016.pdf[↩]
- Bailey RL, Fulgoni VL 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr. 2011 Nov;94(5):1376-81. doi: 10.3945/ajcn.111.020289[↩]
- Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012 Mar;70(3):153-64. doi: 10.1111/j.1753-4887.2011.00465.x[↩]
- Azoulay A, Garzon P, Eisenberg MJ. Comparison of the mineral content of tap water and bottled waters. J Gen Intern Med 2001;16:168-75. https://www.ncbi.nlm.nih.gov/pubmed/11318912[↩]
- Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest 1991;88:396-402. https://www.ncbi.nlm.nih.gov/pubmed/1864954[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/[↩]
- Ranade VV, Somberg JC. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am J Ther. 2001 Sep-Oct;8(5):345-57. doi: 10.1097/00045391-200109000-00008[↩][↩]
- Firoz M, Graber M. Bioavailability of US commercial magnesium preparations. Magnes Res. 2001 Dec;14(4):257-62.[↩]
- Mühlbauer B, Schwenk M, Coram WM, Antonin KH, Etienne P, Bieck PR, Douglas FL. Magnesium-L-aspartate-HCl and magnesium-oxide: bioavailability in healthy volunteers. Eur J Clin Pharmacol. 1991;40(4):437-8. doi: 10.1007/BF00265863[↩]
- Lindberg JS, Zobitz MM, Poindexter JR, Pak CY. Magnesium bioavailability from magnesium citrate and magnesium oxide. J Am Coll Nutr. 1990 Feb;9(1):48-55. doi: 10.1080/07315724.1990.10720349[↩]
- Walker AF, Marakis G, Christie S, Byng M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes Res. 2003 Sep;16(3):183-91.[↩]
- Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 1994 Oct;13(5):479-84. doi: 10.1080/07315724.1994.10718438[↩]
- Hendler SS, Rorvik DM. PDR for Nutritional Supplements. Montvale: Thomson Reuters; 2008.[↩][↩]
- Natural Medicines. Magnesium. Professional handout/Drug Interactions. https://naturalmedicines.therapeuticresearch.com[↩][↩][↩][↩]
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013 Jul;6(4):443-51. doi: 10.1586/17512433.2013.811206[↩]
- FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump[↩][↩][↩]
- Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61(5):685-712. doi: 10.2165/00003495-200161050-00013[↩]
- Arayne MS, Sultana N, Hussain F. Interactions between ciprofloxacin and antacids–dissolution and adsorption studies. Drug Metabol Drug Interact. 2005;21(2):117-29. doi: 10.1515/dmdi.2005.21.2.117[↩]
- Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf. 2010 Mar;9(2):259-73. doi: 10.1517/14740330903499257[↩][↩]
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997. https://nap.nationalacademies.org/read/5776/chapter/1[↩][↩][↩][↩][↩]
- Navarro-González JF, Mora-Fernández C, García-Pérez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009 Jan-Feb;22(1):37-44. doi: 10.1111/j.1525-139X.2008.00530.x[↩]
- Drueke TB, Lacour B. Disorders of calcium, phosphate and magnesium metabolism. Feehally J, Floege J, Johnson RJ, eds. Comprehensive Clinical Nephrology. 3rd ed. Philadelphia, Pa: Mosby; 2007. 137-8.[↩]
- Bringhurst FR, Demay MB, Krane SM, et al. Bone and mineral metabolism in health and disease/hypermagnesemia. Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005. 2245.[↩]
- Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin Dial. 2015 Nov-Dec;28(6):564-77. doi: 10.1111/sdi.12411[↩]
- Kutsal E, Aydemir C, Eldes N, Demirel F, Polat R, Taspnar O, Kulah E. Severe hypermagnesemia as a result of excessive cathartic ingestion in a child without renal failure. Pediatr Emerg Care. 2007 Aug;23(8):570-2. doi: 10.1097/PEC.0b013e31812eef1c[↩]
- McGuire JK, Kulkarni MS, Baden HP. Fatal hypermagnesemia in a child treated with megavitamin/megamineral therapy. Pediatrics. 2000 Feb;105(2):E18. doi: 10.1542/peds.105.2.e18[↩]
- Onishi S, Yoshino S. Cathartic-induced fatal hypermagnesemia in the elderly. Intern Med. 2006;45(4):207-10. doi: 10.2169/internalmedicine.45.1482[↩]
- Yamaguchi H, Shimada H, Yoshita K, Tsubata Y, Ikarashi K, Morioka T, Saito N, Sakai S, Narita I. Severe hypermagnesemia induced by magnesium oxide ingestion: a case series. CEN Case Rep. 2019 Feb;8(1):31-37. doi: 10.1007/s13730-018-0359-5[↩]
- Hypermagnesemia. https://emedicine.medscape.com/article/246489-overview#a6[↩][↩]
- Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005 Aug 15;62(16):1663-82. doi: 10.2146/ajhp040300[↩]
- Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res 2010;134:119–29. https://www.ncbi.nlm.nih.gov/pubmed/19629403[↩][↩]
- Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of U.S. adults. J Nutr 2003;133:2879-82. https://www.ncbi.nlm.nih.gov/pubmed/12949381[↩][↩]
- Musso CG Magnesium metabolism in health and disease. Int Urol Nephrol 2009;41:357-62. https://www.ncbi.nlm.nih.gov/pubmed/19274487[↩][↩]
- Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res 2009;22:235-46. https://www.ncbi.nlm.nih.gov/pubmed/20228001[↩][↩]
- Dickinson HO, Nicolson DJ, Campbell F, Cook JV, Beyer FR, Ford GA, Mason J. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004640. doi: 10.1002/14651858.CD004640.pub2[↩]
- Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012 Apr;66(4):411-8. doi: 10.1038/ejcn.2012.4[↩]
- Champagne CM. Dietary interventions on blood pressure: the Dietary Approaches to Stop Hypertension (DASH) trials. Nutr Rev. 2006 Feb;64(2 Pt 2):S53-6. doi: 10.1111/j.1753-4887.2006.tb00234.x[↩]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997 Apr 17;336(16):1117-24. doi: 10.1056/NEJM199704173361601[↩]
- Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension. 2016 Aug;68(2):324-33. doi: 10.1161/HYPERTENSIONAHA.116.07664[↩]
- Dibaba DT, Xun P, Song Y, Rosanoff A, Shechter M, He K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017 Sep;106(3):921-929. doi: 10.3945/ajcn.117.155291[↩]
- U.S. Food and Drug Administration. RE: Petition for a qualified health claim for magnesium and reduced risk of high blood pressure (hypertension) (docket No. FDA-2016-Q-3770) https://www.fda.gov/media/155304/download[↩][↩]
- Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2010;160:464-70. https://www.ncbi.nlm.nih.gov/pubmed/20826254?dopt=Abstract[↩]
- Chiuve SE, Korngold EC, Januzzi Jr JL, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr 2011;93:253-60. https://www.ncbi.nlm.nih.gov/pubmed/21106914?dopt=Abstract[↩]
- Joosten MM, Gansevoort RT, Mukamal KJ, van der Harst P, Geleijnse JM, Feskens EJM, Navis G, Bakker SJL. Urinary and plasma magnesium and risk of ischemic heart disease. Am J Clin Nutr 2013;97:1299-306. https://www.ncbi.nlm.nih.gov/pubmed/23485414?dopt=Abstract[↩]
- Del Gobbo LC, Imamura F, Wu JHY, Otto MCdO, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2013;98:160-73. https://www.ncbi.nlm.nih.gov/pubmed/23719551?dopt=Abstract[↩]
- Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012;95:362-6. https://www.ncbi.nlm.nih.gov/pubmed/22205313?dopt=Abstract[↩]
- Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013 Mar 18;2(2):e000114. doi: 10.1161/JAHA.113.000114[↩]
- Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2013 Jul;98(1):160-73. doi: 10.3945/ajcn.112.053132[↩][↩][↩]
- Fang X, Wang K, Han D, He X, Wei J, Zhao L, Imam MU, Ping Z, Li Y, Xu Y, Min J, Wang F. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med. 2016 Dec 8;14(1):210. doi: 10.1186/s12916-016-0742-z[↩][↩][↩][↩]
- Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012 Feb;95(2):362-6. doi: 10.3945/ajcn.111.022376[↩]
- Nie ZL, Wang ZM, Zhou B, Tang ZP, Wang SK. Magnesium intake and incidence of stroke: meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2013 Mar;23(3):169-76. doi: 10.1016/j.numecd.2012.04.015[↩]
- Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(3):e57720. doi: 10.1371/journal.pone.0057720[↩]
- Xu T, Sun Y, Xu T, Zhang Y. Magnesium intake and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013 Sep 10;167(6):3044-7. doi: 10.1016/j.ijcard.2012.11.090[↩]
- Zhang X, Xia J, Del Gobbo LC, Hruby A, Dai Q, Song Y. Serum magnesium concentrations and all-cause, cardiovascular, and cancer mortality among U.S. adults: Results from the NHANES I Epidemiologic Follow-up Study. Clin Nutr. 2018 Oct;37(5):1541-1549. doi: 10.1016/j.clnu.2017.08.021[↩]
- Angkananard T, Anothaisintawee T, Eursiriwan S, Gorelik O, McEvoy M, Attia J, Thakkinstian A. The association of serum magnesium and mortality outcomes in heart failure patients: A systematic review and meta-analysis. Medicine (Baltimore). 2016 Dec;95(50):e5406. doi: 10.1097/MD.0000000000005406[↩]
- Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998 Sep;136(3):480-90. doi: 10.1016/s0002-8703(98)70224-8[↩]
- Wannamethee SG, Papacosta O, Lennon L, Whincup PH. Serum magnesium and risk of incident heart failure in older men: The British Regional Heart Study. Eur J Epidemiol. 2018 Sep;33(9):873-882. doi: 10.1007/s10654-018-0388-6[↩]
- van den Bergh WM, Algra A, van der Sprenkel JW, Tulleken CA, Rinkel GJ. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003 Feb;52(2):276-81; discussion 281-2. doi: 10.1227/01.neu.0000043984.42487.0e[↩]
- Chen T, Carter BS. Role of magnesium sulfate in aneurysmal subarachnoid hemorrhage management: A meta-analysis of controlled clinical trials. Asian J Neurosurg. 2011 Jan;6(1):26-31. doi: 10.4103/1793-5482.85632[↩]
- Yarad EA, Hammond NE. Intravenous magnesium therapy in adult patients with an aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis. Aust Crit Care. 2013 Aug;26(3):105-17. doi: 10.1016/j.aucc.2013.05.002[↩]
- Golan E, Vasquez DN, Ferguson ND, Adhikari NK, Scales DC. Prophylactic magnesium for improving neurologic outcome after aneurysmal subarachnoid hemorrhage: systematic review and meta-analysis. J Crit Care. 2013 Apr;28(2):173-81. doi: 10.1016/j.jcrc.2012.07.001[↩]
- Kunze E, Lilla N, Stetter C, Ernestus RI, Westermaier T. Magnesium Protects in Episodes of Critical Perfusion after Aneurysmal SAH. Transl Neurosci. 2018 Sep 1;9:99-105. doi: 10.1515/tnsci-2018-0016[↩]
- Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, Kunze E, Matthies C, Ernestus RI, Solymosi L, Roosen K. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med. 2010 May;38(5):1284-90. doi: 10.1097/CCM.0b013e3181d9da1e[↩]
- Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD003611. doi: 10.1002/14651858.CD003611.pub3[↩]
- Fairley JL, Zhang L, Glassford NJ, Bellomo R. Magnesium status and magnesium therapy in cardiac surgery: A systematic review and meta-analysis focusing on arrhythmia prevention. J Crit Care. 2017 Dec;42:69-77. doi: 10.1016/j.jcrc.2017.05.038[↩][↩][↩]
- Wu X, Wang C, Zhu J, Zhang C, Zhang Y, Gao Y. Meta-analysis of randomized controlled trials on magnesium in addition to beta-blocker for prevention of postoperative atrial arrhythmias after coronary artery bypass grafting. BMC Cardiovasc Disord. 2013 Jan 23;13:5. doi: 10.1186/1471-2261-13-5[↩]
- Severino P., Netti L., Mariani M.V., Maraone A., D’Amato A., Scarpati R., Infusino F., Pucci M., LaValle C., Maestrini V., et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019;2019:1–10. doi: 10.1155/2019/4874921[↩]
- Lawson J.H., Mann K.G. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J. Biol. Chem. 1991;266:11317–11327. doi: 10.1016/S0021-9258(18)99165-9[↩]
- Sidonio R.F., Jr., Malec L. Hemophilia B (factor IX deficiency) Hematol. Oncol. Clin. N. Am. 2021;35:1143–1155. doi: 10.1016/j.hoc.2021.07.008[↩]
- Sekiya F., Yoshida M., Yamashita T., Morita T. Magnesium(II) is a crucial constituent of the blood coagulation cascade. Potentiation of coagulant activities of factor IX by Mg2+ ions. J. Biol. Chem. 1996;271:8541–8544. doi: 10.1074/jbc.271.15.8541[↩]
- Gajsiewicz J.M., Nuzzio K.M., Rienstra C.M., Morrissey J.H. Tissue factor residues that modulate magnesium-dependent rate enhancements of the tissue factor/factor VIIa complex. Biochemistry. 2015;54:4665–4671. doi: 10.1021/acs.biochem.5b00608[↩]
- van den Besselaar A.M. Magnesium and manganese ions accelerate tissue factor-induced coagulation independently of factor IX. Blood Coagul. Fibrinolysis. 2002;13:19–23. doi: 10.1097/00001721-200201000-00003[↩]
- Falls L.A., Furie B.C., Jacobs M., Furie B., Rigby A.C. The omega-loop region of the human prothrombin gamma-carboxyglutamic acid domain penetrates anionic phospholipid membranes. J. Biol. Chem. 2001;276:23895–23902. doi: 10.1074/jbc.M008332200[↩]
- Sheu J.R., Hsiao G., Shen M.Y., Fong T.H., Chen Y.W., Lin C.H., Chou D.S. Mechanisms involved in the antiplatelet activity of magnesium in human platelets. Br. J. Haematol. 2002;119:1033–1041. doi: 10.1046/j.1365-2141.2002.03967.x[↩]
- Xie C., Li X., Wu J., Liang Z., Deng F., Xie W., Zhu M., Zhu J., Zhu W., Geng S., et al. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation. 2015;38:1639–1648. doi: 10.1007/s10753-015-0140-2[↩]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735-52. doi: 10.1161/CIRCULATIONAHA.105.169404. Epub 2005 Sep 12. Erratum in: Circulation. 2005 Oct 25;112(17):e297. Erratum in: Circulation. 2005 Oct 25;112(17):e298.[↩]
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012 Nov;35(11):2402-11. doi: 10.2337/dc12-0336[↩]
- Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004 Jan 6;109(1):42-6. doi: 10.1161/01.CIR.0000108926.04022.0C[↩]
- Sung KC, Lee MY, Kim YH, Huh JH, Kim JY, Wild SH, Byrne CD. Obesity and incidence of diabetes: Effect of absence of metabolic syndrome, insulin resistance, inflammation and fatty liver. Atherosclerosis. 2018 Aug;275:50-57. doi: 10.1016/j.atherosclerosis.2018.05.042[↩]
- Moore-Schiltz L, Albert JM, Singer ME, Swain J, Nock NL. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001-2010 data. Br J Nutr. 2015 Sep 28;114(6):924-35. doi: 10.1017/S0007114515002482[↩]
- Dibaba DT, Xun P, Fly AD, Yokota K, He K. Dietary magnesium intake and risk of metabolic syndrome: a meta-analysis. Diabet Med. 2014 Nov;31(11):1301-9. doi: 10.1111/dme.12537[↩]
- Ju SY, Choi WS, Ock SM, Kim CM, Kim DH. Dietary magnesium intake and metabolic syndrome in the adult population: dose-response meta-analysis and meta-regression. Nutrients. 2014 Dec 22;6(12):6005-19. doi: 10.3390/nu6126005[↩]
- Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016 Apr;32(4):409-17. doi: 10.1016/j.nut.2015.09.014[↩][↩]
- La SA, Lee JY, Kim DH, Song EL, Park JH, Ju SY. Low Magnesium Levels in Adults with Metabolic Syndrome: a Meta-Analysis. Biol Trace Elem Res. 2016 Mar;170(1):33-42. doi: 10.1007/s12011-015-0446-9[↩]
- Volpe SL. Magnesium. In: Erdman Jr. JW, Macdonald IA, Ziegler EE, eds. Present Knowledge in Nutrition. 10th ed: ILSI Press; 2012:459-474.[↩][↩][↩]
- Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005 Jun;28(6):1438-44. doi: 10.2337/diacare.28.6.1438[↩]
- Dibaba DT, Xun P, He K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur J Clin Nutr. 2014 Apr;68(4):510-6. doi: 10.1038/ejcn.2014.7. Epub 2014 Feb 12. Erratum in: Eur J Clin Nutr. 2015 Mar;69(3):410.[↩]
- Maier J.A.M. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2011;122:397–407. doi: 10.1042/CS20110506[↩][↩]
- Coman AE, Ceasovschih A, Petroaie AD, Popa E, Lionte C, Bologa C, Haliga RE, Cosmescu A, Slănină AM, Bacușcă AI, Șorodoc V, Șorodoc L. The Significance of Low Magnesium Levels in COVID-19 Patients. Medicina (Kaunas). 2023 Jan 31;59(2):279. doi: 10.3390/medicina59020279[↩][↩]
- Galley H.F., Webster N.R. Physiology of the endothelium. Br. J. Anaesth. 2004;93:105–113. doi: 10.1093/bja/aeh163[↩]
- Ferrè S., Baldoli E., Leidi M., Maier J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim. Biophys. Acta. 2010;1802:952–958. doi: 10.1016/j.bbadis.2010.06.016[↩]
- Esteban F., Ramos-García P., Muñoz M., González-Moles M. Substance P and Neurokinin 1 Receptor in Chronic Inflammation and Cancer of the Head and Neck: A Review of the Literature. Int. J. Environ. Res. Public Health. 2021;19:375. doi: 10.3390/ijerph19010375[↩]
- Weglicki W.B., Chmielinska J.J., Tejero-Taldo I., Kramer J.H., Spurney C.F., Viswalingham K., Lu B., Mak I.T. Neutral endopeptidase inhibition enhances substance P mediated inflammation due to hypomagnesemia. Magnes. Res. 2009;22:167S–173S. doi: 10.1684/mrh.2009.0181[↩]
- Nielsen F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742[↩][↩][↩]
- Mazur A., Maier J.A., Rock E., Gueux E., Nowacki W., Rayssiguier Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2006;458:48–56. doi: 10.1016/j.abb.2006.03.031[↩]
- Chacko S.A., Song Y., Nathan L., Tinker L., de Boer I.H., Tylavsky F., Wallace R., Liu S. Relations of Dietary Magnesium Intake to Biomarkers of Inflammation and Endothelial Dysfunction in an Ethnically Diverse Cohort of Postmenopausal Women. Diabetes Care. 2009;33:304–310. doi: 10.2337/dc09-1402[↩][↩]
- Malpuech-Brugère C., Nowacki W., Daveau M., Gueux E., Linard C., Rock E., Lebreton J.-P., Mazur A., Rayssiguier Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta. 2000;1501:91–98. doi: 10.1016/S0925-4439(00)00018-1[↩]
- Zhu D., You J., Zhao N., Xu H. Magnesium Regulates Endothelial Barrier Functions through TRPM7, MagT1, and S1P1. Adv. Sci. 2019;6:1901166. doi: 10.1002/advs.201901166[↩]
- King D.E., Mainous A.G., III, Geesey M.E., Woolson R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005;24:166. doi: 10.1080/07315724.2005.10719461[↩][↩]
- Mazidi M., Rezaie P., Banach M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 2018;14:707–716. doi: 10.5114/aoms.2018.75719[↩]
- Dibaba D.T., Xun P., He K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014;68:510. doi: 10.1038/ejcn.2014.7[↩]
- Ju S.-Y., Choi W.-S., Ock S.-M., Kim C.-M., Kim D.-H. Dietary Magnesium Intake and Metabolic Syndrome in the Adult Population: Dose-Response Meta-Analysis and Meta-Regression. Nutrients. 2014;6:6005–6019. doi: 10.3390/nu6126005[↩]
- Konstari S., Sares-Jäske L., Heliövaara M., Rissanen H., Knekt P., Arokoski J., Sundvall J., Karppinen J. Dietary magnesium intake, serum high sensitivity C-reactive protein and the risk of incident knee osteoarthritis leading to hospitalization—A cohort study of 4,953 Finns. PLoS ONE. 2019;14:e0214064. doi: 10.1371/journal.pone.0214064[↩]
- Mahabir S. Dietary magnesium and inflammation. Eur. J. Clin. Nutr. 2014;68:970. doi: 10.1038/ejcn.2014.110[↩]
- Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol. Dis. 2006;36:104–107. doi: 10.1016/j.bcmd.2005.12.008[↩]
- Sarrafzadegan N., Khosravi-Boroujeni H., Lotfizadeh M., Pourmogaddas A., Salehi-Abargouei A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2015;32:409–417. doi: 10.1016/j.nut.2015.09.014[↩]
- Kim D.J., Xun P., Liu K., Loria C., Yokota K., Jacobs D.R., He K. Magnesium Intake in Relation to Systemic Inflammation, Insulin Resistance, and the Incidence of Diabetes. Diabetes Care. 2010;33:2604–2610. doi: 10.2337/dc10-0994[↩]
- Dong Y., Chen L., Gutin B., Huang Y., Dong Y., Zhu H. Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents. Nutrients. 2022;14:2882. doi: 10.3390/nu14142882[↩]
- Maier J.A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2020;115:37–44. doi: 10.1016/j.semcdb.2020.11.002[↩]
- Ceasovschih A., Sorodoc V., Onofrei V., Tesloianu D., Tuchilus C., Anisie E., Petris A., Statescu C., Jaba E., Stoica A., et al. Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study. Diagnostics. 2020;10:723. doi: 10.3390/diagnostics10090723[↩]
- Tam M, Gómez S, González-Gross M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003;57:1193–1197. doi: 10.1038/sj.ejcn.1601689[↩]
- Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7-gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009[↩]
- Ravell J, Chaigne-Delalande B, Lenardo M. X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia disease: a combined immune deficiency with magnesium defect. Curr Opin Pediatr. 2014;26:713–719. doi: 10.1097/MOP.0000000000000156[↩]
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011 Jul 27;475(7357):471-6. doi: 10.1038/nature10246[↩]
- Liang RY, Wu W, Huang J, Jiang SP, Lin Y. Magnesium affects the cytokine secretion of CD4+ T lymphocytes in acute asthma. J Asthma. 2012;49:1012–1015. doi: 10.3109/02770903.2012.739240[↩]
- Kubena KS. The role of magnesium in immunity. J Nutr Immunol. 1994;2(3):107–126. doi: 10.1300/J053v02n03_07[↩][↩]
- Mazur A., Maier J.A., Rock E., Gueux E., Nowacki W., Rayssiguier Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031[↩]
- Laires MJ, Monteiro C. Exercise, magnesium and immune function. Magnes Res. 2008;21(2):92–96. doi: 10.1684/mrh.2008.0136[↩][↩]
- Galland L. Magnesium and immune function: an overview. Magnesium. 1988;7(5-6):290-9.[↩][↩][↩]
- Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018;11:25–34. doi: 10.2147/JIR.S136742[↩]
- Nielsen F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742[↩]
- Klein G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules. 2018;8:69. doi: 10.3390/biom8030069[↩]
- Güzel A., Doğan E., Türkçü G., Kuyumcu M., Kaplan İ., Çelik F., Yıldırım Z.B. Dexmedetomidine and Magnesium Sulfate: A Good Combination Treatment for Acute Lung Injury? J. Investig. Surg. 2019;32:331–342. doi: 10.1080/08941939.2017.1422575[↩]
- Tang C.-F., Ding H., Jiao R.-Q., Wu X.-X., Kong L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546[↩]
- Iotti S., Wolf F., Mazur A., Maier J.A. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020;33:21–27. doi: 10.1684/mrh.2020.0465[↩]
- Tang CF, Ding H, Jiao RQ, Xing Xin Wu, Kong LD. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546[↩]
- Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, et al. A cohort study to evaluate the effect of combination vitamin D, magnesium and vitamin B12 (DMB) on progression to severe outcome in older COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.06.01.20112334[↩]
- Pourdowlat G, Mousavinasab SR, Farzanegan B, Kashefizadeh A, Meybodi ZA, Jafarzadeh M, Baniasadi S. Evaluation of the efficacy and safety of inhaled magnesium sulphate in combination with standard treatment in patients with moderate or severe COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:60. doi: 10.1186/s13063-021-05032-y[↩]
- Schmitz C, Anne-Laure P. Molecular, genetic, and nutritional aspects of major and trace minerals. In: Collins J, editor. Magnesium and the immune response. Amsterdam: Elsevier Inc; 2017. pp. 319–331.[↩]
- Petrović J, Stanić D, Dmitrašinović G, Plećaš-Solarović B, Ignjatović S, Batinić B, Popović D, Pešić V. Magnesium Supplementation Diminishes Peripheral Blood Lymphocyte DNA Oxidative Damage in Athletes and Sedentary Young Man. Oxid Med Cell Longev. 2016;2016:2019643. doi: 10.1155/2016/2019643[↩]
- Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23(2):73–80. doi: 10.1684/mrh.2010.0208[↩]
- Moh’d Nour MBY, Alshawabkeh AD, Jadallah ARR, et al. Magnesium sulfate extended infusion as an adjunctive treatment for complicated Covid-19 infected critically ill patients. EAS J Anesthesiol Crit Care. 2020 doi: 10.36349/easjacc.2020.v02i03.17[↩]
- Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med. 2007 Aug;262(2):208-14. doi: 10.1111/j.1365-2796.2007.01840.x[↩][↩][↩]
- Rodríguez-Morán M, Simental Mendía LE, Zambrano Galván G, Guerrero-Romero F. The role of magnesium in type 2 diabetes: a brief based-clinical review. Magnes Res. 2011 Dec;24(4):156-62. doi: 10.1684/mrh.2011.0299[↩][↩]
- Simmons D, Joshi S, Shaw J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res Clin Pract. 2010 Feb;87(2):261-6. doi: 10.1016/j.diabres.2009.11.003[↩]
- Simmons D., Joshi S., Shaw J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res. Clin. Pract. 2010;87:261–266. doi: 10.1016/j.diabres.2009.11.003[↩]
- Fang X., Wang K., Han D., He X., Wei J., Zhao L., Imam M.U., Ping Z., Li Y., Xu Y., et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. BMC Med. 2016;14:210. doi: 10.1186/s12916-016-0742-z[↩][↩]
- Wu J., Xun P., Tang Q., Cai W., He K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Nutr. J. 2017;16:60. doi: 10.1186/s12937-017-0280-3[↩]
- Kim D.J., Xun P., Liu K., Loria C., Yokota K., Jacobs D.R., Jr., He K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33:2604–2610. doi: 10.2337/dc10-0994[↩]
- Villegas R., Gao Y.T., Dai Q., Yang G., Cai H., Li H., Zheng W., Shu X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009;89:1059–1067. doi: 10.3945/ajcn.2008.27182[↩]
- Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007 May 14;167(9):956-65. doi: 10.1001/archinte.167.9.956[↩][↩][↩]
- Fang X, Han H, Li M, Liang C, Fan Z, Aaseth J, He J, Montgomery S, Cao Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients. 2016 Nov 19;8(11):739. doi: 10.3390/nu8110739[↩][↩]
- Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011 Sep;34(9):2116-22. doi: 10.2337/dc11-0518[↩][↩][↩]
- Hruby A, Ngwa JS, Renström F, et al. Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 CHARGE Consortium Studies. J Nutr. 2013 Mar;143(3):345-53. doi: 10.3945/jn.112.172049[↩][↩]
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. Failure of beta-cell function for compensate variation in insulin sensitivity in hypomagnesemic subjects. Magnes Res. 2009 Sep;22(3):151-6.[↩]
- Guerrero-Romero F, Rodríguez-Morán M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trial. Eur J Clin Invest. 2011 Apr;41(4):405-10. doi: 10.1111/j.1365-2362.2010.02422.x[↩]
- Guerrero-Romero F, Simental-Mendía LE, Hernández-Ronquillo G, Rodriguez-Morán M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: A double-blind placebo-controlled randomized trial. Diabetes Metab. 2015 Jun;41(3):202-7. doi: 10.1016/j.diabet.2015.03.010[↩][↩]
- Rodríguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: a randomized double-blind placebo-controlled trial. Arch Med Res. 2014 Jul;45(5):388-93. doi: 10.1016/j.arcmed.2014.05.003[↩]
- Mooren FC, Krüger K, Völker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects – a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab. 2011 Mar;13(3):281-4. doi: 10.1111/j.1463-1326.2010.01332.x[↩]
- Guerrero-Romero F., Nevárez-Sida A. Cost-effectiveness analysis of using oral magnesium supplementation in the treatment of prediabetes. Prim. Care Diabetes. 2022;16:435–439. doi: 10.1016/j.pcd.2022.03.013[↩][↩]
- Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS Jr; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013 Nov;36(11):3821-42. doi: 10.2337/dc13-2042[↩][↩]
- de Lordes Lima M, Cruz T, Pousada JC, Rodrigues LE, Barbosa K, Canguçu V. The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care. 1998 May;21(5):682-6. doi: 10.2337/diacare.21.5.682[↩][↩][↩]
- Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care. 2003 Apr;26(4):1147-52. doi: 10.2337/diacare.26.4.1147[↩]
- de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet Med. 1998 Jun;15(6):503-7. doi: 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M[↩]
- Hierons S.J., Catchpole A., Abbas K., Wong W., Giles M.S., Miller G.V., Ajjan R.A., Stewart A.J. Total plasma magnesium, zinc, copper and selenium concentrations in obese patients before and after bariatric surgery. Biometals. 2023;36:241–253. doi: 10.1007/s10534-022-00368-7[↩]
- Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009 Apr;28(2):131-41. doi: 10.1080/07315724.2009.10719764[↩][↩]
- Vormann J. Magnesium: Nutrition and Homoeostasis. AIMS Public Health. 2016 May 23;3(2):329-340. doi: 10.3934/publichealth.2016.2.329[↩]
- Sojka JE, Weaver CM. Magnesium supplementation and osteoporosis. Nutr Rev. 1995 Mar;53(3):71-4. doi: 10.1111/j.1753-4887.1995.tb01505.x[↩]
- Zheng J, Mao X, Ling J, He Q, Quan J, Jiang H. Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol Trace Elem Res. 2014 Jun;159(1-3):8-14. doi: 10.1007/s12011-014-9961-3[↩]
- Bégin MJ, Ste-Marie LG, Coupal L, Ethier J, Räkel A. Hypomagnesemia During Teriparatide Treatment in Osteoporosis: Incidence and Determinants. J Bone Miner Res. 2018 Aug;33(8):1444-1449. doi: 10.1002/jbmr.3438[↩]
- Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. 2009 Dec;7(4):111-7. doi: 10.1007/s11914-009-0020-5[↩]
- Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res. 2007 Sep-Oct;35(5):692-5. doi: 10.1177/147323000703500514[↩]
- Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999 Apr;69(4):727-36. doi: 10.1093/ajcn/69.4.727[↩]
- Ryder KM, Shorr RI, Bush AJ, Kritchevsky SB, Harris T, Stone K, Cauley J, Tylavsky FA. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J Am Geriatr Soc. 2005 Nov;53(11):1875-80. doi: 10.1111/j.1532-5415.2005.53561.x[↩]
- Dahl C, Søgaard AJ, Tell GS, Flaten TP, Hongve D, Omsland TK, Holvik K, Meyer HE, Aamodt G. Nationwide data on municipal drinking water and hip fracture: could calcium and magnesium be protective? A NOREPOS study. Bone. 2013 Nov;57(1):84-91. doi: 10.1016/j.bone.2013.06.017[↩]
- Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, LaCroix AZ, Wactawski-Wende J, Jackson RD. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr. 2014 Apr;99(4):926-33. doi: 10.3945/ajcn.113.067488[↩][↩]
- Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015 Aug;102(2):376-84. doi: 10.3945/ajcn.114.102723[↩]
- Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res. 1993 Jun;6(2):155-63.[↩]
- Abraham GE, Grewal H. A total dietary program emphasizing magnesium instead of calcium. Effect on the mineral density of calcaneous bone in postmenopausal women on hormonal therapy. J Reprod Med. 1990 May;35(5):503-7.[↩]
- Aydin H, Deyneli O, Yavuz D, Gözü H, Mutlu N, Kaygusuz I, Akalin S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res. 2010 Feb;133(2):136-43. doi: 10.1007/s12011-009-8416-8[↩][↩]
- Nieves JW. Bone. Maximizing bone health–magnesium, BMD and fractures. Nat Rev Endocrinol. 2014 May;10(5):255-6. doi: 10.1038/nrendo.2014.39[↩]
- Reddy V, Sivakumar B. Magnesium-dependent vitamin-D-resistant rickets. Lancet. 1974 May 18;1(7864):963-5. doi: 10.1016/s0140-6736(74)91265-3[↩]
- Brill S, Sedgwick PM, Hamann W, Di Vadi PP. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002 Nov;89(5):711-4.[↩]
- Tanaka M, Shimizu S, Nishimura W, Mine O, Akatsuka M, Inamori K, Mori H. [Relief of neuropathic pain with intravenous magnesium]. Masui. 1998 Sep;47(9):1109-13. Japanese.[↩]
- Pickering G, Morel V, Simen E, Cardot JM, Moustafa F, Delage N, Picard P, Eschalier S, Boulliau S, Dubray C. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011 Jun;24(2):28-35. doi: 10.1684/mrh.2011.0282[↩]
- Delage N, Morel V, Picard P, Marcaillou F, Pereira B, Pickering G. Effect of ketamine combined with magnesium sulfate in neuropathic pain patients (KETAPAIN): study protocol for a randomized controlled trial. Trials. 2017 Nov 3;18(1):517. doi: 10.1186/s13063-017-2254-3[↩]
- Wang SC, Pan PT, Chiu HY, Huang CJ. Neuraxial magnesium sulfate improves postoperative analgesia in Cesarean section delivery women: A meta-analysis of randomized controlled trials. Asian J Anesthesiol. 2017 Sep;55(3):56-67. doi: 10.1016/j.aja.2017.06.005[↩][↩]
- Xiao F, Xu W, Feng Y, Fu F, Zhang X, Zhang Y, Wang L, Chen X. Intrathecal magnesium sulfate does not reduce the ED50 of intrathecal hyperbaric bupivacaine for cesarean delivery in healthy parturients: a prospective, double blinded, randomized dose-response trial using the sequential allocation method. BMC Anesthesiol. 2017 Jan 17;17(1):8. doi: 10.1186/s12871-017-0300-z[↩]
- Paleti S, Prasad PK, Lakshmi BS. A randomized clinical trial of intrathecal magnesium sulfate versus midazolam with epidural administration of 0.75% ropivacaine for patients with preeclampsia scheduled for elective cesarean section. J Anaesthesiol Clin Pharmacol. 2018 Jan-Mar;34(1):23-28. doi: 10.4103/joacp.JOACP_74_17[↩]
- Vlok R, Melhuish TM, Chong C, Ryan T, White LD. Adjuncts to local anaesthetics in tonsillectomy: a systematic review and meta-analysis. J Anesth. 2017 Aug;31(4):608-616. doi: 10.1007/s00540-017-2310-x[↩][↩]
- Cho HK, Park IJ, Yoon HY, Hwang SH. Efficacy of Adjuvant Magnesium for Posttonsillectomy Morbidity in Children: A Meta-analysis. Otolaryngol Head Neck Surg. 2018 Jan;158(1):27-35. doi: 10.1177/0194599817730354[↩]
- Xie M, Li XK, Peng Y. Magnesium sulfate for postoperative complications in children undergoing tonsillectomies: a systematic review and meta-analysis. J Evid Based Med. 2017 Feb;10(1):16-25. doi: 10.1111/jebm.12230[↩][↩][↩]
- Chen C, Tao R. The Impact of Magnesium Sulfate on Pain Control After Laparoscopic Cholecystectomy: A Meta-Analysis of Randomized Controlled Studies. Surg Laparosc Endosc Percutan Tech. 2018 Dec;28(6):349-353. doi: 10.1097/SLE.0000000000000571[↩]
- Imani F, Rahimzadeh P, Faiz HR, Abdullahzadeh-Baghaei A. An Evaluation of the Adding Magnesium Sulfate to Ropivacaine on Ultrasound-Guided Transverse Abdominis Plane Block After Abdominal Hysterectomy. Anesth Pain Med. 2018 Jul 29;8(4):e74124. doi: 10.5812/aapm.74124[↩]
- Abd-Elsalam KA, Fares KM, Mohamed MA, Mohamed MF, El-Rahman AMA, Tohamy MM. Efficacy of Magnesium Sulfate Added to Local Anesthetic in a Transversus Abdominis Plane Block for Analgesia Following Total Abdominal Hysterectomy: A Randomized Trial. Pain Physician. 2017 Nov;20(7):641-647.[↩]
- Martin DP, Samora WP 3rd, Beebe AC, Klamar J, Gill L, Bhalla T, Veneziano G, Thung A, Tumin D, Barry N, Rice J, Tobias JD. Analgesic effects of methadone and magnesium following posterior spinal fusion for idiopathic scoliosis in adolescents: a randomized controlled trial. J Anesth. 2018 Oct;32(5):702-708. doi: 10.1007/s00540-018-2541-5[↩]
- Srivastava VK, Mishra A, Agrawal S, Kumar S, Sharma S, Kumar R. Comparative Evaluation of Dexmedetomidine and Magnesium Sulphate on Propofol Consumption, Haemodynamics and Postoperative Recovery in Spine Surgery: A Prospective, Randomized, Placebo Controlled, Double-blind Study. Adv Pharm Bull. 2016 Mar;6(1):75-81. doi: 10.15171/apb.2016.012[↩]
- Hamed MA. Comparative Study between Magnesium Sulfate and Lidocaine for Controlled Hypotension during Functional Endoscopic Sinus Surgery: A Randomized Controlled Study. Anesth Essays Res. 2018 Jul-Sep;12(3):715-718. doi: 10.4103/aer.AER_103_18[↩]
- Hassan PF, Saleh AH. Dexmedetomidine versus Magnesium Sulfate in Anesthesia for Cochlear Implantation Surgery in Pediatric Patients. Anesth Essays Res. 2017 Oct-Dec;11(4):1064-1069. doi: 10.4103/aer.AER_72_17[↩]
- Hashimoto Y, Nishimura Y, Maeda H, Yokoyama M. Assessment of magnesium status in patients with bronchial asthma. J Asthma. 2000 Sep;37(6):489-96. doi: 10.3109/02770900009055475[↩]
- Irazuzta JE, Chiriboga N. Magnesium sulfate infusion for acute asthma in the emergency department. J Pediatr (Rio J). 2017 Nov-Dec;93 Suppl 1:19-25. doi: 10.1016/j.jped.2017.06.002[↩]
- Su Z, Li R, Gai Z. Intravenous and Nebulized Magnesium Sulfate for Treating Acute Asthma in Children: A Systematic Review and Meta-Analysis. Pediatr Emerg Care. 2018 Jun;34(6):390-395. doi: 10.1097/PEC.0000000000000909[↩]
- Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014 May 28;(5):CD010909. doi: 10.1002/14651858.CD010909.pub2[↩]
- Knightly R, Milan SJ, Hughes R, Knopp-Sihota JA, Rowe BH, Normansell R, Powell C. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2017 Nov 28;11(11):CD003898. doi: 10.1002/14651858.CD003898.pub6[↩]
- Monteleone CA, Sherman AR. Nutrition and asthma. Arch Intern Med. 1997 Jan 13;157(1):23-34.[↩]
- Beasley R, Aldington S. Magnesium in the treatment of asthma. Curr Opin Allergy Clin Immunol. 2007 Feb;7(1):107-10. doi: 10.1097/ACI.0b013e328012ce4b[↩]
- Sun-Edelstein C, Mauskop A. Role of magnesium in the pathogenesis and treatment of migraine. Expert Rev Neurother 2009;9:369–79. https://www.ncbi.nlm.nih.gov/pubmed/19271946?dopt=Abstract[↩][↩]
- Schürks M, Diener H-C, Goadsby P. Update on the prophylaxis of migraine. Cur Treat Options Neurol 2008;10:20–9. https://www.ncbi.nlm.nih.gov/pubmed/18325296?dopt=Abstract[↩]
- Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults. Neurology 2012;78:1346-53. https://www.ncbi.nlm.nih.gov/pubmed/22529203?dopt=Abstract[↩]
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phillips WR, Phipps MG, Silverstein M, Simon MA, Tseng CW. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017 Apr 25;317(16):1661-1667. doi: 10.1001/jama.2017.3439[↩]
- Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013 Oct;71 Suppl 1(0 1):S18-25. doi: 10.1111/nure.12055[↩][↩]
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009 Jun;33(3):130-7. doi: 10.1053/j.semperi.2009.02.010[↩]
- Duley L, Henderson-Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010 Oct 6;(10):CD000128. doi: 10.1002/14651858.CD000128.pub2[↩][↩][↩]
- Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014 Apr 3;2014(4):CD000937. doi: 10.1002/14651858.CD000937.pub2[↩]
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005 Feb;105(2):402-10. doi: 10.1097/01.AOG.0000152351.13671.99[↩]
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, Smith D; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002 Jun 1;359(9321):1877-90. doi: 10.1016/s0140-6736(02)08778-0[↩]
- McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. Int J Gynaecol Obstet. 2012 Aug;118(2):90-6. doi: 10.1016/j.ijgo.2012.01.028[↩]
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Implications and actions.[↩]
- Berhan Y, Berhan A. Should magnesium sulfate be administered to women with mild pre-eclampsia? A systematic review of published reports on eclampsia. J Obstet Gynaecol Res. 2015 Jun;41(6):831-42. doi: 10.1111/jog.12697[↩]
- Vigil-DeGracia P, Ludmir J, Ng J, Reyes-Tejada O, Nova C, Beltré A, Yuen-Chon V, Collantes J, Turcios E, Lewis R, Cabrera S. Is there benefit to continue magnesium sulphate postpartum in women receiving magnesium sulphate before delivery? A randomised controlled study. BJOG. 2018 Sep;125(10):1304-1311. doi: 10.1111/1471-0528.15320[↩]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice Society for Maternal-Fetal Medicine. Committee Opinion No. 573: Magnesium sulfate use in obstetrics. Obstet Gynecol. 2013 Sep;122(3):727-8. doi: 10.1097/01.AOG.0000433994.46087.85[↩]
- Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017 Mar 21;3(3):CD004454. doi: 10.1002/14651858.CD004454.pub3. Update in: Cochrane Database Syst Rev. 2020 Dec 25;12:CD004454.[↩]
- Crowther CA, Brown J, McKinlay CJ, Middleton P. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD001060. doi: 10.1002/14651858.CD001060.pub2[↩]
- McNamara HC, Crowther CA, Brown J. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour. Cochrane Database Syst Rev. 2015 Dec 14;2015(12):CD011200. doi: 10.1002/14651858.CD011200.pub2[↩]
- Wolf HT, Hegaard HK, Greisen G, Huusom L, Hedegaard M. Treatment with magnesium sulphate in pre-term birth: a systematic review and meta-analysis of observational studies. J Obstet Gynaecol. 2012 Feb;32(2):135-40. doi: 10.3109/01443615.2011.638999[↩]
- Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, Marret S, Doyle LW; AMICABLE Group. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med. 2017 Oct 4;14(10):e1002398. doi: 10.1371/journal.pmed.1002398[↩]
- Tagin M, Shah PS, Lee KS. Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinatol. 2013 Sep;33(9):663-9. doi: 10.1038/jp.2013.65[↩]
- Gragossian A, Bashir K, Bhutta BS, et al. Hypomagnesemia. [Updated 2022 Nov 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500003[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ayuk J, Gittoes NJ. How should hypomagnesaemia be investigated and treated? Clin Endocrinol (Oxf). 2011 Dec;75(6):743-6. doi: 10.1111/j.1365-2265.2011.04092.x[↩][↩][↩][↩]
- Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018 Jan 18;11:25-34. doi: 10.2147/JIR.S136742[↩]
- Kim E. Pathogenesis and treatment of electrolyte problems post transplant. Curr Opin Pediatr. 2019 Apr;31(2):213-218. doi: 10.1097/MOP.0000000000000715[↩]
- Fulchiero R, Seo-Mayer P. Bartter Syndrome and Gitelman Syndrome. Pediatr Clin North Am. 2019 Feb;66(1):121-134. doi: 10.1016/j.pcl.2018.08.010[↩]
- Magnesium deficiency. https://medlineplus.gov/ency/article/000315.htm[↩][↩]
- Viering DHHM, de Baaij JHF, Walsh SB, Kleta R, Bockenhauer D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol. 2017 Jul;32(7):1123-1135. doi: 10.1007/s00467-016-3416-3[↩][↩][↩][↩]
- Pham P. C., Pham P. A., Pham S. V., Pham P. T., Pham P. M., Pham P. T. Hypomagnesemia: a clinical perspective. International Journal of Nephrology and Renovascular Disease. 2014;7:219–230. doi: 10.2147/ijnrd.s42054[↩][↩][↩]
- Andreozzi F, Cuminetti G, Karmali R, Kamgang P. Electrolyte Disorders as Triggers for Takotsubo Cardiomyopathy. Eur J Case Rep Intern Med. 2018 Apr 24;5(4):000760. doi: 10.12890/2018_000760[↩]
- Kurstjens S, Bouras H, Overmars-Bos C, Kebieche M, Bindels RJM, Hoenderop JGJ, de Baaij JHF. Diabetes-induced hypomagnesemia is not modulated by metformin treatment in mice. Sci Rep. 2019 Feb 11;9(1):1770. doi: 10.1038/s41598-018-38351-3[↩]
- Brophy C, Woods R, Murphy MS, Sheahan P. Perioperative magnesium levels in total thyroidectomy and relationship to hypocalcemia. Head Neck. 2019 Jun;41(6):1713-1718. doi: 10.1002/hed.25644[↩]
- Schlegel RN, Cuffe JS, Moritz KM, Paravicini TM. Maternal hypomagnesemia causes placental abnormalities and fetal and postnatal mortality. Placenta. 2015;36:750–758. doi: 10.1016/j.placenta.2015.03.011[↩]
- Hall DG. Serum magnesium in pregnancy. Obstet Gynecol. 1957;9:158–162. doi: 10.1097/00006250-195706000-00019[↩]
- Kolisek M, Galaviz-Hernández C, Vázquez-Alaniz F, Sponder G, Javaid S, Kurth K, Nestler A, Rodríguez-Moran M, Verlohren S, Guerrero-Romero F, Aschenbach JR, Vormann J. SLC41A1 is the only magnesium responsive gene significantly overexpressed in placentas of preeclamptic women. Hypertens Pregnancy. 2013 Nov;32(4):378-89. doi: 10.3109/10641955.2013.810237[↩]
- de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014[↩]
- Agus ZS. Mechanisms and causes of hypomagnesemia. Curr Opin Nephrol Hypertens. 2016 Jul;25(4):301-7. doi: 10.1097/MNH.0000000000000238[↩]
- Cheungpasitporn W, Thongprayoon C, Qian Q. Dysmagnesemia in Hospitalized Patients: Prevalence and Prognostic Importance. Mayo Clin Proc. 2015 Aug;90(8):1001-10. doi: 10.1016/j.mayocp.2015.04.023[↩]
- De Marchi S, Cecchin E, Basile A, Bertotti A, Nardini R, Bartoli E. Renal tubular dysfunction in chronic alcohol abuse–effects of abstinence. N Engl J Med. 1993 Dec 23;329(26):1927-34. doi: 10.1056/NEJM199312233292605[↩]
- Workinger J.L., Doyle R.P., Bortz J. Challenges in the Diagnosis of Magnesium Status. Nutrients. 2018;10:1202. doi: 10.3390/nu10091202[↩][↩]
- Williamson M. A., Snyder L. M., Wallach J. B. Wallach’s Interpretation of Diagnostic Tests. 9th. Philadelphia, PA, USA: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. xvi, 1143.[↩][↩][↩][↩]
- Ismail A.A.A., Ismail Y., Ismail A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM. 2018;111:759–763. doi: 10.1093/qjmed/hcx186[↩][↩][↩]
- Gröber U., Schmidt J., Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388[↩][↩][↩][↩][↩]
- Tibbetts D.M., Aub J.C. Magnesium metabolism in health and disease. I. the magnesium and calcium excretion of normal individuals, also the effects of magnesium, chloride, and phosphate ions. J. Clin. Investig. 1937;16:491–501. doi: 10.1172/JCI100874[↩][↩]
- Deng B., Li X., Zhu P., Xu X., Xu Q., Kang Y. Speciation of magnesium in rat plasma using capillary electrophoresis-inductively coupled plasma-atomic emission spectrometry. Electrophoresis. 2008;29:1534–1539. doi: 10.1002/elps.200700423[↩][↩][↩][↩]
- WALSER M. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest. 1961 Apr;40(4):723-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC373170/pdf/jcinvest00303-0131.pdf[↩][↩]
- Peters T., Jr. All about Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press; New York, NY, USA: 1995.[↩][↩]
- Ryan M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991;28:19–26. doi: 10.1177/000456329102800103[↩][↩]
- Alfrey A.C., Miller N.L., Trow R. Effect of age and magnesium depletion on bone magnesium pools in rats. J. Clin. Investig. 1974;54:1074–1081. doi: 10.1172/JCI107851[↩][↩]
- Hansen BA, Bruserud Ø. Hypomagnesemia in critically ill patients. J Intensive Care. 2018 Mar 27;6:21. doi: 10.1186/s40560-018-0291-y[↩][↩][↩]
- Greco DS. Endocrine causes of calcium disorders. Top Companion Anim Med. 2012 Nov;27(4):150-5. doi: 10.1053/j.tcam.2012.11.001[↩][↩]
- Jahnen-Dechent W., Ketteler M. Magnesium Basics. Clin. Kidney J. 2012;5((Suppl. S1[↩][↩][↩][↩][↩][↩]
- Pham P.C.T., Pham P.A.T., Pham S.V., Pham P.T.T., Pham P.M.T., Pham P.T.T. Hypomagnesemia: A Clinical Perspective. Int. J. Nephrol. Renovasc. Dis. 2014;7:219–230. doi: 10.2147/IJNRD.S42054[↩][↩][↩]
- Seo J. W., Park T. J. Magnesium metabolism. Electrolyte Blood Pressure. 2008;6(2):86–95. doi: 10.5049/EBP.2008.6.2.86[↩]
- Bergman C., Gray-Scott D., Chen J. J., Meacham S. What is next for the dietary reference intakes for bone metabolism related nutrients beyond calcium: phosphorus, magnesium, vitamin D, and fluoride? Critical Reviews in Food Science and Nutrition. 2009;49(2):136–144. doi: 10.1080/10408390701764468[↩]
- Koulouridis I, Alfayez M, Tighiouart H, Madias NE, Kent DM, Paulus JK, Jaber BL. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013 Oct;62(4):730-7. doi: 10.1053/j.ajkd.2013.02.373[↩]
- Alawi A., Majoni A.M., Falhammar S.W. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018;2018:9041694. doi: 10.1155/2018/9041694[↩][↩]
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008 Jun;35(2):215-37, v-vi. doi: 10.1016/j.pop.2008.01.007[↩][↩]
- Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11864-9. doi: 10.1073/pnas.1411705111[↩]
- Costello R.B., Nielsen F. Interpreting Magnesium Status to Enhance Clinical Care: Key Indicators. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:504–511. doi: 10.1097/MCO.0000000000000410[↩]
- Gröber U. Micronutrients: Metabolic Tuning-Prevention-Therapy. MedPharm Scientific Publishers; Stuttgart, Germany: 2009.[↩]
- de Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Regulation of Magnesium Balance: Lessons Learned from Human Genetic Disease. Clin. Kidney J. 2012;5((Suppl. S1[↩]
- Sedehizadeh S, Keogh M, Wills AJ. Reversible hypomagnesaemia-induced subacute cerebellar syndrome. Biol Trace Elem Res. 2011 Aug;142(2):127-9. doi: 10.1007/s12011-010-8757-3[↩]
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med. 2005 Jan-Feb;20(1):3-17. doi: 10.1177/0885066604271539[↩]
- Cohen L, Kitzes R. Clinical clues to magnesium deficiency. Isr J Med Sci. 1987 Dec;23(12):1238-41.[↩]
- An G, Du Z, Meng X, Guo T, Shang R, Li J, An F, Li W, Zhang C. Association between low serum magnesium level and major adverse cardiac events in patients treated with drug-eluting stents for acute myocardial infarction. PLoS One. 2014 Jun 5;9(6):e98971. doi: 10.1371/journal.pone.0098971[↩]
- Seller RH, Cangiano J, Kim KE, Mendelssohn S, Brest AN, Swartz C. Digitalis toxicity and hypomagnesemia. Am Heart J. 1970 Jan;79(1):57-68. doi: 10.1016/0002-8703(70)90394-7[↩]
- Paravicini TM, Yogi A, Mazur A, Touyz RM. Dysregulation of vascular TRPM7 and annexin-1 is associated with endothelial dysfunction in inherited hypomagnesemia. Hypertension. 2009 Feb;53(2):423-9. doi: 10.1161/HYPERTENSIONAHA.108.124651[↩]
- Efstratiadis G, Sarigianni M, Gougourelas I. Hypomagnesemia and cardiovascular system. Hippokratia. 2006 Oct;10(4):147-52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2464251[↩]
- Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK. Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab. 1991 Nov;73(5):1067-72. doi: 10.1210/jcem-73-5-1067[↩]
- Millart H, Durlach V, Durlach J. Red blood cell magnesium concentrations: analytical problems and significance. Magnes Res. 1995 Mar;8(1):65-76.[↩]
- Gullestad L., Midtvedt K., Dolva L.O., Norseth J., Kjekshus J. The magnesium loading test: Reference values in healthy subjects. Scand. J. Clin. Lab. Investig. 1994;54:23–31. doi: 10.3109/00365519409086506[↩]
- Holm CN, Jepsen JM, Sjøgaard G, Hessov I. A magnesium load test in the diagnosis of magnesium deficiency. Hum Nutr Clin Nutr. 1987 Jul;41(4):301-6.[↩]
- Costello RB, Nielsen F. Interpreting magnesium status to enhance clinical care: key indicators. Curr Opin Clin Nutr Metab Care. 2017 Nov;20(6):504-511. doi: 10.1097/MCO.0000000000000410[↩][↩][↩][↩]
- Rosner M.H., Ha N., Palmer B.F., Perazella M.A. Acquired Disorders of Hypomagnesemia. Mayo Clin. Proc. 2023;98:581–596. doi: 10.1016/j.mayocp.2022.12.002[↩]
- Elisaf M, Panteli K, Theodorou J, Siamopoulos KC. Fractional excretion of magnesium in normal subjects and in patients with hypomagnesemia. Magnes Res. 1997 Dec;10(4):315-20.[↩][↩]
- Schlingmann KP, Sassen MC, Weber S, et al. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J Am Soc Nephrol. 2005 Oct;16(10):3061-9. doi: 10.1681/ASN.2004110989[↩]
- Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Hypomagnesemia and mortality in patients admitted to intensive care unit: a systematic review and meta-analysis. QJM. 2016 Jul;109(7):453-459. doi: 10.1093/qjmed/hcw048[↩]