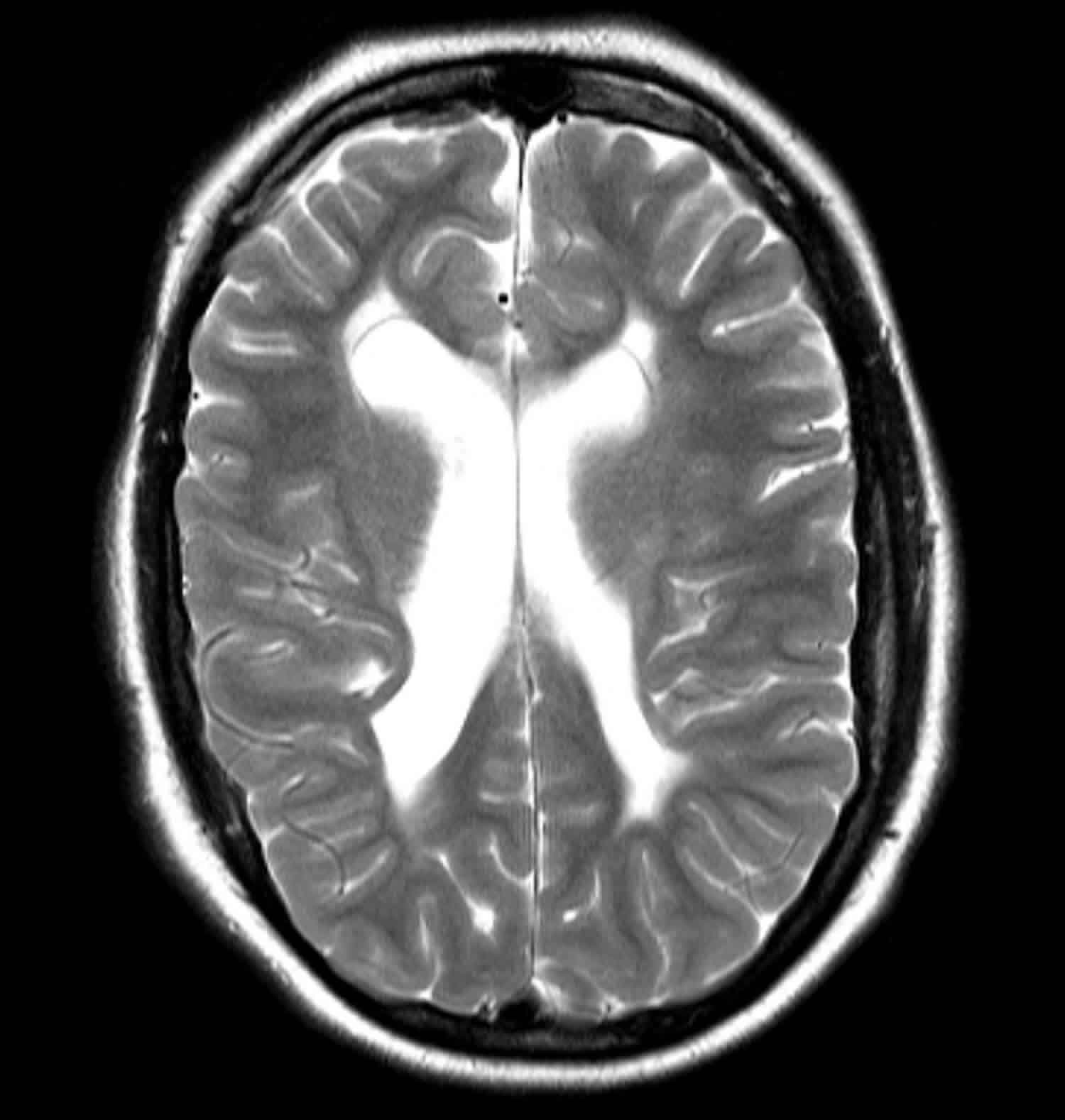
Periventricular leukomalacia
Periventricular leukomalacia (PVL) is characterized by the death of the white matter of the brain due to softening of the brain tissue. Periventricular leukomalacia represent small “holes” in the brain due to the death of small areas of brain tissue around the normal fluid-filled cavities of the brain. Periventricular leukomalacia can affect fetuses or newborns; premature babies are at the greatest risk of periventricular leukomalacia. Periventricular leukomalacia is caused by a lack of oxygen or blood flow to the periventricular area of the brain, which results in the death or loss of brain tissue. The periventricular area-the area around the spaces in the brain called ventricles-contains nerve fibers that carry messages from the brain to the body’s muscles. Although babies with periventricular leukomalacia generally have no outward signs or symptoms of the disorder, they are at risk for motor disorders, delayed mental development, coordination problems, and vision and hearing impairments. Periventricular leukomalacia may be accompanied by a hemorrhage or bleeding in the periventricular-intraventricular area (the area around and inside the ventricles), and can lead to cerebral palsy. Periventricular leukomalacia is diagnosed by ultrasound of the head.
There is no specific treatment for periventricular leukomalacia. Treatment is symptomatic and supportive. Children with periventricular leukomalacia should receive regular medical screenings to determine appropriate interventions.
Figure 1. Periventricular leukomalacia MRI scan
Footnote: This child had a history of a perinatal insult and had significant developmental delay. The lateral ventricles are enlarged with wavy lateral contours demonstrating increase in volume with associated white matter tissue loss typical of moderate to severe periventricular leukomalacia.
Figure 2. Ventricles of the brain
Can periventricular leukomalacia cause migraines?
Migraines or headaches are not specifically described as a feature of periventricular leukomalacia. However, there are two case reports of headaches in individuals with periventricular leukomalacia. Two different studies 1, 2 of children with recurrent headaches each reported one individual with periventricular leukomalacia diagnosed after brain imaging. It is difficult to determine if headaches can be caused by periventricular leukomalacia because there are few reported cases. It is important to keep in mind that although there are often symptoms that are documented in case reports, they are based on the specific individuals that are studied and may differ from one affected person to another.
Periventricular leukomalacia causes
Periventricular leukomalacia is most common in premature infants (less than 34 weeks gestational age with a median gestational age of 30 weeks and <1500 grams at birth) than in full-term infants. A major cause is thought to be changes in blood flow to the area around the ventricles of the brain. This area is fragile and prone to injury, especially before 32 weeks of gestation. Premature babies who have intraventricular hemorrhage are also at increased risk for developing periventricular leukomalacia.
The incidence of periventricular leukomalacia ranges from 4-26% in premature infants in neonatal intensive care units (NICUs). The incidence of periventricular leukomalacia is much higher in reports from autopsy studies of premature infants. As many as 75% of premature infants have evidence of periventricular leukomalacia on postmortem examination.
Periventricular leukomalacia likely occurs as a result of hypoxic-ischemic lesions resulting from impaired perfusion at the watershed areas, which in premature infants are located in a periventricular location. It is likely that infection or vasculitis also play a role in pathogenesis.
- Early: periventricular white matter necrosis
- Subacute: cyst formation
- Late: parenchymal loss and enlargement of the ventricles
Infection around the time of delivery may also play a role in causing periventricular leukomalacia. The risk for periventricular leukomalacia is higher for babies who are more premature and more unstable at birth.
Maternal infection, placental inflammation, and vasculitis are also important in the pathogenesis of periventricular leukomalacia. A link between maternal infection, preterm birth, and central nervous system (CNS) injury has been established by epidemiological studies 3. A role for infection and cytokine-induced injury in periventricular leukomalacia is strengthened by studies that demonstrate the presence of tumor necrosis factor in periventricular leukomalacia lesions 4 and in the cerebrospinal fluid (CSF) of infants with cerebral white matter injury 5.
After the initial insult, either ischemia or inflammation, injury to the immature premyelinating oligodendrocytes occurs by either free radical attack or by excitotoxicity. The preterm infant is particularly sensitive to oxygen free radical attack because of delayed development of superoxide dismutase and catalase 6.
In a 2014 report, Inomata et al suggested that combined elevations in serum levels of interleukin (IL) 6 and C-reactive protein (CRP) at birth are predictive of white matter injury in preterm infants with a fetal inflammatory response 7.
Injury to the premyelinating oligodendrocytes results in astrogliosis and microgliosis. This results in a deficit of mature, myelin-producing oligodendrocytes, which leads to cerebral hypomyelination 8.
Premature infants on mechanical ventilation may develop hypocarbia. Several studies have linked hypocarbia, particularly in the first few days of life, with the development of periventricular leukomalacia 9. Cumulative exposure during the first 7 days of life has been shown to independently increase the risk of periventricular leukomalacia in low birth weight infants 10.
Periventricular leukomalacia symptoms
Periventricular leukomalacia (PVL) occurs most commonly in premature infants born at less than 32 weeks’ gestation who have a birth weight of less than 1500 g. Many of these infants have a history of maternal chorioamnionitis.
Most affected infants experience cardiorespiratory problems, such as respiratory distress syndrome or pneumonia, in association with hypotension or patent ductus arteriosus during their first days of life. Bacterial infection at birth also appears to be a risk factor.
Some children exhibit fairly mild symptoms, while others have significant deficits and disabilities. Periventricular leukomalacia may manifest as cerebral palsy (>50% in the setting of cystic periventricular leukomalacia), intellectual disability or visual disturbance.
Initially, most premature infants are asymptomatic. If symptoms occur, they are usually subtle. Symptoms may include the following:
- Decreased tone in lower extremities
- Increased tone in neck extensors
- Apnea and bradycardia events
- Irritability
- Pseudobulbar palsy with poor feeding
- Clinical seizures (may occur in 10-30% of infants)
Periventricular leukomalacia diagnosis
Tests used to diagnose periventricular leukomalacia include ultrasound and MRI of the head. The traditional diagnostic hallmarks of periventricular leukomalacia are periventricular echodensities or cysts detected by cranial ultrasonography. More recently MRI studies have demonstrated a relatively common diffuse non-cystic form of periventricular leukomalacia in premature infants. Diagnosing periventricular leukomalacia is important because a significant percentage of surviving premature infants develop cerebral palsy (CP), intellectual impairment, or visual disturbances.
Ultrasound
Cranial ultrasound provides a convenient, non-invasive, relatively low-cost screening examination of the haemodynamically-unstable neonate at the bedside. The examination also imparts no radiation exposure. Sonography is sensitive for the detection of hemorrhage, periventricular leukomalacia, and hydrocephalus.
On ultrasound, hyperechoic areas are firstly identified in a distinctive fashion in the periventricular area, more often at the peritrigonal area and in an area anterior and lateral to the frontal horns (periventricular white matter should be less echogenic than the choroid plexus).
These are watershed areas that are sensitive to ischemic injury. Follow-up scans in the more severely affected patients may reveal the development of cysts in these areas, known as cystic periventricular leukomalacia (when cystic periventricular leukomalacia is present, it is considered the most predictive sonographic marker for cerebral palsy).
Periventricular leukomalacia classification
One of the methods used for grading of periventricular leukomalacia based on sonographic appearances is as 11:
- Grade 1: areas of increased periventricular echogenicity without any cyst formation persisting for more than 7 days
- Grade 2: the echogenicity has resolved into small periventricular cysts
- Grade 3: areas of increased periventricular echogenicity, that develop into extensive periventricular cysts in the occipital and frontoparietal region
- Grade 4: areas of increased periventricular echogenicity in the deep white matter developing into extensive subcortical cysts
Periventricular leukomalacia treatment
There is no treatment for periventricular leukomalacia. Premature babies’ heart, lung, intestine, and kidney functions are watched closely and treated in the newborn intensive care unit (NICU). This helps reduce the risk of developing periventricular leukomalacia.
Infants with periventricular leukomalacia require close neurodevelopmental follow-up after discharge from the hospital. Potential consultants include pediatricians, developmental specialists, neurologists, and occupational and physical therapists.
Developmental follow-up
Premature infants with evidence of periventricular leukomalacia (PVL) require close developmental follow-up because of the high association with cerebral palsy (CP).
Early intervention strategies carried out by occupational therapists or physical therapists may decrease symptoms and may increase the infant’s motor function.
Periventricular leukomalacia prognosis
The prognosis for individuals with periventricular leukomalacia depends upon the severity of the brain damage. Periventricular leukomalacia often leads to nervous system and developmental problems in growing babies. These problems most often occur during the first to second year of life. It may cause cerebral palsy (CP), especially tightness or increased muscle tone (spasticity) in the legs.
Mild periventricular leukomalacia is often associated with spastic diplegia. Severe periventricular leukomalacia is associated with quadriplegia. Severe periventricular leukomalacia is also associated with a higher incidence of intelligence deficiencies and visual disturbances.
Babies with periventricular leukomalacia are at risk for major nervous system problems. These are likely to include movements such as sitting, crawling, walking, and moving the arms. Fixation difficulties, nystagmus, strabismus, and blindness have also been associated with periventricular leukomalacia. These babies may need physical therapy. Extremely premature babies may have more problems with learning than with movement.
Some cases of visual dysfunction in association with periventricular leukomalacia occur in the absence of retinopathy of prematurity, suggesting damage to optic radiations as causation.
A baby who is diagnosed with periventricular leukomalacia should be monitored by a developmental pediatrician or a pediatric neurologist. The child should see the regular pediatrician for scheduled exams.
References- Füsun Korkmaz Alehan. Value of neuroimaging in the evaluation of neurologically normal children with recurrent headache. J Child Neurol. November 17, 2002; (11):807-809. http://www.ncbi.nlm.nih.gov/pubmed/12585718
- Todd J. Schwedt, Yifan Guo, David Rothner. “Benign” imaging abnormalities in children and adolescents with headache. Headache. March 21, 2006; 46(3):387-398. http://www.ncbi.nlm.nih.gov/pubmed/16618255
- Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics. June 1996. 97:822-7.
- Kadhim H, Tabarki B, Verellen G, et al. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001 May 22. 56(10):1278-84.
- Ellison VJ, Mocatta TJ, Winterbourn CC, et al. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005 Feb. 57(2):282-6.
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008 Mar. 93(2):F153-61.
- Inomata K, Mizobuchi M, Tanaka S, et al. Patterns of increases in interleukin-6 and C-reactive protein as predictors for white matter injury in preterm infants. Pediatr Int. 2014 Dec. 56(6):851-5.
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011 Jun. 29(4):423-40.
- Okumura A, Hayakawa F, Kato T, et al. Hypocarbia in preterm infants with periventricular leukomalacia: the relation between hypocarbia and mechanical ventilation. Pediatrics. 2001 Mar. 107(3):469-75.
- Resch B, Neubauer K, Hofer N, Resch E, Maurer U, Haas J, et al. Episodes of hypocarbia and early-onset sepsis are risk factors for cystic periventricular leukomalacia in the preterm infant. Early Hum Dev. 2012 Jan. 88(1):27-31.
- Neonatal Hypoxic-Ischemic Encephalopathy: Multimodality Imaging Findings. Christine P. Chao, Christopher G. Zaleski, and Alice C. Patton. RadioGraphics 2006 26:suppl_1, S159-S172.