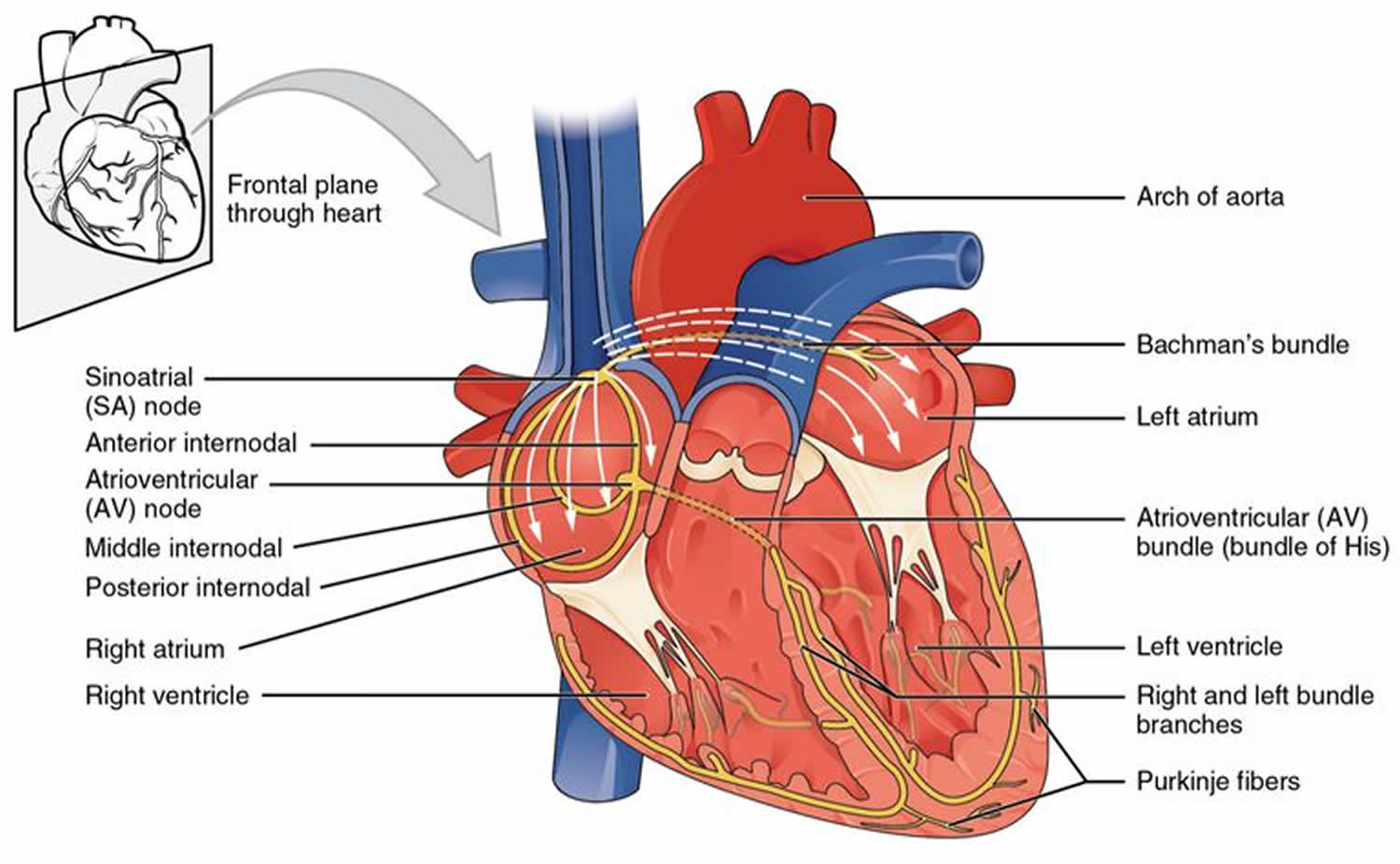
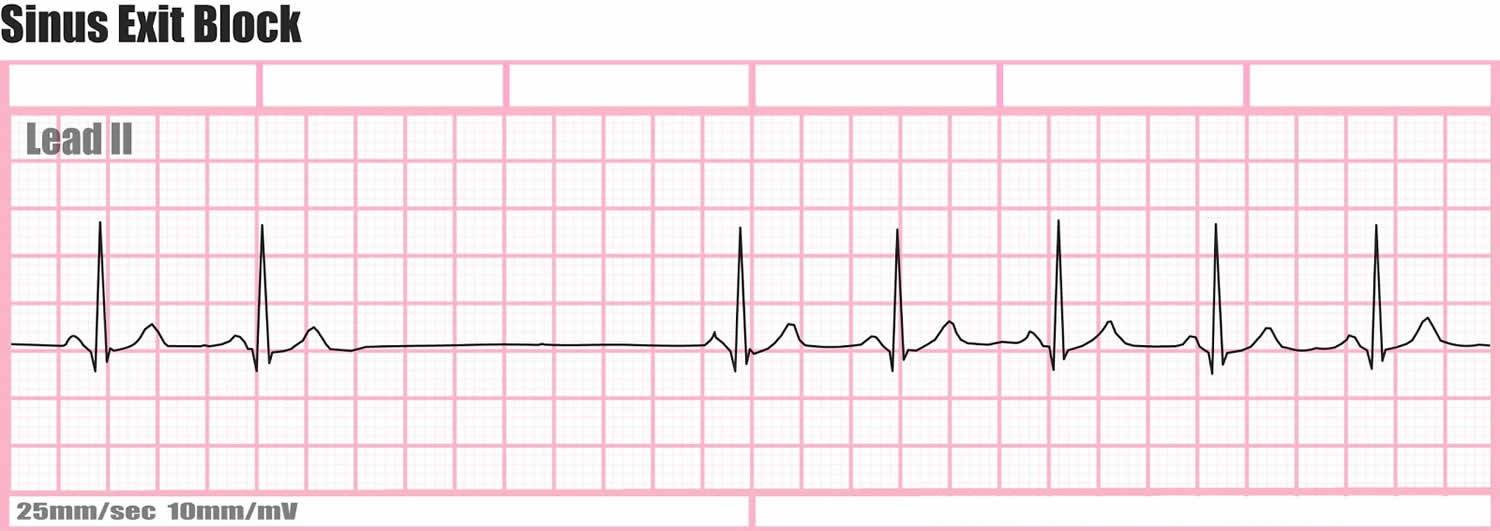
Sinoatrial exit block
Sinoatrial block also known as sinoatrial exit block occurs when the action potential initiated by the sinoatrial node is inhibited or completely blocked before it is able to leave the sinoatrial (SA) node and reach the atrium, and thus no P wave will appear on the ECG.
The sinoatrial node is composed of a collection of atrial myocytes that are specialized into two different functional cells. Pacemaker “P cells” with intrinsic pacemaker function and transitional “T cells” which are responsible for propagating the impulse into the right atrium 1.
Sinoatrial exit block is caused by the failure of the T cells to transmit the impulse 1.
There are 3 degrees of sinoatrial block that parallel atrioventricular (AV) block:
- First-degree sinoatrial block is usually subclinical and undetectable on the surface electrocardiogram (ECG);
- Second-degree sinoatrial exit block is categorized into type 1 and type 2.
- Second-degree-type 1 sinoatrial block (Wenckebach) is characterized by progressive shortening of R-R or P-P interval prior to the dropped P wave. A “sinus pause” ensues afterward and would be shorter than two of the preceding R-R intervals.
- Second-degree-type 2 sinoatrial block refers to a dropped P wave without any preceding change in the R-R or P-P interval. The subsequent “sinus pause” is an exact interval of the preceding R-R intervals (usually two times).
- Third-degree sinoatrial block refers to complete failure of the sinoatrial node to conduct impulses to the atrial tissue and is reflected by the absence of P waves on surface ECG. Third-degree sinoatrial exit block is clinically identical to sinus arrest. When a junctional escape ensues, the rhythm may be confused with a junctional rhythm. If no junctional escape rhythm is present, a long pause resulting in asystole and cardiac arrest can occur.
Sinoatrial node dysfunction is a disease of older adults, although it can occur at any age. The mean age of a patient with sinoatrial node dysfunction is 68 years. Sinoatrial node dysfunction develops in one of every 600 cardiac patients of 65 years of age or older. Males and females are equally affected. SA node dysfunction accounted for more than 50% of the pacemaker implantations in the United States in the 1990s 2.
The natural history of sinus node dysfunction is usually a slow progression over decades. In the early stages, most patients are asymptomatic. When symptoms do develop, they are usually attributed to hypo-perfusion to vital organs with high oxygen demand. Sinus pauses or severe bradycardia results in central nervous system (CNS) underperfusion which manifests in paroxysmal presyncope or syncope. The sudden termination of atrial fibrillation (AF) in tachy-brady syndrome typically results in a prolonged sinus pause and failure to return to sinus rhythm which can manifest in syncope. Renal and gastrointestinal hypoperfusion may result in oliguria and abdominal pain, respectively. Rhythms with AF or atrial flutter pose a significant increased risk of thromboembolism, manifesting as transient ischemic attack or cardioembolic stroke, thus carrying the worst prognosis. Chronotropic incompetence results in fatigue and exercise intolerance. Palpitations are usually felt during episodes of tachycardia, after termination of tachyarrhythmias or due to prolonged pauses. Worsening of angina pectoris or congestive heart failure are also reported.
Figure 1. Sinoatrial exit block ECG
Sinoatrial block causes
The etiologic factors leading to sinus node dysfunction can be classified into two categories: intrinsic pathology to the sinus node itself, typically results from fibrosis of the nodal tissue and external causes that affect the SA node function.
Sinoatrial block causes include:
- Sinus Node Fibrosis: Replacement of the sinus node tissue by fibrous tissue is the most common cause of sinus node dysfunction, the replacement can also include other parts of the conduction system, including the atrioventricular (AV) node 3.
- Medications and toxins: Prescription medications can depress the sinus node function, potentially resulting in sinus node dysfunction include: beta blocker, non-dihydropyridine calcium channel blockers (eg, diltiazem, verapamil), digoxin, antiarrhythmic medications, acetylcholinesterase inhibitors (eg, donepezil, rivastigmine) used in the treatment of Alzheimer’s disease, Ivabradine, parasympathomimetic agents, sympatholytic drugs (eg, methyldopa, clonidine), lithium, poisoning by grayanotoxin, which is produced by some plants (eg, Rhododendron species) and found in certain varieties of honey, has been associated with depressed sinoatrial node function 4
- Infiltrative disease: The SA node tissue can be affected during the disease process of some of the infiltrative diseases such as amyloidosis, sarcoidosis, scleroderma, hemochromatosis, and pericarditis leading to sinus node dysfunction.
- Ischemia: The sinus node is perfused by the sinoatrial nodal artery, which arises from the right coronary artery in 60 % of the time and from the left circumflex artery in 40 % of the time. Narrowing of this artery can lead to impairment of the sinus node function leading to sinus node dysfunction that can be potentially reversible. Almost all such cases are present in inferior myocardial infarction 5. Ischemic cardiac arrest may cause sinus node dysfunction.
- Familial: Sinus node dysfunction in rare cases can be the result of cardiac sodium channel mutations of SCN5A 6 and HCN4 genes 7.
- Miscellaneous: Other disorders that can rarely cause sinus node dysfunction to include hypothyroidism, hypothermia, hypoxia and muscular dystrophies. Some infections (eg, leptospirosis, trichinosis, Salmonella typhi infection) are associated with relative sinus bradycardia; however, these usually do not result in permanent sinoatrial node dysfunction 8. Rheumatic fever is another cause of sinus node dysfunction. Such dysfunction may also result from central nervous system (CNS) disease, which is usually secondary to increased intracranial pressure with a subsequent increase in the parasympathetic tone.
- Surgical causes, especially from operations involving the right atrium. Gradual loss of sinus rhythm occurs after the Mustard, Senning, and all varieties of the Fontan operation. This is thought to be secondary to direct injury to the sinoatrial node during surgery and also due to later, chronic hemodynamic abnormalities. Paroxysmal atrial tachycardias are frequently associated with sinus node dysfunction, and loss of sinus rhythm appears to increase the risk of sudden death. Patients with transposition of the great arteries now undergo the arterial switch operation, which avoids the extensive atrial suture lines that lead to sinoatrial node damage. Sinoatrial node dysfunction was described in 15% of patients who had undergone the Ross operation for aortic valve disease or complex left-sided heart disease, 2.6 to 11 years earlier 9. Other arrhythmias, such as complete AV block and ventricular tachycardia, were present as well after the Ross operation. When repairing atrial septal defects (ASDs), especially sinus venosus ASDs, sinus node dysfunction frequently occurs because of the proximity of the defect with sinoatrial node tissue. Other surgically related causes of sinus node dysfunction include the following:
- Patients who have undergone surgery for endocardial cushion defects may later develop sinus node dysfunction
- Sinus node dysfunction may be caused by a Blalock-Hanlon atrial septectomy
- Sinus node dysfunction may occur after repair of partial anomalous pulmonary venous return (PAPVR) or total anomalous pulmonary venous return (TAPVR)
- Cannulation of the superior vena cava (SVC), usually performed for cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO), may damage sinoatrial node tissue.
- Sinus venosus atrial septal defect (ASD), Ebstein anomaly, and heterotaxy syndromes, particularly left atrial isomerism, can also lead to sinus node dysfunction.
While the most common intrinsic factor leading to sinus node dysfunction is age-related degeneration of the SA node 10, sinoatrial node dysfunction can be a result of congenital disorders, arrhythmias, infiltrative disorders and surgery. Recent studies have identified several mutations in the ion channels explaining familial and congenital forms of sick sinus syndrome. Heart failure and atrial tachyarrhythmias have been shown to induce cellular remodeling of the sinus node in animal models 11. Infiltration of the SA node by sarcoidosis, amyloidosis, hemochromatosis, collagen vascular disease or metastatic cancer results in sinoatrial node dysfunction. Damage to SA node or the sinus nodal artery may occur after cardiothoracic surgery from valve replacement, correction of congenital heart disease or heart transplant. Infectious agents such as bacterial endocarditis and Chagas disease commonly result in atrioventricular conduction problems rather than sinus node dysfunction. Because the sinoatrial node is located within the atrial wall, ischemic injury by atherosclerosis of the arteries feeding the SA node is unusual 12.
Several external causes can also affect the pacing function of the SA node. These can occur in conditions where there is abnormally increased vagal tone such as carotid sinus hypersensitivity, vasovagal syncope, and autonomic dysfunction. Furthermore, metabolic derangements such as hypothyroidism, hyperkalemia, hypokalemia, hypocalcemia, hypoxia, and hypothermia can lead to depression of the pacing function of the SA node. Obstructive sleep apnea may cause bradycardia by profound hypoxia during episodes of apnea. Increased intracranial pressure (Cushing’s reflex) also causes bradycardia. Several pharmacologic and toxic substances can produce a similar effect on the SA node; these include class I to IV antiarrhythmic medications, digoxin, lithium and sympatholytic medications 1.
Sinoatrial block symptoms
The symptoms of sinoatrial node dysfunction are non-specific, primarily due to bradycardia, sinus pause, and sinus arrest 13:
- Lightheadedness,
- Fatigue,
- Palpitations,
- Dyspnea on exertion,
- Chest discomfort
- Presyncope, and syncope.
Patients who have other cardiac comorbidities such as coronary artery disease can present with increasing of their baseline ischemic symptoms such as chest pain and dyspnea.
Symptoms are frequently intermittent with gradual progression in frequency and severity, although some patients may present with profound, persistent symptoms. Rarely, patients with sinus node dysfunction may be asymptomatic and identified on routine electrocardiography (ECG) or ambulatory ECG monitoring.
Patients with symptomatic sinus node dysfunction are usually older with frequent comorbid diseases; they often seek medical attention owing to symptoms of lightheadedness, presyncope, syncope and, in patients with alternating periods of bradycardia and tachycardia, palpitations and/or other symptoms associated with a rapid heart rate.
Patients with coexisting cardiac pathologies such as valvular or ischemic heart disease may notice increasing dyspnea on exertion or worsening chest discomfort related to a lower heart rate and the resulting reduction in cardiac output. Because symptoms may be variable in nature, nonspecific and, frequently, transient, it may be challenging at times to establish this symptom-rhythm relationship. Atrial arrhythmias appear to develop slowly over time, possibly the result of a progressive pathologic process that affects the sinoatrial node and the atrium 14.
Sinoatrial block diagnosis
There are no standardized criteria for establishing a diagnosis of sinus node dysfunction, and the initial clues to the diagnosis are most often gleaned from the patient’s history. However, the symptoms of sinus node dysfunction are nonspecific and the electrocardiographic (ECG) findings may not be diagnostic. Hence, the key to making a diagnosis of sinus node dysfunction is to establish a correlation between the patient’s symptoms and the underlying ECG findings at the time of the symptoms together, ECG finding alone, especially sinus bradycardia, does not indicate the presence of sinus node dysfunction.
Prior to any testing beyond an ECG, a thorough clinical evaluation should be performed for potentially reversible causes, which include medication use (eg, beta blockers, calcium channel blockers, digoxin, antiarrhythmics), myocardial ischemia, systemic illness (eg, hypothyroidism), and autonomic imbalance.
Following a comprehensive history and physical examination, a resting 12-lead ECG, review of previous medical records and ECG tracings, and exercise stress testing are the keys to making a diagnosis of sinus node dysfunction and establishing a symptom-rhythm correlation. A detailed history and ECG findings during symptoms are often sufficient to diagnose sinus node dysfunction. Careful review of prior records, in particular previous ECG tracings, can provide subtle clues to changes in the ECG over time. For patients with clinically suspected sinus node dysfunction in whom the diagnosis remains uncertain following the initial ECG, exercise stress testing is advised.
Carefully evaluate for potentially reversible causes and medication use to exclude remediable etiologies for apparent sinus node dysfunction. In patients with medication use (eg, beta blockers, calcium channel blockers, digoxin, antiarrhythmics, and acetylcholine esterase inhibitors) suspected to result in symptomatic bradycardia, the patient should remain on an ECG monitor while the medications are withdrawn. If symptoms and ECG abnormalities persist following the withdrawal of the medications (ie, after 3-5 half-lives), then sinus node dysfunction/sick sinus syndrome may be diagnosed.
If the diagnosis of sinus node dysfunction cannot be definitively diagnosed following a thorough history and physical examination, an initial 12-lead ECG, and/or an ambulatory ECG monitoring [Holter] for 1 to 14 days, perform an event monitor for up to 4 weeks to identify symptomatic episodes of arrhythmias and to monitor average heart rates over extended periods of surveillance.
Physical examination
The physical examination essentially demonstrates findings of the underlying condition(s). The universal feature, however, is bradycardia.
ECG findings
ECG findings 15:
- Periods of inappropriate and often severe sinus bradycardia.
- Sinus pauses, sinus arrests and sinus exits blocks that can happen with and without appropriate escape rhythm.
- Alternating tachycardia and bradycardia, referred to as a tachy-brady syndrome, which could also be associated with other supraventricular tachycardias.
The key to diagnosing sinus node dysfunction is to establish a correlation between the patient symptoms and the ECG findings at the time of symptoms. It is also beneficial to review previous ECG tracing to check for any changes in the rhythm upon the start of the symptoms.
Exercise stress testing
If the diagnosis could not be made based on history and ECG then exercise stress testing is necessary. Things to look for is the failure of appropriate chronotropic response to exercise, defined as less than 80 percent of the predictable heart rate response to exercise. Also, it will exclude myocardial ischemia and help to program the devices for patients who ultimately receive a permanent pacemaker 16.
Ambulatory ECG monitoring
If all the above failed in making the diagnosis of sinus node dysfunction, then an ambulatory ECG monitoring should be considered. In one study that included 55 symptomatic patients, 24-hour Holter monitor tracking detected the underlying arrhythmia causing the symptoms in 30 patients (55 %) 17. However, longer periods of monitoring might be necessary for patients whom their symptoms are not as frequent. Cardiac event monitors have been shown to be more effective than Holter monitors in detecting the cause of palpitation. In a study involving 43 patients with palpitation, event monitors were twice as likely to provide a diagnostic rhythm strip ECG during symptoms as 48 hours Holter monitors. (67% vs 35%) 18.
Review of potentially reversible causes
Patients who are taking medications that can be contributing to their symptoms (beta blocker, calcium channel blockers, digoxin, antiarrhythmic) should be monitored off of these medications and on the ECG monitor to assess for symptoms reversibility as well as the resolution of the ECG findings 16.
Laboratory studies
Because hypothyroidism and electrolyte imbalances can contribute to sinus node dysfunction, thyroid function testing and serum electrolyte testing (Na+, K+, Ca2+) can be useful. Infiltrative cardiomyopathies (eg, amyloid, sarcoid) can present with evidence of diffuse conduction system disease, but screening is typically reserved for patients in whom specific clinical factors suggest the diagnosis.
Transesophageal atrial pacing
Transesophageal atrial pacing is reserved mainly for pediatric patients. It may be performed safely to determine sinus node recovery time in children who present with dizziness, syncope, or palpitations.
Echocardiography
No specific imaging studies are required in the initial workup of sinus node dysfunction. However, an echocardiogram should be considered because it can document the presence of underlying valvular or ischemic heart disease and may suggest the diagnosis of amyloid when diffuse conduction system findings are present.
Sinoatrial block treatment
The first step in the management of the sinus node dysfunction is to determine whether the patient is hemodynamically stable or no.
Unstable patients
Patients with sinus node dysfunction are rarely unstable for prolonged periods of time, however, if that was the case then one should follow the Advanced Cardiovascular Life Support (ACLS) protocol for symptomatic bradycardia 19. Symptoms include altered mental status, syncope, ischemic chest pain, and hemodynamic instability.
Atropine should be tried first with a dose of 0.5 mg that can be repeated every 3 to 5 minutes with a total dose of 3 mg. However, treatment with atropine should not delay transcutaneous pacing or chronotropic agents.
Chronotropic agent infusion should be tried if atropine failure which includes epinephrine, dopamine or isoproterenol infusions.
Clinicians should initiate transcutaneous pacing in patients who are hemodynamically unstable but should only be a bridge for transvenous pacing.
Stable patients
As discussed above, one should look for the possibility of a reversible cause first; if an offending medication was identified and could be removed or replaced, the patient should undergo monitoring for the reversibility of symptoms and ECG findings. If the offending medications cannot be removed. Then the patient should be managed the same way as if there is no reversible cause. The next step involves determining whether the patient is symptomatic or asymptomatic.
Asymptomatic patient
Observation is recommended in asymptomatic patients, there are no society guidelines that recommend permanent pacemaker for asymptomatic patients with bradycardia or pauses 16.
Symptomatic patients
Symptomatic sinus node dysfunction patients will require placement of a permanent pacemaker. Usually, either single chamber atrial pacemaker (AAI) or dual chamber pacemaker (DDD) should be used. In patients where there are no AV conduction abnormalities, a single chamber atrial pacemaker (AAI) is a reasonable option, however, patients with AV conduction delay or a branch block would benefit from dual chamber pacemaker (DDD) 16.
Discussing the types and modes of pacemaker is beyond the scope of this activity, however, one of the largest trials that looked into single lead atrial pacing (AAI) vs. dual chamber pacing (DDD) in patients with sinus node dysfunction is the DANPACE trial 20 which included 1415 patients with normal AV conduction and the mean follow up was 5.4 years. There was no difference in all-cause mortality between the two groups, however, Single lead atrial pacing was associated with more incidents of paroxysmal atrial fibrillation and a two-fold increase in pacemaker reoperation.
Recommendations for permanent pacing in sinus node dysfunction
On the basis of the American College of Cardiology Foundation, American Heart Association, the Heart Rhythm Society 21 and the European Society of Cardiology 22, general recommendations for cardiac pacing therapy for sinus node dysfunction are outlined below.
Class I recommendations
Permanent pacemaker implantation is indicated for the following:
- Sinus node dysfunction with documented symptomatic bradycardia, including frequent sinus pauses that produce symptoms
- Symptomatic chronotropic incompetence
- Symptomatic sinus bradycardia that results from required drug therapy for medical conditions
Class IIa recommendations
Permanent pacemaker implantation is reasonable for the following:
- Sinus node dysfunction with a heart rate below 40 bpm, when a clear association between significant symptoms consistent with bradycardia and the actual presence of bradycardia has not been documented
- Syncope of unexplained origin, when clinically significant abnormalities of sinoatrial node function are discovered or provoked in electrophysiologic (EP) studies
Class IIb recommendation
- Permanent pacemaker implantation may be considered in minimally symptomatic patients with a chronic heart rate below 40 bpm while awake.
Class III recommendations
Permanent pacemaker implantation is not indicated for sinus node dysfunction in the following individuals:
- Asymptomatic patients
- Those for whom the symptoms suggestive of bradycardia have been clearly documented to occur in the absence of bradycardia
- Those with symptomatic bradycardia due to nonessential drug therapy
Single- versus dual-chamber pacemakers
In patients with sinus node dysfunction, the annual incidence of complete heart block is about 0.6% 23. In the United States, the implantation of dual-chamber pacemakers is preferred in practice because their use anticipates the possible subsequent development of conducting system dysfunction.
This practice is supported by data from the Danish Multicenter Randomized Trial on Single Lead Atrial Pacing versus Dual Chamber Pacing in Sick Sinus Syndrome (DANPACE) trial, in which 9.3% of patients with single-lead atrial pacing (AAI) required upgrade to a dual-chamber pacemaker (DDD) over 5.4 years of follow-up due to new development of significant atrioventricular (AV) conduction abnormalities 24. This was necessary despite the fact that these patients had no significant intraventricular conduction abnormality, had PR intervals below 260 ms, and had no Wenckebach AV block with atrial pacing at 100 bpm at baseline. In addition, the incidence of atrial fibrillation (AF) was higher in patients in AAI mode than those in DDD mode. However, no significant mortality difference was noted between the groups in single-lead atrial pacing (AAI) and dual-chamber pacemaker (DDD) modes 24.
Arguably, a single-chamber atrial pacemaker with single-lead atrial pacing (AAI) mode is an acceptable alternative in patients with sinus node dysfunction and normal AV and intraventricular conduction because of the added expense of, and the potential for, more lead extraction with a dual-chamber pacemaker.
In patients with sinus node dysfunction and known AV conduction abnormality (including bundle branch block and bifascicular block), a dual-chamber pacemaker should be used due to the high risk of AV block (about 36% in a 5-year follow-up study).
In a collaborative pooled-analysis of 10 randomized trials (n = 6639) to evaluate the effect of existing pacing strategies on the risk of postimplantation AF and heart failure (HF) events in sinus node dysfunction patients, Chen et al stratified the pooled-analysis into two subsets—single-lead atrial pacing (AAI) versus dual-chamber pacemaker (DDD), and minimal ventricular pacing (MinVP) versus dual-chamber pacemaker (DDD) —and found that although composite AF and heart failure (HF) events were similar in the AAI versus DDD subset, there was a substantially reduced risk of composite AF and HF in the minimal ventricular pacing group receiving pacemaker as primary treatment 25. Over the long term, however, the rate of AF and heart failure was similar in the minimal ventricular pacing versus DDD subset of patients who were scheduled for device replacement. The investigators indicated their findings supported the use of minimal ventricular pacing over conventional DDD in improving clinical outcomes for sinus node dysfunction patients who received a pacemaker as primary treatment 25.
Pacemaker programming features
Chronic right ventricular pacing has been shown to be associated with an increased incidence of AF, stroke, HF, and probably death 26. A study suggested that right ventricular (RV) pacing is detrimental to left ventricular (LV) function even in patients with a normal LV ejection fraction (LVEF) 27. Therefore, avoiding RV pacing is advantageous in patients with sinus node dysfunction treated with pacemaker therapy.
However, using the intrinsic AV conduction in patients with a very long intrinsic PR interval may not be beneficial clinically, as suggested by a trial in patients with an intracardiac defibrillator (ICD) 24. Theoretically, a very long PR interval may result in pacemaker syndrome during sinus tachycardia or a fast atrial pacing rhythm.
As noted above, in the DANPACE trial, about 65% of patients with a moderate AV delay setting in DDD mode with mean right ventricular (RV) pacing had a lower incidence of AF and no increased rate of HF, as compared with patients in AAI mode 24. The optimal AV delay settings in patients with sinus node dysfunction are remain unknown, although various programming algorithms from different pacemaker companies are very effective in reducing RV pacing.
Mode switch is an important feature to monitor atrial flutter and AF events. Because more than 50% of patients with sinus node dysfunction may develop tachy-brady syndrome over time 28, it is very important to identify these patients through pacemaker monitoring and to anticoagulate them to reduce their risk of stroke. However, the most appropriate anticoagulant therapy is still uncertain for patients in whom AF is detected only as an incidental finding on pacemaker or ICD diagnostics.
Rate response features have been used in patients with sinus node dysfunction, especially in the presence of chronotropic incompetence. However, the clinical benefits of this program feature are still controversial 29.
Anticoagulation
Patients with sinus node dysfunction might have episodes of atrial fibrillation/flutter especially patients with the tachy-brady syndrome. Also, patients who received a permanent pacemaker are at a higher risk of developing atrial fibrillation, thus, frequent device interrogation is recommended. Patients with documented atrial fibrillation should be risk stratified for stroke and bleeding and an informed decision should be made whether to use anticoagulation or no.
Long-term monitoring
Asymptomatic patients with sinus node dysfunction (sinus node dysfunction) should be observed for symptoms. In patients with a pacemaker, carry out the following on routine pacemaker interrogations:
- Monitor leads and battery status
- Ensure adequate heart rate support at rest, during daily activities, and during exercise
- Monitor for pacemaker malfunction
- Ensure minimal right ventricular (RV) pacing.
- Monitor for atrial fibrillation and atrial flutter events
Pregnancy
Patients with sinus node dysfunction who become pregnant and take antiarrhythmic medications should have their medication levels measured because these drug regimens frequently require adjustment. In addition, medication with teratogenic effects (eg, amiodarone, which is associated with fetal thyroid dysfunction) should be avoided.
Patients with sinus node dysfunction who become pregnant and have a pacemaker are advised to perform frequent pacemaker checks and make the appropriate adjustments.
Patients with sinus node dysfunction who become pregnant and have ventricular dysfunction due to cardiomyopathy or a Mustard or Fontan procedure should have regular and close medical follow-ups with their obstetrician and cardiologist. This permits appropriate adjustment and implementation of anti-congestive heart failure (CHF) medication. If the CHF progresses despite medical management and becomes intractable, the mother and fetus are at risk and early delivery may be scheduled.
Sinoatrial block prognosis
The prognosis of patients with sinus node dysfunction is dependent on the underlying associated condition. Sinus node dysfunction is a progressive noncurable but manageable disease. The incidence of sudden cardiac death in patients with sinus node dysfunction is low 28. Complications of sinus node dysfunction without a pacemaker include hypotension and syncope. However, pacemaker therapy does not appear to affect survival in patients with sinus node dysfunction 30.
In a study 31 that followed 52 patients with sinus node dysfunction presenting with sinus bradycardia associated with a sinoatrial block or sinoatrial arrest, it took an average of 13 years to progress to complete the sinoatrial arrest and an escape rhythm. It is unclear if sinus node dysfunction is associated with increased mortality as most patients with sinus node dysfunction have other cardiovascular comorbidities. In a study of 19000 from two cohorts followed for an average of 17 years, 213 persons developed sinus node dysfunction (0.6 events per 1000 person a year). While the development of sinus node dysfunction carried an association with increased mortality, it became attenuated when adjusted for incident cardiovascular disease. Sinus node dysfunction correlates with increased morbidity, but it is unclear if it affects mortality 32.
Patients with tachy-brady syndrome have a worse prognosis than do patients with isolated sinus node dysfunction. The overall prognosis in patients with sinus node dysfunction and additional systemic ventricular dysfunction (eg, numerous postoperative Mustard and Fontan patients) depends on their underlying ventricular dysfunction or degree of congestive heart failure (CHF).
In patients who have undergone a Fontan surgery and developed sinus node dysfunction, endocardial atrial leads can be implanted relatively safely and can permit low-energy thresholds for as long as 5 years after implantation 33.
Morbidity and mortality
The relationship between sinus node dysfunction and mortality is difficult to clearly understand, as many individuals with sinus node dysfunction have preexisting comorbidities (eg, hypertension, diabetes mellitus, atrial fibrillation) that are known to increase all-cause mortality 34.
The complications of sinus node dysfunction include the following:
- Sudden cardiac death
- Syncope
- Thromboembolic events, including stroke
- Congestive heart failure (CHF)
- Atrial tachyarrhythmias
About 50% of patients with sinus node dysfunction develop tachy-brady syndrome over a lifetime; such patients have a higher risk of stroke and death. However, the incidence of sudden death owing directly to sinus node dysfunction is extremely low 28.
References- Dakkak W, Doukky R. Sick Sinus Syndrome. [Updated 2020 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470599
- Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003 Apr 15;67(8):1725-32.
- Thery C, Gosselin B, Lekieffre J, Warembourg H. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. Am. Heart J. 1977 Jun;93(6):735-40.
- Sinus Node Dysfunction. https://emedicine.medscape.com/article/158064-overview#a4
- Hatle L, Bathen J, Rokseth R. Sinoatrial disease in acute myocardial infarction. Long-term prognosis. Br Heart J. 1976 Apr;38(4):410-4.
- Makiyama T, Akao M, Tsuji K, et al. High risk for bradyarrhythmic complications in patients with Brugada syndrome caused by SCN5A gene mutations. J Am Coll Cardiol. 2005 Dec 6. 46(11):2100-6.
- Ishikawa T, Ohno S, Murakami T, et al. Sick sinus syndrome with HCN4 mutations shows early onset and frequent association with atrial fibrillation and left ventricular noncompaction. Heart Rhythm. 2017 May. 14(5):717-24.
- Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000 Dec. 6(12):633-4.
- Pasquali SK, Marino BS, Kaltman JR, et al. Rhythm and conduction disturbances at midterm follow-up after the ross procedure in infants, children, and young adults. Ann Thorac Surg. 2008 Jun. 85(6):2072-8.
- Semelka M, Gera J, Usman S. Sick sinus syndrome: a review. Am Fam Physician. 2013 May 15;87(10):691-6.
- Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007 Apr 10;115(14):1921-32.
- Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N. Engl. J. Med. 2000 Mar 09;342(10):703-9.
- Jabbour F, Kanmanthareddy A. Sinus Node Dysfunction. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544253
- Josephson ME, ed. Sinus node function. Clinical Cardiac Electrophysiology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Kaplan BM, Langendorf R, Lev M, Pick A. Tachycardia-bradycardia syndrome (so-called “sick sinus syndrome”). Pathology, mechanisms and treatment. Am. J. Cardiol. 1973 Apr;31(4):497-508.
- Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019 Aug 20;74(7):e51-e156.
- Lipski J, Cohen L, Espinoza J, Motro M, Dack S, Donoso E. Value of Holter monitoring in assessing cardiac arrhythmias in symptomatic patients. Am. J. Cardiol. 1976 Jan;37(1):102-7.
- Kinlay S, Leitch JW, Neil A, Chapman BL, Hardy DB, Fletcher PJ. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations. A controlled clinical trial. Ann. Intern. Med. 1996 Jan 01;124(1 Pt 1):16-20.
- Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O’Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015 Nov 03;132(18 Suppl 2):S444-64.
- Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV, Christensen PD, Simonsen EH, Eriksen UH, Jensen GV, Svendsen JH, Toff WD, Healey JS, Andersen HR., DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur. Heart J. 2011 Mar;32(6):686-96.
- [Guideline] Epstein AE, DiMarco JP, Ellenbogen KA, et al, for the American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013 Jan 22. 61(3):e6-75
- [Guideline] Brignole M, Auricchio A, Baron-Esquivias G, et al, for the ESC Committee for Practice Guidelines (CPG). 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013 Aug. 34(29):2281-329.
- Rosenqvist M, Obel IW. Atrial pacing and the risk for AV block: is there a time for change in attitude?. Pacing Clin Electrophysiol. 1989 Jan. 12(1 pt 1):97-101.
- Nielsen JC, Thomsen PE, Hojberg S, et al, for the DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011 Mar. 32(6):686-96.
- Chen S, Wang Z, Kiuchi MG, et al. Cardiac pacing strategies and post-implantation risk of atrial fibrillation and heart failure events in sinus node dysfunction patients: a collaborative analysis of over 6000 patients. Clin Res Cardiol. 2016 Aug. 105(8):687-98.
- Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007 Sep 6. 357(10):1000-8.
- Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009 Nov 26. 361(22):2123-34.
- Lamas GA, Lee KL, Sweeney MO, et al, for the Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002 Jun 13. 346(24):1854-62.
- Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity–evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007 Sep. 4(9):1125-32.
- Menozzi C, Brignole M, Alboni P, et al. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol. 1998 Nov 15. 82(10):1205-9.
- Lien WP, Lee YS, Chang FZ, Lee SY, Chen CM, Tsai HC. The sick sinus syndrome: natural history of dysfunction of the sinoatrial node. Chest. 1977 Nov;72(5):628-34.
- Alonso A, Jensen PN, Lopez FL, Chen LY, Psaty BM, Folsom AR, Heckbert SR. Association of sick sinus syndrome with incident cardiovascular disease and mortality: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. PLoS ONE. 2014;9(10):e109662
- Shah MJ, Nehgme R, Carboni M, Murphy JD. Endocardial atrial pacing lead implantation and midterm follow-up in young patients with sinus node dysfunction after the fontan procedure. Pacing Clin Electrophysiol. 2004 Jul. 27(7):949-54.
- [Guideline] Zipes DP, DiMarco JP, Gillette PC, et al. Guidelines for clinical intracardiac electrophysiological and catheter ablation procedures. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Intracardiac Electrophysiologic and Catheter Ablation Procedures), developed in collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 1995 Aug. 26 (2):555-73.