Tumor lysis syndrome
Tumor lysis syndrome is cosidered to be an oncologic emergency that can occur after treatment with chemotherapy or radiotherapy of a fast-growing cancer, especially acute leukemias and lymphomas (cancers of the blood). Tumor lysis syndrome arises most commonly after the start of initial chemotherapy treatment, but may occur following ANY anticancer therapy such steroids, radiation, monoclonal antibodies, targeted agents and spontaneous cases have increasingly been documented in patients with high-grade hematologic malignancies 1. Tumor lysis syndrome can occur spontaneously in highly proliferative tumors— acute lymphoblastic leukemia (ALL) and Burkitt lymphoma 2. As tumor cells die, they break apart and release their contents into the blood. This causes a change in certain chemicals in the blood, which may cause damage to organs, including the kidneys, heart, and liver 3. As evident by its name, tumor cells breakdown with chemotherapy releases potassium, nucleic acids, and phosphorus into the circulation, resulting in rapid development of hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia, and acute renal failure 4. Tumor lysis syndrome is classified according to the 1993 Hande-Garrow classification system into two groups i.e. laboratory tumor lysis syndrome and clinical tumor lysis syndrome. The complications of tumor lysis syndrome include nausea, vomiting, diarrhea, anorexia, hematuria, tachycardia, and muscle cramps. Screening for tumor lysis syndrome is not recommended. However, patients with malignancies undergoing treatment or with acute renal failure should be considered for tumor lysis syndrome workup.
Although tumor lysis syndrome has been reported with virtually every type of tumor, it is typically associated with bulky, rapidly proliferating, treatment-responsive tumors where malignant cells accumulate in the blood and lymph nodes 5, typically, acute leukemias [acute myeloid leukemia (AML) with high white blood cell count and acute lymphoblastic leukemia (ALL)], high-grade non-Hodgkin lymphomas such as Burkitt lymphoma and aggressive B-cell lymphomas 6. Tumor lysis syndrome has also been reported with other hematologic malignancies and with solid tumors such as hepatoblastoma and stage IV neuroblastoma 7.
2010 International Expert Consensus Panel tumor lysis syndrome risk categories 8:
- Low (<1%)
- Most solid tumors
- Multiple myeloma, chronic myeloid leukemia (CML), Hodgkin’s lymphoma
- Chronic lymphocytic leukemia (CLL) treated exclusively with alkylating agents
- Acute myeloid leukemia (AML) with white blood cell count < 25K/mcl and LDH < 2 x upper limit of normal
- Low grade non-Hodgkin’s lymphoma (NHL) and other non-Hodgkin’s lymphoma with normal LDH and non-bulky disease
- Intermediate (1- 5%)
- Specific solid tumors: Neuroblastoma, germ-cell tumors and small-cell lung cancers
- Chronic lymphocytic leukemia (CLL) with white blood cell count ≥ 50K/mcl
- Chronic lymphocytic leukemia (CLL) treated with targeted/biologic therapies (fludarabine, rituximab, venetoclax)
- Acute myeloid leukemia (AML) with LDH >2 x upper limit of normal, white blood cell count ≥ 25K and < 100K/mcl
- T-cell acute lymphoblastic leukemia (T-ALL) with white blood cell count < 100K/mcl and LDH < 2x upper limit of normal
- Diffuse large B-cell lymphoma or Mantle cell lymphoma with elevated LDH and non-bulky disease
- Early stage Burkitt /lymphoblastic lymphoma and LDH < 2 x upper limit of normal
- Low risk lymphomas/leukemias with renal dysfunction
- High (>5%)
- B-cell acute lymphoblastic leukemia (B-ALL) (all)
- T-cell acute lymphoblastic leukemia (T-ALL) with LDH ≥2 x upper limit of normal or white blood cell count ≥100K/mcl
- Acute myeloid leukemia (AML) with white blood cell count ≥ 100K/mcl
- Advanced stage Burkitt /lymphoblastic lymphoma and/or LDH ≥ 2x upper limit of normal
- Diffuse large B-cell lymphoma or Mantle cell lymphoma with bulky disease and elevated LDH
- Any intermediate disease risk malignancy in setting of renal dysfunction, elevated uric acid, phosphorous, or potassium
Because tumor lysis syndrome is potentially lethal, the main principles of management are (1) identification of high-risk patients with initiation of preventive therapy and (2) early recognition of metabolic and renal complications and the prompt administration of supportive care, including aggressive hydration and diuresis, hemodialysis, treating hyperuricaemia with allopurinol prophylaxis and rasburicase and close monitoring of electrolyte abnormalities 2.
Figure 1. Tumor lysis syndrome
Tumor lysis syndrome cause
Tumor lysis syndrome occurs most often in patients with acute leukemia with high white blood cell (WBC) counts and in those with high-grade lymphomas in response to aggressive treatment. Tumor lysis syndrome may also occur in other hematologic malignancies and in a variety of solid tumors such as hepatoblastoma and stage IV neuroblastoma 7. Tumor lysis syndrome has occasionally occurred spontaneously, prior to any form of therapy 9.
Patients at highest risk are those with bulky, rapidly proliferating tumors that are sensitive to treatment. Lactate dehydrogenase (LDH) is a marker for highly proliferative tumors. An elevated pretreatment lactate dehydrogenase (LDH) level, which correlates with high tumor volume, is a strong prognostic indicator for developing clinically significant complications of therapy. The presence of renal insufficiency prior to therapy also correlates with an increased likelihood of tumor lysis syndrome.
An international consensus expert panel has proposed a classification that stratifies cancers into high, intermediate, or low risk for tumor lysis syndrome 8.
The high-risk group of cancers includes the following:
- Advanced Burkitt lymphoma/leukemia or early-stage disease with elevated baseline LDH
- Acute lymphocytic leukemia (ALL) with white blood cell (WBC) count ≥ 100,000/µL, or less if the baseline elevation of LDH is twice the upper limit of normal
- Acute myeloid leukemia (AML) with white blood cell count ≥10000/µL
- Diffuse large B-cell lymphoma with an elevated baseline LDH of twice upper limit of normal and bulky disease
Intermediate-risk malignancies include the following:
- AML with white blood cell of 25,000–100,000/µL
- ALL with white blood cell < 100000/µL and an LDH of less than twice upper limit of normal
- Early stage Burkitt lymphoma/leukemia with an LDH of less than twice upper limit of normal
- Diffuse large B-cell lymphoma with a baseline increase in LDH of twice upper limit of normal but non-bulky disease
Low-risk diseases include the following:
- Indolent lymphomas
- Chronic lymphocytic leukemia
- Chronic myeloid leukemia in the chronic phase
- AML with white blood cell count < 25,000/µL and an LDH elevated to less than twice upper limit of normal
- Multiple myeloma
- Solid cancers
Reports exist of tumor lysis syndrome associated with the administration of radiation therapy 10, corticosteroids, hormonal agents, biologic response modifiers, and monoclonal antibodies.
Agents reported to cause tumor lysis syndrome include the following:
Novel therapies associated with tumor lysis syndrome:
- Alvocidib (0-53%)
- Dasatinib (4%)
- Dinaciclib (15%)
- Ibrutinib (0-7%)
- Carfilzomib (0.4-4%)
- Lenalidomide (0-4%)
- Venetoclax (0-9%)
- Brentuximab (1.7%)
- Obinutuzumab (3-10%)
- CAR T-cells (10%)
The development of tumor lysis syndrome is not limited to the systemic administration of agents; it can occur with intrathecal administration of chemotherapy and with chemo-embolization.
Rare clinical situations in which tumor lysis syndrome has been observed 14 include pregnancy and fever. Patients under general anesthesia have also experienced tumor lysis syndrome.
Tumor lysis syndrome pathophysiology
Rapid tumor cell turnover results in release of intracellular contents into the circulation. This release can inundate renal elimination and cellular buffering mechanisms, leading to numerous metabolic derangements.
Clinically significant tumor lysis syndrome can occur spontaneously, but it is most often seen 48-72 hours after initiation of cancer treatment. Hyperkalemia is often the earliest laboratory manifestation. Hyperkalemia and hyperphosphatemia result directly from rapid cell lysis.
Hypocalcemia is a consequence of acute hyperphosphatemia with subsequent precipitation of calcium phosphate in soft tissues. In acute kidney injury, decreased calcitriol levels also cause hypocalcemia.
Hyperuricemia
Uric acid is the terminal catabolic product of purine metabolism in humans. Nucleic acid purines, which are released by cell breakdown, are ultimately metabolized to uric acid by hepatic xanthine oxidase. This conversion leads to hyperuricemia.
Uric acid is a weak acid with a pKa of approximately 5.4. It is soluble in plasma and is freely filtered at the renal glomeruli. However, uric acid is less soluble in renal tubular and collecting duct fluid due to normally acidic media, thus increasing the possibility of uric acid crystal formation in cases of hyperuricemia.
Acute kidney injury
The kidney is the primary organ involved in the clearance of uric acid, potassium, and phosphate. Preexisting volume depletion or renal dysfunction predisposes patients to worsening metabolic derangements and acute kidney injury. The acute kidney injury is often oliguric and can be multifactorial in etiology.
Uric acid nephropathy, however, is the major cause of acute kidney injury. Its development is due to mechanical obstruction by uric acid crystals in the renal tubules. Uric acid has a pKa of 5.6; uric acid precipitation is enhanced by high acidity and high concentration in the renal tubular fluid, and uric acid becomes less soluble as renal tubule pH decreases. Renal medullary hemoconcentration and decreased tubular flow rate also contribute to crystallization 15.
Another cause of acute kidney injury is acute nephrocalcinosis from calcium phosphate crystal precipitation, which may occur in other tissues. This develops in the setting of hyperphosphatemia and is exacerbated by overzealous iatrogenic alkalinization, because calcium phosphate, unlike uric acid, becomes less soluble at an alkaline pH. Precipitation of xanthine, which is even less soluble in urine than uric acid, or other purine metabolites whose urinary excretion is increased by the use of allopurinol, are other causes of acute kidney injury.
Figure 2. Tumor lysis syndrome pathophyiology
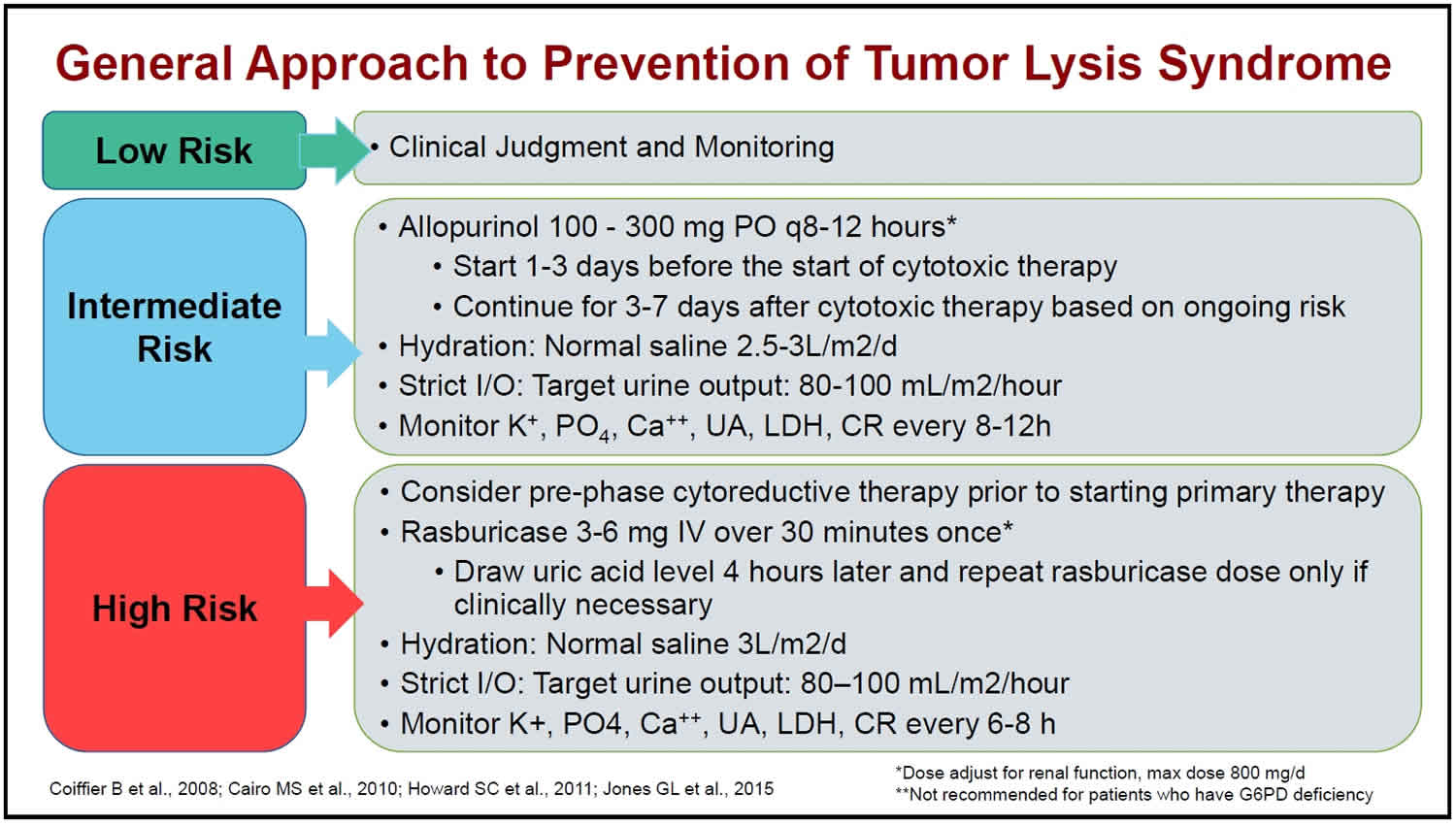
Tumor lysis syndrome prevention
Prevention is key to management of tumor lysis syndrome. Implement prevention strategies PRIOR to initiation of therapy 2:
- Identify patients at risk
- Implement prevention strategies, based on risk, PRIOR to initiation of therapy
- Consider a prephase of low-intensity therapy in highly chemosensitive cancers with high tumor burden
- Hydration to achieve high urine output is the mainstay of prevention
- Allopurinol and/or rasburicase
- Lab monitoring:
- Potassium,
- Phosphorus,
- Uric acid,
- Creatinine,
- Calcium
- LDH not a criterion for tumor lysis syndrome but helpful to measure daily
- Assessment of fluid status and cardiac function
Hyperkalemia is often first abnormality seen (precedes hyperuricemia)-can occur as quickly as 6 hours after start of therapy. Important to identify and treat lab abnormalities promptly.
In high risk patients, the greatest risk is 12-72 hours after cytotoxic therapy.
High urine flow rate most effective strategy to prevent uric acid induced renal injury:
- Sodium bicarbonate is NOT recommended for alkalinization. In animal models, urine alkalinization was not effective to prevent uric acid crystallization in absence of high urine output.
- Urine alkalinization can increase risk of renal injury:
- Xanthine and hypoxanthine less soluble in alkaline urine; can form urinary xanthine crystals
- Calcium phosphate less soluble in alkaline urine; can form calcium phosphate crystals (especially if high phosphate)
For patients with low or intermediate risk, if they have not developed tumor lysis syndrome within 2 days, the likelihood is essentially zero.
- Laboratory tumor lysis syndrome not associated with increased death rate
- Clinical tumor lysis syndrome significantly increases death rate
Cancer-related risk factors for tumor lysis syndrome
- Tumor with high proliferation rate (LDH level >2 x upper limit of normal)
- Tumor bulk (> 10 cm)
- Circulating tumor cells (≥ 25,000 cells/L)
- Sensitivity to cytotoxic therapy
Pre-existing patient factors that predispose to tumor lysis syndrome
- Pre-treatment hyperuricemia
- Dehydration or volume depletion
- Oliguria
- Chronic kidney disease or acute kidney injury
- Acidic urine
- Hypertension
- Left ventricular dysfunction/heart failure (difficult to tolerate hydration needed).
Figure 3. Tumor lysis syndrome
Allopurinol
Allopurinol an xanthine oxidase inhibitor prevents conversion of hypoxanthine to xanthine to uric acid.
- Renal clearance of hypoxanthine and xanthine are 10x higher than uric acid
- Requires 24 -72 hour to effectively prevent de novo formation of uric acid
- Allopurinol should NOT be used for treatment of hyperuricemia. Does not actively reduce existing serum uric acid
- Oral dose: 100-300 mg orally every 8 hpurs, max 800 mg/day
- In practice, 300 mg oral daily often used
- Renal dose adjustment
- If massive tumor lysis syndrome while on allopurinol, risk for xanthine nephropathy or stone formation
- Drug interactions: 6-MP, thiazide diuretics, azathioprine, cyclosporine, cyclophosphamide, and amoxicillin
- Side effects: rash (2-8%), nausea, Stevens-Johnson syndrome ( rare)
Febuxostat
Febuxostat an xanthine oxidase inhibitor prevents conversion of hypoxanthine to xanthine to uric acid.
- NOT FDA approved for tumor lysis syndrome: can consider for patients unable to tolerate allopurinol
- Black Box Warning: Higher rate of cardiovascular death in patients with pre-existing cardiovascular disease
- Reduces uric acid as effectively or more effectively than allopurinol
- Showed no difference in tumor lysis syndrome incidence compared to allopurinol
- Dose: 40-80 mg/day
- No renal dose adjustment needed
- Side effects: liver function abnormalities, nausea, arthralgia, rash, Stevens-Johnson syndrome (rare)
Rasburicase
Rasburicase is a recombinant urate oxidase, it works by oxidizing existing uric acid into allantoin (10x more soluble than uric acid). In contrast to allopurinol, rasburicase reduces preexisting hyperuricemia rapidly.
- FDA approved dose: 0.2 mg/kg IV over 30 minutes daily for up to 5 days
- Alternative fixed dosing: 3-6 mg IV once; may repeat if needed
- Peak effect in 4 hours
- Blood samples for uric acid should be collected in a pre-chilled tube, immediately placed on ice, and the assay completed within four hours
- No renal dose adjustment
- Avoids risk of crystallization in renal tubules
- Contraindication: G6PD deficiency and pregnancy. In G6PD deficiency, erythrocytes are vulnerable to oxidative stress can lead to hemolysis or methemoglobinemia
- Potential adverse effects: nausea, hypersensitivity reactions, methemoglobinemia, hemolysis in G6PD deficiency
Hydration
Volume depletion is a major risk factor for tumor lysis syndrome and must be corrected vigorously. Aggressive IV hydration not only helps to correct electrolyte disturbances by diluting extracellular fluid, it also increases intravascular volume. Increased volume enhances renal blood flow, glomerular filtration rate, and urine volume to decrease the concentration of solutes in the distal nephron and medullary microcirculation.
Ideally, IV hydration in high-risk patients should begin 24-48 hours prior to initiation of cancer therapy and continue for 48-72 hours after completion of chemotherapy.
Continuous infusion rates as high as 4-5 L daily (or 3 L/m² daily), yielding urine volumes of at least 3 L daily, should be given unless the patient’s cardiovascular status indicates impending volume overload.
Diuretics
If optimal state of hydration has been achieved and there is low urine output:
- Consider loop diuretics
- Avoid thiazide diuretics which increase uric acid levels and interact with allopurinol
- Avoid using diuretics if volume deplete
Tumor lysis syndrome signs and symptoms
In tumor lysis syndrome, a constellation of clinical signs and symptoms may develop prior to the initiation of chemotherapy or, more commonly, within 72 hours after administration of cytotoxic therapy 16. Inquiries should be made with regard to the following:
- Time of onset of symptoms of malignancy
- Presence of abdominal pain and distension
- Urinary symptoms – Such as dysuria, oliguria, flank pain, and hematuria
- Symptoms of hypocalcemia – Such as anorexia, vomiting, cramps, seizures, spasms, altered mental status, and tetany
- Neuromuscular: tetany—mild: perioral numbness/tingling, paresthesia of hands/feet, muscle spasm/cramps; severe—carpopedal spasm, laryngospasm, seizures
- Tests for latent tetany: Positive Chvostek (twitch to tapping of cheek bone area) and Trousseau sign (blood pressure cuff inflated higher than systolic blood pressure to occlude brachial artery for 3 minutes elicits spasm of muscles of hand and forearm)
- Cardiac: QT prolongation, arrhythmias
- Hyperphosphatemia: Results in secondary hypocalcemia and symptoms usually result from the hypocalcemia
- Symptoms of hyperkalemia – Such as weakness and paralysis
- Cardiac: arrhythmias, ventricular tachycardia, fibrillation, cardiac arrest
- ECG changes: Tall, peaked T waves with shortened QT interval, followed by progressive lengthening of PR interval and QRS duration
- Neuromuscular: paresthesia, muscle cramps, severe muscle weakness, and paralysis
Other manifestations of tumor lysis syndrome include the following:
- Lethargy
- Edema
- Fluid overload
- Congestive heart failure
- Cardiac dysrhythmias
- Syncope
- Sudden death
Physical Examination
Symptoms reflect the severity of underlying metabolic abnormalities. Hyperkalemia can cause paresthesia, weakness, and fatal cardiac arrhythmias.
Severe hypocalcemia can lead to the following signs and symptoms:
- Paresthesia and tetany with positive Chvostek and Trousseau signs
- Anxiety
- Carpal and pedal spasms
- Bronchospasm
- Seizures
- Cardiac arrest
Deposition of calcium phosphate in various tissues may be responsible for the following signs and symptoms:
- Pruritus
- Gangrenous changes of the skin
- Iritis
- Arthritis
Uremia can produce the following signs and symptoms:
- Fatigue
- Weakness
- Malaise
- Nausea
- Vomiting
- Anorexia
- Metallic taste
- Hiccups
- Neuromuscular irritability
- Difficulty concentrating
- Pruritus
- Restless legs
- Ecchymoses
As uremia progresses, paresthesia and evidence of pericarditis may develop, as well as signs of drug toxicity from medications eliminated by the kidney. Features of volume overload, such as dyspnea, pulmonary rales, edema, and hypertension, may develop.
Elevated uric acid levels may produce lethargy, nausea, and vomiting. Rapidly increasing uric acid levels may lead to arthralgia and renal colic.
Tumor lysis syndrome criteria
The most widely used diagnostic criteria are those proposed by Cairo et al 17 in 2004. Based on this classification, tumor lysis syndrome can be defined as laboratory tumor lysis syndrome, when tumor lysis syndrome is clinically silent and only detected through laboratory work up, and clinical tumor lysis syndrome, when laboratory tumor lysis syndrome is complicated by the clinical manifestations mentioned above. The diagnostic criteria proposed by Cairo et al 17 are presented in Tables 1 and 2. It is necessary to note that laboratory tumor lysis syndrome is defined as the presence of at least two or more biochemical variables within the 3 days before chemotherapy or 7 days after chemotherapy in the face of adequate hydration and use of uric acid lowering agent. Clinical tumor lysis syndrome is defined as the presence of at least one clinical criterion that is not believed to be attributable to the chemotherapy agent 17. However, a group has recently mentioned that this definition is imperfect since radiation therapy may lead to tumor lysis syndrome as well, and tumor lysis syndrome can occur spontaneously in rapidly proliferating and bulky malignancies 18.
Table 1. Cairo-Bishop definition of laboratory tumor lysis syndrome for adults
| Variable | Value | Change from baseline value |
| Uric acid | ≥ 8 mg/dL (476 mmol/L) | 25% increase |
| Potassium | ≥ 6.0 mEq/L (or 6 mmol/L) | 25% increase |
| Phosphorus | ≥ 4.5 mg/dL (1.45 mmol/L) for adults and ≥ 6.5 mg/dL (2.1 mmol/L) for children | 25% increase |
| Calcium | ≤ 7 mg/dL (1.75 mmol/L) | 25% decrease |
Table 2. Cairo-Bishop grading of clinical tumor lysis syndrome for adults
| Variable | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Creatinine | None | 1.5 times upper limits of normal. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 1.5-3.0 times upper limits of normal. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 3.0-6.0 times upper limits of normal. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 6.0 times upper limits of normal. Rise in creatinine is not attributable to chemotherapeutic agent(s) | Death |
| Cardiac arrhythmia | None | Intervention not indicated | Nonurgent medical intervention indicated. Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Symptomatic and incompletely controlled medically or controlled with device (e.g., defibrillator). Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Life-threatening (e.g., arrhythmia associated with heart failure, hypotension, syncope, shock). Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Death |
| Seizures | None | – | One brief, generalized seizure; seizure(s) well controlled by anticonvulsants or infrequent focal motor seizures not interfering with activities of daily living | Seizure in which consciousness is altered; poorly controlled seizure disorder; with breakthrough generalized seizures despite medical intervention | Seizure of any kind which are prolonged, repetitive or difficult to control (e.g., status epilepticus, intractable epilepsy) |
Tumor lysis syndrome treatment
Management of tumor lysis syndrome requires the initiation of preventive measures in high-risk patients prior to cancer treatment, as well as the prompt initiation of supportive care for patients who develop acute tumor lysis syndrome during treatment 19. Conservative management and prevention of tumor lysis syndrome are similar. Identify high-risk patients before treatment by assessing the extent of tumor burden, histopathologic findings, and renal function.
Patients with evidence of pretreatment acute tumor lysis syndrome should be started immediately on therapy for it. If possible, cancer treatment should be withheld until all parameters are corrected.
Patients at high risk and those with evidence of tumor lysis syndrome should have the following levels monitored at least three times daily:
- Blood urea nitrogen (BUN)
- Creatinine
- Uric acid
- Potassium
- Calcium
- Phosphate
- Lactate dehydrogenase (LDH)
Monitoring should continue for the first 48-72 hours after chemotherapy initiation. Some patients may need to be placed on dialysis prior to the initiation of therapy.
Tumor lysis syndrome treatment include:
- Aggressive intravenous fluid; achieve high urine output (≥ 100 mL/m²/hour)
- Frequent monitoring of electrolytes and creatinine every 6-8 hour
- Manage hyperuricemia:
- Rasburicase 3-6 mg IV once
- Check uric acid level 4 hours after rasburicase dose
- Uric acid samples should be drawn into prechilled, heparinized tubes and be promptly placed in an ice bath to avoid falsely low results due to enzymatic degradation. All samples should be analyzed within 4 hours of sample collection
- Correction of hyperkalemia and hyperphosphatemia
- Do not treat asymptomatic hypocalcemia, as it increases risk of calcium phosphate precipitation
- Renal consultation and dialysis if indicated for electrolyte abnormalities
- Cardiac monitoring for hyperkalemia or severe hypocalcemia
Management of electrolyte abnormalities
- Hyperkalemia:
- ≥ 6 mEq/L:
- Remove potassium from IV fluids
- Sodium polystyrene 15g qd-qid (slow effect-hrs to days)
- ECG and continuous cardiac monitoring
- ≥ 6.5 mEq/L: As above, PLUS
- Regular Insulin 10U and 50mL D50 (25g dextrose) IV, then infuse 50-75 mL of 10% dextrose over 1 hour. Monitor blood glucose levels every hour for five to six hours. Promotes redistribution of potassium from extracellular to intracellular space
- Calcium gluconate 1g IV (adults) or 100 mg/kg (children) if K > 6.5 or any ECG changes (cardioprotectant)
- Additional options:
- Loop diuretics- furosemide 40 mg IV (caution if renal dysfunction or hypovolemic)
- Albuterol 20 mg nebulized
- ≥ 6 mEq/L:
- Hyperphosphatemia:
- Phosphorus-restricted diet
- Oral phosphate binders: Sevelamer
- >5.5 mg/dL to <7.5 mg/dL: 800 mg 3 times daily
- ≥7.5 mg/dL to <9 mg/dL: 1,200 to 1,600 mg 3 times daily
- ≥9 mg/dL: 1,600 mg 3 times daily
- Hypocalcemia:
- Asymptomatic: should not be treated to avoid increasing calcium phosphate precipitate in kidney
- Treat only if symptomatic (tetany, seizures)
- Calcium gluconate 50-100 mg/kg IV
Indications for cardiac monitoring or hemofiltration
Indications for cardiac monitoring
- Hyperkalemia: K+ ≥ 6 meQ/dL
- ECG changes- peaked T waves, short P wave, wide QRS
- Hypocalcemia: Ca2+≤ 7 mg/dL
- ECG changes– QT prolongation, bradycardia
Indications for dialysis/ continuous hemofiltration (CRRT):
- Persistent severe hyperkalemia
- Persistent sever hyperphosphatemia
- Severe metabolic acidosis
- Uremia (pericarditis, severe encephalopathy)
- Hyperphosphatemia-induced symptomatic hypocalcemia
- Volume overload unresponsive to diuretic therapy
- Oliguria
Diet
Dietary restrictions are highly dependent on the status of the individual patient. However, patients who are not restricted to a nothing-by-mouth diet could theoretically benefit from restriction of intake of foods that contain high levels of potassium, phosphorus, or uric acid.
Dialysis
If the previously described therapies for the complications of tumor lysis syndrome fail, consider early initiation of dialysis. Dialysis prevents irreversible renal failure and other life-threatening complications. Indications for dialysis include persistent hyperkalemia or hyperphosphatemia despite treatment, volume overload, uremia, symptomatic hypocalcemia, and hyperuricemia.
Hemodialysis is preferred over peritoneal dialysis because of better phosphate and uric acid clearance rates. Continuous hemofiltration also has been used and is effective in correcting electrolyte abnormalities and fluid overload.
Because hyperkalemia can recur after dialysis is initiated and because of the high phosphate burden in some patients with tumor lysis syndrome, electrolyte levels must be monitored frequently and dialysis repeated as needed.
Tumor lysis syndrome guidelines
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. Available at (https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf)
Tumor lysis syndrome prognosis
Early recognition of signs and symptoms of patients at risk for tumor lysis syndrome, including identification of abnormal clinical and laboratory values, can lead to successful prevention of the otherwise life-threatening complications of the condition.
Potential complications of tumor lysis syndrome include uremia and oliguric renal failure due to tubule precipitation of uric acid, calcium phosphate, or hypoxanthine.
Severe electrolyte disturbances, such as hyperkalemia and hypocalcemia, predispose patients to cardiac arrhythmia and seizures.
Iatrogenic complications, such as pulmonary edema from overly vigorous hydration or metabolic alkalosis from excess exogenous administration of bicarbonate, can also occur and are life threatening if not immediately addressed.
Acute kidney injury
Renal tubule precipitation of uric acid, calcium phosphate, or hypoxanthine causes acute kidney injury. This is often oliguric (< 400 mL daily) in nature, leading to volume overload and complications of hypertension and pulmonary edema.
High blood urea nitrogen (BUN) levels due to increased protein catabolism and renal impairment can be severe enough to result in pericarditis, platelet dysfunction, and defective cellular immunity. Renal dysfunction can be severe enough to require dialysis, but with prompt supportive measures, it is usually reversible.
Cardiac arrhythmia
Hyperkalemia can lead to electrocardiographic changes and life-threatening cardiac arrhythmia, including asystole. Severe potassium elevation can cause electrocardiographic alterations such as peaked T waves, flattened P waves, prolonged PR interval, widened QRS complexes, deep S wave, and sine waves. Hypocalcemia can lead to QT interval lengthening, which predisposes patients to ventricular arrhythmia.
Metabolic acidosis
Acute kidney injury and the liberation of large amounts of endogenous intracellular acids from cellular catabolism result in acidemia. This acidemia causes a decrease in serum bicarbonate concentration and a high anion gap acidosis (see the Anion Gap calculator).
Acidemic states can worsen the many electrolyte imbalances already present in tumor lysis syndrome; intracellular uptake of potassium is hindered, uric acid solubility is decreased, and extracellular shift of phosphate is promoted. Calcium phosphate solubility, however, improves in acidic conditions.
The myriad of metabolic disorders must be assessed and treated rapidly. Proper fluid management, alkalinization of the urine, correction of acidosis, and attention to infections are the mainstays of therapy.
References- Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014 Jan. 21(1):18-26.
- Updates in the Management of Tumor Lysis Syndrome. https://education.nccn.org/system/files/Zitella_NCCNhemnf19.pdf
- Williams SM, Killeen AA. Tumor Lysis Syndrome. Arch Pathol Lab Med. 2018 Nov 30.
- Strauss PZ, Hamlin SK, Dang J. Tumor Lysis Syndrome: A Unique Solute Disturbance. Nurs Clin North Am. 2017 Jun. 52 (2):309-320.
- Moore AJ, Vu MA, Strickland SA. Supportive Care in Hematologic Malignancies. In: Greer JP, Arber DA, Glader B, List AF, Means RT Jr, Paraskevas F, Rodgers GM, eds. Wintrobe’s Clinical Hematology. 13th ed. Philadelphia, Pa: Wolters Kluwer/Lippincott Williams & Wilkins; 2003. 1426-66.
- Mirrakhimov AE, Voore P, Khan M, Ali AM. Tumor lysis syndrome: A clinical review. World J Crit Care Med. 2015 May 4. 4 (2):130-8.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997 Nov. 103(5):363-7.
- Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010 May. 149 (4):578-86.
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010 May. 55(5 Suppl 3):S1-13; quiz S14-9.
- Chen SW, Hwang WS, Tsao CJ, et al. Hydroxyurea and splenic irradiation-induced tumour lysis syndrome: a case report and review of the literature. J Clin Pharm Ther. 2005 Dec. 30(6):623-5.
- Lee CC, Wu YH, Chung SH, et al. Acute tumor lysis syndrome after thalidomide therapy in advanced hepatocellular carcinoma. Oncologist. 2006 Jan. 11(1):87-8; author reply 89.
- Jaskiewicz AD, Herrington JD, Wong L. Tumor lysis syndrome after bortezomib therapy for plasma cell leukemia. Pharmacotherapy. 2005 Dec. 25(12):1820-5.
- Kurt M, Onal IK, Elkiran T, et al. Acute tumor lysis syndrome triggered by zoledronic Acid in a patient with metastatic lung adenocarcinoma. Med Oncol. 2005. 22(2):203-6.
- Leibowitz AB, Adamsky C, Gabrilove J, Labow DM. Intraoperative acute tumor lysis syndrome during laparoscopic splenectomy preceded by splenic artery embolization. Surg Laparosc Endosc Percutan Tech. 2007 Jun. 17(3):210-1.
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010 May. 55(5 Suppl 3):S1-13; quiz S14-9
- Mughal TI, Ejaz AA, Foringer JR, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010 Apr. 36(2):164-76.
- Cairo, M.S. and Bishop, M. (2004), Tumour lysis syndrome: new therapeutic strategies and classification. British Journal of Haematology, 127: 3-11. doi:10.1111/j.1365-2141.2004.05094.x https://doi.org/10.1111/j.1365-2141.2004.05094.x
- Tumor Lysis Syndrome in Solid Tumors: An up to Date Review of the Literature. Mirrakhimov AE, Ali AM, Khan M, Barbaryan A. Rare Tumors. 2014 May 13; 6(2):5389.
- [Guideline] Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008 Jun 1. 26(16):2767-78.