Vitamin K2
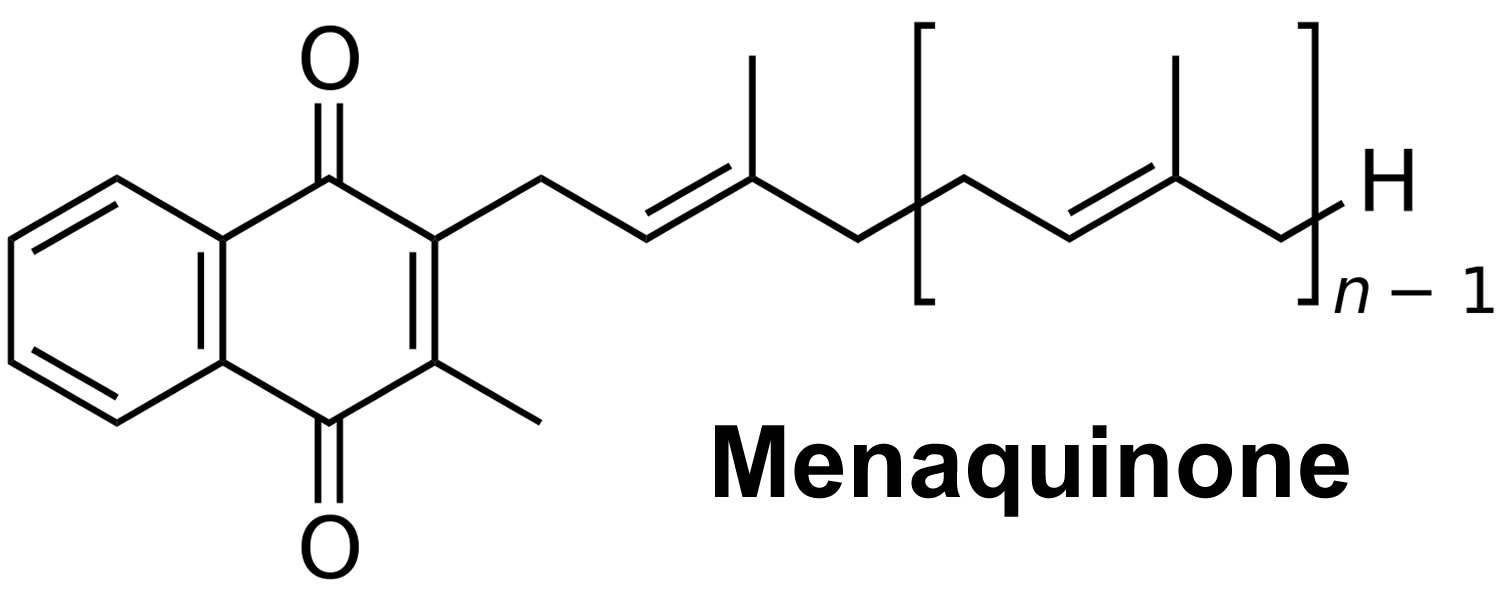
Vitamin K2 also known as menaquinones designated as MK-4 through MK-13 are the product of bacterial production or conversion from dietary vitamin K1 (phylloquinone) and are also found in fermented foods (e.g., cheeses and the Japanese soybean product natto) (Figures 1 and 2) 1, 2. Vitamin K2 (menaquinones) exist in multiple forms; however, the tendency in the literature to group all menaquinones under the term vitamin K2 has erroneously led many to assume that all vitamin K2 (menaquinones) are similar in origin and function. Menaquinones are primarily of bacterial origin, and differ in structure from vitamin K1 (phylloquinone) in their 3-substituted lipophilic side chain. The major vitamin K2 (menaquinones) contain 4–10 repeating isoprenyl side chains and are designated as MK-4 through MK-13, based on the length of their side chain 3, 4. MK-4, MK-7, and MK-9 are the most well-studied menaquinones. Food composition databases are limited for vitamin K2 (menaquinones) and their presence in foods varies by region. Dietary intakes of all forms of vitamin K vary widely among age groups and population subgroups. Similarly, the utilization of vitamin K from different forms and food sources appear to vary, although our understanding of vitamin K is still rudimentary in light of new developments regarding the vitamin K2 (menaquinones) 5. Pharmacological doses of menaquinone-4 (MK-4; brand name, menatetrenone) are currently used in Japan in the treatment of osteoporosis 6. Accordingly, most intervention trials investigating the effect of high-dose MK-4 on bone loss have been conducted in Japanese postmenopausal women. In a three-year placebo-controlled trial among postmenopausal women with osteopenia, adding a MK-7 supplement (375 mcg/day) to combined calcium-vitamin D supplementation did not affect bone mineral density (BMD) or other bone health parameters despite reductions in serum undercarboxylated osteocalcin (ucOC) 7. At present, the potential role for supplemental menaquinones on bone health still needs to be established in large, randomized, and well-controlled trials.
Almost all vitamin K2 (Menaquinones) in particular the long-chain menaquinones are created in the gut by bacteria 8, 9. Gut flora converts vitamin K1 (phylloquinone) into vitamin K2 (menaquinone). A range of vitamin K2 forms can be created. This transformation takes place via the gut bacteria lengthening the isoprenoid side chain during anaerobic respiration. Vitamin K2 (menaquinones) differ in structure from vitamin K1 (phylloquinone) due to the 3-substituted lipophilic side chain. The most important forms of vitamin K2 (menaquinones) contain 4 to 10 repeating isoprenoid units. These are indicated by MK-4 to MK-10. In the human large intestine, the major forms of vitamin K2 (menaquinones) found to be present, including MK-6, MK7, MK-8, MK-10, and MK11, are produced by several types of enterobacteria such as Bacteroides, Enterobacteria, Eubacterium lentum, and Veillonella 10. Although intestinal bacteria synthesis is described to contribute to vitamin K requirements 11, it is not yet clear its true contribution to human vitamin K2 nutrition, and there is a need for further progress in this area 12. The MK-7 and other bacterially derived forms of vitamin K2 exhibit vitamin K activity in animals. The synthetic type of vitamin K, vitamin K3 (menadione), interferes with glutathione, which causes toxicity to animals. For this reason, vitamin K3 is no longer a viable treatment for vitamin K deficiency 3.
Menaquinone-4 (MK-4) is unique among the vitamin K2 (menaquinones) in that it is produced by the body from vitamin K1 (phylloquinone) via a conversion process that does not involve bacterial action. Instead, menaquinone-4 (MK-4) is formed by a realkylation step from vitamin K3 (menadione) present in animal feeds or is the product of tissue-specific conversion directly from dietary vitamin K1 (phylloquinone) 13, 14, 15. In the United States, vitamin K3 (menadione) is the synthetic form of vitamin K used in poultry feed. As such, MK-4 formed from vitamin K3 (menadione) is present in poultry products in the US food supply 16. However, MK-4 formed from vitamin K1 (phylloquinone) is limited to organs not commonly consumed in the diet including kidney. The exceptions are dairy products with menaquinone-4 (MK-4) found in milk, butter, and cheese, albeit in modest amounts. Therefore it is unlikely that menaquinone-4 (MK-4) is an important dietary source of vitamin K in food supplies that do not use vitamin K3 (menadione) for poultry feed nor are rich in dairy products.
There is growing interest in the health benefits of longer-chain menaquinones (vitamin K2), which are limited to certain foods such as dairy products, meats, and fermented foods 3. Menaquinone-7 (MK-7) is primarily the product of fermentation using bacillus subtilis natto and is present in a traditional Japanese soybean-based product called natto 17. Natto contains approximately 2.5 times more MK-7 compared to the vitamin K1 (phylloquinone) content of spinach. Natto also contains MK-8 and phylloquinone (84 and 35 µg/100g, respectively), although both are modest in concentration compared to MK-7 18. Some cheeses also contain MK-8 and MK-9 18, but these are dependent on cheese production practices, hence the food composition databases are limited in their ability to characterize vitamin K2 (menaquinone) intake across different food supplies.
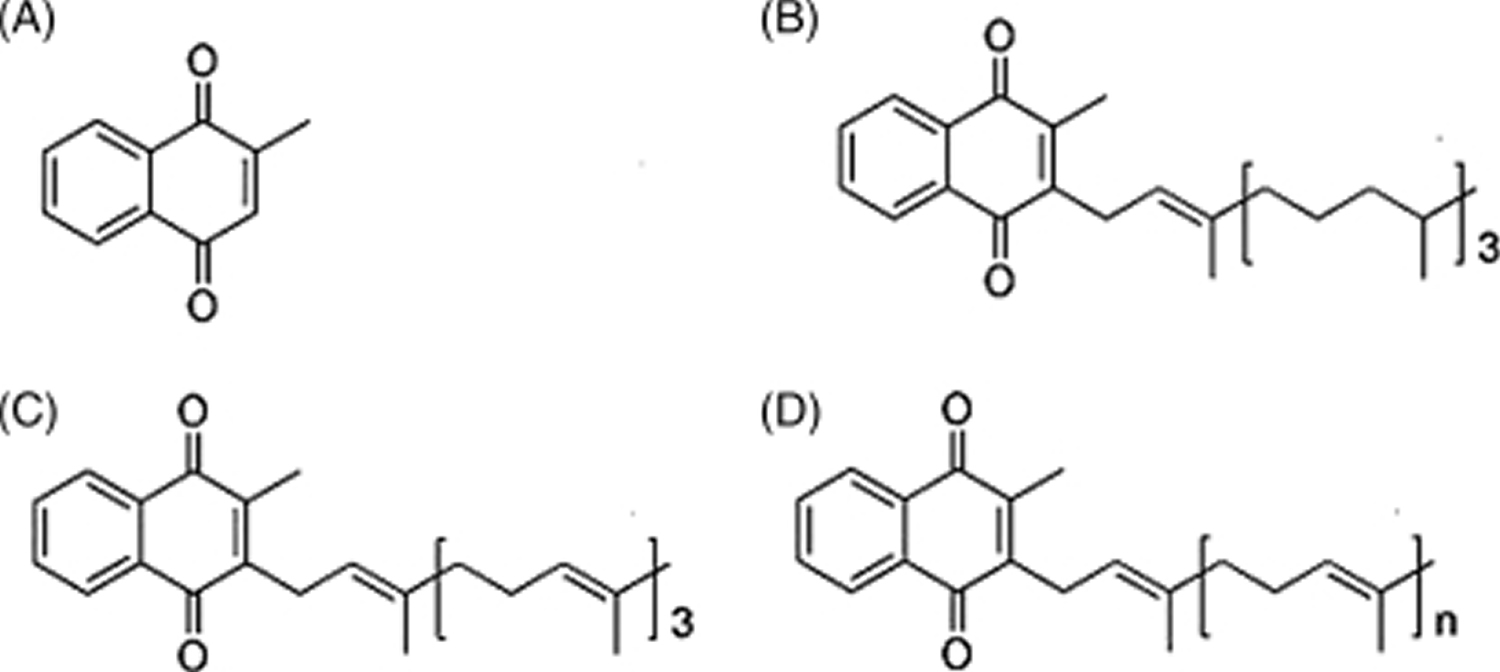
Figure 1. Vitamin K chemical structure
Footnote: Forms of vitamin K. (A) Menadione (vitamin K3) represents the basic structure common to vitamin K1 and vitamin K2; (B) phylloquinone (vitamin K1), which is the primary dietary source; (C) menaquinone-4 (MK-4), which is a conversion product from menadione (vitamin K3) or phylloquinone (vitamin K1); and (D) menaquinones (vitamin K2), which can vary in length from MK-4 to MK-13.
[Source 3 ]Figure 2. Vitamin K2 chemical structure
What does Vitamin K2 do?
The primary function of vitamin K2 (menaquinone) is adding carboxylic acid groups (carboxylation) to glutamate (Glu) residues to form gamma-carboxyglutamate (Gla) residues during the creation of clotting factors (clotting factor II, VII, IX, X) (Figure 3 Vitamin K Cycle) 19. The presence of two carboxylic acid groups on a single carbon that resides in the gamma-carboxyglutamate (Gla) residue allows for the chelation of calcium ions. The binding of calcium ions in this fashion is of crucial importance for vitamin K-dependent clotting factors, permitting the perpetuation of the clotting cascades. Vitamin K is also responsible as a factor in synthesizing prothrombin, factor VII, factor IX, and factor X 20.
Carboxylation is catalyzed by the enzyme gamma-glutamyl carboxylase (GGCX), which utilizes a reduced form of vitamin K hydroquinone, carbon dioxide and oxygen as cofactors (Figure 3). Concomitant with each glutamate modification, vitamin K hydroquinone is oxidized to vitamin K 2,3-epoxide (vitamin K epoxide) 21. Vitamin K epoxide is converted back to vitamin K hydroquinone through a two-step reduction (first to vitamin K, then to vitamin K hydroquinone) using the enzymes vitamin K epoxide reductase (VKOR) and vitamin K reductase (VKR) in a pathway known as the vitamin K cycle (Figure 3). Importantly, warfarin interferes with regeneration of vitamin K 2,3-epoxide (vitamin K epoxide) to vitamin K hydroquinone, thus impairing gamma-carboxylation and the activity of vitamin K dependent proteins, including the extra-hepatic proteins Osteocalcin (bone Gla-protein) and matrix Gla-protein (MGP), respectively involved in bone mineralization and inhibition of vascular calcifications. If vitamin K is deficient, vitamin K dependent proteins cannot increase their carboxylation status (and they become significantly undercarboxylated), losing their capacity to bind calcium, so that bone metabolism may be impaired and the process of vascular calcification enhanced 22.
Vitamin K-dependent carboxylation was originally observed in clotting factors 23. In all clotting factors, 10 to 12 gamma-carboxyglutamate (Gla) residues are located in a homologous amino-terminal region referred to as the Gla domain 24. The multiple gamma-carboxyglutamate (Gla) residues in this domain adopt a calcium-dependent conformation that promotes clotting factors binding to a membrane surface 25, such as damaged vascular endothelial cells or activated platelets, which allows the localization of clotting factors near the site of vascular injury to either promote or regulate clotting.
With the discovery of new gamma-carboxyglutamate (Gla) proteins, vitamin K-dependent carboxylation has been implicated in a number of biological functions beyond coagulation. Osteocalcin is a Gla protein produced by osteoblasts and is important for bone formation 26. Recent studies suggest that osteocalcin also functions as a hormone affecting glucose metabolism in mice 27; whether this is the case in humans still needs to be clarified 28. The second Gla protein found in bone is matrix Gla protein (MGP), which functions as a strong inhibitor of vascular calcification and connective tissue mineralization 29. Defects of matrix Gla protein (MGP) carboxylation have been associated with cardiovascular diseases and pseudoxanthoma elasticum syndrome 30. In addition, MGP has been described as a critical regulator of endothelial cell function, regulating both physiological and tumor-related angiogenesis 31. Other vitamin K-dependent proteins that are not involved in coagulation include Gas6 (growth arrest-specific protein 6), PRGPs (proline-rich Gla proteins) and TMGs (transmembrane Gla proteins). In the nervous system, vitamin K2 (menaquinones) plays a crucial role in regulating many functions, such as sphingolipid synthesis 32, Growth arrest-specific protein 6 (Gas6)—associated-signaling 33 and cognition 34. These functions are associated with gamma-glutamyl carboxylase (GGCX). In the human brain, menaquinone-4 (MK-4) is the most abundant form of vitamin K 35. A better understanding of the structure-function relationship of the enzymes in the vitamin K cycle will help you to better understand and control a variety of biological processes (see Figure 3 below).
Lately, researchers have demonstrated that vitamin K is also involved in building bone. Low levels of circulating vitamin K have been linked with low bone density, and supplementation with vitamin K shows improvements in biochemical measures of bone health 36. There is a consistent line of evidence in human epidemiologic and intervention studies that clearly demonstrates that vitamin K can improve bone health. The human intervention studies have demonstrated that vitamin K can not only increase bone mineral density in osteoporotic people but also actually reduce fracture rates. Further, there is evidence in human intervention studies that vitamins K and D, a classic in bone metabolism, works synergistically on bone density 36. Several mechanisms are suggested by which vitamin K can modulate bone metabolism. Besides the gamma-carboxylation of osteocalcin, a protein believed to be involved in bone mineralization, there is increasing evidence that vitamin K also positively affects calcium balance, a key mineral in bone metabolism. The Institute of Medicine recently has increased the dietary reference intakes of vitamin K to 90 microg/d for females and 120 microg/d for males, which is an increase of approximately 50% from previous recommendations 36. A report from the Nurses’ Health Study suggests that women who get at least 110 micrograms of vitamin K a day are 30 percent less likely to break a hip than women who get less than that 4. Among the nurses, eating a serving of lettuce or other green, leafy vegetable a day cut the risk of hip fracture in half when compared with eating one serving a week. Data from the Framingham Heart Study also shows an association between high vitamin K intake and reduced risk of hip fracture in men and women and increased bone mineral density in women 37, 38.
Pharmacological doses of menaquinone-4 (MK-4; brand name, menatetrenone) are currently used in Japan in the treatment of osteoporosis 6. Accordingly, most intervention trials investigating the effect of high-dose MK-4 on bone loss have been conducted in Japanese postmenopausal women. In a three-year placebo-controlled trial among postmenopausal women with osteopenia, adding a MK-7 supplement (375 mcg/day) to combined calcium-vitamin D supplementation did not affect bone mineral density (BMD) or other bone health parameters despite reductions in serum undercarboxylated osteocalcin (ucOC) 7. At present, the potential role for supplemental menaquinones on bone health still needs to be established in large, randomized, and well-controlled trials.
A 2019 systematic review and meta-analysis of clinical trials in postmenopausal women or women with osteoporosis found vitamin K supplementation — of any form — lowered risk of clinical fracture compared to controls (9 trials varying from 6 months to 2 years: 3 using phylloquinone, 5 using MK-4, and 1 using MK-7) 39. However, no benefit of vitamin K supplementation was seen for vertebral fractures (7 trials) or bone mineral density (BMD) (22 trials) 39. The authors of this meta-analysis noted that the high heterogeneity of the included trials (i.e., form and dose of vitamin K, use of other supplements and drugs by participants, and treatment length) makes it difficult to inform clinical recommendations 39.
Vitamin K Cycle
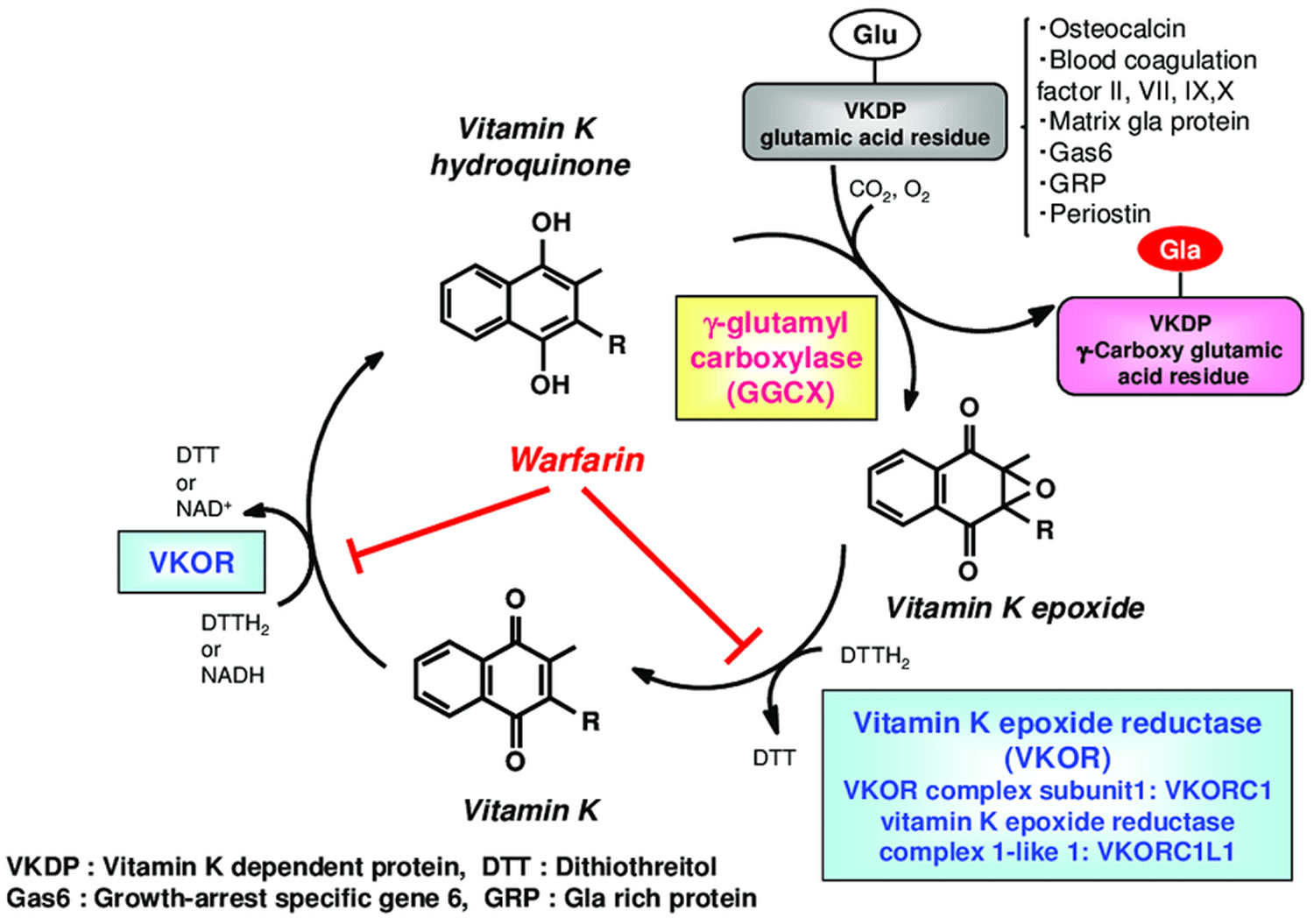
Vitamin K obtained from the diet is considered to reach the target tissues via lipid absorption and the transport system 40. Once transferred to the cells of the target tissue, vitamin K is metabolized by redox (reduction–oxidation) cycling in the intracellular endoplasmic reticulum body, in a process known as the “vitamin K cycle”, “vitamin K oxidation-reduction cycle” or “vitamin K-epoxide cycle” (Figure 3) 41. This series of oxidation-reduction reactions begins with conversion of vitamin K from a stable oxidized form (quinone form [KO]) to a vitamin K hydroquinone (reduced form [KH2]) by vitamin K-epoxide reductase (VKOR) 42, 43. Gamma-glutamyl carboxylase (GGCX) carboxylates the glutamic acid residues of vitamin K-dependent proteins (VKDP) to gamma-carboxyglutamate (Gla) using reduced vitamin K (vitamin K hydroquinone), while simultaneously oxidizing the reduced form of vitamin K to a vitamin K epoxide (oxidized form). These reactions that gamma-glutamyl carboxylase (GGCX) catalyzes proceed on the GGCX protein molecule using CO2 and O2; however, the detailed molecular mechanisms are not clear. The epoxide form of vitamin K (oxidized form) is reduced by epoxide reductase (vitamin K epoxide reductase complex 1; VKORC1 or vitamin K epoxide reductase complex 1-like 1; VKORC1L1) to a reduced form and then to the reduced hydroquinone form 44. This reuse system allows for a very small amount of vitamin K in cells to act efficiently as a cofactor of gamma-glutamyl carboxylase (GGCX) in the post-translational carboxylation of vitamin K-dependent proteins (VKDPs). Warfarin, an oral anticoagulant drug, inhibits vitamin K epoxide reductase (VKOR), stops the vitamin K cycle, and prevents the gamma-glutamyl-carboxylated (Gla) conversion of the blood coagulation factors, thus inhibiting coagulation (Figure 3). Activity of both gamma-glutamyl carboxylase (GGCX) and vitamin K epoxide reductase (VKOR) are regulated by calumenin 45. Recent evidence has revealed that GGCX is the only enzyme involved in Gla formation, based on structure and function analyses of GGCX at the gene level and animal studies showing that GGCX gene deficiency causes embryonic lethality from systemic bleeding.
Some oral anticoagulants, such as warfarin (Jantoven, formerly known as Coumadin), inhibit coagulation by inhibiting the action of vitamin K. Warfarin prevents the recycling of vitamin K by blocking vitamin K-epoxide reductase (VKOR) activity, hence preventing vitamin K recycling and therefore creating a functional vitamin K deficiency (Figure 3) 46. Inadequate gamma-carboxylation of vitamin K-dependent coagulation proteins interferes with the coagulation cascade, which inhibits blood clot formation. Large quantities of dietary or supplemental vitamin K can overcome the anticoagulant effect of vitamin K antagonists; thus, patients taking oral anticoagulants are cautioned against consuming very large or highly variable quantities of vitamin K (see Drug interactions). Experts now advise a reasonably constant dietary intake of vitamin K that meets current dietary recommendations (90-120 mcμg/day) for patients taking vitamin K antagonists like warfarin 47, 48.
Vitamin K acts as a cofactor for gamma-glutamyl carboxylase (GGCX) via the vitamin K cycle and exerts physiological effects through its regulation of vitamin K-dependent proteins (VKDPs) 49. More than 20 VKDPs have been found. Osteocalcin promotes bone formation, and blood coagulation factors II, VII, IX, and X activate blood coagulation. Matrix Gla protein suppresses cardiovascular calcification, and brain-expressed Gas 6 (growth arrest-specific protein 6) promotes neural differentiation for signaling and cognitive functions in the brain 50, 49. Gamma-glutamyl carboxylase (GGCX) is an enzyme that converts glutamic acid (Glu) residues to gamma-carboxyglutamate (Gla) residues, so that the Gla-containing proteins can exert various physiological actions such as blood coagulation and bone formation.
Few studies, however, have addressed derivatives targeting GGCX. Vermeer et al. 51 found increased gamma-glutamyl carboxylase (GGCX) activity with modifying the side-chain structure of vitamin K to a saturated alkyl side chain with an amide bond. Further modification of the side-chain structure is anticipated from synthesis of vitamin K derivatives that yield stronger GGCX activity. Development of such new derivatives may lead to drug discovery that can enhance the physiological effects associated with GGCX and other factors 52.
Figure 3. Vitamin K oxidation-reduction cycle
Footnotes: During vitamin K-dependent carboxylation, glutamate (Glu) is converted to gamma-carboxyglutamte (Gla) by gamma-glutamyl carboxylase (GGCX) using a reduced form of vitamin K hydroquinone, carbon dioxide, and oxygen as cofactors. Vitamin K hydroquinone is oxidized to vitamin K epoxide. Vitamin K epoxide is reduced to vitamin K by vitamin K epoxide reductase (VKOR). The reduction of vitamin K to vitamin K hydroquinone is carried out by vitamin K epoxide reductase (VKOR) and an as-yet-unidentified vitamin K reductase (VKR).
[Source 53 ]Vitamin K derivatives targeting steroid and xenobiotic receptor (SXR)
Both vitamin K1 (phylloquinone) and vitamin K2 (menaquinones) may activate the steroid and xenobiotic receptor (SXR) 54. Steroid and xenobiotic receptor (SXR) is a nuclear receptor involved in the transcriptional regulation of enzymes such as cytochrome P450 (in particular the CYP3A4 isoform) 55.
Tabb et al. 56 revealed that menaquinone-4 (MK-4) regulates gene expression as a ligand of the nuclear receptor SXR. Steroid and xenobiotic receptor (SXR) is mainly expressed in the liver and intestine and regulates expression of genes encoding enzymes involved in steroid metabolism and detoxification of xenobiotics and of various drugs 56. When bound to a ligand, SXR forms a heterodimer with retinoid X receptor, and the resulting complex then binds to an SXR responsive element on the target gene promoter via the DNA-binding domain to exert transcription regulation 57. In addition to bile acids (e.g., lithocholic acid), drugs such as rifampicin, SR12813, and hyperforin are ligands involved in this process. Among the vitamin K homologs, menaquinone-4 (MK-4) can activate transcription of the SXR target gene CYP3A4, as a ligand of SXR 57. MK-4 plays an important role in osteoblast formation by inducing expression of genes such as matrilin-2 and tsukushi, which are involved in collagen accumulation via SXR 58. An in vitro study further showed that overexpression of SXR and its activation by MK-4 inhibits proliferation and migration of liver cancer cells 59. More recently, the data showed that vitamin K2 (menaquinone) has a differentiation-promoting effect on myeloid progenitors and an anti-apoptotic effect on erythroid progenitors 60.
Thus, menaquinone-4 (MK-4) works via SXR to regulate the expression of various genes at the transcriptional level, resulting in broad physiological effects such as bone formation and liver cancer suppression as well as drug metabolism 53. However, to date, MK-4 is the only vitamin K homolog known to exert its activities via SXR, and further research is needed to clarify whether other vitamin K homologs act as SXR ligands. X-ray crystal structure analysis of complexes of human pregnane X receptor (PXR) and ligands (such as rifampicin) have demonstrated that the ligand-binding region of SXR is large, with substantial flexibility 61, suggesting that other vitamin K congeners likely could act as SXR ligands.
Because menaquinone-4 (MK-4) is present in the brain at a relatively high concentration, it is thought to have important roles in the brain 62. Vitamin K protects neural cells from oxidative stress; however, a crucial role for vitamin K in the brain has not been elucidated 53. Neural stem cells engage in continuous self-replication while maintaining the ability to differentiate into neurons and glial cells in the early embryonic and late fetal stages. Neural stem cells can differentiate into neuronal precursor cells and glial precursor cells, and each progenitor cell differentiates into neurons, astrocytes, and oligodendrocytes. The neural stem cells that do not differentiate into neurons differentiate into glial cells before and after birth, at which point differentiation into neurons is complete 63.
Neuronal progenitor cells differentiate into neurons, while glial progenitor cells differentiate into astrocytes and oligodendrocytes 64. Scientists recently reported that vitamin K2 (menaquinone) show weak activity in driving the differentiation of progenitor cells to neuronal cells 65, which depended on the number of isoprene units of the side chain in the vitamin K homolog. If this differentiation activity can be increased by modification of the chemical structure, it would be possible to regulate it with a specific neuronal differentiation inducer based on a low-molecular-weight compound. Such a compound could provide an alternate strategy to conventional gene induction methods used in induced pluripotent stem cells and other types of stem and progenitor cells. Researchers are focusing on the role of vitamin K in the regeneration of neurons, and synthesized vitamin K analogs that could differentiate progenitor cells into neuronal cells.
How much vitamin K2 do I need?
The amount of vitamin K you need depends on your age and sex. Average daily recommended amounts are listed below in micrograms (mcg) (Table 1). The current US dietary guidelines for intakes of vitamin K are 90 and 120 mcg/day for women and men, respectively 66. These guidelines are termed adequate intakes (AI) because the Institute of Medicine concluded in 2001 that there were insufficient data available to generate a precise recommendation for vitamin K 37. The adequate intake (AI) values for vitamin K were generated from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) and based on the median vitamin K1 (phylloquinone) intake in the United States for each age and gender category 66. In the absence of abnormal bleeding associated with low vitamin K intakes among adults, it was assumed that the current intakes are adequate. However, the adequacy of intake defined by an absence of bleeding is controversial. Furthermore, the elderly report median intakes below the current adequate intake (AI) for adults. There is controversy regarding biochemical measures of subclinical vitamin K deficiency and as a consequence, the true dietary requirement of vitamin K is unknown 67. By comparison, the guidelines in the United Kingdom are 1 mcg/kg body weight per day 68 and are set at 75 mcg/day for adult men, 60 mcg/day for women, aged 18–29 year, and 65 mcg/day for women 30 years and over in Japan 69.
Very little is known about the contribution of dietary vitamin K2 (menaquinones) to overall vitamin K nutrition and although it has been stated that approximately 50% of the daily requirement for vitamin K is supplied by the gut flora through the production of vitamin K2 (menaquinones), there is little evidence to support this estimate 12. In one study among adults with acute bacterial overgrowth as induced by omeprazole, vitamin K2 (menaquinones) produced by these bacteria had some contribution to vitamin K status during dietary vitamin K1 (phylloquinone) restriction, but not enough to restore biochemical measures of vitamin K back to normal range 70.
Furthermore, there are regional differences in the forms and content of vitamin K2 (menaquinones) in the food supply. For example, natto is unique to a traditional Japanese diet whereas the cheeses that contain high concentrations of MK-8 and MK-9 appear to be most prevalent in European dairy producing food supplies 3. Although there are reported vitamin K2 (menaquinones) intakes, these are limited to studies from Japan 69 and the Netherlands 71 and are low compared to vitamin K1 (phylloquinone) intakes. In the United States, vitamin K2 (menaquinones) are limited in the food supply and have not been systematically assessed.
Table 1. Adequate Intakes for Vitamin K
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 2.0 mcg |
| 7–12 months | 2.5 mcg |
| 1–3 years | 30 mcg |
| 4–8 years | 55 mcg |
| 9–13 years | 60 mcg |
| 14–18 years | 75 mcg |
| Adult men 19 years and older | 120 mcg |
| Adult women 19 years and older | 90 mcg |
| Pregnant or breastfeeding teens | 75 mcg |
| Pregnant or breastfeeding women | 90 mcg |
Vitamin K2 food sources
Vitamin K2 (menaquinones) are primarily found in dairy products, meats, and fermented foods (Table 2) 3. In addition, egg yolks and high-fat dairy products, such as hard cheeses, provide appreciated amounts of vitamin K2 (menaquinones) 3. Of note, cheese was found to be the most important source of dietary long-chain menaquinones (MK-8 and MK-9) 73. In particular, propionibacteria-fermented cheese, such as Norwegian Jarlsberg cheese and Swiss Emmental cheese, were shown to have the highest concentration of vitamin K2 in the form of tetrahydromenaquinone-9 [MK-9(4H)] 74. Another important dietary source of vitamin K2 (menaquinones), with interest for the industry, are fermented plant foods, such as natto. Natto is a traditional Japanese soybean food produced by fermenting cooked soybean with Bacillus subtilis natto and considered one of the most relevant dietary sources of MK-7 (around 1000 mcg/100 g natto) 75. However, the vitamin K2 (menaquinones) and vitamin K1 (phylloquinone) contents of many food products are unknown 76.
Current knowledge indicates that dairy products are the primary contributors to dietary vitamin K2 (menaquinones) intake, and industrial application of vitamin K2 (menaquinones)-producing bacteria in fermented dairy products may provide an effective means of increasing vitamin K2 (menaquinones) in the food supply. Several lactic acid bacteria commonly used for making fermented food products, and generally recognized as safe (GRAS), have been used for the biosynthetic production of vitamin K2 (menaquinones) for the last few decades, with significant production amounts of menaquinones (MK-7 to MK-10) 77. Nevertheless, some genera of bacteria widely used in the food industry, including Lactobacillus and Streptococcus, have lost the functional ability to produce vitamin K2. Due to this, the vitamin K2 (menaquinones) content of food products using these bacteria is almost undetectable 78. A study examining the capacity of several bacterial strains to produce vitamin K compounds selected three strains of Lactococcus lactis ssp. cremoris, two strains of Lactococcus lactis ssp. lactis, and Leuconostoc lactis as high producers able to deliver more than 230 nmol/g dried cells of MK-7 to MK-10 77. In fact, several other bacterial species including Brevibacterium linens, Brochontrix thermosphacta, Hafnia alvei, Staphylococcus xylosus, Staphylococcus equorum, and Arthrobacter nicotinae, which are commonly used in industrial food fermentations, are well-known to produce several forms of vitamin K2, from MK-5 to MK-9, in different amounts 76.
The specific bacterial strains used and production conditions during fermentation (i.e., pH, temperature, duration) likely determine the concentrations and forms of vitamin K2 (menaquinones) found in fermented food products. For example, tetrahydromenaquinone [MK-9(4H)], produced by propionibacteria and formed from partial hydrogenation of the isoprenoid chain in MK-9 79, has been measured in different varieties of cheese in which propionibacteria are used in starter cultures 74. High concentrations of MK-8 and MK-9 measured in Edam type cheese 80 is consistent with the use of the MK-8– and MK-9–producing lactic acid bacteria strains Lactococcus lactis ssp. lactis and L. lactis ssp. cremoris as starter cultures for these cheeses. However, few studies have used validated HPLC methods to quantify vitamin K2 (menaquinones) in fermented foods 78. The available literature shows that MK-8 and MK-9 are the most common bacterially synthesized vitamin K2 (menaquinones) found in fermented dairy products, with the presence of other vitamin K2 (menaquinones) being more limited (Table 1). In a recent report, total long-chain menaquinone concentrations ranged from nondetectable to 118 mcg/100 g, with a median concentration of 15 mcg/100 g in 62 European fermented dairy products 78. Long-chain menaquinones have also been measured in fermented plant-based foods such as sauerkraut and natto, a fermented soybean product popular in certain regions of Japan but not widely consumed elsewhere 69. The long-chain menaquinone contents of meat and fish products are generally low 69 and likely have little public health importance. As such, the evidence available to date suggests that dairy products are likely the predominant dietary sources of long-chain menaquinones. In support of this claim, cheese and milk products were estimated to contribute to 54% and 22% of total vitamin K2 (menaquinones) intake, respectively, in a cohort of Dutch women in whom long-chain menaquinones were estimated to account for 9% of total vitamin K intake 81. However, the absence of comprehensive data on food vitamin K2 (menaquinones) contents and regional differences in dairy consumption patterns indicate that much more research is needed to accurately quantify vitamin K2 (menaquinones) intake at the individual and population levels 76.
Table 2. Vitamin K2 (menaquinones) concentration in dairy foods and fermented food products
| Vitamin K2 (menaquinones) 2 | |||||||
|---|---|---|---|---|---|---|---|
| Food | MK-4 | MK-5 | MK-6 | MK-7 | MK-8 | MK-9 | MK-10 |
| Milk (mcg/100 mL) | |||||||
| Whole | 0.8 | 0.1 | ND | ND–2.04 | ND | ND | ND |
| Buttermilk | 0.2 | 0.1 | 0.1 | 0.1 | 0.6 | 1.4 | ND |
| Yogurt (mcg/100 g) | |||||||
| Whole | 0.6–1.0 | 0.1–0.3 | ND–0.2 | ND–0.4 | 0.2–2.0 | ND–4.7 | ND |
| Skimmed | ND | ND | ND | ND | ND–0.1 | ND | ND |
| Cheese (mcg/100 g) | |||||||
| Curd | 0.4 | 0.1 | 0.2 | 0.3 | 5.1 | 18.7 | ND |
| Hard | 4.7–10.2 | 1.5 | ND–3.0 | ND–2.3 | ND–16.9 | ND–51.1 | ND–6.5 |
| Semihard | NR | NR | 1.0–3.5 | ND–2.1 | 2.5–7.3 | 10.0–32.1 | ND–13.8 |
| Soft | 3.7 | 0.3 | 0.4–2.6 | ND–1.7 | 2.1–14.0 | 6.6–94.0 | ND–5.7 |
| Other (mcg/100 g) | |||||||
| Salami | 9 | ND | ND | ND | ND | ND | ND |
| Sauerkraut | 0.4 | 0.8 | 1.5 | 0.2 | 0.8 | 1.1 | ND |
| Natto | ND–2.0 | 7.5 | 13.8 | 939–998 | 84.1 | ND | ND |
Footnotes:
2 Values represent the mean concentration (if 1 study), or lowest and highest mean concentrations (if ≥2 studies or foods within the same category) reported in representative studies from the United States, Europe and Japan. Studies reported values as a range or mean of multiple samples for each food type. MK 11–13 concentrations were not reported in any study.
3 In mcg/100 mL.
4 In mcg/100 g.
Abbreviations: MK = menaquinone; ND = not detectable; NR = not reported.
[Source 76 ]Vitamin K2 health benefits
Main vitamin K actions in humans include 54:
- Regulation of blood coagulation activity
- Bone protection; prevention of osteoporosis and bone fracture
- Prevention of vascular calcifications
- Prevention of cancer
- Prevention of inflammation
The primary function of vitamin K2 is adding carboxylic acid groups to glutamate residues (Glu) to form gamma-carboxyglutamate residues (Gla) during the creation of clotting factors 82. The presence of two carboxylic acid groups on a single carbon that resides in the gamma-carboxyglutamate residue (Gla) allows for the chelation of calcium ions. The binding of calcium ions in this fashion is of crucial importance for vitamin K-dependent clotting factors, permitting the perpetuation of the clotting cascades. Vitamin K is also responsible as a factor in synthesizing prothrombin, factor VII, factor IX, and factor X 83, 84.
Several studies suggest that low vitamin K levels are related to osteoporosis, pathological fractures and vascular calcifications 54. Supplementing menaquinone-7 (MK-7) at the dose of at least 200 mcg per day might help protecting from vascular calcification, osteoporosis and cancer 85. Moreover, supplementation of 5 mg daily phylloquinone (vitamin K1) in 440 postmenopausal women with osteopenia for 2 years in a randomized, placebo-controlled, double-blind trial caused a > 50% reduction in clinical fractures vs. placebo, although no protection against the age-related decline in bone mineral density was observed 86.
A meta-analysis has shown that in seven Japanese trials reporting fractures, vitamin K2 (menaquinones) administration significantly reduced the risk of hip (77% reduction), vertebral (60% reduction) and all non-vertebral fractures (81% reduction) 87. Vitamin K administration also significantly delayed the progression of coronary artery calcifications and the deterioration of arterial elasticity 88. A lower risk of coronary heart disease and severe aortic calcifications was observed with higher menaquinones intake, but not with phylloquinone intake. This finding suggests that the dietary phylloquinone intake, without menaquinones, may not be sufficient to suppress arterial calcifications 89.
Vitamin K2 (menaquinones) have also been shown to play an important role in cancer. In a small (40 patients) randomized study the administration of vitamin K2 (menaquinones) 45 mg/day reduced the development of hepatocellular carcinoma in patients with liver cirrhosis: the risk ratio for the development of hepatocellular carcinoma in patients given menaquinones was 0.13 90.
Vitamin K2 (menaquinones) have additional properties in certain cell and tissue types, particularly in bone tissue and in the immune system. Much of the available evidence relates specifically to menaquinone-4 (MK-4), which was found to have a role in bone health since the 1990s. Low circulating levels of vitamin K2 (menaquinones) are associated with osteoporotic fractures in the elderly 91 and vitamin K2 (menaquinones) improved bone mineral density in Japanese women 92. In an experimental setting, MK-4 reduced bone losses caused by either estrogen withdrawal or corticosteroid treatment in experimental model on rats 93, 94. Moreover, other in vitro studies showed that menaquinone-4 (MK-4) inhibits the synthesis of prostaglandin E2 (PGE2), a bone reabsorption-inducing agent, in cultured osteoblasts 95 and inhibits the formation of osteoclast-like cells in bone marrow-derived cultures 96. Finally, experimental data suggests a possible role of MK-4 on pancreatic exocrine cells metabolism. Stimulation of pancreatic acinar cells with secretagogues cholecystokinin-8 and secretin induces secretion of MK-4, along with phospholipase and the membrane trafficking protein caveolin-1 97, although a well-defined function of MK-4 in this setting remains unclear.
In the Vitamin K Italian (VIKI) study 98, a comprehensive assessment of vitamin K status was carried out in a cohort of hemodialysis patients and in healthy controls, including most vitamin K subtypes (in particular phylloquinone [vitamin K1], MK-4, MK-5, MK-6, and MK-7), adjusted for triglycerides levels. Vitamin K deficiency was found in 35.4% of hemodialysis patients for MK-7, 23.5% for PK and 14.5% for MK-4. With the limitations of its observational nature, this is the first study to relate vitamin K1 (phylloquinone) and vitamin K2 (menaquinones) deficiency directly both to vertebral fractures and vascular calcification in the dialysis population 98. In particular, vitamin K1 (phylloquinone) deficiency was the strongest predictor of vertebral fractures, while lower MK-4 and MK-7 levels were associated with vascular calcification. The results in hemodialysis patients may point out a possible role of vitamin K deficiency as a cause of bone and vascular disease also in the general population. It can be hypothesized that a diet rich in vitamin K and/or vitamin K supplements might be of help in preventing bone disease and avoiding vascular calcifications, opening interesting perspectives for research in human health.
The frequent use of warfarin enhances the problem of vitamin K deficiency and its role on bone and vascular disease 99. Warfarin may predispose to bone fractures and vascular calcification by different mechanisms: directly, by inhibition of gamma-carboxylation of osteocalcin and other bone matrix proteins; indirectly, because patients treated with warfarin may limit their dietary intake of foods rich in vitamin K. New oral anticoagulant seems to have less influence on bone metabolism, but their long-term effects need more studies 100, 101.
Finally, vitamin K status was found to be inversely and significantly related to individual inflammatory markers and to the inflammatory process in a human population study based on the Framingham Offspring Study cohort 102. This finding is supported by studies on rats demonstrating that animals with vitamin K-deficient diets had an enhanced expression of genes involved in acute inflammatory response compared to those with normal or phylloquinone-supplemented diets and that a supplemented diet suppressed the inflammatory response 103.
Bone health
Considerable attention has been given to the role of vitamin K in bone health, with an emphasis on vitamin K2 (menaquinones) 104. The biological basis for this has been the presence of vitamin K in bone and the dependence on vitamin K for carboxylation of multiple Gla-containing proteins synthesized in bone. The most notable of these proteins is osteocalcin, a vitamin K–dependent protein synthesized by osteoblasts during bone formation and the predominant noncollagenous protein in bone 105. Chronically low vitamin K intake results in suboptimal carboxylation of osteocalcin, which is thought to lead to decreased bone mineralization and increased risk of fracture and osteoporosis 106. However, the relationship of osteocalcin carboxylation status to bone health is unclear 104. More recently, some have proposed mechanisms that are independent of vitamin K’s role as an enzyme cofactor and that may be exclusive to MKs. Numerous in vitro and in vivo studies have been conducted, but overall, there is a lack of consensus regarding differential effects of vitamin K1 (phylloquinone) and vitamin K2 (menaquinones) on bone health 104.
In 2009, the European Food Safety Authority issued a scientific opinion concluding that “a cause and effect relationship has been established between the dietary intake of vitamin K and the maintenance of normal bone” 107. However, although both menaquinone-4 (MK-4) and menaquinone-7 (MK-7) supplementation consistently reduce the proportion of osteocalcin that is undercarboxylated 108, whether this effect favorably influences bone health is less clear 104. The first meta-analysis of MK-4 supplementation and bone health outcomes reported an overall benefit of MK-4 supplementation on reducing fracture risk 109. However, the authors articulated many caveats regarding their analysis, including geographic homogeneity, varied supplementation with other nutrients and medications, and heterogeneous study designs. In addition, the majority of these studies focused on MK-4 given therapeutically in daily doses of 45 mg, an amount unattainable through diet alone. A more recent meta-analysis combined findings of studies that used either vitamin K1 (phylloquinone) or vitamin K2 (menaquinones) supplementation and examined bone mineral density 110. The authors concluded that there were modest improvements in bone mineral density with vitamin K treatment, but that the results should be interpreted with caution, especially as vitamin K1 (phylloquinone), MK-4, and MK-7 were combined in the analysis, which assumes equivalent bioavailability. Findings from the 2 included studies in which MK-7 supplementation alone was examined were inconsistent with bone health benefits documented at MK-7 doses of 180 mcg/day over 1 year in 1 study 111, but not at 360 mcg/day over 1 year in another 112.
Several relevant studies have been published since these meta-analyses. In a large clinical trial of >4000 postmenopausal women, supplementation with MK-4 and calcium compared with calcium supplementation alone provided no additional protection against bone fracture over 3 years 113. Kanellakis et al. 114: the Postmenopausal Health Study II. Calcif Tissue Int. 2012 Apr;90(4):251-62. doi: 10.1007/s00223-012-9571-z)) examined bone markers during a 1-year regimen in which postmenopausal women were randomized to daily consumption of dairy products fortified with calcium, vitamin D3, and either no vitamin K, 100 mcg vitamin K1 (phylloquinone), or 100 μg MK-7. After 1 year, all 3 interventions improved total bone mineral density relative to a group receiving no intervention. Although no additional benefit attributable to vitamin K1 (phylloquinone) or MK-7 consumption was observed on total bone mineral density, both vitamin K1 (phylloquinone) and MK-7 were shown to have favorable effects on lumbar spine bone mineral density relative to the control and calcium + vitamin D groups. In the longest clinical trial of MK-7 supplementation conducted to date, Knapen et al. 115 assessed bone health and strength in >200 postpmenopausal women randomized to receive a supplement containing 180 mcg/day MK-7 or a placebo over 3 years. Site-specific effects of MK-7 were reported, with marginal attenuation of reductions in bone mineral content and bone mineral density observed at the lumbar spine and femoral neck. No effects of MK-7 supplementation were documented for the total hip, and the effects of MK-7 on bone strength indices were inconsistent. Limited evidence of favorable bone health effects when lower amounts of MK-7 are consumed from dietary sources also containing calcium and/or vitamin D suggests that the combination of nutrients rather than MK-7 alone may have greater efficacy in improving bone health 116 or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int. 2012 Apr;90(4):251-62. doi: 10.1007/s00223-012-9571-z)). In this regard, it is also important to note that, to date, there is no indication of greater efficacy of dietary vitamin K2 (menaquinones) relative to vitamin K1 (phylloquinone) 117. Furthermore, supplemental MK-7 has adverse effects on coagulation parameters in individuals receiving oral anticoagulant therapy 118. Therefore, any presumed benefit of MK-7 supplementation on bone health will need to be balanced with potential safety concerns regarding stability of oral anticoagulant therapy among patients on vitamin K–antagonists.
Anticancer activity
Although the mechanisms are still being sort out, in vitro (test tube) studies suggest that vitamin K2 (menaquinones) may arrest cell growth and induce apoptosis 119, 120, 121, 122, 123. A small number of in vivo (human) studies have investigated the efficacy of MK-4 supplementation in doses far in excess of what could be achieved in the diet for preventing hepatocellular carcinoma in high-risk patients. In a secondary analysis of a trial investigating MK-4 supplementation and bone loss, 45 mg/day of MK-4 was shown to reduce hepatocellular carcinoma risk in women with viral cirrhosis 124. However, results from subsequent studies investigating the efficacy of MK-4 supplementation for preventing hepatocellular carcinoma recurrence have been inconclusive 125. Vitamin K2 (menaquinones) have not been tested as an add-on cancer treatment. The most likely explanation for this is that vitamin K supplementation is contraindicated in cancer patients who are at increased risk of venous thromboembolism and are often prescribed vitamin K antagonists 126. Limited evidence is available to suggest that vitamin K intake reduces cancer risk in healthy adults. In 1 large prospective cohort, inverse relationships between dietary vitamin K2 (menaquinones) intake with incidence of prostate cancer, overall cancer incidence, and cancer mortality were reported 127. However, replication of these findings is needed.
Cardiovascular health
Calcification of vessel walls reduces their elasticity, increasing cardiovascular disease risk 128. There is growing recognition that oral anticoagulants such as warfarin may be associated with increased arterial calcification 129. Anticoagulants inhibit the vitamin K–dependent carboxylation of matrix-Gla protein (MGP) in vascular smooth-muscle cells 130. In its carboxylated form, MGP functions as a calcification inhibitor. As such, anticoagulants are thought to impair the calcium-regulating activity of MGP, thereby leading to increased calcium deposition in the vessel walls. The functional dependence of MGP on vitamin K and associations between anticoagulant use and calcification underpin current speculation that vitamin K may be involved in the progression of cardiovascular disease.
Only 1 randomized clinical trial examining vitamin K supplementation and coronary artery calcification has been published to date. In this trial 131, vitamin K1 (phylloquinone) supplementation reduced progression of existing coronary artery calcification, although there was no effect on incidence of coronary artery calcification. These findings are consistent with the proposed biological mechanism of matrix-Gla protein (MGP), which inhibits further calcification 132. In another study 133, postmenopausal women receiving a supplement containing vitamin K1 (phylloquinone) demonstrated better carotid artery compliance and elasticity after 3 years than those receiving a similar supplement with no vitamin K1 (phylloquinone). However, although suggestive, no direct measures of calcification were reported.
One would predict that the impact of vitamin K1 (phylloquinone) and vitamin K2 (menaquinones) supplementation on reducing progression of vascular calcification should be similar given that both forms support the gamma-carboxylation of matrix-Gla protein (MGP). However, a series of observational studies suggest possible differential effects of vitamin K2 (menaquinones) and vitamin K1 (phylloquinone) on arterial calcification and coronary heart disease risk. For example, inverse associations between vitamin K2 (menaquinones) intake and severe aortic calcification 134, 81, the risk of coronary heart disease 135 and the risk of coronary heart disease mortality and all-cause mortality 134 have been reported. In these studies, estimated vitamin K2 (menaquinones) intakes were up to 10 times lower than vitamin K1 (phylloquinone) intakes, and vitamin K1 (phylloquinone) intakes were not associated with disease risk. In the Dutch PROSPECT study cohort 76, the reduction in coronary heart disease risk was mainly driven by MK-7, MK-8, and MK-9 consumption.
Although these findings are intriguing and have stimulated considerable interest in a potential therapeutic role for vitamin K2 (menaquinones) in the progression of coronary artery calcification and coronary artery disease, these observational data should be interpreted with caution. Unlike vitamin K1 (phylloquinone), long-chain menaquinones are not detectable in circulation unless provided in high doses, either in the form of a supplement 136 or a concentrated food source, such as natto 137. Interestingly, MK-4 is often not detectable in the circulation, even after administration with doses as high as 420 mcg 136. This is problematic for validation of self-reported intakes of vitamin K2 (menaquinones) as there is no comparable biomarker. Use of vitamin K biomarkers that measure the proportion of carboxylation of vitamin K–dependent proteins reflect the dietary contribution of all forms of vitamin K and cannot be used to validate intakes of a single vitamin K2 (menaquinones). Because food composition databases for long-chain menaquinones are not available, it is unlikely that the epidemiological data can be replicated in other countries in the near future. Alternatively, the findings may reflect the use of vitamin K2 (menaquinones) as a marker of another nutrient or food constituent that has heart-healthy properties 138. For example, dairy products, which were the primary dietary source of vitamin K2 (menaquinones) in the Dutch PROSPECT study cohort, contain specific fatty acids that may confer protective effects on coronary artery disease risk 139. Notably an inverse association between coronary artery disease risk and the consumption of meat, milk, and cheese, among the richest sources for vitamin K2 (menaquinones) in the Dutch food supply, was also observed 135, 137. Given the challenges in interpreting the results of these studies, there is clearly a need for randomized clinical trials that isolate any putative effect of individual vitamin K2 (menaquinones) on coronary artery calcification. There have been recent media reports that nutritional doses of MK-7 (180 mcg/day) taken over a 3-years period prevented age-related arterial stiffening in postmenopausal women 140. However, the results of the clinical trial were not available in the peer-reviewed literature and the unique protective role of vitamin K2 (menaquinones) in heart health remains speculative 76.
Vitamin K2 side effects
Currently, there is no known toxicity is associated with high doses of vitamin K1 (phylloquinone) or vitamin K2 (menaquinones) 19. Therefore, there is no designated Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) 141. Despite this, an allergic reaction is possible with either version of vitamin K. Vitamin K1 has had documented associations with bronchospasm and cardiac arrest with IV administration. The oral form of vitamin K does not seem to cause severe reactions 142.
Vitamin K2 also does not display any adverse effects when ingested orally. Studies have shown that coagulation studies in humans did not show an increased risk of blood clots when ingesting 45 mg per day of vitamin K2 (as MK-4). Researchers observed this in a patient who took upwards of 135 mg per day (45 mg three times per day) 143, 144.
References- Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson-Hughes B, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000 May;71(5):1201-8. doi: 10.1093/ajcn/71.5.1201
- Thane CW, Paul AA, Bates CJ, Bolton-Smith C, Prentice A, Shearer MJ. Intake and sources of phylloquinone (vitamin K1): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr. 2002 Jun;87(6):605-13. doi: 10.1079/BJNBJN2002583
- Booth S. L. (2012). Vitamin K: food composition and dietary intakes. Food & nutrition research, 56, 10.3402/fnr.v56i0.5505. https://doi.org/10.3402/fnr.v56i0.5505
- Ferland G. Vitamin K. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:230-47.
- National Institute of Health, Office of Dietary Supplements. Vitamin K. https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/
- Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S. Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos. 2012;7(1):3-20. doi: 10.1007/s11657-012-0109-9
- Rønn SH, Harsløf T, Oei L, Pedersen SB, Langdahl BL. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int. 2021 Jan;32(1):185-191. doi: 10.1007/s00198-020-05638-z
- Suttie JW. Vitamin K. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:851-60.
- Conly JM, Stein K, Worobetz L, Rutledge-Harding S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994 Jun;89(6):915-23.
- Conly JM, Stein K. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am J Gastroenterol. 1992 Mar;87(3):311-6.
- Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci. 1992 Oct-Dec;16(4):307-43.
- Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399-417. doi: 10.1146/annurev.nu.15.070195.002151
- Suttie JW. Vitamin K. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:305-16.
- Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. Davidson RT, Foley AL, Engelke JA, Suttie JW. J Nutr. 1998 Feb; 128(2):220-3. https://www.ncbi.nlm.nih.gov/pubmed/9446847/
- Menadione is a metabolite of oral vitamin K. Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Br J Nutr. 2006 Feb; 95(2):260-6. https://www.ncbi.nlm.nih.gov/pubmed/16469140/
- Vitamin K contents of meat, dairy, and fast food in the U.S. Diet. Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. J Agric Food Chem. 2006 Jan 25; 54(2):463-7. https://www.ncbi.nlm.nih.gov/pubmed/16417305/
- Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012;56. doi: 10.3402/fnr.v56i0.5505
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307. https://www.ncbi.nlm.nih.gov/pubmed/11356998
- Imbrescia K, Moszczynski Z. Vitamin K. [Updated 2021 Jul 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551578
- Tie, J. K., & Stafford, D. W. (2016). Structural and functional insights into enzymes of the vitamin K cycle. Journal of thrombosis and haemostasis : JTH, 14(2), 236–247. https://doi.org/10.1111/jth.13217
- Oldenburg J, Marinova M, Müller-Reible C, Watzka M. The vitamin K cycle. Vitam Horm 2008;78:35–62.
- Fusaro M, Crepaldi G, Maggi S, Galli F, D’Angelo A, Calò L, et al. Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: an important but poorly studied relationship. J Endocrinol Invest 2011;34:317–23.
- Nelsestuen GL, Zytkovicz TH, Howard JB. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J Biol Chem. 1974 Oct 10;249(19):6347-50.
- Vermeer C. (1990). Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. The Biochemical journal, 266(3), 625–636. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1131186/pdf/biochemj00187-0011.pdf
- Nelsestuen GL. Enhancement of vitamin-K-dependent protein function by modification of the gamma-carboxyglutamic acid domain: studies of protein C and factor VII. Trends Cardiovasc Med. 1999 Aug;9(6):162-7. doi: 10.1016/s1050-1738(99)00024-9
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996 Aug 1;382(6590):448-52. doi: 10.1038/382448a0
- Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014 Nov 1;561:137-46. doi: 10.1016/j.abb.2014.05.022
- Booth, S. L., Centi, A., Smith, S. R., & Gundberg, C. (2013). The role of osteocalcin in human glucose metabolism: marker or mediator?. Nature reviews. Endocrinology, 9(1), 43–55. https://doi.org/10.1038/nrendo.2012.201
- Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008 Oct;100(4):593-603.
- Willems BA, Vermeer C, Reutelingsperger CP, Schurgers LJ. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. 2014 Aug;58(8):1620-35. doi: 10.1002/mnfr.201300743
- Sharma, B., & Albig, A. R. (2013). Matrix Gla protein reinforces angiogenic resolution. Microvascular research, 85, 24–33. https://doi.org/10.1016/j.mvr.2012.10.005
- Sundaram K.S., Lev M. Warfarin administration reduces synthesis of sulfatides and other sphingolipids in mouse brain. J. Lipid Res. 1988;29:1475–1479. doi: 10.1016/S0022-2275(20)38426-1.
- Shafit-Zagardo, B., Gruber, R. C., & DuBois, J. C. (2018). The role of TAM family receptors and ligands in the nervous system: From development to pathobiology. Pharmacology & therapeutics, 188, 97–117. https://doi.org/10.1016/j.pharmthera.2018.03.002
- Simes, D. C., Viegas, C., Araújo, N., & Marreiros, C. (2020). Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients, 12(1), 138. https://doi.org/10.3390/nu12010138
- Ferland G. (2012). Vitamin K and the nervous system: an overview of its actions. Advances in nutrition (Bethesda, Md.), 3(2), 204–212. https://doi.org/10.3945/an.111.001784
- Weber P. Vitamin K and bone health. Nutrition. 2001 Oct;17(10):880-7. doi: 10.1016/s0899-9007(01)00709-2. Erratum in: Nutrition 2001 Nov-Dec;17(11-12):1024
- Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin K contents of meat, dairy, and fast food in the U.S. Diet. J Agric Food Chem 2006;54:463-7. https://www.ncbi.nlm.nih.gov/pubmed/16417305?dopt=Abstract
- Mott A, Bradley T, Wright K, Cockayne ES, Shearer MJ, Adamson J, Lanham-New SA, Torgerson DJ. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019 Aug;30(8):1543-1559. doi: 10.1007/s00198-019-04949-0. Epub 2019 May 10. Erratum in: Osteoporos Int. 2020 Nov;31(11):2269-2270.
- Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med. 2015 Feb 18;7(275):275ra23. doi: 10.1126/scitranslmed.3010329
- Tie JK, Stafford DW. Functional Study of the Vitamin K Cycle Enzymes in Live Cells. Methods Enzymol. 2017;584:349-394. doi: 10.1016/bs.mie.2016.10.015
- Rishavy MA, Hallgren KW, Wilson LA, Usubalieva A, Runge KW, Berkner KL. The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation. J Biol Chem. 2013 Nov 1;288(44):31556-66. doi: 10.1074/jbc.M113.497297
- Tie JK, Jin DY, Straight DL, Stafford DW. Functional study of the vitamin K cycle in mammalian cells. Blood. 2011 Mar 10;117(10):2967-74. doi: 10.1182/blood-2010-08-304303
- Lacombe, J., Rishavy, M. A., Berkner, K. L., & Ferron, M. (2018). VKOR paralog VKORC1L1 supports vitamin K-dependent protein carboxylation in vivo. JCI insight, 3(1), e96501. https://doi.org/10.1172/jci.insight.96501
- Wajih, N.; Owen, J.; Wallin, R. Enhanced functional recombinant factor VII production by HEK 293 cells stably transfected with VKORC1 where the gamma-carboxylase inhibitor calumenin is stably suppressed by shRNA transfection. Thromb. Res. 2008, 122, 405–410.
- Vitamin K. https://lpi.oregonstate.edu/mic/vitamins/vitamin-K
- Holmes MV, Hunt BJ, Shearer MJ. The role of dietary vitamin K in the management of oral vitamin K antagonists. Blood Rev. 2012 Jan;26(1):1-14. doi: 10.1016/j.blre.2011.07.002
- Violi F, Lip GY, Pignatelli P, Pastori D. Interaction Between Dietary Vitamin K Intake and Anticoagulation by Vitamin K Antagonists: Is It Really True?: A Systematic Review. Medicine (Baltimore). 2016 Mar;95(10):e2895. doi: 10.1097/MD.0000000000002895
- Thijssen, H.H.; Vervoort, L.M.; Schurgers, L.J.; Shearer, M.J. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 2006, 95, 260–266.
- Alisi, L., Cao, R., De Angelis, C., Cafolla, A., Caramia, F., Cartocci, G., Librando, A., & Fiorelli, M. (2019). The Relationships Between Vitamin K and Cognition: A Review of Current Evidence. Frontiers in neurology, 10, 239. https://doi.org/10.3389/fneur.2019.00239
- Vermeer, C.; van’t Hoofd, C.; Knapen, M.H.J.; Xanthoulea, S. Synthesis of 2-methyl-1,4-naphthoquinones with higher gamma-glutamyl carboxylase activity than MK-4 both in vitro and in vivo. Bioorg. Med. Chem. Lett. 2017, 27, 208–211.
- Fujii S, Shimizu A, Takeda N, Oguchi K, Katsurai T, Shirakawa H, Komai M, Kagechika H. Systematic synthesis and anti-inflammatory activity of ω-carboxylated menaquinone derivatives–Investigations on identified and putative vitamin K₂ metabolites. Bioorg Med Chem. 2015 May 15;23(10):2344-52. doi: 10.1016/j.bmc.2015.03.070
- Hirota, Yoshihisa & Suhara, Yoshitomo. (2019). New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. International Journal of Molecular Sciences. 2019, 20(12), 3006; https://doi.org/10.3390/ijms20123006
- Fusaro M, Gallieni M, Rizzo MA, Stucchi A, Delanaye P, Cavalier E, Moysés RMA, Jorgetti V, Iervasi G, Giannini S, Fabris F, Aghi A, Sella S, Galli F, Viola V, Plebani M. Vitamin K plasma levels determination in human health. Clin Chem Lab Med. 2017 May 1;55(6):789-799. doi: 10.1515/cclm-2016-0783
- Traber MG. Vitamin E and K interactions: a 50-year-old problem. Nutr Rev 2008;66:624–9.
- Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003 Nov 7;278(45):43919-27. doi: 10.1074/jbc.M303136200
- Xie, W.; Barwick, J.L.; Downes, M.; Blumberg, B.; Simon, C.M.; Nelson, M.C.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Guzelian, P.S.; Evans, R.M. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 2000, 406, 435–439.
- Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006 Jun 23;281(25):16927-16934. doi: 10.1074/jbc.M600896200
- Azuma K, Urano T, Ouchi Y, Inoue S. Vitamin K2 suppresses proliferation and motility of hepatocellular carcinoma cells by activating steroid and xenobiotic receptor. Endocr J. 2009;56(7):843-9. doi: 10.1507/endocrj.k09e-108
- Sada E, Abe Y, Ohba R, Tachikawa Y, Nagasawa E, Shiratsuchi M, Takayanagi R. Vitamin K2 modulates differentiation and apoptosis of both myeloid and erythroid lineages. Eur J Haematol. 2010 Dec;85(6):538-48. doi: 10.1111/j.1600-0609.2010.01530.x
- Ekins, S., Kortagere, S., Iyer, M., Reschly, E. J., Lill, M. A., Redinbo, M. R., & Krasowski, M. D. (2009). Challenges predicting ligand-receptor interactions of promiscuous proteins: the nuclear receptor PXR. PLoS computational biology, 5(12), e1000594. https://doi.org/10.1371/journal.pcbi.1000594
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008, 283, 11270–11279.
- Hirabayashi Y, Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005 Apr;51(4):331-6. doi: 10.1016/j.neures.2005.01.004
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998 Nov;4(11):1313-7. doi: 10.1038/3305
- Sakane, R.; Kimura, K.; Hirota, Y.; Ishizawa, M.; Takagi, Y.; Wada, A.; Kuwahara, S.; Makishima, M.; Suhara, Y. Synthesis of novel vitamin K derivatives with alkylated phenyl groups introduced at the ù-terminal side chain and evaluation of their neural differentiation activities. Bioorg. Med. Chem. Lett. 2017, 27, 4881–4884.
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001 Mar;101(3):294-301. doi: 10.1016/S0002-8223(01)00078-5
- Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89-110. doi: 10.1146/annurev-nutr-080508-141217
- Dietary reference values for food energy and nutrients for the United Kingdom. Report on health and social subjects no. 41. London: HMSO; 1991.
- Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol (Tokyo). 2007 Dec;53(6):464-70. doi: 10.3177/jnsv.53.464
- Paiva SA, Sepe TE, Booth SL, Camilo ME, O’Brien ME, Davidson KW, Sadowski JA, Russell RM. Interaction between vitamin K nutriture and bacterial overgrowth in hypochlorhydria induced by omeprazole. Am J Clin Nutr. 1998 Sep;68(3):699-704. doi: 10.1093/ajcn/68.3.699
- Schurgers L, Geleijnse J, Grobbee D, Pols H, Hofman A, Witteman J, et al. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands. J Nutr Environ Med. 1999;9:115–22.
- Vitamin K. https://ods.od.nih.gov/factsheets/VitaminK-Consumer
- Vermeer, C., Raes, J., van ‘t Hoofd, C., Knapen, M., & Xanthoulea, S. (2018). Menaquinone Content of Cheese. Nutrients, 10(4), 446. https://doi.org/10.3390/nu10040446
- Hojo K, Watanabe R, Mori T, Taketomo N. Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria. J Dairy Sci. 2007 Sep;90(9):4078-83. doi: 10.3168/jds.2006-892
- Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition. 2001 Apr;17(4):315-21. doi: 10.1016/s0899-9007(00)00554-2. Erratum in: Nutrition. 2006 Oct;22(10):1075.
- Walther, B., Karl, J. P., Booth, S. L., & Boyaval, P. (2013). Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Advances in nutrition (Bethesda, Md.), 4(4), 463–473. https://doi.org/10.3945/an.113.003855
- Morishita T, Tamura N, Makino T, Kudo S. Production of menaquinones by lactic acid bacteria. J Dairy Sci. 1999 Sep;82(9):1897-903. doi: 10.3168/jds.S0022-0302(99)75424-X
- Manoury E, Jourdon K, Boyaval P, Fourcassié P. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J Dairy Sci. 2013 Mar;96(3):1335-46. doi: 10.3168/jds.2012-5494
- Furuichi K, Hojo K, Katakura Y, Ninomiya K, Shioya S. Aerobic culture of Propionibacterium freudenreichii ET-3 can increase production ratio of 1,4-dihydroxy-2-naphthoic acid to menaquinone. J Biosci Bioeng. 2006 Jun;101(6):464-70. doi: 10.1263/jbb.101.464
- Koivu-Tikkanen TJ, Ollilainen V, Piironen VI. Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. J Agric Food Chem. 2000 Dec;48(12):6325-31. doi: 10.1021/jf000638u
- Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009 Apr;203(2):489-93. doi: 10.1016/j.atherosclerosis.2008.07.010
- Imbrescia K, Moszczynski Z. Vitamin K. [Updated 2023 Feb 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551578
- Tie JK, Stafford DW. Structural and functional insights into enzymes of the vitamin K cycle. J Thromb Haemost. 2016 Feb;14(2):236-47. doi: 10.1111/jth.13217
- Oldenburg J, Bevans CG, Müller CR, Watzka M. Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle. Antioxid Redox Signal. 2006 Mar-Apr;8(3-4):347-53. doi: 10.1089/ars.2006.8.347
- Vermeer C. Vitamin K: the effect on health beyond coagulation – an overview. Food Nutr Res 2012;56. doi: 10.3402/fnr.v56i0.5329
- Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 2008;5:e196.
- Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256–61.
- Braam LA, Hoeks AP, Brouns F, Hamulyák K, Gerichhausen MJ, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost 2004;91:373–80.
- Villines TC, Hatzigeorgiou C, Feuerstein IM, O’Malley PG, Taylor AJ. Vitamin K1 intake and coronary calcification. Coron Artery Dis 2005;16:199–203.
- Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, et al. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. J Am Med Assoc 2004;292:358–61.
- Hodges SJ, Pilkington MJ, Stamp TC. Depressed levels of circulating menaquinones in patients with osteoporotic fractures of the spine and femoral neck. Bone 1991;12:387–9.
- Orimo H, Shiraki M, Fujita T, Onomura T, Inoue T, Kushida K. Clinical evaluation of menatetrenone in the treatment of involutional osteoporosis-a double blind multicenter comparative study with 1α hydroxyvitamin D3. J Bone Miner Res 1992 (Suppl1);7:S122 (Abstract).
- Hara K, Akiyama Y, Ohkawa I, Tajima T. Effects of menatetrenone on prednisolone- induced bone loss in rats. Bone 1993;14:813–8.
- Akiyama Y, Hara K, Ohkawa I, Tajima T. Effects of menatetrenone on bone loss induced by ovariectomy in rats. Jpn J Pharmacol 1993;62:145–53.
- Koshihara Y, Hoshi K, Shiraki M. Vitamin K2 (menatetrenone) inhibits prostaglandin synthesis in cultured human osteoblast-like periosteal cells by inhibiting prostaglandin H synthase activity. Biochem Pharmacol 1993;46:1355–62.
- Akiyama Y, Hara K, Tajima T, Murota S, Morita I. Effect of vitamin K2 (menatetrenone) on osteoclast-like cell formation in mouse bone marrow cultures. Eur J Pharmacol 1994;263:181–5.
- Thomas DD, Krzuykowski KJ, Engelke JA, Groblewski GE. Exocrine pancreatic secretion of phospholipid, menaquinone-4, and caveolin-1 in vivo. Biochem Biophys Res Commun 2004;319:974–9.
- Fusaro M, Noale M, Viola V, Galli F, Tripepi G, Vajente N, et al. Vitamin K, vertebral fractures, vascular calcifications and mortality: VItamin K Italian (VIKI) dialysis study. J Bone Mineral Res 2012;27:2271–8.
- Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol 2008;3:1504–10.
- Tufano A, Coppola A, Contaldi P, Franchini M, Minno GD. Oral anticoagulant drugs and the risk of osteoporosis: new anticoagulants better than old? Semin Thromb Hemost 2015;41:382–8.
- Fusaro M, Dalle Carbonare L, Dusso A, Arcidiacono MV, Valenti MT, Aghi A, et al. Differential effects of dabigatran and warfarin on bone volume and structure in rats with normal renal function. PLoS One 2015;10:e0133847.
- Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB Sr, Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 2008;167:313–20.
- Ohsaki Y, Shirakawa H, Hiwatashi K, Furokawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem 2006;70:926–32.
- Gundberg, C. M., Lian, J. B., & Booth, S. L. (2012). Vitamin K-dependent carboxylation of osteocalcin: friend or foe?. Advances in nutrition (Bethesda, Md.), 3(2), 149–157. https://doi.org/10.3945/an.112.001834
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989 Jul;69(3):990-1047. doi: 10.1152/physrev.1989.69.3.990
- Vermeer C, Theuwissen E. Vitamin K, osteoporosis and degenerative diseases of ageing. Menopause Int. 2011 Mar;17(1):19-23. doi: 10.1258/mi.2011.011006
- EFSA Panel on Dietetic Products Nutrition, and Allergies (NDA). Scientific opinion on the substantiation of health claims related to vitamin K and maintenance of bones (ID 123, 127, 128 and 2879), blood coagulation (ID 124 and 126), and function of the heart and blood vessels (ID 124, 125 and 2880) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA J. 2009;7:1228.
- Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007 Apr 15;109(8):3279-83. doi: 10.1182/blood-2006-08-040709
- Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006 Jun 26;166(12):1256-61. doi: 10.1001/archinte.166.12.1256. Erratum in: JAMA Intern Med. 2018 Jun 1;178(6):875-876.
- Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2012 Jan;30(1):60-8. doi: 10.1007/s00774-011-0287-3
- Forli L, Bollerslev J, Simonsen S, Isaksen GA, Kvamsdal KE, Godang K, Gadeholt G, Pripp AH, Bjortuft O. Dietary vitamin K2 supplement improves bone status after lung and heart transplantation. Transplantation. 2010 Feb 27;89(4):458-64. doi: 10.1097/TP.0b013e3181c46b69
- Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fønnebø V. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. 2010 Oct;21(10):1731-40. doi: 10.1007/s00198-009-1126-4
- Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Nakamura T, Sato T, Yamazaki K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab. 2009;27(1):66-75. doi: 10.1007/s00774-008-0008-8
- Kanellakis S, Moschonis G, Tenta R, Schaafsma A, van den Heuvel EG, Papaioannou N, Lyritis G, Manios Y. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K1) or menaquinone-7 (vitamin K (2
- Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013 Sep;24(9):2499-507. doi: 10.1007/s00198-013-2325-6
- Kanellakis S, Moschonis G, Tenta R, Schaafsma A, van den Heuvel EG, Papaioannou N, Lyritis G, Manios Y. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1
- Apalset EM, Gjesdal CG, Eide GE, Tell GS. Intake of vitamin K1 and K2 and risk of hip fractures: The Hordaland Health Study. Bone. 2011 Nov;49(5):990-5. doi: 10.1016/j.bone.2011.07.035
- Theuwissen E, Teunissen KJ, Spronk HM, Hamulyák K, Ten Cate H, Shearer MJ, Vermeer C, Schurgers LJ. Effect of low-dose supplements of menaquinone-7 (vitamin K2 ) on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteers. J Thromb Haemost. 2013 Jun;11(6):1085-92. doi: 10.1111/jth.12203
- Wei, G., Wang, M., Hyslop, T., Wang, Z., & Carr, B. I. (2010). Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. International journal of cancer, 127(12), 2949–2958. https://doi.org/10.1002/ijc.25498
- Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008 Oct;100(4):530-47.
- Tokita H, Tsuchida A, Miyazawa K, Ohyashiki K, Katayanagi S, Sudo H, Enomoto M, Takagi Y, Aoki T. Vitamin K2-induced antitumor effects via cell-cycle arrest and apoptosis in gastric cancer cell lines. Int J Mol Med. 2006 Feb;17(2):235-43.
- Yoshida T, Miyazawa K, Kasuga I, Yokoyama T, Minemura K, Ustumi K, Aoshima M, Ohyashiki K. Apoptosis induction of vitamin K2 in lung carcinoma cell lines: the possibility of vitamin K2 therapy for lung cancer. Int J Oncol. 2003 Sep;23(3):627-32.
- Karasawa S, Azuma M, Kasama T, Sakamoto S, Kabe Y, Imai T, Yamaguchi Y, Miyazawa K, Handa H. Vitamin K2 covalently binds to Bak and induces Bak-mediated apoptosis. Mol Pharmacol. 2013 Mar;83(3):613-20. doi: 10.1124/mol.112.082602
- Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004 Jul 21;292(3):358-61. doi: 10.1001/jama.292.3.358
- Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, Kawabe T, Kawazoe S, Kobashi H, Kasugai H, Osaki Y, Araki Y, Izumi N, Oka H, Tsuji K, Toyota J, Seki T, Osawa T, Masaki N, Ichinose M, Seike M, Ishikawa A, Ueno Y, Tagawa K, Kuromatsu R, Sakisaka S, Ikeda H, Kuroda H, Kokuryu H, Yamashita T, Sakaida I, Katamoto T, Kikuchi K, Nomoto M, Omata M. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011 Aug;54(2):532-40. doi: 10.1002/hep.24430
- Verso M, Agnelli G. New and old anticoagulants in cancer. Thromb Res. 2012 Apr;129 Suppl 1:S101-5. doi: 10.1016/S0049-3848(12)70027-0
- Nimptsch K, Rohrmann S, Kaaks R, Linseisen J. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr. 2010 May;91(5):1348-58. doi: 10.3945/ajcn.2009.28691
- Karwowski, W., Naumnik, B., Szczepański, M., & Myśliwiec, M. (2012). The mechanism of vascular calcification – a systematic review. Medical science monitor : international medical journal of experimental and clinical research, 18(1), RA1–RA11. https://doi.org/10.12659/msm.882181
- Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012 Jul;26(4):155-66. doi: 10.1016/j.blre.2012.03.002
- Theuwissen, E., Smit, E., & Vermeer, C. (2012). The role of vitamin K in soft-tissue calcification. Advances in nutrition (Bethesda, Md.), 3(2), 166–173. https://doi.org/10.3945/an.111.001628
- Shea, M. K., O’Donnell, C. J., Hoffmann, U., Dallal, G. E., Dawson-Hughes, B., Ordovas, J. M., Price, P. A., Williamson, M. K., & Booth, S. L. (2009). Vitamin K supplementation and progression of coronary artery calcium in older men and women. The American journal of clinical nutrition, 89(6), 1799–1807. https://doi.org/10.3945/ajcn.2008.27338
- Shanahan CM, Proudfoot D, Farzaneh-Far A, Weissberg PL. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8(3-4):357-75. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60
- Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, Booth S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc. 2004 Sep;104(9):1410-4. doi: 10.1016/j.jada.2004.06.021
- Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004 Nov;134(11):3100-5. doi: 10.1093/jn/134.11.3100
- Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009 Sep;19(7):504-10. doi: 10.1016/j.numecd.2008.10.004
- Sato, T., Schurgers, L. J., & Uenishi, K. (2012). Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutrition journal, 11, 93. https://doi.org/10.1186/1475-2891-11-93
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000 Nov-Dec;30(6):298-307. doi: 10.1159/000054147
- Erkkilä AT, Booth SL. Vitamin K intake and atherosclerosis. Curr Opin Lipidol. 2008 Feb;19(1):39-42. doi: 10.1097/MOL.0b013e3282f1c57f
- German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46(1):57-92. doi: 10.1080/10408690590957098
- NattoPharma.com[Internet] MENAQ7®: 3 year human study with vitamin K2 demonstrates that MenaQ7® significantly improves bone strength, and prevents cardiovascular aging.
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222310
- Britt, R. B., & Brown, J. N. (2018). Characterizing the Severe Reactions of Parenteral Vitamin K1. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 24(1), 5–12. https://doi.org/10.1177/1076029616674825
- Asakura H, Myou S, Ontachi Y, Mizutani T, Kato M, Saito M, Morishita E, Yamazaki M, Nakao S. Vitamin K administration to elderly patients with osteoporosis induces no hemostatic activation, even in those with suspected vitamin K deficiency. Osteoporos Int. 2001 Dec;12(12):996-1000. doi: 10.1007/s001980170007
- Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas. 2002 Mar 25;41(3):211-21. doi: 10.1016/s0378-5122(01)00275-4