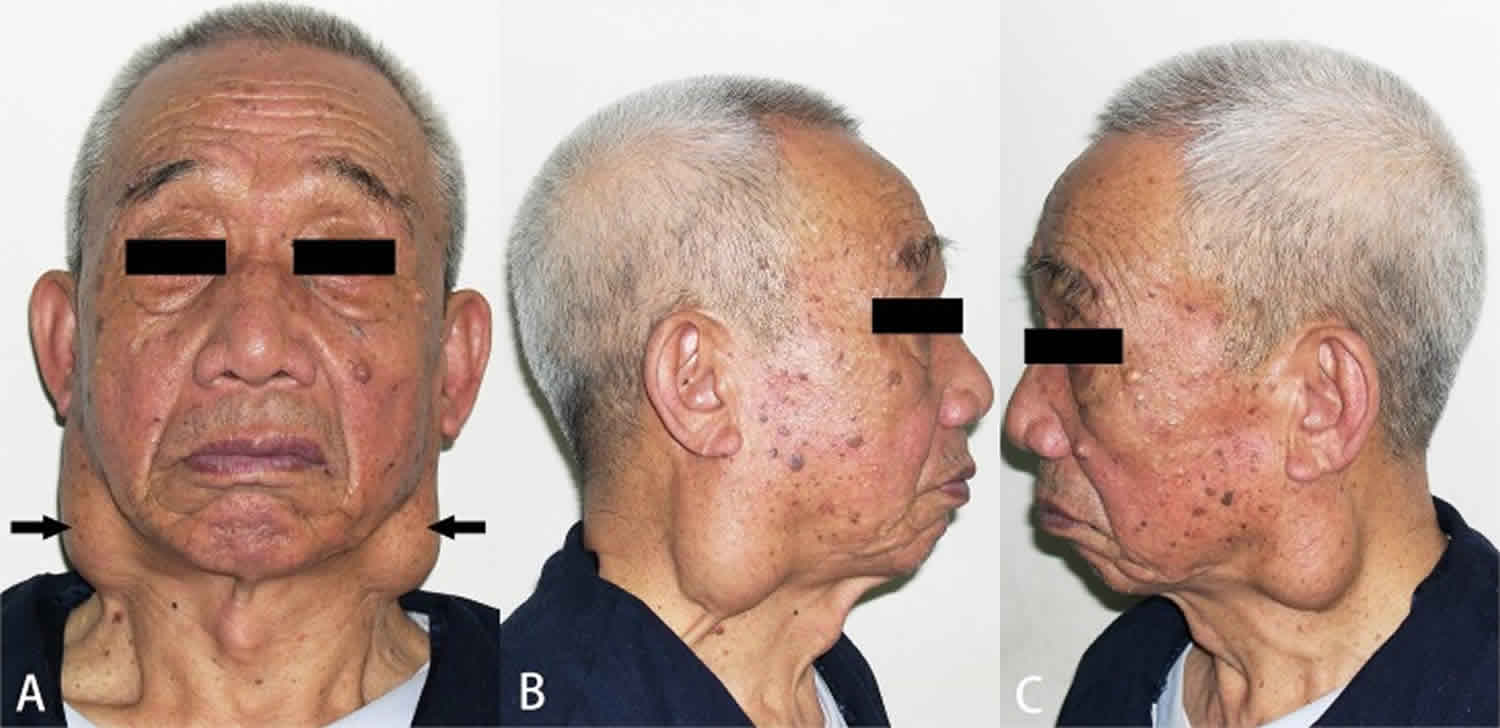
Warthin tumor
Warthin tumor also called papillary cystadenoma lymphomatosum or adenolymphoma, is a common, benign (noncancerous), painless salivary gland neoplasm that consists of a bilayered oncocytic epithelium lining a ductal, papillary, and cystic arrangement in an abundant lymphoid stroma 1. Warthin’s tumors mainly occur in the parotid gland and affect males in their sixth to seventh decade of life 2. Warthin’s tumors can show squamous metaplasia of the tumor epithelium; Seifert et al. 3 have reported metaplastic Warthin’s tumors with large areas of squamous metaplasia. Rarely, Warthin tumors show large areas of squamous metaplasia; such Warthin tumors are called metaplastic or infarcted Warthin tumors because they are occasionally accompanied with tumor necrosis. Metaplastic Warthin’s tumors are rare, and their incidence ranges from 0% (0/278 cases) 4 to 7.6% (21/275 cases) 3. Metaplastic Warthin’s tumors can also be called infarcted Warthin’s tumors because they are occasionally accompanied by extensive necrosis, fibrosis, and inflammation 5. Metaplastic Warthin’s tumors may develop mucinous epithelial metaplasia 6, which can pathologically mimic a low-grade mucoepidermoid carcinoma (MEC). Warthin’s tumors rarely coexist with malignant epithelial neoplasms, such as the squamous cell carcinoma (the most common) and mucoepidermoid carcinoma 7; 27 cases of mucoepidermoid carcinoma in or coexisting with Warthin’s tumor have been described 8.
Warthin’s tumor key points
- Second most common benign parotid tumor (5%)
- Most common bilateral benign neoplasm of the parotid
- Marked male as compared to female predominance
- Occurs later in life (sixth and seventh decades)
- Presents as a lymphocytic infiltrate and cystic epithelial proliferation
- May represent heterotopic salivary gland epithelial tissue trapped within intraparotid lymph nodes
- Incidence of bilaterality and multicentricity of 10%
- Malignant transformation rare (almost unheard of).
Figure 1. Parotid gland
Figure 2. Parotid gland and facial nerve – Facial nerve is an important nerve that passes through the parotid gland and its function is to move facial muscles.
Warthin’s tumor causes
The exact underlying cause of Warthin tumor is currently unknown. However, smoking is thought to increase the risk of developing the tumor. Some studies suggest that radiation exposure and autoimmune disorders may also be associated with Warthin tumor 9.
A retrospective study by Kadletz et al 10 suggested that a high body mass index (BMI) and metabolic syndrome may increase the risk of Warthin tumor formation. The investigators found a BMI of 29.1 in patients with Warthin tumor, compared with 26.2 in persons with other types of benign parotid tumors. Moreover, patients with Warthin tumor had a 62.4% rate of metabolic syndrome–associated comorbidities, compared with 35.2% in the others 10.
Warthin’s tumor symptoms
Warthin’s tumor is a benign (noncancerous) tumor of the salivary glands. Warthin’s tumors most commonly arise in the parotid glands, the largest salivary glands which are located in each cheek above the jaw in front of the ears. Approximately 5-14% of cases are bilateral and 12-20% of affected people experience multicentric (more than one tumor which formed separately from one another) disease 11.
Warthin’s tumor first symptom is usually a firm, painless bump. Without treatment, the swelling may gradually increase overtime which can cause facial nerve palsy (difficulty moving one side of the face) 12.
Warthin’s tumor diagnosis
A diagnosis of Warthin tumor is often suspected based on the presence of characteristic signs and symptoms.
A thorough history and physical examination is important in the workup of parotid masses. The major goal in the evaluation is to determine or exclude the diagnosis of malignancy. History often is the most useful tool in distinguishing inflammatory from neoplastic masses.
Characteristics of inflammatory conditions are sudden onset, pain, and systemic infection. The most common presentation is that of an asymptomatic mass (81%) noted incidentally while washing or shaving the face 13. Pain (12%) or facial nerve paralysis (7%) is less frequent. Facial nerve paralysis is more commonly due to malignancy in the presence of a parotid mass, but most facial nerve paralysis is due to Bell palsy. Parotid masses occur most commonly in the lower pole, or tail, and in the superficial lobe of the gland.
Physical examination most often reveals a mobile nontender mass that is firm and solitary. Evaluate the possibility of a deep tumor by intraoral examination, with attention directed to the tonsillar fossa and soft palate. Inspect the Stensen duct for the character of the salivary flow (clarity, consistency, purulence) and notation of redness, bulging, and irritation of the ductal orifice as part of the physical examination. Evaluate the skin, oral cavity, oropharynx, and neck for possible primary lesions or nodal disease.
The following tests may then be ordered to confirm the diagnosis and rule out other conditions that cause similar features:
- X-rays of the salivary gland (called a ptyalogram or sialogram)
- CT scan, MRI and/or ultrasound
- Salivary gland biopsy
Laboratory studies
Hematologic and serologic tests are of little importance in the workup of salivary gland tumors.
Radiologic studies
Radiologic studies are involved minimally in the workup of an asymptomatic mass, as follows:
- Plain radiography findings can help the clinician exclude calculi
- Sialography is rarely used. When used, it helps the clinician delineate disorders of ductal function or anatomy
- Computed tomography (CT) scanning is almost 100% sensitive in detecting a salivary gland mass; it is most helpful in specifying the size and anatomic extent of a tumor
- MRI is superior in demonstrating benign tumors of the parotid gland because of its greater contrast than CT scan
- Positron emission tomography (PET) scans may be of value in assessing malignant tumors, with attention to metastatic adenopathy and distant metastases
Evidence indicates that for the most part, at least, CT scanning cannot help the clinician to distinguish a benign mass from a malignant one. However, a study by Kim and Lee reported that enhanced, multidetector CT scanning with a 50-second delay between contrast injection and scan can be used to differentiate Warthin tumors from pleomorphic adenomas and malignant tumors. The investigators found that unlike with the other lesions, the 50-second interval led to peak enhancement of the Warthin tumors 14.
Ultrasonography
A study by Rong et al 15 identified differences between the ultrasonographic characteristics of Warthin tumors and those of pleomorphic adenomas, including with regard to shape, vascularity, and the prevalence of cystic areas. The study involved 93 Warthin tumors (61 patients) and 77 pleomorphic adenomas (70 patients), with lobulated lesions representing 38.7% of Warthin tumors and 63.6% of pleomorphic adenomas. Grade 2 or 3 vascularity was identified in the majority of Warthin tumors (73.1%), while grade 0 or 1 vascularity was present in most of the pleomorphic adenomas (77.9%); vessel distribution also varied significantly between the two types of tumors. In addition, cystic areas were identified in 45.2% of the Warthin tumors but in only 20.8% of the pleomorphic adenomas 15.
Biopsy
Fine-needle aspiration may be a valuable pretreatment diagnostic test. Its overall accuracy is greater than 96%, with a sensitivity for benign tumors of 88-98% and a specificity of 94%. Its sensitivity for detecting malignant tumors ranges from 58-96%, and its specificity is 71-88%. Frozen sections are 93% accurate when performed at surgery, but their use is controversial, since diagnosis depends on the experience of the pathologist with regard to salivary gland tumors.
The standard biopsy approach is a superficial parotidectomy with preservation of the facial nerve. For 80-90% of parotid neoplasms, this procedure is both diagnostic and therapeutic. For this reason, preoperative fine-needle aspiration biopsy is recommended, since it can change the clinical approach in up to 35% of patients 16. Lymph nodes can be enucleated 17, as can Warthin tumors, and sialadenitis does not require surgical intervention in most cases.
Warthin tumor treatment
Treatment of Warthin tumor generally includes surgery to remove the tumor or careful observation to watch for changes in the tumor over time. Because Warthin tumor is almost always benign, additional treatment (i.e. radiation therapy and/or chemotherapy) is rarely needed 9.
Published in 2016, national multidisciplinary guidelines from the United Kingdom 18 on the management of salivary gland tumors included a recommendation that benign parotid tumors undergo complete excision, a strategy that, according to the guidelines, leads to good cure rates.
Superficial parotidectomy is the treatment of choice for most benign tumors in the superficial lobe. Make every effort to preserve the facial nerve. In order to preserve the facial nerve, it is important to try to determine the proximity of the nerve to the capsule of the tumor prior to surgery. Results of a retrospective review showed that malignant tumors were likely to have a positive facial nerve margin 19.
Avoid enucleation (except for Warthin tumors and lymph nodes), since it greatly increases the likelihood of recurrence (up to 80%) and nerve damage. Deep lobe tumors demand total parotidectomy with preservation of the facial nerve. For recurrences, postoperative radiotherapy may be administered, with local control rates exceeding 95%.
A study by Cristofaro et al 20 suggested that extracapsular dissection may be superior to superficial parotidectomy in the treatment of pleomorphic adenoma, with extracapsular dissection leading to fewer side effects. The study involved 198 patients with pleomorphic adenomas of the parotid gland, including 153 patients who underwent extracapsular dissection (mean follow-up 61.02 months) and 45 who underwent superficial parotidectomy (mean follow-up 66.4 months). The investigators found that although both techniques were comparably effective, superficial parotidectomy was associated with a significantly greater rate of transient facial nerve injury and facial paralysis than was the other procedure 20.
Warthin’s tumor surgery
Parotidectomy incision should allow for adequate exposure and the most aesthetic result. The incision begins anterior to the superior root of the helix and descends anterior to the tragus. It then is directed behind the lobule of the pinna and can be carried down anteriorly onto the neck as dictated by the need for exposure.
If a large soft tissue defect is created by the excision of the parotid tumor, numerous autologous or allograft tissues (ie, dermal grafts, fascial grafts, fat grafts, AlloDerm) or synthetic substances may be used for filling these defects. Try to preserve a layer of tissue (the parotid fascia or SMAS layer) if it does not compromise the capsule of the tumor. This preservation is important so that a layer of tissue interposes between the cut salivary tissue and the skin. This has been shown to reduce the incidence of Frey syndrome (gustatory sweating).
Complications
Parotidectomy can be performed with little morbidity and no mortality. Most serious complications result from damage to the facial nerve (either temporary or permanent paralysis). Injury to the greater auricular nerve results in hypesthesia of the ear. A slight loss of fullness and an increased prominence of the angle of the mandible may occur after superficial parotidectomy. Uncommon sequelae include salivary fistula, seroma, hematoma, and infection.
Frey (auriculotemporal) syndrome results from aberrant regeneration of auriculotemporal nerve fibers to sweat glands in the skin. The result is sweating on the affected side of the face during mastication. The incidence of this complication is variable, depending upon whether the examiner performs a starch-iodine test. Its incidence may be decreased by interposing a layer of tissue (either preserving the SMAS layer and replacing it on the surface of the parotid gland before closing the incision or placing a layer of allograft in a similar position).
Warthin’s tumor prognosis
The long-term outlook for people with Warthin tumor is generally good. The tumor is almost always benign and is generally cured with surgery. The risk of recurrence (the tumor returning) is thought to be 2% or less 11.
References- Yorita K, Nakagawa H, Miyazaki K, Fukuda J, Ito S, Kosai M. Infarcted Warthin tumor with mucoepidermoid carcinoma-like metaplasia: a case report and review of the literature. J Med Case Rep. 2019;13(1):12. Published 2019 Jan 14. doi:10.1186/s13256-018-1941-3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330755
- Nagao T, Gnepp DR, Simpson RHW, Vielh P. Warthin tumour. In: El-Naggar AK, JKC C, Grandis JR, Takata T, Slootweg PJ, editors. World Health Organization classification of head and neck tumors. Lyon: International Agency for Research on Cancer; 2017. pp. 188–189.
- Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol. 1980;388:13–38. doi: 10.1007/BF00430674
- Eveson JW, Cawson RA. Warthin’s tumor (cystadenolymphoma) of salivary glands. A clinicopathologic investigation of 278 cases. Oral Surg Oral Med Oral Pathol. 1986;61:256–262. doi: 10.1016/0030-4220(86)90371-3
- Eveson JW, Cawson RA. Infarcted (‘infected’) adenolymphomas. A clinicopathological study of 20 cases. Clin Otolaryngol Allied Sci. 1989;14:205–210. doi: 10.1111/j.1365-2273.1989.tb00362.x
- Di Palma S, Simpson RH, Skalova A, Michal M. Metaplastic (infarcted) Warthin’s tumour of the parotid gland: a possible consequence of fine needle aspiration biopsy. Histopathology. 1999;35:432–438. doi: 10.1046/j.1365-2559.1999.035005432.x
- Smolka W, Markowski J, Piotrowska-Seweryn A, Palen P, Dobrosz Z, Dziubdziela W. Mucoepidermoid carcinoma in Warthin tumor of the parotid gland. Arch Med Sci. 2015;11:691–695. doi: 10.5114/aoms.2015.52379
- Yu C, Song Z, Xiao Z, Lin Q, Dong X. Mucoepidermoid carcinoma arising in Warthin’s tumor of the parotid gland: Clinicopathological characteristics and immunophenotypes. Sci Rep. 2016;6:30149. doi: 10.1038/srep30149
- Benign Parotid Tumors. https://emedicine.medscape.com/article/1289560-overview#showall
- Kadletz L, Grasl S, Perisanidis C, Grasl MC, Erovic BM. Rising incidences of Warthin’s tumors may be linked to obesity: a single-institutional experience. Eur Arch Otorhinolaryngol. 2019 Apr. 276 (4):1191-6.
- Iwai T, Baba J, Murata S, Mitsudo K, Maegawa J, Nagahama K, Tohnai I. Warthin tumor arising from the minor salivary gland. J Craniofac Surg. September 2012; 23(5):374-376. https://www.ncbi.nlm.nih.gov/pubmed/22976673
- Salivary gland tumors. https://medlineplus.gov/ency/article/001040.htm
- Byrne MN, Spector JG. Parotid masses: evaluation, analysis, and current management. Laryngoscope. 1988 Jan. 98(1):99-105.
- Kim TY, Lee Y. Contrast-enhanced Multi-detector CT Examination of Parotid Gland Tumors: Determination of the Most Helpful Scanning Delay for Predicting Histologic Subtypes. J Belg Soc Radiol. 2019 Jan 3. 103 (1):2.
- Rong X, Zhu Q, Ji H, et al. Differentiation of pleomorphic adenoma and Warthin’s tumor of the parotid gland: ultrasonographic features. Acta Radiol. 2014 Dec. 55(10):1203-9.
- Heller KS, Dubner S, Chess Q, Attie JN. Value of fine needle aspiration biopsy of salivary gland masses in clinical decision-making. Am J Surg. 1992 Dec. 164(6):667-70.
- Ascani G, Messi M, Balercia P. [Surgical management of pleomorphic adenoma of the salivary glands: our experience]. G Chir. 2008 Aug-Sep. 29(8-9):343-6.
- [Guideline] Sood S, McGurk M, Vaz F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016 May. 130 (S2):S142-9.
- Domenick NA, Johnson JT. Parotid tumor size predicts proximity to the facial nerve. Laryngoscope. 2011 Nov. 121(11):2366-70.
- Cristofaro MG, Allegra E, Giudice A, et al. Pleomorphic adenoma of the parotid: extracapsular dissection compared with superficial parotidectomy–a 10-year retrospective cohort study. ScientificWorldJournal. 2014. 2014:564053