The pineal gland
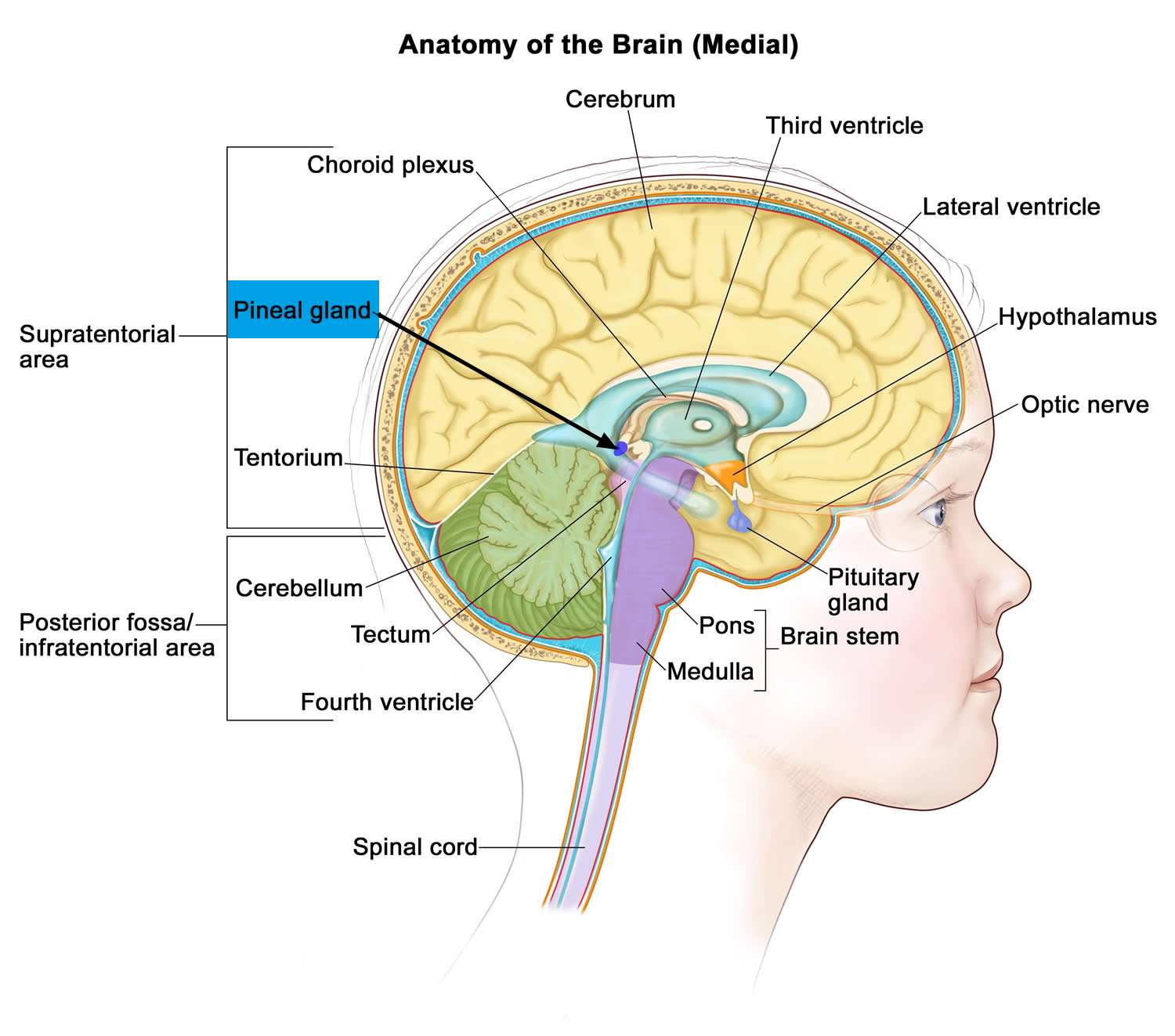
The pineal gland is a small pine cone like shape of highly vascularized structure weighing 100-150mg located deep between the cerebral hemispheres but outside the blood-brain barrier where it is attached to the upper part of the thalamus near the roof of the third ventricle by a short stalk (see Figures 1 and 2) 1. The normal pineal gland appears as a small reddish-brown structure and the normal size ranges between 10 and 14 mm 2. Two primary cell types make up the pineal gland. The pineocyte is the principal parenchyma cell and comprises 95% of the pineal gland. The other 5% are supporting cells referred to as astrocytes. Together, these two cell types are arranged in lobules, which are separated by a fibrovascular stroma. Calcifications commonly occur within the pineal gland and are often associated with increasing age 3, 4.
It was reported that several physiological or pathological conditions alter the morphology of the pineal glands 3. For example, the pineal gland of obese individuals is usually significantly smaller than that in a lean subject 5. The pineal gland volume is also significantly reduced in patients with primary insomnia compared to healthy controls and further studies are needed to clarify whether low pineal volume is the basis or a consequence of a functional sleep disorder 6. These observations indicate that the phenotype of the pineal gland may be changeable by health status or by environmental factors, even in humans 3. The largest pineal gland was recorded in newborn South Pole seals; it occupies one third of their entire brain 7. The pineal size decreases as they grow. Even in the adult seal, however, the pineal gland is considerably large and its weight can reach up to approximately 4000 mg, 27 times larger than that of a human. This huge pineal gland is attributed to the harsh survival environments these animals experience 8.
To the present day, the functions of the pineal gland are not fully understood 9. Current knowledge indicates that by secretion of melatonin (N-acetyl-5-hydroxytryptamine), the pineal gland plays an important role in the regulation of the sleep-wake cycle and of reproductive function (e.g. onset of puberty) 10, with melatonin also acting as a neuroprotector or antioxidant 11, 12.
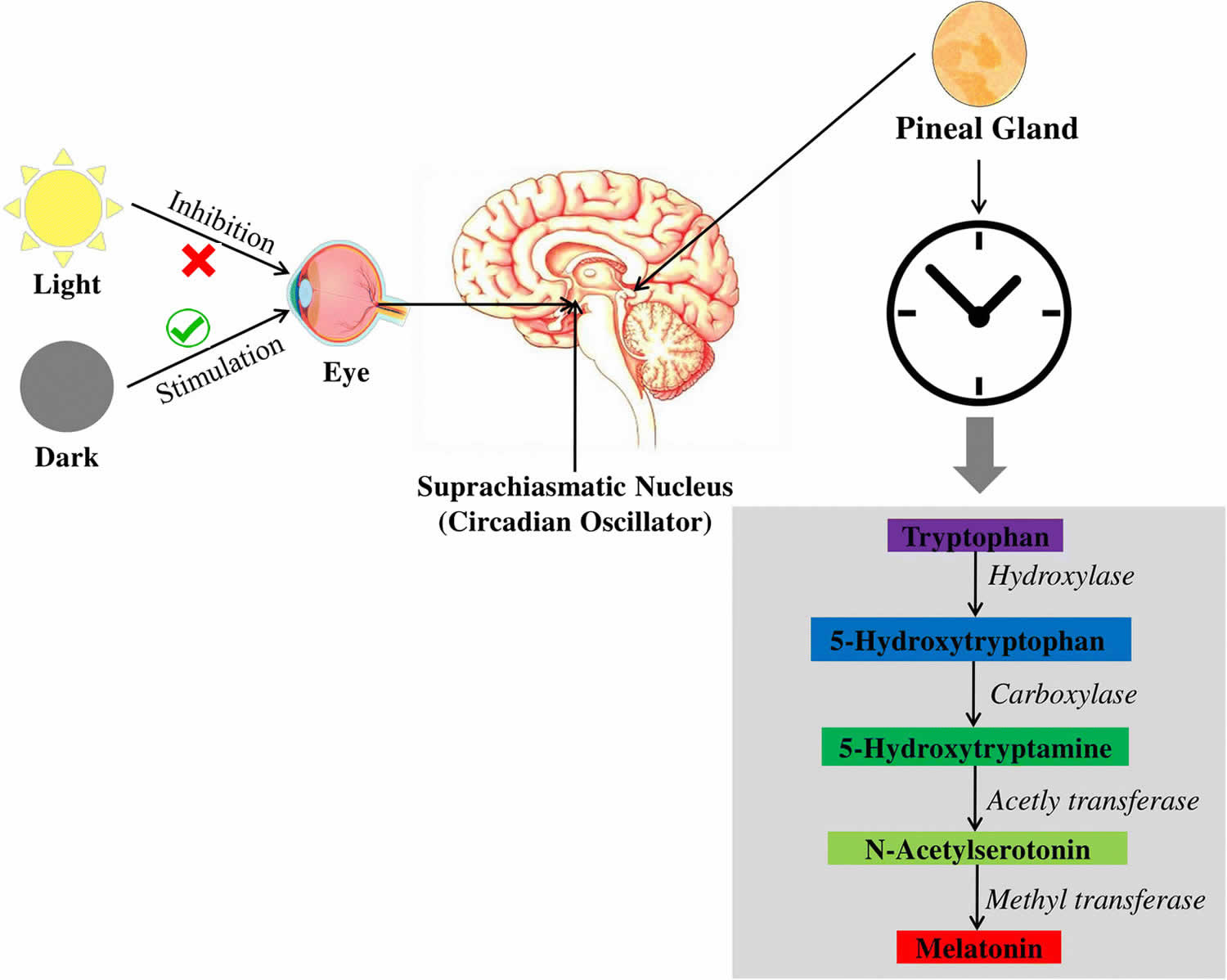
The pineal gland translates the rhythmic cycles of night and day encoded by the retina into hormonal signals that are transmitted to the rest of the neuronal system in the form of serotonin and melatonin synthesis and release 13. The pineal gland secretes the hormone melatonin in response to changing light conditions outside the body (see Figure 3 below). Impulses originating in the retinas of the eyes are conducted along a complex pathway that eventually reaches the pineal gland. Melatonin secretion is suppressed during the day and increases in the dark of night.
Melatonin may help to regulate circadian rhythms. Circadian rhythms are patterns of repeated activity associated with the environmental cycles of day and night, including the sleep–wake cycle. The fact that melatonin secretion responds to day length may explain why traveling across several time zones produces the temporary insomnia of jet lag.
Previous studies have suggested a decline of melatonin secretion with age and an association between melatonin decrease and neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease 14, 15, 16, 17. The amount of uncalcified pineal tissue was shown to predict total melatonin excretion with lack of melatonin being hypothesized to result from pineal gland calcification 18, 19. As a consequence, detection and measurement of pineal gland calcification might be of clinical interest by identifying patients with possible melatonin deficits and a risk for the development of neurodegenerative diseases 18, 19.
In addition, the pineal gland also produces some peptides 20 and other methylated molecules, for example, N,N-dimethyltryptamine (DMT or N,N-DMT) 21, a potent psychedelic. This chemical was suggested to be exclusively generated by the pineal gland at birth, during dreaming, and/or near death to produce “out of body” experiences 22. However, the exact biological consequences (if any) of these substances remain to be clarified. Recently, it was reported that pineal gland is an important organ to synthesize neurosteroids from cholesterol. These neurosteroids include testosterone, 5α- and 5β-dihydrotestosterone (5α- and 5β-DHT), 7α-hydroxypregnenolone (7α-OH PREG) and estradiol-17β (E2). The machinery for synthesis of these steroids has been identified in the pineal gland. 7α-hydroxypregnenolone (7α-OH PREG) is the major neurosteroid synthesized by the pineal gland. Its synthesis and release from the pineal gland exhibits a circadian rhythm and it is regulates the locomote activities of some vertebrates, especially in birds 23. These observations opened a new avenue for functional research on pineal gland; the observations require further confirmation.
Figure 1. Pineal gland
Note: The pineal gland is commonly located along the midline above the superior colliculi and inferior to the splenium of the corpus callosum. It is attached to the superior aspect of the posterior border of the third ventricle.
Figure 2. Pineal gland
Biological rhythms
Biological rhythms are changes that systematically recur in organisms. The period of any rhythm is the duration of one complete cycle. The frequency of a rhythm is the number of cycles per time unit.
Three common types of rhythms in humans are:
- Ultradian rhythm: Ultradian rhythms have periods shorter than 24 hours and include the cardiac cycle and the breathing cycle,
- Infradian rhythm: Periods of infradian rhythms, such as the female reproductive cycle, are longer than 24 hours,
- Circadian rhythm: Periods of circadian rhythms, such as the sleep–wake cycle, variation in body temperature, and changes in hormone secretion, are approximately 24 hours.
Both external (exogenous) and internal (endogenous) factors regulate human biological rhythms. Exogenous factors are environmental components, such as daily temperature changes and the light–dark cycle. Endogenous factors include “clock” genes. Many members of an extended family in Utah, for example, have “advanced sleep phase syndrome” due to a mutation in a gene called “period.” The effect is striking—they promptly fall asleep at 7:30 each night and awaken suddenly at 4:30 a.m 24.
The sleep–wake cycle is the most obvious circadian rhythm in humans. It is largely controlled by the pattern of daylight and night, but under laboratory conditions of constant light or dark, the human body eventually follows an approximately 25-hour cycle.
Using a backlit electronic device, such as a smartphone or tablet, can delay falling asleep long after the device is shut off. Experiments show that such light exposure decreases melatonin production by about 22 percent. Body temperature is mostly endogenously regulated, but light exposure and physical activity help
keep this rhythm on a 24- rather than 25-hour cycle. Body temperature is usually lowest between 4 and 6 a.m., and then increases and peaks between 5 and 11 p.m. It drops during the late evening hours and into the night.
Platelet cohesion, blood pressure, and pulse rate are typically highest 2 hours after awakening. This may explain why heart attacks and strokes are more likely to occur between 6 a.m. and noon than at other times. Plasma cortisol surges and peaks at about 6 a.m., and then gradually declines to its minimum level in late evening before increasing again in the early morning. Growth hormone secretion peaks during the night. Antidiuretic hormone secretion is greater at night, when it decreases urine formation.
Pineal gland function
The main function of the pineal gland is to receive and convey information about the current light-dark cycle from the environment and, consequently produce and secrete melatonin cyclically at night (dark period) 1. Melatonin is the main hormone secreted by the pineal gland. Extrapineal sources of melatonin were reported in the retina, bone marrow cells, platelets, skin, lymphocytes, Harderian gland, cerebellum, and especially in the gastrointestinal tract of vertebrate species 25. Indeed, melatonin is present but can also be synthesized in the enterochromaffin cells; the release of gastrointestinal melatonin into the circulation seems to follow the periodicity of food intake, particularly tryptophan intake 26. It is noteworthy that the concentration of melatonin in the gastrointestinal tract surpasses blood levels by 10-100 times and there is at least 400 times more melatonin in the gastrointestinal tract than in the pineal gland 26. Melatonin in the gastrointestinal tract of newborn and infant mammals is of maternal origin given that melatonin penetrates easily the placenta and is after secreted into the mother’s milk 27. It has even been suggested that melatonin is involved in the production of mekonium 26. Melatonin in human breast milk follows a circadian rhythm in both preterm and term milk, with high levels during the night and undetectable levels during the day 28. No correlation was found between gestational age and concentration of melatonin. It is noteworthy that bottle milk composition does not contain melatonin in powder formula. Also, human colostrum, during the first 4 or 5 days after birth, contains immune – competent cells (colostral mononuclear cells) which are able to synthesize melatonin in an autocrine manner 29.
Melatonin (N-acetyl-5-hydroxytryptamine) is mainly synthesized by the pinealocytes from the amino acid tryptophan, which is hydroxylated (by the tryptophan-5-hydroxylase) into 5-hydroxytryptophan (5-HTP), then decarboxylated (by the 5-hydroxytryptophan decarboxylase) into serotonin (5-hydroxytryptamine or 5-HT) (see Figure 5). Two enzymes, found mainly in the pineal gland, transform serotonin (5-hydroxytryptamine or 5-HT) to melatonin (N-acetyl-5-hydroxytryptamine) 30: serotonin is first acetylated to form N-acetylserotonin by arylalkylamine-N-acetyltransferase (AA-NAT, also called “Timezyme”, is the rate-limiting enzyme for melatonin synthesis), and then N-acetylserotonin is methylated by acetylserotonin-O-methyltransferase (ASMT, also called hydroxyindole-O- methyltransferase or HIOMT) to form melatonin (Figure 2). Both AA-NAT and ASMT activities are controlled by noradrenergic and neuropeptidergic projections to the pineal gland 31. Norepinephrine, also called noradrenaline, activates adenylate cyclase which in turn promotes the melatonin biosynthesis enzymes, especially AA-NAT 32. Once synthesized, melatonin is quickly released into the systemic circulation to reach central and peripheral target tissues.
Melatonin synthesis and secretion is enhanced by darkness and inhibited by light (Figure 3) 33. Luminous information is transmitted from the retina to pineal gland through the suprachiasmatic nucleus (SCN) of the hypothalamus. In humans, its secretion starts soon after sundown, reaches a peak in the middle of the night (between 2 and 4 in the morning) and decreases gradually during the second half of the night 34. Nearly 80% of the melatonin is synthesized at night, with serum concentrations varying between 80 and 120 pg/ml. During daylight hours, serum concentrations are low (10-20 pg/ml) 35.
Serum concentrations of melatonin vary considerably with age, and infants secrete very low levels of melatonin before 3 months of age. Melatonin secretion increases and becomes circadian along with child development: Sadeh 36 reported an association between melatonin secretion and organization of sleep-wake rhythm from 6 months of age. However, more recent studies suggest that melatonin rhythm is set around 3 months of age in typical development, at the same time that infants begin to have more regular sleep–wake cycles associated with nighttime sleep lasting 6-8 h 37. In 3-years-old children, a stabilization of the sleep-wake rhythm is observed, which corresponds to a regular melatonin secretion rhythm. Nocturnal concentration peaks are the highest between the 4th and 7th years of age 38 and then decline progressively 39.
Both serotonin-N-acetyltransferase (NAT) and serotonin availability play a role limiting melatonin production 1. Serotonin-N-acetyltransferase (NAT) mRNA is expressed mainly in the pineal gland, retina, and to a lesser extent in some other brain areas, pituitary, and testis. Serotonin-N-acetyltransferase (NAT) activation is trigger by the activation of β1 and α1b adrenergic receptors by norepinephrine 40. Norepinephrine is the major transmitter via β-1 adrenoceptors with potentiation by α-1 stimulation. Norepinephrine levels are higher at night, approximately 180 degrees out of phase with the serotonin rhythm. Both availability of norepinephrine and serotonin are stimulatory for melatonin synthesis. Pathological or traumatic sympathetic denervation of the pineal gland or administration of β-adrenergic antagonists abolishes the rhythmic synthesis of melatonin and the light-dark control of its production.
Recently, it has been found that almost all organs, tissues and cells tested have the ability to synthesize melatonin using the same pathway and enzymes the pineal uses 41. These include, but not limited to, skin, lens, ciliary body, retina, gastrointestinal tract, testis, ovary, uterus, bone marrow, placenta, oocytes, red blood cells, platelets, lymphocytes, astrocytes, glia cells, mast cells and neurons 3, acting in an autocrine or paracrine manner 42. In the autocrine signaling process, molecules act on the same cells that produce them. In paracrine signaling, they act on nearby cells. Autocrine signals include extracellular matrix molecules and various factors that stimulate cell growth. Nevertheless, except for the pineal gland, these structures contribute little to circulating concentrations in mammals, since after pinealectomy, melatonin levels remain undetectable 43. It was calculated that the amounts of extrapineal derived melatonin is much greater than that produced by the pineal 44. However, the extra pineal-derived melatonin cannot replace/compensate for the role played by the pineal-derived melatonin in terms of circadian rhythm regulation. As scientists know pineal melatonin exhibits a circadian rhythm in circulation and in the CSF with a secretory peak at night and low level during the day 45; thus, the primary function of the pineal-derived melatonin is as a chemical signal of darkness for vertebrates 46. This melatonin signal helps the animals to cope with the light/dark circadian changes to synchronize their daily physiological activities (feeding, metabolism, reproduction, sleep, etc.).
Several other factors, summarized in Table 1, have been related to the secretion and production of melatonin 47.
After intravenous or oral administration, melatonin is quickly metabolized, mainly in the liver and secondarily in the kidney. However, after intravenous administration, the hepatic bio-degradation is less important due to the absence of hepatic first pass. It undergoes hydroxylation to 6-hydroxymelatonin by the action of the cytochrome P450 enzyme CYP1A2, followed by conjugation with sulfuric acid (90%) or glucuronic acid (10%) and is excreted in the urine. About 5% of serum melatonin is excreted unmetabolized also in urine. The principal metabolite, the 6-sulfatoxy-melatonin, is inactive, and its urinary excretion reflects melatonin plasma concentrations 48. Plasma levels can be also measured directly or indirectly assessed through salivary measures. A reverse relation between bioavailability of melatonin and the 6-sulfatoxy-melatonin concentrations area under the curve has been shown, the low bioavailability being explained by an important hepatic first pass 49.
Table 1. Factors influencing human melatonin secretion and production
| Factor | Effect(s) on melatonin | Comment |
| Light | Suppression | >30 lux white 460-480 nm most effective |
| Light | Phase-shift/ Synchronization | Short wavelengths most effective |
| Sleep timing | Phase-shift | Partly secondary to light exposure |
| Posture | ↑ standing (night) | |
| Exercise | ↑ phase shifts | Hard exercise |
| ß-adrenoceptor-antagonist | ↓ synthesis | Anti-hypertensives |
| Serotonin (5-hydroxytryptamine or 5HT) uptake inhibitor | ↑ fluvoxamine | Metabolic effect |
| Norepinephrine uptake inhibitor | ↑ change in timing | Antidepressants |
| MAOA inhibitor | ↑ may change phase | Antidepressants |
| α-adrenoceptor-antagonist | ↓ alpha-1, ↑ alpha-2 | |
| Benzodiazepines | Variable↓ diazepam, alprazolam | GABA mechanisms |
| Testosterone | ↓ | Treatment |
| Oral contraceptives | ↑ | |
| Estradiol | ↓? Not clear | |
| Menstrual cycle | Inconsistent | ↑ amenorrhea |
| Smoking | Possible changes ↑↓ ? | |
| Alcohol | ↓ | Dose dependent |
| Caffeine | ↑ | Delays clearance (exogenous) |
| Aspirin, Ibuprofen | ↓ | |
| Chlorpromazine | ↑ | Metabolic effect |
| Benserazide | Possible phase change, Parkinson patients | Aromatic amino-acid decarboxylase-inhibitor |
Abbreviations: A: antagonist, U: uptake, I: inhibitor, MAO: monoamine oxidase, OC: oral contraceptives, 5HT: 5-hydroxytryptamine.
[Source 1 ]Figure 3. Melatonin plasma concentrations – Circadian profile (in grey is represented the period of darkness)
Footnote: Melatonin (N-acetyl-5-hydroxytryptamine) is synthesized within the pinealocytes from tryptophan, mostly occurring during the dark phase of the day. The duration of melatonin secretion each day is directly proportional to the length of the night. The mechanism behind this pattern of melatonin secretion during the night (dark cycle) is that activity of the rate-limiting enzyme in melatonin synthesis – serotonin N-acetyltransferase (NAT) – responsible for the transformation of 5-hydroxytryptamine (5HT, serotonin) to N-acetylserotonin (NAS), is low during daylight and peaks during the dark phase. Finally, N-acetylserotonin is converted to melatonin by acetylserotonin O-methyltransferase.
Figure 4. Melatonin chemical structure
Figure 5. Melatonin synthesis
Footnote: AA-NAT = arylalkylamine-N-acetyl-transferase; ASMT = acetylserotonin-O-methyltransferase
[Source 50 ]Footnote: In higher vertebrates, light is sensed by the inner retina (retinal ganglion cells) that send neural signals to the visual areas of the brain. However, a few retinal ganglion cells contain melanopsin and have intrinsic photoreceptor capability that send neural signals to non-image forming areas of the brain, including the pineal gland through complex neuronal connections. The photic information from the retina is sent to the suprachiasmatic nucleus (SCN), the major rhythm-generating system or “clock” in mammals, and from there to the hypothalamus. When the light signal is positive, the suprachiasmatic nucleus (SCN) secretes gamma-amino butyric acid (GABA), responsible for the inhibition of the neurons that synapse in the paraventricular nucleus (PVN) of the hypothalamus, consequently the signal to the pineal gland is interrupted and melatonin is not synthesized. On the contrary, when there is no light (darkness), the suprachiasmatic nucleus (SCN) secretes glutamate, responsible for the paraventricular nucleus (PVN) transmission of the signal along the pathway to the pineal gland. The paraventricular nucleus (PVN) communicates with higher thoracic segments of spinal column, conveying information to the superior cervical ganglion that transmits the final signal to the pineal gland through sympathetic postsynaptic fibers by releasing norepinephrine (NE). Norepinephrine (noradrenaline) is the trigger for the pinealocytes to produce melatonin by activating the transcription of the mRNA encoding the enzyme serotonin-N-acetyltransferase (arylalkylamine N-acetyltransferase, AA-NAT), the first molecular step for melatonin synthesis 51.
Control of melatonin synthesis
The rhythm of melatonin production is internally generated and controlled by interacting networks of “clock genes” in the bilateral suprachiasmatic nucleus (SCN) 52. Damage to the suprachiasmatic nucleus (SCN) leads to a loss of the majority of circadian rhythms. The suprachiasmatic nucleus (SCN) rhythm is synchronized to 24 hours mainly by the light-dark cycle acting via the retina and the retinohypothalamic projection to the suprachiasmatic nucleus (SCN); the longer the night the longer the duration of secretion is, and the ocular light serves to synchronize the rhythm to 24 hour and to suppress secretion at the end of the dark phase, as explained above. Light exposure is the most important factor related to pineal gland function and melatonin secretion. A single daily light pulse of suitable intensity and duration in otherwise constant darkness is enough to phase shift and to synchronize the melatonin rhythm to 24 hour 53. The amount of light required at night to suppress melatonin secretion varies across species. In humans, intensities of 2500 lux full spectrum light (domestic light is around 100 to 500 lux) or light preferably in the blue range (460 to 480 nm) are required to completely suppress melatonin at night and shift the rhythm, but lower intensities < 200 lux might suppress secretion 54. Furthermore, the degree of light perception between individuals is related with the incidence of circadian desynchrony; along these lines, blind people with unconscious light perception show abnormally synchronized melatonin and other circadian rhythms 55. Some blind subjects retain an intact retinohypothalamic tract and therefore a normal melatonin response despite a lack of conscious light perception 56. It seems clear that an intact innervated pineal gland is necessary for the perfection of photoperiod change 57. Melatonin functions as a paracrine signal within the retina, it enhances retinal function in low intensity light by inducing photomechanical changes and provides a closed-loop to the pineal-retina-suprachiasmatic nucleus (SCN) system. All together, they are the basic structures to perceive and transduce non-visual effects of light, and to generate the melatonin rhythm by a closed-loop negative feedback (Clock, “Circadian locomotor output cycles kaput” and Bmal, “Brain and muscle ARNT-like” genes), positive stimulatory elements (Per, “period” and Cry, “Cryptochrome” genes), and negative elements (CCG, clock-controlled genes) of clock gene expression in the suprachiasmatic nucleus (SCN).
Low melatonin level is considered as a biomarker of aging 58. As to the association between the aging and melatonin production, in most vertebrates, melatonin production wanes with aging. The reasons for this may be two-fold. Melatonin synthetic capacity is dampened during aging due to the reduced density of β-adrenergic receptors in the pineal gland 59 and the downregulation of gene expression or phosphorylation of arylalkylamine N-acetyltransferase (AANAT) or serotonin-N-acetyltransferase (NAT) 60. A second reason is the increased consumption of melatonin. This is due to the metabolic alterations. For example, more reactive oxygen species (ROS) are generated by the aged cells than in the young cells and melatonin as the endogenous antioxidant is used to neutralize the overproduced ROS in aging organisms. Both of these effects may cause its low levels in the aged vertebrates. When melatonin production was depressed by pinealectomy in rats, accumulation of oxidatively-damaged products accelerated their aging process 61. In contrast, when young pineal glands were grafted to the old animals or exogenous melatonin was supplemented, both significantly increased the life span of experimental animals 62.
Physiological effects of melatonin
Melatonin regulates circadian rhythms such as the sleep-wake rhythm, neuroendocrine rhythms or body temperature cycles through its action on melatonin receptors (MT-1 and MT-2) 63. Ingestion of melatonin induces fatigue, sleepiness and a diminution of sleep latency 64. Disturbed circadian rhythms are associated with sleep disorders and impaired health 65. For example, children with multiple developmental, neuro-psychiatric and health difficulties often show melatonin deficiency 66. When circadian rhythms are restored, behavior, mood, development, intellectual function, health, and even seizure control may improve 65. It should be noted that according to several studies, circadian rhythms are important for typical (normal) neurodevelopment and their absence suppresses neurogenesis in animal models 67.
Finally, melatonin may be involved in early fetal development, with direct effects on placenta, glial and neuronal development, and could play an ontogenic role in the establishment of diurnal rhythms and synchronization of the fetal biological clock 68. Iwasaki et al. 68 investigated the expression of the two enzymes involved in the conversion of serotonin to melatonin (AA-NAT and ASMT) (see Figure 3 above) and found that transcripts of these enzymes were present in the first-trimester human placenta. Moreover, they found also that melatonin significantly potentiated hCG (human chorionic gonadotropin) secretion at optimal concentrations on cultured human trophoblast cells. These results suggest that melatonin regulates in a paracrine/autocrine way human placental function with a potential role in human reproduction. Test tube studies have shown that neural stem/progenitor cells express melatonin MT1 receptors and melatonin induces glial cell-line derived neurotrophic factor (GDNF) expression in neural stem cells, suggesting an early role for melatonin in central nervous system development. Indeed, as indicated previously, melatonin of maternal origin crosses the placenta and can therefore influence fetal development. Studies in humans have repeatedly confirmed that the cycle of melatonin in maternal blood occurs also in fetal circulation 69. The maturation and synchronization of the fetal circadian system have not been thoroughly studied. However, studies in nonhuman primate fetus have shown that maternal melatonin stimulates growth of the primate fetal adrenal gland and entrains fetal circadian rhythms, including suprachiasmatic nucleus (SCN) rhythms 70. Furthermore, in mice, the suppression of melatonin rhythm by maternal exposure to constant light changes the rhythmic expression in fetal clock genes; these changes are reversed when melatonin is injected daily to the mother 71. These results document that the fetal clock is imprinted by melatonin, which under normal circumstances is of maternal origin. In addition, some studies in humans and nonhuman primates show 24h rhythms in fetal heart rate and respiratory movements during the latter half of pregnancy. Whether the circadian system of the human fetus, particularly in late pregnancy, is under the influence of maternal suprachiasmatic nucleus (SCN) remains to be better ascertained 72.
Besides the well-known effects of melatonin on the regulation of sleep-wake rhythms, melatonin is considered as an endogenous synchronizer and a chronobiotic molecule, i.e. a substance that reinforces oscillations or adjusts the timing of the central biological clock located in the suprachiasmatic nuclei of the hypothalamus to stabilize bodily rhythms 73. Furthermore, Pevet and Challet 74 view melatonin as both the master clock output and internal time-giver in the complex circadian clocks network: as a major hormonal output, melatonin distributes, through its daily rhythm of secretion, temporal cues to the numerous tissue targets with melatonin receptors, driving circadian rhythms in some tissue structures such as the adenohypophysis or synchronizing peripheral oscillators such as the fetal adrenal gland but also many other peripheral tissues (pancreas, liver, kidney, heart, lung, fat, gut, etc.). Circadian rhythms, and more precisely the circadian clocks network, allow temporal organization of biological functions in relation to periodic environmental changes and therefore reflect adaptation to the environment. Thus, the sleep–wake rhythm associated with biological circadian rhythms can be seen as an adaptation to the day–night cycle. Moreover, the synchronization by melatonin of peripheral oscillators reflects adaptation of the individual to his/her internal and external environment (for example, the synchronized effects of melatonin on cortisol and insulin secretion allow the individual to be fully awake at 8am and able to start the day by eating and getting some energy from food intake). Given the major synchronizing effects of melatonin on central and peripheral oscillators, measures of melatonin are considered the best peripheral indices of human circadian timing 75.
Futhermore, melatonin is involved in blood pressure and autonomic cardiovascular regulation, immune system regulation but also various physiological functions such as retinal functions, detoxification of free radicals and antioxidant actions through its action on melatonin MT3 receptors protecting the brain from oxidative stress 76. A through its action on MT3 receptors specific section is developed below on melatonin and brain protection. The antioxidant actions of melatonin protect also the gastrointestinal tract from ulcerations by reducing secretion of hydrochloric acid and the oxidative effects of bile acids on the intestinal epithelium, and by increasing duodenal mucosal secretion of bicarbonate through its action on MT2 receptors (this alkaline secretion is an important mechanism for duodenal protection against gastric acid); melatonin prevents also ulcerations of gastrointestinal tract by increasing microcirculation and fostering epithelial regeneration 77. Concerning the role of melatonin in immune regulation, melatonin has direct immuno-enhancement effects in animals and humans 78. Indeed, melatonin stimulates the production of cytokines and more specifically interleukins (IL-2, IL-6, IL-12) 79. In addition, melatonin enhances T helper immune responses 80. Furthermore, the melatonin antioxidant actions contribute to its immuno-enhancing effects 79 and have also an indirect effect by reducing nitric oxide formation which facilitates the decrease of the inflammatory response 81. As suggested by Esquifino et al. 82, melatonin might provide a time-related signal to the immune network.
In addition, effects of melatonin on body mass and bone mass regulation have been reported. Melatonin is known for its role in energy expenditure and body mass regulation in mammals by preventing the increase in body fat with age 83. These effects are mediated by MT2 receptors in adipose tissue 84. Moreover, melatonin increases bone mass by promoting osteoblast cell differentiation and bone formation 85. In humans, melatonin stimulates bone cell proliferation and Type I collagen synthesis in these cells, and inhibits bone resorption through down-regulation of the RANKL-mediated osteoclast formation and activation 86. Also, a deficit of melatonin has been found to be associated with animal scoliosis following pinealectomy and human idiopathic scoliosis 87.
Finally, melatonin has physiological effects on reproduction and sexual maturation in mammals through down-regulation of gonadotropin-releasing hormone (GnRH) gene expression in a cyclical pattern over a 24-hour period 88. The rhythmic release of GnRH controls luteinizing hormone (LH) and follicule-stimulating hormone (FSH) secretion. The daily profile of melatonin secretion conveys internal information used for both circadian and seasonal temporal organization 74. The melatonin rhythmic pattern entrains the reproductive rhythm via the influence of photoperiod on LH pulsatile secretion and therefore mediates the seasonal fluctuations of reproduction clearly observed in animals (seasonal breading as species-specific seasons for reproduction) and moderately observed in humans 89.
Pineal gland calcification
Pineal gland calcification also known as “brain sand”, “psammoma bodies”, pineal concretions, corpora arenacea or acervuli, is calcium deposition (hydroxyapatite deposits [Ca10(PO4)6(OH)2]) in pineal gland and was observed as early as in 1653 in humans 90 and is very common with a reported prevalence of approximately 68–75% in adults 91, 92, 93. Apart from humans, pineal calcification was also identified in a wide range of species including ox, sheep, horse, donkey, monkey, cow, gerbil, rat, guinea pig, chicken and turkey 94. Thus, pineal calcium metabolism and pineal calcification are wide spread phenomenon across species. In all population groups, calcification of the pineal gland was found to increase with age 91, 93 and in some species the pineal calcification rates are as high as 100% with age 95, 96. Ironically, pineal calcification also occurs in neonatal humans 97, 98. Studies have shown that pineal gland calcification can be noted in children as young as 5 years 99. The degree and frequency of pineal calcification have been noted to increase with age 100. Pineal gland calcification also depends on environmental factors, such as altitude and sunlight exposure 100. And it has been hypothesized that increased pineal gland calcification results in decreased melatonin production 101. Pineal gland calcification reduces CSF melatonin levels and dampens its rhythm resulting in chronological disturbance including insomnia and migraine. The low levels of CSF melatonin also elevate neuronal damage from reactive oxygen species (ROS), thus, accelerating the neurodegenerative disorders 3.
Pineal calcification occurs when calcareous deposits form within the connective tissue of the pineal gland stroma 102. Unlike kidney stones, the main component of pineal calcification is hydroxyapatite [Ca10(PO4)6(OH)2] and the Calcium/Phosphorus molar ratio in pineal calcification is similar to that found in the enamel and dentine of teeth 103. Pineal gland calcification is frequently detected on computed tomography (CT) scans 9. As CT causes substantial radiation exposure, it would be of advantage to identify pineal gland calcification with MRI instead. Apart from the absence of ionizing radiation, MRI provides superior soft-tissue contrast and is the modality of choice to evaluate the pineal region, as it enables an accurate delineation of pineal tumors before surgery. However, in the case of calcifications of the pineal gland or tumor calcifications in the pineal region, conventional MRI sequences do not allow for a reliable identification and have a poor sensitivity, as calcifications appear hypointense on T1, T2 and T2*weighted sequences and consequently cannot be reliably differentiated from e.g. soft tissue artifacts or microbleeds 9.
Some researchers believe that pineal calcification was associated with certain neurodegenerative diseases (Alzheimer’s disease, multiple sclerosis), schizophrenia 104, bipolar disorder 105, migraine, symptomatic intracerebral hemorrhage, symptomatic cerebral infarction 106, sleep disorders 107, defective sense of direction, pediatric primary brain tumor 108 and breast cancer 109, 110, 111, 112, 113, 114. Others feel that it is a natural process and has no consequences for human physiopathology since this process occurs early in childhood 115 and it also may not impact the melatonin synthetic ability of the pineal gland in some animals 116, 117. Recently, additional studies have shown that reduced pineal gland volume and pineal calcification jeopardizes the melatonin production in humans due to the decreased function in the pineal gland tissue 118 and resulting in altered sleep patterns 107.
A few studies reported that pineal calcification trigger severe sleep disorders by disturbing melatonin secretion in the pineal gland 119, 118 and pineal cysts 120. In clinical studies, patients with primary insomnia showed reduced plasma melatonin levels during the daytime 121.
Decades ago several studies pointed out the relationship between the pineal calcification and schizophrenia 122, 123. The highest pineal calcium content was detected in the pineal gland of patients who died of kidney disease associated with hypertension among other diseases 124. Currently, additional studies have reported the strong association of pineal gland calcification and neurodegenerative diseases, particularly Alzheimer’s disease 101 accompanied by cognitive decline and sleep disturbances 125 and aging 100.
This association is connected with the melatonin levels synthesized by this gland. It is well established that melatonin is a neuroprotector with its potent antioxidant function and anti-inflammatory activity 126, 127, 128. The brain is rich in lipid, lacks the antioxidative enzyme, catalase, and consumes large quantity of oxygen (roughly 20% of the total oxygen consumed by the brain with 1% of the total body weight). This makes the brain more vulnerable to the oxidative stress than other organs. Decrease of endogenous melatonin will result in the neurons being less resistance to the oxidative stress or brain inflammation. The mechanistic investigations uncovered that in addition to its antioxidant and anti-inflammatory activities, melatonin directly inhibits the secretion and deposition of the β amyloid protein (Alzheimer’s disease plague) 129, 130 which is the hallmark of this disease; it also suppresses tau protein hyperphosphorylation thereby reducing intracellular neurotangles 131, 132, another biomarker of Alzheimer’s disease.
In Alzheimer’s disease, reduced pineal size, pineal gland dysfunction, and pineal calcification have been reported 133 and decreased melatonin levels have been detected in serum 134 and urine 135 of Alzheimer’s disease patients. Previous researches demonstrated that the reduction of melatonin levels in cerebrospinal fluid (CSF) and serum leads to the aggravation of Alzheimer’s disease neuropathology 136, 133, 137. A recent computed tomography study clearly observed pineal calcification in Alzheimer’s disease patients 101. However, the detailed mechanisms on pineal gland calcification and pineal gland dysfunction in Alzheimer’s disease are not fully understood yet 125. The majority of the small scale clinical trials support that melatonin application improved the symptoms of sundowning syndrome and retarded the progress of Alzheimer’s disease 138, 139.
The most suggestive results come from the animal studies. In single, double or triple gene mutated Alzheimer’s disease animal models large doses of melatonin (100 mg/L drinking water or 10 mg/kg body weight/day) prolonged their life span, positively modulated the biochemical and morphological alterations and improved their cognitive performance 140, 141, 142. To date, the large doses of melatonin used in animal studies have not been applied in human clinical trials of Alzheimer’s disease or for dementia prevention, though several trials have examined its short-term effects on cognitive function. Clinical trials in humans have reported mixed effects of melatonin on short-term cognitive functions. In one trial, melatonin improved verbal memory, with slight improvements in other cognitive tests 143. Another trial showed that a single dose of melatonin enhanced memory functions while under stress, but not after stress 144. However, in a third trial, melatonin cream 12.5% did not result in significant effects on cognition 145. Other clinical trials have found that melatonin treatment significantly lowered the risk of delirium, which is a risk factor for dementia 146, 147. But a trial of 452 patients found that treatment with melatonin after surgery did not reduce the incidence of delirium 148.
Clinical trials have not shown that melatonin can slow disease progression or improve cognitive function in patients with dementia or mild cognitive impairment. A recent meta-analysis of patients with Alzheimer’s and other dementias concluded that there are no significant benefits of melatonin on cognitive scores or measures of sleep 149. In 2015, the American Academy of Sleep Medicine Clinical Practice Guideline 150 recommended against the use of melatonin and sleep-promoting medications for elderly people with dementia due to increased risks of falls and other adverse events.
No clinical studies have tested whether melatonin effects are different in APOE4 carriers. One preclinical study tentatively reported that melatonin could protect from possible toxicity from APOE4 151 but the findings have not yet been replicated.
What causes pineal gland calcification?
The exact cause, formation processes and mechanisms of pineal gland calcification is currently unknown. Here, we summarize several opinions, hypothesis and speculations on the potential mechanisms of pineal gland calcification formation. Large amounts of evidence suggests that the pineal gland calcification was associated with human pathological disorders and aging. A great deal of attention has recently been given to the relationship of decreased melatonin levels in neurodegenerative diseases and aging associated pineal calcification. With the increased use of the PET scan, susceptibility-weighted magnetic resonance imaging or other advanced technologies, even very small pineal concretions can be identified in patients or animals, which could not be seen previously. It was found that the rates of pineal calcification have been significantly underestimated previously. For example, in non-specifically targeted patients with the average age of 58.7 ± 17.4 years, 214 out of 346 showed gland calcification on CT scans (62%) 152; the data of 12,000 healthy subjects from Turkey indicated that the highest intracranial calcifications occurred in the pineal gland with an incidence of 71.6% 153. Pineal gland calcification appears to occur without significant differences among countries, regions and races. For example, in Iran the gland calcification incidence is around 71% 154 and in African (Ethiopia), it is roughly 72% 155 and in black people in the US it is 70% 156.
Some studies found that pineal gland calcification was restricted to the connective tissue. The mechanisms involved the formation of calcareous deposits within the connective tissue stroma of the gland 157. These deposits represent the aging-related calcium accumulation within the connective tissue. This type of calcification is similar to that found in the habenular commissure and choroid plexus 158. The connective tissue derived pineal gland calcification is predominant in the rat 159 and Pirbright white guinea pig 160. In analysis of the specimens of human pineal gland, Maslinska et al. 97 reported that the initiation of pineal gland calcification was associated with the tryptase-containing mast cells. During the systemic or local pathological conditions, the tryptase-containing mast cells infiltrate into the pineal gland where they release biologically active substances including tryptase which participates in calcification. This process is pathological but not age related since it also occurs in the children.
The high accumulation of fluoride in pineal gland hydroxyapatite (among those chronically exposed) points to a plausible mechanism by which fluoride may influence sleep patterns 161. In adults, pineal gland fluoride concentrations have been shown to strongly correlate with degree of pineal gland calcification 162, 163. Interestingly, greater degree of pineal calcification among older adolescents and/or adults is associated with decreased melatonin production 164, lower REM sleep percentage, decreased total sleep time, poorer sleep efficiency 165, greater sleep disturbances and greater daytime tiredness 166. While there are no existing human studies on fluoride exposure and melatonin production or sleep behaviors, findings from a doctoral dissertation demonstrated that gerbils fed a high fluoride diet had lower nighttime melatonin production than those fed a low fluoride diet 167. Moreover, their melatonin production was lower than normal for their developmental stage 168. Therefore, it is possible that excess fluoride exposure may contribute to increased pineal gland calcification and subsequent decreases in nighttime melatonin production that contribute to sleep disturbances 167. Additional animal and prospective human studies are needed to explore this hypothesis.
As to the pineal gland calcification of pinealocyte-origin, two speculations should be mentioned. One is proposed by Lukaszyk and Reiter 169. They reported that the pinealocytes extruded polypeptides into the extracellular space in conjunction with their hypothetic carrier protein, neuroepiphysin. The pineal polypeptides of exocytotic microvesicles were actively exchanged for the calcium. The calcium-carrier complex then is formed and deposited on the surface of adjacent mutilayed concretions. Thus, the concretion formation is related to the secretory function of pineal gland. For example, in the gerbil following the superior cervical ganglionectomy, the pineal gland calcification are completely inhibited; this was attributed to a decrease in the functional activity of the gland 170. However, this cannot explain the observation of intracellular calcification of the pinealocytes 171. Krstić 172 proposed another mechanism to explain the origin of pineal gland calcification from pinealocytes. He speculated that the cytoplasmic matrix, vacuoles, mitochondria and the endoplasmic reticulum of large clear pinealocytes were the initial intracellular calcification sites. These loci, and particularly those within the cytoplasmic matrix, transformed into acervuli by a further addition of hydroxyapatite crystals. The cells gradually degenerated, died, broke down, and the acervuli reached the extracellular space. High intracellular calcium levels could be a situation that is responsible for eliminating calcium from the cell, with the hypercalcemic intracellular milieu promoting the initial crystallization. The failure of Ca2+-ATPase could be a natural process of aging or pathological conditions 173. Hence, pineal gland calcification does not occur under normal conditions and it is a result of altered molecular processes in vertebrates. These speculations; however, cannot completely explain the mechanisms of the pineal gland calcification formation. In another speculation, which is a complementary of the previous suggestions, it seems that the pineal gland calcification in some cases is an active rather than a passive process. Tan et al 174 previously hypothesized that the pineal gland may have a blood filtration function like the kidney since its vascular structures as well as its blood flow rate are similar to the kidney. The question is whether they share a similarity to the calcification this is observed in both organs. It is well documented that the compositions of pineal gland calcification is totally different from the kidney stone 3. Kidney stones are primarily composed of calcium oxalate and its formation is simply a sedimentary process caused by high concentrations of both calcium and oxalate 175. A main component of a pineal gland calcification is hydroxyapatite [Ca10(PO4)6(OH)2] 103 which is the chief structural element of vertebrate bone. The Ca/P molar ratio in pineal concretions is similar to the enamel and dentine 176 and these authors pointed out that the nature and crystallinity of the inorganic tissue of the pineal concretions lead one to think of a physiological rather than pathological ossification type with characteristics between enamel and dentine. It is not very clear how the hydroxyapatite is formed in the bone but there is little doubt that its formation involves the collaboration of bone cells and it is a programmed process. In addition, the concentric laminated pineal concretions are frequent observed 177 to be structurally similar the osteons, the major unit of compact bone.
The laminated pineal stone indicates its formation is not random but organized and programmed 3. For example, in humans, laminated pineal stones are associated with aging. The older the individual, the larger number of lamellae 177. Tan et al 3 hypothesis is that the pineal calcification, at least partially, may be similar to the bone formation that is, the pineal calcium deposit may be formed by differentiated bone cells under certain conditions. Recently, numerous studies have reported that melatonin facilitates the capacity of mesenchymal stem cells (MSCs) to differentiate into osteoblast-like cells under in vivo or in vitro conditions 178. Mesenchymal cells are found in the early stage of pineal development in birds and in rats 179. Mesenchymal cells have an important role in pineal follicular formation later during development of the pineal gland. It was also documented that the striated muscle fibers are present in the pig 180 and rat pineal gland 181. These striated muscle fibers are of mesenchymal rather than ectodermal origin 181. These observations indicate that the mesenchymal stem cells are present in the pineal gland and they have the capacity to differentiate into different cell types including muscle as well as probably the osteoblasts and even the osteocytes. The mesenchymal stem cells in the pineal gland may be retained from its early embryonic stage of mesenchymal tissue and/or they may be of vasculature origin. The differentiation from mesenchymal stem cells into osteoblasts/osteocytes seems to be melatonin dependent. The signal transduction pathway of this transition is probably mediated by melatonin membrane receptor 2 (MT2) 181. The detailed mechanism was proposed by Maria and Witt-Enderby 182. Simply, melatonin binds to the MT2 of mesenchymal stem cells to promote them to differentiate into pre-osteoblasts. At the same time melatonin increases the levels of parathyroid hormone (PTH); type I collagen and alkaline phosphatase (ALP) and these factors further promote pre-osteoblasts to form osteoblasts. Finally, melatonin upregulates the gene expression of the osteopontin (OSP), bone morphogenetic protein 2 (BMP-2), osteocalcin (OCN) and ALP and facilitates the osteoblast proliferation, osteocyte formation, mineralization and bone formation.
Transgenic knockout of the membrane receptor 2 (MT2) in mice inhibited the osteoblast proliferation and bone formation 183. This indicates that the pineal gland has the capacity to form the bone like structure (calcification) by the pathway including mesenchymal stem cells. The promotor is the high levels of melatonin generated by this gland. The process of pineal gland calcification in bird (turkey) resembles the bone formation which strongly supports our hypothesis. It requires a microenvironment which includes collagen fibrils, phosphate and calcium. The osteocyte-like cells are found in the center of the pineal concretion and the peripheral part contains the osteoblast-like cells and densely packed collagen fibrils 184. The intermediate portion is the place of mineralization as bone.
Pineal Gland Cyst
Pineal gland cysts are common. Pineal cysts are relatively common and may be found by chance in up to 10% of people who have a head CT scan or MRI 185. Most people with a pineal cyst do not have any signs or symptoms 186. Rarely, a pineal cyst may cause headaches, hydrocephalus, obstruction of the vein of Galen (a vein at the base of the brain), Parinaud syndrome (also known as dorsal midbrain syndrome, which leads to difficulty of upward vertical gaze, mydriasis, blepharospasm and impaired ocular convergence), or other symptoms 187, 188. The exact cause of pineal cysts is unknown. Treatment is usually only considered when a cyst causes symptoms. In most cases, no treatment is necessary for a pineal gland cyst 189. Treatment may involve open or stereotactic (a surgical technique for precisely directing the tip of a delicate instrument (e.g a needle) or beam of radiation in three planes using coordinates provided by medical imaging) removal of the cyst, stereotactic aspiration, and/or CSF diversion (a procedure used to drain fluid from the brain) 188.
A cyst is a sac that can form in any part of the body. Often cysts are filled with air, fluid or other material. Cysts that occur in the pineal gland almost never cause symptoms. So, it is unlikely that headaches are the result of a pineal gland cyst. In most cases, these cysts are discovered when a brain scan is done for an unrelated reason, such as a head trauma, migraine headaches or dizzy spells. Pineal gland cysts are most commonly found in women 20 to 30 years old, and are very rare before puberty or after menopause. This suggests hormones may play a role in causing the cysts.
Because they do not usually cause symptoms or lead to complications, the vast majority of pineal gland cysts do not require surgery or other treatment. Pineal cysts are best seen on brain magnetic resonance imaging (MRI). This type of brain imaging is typically reviewed by a specialist, such as a neuroradiologist, who is experienced in evaluating brain cysts and tumors. That physician can tell the difference between a simple pineal gland cyst and another condition that may require treatment, such as a pineal gland tumor.
In contrast to cysts, tumors are an abnormal mass of tissue. They can be either noncancerous or cancerous. If a pineal gland tumor is found, treatment depends on the specific type, size and location of the tumor, as well as the individual’s overall health and preferences. In many cases, surgery is often the first step in treating pineal gland tumors.
Pineoblastoma
Pineoblastoma is a rare, aggressive type of cancer that begins in the cells of the brain’s pineal gland 190. Your pineal gland, located in the center of your brain, produces a hormone (melatonin) that plays a role in your natural sleep-wake cycle.
Pineoblastoma can occur at any age, but it tends to occur most often in young children 190. Pineoblastoma may cause headaches, sleepiness and subtle changes in the way the eyes move 190.
Pineoblastoma can be very difficult to treat. It can spread within the brain and the fluid (cerebrospinal fluid) around the brain, but it rarely spreads beyond the central nervous system. Treatment usually involves surgery to remove as much of the cancer as possible. Additional treatments may also be recommended.
Diagnosis of pineoblastoma
Tests and procedures used to diagnose pineoblastoma include:
- Imaging tests. Imaging tests can help your doctor determine the location and size of your child’s brain tumor. Magnetic resonance imaging (MRI) is often used to diagnose brain tumors, and advanced techniques, such as perfusion MRI and magnetic resonance spectroscopy, may also be used.
Additional tests might include computerized tomography (CT) and positron emission tomography (PET).
- Removing a sample of tissue for testing (biopsy). A biopsy can be done with a needle before surgery or during surgery to remove the pineoblastoma. The sample of suspicious tissue is analyzed in a laboratory to determine the types of cells and their level of aggressiveness.
- Removing cerebrospinal fluid for testing (lumbar puncture). Also called a spinal tap, this procedure involves inserting a needle between two bones in the lower spine to draw out cerebrospinal fluid from around the spinal cord. The fluid is tested to look for tumor cells or other abnormalities. In certain situations, cerebrospinal fluid may instead be collected during a biopsy procedure to remove suspicious tissue from the brain.
Treatment for pineoblastoma
Pineoblastoma treatment options include:
- Surgery to relieve fluid buildup in the brain. A pineoblastoma may grow to block the flow of cerebrospinal fluid, which can cause a buildup of fluid that puts pressure on the brain (hydrocephalus). An operation to create a way for the fluid to flow out of the brain may be recommended. Sometimes this procedure can be combined with a biopsy or surgery to remove the tumor.
- Surgery to remove the pineoblastoma. The brain surgeon (neurosurgeon) will work to remove the pineoblastoma with the goal of removing as much of the tumor as possible. But it’s often impossible to remove the tumor entirely because pineoblastoma forms near critical structures deep within the brain. Most children with pineoblastoma receive additional treatments after surgery to target the remaining cells.
- Radiation therapy. Radiation therapy uses high-energy beams, such as X-rays or protons, to kill cancer cells. During radiation therapy, your child lies on a table while a machine moves around him or her, directing beams to the brain and spinal cord, with additional radiation to the tumor. Because there is a high risk the tumor cells can spread beyond the initial site to other areas of the central nervous system, radiation therapy directed to the entire brain and spinal cord is recommended for children older than 3.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. Chemotherapy may be recommended after surgery or radiation therapy in children with pineoblastoma. In some cases, it’s used at the same time as radiation therapy. For larger tumors, chemotherapy may be used before surgery to shrink the tumor and make it easier to remove.
- Radiosurgery. Technically a type of radiation and not an operation, stereotactic radiosurgery focuses multiple beams of radiation on precise points to kill the tumor cells. Radiosurgery is sometimes used to treat pineoblastoma that recurs.
- Clinical trials. Clinical trials are studies of new treatments. These studies give you a chance to try the latest treatment options, but the risk of side effects may not be known. Ask your doctor whether your child might be eligible to participate in a clinical trial.
Melatonin supplement
Melatonin is a hormone secreted by the pineal gland in the brain. It helps regulate other hormones and maintains the body’s circadian rhythm. The circadian rhythm is an internal 24-hour “clock” that plays a critical role in when you fall asleep and when you wake up. When it is dark, your body produces more melatonin. When it is light, the production of melatonin drops. Being exposed to bright lights in the evening, or too little light during the day, can disrupt the body’s normal melatonin cycles. For example, jet lag, shift work, and poor vision can disrupt melatonin cycles.
Some scientific evidence supports use of melatonin to minimize the effects of jet lag, especially in people traveling eastward over 2 to 5 time zones 191, 192. However, in one well-designed study, melatonin supplements did not relieve symptoms of jet lag 193 and only a few small studies suggest that these supplements can relieve jet lag symptoms 194, 195, indicating that clinical trial results are inconsistent.
Standard dosage is not established and ranges from 0.5 to 5 mg orally taken 1 hour before usual bedtime on the day of travel and 2 to 4 nights after arrival. Evidence supporting use of melatonin as a sleep aid in adults and children with neuropsychiatric disorders (eg, pervasive developmental disorders) is less strong.
Melatonin also helps control the timing and release of female reproductive hormones 196. It helps determine when a woman starts to menstruate, the frequency and duration of menstrual cycles, and when a woman stops menstruating (menopause). Preliminary research suggests low levels of melatonin help identify women at risk of a pregnancy complication called pre-eclampsia 196.
Some researchers also believe that melatonin levels may be related to aging 196. For example, young children have the highest levels of nighttime melatonin. Researchers believe these levels drop as we age. Some people think lower levels of melatonin may explain why some older adults have sleep problems and tend to go to bed and wake up earlier than when they were younger. However, newer research calls this theory into question.
Melatonin has strong antioxidant effects. Preliminary evidence suggests that it may help strengthen the immune system 196.
Melatonin levels may also play a role in 196, 197:
- regulating the immune response or immune system function
- regulating development and aging
- temperature homeostasis
- the development of cardiovascular diseases, obesity, metabolic syndrome, and osteoporosis
- mood
However, it is not confirmed whether melatonin levels cause, or are a consequence of, specific conditions because they may strongly influence sleep 197. The most common use of melatonin as a supplement is to aid in sleep 198.
There is very limited information in the literature about the influence of a pineal cyst on melatonin secretion. A recent study concluded that their patients with a pineal cyst retained a pattern of melatonin secretion comparable to those without a pineal cyst. However, this study had several limitations, including a small sample size of 4 patients. It should be noted that those who have a pineal cyst removed will no longer produce melatonin. Possible consequences of melatonin deficiency other than expected sleep disturbances are difficult to identify 197.
If you are considering using melatonin supplements, talk to your doctor first.
How much melatonin should I take?
The typical adult dose ranges from 0.3 mg to 5 mg at bedtime. Lower doses often work as well as higher doses. Intake of an usual dose (i.e., 1 to 5 mg), allows within the hour after ingestion, melatonin concentrations 10 to 100 times higher than the physiological nocturnal peak to be obtained, with a return to basal concentrations in 4 to 8 hours 50. A bioavailability study in four male healthy volunteers 199 showed a plasma melatonin peak varying between 2 and 395 nmol/L and an elimination half-life of 47± 3 min (mean ± SD) after oral administration of a 0.5 mg dose. Bioavailability varied from 10 to 56% (mean 33%).
Read the directions on the label of the pill bottle. These will tell you how much melatonin to take and how often to take it. If you have questions about how to take melatonin, call your doctor or pharmacist. Do not take more than the recommended amount. Taking more melatonin does not make it work quicker or better. Overdosing on any medicine can be dangerous.
Keep a record of all medicines and supplements you take and when you take them. Take this list with you when you go to the doctor. Ask your doctor if it’s okay to take melatonin if:
- You take other prescription or over the counter (OTC) medicines.
- You have ongoing health problems.
- You are pregnant or nursing (it is unclear what effect melatonin can have on an unborn baby or nursing infant).
Melatonin for sleep
How to Take Melatonin for Sleep (Insomnia):
Dosage: Take melatonin 0.1 mg to 0.5 mg thirty minutes before bedtime. The most effective dose and length of treatment vary by individual. Treatment can range from a few days (for jet lag) to nine months (for trouble falling asleep) and should be overseen by a physician. Studies suggest melatonin for sleep may be effective in promoting but not maintaining sleep (early morning awakening).
How to Take Melatonin for Shift-Work Sleep Disorders
Dosage: Take melatonin 1.8 mg to 3 mg thirty minutes prior to the desired onset of daytime sleep; melatonin may NOT lead to improved alertness during the nighttime work shift and may only improve daytime sleep time by about 30 minutes.
How to Take Melatonin for Delayed Sleep Phase Disorder
Delayed sleep phase disorder most often occurs in adolescents, possibly due to reduced melatonin production and melatonin deficiency at this age. Sleep onset is delayed by 3 to 6 hours compared with conventional bedtimes (10 to 11 pm). Delayed sleep phase disorder can negatively affect school performance, daily activities, and lead to morning drowsiness which can be dangerous for teen drivers. Any sleep disorder in an adolescent should be evaluated by a physician.
Dosage: Take melatonin 1 mg four to six hours before set bedtime. Once a set bedtime is achieved, use maintenance doses of 0.5 mg melatonin 2 hours before expected sleep onset. Bright light therapy and behavioral management may enhance results. Be aware drowsiness may occur after melatonin dose, so avoid hazardous activities such as driving.
How to Take Melatonin for Non-24-Hour Sleep Wake Disorder (Non-24)
More than 70% of people who are totally blind have Non-24, a circadian rhythm disorder. For people who are totally blind, there are no light cues to help reset the biological clock. The sleep time and wake up time of people who have Non-24-Hour Sleep Wake Disorder shifts a little later every day. Sleep times go in and out of alignment compared to a normal sleep-wake phase. Extra minutes add up each day by day and disrupt the normal wake-sleep pattern.
Use of melatonin in Non-24 is to aid in stimulation to reset the biological clock with one long sleep time at night and one long awake time during the day. However, no large-scale clinical trials of melatonin therapy for Non-24 have been conducted to date.
Dosage: Studies on the blind suggest that 0.5 mg/day melatonin is an effective dose.
Hetlioz, a prescription-only melatonin agonist is also approved for use in Non-24-Hour Sleep Wake Disorder in blind individuals.
Hetlioz (tasimelteon)
Fast-dissolving Melatonin
Some melatonin tablets are available in fast-dissolving formulations in the U.S. To take the orally disintegrating tablet:
- Use dry hands to remove the tablet and place it in your mouth.
- Do not swallow the tablet whole. Allow it to dissolve in your mouth without chewing. If desired, you may drink liquid to help swallow the dissolved tablet.
Call your doctor if the condition you are treating with melatonin does not improve, or if it gets worse while using this product.
Store at room temperature away from moisture and heat.
Melatonin for children
Parents may consider using melatonin to help their child who has a trouble falling asleep. Only use melatonin for your child under the care of a pediatrician or other medical sleep specialist. Insomnia or other sleeping disorders in children should always be evaluated by a medical professional. Children 6 months to 14 years of age with sleep disorders : Melatonin 2 to 5 mg has been used.
Melatonin should not be used as a substitute for good sleep hygiene and consistent bedtime routines in children.
Products containing lower-dose melatonin for kids do exist on the U.S. market. However, long-term use of melatonin has not been studied in children and possible side effects with prolonged use are not known. Use for children with autism spectrum disorder or attention-deficit hyperactivity disorder should involve behavioral interventions and should be directed by a physician.
Delayed sleep phase disorder often occurs in teenagers and young adults, possibly due to alterations in endogenous melatonin production. Sleep onset is delayed by 3 to 6 hours compared with normal bedtime hours of 10 to 11 PM. Maintaining a consistent bedtime free of electronics for at least one hour prior to bedtime is especially important for children and adolescents.
Melatonin Side Effects in Children
The most common melatonin side effect in children is morning drowsiness. Other common side effects in children include:
- Bedwetting
- Headache
- Dizziness
- Nausea
- Diarrhea
- Possible increased risk for seizures in children with severe neurological disorders.
Dietary melatonin supplements can still have drug interactions or health risks if you have certain medical conditions, upcoming surgery, or other health concerns.
Melatonin benefits
Melatonin supplements may help some people with certain sleep disorders, including jet lag, sleep problems related to shift work, and delayed sleep phase disorder (one in which people go to bed but can’t fall asleep until hours later), and insomnia. Unlike many other sleep medications, with melatonin you are unlikely to become dependent, have a diminished response after repeated use (habituation), or experience a hangover effect.
If melatonin for sleep isn’t helping after a week or two, stop using it. And if your sleep problems continue, talk with your health care provider.
If melatonin does seem to help, it’s safe for most people to take nightly for one to two months. After that, stop and see how your sleep is. Be sure you’re also relaxing before bed, keeping the lights low and sleeping in a cool, dark, comfortable bedroom for optimal results.
Sleep Disorders
Studies suggest that melatonin may help with certain sleep disorders, such as jet lag, delayed sleep phase disorder (a disruption of the body’s biological clock in which a person’s sleep-wake timing cycle is delayed by 3 to 6 hours), sleep problems related to shift work, and some sleep disorders in children. It’s also been shown to be helpful for a circadian rhythm sleep disorders in the blind that causes changes in blind peoples’ sleep and wake times. Melatonin can help improve these disorders in adults and children.
However, study results are mixed on whether melatonin is effective for insomnia in adults, but some studies suggest it may slightly reduce the time it takes to fall asleep.
Jet lag
Jet lag is caused by rapid travel across several time zones; its symptoms include disturbed sleep, daytime fatigue, indigestion, and a general feeling of discomfort. To ease jet lag, try taking melatonin two hours before your bedtime at your destination, starting a few days before your trip.
- In a 2009 research review, results from six small studies and two large studies suggested that melatonin may ease jet lag symptoms, such as alertness.
- In a 2007 clinical practice guideline, the American Academy of Sleep Medicine supported using melatonin to reduce jet lag symptoms and improve sleep after traveling across more than one time zone.
You can also adjust your sleep-wake schedule to be in sync with your new time zone by simply staying awake when you reach your destination—delaying sleep until your usual bedtime in the new time zone. Also, get outside for natural light exposure.
Melatonin for Jet Lag:
- Eastbound: If you are traveling east, say from the US to Europe, take melatonin after dark, 30 minutes before bedtime in the new time zone or if you are on the plane. Then take it for the next 4 nights in the new time zone, after dark, 30 minutes before bedtime. If drowsy the day after melatonin use, try a lower dose.
- Westbound: If you are heading west, for example, from the US to Australia, a dose is not needed for your first travel night, but you then may take it for the next 4 nights in the new time zone, after dark, 30 minutes before bedtime. Melatonin may not always be needed for westbound travel.
Given enough time (usually 3 to 5 days), jet lag will usually resolve on its own, but this is not always optimal when traveling.
Delayed Sleep Phase Disorder
In this disorder your sleep pattern is delayed two hours or more from a conventional sleep pattern, causing you to go to sleep later and wake up later. Adults and teens with delayed sleep-wake phase sleep disorder have trouble falling asleep before 2 a.m. and have trouble waking up in the morning. Research shows that melatonin reduces the length of time needed to fall asleep and advances the start of sleep in young adults and children with this condition. Talk to your child’s doctor before giving melatonin to a child.
- In a 2007 review of the literature, researchers suggested that a combination of melatonin supplements, a behavioral approach to delay sleep and wake times until the desired sleep time is achieved, and reduced evening light may even out sleep cycles in people with this sleep disorder.
- In a 2007 clinical practice guideline, the American Academy of Sleep Medicine recommended timed melatonin supplementation for this sleep disorder.
Shift Work Disorder
Shift work refers to job-related duties conducted outside of morning to evening working hours. About 2 million Americans who work afternoon to nighttime or nighttime to early morning hours are affected by shift work disorder.
- A 2007 clinical practice guideline and 2010 review of the evidence concluded that melatonin may improve daytime sleep quality and duration, but not nighttime alertness, in people whose jobs require them to work outside the traditional morning to evening schedule.
- The American Academy of Sleep Medicine recommended taking melatonin prior to daytime sleep for night shift workers with shift work disorder to enhance daytime sleep.
Insomnia
Insomnia is a general term for a group of problems characterized by an inability to fall asleep and stay asleep. Research suggests that melatonin might provide relief from the inability to fall asleep and stay asleep (insomnia) by slightly improving your total sleep time, sleep quality and how long it takes you to fall asleep.
- In adults. A 2013 analysis of 19 studies of people with primary sleep disorders found that melatonin slightly improved time to fall asleep, total sleep time, and overall sleep quality. In a 2007 study of people with insomnia, aged 55 years or older, researchers found that prolonged-release melatonin significantly improved quality of sleep and morning alertness.
- In children. There’s limited evidence from rigorous studies of melatonin for sleep disorders among young people. A 2011 literature review suggested a benefit with minimal side effects in healthy children as well as youth with attention-deficit hyperactivity disorder, autism, and several other populations. There’s insufficient information to make conclusions about the safety and effectiveness of long-term melatonin use.
Sleep-wake cycle disturbances in children
Sleep problems are one of the most common problems parents encounter with their children. There are some simple steps parents can take to improve their children’s sleep, such as having a set bedtime and bedtime routine, avoiding foods or drinks with caffeine, and limiting the amount of screen time. Melatonin can help treat these sleep-wake cycle disturbances in children with a number of disabilities. For example, children with multiple developmental, neuro-psychiatric and health difficulties often show melatonin deficiency 66. When circadian rhythms are restored, behavior, mood, development, intellectual function, health, and even seizure control may improve 65.
Other therapeutic effects of melatonin
Therapeutic effects of melatonin have been reported in several disorders such as certain tumors, cardiovascular diseases or psychiatric disorders. The part concerning melatonin and psychiatric disorders is in particular developed given our past and current work on this topic.
Indeed, oncostatic effects of melatonin have been reported in several tumors (breast cancer, ovarian and endometrial carcinoma, human uveal melanoma, prostate cancer, hepatomas, and intestinal tumors) 200. These oncostatic effects have been attributed to the anti-oxidative role of melatonin given that oxidative stress is involved in the initiation, promotion and progression of carcinogenesis 201. Also, decreased melatonin levels (measures of blood melatonin or urinary excretion of 6-SM) were reported in patients with cardiovascular diseases 202. Inversely, melatonin treatment reduces blood pressure in patients with hypertension 203.
Concerning psychiatric disorders, secretion disturbances of the pineal gland have been described in child and adult psychiatry, with notably in most studies a decreased nocturnal melatonin secretion observed in major depressive disorder, bipolar disorder, schizophrenia or autism spectrum disorder 204.
Also, a phase-shift of melatonin has been reported in major depressive disorder and bipolar disorder, including in particular a delayed melatonin peak secretion 205. It is noteworthy that increased melatonin levels (measures of blood melatonin and urinary excretion of 6-SM) were found when clinical therapeutic benefits were observed following the use of antidepressants 206. Furthermore, significant improvement of major depressive disorder and anxiety was described following administration of 25mg per day of agomelatine, a MT1/MT2 melatonin agonist and selective antagonist of 5-HT2C receptors 207.
Autism spectrum disorder
Concerning autism spectrum disorder (ASD), abnormalities in the serotoninergic system and sleep-wake rhythm disturbances observed in children with autism spectrum disorder suggest altered melatonin secretion in autism 208. Sleep disorders (mostly increased sleep latency, reduced total sleep and nocturnal awakenings with insomnia) are observed in 50-80% of individuals with autism 209. It is noteworthy that sleep problems are not specific of autism and are also observed in children with intellectual disability associated or not with autism 210. However, melatonin measures in children with intellectual disability not associated with autism, such as some children with Down syndrome and Fragile X syndrome, showed respectively normal melatonin production despite delayed nocturnal melatonin peak secretion and increased levels of melatonin 211, whereas decreased nocturnal melatonin secretion was mostly observed in children with autism 210. Scientists reported in two different large samples of children with autism significant relationships between decreased nocturnal urinary excretion and severity of autistic impairments in social communication 212, 213. These results suggest that abnormalities in melatonin physiology might contribute not only to sleep problems in autism, but also to biological and psychopathological mechanisms involved in the development of autism spectrum disorder (for example, certain immunological abnormalities found in autism, such as a decrease number of T lymphocytes, might be explained by the hypo-functioning of the melatonin system).
Schizophrenia
Concerning schizophrenia, as suggested by Morera-Fumero and Abreu-Gonzalez 204, a possible explanation for the “low melatonin syndrome” present in some individuals with schizophrenia may stem from the study of the melatonin-synthesizing enzymes, the AA-NAT and ASMT. Furthermore, according to some authors, MT3 might be involved in the melatonin disturbances observed in schiozophrenia 214. Finally, melatonin secretion was also studied in obsessive compulsive disorder but no abnormalities in melatonin levels were reported.
Brain Protection
Neurological and neuropsychological disabilities caused by brain injuries are a major public health concern. Thus, reducing deficits after a stroke is a major issue. In this line, a number of recent studies have reported the important role of melatonin in neuroprotection in animal models of stroke 215. Indeed, melatonin administration after an experimental stroke in animals reduces infarction volume 216. Such a protective effect can be seen in both gray and white matter 217 and melatonin reduces also inflammatory response 218, cerebral oedema formation 219, and blood-brain barrier permeability 220. In addition, Kilic et al. 221 investigated how sub-acute delivery of melatonin, starting at 24 hours after stroke onset, and continuing for 29 days can influences neuronal survival, endogenous neurogenesis, motor recovery and locomotor activity in mice submitted to an occlusion of the middle cerebral artery during 30 minutes. Furthermore, melatonin improved neuronal survival and enhanced neurogenesis, even when applied one day after stroke. In addition, the authors showed both motor as well as behavioral improvements after melatonin administration. Indeed, the results indicate that cell survival was associated with a long-lasting improvement of motor and coordination deficits as well as with attenuation of hyperactivity and anxiety of the animals as revealed in open field tests. Its neuroprotective activity in animal models of ischemic stroke, as well as its lack of serious toxicity suggests that melatonin could be used for human stroke treatment in the future.
In addition to its protective effect after stroke, experimental data obtained in various independent animal models of brain lesions in neonates support the notion of a neuroprotective effect of melatonin in preterm neonates {see for a review, Biran et al., 222]. In infants, a major source of brain injury is preterm birth, often associated to long-term neurological, cognitive, educational, and social problems. Neurodevelopmental disorders are not only seen in extremely preterm birth 223 but also in late prematurity 224. A large number of infants who survive very preterm birth develop cerebral palsy 225 with a high occurrence of associated motor, perceptual and cognitive deficiencies in childhood 226. Nowadays, the most common brain damage observed in preterm children is diffuse white matter damage as well as reduced neural connectivity 227 in the context of infection, inflammation, and hypoxia-ischemia 228. Although a number of treatments have been tested in preclinical animal models of perinatal brain injury, none of them had been proved to be efficient as a neuroprotector nor translated in clinical practice. Among the molecules proposed, melatonin is a very good candidate, given its effect on brain development, neuroprotection as well as regarding its absence of adverse effects 229. As discussed by Biran et al., 222, in addition to its good safety profile, melatonin easily crosses the placenta as the blood-brain barriers and blocks oxidative, excitotoxic and inflammatory pathways, all involved in the pathogenesis of perinatal brain damage caused by preterm birth. However, only a few studies have looked at the synthesis of melatonin in preterm and term neonates. These studies point to a reduced urinary concentration of melatonin during the first 3 months after birth in preterm infants 222. As these authors discussed, compared with term neonates, preterm neonates show a delayed secretion of melatonin which persists after correction for gestational age up to 8 to 9 months of age. In the absence of maternal melatonin, the appearance of circadian rhythms depends principally on neurological maturation, and very little on the environment 230.
Since, melatonin easily crosses the blood–brain and placental barriers, it can be administered antenatally in order to reduce or prevent the impact of
brain lesions in preterm neonates. Currently, two therapeutic trials testing the neuroprotective properties of melatonin administration in the immediate prepartum period in very preterm infants are under way in France and in the United Kingdom222. The French trial aims to determine the dose of melatonin to be administered in prepartum by parenteral route to mothers at risk of preterm delivery, to decrease the extent of white matter damage detected by diffusion tensor imaging in infants born preterm. The objective of the English trial is to prove that melatonin is capable of reducing brain injury and white matter disease as defined by magnetic resonance imaging at term. These trials will probably lead to a clinical use of melatonin before preterm birth (in case of at risk mother) of just after birth in preterm neonates in order to prevent neurodevelopmental deficits in these children.
Interestingly, from a functional point of view, abnormalities in melatonin physiology associated with sleep disorders, and in particular sleep deprivation, are seen to endanger cerebral and more specifically hippocampal integrity, leading to cognitive dysfunction and contributing to the development of mood disorders 231. The involvement of melatonin in the development of mood disorders was discussed in the previous section.
Finally, based on the brain protective role of melatonin against oxidative stress previously described in this article, there is also increased experimental evidence showing the therapeutic potential of melatonin in neurodegenerative conditions such as Alzheimer disease, Parkinson disease, Huntington’s disease and amyotrophic lateral sclerosis 232. Additional studies and clinical trials are now required both in preterm neonates and aging adults to test the clinical efficacy of melatonin supplementation in such disorders, and to identify the specific therapeutic concentrations needed regarding the subject’s age, disease and brain lesion as well as the short and long-term effects of melatonin both on physiological, functional and cognitive outcomes.
Uses of melatonin supplement
- Insomnia
Studies suggest that melatonin supplements may help people with disrupted circadian rhythms (such as people with jet lag or those who work the night shift), and those with low melatonin levels (such as some seniors and people with schizophrenia) to sleep better 233. A review of the scientific literature suggests that melatonin supplements may help prevent jet lag, particularly in people who cross 5 or more time zones.
A few clinical studies suggest that, when taken for short periods of time (days to weeks), melatonin is more effective than a placebo in reducing the time it takes to fall asleep, increasing the number of sleeping hours, and boosting daytime alertness. It is not clear how well melatonin works, however. Some studies suggest that it only reduces the amount of time to fall asleep by a few minutes.
Several human studies have measured the effects of melatonin supplements on sleep in healthy people. A wide range of doses has been used, often taken by mouth 30 to 60 minutes prior to sleep time. Results have been mixed. Some evidence suggests that melatonin may work best for people over 55 who have insomnia 234. One study of 334 people aged 55 and older found that sustained-release melatonin seemed to help people with primary insomnia fall asleep faster, sleep better, be more alert in the morning, and improve quality of life in people with primary insomnia 235.
- Heart Disease
Several studies show melatonin has cardioprotective properties, including antioxidant and anti-inflammatory effects 236, 237. Research also suggests that melatonin may help lower blood pressure levels and improve cholesterol profiles 237. More research is needed.
- Menopause
Melatonin supplements may improve sleep problems associated with menopause. Other studies suggest it may help restore quality of life 238 and prevent bone loss among perimenopausal women 239. However, it does not appear to relieve other symptoms of menopause, such as hot flashes. Peri- or postmenopausal women who use melatonin supplements should do so only for a short period of time since long-term effects are not known.
- Benzodiazepine Withdrawal
Some research suggests that melatonin may help elderly people with insomnia who are tapering off or stopping benzodiazepines such as diazepam (Valium), alprazolam (Xanax), or lorazepam (Ativan). Taking controlled-release melatonin improved sleep quality in those stopping benzodiazepine use. More research is needed. You should never combine melatonin with sedative medications unless you are under the strict supervision of a health care provider.
- Breast Cancer
Several studies suggest that low melatonin levels may be associated with breast cancer risk 240, 241. For example, women with breast cancer tend to have lower levels of melatonin than those without the disease 242. Laboratory experiments have found that low levels of melatonin stimulate the growth of certain types of breast cancer cells, while adding melatonin to these cells slows their growth 243. Preliminary evidence also suggests that melatonin may strengthen the effects of some chemotherapy drugs used to treat breast cancer 244. In a study that included a small number of women with breast cancer, melatonin (given 7 days before beginning chemotherapy) prevented the lowering of platelets in the blood 245. This is a common complication of chemotherapy that can lead to bleeding.
In another small study of women who were taking tamoxifen for breast cancer but seeing no improvement, adding melatonin caused tumors to modestly shrink in more than 28% of the women 246. Women with breast cancer should ask their doctors before taking melatonin.
- Prostate Cancer
Studies show that men with prostate cancer have lower melatonin levels than men without the disease 247, 248. In test tube studies, melatonin blocks the growth of prostate cancer cells. In one small-scale study, melatonin, combined with conventional medical treatment, improved survival rates in 9 out of 14 men with metastatic prostate cancer. Interestingly, since meditation may cause melatonin levels to rise it appears to be a valuable addition to the treatment of prostate cancer. More research is needed before doctors can make recommendations in this area. Men with prostate cancer should talk to their doctor before taking medication.
- Attention Deficit Hyperactivity Disorder (ADHD) and Autism
Some evidence suggests that melatonin may help promote sleep in children with ADHD 249 or autism, although it does not seem to improve the behavioral symptoms of ADHD or autism 250, 251.
- Fibromyalgia and Chronic Pain
A randomized, placebo-controlled study found that people with fibromyalgia experienced a significant reduction in their symptoms when they took a melatonin supplement either alone or in conjunction with fluoxetine (Prozac) 252. Other studies suggest that melatonin may play a role in other painful conditions, such as migraines 253. People with chronic pain should speak to their physicians before using melatonin as it can interact with some medications.
Other Uses
- Sunburn. Preliminary studies suggest that gels, lotions, or ointments containing melatonin may protect against sunburn and other skin damage. Studies examined using melatonin alone or combined with topical vitamin E prior to UV light exposure from the sun.
- Irritable Bowel Syndrome (IBS). Preliminary research suggests that people with IBS who take melatonin reduce some symptoms, such as abdominal pain 254. Results are mixed as to whether melatonin may help improve other symptoms, such as bloating and frequency of bowel movements.
- Epilepsy. Some studies suggest melatonin may reduce the frequency and duration of seizures in children with epilepsy, but other studies suggest melatonin may increase the frequency of seizures 255, 256. DO NOT take melatonin for epilepsy, or give it to a child, without talking to your doctor first.
- Sarcoidosis. Some researchers suggest that melatonin may be effective in the treatment of pulmonary sarcoidosis 257. Talk to your doctor.
- Assisted Reproduction. Interestingly, preliminary studies suggest melatonin supplementation in the eggs of women with polycystic ovarian syndrome could improve egg maturation and pregnancy rates 258.
- Other Uses. Preliminary evidence suggests that melatonin may play a role in pain modulation and digestive function. More research is needed.
Available Forms
Melatonin is available as tablets, capsules, cream, and lozenges that dissolve under the tongue.
How to take melatonin
There is currently no recommended dose for melatonin supplements. Different people will have different responses to its effects. Lower doses appear to work better in people who are especially sensitive. Higher doses may cause anxiety and irritability.
The best approach for any condition is to begin with very low doses of melatonin. Keep the dose close to the amount that our bodies normally produce (< 0.3 mg per day). You should only use the lowest amount possible to achieve the desired effect. Your doctor can help you determine the most appropriate dose for your situation, including how to increase the amount, if needed.
Effective starting doses for jet lag range from 0.3 to 0.5 mg. One milligram tablets can be cut in half to achieve a 0.5 mg dose if smaller doses are not available for purchase. Lower doses may work for some people, while others may need a higher dose, up to 3 to 5 mg. However, higher doses may be associated with more side effects such as headache, next day grogginess, or vivid dreams.
Always start with the lowest melatonin dose. According to a Cochrane review 259, doses over 5 mg appear to be no more effective than lower doses. It is important to note that much higher doses are available for sale in the U.S., but these doses may result in excessively high levels of physiologic melatonin.
Pediatric
Always ask your child’s doctor before giving melatonin to a child. In fact, doses between 1 to 5 mg may cause seizures in this age group.
Adult
You should work with your doctor to find the safest and most effective dose for you. The right dose for you should produce restful sleep with no daytime irritability or fatigue.
Jet lag: 0.5 to 5 mg of melatonin 1 hour prior to bedtime at final destination has been used in several studies. Another approach that has been used is 1 to 5 mg 1 hour before bedtime for 2 days prior to departure and for 2 to 3 days upon arrival at final destination.
Precautions
Because of the potential for side effects and interactions with medications, people should take dietary supplements only under the supervision of a knowledgeable health care provider.
Some people may have vivid dreams or nightmares when they take melatonin. Taking too much melatonin may disrupt circadian rhythms (your “body clock”).
Melatonin can cause drowsiness if taken during the day. If you are drowsy the morning after taking melatonin, try taking a lower dose.
Additional side effects include stomach cramps, dizziness, headache, irritability, decreased libido, breast enlargement in men (called gynecomastia), and reduced sperm count.
Pregnant or nursing women should not take melatonin because it could interfere with their fertility, or their pregnancy.
Melatonin is a hormone so patients with a history of hormonal-related issues should only use melatonin under the supervision of their physicians.
Some studies show that melatonin supplements worsened symptoms of depression. For this reason, people with depression should consult their doctor before using melatonin supplements.
Although many researchers believe that melatonin levels go down with age, newer evidence has brought this theory into question. People older than 65 should ask their doctor before taking melatonin supplements, so blood levels of this hormone can be monitored.
Possible Interactions
If you are taking prescription medications, you should not use melatonin without first discussing it with your health care provider. Below is a partial list of medications that may interact with melatonin.
- Antidepressant medications. In an animal study, melatonin supplements reduced the antidepressant effects of desipramine and fluoxetine (Prozac). More research is needed to know if the same thing would happen in people. In addition, fluoxetine (a member of a class of drugs called selective serotonin reuptake inhibitors, or SSRIs) can cause low levels of melatonin in people.
- Antipsychotic medications. A common side effect of antipsychotic medications used to treat schizophrenia is a condition called tardive dyskinesia, which causes involuntary movements. In a study of 22 people with schizophrenia and tardive dyskinesia caused by antipsychotic medications, those who took melatonin supplements had fewer symptoms compared to those who did not take the supplements.
- Benzodiazepines. The combination of melatonin and triazolam (Halcion) improved sleep quality in one study. In addition, a few reports have suggested that melatonin supplements may help people stop using long-term benzodiazepine therapy. (Benzodiazepines are habit forming.)
- Birth control pills. Birth control pills may increase the amount of melatonin your body makes. Taking additional melatonin could increase your levels of melatonin above the healthy range.
- Blood pressure medications. Melatonin may make blood pressure medications like methoxamine (Vasoxyl) and clonidine (Catopres) less effective. In addition, medications in a class called calcium channel blockers may lower melatonin levels. Calcium channel blockers include:
- Nifedipine (Procardia)
- Amlodipine (Norvasc)
- Verapamil (Calan, Isoptin)
- Diltiazem (Cardizem)
- Felodipine (Plendil)
- Nisoldipine (Sular)
- Bepridil (Vascor)
Beta-blockers. Use of beta-blockers may lower melatonin levels in the body. Beta-blockers include:
- Acebutolol (Sectral)
- Atenolol (Tenormin)
- Bisoprolol (Zebeta)
- Carteolol (Cartrol)
- Metoprolol (Lopressor, Toprol XL)
- Nadolol (Corgard)
- Propranolol (Inderal)
Blood-thinning medications (anticoagulants). Melatonin may increase the risk of bleeding from anticoagulant medications such as warfarin (Coumadin).
Interleukin-2. In one study of 80 cancer patients, use of melatonin along with interleukin-2 led to more tumor regression and better survival rates than treatment with interleukin-2 alone.
Nonsteroidal anti-inflammatory drugs (NSAIDs). NSAIDs such as ibuprofen (Advil, Motrin) may lower levels of melatonin in the blood.
Steroids and immunosuppressant medications. Melatonin may cause these medication to lose their effectiveness. DO NOT take melatonin with corticosteroids or other medications used to suppress the immune system.
Tamoxifen. Preliminary research suggests that the combination of tamoxifen (a chemotherapy drug) and melatonin may benefit some people with breast and other cancers. More research is needed to confirm these results.
Other. Caffeine, tobacco, and alcohol can all lower levels of melatonin in the body.
Melatonin side effects
Melatonin taken orally in appropriate amounts is generally safe. Side effects of melatonin are uncommon but can include:
- drowsiness,
- a “heavy-head” feeling,
- headache,
- dizziness,
- feeling hungover,
- nausea.
There have been no reports of significant side effects of melatonin in children.
Other, less common melatonin side effects might include short-lasting feelings of depression, mild tremor, mild anxiety, abdominal cramps, irritability, reduced alertness, confusion or disorientation, abnormally low blood pressure (hypotension) and lower body temperature (hypothermia) 260. Because melatonin can cause daytime drowsiness, don’t drive or use machinery within five hours of taking the supplement.
Melatonin may also be unsafe in people with orthostatic hypotension, bleeding disorders, diabetes, depression, autoimmune diseases, seizure disorders, and transplant recipients 261. In elderly patients with dementia, melatonin treatment has been shown to worsen caregiver ratings of patient mood 262. It may also interfere with the action of other drugs.
- Do not use melatonin if you are pregnant or breastfeeding or have an autoimmune disorder, a seizure disorder or depression. Talk to your health care provider if you have diabetes or high blood pressure. Melatonin supplements may also raise blood-sugar levels and increase blood pressure levels in people taking some hypertension medications.
In addition, melatonin supplements can interact with various medications, including:
- Anticoagulants and anti-platelet drugs. These types of drugs, herbs and supplements reduce blood clotting. Combining use of melatonin with them might increase the risk of bleeding.
- Anticonvulsants. Melatonin might inhibit the effects of anticonvulsants in neurologically disabled children.
- Blood pressure drugs. Melatonin might worsen blood pressure in people taking blood pressure medications.
- CNS depressants. Melatonin use with use of these medications might cause an additive sedative effect.
- Diabetes medications. Melatonin might affect sugar levels. If you use diabetes medications, use melatonin cautiously.
- Contraceptive drugs. Use of contraceptive drugs with melatonin might increase the effects and possible side effects of melatonin.
- Cytochrome P450 1A2 (CYP1A2) and cytochrome P450 2C19 (CPY2C19) substrates. Use melatonin cautiously if you take drugs such as diazepam (Valium) and others that are affected by these enzymes.
- Fluvoxamine (Luvox). This selective serotonin reuptake inhibitor can increase melatonin levels, causing unwanted excessive drowsiness.
- Medications that suppress the immune system (immunosuppressants). Melatonin can stimulate immune function and interfere with immunosuppressive therapy.
- Seizure threshold lowering drugs. Taking melatonin with these drugs might increase the risk of seizures.
If you’re considering taking melatonin supplements, check with your doctor first — especially if you have any health conditions. He or she can help you determine if melatonin is right for you.
Is melatonin safe?
Melatonin supplements appear to be safe when used short-term by most healthy people; less is known about its long-term safety 263, 264, 265. Further study is needed to find out more about melatonin’s side effects, especially the delayed or long-term effects. It is unknown if melatonin causes problems when taken with other medicines. It also is unknown if melatonin affects people who have certain diseases and conditions. The American Academy of Sleep Medicine, however, recommends against the use of melatonin and sleep-promoting medications for demented elderly patients due to increased risks of falls and other adverse events 150.
- In one study, researchers noted that melatonin supplements may worsen mood in people with dementia.
- The U.S. Food and Drug Administration (FDA) regulates dietary supplements such as melatonin, but the regulations for dietary supplements are different and less strict than those for prescription or over-the-counter drugs. In 2011, the U.S. Food and Drug Administration (FDA) issued a warning to a company that makes and sells “relaxation brownies,” stating that the melatonin in them hasn’t been deemed a safe food additive.
- Most dietary supplements haven’t been tested in pregnant women, nursing mothers, or children. If you’re pregnant or nursing a child, it’s especially important to see your health care provider before taking any medication or supplement, including melatonin.
Melatonin overdose
Melatonin is thought to be very safe in the short-term with a low risk for overdose. Melatonin is not known to be a potential cause of death, but you need to be aware that it could lead to certain complications if you overdose. Taking too much melatonin can disrupt your circadian rhythms (sleep-wake cycle). It may also cause other unwanted side effects. So, yes, you can technically overdose on melatonin. However, a melatonin overdose can be hard to define since there isn’t an official standard safe dose for everyone.
The acute toxicity of melatonin as seen in both animal and human studies is extremely low. Melatonin may cause minor adverse effects, such as headache, insomnia, rash, upset stomach, and nightmares 266. In animals, an LD50 (the lethal dose which suggests that this is an amount of which at least 50% of the experimental animals (rat or mouse) would die of exposure) could not be established 266. Even 800 mg/kg bodyweight (high dose) was not lethal 267. Studies of human subjects given varying doses of melatonin (1–6.6 g/day) for 30–45 days, and followed with an elaborate battery of biochemical tests to detect potential toxicity, have concluded that, aside from drowsiness, all findings were normal at the end of the test period 268.
Melatonin is widely available as an over-the-counter supplement marketed by different companies. These supplements may not be similar in dosage and/or composition, and some of them may contain additional vitamins. Moreover, melatonin may interact with other over-the-counter drugs, although such interactions have not been systematically evaluated and, therefore, remain unreported. Some people are more sensitive than others to the effects of melatonin. A dose that might trigger side effects in one person may have little effect on someone else.
Young children should avoid melatonin unless otherwise directed by a doctor. Doses between 1 and 5 milligrams (mg) may cause seizures or other complications for young children. In adults, doses in the 30-mg range may be harmful. In general, it’s better to start low and move up slowly and carefully if you see encouraging results.
Some people can have side effects from melatonin that may include:
- daytime drowsiness, dizziness, weakness, or confusion
- vivid dreams, nightmares
- feeling depressed, anxious, irritable
- headache
- loss of appetite, diarrhea, nausea, stomach pain
- blood pressure changes
- joint or back pain
- elevated risk for seizures
Animal studies suggest that melatonin can downregulate the pituitary/gonadal axis resulting in hypogonadism and/or delayed puberty. However chronic administration of low-dose melatonin in men did not alter blood levels of testosterone or luteinizing hormone 269. One case of extremely high melatonin levels associated with delayed puberty and hypogonadism has been reported 270. Pubertal development and resolution of the hypogonadism occurred spontaneously as melatonin levels declined over several years. Recent experimental evidence demonstrates that melatonin reduces sperm motility 271 and that long-term administration inhibits testicular aromatase levels 272.
Melatonin has also been suggested for use as a contraceptive for women 273, which might raise the question of whether melatonin damages the female reproductive system. Notably, no side effects were reported in a report of a phase 2 clinical trial in which 1400 women were treated with 75 mg of melatonin nightly for 4 years 273.
Preliminary animal studies suggest that melatonin may accelerate the development of autoimmune conditions 274. Melatonin transiently exacerbated neurologic symptoms in 1 patient with multiple sclerosis 275.
Although melatonin is a potential adjunctive agent in the treatment of cancer and immune deficiency, poorly timed administration can produce opposite effects. Melatonin injections given in the morning stimulate tumor growth 276, whereas the same doses in mid-afternoon have no effect but in the evening have a retarding effect. And although some people with depression may suffer from a “low melatonin syndrome” 277, melatonin administration that unduly prolongs the nocturnal melatonin rise, or that is given throughout the day, may exacerbate seasonal affective disorder 278 and bipolar and classic depression 279. Finally, animal studies have shown that moderately large doses of melatonin (equivalent in one study to about 30 mg in adult humans) increased light-induced damage to retinal photoreceptors 280.
There is also some concern regarding increased atherosclerosis in the aorta in hypercholesterolemic rats caused by melatonin 281. Moreover, in these animals LDL “bad” cholesterol were less well recognized by LDL-receptor metabolic pathways when melatonin was administered.
- Aulinas A. Physiology of the Pineal Gland and Melatonin. [Updated 2019 Dec 10]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK550972[↩][↩][↩][↩]
- Development of the pineal gland: measurement with MR. Sumida M, Barkovich AJ, Newton TH. AJNR Am J Neuroradiol. 1996 Feb; 17(2):233-6. http://www.ajnr.org/content/17/2/233.long[↩]
- Tan, D. X., Xu, B., Zhou, X., & Reiter, R. J. (2018). Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules (Basel, Switzerland), 23(2), 301. https://doi.org/10.3390/molecules23020301[↩][↩][↩][↩][↩][↩][↩][↩]
- Age-related incidence of pineal calcification detected by computed tomography. Zimmerman RA, Bilaniuk LT. Radiology. 1982 Mar; 142(3):659-62. https://www.ncbi.nlm.nih.gov/pubmed/7063680/[↩]
- Grosshans M, Vollmert C, Vollstaedt-Klein S, Nolte I, Schwarz E, Wagner X, Leweke M, Mutschler J, Kiefer F, Bumb JM. The association of pineal gland volume and body mass in obese and normal weight individuals: a pilot study. Psychiatr Danub. 2016 Sep;28(3):220-224.[↩]
- Bumb JM, Schilling C, Enning F, Haddad L, Paul F, Lederbogen F, Deuschle M, Schredl M, Nolte I. Pineal gland volume in primary insomnia and healthy controls: a magnetic resonance imaging study. J Sleep Res. 2014 Jun;23(3):274-80. doi: 10.1111/jsr.12125[↩]
- Bryden MM, Griffiths DJ, Kennaway DJ, Ledingham J. The pineal gland is very large and active in newborn antarctic seals. Experientia. 1986 May 15;42(5):564-6. doi: 10.1007/BF01946705[↩]
- Tan DX, Manchester LC, Sainz RM, Mayo JC, León J, Reiter RJ. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: a hypothesis. Endocrine. 2005 Jul;27(2):149-58. doi: 10.1385/endo:27:2:149[↩]
- Adams LC, Böker SM, Bender YY, et al. Diagnostic accuracy of susceptibility-weighted magnetic resonance imaging for the evaluation of pineal gland calcification. Jiang Q, ed. PLoS ONE. 2017;12(3):e0172764. doi:10.1371/journal.pone.0172764. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5344338/[↩][↩][↩]
- Melatonin and male reproductive health: relevance of darkness and antioxidant properties. Rocha CS, Rato L, Martins AD, Alves MG, Oliveira PF. Curr Mol Med. 2015; 15(4):299-311. https://www.ncbi.nlm.nih.gov/pubmed/25941822/[↩]
- Pineal calcification in Alzheimer’s disease: an in vivo study using computed tomography. Mahlberg R, Walther S, Kalus P, Bohner G, Haedel S, Reischies FM, Kühl KP, Hellweg R, Kunz D. Neurobiol Aging. 2008 Feb; 29(2):203-9. https://www.ncbi.nlm.nih.gov/pubmed/17097768/[↩]
- Melatonin, immune function and cancer. Srinivasan V, Pandi-Perumal SR, Brzezinski A, Bhatnagar KP, Cardinali DP. Recent Pat Endocr Metab Immune Drug Discov. 2011 May; 5(2):109-23. https://www.ncbi.nlm.nih.gov/pubmed/22074586/[↩]
- González S, Moreno-Delgado D, Moreno E, et al. Circadian-Related Heteromerization of Adrenergic and Dopamine D4 Receptors Modulates Melatonin Synthesis and Release in the Pineal Gland. Schibler U, ed. PLoS Biology. 2012;10(6):e1001347. doi:10.1371/journal.pbio.1001347. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3378626/[↩]
- Mahlberg R, Tilmann A, Salewski L, Kunz D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 2006;31(5):634–41. doi: 10.1016/j.psyneuen.2006.01.009 https://www.ncbi.nlm.nih.gov/pubmed/16584848[↩]
- Zhou JN, Liu RY, van Heerikhuize J, Hofman MA, Swaab DF. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. Journal of pineal research. 2003;34(1):11–6. https://www.ncbi.nlm.nih.gov/pubmed/12485366[↩]
- Mayo JC, Sainz RM, Tan DX, Antolin I, Rodriguez C, Reiter RJ. Melatonin and Parkinson’s disease. Endocrine. 2005;27(2):169–78. doi: 10.1385/ENDO:27:2:169. https://www.ncbi.nlm.nih.gov/pubmed/16217130[↩]
- Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. The Journal of clinical endocrinology and metabolism. 1999;84(1):323–7. doi: 10.1210/jcem.84.1.5394. https://www.ncbi.nlm.nih.gov/pubmed/9920102[↩]
- Mahlberg R, Kienast T, Hadel S, Heidenreich JO, Schmitz S, Kunz D. Degree of pineal calcification (DOC) is associated with polysomnographic sleep measures in primary insomnia patients. Sleep medicine. 2009;10(4):439–45. doi: 10.1016/j.sleep.2008.05.003. https://www.ncbi.nlm.nih.gov/pubmed/18755628[↩][↩]
- Maslinska D, Laure-Kamionowska M, Deregowski K, Maslinski S. Association of mast cells with calcification in the human pineal gland. Folia Neuropathol. 2010;48(4):276–82. https://www.ncbi.nlm.nih.gov/pubmed/21225510[↩][↩]
- Khavinson VK, Kopylov AT, Vaskovsky BV, Ryzhak GA, Lin’kova NS. Identification of Peptide AEDG in the Polypeptide Complex of the Pineal Gland. Bull Exp Biol Med. 2017 Nov;164(1):41-43. doi: 10.1007/s10517-017-3922-8[↩]
- Hill DR, Persinger MA. Application of transcerebral, weak (1 microT) complex magnetic fields and mystical experiences: are they generated by field-induced dimethyltryptamine release from the pineal organ? Percept Mot Skills. 2003 Dec;97(3 Pt 2):1049-50. doi: 10.2466/pms.2003.97.3f.1049[↩]
- Nichols DE. N,N-dimethyltryptamine and the pineal gland: Separating fact from myth. J Psychopharmacol. 2018 Jan;32(1):30-36. doi: 10.1177/0269881117736919[↩]
- Tsutsui K, Haraguchi S, Vaudry H. 7α-Hydroxypregnenolone regulating locomotor behavior identified in the brain and pineal gland across vertebrates. Gen Comp Endocrinol. 2018 Sep 1;265:97-105. doi: 10.1016/j.ygcen.2017.09.014[↩]
- Hole’s Essentials of Human Anatomy and Physiology 13th edition. Published by McGraw-Hill Education, 2 Penn Plaza, New York, NY 10121.[↩]
- Carrillo-Vico A., Calvo J.R., Abreu P., Lardone P.J., Garcia-Maurino S., Reiter R.J., Guerrero J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537–539.[↩]
- Bubenik G.A. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 2002;47:2336–2348.[↩][↩][↩]
- Reiter R.J., Tan D.X., Maldonado M.D. Melatonin as an antioxidant: physiology versus pharmacology. J. Pineal Res. 2005;39:215–216.[↩]
- Katzer D., Pauli L., Mueller A., Reutter H., Reinsberg J., Fimmers R., Bartmann P., Bagci S. Melatonin concentrations and antioxidative capacity of human breast milk according to gestational age and the time of the day. 2016.[↩]
- Pires-Lapa M.A., Tamura E.K., Salustiano E.M., Markus R.P. Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. 2013.[↩]
- Coon S.L., Roseboom P.H., Baler R., Weller J.L., Namboodiri M.A., Koonin E.V., Klein D.C. Pineal serotonin N-acetyltrans- ferase: expression cloning and molecular analysis. Science. 1995;270(5242):1681–1683[↩]
- Simonneaux V., Ribelayga C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003;55:325–395.[↩]
- Klein D.C. 2004.[↩]
- Touitou Y. La mélatonine: hormone et médicament. C. R. Soc. Biol. 1998;192:643–657[↩]
- Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336(3):186–195[↩]
- Karasek K., Winczyk K. Melatonin in humans. J. Physiol. Pharmacol. 2006;57(5):19–39[↩]
- Sadeh A. Sleep and melatonin in infants: a preliminary study. Sleep. 1997;20(3):185–191[↩]
- Joseph D., Chong N.W., Shanks M.E., Rosato E., Taub N.A., Petersen S.A. Get- ting rhythm: how do babies do it? Arch. Dis. Child. Fetal Neonatal Ed. 2014;100(1):F50–F54. doi: 10.1136/archdischild-204-306104.[↩]
- Karasek K., Winczyk K. Melatonin in humans. J. Physiol. Pharmacol. 2006;57(5):19–39.[↩]
- Waldhauser F., Weiszenbacher G., Frisch H., Zeitlhuber U., Waldhauser M., Wurtman R.J. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1(8373):362–365.[↩]
- Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998 Feb 27;279(5355):1358-60. doi: 10.1126/science.279.5355.1358[↩]
- Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res. 2016 Aug;61(1):27-40. doi: 10.1111/jpi.12336[↩]
- Bubenik GA. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signals Recept. 2001 Nov-Dec;10(6):350-66. doi: 10.1159/000046903[↩]
- Lewy AJ, Tetsuo M, Markey SP, Goodwin FK, Kopin IJ. Pinealectomy abolishes plasma melatonin in the rat. J Clin Endocrinol Metab. 1980 Jan;50(1):204-5. doi: 10.1210/jcem-50-1-204[↩]
- Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014 Aug;71(16):2997-3025. doi: 10.1007/s00018-014-1579-2[↩]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991 May;12(2):151-80. doi: 10.1210/edrv-12-2-151[↩]
- Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philos Soc. 2010 Aug;85(3):607-23. doi: 10.1111/j.1469-185X.2009.00118.x[↩]
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009 May-Jun;61(3):383-410. doi: 10.1016/s1734-1140(09)70081-7[↩]
- Lynch H.J., Wurtman R.J., Moskowitz M.A., Archer M.C., Ho M.H. Daily rhythm in human urinary melatonin. Science. 1975;187(4172):169–171[↩]
- Di W.L., Kadva A., Johnston A., Silman R. Variable bioavailability of oral melatonin. N. Engl. J. Med. 1997;336(14):1028–1029[↩]
- Tordjman S, Chokron S, Delorme R, et al. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Current Neuropharmacology. 2017;15(3):434-443. doi:10.2174/1570159X14666161228122115. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5405617/[↩][↩]
- Cipolla-Neto J, Amaral FGD. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev. 2018 Dec 1;39(6):990-1028. doi: 10.1210/er.2018-00084[↩]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999 Jun 25;284(5423):2177-81. doi: 10.1126/science.284.5423.2177[↩]
- Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF 3rd, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998 Apr;274(4 Pt 2):R991-6. doi: 10.1152/ajpregu.1998.274.4.r991[↩]
- Gooley, J. J., Chamberlain, K., Smith, K. A., Khalsa, S. B., Rajaratnam, S. M., Van Reen, E., Zeitzer, J. M., Czeisler, C. A., & Lockley, S. W. (2011). Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. The Journal of clinical endocrinology and metabolism, 96(3), E463–E472. https://doi.org/10.1210/jc.2010-2098[↩]
- Lewy AJ, Newsome DA. Different types of melatonin circadian secretory rhythms in some blind subjects. J Clin Endocrinol Metab. 1983 Jun;56(6):1103-7. doi: 10.1210/jcem-56-6-1103[↩]
- Hull JT, Czeisler CA, Lockley SW. Suppression of Melatonin Secretion in Totally Visually Blind People by Ocular Exposure to White Light: Clinical Characteristics. Ophthalmology. 2018 Aug;125(8):1160-1171. doi: 10.1016/j.ophtha.2018.01.036[↩]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms. 2001 Aug;16(4):336-47. doi: 10.1177/074873001129002051[↩]
- Brazão V., Santello F.H., Colato R.P., Mazotti T.T., Tazinafo L.F., Toldo M.P.A., do Vale G.T., Tirapelli C.R., do Prado J.C. Melatonin: Antioxidant and modulatory properties in age-related changes during Trypanosoma cruzi infection. J. Pineal Res. 2017;63:e12409. doi: 10.1111/jpi.12409[↩]
- Henden T., Stokkan K.A., Reiter R.J., Nonaka K.O., Lerchl A., Jones D.J. Age-associated reduction in pineal beta-adrenergic receptor density is prevented by life-long food restriction in rats. Biol. Signals. 1992;1:34–39. doi: 10.1159/000109343[↩]
- Paltsev M.A., Polyakova V.O., Kvetnoy I.M., Anderson G., Kvetnaia T.V., Linkova N.S., Paltseva E.M., Rubino R., De Cosmo S., De Cata A., et al. Morphofunctional and signaling molecules overlap of the pineal gland and thymus: Role and significance in aging. Oncotarget. 2016;7:11972–11983. doi: 10.18632/oncotarget.7863[↩]
- Reiter R.J., Tan D., Kim S.J., Manchester L.C., Qi W., Garcia J.J., Cabrera J.C., El-Sokkary G., Rouvier-Garay V. Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech. Ageing Dev. 1999;110:157–173. doi: 10.1016/S0047-6374(99)00058-5[↩]
- Pierpaoli W., Regelson W. Pineal control of aging: Effect of melatonin and pineal grafting on aging mice. Proc. Natl. Acad. Sci. USA. 1994;91:787–791. doi: 10.1073/pnas.91.2.787[↩]
- Karasek K., Winczyk K. Melatonin in humans. J. Physiol. Pharmacol. 2006;57(5):19–39. https://www.ncbi.nlm.nih.gov/pubmed/17218758[↩]
- Zhdanova I., Lynch H., Wurtman R. Melatonin: a sleep- promoting hormone. Sleep. 1997;20:899–907 https://www.ncbi.nlm.nih.gov/pubmed/9415953[↩]
- Arendt J. Melatonin: characteristics, concerns, and prospects. 2005. https://www.ncbi.nlm.nih.gov/pubmed/16077149[↩][↩][↩]
- Jan J.E., Bax M.C., Owens J.A., Ipsiroglu O.S., Wasdell M.B. Neurophysiology of circadian rhythm sleep disorders of children with neurodevelopmental disabilities. Eur. J. Paediatr. Neurol. 2012;16(5):403–412. https://www.ncbi.nlm.nih.gov/pubmed/22264650[↩][↩]
- Guzman-Marin R., Suntsova N., Methippara M., Greiffenstein R., Szymusiak R., McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. 2005 https://www.ncbi.nlm.nih.gov/pubmed/16262649[↩]
- Iwasaki S., Nakazawa K., Sakai J., Kometani K., Iwashita M., Yoshimura Y., Maruyama T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005;39:261–265. https://www.ncbi.nlm.nih.gov/pubmed/16150106[↩][↩]
- Okatani Y., Morioka N., Hayashi K. Changes in nocturnal pineal melatonin synthesis during the perimenopausal period: relation to estrogen levels in female rats. J. Pineal Res. 1999;27(2):65–72.[↩]
- Torres-Farfan C., Valenzuela F.J., Germain A.M., Viale M.L., Campino C., Torrealba F., Valenzuela G.J., Richter H.G., Serón-Ferré M. Maternal melatonin stimulates growth and prevents maturation of the capuchin monkey fetal adrenal gland. 2006[↩]
- Torres-Farfan C., Serón-Ferré M., Dinet V., Korf H.W. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 2006;40(1):64–70.[↩]
- Reiter R.J., Tan D.X., Korkmaz A., Rosales-Corral S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 2014;20(2):293–307 [↩]
- Pandi-Perumal S.R., Srinivasan V., Maestroni G.J., Cardinali D.P., Poeggeler B., Hardeland R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838.[↩]
- Pevet P., Challet E. 2011.[↩][↩]
- Arendt J. Melatonin and human rhythms. Chronobiol. Int. 2006;29:21–37.[↩]
- Reiter R.J., Tan D.X., Maldonado M.D. Melatonin as an antioxidant: physiology versus pharmacology. J. Pineal Res. 2005;39:215–216[↩]
- Bubenik G.A. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 2002;47:2336–2348[↩]
- Pandi-Perumal S.R., Esquifino A.I., Cardinali D.P. The role of melatonin in immunoenhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006;87:81–87.[↩]
- Srinivasan V., Pandi-Perumal S.R., Maestroni G.J., Esquifino A.I., Hardeland R., Cardinali D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005;7:293–318.[↩][↩]
- Carrillo-Vico A., Guerrero J.M., Lardone P.J., Reiter R.J. A review of the multiple actions of melatonin on the immune system. 2005.[↩]
- Hardeland R., Pandi-Perumal S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005;2:22.[↩]
- Esquifino A.I., Pandi-Perumal S.R., Cardinali D.P. Circadian organization of the immune response: a role for melatonin. 2004.[↩]
- Bartness T.J., Demas G.E., Song C.K. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med. (Maywood) 2002;227:363–376.[↩]
- Brydon L., Petit L., Delagrange P., Strosberg A.D., Jockers R. Functional expression of mt2 (mel1b) melatonin receptors in human paz6 adipocytes. Endocrinology. 2001;142:4264–4271.[↩]
- Ladizesky M.G., Cutrera R.A., Boggio V., Somoza J., Centrella J.M., Mautalen C., Cardinali D.P. Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci. 2001;70:557–565.[↩]
- Koyama H., Nakade O., Takada Y., Kaku T., Lau K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption throught downregulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002;17:1219–1229.[↩]
- Fjelldal P.G., Grotmol S., Kryvi H., Gjerdet N.R., Taranger G.L., Hansen T., Porter M.J., Totland G.K. Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar. J. Pineal Res. 2004;36:132–139.[↩]
- Balik A., Kretschmannova K., Mazna P., Svobodova I., Zemkova H. Melatonin action in neonatal gonadotrophs. Physiol. Res. 2004;53(1):S153–S166.[↩]
- Barrell G.K., Thrun L.A., Brown M.E., Viguie C., Karsch F.J. Importance of photoperiodic signal quality to entrainment of the circannual reproductive rhythm of the Ewe. Biol. Reprod. 2000;63:769–774.[↩]
- Del Rio-Hortega P. Cytology and cellular pathology of the nervous system. In: Penfield W., editor. Pineal Gland. Hoeber; New York, NY, USA: 1932. pp. 637–703.[↩]
- Yalcin A, Ceylan M, Bayraktutan OF, Sonkaya AR, Yuce I. Age and gender related prevalence of intracranial calcifications in CT imaging; data from 12,000 healthy subjects. Journal of chemical neuroanatomy. 2016;78:20–4. doi: 10.1016/j.jchemneu.2016.07.008. https://www.ncbi.nlm.nih.gov/pubmed/27475519[↩][↩]
- Admassie D, Mekonnen A. Incidence of normal pineal and chroids plexus calcification on brain CT (computerized tomography) at Tikur Anbessa Teaching Hospital Addis Ababa, Ethiopia. Ethiopian medical journal. 2009;47(1):55–60. https://www.ncbi.nlm.nih.gov/pubmed/19743781[↩]
- Turgut AT, Karakas HM, Ozsunar Y, Altin L, Ceken K, Alicioglu B, et al. Age-related changes in the incidence of pineal gland calcification in Turkey: A prospective multicenter CT study. Pathophysiology: the official journal of the International Society for Pathophysiology. 2008;15(1):41–8. https://www.ncbi.nlm.nih.gov/pubmed/18420391[↩][↩]
- Welsh M.G. Pineal calcification: Structural and functional aspects. Pineal Res. Rev. 1985;3:41–68.[↩]
- Przybylska-Gornowicz B, Lewczuk B, Prusik M, Bulc M. Pineal concretions in turkey (Meleagris gallopavo) as a result of collagen-mediated calcification. Histol Histopathol. 2009 Apr;24(4):407-15. doi: 10.14670/HH-24.407[↩]
- Japha JL, Eder TJ, Goldsmith ED. Calcified inclusions in the superficial pineal gland of the mongolian gerbil, Meriones unguiculatus. Acta Anat (Basel). 1976;94(4):533-44.[↩]
- Maślińska D, Laure-Kamionowska M, Deręgowski K, Maśliński S. Association of mast cells with calcification in the human pineal gland. Folia Neuropathol. 2010;48(4):276-82.[↩][↩]
- Winkler P, Helmke K. Age-related incidence of pineal gland calcification in children: a roentgenological study of 1,044 skull films and a review of the literature. J Pineal Res. 1987;4(3):247-52. doi: 10.1111/j.1600-079x.1987.tb00862.x[↩]
- Whitehead MT, Oh C, Raju A, Choudhri AF. Physiologic pineal region, choroid plexus, and dural calcifications in the first decade of life. AJNR Am J Neuroradiol. 2015 Mar;36(3):575-80. doi: 10.3174/ajnr.A4153[↩]
- Turgut AT, Karakaş HM, Ozsunar Y, Altın L, Ceken K, Alıcıoğlu B, Sönmez I, Alparslan A, Yürümez B, Celik T, Kazak E, Geyik PÖ, Koşar U. Age-related changes in the incidence of pineal gland calcification in Turkey: A prospective multicenter CT study. Pathophysiology. 2008 Jun;15(1):41-8. doi: 10.1016/j.pathophys.2008.02.001[↩][↩][↩]
- Mahlberg R, Walther S, Kalus P, Bohner G, Haedel S, Reischies FM, Kühl KP, Hellweg R, Kunz D. Pineal calcification in Alzheimer’s disease: an in vivo study using computed tomography. Neurobiol Aging. 2008 Feb;29(2):203-9. doi: 10.1016/j.neurobiolaging.2006.10.003[↩][↩][↩]
- Macpherson P, Matheson MS. Comparison of calcification of pineal, habenular commissure and choroid plexus on plain films and computed tomography. Neuroradiology. 1979 Aug 15;18(2):67-72. doi: 10.1007/BF00344824[↩]
- Kodaka T, Mori R, Debari K, Yamada M. Scanning electron microscopy and electron probe microanalysis studies of human pineal concretions. J Electron Microsc (Tokyo). 1994 Oct;43(5):307-17.[↩][↩]
- Sandyk R, Kay SR. Abnormal EEG and calcification of the pineal gland in schizophrenia. Int J Neurosci. 1992 Jan;62(1-2):107-11. doi: 10.3109/00207459108999764[↩]
- Sandyk R, Pardeshi R. The relationship between ECT nonresponsiveness and calcification of the pineal gland in bipolar patients. Int J Neurosci. 1990 Oct;54(3-4):301-6. doi: 10.3109/00207459008986648[↩]
- Kitkhuandee A, Sawanyawisuth K, Johns NP, Kanpittaya J, Johns J. Pineal calcification is associated with symptomatic cerebral infarction. J Stroke Cerebrovasc Dis. 2014 Feb;23(2):249-53. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.009[↩]
- Kunz D, Bes F, Schlattmann P, Herrmann WM. On pineal calcification and its relation to subjective sleep perception: a hypothesis-driven pilot study. Psychiatry Res. 1998 Jun 30;82(3):187-91. doi: 10.1016/s0925-4927(98)00013-4[↩][↩]
- Tuntapakul S, Kitkhuandee A, Kanpittaya J, Johns J, Johns NP. Pineal calcification is associated with pediatric primary brain tumor. Asia Pac J Clin Oncol. 2016 Dec;12(4):e405-e410. doi: 10.1111/ajco.12519[↩]
- DREXLER J, MEANEY TF, McCORMACK LJ. The calcified pineal body and carcinoma. Cleve Clin Q. 1957 Oct;24(4):242-7. doi: 10.3949/ccjm.24.4.242[↩]
- DREXLER J. THE CALCIFIED PINEAL BODY AND MAMMARY CARCINOMA. Cancer. 1963 Dec;16:1616-7. doi: 10.1002/1097-0142(196312)16:12<1616::aid-cncr2820161216>3.0.co;2-0[↩]
- Sandyk R. The relationship of thought disorder to third ventricle width and calcification of the pineal gland in chronic schizophrenia. Int. J. Neurosci. 1993;68:53–59. doi: 10.3109/00207459308994259[↩]
- Sandyk R. Pineal calcification in relation to menopause in schizophrenia. Int. J. Neurosci. 1992;67:1–8. doi: 10.3109/00207459208994771[↩]
- Bersani G., Garavini A., Taddei I., Tanfani G., Nordio M., Pancheri P. Computed tomography study of pineal calcification in schizophrenia. Eur. Psychiatry. 1999;14:163–166. doi: 10.1016/S0924-9338(99)80735-4[↩]
- Sandyk R., Kay S.R. Pineal calcification in schizophrenia. Relationship to age of onset and tardive dyskinesia. Schizophr. Res. 1991;5:85–86. doi: 10.1016/0920-9964(91)90057-X[↩]
- Doyle AJ, Anderson GD. Physiologic calcification of the pineal gland in children on computed tomography: prevalence, observer reliability and association with choroid plexus calcification. Acad Radiol. 2006 Jul;13(7):822-6. doi: 10.1016/j.acra.2006.04.004[↩]
- Bojkowski CJ, Arendt J. Factors influencing urinary 6-sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin Endocrinol (Oxf). 1990 Oct;33(4):435-44. doi: 10.1111/j.1365-2265.1990.tb03882.x[↩]
- Commentz J.C., Fischer P., Stegner H., Winkler P., Helmake K., Willig R.P. Pineal calcification does not affect melatonin production. J. Neural Transm. Suppl. 1986;21:481–502.[↩]
- Nölte I, Lütkhoff AT, Stuck BA, Lemmer B, Schredl M, Findeisen P, Groden C. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging. 2009 Sep;30(3):499-505. doi: 10.1002/jmri.21872[↩][↩]
- Mahlberg R, Kienast T, Hädel S, Heidenreich JO, Schmitz S, Kunz D. Degree of pineal calcification (DOC) is associated with polysomnographic sleep measures in primary insomnia patients. Sleep Med. 2009 Apr;10(4):439-45. doi: 10.1016/j.sleep.2008.05.003[↩]
- Bumb JM, Brockmann MA, Groden C, Al-Zghloul M, Nölte I. TrueFISP of the pediatric pineal gland: volumetric and microstructural analysis. Clin Neuroradiol. 2012 Mar;22(1):69-77. doi: 10.1007/s00062-011-0110-5[↩]
- Riemann D, Klein T, Rodenbeck A, Feige B, Horny A, Hummel R, Weske G, Al-Shajlawi A, Voderholzer U. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002 Dec 15;113(1-2):17-27. doi: 10.1016/s0165-1781(02)00249-4[↩]
- Sandyk R., Kay S.R. Abnormal EEG and calcification of the pineal gland in schizophrenia. Int. J. Neurosci. 1992;62:107–111. doi: 10.3109/00207459108999764[↩]
- Sandyk R., Kay S.R. The relationship of pineal calcification and melatonin secretion to the pathophysiology of tardive dyskinesia and Tourette’s syndrome. Int. J. Neurosci. 1991;58:215–247. doi: 10.3109/00207459108985437[↩]
- Hinterberger H, Pickering J. Catecholamine, indolealkylamine and calcium levels of human pineal glands in various clinical conditions. Pathology. 1976 Jul;8(3):221-9. doi: 10.3109/00313027609059003[↩]
- Song J. (2019). Pineal gland dysfunction in Alzheimer’s disease: relationship with the immune-pineal axis, sleep disturbance, and neurogenesis. Molecular neurodegeneration, 14(1), 28. https://doi.org/10.1186/s13024-019-0330-8[↩][↩]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016 Oct;61(3):253-78. doi: 10.1111/jpi.12360[↩]
- Berkiks I, Benmhammed H, Mesfioui A, Ouichou A, El Hasnaoui A, Mouden S, Touil T, Bahbiti Y, Nakache R, El Hessni A. Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress. Int J Neurosci. 2018 Jun;128(6):495-504. doi: 10.1080/00207454.2017.1398156[↩]
- Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S, Sun C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017 Aug;63(1). doi: 10.1111/jpi.12414[↩]
- Lahiri DK. Melatonin affects the metabolism of the beta-amyloid precursor protein in different cell types. J Pineal Res. 1999 Apr;26(3):137-46. doi: 10.1111/j.1600-079x.1999.tb00575.x[↩]
- Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, Frangione B, Ghiso J. Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem. 1998 Mar 27;273(13):7185-8. doi: 10.1074/jbc.273.13.7185[↩]
- Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol Sin. 2005 May;26(5):519-26. doi: 10.1111/j.1745-7254.2005.00102.x[↩]
- Li XC, Wang ZF, Zhang JX, Wang Q, Wang JZ. Effect of melatonin on calyculin A-induced tau hyperphosphorylation. Eur J Pharmacol. 2005 Mar 7;510(1-2):25-30. doi: 10.1016/j.ejphar.2005.01.023[↩]
- Wu YH, Feenstra MG, Zhou JN, Liu RY, Toranõ JS, Van Kan HJ, Fischer DF, Ravid R, Swaab DF. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003 Dec;88(12):5898-906. doi: 10.1210/jc.2003-030833[↩][↩]
- Ohashi Y, Okamoto N, Uchida K, Iyo M, Mori N, Morita Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol Psychiatry. 1999 Jun 15;45(12):1646-52. doi: 10.1016/s0006-3223(98)00255-8[↩]
- Luboshitzky R, Shen-Orr Z, Tzischichinsky O, Maldonado M, Herer P, Lavie P. Actigraphic sleep-wake patterns and urinary 6-sulfatoxymelatonin excretion in patients with Alzheimer’s disease. Chronobiol Int. 2001 May;18(3):513-24. doi: 10.1081/cbi-100103973[↩]
- Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003 Sep;35(2):125-30. doi: 10.1034/j.1600-079x.2003.00065.x[↩]
- Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab. 1999 Jan;84(1):323-7. doi: 10.1210/jcem.84.1.5394[↩]
- Wade, A. G., Farmer, M., Harari, G., Fund, N., Laudon, M., Nir, T., Frydman-Marom, A., & Zisapel, N. (2014). Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clinical interventions in aging, 9, 947–961. https://doi.org/10.2147/CIA.S65625[↩]
- Mahlberg R, Kunz D, Sutej I, Kühl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol. 2004 Aug;24(4):456-9. doi: 10.1097/01.jcp.0000132443.12607.fd[↩]
- Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, Arendash GW. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009 Aug;47(1):82-96. doi: 10.1111/j.1600-079X.2009.00692.x[↩]
- Nie L, Wei G, Peng S, Qu Z, Yang Y, Yang Q, Huang X, Liu J, Zhuang Z, Yang X. Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer’s disease. Biofactors. 2017 Jul 8;43(4):593-611. doi: 10.1002/biof.1369[↩]
- Di Paolo C, Reverte I, Colomina MT, Domingo JL, Gómez M. Chronic exposure to aluminum and melatonin through the diet: neurobehavioral effects in a transgenic mouse model of Alzheimer disease. Food Chem Toxicol. 2014 Jul;69:320-9. doi: 10.1016/j.fct.2014.04.022[↩]
- Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am J Geriatr Psychiatry. 2004 Jul-Aug;12(4):432-6. doi: 10.1176/appi.ajgp.12.4.432[↩]
- Rimmele U, Spillmann M, Bärtschi C, Wolf OT, Weber CS, Ehlert U, Wirtz PH. Melatonin improves memory acquisition under stress independent of stress hormone release. Psychopharmacology (Berl). 2009 Mar;202(4):663-72. doi: 10.1007/s00213-008-1344-z[↩]
- Scheuer C, Pommergaard HC, Rosenberg J, Gögenur I. Effect of topical application of melatonin cream 12.5% on cognitive parameters: A randomized, placebo-controlled, double-blind crossover study in healthy volunteers. J Dermatolog Treat. 2016 Nov;27(6):488-494. doi: 10.3109/09546634.2016.1161154[↩]
- Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011 Jul;26(7):687-94. doi: 10.1002/gps.2582. Epub 2010 Sep 15. Erratum in: Int J Geriatr Psychiatry. 2014 May;29(5):550.[↩]
- Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014 Apr;71(4):397-403. doi: 10.1001/jamapsychiatry.2013.3320[↩]
- de Jonghe A, van Munster BC, Goslings JC, Kloen P, van Rees C, Wolvius R, van Velde R, Levi M, de Haan RJ, de Rooij SE; Amsterdam Delirium Study Group. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014 Oct 7;186(14):E547-56. doi: 10.1503/cmaj.140495[↩]
- Trotti LM, Karroum EG. Melatonin for Sleep Disorders in Patients with Neurodegenerative Diseases. Curr Neurol Neurosci Rep. 2016 Jul;16(7):63. doi: 10.1007/s11910-016-0664-3[↩]
- Auger, R. R., Burgess, H. J., Emens, J. S., Deriy, L. V., Thomas, S. M., & Sharkey, K. M. (2015). Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 11(10), 1199–1236. https://doi.org/10.5664/jcsm.5100[↩][↩]
- Poeggeler B, Miravalle L, Zagorski MG, Wisniewski T, Chyan YJ, Zhang Y, Shao H, Bryant-Thomas T, Vidal R, Frangione B, Ghiso J, Pappolla MA. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry. 2001 Dec 11;40(49):14995-5001. doi: 10.1021/bi0114269[↩]
- Adams L.C., Böker S.M., Bender Y.Y., Diederichs G., Fallenberg E.M., Wagner M., Hamm B., Makowski M.R. Diagnostic accuracy of susceptibility-weighted magnetic resonance imaging for the evaluation of pineal gland calcification. PLoS ONE. 2017;12:e0172764. doi: 10.1371/journal.pone.0172764[↩]
- Yalcin A., Ceylan M., Bayraktutan O.F., Sonkaya A.R., Yuce I. Age and gender related prevalence of intracranial calcifications in CT imaging; data from 12,000 healthy subjects. J. Chem. Neuroanat. 2016;78:20–24. doi: 10.1016/j.jchemneu.2016.07.008[↩]
- Daghighi M.H., Rezaei V., Zarrintan S., Pourfathi H. Intracranial physiological calcifications in adults on computed tomography in Tabriz, Iran. Folia Morphol. 2007;66:115–119.[↩]
- Admassie D., Mekonnen A. Incidence of normal pineal and chroids plexus calcification on brain CT (computerized tomography) at Tikur Anbessa Teaching Hospital Addis Ababa, Ethiopia. Ethiop. Med. J. 2009;47:55–60.[↩]
- Fan K. J. (1983). Pineal calcification among black patients. Journal of the National Medical Association, 75(8), 765–769. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2561516/pdf/jnma00231-0037.pdf[↩]
- Quay W.B. Pineal Chemistry. Charles C. Thomas; Springfield, IL, USA: 1974. pp. 1–430.[↩]
- Macpherson P., Matheson M.S. Comparison of calcification of pineal, habenular commissure and choroid plexus on plain films and computed tomography. Neuroradiology. 1979;18:67–72. doi: 10.1007/BF00344824[↩]
- Diehl B.J. Occurrence and regional distribution of calcareous concretions in the rat pineal gland. Cell Tissue Res. 1978;195:359–366. doi: 10.1007/BF00236732[↩]
- Jung D., Vollrath L. Structural dissimilarities in different regions of the pineal gland of Pirbright white guinea-pigs. J. Neural Transm. 1982;54:117–128. doi: 10.1007/BF01249285[↩]
- Kalisinska, E., Bosiacka-Baranowska, I., Lanocha, N., Kosik-Bogacka, D., Krolaczyk, K., Wilk, A., Kavetska, K., Budis, H., Gutowska, I., & Chlubek, D. (2014). Fluoride concentrations in the pineal gland, brain and bone of goosander (Mergus merganser) and its prey in Odra River estuary in Poland. Environmental geochemistry and health, 36(6), 1063–1077. https://doi.org/10.1007/s10653-014-9615-6[↩]
- Tharnpanich T, et al. Association between high pineal fluoride content and pineal calcificatin in a low fluoride area. Fluoride. 2016;49(2):472–484.[↩]
- Luke J. Fluoride deposition in the aged human pineal gland. Caries Res. 2001 Mar-Apr;35(2):125-8. doi: 10.1159/000047443[↩]
- Kunz D, et al. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacol. 1999;21(6):765–772. doi: 10.1016/S0893-133X(99)00069-X[↩]
- Mahlberg R, et al. Degree of pineal calcification (DOC) is associated with polysomnographic sleep measures in primary insomnia patients. Sleep Med. 2009;10(4):439–445. doi: 10.1016/j.sleep.2008.05.003[↩]
- Kunz D, et al. On pineal calcification and its relation to subjective sleep perception: a hypothesis-driven pilot study. Psychiatry Res. 1998;82(3):187–191. doi: 10.1016/S0925-4927(98)00013-4[↩]
- Malin, A. J., Bose, S., Busgang, S. A., Gennings, C., Thorpy, M., Wright, R. O., Wright, R. J., & Arora, M. (2019). Fluoride exposure and sleep patterns among older adolescents in the United States: a cross-sectional study of NHANES 2015-2016. Environmental health : a global access science source, 18(1), 106. https://doi.org/10.1186/s12940-019-0546-7[↩][↩]
- Luke J. The effect of fluoride on the physiology of the pineal gland. University of Surrey: Guildford; 1997.[↩]
- Lukaszyk A, Reiter RJ. Morfologiczne podstawy dla sekrecji hormonów polipeptydowych w szyszynce małlpy (maccacus rhesus) [Morphological basis for polypeptide hormone secretion by the pineal body of macacus rhesus]. Endokrynol Pol. 1975 Nov-Dec;26(6):603-11. Polish.[↩]
- Reiter R.J., Welsh M.G., Vaughan M.K. Age-related changes in the intact and sympathetically denervated gerbil pineal gland. Am. J. Anat. 1976;146:427–432. doi: 10.1002/aja.1001460405[↩]
- Chau Y.P., Liao K.K., Kao M.H., Huang B.N., Kao Y.S., Lu K.S. Ultrastructure, ZIO-staining and chromaffinity of gerbil pinealocytes. Gaoxiong Yi Xue Ke Xue Za Zhi. 1994;10:613–623.[↩]
- Krstić R. A combined scanning and transmission electron microscopic study and electron probe microanalysis of human pineal acervuli. Cell Tissue Res. 1976;174:129–137. doi: 10.1007/BF00222155[↩]
- Krstić R. Pineal calcification: Its mechanism and significance. J. Neural Transm. Suppl. 1986;21:415–432.[↩]
- Tan D.-X., Manchester L.C., Reiter R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses. 2016;86:3–9. doi: 10.1016/j.mehy.2015.11.018[↩]
- Khan S.R., Pearle M.S., Robertson W.G., Gambaro G., Canales B.K., Doizi S., Traxer O., Tiselius H.-G. Kidney stones. Nat. Rev. Dis. Prim. 2016;2:16008. doi: 10.1038/nrdp.2016.8[↩]
- Bocchi G., Valdre G. Physical, chemical, and mineralogical characterization of carbonate-hydroxyapatite concretions of the human pineal gland. J. Inorg. Biochem. 1993;49:209–220. doi: 10.1016/0162-0134(93)80006-U[↩]
- Schmid H.A., Raykhtsaum G. Age-related differences in the structure of human pineal calcium deposits: Results of transmission electron microscopy and mineralographic microanalysis. J. Pineal Res. 1995;18:12–20. doi: 10.1111/j.1600-079X.1995.tb00134.x[↩][↩]
- Maria S., Samsonraj R.M., Munmun F., Glas J., Silvestros M., Kotlarczyk M.P., Rylands R., Dudakovic A., van Wijnen A.J., Enderby L.T., et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 2017 doi: 10.1111/jpi.12465[↩]
- Shimauchi Y., Yahata T., Matsubara S., Araki M. Role of tissue interaction between pineal primordium and neighboring tissues in avian pineal morphogenesis studied by intraocular transplantation. Dev. Genes Evol. 2002;212:319–329. doi: 10.1007/s00427-002-0236-1[↩]
- Hayano M., Sung J.H., Mastri A.R., Hill E.G. Striated muscle in the pineal gland of swine. J. Neuropathol. Exp. Neurol. 1976;35:613–621. doi: 10.1097/00005072-197611000-00003[↩]
- Diehl B.J. Occurrence and regional distribution of striated muscle fibers in the rat pineal gland. Cell Tissue Res. 1978;190:349–355. doi: 10.1007/BF00218180[↩][↩][↩]
- Maria S, Witt-Enderby PA. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res. 2014 Mar;56(2):115-25. doi: 10.1111/jpi.12116[↩]
- Sharan K., Lewis K., Furukawa T., Yadav V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal Res. 2017;63:e12423. doi: 10.1111/jpi.12423[↩]
- Przybylska-Gornowicz B., Lewczuk B., Prusik M., Bulc M. Pineal concretions in turkey (Meleagris gallopavo) as a result of collagen-mediated calcification. Histol. Histopathol. 2009;24:407–415.[↩]
- Fakhran S, Escott EJ. Pineocytoma mimicking a pineal cyst on imaging: true diagnostic dilemma or a case of incomplete imaging?. American Journal of Neuroradiology. 2008; 29:159-163. http://www.ncbi.nlm.nih.gov/pubmed/17925371.[↩]
- Fakhran S, Escott EJ. Pineocytoma mimicking a pineal cyst on imaging: true diagnostic dilemma or a case of incomplete imaging?. American Journal of Neuroradiology. 2008; 29:159-163. http://www.ncbi.nlm.nih.gov/pubmed/17925371[↩]
- Al-Holou WN, Maher CO, Muraszko KM, Garton HJL. The natural history of pineal cysts in children and young adults. J. Neurosurg. Pediatrics. February 2010; 5(2):162-166. https://www.ncbi.nlm.nih.gov/pubmed/20121364[↩]
- Moschovi M and Chrousos GP. Pineal gland masses. UpToDate. Waltham, MA: UpToDate; July 28, 2016; https://www.uptodate.com/contents/pineal-gland-masses[↩][↩]
- Mayo Clinic. Pineal Gland Cysts Are Common but Don’t Normally Cause Headaches. https://newsnetwork.mayoclinic.org/discussion/pineal-gland-cysts-are-common-but-dont-normally-cause-headaches/[↩]
- Mayo Foundation for Medical Education and Research. Pineoblastoma. http://www.mayoclinic.org/diseases-conditions/pineoblastoma/cdc-20339467[↩][↩][↩]
- Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev(2):CD001520, 2002. https://www.ncbi.nlm.nih.gov/pubmed/12076414[↩]
- Buscemi N, Vandermeer B, Pandya R, et al. Melatonin for Treatment of Sleep Disorders. AHRQ Publication No. 05-E002-2, 2004. https://archive.ahrq.gov/downloads/pub/evidence/pdf/melatonin/melatonin.pdf[↩]
- Edwards BJ, Atkinson G, Waterhouse J, et al. Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics 43(10):1501-1513, 2000. https://www.ncbi.nlm.nih.gov/pubmed/11083131[↩]
- Claustrat B, Brun J, David M, et al. Melatonin and jet lag: confirmatory result using a simplified protocol. Biol Psychiatry 3(8):705-711, 1992. https://www.ncbi.nlm.nih.gov/pubmed/1457626[↩]
- Petrie K, Dawson AG, Thompson L, et al. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol Psychiatry 33(7):526-530, 1993. https://www.ncbi.nlm.nih.gov/pubmed/8513037[↩]
- University of Maryland Medical Center. Melatonin. http://www.umm.edu/health/medical/altmed/supplement/melatonin[↩][↩][↩][↩][↩]
- Májovský M, Rezácová L, Sumová A, Pospíšilová L, Netuka D, Bradác O, Beneš V. Melatonin and cortisol secretion profile in patients with pineal cyst before and after pineal cyst resection. J Clin Neurosci. May, 2017; 39:155-163. https://www.ncbi.nlm.nih.gov/pubmed/28209308[↩][↩][↩]
- Mayo Clinic. Melatonin (N-acetyl-5-methoxytryptamine). http://www.mayoclinic.org/drugs-supplements/melatonin/background/hrb-20059770[↩]
- Di W.L., Kadva A., Johnston A., Silman R. Variable bioavailability of oral melatonin. N. Engl. J. Med. 1997;336(14):1028–1029. http://www.nejm.org/doi/10.1056/NEJM199704033361418[↩]
- Blask D.E., Dauchy R.T., Sauer L.A. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–188.[↩]
- Karbownik M., Lewinski A., Reiter R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. Int. J. Biochem. Cell Biol. 2001;33:735–753.[↩]
- Yaprak M., Altun A., Vardar A., Aktoz M., Ciftci S., Ozbay G. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int. J. Cardiol. 2003;89:103–107.[↩]
- Scheer F.A. Potential use of melatonin as adjunct antihypertensive therapy. 2005.[↩]
- Morera-Fumero A.L., Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int. J. Mol. Sci. 2013;14:9037–9050. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3676771/[↩][↩]
- Milhiet V., Etain B., Boudebesse C., Bellivier F. Circadian biomakers, circadian genes and bipolar disorders. J. Physiol. 2011;105:183–189.[↩]
- Thompson C., Mezey G., Corn T., Franey C., English J., Arendt J., Checkley S.A. The effect of desipramine upon melatonin and cortisol secretion in depressed and normal subjects. 1985.[↩]
- Den Boer J.A., Bosker F.J., Meesters Y. Clinical efficacy of agomelatine in depression: the evidence. 2006.[↩]
- Kotagal S., Broomall E. Sleep in children with autisms pectrum disorder. J. Pediatr. Neurol. 2012;47(4):242–251. doi: 10.1016/j.pediatrneurol.2012.05.007. [↩]
- Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910.[↩]
- Tordjman S., Najjar I., Bellissant E., Anderson G.M., Barburoth M., Cohen D., Nemat Jaafari N., Schischmanoff O., Fagard R., Lagdas E., Kermarrec S., Ribardiere R., Botbol M., Fougerou C., Bronsard G., Vernay-Leconte J. Advances in the research of melatonin in autism spectrum disorders: literature review and new perspectives. Int. J. Mol. Sci. 2013;14(10):20508–20542. doi: 10.3390/ijms141020508. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3821628/[↩][↩]
- Gould E.L., Loesch D.Z., Martin M.J., Hagerman R.J., Amstrong S.M., Huggins R.M. Melatonin profiles and sleep characteristics in boys with fragile X syndrome: a preliminary study. 2000.[↩]
- Tordjman S., Anderson G.M., Pichard N., Charbuy H., Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry. 2005;57:134–138. https://www.ncbi.nlm.nih.gov/pubmed/15652871[↩]
- Tordjman S., Anderson G.M., Bellissant E., Botbol M., Charbuy H., Camus F., Graignic R., Kermarrec S., Fougerou C., Cohen D., Touitou Y. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology. 2012;37:1996–1997. https://www.ncbi.nlm.nih.gov/pubmed/22613035[↩]
- Jockers R., Maurice P., Boutin J.A., Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br. J. Pharmacol. 2008;154(6):1182–1195 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2483381/[↩]
- Kilic E., Ozdemir Y.G., Bolay H., Kelestimur H., Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 1999;19:511–516. https://www.ncbi.nlm.nih.gov/pubmed/10326718[↩]
- Pei Z., Pang S.F., Cheung R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. http://stroke.ahajournals.org/content/34/3/770.long[↩]
- Lee E.J., Lee M.Y., Chen H.Y., Hsu Y.S., Wu T.S., Chen S.T., Chang G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005;38:42–52 https://www.ncbi.nlm.nih.gov/pubmed/15617536[↩]
- Lee M.Y., Kuan Y.H., Chen H.Y., Chen T.Y., Chen S.T., Huang C.C., Yang I.P., Hsu Y.S., Wu T.S., Lee E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. 2007 https://www.ncbi.nlm.nih.gov/pubmed/17349029[↩]
- Kondoh T., Uneyama H., Nishino H., Torii K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–590 https://www.ncbi.nlm.nih.gov/pubmed/12467899[↩]
- Chen T.Y., Lee M.Y., Chen H.Y., Kuo Y.L., Lin S.C., Wu T.S., Lee E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006;40:242–250. https://www.ncbi.nlm.nih.gov/pubmed/16499561[↩]
- Kilic E., Kilic U., Bacigaluppi M., Guo Z., Ben Abdallah N., Wolfer D.P., Reiter R.J., Hermann D.M., Bassetti C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008;45:142–148. https://www.ncbi.nlm.nih.gov/pubmed/18284547[↩]
- Biran V., Phan Duy A., Decobert F., Bednarek N., Alberti C., Baud O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev. Med. Child Neurol. 2014;56(8):717–723. https://www.ncbi.nlm.nih.gov/pubmed/24575840[↩][↩][↩][↩]
- Arpino C., Compagnone E., Montanaro M.L., Cacciatore D., De Luca A., Cerulli A., Di Girolamo S., Curatolo P. 2010.[↩]
- Machado J. L.C.; Passini, Júnior, R.; Rodrigues, M. R. I. Late prematurity: a systematic review. J. Pediatr. (Rio J.) 2014;90(3):221–231 https://www.ncbi.nlm.nih.gov/pubmed/24508009[↩]
- Allen M.C. Neurodevelopmental outcomes of preterm infants. 2008. https://www.ncbi.nlm.nih.gov/pubmed/18317268[↩]
- Vincer M.J., Allen A.C., Allen V.M., Baskett T.F., O’Connell C.M. Trends in the prevalence of cerebral palsy among very preterm infants. Paediatr. Child Health. 2014;19(4):185–189. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4028643/[↩]
- Pineda R.G., Neil J., Dierker D., Smyser C.D., Wallendorf M., Kidokoro H., Reynolds L.C., Walker S., Rogers C., Mathur A.M., Van Essen D.C., Inder T. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 2014;164(1):52–60.e2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3872171/[↩]
- Benders M.J., Kersbergen K.J., de Vries L.S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. 2014. https://www.ncbi.nlm.nih.gov/pubmed/24524447[↩]
- Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29(5):325–333. https://www.ncbi.nlm.nih.gov/pubmed/25180262[↩]
- Gertner S., Greenbaum C.W., Sadeh A., Dolfin Z., Sirota L., Ben-Nun Y. Sleep-wake patterns in preterm infants and 6 month’s home environment: implications for early cognitive development. 2002. https://www.ncbi.nlm.nih.gov/pubmed/12113995[↩]
- Meerlo P., Mistlberger R.E., Jacobs B.L., Heller H.C., McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 2009;13(3):187–194 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2771197/[↩]
- Polimeni G., Esposito E., Bevelacqua V., Guarneri C., Cuzzocrea S. Role of melatonin supplementation in neurodegenerative disorders. Front. Biosci. (Landmark Ed.) 2014;19:429–446. https://www.ncbi.nlm.nih.gov/pubmed/24389194[↩]
- Attele AS, Xie JT, Yuan CS. Treatment of insomnia: an alternative approach.Altern Med Rev. 2000;5(3):249-59.[↩]
- Rondanelli M, Opizzi A, Monteferrario F, Antoniello N, Manni R, Klersy C. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: a double-blind, placebo-controlled clinical trial. J Am Geriatr Soc. 2011;59(1):82-90. doi: 10.1111/j.1532-5415.2010.03232.x.[↩]
- Lyseng-Williamson KA. Melatonin prolonged release: in the treatment of insomnia in patients ages >55 years. Drugs Aging. 2012;29(11):911-23.[↩]
- Dominguez-Rodriguez A. Melatonin in cardiovascular disease. Expert Opin Investig Drugs. 2012;21(11):1593-6.[↩]
- Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br J Clin Pharmacol. 2000;49(5):423-7.[↩][↩]
- Nagtagaal JE, Laurant MW, Kerkhof GA, Smits MG, van der Meer YG, Coenen AM. Effects of melatonin on the quality of life in patients with delayed sleep phase syndrome. J Psychosom Res. 2000;48(1):45-50.[↩]
- Kotlarczyk MP, Lassila HC, O’Neil CK, et al. Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res. 2012;52(4):414-26.[↩]
- Barcelo E. melatonin and mammary cancer: a short review. Endocrine-Related Cancer. 2003;10:153-9.[↩]
- Jacobson JS, Workman SB, Kronenberg F. Research on complementary/alternative medicine for patients with breast cancer: a review of the biomedical literature. J Clin Onc. 2000;18(3):668-83.[↩]
- Schernhammer E, Hankinson S. Urinary melatonin levels and breast cancer risk. J Nat Canc Instit. 2005;97(14):1084-7.[↩]
- Eck-Enriquez K, Kiefer TL, Spriggs LL, Hill SM. Pathways through which a regimen of melatonin and retinoic acid induces apoptosis in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 2000;61(3):229-39.[↩]
- Low Dog T, Riley D, Carter T. Traditional and alternative therapies for breast cancer. Alt Ther. 2001;7(3):36-47.[↩]
- Barcelo E. Melatonin — estrogen interactions in breast cancer. J of Pineal Res. 2005;38:217-22.[↩]
- Cos S, Sanchez-Barcelo EJ. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histo Histopath. 2000;15:637-47.[↩]
- Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000;7(2):347-51.[↩]
- Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008 Sep;7(3):189-203. Review.[↩]
- Bendz LM, Scates AC. Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder. [Review]. Ann Pharmacother. 2010;44(1):185-91. Epub 2009 Dec 22.[↩]
- Rossignol DA, Frye RE. Melatonin in autism spectrum disorders. Curr Clin Pharmacol. 2014;9(4):326-34.[↩]
- Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53(9):783-92. doi: 10.1111/j.1469-8749.2011.03980.x.[↩]
- Hussain SA, Al-Khalifa II, Jasim NA, Gorial FI. Adjuvant use of melatonin for treatment of fibromyalgia. J Pineal Res. 2011;50(3):267-71. doi: 10.1111/j.1600-079X.2010.00836.x.[↩]
- Alstadhaug KB, Odeh F, Salvesen R, Bekkelund SI. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75(17):1527-32.[↩]
- Chang FY, Lu CL. Treatment of irritable bowel syndrome using complementary and alternative medicine. J Chin Med Assoc. 2009 Jun;72(6):294-300. Review.[↩]
- Bazil CW, Short D, Crispin D, Zheng W. Patients with intractable epilepsy have low melatonin, which increases following seizures. Neurology. 2000;55(11):1746-8.[↩]
- Motta E, Czuczwar SJ, Ostrowska Z, et al. Circadian profile of salivary melatonin secretion and its concentration after epileptic seizure in patients with drug-resistant epilepsy–preliminary report. Pharmacol Rep. 2014;66(3):492-8.[↩]
- Pignone AM, Rosso AD, Fiori G, et al. Melatonin is a safe and effective treatment for chronic pulmonary and extrapulmonary sarcoidosis. J Pineal Res. 2006 Sep;41(2):95-100.[↩]
- Reiter RJ, Tamura H, Tan DX, Xu XY. Melatonin and the circadian system: contributions to successful female reproduction. Fertil Steril. 2014;102(2):321-8.[↩]
- Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No.: CD001520. DOI: 10.1002/14651858.CD001520.[↩]
- Buscemi, N., Vandermeer, B., Hooton, N., Pandya, R., Tjosvold, L., Hartling, L., Vohra, S., Klassen, T. P., & Baker, G. (2006). Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ (Clinical research ed.), 332(7538), 385–393. https://doi.org/10.1136/bmj.38731.532766.F6[↩]
- Ray C. A. (2003). Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. The Journal of physiology, 551(Pt 3), 1043–1048. https://doi.org/10.1113/jphysiol.2003.043182[↩]
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008 Jun 11;299(22):2642-55. doi: 10.1001/jama.299.22.2642[↩]
- American Academy of Sleep Medicine Clinical Practice Guideline 2017. https://aasm.org/resources/clinicalguidelines/040515.pdf[↩]
- McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in Alzheimer’s disease. Cochrane Database Syst Rev. 2014 Mar 21;(3):CD009178. doi: 10.1002/14651858.CD009178.pub2. Update in: Cochrane Database Syst Rev. 2016 Nov 16;11:CD009178.[↩]
- Xu J, Wang LL, Dammer EB, Li CB, Xu G, Chen SD, Wang G. Melatonin for sleep disorders and cognition in dementia: a meta-analysis of randomized controlled trials. Am J Alzheimers Dis Other Demen. 2015 Aug;30(5):439-47. doi: 10.1177/1533317514568005[↩]
- Malhotra S, Sawhney G, Pandhi P. The Therapeutic Potential of Melatonin: A Review of the Science. Medscape General Medicine. 2004;6(2):46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1395802/[↩][↩]
- Barchas J, DaCosta F, Spector S. Acute pharmacology of melatonin. Nature. 1967; 214: 919-920. https://www.ncbi.nlm.nih.gov/pubmed/6054984[↩]
- Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. 1977; 45: 768-774 https://www.ncbi.nlm.nih.gov/pubmed/914981[↩]
- Wright J, Aldhous M, Franey C, English J, Arendt J. The effect of exogenous melatonin in endocrine function in man. Clin Endocrinol. 1986; 24: 375-382. https://www.ncbi.nlm.nih.gov/pubmed/3742833[↩]
- Puig-Domingo M, Webb SM, Serrano J, et al. Brief report: melatonin-related hypogonadotropic hypogonadism. N Engl J Med. 1992; 327: 1356-1359. http://www.nejm.org/doi/10.1056/NEJM199211053271905[↩]
- Gwayi N, Bernard RT. The effects of melatonin on sperm motility in vitro in Wistar rats. Andrologia. 2002; 34: 391-396 https://www.ncbi.nlm.nih.gov/pubmed/12472624[↩]
- Luboshitzky R, Shen-Orr Z, Nave R, Lavi S, Lavie P. Melatonin administration alters semen quality in healthy men. J Androl. 2002; 23: 572-578. https://www.ncbi.nlm.nih.gov/pubmed/12065466[↩]
- Silman RE. Melatonin: a contraceptive for the nineties. Eur J Obstet Gynecol Reprod Biol. 1993; 49: 3-9. https://www.ncbi.nlm.nih.gov/pubmed/8365512[↩][↩]
- Mattsson R, Hannsson I, Holmdahl R. Pineal gland in autoimmunity: melatonin-dependent exaggeration of collagen-induced arthritis in mice. Autoimmunity. 1994; 17: 83-86. https://www.ncbi.nlm.nih.gov/pubmed/8025216[↩]
- Sandyk R. Successful treatment of multiple sclerosis with magnetic fields. Int J Neurosci. 1992; 66: 237-250 https://www.ncbi.nlm.nih.gov/pubmed/1305621[↩]
- Blask DE, Cos S, Hill SM, Burns DM, Lemus-Wilson A, Grosso DS. Melatonin action on oncogenesis. In: Fraschini, F, Reiter, RJ, eds. Role of Melatonin and Pineal Peptides in Neuroimmunomodulation. New York, NY: Plenum; 1991:233-240.[↩]
- Beck-Friis J, Kjellman BF, Aperia B, et al. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand. 1985; 71: 319-330 https://www.ncbi.nlm.nih.gov/pubmed/4039876[↩]
- Rosenthal NE, James SP, Sack DA, et al. Seasonal affective disorder and phototherapy. N Y Acad Sci Ann. 1985; 453: 260-269. https://www.ncbi.nlm.nih.gov/pubmed/3865586[↩]
- Carman JS, Post RM, Buswell R, Goodwin FK. Negative effects of melatonin on depression. Am J Psychiatry. 1976; 133: 1181-1186 https://www.ncbi.nlm.nih.gov/pubmed/788529[↩]
- Wiechmann AF, O’Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Visual Sci. 1992; 33: 1894-1902. https://www.ncbi.nlm.nih.gov/pubmed/1582795[↩]
- Tailleux A, Torpier G, Bonnefont-Rousselot D, et al. Daily melatonin supplementation in mice increases atherosclerosis in proximal aorta. Biochem Biophys Res Commun. 2002; 293: 1114-1123. https://www.ncbi.nlm.nih.gov/pubmed/12051775[↩]