Hashimoto’s disease
Hashimoto’s disease also called Hashimoto’s thyroidits, chronic lymphocytic thyroiditis, autoimmune thyroiditis or chronic thyroiditis, is an autoimmune disease that causes chronic inflammation of your thyroid gland or thyroidits. The term “thyroiditis” refers to “inflammation of the thyroid gland”. Hashimoto’s thyroiditis or Hashimoto’s disease was first described by Hashimoto as Struma lymphomatosa in 1912 1. At present, Hashimoto’s disease or Hashimoto’s thyroidits is the most common type of autoimmune endocrine disorder and underactive thyroid (hypothyroidism) in the United States 2, 3, 4. Approximately 2% of the general population are affected by Hashimoto’s disease or Hashimoto’s thyroiditis with a high prevalence in middle-aged individuals and in women, but can be seen at any age, and can also affect men and children 5, 6, 7. Hashimoto’s disease also called Hashimoto’s thyroidits tends to run in families. Over time, the ability of the thyroid gland to produce thyroid hormones often becomes impaired and leads to a gradual decline in function and eventually hypothyroidism (an underactive thyroid).
Hashimoto’s thyroiditis is an autoimmune disorder in which antibodies attacks your thyroid gland, preventing it from producing enough thyroid hormones (tri-iodothyronine [T3] and thyroxine [T4]). Hashimoto’s thyroiditis is distinguished by the presence of autoantibodies against thyroid peroxidase (anti-TPO or antibody to thyroid peroxidase) and thyroglobulin (anti-Tg or thyroglobulin antibody) 8. Low thyroid hormone levels may cause hypothyroidism with a range of symptoms, such as tiredness or fatigue, weight gain, intolerance to cold temperatures, dry skin or dry thinning hair, slowed heart rate, heavy or irregular menstrual periods or fertility problems. Rarely, early in the course of the disease, thyroid gland damage may lead to the release of too much thyroid hormone into your blood, causing symptoms of hyperthyroidism called Hashitoxicosis 9, 10. Hashitoxicosis occurs as a result of the biochemical release of preformed thyroid hormone due to autoimmune destruction of the thyroid. Too much thyroid hormone (hyperthyroidism) can cause weight loss, despite an increased appetite. You might also feel anxious and find it difficult to relax. Hashitoxicosis precedes the signs and symptoms of the hypothyroid state associated with Hashimoto’s thyroiditis and lasts a few weeks to months 9.
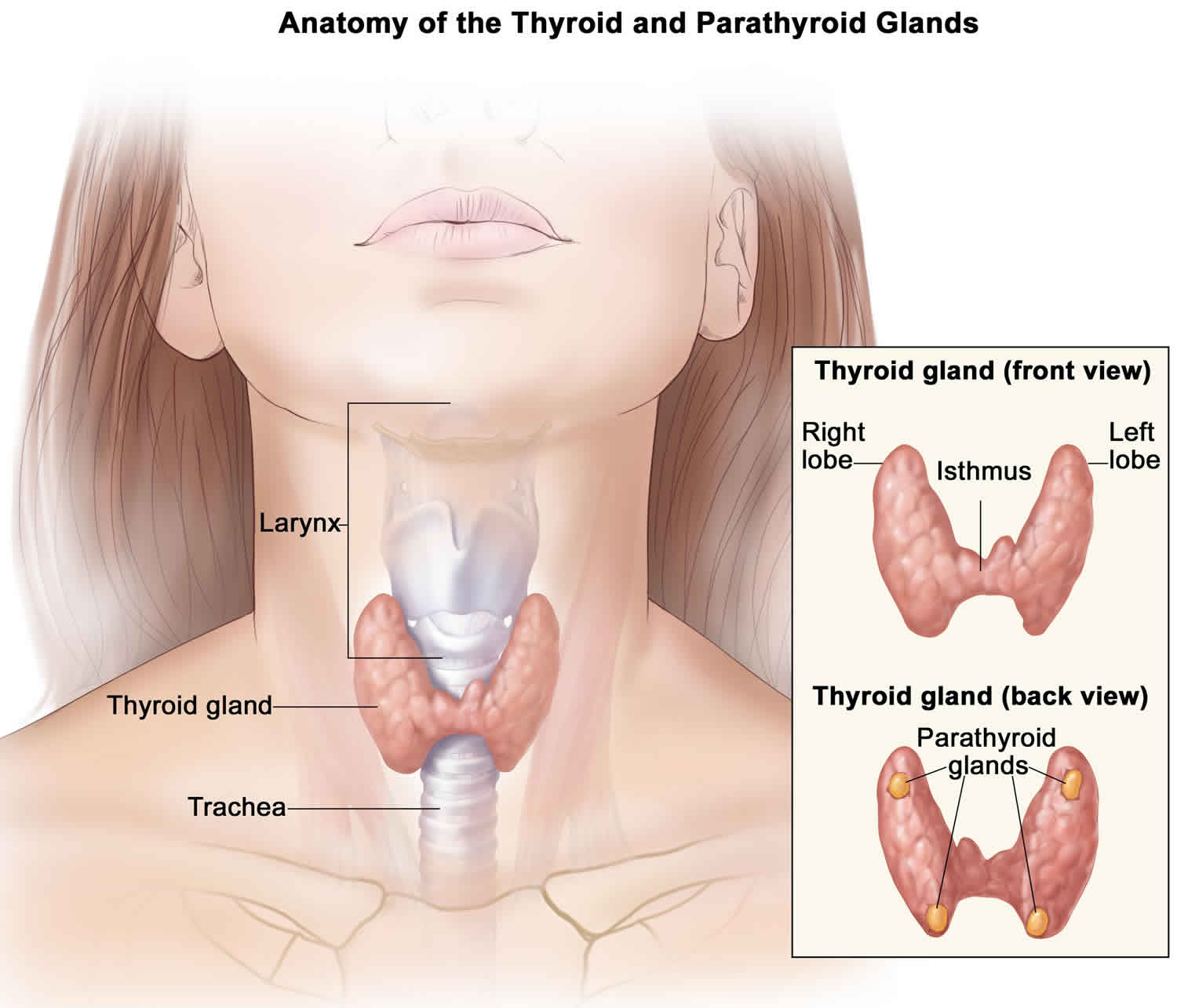
Your thyroid gland is a butterfly-shaped gland with 2 lobes (the right lobe and the left lobe — joined by a narrow piece of the thyroid gland called the isthmus) that is located in front of your neck near the base of your throat, beneath the larynx (voice box or Adam’s apple) (Figure 1). In most people, the thyroid gland cannot be seen or felt. Your thyroid gland produces thyroid hormones, tri-iodothyronine (T3) and thyroxine (T4) (the main hormones that your thyroid gland makes) and calcitonin. The thyroid hormones, T3 (tri-iodothyronine) and T4 (thyroxine) influence important body processes such as body temperature, energy levels, growth, your digestion, muscles and heart. Thyroid hormones are important for how your body uses energy, your metabolism, so thyroid hormones affect nearly every organ in your body even the way your heart beats. You might put on weight and feel very tired and lacking in energy if your thyroid gland doesn’t make enough T3 (tri-iodothyronine) and T4 (thyroxine).
In people with Hashimoto’s disease:
- the immune system makes antibodies that attack the thyroid gland (autoimmune disorder). Usually in Hashimoto’s disease, the immune system produces an antibody to thyroid peroxidase (TPO), a protein that plays an important part in thyroid hormone production. Most people with Hashimoto’s disease will have TPO (thyroid peroxidase) antibodies in their blood. Lab tests for other antibodies associated with Hashimoto’s disease may need to be done.
- large numbers of white blood cells, which are part of the immune system, build up in the thyroid gland
- the thyroid gland becomes damaged and can’t make enough thyroid hormones (hypothyroidism)
Many people who have Hashimoto’s disease have no symptoms at all. If the disease does enough damage to the thyroid, it can cause hypothyroidism (underactive thyroid disease). This is because the attack on the thyroid causes the gland to produce fewer hormones. Symptoms of Hashimoto’s disease include:
- Fatigue.
- Weight gain.
- Increased sensitivity to cold.
- Joint and muscle pain or stiffness.
- Pale, dry skin.
- Dry skin, thin hair and / or brittle nails.
- Puffy face.
- Hoarse voice.
- Constipation.
- Heavier than normal periods in women.
- Elevated cholesterol.
- Depression.
- Visibly enlarged thyroid.
- Forgetfulness or memory problems.
- Low sex drive (libido)
Hashimoto’s disease can also cause cognitive symptoms including:
- depression or low mood
- an inability to concentrate
- poor memory
In some cases, your thyroid gland may become noticeably larger (called a goiter) or it may shrink. Lumps or nodules may also develop in your thyroid gland.
Although Hashimoto’s disease can affect people of all ages, it’s most common in women in their 30s and 40s 11. The female-to-male ratio is at least 10:1 10. If someone in your family has had thyroid disease, you may have an increased risk for Hashimoto’s disease. No one is sure why people get Hashimoto’s disease.
Calcitonin is another hormone produced by the thyroid gland. Calcitonin helps to control the amount of calcium circulating in your blood. Calcitonin works with a hormone called parathyroid hormone (PTH) to do this. Parathyroid hormone is made by parathyroid glands. These sit behind and are attached to the thyroid gland (see Figure 1).
If you have symptoms of hypothyroidism, see your doctor. Your doctor will examine you and may run blood tests, including testing your thyroid hormone levels.
If left untreated, hypothyroidism can lead to problems including goiter (an increase in the size of the thyroid gland), heart problems or mental health problems. Occasionally, it can lead to a potentially life-threatening disorder called myxedema coma.
While there is no cure for Hashimoto’s disease, hypothyroidism can be treated. The primary treatment of Hashimoto’s disease is thyroid hormone replacement. Most people with Hashimoto’s disease take a synthetic thyroid hormone medication called levothyroxine (Levoxyl, Synthroid, others) to treat hypothyroidism. The synthetic thyroid hormone works like the T4 hormone naturally produced by the thyroid. Your hypothyroidism can be well-controlled with thyroid hormone medicine, as long as you take the medicine as instructed by your doctor and have regular follow-up blood tests.
If you have mild hypothyroidism, you may not need to have treatment but get regular thyroid stimulating hormone (TSH) tests to monitor thyroid hormone levels.
How common is Hashimoto’s disease?
The number of people who have Hashimoto’s disease in the United States is unknown. However, Hashimoto’s disease is the most common cause of hypothyroidism the United States, which affects about 5 in 100 Americans 12.
Hashimoto is also the most common cause of hypothyroidism in those areas of the world where iodine intake is adequate. The incidence is estimated to be 0.8 per 1000 per year in men and 3.5 per 1000 per year in women 10. The prevalence of thyroid disease, in general, increases with age.
How does eating, diet, and nutrition affect Hashimoto’s disease?
The thyroid gland uses iodine, a mineral in some foods, to make thyroid hormones. However, if you have Hashimoto’s disease or other types of autoimmune thyroid disorders, you may be sensitive to harmful side effects from iodine. Eating foods that have large amounts of iodine—such as kelp, dulse, or other kinds of seaweed, and certain iodine-rich medicines—may cause hypothyroidism or make it worse. Taking iodine supplements can have the same effect.
Talk with members of your health care team about:
- what foods and beverages to limit or avoid
- whether you take iodine supplements
- any cough syrups you take that may contain iodine
However, if you are pregnant, you need to take enough iodine because the baby gets iodine from your diet. Too much iodine can cause problems as well, such as a goiter in the baby. If you are pregnant, talk with your doctor about how much iodine you need.
Researchers are looking at other ways in which diet and supplements such as vitamin D and selenium may affect Hashimoto’s disease 13. However, no specific guidance is currently available 10.
How much iodine do I need?
The amount of iodine you need each day depends on your age. Average daily recommended amounts are listed below in micrograms (mcg).
Table 1 lists the current Recommended Dietary Allowances (RDA – the average daily level of intake sufficient to meet the nutrient requirements of nearly all [97%–98%] healthy individuals; often used to plan nutritionally adequate diets for individuals) for iodine 14. For infants from birth to 12 months, the Food and Nutrition Board at the Institute of Medicine of the National Academies established an Adequate Intake (AI) for iodine that is equivalent to the mean intake of iodine in healthy, breastfed infants in the United States.
The World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) recommend a slightly higher iodine intake for pregnant women of 250 mcg per day 15, 16.
Table 1. Recommended Dietary Allowances (RDAs) for Iodine
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months | 110 mcg* | 110 mcg* | ||
| 7–12 months | 130 mcg* | 130 mcg* | ||
| 1–3 years | 90 mcg | 90 mcg | ||
| 4–8 years | 90 mcg | 90 mcg | ||
| 9–13 years | 120 mcg | 120 mcg | ||
| 14–18 years | 150 mcg | 150 mcg | 220 mcg | 290 mcg |
| 19+ years | 150 mcg | 150 mcg | 220 mcg | 290 mcg |
Footnote: * Adequate Intake (AI)
What foods are good source for iodine?
Seaweed (such as kelp, nori, kombu, and wakame) is one of the best food sources of iodine 17. Other good sources include fish and other seafood, as well as eggs (see Table 2). Iodine is also present in human breast milk 14 and infant formulas 18. The U.S. Department of Agriculture (USDA) lists the iodine content of numerous foods and beverages 18.
Dairy products contain iodine. However, the amount of iodine in dairy products varies by whether the cows received iodine feed supplements and whether iodophor sanitizing agents were used to clean the cows and milk-processing equipment 19. For example, an analysis of 44 samples of nonfat milk found a range of 38 to 159 mcg per cup (with an average of 85 mcg/cup used for Table 2) 18. Plant-based beverages used as milk substitutes, such as soy and almond beverages, contain relatively small amounts of iodine.
Most commercially prepared bread contains very little iodine unless the manufacturer has used potassium iodate or calcium iodate as a dough conditioner 20. Manufacturers list dough conditioners as an ingredient on product labels but are not required to include iodine on the Nutrition Facts label 21, even though these conditioners provide a substantial amount of iodine. According to 2019 data from the USDA Branded Food Products Database, approximately 20% of ingredient labels for white bread, whole-wheat bread, hamburger buns, and hot dog buns listed iodate. Pasta is not a source of iodine unless it is prepared in water containing iodized salt because it absorbs some of the iodine 22.
Most fruits and vegetables are poor sources of iodine, and the amounts they contain are affected by the iodine content of the soil, fertilizer use, and irrigation practices 20. This variability affects the iodine content of meat and animal products because of its impact on the iodine content of foods that the animals consume 23. The iodine amounts in different seaweed species also vary greatly. For example, commercially available seaweeds in whole or sheet form have iodine concentrations ranging from 16 mcg/g to 2,984 mcg/g 24. For these reasons, the values for the foods listed in Table 2 are approximate but can be used as a guide for estimating iodine intakes.
Table 2. Iodine Content of Selected Foods
| Food | Micrograms (mcg) per serving | Percent DV* |
| Seaweed, nori, dried, 10 g | 232 | 155 |
| Bread, whole-wheat, made with iodate dough conditioner, 1 slice | 198 | 132 |
| Bread, white, enriched, made with iodate dough conditioner, 1 slice | 185 | 123 |
| Cod, baked, 3 ounces | 158 | 106 |
| Yogurt, Greek, plain, nonfat, 1 cup | 116 | 77 |
| Oysters, cooked, 3 ounces | 93 | 62 |
| Milk, nonfat, 1 cup | 85 | 57 |
| Iodized table salt, 1.5 g (approx. ¼ teaspoon) | 76 | 51 |
| Fish sticks, cooked, 3 ounces | 58 | 39 |
| Pasta, enriched, boiled in water with iodized salt, 1 cup | 36 | 24 |
| Egg, hard boiled, 1 large | 26 | 17 |
| Ice cream, chocolate, ½ cup | 21 | 14 |
| Liver, beef, cooked, 3 ounces | 14 | 9 |
| Cheese, cheddar, 1 ounce | 14 | 9 |
| Shrimp, cooked, 3 ounces | 13 | 9 |
| Tuna, canned in water, drained, 3 ounces | 7 | 5 |
| Soy beverage, 1 cup | 7 | 5 |
| Fruit cocktail in light syrup, canned, ½ cup | 6 | 4 |
| Beef, chuck, roasted, 3 ounces | 3 | 2 |
| Chicken breast, roasted, 3 ounces | 2 | 1 |
| Almond beverage, 1 cup | 2 | 1 |
| Apple juice, 1 cup | 1 | 1 |
| Bread, whole-wheat, made without iodate dough conditioner, 1 slice | 1 | 1 |
| Bread, white, enriched, made without iodate dough conditioner, 1 slice | 1 | 1 |
| Raisin bran cereal, 1 cup | 1 | 1 |
| Rice, brown, cooked, ½ cup | 1 | 1 |
| Corn, canned, ½ cup | 1 | 1 |
| Sea salt, non-iodized, 1.5 g (approx. ¼ teaspoon) | <1 | <1 |
| Broccoli, boiled, ½ cup | 0 | 0 |
| Banana, 1 medium | 0 | 0 |
| Lima beans, mature, boiled, ½ cup | 0 | 0 |
| Green peas, frozen, boiled, ½ cup | 0 | 0 |
| Pasta, enriched, boiled in water without iodized salt, 1 cup | 0 | 0 |
Footnotes: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for iodine is 150 mcg for adults and children aged 4 years and older. FDA does not require food labels to list iodine content unless iodine has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 18 ]Thyroid gland
The thyroid gland is the largest adult gland to have a purely endocrine function, weighing about 25-30 g. The thyroid gland is a small butterfly shaped gland with 2 lobes, the right lobe and the left lobe joined by a narrow piece of the thyroid gland called the isthmus, that is located in front of your neck near the base of your throat, beneath the larynx (voice box or Adam’s apple). About 50% of thyroid glands have a small third lobe, called the pyramidal lobe. It extends superiorly from the isthmus. The thyroid gland makes and releases hormones. You can’t usually feel a thyroid gland that is normal.
The thyroid gland has 2 main types of cells:
- Follicular cells use iodine from the blood to make thyroid hormones, which help regulate a person’s metabolism. Having too much thyroid hormone (hyperthyroidism) can cause a fast or irregular heartbeat, trouble sleeping, nervousness, hunger, weight loss, and a feeling of being too warm. Having too little thyroid hormone (hypothyroidism) causes a person to slow down, feel tired, and gain weight. The amount of thyroid hormone released by the thyroid gland is regulated by the pituitary gland at the base of the brain, which makes a substance called thyroid-stimulating hormone (TSH) (see Figure 5).
- C cells also called parafollicular cells at the periphery of the follicles that make calcitonin, a hormone that helps control how your body uses calcium. The parafollicular cells (C cells) respond to rising levels of blood calcium by secreting the hormone calcitonin. Calcitonin antagonizes (blocks) parathyroid hormone (PTH) and stimulates osteoblast activity, thus promoting calcium deposition and bone formation. It is important mainly in children, having relatively little effect in adults.
Other, less common cells in the thyroid gland include immune system cells (lymphocytes) and supportive (stromal) cells.
Thyroid hormone is secreted or inhibited in response to fluctuations in metabolic rate. The brain monitors the body’s metabolic rate and stimulates thyroid hormone secretion through the action of thyrotropin-releasing hormone (TRH) and thyroid stimulating hormone (TSH) as depicted in figure 5.
The primary effect of thyroid hormone (TH) is to increase one’s metabolic rate. As a result, it raises oxygen consumption and has a calorigenic effect—it increases heat production. To ensure an adequate blood and oxygen supply to meet this increased metabolic demand, thyroid hormone also raises the breathing (respiratory) rate, heart rate, and strength of the heartbeat. It stimulates the appetite and accelerates the breakdown of carbohydrates, fats, and protein for fuel. Thyroid hormone also promotes alertness and quicker reflexes; growth hormone secretion; growth of the bones, skin, hair, nails, and teeth; and development of the fetal nervous system.
Figure 1. Thyroid gland and parathyroid gland
Footnotes: Anatomy of the thyroid and parathyroid glands. The thyroid gland lies at the base of the throat near the trachea. It is shaped like a butterfly, with the right lobe and left lobe connected by a thin piece of tissue called the isthmus. The parathyroid glands are four pea-sized organs found in the neck near the thyroid. The thyroid and parathyroid glands make hormones.
Figure 2. Thyroid gland location
Figure 3. Thyroid gland anatomy
Footnote: (a) Gross anatomy, anterior view. (b) Histology, showing the saccular thyroid follicles (the source of thyroid hormone) and nests of C cells (the source of calcitonin).
What does the thyroid gland do?
Formation, storage, and release of thyroid hormones
The thyroid gland is the only endocrine gland that stores its secretory product in large quantities—normally about a 100-day supply. Synthesis and secretion of triiodothyronine (T3) and thyroxine or tetraiodothyronine (T4) occurs as follows:
- Iodide trapping. Thyroid follicular cells trap iodide ions (I −) by actively transporting them from the blood into the cytosol. As a result, the thyroid gland normally contains most of the iodide in the body.
- Synthesis of thyroglobulin. While the follicular cells are trapping I −, they are also synthesizing thyroglobulin (TGB), a large glycoprotein that is produced in the rough endoplasmic reticulum, modified in the Golgi complex, and packaged into secretory vesicles. The vesicles then undergo exocytosis, which releases thyroglobulin into the lumen of the follicle.
- Oxidation of iodide. Some of the amino acids in thyroglobulin are tyrosines that will become iodinated. However, negatively charged iodide (I −) ions cannot bind to tyrosine until they undergo oxidation (removal of electrons) to iodine: I −→ I. As the iodide ions are being oxidized, they pass through the membrane into the lumen of the follicle.
- Iodination of tyrosine. As iodine atoms (I) form, they react with tyrosines that are part of thyroglobulin molecules. Binding of one iodine atom yields monoiodotyrosine (T1), and a second iodination produces diiodotyrosine (T2). The thyroglobulin with attached iodine atoms, a sticky material that accumulates and is stored in the lumen of the thyroid follicle, is termed colloid.
- Coupling of monoiodotyrosine (T1) and diiodotyrosine (T2). During the last step in the synthesis of thyroid hormone, two diiodotyrosine (T2) molecules join to form thyroxine (T4) or one T1 and one T2 join to form triiodothyronine (T3).
- Pinocytosis and digestion of colloid. Droplets of colloid reenter follicular cells by pinocytosis and merge with lysosomes. Digestive enzymes in the lysosomes break down thyroglobulin, cleaving off molecules of triiodothyronine (T3) and thyroxine (T4).
- Secretion of thyroid hormones. Because T3 and T4 are lipid soluble, they diffuse through the plasma membrane into interstitial fluid and then into the blood. T4 normally is secreted in greater quantity than T3, but T3 is several times more potent. Moreover, after T4 enters a body cell, most of it is converted to T3 by removal of one iodine.
- Transport thyroid hormones in the blood. More than 99% of both the T3 and the T4 combine with transport proteins in the blood, mainly thyroxine binding globulin (TBG).
Figure 4. Thyroid hormones
Actions of thyroid hormones
Because most body cells have receptors for thyroid hormones, triiodothyronine (T3) and thyroxine (T4) affect tissues throughout the body. Thyroid hormones act on their target cells mainly by inducing gene transcription and protein synthesis. The newly formed proteins in turn carry out the cellular response.
Functions of thyroid hormones include the following:
- Increase basal metabolic rate. Thyroid hormones raise the basal metabolic rate (BMR), the rate of energy expenditure under standard or basal conditions (awake, at rest, and fasting). When basal metabolic rate increases, cellular metabolism of carbohydrates, lipids, and proteins increases. Thyroid hormones increase BMR in several ways: (1) They stimulate synthesis of additional Na+/K+ ATPases, which use large amounts of ATP to continually eject sodium ions (Na+) from cytosol into extracellular fluid and potassium ions (K+) from extracellular fluid into cytosol; (2) they increase the concentrations of enzymes involved in cellular respiration, which increases the breakdown of organic fuels and ATP production; and (3) they increase the number and activity of mitochondria in cells, which also increases ATP production. As cells produce and use more ATP, basal metabolic rate increases, more heat is given off and body temperature rises, a phenomenon called the calorigenic effect. In this way, thyroid hormones play an important role in the maintenance of normal body temperature. Normal mammals can survive in freezing temperatures, but those whose thyroid glands have been removed cannot.
- Enhance actions of catechlolamines. Thyroid hormones have permissive effects on the catecholamines (epinephrine and norepinephrine) because they up-regulate β-adrenergic receptors. Catecholamines bind to β-adrenergic receptors, promoting sympathetic responses. Therefore, symptoms of excess levels of thyroid hormone include increased heart rate, more forceful heartbeats, and increased blood pressure.
- Regulate development and growth of nervous tissue and bones. Thyroid hormones are necessary for the development of the nervous system: They promote synapse formation, myelin production, and growth of dendrites. Thyroid hormones are also required for growth of the skeletal system: They promote formation of ossification centers in developing bones, synthesis of many bone proteins, and secretion of growth hormone (GH) and insulin-like growth factors (IGFs). Deficiency of thyroid hormones during fetal development, infancy, or childhood causes severe mental retardation and stunted bone growth.
Control of thyroid hormone secretion
Thyrotropin-releasing hormone (TRH) from the hypothalamus and thyroid-stimulating hormone (TSH) from the anterior pituitary stimulate secretion of thyroid hormones, as shown in Figure 5:
- Low blood levels of T3 and T4 or low metabolic rate stimulate the hypothalamus to secrete thyrotropin-releasing hormone (TRH).
- Thyrotropin-releasing hormone (TRH) enters the hypothalamic–hypophyseal portal system and flows to the anterior pituitary, where it stimulates thyrotrophs to secrete thyroid stimulating hormone (TSH).
- Thyroid stimulating hormone (TSH) stimulates virtually all aspects of thyroid follicular cell activity, including iodide trapping, hormone synthesis and secretion, and growth of the follicular cells.
- The thyroid follicular cells release T3 and T4 into the blood until the metabolic rate returns to normal.
- An elevated level of T3 inhibits release of TRH and TSH (negative feedback inhibition).
Conditions that increase ATP demand—a cold environment, hypoglycemia, high altitude, and pregnancy—increase the secretion of the thyroid hormones.
Figure 5. Control of thyroid hormone secretion
Footnote: Negative Feedback Inhibition of the Anterior Pituitary Gland by the Thyroid Gland
Control of calcium balance
The hormone produced by the parafollicular cells of the thyroid gland is calcitonin. Calcitonin can decrease the level of calcium in the blood by inhibiting the action of osteoclasts, the cells that break down bone extracellular matrix. The secretion of calcitonin is controlled by a negative feedback system (see Figure 7).
Calcitonin is produced by C cells (clear cells) of the thyroid gland. It is secreted when the blood calcium concentration rises too high, and it lowers the concentration by two principal mechanisms:
- Osteoclast inhibition. Within 15 minutes after it is secreted, calcitonin reduces osteoclast activity by as much as 70%, so osteoclasts liberate less calcium from the skeleton.
- Osteoblast stimulation. Within an hour, calcitonin increases the number and activity of osteoblasts, which deposit calcium into the skeleton.
Calcitonin plays an important role in children but has only a weak effect in most adults. The osteoclasts of children are highly active in skeletal remodeling and release 5 g or more of calcium into the blood each day. By inhibiting this activity, calcitonin can significantly lower the blood calcium level in children. In adults, however, the osteoclasts release only about 0.8 g of calcium per day. Calcitonin cannot change adult blood calcium very much by suppressing this lesser contribution. Calcitonin deficiency is not known to cause any adult disease. Calcitonin may, however, inhibit bone loss in pregnant and lactating women. Miacalcin, a calcitonin extract derived from salmon that is 10 times more potent than human calcitonin, is prescribed to treat osteoporosis.
Figure 6. Hormonal control of calcium balance
Footnote: The central panel represents the blood reservoir of calcium and shows its normal (safe) range. Calcitriol and Parathyroid Hormone (PTH) regulate calcium exchanges between the blood and the small intestine and kidneys (left). Calcitonin, calcitriol, and Parathyroid Hormone (PTH) regulate calcium exchanges between blood and bone (right).
Hashimoto’s disease causes
The cause of Hashimoto’s disease or Hashimoto’s thyroidits is poorly understood 25. Hashimoto’s thyroiditis is an autoimmune disease that destroys thyroid cells by cell and antibody-mediated immune processes. Your immune system creates antibodies that attack thyroid cells as if they were bacteria, viruses or some other foreign body. Your immune system wrongly enlists disease-fighting agents that damage cells and lead to cell death. Most Hashimoto’s disease patients develop antibodies to a variety of thyroid antigens, the most common of which is anti-thyroid peroxidase (anti-TPO or antibody to thyroid peroxidase). Many also form antithyroglobulin (anti-Tg or thyroglobulin antibody) and TSH receptor-blocking antibodies (TBII) 25. These antibodies attack your thyroid tissue, eventually leading to inadequate production of thyroid hormone. There is a small subset of the population, no more than 10-15% with the clinically evident disease, that are serum antibody-negative. Positive anti-thyroid peroxidase (anti-TPO) antibodies point to the clinical syndrome 26, 27.
What causes your immune system to attack thyroid cells is not clear. Multiple factors from the external environment and the genetic background contribute to the pathogenesis of Hashimoto’s disease 28. These genetic, environmental, and existential factors provoke the immune system to produce antibodies to thyroid antigens 29, 30, 31, 32, 33, 34, 35, 36. The most important factors associated with Hashimoto’s thyroiditis are summarized in Table 3 below.
Hashimoto’s disease or Hashimoto’s thyroidits can also be part of the Polyglandular Autoimmune Syndrome type 2 with autoimmune adrenal deficiency and type-1 diabetes 37. Hashimoto thyroiditis is also related to several other autoimmune diseases such as pernicious anemia, adrenal insufficiency, and celiac disease. Ruggeri et al. 38 found that Hashimoto disease is associated with a variety of different non-thyroidal autoimmune diseases (NTADs) and diagnosis in adulthood made these even more prevalent.
The onset of Hashimoto’s disease may be related to 26, 27, 39, 40:
- Genetic factors. Twin studies have shown an increased concordance of autoimmune thyroiditis in monozygotic twins as compared with dizygotic twins. Danish studies have demonstrated concordance rates of 55% in monozygotic twins, compared with only 3% in dizygotic twins 41. This data suggests that 79% of predisposition is due to genetic factors, allotting 21% for environmental and sex hormone influences.
- Environmental triggers, such as infection, stress or radiation exposure
- Interactions between environmental and genetic factors.
Hypothyroidism can also be caused by:
- some medicines used to treat bipolar disorder or other mental health problems
- iodine-containing medicines used to treat abnormal heart rhythm
- exposure to toxins, such as nuclear radiation
- viruses, such as hepatitis C
Several genes have been involved in Hashimoto’s disease pathogenesis, including genes of the immune response (coded in the Human Leukocyte Antigen (HLA) complex) and thyroid function 28. Other immunoregulatory genes are involved in the development of Hashimoto’s disease, including the single nucleotide polymorphisms (SNPs) in cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), protein tyrosine phosphatase non-receptor type 22 (PTPN22), and CD40 42, 29, 34, 43.
Among the environmental factors are inadequate or excessive iodine intake, infections, or the intake of certain medications 29, 34, 33, 35, 44. Several of the currently used anticancer drugs, such as interferon-alpha, may cause autoimmune thyroid dysfunction 45, 36. The role of smoking and alcohol consumption in the etiopathogenesis of Hashimoto’s disease is still not clear 28. The data suggest that moderate alcohol consumption may protect against Hashimoto’s disease and the development of overt hypothyroidism 36, 46, 47. Furthermore, some studies indicate that smoking decreases the levels of thyroid autoantibodies and the risk of hypothyroidism. However, the mechanism for these protective effects of smoking and drinking remains unclear and must be clarified with future studies 36, 46, 47. In recent years, the influence of stress on the development and course of Hashimoto’s disease has also been investigated. Some studies suggest that stress is involved in the pathogenesis of Hashimoto’s disease, while other evidence indicates that it has no effect 36, 48. A randomized controlled trial by Markomanolaki et al. 49 showed that managing stress is also important in treating Hashimoto’s disease patients. After eight weeks of stress management intervention, patients demonstrated a reduction in antithyroglobulin (anti-Tg) titers, decreased levels of stress, depression, anxiety and improved lifestyle 49. Additionally, the adequate levels of vitamin D and selenium may help prevent or delay the onset of Hashimoto’s disease 33, 35, 50, 51. Moreover, the risk of Hashimoto’s disease is increased in other autoimmune diseases 52, 36.
Most Hashimoto’s disease patients develop antibodies to a variety of thyroid antigens, the most common of which is anti-thyroid peroxidase (anti-TPO). Many also form antithyroglobulin (anti-Tg) and TSH receptor-blocking antibodies (TBII) 10. These antibodies attack the thyroid tissue, eventually leading to inadequate production of thyroid hormone. There is a small subset of the population, no more than 10-15% with the clinically evident disease, that are serum antibody-negative 10.
Table 3. Genetic, environmental and existential factors associated with Hashimoto’s thyroiditis
| Genetic Factors | Environmental Factors | Existential Factors |
|---|---|---|
| Histocompatibility genes (HLA class I and II) | Iodine | Sex |
| Immunoregulatory genes (SNPs in HLA, CTLA-4, PTPN22, CD40 genes) | Medications (e.g., interferon-α, lithium, amiodarone) | Associated diseases (e.g., type 1 diabetes mellitus, pernicious anaemia, coeliac disease, myasthenia gravis) |
| Thyroid-specific genes | Infections (e.g., hepatitis C virus) | Age |
| Genes associated with thyroid peroxidase antibody synthesis | Smoking | Pregnancy |
| Selenium | Down’s syndrome | |
| Vitamin D | Microbiome composition | |
| Alcohol | Familial aggregation | |
| Radiation Exposure |
Risk factors for Hashimoto’s disease
The following factors are associated with an increased risk of Hashimoto’s disease 13:
- Sex. Women are much more likely to get Hashimoto’s disease.
- Age. Hashimoto’s disease can occur at any age but more commonly occurs during middle age.
- Other autoimmune disease. Having another autoimmune disease — such as rheumatoid arthritis, type 1 diabetes or lupus — increases your risk of developing Hashimoto’s disease.
- Genetics and family history. You’re at higher risk for Hashimoto’s disease if others in your family have thyroid disorders or other autoimmune diseases.
- Pregnancy. Typical changes in immune function during pregnancy may be a factor in Hashimoto’s disease that begins after pregnancy.
- Excessive iodine intake. Too much iodine in the diet may function as a trigger among people already at risk for Hashimoto’s disease.
- Radiation exposure. People exposed to excessive levels of environmental radiation are more prone to Hashimoto’s disease.
Hashimoto’s disease symptoms
Signs and symptoms of Hashimoto’s disease vary widely and are not specific to the disorder. Hashimoto’s disease progresses slowly over the years. Many people with Hashimoto’s disease may not notice signs or symptoms of the disease at first. An ordinary blood test may just show a thyroid hormone imbalance. Because the thyroid gland may grow and get larger, you may have a feeling of fullness or tightness in your throat, though it is usually not painful. You may have trouble swallowing food or liquids. You might have a swelling (a bump) in the front of your neck, the enlarged thyroid is called a goiter. After many years, or even decades, damage to the thyroid may cause the gland to shrink and the goiter to disappear.
Some people with Hashimoto’s disease have symptoms such as tiredness, forgetfulness, depression, coarse dry skin, slow heartbeat, weight gain, constipation and intolerance to cold. A blood test can tell if your thyroid gland is underactive. Other blood tests can be done to look for Hashimoto’s disease.
Eventually, the decline in thyroid hormone production can result in hypothyroidism with any of the following:
- Fatigue and sluggishness
- Increased sensitivity to cold
- Increased sleepiness
- Dry skin
- Constipation
- Muscle weakness
- Muscle aches, tenderness and stiffness
- Joint pain and stiffness
- Irregular or excessive menstrual bleeding
- Depression
- Problems with memory or concentration
- Swelling of the thyroid (goiter)
- A puffy face
- Brittle nails
- Hair loss
- Enlargement of the tongue
Because these symptoms could result from any number of disorders, it’s important to see your doctor as soon as possible for a timely and accurate diagnosis.
See your doctor if you develop these signs and symptoms:
- Tiredness for no apparent reason
- Dry skin
- Pale, puffy face
- Constipation
You’ll also need to see your doctor for periodic testing of your thyroid function if:
- You’ve had thyroid surgery
- You’ve had treatment with radioactive iodine or anti-thyroid medications
- You’ve had radiation therapy to your head, neck or upper chest
If you have high blood cholesterol, talk to your doctor about whether hypothyroidism may be a cause.
And if you’re receiving hormone therapy for hypothyroidism caused by Hashimoto’s thyroiditis, schedule follow-up visits as often as your doctor recommends. It’s important to make sure you’re receiving the correct dose of medicine. Over time, the dose you need to adequately replace your thyroid function may change.
Hashimoto’s disease complications
Thyroid hormones are essential for the healthy function of many body systems. Therefore, when Hashimoto’s disease and hypothyroidism are left untreated, many complications can occur. These include:
- Goiter. A goiter is enlargement of the thyroid. As thyroid hormone production declines due to Hashimoto’s disease, the thyroid receives signals from the pituitary gland to make more. This cycle may result in a goiter. It’s generally not uncomfortable, but a large goiter can affect your appearance and may interfere with swallowing or breathing.
- Heart problems. Hypothyroidism can result in poor heart function, an enlarged heart and irregular heartbeats. It can also result in high levels of low-density lipoprotein (LDL) cholesterol — the “bad” cholesterol — that is a risk factor for cardiovascular disease and heart failure.
- Peripheral neuropathy. Hypothyroidism that goes without treatment for a long time can damage the peripheral nerves. These are the nerves that carry information from the brain and spinal cord to the rest of the body. Peripheral neuropathy may cause pain, numbness and tingling in the arms and legs.
- Infertility. Low levels of thyroid hormone can interfere with ovulation, which can limit fertility. Some of the causes of hypothyroidism, such as autoimmune disorders, also can harm fertility.
- Mental health issues. Depression or other mental health disorders may occur early in Hashimoto’s disease and may become more severe over time.
- Sexual and reproductive dysfunction. In women, hypothyroidism can result in a reduced sexual desire (libido), an inability to ovulate, and irregular and excessive menstrual bleeding. Men with hypothyroidism may have a reduced libido, erectile dysfunction and a lowered sperm count.
- Poor pregnancy outcomes. Hypothyroidism during pregnancy may increase the risk of a miscarriage or preterm birth. Babies born to women with untreated hypothyroidism are at risk for decreased intellectual abilities, autism, speech delays and other developmental disorders.
- Birth defects. Babies born to people with untreated thyroid disease may have a higher risk of birth defects compared with babies born to mothers who do not have thyroid disease. Infants with hypothyroidism present at birth that goes untreated are at risk of serious physical and mental development problems. But if the condition is diagnosed within the first few months of life, the chances of typical development are excellent.
- Myxedema coma. This rare, life-threatening condition can develop due to long-term, severe, untreated hypothyroidism. Its signs and symptoms include drowsiness followed by profound lethargy and unconsciousness. A myxedema coma may be triggered by exposure to cold, sedatives, infection or other stress on your body. Myxedema requires immediate emergency medical treatment.
Hashimoto’s disease diagnosis
In general, your doctor may test for Hashimoto’s thyroiditis if you’re feeling increasingly tired or sluggish, have dry skin, constipation, and a hoarse voice, or have had previous thyroid problems or goiter.
Diagnosis of Hashimoto’s thyroiditis is based on your signs and symptoms and the results of blood tests that measure levels of thyroid hormone and thyroid-stimulating hormone (TSH) produced in the pituitary gland. These may include:
- A hormone test. Blood tests can determine the amount of hormones produced by your thyroid and pituitary glands. If your thyroid is underactive, the level of thyroid hormone is low. At the same time, the level of TSH is elevated because your pituitary gland tries to stimulate your thyroid gland to produce more thyroid hormone.
- An antibody test. Because Hashimoto’s thyroiditis is an autoimmune disorder, the cause involves production of abnormal antibodies. A blood test may confirm the presence of antibodies against thyroid peroxidase (TPO antibodies), an enzyme normally found in the thyroid gland that plays an important role in the production of thyroid hormones.
In the past, doctors weren’t able to detect an underactive thyroid (hypothyroidism), the main indicator of Hashimoto’s thyroiditis, until symptoms were fairly advanced. But by using the sensitive TSH test, doctors can diagnose thyroid disorders much earlier, often before you experience symptoms.
Because the TSH test is the best screening test, your doctor will likely check TSH first and follow with a thyroid hormone test if needed. TSH tests also play an important role in managing hypothyroidism. These tests also help your doctor determine the right dosage of medication, both initially and over time.
Testing thyroid function
To determine if hypothyroidism is the cause of your symptoms, your doctor will order blood tests that may include the following:
- Thyroid stimulating hormone (TSH) test. Thyroid stimulating hormone (TSH) is produced by the pituitary gland. When the pituitary detects low thyroid hormones in the blood, it sends TSH to the thyroid to prompt an increase in thyroid hormone production. High TSH levels in the blood indicates hypothyroidism.
- Thyroxine (T4) tests. The main thyroid hormone is thyroxine (T4). A low blood level of T4 confirms the findings of a TSH (thyroid stimulating hormone) test and indicates the problem is within the thyroid itself.
Antibody tests
More than one disease process can lead to hypothyroidism. To determine if Hashimoto’s disease is the cause of hypothyroidism, your doctor will order an antibody test.
The intended purpose of an antibody is to flag disease-causing foreign agents that need to be destroyed by other actors in the immune system. In an autoimmune disorder, the immune system produces rogue antibodies that target healthy cells or proteins in the body.
Usually in Hashimoto’s disease, the immune system produces an antibody to thyroid peroxidase (anti-TPO), a protein that plays an important part in thyroid hormone production. Most people with Hashimoto’s disease will have TPO antibodies (anti-TPO) in their blood. Lab tests for other antibodies associated with Hashimoto’s disease may also need to be done.
Thyroglobulin antibodies (anti-Tg) can also be a sign of Hashimoto disease. Most people with Hashimoto disease have high levels of both thyroglobulin antibodies (anti-Tg) and TPO antibodies (anti-TPO).
Circulating antibody to thyroid peroxidase (anti-TPO) are found in about 90% of Hashimoto’s disease patients. Anti-thyroglobulin antibodies (anti-Tg) are less sensitive (positive in about 60–80% of patients) and less specific than antibody to thyroid peroxidase (anti-TPO) 53, 29, 54.
You probably won’t need other tests to confirm you have Hashimoto’s disease. However, if your doctor suspects Hashimoto’s disease but you don’t have antithyroid antibodies in your blood, you may have an ultrasound of your thyroid. The ultrasound images can show the size of your thyroid and other features of Hashimoto’s disease. The ultrasound also can rule out other causes of an enlarged thyroid, such as thyroid nodules—small lumps in the thyroid gland.
Hashimoto’s disease treatment
How your doctors treat Hashimoto’s disease usually depends on whether your thyroid is damaged enough to cause hypothyroidism. If you don’t have hypothyroidism or you have mild hypothyroidism, your doctor may choose to simply check your symptoms and do regular thyroid stimulating hormone (TSH) tests to monitor your thyroid hormone levels.
Most people with Hashimoto’s disease need take a synthetic thyroid hormone medication called levothyroxine (Levoxyl, Synthroid, others) to treat hypothyroidism. The synthetic thyroid hormone works like the thyroxine (T4) hormone naturally produced by your thyroid. Prescribed in pill form for many years, this medicine is now also available as a liquid and in a soft gel capsule 13. These newer formulas may be helpful to people with digestive problems that affect how the thyroid hormone pill is absorbed.
Some foods and supplements can affect how well your body absorbs levothyroxine. Examples include grapefruit juice, espresso coffee, soy, and multivitamins that contain iron or calcium 12, 55. Taking levothyroxine on an empty stomach can prevent this from happening. Your doctor may ask you to take the levothyroxine in the morning, 30 to 60 minutes before you eat your first meal.
Your doctor will give you a blood test about 6 to 8 weeks after you begin taking levothyroxine and adjust your dose if needed. Each time you change your dose, you’ll have another blood test. Once you’ve reached a dose that’s working for you, your doctor will likely repeat the blood test in 6 months and then once a year.
Never stop taking your levothyroxine or take a higher dose without talking with your doctor first. Taking too much thyroid hormone medicine can cause serious problems, such as atrial fibrillation or osteoporosis 39.
Thyroxine (T4) hormone replacement therapy
Hypothyroidism associated with Hashimoto’s disease is treated with a synthetic hormone called levothyroxine (Levoxyl, Synthroid, others). The recommended dose of levothyroxine is 1.6 to 1.8 mcg/kg/day 10. The synthetic hormone works like the thyroxine (T4) hormone naturally produced by the thyroid. The treatment goal is to restore and maintain adequate thyroxine (T4) hormone levels and improve symptoms of hypothyroidism. You will need this treatment for the rest of your life.
Monitoring the dosage
Your doctor will determine a dosage of levothyroxine that’s appropriate for your age, weight, current thyroid production, other medical conditions and other factors. Your doctor will retest your TSH (thyroid stimulating hormone) levels about 6 to 10 weeks later and adjust the dosage as necessary.
Once the best dosage is determined, you will continue to take the medication once a day. You’ll need follow-up tests once a year to monitor TSH (thyroid stimulating hormone) levels or any time after your doctor changes your dosage.
A levothyroxine pill is usually taken in the morning before you eat. Talk to your doctor if you have any questions about when or how to take the pill. Also, ask what to do if you accidentally skip a dose. If your health insurance requires you to switch to a generic drug or a different brand, talk to your doctor.
Precautions
Because levothyroxine acts like natural thyroxine (T4) in your body, there are generally no side effects as long as the treatment is resulting in “natural” levels of thyroxine (T4) for your body.
Too much thyroid hormone can worsen bone loss that causes weak, brittle bones (osteoporosis) or cause irregular heartbeats (arrhythmias) the most common being atrial fibrillation.
Effects of other substances
Certain medications, supplements and foods may affect your ability to absorb levothyroxine. It may be necessary to take levothyroxine at least four hours before these substances. Talk to your doctor about any of the following:
- Soy products
- High-fiber foods
- Iron supplements, including multivitamins that contain iron
- Cholestyramine (Prevalite), a medication used to lower blood cholesterol levels
- Aluminum hydroxide, which is found in some antacids
- Sucralfate, an ulcer medication
- Calcium supplements
Triiodothyronine (T3) hormone replacement therapy
Naturally produced thyroxine (T4) is converted into another thyroid hormone called triiodothyronine (T3). The thyroxine (T4) replacement hormone is also converted into triiodothyronine (T3), and for most people the thyroxine (T4) replacement therapy results in an adequate supply of triiodothyronine (T3) for the body.
For people who need better symptom control, a doctor also may prescribe a synthetic triiodothyronine (T3) (Cytomel) or a synthetic T4 and T3 combination. Side effects of triiodothyronine (T3) hormone replacement include rapid heartbeat, insomnia and anxiety. These treatments may be tested with a trial period of 3 to 6 months.
Is a combination of hormones needed?
Levothyroxine is the synthetic form of the natural thyroxine (T4). Thyroxine (T4) is converted into Triiodothyronine (T3) in the body. While most people are treated successfully with levothyroxine alone, some people don’t feel completely normal on levothyroxine.
Researchers have investigated whether adjusting standard hypothyroidism treatment to replace some thyroxine (T4) with small amounts of triiodothyronine (T3) may offer benefit. But, the majority of studies have determined that the addition of triiodothyronine (T3) does not offer any advantage over treatment with thyroxine (T4) alone.
There is some evidence that triiodothyronine (T3) may offer benefit to certain subsets of people, such as people who have had their thyroid surgically removed (thyroidectomy). Research is ongoing.
Triiodothyronine (T3) can be given alone as liothyronine (Cytomel) or in combination with thyroxine (T4) as liotrix (Thyrolar). Taking a combination T4 and T3 ends up producing higher than normal levels of triiodothyronine (T3), especially soon after the medication is taken. This can cause a fast heart rate, anxiety and trouble sleeping.
But, for those who haven’t gotten enough relief from thyroxine (T4) alone, adding Cytomel to standard levothyroxine treatment for a three- to six-month trial is a long enough period to see if the combination helps you.
Alternative medicine
Products with triiodothyronine (T3) and thyroxine (T4) hormones derived from pigs or other animals are available as prescriptions or as dietary supplements, such as Armour Thyroid, in the United States. Concerns about these products include the following:
- The balance of thyroxine (T4) and triiodothyronine (T3) in animals isn’t the same as in humans.
- The exact amount of thyroxine (T4) and triiodothyronine (T3) in each batch of a natural extract product can vary, leading to unpredictable levels of these hormones in your blood.
Anti-inflammatory diet
An anti-inflammatory diet rich in vitamins, minerals and polyphenols is recommended as diet therapy for Hashimoto’s disease 56, 44, 57. The theory behind the inflammation has to do with the leaky gut syndrome, where there is an insult to the gut mucosa, which allows the penetrance of proteins that do not typically enter the bloodstream via transporters in the gut mucosa. It is theorized that a response similar to molecular mimicry occurs, and antibodies are produced against the antigens. Unfortunately, the antigen may be very structurally similar to thyroid peroxidase, leading to antibody formation against this enzyme. The concept of an autoimmune diet is based on healing the gut and decreasing the severity of the autoimmune response.
Natural antioxidants like vitamin A, vitamin C and vitamin E are found in products of plant origin, including a wide variety of vegetables and fruits. Sources of vitamin C include broccoli, peppers, black currant, strawberries, lemons, spinach, kiwifruit, oranges, grapefruit, limes, tomatoes, raspberries, asparagus, pineapples, fennel and parsley. The best source of vitamin E is avocado, nuts, seeds, egg, milk and whole grains. In addition, vitamin A is present in foods such as liver, carrot, broccoli, butter, pumpkin, cheese, egg, mango and milk 58. According to the current findings, the Mediterranean diet may show the most benefits for Hashimoto’s disease patients with its antioxidant properties 59.
One study by Ostrowska et al. 60 assessed the effectiveness of two “reducing diets” and their effect on thyroid parameters in female obese patients with Hashimoto’s disease. All women who received levothyroxine, selenium and zinc were randomly assigned to the study group following individually balanced elimination/reducing diets, in accordance with the previously performer food sensitivity tests, and the control group following reducing diets with the same caloric content, but without product elimination. The anthropometric and thyroid parameters have changed in both groups during the nutritional intervention. This research showed that weight reduction may improve thyroid function in patients suffering from obesity and Hashimoto’s disease 60. Moreover, an individually selected elimination reducing diet was more effective than classic reducing diets with the same energy intake and macronutrient content and can lead to better therapeutic outcomes, which may cause an anti-inflammatory effect 60.
One case report 61 showed a novel approach that led to the improvement of symptoms and a reduction of thyroid antibodies in a 23-year-old woman with Hashimoto’s disease. The woman presented with symptoms of fatigue, hair loss, energy and mood disturbance, problems with insomnia and daytime napping. The thyroid antibodies were strongly positive, with a normal TSH level. Integrative treatment was started, which involved nutritional changes and micronutrient supplementation 61. This supplementation supported the methylation cycle, anti-oxidant capacity and stress management, and included vitamin C, vitamin B1, vitamin B2, vitamin B5, vitamin B6, Pyridoxal-5 Phosphate, zinc picolonate, L-5 methyltetrahydrofolate, magnesium glycinate, selenomethionine, N- Acetyl Cysteine and methylcobalamin (vitamin B12). The patient followed a paleo-style diet without grains and dairy products and increased consumption of bone broth and fermented foods as well as organic animal protein as tolerated. In addition, daily meditation and mindfulness techniques were recommended, and gentle exercise three times a week was added. After 15 months of treatment, there was a reduction in antithyroid antibodies and a significant relief of symptoms. This case demonstrated the potential benefits of an integrative approach to autoimmunity and oxidative stress in Hashimoto’s disease 61.
In a pilot study by Abbott et al. 62, women participated in a 10-week online health coaching program focused on implementing an “autoimmune protocol diet”. They applied a modified paleolithic diet. In the referred study, there were no significant changes in thyroid function markers, as well as serum antithyroid antibody concentrations, although the number of immune cells and an inflammatory processes marker (high sensitivity CRP) were decreased. These results suggest that an “autoimmune protocol” may decrease inflammation and modulate the immune system. Moreover, the therapy improves health-related quality of life (measured by 36-Item Short-Form Health Survey) and reduces symptoms of the diseases (measured by the Medical Symptoms Questionnaire) 62. A case study with a 49-year-old obese Hashimoto’s disease woman indicated that a modified autoimmune paleo low-calorie diet might improve TSH, anti-TPO antibody, body composition and lipid profile 63.
- KINNEY FJ, HERRMANN RE. Increasing occurrence of thyroiditis in the Rocky Mountain area. Rocky Mt Med J. 1962 Aug;59:35-7 passim.[↩]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412[↩]
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252–265. doi: 10.1007/s12020-012-9703-2[↩]
- Delemer B, Aubert JP, Nys P, Landron F, Bouée S. An observational study of the initial management of hypothyroidism in France: The ORCHIDÉE study. Eur J Endocrinol. 2012;167:817–823. doi: 10.1530/EJE-11-1041[↩]
- Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291[↩]
- Chistiakov DA. Immunogenetics of Hashimoto’s thyroiditis. J Autoimmune Dis. 2005;2:1. doi: 10.1186/1740-2557-2-1[↩]
- Ekambaram M, Kumar B, Chowdhary N, Siddaraju N, Kumar S. Significance of eosinophils in diagnosing Hashimoto’s thyroiditis on fine-needle aspiration cytology. Indian J Pathol Microbiol. 2010 Jul-Sep;53(3):476-9. doi: 10.4103/0377-4929.68282[↩]
- Vargas-Uricoechea H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells. 2023 Mar 16;12(6):918. doi: 10.3390/cells12060918[↩]
- Unnikrishnan, A. G.. Hashitoxicosis: A clinical perspective. Thyroid Research and Practice 10(Suppl 1):p S5-S6, February 2013. DOI: 10.4103/0973-0354.106803[↩][↩]
- Mincer DL, Jialal I. Hashimoto Thyroiditis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459262[↩][↩][↩][↩][↩][↩][↩]
- Hashimoto’s Disease: What It Is and How It’s Treated. https://www.aafp.org/pubs/afp/issues/2000/0215/p1054.html[↩]
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012 Nov-Dec;18(6):988-1028. doi: 10.4158/EP12280.GL. Erratum in: Endocr Pract. 2013 Jan-Feb;19(1):175.[↩][↩]
- Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, Churilov LP, Ferrari SM, Antonelli A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019 Dec;33(6):101367. doi: 10.1016/j.beem.2019.101367[↩][↩][↩]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001. https://www.nap.edu/read/10026/chapter/2[↩][↩]
- World Health Organization. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva, Switzerland: WHO, 2007. http://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf;jsessionid=2E9F56538AEFD33C83934FB34BD4E8C4[↩]
- WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007 Dec;10(12A):1606-11. doi: 10.1017/S1368980007361004. Erratum in: Public Health Nutr. 2008 Mar;11(3):327.[↩]
- Zimmermann MB. Iodine deficiency. Endocr Rev. 2009 Jun;30(4):376-408. doi: 10.1210/er.2009-0011[↩]
- USDA, FDA, and ODS-NIH Database for the Iodine Content of Common Foods Release 1.0. 2020. https://www.ars.usda.gov/ARSUSERFILES/80400535/DATA/IODINE/IODINE_DATABASE_PDFVersion_2020.PDF[↩][↩][↩][↩]
- Pennington JA, Young B. Iron, zinc, copper, manganese, selenium, and iodine in foods from the United States Total Diet Studyexternal link disclaimer. J Food Compost Anal. 1990 June;3(2):166-184. https://www.sciencedirect.com/science/article/abs/pii/088915759090022E[↩]
- Ershow AG, Skeaff SA, Merkel JM, Pehrsson PR. Development of Databases on Iodine in Foods and Dietary Supplements. Nutrients. 2018 Jan 17;10(1):100. doi: 10.3390/nu10010100[↩][↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels[↩]
- Patterson KY, Spungen JH, Roseland JM, Pehrsson PR, Ershow AG, Gahche JJ. USDA-FDA-ODS database for the iodine content of common foods (release one). Iodine database PDF. Methods and Application of Food Composition Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville MD. July 2020. https://www.ars.usda.gov/ARSUSERFILES/80400535/DATA/IODINE/IODINE_DATABASE.PDF[↩]
- Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of Core Foods of the U.S. Food Supply, 1982-1991. III. Copper, Manganese, Selenium, and Iodine. J Food Comp Anal. 1995;8(2):171-217. https://www.sciencedirect.com/science/article/abs/pii/S0889157585710149[↩]
- Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004 Oct;14(10):836-41. doi: 10.1089/thy.2004.14.836[↩]
- Mincer DL, Jialal I. Hashimoto Thyroiditis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459262[↩][↩]
- Leung AKC, Leung AAC. Evaluation and management of the child with hypothyroidism. World J Pediatr. 2019 Apr;15(2):124-134. doi: 10.1007/s12519-019-00230-w[↩][↩]
- Yuan J, Sun C, Jiang S, Lu Y, Zhang Y, Gao XH, Wu Y, Chen HD. The Prevalence of Thyroid Disorders in Patients With Vitiligo: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2019 Jan 15;9:803. doi: 10.3389/fendo.2018.00803[↩][↩]
- Mikulska AA, Karaźniewicz-Łada M, Filipowicz D, Ruchała M, Główka FK. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management-An Overview. Int J Mol Sci. 2022 Jun 13;23(12):6580. doi: 10.3390/ijms23126580[↩][↩][↩][↩]
- Ragusa F., Fallahi P., Elia G., Gonnella D., Paparo S.R., Giusti C., Churilov L.P., Ferrari S.M., Antonelli A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33:101367. doi: 10.1016/j.beem.2019.101367[↩][↩][↩][↩]
- Shukla S.K., Singh G., Ahmad S., Pant P. Infections, Genetic and Environmental Factors in Pathogenesis of Autoimmune Thyroid Diseases. Microb. Pathog. 2018;116:279–288. doi: 10.1016/j.micpath.2018.01.004[↩]
- Weetman A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Invest. 2021;44:883–890. doi: 10.1007/s40618-020-01477-1[↩]
- Ferrari S.M., Fallahi P., Antonelli A., Benvenga S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017;8:50. doi: 10.3389/fendo.2017.00050[↩]
- Wiersinga W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. 2016;31:213–222. doi: 10.3803/EnM.2016.31.2.213[↩][↩][↩]
- Ralli M., Angeletti D., Fiore M., D’Aguanno V., Lambiase A., Artico M., de Vincentiis M., Greco A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020;19:102649. doi: 10.1016/j.autrev.2020.102649[↩][↩][↩]
- Effraimidis G., Wiersinga W.M. Mechanisms in Endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur. J. Endocrinol. 2014;170:R241–R252. doi: 10.1530/EJE-14-0047[↩][↩][↩]
- Ajjan R.A., Weetman A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in Our Understanding. Horm. Metab. Res. 2015;47:702–710. doi: 10.1055/s-0035-1548832[↩][↩][↩][↩][↩][↩]
- Singh G, Jialal I. Polyglandular Autoimmune Syndrome Type II. [Updated 2023 Jan 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525992[↩]
- R M Ruggeri, F Trimarchi, G Giuffrida, R Certo, E Cama, A Campennì, A Alibrandi, F De Luca, M Wasniewska, Autoimmune comorbidities in Hashimoto’s thyroiditis: different patterns of association in adulthood and childhood/adolescence, European Journal of Endocrinology, Volume 176, Issue 2, Feb 2017, Pages 133–141, https://doi.org/10.1530/EJE-16-0737[↩]
- Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017 Sep 23;390(10101):1550-1562. doi: 10.1016/S0140-6736(17)30703-1[↩][↩]
- Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, Hermann M. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011 Feb;21(2):161-7. doi: 10.1089/thy.2010.0191. Epub 2010 Dec 27. Erratum in: Thyroid. 2011 Apr;21(4):467.[↩]
- Brix TH, Hegedüs L, Gardas A, Banga JP, Nielsen CH. Monozygotic twin pairs discordant for Hashimoto’s thyroiditis share a high proportion of thyroid peroxidase autoantibodies to the immunodominant region A. Further evidence for genetic transmission of epitopic “fingerprints”. Autoimmunity. 2011 May;44(3):188-94. doi: 10.3109/08916934.2010.518575[↩]
- Klubo-Gwiezdzinska J., Wartofsky L. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022;132:16222. doi: 10.20452/pamw.16222[↩]
- Kust D., Matesa N. The Impact of Familial Predisposition on the Development of Hashimoto’s Thyroiditis. Acta Clin. Belg. 2020;75:104–108. doi: 10.1080/17843286.2018.1555115[↩]
- Ihnatowicz P., Drywień M., Wątor P., Wojsiat J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020;27:184–193. doi: 10.26444/aaem/112331[↩][↩]
- Torino F., Barnabei A., Paragliola R., Baldelli R., Appetecchia M., Corsello S.M. Thyroid Dysfunction as an Unintended Side Effect of Anticancer Drugs. Thyroid. 2013;23:1345–1366. doi: 10.1089/thy.2013.0241[↩]
- Carlé A., Pedersen I.B., Knudsen N., Perrild H., Ovesen L., Rasmussen L.B., Jørgensen T., Laurberg P. Moderate Alcohol Consumption May Protect against Overt Autoimmune Hypothyroidism: A Population-Based Case-Control Study. Eur. J. Endocrinol. 2012;167:483–490. doi: 10.1530/EJE-12-0356[↩][↩]
- Effraimidis G., Tijssen J.G.P., Wiersinga W.M. Alcohol Consumption as a Risk Factor for Autoimmune Thyroid Disease: A Prospective Study. Eur. Thyroid J. 2012;1:99–104. doi: 10.1159/000338920[↩][↩]
- Effraimidis G., Tijssen J.G.P., Brosschot J.F., Wiersinga W.M. Involvement of Stress in the Pathogenesis of Autoimmune Thyroid Disease: A Prospective Study. Psychoneuroendocrinology. 2012;37:1191–1198. doi: 10.1016/j.psyneuen.2011.12.009[↩]
- Markomanolaki ZS, Tigani X, Siamatras T, Bacopoulou F, Tsartsalis A, Artemiadis A, Megalooikonomou V, Vlachakis D, Chrousos GP, Darviri C. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J Mol Biochem. 2019;8(1):3-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688766[↩][↩]
- Rostami R., Nourooz-Zadeh S., Mohammadi A., Khalkhali H.R., Ferns G., Nourooz-Zadeh J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants. 2020;9:1070. doi: 10.3390/antiox9111070[↩]
- Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med. 2015 Sep-Dec;18(3):222-7.[↩]
- Wiebolt J., Achterbergh R., den Boer A., van der Leij S., Marsch E., Suelmann B., de Vries R., van Haeften T.W. Clustering of Additional Autoimmunity Behaves Differently in Hashimoto’s Patients Compared with Graves’ Patients. Eur. J. Endocrinol. 2011;164:789–794. doi: 10.1530/EJE-10-1172[↩]
- Caturegli P., De Remigis A., Rose N.R. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun. Rev. 2014;13:391–397. doi: 10.1016/j.autrev.2014.01.007[↩]
- Iddah M.A., Macharia B.N. Autoimmune Thyroid Disorders. ISRN Endocrinol. 2013;2013:e509764. doi: 10.1155/2013/509764[↩]
- Burch HB. Drug Effects on the Thyroid. N Engl J Med. 2019 Aug 22;381(8):749-761. doi: 10.1056/NEJMra1901214[↩]
- Kawicka A., Regulska-Ilow B., Regulska-Ilow B. Metabolic Disorders and Nutritional Status in Autoimmune Thyroid Diseases. Postepy Hig. Med. Doswiadczalnej Online. 2015;69:80–90. doi: 10.5604/17322693.1136383[↩]
- Szczuko M., Syrenicz A., Szymkowiak K., Przybylska A., Szczuko U., Pobłocki J., Kulpa D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients. 2022;14:1727. doi: 10.3390/nu14091727[↩]
- Landete J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013;53:706–721. doi: 10.1080/10408398.2011.555018[↩]
- Ruggeri R.M., Giovinazzo S., Barbalace M.C., Cristani M., Alibrandi A., Vicchio T.M., Giuffrida G., Aguennouz M.H., Malaguti M., Angeloni C., et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid. 2021;31:96–105. doi: 10.1089/thy.2020.0299[↩]
- Ostrowska L., Gier D., Zyśk B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients. 2021;13:862. doi: 10.3390/nu13030862[↩][↩][↩]
- Avard N., Grant S. A Case Report of a Novel, Integrative Approach to Hashimoto’s Thyroiditis with Unexpected Results. Adv. Integr. Med. 2018;5:75–79. doi: 10.1016/j.aimed.2018.03.003[↩][↩][↩]
- Abbott R.D., Sadowski A., Alt A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-Disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus. 2019;11:e4556. doi: 10.7759/cureus.4556[↩][↩]
- Al-Bayyari N.S. Successful Dietary Intervention Plan for Hashimoto’s Thyroiditis: A Case Study. Rom. J. Diabetes Nutr. Metab. Dis. 2020;27:381–385.[↩]