Zinc deficiency
Zinc deficiency is a lack of sufficient zinc to maintain optimal health. Zinc deficiency can be caused by acquired zinc deficiency or a result of an inherited zinc transporter defect (e.g., acrodermatitis enteropathica caused by mutations in the SLC39A4 gene) 1, 2. Acquired zinc deficiency is typically caused by inadequate intake, increased demand, malabsorption disorders, or excessive losses (e.g., celiac and Crohn’s disease, premature, low birth weight, and those who received exclusive parenteral low zinc breast milk feeding). Acrodermatitis enteropathica is an autosomal recessive disorder caused by a mutation in the SLC39A4 gene that encodes the zinc-iron-regulated transporter-like protein 4 that reduces the uptake and transport of zinc, particularly across the small intestinal mucosa 3, 4. Human milk usually contains enough zinc to maintain adequate levels despite zinc-iron transporter defects; therefore, acrodermatitis enteropathica tends to present when weaning from breast milk 5.
Zinc is a key micronutrient important in growth and development, immune function, taste, smell, wound healing, protein synthesis, and maintenance of skin and hair. Because zinc has many functions throughout the body, zinc deficiency affects many different tissues and organs 6. Zinc deficiency can affect, for example, skin; bones; and the digestive, reproductive, central nervous, and immune systems 6. The signs and symptoms of zinc deficiency vary by age 6. In infants and children, diarrhea is a common sign. In older children, hair loss (alopecia), delayed growth, and frequent infections become more prevalent. In both infants and children, zinc deficiency can impair growth and lead to a loss of appetite and reproductive problems when they reach adulthood 7, 8, 9, 10. In populations with low intakes of absorbable zinc (e.g., from meat and fish), including many low-income and middle-income countries, zinc deficiency affects the health of pregnant women and their infants by increasing the risk of child morbidity (including premature birth and low birthweight) and mortality, maternal morbidity, and adverse birth outcomes 9. In addition, zinc deficiency can interfere with the senses of taste and smell 11. Zinc deficiency in older adults can cause delays in wound healing and changes in cognitive and psychological function 6.
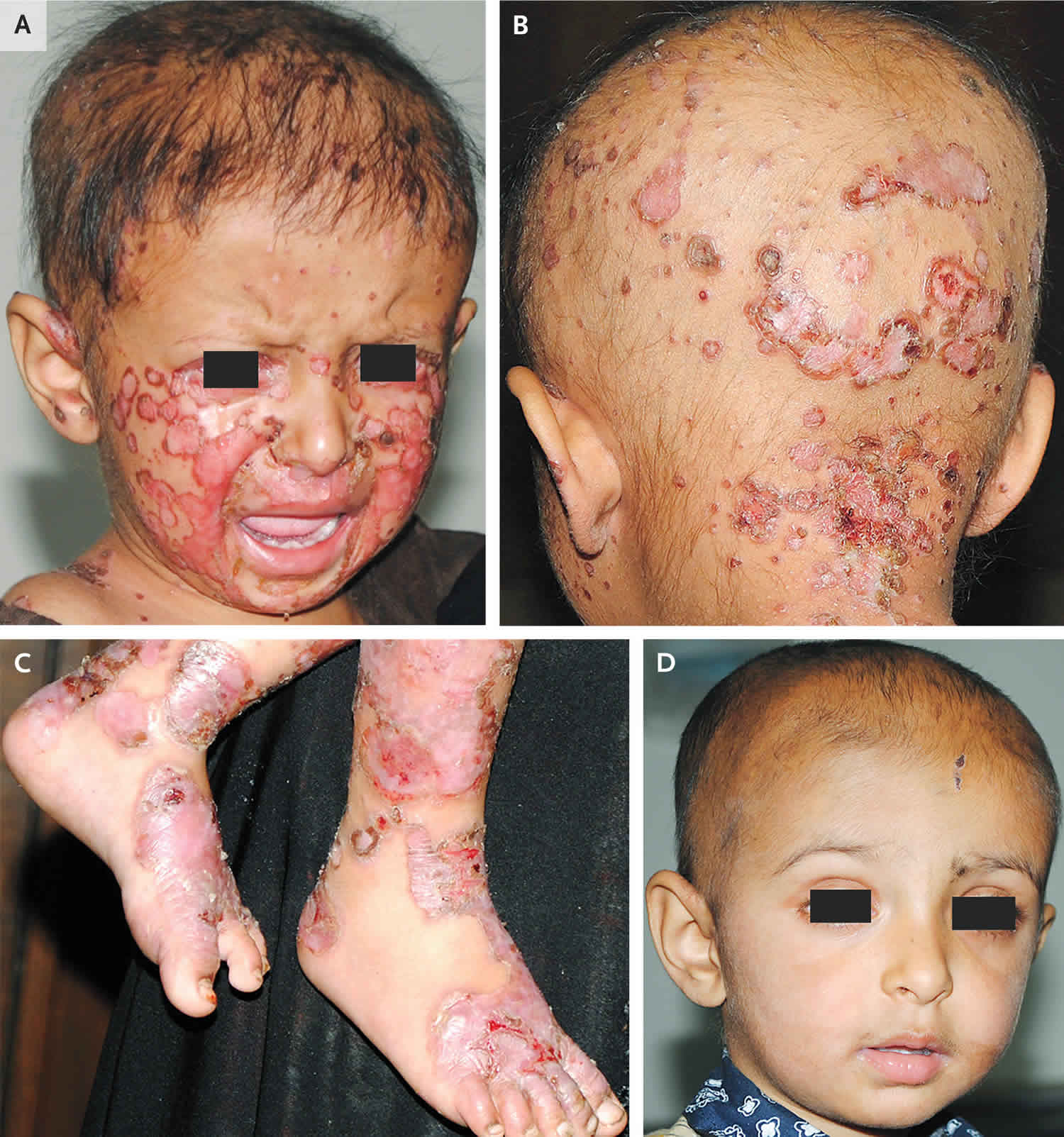
In general, individuals with zinc deficiency have compromised skin integrity because of decreased keratinocyte proliferation, differentiation, and survival 12. Zinc deficiency classically presents with a perioral facial rash in a U-shaped pattern, with cheeks to chin involvement and sparing of the upper lip (see Figures 1 to 3). There is sharp demarcation between the affected area and normal skin. A symmetrical rash with excoriation can also be prominent in the perineal, gluteal, and perianal areas 13. Zinc deficiency is often associated with hair loss (alopecia), diarrhea, and nails that tend to be soft with bridging, dystrophy, and paronychia (infection of the tissue folds around the nails). Other symptoms of zinc deficiency may include conjunctivitis (pink eye or inflammation or infection of the outer membrane of the eyeball and the inner eyelid) and sensitivity to light, loss of appetite, irritability, and impeded growth 14, 15, 16.
Endemic zinc deficiency is common in up to one-third of the population in various parts of the world, primarily in Southeast Asia and sub-Saharan Africa 17, 18. It is estimated that up to 17% of the global population is at risk for inadequate zinc intake, while in South Asia, up to 30% of the population may be deficient. Other areas at risk include sub-Saharan Africa and Central America 17. Zinc deficiency is also prevalent in Iran, Egypt, and Turkey, secondary to high phytate intake.
Clay eating or ‘pica’ is commonly seen in children of certain communities and regions. Clay effectively binds zinc, causing a dramatic decrease in the bioavailability of zinc. Approximately two billion people in developing regions are deficient in zinc to some extent. The at-risk population is comprised of children and elderly adults 19, 20, 21.
Worldwide trends and prevalence of zinc deficiency have largely been stable; however, notable reductions have been seen exemplified by countries like China with a decrease of prevalence from 17% to 8% recorded in 2005 22, 23.
The treatment for zinc deficiency is supplementation with elemental zinc (0.5 to 1 mg per kg per day) until the symptoms resolve 24. Treatment for acrodermatitis enteropathica is lifelong supplementation with elemental zinc (3 mg per kg per day); a higher dosage is needed to overcome the zinc transporter defect 14. The only known adverse effect of zinc supplementation is copper deficiency. High zinc levels inhibit copper absorption by competitively inhibiting a common cationic transporter; therefore, copper levels should be monitored during treatment of zinc deficiency.
Figure 1. Zinc deficiency in a preterm baby
Footnotes: (A) 6-month-old female, born at 27 weeks of gestational age presented with sharply demarcated, eczematous, pinkish-orange, hyperkeratotic papules with “scald-like” in appearance, and plaques in a U-shaped distribution, extending from her cheeks to her chin and notably sparing the lips and eyebrow hair (rash also found on her fingers, legs, and perianal area). Fingernails were slightly ridged and soft. Mouth, tongue, and muscle tone were normal. Baby’s clinical course from initial presentation to pediatric dermatology to 5 days post-zinc supplementation. (B) Baby’s rapid response to 24 hours of oral zinc supplementation. (C) Near-total clinical resolution after only 5 days of oral zinc supplementation.
[Source 25 ]Figure 2. Zinc deficiency in a 5 month old baby
[Source 13 ]Figure 3. Dietary zinc deficiency
Footnotes: A 2-year-old boy was referred to the dermatology clinic with an 8-month history of rash, hair loss, and watery diarrhea. The child had been exclusively breast-fed until 6 months of age, at which time a vegetarian solid-food diet of produce and natural grains had been introduced. When the child was weaned from breast milk to cow’s milk at 16 months of age, the symptoms had started. On examination, the child was irritable; he had angular cheilitis, alopecia, and erosions with crusted borders across the face and scalp and around the mouth, eyes, and ears (Panels A and B). The rash also involved the perianal region, trunk, and all four limbs (Panel C). Laboratory testing showed a serum zinc level of 0.32 mcg per milliliter (reference range, 0.65 to 1.10) and a low alkaline phosphatase level. Zinc is a cofactor for alkaline phosphatase activity, and therefore a low alkaline phosphatase level may be seen in a patient with zinc deficiency. A diagnosis of dietary zinc deficiency–associated dermatitis was made. Oral zinc supplementation and dietary changes were recommended. At a 3-week follow-up visit, the alopecia and dermatitis had abated (Panel D). When zinc supplementation was stopped 3 months later, the symptoms did not recur.
[Source 26 ]Figure 4. Acquired zinc deficiency
Footnotes: A 66-year-old man with a history of diabetes mellitus experienced anorexia six months before admission. He had lost weight (52 kg → 42 kg), and his energy levels had fallen. His vital signs were unremarkable. On physical examination, he had redness with many wet yellowish scales over the entire face, except the eyelids (A), and slow healing decubitus. Alopecia, diarrhea, aphthous ulcer, or taste disorder was not observed. Laboratory testing revealed a zinc level of 35 (80-130) mcg/dL. Based on clinical and serological findings, the patient was diagnosed with acquired zinc deficiency. The patient was administered 100 mg/day of zinc acetate dihydrate, and his appetite and facial rash improved immediately (B). After 14 days, the patient’s zinc level increased to 92 mcg/dL. The patient’s nutritional status improved, and he was discharged. The patient’s zinc intake has remained satisfactory. His vitality improved.
[Source 27 ]What is Zinc
Zinc is an essential mineral that is naturally present in some foods, added to others, and available as a dietary supplement. Zinc is also found in many cold lozenges and some over-the-counter drugs sold as cold remedies. Zinc is a nutrient that people need to stay healthy. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 28. Absorption of zinc occurs in the jejunum of the small intestine 29.
Zinc is found in cells throughout the body, found mainly in bones, teeth, hair, skin, liver, muscle, leukocytes, and testes 30. Zinc plays an important role in a variety of cellular processes, including protein synthesis, wound healing, and immune function 31. Zinc helps the immune system fight off invading bacteria and viruses. The body also needs zinc to make proteins and DNA, the genetic material in all cells. During pregnancy, infancy, and childhood, the body needs zinc to grow and develop properly. Zinc also helps wounds heal and is important for proper senses of taste and smell.
Animal proteins are a good source of zinc. Beef, pork, and lamb contain more zinc than fish. The dark meat of a chicken has more zinc than the light meat. Other good sources of zinc are nuts, whole grains, legumes, and yeast.
Dietary sources of zinc include human breast milk, meat, shellfish, chickpeas, cashews, and pumpkin seeds.
Fruits and vegetables are not good sources, because the zinc in plant foods is not as available for use by the body as the zinc from animal sources. Therefore, low-protein diets and vegetarian diets tend to be low in zinc.
Most Americans get enough zinc from the foods they eat.
However, certain groups of people are more likely than others to have trouble getting enough zinc:
- People who have had gastrointestinal surgery, such as weight loss surgery, or who have digestive disorders, such as ulcerative colitis or Crohn’s disease. These conditions can both decrease the amount of zinc that the body absorbs and increase the amount lost in the urine.
- Vegetarians or vegans because they do not eat meat, which is a good source of zinc. Also, the beans and grains they typically eat have compounds that keep zinc from being fully absorbed by the body. For this reason, vegetarians might need to eat as much as 50% more zinc than the recommended amounts.
- Women who are pregnant or breastfeeding because they need more zinc for their growing baby and to make breast milk.
- Older infants who are breastfed because breast milk does not have enough zinc for infants over 6 months of age. Older infants who do not take formula should be given foods that have zinc such as pureed meats. Formula-fed infants get enough zinc from infant formula.
- Alcoholics because alcoholic beverages decrease the amount of zinc that the body absorbs and increase the amount lost in the urine. Also, many alcoholics eat a limited amount and variety of food, so they may not get enough zinc.
- Children and individuals who have sickle cell disease, possibly because the medications they take can cause low levels of zinc. These people might benefit from taking zinc supplements.
Clinical zinc deficiency in humans was first described in 1961, when the consumption of diets with low zinc bioavailability due to high phytate content was associated with “adolescent nutritional dwarfism” in the Middle East 32. Since then, zinc insufficiency has been recognized by a number of experts as an important public health issue, especially in low-resource countries 33. Severe zinc deficiency is rare and caused by an inherited condition called acrodermatitis enteropathica. Acquired zinc deficiency is primarily due to malabsorption syndromes and chronic alcoholism.
Serum or plasma zinc levels are typically used in clinical practice to assess zinc status 34. In healthy people, the amount of zinc in serum or plasma is 80 to 120 microgram (mcg)/dL (12 to 18 micromol/L) 7. Serum zinc levels below 70 mcg/dL in women and 74 mcg/dL in men indicate zinc deficiency 34. However, both serum and plasma measures have important limitations. Zinc concentrations in serum are associated with the patient’s sex and age as well as the time of the blood draw (morning vs. evening) and do not always correlate with dietary or supplemental zinc intakes 35. Zinc levels also fluctuate in response to other factors, including infections, changes in steroid hormones, stress and muscle catabolism during weight loss or illness 10, 36, 37, 8. Doctors consider risk factors (such as inadequate caloric intake, chronic alcohol use, and malabsorptive digestive diseases) and signs of zinc deficiency (such as impaired growth in infants and children) when they assess a patient’s zinc status 37.
Zinc key points
- Zinc is a nutritionally essential mineral needed for catalytic, structural, and regulatory functions in the body.
- Severe zinc deficiency is rare and caused by an inherited condition called acrodermatitis enteropathica. Acquired zinc deficiency is primarily due to malabsorption syndromes and chronic alcoholism.
- Dietary zinc deficiency is quite common in the developing world, affecting an estimated 2 billion people. Consumption of diets high in phytate and lacking foods from animal origin drive zinc deficiency in these populations.
- The recommended dietary allowance (RDA) for adult men and women is 11 mg/day and 8 mg/day of zinc, respectively.
- Long-term consumption of zinc in excess of the tolerable upper intake level (UL) is 40 mg/day for adults can result in copper deficiency 37. Copper deficiency has also been reported following chronic use of excessive amounts of zinc-containing denture creams (≥2 tubes per week containing 17-34 mg/g of zinc) 38.
- Dietary zinc deficiency has been associated with impaired growth and development in children, pregnancy complications, and immune dysfunction with increased susceptibility to infections.
- Supplementation with doses of zinc in excess of the upper intake level (40 mg/day for adults) is effective to reduce the duration of common cold symptoms.
- Milder gastrointestinal distress has been reported at doses of 50 to 150 mg/day of supplemental zinc. Single doses of 225 to 450 mg of zinc usually induce vomiting.
- Current evidence suggests that supplemental zinc may be useful in the management of chronic conditions, such as age-related macular degeneration, diabetes mellitus, Wilson’s disease, and HIV/AIDS.
- Zinc bioavailability is relatively high in meat, eggs, and seafood; zinc is less bioavailable from whole grains and legumes due to their high content in phytate that inhibits zinc absorption.
What is the function of Zinc?
Zinc is involved in numerous aspects of cellular metabolism. Zinc is required for the catalytic activity of approximately 100 enzymes, including many nicotinamide adenine dinucleotide (NADH) dehydrogenases, RNA and DNA polymerases, and DNA transcription factors as well as alkaline phosphatase, superoxide dismutase, and carbonic anhydrase 39, 40, 12, 41 and it plays a role in immune function 42, 43, protein synthesis 43, wound healing 44, DNA synthesis for cell growth 45, and cell division 43 and the breakdown of carbohydrates. Zinc also supports normal growth and development during pregnancy, infancy, childhood, and adolescence 46 and is required for proper sense of taste and smell 47. Zinc also enhances the action of insulin. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 28.
Information from an expert review on zinc supplements showed that 48, 49, 50:
- When taken for at least 5 months, zinc may reduce your risk of becoming sick with the common cold. Starting to take zinc supplements within 24 hours after cold symptoms begin may reduce how long the symptoms last and make the symptoms less severe. However, supplementation beyond the recommended dietary allowance (RDA) and adequate intake (AI) is not recommended at this time.
People commonly use zinc for zinc deficiency, diarrhea, and Wilson disease. Zinc is also used for acne, diabetes, anorexia, burns, and many other purposes. There is some scientific evidence to support its use for some of these conditions. But for most, there is no good scientific evidence to support its use 51. There is also no good evidence to support using zinc for COVID-19.
Zinc supplements taken in large amounts may cause diarrhea, abdominal cramps, and vomiting. These symptoms most often appear within 3 to 10 hours of swallowing the supplements. The symptoms go away within a short period of time after stopping the supplements. An excess intake of zinc can lead to copper or iron deficiency.
People who use nasal sprays and gels that contain zinc may have side effects, such as losing their sense of smell.
How much zinc do I need?
The amount of zinc you need each day depends on your age. Average daily recommended amounts for different ages are listed below in milligrams (mg). Intake recommendations for zinc and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies 41. Dietary Reference Intake is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and gender 41, include the following:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects 41.
The current RDAs for zinc are listed in Table 1 41. For infants aged 0 to 6 months, the FNB established an AI for zinc that is equivalent to the mean intake of zinc in healthy, breastfed infants.
Most infants (especially those who are formula fed), children, and adults in the United States consume recommended amounts of zinc according to two national surveys, the 1988–1991 National Health and Nutrition Examination Survey 52 and the 1994 Continuing Survey of Food Intakes of Individuals 53.
However, some evidence suggests that zinc intakes among older adults might be marginal. An analysis of National Health and Nutrition Examination Survey data found that 35%–45% of adults aged 60 years or older had zinc intakes below the estimated average requirement of 6.8 mg/day for elderly females and 9.4 mg/day for elderly males. When the investigators considered intakes from both food and dietary supplements, they found that 20%–25% of older adults still had inadequate zinc intakes 54.
Zinc intakes might also be low in older adults from the 2%–4% of U.S. households that are food insufficient (sometimes or often not having enough food) 55. Data from National Health and Nutrition Examination Survey indicate that adults aged 60 years or older from food-insufficient families had lower intakes of zinc and several other nutrients and were more likely to have zinc intakes below 50% of the RDA on a given day than those from food-sufficient families 56.
Table 1. Recommended Dietary Allowances for Zinc
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 2 mg |
| Infants 7–12 months | 3 mg |
| Children 1–3 years | 3 mg |
| Children 4–8 years | 5 mg |
| Children 9–13 years | 8 mg |
| Teens 14–18 years (boys) | 11 mg |
| Teens 14–18 years (girls) | 9 mg |
| Adults (men) | 11 mg |
| Adults (women) | 8 mg |
| Pregnant teens | 12 mg |
| Pregnant women | 11 mg |
| Breastfeeding teens | 13 mg |
| Breastfeeding women | 12 mg |
What foods provide zinc?
Zinc is found in a wide variety of foods. You can get recommended amounts of zinc by eating a variety of foods including the following:
- Oysters, which are the best source of zinc. Oysters contain more zinc per serving than any other food.
- Red meat, poultry, seafood such as crab and lobsters, and fortified breakfast cereals, which are also good sources of zinc. They provide the majority of zinc in the American diet.
- Beans, nuts, whole grains, and dairy products, which provide some zinc.
Animal proteins are a good source of zinc. Beef, pork, and lamb contain more zinc than fish. The dark meat of a chicken has more zinc than the light meat. Other good sources of zinc are nuts, whole grains, legumes, and yeast.
Fruits and vegetables are not good sources, because the zinc in plant foods is not as available for use by the body as the zinc from animal sources. Therefore, low-protein diets and vegetarian diets tend to be low in zinc.
Phytates (is the principal storage form of phosphorus in many plant tissues, especially bran and seeds), which are present in whole-grain breads, cereals, legumes, and other foods—bind zinc and inhibit its absorption 41, 58, 59. Therefore, the bioavailability of zinc from grains and plant foods is lower than that from animal foods, although many grain- and plant-based foods are still good sources of zinc 41.
Although breast milk typically provides adequate concentrations of zinc for full-term infants, it may be insufficient for preterm infants given their relatively increased metabolic rate and limited liver zinc storage from the third trimester of pregnancy 60, 61.
The U.S. Department of Agriculture’s (USDA’s) Nutrient Database website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing zinc arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Zinc-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Zinc-Food.pdf).
Table 2. Food Sources of Zinc
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Oysters, cooked, breaded and fried, 3 ounces | 74.0 | 493 |
| Beef chuck roast, braised, 3 ounces | 7.0 | 47 |
| Crab, Alaska king, cooked, 3 ounces | 6.5 | 43 |
| Beef patty, broiled, 3 ounces | 5.3 | 35 |
| Breakfast cereal, fortified with 25% of the DV for zinc, ¾ cup serving | 3.8 | 25 |
| Lobster, cooked, 3 ounces | 3.4 | 23 |
| Pork chop, loin, cooked, 3 ounces | 2.9 | 19 |
| Baked beans, canned, plain or vegetarian, ½ cup | 2.9 | 19 |
| Chicken, dark meat, cooked, 3 ounces | 2.4 | 16 |
| Yogurt, fruit, low fat, 8 ounces | 1.7 | 11 |
| Cashews, dry roasted, 1 ounce | 1.6 | 11 |
| Chickpeas, cooked, ½ cup | 1.3 | 9 |
| Cheese, Swiss, 1 ounce | 1.2 | 8 |
| Oatmeal, instant, plain, prepared with water, 1 packet | 1.1 | 7 |
| Milk, low-fat or non fat, 1 cup | 1.0 | 7 |
| Almonds, dry roasted, 1 ounce | 0.9 | 6 |
| Kidney beans, cooked, ½ cup | 0.9 | 6 |
| Chicken breast, roasted, skin removed, ½ breast | 0.9 | 6 |
| Cheese, cheddar or mozzarella, 1 ounce | 0.9 | 6 |
| Peas, green, frozen, cooked, ½ cup | 0.5 | 3 |
| Flounder or sole, cooked, 3 ounces | 0.3 | 2 |
Footnote: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents of products within the context of a total diet. The DV for zinc is 15 mg for adults and children age 4 and older. Food labels, however, are not required to list zinc content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 62]Can too much zinc be harmful?
Yes, if you get too much. Signs of too much zinc include nausea, vomiting, dizziness, headaches, gastric distress, loss of appetite, stomach cramps and diarrhea 7, 8. When people take too much zinc for a long time, at doses of 50 mg zinc or more—typically from supplements or excessive use of denture adhesive creams that contain zinc, they sometimes have problems such as low copper levels, lower immunity, and low levels of HDL cholesterol (the “good” cholesterol) 7, 8, 63. According to a few reports, overuse of denture adhesive creams containing up to 34 mg zinc per gram of product can lead to neurological symptoms (including sensory ataxia and myelopathy) and anemia. Zinc-free formulations are available to prevent these effects 7, 38, 64.
One case report cited severe nausea and vomiting within 30 minutes of ingesting 4 g of zinc gluconate (570 mg elemental zinc) 65. Intakes of 150–450 mg of zinc per day have been associated with such chronic effects as low copper status, altered iron function, reduced immune function, and reduced levels of high-density lipoproteins 66. Reductions in a copper-containing enzyme, a marker of copper status, have been reported with even moderately high zinc intakes of approximately 60 mg/day for up to 10 weeks 41. The doses of zinc used in the AREDS study (80 mg per day of zinc in the form of zinc oxide for 6.3 years, on average) have been associated with a significant increase in hospitalizations for genitourinary causes, raising the possibility that chronically high intakes of zinc adversely affect some aspects of urinary physiology 67. Very high doses of zinc from supplements (142 mg/day) might also interfere with magnesium absorption and disrupt magnesium balance 68.
The amount of zinc obtained from food is rarely as high as 50 mg, so the zinc in foods is unlikely to cause zinc toxicity 34.
In order to prevent copper deficiency, the US Food and Nutrition Board set the tolerable upper intake level (UL) for zinc from foods, beverages, supplements, and medications for healthy adults at 40 mg/day 37. Table 3 below lists the tolerable upper intake levels (ULs) for zinc from foods, beverages, supplements, and medications for healthy adults by age group. The tolerable upper intake levels (ULs) do not apply to individuals receiving zinc for medical treatment, but such individuals should be under the care of a physician.
Table 3. Tolerable Upper Intake Levels (ULs) for Zinc
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | 4 mg | 4 mg | ||
| 7–12 months | 5 mg | 5 mg | ||
| 1–3 years | 7 mg | 7 mg | ||
| 4–8 years | 12 mg | 12 mg | ||
| 9–13 years | 23 mg | 23 mg | ||
| 14–18 years | 34 mg | 34 mg | 34 mg | 34 mg |
| 19+ years | 40 mg | 40 mg | 40 mg | 40 mg |
Zinc deficiency causes
Zinc deficiency can be caused by acquired zinc deficiency or a result of an inherited zinc transporter defect (e.g., acrodermatitis enteropathica caused by mutations in the SLC39A4 gene) 1, 2, 69. Acquired zinc deficiency is typically caused by inadequate intake, increased demand, malabsorption disorders, or excessive losses (e.g., celiac and Crohn’s disease, premature, low birth weight, and those who received exclusive parenteral low zinc breast milk feeding) 8. Inadequate dietary intake of absorbable zinc is the primary cause of zinc deficiency in most situations 70. This may result from low dietary intake or heavy reliance on foods with little or poorly absorbable zinc. Inadequate dietary zinc intake is common in many parts of the world. It is often exacerbated by physiologic conditions associated with elevated zinc requirements 70, 71.
Malabsorption of zinc may occur in a number of situations for example, acrodermatitis enteropathica 72. Malabsorption syndromes and inflammatory diseases of the bowel, resulting in poor absorption and loss of zinc, may lead to secondary zinc deficiency particularly in the presence of marginal dietary intakes 8. Utilization of zinc is impaired in the presence of infection as decreased circulation of zinc reduces the availability of zinc to the tissues.
Acrodermatitis enteropathica caused is an autosomal recessive disorder caused by a mutation in the SLC39A4 gene that encodes the zinc-iron-regulated transporter-like protein 4 that reduces the uptake and transport of zinc, particularly across the small intestinal mucosa 3, 4. Human milk usually contains enough zinc to maintain adequate levels despite zinc-iron transporter defects; therefore, acrodermatitis enteropathica tends to present when weaning from breast milk 5.
Conditions of impaired intestinal integrity not only reduce absorption, but also result in increased endogenous losses of zinc. Fecal excretion of zinc is increased during acute diarrhea 73. It is not clear to what extent this represents unabsorbed zinc or zinc of endogenous origin 74. Diarrheal diseases are common in many low-income countries. The fact that zinc deficiency increases the susceptibility to childhood diarrhea while increased losses of endogenous zinc associated with diarrhea further deplete body zinc, results in a vicious cycle that merits further study 75.
Inherited zinc deficiency
Much of what is known about severe zinc deficiency was derived from the study of individuals born with acrodermatitis enteropathica, a rare genetic disorder resulting from the impaired uptake and transport of zinc 76, 72. The symptoms of severe zinc deficiency include the slowing or cessation of growth and development, delayed sexual maturation, characteristic skin rashes, chronic and severe diarrhea, immune system deficiencies, impaired wound healing, diminished appetite, impaired taste sensation, night blindness, swelling and clouding of the cornea, and behavioral disturbances. Before the cause of acrodermatitis enteropathica was known, patients typically died in infancy. Oral zinc therapy results in the complete remission of symptoms, though it must be maintained indefinitely in individuals with the genetic disorder 76, 69.
Acquired zinc deficiency
In modern society, acquired zinc deficiency is common, occurring in approximately 17% of the world population 77; vegetarians, alcoholics, malnourished individuals, and premature infants are most at risk 14.
It is now recognized that milder zinc deficiency contributes to a number of health problems, especially common in children who live in low-resource countries. An estimated 2 billion people worldwide are affected by dietary zinc deficiency 33. The lack of a sensitive and specific indicator of marginal zinc deficiency hinders the scientific study of its health implications 78. However, controlled trials of moderate zinc supplementation have demonstrated that marginal zinc deficiency contributes to impaired physical and neuropsychological development and increased susceptibility to life-threatening infections in young children 69. In fact, zinc deficiency has been estimated to cause more than 450,000 deaths annually in children under five years of age, comprising 4.4% of global childhood deaths 79.
In industrialized countries, dietary zinc deficiency is unlikely to cause severe zinc deficiency in individuals without a genetic disorder, zinc malabsorption or conditions of increased zinc loss, such as severe burns or prolonged diarrhea. Severe zinc deficiency has also been reported in individuals undergoing total parenteral nutrition without zinc, in those who abuse alcohol, and in those who are taking certain medications like penicillamine 80.
Individuals at risk of zinc deficiency 8, 80, 74, 10:
- Premature and low-birth-weight infants
- Older breast-fed infants and toddlers with inadequate intake of zinc-rich complementary foods
- Children and adolescents
- Pregnant and lactating (breast-feeding) women, especially adolescents
- Patients receiving total parenteral nutrition (intravenous feedings)
- Malnourished individuals, including those with protein-energy malnutrition and anorexia nervosa
- Individuals with severe or persistent diarrhea
- Individuals with malabsorption syndromes, including celiac disease and short bowel syndrome
- Individuals with inflammatory bowel disease, including Crohn’s disease and ulcerative colitis
- Alcoholics and those with alcoholic liver disease who have increased urinary zinc excretion and low liver zinc levels
- Individuals with chronic renal disease
- Individuals with sickle cell anemia
- Individuals who use medications that decrease intestinal zinc absorption, increase zinc excretion, or impair zinc utilization
- Older adults (65 years and older)
- Vegetarians: The requirement for dietary zinc may be as much as 50% greater for vegetarians whose major food staples are grains and legumes, because high levels of phytate in these foods reduce zinc absorption 37.
Groups at risk of zinc deficiency
In North America, overt zinc deficiency is uncommon 41. When zinc deficiency does occur, it is usually due to inadequate zinc intake or absorption, increased losses of zinc from the body, or increased requirements for zinc 81, 82, 80. People at risk of zinc deficiency or inadequacy need to include good sources of zinc in their daily diets. Supplemental zinc might also be appropriate in certain situations.
The following groups are among those most likely to have inadequate zinc status.
People with gastrointestinal disorders or who have had weight loss surgery (bariatric surgery)
Zinc deficiency is common in people with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease or have had weight loss surgery (bariatric surgery) involving resection of the gastrointestinal tract because of poor dietary intakes, decreased absorption, or increased urinary excretion as a result of inflammation 83, 84. Approximately 15% to 40% of people with inflammatory bowel disease (ulcerative colitis and Crohn’s disease) have zinc deficiency during active disease states and while in remission 83, 84. In patients with zinc deficiency, the risk of inflammatory bowel disease-related symptoms (including anemia, hemorrhage, and abdominal or perianal fistula) increases, and these patients are more likely to need hospitalization or surgery. Zinc supplementation might reduce these risks 83.
Approximately 50% of people with newly diagnosed celiac disease have a high risk of zinc inadequacy or deficiency; potential contributors to this risk might include zinc malabsorption and mucosal inflammation 85, 86. Zinc deficiency sometimes persist even when people with celiac disease avoid foods containing gluten 86.
Vegetarians (especially vegans)
The bioavailability of zinc from vegetarian diets is lower than from non-vegetarian diets because vegetarians typically eat large amounts of legumes and whole grains, which contain phytates that bind zinc and inhibit its absorption 7. In addition, meat is high in bioavailable zinc 87. As a result, vegetarians and vegans usually have lower dietary intakes of zinc and lower serum zinc levels than non-vegetarians 88.
Vegetarians and vegans might benefit from using certain food preparation techniques that reduce the binding of zinc by phytates and increase its bioavailability, such as soaking beans, grains, and seeds in water for several hours before cooking them 89. In addition, organic acids in fermented foods might increase zinc absorption 89. Vegetarians and vegans might also benefit from zinc supplements 90.
Women who are pregnant or lactating
During pregnancy, the amount of zinc needed increases to accommodate fetal growth, and the Food and Nutrition Board therefore recommends that pregnant people consume 3 mg/day more zinc than nonpregnant people in the same age group 37, 8. Similarly, the zinc requirement increases by 4 mg/day during lactation.
The National Health and Nutrition Examination Survey (NHANES) data from 2001–2014 show that 11% of pregnant women in the United States have total zinc intakes from foods and supplements that are below the Estimated Average Requirement (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) 91. Low serum zinc concentrations during pregnancy might increase the risk of preeclampsia and low-birthweight infants 92, 93. Routine zinc supplementation during pregnancy does not appear to reduce the risk of low birthweight, stillbirth, or neonatal death, but it might lower the risk of preterm birth 94.
During lactation, some but not all studies show that adequate intakes of foods rich in zinc increase concentrations of the mineral in breast milk 95, 96, 97. Evidence is also conflicting on whether zinc supplementation during lactation increases the zinc content of breast milk 98, 99.
Older infants who are exclusively breastfed
Zinc concentrations in breast milk peak during the first month after birth and then decline by approximately 75% by the ninth month 8. Because of this sharp drop, human breast milk alone is not sufficient to meet the infant’s zinc requirement after age 6 months 8, 61. The Food and Nutrition Board recommends that in addition to breast milk, infants aged 7–12 months consume age-appropriate foods or formula containing zinc 37.
Children with sickle cell disease
Children with sickle cell disease have a high risk of zinc insufficiency or deficiency, possibly as a result of the chelation therapy used to treat iron overload 8, 100. Children with sickle cell disease and low zinc status often are shorter and weigh less than age-matched peers, and they also have a higher risk of maturation delays, vaso-occlusive pain crises (blockages of blood flow to an area of the body), and associated hospitalizations 100. Supplemental zinc might enhance growth in children with sickle cell disease and decrease the risk of bacterial infections, hospitalizations, and vaso-occlusive pain crises 101, 8, 100.
People with alcohol use disorder
Low zinc status has been observed in 30% to 50% of people with alcohol use disorder 37, 102. Ethanol consumption decreases intestinal absorption of zinc and increases urinary zinc excretion 37, 102, 103, 104. In addition, the variety and amount of food consumed by many people with alcohol use disorder is limited, leading to inadequate zinc intake 105, 106.
Zinc deficiency prevention
People should get most of their nutrients from food and beverages, according to the federal government’s Dietary Guidelines for Americans. The federal government’s 2020-2025 Dietary Guidelines for Americans notes that “Because foods contain vitamins, minerals, dietary fiber, and other components that have benefits for health, nutritional needs should be met primarily through foods. … In some cases, fortified foods and dietary supplements are useful when it is not possible otherwise to meet needs for one or more nutrients (for example, during specific life stages such as pregnancy).”
Numerous zinc supplementation trials have shown that a wide range of health benefits can be realized by increasing the intake of zinc where diets are inadequate in zinc 46, 107. The results of these trials strongly argue for the development of programs to improve zinc status in high-risk populations. The major intervention strategies are dietary diversification/modification, zinc supplementation, food fortification, and bio-fortification. These strategies are not mutually exclusive but can be used in a complementary way. Their choice depends upon the available resources and technical feasibility.
Zinc supplementation
Supplements contain several forms of zinc, including zinc gluconate, zinc sulfate, and zinc acetate 34. The percentage of elemental zinc varies by form. Zinc oxide, zinc acetate, zinc sulfate, and zinc gluconate, contain elemental zinc percentages of 80%, 30%, 23%, and 14.3%, respectively 108. For example, approximately 23% of zinc sulfate consists of elemental zinc; thus, 220 mg of zinc sulfate contains 50 mg of elemental zinc. The elemental zinc content appears in the Supplement Facts panel on the supplement container. Research has not determined whether differences exist among forms of zinc in absorption, bioavailability, or tolerability 109.
Supplementation programs are useful for targeting vulnerable population subgroups, which are at a particular high-risk of micronutrient deficiencies. The easiest way to supplement zinc could be to include it in programs already delivering daily or weekly nutrient supplements for the prevention of iron deficiency anemia and other micronutrient deficiencies. The recommended zinc dosages are 5 mg/day for children between 7 months and 3 years and 10 mg/day for older children 110, 111. When formulating multi-nutrient supplements, it is recommended that salts providing readily absorbable zinc, like zinc sulfate (ZnSO4), zinc gluconate or zinc acetate are used because they are absorbed more efficiently 74.
Supplemental zinc is also recommended as an add-on therapy during the treatment of diarrhea in children 112. The recommended daily dosage is twice the age-specific Recommended Dietary Allowance (RDA) per day for 14 days; that is 10 mg/day for children under 3 years and 20 mg for older children. Several clinical trials have demonstrated that zinc supplements reduce the severity and duration of acute and persistent diarrhea 110, 107.
Food fortification
Food fortification is a more cost-effective and sustainable strategy to overcome micronutrient malnutrition than supplementation 6. Where micronutrient deficiency is widely distributed in a population and dietary modification or diversification is difficult to achieve, fortification of centrally processed foods is an appropriate alternative. Mexico provides an example of a country with a nationwide zinc fortification program. Apart from zinc, other micronutrients are added to wheat and corn flours that are used in preparing bread and tortilla, the two principal staple foods in the country. For such multiple interventions synergistic and antagonistic interactions between micronutrients have to be taken into account during the development of appropriate formulations 113.
Fortification programs can also be specifically targeted to increase the intake of zinc in groups of high-risk such as infants and young children who consume particular type of food. In many countries, infant formulas and complementary foods are currently fortified with zinc and other micronutrients. Commercially available standard infant formulas contain zinc in concentrations of around 1 mg/L, following current recommendations. In general, the food selected for fortification should be one that is widely consumed in stable and predictable amounts. Among several zinc compounds that are available for fortification, zinc oxide and zinc sulfate are least expensive and most commonly used by the food industry. Suggested levels for fortification of flour are 30-70 mg zinc/kg 114. Zinc sulfate theoretically provides more absorbable zinc because of its greater solubility 115, but it is more expensive 74. However, there are studies in humans that do not show a difference in the absorption of zinc from zinc oxide and zinc sulfate 116. Further information is required on the bio-availability of zinc, acceptability and cost of fortifying food products with different chemical forms of zinc.
Bio-fortification
Recently, plant breeding or genetic engineering strategies that either increase the level of zinc, reduce the content of inhibitors (e.g., phytate), or increase the expression of compounds that enhance zinc absorption (e.g., amino acids) have been considered to improve the bio-availability of zinc from plant foods 117. Bio-fortification differs from ordinary fortification because it focuses on intrinsic enrichment of micronutrients in plant parts that are used for food while the plants are still growing, rather than having nutrients from external resources added to the foods when they are being processed 118. This is an improvement on ordinary fortification when it comes to providing nutrients for the rural poor, who rarely have access to commercially fortified foods 118. As such, bio-fortification is seen as an upcoming strategy for dealing with deficiencies of micronutrients in the developing world 6. Its additional benefits include higher yield where micronutrients are limiting plant growth and increased vitality of seedlings emerging from zinc-enriched seeds 6.
Dietary diversification or modification
Dietary diversification or modification is a sustainable long-term approach to improving the intake of several nutrients simultaneously. Dietary diversification or modification strategies at the community or household level have the potential to increase the intake of bio-available zinc. Such strategies include (1) Agricultural interventions (2) Production and promotion of animal-source foods through animal husbandry or aquaculture (3) Processing strategies at the commercial or household level to enhance zinc absorption from plant-based diets 119. Agricultural interventions focused on plant-based foods may have little impact on intake of bio-available zinc. Some benefit may be realized if accompanied by processing strategies to reduce the levels of substances that inhibit zinc absorption, such as phytate, however, this is likely to be insufficient to meet zinc needs of infants and young children. Animal husbandry efforts that increase red meat or liver consumption by infants and young children can have a positive impact. Milk and cheese are also important sources of dietary zinc 120. These foods generally have a higher content of readily absorbed zinc than poultry, eggs, or fish. Nutrition education to promote dietary diversification or modification can lead to greater intakes of animal-source foods and thus bioavailable zinc. Care must be taken to avoid potential adverse effects of the above strategies, such as aflatoxin contamination of germinated cereals, loss of water-soluble nutrients from soaking cereal flours, and displacement of breast milk by increased intakes of other foods 119. However, more information is required on zinc content and zinc absorption modifiers in local foods to identify suitable sources of absorbable zinc. Although, dietary modification and diversification is the most sustainable approach, change of the dietary practices and preferences is difficult and foods that provide highly bio-available zinc (such as red meat) are generally expensive.
Zinc deficiency signs and symptoms
Because zinc has many functions throughout the body, zinc deficiency affects many different tissues and organs resulting in a broad range of signs and symptoms of zinc deficiency 6. Organ systems known to be affected clinically by zinc deficiency states include the skin, bones, and the digestive, reproductive, central nervous, and immune systems 121, 6.
The signs and symptoms of zinc deficiency vary by age 6. In infants and children, diarrhea is a common sign. In older children, hair loss (alopecia), delayed growth, and frequent infections become more prevalent. In both infants and children, zinc deficiency can impair growth and lead to a loss of appetite and reproductive problems when they reach adulthood 7, 8, 9, 10. In populations with low intakes of absorbable zinc (e.g., from meat and fish), including many low-income and middle-income countries, zinc deficiency affects the health of pregnant women and their infants by increasing the risk of child morbidity (including premature birth and low birthweight) and mortality, maternal morbidity, and adverse birth outcomes 9. In addition, zinc deficiency can interfere with the senses of taste and smell 11. Zinc deficiency in older adults can cause delays in wound healing and changes in cognitive and psychological function 6.
Zinc deficiency symptoms may include:
- Frequent infections
- Hypogonadism in males
- Loss of hair (alopecia)
- Poor appetite
- Problems with the sense of taste
- Problems with the sense of smell
- Skin sores
- Slow growth
- Trouble seeing in the dark
- Wounds that take a long time to heal
Dermatitis (eczema), hair loss (alopecia), and diarrhea are typical symptoms of acquired zinc deficiency, in addition to impaired wound healing, taste disorder (dysgeusia), and night blindness 27. As with hereditary zinc deficiency, dermatitis of acquired zinc deficiency typically occurs in perioral and acral areas; skin findings are erythematous, scaly, and crusted plaques and erosions 122. There are few case reports of zinc deficiency with a facial rash 123, 124. Clinicians should consider zinc deficiency as a differential diagnosis of loss of appetite with various facial rashes.
Impaired immune system function
Adequate zinc intake is essential in maintaining the integrity of the immune system 125, specifically for normal development and function of cells that mediate both innate (neutrophils, macrophages, and natural killer cells) and adaptive (B-lymphocytes and T-lymphocytes) immune responses 126. Because pathogens also require zinc to thrive and invade, a well-established antimicrobial defense mechanism in the body sequesters free zinc away from microbes 127. Another opposite mechanism consists in intoxicating intracellular microbes within macrophages with excess zinc 128. Through weakening innate and adaptive immune responses, zinc deficiency diminishes the capacity of the body to combat pathogens 127. As a consequence, zinc-deficient individuals experience an increased susceptibility to a variety of infectious agents 20.
Diarrhea
Zinc promotes mucosal resistance to infections by supporting the activity of immune cells and the production of antibodies against invading pathogens 127. Therefore, a deficiency in zinc increases the susceptibility to intestinal infections and constitutes a major contributor to diarrheal diseases in children 20. In turn, persistent diarrhea contributes to zinc deficiency and malnutrition 20. Research indicates that zinc deficiency may also potentiate the effects of toxins produced by diarrhea-causing bacteria like E. coli 129. It is estimated that diarrheal diseases are responsible for the deaths of about 500,000 children under five years of age annually in low- and middle-income countries 130. Zinc supplementation in combination with oral rehydration therapy has been shown to significantly reduce the duration and severity of acute and persistent childhood diarrhea and to increase survival in a number of randomized controlled trials 131. A 2016 meta-analysis of randomized controlled trials found that zinc supplementation reduced the duration of acute diarrhea by one day in children aged >6 months who presented signs of malnutrition (5 trials; 419 children) 132. However, there was little evidence to suggest that zinc could be as efficacious to reduce the duration of acute diarrheal episodes in children aged <6 months and in well-nourished children aged >6 months. Zinc supplementation also reduced the duration of persistent diarrhea in children by more than half a day (5 trials; 529 children) 132.
The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) currently recommend supplementing young children with 10 to 20 mg/day of zinc as part of the treatment for acute diarrheal episodes and to prevent further episodes in the two to three months following zinc supplementation 133.
Pneumonia
Pneumonia — caused by lower respiratory tract viral or bacterial infections (LRTIs) — accounts for nearly 1 million deaths among children annually, primarily in low-and middle-income countries 130. Vaccinations against Haemophilus influenzae type B, pneumococcus, pertussis (whooping cough), and measles can help prevent pneumonia 134. According to a 2009 WHO report on disease risk factors, zinc deficiency may be responsible for 13% of all LRTI cases, primarily pneumonia and flu cases, in children younger than 5 years 135. Accordingly, a 2016 meta-analysis of six trials found that zinc supplementation in children under 5 years old reduced the risk of pneumonia by 13% 136. However, it remains unclear whether supplemental zinc, in conjunction with antibiotic therapy, is beneficial in the treatment of pneumonia. A recent randomized, placebo-controlled trial conducted in Gambian children who were not zinc deficient failed to show any benefit of zinc supplementation (10 mg/day or 20 mg/day [depending on child’s age] for 7 days) given alongside antibiotics in the treatment of severe pneumonia 137. A 2018 meta-analysis of five trials (1,822 participants) found no improvement when zinc was used as an adjunct to antibiotic treatment in children with pneumonia 138. There was, however, evidence that supplemental zinc reduced the risk of pneumonia-related mortality (3 trials; 1,318 participants) 138.
Delayed mental and psychomotor development in young children
Adequate nutrition in essential for brain growth and development, especially during the first 1,000 days of life — a critical period of development for all organs and systems spanning from conception to 24 months of age 139. Animal studies 140 have established that zinc deficiency in early life interferes with normal brain development and cognitive functions. Data on the effect of zinc supplementation during pregnancy on infants’ neurologic and psychomotor outcomes is very limited. In a randomized, placebo-controlled trial in African-American women, daily maternal supplementation with 25 mg of zinc from about 19 weeks’ gestation had no effect on neurologic development test scores in their children at five years of age 141.
Several studies have reported on the effect of postnatal zinc supplementation on mental and motor development. Two early randomized controlled trials, one conducted in India and the other in Guatemala, suggested that postnatal supplementation with 10 mg/day of zinc resulted in toddlers being more vigorous 142 and functionally active 143. In one trial conducted in Brazilian newborns from low-income families and weighing between 1,500 g and 2,499 g at birth 144, neither zinc supplementation for eight weeks with 1 mg/day or 5 mg/day improved mental and psychomotor development at 6 or 12 months of age compared to a placebo and assessed using the Bayley Scales of Infant Development (BSID) for Mental Development Index (MDI) and Psychomotor Development Index (PDI). Additionally, a randomized, placebo-controlled, double-blind trial in Chilean newborns (birth weights >2,300 g) from low-income families reported no effect of zinc supplementation (5 mg/day) on mental and psychomotor development indices at 6 and 12 months 145. Two other trials found that supplemental zinc failed to improve MDI or PDI at 12 months of age when zinc (10 mg/day) was given to six-month-old infants for six months 146 or at the end of the intervention in toddlers aged 12-18 months when zinc (30 mg/day) was given for four months 147. A 2012 Cochrane review of eight clinical trials found no evidence that postnatal zinc supplementation improves mental or motor development of infants and children from populations with presumably inadequate zinc status 148.
Impaired growth, growth retardation and development
Significant delays in linear growth and weight gain, known as growth retardation or failure to thrive, are common features of mild zinc deficiency in children. In the 1970s and 1980s, several randomized, placebo-controlled studies of zinc supplementation in young children with significant growth delays were conducted in Denver, Colorado. Modest zinc supplementation (5.7 mg/day) resulted in increased growth rates compared to placebo 149. Several meta-analyses of growth data from zinc intervention trials have confirmed the widespread occurrence of growth-limiting zinc deficiency in young children, especially in low- and middle-income countries 150. A 2018 systematic review and meta-analysis identified 54 trials 151 that examined the impact of zinc supplementation during infancy (on average, 7.6 mg/day for 30.9 weeks) or childhood (on average, 8.5 mg/day for 38.9 weeks) on child anthropometric measurements. There was evidence of a positive effect of supplemental zinc on children’s height, weight, and weight-for-age Z score (WAZ), but neither on height-for-age Z score (HAZ) or weight-for-height Z score (WHZ). In addition, zinc supplementation did not reduce the risks of underweight (WAZ<-2 standard deviation [SD]), wasting (WHZ<-2 SD), or stunting (HAZ<-2 SD) in children 151. Although the exact mechanisms for the growth-limiting effect of zinc deficiency are not known, research indicates that zinc availability affects cell-signaling systems that coordinate the response to the growth-regulating hormone, insulin-like growth factor-1 (IGF-1) 152.
Pregnancy complications and adverse pregnancy outcomes
Estimates based on national food supply indicate that dietary zinc intake is likely inadequate in most low- and middle-income countries, especially those in Sub-Saharan Africa and South Asia 153. Inadequate zinc status during pregnancy interferes with fetal development, and preterm neonates from zinc-deficient mothers suffer from growth retardation and dermatitis and are at risk of infections, necrotizing enterocolitis, chronic lung disease, and retinopathy of prematurity 154. Maternal zinc deficiency has also been associated with a number of pregnancy complications and poor outcomes. A recent case-control study conducted in an Iranian hospital reported higher odds of congenital malformations in newborns of mothers with low serum zinc concentrations during the last month of pregnancy 155. A 2016 review of 64 observational studies 92 found an inverse relationship between maternal zinc status and the severity of preeclampsia, as well as between maternal zinc intake and the risk of low-birth-weight newborns. There were no apparent associations between maternal zinc status and the risk of gestational diabetes mellitus and preterm birth. However, the conclusions of this analysis were limited by the fact that most observational studies were conducted in women from populations not at risk for zinc deficiency 92.
To date, available evidence from maternal zinc intervention trials conducted worldwide does not support the recommendation of routine zinc supplementation during pregnancy. A 2015 systematic review and meta-analysis of 21 randomized controlled trials in over 17,000 women and their babies found a 14% reduction in premature deliveries with zinc supplementation during pregnancy, mainly in low-income women 156. This analysis, however, did not find zinc supplementation to benefit other indicators of maternal or infant health, including stillbirth or neonatal death, low birth weight, small-for-gestational age, and pregnancy-induced hypertension. There was also no effect of supplemental zinc on postpartum hemorrhage, maternal infections, congenital malformations, and child development outcomes 156. A recent review of 17 trials (of which 15 were conducted in low- and middle-income countries) found that maternal supplementation with multiple micronutrients (including, among others, zinc, iron, and folic acid) reduced the risk of low-birth-weight newborns and small-for-gestational age infants when compared to supplemental iron with or without folic acid 157. While multiple micronutrient supplementation would likely benefit pregnant women with coexisting micronutrient deficiencies in low- and middle-income countries, there is no evidence to recommend zinc supplementation in isolation in pregnant women from any settings 158.
Zinc deficiency complications
Prolonged and severe zinc deficiency can lead to many complications, such as:
- Growth failure – Untreated zinc deficiency is often linked with permanently stunted growth and development 150.
- Hypogonadism
- Recurrent infections – Zinc deficiency can aggravate both acute and chronic infections and these infections, in turn, can exacerbate zinc deficiency themselves. It is established to give zinc in diarrhoeal illness; however, there is limited but growing evidence of its role in other infections, such as malaria and pneumonia 132, 159.
- Diarrhea
- Skin conditions – Skin conditions associated with zinc deficiency include acrodermatitis enteropathica, cheilitis, and dermatitis.
- Zinc deficiency is also considered a risk factor for diabetes mellitus and obesity 160. However, the causative role of zinc deficiency in these endocrine disorders is still a subject of early research.
- Delayed wound healing
- Low bone mineral density – The effect of the deficiency of zinc on bone density is not well understood. There is limited evidence adding calcium to zinc supplementation is more beneficial than giving calcium alone 161.
Zinc deficiency diagnosis
Zinc deficiency may be diagnosed by clinical history and examination 162, 18. Laboratory testing, response to zinc supplementation, and cutaneous histopathology can help to confirm the diagnosis 108. In healthy people, the amount of zinc in serum or plasma is 80 to 120 mcg/dL (12 to 18 micromol/L) 7. Serum zinc levels below 70 mcg/dL in women and 74 mcg/dL in men indicate inadequate zinc status 7. However, both serum and plasma measures have important limitations. Zinc concentrations in serum are associated with the patient’s sex and age as well as the time of the blood draw (morning vs. evening) and do not always correlate with dietary or supplemental zinc intakes 35. Zinc levels also fluctuate in response to other factors, including infections, changes in steroid hormones, and muscle catabolism during weight loss or illness 37, 8. Clinicians consider risk factors (such as inadequate caloric intake, chronic alcohol use, and malabsorptive digestive diseases) and signs of zinc deficiency (such as impaired growth in infants and children) when they assess a patient’s zinc status 37.
Serum zinc concentrations fluctuate by as much as 20% during a 24-hour period, largely due to the effects of food ingestion 163. Following a meal, there is an immediate initial increase, after which the concentration declines progressively for the next 4 hour and then rises until food is eaten again. During an overnight fast, the concentration of serum zinc increases slightly, so the highest levels of the day are generally seen in the morning 164, 165. However, diurnal variations in serum zinc concentration among fasted individuals have also been observed, whereby serum zinc decreased from morning to mid-afternoon and then began to rise again to morning levels 166.
Low serum zinc concentrations can occur in the presence of several conditions, representing a normal physiologic response and not necessarily indicative of low zinc status. Serum zinc concentrations are reduced during acute infections and inflammation, which is likely due to the redistribution of zinc from the plasma to the liver 167; cytokines released during the acute phase response activate hepatic metallothionein synthesis 168, a metal-binding protein which appears to alter the hepatic uptake of zinc 169. Elevated concentrations of C-reactive protein (CRP) or other markers of the acute phase response can be used to indicate the presence of infection and should be considered in the interpretation of results. Stress and heart attack (myocardial infarction) also reduce serum zinc levels 170. Because zinc is transported in plasma bound to albumin, diseases, such as cirrhosis and protein-energy malnutrition, that produce hypoalbuminemia result in lower serum zinc concentrations 171. Hemodilution, as observed during pregnancy, oral contraceptive use, and other hormonal treatments, also results in a lower serum zinc concentration 172. On the other hand, conditions resulting in intrinsic or extrinsic hemolysis of blood cells can result in extremely high serum zinc levels because the concentration of intracellular zinc is considerably greater than in serum.
Zinc is a cofactor for alkaline phosphatase (ALP) activity, so decreased serum alkaline phosphatase (ALP) levels may provide supportive evidence of zinc deficiency 173.
Punch biopsy and histopathology of affected skin can support the diagnosis of necrolysis seen as cytoplasmic pallor, vacuolization, ballooning degeneration, and confluent necrosis of keratinocytes in the upper epidermis. Confluent parakeratosis is often present with loss of the granular layer and with dermal edema. An associated neutrophilic crust may be present. Individual keratinocytes often have pyknotic nuclei. These findings are non-specific and are often seen with pellagra and necrolytic migratory erythema. Late lesions of zinc deficiency may mimic psoriasis. Clinical improvement to zinc supplementation can also be confirmatory 33.
Zinc deficiency treatment
Zinc deficiency treatment begins with oral zinc replacement 174. In adults, zinc 2 to 3 mg/kg per day or 20-40 mg daily dose often cures all zinc deficiency clinical manifestations within 1 to 2 weeks. Even in patients with acrodermatitis enteropathica, a disease of malabsorption, oral zinc replacement with 1 to 2 mg/kg per day is still the standard of therapy with life-long zinc supplementation 175, 176.
Formulations of zinc supplements include 174:
- Zinc sulfate
- Zinc acetate
- Zinc aspartate
- Zinc orotate
- Zinc gluconate
Recommended daily elemental zinc intake is 174:
- 3 mg/day for children less than 4 years
- 5 mg/day for children between 4 and 8 years
- 8 mg/day for children between 9 and 13 years
- 9 mg/day for women (non-pregnant and non-lactating)
- 11 mg/day for men
- 11 to 12 mg/day in pregnant and lactating women
In patients with severe zinc deficiency because of malnutrition or malabsorption in disorders such as Crohn disease or short bowel syndrome, higher doses of zinc (more than 50 mg/day) may be acutely needed 174.
For preterm infants with zinc deficiency, normal breastfeeding is usually sufficient for correction, and the zinc deficiency usually resolves within weeks with no clinical symptoms. However, maternal breast milk can be zinc deficient if the mother’s stores are depleted. Also, low maternal breast milk secretion of zinc from the SLC30A2 mutation can occur. If breast secretion is low, the infant will need zinc supplemental replacement.
Overcorrection with zinc supplementation is rare but very large doses can cause severe side effects. Zinc supplements at doses more than 50 mg/day, gastrointestinal symptoms, such as nausea, vomiting, abdominal discomfort, gastric hemorrhage and diarrhea commonly occur 17. Furthermore, zinc doses more than 150 mg/day may adversely affect the immune status and lipid profile. These higher zinc supplements doses can also impair the absorption of iron and copper and over-treatment can lead to copper deficiency and can potentially lead to genito-urinary problems; therefore when zinc is given for extended periods of time, particularly at high doses, it is important to monitor the levels of copper in the blood.
Patients should be monitored for response to therapy and serum zinc levels should be checked after three to six months of supplementation. If there is an inadequate response, the zinc dose may be increased; however, close monitoring for toxicity should be done on higher doses.
In acrodermatitis enteropathica, zinc supplementation is taken for life. The dose of zinc should be individualized in cases where long-term zinc supplementation may be needed and it should be guided by serial serum zinc levels. Additionally, regular assessment of copper and concurrent copper supplementation may become necessary as zinc competes with copper absorption.
Parenteral zinc becomes necessary rarely, except for cases where there is an intestinal failure and/or the patient is on prolonged total parenteral nutrition 177.
Zinc deficiency prognosis
Typically cases of zinc deficiency respond to zinc supplementation and correction of any dietary factors that might lead to the condition. With treatment, there is often a rapid improvement of symptoms. Diarrhea may resolve within 24 hours, and skin lesions often heal within 1 to 2 weeks. Patients with inherited zinc deficiencies should have zinc levels, and alkaline phosphatase should be monitored 3 to 6 months after initiation of zinc replacement therapy and the zinc dose adjusted accordingly 178, 179.
Approximately 70% of patients with zinc deficiency respond positively to zinc supplementation if initiated within 6 months of onset 108. The skin lesions heal without permanent complication, but extended periods of zinc deficiency may have permanent effects on growth and development in children 108. Studies of zinc deprivation on rats demonstrated reduced motor activity, decreased brain mass, and short-term memory loss persisting from early life to adulthood. Similar experiments in mice showed stunted growth.
Untreated patients with classic acrodermatitis enteropathica usually die in the first few years of life 180, 181. Untreated infants exhibit severe growth retardation, dermatitis, alopecia, secondary bacterial and fungal infections, and neurologic and behavioral changes. However, all symptoms are reversible with zinc therapy 180, 181. Patients with acrodermatitis enteropathica uniformly respond to zinc therapy with a 100% survival rate. With zinc supplementation, various symptoms completely resolve or substantially improve.
References- Perafán-Riveros C, França LF, Alves AC, Sanches JA., Jr Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol 2002. Sep-Oct;19(5):426-431. 10.1046/j.1525-1470.2002.00200.x
- Kaur S, Sangwan A, Sahu P, Dayal S, Jain VK. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol 2016. Jan;17(1):35 . 10.4103/2319-7250.173153
- Acrodermatitis enteropathica. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica
- Acrodermatitis enteropathica. https://rarediseases.info.nih.gov/diseases/5723/acrodermatitis-enteropathica
- Küry S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002 Jul;31(3):239-40. doi: 10.1038/ng913
- Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013 Feb;18(2):144-57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3724376
- Ryu M-S, Aydemir TB. Zinc. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:393-408.
- King JC, Cousins RJ. Zinc. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:189-205.
- Gupta S, Brazier AKM, Lowe NM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020 Oct;33(5):624-643. doi: 10.1111/jhn.12791
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 Suppl 1:19-29. doi: 10.1159/000348261
- Kumbargere Nagraj S, George RP, Shetty N, Levenson D, Ferraiolo DM, Shrestha A. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017 Dec 20;12(12):CD010470. doi: 10.1002/14651858.CD010470.pub3
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016 Dec 1;611:113-119. doi: 10.1016/j.abb.2016.06.003
- Snow C, Heard S, Karnes J, Harpell G. Progressive Rash in an Infant. Am Fam Physician. 2022 Mar 1;105(3):319-320. https://www.aafp.org/pubs/afp/issues/2022/0300/p319.html
- Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007 Jan;56(1):116-24. doi: 10.1016/j.jaad.2006.08.015
- Al Naamani A, Al Lawati T. Acrodermatitis Enteropathica: A Case Report. Oman Med J. 2020 Nov 23;35(6):e201. doi: 10.5001/omj.2020.97
- Al-Mendalawi MD. Transient Symptomatic Zinc Deficiency: An Overlooked Diagnosis in Acrodermatitis Enteropathica like Eruption in an Exclusively Breastfed Preterm Infant. Oman Med J. 2022 Mar 22;37(2):e364. doi: 10.5001/omj.2022.51
- Skalny AV, Aschner M, Tinkov AA. Zinc. Adv Food Nutr Res. 2021;96:251-310. doi: 10.1016/bs.afnr.2021.01.003
- Hess SY. National Risk of Zinc Deficiency as Estimated by National Surveys. Food Nutr Bull. 2017 Mar;38(1):3-17. doi: 10.1177/0379572116689000
- Oldewage-Theron WH, Samuel FO, Venter CS. Zinc deficiency among the elderly attending a care centre in Sharpeville, South Africa. J Hum Nutr Diet. 2008 Dec;21(6):566-74. doi: 10.1111/j.1365-277X.2008.00914.x
- Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255-75. doi: 10.1146/annurev.nutr.23.011702.073054
- Schneider JM, Fujii ML, Lamp CL, Lönnerdal B, Zidenberg-Cherr S. The prevalence of low serum zinc and copper levels and dietary habits associated with serum zinc and copper in 12- to 36-month-old children from low-income families at risk for iron deficiency. J Am Diet Assoc. 2007 Nov;107(11):1924-9. doi: 10.1016/j.jada.2007.08.011
- Pitchik HO, Fawzi WW, McCoy DC, Darling AM, Abioye AI, Tesha F, Smith ER, Mugusi F, Sudfeld CR. Prenatal nutrition, stimulation, and exposure to punishment are associated with early child motor, cognitive, language, and socioemotional development in Dar es Salaam, Tanzania. Child Care Health Dev. 2018 Nov;44(6):841-849. doi: 10.1111/cch.12605
- Vuralli D, Tumer L, Hasanoglu A. Zinc deficiency in the pediatric age group is common but underevaluated. World J Pediatr. 2017 Aug;13(4):360-366. doi: 10.1007/s12519-017-0007-8
- Trace elements. In: Kleinman RE, Greer FR; American Academy of Pediatrics Committee on Nutrition. Pediatric Nutrition. 8th ed. American Academy of Pediatrics; 2020:591. https://ebin.pub/qdownload/pediatric-nutrition-8nbsped-1610023609-9781610023603-2018968584-9781610023610.html
- Zaladonis CA, Safeer LZ, Hanson DC, Erickson-Parsons L, Krakowski AC. Zinc Deficiency in a Preterm Infant. J Pediatr. 2022 Jan;240:304-306. https://doi.org/10.1016/j.jpeds.2021.08.020
- Kothari R, Khare S. Dietary Zinc Deficiency-Associated Dermatitis in a Child. N Engl J Med. 2023 May 11;388(19):1799. https://www.nejm.org/doi/full/10.1056/NEJMicm2213071
- Kunitomo, K. (2020), Acquired zinc deficiency. J Gen Fam Med, 21: 82-83. https://doi.org/10.1002/jgf2.303
- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000 Nov;59(4):541-52. doi: 10.1017/s0029665100000781
- Lee HH, Prasad AS, Brewer GJ, Owyang C. Zinc absorption in human small intestine. Am J Physiol. 1989 Jan;256(1 Pt 1):G87-91. doi: 10.1152/ajpgi.1989.256.1.G87
- Merck Sharp & Dohme Corp., Merck Manual. Zinc. https://www.merckmanuals.com/professional/nutritional-disorders/mineral-deficiency-and-toxicity/zinc
- Andreini C, Bertini I. A bioinformatics view of zinc enzymes. J Inorg Biochem. 2012 Jun;111:150-6. doi: 10.1016/j.jinorgbio.2011.11.020
- PRASAD AS, HALSTED JA, NADIMI M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961 Oct;31:532-46. doi: 10.1016/0002-9343(61)90137-1
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol. 2014 Oct;28(4):357-63. doi: 10.1016/j.jtemb.2014.09.002
- Zinc. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional
- Hennigar SR, Lieberman HR, Fulgoni VL 3rd, McClung JP. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J Nutr. 2018 Aug 1;148(8):1341-1351. doi: 10.1093/jn/nxy105
- Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008 Jun;99 Suppl 3:S14-23. doi: 10.1017/S0007114508006818
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222310
- Nations SP, Boyer PJ, Love LA, Burritt MF, Butz JA, Wolfe GI, Hynan LS, Reisch J, Trivedi JR. Denture cream: an unusual source of excess zinc, leading to hypocupremia and neurologic disease. Neurology. 2008 Aug 26;71(9):639-43. doi: 10.1212/01.wnl.0000312375.79881.94
- Sandstead HH. Understanding zinc: recent observations and interpretations. J Lab Clin Med. 1994 Sep;124(3):322-7.
- Vaughn AR, Foolad N, Maarouf M, Tran KA, Shi VY. Micronutrients in Atopic Dermatitis: A Systematic Review. J Altern Complement Med. 2019 Jun;25(6):567-577. doi: 10.1089/acm.2018.0363
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001). Washington, DC: National Academy Press, 2001. https://www.nap.edu/catalog/10026/dietary-reference-intakes-for-vitamin-a-vitamin-k-arsenic-boron-chromium-copper-iodine-iron-manganese-molybdenum-nickel-silicon-vanadium-and-zinc
- Solomons NW. Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev. 1998 Jan;56(1 Pt 1):27-8. doi: 10.1111/j.1753-4887.1998.tb01656.x
- Prasad AS. Zinc: an overview. Nutrition. 1995 Jan-Feb;11(1 Suppl):93-9.
- Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996 Feb;30(2):186-7. doi: 10.1177/106002809603000215
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001) https://doi.org/10.17226/10026
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20(1):3-18. doi: 10.1016/j.jtemb.2006.01.006
- Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians. 1997 Jan;109(1):68-77.
- Markell M, Siddiqi HA. Vitamins and trace elements. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Philadelphia, PA: Elsevier; 2022:chap 27.
- Mason JB, Booth SL. Vitamins, trace minerals, and other micronutrients. In: Goldman L, Schafer AI, eds. Goldman-Cecil Medicine. 26th ed. Philadelphia, PA: Elsevier; 2020:chap 205.
- Ramu A, Neild P. Diet and nutrition. In: Naish J, Syndercombe Court D, eds. Medical Sciences. 3rd ed. Philadelphia, PA: Elsevier; 2019:chap 16.
- Zinc. https://medlineplus.gov/druginfo/natural/982.html
- Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1986-91. https://www.cdc.gov/nchs/data/ad/ad258.pdf
- Interagency Board for Nutrition Monitoring and Related Research. Third Report on Nutrition Monitoring in the United States. Washington, DC: U.S. Government Printing Office, 1995.
- Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr 2002;132:3422-7. https://www.ncbi.nlm.nih.gov/pubmed/12421862?dopt=Abstract
- Ribar DS, Hamrick KS. Dynamics of Poverty and Food Sufficiency. Food Assistance and Nutrition Report Number 36, 2003. Washington, DC: U.S. Department of Agriculture, Economic Research Service. https://www.ers.usda.gov/publications/pub-details/?pubid=46766
- Dixon LB, Winkleby MA, Radimer KL. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 2001;131:1232-46. https://www.ncbi.nlm.nih.gov/pubmed/11285332?dopt=Abstract
- Zinc. https://ods.od.nih.gov/factsheets/Zinc-Consumer
- Sandström B. Bioavailability of zinc. Eur J Clin Nutr. 1997 Jan;51 Suppl 1:S17-9.
- Wise A. Phytate and zinc bioavailability. Int J Food Sci Nutr. 1995 Feb;46(1):53-63. doi: 10.3109/09637489509003386
- Brion LP, Heyne R, Lair CS. Role of zinc in neonatal growth and brain growth: review and scoping review. Pediatr Res. 2021 May;89(7):1627-1640. doi: 10.1038/s41390-020-01181-z. Erratum in: Pediatr Res. 2021 Mar 2
- Ackland ML, Michalczyk AA. Zinc and infant nutrition. Arch Biochem Biophys. 2016 Dec 1;611:51-57. doi: 10.1016/j.abb.2016.06.011
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010 Apr;7(4):1342-65. doi: 10.3390/ijerph7041342
- Doherty K, Connor M, Cruickshank R. Zinc-containing denture adhesive: a potential source of excess zinc resulting in copper deficiency myelopathy. Br Dent J. 2011 Jun 10;210(11):523-5. doi: 10.1038/sj.bdj.2011.428
- Lewis MR, Kokan L. Zinc gluconate: acute ingestion. J Toxicol Clin Toxicol. 1998;36(1-2):99-101. doi: 10.3109/15563659809162595
- Hooper PL, Visconti L, Garry PJ, Johnson GE. Zinc lowers high-density lipoprotein-cholesterol levels. JAMA. 1980 Oct 24-31;244(17):1960-1.
- Johnson AR, Munoz A, Gottlieb JL, Jarrard DF. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007 Feb;177(2):639-43. doi: 10.1016/j.juro.2006.09.047
- Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 1994 Oct;13(5):479-84. doi: 10.1080/07315724.1994.10718438
- Hambidge M. Human zinc deficiency. J Nutr. 2000 May;130(5S Suppl):1344S-9S. doi: 10.1093/jn/130.5.1344S
- Lönnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000 May;130(5S Suppl):1378S-83S. doi: 10.1093/jn/130.5.1378S
- Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandolini-Bunlon M, Polito A, O’Connor JM, Ferry M, Coudray C, Roussel AM. Zinc intake and status in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005 Nov;59 Suppl 2:S37-41. doi: 10.1038/sj.ejcn.1602296
- Van Wouwe JP. Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr. 1989 Oct;149(1):2-8. doi: 10.1007/BF02024322
- Brown KH, Peerson JM, Allen LH. Effect of zinc supplementation on children’s growth: A meta-analysis of intervention trials. In: Sandström B, Walter P, editors. Role of Trace Elements for Health Promotion and Disease Prevention. California: S Karger Pub; 1998. pp. 76–83.
- International Zinc Nutrition Consultative Group (IZiNCG); Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandtröm B, Wasantwisut E, Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004 Mar;25(1 Suppl 2):S99-203.
- Maret W. Zinc biochemistry, physiology, and homeostasis: Recent insights and current trends. BioMetals. 2001;14:187–90.
- Ciampo I, Sawamura R, Ciampo LAD, Fernandes MIM. Acrodematitis enteropathica: clinical manifestations and pediatric diagnosis. Rev Paul Pediatr. 2018;36(2):238-241. doi: 10.1590/1984-0462/;2018;36;2;00010
- Glutsch V, Hamm H, Goebeler M. Zinc and skin: an update. J Dtsch Dermatol Ges. 2019; 17(6): 589–96.
- King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011 Aug;94(2):679S-84S. doi: 10.3945/ajcn.110.005744
- Fischer Walker CL, Ezzati M, Black RE. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur J Clin Nutr. 2009 May;63(5):591-7. doi: 10.1038/ejcn.2008.9
- Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012 Jun;26(2-3):66-9. doi: 10.1016/j.jtemb.2012.04.004
- Hambidge KM, Mild zinc deficiency in human subjects. In: Mills CF, ed. Zinc in Human Biology. New York, NY: Springer-Verlag, 1989:281-96.
- King JC, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins, RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005:271-85.
- Siva S, Rubin DT, Gulotta G, Wroblewski K, Pekow J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Jan;23(1):152-157. doi: 10.1097/MIB.0000000000000989
- Ehrlich S, Mark AG, Rinawi F, Shamir R, Assa A. Micronutrient Deficiencies in Children With Inflammatory Bowel Diseases. Nutr Clin Pract. 2020 Apr;35(2):315-322. doi: 10.1002/ncp.10373
- Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013 Sep 30;5(10):3975-92. doi: 10.3390/nu5103975
- Rondanelli M, Faliva MA, Gasparri C, Peroni G, Naso M, Picciotto G, Riva A, Nichetti M, Infantino V, Alalwan TA, Perna S. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina (Kaunas). 2019 Jul 3;55(7):337. doi: 10.3390/medicina55070337
- Bakaloudi DR, Halloran A, Rippin HL, Oikonomidou AC, Dardavesis TI, Williams J, Wickramasinghe K, Breda J, Chourdakis M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin Nutr. 2021 May;40(5):3503-3521. doi: 10.1016/j.clnu.2020.11.035
- Foster M, Chu A, Petocz P, Samman S. Effect of vegetarian diets on zinc status: a systematic review and meta-analysis of studies in humans. J Sci Food Agric. 2013 Aug 15;93(10):2362-71. doi: 10.1002/jsfa.6179
- Agnoli C, Baroni L, Bertini I, Ciappellano S, Fabbri A, Papa M, Pellegrini N, Sbarbati R, Scarino ML, Siani V, Sieri S. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis. 2017 Dec;27(12):1037-1052. doi: 10.1016/j.numecd.2017.10.020
- Foster M, Samman S. Vegetarian diets across the lifecycle: impact on zinc intake and status. Adv Food Nutr Res. 2015;74:93-131. doi: 10.1016/bs.afnr.2014.11.003
- Bailey RL, Pac SG, Fulgoni VL 3rd, Reidy KC, Catalano PM. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw Open. 2019 Jun 5;2(6):e195967. doi: 10.1001/jamanetworkopen.2019.5967
- Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients. 2016 Oct 15;8(10):641. doi: 10.3390/nu8100641
- He L, Lang L, Li Y, Liu Q, Yao Y. Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertens Pregnancy. 2016 May;35(2):202-9. doi: 10.3109/10641955.2015.1137584
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015 Feb 2;2015(2):CD000230. doi: 10.1002/14651858.CD000230.pub5. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD000230
- Bzikowska-Jura A, Sobieraj P, Michalska-Kacymirow M, Wesołowska A. Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland. Nutrients. 2021 Jan 21;13(2):303. doi: 10.3390/nu13020303
- Keikha M, Shayan-Moghadam R, Bahreynian M, Kelishadi R. Nutritional supplements and mother’s milk composition: a systematic review of interventional studies. Int Breastfeed J. 2021 Jan 4;16(1):1. doi: 10.1186/s13006-020-00354-0
- Aumeistere L, Ciproviča I, Zavadska D, Bavrins K, Borisova A. Zinc Content in Breast Milk and Its Association with Maternal Diet. Nutrients. 2018 Oct 5;10(10):1438. doi: 10.3390/nu10101438
- Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev. 2016 Feb 18;2(2):CD010647. doi: 10.1002/14651858.CD010647.pub2
- Katayama K, Hosui A, Sakai Y, Itou M, Matsuzaki Y, Takamori Y, Hosho K, Tsuru T, Takikawa Y, Michitaka K, Ogawa E, Miyoshi Y, Ito T, Ida S, Hamada I, Miyoshi K, Kodama H, Takehara T. Effects of Zinc Acetate on Serum Zinc Concentrations in Chronic Liver Diseases: a Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial and a Dose Adjustment Trial. Biol Trace Elem Res. 2020 May;195(1):71-81. doi: 10.1007/s12011-019-01851-y
- Martyres DJ, Vijenthira A, Barrowman N, Harris-Janz S, Chretien C, Klaassen RJ. Nutrient Insufficiencies/Deficiencies in Children With Sickle Cell Disease and Its Association With Increased Disease Severity. Pediatr Blood Cancer. 2016 Jun;63(6):1060-4. doi: 10.1002/pbc.25940
- Swe KM, Abas AB, Bhardwaj A, Barua A, Nair NS. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst Rev. 2013 Jun 28;2013(6):CD009415. doi: 10.1002/14651858.CD009415.pub2
- Skalny AV, Skalnaya MG, Grabeklis AR, Skalnaya AA, Tinkov AA. Zinc deficiency as a mediator of toxic effects of alcohol abuse. Eur J Nutr. 2018 Oct;57(7):2313-2322. doi: 10.1007/s00394-017-1584-y
- Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005 Aug-Oct;26(4-5):391-404. doi: 10.1016/j.mam.2005.07.002
- McClain C, Vatsalya V, Cave M. Role of Zinc in the Development/Progression of Alcoholic Liver Disease. Curr Treat Options Gastroenterol. 2017 Jun;15(2):285-295. doi: 10.1007/s11938-017-0132-4
- Navarro S, Valderrama R, To-Figueras J, Giménez A, López JM, Campo E, Fernandez-Cruz L, Ros E, Caballería J, Parés A. Role of zinc in the process of pancreatic fibrosis in chronic alcoholic pancreatitis. Pancreas. 1994 Mar;9(2):270-4. doi: 10.1097/00006676-199403000-00020
- Menzano E, Carlen PL. Zinc deficiency and corticosteroids in the pathogenesis of alcoholic brain dysfunction–a review. Alcohol Clin Exp Res. 1994 Aug;18(4):895-901. doi: 10.1111/j.1530-0277.1994.tb00057.x
- Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Bannon D, Tielsch JM, West KP Jr, Alpers MP. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000 Jun;62(6):663-9. doi: 10.4269/ajtmh.2000.62.663
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol. 2013 Oct;69(4):616-624.e1. https://doi.org/10.1016/j.jaad.2013.04.028
- National Institutes of Health. Office of Dietary Supplements. Zinc. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
- Müller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, Gbangou A, Kouyate B, Garenne M. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. BMJ. 2001 Jun 30;322(7302):1567. doi: 10.1136/bmj.322.7302.1567
- Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002 Jun;75(6):1062-71. doi: 10.1093/ajcn/75.6.1062
- Fontaine O. Effect of zinc supplementation on clinical course of acute diarrhoea. J Health Popul Nutr. 2001 Dec;19(4):339-46.
- 2nd ed. Bangkok, Thailand: 2004. FAO/WHO. Expert Consultation on Human Vitamin and Mineral Requirements, Vitamin and mineral requirements in human nutrition: Report of joint FAO/WHO expert consolation; p. 341.
- Guideline: fortification of wheat flour with vitamins and minerals as a public health strategy. https://www.who.int/publications/i/item/9789240043398
- Wolfe SA, Gibson RS, Gadowsky SL, O’Connor DL. Zinc status of a group of pregnant adolescents at 36 weeks gestation living in southern Ontario. J Am Coll Nutr. 1994 Apr;13(2):154-64. doi: 10.1080/07315724.1994.10718389
- Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. J Nutr. 2005 May;135(5):1102-5. doi: 10.1093/jn/135.5.1102
- Lönnerdal B. Genetically modified plants for improved trace element nutrition. J Nutr. 2003 May;133(5 Suppl 1):1490S-3S. doi: 10.1093/jn/133.5.1490S
- Banuelos G, Lin ZQ. Florida: CRC Press, Boca Raton; 2009. Phytoremediation of selenium-contaminated soil and water produces biofortified products and new agricultural byproducts. Development and Uses of Biofortified Agricultural Products; pp. 57–70.
- Gibson RS, Anderson VP. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr Bull. 2009 Mar;30(1 Suppl):S108-43. doi: 10.1177/15648265090301S107
- Roohani N, Hurrell R, Wegmueller R, Schulin R. Zinc and phytic acid in major foods consumed by a rural and a suburban population in central Iran. J Food Comp Anal. 2012;28:8–15.
- Hambidge KM, Walravens PA. Disorders of mineral metabolism. Clin Gastroenterol. 1982 Jan;11(1):87-117.
- Glutsch V, Hamm H, Goebeler M. Zinc and skin: an update. J Dtsch Dermatol Ges. 2019 Jun;17(6):589-596. doi: 10.1111/ddg.13811
- Weismann K, Wanscher B, Knudsen L. Diagnose und Therapie des Zinkmangelsyndroms während totaler parenteraler Ernährung. Eine Ubersicht mit einem Fallbericht [Diagnosis and therapy of the zinc deficiency syndrome during total parenteral feeding. Review with report of a case]. Hautarzt. 1977 Nov;28(11):578-82.
- Bernstein B, Leyden JJ. Zinc deficiency and acrodermatitis after intravenous hyperalimentation. Arch Dermatol. 1978 Jul;114(7):1070-2.
- Baum MK, Shor-Posner G, Campa A. Zinc status in human immunodeficiency virus infection. J Nutr. 2000 May;130(5S Suppl):1421S-3S. doi: 10.1093/jn/130.5.1421S
- Maares M, Haase H. Zinc and immunity: An essential interrelation. Arch Biochem Biophys. 2016 Dec 1;611:58-65. doi: 10.1016/j.abb.2016.03.022
- Subramanian Vignesh K, Deepe GS Jr. Immunological orchestration of zinc homeostasis: The battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016 Dec 1;611:66-78. doi: 10.1016/j.abb.2016.02.020
- Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013 Oct 17;39(4):697-710. doi: 10.1016/j.immuni.2013.09.006
- Wapnir RA. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000 May;130(5S Suppl):1388S-92S. doi: 10.1093/jn/130.5.1388S
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015 Jan 31;385(9966):430-40. doi: 10.1016/S0140-6736(14)61698-6. Epub 2014 Sep 30. Erratum in: Lancet. 2015 Jan 31;385(9966):420. Erratum in: Lancet. 2016 Jun 18;387(10037):2506
- Black RE. Progress in the use of ORS and zinc for the treatment of childhood diarrhea. J Glob Health. 2019 Jun;9(1):010101. doi: 10.7189/09.010101
- Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016 Dec 20;12(12):CD005436. doi: 10.1002/14651858.CD005436.pub5
- Clinical management of acute diarrhoea. WHO/Unicef joint statement 2004. https://www.who.int/maternal_child_adolescent/documents/who_fch_cah_04_7/en
- Pneumonia. https://www.who.int/news-room/fact-sheets/detail/pneumonia
- World Health Organization. (2009). Global health risks : mortality and burden of disease attributable to selected major risks. World Health Organization. https://apps.who.int/iris/handle/10665/44203
- Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016 Dec 4;12(12):CD005978. doi: 10.1002/14651858.CD005978.pub3
- Howie S, Bottomley C, Chimah O, Ideh R, Ebruke B, Okomo U, Onyeama C, Donkor S, Rodrigues O, Tapgun M, Janneh M, Oluwalana C, Kuti B, Enwere G, Esangbedo P, Doherty C, Mackenzie G, Greenwood B, Corrah T, Prentice A, Adegbola R, Zaman S. Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: randomized controlled trial. J Glob Health. 2018 Jun;8(1):010418. doi: 10.7189/jogh.08.010418
- Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: A meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J. 2018 Mar;12(3):857-864. doi: 10.1111/crj.12646
- https://thousanddays.org/
- Bhatnagar S, Taneja S. Zinc and cognitive development. Br J Nutr. 2001 May;85 Suppl 2:S139-45. doi: 10.1079/bjn2000306
- Tamura T, Goldenberg RL, Ramey SL, Nelson KG, Chapman VR. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. Am J Clin Nutr. 2003 Jun;77(6):1512-6. doi: 10.1093/ajcn/77.6.1512
- Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK. Effect of zinc supplementation on observed activity in low socioeconomic Indian preschool children. Pediatrics. 1996 Dec;98(6 Pt 1):1132-7.
- Bentley ME, Caulfield LE, Ram M, Santizo MC, Hurtado E, Rivera JA, Ruel MT, Brown KH. Zinc supplementation affects the activity patterns of rural Guatemalan infants. J Nutr. 1997 Jul;127(7):1333-8. doi: 10.1093/jn/127.7.1333
- Ashworth A, Morris SS, Lira PI, Grantham-McGregor SM. Zinc supplementation, mental development and behaviour in low birth weight term infants in northeast Brazil. Eur J Clin Nutr. 1998 Mar;52(3):223-7. doi: 10.1038/sj.ejcn.1600553
- Castillo-Durán C, Perales CG, Hertrampf ED, Marín VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. J Pediatr. 2001 Feb;138(2):229-35. doi: 10.1067/mpd.2001.110530
- Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson LA. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004 Sep;80(3):729-36. doi: 10.1093/ajcn/80.3.729
- Taneja S, Bhandari N, Bahl R, Bhan MK. Impact of zinc supplementation on mental and psychomotor scores of children aged 12 to 18 months: a randomized, double-blind trial. J Pediatr. 2005 Apr;146(4):506-11. doi: 10.1016/j.jpeds.2004.10.061
- Gogia S, Sachdev HS. Zinc supplementation for mental and motor development in children. Cochrane Database Syst Rev. 2012 Dec 12;12:CD007991. doi: 10.1002/14651858.CD007991.pub2
- Walravens PA, Hambidge KM, Koepfer DM. Zinc supplementation in infants with a nutritional pattern of failure to thrive: a double-blind, controlled study. Pediatrics. 1989 Apr;83(4):532-8.
- Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S22. doi: 10.1186/1471-2458-11-S3-S22
- Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D, Fawzi WW. Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients. 2018 Mar 20;10(3):377. doi: 10.3390/nu10030377
- MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000 May;130(5S Suppl):1500S-8S. doi: 10.1093/jn/130.5.1500S
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. doi: 10.1371/journal.pone.0050568
- Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, De Curtis M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients. 2015 Dec 11;7(12):10427-46. doi: 10.3390/nu7125542
- Moghimi M, Ashrafzadeh S, Rassi S, Naseh A. Maternal zinc deficiency and congenital anomalies in newborns. Pediatr Int. 2017 Apr;59(4):443-446. doi: 10.1111/ped.13176
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015 Feb 2;2015(2):CD000230. https://doi.org/10.1002/14651858.CD000230.pub5
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2017 Apr 13;4(4):CD004905. doi: 10.1002/14651858.CD004905.pub5. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD004905
- Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients. 2016 Nov 30;8(12):773. doi: 10.3390/nu8120773
- Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull. 2009 Mar;30(1 Suppl):S41-59. doi: 10.1177/15648265090301S104
- Fukunaka A, Fujitani Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int J Mol Sci. 2018 Feb 6;19(2):476. doi: 10.3390/ijms19020476
- Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46(8):621-8. doi: 10.1080/10408390500466174
- Han YM, Yoon H, Lim S, Sung MK, Shin CM, Park YS, Kim N, Lee DH, Kim JS. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver. 2017 May 15;11(3):363-369. doi: 10.5009/gnl16333
- Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis. 1989 Mar;3(1):55-7.
- Couturier E, van Onderbergen A, Bosson D, Neve J. Circadian variations in plasma zinc and cortisol in man. J Trace Elem Electrolytes Health Dis. 1988 Dec;2(4):245-9.
- Goode HF, Robertson DA, Kelleher J, Walker BE. Effect of fasting, self-selected and isocaloric glucose and fat meals and intravenous feeding on plasma zinc concentrations. Ann Clin Biochem. 1991 Sep;28 ( Pt 5):442-5. doi: 10.1177/000456329102800503
- Guillard O, Piriou A, Gombert J, Reiss D. Diurnal variations of zinc, copper and magnesium in the serum of normal fasting adults. Biomedicine. 1979 Nov;31(7):193-4.
- Singh A, Smoak BL, Patterson KY, LeMay LG, Veillon C, Deuster PA. Biochemical indices of selected trace minerals in men: effect of stress. Am J Clin Nutr. 1991 Jan;53(1):126-31. doi: 10.1093/ajcn/53.1.126
- Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3137-41. doi: 10.1073/pnas.87.8.3137
- Rofe AM, Philcox JC, Coyle P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J. 1996 Mar 15;314 ( Pt 3)(Pt 3):793-7. doi: 10.1042/bj3140793
- Prasad AS. Laboratory diagnosis of zinc deficiency. J Am Coll Nutr. 1985;4(6):591-8. doi: 10.1080/07315724.1985.10720101
- Solomons NW. On the assessment of zinc and copper nutriture in man. Am J Clin Nutr. 1979 Apr;32(4):856-71. doi: 10.1093/ajcn/32.4.856
- Hobisch-Hagen P, Mörtl M, Schobersberger W. Hemostatic disorders in pregnancy and the peripartum period. Acta Anaesthesiol Scand Suppl. 1997;111:216-7.
- Lombeck I, Fuchs A. Zinc and copper in infants fed breast-milk or different formula. Eur J Pediatr. 1994 Oct;153(10):770-6. doi: 10.1007/BF01954500
- Maxfield L, Shukla S, Crane JS. Zinc Deficiency. [Updated 2022 Nov 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493231
- Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017 May;13(5):727-741. doi: 10.1016/j.soard.2016.12.018
- Freitas BA, Lima LM, Moreira ME, Priore SE, Henriques BD, Carlos CF, Sabino JS, Franceschini Sdo C. Micronutrient supplementation adherence and influence on the prevalences of anemia and iron, zinc and vitamin A deficiencies in preemies with a corrected age of six months. Clinics (Sao Paulo). 2016 Aug;71(8):440-8. doi: 10.6061/clinics/2016(08)06
- Sriram K, Lonchyna VA. Micronutrient supplementation in adult nutrition therapy: practical considerations. JPEN J Parenter Enteral Nutr. 2009 Sep-Oct;33(5):548-62. doi: 10.1177/0148607108328470
- Narváez-Caicedo C, Moreano G, Sandoval BA, Jara-Palacios MÁ. Zinc Deficiency among Lactating Mothers from a Peri-Urban Community of the Ecuadorian Andean Region: An Initial Approach to the Need of Zinc Supplementation. Nutrients. 2018 Jul 5;10(7):869. doi: 10.3390/nu10070869
- Dao DT, Anez-Bustillos L, Cho BS, Li Z, Puder M, Gura KM. Assessment of Micronutrient Status in Critically Ill Children: Challenges and Opportunities. Nutrients. 2017 Oct 28;9(11):1185. doi: 10.3390/nu9111185
- Pediatric Acrodermatitis Enteropathica. https://emedicine.medscape.com/article/912075-overview#a2
- Acrodermatitis Enteropathica. https://emedicine.medscape.com/article/1102575-overview#a2