Acute chest syndrome
Acute chest syndrome is a term used to cover conditions characterized by fever and/or new respiratory symptoms such as chest pain, cough, hypoxia (low oxygen level) accompanied by the presence of a new pulmonary infiltrate on chest X-ray 1. Acute chest syndrome is a frequent cause of acute lung disease in children with sickle cell disease. Patients may present with acute chest syndrome or may develop this complication during the course of a hospitalization for acute vaso-occlusive crises 1. Acute chest syndrome may be the result of sickling in the small blood vessels in the lungs causing a pulmonary infarction/emboli or viral or bacterial pneumonia. Acute chest syndrome is associated with prolonged hospitalization, increased risk of respiratory failure, and the potential for developing chronic lung disease. Acute chest syndrome is one of the most common causes of death in sickle cell anemia patients.
The cause of acute chest syndrome is often multifactorial 1. One of the proposed mechanisms involves increased adhesion of sickle red cells to pulmonary microvasculature in the presence of hypoxia. Other commonly associated causes include infection, pulmonary fat embolism, and infarction. Infection is a common cause in children, whereas adults usually present with pain crises. Several risk factors have been identified in children to be associated with increased incidence of acute chest syndrome. These include younger age, severe sickle cell disease genotypes (SS or Sβ0 thalassemia), lower fetal hemoglobin concentrations, higher steady-state hemoglobin levels, higher steady-state white blood cell counts, history of asthma, and tobacco smoke exposure. Opiate overdose and resulting hypoventilation can also trigger acute chest syndrome.
Acute chest syndrome may develop as a single event, or during a painful vaso-occlusive crisis. The clinical course is usually self-limited when small areas of the lung tissue are involved, but without proper care, acute chest syndrome can rapidly progress and result in death. The spectrum of clinical manifestations can range from mild respiratory illness to acute respiratory distress syndrome. The presence of severe hypoxemia is a useful predictor of severity and outcome.
Chest pain when breathing is the most common presenting complaint in adults. Fever, cough, tachypnea (abnormally rapid breathing), hypoxemia (an unusually low concentration of oxygen in the blood), or abdominal pain are common presentations for infants and children.
It is always best to rule out infection in these cases and obtain appropriate blood cultures and serologic studies. There may or may not be radiographic evidence (X-ray) of pulmonary infiltrates at the initial time of symptoms. Rib infarction, stomach ulcer, or gallbladder problems can also result in chest pain and should be checked as well.
Prompt diagnosis and management with intravenous fluids, analgesics, aggressive incentive spirometry, supplemental oxygen or respiratory support, antibiotics, and transfusion therapy, are key to the prevention of clinical deterioration. Bronchodilators should be considered if there is history of asthma or in the presence of acute bronchospasm. Treatment with hydroxyurea should be considered for prevention of recurrent episodes.
Acute chest syndrome key points 2:
- Acute chest syndrome is defined as an acute illness characterized by fever and/or respiratory symptoms, accompanied by a new pulmonary infiltrate on chest X‐ray. Severe hypoxia is a useful predictor of severity and outcome.
- Acute chest syndrome has a multifactorial aetiology and an infective cause is common and this should be considered in treatment algorithms.
- Patients with sickle cell disease can present with acute chest syndrome, or it may develop sometime after onset of severe pain. Therefore, vigilance should be maintained throughout hospital admission.
- Clinicians should maintain a high index of suspicion of acute chest syndrome in patients who have chest symptoms and signs, especially if hypoxic, even in the presence of a normal chest X‐ray.
- Pulmonary embolism, fluid overload, opiate narcosis and hypoventilation may cause or trigger acute chest syndrome and should be considered when a diagnosis of acute chest syndrome is made as these conditions may require additional treatment.
- Acute chest syndrome can be a severe life‐threatening condition. Early recognition of progression to acute respiratory failure is vital.
- Patients should be monitored for predictors of severity which include worsening hypoxia, increasing respiratory rate, decreasing platelet count, decreasing haemoglobin concentration, multilobar involvement on chest X‐ray and neurological complications.
- Patients should be treated aggressively irrespective of their sickle genotype.
- Essential investigations for the diagnosis and management of acute chest syndrome are plain chest X‐ray, full blood count, basic biochemistry tests (creatinine and liver function) and blood group and screen (or crossmatch). Blood cultures, sputum for microscopy and culture and sputum and nasopharyngeal aspirate for viral testing including influenza A (and H1N1 subtype), influenza B, metapneumovirus, adenovirus, parainfluenza and respiratory syncytical virus should also be performed if clinically indicated.
- Arterial blood gases analysis should be performed in adults with oxygen saturation (SpO2) ≤94% on air.
- Computerized tomography (CT) of the chest and V/Q scanning are not helpful in the acute setting. A CT pulmonary angiogram (CTPA) is recommended if there is a high clinical suspicion of pulmonary embolism.
- All hospitals should have a treatment pathway for acute chest syndrome, which should include a referral pathway to the high dependency unit (HDU‐level 2) and intensive care unit (ICU – level 3).
- All patients with acute chest syndrome should be given prompt and adequate pain relief.
- Incentive spirometry has proven benefit in preventing acute chest syndrome in patients with chest or rib pain and should be also considered in all patients with acute chest syndrome.
- Antibiotics, with cover for atypical organisms, should be used even if blood cultures and sputum cultures are negative. Anti‐viral agents should be used if there is a clinical suspicion of H1N1 infection.
- Early simple (‘top‐up’) transfusion should be considered early in the hypoxic patient but exchange transfusion is necessary if there are severe clinical features or evidence of progression despite initial simple transfusion.
- Blood should be sickle‐negative and fully matched for Rh (C, D and E type) and Kell. A history of previous red cell antibodies should be sought and appropriate antigen‐negative blood given.
- The critical care team should be consulted early for respiratory support.
- Bronchodilators should be used if there are clinical features suggestive of a history of asthma or evidence of acute bronchospasm.
- Ensure that all patients are offered penicillin V prophylaxis and pneumococcal polysaccharide vaccination in addition to pneumococcal conjugate vaccine, and appropriate seasonal vaccination.
- Hydroxycarbamide should be recommended for prevention of recurrent acute chest syndrome.
- Consider chronic transfusion for prevention of recurrent acute chest syndrome if hydroxycarbamide therapy is not effective.
- In children, consider stem cell transplantation for prevention of recurrent acute chest syndrome if hydroxycarbamide therapy is not effective.
Acute chest syndrome causes
Acute chest syndrome is a form of acute lung injury in sickle cell disease. Acute chest syndrome can occur due to multiple factors. A low level of oxygen inside the lungs makes it easier for sickled red blood cells to stick to blood vessels, resulting in decreased blood flow. This may cause a blockage in small blood vessels, leading to more severe hypoxia. Other causes include infections, pulmonary fat embolism (a condition in which fat particles enter the blood vessels in the lung), and infarction (tissue death due to blocked blood supply).
Acute chest syndrome pathophysiology
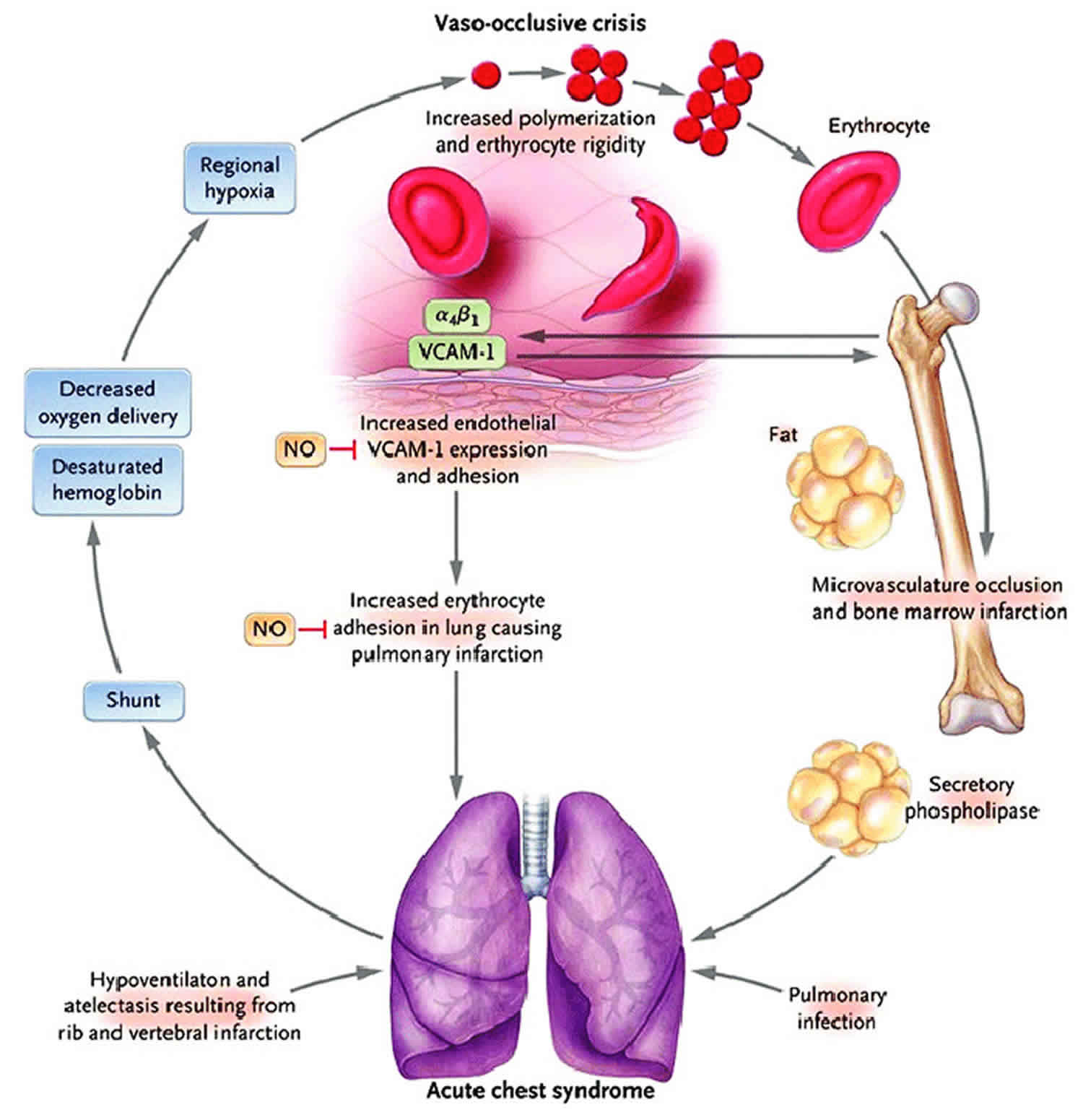
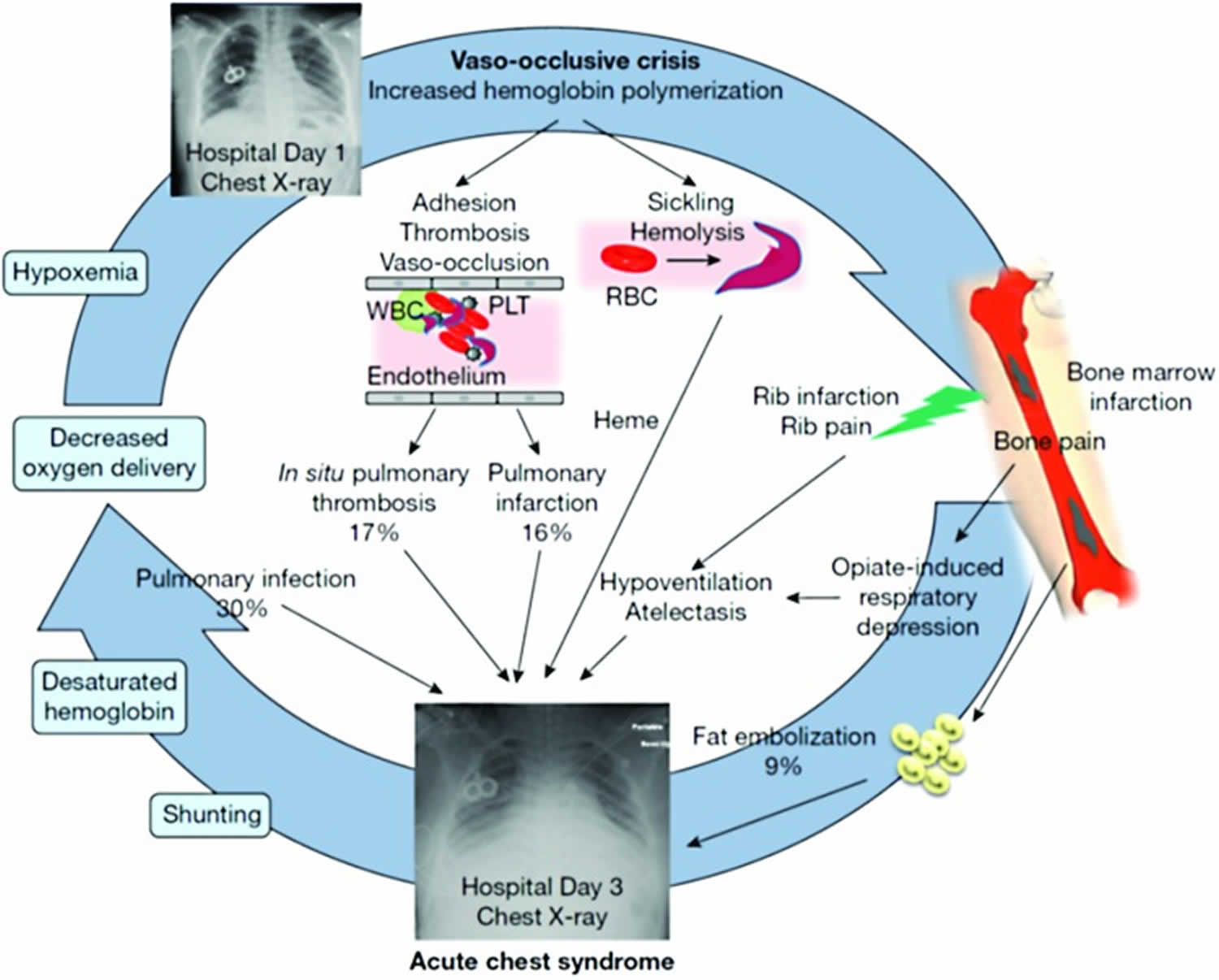
The development of acute chest syndrome represents a vicious cycle of lung infarction, inflammation, and atelectasis leading to ventilation–perfusion mismatch, hypoxemia, and acute increases in pulmonary artery and right ventricular pressures 3. At the cellular level, in the presence of low alveolar oxygen tension, abnormal rheology of the sickled red blood cells facilitates adhesion to each other, leukocytes, and the vascular endothelium, resulting in vaso-occlusion and tissue hypoxia. These interactions also cause the release of inflammatory cytokines which promote acute and chronic inflammation in the airways by virtue of being in close proximity with the vasculature 4. Evidence for this pathophysiological mechanism comes from markedly elevated plasma levels of vascular cell adhesion molecule-1, an adhesion receptor on endothelial cells, in patients with acute chest syndrome along with significantly lower levels of cytoprotective factors such as nitric oxide (NO) metabolites 5. The three major identifiable etiologies for the development of acute chest syndrome are pulmonary infections, pulmonary fat embolism from necrotic bone marrow, and pulmonary infarction as outlined in Figure 1.
Figure 1. Pathogenesis of acute chest syndrome
Footnote: A vicious cycle in the pathogenesis of acute chest syndrome, involving red blood cell sickling, cellular adhesion, hemolysis, and vaso-occlusion. Common causes for the development of ACS include fat embolism, pulmonary infection, pulmonary infraction, and hypoventilation.
Abbreviations: ACS = acute chest syndrome; PLT = platelet; RBC = red blood cell; WBC = white blood cell.
[Source 6 ]Pulmonary infection
The multicenter National Acute Chest Syndrome Study (NACSS) evaluated 671 acute chest syndrome episodes in 538 patients and nearly half of the patients were children and adolescents 6. A specific cause for acute chest syndrome was found in only 38% of episodes with infection as a leading cause in children. The infectious organisms which were identified in the study included Chlamydia pneumoniae, Mycoplasma pneumoniae, respiratory syncytial virus (RSV), Staphylococcus aureus, Streptococcus pneumoniae, and other pathogens in decreasing order of frequency. Viral infections were more common in children <10 years of age. Infections can precipitate an acute chest syndrome episode in a susceptible sickle cell disease patient by inducing an excessive inflammatory lung injury response in the presence of a damaged lung microvasculature 7. This has been further demonstrated in transgenic mouse models of homozygous sickle cell disease (HbSS), which develop lung injury at low endotoxin levels that do not adversely affect the wild-type mice 8. The incidence of S. pneumoniae infection in the recent years has decreased as more effective vaccines are available and widely used. Influenza, notably the H1N1 strain, also produces seasonal outbreaks of acute chest syndrome and may cause severe disease 9. Parvovirus B19 through its association with bone marrow infarction/necrosis and potentially pulmonary fat embolism can also result in acute chest syndrome 10.
Pulmonary fat embolism
Pulmonary fat embolism, with or without pulmonary infection, can cause acute chest syndrome in both adults and children 6. During acute pain episodes, infarcted and necrosed bone marrow (especially of the pelvis and femur) release fat droplets in the bloodstream, which embolize to the lungs. Fat emboli in the lung vasculature are metabolized to free fatty acids, including secretory phospholipase A2 (sPLA2), which mediate alveolar inflammation and endothelial injury 11. Diagnosis of pulmonary fat embolism in National Acute Chest Syndrome Study (NACSS) was based on quantitative evaluation of oil red-positive fat-laden alveolar macrophages in bronchoalveolar lavage (BAL) or induced sputum specimens. Although this method is sensitive for the diagnosis of pulmonary fat embolism, its specificity and clinical usefulness is questionable since fat-laden alveolar macrophages are also associated with microaspiration, gastroesophageal reflux-related respiratory disease, and hyperlipidemia 12. The presence of these additional factors can influence the detection of the fat-laden macrophages.
Pulmonary infarction and in situ thrombosis
Pulmonary infarction is another major contributor to the development of acute chest syndrome and was determined to be the cause in 16% of acute chest syndrome episodes in National Acute Chest Syndrome Study (NACSS). This occurs as a result of increased adhesion of sickled red blood cells to the endothelium causing vaso-occlusion, which leads to ventilation–perfusion mismatch and exacerbation of hypoxemia 13. In a small subset of patients, computed tomography (CT)–pulmonary angiography identified subsegmental thromboembolism in 17% of acute chest syndrome episodes without associated peripheral thrombosis, possibly suggestive of in situ thrombosis leading to infarction 14. Furthermore, there is a high prevalence of pulmonary embolism in patients with sickle cell disease associated with thrombosis in the distal venous circulation 15. This underappreciated entity may contribute to the pulmonary symptoms associated with acute chest syndrome and also increase the risk of mortality 16.
Hypoventilation
Splinting caused by rib infarction can lead to decreased respiratory effort resulting in hypoventilation and atelectasis 17. This may be further exacerbated by excessive opioid administration or following general anesthesia 18. As a result, pooling of secretions at lung bases occurs and predisposes to ventilation–perfusion mismatch and tissue hypoxia.
Biomarkers of acute chest syndrome
Some investigators have sought to define objective biomarkers to predict the occurrence of acute chest syndrome based on the understanding of the pathophysiology. Serum concentration of sPLA2 was shown to be elevated in a small group of patients with acute chest syndrome and also correlated with clinical severity 11. In a follow-up study, elevation was detected even 24–48 hours before clinical onset of acute chest syndrome in children during hospitalization for vaso-occlusive crises 19. These findings have been variably replicated with elevated levels found in 80% of subjects with acute chest syndrome participating in a multicenter trial 20, but with a poor positive predictive value of 24% in another cohort of patients hospitalized for acute pain episodes 21. Moreover, the time it takes to perform the test makes it impractical for clinical use as enzyme-linked immunosorbent assay-based assays are not widely available in clinical laboratories 11.
C-reactive protein (CRP), an acute-phase reactant, was suggested as a surrogate for sPLA2 and was found to have serum concentrations that paralleled those of sPLA2 during the hospital course of 20 patients with sickle cell disease admitted for vaso-occlusive crises and acute chest syndrome 22. Whether CRP can be used as a substitute for predicting development of acute chest syndrome in patients with vaso-occlusive crises is not clear. Another acute-phase reactant, pentraxin-related protein (PTX3), has been found to be elevated in sickle cell disease patients with vaso-occlusive crises, similar to the levels seen in other inflammatory and ischemic states 23. Patients with higher initial PTX3 concentrations have been found to be more likely to develop acute chest syndrome and were observed to have further increase in PTX3 levels upon progression to acute chest syndrome 24. But there is a lack of feasibility in using these biomarkers in the clinical setting to predict occurrence of acute chest syndrome as these tests are not widely available.
Risk factors for developing acute chest syndrome
Several prospective and retrospective studies have attempted to identify risk factors for the prediction of development, recurrence, and severity of acute chest syndrome. Based on these data, Table 1 lists risk factors that have been found to be associated with the development of acute chest syndrome episodes.
Table 1. Clinical and laboratory predictors of development of acute chest syndrome
| Risk factor | References |
|---|---|
| Young age | 25 |
| Low HbF | 25 |
| High steady-state Hb | 25 |
| High steady-state WBC | 26 |
| Severe genotypes (HbSS and HbSβ0 thalassemia) | 25 |
| >3 severe VOC episodes in the preceding year | 26 |
| Asthma/airway hyperreactivity | 26 |
| Tobacco smoke exposure | 27 |
| Recent surgery | 28 |
Abbreviations: Hb = hemoglobin; HbF = fetal hemoglobin; HbS = sickle hemoglobin; HbSS = homozygous sickle cell disease; VOC = vaso-occlusive crises; WBC = white blood cell.
[Source 1 ]In the prospective multicenter Cooperative Study of Sickle Cell Disease (CSSCD), younger age, lower fetal hemoglobin (HbF) concentration, higher steady-state Hb, and higher steady-state white blood cell (white blood cell) counts were found to be significant risk factors for acute chest syndrome 25. Those with more severe genotypes (HbSS and HbSβ0 thalassemia) had a greater risk of developing acute chest syndrome than the milder ones (HbSC and HbSβ+ thalassemia) 25. In a retrospective study on 175 pediatric patients hospitalized for vaso-occlusive crisis, a significantly higher white blood cell (19.1 ± 4.8/cmm versus 13.2 ± 5.2/cmm) and lower admitting Hb level from baseline (7.7 ± 1.2 g/dL versus 9.3 ± 2.0 g/dL) were found in those who developed acute chest syndrome 29. These data suggest that a higher steady-state Hb level likely promotes vaso-occlusive crisis by increasing blood viscosity, and acute hemolysis following vaso-occlusive crisis may contribute to the development of lung injury.
Higher levels of free heme (nonprotein bound) in plasma, a byproduct of hemolysis, were associated with increased odds of developing acute chest syndrome [odds ratio (OR) = 2.56] in a cohort of children with sickle cell disease 30. This observation is supported by the recent findings of a polymorphism in the promoter region of the heme oxygenase-1 gene, involved in catabolism of heme, to be associated with degree of hemolysis and severity of acute chest syndrome 31. A recent study in sickle cell mouse model suggests that extracellular hemin (the oxidized prosthetic moiety of Hb) may contribute to acute chest syndrome pathogenesis through toll-like receptor and provides a proof of principle for using recombinant hemopexin (a scavenger protein of free heme) as a therapeutic strategy for acute chest syndrome in mice 32.
In a large cross-sectional study, Pulmonary Hypertension and the Hypoxic Response in sickle cell disease, involving pediatric patients with HbSS (homozygous sickle cell disease) and other sickle cell genotypes (N = 503), acute pulmonary events (either acute chest syndrome or pneumonia) were found to be independently associated with >3 severe pain episodes in the preceding year irrespective of the genotype 26. In patients with HbSS, significant associations for development of acute pulmonary events were also found with history of asthma, higher steady-state tricuspid regurgitant velocity (TRV), and higher white blood cell count. The prognostic significance of higher tricuspid regurgitant velocity in children is unclear as it has not been found to be particularly associated with acute chest syndrome in other studies 33.
Another risk factor that has been shown to predict recurrence of acute chest syndrome episodes is a history of acute chest syndrome in younger age, especially in the first 3 years of life, as reported by two independent cohorts of pediatric patients 34. Moreover, the highest incidence rate of recurrence has been reported to be within 6 months from the last event 35. Perhaps pulmonary injury from previous acute chest syndrome episodes predisposes to increased susceptibility to recurrent events or alternatively a genetic predisposition underlies the development of frequent acute chest syndrome episodes 36. A genetic association with acute chest syndrome in patients with sickle cell disease, particularly in the younger age group, has been identified in the form of a single nucleotide involving COMMd7, which is a gene highly expressed in the lung that interacts with nuclear factor-κB signaling to control inflammatory responses 37.
Certain risk factors are danger signals for a severe disease course, such as an arterial:alveolar oxygen gradient of >30 (on room air) as reported from a small series in children 38. Multilobar involvement, platelet count of <200,000/cumm at diagnosis, and history of cardiac disease were found to be independent predictors of respiratory failure in the National Acute Chest Syndrome Study 6. A decrease in platelet count of more than 10% from steady state when admitted for vaso-occlusive crisis was also reported to be a significant predictor for developing acute chest syndrome (OR = 6.90) in a retrospective study of adult patients with sickle cell disease 39.
General anesthesia, asthma/airway hyperreactivity, and smoking may act as triggers for the development of acute chest syndrome in sickle cell disease. Patients undergoing an abdominal surgery, such as splenectomy and/or cholecystectomy, are especially at risk for developing acute chest syndrome postoperatively secondary to hypoventilation/atelectasis 28. Tobacco smoke exposure (TSE) either primary in adolescents and adults or secondary (at all ages) has been shown to be associated with increased rates of vaso-occlusive crisis and acute chest syndrome 27. The mechanisms underlying the association between tobacco smoke exposure and acute chest syndrome, particularly in young children, could be related to impairment of lung growth/development in utero from secondhand tobacco smoke exposure, increased viral respiratory illnesses, airway inflammation, and increased expression of adhesion markers. Secondhand smoke exposure can also cause pulmonary function abnormalities such as lower airway obstruction in children with sickle cell disease 40.
Asthma or airway hyperreactivity and acute chest syndrome
Asthma is common in children with sickle cell disease with occurrence estimates of 15%–28% 41 and at least 55% have airway hyperreactivity without a clinical diagnosis of asthma 42. Asthma exacerbations in children with sickle cell disease have been associated with increased incidence of acute chest syndrome episodes at a young age (median age of 2.4 years) 43. Those with asthma are five times more likely (OR = 4.9) to present with respiratory symptoms at the time of a vaso-occlusive crisis 44. Several plausible explanations for the relationship between asthma and acute chest syndrome or vaso-occlusive crisis are, ventilation–perfusion mismatch, synergistic inflammatory response, and vascular leak. Although the importance of comorbid asthma in sickle cell disease outcomes is being increasingly recognized, there still remain substantial gaps in diagnosis and management.
Acute chest syndrome prevention
Acute chest syndrome is the second most common cause for hospitalization after vaso-occlusive crisis in patients with sickle cell disease. Recurrent episodes of acute chest syndrome in childhood may lead to sequelae such as obstructive and/or restrictive abnormalities, fibrosis, chronic hypoxemia, and early mortality 45. Thus, it is imperative to reduce the frequency of future episodes by implementing preventive interventions.
Prophylactic antibiotic administration with Penicillin VK, annual influenza (including H1N1) vaccination from 6 months of age, 23-valent pneumococcal polysaccharide vaccine (≥2 years of age), 13-valent pneumococcal conjugate vaccine (shortly after birth), and avoidance of tobacco smoke exposure may be beneficial in decreasing risk of developing acute chest syndrome. All hospitalized patients with sickle cell disease should be carefully examined for respiratory symptoms and be continuously monitored by pulse oximetry to detect changes in oxygen saturation. The routine use of incentive spirometry at a prescribed regimen of 10 puffs every 2 hour (while awake), in the setting of acute vaso-occlusive crisis and postsurgical state, has been shown to be effective in preventing acute chest syndrome 46. Patients hospitalized for chest and back pain often require opioids to achieve analgesia to reduce respiratory splinting, doses of which should be optimally titrated to avoid causing oversedation and atelectasis. The secondary effects of hypoventilation and atelectasis resulting from opioids can be overcome by incentive spirometry. Preoperative blood transfusion is generally recommended in patients undergoing major surgery involving general anesthesia to prevent acute chest syndrome postoperatively 47.
Asthma, chronic obstructive pulmonary disease (COPD), restrictive lung disease, and obstructive sleep apnea are prevalent in 20%–48% in sickle cell disease individuals 48. These respiratory disorders are associated with increased morbidity such as higher rates of acute chest syndrome and decreasing lung function over time 49. There is also strong evidence for asthma and decreased FEV1 percent to be independent risk factors for mortality in sickle cell disease 50. Keeping this in mind, expert panel report from National Heart, Lung, and Blood Institute recommends assessing for signs and symptoms of respiratory problems by history and physical in patients with sickle cell disease during routine clinic visits. If found to be symptomatic, objective assessment using pulmonary function tests should be done to determine the cause and institute appropriate treatment of the identified problem 51.
A single episode of acute chest syndrome at any age should prompt discussion with the family regarding therapy with hydroxyurea, a potent inducer of HbF. It has been shown to be safe in both adults and children, including infants, and higher HbF levels have been inversely correlated with the development of acute chest syndrome 52. In addition, hydroxyurea decreases the white blood cell count, which likely has a positive effect on decreasing endothelial inflammation and vaso-occlusion 53. Chronic transfusion therapy may also have a protective effect for acute chest syndrome especially in those children with recurrent episodes of acute chest syndrome, defined as two or more episodes within a 2-year period, and who do not respond or are not compliant with hydroxyurea 54. This effect has been demonstrated during the randomized Stroke Prevention Trial in which a dramatic reduction in acute chest syndrome events was seen in the chronically transfused versus observation group (2.2 versus 15.7 events per 100 patient years) 55. However, the use of chronic transfusions is also associated with additional morbidity of transfusional hemosiderosis, potential for alloimmunization, and includes risks of transmission of infections, and may not be an optimal long-term strategy. Lastly, stem cell transplant may be considered for recurrent acute chest syndrome that is refractory to hydroxyurea while weighing in the risks versus benefits, donor availability, and patient age 56.
Acute chest syndrome symptoms
Acute chest syndrome is characterized by fever, cough, chest pain and shortness of breath, hypoxia, and lung infiltrates. The condition may progress rapidly from mild hypoxia to respiratory failure and death. Symptoms can differ with age. Pain and difficulty breathing are the predominant symptoms in adults; fever, coughing, and wheezing are more common in children.
The severity of the condition can be predicted by indicators such as worsening hypoxia, increased respiratory rate, decreased platelet count, and decreased hemoglobin concentration.
Table 2. Signs and symptoms of acute chest sydrome in children and adults
| Symptoms | Children | Adults |
|---|---|---|
| Fever | +++ | ++ |
| Cough | ++ | ++ |
| Chest pain | + | ++ |
| Dyspnoea | + | ++ |
| Tachypnoea | + | + |
| Wheezing | + | +/− |
| Intercostal recession/nasal flaring | + | +/− |
| Skeletal pain | + | ++ |
| Hypoxia | ++ | +++ |
| Hemoptysis | +/− | + |
Footnote: +++ frequent (>80%), ++ common (50–79), +less common (10–49%), +/− infrequent (<10%).
[Source 57 ]Acute chest syndrome features include:
- Hypoxia – Evidence of acute chest syndrome should be sought in any patient who is unwell and hypoxic. Oxygen saturation (SpO2) ≤94% (on air) on pulse oximetry or a fall in SpO2 of 3% or more from baseline steady state values should prompt further action. Oxygen desaturation may not always correlate with degree of hypoxia and can be influenced by other factors, including chronic anaemia, so hypoxia may need to be confirmed by arterial blood gas (ABG) measurement. A number of patients are chronically hypoxic for reasons such as pulmonary hypertension and chronic sickle lung disease and any further deterioration should be considered significant. Hypoxia may precede clinical signs and chest X‐ray abnormalities.
- Fever.
- Tachypnea.
- Tachycardia.
- Wheeze.
- Chest signs including dullness to percussion, reduced air entry, crepitations, bronchial breath sounds, rhonchi and pleural rubs.
- Pleural effusions (more common in adults).
- Intercostal recession, nasal flaring and other signs of increased work of breathing may be may be seen in children.
Clinical signs often precede the chest X‐ray findings. In addition to the rather general nature of these signs, which could indicate a primary bacterial or viral pneumonic process, the chest examination may be normal. It is important if this is the case, that the diagnosis is not excluded at this stage. If there is strong clinical suspicion, close monitoring should be undertaken and, perhaps, therapy initiated. A normal chest examination is more likely to be encountered in children.
A classical episode of acute chest syndrome is defined as an acute illness characterized by the finding of a new segmental pulmonary infiltrate consistent with consolidation, but not atelectasis along with one or more new respiratory symptoms or signs, such as cough, chest pain, fever (>38.5°C), hypoxemia, and tachypnea 7. Various other definitions of acute chest syndrome have been used in clinical practice and research, but none considers rate of decline of respiratory status as a diagnostic criteria and thus lack specificity 58. Acute chest syndrome is often a clinical diagnosis especially in the presence of hypoxemia as radiographic changes may lag behind clinical findings. It can mimic bacterial pneumonia, asthma exacerbation, or bronchiolitis at presentation. It can also progress rapidly from mild hypoxemia to acute respiratory failure within 24 hour of onset of respiratory symptoms, which in itself constitutes a distinct clinical phenotype of acute chest syndrome 59. This form of rapidly progressive acute chest syndrome is seen more commonly in adults as compared with children with sickle cell disease, and is frequently associated with thrombocytopenia and multiorgan failure 59. The cause and underlying pathophysiological mechanisms of this subgroup of acute chest syndrome are unclear, but shares resemblance with other thrombotic microangiopathic syndromes 59.
The presentation, causal mechanisms, and natural course of acute chest syndrome differ considerably with age. The highest incidence of acute chest syndrome is in children <10 years of age 25 with frequently occurring triggers in the form of pulmonary infections 6. As a result, they usually present with fever, cough, and wheeze. Older children and adults more frequently present with dyspnea and chest pain and tend to follow a more severe course 6. Adults also tend to present with lower mean oxygen saturations because of a higher prevalence of pulmonary fat embolism 6. Children usually present with isolated upper or middle lobe involvement as compared with bilateral lower or multiple lobes in adults 6.
A close temporal relationship exists between acute chest syndrome and vaso-occlusive crisis. Nearly half of all acute chest syndrome episodes occur between 1 and 3 days after admission for severe vaso-occlusive crisis 6, suggesting that vaso-occlusive crisis may be a prodromal event for the development of acute chest syndrome. A retrospective report from a single institution revealed that approximately a third of patients who developed acute chest syndrome had sought acute care for painful vaso-occlusive crisiss (mostly chest and back) within 2 weeks of hospitalization for acute chest syndrome 60. Buchanan et al. 29 have suggested that opioid selection for the treatment of vaso-occlusive crisis episodes may have an impact on the development of acute chest syndrome, with patients receiving Nubain (Nalbuphine) less likely to develop acute chest syndrome; however, their results were confounded by the use of continuous infusion analgesia. This observation has not been studied in a prospective clinical trial.
Neurological complications, such as infarctive stroke, silent cerebral infarcts, and posterior reversible leukoencephalopathy syndrome, have been shown to be higher after severe episodes of acute chest syndrome in children 61. These neurological events have also been seen with platelet counts <200,000 per cumm in children hospitalized with acute chest syndrome 6.
Acute chest syndrome diagnosis
Acute chest syndrome can be diagnosed following thorough investigations that include a chest X‐ray, full blood count, and blood and liver function tests. Blood and sputum should also be tested to identify bacterial or viral infections. Arterial blood gas analyses should be performed to determine hypoxia, a useful predictor of the severity and outcome of the condition.
Challenges in the diagnosis of acute chest syndrome
Clinical features overlap with those of pneumonia in a patient without sickle cell disease. Acute chest syndrome is often precipitated by infection but treating acute chest syndrome as a purely infective episode may lead to progression and rapid clinical deterioration.
Other considerations when making a diagnosis of acute chest syndrome should include:
- Pulmonary embolism. May present with chest pain, dyspnoea and hypoxia. D‐dimers are unhelpful in sickle cell disease as levels are usually elevated, so testing should be avoided. If there is a high clinical suspicion of pulmonary embolism (i.e. sudden onset unilateral pleuritic pain that is not typical of sickle pain) treat for both conditions pending a computerized tomography pulmonary angiogram (CTPA). Acute chest syndrome may be complicated by pulmonary embolism or may occur secondary to pulmonary embolism and treatment will be required for both conditions simultaneously.
- Fluid overload. Fluid replacement is an integral part of the management of acute chest syndrome. However, overhydration may lead to pulmonary vascular congestion and pulmonary edema, especially in patients with decreased cardiac function. Close attention should be paid to fluid balance and a fluid balance chart must be maintained. Acute deterioration in a patient after blood transfusion should prompt consideration of this complication or transfusion‐related acute lung injury (TRALI).
- Opiate narcosis. Careful attention should be paid to avoid this untoward effect of opiates. Monitoring of respiratory rate, sedation and pain scores should be in place, with opiate narcosis being associated with a falling respiratory rate. Dose modification or discontinuation may be necessary and naloxone may be required if there is evidence of opiate toxicity. Opiate narcosis may trigger or worsen acute chest syndrome.
- Alveolar hypoventilation due to pain. Effective analgesia is necessary to prevent hypoxia and hypercapnia developing due to a restrictive ventilatory defect as a consequence of ongoing chest pain, in particular, in the context of patients with chronic sickle lung disease. This may contribute to the development of acute chest syndrome.
Essential investigations
The investigations required for the diagnosis and management of acute chest syndrome are standard tests available in acute district general hospitals. It should be stressed that clinical suspicion is vital to early diagnosis.
The following investigations are essential and should be performed on all patients with suspected acute chest syndrome:
- Chest radiograph.
- Full blood count.
- Basic biochemistry (creatinine and liver function tests).
- Blood group and screen (or crossmatch).
- Blood cultures.
- ABG measurement on room air in adults (if SpO2 ≤ 94% on room air). This should not be done on room air if patient is in obvious respiratory distress or if SpO2 saturations fall to <85% if oxygen is stopped briefly.
- Serology for atypical respiratory organisms and urine for Pneumococcal and Legionella antigen.
- Sputum for bacterial culture and sputum and nasopharyngeal aspirate for immunofluorescence or polymerase chain reaction (PCR) for viruses in patients with coryzal symptoms.
Chest X‐ray
Typical findings on plain chest radiography in acute chest syndrome are segmental, lobar or multilobar consolidation usually involving the lower lobes, or collapse with or without pleural effusion 62. Children are less likely to have pleural effusions, but are more likely to have upper or middle lobe disease 63. Radiological signs are often absent early in the illness 64, or if present, may underestimate the severity of hypoxia 65 and treatment must not be delayed because the changes on chest X‐ray are unremarkable. If initial chest X‐ray is normal, a repeat chest X‐ray should be performed if there is ongoing clinical suspicion. In the situation of unexplained hypoxia (no clinical features and no radiological signs on chest X‐ray), a computerized tomography (CT) scan of the pulmonary arteries should be considered. This will provide clinical data on the pulmonary vasculature as well as the lung parenchyma.
Full blood count
An acute fall in haemoglobin concentration and platelet count are often seen in acute chest syndrome. These are markers of disease severity. A decreasing platelet count to less than 200 × 109/l is an independent risk factor for neurological complications and the need for mechanical ventilation 66. Measurement of reticulocytes will confirm adequate bone marrow function and exclude red cell aplasia (erythrovirus B19 infection).
Biochemical tests
Patients with acute chest syndrome are at risk of developing multi‐organ failure as a result of systemic fat emboli and require monitoring of their renal and liver function. C‐reactive protein (CRP) levels, if elevated, may be used to follow the clinical progress of patients with acute chest syndrome. If there is evidence of co‐existent infection, the sensitivity and specificity of CRP levels as a biomarker of vaso‐occlusion is reduced, but an increasing CRP is still a useful marker of a worsening clinical condition.
Blood group and screen/crossmatch
Blood must be urgently ABO‐, Rh‐ (C, D and E) and Kell‐typed with antibody screening for all sickle cell patients at increased risk of acute chest syndrome (severe vaso‐occlusive crisis, previous acute chest syndrome), and for those with mild acute chest syndrome as their clinical condition may suddenly deteriorate and require emergency transfusion. Red cell alloimmunization is a common problem in sickle cell disease patients and can pose significant difficulties in their management 67. Blood for transfusion should be fully matched for Rh (C, D and E) and Kell type and should be sickle‐negative. If the patient is not known to the hospital, they should be asked if they are carrying an antibody card with details of their latest alloantibody profile and any previous alloantibodies detected. In addition every effort must be made to obtain the red cell phenotype and latest red cell alloantibody profile from the hospital where their usual care is provided. This is especially important because red cell alloantibodies often become undetectable over time in sickle cell patients 67, thereby increasing the risk of delayed haemolytic transfusion reaction if antigen positive blood is inadvertently transfused.
Blood culture
This must be performed in all patients with acute chest syndrome who are febrile although the yield is low 68.
Arterial blood gas sampling on room air (if SpO2 ≤ 94% on room air)
Although pulse oximetry can be used to provide a reliable estimate of arterial SpO2 in patients with sickle cell anaemia 69, ABG measurement is the gold standard in determining the partial pressure of oxygen and partial pressure of carbon dioxide in adults with suspected acute chest syndrome. An ABG on room air is useful for making the diagnosis of acute chest syndrome, assessing severity and for guiding decisions about the need for HDU/ITU admission and for urgent blood transfusion and is the investigation of choice for this reason in patients with SpO2 of ≤94% on room air, even if they have higher SpO2 with oxygen therapy. Patients in clear respiratory distress or in whom SpO2 falls rapidly to <85% when oxygen is removed need immediate medical review and escalation of therapy and ABGs on room air are unlikely to be needed to confirm a diagnosis or influence therapy. If samples for ABG measurement are taken whilst the patient is breathing oxygen then this must be acknowledged and the arterial‐alveolar (A‐a) gradient calculated to determine the degree of shunting and alveolar hypoventilation. This is unlikely to be feasible outside the HDU/ITU setting, but may be useful to guide the need for escalation of respiratory support. Arterial puncture is often very distressing to children and thus pulse oximetry is the mainstay of monitoring oxygenation. Venous or capillary samples give similar values to ABG in terms of pH, PaCO2, base excess and bicarbonate but poor correlation with PaO2 70.
A lower than normal PaO2 (11 kPa) on room air is common in clinical studies, but the prevalence varies with some studies reporting a prevalence of around 70% of cases 71. The mean PaO2 of patients with acute chest syndrome was 9·3 kPa (70 mmHg) and 9·2 kPa (69 mmHg) in two large studies 72, and in another study, nearly one‐fifth of patients had a PaO2 less than 8·0 kPa 68. In the majority of studies PaCO2 was in the normal range with a mean value of 4·7 kPa being recorded in 141 of 252 of first acute chest syndrome events in adults 68. However, an unexpectedly high prevalence of hypercapnia (46%) was reported in one study among adult patients with no evidence of opioid abuse 63. Experts suggest that a PaO2 less than 8 kPa on room air should be accepted as severe hypoxia and a PaCO2 of greater than 6 kPa as hypercapnia.
Infectious disease testing
Acute and convalescent samples for antibodies against atypical respiratory organisms, including M. pneumoniae, Chlamyophila pneumoniae and Legionella should be sent, if feasible, and for erythrovirus B19 if indicated by the clinical and haematological features 57. Mycoplasma infection may be suggested by red cell agglutination on a stained blood film and the presence of cold agglutinins in serum. The usual screening test for Legionella is by testing for the urinary antigen. PCR/Immunofluorescence testing for viruses in sputum and nasopharyngeal aspirate are generally performed as a virus panel for a range of viruses. This may include testing for influenza A (including H1N1 subtype), influenza B, metapneumovirus, adenovirus, parainfluenza and RSV but will be dictated by the clinical picture and local microbiological advice. Identification of specific infective organisms will help to guide antibiotic prescription and appropriate use of isolation facilities.
Other investigations
High‐resolution computerized tomography of the chest
In a study of ten children with moderate to severe acute chest syndrome, high‐resolution computerized tomography (HRCT) performed within 48 h of presentation accurately detected microvascular occlusion in areas of the lungs that looked normal on chest X‐ray, with an average sensitivity and specificity of 84% and 97% respectively 65. In contrast to chest X‐ray, the extent of microvascular occlusion on HRCT correlated with the clinical severity and degree of hypoxia 65.
However, in view of the high radiation dose delivered by CT, the use of this modality for diagnosis of acute chest syndrome is not recommended. The routine use of CT is also to be discouraged because of the tendency of acute chest syndrome to recur and also because people with sickle cell disease frequently require other radiological tests for a range of other indications. These considerations should not, however, detract from the use of HRCT where it is judged to be clinically appropriate. Likewise, a CTPA should be done if there is a clinical suspicion of pulmonary embolism.
Ventilation/perfusion (V/Q) lung scan
This usually reveals widespread perfusion defects with normal ventilation 73. However, the appearances may be confused with pulmonary emboli or diminished by the presence of other pathology, such as pleural effusions. The role of V/Q lung scanning in the diagnosis of acute chest syndrome has not been formally investigated in prospective studies and therefore it has a limited role in the investigation of this syndrome.
Bronchoalveolar lavage for bacterial and viral studies
The complication rate of bronchoscopy in patients with acute chest syndrome has been reported as 13%. The commonest reported complication was a transient fall on SpO2, but there was a small risk of mechanical ventilation following the procedure 57. In practice, bronchoalveolar lavage is only ever likely to be done in patients who have progressed to intubation and ventilation. These data, combined with the lack of immediate availability of bronchoscopy in most hospitals, support the use of bronchoscopy only to answer specific clinical questions based on the clinical features and first line investigations.
Additional investigations
Secretory phospholipase A2 (sPLA2) is an enzyme that is thought to release inflammatory free fatty acids from bone marrow lipid 74 and it was initially shown to be elevated in acute chest syndrome with the levels of phospholipase A2 correlating with the severity of acute chest syndrome. It has been suggested that measurement of levels of this enzyme may be useful in diagnosis of acute chest syndrome, but the test is not widely available 75 and more recent studies have shown a positive predictive value of only 24% in acute chest syndrome 76.
Oil‐Red‐O staining of bronchoalveolar lavage samples has been used to diagnose pulmonary fat embolism 63. Although acute chest syndrome patients with pulmonary fat embolism have a more severe clinical course 77, this is not standard practice as it is not necessary to demonstrate the occurrence of fat embolism in routine clinical practice.
Acute chest syndrome treatment
All patients with acute chest syndrome should be hospitalized for careful monitoring of their oxygenation and clinical status. Acute chest syndrome in sickle cell disease should be treated promptly with pain relief medication, intravenous fluids, aggressive incentive spirometry, which improves lung function, supplemental oxygen or respiratory support, antibiotics, and transfusion therapy. If the patient has a history of asthma, bronchodilators should be considered. Recurrent episodes can be prevented with hydroxyurea treatment.
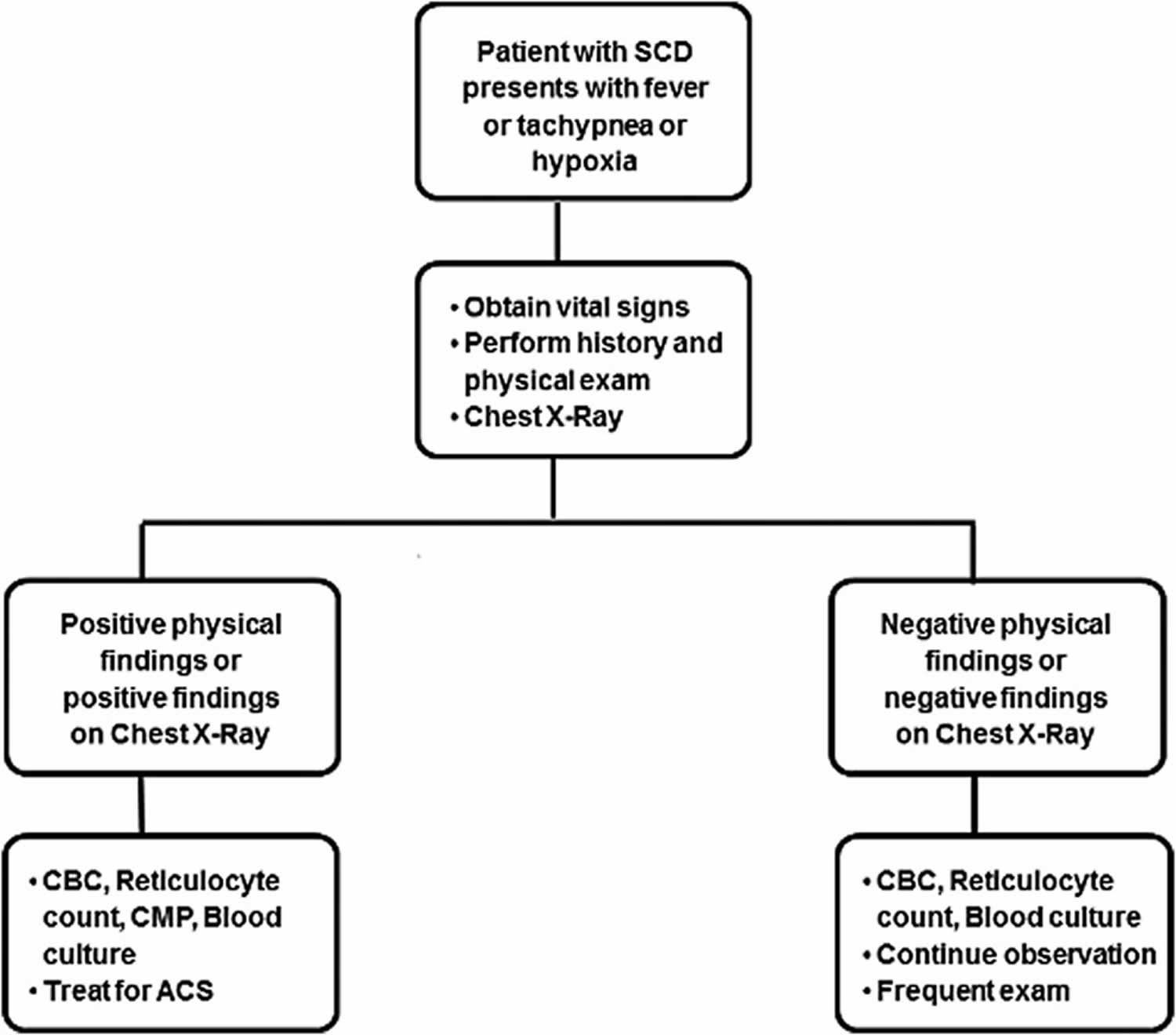
Patients benefit from early, aggressive intervention especially in the presence of risk factors such as multilobar involvement, coexisting respiratory diseases, and for whom blood is not readily available. Table 3 offers guidance for management based on an expert panel report from National Heart, Lung, and Blood Institute 51, which can be effectively put into clinical practice and lead to rapid resolution in majority of patients. As part of the initial workup, a child with sickle cell disease presenting with fever should have a complete blood count (CBC), reticulocyte count, blood culture, basic biochemistry, type and crossmatch, and a chest X-ray, irrespective of respiratory symptoms. A screening algorithm for acute chest syndrome in pediatric patients with sickle cell disease is presented schematically in Figure 2. Rapid screening tests in nasopharyngeal aspirates for RSV, influenza A (including H1N1 subtype), and influenza B, in season should be performed.
General principles of management in all patients with acute chest syndrome are supportive care with hydration, antibiotics, pain control, and maintenance of oxygenation and ventilation. Hydration is generally given with intravenously administered hypotonic fluids at approximately three-fourths of a maintenance rate as patients with acute chest syndrome may be at risk for pulmonary edema 78.
Figure 2. Screening algorithm for acute chest syndrome
Footnote: Screening algorithm for acute chest syndrome in pediatric patients with sickle cell disease.
Abbreviations: CBC = complete blood count, CMP = comprehensive metabolic panel; SCD = sickle cell disease.
[Source 1 ]The use of aggressive incentive spirometry while awake to prevent atelectasis is an important aspect of management 79. Alternatively, the use of mechanical chest physiotherapy maneuvers, such as positive expiratory pressure devices, may be considered in younger children who may not be able to coordinate incentive spirometry or is limited by chest wall pain 80. The use of respiratory therapy warrants further study in patients with sickle cell disease at high risk for acute chest syndrome. Supplemental oxygen should be given for oxygen saturation ≤94% and/or tachypnea. It is important to note that patients might have chronic hypoxemia as a result of conditions such as pulmonary hypertension and chronic sickle lung disease, but any deterioration in oxygen saturation from baseline steady state warrants prompt action. Noninvasive ventilation such as bilevel positive airway pressure may be used to improve oxygenation and decrease work of breathing in patients with acute chest syndrome. These benefits may prevent progression to respiratory failure and reduce the need for intubation and mechanical ventilation, but there is currently limited evidence regarding its precise role in managing children with acute chest syndrome 81. Indications for more intensive respiratory support include worsening hypoxemia, severe dyspnea, and respiratory acidosis (pH <7.35).
All patients with acute chest syndrome should be started on empiric antibiotic coverage for pneumococcal infection, commonly with third-generation cephalosporins. Vancomycin should be added for coverage of Methicillin-resistant S. aureus infection in severely ill patients. Coverage for atypical bacteria with macrolide or another antibiotic is also indicated in the treatment for acute chest syndrome, considering that these are the most common infectious agents and also contribute to severe disease 82. There are no clinical trials comparing the various antibiotic regimens 83. In the influenza season, empiric treatment with antiviral medication oseltamivir should be included, ideally started within 48 hour of onset of symptoms.
Pain arising from thoracic bone infarction (ribs, sternum, and thoracic spine) leads to alveolar hypoventilation 84 and is highly correlated with development of acute chest syndrome 85. The goal of effective pain management should be to achieve a balance between analgesia to reduce respiratory splinting and hypoventilation, while avoiding respiratory depression 86. The presence of chest pain in adults with sickle cell disease, as compared with nonchest pain in sickle cell disease, has been shown to be associated with the development of shallower breathing patterns along with reduced tidal volumes and resolution of the differences in tidal volumes after instituting opioid analgesia 87. Evidence from several randomized controlled trials (RCTs) and observational studies supports the use of around-the-clock administration of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids to achieve adequate analgesia 88. Recommendations regarding types of analgesics and administration protocols may be referred to those developed by National Heart, Lung, and Blood Institute based on expertise of the study panel and some were adapted from published guidelines from American Pain Society 51. NSAIDs solely can be used to treat mild–moderate pain with rapid addition of opioids if there is progression to severe pain. The NSAID, Ketorolac, is widely used, and is administered parenterally, but can only be used for 5 days. Morphine and Hydromorphone are commonly used as first-choice opioid analgesics. Patients with acute chest syndrome should be monitored for exacerbation of pain episodes and pain scores should be recorded along with vital signs to titrate analgesia. While not typical, the input of pain management specialists may be considered to guide pain management, particularly for patients who are sensitive to the sedative effects of opioids, or have chronic pain with tolerance to opioids.
Early and aggressive packed RBC (pRBC) transfusions, simple or exchange, (extended matching to D, C, E, and Kell antigens) result in improvements in oxygen saturation and A-a oxygen gradient 89. Transfusions have considerably improved outcomes as demonstrated in the DeNOVO trial in which none of the 30 patients who received packed red blood cells required mechanical ventilation 90. Recommendation for transfusion in acute chest syndrome from the National Heart, Lung, and Blood Institute expert panel is to administer simple transfusion (10 mL/kg packed red blood cells) if Hb is more than 1 g/dL below baseline, especially if baseline Hb is <9 g/dL 51. The goal should be to either increase the Hb concentration to 9–11 g/dL or reduce Hb S levels to <30%. It has been noted in observational studies that simple transfusion is as effective as exchange transfusion in improving clinical outcomes and is adequate in most circumstances as front-line therapy for acute chest syndrome 6. However, exchange transfusion should be considered in patients with severe or rapidly progressive illness requiring mechanical ventilation.
Children with sickle cell disease with history of acute chest syndrome have worse pulmonary lung functions than age-matched children with sickle cell disease who have not experienced acute chest syndrome. Various studies have reported a significant decrease in the percentage predicted FEV1 and FEV1/FVC ratio suggesting lower airway obstructive lung disease to be a common abnormality in children with acute chest syndrome 91. Thus, for patients with airway hyperreactivity/asthma, use of bronchodilator therapy every 4 hour is recommended irrespective of the presence of wheezing, even though its efficacy has not been tested in any randomized controlled trials 92. A short course of low-dose steroids has shown efficacy in attenuating the course of acute chest syndrome 93 and also decrease requirement for transfusion therapy 94. However, routine corticosteroid therapy for acute chest syndrome is controversial in patients with sickle cell disease as its use has been associated with rebound pain crisis, hospitalization 95 and hemorrhagic stroke 96. It is noteworthy that risk of readmission for pain with steroids was reduced if patients had received transfusion therapy during the episode 97.
If a patient does not improve with supportive care and empiric antibiotics, consider diagnostic bronchoscopy with bronchoalveolar lavage (BAL) to evaluate for other sources of infection and possible thoracocentesis if large effusions are present. In the presence of unexplained hypoxemia with absent clinical and X-ray findings, consider obtaining CT chest to evaluate pulmonary vasculature as well as lung parenchyma. In the presence of pulmonary embolism, treatment guidelines are the same as for the general population, although clinical research is ongoing to establish the role of anticoagulation for in situ thrombosis. The effectiveness of vasodilators such as inhaled nitric oxide has not been fully ascertained in clinical trials, but has shown some benefit in several case reports 98. Doppler echocardiogram should be performed in every patient admitted to the intensive care unit with acute chest syndrome to diagnose right ventricular failure secondary to pulmonary hypertension which is associated with high mortality 99. Although this is more common in the adult population, but may present in late adolescence.
Acute chest syndrome treatment guidelines
Table 3. General Guidelines for Management of Acute Chest Syndrome in Sickle Cell Disease
| A. Supportive care | |
| Goals | |
| 1. Adequate oxygenation | Maintain oxygen saturations >94% |
| Use noninvasive or mechanical ventilation if needed | |
| 2. Prevention of atelectasis | Incentive spirometry |
| 10 puffs q2 hours when awake | |
| 10 puffs q4 hours when sleeping | |
| Patient mobilization | |
| Chest physiotherapy | |
| 3. Hydration | Maintain fluids ¾ 1 × maintenance to avoid pulmonary edema |
| Avoid bolus IV fluids, unless hypotensive | |
| B. Transfusion therapy | |
| 1. Increase the Hb concentration to 9–11 g/dL or reduce HbS levels to <30% | Prompt institution of simple packed red blood cell transfusion |
| Consider exchange transfusion with severe hypoxemia (PaO2 < 70 mmHg), multilobar disease, or rapidly progressive disease | |
| C. Medications | |
| 1. Pain control to prevent splinting and atelectasis | Analgesia (NSAIDs ± Opioids) |
| 2. Respiratory infections | Empiric coverage for pneumococcus and atypical organisms (third-generation cephalosporin + Macrolide) ± Vancomycin if clinically unstable |
| Antivirals: Oseltamivir (Influenza season) | |
| 3. Presence of wheezing/ history of reactive airway disease | Bronchodilators |
Abbreviations: NSAID = nonsteroidal anti-inflammatory drug; pRBC = packed red blood cell.
[Source 51 ]Acute chest syndrome prognosis
The severity of acute chest syndrome is variable, ranging from a mild illness to a severe life‐threatening condition. In addition to the age‐dependent variation of clinical presentation, the age of the patient also influences severity. Although acute chest syndrome is more common in children (21 events per 100 person years in children vs. 8·7 events per 100 person years in adults) it tends to follow a milder course with infection frequently implicated in the aetiology. In contrast, acute chest syndrome in adults tends to be a more severe illness marked by severe hypoxia, a higher requirement for transfusion and higher mortality. It can be considered as a form of acute lung injury that can progress to acute respiratory distress syndrome progressing, albeit infrequently, to acute multi‐organ failure.
Acute chest syndrome can be severe in all sickle genotypes, with similar death rates per event in HbSS and HbSC 68. All patients should be treated aggressively, irrespective of their sickle genotype.
Hospital stay is often shorter in children (5 days vs. 10 days in adults) 68. Recurrence is a feature and some patients have multiple episodes with previous episodes increasing the likelihood of further similar events.
Acute respiratory failure (partial pressure of arterial oxygen on room air ≤8 kPa)
All patients, in particular, those with chest signs and symptoms can progress rapidly during acute chest syndrome to acute hypoxic respiratory failure and therefore regular SpO2 monitoring is essential. Predictors of acute respiratory failure include extensive lobar involvement and a history of cardiac disease 57.
Invasive and non‐invasive (including continuous positive airway pressure) mechanical ventilation
Mechanical ventilation has been reported as necessary in up to 20% of cases and it is more likely to be required in older patients. In the large multicentre National Acute Chest Syndrome Study, mechanical ventilation was provided for 13% of patients, of whom 81% recovered 57.
Other morbidity
Neurological features, such as altered mental status, seizures and strokes, may be associated with acute chest syndrome. Patients with neurological symptoms more often progress to acute respiratory failure and have a significantly higher mortality compared to those without neurological features 57. A recent history of acute chest syndrome is a risk factor for overt stroke, silent stroke and posterior reversible encephalopathy syndrome in children100.
An acute drop in haemoglobin concentration with an associated increase in markers of haemolysis prior to the onset of acute chest syndrome is common. Reported falls from steady state haemoglobin values have varied from 7 g/l for all genotypes 68, to 16–22·5 g/l depending on genotype 63. A greater fall in haemoglobin values has been documented for cases with bilateral lung involvement than for unilateral disease (28·3 g/l vs. 13·3 g/l) 101.
Mortality
Acute chest syndrome remains a leading cause of premature mortality in sickle cell disease. In a recent national survey in the United Kingdom, acute chest syndrome was the third most common cause of death reported in adults 102. It is a recognized risk factor for early death in HbSS patients above the age of 20 years 103. Respiratory failure is the most common cause of death. Other causes of death in these patients included pulmonary haemorrhage, cor pulmonale, overwhelming sepsis and cerebrovascular events.
Mortality rates in acute chest syndrome will be dependent in part on the appropriateness of medical management. Even with good medical treatment, overall mortality rates of up to 3% are reported, with the overall death rate in adults being four times higher than in children 57.
References- Jain S, Bakshi N, Krishnamurti L. Acute Chest Syndrome in Children with Sickle Cell Disease. Pediatr Allergy Immunol Pulmonol. 2017;30(4):191–201. doi:10.1089/ped.2017.0814 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733742
- Guideline on the management of acute chest syndrome in sickle cell disease. British Journal of Haematology, 2015,169,492–505 https://doi.org/10.1111/bjh.13348
- Mekontso Dessap A, et al. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med 2008; 177:646–653
- Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest 2000; 106:337–338
- Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood 1999; 94:1555–1560
- Vichinsky EP, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med 2000; 342:1855–1865
- Ballas SK, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol 2010; 85:6–13
- Sabaa N, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest 2008; 118:1924–1933
- Strouse JJ, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010; 116:3431–3434
- Godeau B, et al. Aplastic crisis due to extensive bone marrow necrosis and human parvovirus infection in sickle cell disease. Am J Med 1991; 91:557–558
- Styles LA, et al. Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood 1996; 87:2573–2578
- Gibeon D, et al. Lipid-laden bronchoalveolar macrophages in asthma and chronic cough. Respir Med 2014; 108:71–77
- Bope ET, Kellerman RD. Conn’s current therapy 2016. Philadelphia, PA: Elsevier Health Sciences, 2015
- Mekontso Dessap A, et al. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med 2011; 184:1022–1029
- Brunson A, et al. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol 2017; 178:319–326
- Manci EA, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol 2003; 123:359–365
- Gelfand MJ, Daya SA, Rucknagel DL, Kalinyak KA, Paltiel HJ. Simultaneous occurrence of rib infarction and pulmonary infiltrates in sickle cell disease patients with acute chest syndrome. J Nucl Med 1993; 34:614–618
- Kopecky EA, Jacobson S, Joshi P, Koren G. Systemic exposure to morphine and the risk of acute chest syndrome in sickle cell disease. Clin Pharmacol Ther 2004; 75:140–146
- Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA. Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease. Blood 2000; 96:3276–3278
- Ballas SK, et al. Secretory phospholipase A2 levels in patients with sickle cell disease and acute chest syndrome. Hemoglobin 2006; 30:165–170
- Styles L, et al. Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: results of a feasibility study (PROACTIVE). Br J Haematol 2012; 157:627–636
- Bargoma EM, et al. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vasoocclusive crisis or acute chest syndrome. Blood 2005; 105:3384–3385
- Nur E, et al. Plasma levels of pentraxin-3, an acute phase protein, are increased during sickle cell painful crisis. Blood Cells Mol Dis 2011; 46:189–194
- Elshazly SA, Heiba NM, Abdelmageed WM. Plasma PTX3 levels in sickle cell disease patients, during vaso occlusion and acute chest syndrome (data from Saudi population). Hematology 2014; 19:52–59
- Castro O, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994; 84:643–649
- Paul R, et al. Clinical correlates of acute pulmonary events in children and adolescents with sickle cell disease. Eur J Haematol 2013; 91:62–68
- Sadreameli SC, Eakin MN, Robinson KT, Alade RO, Strouse JJ. Secondhand smoke is associated with more frequent hospitalizations in children with sickle cell disease. Am J Hematol 2016; 91:313–317
- Vichinsky EP, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213
- Buchanan ID, Woodward M, Reed GW. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatr Blood Cancer 2005; 45:716–724
- Adisa OA, et al. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol 2013; 162:02–705
- Bean CJ, et al. Heme oxygenase-1 gene promoter polymorphism is associated with reduced incidence of acute chest syndrome among children with sickle cell disease. Blood 2012; 120:3822–3828
- Ghosh S, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest 2013; 123:4809–4820
- Chaudry RA, et al. Paediatric sickle cell disease: pulmonary hypertension but normal vascular resistance. Arch Dis Child 2011; 96:131–136
- Araujo JG, et al. Risk factors for acute chest syndrome in patients from low socioeconomic background: a cohort study from Sergipe, Brazil. J Pediatr Hematol Oncol 2011; 33:421–423
- Vance LD, et al. Increased risk of severe vaso-occlusive episodes after initial acute chest syndrome in children with sickle cell anemia less than 4 years old: sleep and asthma cohort. Am J Hematol 2015; 90:371–375
- Fertrin KY, Costa FF. Genomic polymorphisms in sickle cell disease: implications for clinical diversity and treatment. Expert Rev Hematol 2010; 3:443–458
- Galarneau G, et al. Gene-centric association study of acute chest syndrome and painful crisis in sickle cell disease patients. Blood 2013; 122:434–442
- Emre U, Miller ST, Rao SP, Rao M. Alveolar-arterial oxygen gradient in acute chest syndrome of sickle cell disease. J Pediatr 1993; 123:272–275
- Alhandalous CH, et al. Platelets decline during vaso-occlusive crisis as a predictor of acute chest syndrome in sickle cell disease. Am J Hematol 2015; 90:E228–E229
- Cohen RT, et al. Environmental tobacco smoke and airway obstruction in children with sickle cell anemia. Chest 2013; 144:1323–1329
- Strunk RC, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr 2014; 164:821..e1–826.e1
- Field JJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest 2011; 139:563–568
- Poulter EY, Truszkowski P, Thompson AA, Liem RI. Acute chest syndrome is associated with history of asthma in hemoglobin SC disease. Pediatr Blood Cancer 2011; 57:289–293
- Glassberg J, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol 2006; 28:481–485
- Santoli F, et al. Pulmonary function in sickle cell disease with or without acute chest syndrome. Eur Respir J 1998; 12:1124–1129
- Merrill WW. Incentive spirometry in sickle cell crisis. N Engl J Med 1996; 334:124–125
- Howard J, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938
- Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood 2006; 108:2923–2927
- Anim SO, Strunk RC, DeBaun MR. Asthma morbidity and treatment in children with sickle cell disease. Expert Rev Respir Med 2011; 5:635–645
- Kassim AA, et al. Low forced expiratory volume is associated with earlier death in sickle cell anemia. Blood 2015; 126:1544–1550
- Yawn BP, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014; 312:1033–1048
- Hankins JS, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood 2005; 106:2269–2275
- Lemonne N, et al. Effects of hydroxyurea on blood rheology in sickle cell anemia: a two-years follow-up study. Clin Hemorheol Microcirc 2017. [Epub ahead of print]; doi:10.3233/CH-170280
- Hankins J, et al. Chronic transfusion therapy for children with sickle cell disease and recurrent acute chest syndrome. J Pediatr Hematol Oncol 2005; 27:158–161
- Miller ST, et al. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr 2001; 139:785–789
- Hsieh MM, Fitzhugh CD, Tisdale JF. Allogeneic hematopoietic stem cell transplantation for sickle cell disease: the time is now. Blood 2011; 118:1197–1207
- Vichinsky, E.P., Neumayr, L.D., Earles, A.N., Williams, R., Lennette, E.T., Dean, D., Nickerson, B., Orringer, E., McKie, V., Bellevue, R., Daeschner, C. & Manci, E.A. (2000) Causes and outcomes of the acute chest syndrome in sickle cell disease. The New England Journal of Medicine, 342, 1855–1865.
- DeBaun MR, Strunk RC. The intersection between asthma and acute chest syndrome in children with sickle-cell anaemia. Lancet 2016; 387:2545–2553
- Chaturvedi S, et al. Rapidly progressive acute chest syndrome in individuals with sickle cell anemia: a distinct acute chest syndrome phenotype. Am J Hematol 2016; 91:1185–1190
- Creary SE, Krishnamurti L. Prodromal illness before acute chest syndrome in pediatric patients with sickle cell disease. J Pediatr Hematol Oncol 2014; 36:480–483
- Quinn CT, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol 2013; 70:58–65
- Madani, G., Papadopoulou, A.M., Holloway, B., Robins, A., Davis, J. & Murray, D. (2007) The radiological manifestations of sickle cell disease. Clinical Radiology, 62, 528–538.
- Maitre, B., Habibi, A., Roudot‐Thoraval, F., Bachir, D., Belghiti, D.D., Galacteros, F. & Godeau, B. (2000) Acute chest syndrome in adults with sickle cell disease. Chest, 117, 1386–1392.
- van Agtmael, M.A., Cheng, J.D. & Nossent, H.C. (1994) Acute chest syndrome in adult Afro‐Carribean patients with sickle cell disease. Analysis of 81 episodes among 53 patients. Archives of Internal Medicine, 154, 557–561.
- Bhalla, M., Abboud, M.R., McLoud, T.C., Shepard, J.A., Munden, M.M., Jackson, S.M., Beaty, J.R. & Laver, J.H. (1993) Acute chest syndrome in sickle cell disease: CT evidence of microvascular occlusion. Radiology, 187, 45–49.
- Velasquez, M.P., Mariscalco, M.M., Goldstein, S.L. & Airewele, G.E. (2009) Erythrocytapheresis in children with sickle cell disease and acute chest syndrome. Pediatric Blood & Cancer, 53, 1060–1063.
- Vichinsky, E.P. (2001) Current issues with blood transfusion in sickle cell disease. Seminars in Hematology, 38, 14–22.
- Vichinsky, E.P., Styles, L.A., Colangleo, L.H., Wright, E.C., Castro, O. & Nickerson, B. (1997) Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood, 89, 1787–1792.
- Fitzgerald, R.K. & Johnson, A. (2001) Pulse oximetry in sickle cell anemia. Critical Care Medicine, 29, 1803–1806.
- Yildizdaş, D., Yapicioğlu, H., Yilmaz, H.L. & Sertdemir, Y. (2004) Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Archives of Disease in Childhood, 89, 176–180.
- Bernini, J.C., Rogers, Z.R., Sandler, E.S., Reisch, J.S., Quinn, C.T. & Buchanan, G.R. (1998) Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood, 92, 3082–3089.
- Fartoukh, M., Lefort, Y., Habibi, A., Bachir, D., Galacteros, F., Godeau, B., Maitre, B. & Brochard, L. (2010) Early intermittent noninvasive ventilation for acute chest syndrome in adults with sickle cell disease: a pilot study. Intensive Care Medicine, 36, 1355–1362.
- Kaur, N., Motwani, B., Sivasubramaniam, D., Feldman, L., Allen, S., Ferguson, R., Shiomoto, G., Ansell, D., Westerman, M. & Feldman, L. (2004) Potential role of the ventilation and perfusion (V/Q) lung scan in the diagnosis of acute chest syndrome in adults with sickle cell disease. American Journal of Hematology, 77, 407–409.
- Styles, L.A., Schalkwijk, C.G., Aarsman, A.J., Vichinsky, E.P., Lubin, B.H. & Kuypers, F.A. (1996) Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood, 87, 2573–2578.
- Styles, L.A., Abboud, M., Larkin, S., Lo, M. & Kuypers, F.A. (2007) Transfuion prevents acute chest syndrome predicted by elevated secretory phospholipase A2. British Journal of Haematology, 136, 343–344.
- Styles, L., Wager, C.G., Labotka, R.J., Smith‐Whitley, K., Thompson, A.A., Lane, P.A., McMahon, L.E., Miller, R., Roseff, S.D., Iyer, R.V., Hsu, L.L., Castro, O.L., Ataga, K.I., Onyekwere, O., Okam, M., Bellevue, R., Miller, S.T.; Sickle Cell Disease Clinical Research Network (SCDCRN). (2012) Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: results of a feasibility study (PROACTIVE). British Journal of Haematology, 157, 627–636.
- Vichinsky, E., Williams, R., Das, M., Earles, A.N., Lewis, N., Adler, A. & McQuitty, J. (1994) Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood, 83, 3107–3112.
- Haynes J, Jr., Allison RC. Pulmonary edema. Complication in the management of sickle cell pain crisis. Am J Med 1986; 80:833–840
- Ahmad FA, Macias CG, Allen JY. The use of incentive spirometry in pediatric patients with sickle cell disease to reduce the incidence of acute chest syndrome. J Pediatr Hematol Oncol 2011; 33:415–420
- Crabtree EA, et al. Improving care for children with sickle cell disease/acute chest syndrome. Pediatrics 2011; 127:e480–e488
- Padman R, Henry M. The use of bilevel positive airway pressure for the treatment of acute chest syndrome of sickle cell disease. Del Med J 2004; 76:199–203
- Dean D, et al. Chlamydia pneumoniae and acute chest syndrome in patients with sickle cell disease. J Pediatr Hematol Oncol 2003; 25:46–55
- Marti-Carvajal AJ, Conterno LO, Knight-Madden JM. Antibiotics for treating acute chest syndrome in people with sickle cell disease. Cochrane Database Syst Rev 2013; 2:CD006110
- Howard J, et al. Prevention of morbidity in sickle cell disease—qualitative outcomes, pain and quality of life in a randomised cross-over pilot trial of overnight supplementary oxygen and auto-adjusting continuous positive airways pressure (POMS2a): study protocol for a randomised controlled trial. Trials 2015; 16:376
- Rucknagel DL. The role of rib infarcts in the acute chest syndrome of sickle cell diseases. Pediatr Pathol Mol Med 2001; 20:137–154
- Miller ST. How I treat acute chest syndrome in children with sickle cell disease. Blood 2011; 117:5297–5305
- Needleman JP, Benjamin LJ, Sykes JA, Aldrich TK. Breathing patterns during vaso-occlusive crisis of sickle cell disease. Chest 2002; 122:43–46
- Uzun B, Kekec Z, Gurkan E. Efficacy of tramadol vs meperidine in vasoocclusive sickle cell crisis. Am J Emerg Med 2010; 28:445–449
- Emre U, et al. Effect of transfusion in acute chest syndrome of sickle cell disease. J Pediatr 1995; 127:901–904
- Gladwin MT, et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 2011; 305:893–902
- Ozbek OY, et al. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol 2007; 42:1187–1192
- Knight-Madden JM, Hambleton IR. Inhaled bronchodilators for acute chest syndrome in people with sickle cell disease. Cochrane Database Syst Rev 2012; 7:CD003733
- Bernini JC, et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood 1998; 92:3082–3089
- Kumar R, Qureshi S, Mohanty P, Rao SP, Miller ST. A short course of prednisone in the management of acute chest syndrome of sickle cell disease. J Pediatr Hematol Oncol 2010; 32:e91–e94
- Sobota A, Graham DA, Heeney MM, Neufeld EJ. Corticosteroids for acute chest syndrome in children with sickle cell disease: variation in use and association with length of stay and readmission. Am J Hematol 2010; 85:24–28
- Strouse JJ, Hulbert ML, DeBaun MR, Jordan LC, Casella JF. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics 2006; 118:1916–1924
- Strouse JJ, Takemoto CM, Keefer JR, Kato GJ, Casella JF. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer 2008; 50:1006–1012
- Chang WL, Corate LM, Sinclair JM, van der Heyde HC. Continuous inhaled nitric oxide therapy in a case of sickle cell disease with multiorgan involvement. J Investig Med 2008; 56:1023–1027
- Gladwin MT, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004; 350:886–895
- Henderson, J.N., Noetzel, M.J., McKinstry, R.C., White, D.A., Armstrong, M. & DeBaun, M.R. (2003) Reversible posterior leukoencephalopathy syndrome and silent cerebral infarcts are associated with severe acute chest syndrome in children with sickle cell disease. Blood, 101, 415–419.
- Davies, S.C., Luce, P.J., Win, A.A., Riordan, J.F. & Brozovic, M. (1984) Acute chest syndrome in sickle cell disease. Lancet, 8367, 36–38.
- Lucas, S.B., Mason, D.G., Mason, M. & Weyman, D.; on behalf of NCEPOD. (2008) A Sickle Crisis? A Report of the National Confidential Enquiry in Patient Outcome and Death. https://www.ncepod.org.uk/2008report1/Downloads/Sickle_report.pdf
- Platt, O.S., Brambilla, D.J., Rosse, W.F., Milner, P.F., Castro, O., Steinberg, M.H. & Klug, P.P. (1994) Mortality in sickle cell disease: life expectancy and risk factors for early death. New England Journal of Medicine, 330, 1639–1644.