What is aflatoxin
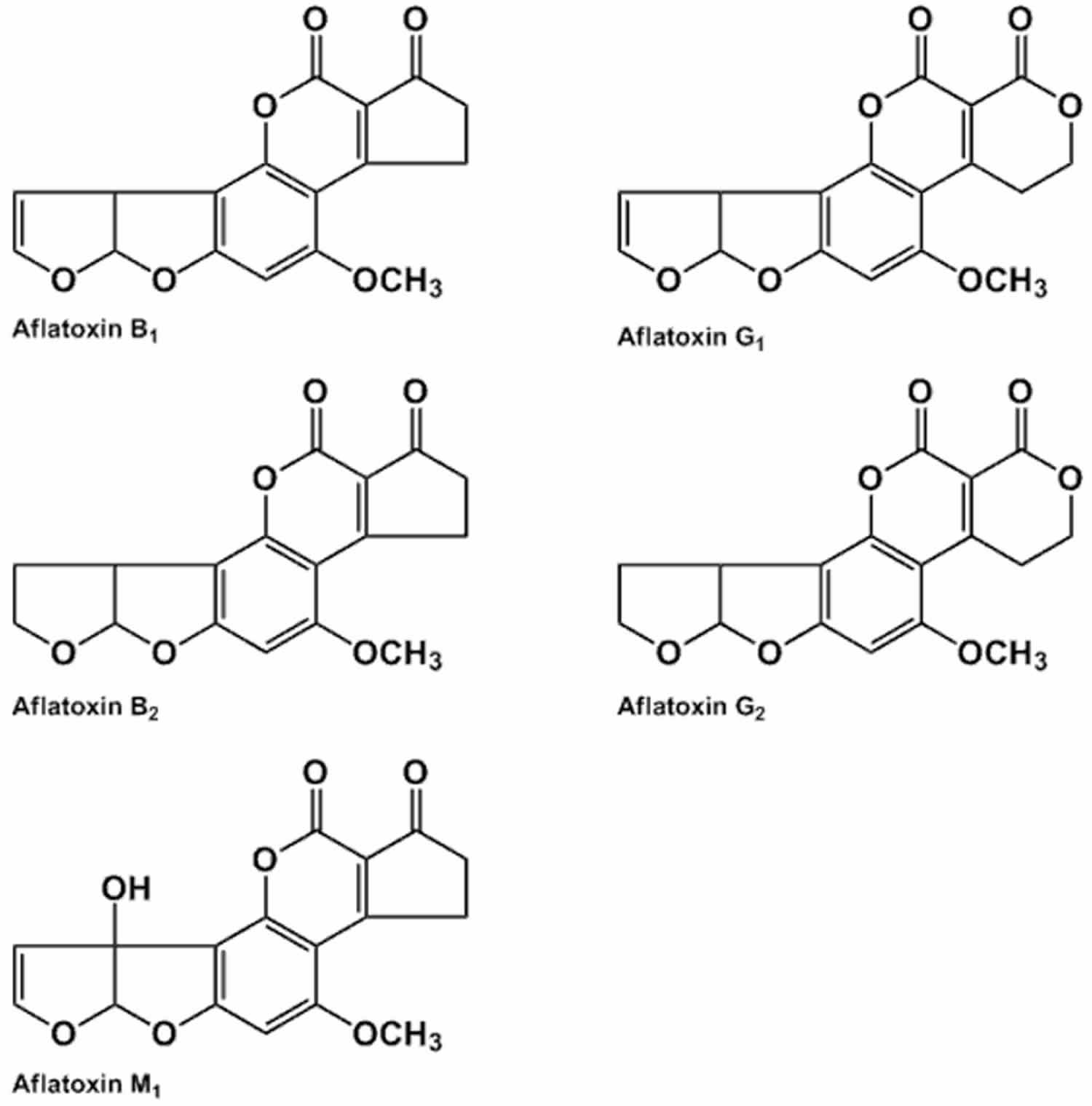
Aflatoxins are toxins produced by a Aspergillus flavus and and the closely related species Aspergillus parasiticus fungus, one of the most abundant soil-borne molds on earth, that grows in nuts, seeds, and legumes. Most Aspergillus flavus produces aflatoxins B1 and B2 whereas Aspergillus parasiticus, produces aflatoxins B1, B2, G1, and G2 1. These four major aflatoxins are named based on their blue (B) or green (G) fluorescence under ultraviolet light, and their relative mobility by thin-layer chromatography on silica gel. Aflatoxin M1 is a hydroxylated derivative metabolized from aflatoxin B1 by cows and secreted in milk 2. In addition to aflatoxins B1 and B2, Aspergillus flavus also produces many other mycotoxins such as cyclopiazonic acid, kojic acid, beta-nitropropionic acid, aspertoxin, aflatrem and aspergillic acid 3.
Aflatoxin B1 is the most potent known natural carcinogen and is the most abundant of the aflatoxins. The International Agency for Research on Cancer has classified Aflatoxin B1 as a group 1 carcinogen and aflatoxin M1 as a group 2b carcinogen (carcinogenic to laboratory animals and possibly carcinogenic to humans, respectively). Combined exposure to aflatoxin and hepatitis B increases the risk for development of human hepatocellular carcinoma (HCC).
People can be exposed to aflatoxins by eating contaminated plant products (such as peanuts) or by consuming meat or dairy products from animals that ate contaminated feed. Farmers and other agricultural workers may be exposed by inhaling dust generated during the handling and processing of contaminated crops and feeds.
The combination of hepatitis B infection and eating foods contaminated with aflatoxin appears to make the risk of liver cancer especially high. Foods in which aflatoxins commonly are found(unless regulations and inspections prevent it,as in the U.S.) include corn, sorghum, rice, cottonseed, peanuts, tree nuts, dried coconut meat,cocoa beans, figs, ginger,and nutmeg.
Aflatoxins can cause illness in animals and contaminated pet foods caused outbreaks and deaths among U.S. dogs and cats in 1998 and 2005. Cows are able to metabolize–process–aflatoxin. The substance (metabolite) that results after the cow processes the aflatoxin then may appear in the cow’s milk, but is less toxic than the aflatoxin itself. Milk is routinely tested for this substance. In some developing countries, this metabolite also is found in the breast milk of human mothers who eat aflatoxin‐contaminated foods.
Aspergillus flavus fungus is a saprobe that is capable of surviving on many organic nutrient sources like plant debris, animal fodder, cotton, compost piles, dead insects and animal carcasses, stored grains, and even immunocompromised humans and animals 4. Although aflatoxins are known to cause cancer in animals, the U.S. Food and Drug Administration (FDA) allows them at low levels in nuts, seeds, and legumes because they are considered “unavoidable contaminants” 5. The FDA believes occasionally eating small amounts of aflatoxin poses little risk over a lifetime. It is not practical to attempt to remove aflatoxin from food products in order to make them safer.
Aspergillus flavus fungus has the ability to survive temperatures ranging from 12 °C to 48 °C, but the optimal growth temperature ranges from 28 °C to 37 °C 1. Aspergillus flavus fungus has the ability to grow at relatively high temperatures contributes to its pathogenicity toward humans and other warm-blooded animals 1. For most of its lifecycle, the Aspergillus flavus fungus exists in the form of mycelium or asexual spores known as conidia. Under adverse conditions such as lack of adequated nutrients or water, the fungal mycelium will transform to resistant structures called sclerotia, which can survive in extremely harsh environmental conditions. The fungus overwinters either as spores, sclerotia, or as mycelium in debris. When conditions become favorable, the sclerotia germinate directly to produce new colonies or conidiophores with conidia 6.
Aflatoxin B1
Aflatoxin B1, among the four major types of aflatoxins, is the most toxic and the most potent carcinogen in humans and animals including nonhuman primates, birds, fish, and rodents 7. Chronic exposure can result in suppressed immune response, malnutrition, proliferation of the bile duct, centrilobular necrosis and fatty infiltration of the liver, hepatic lesions, and even hepatomas 7. In animal models, aflatoxin B1 is modified into a more toxic and carcinogenic by-product during detoxification by a cytochrome P450 monooxygenase in liver 8. The epoxide form of aflatoxin binds to guanine residues in DNA, forms guanyl-N7 adducts, and induces mutations. One mutation, a G to T transversion 9 at the third base of codon 249, a mutation hot spot of the p53 tumor suppressor gene and is generally believed to be the mechanism for initiating hepatocarcinoma formation 10. The p53 gene encodes a transcription factor involved in cell cycle regulation. It is commonly mutated in human liver cancers 11. Aflatoxin B1 is also a potential immunosuppressive agent 12. Chronic low level exposure of growing vertebrates to aflatoxins may enhance their susceptibility to infection and tumorigenesis 12. Aflatoxin B1 also affects other organs and tissues, such as the lungs and the entire respiratory system 13. Human hepatocarcinomas are also associated with hepatitis B virus (HBV) and C virus (HCV) infections 14. Together with aflatoxins these viruses significantly increased the risk of hepatoma in hepatitis patients 15.
Aflatoxin M1 and M2
The aflatoxin M1 and M2 are mammalian bioconversion products of aflatoxin B1 and aflatoxin B2 respectively and are originally isolated and identified from bovine milk 16. After entering the mammaliam body (human or animals), aflatoxins are metabolized by the liver cytochrome P450 enzymes to a reactive epoxide intermediate which becomes more carcinogenic, or be hydroxylated and become the less harmful aflatoxins M1 and M2. However, recent studies by feeding with aspertoxin (12c-hydroxy-OMST) 17 indicated that Aspergillus parasiticus produces the minor aflatoxins M1 (AFM1), M2 (AFM2), GM1 (AFGM1), and GM2 (AFGM2), as well as the major aflatoxins B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2). It demonstrated that aspertoxin is a precursor of aflatoxins M1 and aflatoxins GM1. Feeding of the same fungus with O-methylsterigmatocystin (OMST), aflatoxins M1 and aflatoxins GM1 were formed together with aflatoxin B1 and aflatoxin G1; feeding with dihydro-OMST (DHOMST), aflatoxin M2 and aflatoxin GM2 were formed together with aflatoxin B2 and aflatoxin G2. It showed that the enzyme OrdA catalyzes both 12c-hydroxylation reaction from O-methylsterigmatocystin to aspertoxin and the successive reaction from aspertoxin to aflatoxin M1 and the aflatoxin B1 is not a precursor of aflatoxin M1.
Aflatoxin toxic dose
The toxic level of aflatoxin in humans is largely unknown. In one example, a laboratory worker who intentionally ingested aflatoxin B1 at 12 μg/kg body weight for 2 days developed a rash, nausea, and headache, but recovered without ill effect. In a 14-year follow-up of the worker, a physical examination and blood chemistry, including tests for liver function, were normal.
- From acute exposure: Acute exposure to high doses of aflatoxins can result in aflatoxicosis, with the target organ being the liver, leading to serious liver damage. aflatoxins inhibit the normal functions of the liver, including carbohydrate and lipid metabolism and protein synthesis.
- From chronic exposure at sublethal doses: cancer, impaired protein formation, impaired blood coagulation, toxic hepatitis, and probable immunosuppression. In animals, aflatoxins may cause, in addition, reduced weight gain and reduced feed-conversion efficiency.
Other significant health effects of aflatoxin exposure follow from the finding that they are probably immunosuppressive in humans. aflatoxins have been shown primarily to affect the cellular immune processes in most of the laboratory animal species studied. Some animals exhibit a decrease in antibody formation, and there is evidence of transplacental movement of aflatoxins, allowing embryonic exposure and reducing immune responses in offspring.
Aflatoxin in food
The Aspergillus flavus (and Aspergillus parasiticus) mold that produces aflatoxin may be found in the following foods:
- Peanuts and peanut butter
- Tree nuts such as pecans
- Corn
- Wheat
- Oil seeds such as cottonseed
With maize, peanuts, and cottonseed, invasion of plants and developing seed or nut by Aspergillus spp. may occur before harvest, resulting in potentially high levels of aflatoxins in these commodities and the continuing difficulty to eliminate aflatoxins from these products. With other crops, prevention of the formation of aflatoxins relies mainly on avoidance of contamination after harvest by use of rapid drying and good storage practice 18.
Food and feed contamination by aflatoxins is a significant food safety issue in the developing countries sometimes because of lack of detection, monitoring and regulating measures to safe guard the food supply. It is estimated that approximately 4.5 billion people living in developing countries are chronically exposed to largely uncontrolled amounts of aflatoxin that severely results in changes in immunity and nutrition 19. Major outbreaks of acute aflatoxicosis from contaminated food in humans have been documented in developing countries 8. For example, in western India in 1974, 108 persons among 397 people affected, died from aflatoxin poisoning 20. A more recent incident of aflatoxin poisoning occurred in Kenya in July 2004 leading to the death of 125 people among the 317 reported illnesses due to consumption of aflatoxin contaminated maize (corn) 20. In the Kenia case, the aflatoxins were produced by Aspergillus parvisclerotigenus instead of Aspergillus flavus. Acute toxicosis is not the only concern. The world health authorities warn that low doses with long-term dietary exposure to aflatoxins is also a major risk as they can lead to hepatocellular carcinoma 21. International Agency for Research on Cancer (IARC) has designated aflatoxin as a human liver carcinogen 22. This food poisoning problem is rarely observed in the U.S. in humans but does occasionally occur in animals. The most notable recent case involved the reported death of over 100 dogs in 2006 that had consumed tainted dog feed.
To minimize potential exposure to aflatoxins, maximum levels of aflatoxins in many commodities have been set at levels below 20 ppb by most countries 23. Regulatory guidelines of the U.S. Food and Drug Administration (FDA) specifically prevent the sale of commodities if contamination by aflatoxins exceeds 20 ppb total aflatoxins for interstate commerce of food and feedstuff and 0.5 ppb aflatoxin M1 in milk. The European Commission has set the limits on groundnuts subject to further processing at 15 ppb for total aflatoxins and 8 ppb for aflatoxin B1, and for nuts and dried fruits subject to further processing at 10 ppb for total aflatoxins and 5 ppb for aflatoxin B1. The aflatoxin standards for cereals, dried fruits, and nuts intended for direct human consumption are even more stringent, and the limit for total aflatoxins is 4 ppb and 2 ppb for aflatoxin B1 24.
Due to restrictions limiting the trade of contaminated crops, aflatoxin contamination of agricultural commodities is not only a serious food safety concern, but it has significant economic implications for the agricultural industry worldwide.
Aflatoxin test
Since the late 1970s, aflatoxin-specific antibodies have been developed. The antibody development has led to the development of enzyme-linked immunosorbent assays (ELISAs) for aflatoxins. The ELISAs are mainly used in screening methods.
With advances in instrumentation, chromatographic methods for aflatoxins have expanded from thin-layer chromatography (TLC) to high-performance liquid chromatography (LC) with fluorescence detection. Hyphenated methods, such as liquid chromatography/mass spectrometry (MS) or LC/MS, have also been developed for aflatoxin quantitation and confirmation of identities.
Emerging analytical technologies for aflatoxin include solid-phase micro-extraction, surface-plasmon resonance, fiber-optic sensors, electrochemical immunosensors, fluorescence-based immunoassays, and the use of molecularly imprinted polymers for binding the aflatoxins. Recently, non-invasive analyses, such as near-infrared spectrometry, have been used, with limited success, for detecting the occurrence of Aspergillus flavus-infected corn kernels and correlating these occurrences with aflatoxin levels.
How to avoid aflatoxin
You can reduce your aflatoxin exposure by buying only major commercial brands of nuts and nut butters and by discarding nuts that look moldy, discolored, or shriveled. To help minimize risk, the U.S. Food and Drug Administration (FDA) tests foods that may contain aflatoxins. Peanuts and peanut butter are some of the most rigorously tested products because they often contain aflatoxins and are widely eaten. To date, no outbreak of human illness caused by aflatoxins has been reported in the United States, but such outbreaks have occurred in some developing countries.
You can reduce aflatoxin intake by:
- Buying only major brands of nuts and nut butters
- Discarding any nuts that look moldy, discolored, or shriveled
Aflatoxin symptoms
The disruption and inhibition of carbohydrate and lipid metabolism and protein synthesis associated with aflatoxicosis can lead to hemorrhaging, jaundice, premature cell death, and tissue necrosis in liver and, possibly, other organs. Other general symptoms include edema of the lower extremities, abdominal pain, and vomiting.
Aflatoxins ingested in large mounts may cause acute liver damage. Chronic aflatoxin intoxication may lead to weight gain or weight loss, loss of appetite, or infertility in men.
Aflatoxin Disease
Chronic exposure to aflatoxin well above the FDA guideline affects many organs; however, the major target is the liver. Aflatoxins are hepatotoxic in humans and animals. Food-related exposures to aflatoxins and the resulting aflatoxicosis can range from acute to chronic, and illness can range from mild to severe, including development of cirrhosis (severe liver damage) and may result in development of liver cancer. aflatoxinB1 is the most potent known natural carcinogen.
It is difficult to prove that a disease is caused by aflatoxins. It is possible to test tumor tissue for biomarkers or characteristic genetic damage. Even in cases where aflatoxin exposures have been of long duration and are well above the U.S. limits, it is unlikely that they are the only agents responsible for the outcome. However, there is reliable evidence, from animal studies and case reports and long-term studies of human health outcomes, that aflatoxins pose an important danger to human and animal health unless properly regulated.
Mortality: Documented epidemics of aflatoxin poisoning in the following countries illustrate mortality rates from outbreaks:
- In northwest India, in 1974, there were 108 fatalities from 397 illnesses. aflatoxin levels of 0.25 to 15 mg/kg were found in corn.
- In 1982, in Kenya, there were 20 hospital admissions, with a 60% mortality rate, with aflatoxin intake at 38 μg/kg of body weight.
- In 1988, in Malaysia, 13 Chinese children died of acute hepatic encephalopathy after eating Chinese noodles. Aflatoxins were confirmed in postmortem samples from the patients.
- In 2004 and 2005, one of the largest aflatoxicosis outbreaks on record occurred in rural Kenya, resulting in illness in 317 people, 125 of whom died. aflatoxin-contaminated homegrown maize with an average concentration of 354 ng/g was the source of the outbreak.
Aflatoxin poisoning diagnosis
People who have aflatoxicosis might exhibit the following characteristics.
- Liver damage may be evidenced by jaundice and its characteristic yellowing of tissues.
- Gall bladder may become swollen.
- Immunosuppression may provide an opportunity for secondary infections.
- Vitamin K functions may decrease.
- High levels of aflatoxinB1-albumin adducts may be present in plasma.
Aflatoxin exposure can be monitored through the use of biomarkers that detect the presence of aflatoxin metabolites in blood, milk, and urine, and excreted DNA adducts and blood-protein adducts. Aflatoxin B1-albumin adducts can be measured in blood; aflatoxin M1 and aflatoxin B1-DNA adduct (aflatoxin B1-guanine adduct) can be detected in the urine of people consuming sufficient amounts of aflatoxin B1.
Aflatoxin cancer
Exposure to aflatoxins is associated with an increased risk of liver cancer.
Liver cancer
Aflatoxins were evaluated in International Agency for Research on Cancer (IARC) and confirmed as a Group-1 agent. The weight of evidence for the classification of the aflatoxins as Group-1 carcinogens was driven by statistically significantly increased risks for hepatocellular carcinoma in individuals exposed to aflatoxins, as measured by aflatoxin-specific biomarkers in cohort studies in Shanghai and Taiwan, China 25. This effect was independent of exposure to hepatitis B virus (HBV); however, when HBV (hepatitis B virus) status was included in the analysis, a greater than multiplicative interaction between aflatoxin exposure and hepatitis B virus infection was found. The cohort of Wang et al. 25 has been extensively updated in three subsequent reports 26. In these studies 26, 27, the risk for liver cancer was significantly elevated for subjects with high concentrations of aflatoxin metabolites in the urine. Subjects who were seropositive for the hepatitis-B surface antigen (HBsAg) and had high aflatoxin exposure were at higher risk than those with high aflatoxin exposure only, or HBsAg-seropositivity only. There seemed to be no correlation with polycyclic aromatic hydrocarbon-albumin-adduct formation 28. The risk was elevated in those with urinary concentrations of the biomarker 8-oxodeoxyguanosine (8-oxodG) above the median, who were also HBsAg-positive 29. In one small cohort the risk for liver cancer from aflatoxin exposure was also elevated 30.
References- Yu J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins (Basel). 2012;4(11):1024-57. Published 2012 Oct 25. doi:10.3390/toxins4111024 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509697/
- Current situation on regulations for mycotoxins. Overview of tolerances and status of standard methods of sampling and analysis. Van Egmond HP. Food Addit Contam. 1989 Apr-Jun; 6(2):139-88.
- Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Goto T, Wicklow DT, Ito Y. Appl Environ Microbiol. 1996 Nov; 62(11):4036-8.
- Soil fungi of some low-altitude desert cotton fields and ability of their extracts to inhibit Aspergillus flavus. Klich MA. Mycopathologia. 1998; 142(2):97-100.
- https://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/UCM297627.pdf
- Chang P.K., Bennett J.W., Cotty P.J. Association of aflatoxin biosynthesis and sclerotial development in Aspergillus parasiticus. Mycopathologia. 2002;153:41–48. doi: 10.1023/A:1015211915310
- Yu J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins (Basel). 2012;4(11):1024-57. Published 2012 Oct 25. doi:10.3390/toxins4111024 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509697
- Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., Nyamongo J., Backer L., Dahiye A.M., Misore A., et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005;113:1763–1767. doi: 10.1289/ehp.7998
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431
- Coursaget P., Depril N., Chabaud M., Nandi R., Mayelo V., LeCann P., Yvonnet B. High prevalence of mutations at codon 249 of the p53 gene in hyptocellular carcinomas from Senegal. Br. J. Cancer. 1993;67:1395–1397. doi: 10.1038/bjc.1993.258.
- Groopman J.D., Wogan G.N., Roebuck B.D., Kensler T.W. Molecular biomarkers for aflatoxins and their application to human cancer prevention. Cancer Res. 1994;54:190–191
- Raisuddin S., Singh K.P., Zaidi S.I., Paul B.N., Ray P.K. Immunosuppressive effects of aflatoxin in growing rats. Mycopathologia. 1993;124:189–194. doi: 10.1007/BF01103737
- Kelly J.D., Eaton D.L., Guengerich F.P., Coulombe R.A., Jr. Aflatoxin B1 activation in human lung. Toxicol. Appl. Pharmacol. 1997;144:88–95. doi: 10.1006/taap.1997.8117
- Wild C.P., Shrestha S.M., Anwar W.A., Montesano R. Field studies of aflatoxin exposure, metabolism and induction of genetic alterations in relation to HBV infection and hepatocellular carcinoma in the Gambia and Thailand. Toxicol. Lett. 1992;64-65:455–461. doi: 10.1016/0378-4274(92)90219-A
- Arsura M., Cavin L.G. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005;229:157–169. doi: 10.1016/j.canlet.2005.07.008.
- Garrido N.S., Iha M.H., Santos Ortolani M.R., Duarte Fávaro R.M. Occurrence of aflatoxin M1 and aflatoxin M2 in milk commercialized in Ribeirão Preto-SP, Brazil. J. Food Addit. Contam. 2003;20:70–73. doi: 10.1080/0265203021000035371
- Yabe K., Chihaya N., Hatabayashi H., Kito M., Hoshino S., Zeng H., Cai J., Nakajima H. Production of M-/GM-group aflatoxins catalyzed by the OrdA enzyme in aflatoxin biosynthesis. Fungal Genet. Biol. 2012;49:744–754. doi: 10.1016/j.fgb.2012.06.011
- https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100F-23.pdf
- Williams J.H., Phillips T.D., Jolly P.E., Stiles J.K., Jolly C.M., Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004;80:1106–1122
- Krishnamachari K.A., Bhat R.V., Nagarajan V., Tilak T.B. Hepatitis due to aflatoxicosis: An outbreak of hepatitis in parts of western India. Lancet. 1975;1:1061–1063.
- Coursaget P., Depril N., Chabaud M., Nandi R., Mayelo V., LeCann P., Yvonnet B. High prevalence of mutations at codon 249 of the p53 gene in hyptocellular carcinomas from Senegal. Br. J. Cancer. 1993;67:1395–1397. doi: 10.1038/bjc.1993.258
- Wogan G.N. Impacts of chemicals on liver cancer risk. Semin. Cancer Biol. 2000;10:201–210. doi: 10.1006/scbi.2000.0320
- Van Egmond H.P., Schothorst R.C., Jonker M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007;389:147–157. doi: 10.1007/s00216-007-1317-9
- Van Egmond H.P., Jonker M.A. In: Worldwide Regulations on Aflatoxins. Abbas H.K., editor. CRC Press; Boca Raton, FL, USA: 2005. pp. 77–93
- Wang LY, Hatch M, Chen CJ, et al. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. International Journal of Cancer. 1996;67:620–625.
- Wu HC, Wang Q, Yang HI, et al. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:846–853
- Wu HC, Wang Q, Wang LW, et al. Urinary 8-oxodeoxyguanosine, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis. 2007;28:995–999. b
- Wu HC, Wang Q, Wang LW, et al. Polycyclic aromatic hydrocarbon- and aflatoxin-albumin adducts, hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Cancer Lett. 2007;252:104–114. a.
- Wu HC, Wang Q, Wang LW, et al. Urinary 8-oxodeoxyguanosine, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis. 2007;28:995–999. b.
- Ming L, Thorgeirsson SS, Gail MH, et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214–1220.