What is amusia
Amusia is a neural disorder characterized by severe impairment to perceive music caused by either abnormal brain development (congenital amusia) or brain tissue damage (acquired amusia) 1. Deficit in perceiving fine-grained pitch changes (tone deafness) is the hallmark symptom of amusia, but also other domains of music, such as rhythm, can be affected 2. Congenital amusia is a neurodevelopmental auditory disorder characterized by deficits in pitch perception and production that cannot be attributed to hearing loss or neurophysiological causes 3. Congenital amusia has been estimated to affect 1.5%–4% of the general population with slightly higher rates in females 4, has been shown to be hereditary 5, and does not appear to co-occur with other cognitive disorders 4. Besides having pitch discrimination difficulties 6, individuals with congenital amusia are impaired in pitch contour identification 7, melodic sequence recognition 8, singing in tune 9, as well as in memorizing pitch-based materials 10. However, amusics show normal processing on some other music related attributes such as musical emotion based on temporal or timbral cues 11, but not when emotion was elicited by the tonality of musical excerpts 12. Recent theories have suggested that amusic individuals’ pronounced difficulties in pitch processing may stem from an inability to use the fine spectro-temporal cues in the resolved harmonics in complex tones without peripheral basis 13. Consistent with this view, one recent meta analysis has shown that pitch change is an effective moderator of the effect size of performance gap between amusic and control across studies, supporting the hypothesis that amusia stems from a broad disorder of acoustic pitch processing 14.
The perception and experience of music in the healthy brain is based on the functioning of a large-scale bilateral neural network comprising temporal, frontal, parietal, cerebellar, and subcortical areas 15. The amusic brain shows abnormalities in neural transmission between the auditory cortex and the inferior frontal gyrus in the right cerebral hemisphere.
The two types of amusia, acquired and congenital amusia, may have partly distinct neural basis. Congenital amusia is a developmental deficit and thus impedes acquiring musical syntax 16, whereas acquired amusia represents a shift from a normal to deficiently functioning music processing system caused by a brain lesion. Studying brain lesions and associated cognitive deficits is essential in uncovering crucial brain regions that are causally connected 17. This can be achieved by voxel-based lesion-symptom mapping (VLSM), which is an advanced MRI analysis method investigating the relationship between focal brain damage and behavioral data on a voxel-by-voxel basis 18. Compared to the traditional lesion-led or symptom-led approaches, voxel-based lesion-symptom mapping (VLSM) allows both binary and continuous analyses and does not require patient grouping by lesion site. Voxel-based lesion-symptom mapping (VLSM) utilizes three-dimensional lesion maps formed from MRI images, and evaluates the presence or absence of lesion in each voxel to finally associate this information with the behavioral data.
While congenital amusia is generally described as a deficit in processing pitch, arguably due to an impairment of pitch perception and/or pitch-specific short-term or working memory—the processing of musical rhythm, timbre, and emotions can be affected as well 19.
The majority of the neuroimaging studies examining defective music processing in the brain have been carried out on congenital amusia, a condition affecting 2–4% of the population 20. In contrast, acquired amusia is a relatively common disorder after a middle cerebral artery stroke, with incidence ranging from 35 to 69% 21. Evidence derived from MRI morphometry studies, utilizing e.g., voxel-based morphometry (VBM) 22, an automated method for analyzing gray matter and white matter differences between groups or across time, has implicated reduced white matter concentration in the right inferior frontal gyrus 23 and right superior temporal gyrus 23 in congenital amusics. Additionally, the cortex in both of these areas have been shown to be thicker in congenital amusic subjects than in controls 24. However, the reported results have been contradictory regarding laterality of the observed effect: a recent study showed that congenital amusics had decreased gray matter volume in the left inferior frontal gyrus and superior temporal gyrus with no differences observed in the right homologous areas 25. Taken together, these findings suggest that congenital amusia may be a somewhat heterogeneous condition 26.
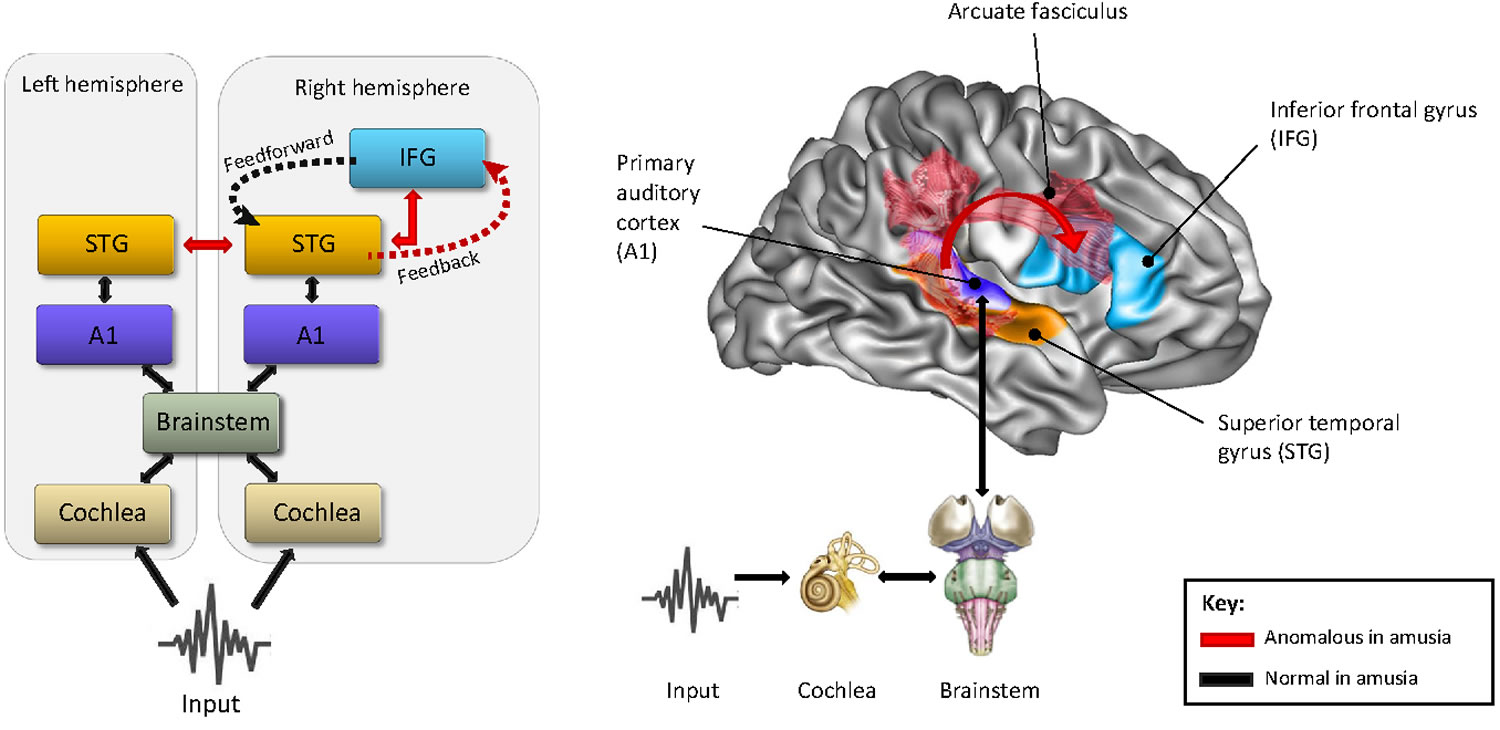
Figure 1. Amusia neural basis
Footnote: Anomalous Recurrent Processing in the Right Frontotemporal Network. Schematic (left panel) and anatomical representation (right panel) of the anomalies (in red) found in functional and structural connectivity between the right and left superior temporal gyrus (STG) and right inferior frontal gyrus (IFG) in the amusic brain as compared to the normal brain. Current evidence suggests that all other connections from the cochlea up to the STG, including the primary auditory cortex (A1), are normal. The proposed altered recurrent processing as a result of poor feedback control between the IFG and STG is represented by dashed lines.
[Source 27 ]Amusia treatment
Amusia treatment involves treating the underlying cause of amusia. For example, all stroke patients should receive standard medical treatment and rehabilitation for their stroke. Congenital amusia is a lifelong condition.
References- Sihvonen AJ, Ripollés P, Rodríguez-Fornells A, Soinila S, Särkämö T. Revisiting the Neural Basis of Acquired Amusia: Lesion Patterns and Structural Changes Underlying Amusia Recovery. Front Neurosci. 2017;11:426. Published 2017 Jul 25. doi:10.3389/fnins.2017.00426 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5524924
- Music and the brain: disorders of musical listening. Stewart L, von Kriegstein K, Warren JD, Griffiths TD. Brain. 2006 Oct; 129(Pt 10):2533-53.
- Peretz I. The biological foundations of music: Insights from congenital amusia In: Deutsch D, editor. The psychology of music. 3rd ed San Diego, CA: Elsevier; 2013. pp. 551–64.
- Prevalence of congenital amusia. Peretz I, Vuvan DT. Eur J Hum Genet. 2017 May; 25(5):625-630.
- The genetics of congenital amusia (tone deafness): a family-aggregation study. Peretz I, Cummings S, Dubé MP. Am J Hum Genet. 2007 Sep; 81(3):582-8.
- Learning for pitch and melody discrimination in congenital amusia. Whiteford KL, Oxenham AJ. Cortex. 2018 Jun; 103():164-178.
- Congenital amusias. Tillmann B, Albouy P, Caclin A. Handb Clin Neurol. 2015; 129():589-605.
- Congenital amusia: a group study of adults afflicted with a music-specific disorder. Ayotte J, Peretz I, Hyde K. Brain. 2002 Feb; 125(Pt 2):238-51.
- Singing in congenital amusia. Dalla Bella S, Giguère JF, Peretz I. J Acoust Soc Am. 2009 Jul; 126(1):414-24.
- Impaired short-term memory for pitch in congenital amusia. Tillmann B, Lévêque Y, Fornoni L, Albouy P, Caclin A. Brain Res. 2016 Jun 1; 1640(Pt B):251-63.
- Sensitivity to musical emotions in congenital amusia. Gosselin N, Paquette S, Peretz I. Cortex. 2015 Oct; 71():171-82.
- Sensitivity to musical emotion is influenced by tonal structure in congenital amusia. Jiang C, Liu F, Wong PCM. Sci Rep. 2017 Aug 8; 7(1):7624.
- Congenital amusia: a cognitive disorder limited to resolved harmonics and with no peripheral basis. Cousineau M, Oxenham AJ, Peretz I. Neuropsychologia. 2015 Jan; 66():293-301.
- Meta-analytic evidence for the non-modularity of pitch processing in congenital amusia. Vuvan DT, Nunes-Silva M, Peretz I. Cortex. 2015 Aug; 69():186-200.
- Brain correlates of music-evoked emotions. Koelsch S. Nat Rev Neurosci. 2014 Mar; 15(3):170-80.
- Fractionating the musical mind: insights from congenital amusia. Stewart L. Curr Opin Neurobiol. 2008 Apr; 18(2):127-30.
- Using human brain lesions to infer function: a relic from a past era in the fMRI age? Rorden C, Karnath HO. Nat Rev Neurosci. 2004 Oct; 5(10):813-9.
- Voxel-based lesion-symptom mapping. Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Nat Neurosci. 2003 May; 6(5):448-50.
- Auditory deficits in amusia extend beyond poor pitch perception. Whiteford KL, Oxenham AJ. Neuropsychologia. 2017 May; 99():213-224.
- Henry M., McAuley J. (2010). On the prevalence of congenital amusia. Music Percept. 27, 413–418. 10.1525/mp.2010.27.5.413
- Neural Basis of Acquired Amusia and Its Recovery after Stroke. Sihvonen AJ, Ripollés P, Leo V, Rodríguez-Fornells A, Soinila S, Särkämö T. J Neurosci. 2016 Aug 24; 36(34):8872-81.
- Voxel-based morphometry–the methods. Ashburner J, Friston KJ. Neuroimage. 2000 Jun; 11(6 Pt 1):805-21.
- Impaired pitch perception and memory in congenital amusia: the deficit starts in the auditory cortex. Albouy P, Mattout J, Bouet R, Maby E, Sanchez G, Aguera PE, Daligault S, Delpuech C, Bertrand O, Caclin A, Tillmann B. Brain. 2013 May; 136(Pt 5):1639-61.
- Cortical thickness in congenital amusia: when less is better than more. Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. J Neurosci. 2007 Nov 21; 27(47):13028-32.
- Congenital amusia: an auditory-motor feedback disorder? Mandell J, Schulze K, Schlaug G. Restor Neurol Neurosci. 2007; 25(3-4):323-34.
- Omigie D., Müllensiefen D., Stewart L. (2012). The experience of music in congenital amusia. Music Percept. 30, 1–18. 10.1525/mp.2012.30.1.1
- Peretz, I. (2016). Neurobiology of Congenital Amusia. Trends in Cognitive Sciences, 20, 857-867.