What is oliguria Oliguria is decreased production of urine. Oliguria can be defined as a urine output that is less than 500 mL/day or urine output
What is shortness of breath Shortness of breath is also called dyspnea or breathlessness, is an intense tightening in your chest, air hunger, a feeling
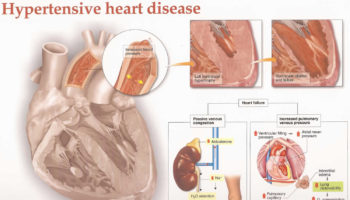
What is hypertensive heart disease Hypertensive heart disease refers to heart problems that occur because of high blood pressure that is present over a long
What is hypersensitivity pneumonitis Hypersensitivity pneumonitis also known as extrinsic allergic alveolitis, bird fancier’s lung, farmer’s lung, hot tub lung or humidifier lung, is a
What is a hip flexor strain A hip flexor strain or a ‘pulled hip flexor’, occurs when one of the hip flexor muscles (iliacus and
What are granulocytes Granulocytes are mature infection-fighting white blood cells that have granules in their cytoplasm that show up as spots under the microscope. Granulocytes
What is gestational age Gestational age is the age of a fetus or baby that starts on the first day of the mother's last menstrual
What are H2 blockers H2 blockers also called H2 histamine blockers or H2 antagonists, are medicines that work by reducing the amount of stomach acid
What is Goodpasture syndrome Goodpasture syndrome also called pulmonary-renal syndrome, is a rare autoimmune disorder that involves your kidneys and lungs. Goodpasture syndrome is fatal unless
What is heat intolerance Heat intolerance is a feeling of being overheated when the temperature around you rises. Heat intolerance is also defined as the