What are granulocytes
Granulocytes are mature infection-fighting white blood cells that have granules in their cytoplasm that show up as spots under the microscope. Granulocytes have granules that contain enzymes and other substances that are released as part of an immune response that can destroy germs, such as bacteria. Granulocytes develop from myeloblasts, a type of blood-forming cell in the bone marrow. The three types of granulocytes include:
- Neutrophils normally make up the largest number of circulating white blood cells, about 40–80% of the total white blood cell count. Neutrophils move into an area of damaged or infected tissue, where they engulf and destroy bacteria or sometimes fungi.
- Eosinophils respond to infections caused by parasites, play a role in allergic reactions (hypersensitivities), and control the extent of immune responses and inflammation. Eosinophils, normally about 1-3% of the total white blood cell count, are believed to function in allergic responses and in resisting some infections.
- Basophils usually make up the fewest number of circulating white blood cells and are thought to be involved in allergic reactions. Basophils normally constitute 1% or less of the total white blood cell count but may increase or decrease in certain diseases.
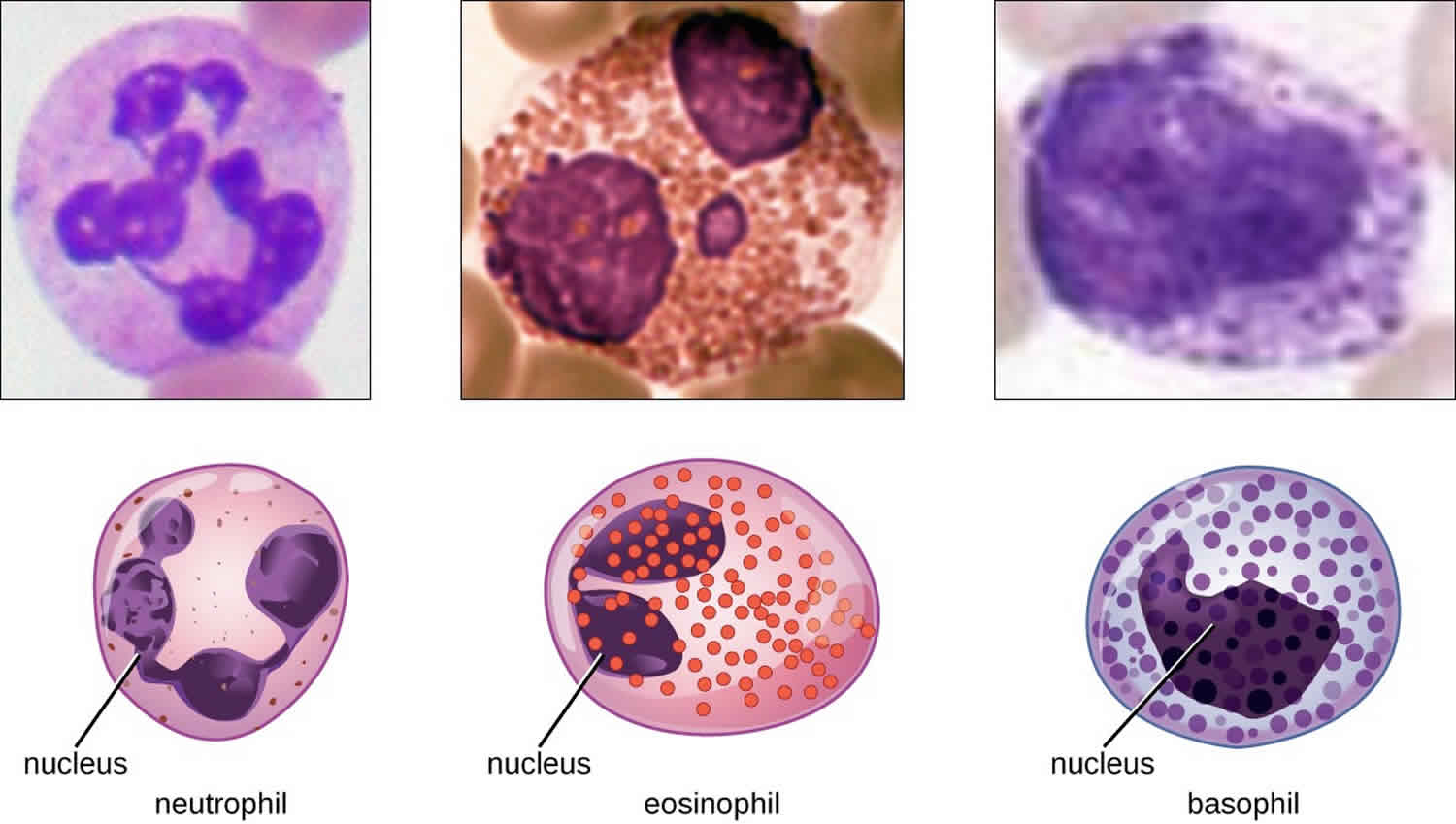
The various types of granulocytes can be distinguished from one another in a blood smear by the appearance of their nuclei and the contents of their granules, which confer different traits, functions, and staining properties. The neutrophils, also called polymorphonuclear neutrophils (PMNs), have a nucleus with three to five lobes and small, numerous, lilac-colored granules. Each lobe of the nucleus is connected by a thin strand of material to the other lobes. The eosinophils have fewer lobes in the nucleus (typically 2–3) and larger granules that stain reddish-orange. The basophils have a two-lobed nucleus and large granules that stain dark blue or purple (Figure 1).
When there is an infection or an inflammatory process somewhere in your body, your bone marrow produces more white blood cells, releasing them into the blood. Depending on the cause of infection or inflammation, one particular type of white blood cell may be increased as opposed to other types. As the condition resolves, the production of that type of white blood cell subsides and the number drops to normal levels again.
In addition to infections and inflammation, there are a variety of conditions that can affect the production of white blood cells by the bone marrow or their survival in the blood, resulting in either increased or decreased numbers. The differential, along with the other components of the complete blood count (CBC), alerts the healthcare provider to possible health issues. Results are often interpreted in conjunction with additional tests such as a blood smear review, which can reveal the presence of abnormal and/or immature populations of white blood cells.
In a few serious diseases, some immature forms of the cells are released from the bone marrow into the circulation and may be detected by the white blood cell differential. This may occur with bacterial infection, leukemia, bone marrow involvement by solid tumor, myelodysplastic syndrome, or myeloproliferative neoplasms, for example. Some immature cells that may be detected include metamyelocytes, myelocytes, promyelocytes, and/or blasts.
If results indicate a problem, a wide variety of other tests may be performed in order to help determine the cause. A healthcare provider will typically consider an individual’s signs and symptoms, medical history, and results of a physical examination to decide what other tests may be necessary. For example, as needed, a bone marrow biopsy will be performed to evaluate the bone marrow status.
Figure 1. Granulocytes
Neutrophils
Neutrophils also called polymorphonuclear neutrophils, are frequently involved in the elimination and destruction of extracellular bacteria. They are capable of migrating through the walls of blood vessels to areas of bacterial infection and tissue damage, where they seek out and kill infectious bacteria. Neutrophil granules contain a variety of defensins and hydrolytic enzymes that help them destroy bacteria through phagocytosis. In addition, when many neutrophils are brought into an infected area, they can be stimulated to release toxic molecules into the surrounding tissue to better clear infectious agents. This is called degranulation.
Another mechanism used by neutrophils is neutrophil extracellular traps (NETs), which are extruded meshes of chromatin that are closely associated with antimicrobial granule proteins and components. Chromatin is DNA with associated proteins (usually histone proteins, around which DNA wraps for organization and packing within a cell). By creating and releasing a mesh or lattice-like structure of chromatin that is coupled with antimicrobial proteins, the neutrophils can mount a highly concentrated and efficient attack against nearby pathogens. Proteins frequently associated with NETs include lactoferrin, gelatinase, cathepsin G, and myeloperoxidase. Each has a different means of promoting antimicrobial activity, helping neutrophils eliminate pathogens. The toxic proteins in NETs may kill some of the body’s own cells along with invading pathogens. However, this collateral damage can be repaired after the danger of the infection has been eliminated.
As neutrophils fight an infection, a visible accumulation of leukocytes, cellular debris, and bacteria at the site of infection can be observed. This buildup is what you call pus (also known as purulent or suppurative discharge or drainage).
Eosinophils
Eosinophils are granulocytes that protect against protozoa and helminths; they also play a role in allergic reactions. The granules of eosinophils, which readily absorb the acidic reddish dye eosin, contain histamine, degradative enzymes, and a compound known as major basic protein. The major basic protein binds to the surface carbohydrates of parasites, and this binding is associated with disruption of the cell membrane and membrane permeability.
Basophils
Basophils have cytoplasmic granules of varied size and are named for their granules’ ability to absorb the basic dye methylene blue. Their stimulation and degranulation can result from multiple triggering events. Activated complement fragments C3a and C5a, produced in the activation cascades of complement proteins, act as anaphylatoxins by inducing degranulation of basophils and inflammatory responses. This cell type is important in allergic reactions and other responses that involve inflammation. One of the most abundant components of basophil granules is histamine, which is released along with other chemical factors when the basophil is stimulated. These chemicals can be chemotactic and can help to open the gaps between cells in the blood vessels. Other mechanisms for basophil triggering require the assistance of antibodies.
Granulocyte transfusion
Cancer patients, transplant patients and other sick patients often can’t make enough of their own granulocytes to control infections during treatment. Granulocyte transfusion from donors can be critical to control infections after chemotherapy, transplantation and other treatment. Clinical center physicians rely on donated granulocytes to treat patients with life-threatening infections or severely impaired immune systems.
Granulocyte donors receive medication the day before donation to increase cell production. The next day, granulocytes are donated using a blood separation process called aspheresis. Much like platelet donation, granulocytes donation takes approximately two to three hours, during which donor blood passes through a sterile kit attached to a cell separator device. This device separates blood cells, keeping the granulocytes and returning the other blood cells to the donor. Learn more about the donation process.
What is granulocyte stimulating factor
Granulocyte-colony stimulating factor (G-CSF) is a monocyte-derived cytokine that increases circulating neutrophils numbers, cell surface expression of antimicrobial receptors, and enhances phagocytosis, respiratory burst activity and chemotaxis 1. In healthy individuals, granulocyte-colony stimulating factor (G-CSF) is produced mainly in vascular endothelial cells, fibroblasts, monocytes, and macrophages 2. Recombinant human granulocyte colony stimulating factor (G-CSF) is the only agent currently approved that can treat or prevent chemotherapy-induced neutropenia and therefore reduce its associated complications.
What is granulocyte macrophage colony stimulating factor
Granulocyte-macrophage colony-stimulating factor (GM-CSF) also called colony-stimulating factor 2 (CSF-2) is an important hematopoietic growth factor and immune modulator 3. Granulocyte-macrophage colony-stimulating factor (GM-CSF) also has profound effects on the functional activities of various circulating leukocytes. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has low basal circulating levels under homeostatic conditions, but its levels can quickly become elevated during infection or inflammation 4. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is produced during inflammatory/autoimmune reactions by a variety of cells, including fibroblasts, endothelial cells, macrophages, dendritic cells (antigen presenting cells), T cells, neutrophils, eosinophils, resident tissue cells, and cancer cells 5. Expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) is induced by proinflammatory cytokines, such as IL-1α, IL-1β, TNF-α, and IL-12, whereas IL-4, IFN-γ, and IL-10 suppress it 6. At sites of inflammation, granulocyte-macrophage colony-stimulating factor (GM-CSF) has proinflammatory effects through recruitment of myeloid cells and by enhancing their survival and activation 7. In several murine models of autoimmunity/inflammation, granulocyte-macrophage colony-stimulating factor (GM-CSF) blockade led to reduced levels of monocyte and neutrophil recruitment with corresponding alleviation of disease severity, whereas in vivo administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) resulted in mobilization of monocytes from the bone marrow into the bloodstream 8. Although the in vivo kinetics of granulocyte-macrophage colony-stimulating factor (GM-CSF)-mediated chemokine production responsible for immune cell recruitment remains to be elucidated, there is evidence indicating that granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates the production of the chemokines CCL3 and CCL2 by neutrophils and macrophages 9. These observations contrast with the more “homeostatic” role of M-CSF, as granulocyte-macrophage colony-stimulating factor (GM-CSF) not only promotes the survival of macrophages but also supports their differentiation toward a proinflammatory phenotype.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) shifts the phenotype of macrophages in a proinflammatory direction. It promotes this through induction of proinflammatory cytokines, such as TNF-α, IL-6, IL-12p70, IL-23, and IL-1β, and chemokines, such as CCL22, CCL24, CCL5, and CCL1, which promote leukocyte recruitment 10. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-treated monocytes/macrophages have been linked to the M1 activation state of macrophages, as many of the cytokines induced by granulocyte-macrophage colony-stimulating factor (GM-CSF) are also induced by IFN-γ. However, as mentioned above, such terminology should be applied with caution. Because of their excellent antigen-presenting capacity, granulocyte-macrophage colony-stimulating factor (GM-CSF)-treated bone marrow cells are often referred to as dendritic cells (antigen presenting cells). It is important to note, however, that granulocyte-macrophage colony-stimulating factor (GM-CSF)-cultured bone marrow cells express a mixture of macrophage and dendritic cell-specific markers. For example, both GM-BMMs and M-BMMs express common macrophage markers, such as CD11b (Mac-1), F4/80, and c-Fms (M-CSFR), whereas only GM-BMM expresses the dendritic cell-associated marker CD11c 11. Unlike dendritic cells (antigen presenting cells), GM-BMMs are good osteoclast precursors and are phagocytic 12. Additionally, despite significant differences in their cytokine and chemokine production repertoire, meta analysis of mouse microarray data indicate that granulocyte-macrophage colony-stimulating factor (GM-CSF)- and M-CSF-stimulated cells show more similarities than differences 12. Because of this, the molecular nature of the mediators that define the proinflammatory phenotype of GM-BMMs has long been elusive. The proinflammatory nature of granulocyte-macrophage colony-stimulating factor (GM-CSF)-treated myeloid cells may be explained by the observation that IFN regulatory factor 5 plays a key role in granulocyte-macrophage colony-stimulating factor (GM-CSF)-mediated macrophage polarization driving the expression of IL-12p70 and IL-23 cytokines, while suppressing expression of IL-10 13. Additionally, activin A has been identified recently as an emerging player in the regulation of M- and GM-BMM polarization. One study has shown that treating M-BMMs with activin A prevents LPS-induced production of IL-10, and conversely, blockade of activin A suppressed the acquisition of proinflammatory markers by human monocytes cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) 14. Thus, the latter study suggests that activin A may be a key molecule inducing the proinflammatory phenotype in macrophages.
Role of granulocyte-macrophage colony-stimulating factor in dendritic cell development
Despite the ability of granulocyte-macrophage colony-stimulating factor (GM-CSF) to generate cells with dendritic cell (DC) characteristics from monocytes or bone marrow cells in vitro, the deficiency of granulocyte-macrophage colony-stimulating factor (GM-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF)R in mice resulted in only minor defects in dendritic cell development in lymphoid organs. Instead, the phenotype of these mice indicates that granulocyte-macrophage colony-stimulating factor (GM-CSF) plays an important role in the maturation of alveolar macrophages 15. In humans, mutations in the granulocyte-macrophage colony-stimulating factor (GM-CSF)R/IL-3R/IL-5R common β chain lead to alveolar macrophage defects, which are associated with proteinosis [103, 104]. granulocyte-macrophage colony-stimulating factor (GM-CSF) is also involved in steady-state development of dermal CD103+CD11b+ DCs and a subset of intestinal lamina propria dendritic cells but suppresses the development of resident CD8+ dendritic cells 16. granulocyte-macrophage colony-stimulating factor (GM-CSF) has also been shown to be involved in the development of monocyte-derived dendritic cells and inflammatory dendritic cells, which accumulate in tissues in response to infection or tissue injury 17.
Granulocyte-macrophage colony-stimulating factor in inflammatory/autoimmune disease
Although granulocyte-macrophage colony-stimulating factor (GM-CSF) is dispensable for the development and maintenance of the most major hematopoietic cell types in spleen, blood, and bone marrow, this cytokine plays an important role in the induction of a wide range of effector functions in many cells of the immune lineage, including stimulation of proliferation and activation of monocytes/macrophages, dendritic cells, T cells, neutrophils, and B cells 18. Mice lacking granulocyte-macrophage colony-stimulating factor (GM-CSF) are more susceptible to pulmonary 19 and intestinal 20 infections, indicating an important role for this cytokine against many pathogens.
Although granulocyte-macrophage colony-stimulating factor (GM-CSF) production in T cells is often associated with Th17 cells, other T cell subsets, including Th1, Th2, and CD8+ T cells also produce granulocyte-macrophage colony-stimulating factor (GM-CSF) upon activation. The expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in T cells is regulated by multiple cytokines. For example, inflammatory cytokines, such as IL-6, TNF-α, and IL-23, induce production of granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas conversely, IL-10, IL-4, and IL-27 inhibit it. CD4+ T and CD8+ T cells from granulocyte-macrophage colony-stimulating factor (GM-CSF)-deficient mice exhibit impaired, proliferative responses and effector functions. Furthermore, several studies have demonstrated that granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing T cells play an essential role in the induction of immune responses against Mycobacterium tuberculosis, EBV, and HIV-1. It is unlikely though that granulocyte-macrophage colony-stimulating factor (GM-CSF) acts directly on T cells, as these cells do not express the granulocyte-macrophage colony-stimulating factor (GM-CSF)R. Instead, T cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes maturation and activation of APCs, which in turn, potentiate T cell functions. The complexity behind the production of T cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) suggests that it represents a powerful immune system mediator in both inflammation and infections.
Moreover, other non-T cell mechanisms against infection, where granulocyte-macrophage colony-stimulating factor (GM-CSF) also participates, have been described. For example, it has been implicated against pneumonia through its ability to activate IgM production in B1a cells 21. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by iNKT cells may be involved in resistance to tuberculosis. The increase of evidence points to a central role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in a proinflammatory cytokine “positive feedback” loop, which often underlies the chronic nature of many inflammatory and autoimmune disorders. Th17 cells have been identified as potent inducers of many inflammatory/autoimmune diseases, including CIA, EAE, and arthritis in the SKG mouse. Th17 cells have been shown to have high “plasticity,” which allows their differentiation into highly pathogenic Th1/Th17 cells (particularly in humans). These cells are characterized by their coproduction of IL-17A, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF), coexpression of retinoid acid receptor-related orphan receptor γt and T-bet, as well as coexpression of the Th1 and Th17 signature chemokine receptors CXCR3 and CCR6. Multiple reports have shown that it is not IL-17A but rather Th cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) that mediates the pathogenic effects observed. Accordingly, studies using granulocyte-macrophage colony-stimulating factor (GM-CSF)-deficient mice have demonstrated that the inability to produce granulocyte-macrophage colony-stimulating factor (GM-CSF) protects these mice from developing multiple autoimmune diseases, including: EAE, CIA, and autoimmune myocarditis. Moreover, in vivo administration of an anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) neutralizing mAb during the early stages of clinical disease completely prevented or strongly ameliorated autoimmune-associated inflammation.
The success of blockade/neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) in several mouse models of autoimmune/inflammatory diseases suggested that neutralizing the granulocyte-macrophage colony-stimulating factor (GM-CSF)/CSF-2R axis could be a useful therapeutic strategy for patients with MS, arthritis, and other indications, such as psoriasis. In clinical trials, an anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) mAb (MOR103) has shown significant improvements in patients with active rheumatoid arthritis. Its clinical efficacy supports further advancement into clinical development 22. Likewise, Phase II clinical trials with mavrilimimab (an anti-granulocyte-macrophage colony-stimulating factor (GM-CSF)Rα mAb) showed rapid improvement in patients with RA with no significant adverse effects 23.
References- Granulocyte colony-stimulating factor and its receptor. Demetri GD, Griffin JD. Blood. 1991 Dec 1; 78(11):2791-808.
- Shimakawa T, Asaka S, Usuda A, et al. Granulocyte-colony stimulating factor (G-CSF)-producing esophageal squamous cell carcinoma: a case report. Int Surg. 2014;99(3):280-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4027914/
- Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006 Feb;16(2):126-33. https://www.nature.com/articles/7310017
- Williamson D. J., Begley C. G., Vadas M. A., Metcalf D. (1988) The detection and initial characterization of colony-stimulating factors in synovial fluid. Clin. Exp. Immunol. 72, 67–73.
- Gasson J. C. (1991) Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood 77, 1131–1145
- Lukens J. R., Barr M. J., Chaplin D. D., Chi H., Kanneganti T. D. (2012) Inflammasome-derived IL-1β regulates the production of GM-CSF by CD4(+) T cells and γδ T cells. J. Immunol. 188, 3107–3115.
- Parajuli B., Sonobe Y., Kawanokuchi J., Doi Y., Noda M., Takeuchi H., Mizuno T., Suzumura A. (2012) GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J. Neuroinflammation 9, 268
- Cook A. D., Turner A. L., Braine E. L., Pobjoy J., Lenzo J. C., Hamilton J. A. (2011) Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum. 63, 2340–2351.
- Shinohara H., Yano S., Bucana C. D., Fidler I. J. (2000) Induction of chemokine secretion and enhancement of contact-dependent macrophage cytotoxicity by engineered expression of granulocyte-macrophage colony-stimulating factor in human colon cancer cells. J. Immunol. 164, 2728–2737
- Lacey D. C., Achuthan A., Fleetwood A. J., Dinh H., Roiniotis J., Scholz G. M., Chang M. W., Beckman S. K., Cook A. D., Hamilton J. A. (2012) Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 188, 5752–5765
- Lari R., Fleetwood A. J., Kitchener P. D., Cook A. D., Pavasovic D., Hertzog P. J., Hamilton J. A. (2007) Macrophage lineage phenotypes and osteoclastogenesis—complexity in the control by GM-CSF and TGF-beta. Bone 40, 323–336.
- Mabbott N. A., Kenneth Baillie J., Hume D. A., Freeman T. C. (2010) Meta-analysis of lineage-specific gene expression signatures in mouse leukocyte populations. Immunobiology 215, 724–736
- Krausgruber T., Blazek K., Smallie T., Alzabin S., Lockstone H., Sahgal N., Hussell T., Feldmann M., Udalova I. A. (2011) IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 12, 231–238.
- Sierra-Filardi E., Puig-Kröger A., Blanco F. J., Nieto C., Bragado R., Palomero M. I., Bernabéu C., Vega M. A., Corbí A. L. (2011) Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 117, 5092–5101
- Shibata Y., Berclaz P. Y., Chroneos Z. C., Yoshida M., Whitsett J. A., Trapnell B. C. (2001) GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15, 557–567
- King I. L., Kroenke M. A., Segal B. M. (2010) GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 207, 953–961.
- Zhan Y., Xu Y., Lew A. M. (2012) The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol. Immunol. 52, 30–37.
- Burgess A. W., Camakaris J., Metcalf D. (1977) Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J. Biol. Chem. 252, 1998–2003
- LeVine A. M., Reed J. A., Kurak K. E., Cianciolo E., Whitsett J. A. (1999) GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J. Clin. Invest. 103, 563–569.
- Hirata Y., Egea L., Dann S. M., Eckmann L., Kagnoff M. F. (2010) GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe 7, 151–163.
- Weber G. F., Chousterman B. G., Hilgendorf I., Robbins C. S., Theurl I., Gerhardt L. M., Iwamoto Y., Quach T. D., Ali M., Chen J. W., Rothstein T. L., Nahrendorf M., Weissleder R., Swirski F. K. (2014) Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 211, 1243–1256
- Behrens F., Tak P. P., Burkhardt H. et al. (2015) MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 74, 1058–1064.
- Takeuchi T., Tanaka Y., Close D., Godwood A., Wu C. Y., Saurigny D. (2015) Efficacy and safety of mavrilimumab in Japanese subjects with rheumatoid arthritis: findings from a Phase IIa study. Mod. Rheumatol. 25, 21–30