What is carnauba wax Carnauba wax also called Brazilian wax and palm wax, is a wax obtained from the leaf buds and leaves of the
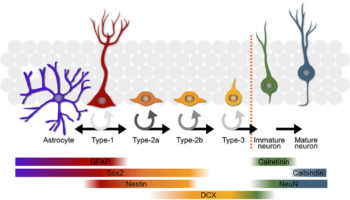
What is neurogenesis Adult neurogenesis is the biological process of continuously generating new neurons that can functionally integrate into the adult mammalian brain throughout life
What is Erb's palsy Erb's palsy is also called Erb-Duchenne palsy refers to paralysis of the upper brachial plexus caused by injury to the superior
What is lactase Lactase is an enzyme that breaks down lactose (milk sugar) into two sugars called glucose and galactose, which can be easily absorbed
What is BNP test BNP test is a test for B-type natriuretic peptide (BNP or brain natriuretic peptide) and N-terminal pro b-type natriuretic peptide (NT-proBNP),
What is clostridium difficile Clostridium difficile (C. difficile) is a Gram-positive obligate anaerobic bacterium that causes antibiotic-associated diarrhea and more serious intestinal conditions such as
What is luteinizing hormone Luteinizing hormone (LH) is a hormone associated with reproduction and the stimulation of the release of an egg from the ovary
What is mental retardation Mental retardation is now called "intellectual disability" sometimes also called cognitive disabilities. According to American Association of Intellectual and Developmental Disabilities
What is AMH AMH is short for antimullerian hormone, also known as mullerian-inhibiting substance, is a dimeric glycoprotein hormone produced by reproductive tissues, including the testicles
What is pepsin Pepsin is an aspartic acid protease enzyme that uses aspartic acid residues in the active center ((Cooper JB. Aspartic proteinases in disease: