What is neurogenesis
Adult neurogenesis is the biological process of continuously generating new neurons that can functionally integrate into the adult mammalian brain throughout life 1. Neurogenesis is an endogenous process that involves coordinated proliferation, differentiation, and migration of neural precursor cells 2. Neurogenesis determines brain formation during embryonic development and persists in localized regions of the adult brain or neurogenic niches 3. As a result of aging, brain injury, and genetic mutations, the progressive loss of structure, function, and depletion of neural precursors may contribute to neurodegenerative disorders including Alzheimer’s disease and Parkinson’s diseases 4. Neurogenesis was traditionally viewed to occur only during embryonic and perinatal stages in mammals 5. Altman’s pioneering studies in the 1960s provided the first anatomical evidence for the presence of newly generated dentate granule cells in the postnatal rat hippocampus 6, followed by Kaplan and Hinds in the 1970s 7, provided evidence for the birth of new neurons in the adult brain. Functional integration of new neurons in the adult central nervous system (CNS) was first shown in songbirds 8. Multipotent neural stem cells were later derived from the adult mammalian brain 9. The field of adult neurogenesis took off after the introduction of bromodeoxyuridine, a nucleotide analog as a lineage tracer 10 and demonstrations of life-long continuous neurogenesis in almost all mammals examined, including in humans 2. The most relevant question, that yet remains to be fully answered, is the one regarding the role new neurons play in the functional activity of the mature brain and whether these cells display any clinical relevance 11. Due to the technical limitations of human studies, our understanding of the functional role of adult hippocampal neurogenesis in humans relies on retrospective analyses using post-mortem tissues 12. Therefore, it remains unclear how adult-born dentate granule cells of the hippocampus functionally modulate complex behavior and how dysregulation of adult neurogenesis mediates brain disorders in the human brain 12.
A large body of evidence now suggests that impaired adult neurogenesis in the hippocampus is associated with various mental disorders, including major depression, schizophrenia, mood, and anxiety disorders as well as addictive behaviors. Importantly, decreased adult neurogenesis correlates with reduced cognitive and affective functions, a common symptom in patients and a frequent phenotype in animal models of these diseases, and often coincides with a decrease in cell proliferation in the dentate gyrus and reduced hippocampal volume 13. An emerging hypothesis linking neurogenesis modulation with the onset and subsequent cure of depression has received much attention 14. This is partly due to the fact that chronic administration of most antidepressants leads to an increase in neurogenesis. As adult hippocampal neurogenesis is directly linked to the action of antidepressants, it has been suggested that adult neurogenesis could be a target for treatment of depression 15. Some researchers have even proposed that neurogenesis is a “requirement” for antidepressant behavioral effects 16, but it is still unknown if diminished neurogenesis could be a cause, consequence, or correlate of depression 17. In fact, despite a plethora of discovery-driven research, the functional contribution of adult neurogenesis remains elusive. As a result, it continues to be difficult to draw conclusions regarding the possibility of an evidence-based link between neurogenesis and depression 14. While it is probable that modulation of hippocampal neurogenesis contributes to the onset and alleviation of depression 14, much investigation is still needed into the mechanisms that may regulate this process.
Where does neurogenesis occur?
Although there is evidence that new neurons may be added to several other neural regions under some conditions, in the normal adult brain, neurogenesis appears to be restricted to three areas, each with a focal population of progenitor cells and a characteristic pattern of differentiation and migration of new neurons 18.
Active adult neurogenesis is spatially restricted under normal conditions to two specific “neurogenic” brain regions, the subgranular zone (SGZ) in the dentate gyrus of the hippocampus where new dentate granule cells are generated, and the subventricular zone (SVZ) of the lateral ventricles where new neurons are generated and then migrate through the rostral migratory stream (RMS) to the olfactory bulb to become interneurons (Figure 3A) 19.
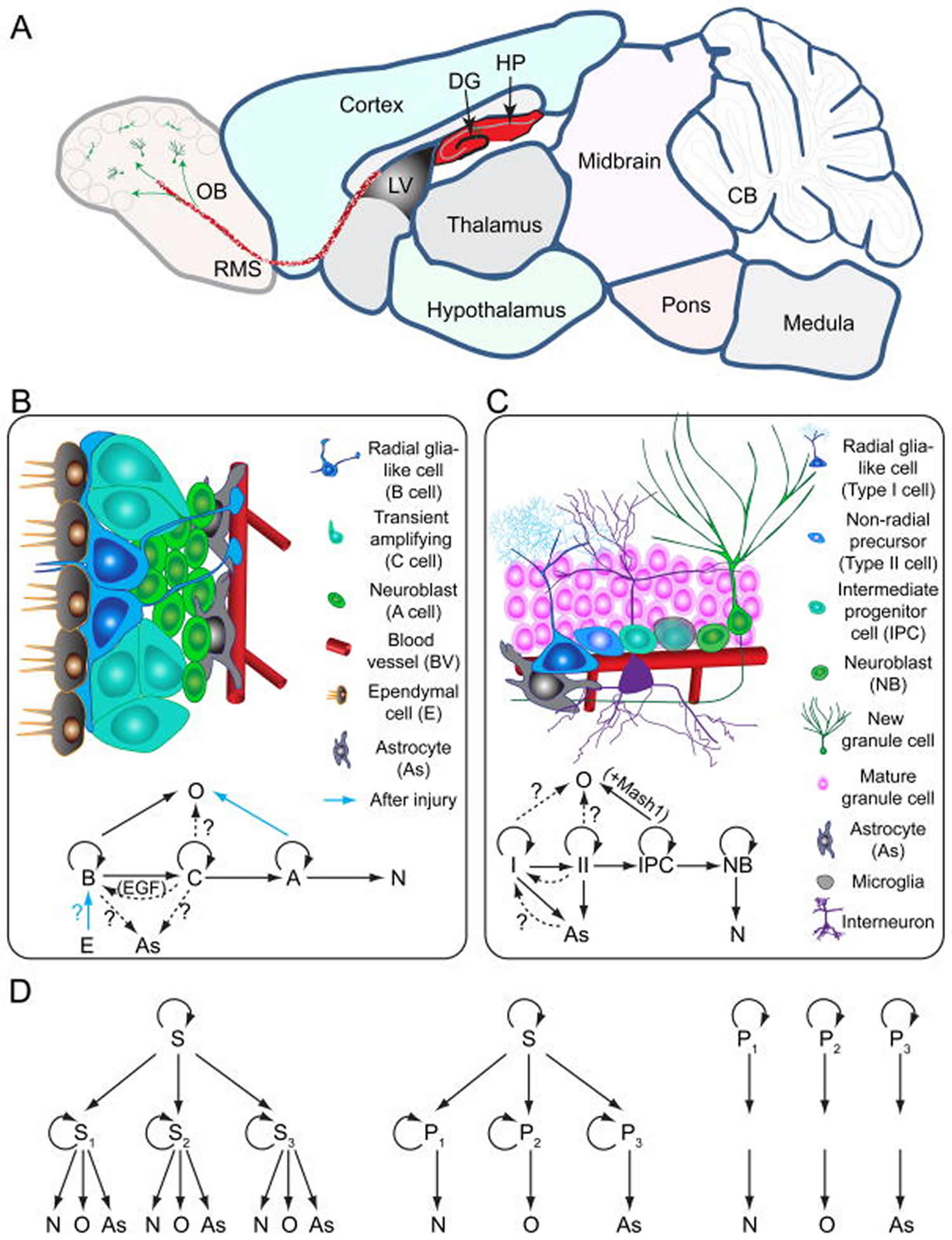
Figure 1. Medial aspect of the human brain
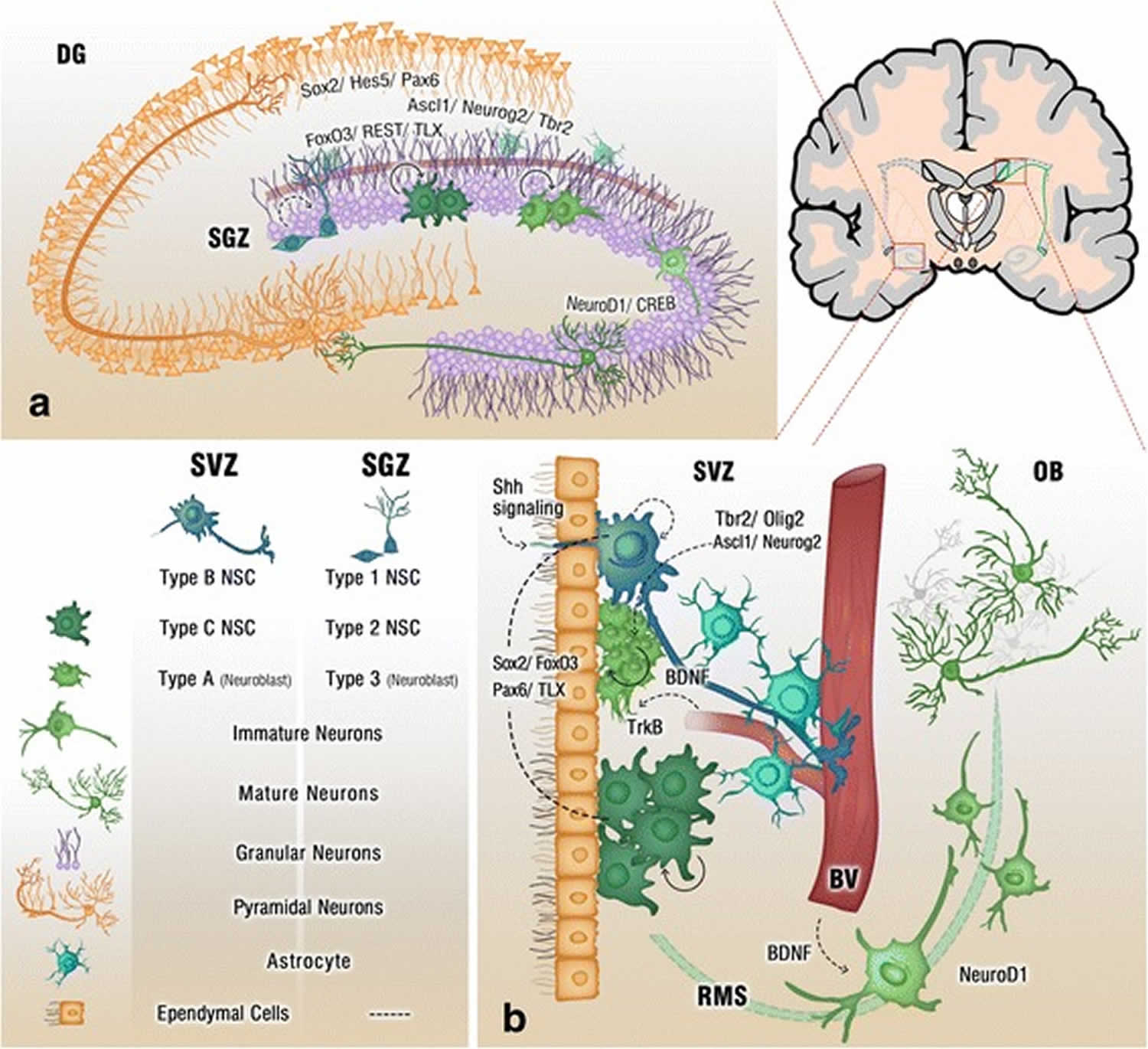
Footnote: Cross section of the adult brain showing regions of subgranular zone (SGZ) and subventricular zone (SVZ), where neurogenesis takes place. The schematic illustrates neurogenesis involving neural stem cells (NSCs) development into mature neurons and the neurogenic niches of Blood Vessels (BV), astrocytes and cilia, as well as transcription programs in the SGZ (a) and SVZ (b). Neuronal migration from the SVZ to the olfactory bulb (OB) via the rostral migratory stream (RMS) is also shown in (b)
[Source 3 ]Figure 3. Neurogenesis brain regions
Footnotes:
Models of neural stem cells and lineage relationship in the adult dentate gyrus and subventricular zone
(A) A sagittal section view of an adult rodent brain highlighting the two restricted regions that exhibit active adult neurogenesis: dentate gyrus (DG) in the hippocampal formation (HP); the lateral ventricle (LV) to the rostral migratory stream (RMS) to the olfactory bulb (OB).
(B) A schematic illustration of the neural stem cell niche in the subventricular zone (SVZ) and a model of potential lineage relationship under basal (solid arrows) and injury conditions (blue arrows). N: immature neurons
(C) A schematic illustration of the neural stem cell niche in the subgranular zone (SGZ) in the dentate gyrus and a model of potential lineage relationship.
(D) Three lineage models of neural precursors in the adult mammalian brain. In the first model (left), adult neural stem cells (S1,2,3…) generated from primitive neural stem cells (S) are intrinsically diverse, exhibiting vastly different developmental potential depending on their regions of distribution and developmental origins. In the second model (middle), adult neural stem cells (S) are relatively homogenous and give rise to a heterogeneous population of lineage-restricted progenitors (P1,2,3…). In the third model (right), only lineage-restricted neural progenitors (P1,2,3…) are present in the adult brain; self-renewal and multi-lineage differentiation represent a collective property of a mixture of different lineage-restricted neural progenitors. N: neurons; O: oligodendrocytes; As: astrocytes.
[Source 20 ]1. Neurogenesis in the Hippocampus
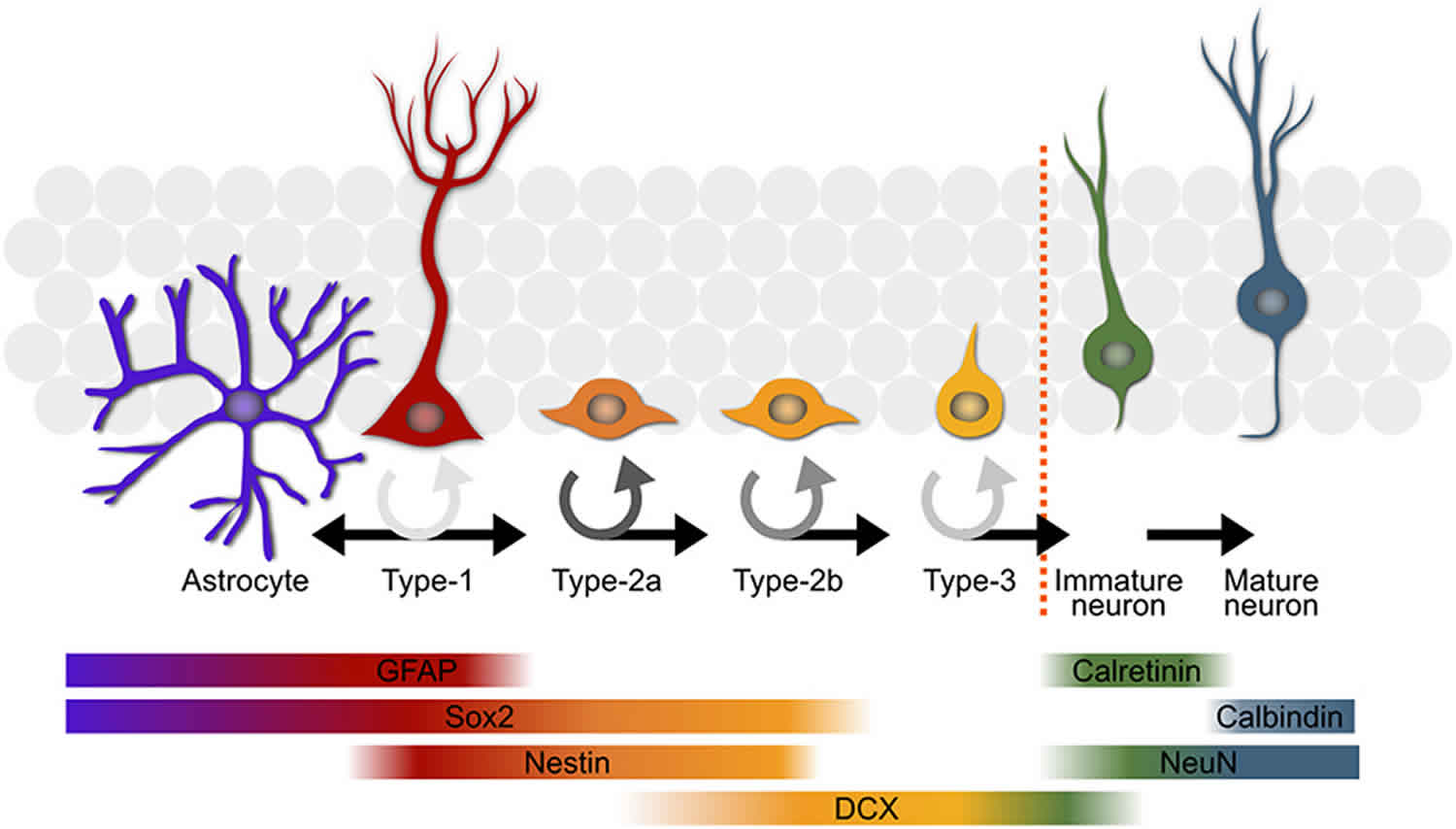
Adult hippocampal neurogenesis is a process that describes the generation of new functional dentate granule cells from adult neural stem cells through the amplification of intermediate progenitors and neuroblasts, as well as the integration of these new neurons into the existing neural circuits 12. Adult hippocampal neural stem cells (radial glia-like cells, RGLs; Type 1 cells) exist in the subgranular zone (SGZ) of the dentate gyrus. Various forms of activation of the environmental niche stimulate quiescent radial glia-like cells and facilitate their proliferation. Active radial glia-like cells self-renew and also generate intermediate neural progenitors that subsequently differentiate into neuroblasts and finally give rise to dentate granule cells or, to a lesser extent, to astrocytes 12. These processes, including proliferation, differentiation, migration, neurite extension and synaptic integration, are regulated by a number of signals from the environmental niche and local neural circuits, which are summarized in Table 1.
Neural progenitor cells in the hippocampus are located in the subgranular zone (SGZ) at the border between the granule cell layer and hilus of the dentate gyrus 21. Some of the daughter cells produced by division of those precursor cells differentiate into neurons and develop the prominent apical dendrite that characterizes dentate granule neurons as they move into the granule cell layer. Adult-born neurons project axons to the primary target of dentate granule neurons, the stratum lucidum of area CA3, as early as 4 to 10 days after their final mitosis 22, are integrated into the hippocampal circuitry, and are electrophysiologically comparable to earlier born granule neurons within several weeks 23. The structural 22 and functional 24 development of adult-born granule neurons is slightly slowed, however, compared to the development of those born at the developmental peak of genesis.
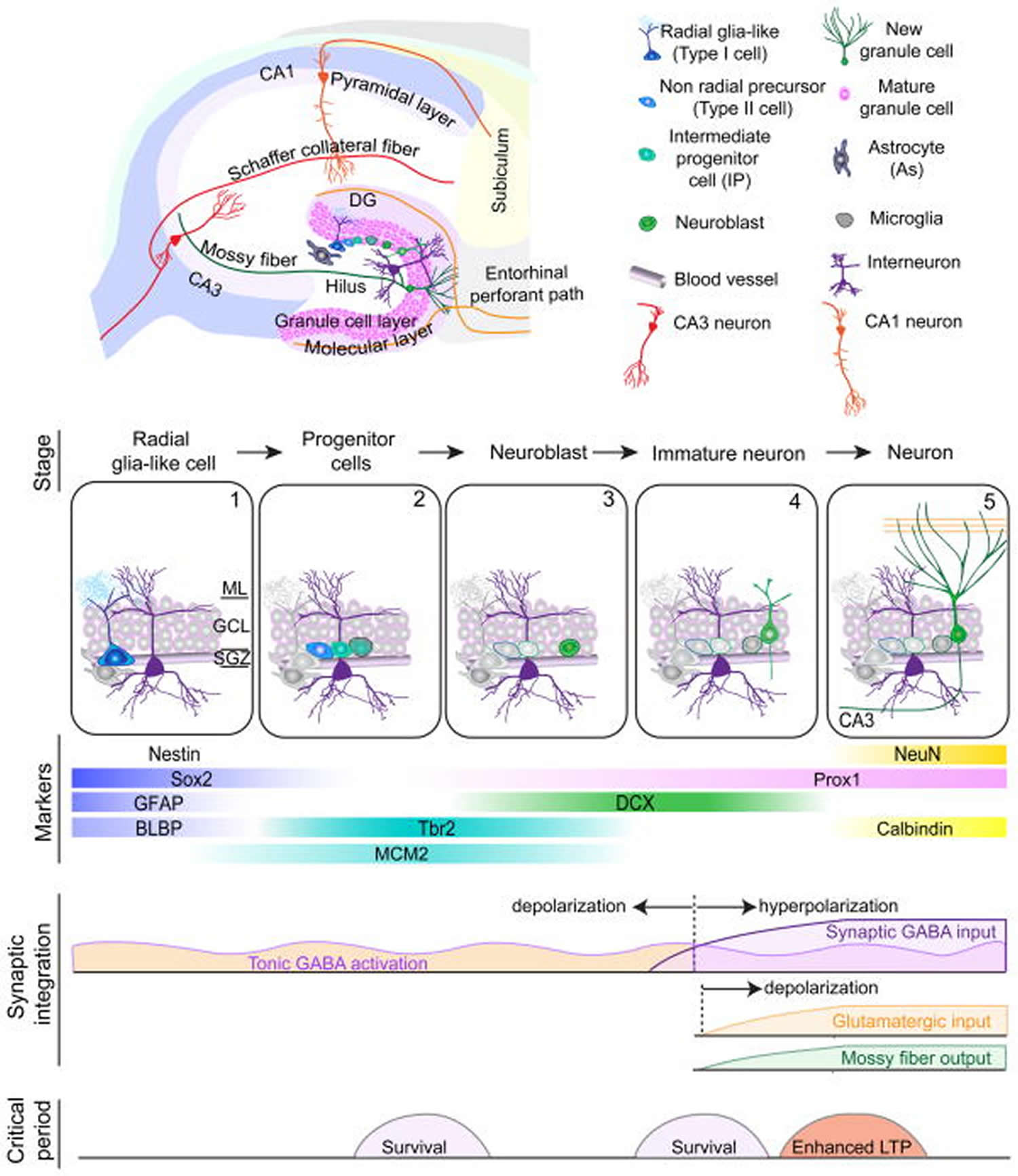
Figure 4. Neurogenesis in the dentate gyrus of the hippocampus
Footnote: Summary of five developmental stages during adult hippocampal neurogenesis: (1) activation of quiescent radial glia-like cell in the subgranular zone (SGZ); (2) proliferation of non radial precursor and intermediate progenitors; (3) generation of neuroblasts; (4) integration of immature neurons; (5) maturation of adult-born dentate granule cells. Also shown are expression of stage-specific markers, sequential process of synaptic integration, and critical periods regulating survival and plasticity.
Abbreviations: ML = molecular layer; GCL= granule cell layer; SGZ = subgranular zone; GFAP = glial fibrillary acidic protein; BLBP = brain lipid-binding protein; DCX = doublecortin; NeuN = neuronal nuclei; LTP = long-term potentiation.
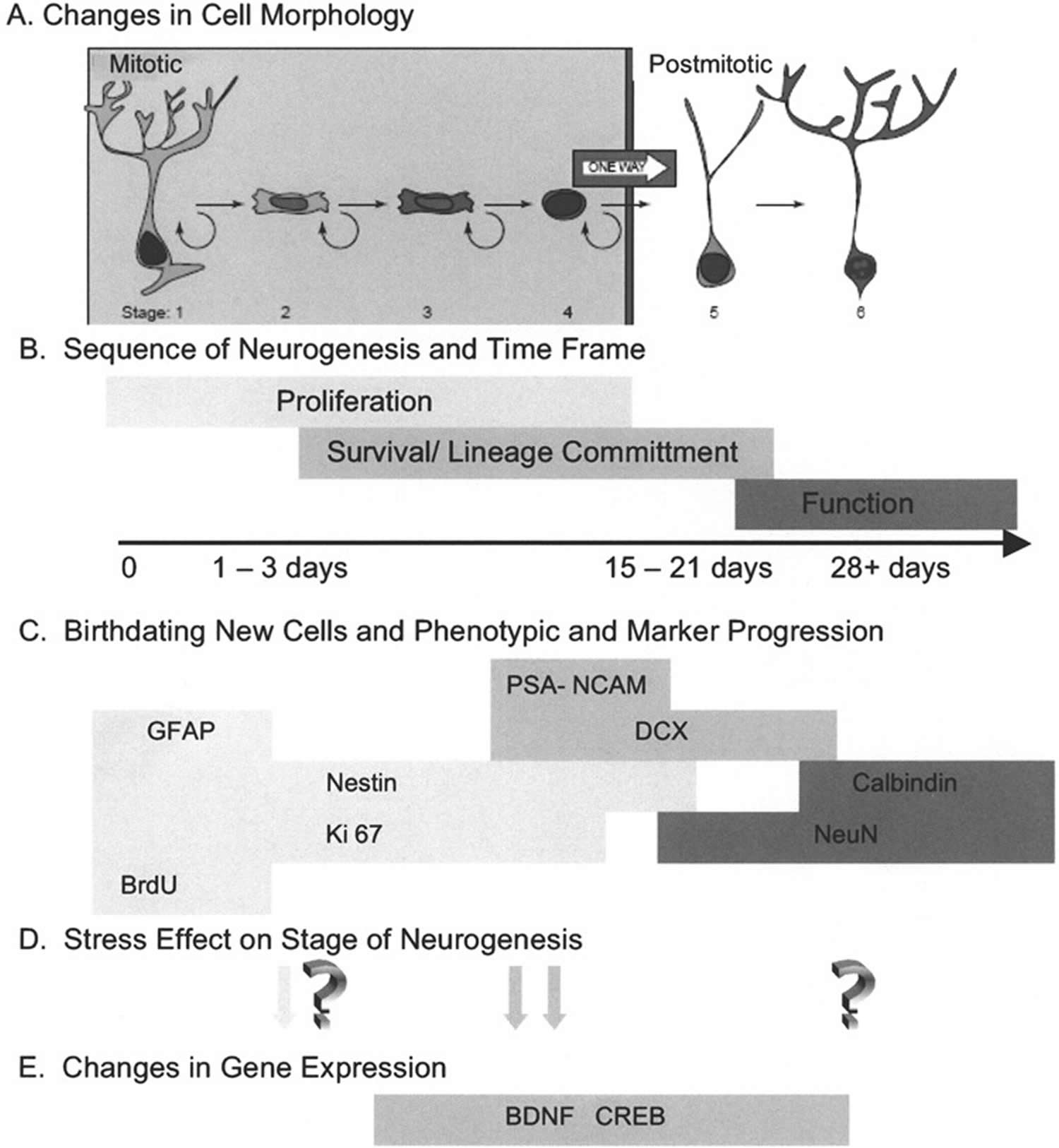
[Source 20 ]Figure 5. Progression of adult hippocampal neurogenesis
Footnotes: From their initial generation until their maturation as a functioning neuron, emerging evidence suggests that the process of neurogenesis consists of sequential progression of the new cell through distinct stages that are identified by a variety of detection methods. (A) Changes in cell morphology and location relative to the granule cell layer formed the original observations that newly generated cells underwent a specific maturation process en route to expressing the morphology of mature neurons. In conjunction with the phenotypic markers discussed below, Kempermann et al. 25 have proposed six specific stages of cell maturation. (B) While it was initially envisioned that proliferating cells terminally exited cell cycle before beginning lineage commitment, it is now recognized that cells expressing early markers of lineage commitment can continue to proliferate. Likewise, there is a transition between expression of lineage commitment markers and the initiation of functional neuronal properties. The population of new cells in the hippocampus is not synchronized, but it appears that any individual cell would progress from mitosis to full maturation in an interval of 3–4 weeks. It is important to note that not all cells will progress to maturity. Some will remain proliferative and constitute a pool of amplifying neural progenitor cells. Other cells will fail to survive the process of lineage commitment and this interval also appears to be critical for regulating the extent of neurogenesis. (C) Identification of progression through the stages of neurogenesis has been facilitated by the characterization of markers that have restricted expression. In the absence of a definitive neural stem cell marker, these cells are identified by the coexpression of markers such as glial fibrillary acidic protein (GFAP), which has been shown to be present in neural stem cells 26. Detection of proliferating cells can be done with exogenous administration of thymidine analogs such as BrdU, labeling a cohort of dividing cells, or by marking the presence of endogenous cell cycle proteins such as Ki-67. Multiple immunohistochemical labeling makes it possible to stage a cell’s progress through neurogenesis by the combinatorial expression of early lineage commitment markers such as nestin, polysialylated neural cell adhesion molecule (PSA-NCAM), and doublecortin (DCX), and to distinguish these from more mature markers of neurons, such as NeuN and calbindin. (D) The transition through stages of neurogenesis provides an opportunity to determine at what point in the process an experimental manipulation exerts its effect. In the case of animal models of stress, early studies suggested that proliferation was altered under stress conditions, while more recent studies have identified the period of lineage commitment and survival as vulnerable to stress. While there is evidence of plasticity of mature hippocampal neurons in response to stress, it is yet to be determined if these alterations are shared by adult-generated neurons. (E) Identifying the stage of neurogenesis that is altered provides an opportunity to examine specific regulatory mechanisms that may participate in the response to stress. As discussed in the text, evidence is emerging that BDNF (brain-derived neurotrophic factor) and CREB (cAMP response element-binding protein) may be important mechanisms of regulating the response to stress. The onset and duration of expression of BDNF (brain-derived neurotrophic factor) and CREB (cAMP response element-binding protein) is still under investigation.
[Source 14 ]Table 1. Signals regulating adult hippocampal neurogenesis
| Stages | Regulators | |
|---|---|---|
| Secreted factors and downstream effectors (Morphogens, growth factors, cytokines, etc) | Type 1 (RGLs) | Maintenance of RGLs BMPs, VEGF, Shh Proliferation of RGLs/NPs IGF2 |
| Type2a, 2b | Proliferation of NPs FGF2, IGF-2, EGF, ERK5, estrogen Promoting differentiation Wnt, IGF-1, VEGF, BDNF/NT-3, BMPs Inhibition of proliferation Cortisol, Chronic Opioid Use, ApoE4 | |
| Neuroblasts & immature neurons | Promoting neuronal maturation Wnt/PCP, BDNF/NT-3, TIMP2 Inhibition of proliferation CCL11, β2M | |
| Adhesion molecules | Type 1, 2a & 2b | Maintenance of RGLs Notch Inhibition of proliferation Integrin Promoting differentiation Eph-Ephrin, |
| Neuroblasts & immature neurons | Neuronal migration and synaptogenesis Semaphorin/Plexin Inhibition of proliferation Tenasin-R | |
| Transcription factors | Type 1 | Maintenance of RGLs REST, Sox2, Hes5, FoxO, NFIX, NFIB Activation of RGLs Ascl1 |
| Type 2a | Maintenance of NPs Sox2, TLX1, REST Differentiation of NPs Ascl1 | |
| Type 2b | Differentiation into intermediate progenitors Tbr2 Neuronal differentiation Neurog2, NeuroD1 | |
| Neuroblasts | Neuronal differentiation NeuroD1 | |
| Immature neurons | Neuronal maturation Prox1, CREB, Klf9 | |
| Epigenetic modifiers | Type 1, 2a & 2b | Proliferation of RGLs/NPs GADD45b, TET1, miR-137, miR-17-92, Nup153 Differentiation of RGLs/NPs MBD1, HDAC1, HDAC2, MeCP2, miR-184, miR-199 |
| Immature neurons | Synaptogenesis MeCP2, HDAC2 Neuronal migration/dendritic growth miR19, miR-132 | |
| Neurotransmitters | Type 1 | Activation of RGLs GABA, Glutamate |
| Type2a, 2b & neuroblasts | Proliferation of NPs GABA, Dopamine, Serotonin, Norepinephrine, Acetylcholine Inhibition of proliferation Chronic opioid use | |
| Immature neurons | Activation of immature neurons GABA, Glutamate, Acetylcholine, Dopamine |
Abbreviations: RGLs = radial glia-like cells, NPs = neural progenitors
[Source 12 ]2. Neurogenesis in the Subventricular Zone and Rostral Migratory Stream
Quantitatively, the extent of adult neurogenesis in the hippocampus is only a fraction of that in the anterior portion of the adult subventricular zone, a thin, persistent remnant of the secondary proliferative zone of the developing brain. Although not readily identifiable by cell-type specific markers, neural stem cells can be isolated from the adult subventricular zone and shown in culture to be both self-renewing and multipotent, capable of generating both neurons and glia. Studies indicate that the neural stem cells have some characteristics of astrocytes, but clearly not all astrocytes in the region are neural stem cells 27. Extensive analysis of the adult subventricular zone indicates that the region comprises several cell types in addition to the slowly dividing stem cells, including a more rapidly dividing population of transit amplifying progenitor cells, neuroblasts, glial cells, and a monolayer of ependymal cells lining the ventricle 28. Neuroblasts born in the subventricular zone maintain the ability to proliferate as they migrate through the subventricular zone, into the rostral migratory stream, and anteriorly to the olfactory bulb, finally differentiating into interneurons 29. Throughout their migration, chains of neuroblasts are ensheathed by slowly proliferating astrocytes, which presumably help maintain an appropriate microenvironment for migration and cell division. The division of neuroblasts within the rostral migratory stream, far from the subventricular zone, demonstrates that the environment that supports the division of neuronal progenitors is much more extensive in the subventricular zone/rostral migratory stream than in the dentate gyrus, where the division of progenitor cells is spatially restricted. It also is important to note that the stem/progenitor cell populations differ between the subventricular zone and the subgranular zone 30, and that the progenitor population in the adult hippocampus lacks true stem cells, containing only more restricted progenitor cells 31.
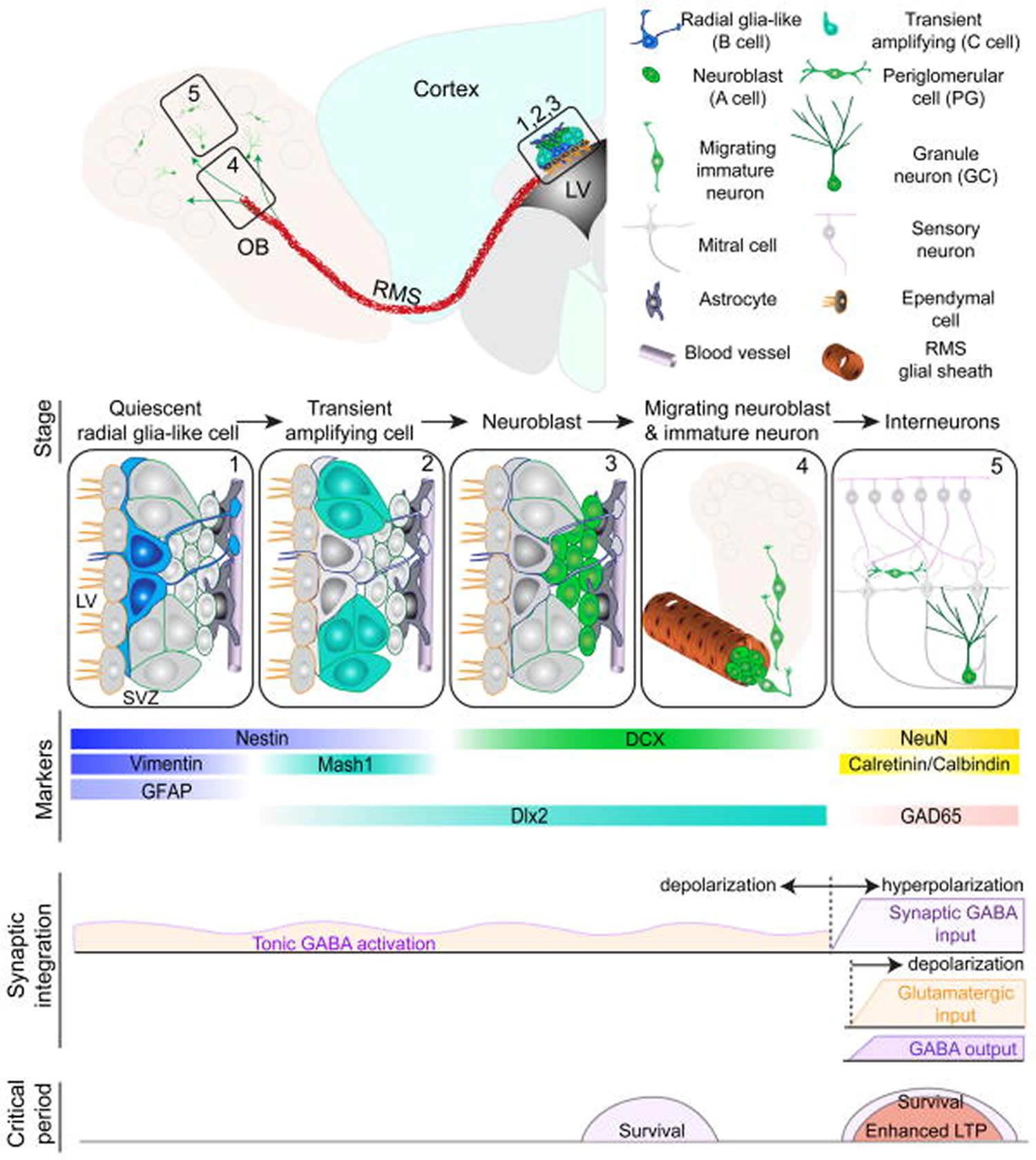
Figure 6. Neurogenesis in the subventricular zone of the lateral ventricle and olfactory bulb
Footnote: Summary of five developmental stages during adult subventricular zone (SVZ) neurogenesis: (1) activation of radial glia-like cells in the subventricular zone in the lateral ventricle (LV); (2) proliferation of transient amplifying cells; (3) generation of neuroblasts; (4) chain migration of neuroblasts within the rostral migratory stream (RMS) and radial migration of immature neurons in the olfactory bulb (OB); (5) Synaptic integration and maturation of granule cells (GC) and periglomerular neurons (PG) in the olfactory bulb. Also shown are expression of stage-specific markers, sequential process of synaptic integration, and critical periods regulating survival and plasticity of newborn neurons.
Abbreviations: GFAP = glial fibrillary acidic protein; DCX = doublecortin; NeuN = neuronal nuclei; LTP = long-term potentiation.
[Source 20 ]3. Neurogenesis in the Olfactory Epithelium
Concurrent with the early demonstrations of neuronal addition in the adult hippocampus and olfactory bulb, several laboratories described the birth of new olfactory receptor neurons within the adult olfactory epithelium 32. Progenitor cells in the basal layer of the olfactory epithelium give rise to new receptor neurons that migrate superficially as they develop their characteristic apical dendrite and project an axon to the glomerular layer of the olfactory bulb. Quantitative studies indicate that olfactory receptor neurons may have lifespans as short as a few weeks or months (influenced in part by ongoing damage to the exposed olfactory mucosa); thus, neurogenesis in the adult olfactory epithelium supports a process of wholesale turnover, compared to the more selective replacement of new neurons within the granule cell and interneuron populations of the dentate gyrus and olfactory bulb. Although less extensively studied than the rostral migratory stream/subventricular zone and hippocampus, the mechanisms of regulation on neurogenesis in the peripheral olfactory system are beginning to be elucidated 32. Because olfactory loss may be an early indicator of age-related neural decline and Alzheimer’s disease pathogenesis 33, understanding aging-related changes in the peripheral olfactory system may provide particularly important translational and clinical benefits.
4. Neurogenesis in Other Neural Regions?
Although it generally is agreed that the three regions above are the only sites of (relatively) large-scale, ongoing neuronal replacement in the normal adult brain, there is evidence that at least the potential for adult neurogenesis is more widespread 18. Cells with properties similar to the stem cells obtained from the hippocampus and anterior subventricular zone have been isolated from other brain regions, including the striatum, cerebral cortex, septum, spinal cord, hypothalamus, and even white matter 34. These cells show at least some capacity for self-renewal in culture, as well as the ability to give rise to both neuronal and glial lineages. Despite the apparently wide distribution of such progenitor cells, however, evidence for constitutive neuronal replacement in areas other than the three regions above remains controversial, in part because of methodological challenges in analyzing cells that divide slowly or seldom and critical questions of what constitutes adequate proof that a particular cell is newly born and that a cell identified as newly born is a neuron 35. Such debates notwithstanding, it is clear that if neurogenesis does occur in the cerebral cortex 36, amygdala 37, spinal cord 38, or other regions, it is at a level that is orders of magnitude below that in the dentate gyrus and subventricular zone/rostral migratory stream.
There is more compelling evidence that neurogenesis may be induced in normally nonneurogenic regions of the adult brain in response to injury and neuronal death 18. There are reports of both injury-induced activation of local precursor cells to generate new neurons and migration of precursor cells from neurogenic to nonneurogenic regions, upon injury to the latter 18. Regions of the brain exhibiting such induced neurogenesis include the cerebral cortex, striatum, and CA1 region of the hippocampus, with neurogenesis occurring in response to focal neuronal degeneration and ischemia 39. Whether such induced neurogenesis is or can be made sufficient to facilitate functional recovery remains to be established, but it offers exciting translational and clinical possibilities 40.
The isolation of progenitor cells from, and presence of “inducible” neurogenesis within, normally nonneurogenic regions of the adult brain illustrates that the characteristically neurogenic regions differ from the remainder of the brain primarily in their permissiveness for neurogenesis, rather than simply representing the sole repositories of neuronal progenitor cells 18. Elucidating the aspects of the cellular microenvironment that are critical for permitting or promoting the generation of new neurons is a fundamental challenge in understanding the regulation of neurogenesis and how that regulation is affected under a variety of physiological conditions, including aging. This task is made particularly challenging by the recognition that the neurogenic microenvironment reflects a complex and dynamic molecular state, rather than a fixed cellular environment 40. Changes in the neurogenic environment represent one possible contributor to the profound decrease in neurogenesis that occurs with brain aging.
Regulators of neurogenesis in the adult and aging brain
The list of intrinsic factors (e.g., transcription factors and cell cycle regulators) and extracellular growth factors, hormones, and neurotransmitters that influence neurogenesis in the adult neurogenic zones is large, diverse, and ever-growing 41. Intrinsic cell cycle regulators have been studied primarily in the developing nervous system, only recently in adults 41, and not at all in the aging brain. Importantly, many recent studies indicate that the aging-related decline in neurogenesis develops primarily from changes in the neurogenic microenvironment and in the factors that control the division of stem and progenitor cells, not from loss of, or changes intrinsic to, those precursors 18. Among the most striking recent advances in the understanding of adult neurogenesis was the observation that proliferation does not occur randomly or homogeneously throughout the neurogenic regions, but rather that dividing progenitor cells are found in close association with the microvasculature, in a “vascular niche,” and that neurogenesis is associated with a process of active vasculogenesis and remodeling 42. This view provides hope that aging-related changes may be reversed by experimental modulation of the microenvironment if the critical cellular and molecular factors that define that environment can be identified. A complete discussion of the neurogenic niche and of all the extrinsic factors that might influence adult neurogenesis is beyond the scope of this post; the discussion here focuses on factors for which there is evidence of an important role in mediating aging-related changes in neurogenesis in the hippocampus and subventricular zone 32.
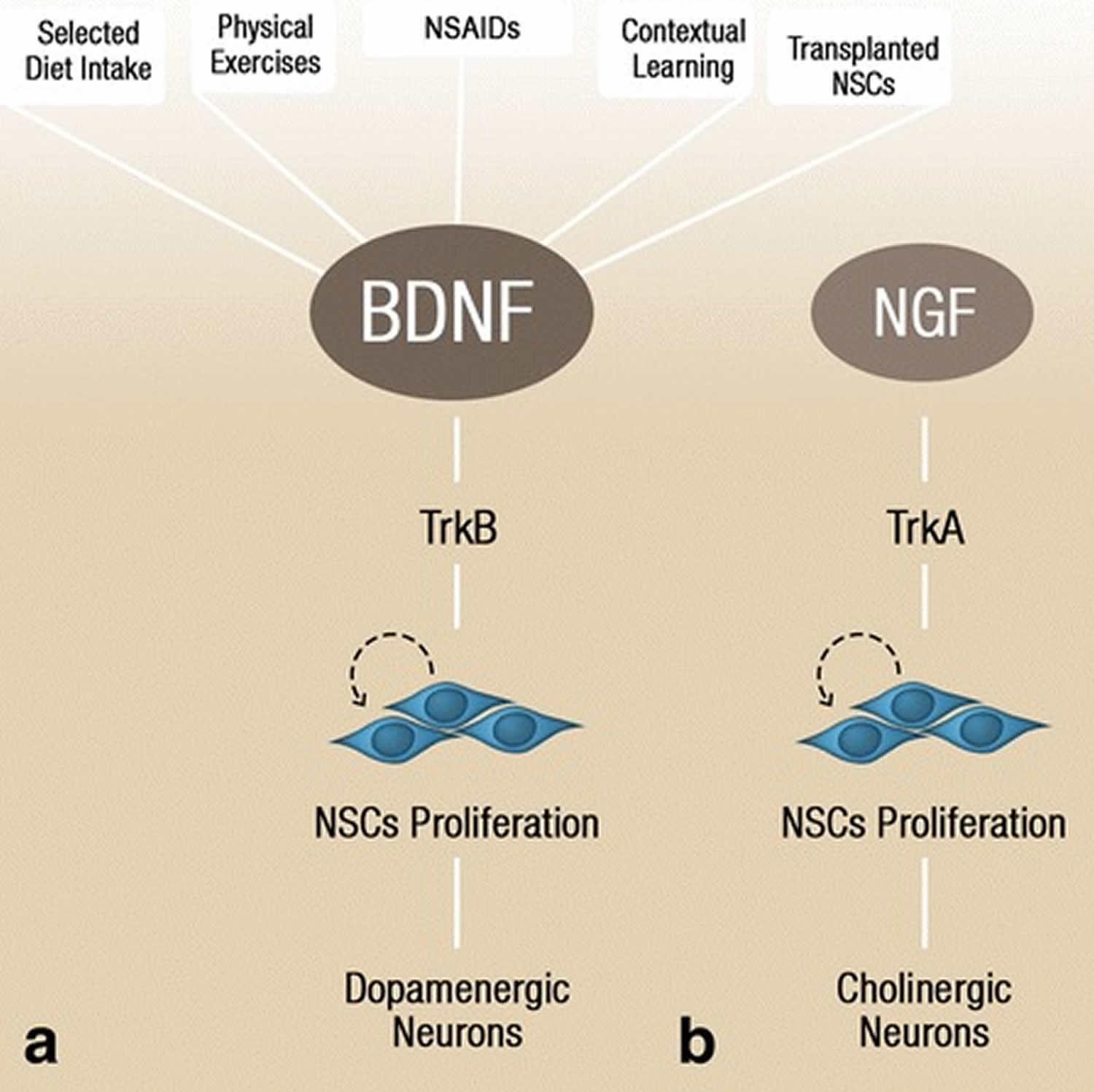
Figure 7. Factors upregulating neurotrophins
Footnote: BDNF levels and consequently initiate neural stem cell proliferation via activation of the TrkB receptor, which later differentiates into dopaminergic neurons (a). Nerve growth factor, through its downstream receptor TrkA, initiates neural stem cell proliferation that results in cholinergic neurons formation (b). The dopaminergic and cholinergic neuronal differentiation occurs primarily during developmental neurogenesis, however, environmental factors, stem cell transplantation, and anti-inflammatory drugs could potentially induce these processes in adult neurogenesis.
Abbreviations: BDNF = brain-derived neurotrophic factor; NSC = neural stem cell; TrkA = tropomyosin-related kinase A; TrkB = tropomyosin-related kinase B; NGF = nerve growth factor
[Source 3 ]Hormones and Growth Factors
1. Stress Hormones
Stress and corticosteroids were among the first studied regulators of adult neurogenesis 43. The idea that stress and glucocorticoids contribute to the aging-related decline in neurogenesis is particularly attractive, given widespread evidence that stress influences brain aging and cognitive function 44, and that aging is associated with elevated levels of corticosteroids 45. Stress-induced depression of proliferation in the dentate gyrus has been demonstrated in several species (shrews and marmosets, as well as rats and mice) and in response to a wide variety of stressors) 46. The effects of stress on neurogenesis overall are more complex than the effects on proliferation. Some studies indicate that stress-induced depression of proliferation is accompanied by a decrease in neuronal production 47. Others, however, have shown that the stress-induced reduction in proliferation is followed, after a short period, by an increase in cell survival, such that the overall addition of new neurons remains largely unchanged 48. Whatever the complexity of stress-induced changes, clearly they are mediated, at least in part, by glucocorticoids 18. Depletion of glucocorticoids by adrenalectomy increases the number of BrdU-labeled cells in the dentate gyrus in both young adult and old rats 49, and increasing glucocorticoid activity decreases proliferation 43. The relationship between glucocorticoid levels and neurogenesis is not simple, however, because physical activity, living in an enriched environment, and training on learning paradigms all increase glucocorticoids but also increase neurogenesis 46. Moreover, despite the profound effects of glucocorticoids on neurogenesis in the dentate gyrus, proliferation in the subventricular zone remains unchanged following adrenalectomy 50. Progress in investigating the expression of glucocorticoid and mineralocorticoid receptors on neural precursor cell populations 51 is beginning to clarify the cellular targets of stress hormones within neurogenic regions and offers promise that the specific role of those factors in aging-related changes in neurogenesis soon will be clearer.
2. Growth Hormone/Insulin-Like Growth Factor-1 Axis
Interest in the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis as a mediator of aging-related changes in the brain and other organ systems developed from the recognition that a substantial decline in serum growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels is one of the most robust hallmarks of mammalian aging (reviewed in 52. Given the pleiotropic effects of IGF-1 in the brain and increasing evidence that growth hormone (GH) has direct effects in the brain, as well as regulating IGF-1 levels, it is reasonable to expect that the aging-related decline in GH/IGF-1 activity is significant for brain structure and function. There is experimental evidence that restoring GH and/or IGF-1 in older animals ameliorates many aging-related neural changes 52. With respect to neurogenesis, several lines of evidence implicate the GH/IGF-1 axis in aging-related changes. The regulation of adult neurogenesis appears to be linked to the regulation of angiogenesis 53, which is modulated in the aging brain by the GH/IGF-1 axis 54. In addition, several laboratories have demonstrated that modulating IGF-1 levels in adult rodents alters hippocampal neurogenesis 52. Neurogenesis is decreased by hypophysectomy, which decreases levels of GH (and other pituitary hormones) and IGF-1, and neurogenesis in hypophysectomy animals is restored by peripheral infusion of IGF-1 55. IGF-1 also mediates the ability of physical exercise to increase neurogenesis 56. Finally, direct intracerebral ventricular infusion of IGF-1 into aged rats ameliorates the aging-related decline in neurogenesis 57.
Although the evidence that the GH/IGF-1 axis plays some role in the regulation of adult neurogenesis and contributes to aging-related changes is intriguing, the mechanisms of such regulation remain poorly understood, particularly with respect to which endogenous source or sources of IGF-1 are most important for regulating adult neurogenesis and which aspects of neurogenesis are regulated by the GH/IGF-1 axis. IGF-1 is available to cells in the CNS from at least three sources: from the plasma across the blood-brain barrier 58, following production by cells of the cerebral vasculature 59, and from local production by neurons and glia within the brain parenchyma 60. Teasing apart the endocrine, paracrine, and autocrine effects of IGF-1 on neurogenesis remains a significant challenge, but some insight was provided by the initially paradoxical observation that hippocampal neurogenesis increases in Ames dwarf mice, a model of GH/IGF-1 deficiency. Although Ames mice have profound deficits in circulating GH and IGF-1, analysis revealed greatly increased IGF-1 levels in the hippocampus, presumably accounting for the increase in neurogenesis 61. Thus, at least for the regulation of adult neurogenesis, local production of IGF-1 may be more important than endocrine levels. Quantitative data on levels and production of IGF-1 within the brain parenchyma are limited, but IGF-1 levels within the rat hippocampus decline significantly by middle age and then only slightly, if at all, during later senescence 62, a temporal pattern that correlates with the aging-related change in neurogenesis.
Even as the regional regulation of IGF-1 production and activity becomes clearer, it is not yet established that IGF-1 modulates the same aspects of neurogenesis that are most affected by aging, and one must consider the possibility that changes in neurogenesis in response to experimental modulation of IGF-1 levels reflect a pharmacological effect rather than normal physiological regulation. As discussed, the aging-related changes in neurogenesis include both decreased proliferation and changes in commitment and/or differentiation, whereas effects on survival are less clear. The reported increase in the number of BrdU-labeled cells in the subgranular zone following restoration of IGF-1 by icv infusion in aged rats appears to involve increased proliferation 57, but because BrdU was injected over several days in that study, one cannot exclude the possibility that IGF-1 affected survival. Significantly, icv infusion of IGF-1 has no effect on the percentage of newborn cells that committed to neuronal differentiation; thus, it is unlikely that decreased GH/IGF-1 activity accounts for the aging-related decline in neuronal commitment in the dentate gyrus. A recent study of neurogenesis in a model of adult-onset GH/IGF-1 deficiency indicates that IGF-1 modulates the survival of newborn neurons 63, but, as noted above, the effects of aging on survival remain unclear. Taken together, the available data suggest that the aging-related decline in the activity of the GH/IGF-1 axis contributes to, but cannot fully account for, aging-related changes in neurogenesis. Additional factors clearly are involved.
3. Fibroblast Growth Factor
Fibroblast growth factor 2 (FGF-2), also known as basic fibroblast growth factor (bFGF), declines in the aging hippocampus and, like the declines in IGF-1 and in neurogenesis, the decrease in FGF-2 occurs by middle age 62. The aging-related decline in FGF-2 appears to be due, at least in part, to decreased local production, because both the number of FGF-positive cells and the intensity of immunolabeling is reduced in middle-aged and old mice compared to young adults 62. There is more than correlative evidence that FGF-2 contributes to the aging-related decline in neurogenesis; infusion (icv) of FGF-2 into old mice partially ameliorates the aging-related decline in BrdU labeling 64. In the subgranular zone, the number of BrdU-labeled cells in FGF-2 infused old mice is 20% of that seen in normal, young adults, compared to the 10% seen in control aged mice. The effects of FGF-2 infusion are greater in the subventricular zone, where the number of BrdU-labeled cells in FGF-treated aged animals approaches the level seen in normal, young adults. The effects of FGF-2 treatment of aged animals, like the effects of IGF-1, not only suggest the factors may mediate (in part) the aging-related decline in neurogenesis, but they also demonstrate that the aged brain retains the capacity to respond to growth factors with increased neurogenesis, an important consideration as one contemplates the therapeutic potential of modulating neurogenesis to ameliorate aging-related cognitive deficits or neurodegeneration.
4. Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF) shows the same pattern of aging-related decline in the hippocampus as IGF-1 and FGF-2; levels of VEGF in middle-aged and aged animals are only about half that in young adults 62. Vascular endothelial growth factor (VEGF) is of particular interest with respect to aging-dependent regulation of neurogenesis because adult neurogenesis and angiogenesis may be linked 53, but there are multiple mechanisms by which VEGF might influence neurogenesis, some of which need not involve the vasculature. Reports that VEGF receptors are expressed by neurons, as well as by vascular endothelial cells 65, and that neurogenesis is increased in response to doses of VEGF that are too low to induce endothelial proliferation, indicate that VEGF may influence neurogenesis independently of its ability to promote angiogenesis 66. Although the ability of VEGF to restore neurogenesis in aged animals has not been tested directly, there is evidence that modulating VEGF activity in young adults alters neurogenesis in both the dentate gyrus and subventricular zone/rostral migratory stream. VEGF knockout mice show reduced neurogenesis in both neurogenic regions and icv infusion of VEGF increases neurogenesis 67. Significantly, there is accumulating evidence that VEGF mediates some of the effects of stress 68, of exercise 69, and of complex environments and learning on adult neurogenesis 70.
5. Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) is expressed at high levels in the adult hippocampus, including the dentate gyrus 71, and a variety of evidence implicates BDNF as an important mediator of aging-related neural changes in the brain 72. The effects of aging on hippocampal BDNF levels remain controversial, with some laboratories reporting an aging-related decrease 73 but others finding little or no change 74. The specific role of brain-derived neurotrophic factor (BDNF) in modulating neurogenesis in the normal adult and aging brain also remains unclear. Consistent with the hypothesis that BDNF is a proneurogenic factor is evidence that neurogenesis decreases in BDNF knockout mice 75 and that increasing BDNF levels in the subventricular zone by adenovirus 76 or intracerebral ventricular infusion 77 increases the number of neuroblasts and new neurons in the subventricular zone and olfactory bulb. Direct unilateral intrahippocampal infusion of BDNF increases neurogenesis in the dentate gyrus, but the effect must be indirect and not by direct stimulation of progenitor cells because increased neurogenesis is evident bilaterally while exogenous BDNF appears to remain restricted to the infused hemisphere 78. The effects of BDNF on proliferation and neurogenesis also are influenced by other factors. In apparent contrast to the evidence that BDNF promotes neurogenesis in the normal adult brain, infusion of the factor following an ischemic event reduces the increase in neurogenesis that usually follows ischemia 79. Moreover, reducing endogenous BDNF following ischemia promotes, rather than decreases, neurogenesis 80. Additional evidence for complex, differential regulation of BDNF and its effects comes from reports that one form of dietary restriction, every-other-day feeding, increases adult neurogenesis by increasing BDNF 81, whereas another form of dietary restriction, 40% calorie reduction, does not affect BDNF levels and does not increase neurogenesis 74.
6. Epidermal Growth Factor Family
Members of the epidermal growth factor (EGF) family of growth factors and receptors have been implicated in the regulation of both embryonic and adult neurogenesis 82. Adult transforming growth factor-alpha knockout mice show decreased proliferation in the subventricular zone and fewer new neurons migrating to the olfactory bulb 83. Aged mice show reduced EGF receptor signaling in the subventricular zone and decreased neuronal replacement in the olfactory bulb, along with deficits in olfactory discrimination 84. Infusion of EGF increases proliferation in the subventricular zone, but also reduces neuronal replacement in the olfactory bulb 85. Thus, EGF not only promotes proliferation of precursors but also directs differentiating cells away from differentiation as neurons. The capacity to respond to EGF with increased proliferation in the subventricular zone is retained in old animals 86, in which increased proliferation is associated with improved performance in a passive avoidance learning task 87. EGF does not promote proliferation in the adult hippocampus as it does in the subventricular zone 85, but another family member, heparin-binding EGF (HB-EGF), increases both BrdU labeling and the number of doublecortin-positive cells in the subventricular zone and the subgranular zone 64. As with all the growth factors discussed above, the roles of EGF family members in regulating neurogenesis in the adult and aging brain are only beginning to be elucidated.
7. Transforming Growth Factor-β Family
Members of the Transforming Growth Factor-β family of growth factors, particularly TGF-β and bone morphogenetic proteins, are powerful regulators of neural stem cells and of neurogenesis in both developing and adult brains. Much like BDNF, the effects of TGF-β on neurogenesis appear to be context dependent. Intracerebroventricular infusion of TGF-β reduces proliferation of progenitor cells in both the dentate gyrus and subventricular zone of adult mice 88, and chronic overexpression of TGF-β in aged transgenic mice virtually eliminates neurogenesis 89, but TGF-β mediates proneurogenic effects of microglia following adrenalectomy. The question of whether changes in TGF-β signaling contribute to changes in neurogenesis in normally aging mice has not yet been addressed. Whether changes in bone morphogenetic protein signaling contribute to aging-related changes in neurogenesis similarly is unknown, but bone morphogenetic proteins influence adult neural stem cells in much the same way that they influence stem cells in the developing brain. In the adult subventricular zone, in the absence of other factors, bone morphogenetic proteins direct stem cells toward a glial fate, but that gliogenic signal is blocked by the bone morphogenetic protein inhibitor noggin, which is expressed by ependymal cells 90. Thus, a change in the balance of subventricular zon signaling and inhibitors could contribute to the aging-related decrease in the percentage of newborn cells that differentiate as neurons.
8. Retinoic Acid
Retinoic acid, a member of the steroid/thyroid hormone super family, is an essential growth factor and regulates many aspects of embryonic neural development 18. Recent evidence indicates that retinoic acid continues to influence plasticity and regeneration in the adult brain and may influence neurogenesis in the olfactory epithelium, subventricular zone, and hippocampus, with possible effects on both proliferation of progenitor cells and differentiation of newborn neurons 91. Changes in retinoic acid signaling may be particularly important in the aging olfactory system, with effects in both the OE and the subventricular zone 92. retinoic acid critically regulates the initial development of the peripheral olfactory system and continues to influence olfactory progenitor cells in the adult 93. In addition to activating basal stem cells in the olfactory epithelium, retinoic acid influences a small population of cells in the subventricular zone that appear to be stem cells (i.e., they are slowly dividing, express glial fibrillary acidic protein (GFAP), and form neurospheres when isolated in culture). A variety of studies in humans and animal models suggest that retinoic acid promotes recovery of olfactory function in aged individuals and following injury to the peripheral olfactory system 92. Moreover, retinoic acid improves performance on an odor-mediated learning task when administered to senescent mice 94. Perhaps most intriguingly, because dietary vitamin A is the primary source of retinoids, retinoic acid could play the central role in a feed-forward cycle in which a modest aging-related decline in olfactory function contributes to reduced appetite, which leads to reduced food intake and sub-optimal vitamin A levels, resulting in decreased retinoic acid signaling and thereby to further deficits in olfactory neurogenesis 92. Further investigation may establish retinoic acid as an important therapeutic target for ameliorating the age-related decline in olfactory function.
Neurotransmitters
Several neurotransmitters have been implicated in the regulation of adult neurogenesis, consistent with the growing awareness that neurotransmitters can act as trophic factors as well as synaptic messengers and with evidence that activity within the adult hippocampus and olfactory system influences the production and integration of new neurons. In the olfactory bulb, centrifugal projections of cholinergic, serotonergic, and catecholaminergic inputs each can influence the differentiation and survival of new neurons 90. Cholinergic neurons in the basal forebrain project to both the dentate gyrus and the olfactory bulb, and the number of newborn granule neurons decreases in both regions following lesion of that cholinergic projection 95. GABA plays a key role in the integration of newborn neurons in the adult brain 96, and additional influences of serotonin may link changes in neurogenesis to the development of depression 97. Few studies have investigated neurotransmitter-dependent regulation of neurogenesis in aged animals, but loss of dopaminergic projections from the substantia nigra to the subventricular zone further decreases the number of proliferating progenitors and developing neurons in the subventricular zone of aged primates 98, and treatment with antagonists to the NMDA type glutamate receptor increases neurogenesis in aged rats 99, the latter suggesting that the decline in neurogenesis may be one of several deficits influenced by aging-related changes in glutamate signaling.
Inflammatory Mediators
Evidence from many laboratories and studies suggests that aging-related increases in inflammatory processes contribute to the development of neural deficits 100. This hypothesis is based on evidence that (1) basal levels of many pro-inflammatory cytokines increase in older brains and (2) those cytokines affect the function, and even the survival, of neurons, glia, and progenitor cells. Although not yet established, it is reasonable to suspect that aging-related activation of microglia suppresses neurogenesis (and thereby cognitive function), because neurogenesis is profoundly affected by changes in the neurogenic microenvironment that are mediated by reactive microglia and inflammatory cytokines. Inducing an inflammatory response by infusion of the bacterial toxin lipopolysaccharide (LPS) virtually eliminates neurogenesis in the adult brain, with the extent of reduction in individuals well correlated with the number of activated microglia 101. Neurogenesis is restored in bacterial toxin lipopolysaccharide-infused animals by treatment with minocycline, a tetracycline derivative that inhibits the activation of microglia. Studies using brain irradiation to induce microglia activation and a sustained inflammatory response demonstrate that inflammatory-induced changes in neurogenesis arise as a result changes in the neurogenic environment, rather than intrinsic changes in progenitor cells. Hippocampal progenitor cells can be cultured from irradiated brains, divide normally, and produce a normal array of cell types in culture, but hippocampal progenitor cells from non-irradiated brains do not divide and produce neurons when transplanted into an irradiated brain, as they do when transplanted into a nonirradiated brain 102. Significantly, these deleterious changes in the progenitor cell niche arise, at least in part, from the radiation-induced inflammatory response, because treatment with non-steroidal anti-inflammatory drugs (NSAIDs) ameliorates the deleterious effects of whole-brain irradiation on hippocampal neurogenesis 103.
Thus, to the extent that microglial activation and a chronic inflammatory response accompany brain aging 104, one would expect they contribute to the aging-related decline in neurogenesis, and possibly to cognitive deficits. It must be recognized, however, that the “activation” of microglial cells and their relationship to other cell types is significantly more complex than previously appreciated 105, and that activated microglia are not always antineurogenic. Following adrenalectomy, microglia increase neurogenesis via TGF-β 106 and microglia activated by anti-inflammatory cytokines associated with T-helper cells also increase neurogenesis 107, in contrast to endotoxin-activated microglia, which inhibit neurogenesis 108. The balance of pro- and anti-inflammatory factors produced by microglial cells under specific conditions determines their effect on the neurogenic microenvironment, and clarification of the role of inflammation in aging-related changes in neurogenesis awaits a clearer understanding of aging and aged microglia 109.
How to increase neurogenesis
Environmental Factors
An intriguing feature of adult hippocampal neurogenesis is that the process is regulated by such factors as the environment and an individual’s emotional or physiological status 12. In other words, adult-born dentate granule cells can in theory be generated on demand in response to environmental signals, which could provide a degree of meta-plasticity in the adult hippocampal neurogenesis-dependent reorganization of hippocampal circuits. An enriched environment, including a larger cage area, novel objects, and running wheels, has been shown to significantly increase the number of adult-born neurons and the volume of the granule cell layer and to improve the speed of spatial learning in rodents 110. A follow-up study revealed that voluntary running alone selectively increased proliferation of adult neural progenitors/neuroblasts, whereas environmental enrichment promoted the survival of adult-born dentate granule cells through the increased integration of immature neurons 111.
These processes are mediated by several types of signaling, including glutamatergic and GABAergic inputs from local neural networks 112. Glutamatergic inputs through NMDA receptors are critical for the survival of immature neurons 113, and surviving neurons are functionally integrated into existing circuits within one month 114. A short exposure to an enriched environment depolarizes immature neurons through GABAergic inputs that enable activation of NMDA receptors, which in turn allows immature neurons to respond to future glutamatergic synaptic inputs 115. A recent study revealed that the combination of GABAergic inputs from the molecular layer and the granule cell layer in the gamma frequency range evoked action potentials in young adult-born dentate granule cells 116. Furthermore, the study revealed the spatial and temporal integration dynamics of the GABAergic and glutamatergic inputs required to elicit action potentials in young adult-born dentate granule cells. Thus, the oscillatory activity in the hippocampus could regulate the integration of young dentate gyrus neurons into hippocampal neuronal networks through GABAergic signaling. Importantly, the effects of environmental enrichment on the survival and integration of adult-born dentate granule cells are restricted to the first three weeks after the birth of the neurons 117.
Following the survival checkpoint, the time course of neuronal maturation is also modulated by local network activity, which in turn is also modulated by physical activity or exposure to an enriched environment 118. Optogenetic silencing of the dentate during exposure to a novel environment prevents the environmentally induced increase in integration of immature dentate granule cells 119. Furthermore, GABAergic inputs from parvalbumin-positive interneurons are essential for an enriched environment to enhance the integration and maturation of young dentate gyrus neurons 112. The increase in surviving and integrating immature neurons based on environmental inputs could be crucial, as the surviving adult-born dentate granule cells could potentially be tuned to respond to future occurrences of the same experiences that they experience during their maturation periods 117. Intriguingly, an enriched environment can also change the connectivity of adult-born dentate granule cells 120, implying that those neurons may play distinct roles in local neural circuits. Exercise itself also alters the connectivity of the dentate gyrus. Neurogenesis recruits additional inputs from entorhinal cortex but increases the frequency of inhibitory input to mature dentate granule cells, potentially contributing to the overall sparsity of the dentate gyrus network 121. Conversely, stress and aging reduce adult neurogenesis in the dentate gyrus through corticosteroid signaling 122. Importantly, adverse experiences during childhood can have prolonged effects on adult neurogenesis and hippocampus-mediated stress responses 123, suggesting that experience in early life may epigenetically modulate the process of adult hippocampal neurogenesis. In addition, the levels of hormones such as estrogen and thyroid hormones regulate the rate of adult neurogenesis 124. Thus, in addition to environmental stimuli from the external world, the physiological state of an individual plays a prominent role in the regulation of adult hippocampal neurogenesis in the physiological and pathological conditions 12.
Exercise and neurogenesis
There is a robust association between physical exercise and adult neurogenesis, as physical activity is known to upregulate neurogenesis in the subgranular zone (SGZ) and the subventricular zone (SVZ) 125. Early studies that characterized and described this association were carried out by Van Praag et al. 126 in mice. They showed that neither swimming, nor maze training improved cell proliferation and neurogenesis in the dentate gyrus, however, a voluntary exercise in a running wheel doubled the number of proliferating cells and net neurogenesis in the dentate gyrus 126. In contrast, an independent study showed that exposing rats to regular swimming exercise resulted in an increase in the progenitor cell proliferation and maturation in the subventricular zone (SVZ) 127. Species specific differences, that is rats instead of mice, and also the frequency or the duration of swimming exercise may account for the discrepancies in findings on the extent of physical activity required to elicit augmented neurogenesis. Interestingly, the increase in the subventricular zone (SVZ) neurogenesis in rats after regular swimming exercise was associated with an increase in the trophic factor NGF, which may contribute to inducing neurogenesis following physical activity 127. Other work carried out by Kronenberg et al. showed that sustained running in mice resulted in an acute but transient increase in neural stem cell proliferation 128. Moreover, the number of doublecortin-positive immature neurons increased significantly with continued running, despite a return of neural stem cell proliferation rates to baseline levels in the dentate gyrus 128. This increase in neurogenesis in the dentate gyrus following exercise was associated with an enhancement in the spatial memory, suggesting that consistent exercise may improve cognitive function due to augmented neurogenesis 129. In agreement, Shors et al. revealed that newly-born neurons contribute towards acquiring tracing memory 130. Furthermore, physical activity may preserve neuronal plasticity and improve learning as mice demonstrated enhanced performance in water maze tests following wheel running 131. In support of this, inhibition of neurogenesis by focal irradiation removed exercise-stimulated increases in spatial learning 132. These studies highlight the links between exercise and hippocampal neurogenesis and how this could be reflected in improved cognitive function.
Physical activity enhances hippocampal neurogenesis and cognitive function through promoting the increase in the cerebral blood flow 133, blood-brain barrier permeability 134, angiogenesis 135 and the expression of neurotrophic factors 136. In the following we discuss the link between neurotrophic factors as mediators of the effects of physical activity in inducing neurogenesis. It has been demonstrated that physical activity increased levels of neurotrophic factors, such as nerve growth factor 127, IGF-1 137, vascular endothelial growth factor (VEGF) 138, and BDNF 139. The augmented release of these neurotrophic factors may underlie the ability of exercise to enhance adult neurogenesis.
For instance, Lafenêtre et al. 140 generated mice that were genetically modified to have down-regulated cell proliferation in the hippocampus and short-term memory. After running, these mice showed a reverse of a genetic blockade of cell proliferation and improved short-term memory 140. An increase in BrdU- and doublecortin-positive cells were seen in the hippocampus mice after running 140. Interestingly, an upregulation in BDNF receptor, TrKB, was seen in doublecortin-positive cells following running, which may signal the increase in hippocampal neurogenesis 140. The inhibition of IGF-1 signaling, using a specific antibody targeting the IGF-1 receptor, resulted in the loss of exercise enhanced cognition in rats 141. Furthermore, it has been reported that running exercise enhanced IGF-1 uptake by specific neurons in the rat brain, which resulted in a spontaneous firing of neurons and elevated the expression of BDNF 142. In addition to BDNF and IGF-1, VEGF has been shown to have a neurotrophic effect and increased levels have been observed following exercise in rats 143. Peripheral blockade of VEGF prevented augmented neurogenesis induced by running, whereas VEGF blockade did not alter baseline neurogenesis in non-running mice 144 which indicates VEGF contributions to exercise-mediated adult neurogenesis. In a clinical study, investigation of high to moderate intensity exercise groups revealed an increase in BDNF and IGF-1 levels that were associated with enhanced cognitive function when compared to low-intensity exercise groups 145. These studies highlight the increased production of growth factors as underlying the beneficial neurophysiological effects of physical activity. As a result, regular physical exercise could be useful as a non-invasive approach of inducing the endogenous expression of neurotrophic factors, which ultimately induce neurogenesis.
Neurogenesis diet
Dietary intake represents a modifiable behavior and an extrinsic factor that can alter cognitive function and adult neurogenesis 146. Dietary behavior including dietary restriction or intake of certain diet textures or content have been reported to influence adult hippocampal neurogenesis 147. According to Lee and colleagues, dietary restriction for 4 weeks in rodents resulted in increased BrdU- and NeuN-positive cells in the dentate gyrus, which indicates an increase in proliferation and neuronal differentiation, respectively 148. Lee et al. have further revealed that the impact of the dietary restriction on hippocampal neurogenesis was mediated by BDNF 149. In this study, they compared BDNF +/+ control mice and BDNF -/+ heterozygous mice maintained on ab libitum diet (normal diet according to animal needs) or a regimen of dietary restriction for 3 months 149. After 4 weeks of BrdU injection, BrdU-positive cells were decreased in BDNF-/+ mice maintained on ab libitum diet, however, dietary restriction significantly increased BrdU-positive cells in the dentate gyrus of BDNF +/+ mice and to a lesser extent in BDNF -/+ mice 149. This indicates a positive effect of dietary restriction on neural stem cell proliferation that involves BDNF levels 149. In addition, IGF-1 is a neurotrophic factor that has also been suggested to be elevated in rodents maintained on a restricted diet regimen 150. A number of studies have also reported that the effects of dietary restriction extend to behavioral and cognitive functions 151. Therefore, combined exercise and dietary normalization had positive additive effects in enhancing BDNF and NGF expression and improving decreased cognitive function in high fat-induced obese adult rats 152. Pitsikas et al. 153 reported that dietary restriction has significantly improved learning and memory in aged rats. In humans, a study on a cohort of 49 healthy elderly subjects reported that the subjects maintained on a regimen of dietary restriction, in comparison to subjects maintained on ab libitum diet, exhibited enhanced verbal memory, albeit without increased serum levels of BDNF 154. This does not necessarily exclude BDNF from mediating the improved memory of elderly subjects maintained on dietary restriction as peripheral BDNF levels were reported and neural BDNF levels remained unknown 154. In addition to caloric restriction, some reports suggest that diet texture can alter neurogenesis in the hippocampus 155. This was first reported by Aoki and colleagues who demonstrated that rats on soft-diet feeding led to reduced proliferation in the hippocampus 156. However, a reduction in proliferation may not necessarily translate to reduced neurogenesis as Patten et al. 157 similarly reported a decrease in hippocampal proliferation in rats fed a liquid diet but did not observe significant changes in the differentiation and survival of new neurons compared to groups fed a solid diet. Since cell proliferation was decreased, Patten and colleagues postulated that compensatory mechanisms were involved in maintaining hippocampal neurogenesis 157. However, other studies have reported inhibitory effects on neurogenesis in mice 158. In this context, mice fed a soft-textured diet exhibited reduced proliferation and neurogenesis in the hippocampus, when compared to hard-diet feeding of equivalent calories 158. Furthermore, mice fed on hard-diet recovered the impairment in the olfactory function resulted from soft-diet feeding, which was related to the enhancement in neurogenesis 158. Interestingly, the inhibition in the hippocampal neurogenesis after soft-diet feeding was linked to a down-regulation in BDNF expression following a reduction in the mastication activity 159. Thus, the improvement in the hippocampal neurogenesis in rodents fed on hard-diet may be attributed to the physical act of mastication 147. These studies indicate the potential of dietary restriction and diet texture in enhancing the hippocampal neurogenesis through upregulating neurotrophic factors.
Nutritional content may also represent a dietary factor that can influence adult neurogenesis. Diets high in saturated fats and simple sugars can dramatically impair neurogenesis, learning, and memory in rodents and lower BDNF levels expression 160. In contrast, diets rich in poly-unsaturated fatty acids (PUFA) and polyphenols induce neuronal plasticity in the adult hippocampus 146. Supplementation of PUFA in rodents increased proliferation, neural stem cell differentiaton into neurons in the dentate gyrus and increased hippocampal volume, which indicates an increase in the hippocampal neurogenesis 161. The enhancement in hippocampal neurogenesis after PUFA supplementation was also associated with an increase in cognitive function and learning 162. Interestingly, BDNF levels were found to increase after PUFA supplementation in mice, which may mediate PUFA-induced neurogenesis in the hippocampus 161. In addition to PUFA, polyphenols (flavonoids and other subtypes) are additional dietary compounds that may modulate adult hippocampal neurogenesis 146. The flavonoid resveratrol, enriched in the skin of red grapes, amongst other fruits and plants, was shown to enhance hippocampal neurogenesis through up-regulating cyclic AMP response element-binding protein levels to subsequently promote BDNF synthesis in the hippocampus 163. Furthermore, Harada et al. showed that oral administration of resveratrol in mice elevated IGF-1 levels in the hippocampus through stimulation of gastrointestinal tract sensory neurons, thereby improving neurogenesis and cognitive function 164. In addition, blueberries are rich in the flavonoid anthocyanin and other polyphenol subsets, which were able to reverse the neuronal and behavioral impairment related to aging in rats 165. Blueberry supplementation in rats resulted in an enhancement in neuronal plasticity and improved cognitive function that was associated with increased IGF-1 and BDNF levels 166. Similarly, the flavonoids quercetin and kaempferol were found to elevate hippocampal BDNF expression in mice to promote neuronal plasticity 167. Curcumin, found in turmeric 168, is another polyphenolic compound that was reported to improve learning and memory in aged rats 169. Dong et al. 169 reported that curcumin supplementation for 12 weeks in aged rats enhanced hippocampal neurogenesis. According to an epidemiological study, elderly subjects with diets comprising a large amount of turmeric showed an improvement in cognitive function compared to elderly subjects with diets low in turmeric 170. Thus, manipulating dietary content and intake may be one approach to enhance adult neurogenesis. In addition, these dietary studies also highlight naturally occurring compounds that could potentially be exploited therapeutically to enhance cognitive function in clinical settings.
As previously explained; BDNF levels, and other neurotrophic factors, were altered in response to different extrinsic factors including diet and physical exercises (Figure 7), which in return mediate hippocampal neuronal plasticity. Hence, defined nutritional intake and regular physical exercises are recommended lifestyle approaches to enhance neurotrophic factors expression and maintain neural stem cells homeostasis in the hippocampus. These lifestyle interventions may serve to prevent, or significantly improve the status of neurogenesis in aging and neurodegeneration.
References- Adult neurogenesis in the mammalian brain: significant answers and significant questions. Ming GL, Song H. Neuron. 2011 May 26; 70(4):687-702.
- Neurogenesis in the adult human hippocampus. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Nat Med. 1998 Nov; 4(11):1313-7.
- Shohayeb B, Diab M, Ahmed M, Ng DCH. Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener. 2018;7:4. Published 2018 Feb 21. doi:10.1186/s40035-018-0109-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5822640/
- Neurodegenerative disease and adult neurogenesis. Winner B, Kohl Z, Gage FH. Eur J Neurosci. 2011 Mar; 33(6):1139-51.
- Adult neurogenesis in the mammalian central nervous system. Ming GL, Song H. Annu Rev Neurosci. 2005; 28():223-50.
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573.
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092.
- Neurons generated in the adult brain are recruited into functional circuits. Paton JA, Nottebohm FN. Science. 1984 Sep 7; 225(4666):1046-8.
- Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Reynolds BA, Weiss S. Science. 1992 Mar 27; 255(5052):1707-10.
- Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. Kuhn HG, Dickinson-Anson H, Gage FH. J Neurosci. 1996 Mar 15; 16(6):2027-33.
- Baptista P, Andrade JP. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front Neuroanat. 2018;12:44. Published 2018 Jun 5. doi:10.3389/fnana.2018.00044 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5996050/
- Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease[published online ahead of print, 2018 Apr 20]. Mol Psychiatry. 2018;10.1038/s41380-018-0036-2. doi:10.1038/s41380-018-0036-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6195869/
- Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. Noonan MA, Bulin SE, Fuller DC, Eisch AJ. J Neurosci. 2010 Jan 6; 30(1):304-15.
- Thomas RM, Peterson DA. Even neural stem cells get the blues: evidence for a molecular link between modulation of adult neurogenesis and depression. Gene Expr. 2018;14(3):183-93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6042005/
- Adult hippocampal neurogenesis in depression. Sahay A, Hen R. Nat Neurosci. 2007 Sep; 10(9):1110-5.
- Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003 Aug 8; 301(5634):805-9.
- Thomas R. M.; Peterson D. A. A neurogenic theory of depression gains momentum. Mol. Interv. 3(8):441–444; 2003
- Riddle DR, Lichtenwalner RJ. Neurogenesis in the Adult and Aging Brain. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3874
- Mammalian neural stem cells. Gage FH. Science. 2000 Feb 25; 287(5457):1433-8.
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687-702. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3106107/
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199.
- Zhao C, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3.
- Van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030.
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326
- Kempermann G.; Jessberger S.; Steiner B.; Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27(8):447–452; 2004.
- Alvarez-Buylla A.; Lim D. A. For the long run: maintaining germinal niches in the adult brain. Neuron 41(5):683–686; 2004
- Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703.
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046.
- Coskun V, Luskin MB. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J Neurosci Res. 2002;69:795.
- Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31:560.
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815.
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33.
- Rawson NE, LaMantia A-S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp Gerontol. 2007;42:46.
- Emsley JG, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321.
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65.
- Dayer AG, et al. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415
- Bernier PJ, et al. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 2002;99:11464
- Yamamoto S, et al. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115
- Parent JM, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802.
- Emsley JG, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523.
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203
- Lupien SJ, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992;13:171
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233.
- Westenbroek C, et al. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562.
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894
- Rodriguez JJ, et al. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10:2994
- Garcia A, et al. Age-dependent expression of gulcocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363
- Trejo JL, et al. Role of serum insulin-like growth factor 1 in mammalian brain aging. Growth Horm IGF Res. 2004;14:S39
- Ward NL, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870
- Sonntag WE, et al. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515
- Aberg MA, et al. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628
- Lichtenwalner RJ, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603
- Armstrong CS, Wuarin L, Ishii DN. Uptake of circulating insulin-like growth factor-I into the cerebrospinal fluid of normal and diabetic rats and normalization of IGF-II mRNA content in diabetic rat brain. J Neurosci Res. 2000;59:649
- Sonntag WE, et al. Alterations in insulin-like growth factor-1 and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269
- Sun LY, et al. Local expression of GH and IGF-I in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929
- Sun LY, et al. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology. 2005;146:1138
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173
- Lichtenwalner RJ, et al. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83:199
- Jin K, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175
- Galvan V, Greenberg DA, Jin K. The role of vascular endothelial growth factor in neurogenesis in adult brain. Mini Rev Med Chem. 2006;6:667
- Schanzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237
- Sun Y, et al. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329.
- Heine VM, et al. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature of VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304.
- Ding YH, et al. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29
- Yan Q, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431
- Cotman CW. The role of neurotrophins in brain aging: a perspective in honor of Regino Perez-Polo. Neurochem Res. 2005;30:877
- Hattiangady B, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353
- Newton IG, et al. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging. 2005;26:683
- Linnarsson S, Willson CA, Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Brain Res Mol Brain Res. 2000;75:61
- Benraiss A, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718
- Pencea V, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706
- Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348
- Larsson E, et al. Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol. 2002;177:1
- Gustafsson E, Lindvall O, Kakaia Z. Intraventricular infusion of TrkB-Fc fusion protein promotes ischemia-induced neurogenesis in adult rat dentate gyrus. Stroke. 2003;34:2710.
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445.
- Arsenijevic Y, et al. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci. 2001;21:7194
- Tropepe V, et al. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850
- Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354
- Kuhn HG, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820.
- Tirassa P, et al. EGF and NGF injected into the brain of old mice enhance BDNF and ChAT in proliferating subventricular zone. J Neurosci Res. 2003;72:557
- Fiore M, et al. Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol Behav. 2002;77:437
- Wachs FP, et al. Transforming growth factor-β1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol. 2006;65:358
- Buckwalter MS, et al. Chronically increased transforming growth factor-β1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248
- McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66:780.
- Rawson NE, LaMantia A-S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp Gerontol. 2007;42:46
- Haskell GT, Lamantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636
- Etchamendy N, et al. Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci. 2001;21:6423
- Mohapel P, et al. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939.
- Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589.
- Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196.
- Freundlieb N, et al. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321
- Nacher J, et al. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273
- Joseph JA, et al. Oxidative stress and inflammation in brain aging: nutritional considerations. Neurochem Res. 2005;30:927
- Ekdahl CT, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632
- Monje ML, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955.
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760
- Bodles AM, Barger SW. Cytokines and the aging brain — what we don’t know might help us. Trends Neurosci. 2004;27:621
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302.
- Battista D, et al. Neurogenic niche modulation by activated microglia; transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83.
- Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268
- Butovsky O, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495.
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270
- Alvarez DD, Giacomini D, Yang SM, Trinchero MF, Temprana SG, Buttner KA, et al. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science. 2016;354:459–465
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933.
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712.
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33:6614–6622
- Heigele S, Sultan S, Toni N, Bischofberger J. Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nat Neurosci. 2016;19:263–270
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259.
- Goncalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, et al. In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci. 2016;19:788–791
- Kirschen GW, Shen J, Tian M, Schroeder B, Wang J, Man G, et al. Active Dentate Granule Cells Encode Experience to Promote the Addition of Adult-Born Hippocampal Neurons. J Neurosci. 2017;37:4661–4678
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Gobel J, et al. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron. 2015;85:710–717.
- Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage. 2016;131:29–41.
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461.
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846.
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801.
- Niwa A, Nishibori M, Hamasaki S, Kobori T, Liu K, Wake H, Mori S, Yoshino T, Takahashi H. Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct Func. 2015:1–14.
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266. doi: 10.1038/6368
- Chae CH, Jung SL, An SH, Park BY, Kim TW, Wang SW, Kim JH, Lee HC, Kim HT. Swimming exercise stimulates neuro-genesis in the subventricular zone via increase in synapsin I and nerve growth factor levels. Biol Sport. 2014;31(4):309–314. doi: 10.5604/20831862.1132130
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72(6):943–952. doi: 10.1002/dneu.22009
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–1058. doi: 10.1016/j.neuroscience.2008.06.051
- Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol (1985). 1993;75(3):1334–1340. doi: 10.1152/jappl.1993.75.3.1334
- Sharma HS, Cervos-Navarro J, Dey PK. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res. 1991;10(3):211–221. doi: 10.1016/0168-0102(91)90058-7
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/S0306-4522(02)00664-4
- Triviño-Paredes J, Patten AR, Gil-Mohapel J, Christie BR. The effects of hormones and physical exercise on hippocampal structural plasticity. Front Neuroendocrinol. 2016;41:23–43. doi: 10.1016/j.yfrne.2016.03.001
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–1634
- Schobersberger W, Hobisch-Hagen P, Fries D, Wiedermann F, Rieder-Scharinger J, Villiger B, Frey W, Herold M, Fuchs D, Jelkmann W. Increase in immune activation, vascular endothelial growth factor and erythropoietin after an ultramarathon run at moderate altitude. Immunobiology. 2000;201(5):611–620. doi: 10.1016/S0171-2985(00)80078-9
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029
- Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Exercise Can Rescue Recognition Memory Impairment in a Model with Reduced Adult Hippocampal Neurogenesis. Front Behave Neurosci. 2009;3:34
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20(8):2926–2933
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x
- Jeon YK, Ha CH. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med. 2017;22(1):27. doi: 10.1186/s12199-017-0643-6
- Murphy T, Dias GP, Thuret S. Effects of Diet on Brain Plasticity in Animal and Human Studies: Mind the Gap. Neural Plast. 2014;2014:32. doi: 10.1155/2014/563160
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4(4):271–282. doi: 10.1007/s12263-009-0134-5
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038. doi: 10.1038/nature05456
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, Henmi T, Ohtani H, Higami Y, de Cabo R, et al. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol. 2008;43(4):339–346. doi: 10.1016/j.exger.2008.01.008
- Woo J, Shin KO, Park SY, Jang KS, Kang S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013;12(1):144. doi: 10.1186/1476-511X-12-144
- Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: Protective effect of life-long calorie restriction. Neurobiol Aging. 1992;13(3):369–373. doi: 10.1016/0197-4580(92)90110-J
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106
- Yamamoto T, Hirayama A, Hosoe N, Furube M, Hirano S. Soft-diet feeding inhibits adult neurogenesis in hippocampus of mice. Bull Tokyo Dent Coll. 2009;50(3):117–124. doi: 10.2209/tdcpublication.50.117
- Aoki H, Kimoto K, Hori N, Toyoda M. Cell proliferation in the dentate gyrus of rat hippocampus is inhibited by soft diet feeding. J Gerontol. 2005;51(6):369–374
- Patten AR, Moller DJ, Graham J, Gil-Mohapel J, Christie BR. Liquid diets reduce cell proliferation but not neurogenesis in the adult rat hippocampus. Neuroscience. 2013;254:173–184. doi: 10.1016/j.neuroscience.2013.09.024
- Utsugi C, Miyazono S, Osada K, Sasajima H, Noguchi T, Matsuda M, Kashiwayanagi M. Hard-Diet Feeding Recovers Neurogenesis in the Subventricular Zone and Olfactory Functions of Mice Impaired by Soft-Diet Feeding. PLoS ONE. 2014;9(5):e97309. doi: 10.1371/journal.pone.0097309
- Yamamoto T, Hirayama A, Hosoe N, Furube M, Hirano S. Effects of soft-diet feeding on BDNF expression in hippocampus of mice. Bull Tokyo Dent Coll. 2008;49(4):185–190. doi: 10.2209/tdcpublication.49.185
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003
- Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34(2):199–211. doi: 10.1016/j.psyneuen.2008.08.025
- He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA. 2009;106(27):11370–11375. doi: 10.1073/pnas.0904835106
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435(4):597–602. doi: 10.1016/j.bbrc.2013.05.025.
- Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem. 2011;22(12):1150–1159. doi: 10.1016/j.jnutbio.2010.09.016
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19(18):8114–8121
- Rendeiro C, Vauzour D, Kean RJ, Butler LT, Rattray M, Spencer JP, Williams CM. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology (Berl). 2012;223(3):319–330. doi: 10.1007/s00213-012-2719-8.
- Hou Y, Aboukhatwa MA, Lei D-L, Manaye K, Khan I, Luo Y. Antidepressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 2010;58(6):911–920. doi: 10.1016/j.neuropharm.2009.11.002
- Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x
- Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z. Curcumin Enhances Neurogenesis and Cognition in Aged Rats: Implications for Transcriptional Interactions Related to Growth and Synaptic Plasticity. PLoS ONE. 2012;7(2):e31211. doi: 10.1371/journal.pone.0031211
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164(9):898–906. doi: 10.1093/aje/kwj267