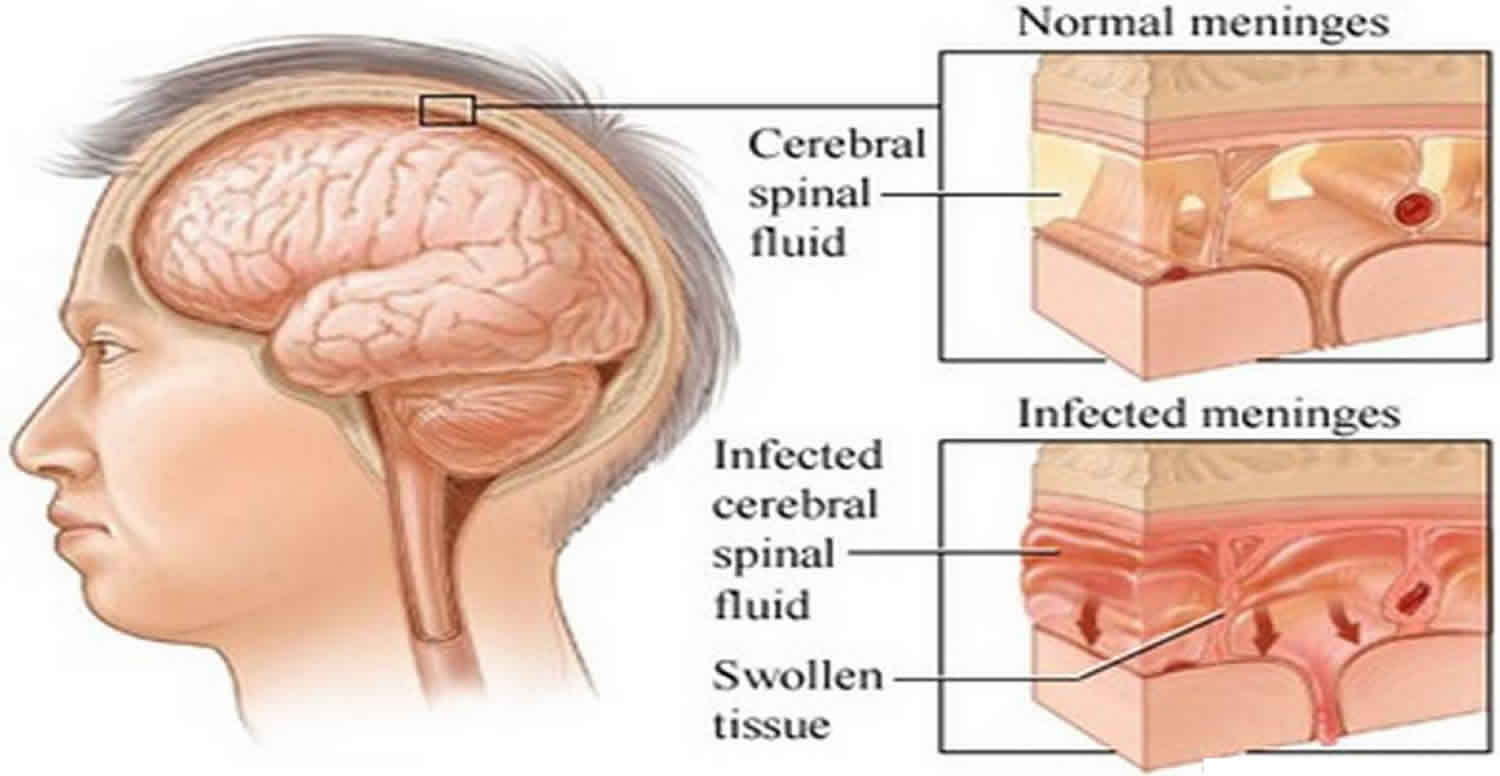
What is aseptic meningitis
Aseptic meningitis describes a clinical syndrome characterized by serous inflammation of the linings of the brain (i.e., meninges) not caused by an identifiable bacterial pathogen in the cerebrospinal fluid (CSF) 1, usually with an accompanying mononuclear pleocytosis 2. Aseptic meningitis also distinguishes a group of disorders that do not typically cause notable parenchymal involvement of the brain (encephalitis) or spinal cord (myelitis) 3. It also bears remembering that aseptic meningitis can also occur in the setting of an underlying connective tissue disorder or malignancy, or following certain drug ingestions or administrations (drug-induced aseptic meningitis) 4, which necessitates a search for the non-infectious causes of aseptic meningitis as well. Aseptic meningitis clinical manifestations vary, with headache and fever predominating. Aseptic meningitis is usually mild and runs its course without treatment; however, some cases can be severe and life threatening.
Aseptic meningitis syndrome is not caused by pyogenic bacteria. Although aseptic meningitis is usually caused by certain viruses, aseptic meningitis has a number of other etiologies as well, both infectious and noninfectious. Hence, the term aseptic meningitis is no longer synonymous with viral meningitis, although the two are still often used interchangeably 2.
The epidemiologic setting (e.g., time of year, geographic locale, exposure to insects, diseases prevalent in the local community) and accompanying systemic manifestations may be helpful in making a presumptive diagnosis. However, with a few exceptions, the clinical and laboratory findings accompanying acute viral meningitis are insufficiently distinct to allow an etiologic diagnosis, and distinguishing these disorders from a number of nonviral diseases may be difficult.
Aseptic meningitis treatment varies with the cause. No specific pharmacologic treatment is available for most cases of viral meningitis; these patients are managed with supportive therapy, which includes analgesics, antinausea medications, intravenous fluids, and prevention and treatment of complications 2.
Is aseptic meningitis contagious?
It depends on what is the cause of aseptic meningitis. See aseptic meningitis causes below.
Aseptic meningitis causes
Aseptic meningitis may be caused by viruses, bacteria, fungi, parasites, drugs, systemic diseases, and miscellaneous other conditions.
Viral causes include the following:
- Enteroviruses – coxsackievirus, echovirus, poliovirus
- Herpes simplex virus (HSV) types 1 and 2 (HSV-1, HSV-2)
- Varicella-zoster virus
- Arboviruses
- Epstein-Barr virus (EBV)
- HIV
- Influenza virus types A and B
- Mumps virus
- Colorado tick fever virus
- Lymphocytic choriomeningitis virus (LCMV)
- Rabies
Bacterial causes are as follows:
- Partially treated bacterial meningitis
- Parameningeal infection
- Endocarditis
- Mycoplasma pneumoniae
- Mycobacterium tuberculosis
- Ehrlichiosis – monocytic, granulocytic
- Borrelia burgdorferi
- Treponema pallidum
- Brucella species
Fungal causes are as follows:
- Cryptococcus neoformans
- Histoplasma capsulatum
- Coccidioides immitis
- Blastomyces dermatitides
Parasites that can cause aseptic meningitis are as follows:
- Toxoplasma gondii
- Taenia solium (cysticercosis)
Drugs that can cause aseptic meningitis include the following:
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Antimicrobials (e.g., trimethoprim-sulfamethoxazole, amoxicillin, isoniazid)
- Muromonab-CD3 (Orthoclone OKT3)
- Azathioprine
- Isotretinoin
- Intravenous immunoglobulin
- Intrathecal methotrexate
- Intrathecal cystine arabinoside
- Vaccines
- Allopurinol
Systemic diseases that can cause aseptic meningitis include the following:
- Sarcoidosis
- Leptomeningeal cancer
- Posttransplantation lymphoproliferative disorder
- Systemic lupus erythematosus (SLE)
- Granulomatosis with polyangiitis previously known as Wegener’s granulomatosis
- CNS vasculitis
- Behçet disease
- Vogt-Koyanagi-Harada syndrome
Miscellaneous causes include the following:
- Arachnoiditis
- Migraine
- Postinfectious syndromes
Viral infection
Overall, viral infection is the most common form of aseptic meningitis, and enteroviruses are the most common viral cause, with more than 90% of all viral meningitis cases are caused by enteroviruses 3. Enteroviruses are small, nonenveloped RNA viruses of the picornavirus family with various serotypes. More than 50 subtypes have been linked with meningitis. Coxsackieviruses and echoviruses, which are enteroviruses, account for approximately half of cases of aseptic meningitis.
Overall enteroviruses occur in a worldwide distribution 5, although only a handful of specific serotypes predominate in particular part of the world in any given year 6. Humans are their only natural reservoir, and they are transmitted primarily by fecal-oral contamination and less commonly in respiratory secretions 5. As such, enteroviruses exhibit a summer-to-fall seasonality in temperate climates and a high year-round incidence in tropical and subtropical areas (ostensibly due to sparse clothing and lower hygiene standards among children in these environments). While enteroviruses are still the most common cause of viral meningitis in adults 7, the majority of cases occur in children under the age of 5 years 8. In the United States, the 15 or so most common serotypes that cause disease cycle with varying periodicity, likely reflecting the birth of new susceptible hosts (i.e., non-immune children) within a given community 9. Occasional outbreaks in adults are caused by those serotypes that have not been present in a community for some time, again because a pool of susceptible hosts without preexisting immunity needs a longer time to develop 10. Among the many enterovirus serotypes that cycle from year to year, certain ones are also more associated with the development of aseptic meningitis than others.
Certain enteroviruses (e.g., coxsackievirus B5, echovirus 6, 9, and 30) are more likely to cause meningitis outbreaks, while others (coxsackie A9, B3, and B4) are mostly endemic 11. The incidence of infections from enteroviruses increases in the summer and early fall. Transmission occurs by hand-to-mouth contact and to a lesser extent by respiratory and fecal routes.
Herpesviruses, both herpes labialis (HSV-1) and genital herpes (HSV-2), can cause meningitis in children and especially infants. Varicella-zoster virus, another herpesvirus, causes encephalitis but only in immunocompromised persons.
Mumps was a common cause of aseptic meningitis in the United States until mumps vaccination came into use. In several countries, mumps virus remains a common pathogen in aseptic meningitis. It is spread by respiratory secretions, with increased incidence in the spring.
Aseptic meningitis from HIV occurs mostly at the time of seroconversion. HIV spreads to the meninges hematogenously, while rabies, polio, and herpesviruses are neurotrophic (i.e., spread through neurons).
Aseptic meningitis from arboviruses follows geographic and seasonal patterns determined by the life cycle of arthropod vectors, animal reservoirs, and their contact with human subjects. Eastern equine encephalitis virus usually is observed in Atlantic and Gulf regions, whereas western equine encephalitis virus is prevalent in the western part of the United States. Western equine encephalitis virus is responsible for more aseptic meningitis cases than eastern equine encephalitis virus.
Approximately 15% of St Louis encephalitis virus infections result in meningitis. In children, this incidence is as high as 60%. St Louis encephalitis virus infection can occur in both rural and urban areas. In the rural setting, the infection from St Louis encephalitis virus tends to follow the same pattern as western equine encephalitis virus infection. Conversely, in urban settings, outbreaks tend to be more explosive.
Approximately 18% of people infected with Colorado tick fever develop meningitis. This disease primarily occurs in the Rocky Mountain region, which is the habitat of Dermacentor andersoni ticks whose bite transmits the virus.
Infection with Venezuelan equine encephalitis virus initially leads to influenza-like illness in most people. Only 3% of infected persons are known to develop acute meningitis. This virus has spread into Florida and some southwestern states. lymphocytic choriomeningitis mammarenavirus, an arenavirus, is an extremely rare cause of meningitis. Transmission of lymphocytic choriomeningitis mammarenavirus infection occurs by contact with dust or food contaminated by excreta of rodents. Cases tend to be more common in the winter. Human infections have been seen in both laboratory and home settings.
Brucellosis
Brucellosis is an infection with a bacterium of one of the Brucella species, usually Brucella abortus (cattle), Brucella melitensis, Brucella ovis (sheep, goats), Brucella suis (pigs), or rarely Brucella canis (dogs). Its distribution is worldwide, but it is most common in the Mediterranean regions, Africa, the Middle East, India, Central Asia, Mexico, and Central and South America.
Persons at highest risk of brucellosis are those who work with animals that are infected, such as veterinarians and ranchers, and persons who consume raw milk or cheeses made with raw milk. Brucellosis also may be transmitted to humans if they are exposed inadvertently to live brucellosis vaccine by a needlestick or other accident.
The incidence in the United States is fewer than 0.5 cases per 100,000 population.
Drug-induced aseptic meningitis
The incidence of drug-induced aseptic meningitis (DIAM) is unknown. Many antimicrobials can cause the disorder (e.g., trimethoprim-sulfamethoxazole, ciprofloxacin, cephalexin, metronidazole, amoxicillin, penicillin, isoniazid). Other drugs that have been associated with drug-induced aseptic meningitis (DIAM) include nonsteroidal anti-inflammatory drugs (NSAIDs), ranitidine, carbamazepine, vaccines against hepatitis B and mumps, immunoglobulins, radiographic agents, and muromonab-CD3.
Drug-induced aseptic meningitis may recur with re-exposure to the offending agent. Green et al reported a case of lamotrigine-induced aseptic meningitis, with a second episode on rechallenge with lamotrigine 12.
The pathogenic mechanisms of drug-induced aseptic meningitis are diverse and presumably differ from drug to drug. There are two proposed mechanisms: direct meningeal irritation by the intrathecal drug and hypersensitivity reactions to the drug (type III and IV). In type III hypersensitivity reactions, the drug or its metabolite forms a complex with antibodies in the serum, in turn activating the complement cascade. In type IV reactions, T helper cells, after previous sensitization, are recruited to the site of inflammation 13.
Drug-induced aseptic meningitis from muromonab (OKT3) is believed to be mediated, at least in part, by cytokine release. Why such reactions are confined selectively to the CSF compartment is unclear.
Aseptic meningitis—along with cerebral vasospasm or ischemic encephalopathy—has been reported with intravenous immunoglobulin (IVIg) therapy 14. Jarius et al strongly suggest that in vivo activation of TNF-alpha–primed neutrophils by atypical antineutrophil cytoplasmic antibodies (ANCAs) of IVIg may contribute to these side effects 15.
Aseptic meningitis in systemic disease
Patients with systemic lupus erythematosus (SLE) are especially susceptible to aseptic meningitis. In these patients, the disorder is often precipitated by drugs.
Vogt-Koyanagi-Harada syndrome
The exact etiology of this condition remains uncertain. Originally, the Vogt-Koyanagi syndrome was described independently of the Harada syndrome. However, the clinical manifestations of both overlap sufficiently to justify their combination into a single entity.
Behçet syndrome
The cause of Behçet syndrome remains uncertain. CNS manifestations occur in 18% of patients with Behçet syndrome.
Mollaret meningitis
Mollaret meningitis is a recurrent disorder whose causative agent remains unknown. However, recent data suggest that herpes simplex virus (HSV-2 and, less frequently, HSV-1) may cause some if not most cases.
Iatrogenic aseptic meningitis
Patients have developed febrile meningeal syndromes shortly after undergoing embolization of cerebral aneurysms with hydrogel-coated coils 16.
Postoperative aseptic meningitis was first described by Cushing in 1925, though the mechanisms remain unclear 17.
Aseptic meningitis prevention
Hand washing and other general good health measures may reduce the risk of developing an infection that can progress to meningitis.
Many of the causes of meningitis are communicable and, if one case of meningitis is diagnosed within a community, appropriate steps may need to be taken immediately to prevent the further spread of the disease. Since viruses that are passed in the stool cause most cases, people diagnosed with aseptic meningitis should be sure to wash their hands thoroughly after using the toilet. Always wash hands after changing diapers.
Effective vaccines are available for polio, measles, mumps, and rubella. Illnesses related to these viruses have declined dramatically in countries with effective vaccination strategies. Vaccination against Japanese encephalitis has been effective in controlling the infection in Asia. Rabies is the only infection in which the vaccine is given after exposure to the virus.
Aseptic meningitis symptoms
The signs and symptoms of most acute viral meningitis may vary with the particular virus. Illness may be biphasic, with nonspecific constitutional symptoms followed by meningitis. The epidemiologic setting (e.g., time of year, geographic locale, exposure to insects, prevalent illnesses in the local community) and accompanying systemic manifestations may be helpful in making a presumptive diagnosis.
A detailed drug history is invaluable for identifying possible drug-induced aseptic meningitis, which has a clinical presentation indistinguishable from infectious meningitis. The drug history must include nonprescription medications such as ibuprofen.
The time course of acute viral meningitis varies. Onset may occur within a matter of hours after exposure or evolve more slowly over a few days. Usually, maximum deficit appears within 3-6 days after exposure. Persons infected with the viruses that commonly cause aseptic meningitis may remain infectious for weeks after contracting the virus.
Characteristic signs of acute viral meningitis include the following:
- Headache
- Fever
- Stiff neck
- Photophobia
- Drowsiness
- Myalgias
- Malaise
- Chills
- Sore throat
- Abdominal pain
- Nausea and vomiting
Focal signs, seizures, and profound lethargy are rarely features of aseptic meningitis. Occasionally, patients may exhibit altered levels of consciousness, including confusion and visual hallucinations.
Physical Examination
Meningeal signs
Neck stiffness in meningitis is tested by gentle forward flexion of the neck with the patient lying in the supine position. Meningeal irritation also can be tested by the jolt accentuation of headache. This is elicited by asking the patient to turn his or her head horizontally at a frequency of 2-3 rotations per second. Worsening of a baseline headache represents a positive sign.
Severe meningeal irritation may result in the patient assuming the tripod position (termed Amoss sign or Hoyne sign) with the knees and hips flexed, the back arched lordotically, the neck extended, and the arms brought back to support the thorax.
When passive neck flexion in a supine patient results in flexion of the knees and hips, the Brudzinski sign is positive. Yet another Brudzinski sign, the contralateral reflex, is present if passive flexion of one hip and knee causes flexion of the contralateral leg.
Kernig sign is elicited with the patient lying supine and the hip flexed at 90°. A positive sign is present when extension of the knee from this position elicits resistance or pain in the lower back or posterior thigh.
Papilledema or absence of venous pulsations upon funduscopic examination indicates increased intracranial pressure.
Rash
Skin manifestations may suggest the diagnosis of aseptic meningitis from certain causes. Examples include the rash of varicella zoster, the genital lesions of herpes simplex virus type 2 (HSV-2), or a mild maculopapular rash occurring in the summer and fall months with some enteroviruses.
Rash from enteroviral infections usually accompanies the onset of fever and persists for 4-10 days. In infections due to coxsackieviruses A5, 9, or 16 or echoviruses 4, 6, 9, 16, or 30, the rash is typically maculopapular and nonpruritic, may be confined to the face and trunk, or may involve extremities, including the palms and soles.
In coxsackievirus A16 and, rarely, in other group A serotype infections, a vesicular rash may involve the hands, feet, and oropharynx. Herpangina, characterized by gray vesicular lesions on the tonsillar fossae, soft palate, and uvula, can accompany infection caused by group A coxsackievirus. With echovirus 9 infections, a petechial rash resembling meningococcemia typically is observed.
Aseptic meningitis complications
Seizures sometimes can complicate meningitis. Encephalitis may develop in some patients. The most common sequela following mumps meningoencephalitis is sensorineural deafness. Hydrocephalus from aqueductal stenosis has been reported as a late sequela of mumps meningitis and encephalitis in children.
Aseptic meningitis diagnosis
Spinal MRI imaging begins the evaluation of any patient with an acute myelopathy in order to exclude an extramedullary or extradural compressive lesion. In cases of acute viral myelitis, spinal MRI scans can reveal a wide range of findings from a normal appearing spinal cord to a swollen, enhancing cord lesion that extends over multiple spinal levels. Myelitis is usually distinguished from spinal multiple sclerosis (MS) plaques on radiographic grounds by virtue of involving a greater cross sectional area of the cord and by extending over more than 2 contiguous spinal levels. Viral myelitis, however, can be very difficult to distinguish from the spinal onset of neuromyelitis optica based on MRI findings. Contrast enhancement of the lesion is usually seen in the earliest stages of disease, but may disappear within days to a few weeks. Hyperintensity of the cord on non-contrast T1-weighted sequences suggests the presence of hemorrhagic necrosis. Imaging of the brain is also commonly advised to exclude a multifocal process such as ADEM (acute disseminated encephalomyelitis) or MS (multiple sclerosis).
The presence of inflammation is suggested by gadolinium enhancement on MRI and confirmed by lumbar puncture and CSF analysis. A mononuclear cell pleocytosis with elevated total protein content are the expected findings in viral myelitis, but this profile does not exclude other non-viral or non-infectious etiologies. Furthermore, some confirmed cases of viral myelitis are accompanied by a high proportion of neutrophils in the CSF (cytomegalovirus and West Nile virus, in particular), while others (~3–5%) do not show any evidence of a pleocytosis at all. Hypoglycorrhachia (<40 mg/dl) is uncommon with all CNS viral infections, but it can occasionally be seen in those disorders that also elicit a neutrophilic pleocytosis. Direct isolation of a viral pathogen from the CSF is rarely accomplished in either acute transverse myelitis or acute flaccid paralysis. Instead, PCR for DNA viruses and RT-PCR for RNA viruses are now the standard methods used in the rapid identification of a specific viral pathogen from clinical samples. These assays are routinely available in most institutions for herpes simplex virus, cytomegalovirus, varicella zoster virus, Epstein–Barr virus, enteroviruses and HIV, and are becoming more commonplace for West Nile virus and human T-lymphotropic virus 1 (HTLV-1). One important general limitation of these PCR tests is that viral replication often peaks early in CNS viral infection then declines rapidly to undetectable levels; one study found that the incidence of a positive PCR assay was highest when CSF samples were obtained 5 days after symptom onset 18. A diagnosis can also be confirmed by detecting virus-specific IgM in CSF, since these large molecules do not readily cross the blood-brain barrier (BBB) and thus indicate intrathecal synthesis. Measurement of serum IgM titers can also sometimes be useful, as is the demonstration of a four-fold higher IgG titer during the acute compared to the convalescent phase of disease. This latter approach, of course, is only useful in establishing a retrospective diagnosis, since convalescent samples should be obtained at least 6 weeks after the acute illness.
Aseptic meningitis treatment
Many patients who have aseptic meningitis can be cared for on an outpatient basis, but those who have profound headache, nausea, vomiting, or CSF pleocytosis with a polymorphonuclear leukocyte predominance should be admitted for observation. Antibiotic coverage for bacterial meningitis may be given, at the discretion of the managing clinician. Given the potential for serious neurological morbidity and the persistently high mortality rates of bacterial meningitis, rapid institution of antibiotic coverage is essential when the diagnosis of bacterial meningitis is suspected. A third-generation intravenous cephalosporin is the customary choice.
A consensus conference recommended empirical antibiotic therapy for all patients with postoperative meningitis and treatment withdrawal after 48 or 72 hours if CSF culture results are negative 19. This concept is not universally accepted. Zarrouk et al 20 found that stopping antibiotic treatment after 3 days is effective and safe for patients with postoperative meningitis whose CSF culture results are negative.
No specific treatment exists for most of the viruses that cause meningitis; therefore, management, for the most part, is supportive and includes analgesics, antinausea medications, intravenous fluids, and prevention and treatment of complications.
Headache and fever usually can be treated with judicious doses of acetaminophen. Severe hyperthermia (>104 °F [>40°C]) may require vigorous therapy, but mild temperature elevation may serve as a natural defense mechanism, and some authors believe it should be left untreated.
Strict isolation is not necessary. When enteroviral infection is suspected, take precautions in handling stools and wash hands carefully. In patients with meningitis from measles, chickenpox, rubella, or mumps virus infections, the usual precaution of isolation from susceptible individuals should be observed.
For severe cases, meticulous care in an intensive care setting with respiratory and nutritional support is warranted. Remarkable recovery may be achieved in some patients who become comatose. Vigorous support and avoidance of complications are very important in these patients.
Effective antiviral therapy is available against herpes simplex virus type 1 (HSV-1), varicella, and cytomegalovirus. In immunosuppressed patients, long-term therapy may be necessary.
Acyclovir is recommended for immunocompetent hosts with herpes simplex virus type 2 (HSV-2) meningitis and a primary genital herpes infection. Valacyclovir and foscarnet are alternative antiviral agents.
Although formal clinical trial evidence of efficacy is lacking given the rarity of these diseases, it is advisable to administer intravenous acyclovir, 10–15 mg/kg every 8 hours for 10–14 days, to patients with herpes simplex virus and varicella zoster virus myelitis as soon as a pathogen is confirmed in CSF by PCR or even empirically if the clinical suspicion is high enough (i.e., recent zoster rash or recurrent genital herpes outbreaks) 21. Ganciclovir (5 mg/kg every 12 hours) and foscarnet (90 mg/kg every 12 hours) both have been used in cases of cytomegalovirus myelitis with some effectiveness, although overall outcome from this disorder remains poor even when drug treatment is continued over 2–3 weeks 21. Spinal Epstein–Barr virus infections have not responded well to either of these two therapies. There is no proven antiviral therapy for spinal poliomyelitis, although pleconaril can be made available from the manufacturer (ViroPharma) on a compassionate use basis. The drug was felt to be of some use in 2 of 3 cases of vaccine-associated poliomyelitis in an open-label study 22.
Another approach being taken in patients with neuroinvasive West Nile virus infections is the administration of IVIg pooled from donors where the virus is endemic and high titers of neutralizing antibodies are found. Such passively transferred antibodies would be presumed to cross the BBB and facilitate viral clearance from the CNS, as it does in animal models of this infection 23. Unfortunately, however, results of a Phase I/II clinical trial have not yet reported any efficacy compared to a placebo. Other immunotherapeutic interventions are also conceivable in the setting of efficacy in animal models of alphavirus and flavivirus myelitis 24, but these will similarly require further testing before any use in humans is advised. The application of high-dose corticosteroids, either as an anti-inflammatory intervention or through actions via some other mechanism, has not been systematically studied in viral myelitis, although anecdotal evidence has reported benefit in 4 patients with enterovirus-71 infections extending into the brainstem to ameliorate long-term deficits 25.
In patients with Mollaret meningitis, acyclovir (intravenous or oral) or valacyclovir (oral only) are worthy of consideration for both therapy and prophylaxis.
Specific antibacterial therapy
For meningitis from the following pathogens, these specific agents are appropriate:
- Tuberculosis- triple drug therapy with rifampin/isoniazid/pyrazinamide
- Actinomycetes and spirochetes – penicillin and ceftriaxone
- Brucella – doxycycline or rifampin
- Pasteurella tularensis – gentamicin
Antifungal therapy
Antifungal agents of choice include amphotericin B, fluconazole, and flucytosine.
Steroids
In general, corticosteroids are avoided in aseptic meningitis because of their inhibitory effects on host immune responses. Occasionally, glucocorticoids, such as dexamethasone, are useful when meningitis is associated with signs of increased intracranial pressure.
Meningitis from Vogt-Koyanagi-Harada syndrome responds to prednisone in moderate to high doses.
Treatment of complications
Seizures sometimes can complicate meningitis; however, prophylactic anticonvulsants are not recommended routinely. If seizures develop, they can be controlled with phenytoin and phenobarbital. If status epilepticus develops, appropriate therapy should be provided to prevent secondary brain injury.
If persistent cognitive problems occur after recovery from acute meningitis, especially residual suboptimal functioning in the workplace or in school, referral for formal neuropsychological testing clarifies the nature of the complaint both to the physician and to the patient and helps separate psychological adjustment factors from organic dysfunction.
Aseptic meningitis prognosis
Aseptic meningitis is usually a benign disease. Rates of morbidity and mortality are low, except among neonates. Most patients experience full recovery in 5-14 days after onset of symptoms. Fatigue, light-headedness, and asthenia may persist for months in some cases, however.
References- [Etiology of meningoencephalitis in children, especially the syndrome of acute aseptic meningitis]. WALLGREN A. Acta Paediatr. 1951 Nov; 40(6):541-65.
- Aseptic meningitis. https://emedicine.medscape.com/article/1169489-overview
- Irani DN. Aseptic meningitis and viral myelitis. Neurol Clin. 2008;26(3):635–viii. doi:10.1016/j.ncl.2008.03.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2728900
- Acute aseptic meningitis during isotretinoin treatment for nodular acne solely presenting with headache: case report and brief review of the literature. International Journal of Neuroscience Volume 129, 2019 – Issue 2 https://doi.org/10.1080/00207454.2018.1517763
- Melnick JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 655–712
- Strikas RA, Anderson LJ, Parker RA. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351
- Rotbart HA, Brennan PJ, Fife KH, Romero JR, Griffin JA, McKinlay MA, Hayden FG. Enterovirus meningitis in adults. Clin Infect Dis. 1998;27:896–898.
- Rotbart HA. Viral meningitis. Semin Neurol. 2000;20:277–292.
- Strikas RA, Anderson LJ, Parker RA. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351.
- Kinnunen E, Hovi T, Stenvik M, Hellstrom O, Porras J, Kleemola M, Kantanen MC. Localized outbreak of enteroviral meningitis in adults. Acta Neurol Scand. 1987;74:346–351
- Pabbaraju K, Wong S, Chan EN, Tellier R. Genetic characterization of a Coxsackie A9 virus associated with aseptic meningitis in Alberta, Canada in 2010. Virol J. 2013 Mar 22. 10:93.
- Green MA, Abraham MN, Horn AJ, Yates TE, Egbert M; Sharma A. Lamotrigine-induced aseptic meningitis: a case report. International Clinical Psychopharmacology. May, 2009. 24(3):159-161.
- Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000 Mar. 22(3):215-26.
- Bhatt GC, Sharma T. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2012 Sep. 7(3):242-3
- Jarius S, Eichhorn P, Albert MH, Wagenpfeil S, Wick M, Belohradsky BH. Intravenous immunoglobulins contain naturally occurring antibodies that mimic antineutrophil cytoplasmic antibodies and activate neutrophils in a TNFalpha-dependent and Fc-receptor-independent way. Blood. 2007 May 15. 109(10):4376-82
- Im SH, Han MH, Kwon BJ, Jung C, Kim JE, Han DH. Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils: report of three cases. AJNR Am J Neuroradiol. 2007 Mar. 28(3):511-2.
- Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988 Nov. 69(5):669-74.
- Davies NW, Brown LJ, Irish D, Robinson RO, Swan AV, Banatvala J, Howard RS, Sharief MK, Muir P. Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J Neurol Neurosurg Psychiatry. 2005;76:82–87
- The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. Br J Neurosurg. 2000 Feb. 14(1):7-12.
- Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007 Jun 15. 44(12):1555-9.
- Kincaid O, Lipton HL. Viral myelitis: an update. Curr Neurol Neurosci Rep. 2006;6:469–475.
- Rotbart HA, Webster AD Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–235
- Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300.
- Irani DN, Prow NA. Neuroprotective interventions targeting detrimental host immune responses protect mice from fatal alphavirus encephalitis. J Neuropathol Exp Neurol. 2007;66:533–544.
- Nolan MA, Craig ME, Lahra MM, Rawlinson WD, Prager PC, Williams GD, Bye AM, Andrews PI. Survival after pulmonary edema due to enterovirus 71. Neurology. 2006;60:1651–1656.