What is a baclofen pump
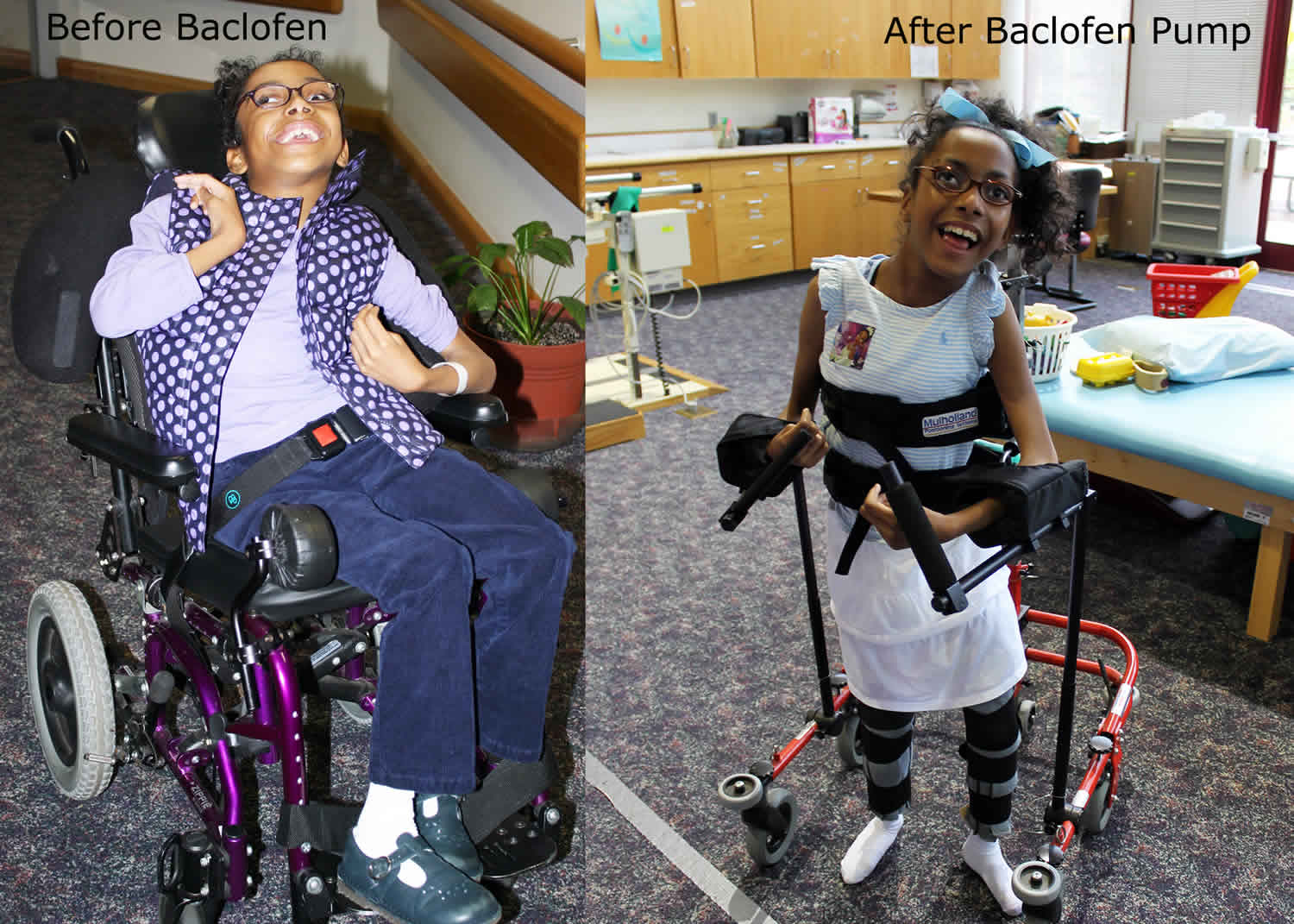
Intrathecal baclofen pump is used in patients who have intractable spasticity uncontrolled by drug therapy or who experience intolerable side effects to oral baclofen 1. The baclofen pump is most appropriate for individuals with chronic, severe stiffness or uncontrolled muscle movement throughout the body. Intrathecal baclofen pump relieves the spasms, cramping, and tightness of muscles caused by medical problems such as multiple sclerosis, cerebral palsy, or certain injuries to the spine. Baclofen is a skeletal muscle relaxant acting centrally as a presynaptic gamma-amino-butyric acid-B (GABA-B) receptor agonist that is most frequently used for the treatment for spasticity 2. Baclofen decreases the excitability of nerve cells in the spinal cord, which then reduces muscle spasticity throughout the body. However, the use of baclofen has been limited due to systemic side effects such as drowsiness, confusion, and headache 3. Intrathecal baclofen (into the fluid surrounding the spinal cord) administration has been introduced, therefore, to maximize baclofen’s effects and minimize its side effects 4. The intrathecal baclofen pump therapy can directly and effectively control spasticity by selectively acting as a GABA receptor agonist at the phase of the spinal cord with less systemic side effects 5. Intrathecal baclofen pump has been shown to be useful in the majority of patients to treat severe or intractable spasticity and dystonia leading to a decrease in muscle spasms, pain, and an increase in functional motor activity and control 6. The intrathecal baclofen pump can be adjusted if muscle tone is worse at certain times of the day or night.
“Spasticity” is defined as hypertonia in which one or both of the following signs are present (1) resistance to externally imposed movement that increases with increasing speed of stretch and varies with the direction of joint movement, and/or (2) resistance to externally imposed movement rises rapidly above a threshold speed or joint angle 7. Spasticity is a common phenomenon in patients with a wide variety of neurological disorders like cerebral palsy, multiple sclerosis, cardiovascular accidents, strokes, traumatic brain, and spinal cord injury 8. These patients not only suffer from severe contractures and deformities but also severe pain that incapacitates them. Although spasticity is part of the upper motor neuron syndrome, it is frequently tied to the other presentations of the said syndrome. Contracture, hypertonia, weakness, and movement disorders can all coexist as a result of the upper motor neuron syndrome 9.
A doctor will surgically place the baclofen pump and monitor the dose of the medication that is delivered by the pump. The dose of intrathecal baclofen will be different for different patients and will depend on the type of muscle tightness that you have.
Intrathecal baclofen is given only by or under the direct supervision of a doctor.
Intrathecal administration of baclofen is initiated by screening. The patient receives a single dose, typically 50 mcg baclofen and is observed for 4 to 8 hours to assess its efficacy 10. The screening dose that gives a positive response for the initial 24 hours will then be doubled and administrated via an implantable pump with an intrathecal catheter. Daily dose adjustments will be made gradually on the order of 10% to 30% for spasticity of spinal cord origin and 5% to 15% for spasticity of cerebral origin until a positive response is achieved. The dose may be increased or decreased slightly to obtain an optimum daily dose. Spasticity of cerebral origin is usually adequately managed on 90 to 703 mcg daily. Spasticity related to the spinal cord usually requires 300 to 800 mcg of baclofen daily. However, the lowest dose that produces optimal response should be maintained. The reservoir in the pump is filled by percutaneous injections on a regular basis, and the pump is programmed to deliver a certain dose automatically, via simple continuous or bolus dosing.
Candidates for intrathecal baclofen infusion are patients with spasticity who have intractable spasticity uncontrolled by drug therapy, or who experience intolerable side effects from oral baclofen 11.
Advantages of intrathecal baclofen infusion are:
- Direct drug administration to the cerebrospinal fluid (CSF)
- The central side effects of oral baclofen, such as drowsiness or confusion, appear to be minimized with intrathecal administration.
- The intrathecal delivery of baclofen concentrates the drug in the CSF at higher levels than those attainable via the oral route.
- Intrathecal administration can use concentrations of baclofen of less than one hundredth of those used orally 12
- Adjustable/programmable continuous infusion makes it possible to finely titrate patients’ doses and to vary the doses over the hours of the day. For example, the dose can be relatively low to give the patients the extensor tone needed for ambulation during the day and increased at night, thereby improving quality of sleep.
- Reversible (in contrast to surgery).
A patient who is a candidate for intrathecal baclofen infusion must have no contraindications to the insertion of an intrathecal catheter (e.g., anticoagulant therapy, coagulopathy, local or systemic infection, anatomical abnormality of the spine).
Baclofen pump placement
Before surgery to implant the baclofen pump, you or your child will be treated with one test dose of baclofen injected into the spinal fluid, or with a continuous infusion using an external pump. These test injections will be administered by a trained nurse in the hospital, and you or your child will be kept overnight for observation.
The test doses allow the doctor to see how you or your child’s movement symptoms are affected by the medication and whether there are any negative side effects.
The baclofen pump is implanted using a surgical procedure performed while you or your child is under general anesthesia.
- Baclofen pumps are placed in to the lower abdominal wall. Placing the pump requires surgery.
- A catheter (tube) attached to the pump is placed into the spinal column.
- The pump delivers small amounts of baclofen into the fluid around the spinal cord causing inhibition at the spinal level.
- The surgery itself usually takes about two hours.
The pump is a round metallic disc, about 3 inches across and 1 inch thick. It is typically placed just beneath the skin of the abdomen, to the left or right of the belly button. The catheter to deliver the medication into the spinal fluid is fed from the pump to the point near the spine or in the brain where the dosage of baclofen will most effectively address your child’s symptoms.
How is the baclofen pump refilled?
You must make an appointment in the clinic to have the baclofen pump refilled. In the clinic, your care team will locate the place on the pump to fill it. A needle will be used to enter the baclofen pump and refill it with medication using a syringe.
Baclofen pump surgery recovery time
You or your child will be in the hospital from 1-7 days after getting the baclofen pump, depending on recovery and how soon the medicine can be adjusted.
While your child is at the hospital, the medical team may make initial adjustments to the dose of medication to control your child’s movement symptoms, and will watch for any negative side effects. The baclofen pump is controlled by a remote computerized device, using a wand placed over the skin.
Physical and occupational therapists may begin to work with your child on improving their range of motion, and decreasing spastic and/or dystonic movements that they may not have had before the baclofen treatment. You and your child will learn how to continue these exercises at home.
Before your child is released to go home, your medical team will review what you need to know about managing the pump, caring for the incision while it heals, recognizing the signs of baclofen withdrawal or overdose, and what activities should be encouraged or avoided in the first few weeks. Arrangements may be made for local physical and occupational therapist to come to your home.
Going home with a baclofen pump
You will be asked to bring your child back for regular follow-up visits to assess progress, adjust the medication dosage, and refill the pump with baclofen as needed. The pump is refilled every one to six months by inserting a needle through the skin into a refill port on the pump.
It may take several weeks or a few months to get to the optimal dose levels and schedule. Changes to the baclofen doses are made gradually, with careful monitoring of the results.
Once the optimum dose is found, the pump can be programmed to vary the dose at different times of day or for specific activities.
It will be important to maintain the pump and monitor its supply of baclofen to avoid lapses in medication, which can lead to withdrawal systems. You will be expected to bring your child to all scheduled medical appointments, and to keep a supply of oral baclofen on hand in case of an unexpected interruption in the pump’s delivery of medication.
The pump will likely be visible as a small protrusion in the skin and may be visible under close-fitting clothes.
- Frequent hand hygiene is the most important way to prevent the spread of germs. Always wash hands well with soap and water for at least 15 seconds, or use an alcohol hand sanitizer, such as Purell®.
- Keep the dressing placed in the hospital until 4 days after the surgery. If there is any bleeding or drainage on the dressing, call the clinic.
- Remove the dressing after four days to allow the wound to breathe.
- The wound should be kept clean and dry for 7 days after surgery before full immersion underwater (e.g. bathing, swimming, etc.).
- May shower after 4 days, and no full immersion underwater for at least 1 week after surgery.
- Patients with pumps need ongoing follow-up at outpatient rehab for pump and medication adjustment.
When should I call the clinic?
- Temperature higher than 102° F (38.9°C)
- Any concerns of hardware exposure or leakage should be reported to neurosurgery immediately
- Drainage from the wound
- Any signs of not getting enough baclofen or getting too much
Baclofen pump side effects
Your doctor should check your progress at regular visits, especially during the first few weeks of treatment with baclofen pump. During this time, the amount of baclofen you are using may have to be changed often to meet your individual needs.
Make sure to keep all appointments to refill the baclofen pump. If the baclofen pump is not refilled on time, you may experience return of your muscle tightness and early withdrawal symptoms which might include:
- itching of the skin
- decreased blood pressure
- blurred vision
- confusion
- dizziness, faintness, or lightheadedness when getting up from a lying or sitting position suddenly
- sweating
- unusual tiredness or weakness
- burning, crawling, itching, numbness, prickling, “pins and needles” , or tingling feelings
- seizures
Intrathecal baclofen will add to the effects of alcohol and other CNS depressants (medicines that may make you drowsy or less alert). Some examples of CNS depressants are antihistamines or medicine for hay fever, other allergies, or colds; sedatives, tranquilizers, or sleeping medicine; prescription pain medicine or narcotics; barbiturates; medicine for seizures; other muscle relaxants; and anesthetics, including some dental anesthetics. Check with your doctor before taking any of the above while you are using intrathecal baclofen.
Intrathecal baclofen may cause dizziness, drowsiness, false sense of well-being, lightheadedness, vision problems, or clumsiness or unsteadiness in some people. Make sure you know how you react to this medicine before you drive, use machines, or do anything else that could be dangerous if you are not alert, well-coordinated, and able to see well.
Intrathecal baclofen may cause dryness of the mouth. For temporary relief, use sugarless candy or gum, melt bits of ice in your mouth, or use a saliva substitute. However, if dry mouth continues for more than 2 weeks, check with your medical doctor or dentist. Continuing dryness of the mouth may increase the chance of dental disease, including tooth decay, gum disease, and fungus infections.
Dizziness, lightheadedness, or fainting may occur when you get up suddenly from a lying or sitting position. Getting up slowly may help lessen this problem.
Check with your doctor as soon as possible if any of the following side effects occur:
More common
- Convulsions (seizures)
Less common or rare
- blurred vision or double vision
- fainting
- mental depression
- muscle weakness
- ringing or buzzing in ears
- seeing, hearing, or feeling things that are not there
- shortness of breath or troubled breathing
Symptoms of baclofen overdose
- convulsions (seizures)
- dizziness, drowsiness, or lightheadedness
- increased watering of the mouth
- mental confusion
- muscle weakness
- nausea and/or vomiting
- shortness of breath or troubled breathing
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- constipation
- difficult urination
- dizziness
- headache
- nausea and/or vomiting
- numbness or tingling in hands or feet
- sleepiness
Less common
- clumsiness, unsteadiness, trembling, or other problems with muscle control
- diarrhea
- difficulty sleeping
- dizziness or lightheadedness, especially when getting up from a lying or sitting position
- dry mouth
- frequent urge to urinate
- irritation of the skin at the site where the pump is located
- itching of the skin
- sexual problems
- slurred speech or other speech problems
- swelling of ankles, feet, or lower legs
- trembling or shaking
After you stop using this medicine, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Baclofen pump complications
Although generally safe, baclofen pumps are nevertheless mechanical devices that may malfunction. The malfunction of the drug pump allowed much of the administered baclofen to leak into the subarachnoid space at once and threatened a patient’s life. Potentially life-threatening complications of intrathecal baclofen therapy include overdose and withdrawal syndrome 13. Baclofen toxicity due to pump failure can cause coma as well as a generalized seizure developing within minutes of the filling of the pump with baclofen. In addition, infections, wound dehiscence, seroma, cerebrospinal fluid leak, and mechanical problems related to the pump and catheter can occur, which can cause life-threatening complications 14. However, pump-related complications (i.e, mechanical device failures) are said to be rare in currently available systems 15. Review of the literature showed that baclofen withdrawal syndromes are the most common cause of life-threatening complications of intrathecal baclofen pump.
Empty pump reservoir, catheter leaks or displacement, pump malfunction, programming error and refill of pump with improper drug concentration are the possible mechanisms which could lead to an intrathecal baclofen withdrawal syndrome 16. Regular check-up of the intrathecal baclofen pump by a specialist, educating patients and their caregivers may decrease the incidence of intrathecal baclofen withdrawal syndrome. Oral baclofen replacement may not be an effective method to treat or prevent intrathecal baclofen withdrawal syndrome. Early recognition of syndrome, high-dose benzodiazepines, prompt analysis of the intrathecal baclofen pump with reinstitution of baclofen, and proper intensive care management are mainstays for the management of intrathecal baclofen withdrawal syndrome.
Baclofen withdrawal syndrome
Abrupt discontinuation of intrathecal baclofen, regardless of the cause, has resulted in complications that include high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure and death.
Long-term intrathecal baclofen pump infusion causes down-regulation of GABA-B receptors in the central nervous system (CNS) and spinal cord 16. Down-regulation of GABA-B receptors accounts for the decreased sensitivity to the baclofen over time. Although GABA-B receptors are down-regulated, it is the baclofen itself that causes increased inhibitory tone in the CNS and spinal cord 17. Therefore, abrupt intrathecal baclofen pump withdrawal results in a predominance of excitatory effects and simulates other conditions that are associated with CNS hyperexcitability and severe spasticity. Sudden cessation of intrathecal baclofen pump administration can cause mild symptoms like reappearance of baseline level of spasticity associated with pruritis, anxiety and disorientation. These mild symptoms represent “loss of drug effect”. All patients experience “loss of drug effect” when intrathecal baclofen pump is discontinued. However, more severe symptoms like hyperthermia (109.4°F), myoclonus, seizures 18, rhabdomyolysis, disseminated intravascular coagulation, multisystem organ failure 19, cardiac arrest, coma and death 20 have been well reported, and represents a full-blown life-threatening intrathecal baclofen pump withdrawal syndrome. Food and drug administration (FDA) of USA has included a drug label warning for baclofen withdrawal syndrome in April 2002 21. Differential diagnoses include malignant hyperthermia, neuroleptic-malignant syndrome, autonomic dysreflexia, sepsis and meningitis. intrathecal baclofen pump withdrawal syndrome has been fatal in some cases. Six patients have died out of 27 cases reported to FDA 21. Most reported episodes of intrathecal baclofen pump withdrawal were caused by preventable human errors or oversights. However, catheter dislodgement, catheter migration and kinks, and other catheter-related issues might be more common than pump-related malfunctions 22. Close attention to pump refilling and programming procedures may reduce the incidence of intrathecal baclofen pump withdrawal syndrome.
Benzodiazepines are helpful in controlling spasticity and seizures during intrathecal baclofen pump withdrawal syndrome 20. Benzodiazepines activate central receptors and GABA-A receptors of spinal cord by different mechanisms 21. Therefore, intrathecal baclofen pump induced down-regulation of GABA-B receptors do not interfere with benzodiazepine’s mechanism of action. During a planned removal of intrathecal baclofen pump due to infection or other causes, premedication with high doses of benzodiazepines and augmented oral baclofen is usually administered in the hospitals to prevent spasticity. Similarly, high doses of oral baclofen is also tried in some cases of intrathecal baclofen pump withdrawal syndrome 23. But failure of high doses of oral baclofen (80 mg three times daily) have been reported recently 24. High doses of oral baclofen may not be adequate to treat or prevent intrathecal baclofen pump withdrawal because of down-regulation of central GABA-B receptors due to chronic intrathecal baclofen pump administration. Moreover, it has been suggested that it may take many hundreds of grams of oral baclofen to achieve a therapeutic baclofen level in the cerebrospinal fluid, compared to the patients who had effective spasticity control with an intrathecal baclofen pump pump 24. Although, our patient received oral baclofen (120 mg daily in four divided doses) initially but these doses may be low enough to prevent intrathecal baclofen pump withdrawal syndrome. Failure of high doses of oral baclofen suggests that resumption of GABA-B receptor agonist by prompt restoration of intrathecal baclofen pump pump and proper supportive care might be the best treatment. Similarly, high-dose benzodiazepines may be effective because of similar mechanism of action on widespread CNS GABA-A receptors. High-dose benzodiazepines could be an initial life saving strategy even before analysis and restoration of intrathecal baclofen pump is achieved, or in cases, where resumption of intrathecal baclofen pump administration is not as simple as correcting a programming error 16.
References- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A-S, McNamara JO, et al., editors. Neuroscience. 3rd edition. Sunderland, Mass, USA: Sinauer Publishers; 2004. Upper motor neuron control of the brainstem and spinal cord; pp. 414–415.
- Baclofen toxicity in an 8-year-old with an intrathecal baclofen pump. Yeh RN, Nypaver MM, Deegan TJ, Ayyangar R. J Emerg Med. 2004 Feb; 26(2):163-7.
- Clinical assessment and management of spasticity: a review. Rekand T. Acta Neurol Scand Suppl. 2010; (190):62-6.
- Awaad Y, Rizk T, Siddiqui I, Roosen N, McIntosh K, Waines GM. Complications of intrathecal baclofen pump: prevention and cure. ISRN Neurol. 2012;2012:575168. doi:10.5402/2012/575168 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3323842/
- Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain. Brennan PM, Whittle IR. Br J Neurosurg. 2008 Aug; 22(4):508-19.
- Awaad Y, Rizk T. Synergetic effect of intrathecal baclofen and deep brain stimulation in treating dystonia. Journal of Pediatric Neurology. 2011;9(2):221–225.
- Classification and definition of disorders causing hypertonia in childhood. Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW, Task Force on Childhood Motor Disorders. Pediatrics. 2003 Jan; 111(1):e89-97.
- Spasticity-assessment: a review. Biering-Sørensen F, Nielsen JB, Klinge K. Spinal Cord. 2006 Dec; 44(12):708-22.
- Spasticity: the misunderstood part of the upper motor neuron syndrome. Ivanhoe CB, Reistetter TA. Am J Phys Med Rehabil. 2004 Oct; 83(10 Suppl):S3-9.
- Ghanavatian S, Derian A. Baclofen. [Updated 2018 Dec 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526037
- Medical Advisory Secretariat. Intrathecal baclofen pump for spasticity: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(7):1–93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3382401/
- Nance P, Schryvers O, Schmidt B. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci. 1995;22:22–29
- Tunali Y, Hanimoglu H, Tanriverdi T, Hanci L, Hanci M. Intrathecal baclofen toxicity and deep coma in minutes. J Spinal Cord Med. 2006;29(3):237–239. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1864812/
- Penn RD. Intrathecal baclofen therapy. Op Tech Neurosurg. 2004;7:124–127.
- A prospective study of catheter-related complications of intrathecal drug delivery systems. Follett KA, Naumann CP. J Pain Symptom Manage. 2000 Mar; 19(3):209-15.
- Mohammed I, Hussain A. Intrathecal baclofen withdrawal syndrome- a life-threatening complication of baclofen pump: a case report. BMC Clin Pharmacol. 2004;4:6. Published 2004 Aug 9. doi:10.1186/1472-6904-4-6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC514562
- Kroin JS, Bianchi GD, Penn RD. Intrathecal baclofen down-regulates GABAB receptors in the rat substantia gelatinosa. J Neurosurg. 1993;79:544–549
- Green LB, Nelson VS. Death after acute withdrawal of intrathecal baclofen: case report and literature review. Arch Phys Med Rehabil. 1999;80:1600–1604. doi: 10.1016/S0003-9993(99)90337-4
- Meinck HM, Tronnier V, Rieke K, Wirtz CR, Flugel D, Schwab S. Intrathecal baclofen treatment for stiff-man syndrome: pump failure may be fatal. Neurology. 1994;44:2209–2210
- Sampathkumar P, Scanlon PD, Plevak DJ. Baclofen withdrawal presenting as multiorgan system failure. Anesth Analg. 1998;87:562–563. doi: 10.1097/00000539-199809000-00011
- Coffey RJ, Edgar TS, Francisco GE, Graziani V, Meythaler JM, Ridgely PM, Sadiq SA, Turner MS. Abrupt withdrawal from intrathecal baclofen: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83:735–741. doi: 10.1053/apmr.2002.32820
- Kao LW, Amin Y, Kirk MA, Turner MS. Intrathecal baclofen withdrawal mimicking sepsis. J Emerg Med. 2003;24:423–427. doi: 10.1016/S0736-4679(03)00039-8
- Al-Khodairy AT, Vuagnat H, Uebelhart D. Symptoms of recurrent intrathecal baclofen withdrawal resulting from drug delivery failure: a case report. Am J Phys Med Rehabil. 1999;78:272–277. doi: 10.1097/00002060-199905000-00018.
- Greenberg MI, Hendrickson RG. Baclofen withdrawal following removal of an intrathecal baclofen pump despite oral baclofen replacement. J Toxicol Clin Toxicol. 2003;41:83–85. doi: 10.1081/CLT-120018277