Butterflies in stomach
Butterflies in the stomach is the physical sensation in humans characterized by the physical sensation of an unpleasant “fluttery” or “tickling” (hence butterflies) feeling in the stomach. Some believe that this is caused by the release of epinephrine, or adrenaline, when one is nervous, pulling blood away from the stomach and sending it to the muscles 1. This in turn causes the stomach to temporarily shut down, possibly the reason for loss of appetite when one is “love sick” 2, 3.
It’s normal to feel nervous in some social situations. Butterflies in the stomach is most often experienced prior to important events, when stress is induced, but can be experienced in situations of impending danger. It can be a symptom of social anxiety disorder also called social phobia. For example, going on a date or giving a presentation may cause that feeling of butterflies in your stomach. But in social anxiety disorder, everyday interactions cause significant anxiety, fear, self-consciousness and embarrassment because you fear being scrutinized or judged by others.
In social anxiety disorder, fear and anxiety lead to avoidance that can disrupt your life. Severe stress can affect your daily routine, work, school or other activities.
Social anxiety disorder is a chronic mental health condition, but learning coping skills in psychotherapy and taking medications can help you gain confidence and improve your ability to interact with others.
Feelings of shyness or discomfort in certain situations aren’t necessarily signs of social anxiety disorder, particularly in children. Comfort levels in social situations vary, depending on personality traits and life experiences. Some people are naturally reserved and others are more outgoing.
In contrast to everyday nervousness, social anxiety disorder includes fear, anxiety and avoidance that interfere with daily routine, work, school or other activities. Social anxiety disorder typically begins in the early to mid-teens, though it can sometimes start in younger children or in adults.
Emotional and behavioral symptoms
Signs and symptoms of social anxiety disorder can include persistent:
- Fear of situations in which you may be judged
- Worrying about embarrassing or humiliating yourself
- Intense fear of interacting or talking with strangers
- Fear that others will notice that you look anxious
- Fear of physical symptoms that may cause you embarrassment, such as blushing, sweating, trembling or having a shaky voice
- Avoiding doing things or speaking to people out of fear of embarrassment
- Avoiding situations where you might be the center of attention
- Having anxiety in anticipation of a feared activity or event
- Enduring a social situation with intense fear or anxiety
- Spending time after a social situation analyzing your performance and identifying flaws in your interactions
- Expecting the worst possible consequences from a negative experience during a social situation
For children, anxiety about interacting with adults or peers may be shown by crying, having temper tantrums, clinging to parents or refusing to speak in social situations.
Performance type of social anxiety disorder is when you experience intense fear and anxiety only during speaking or performing in public, but not in other types of social situations.
Physical symptoms
Physical signs and symptoms can sometimes accompany social anxiety disorder and may include:
- Blushing
- Fast heartbeat
- Trembling
- Sweating
- Upset stomach or nausea
- Trouble catching your breath
- Dizziness or lightheadedness
- Feeling that your mind has gone blank
- Muscle tension
Avoiding common social situations
Common, everyday experiences that may be hard to endure when you have social anxiety disorder include, for example:
- Interacting with unfamiliar people or strangers
- Attending parties or social gatherings
- Going to work or school
- Starting conversations
- Making eye contact
- Dating
- Entering a room in which people are already seated
- Returning items to a store
- Eating in front of others
- Using a public restroom
Social anxiety disorder symptoms can change over time. They may flare up if you’re facing a lot of stress or demands. Although avoiding situations that produce anxiety may make you feel better in the short term, your anxiety is likely to continue over the long term if you don’t get treatment.
See your doctor or mental health professional if you fear and avoid normal social situations because they cause embarrassment, worry or panic.
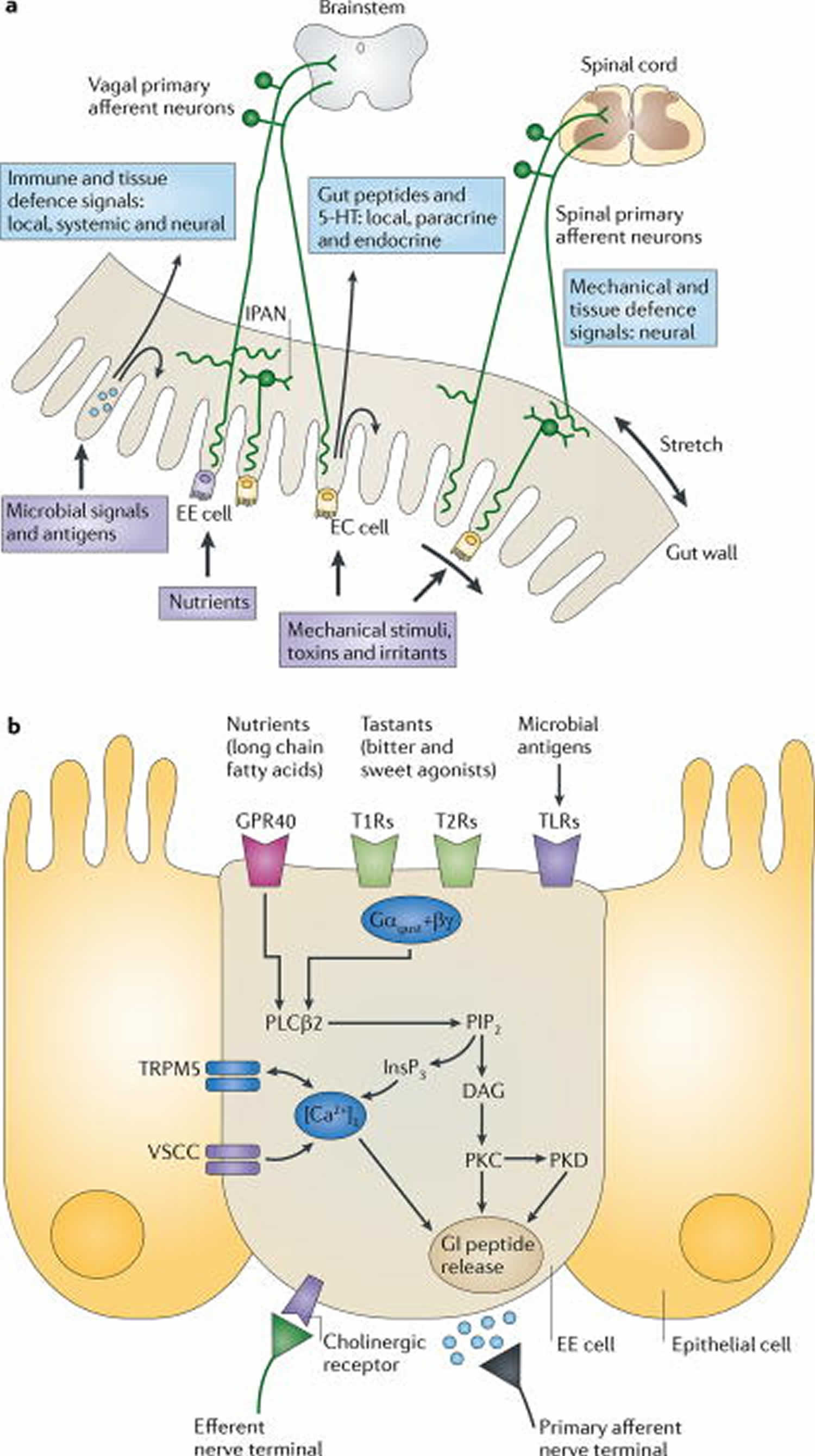
Figure 1. Gut to brain communication
Footnotes:
a) Endocrine, immune and neuronal afferent signalling from the gut to the CNS. Information about luminal factors and conditions of the gut are signalled through extrinsic vagal and spinal afferents to the brain stem and spinal cord, respectively. Mechanical stimuli (stretch, pressure, distortion and shearing forces) can activate spinal, vagal and intrinsic primary afferents (IPANs) directly, without intermediary cells such as the enteroendocrine (EE) cells. Although no synaptic connections have been found between intrinsic primary afferents (IPANs) and extrinsic afferents, the latter form networks around myenteric ganglia (intraganglionic laminar endings27), many of which receive synaptic input from intrinsic primary afferents (IPANs). Signalling molecules (including proteases, histamine, serotonin and cytokines) that are produced by immune cells in Peyer’s patches and within the gut epithelium can activate their respective receptors on vagal and spinal afferents. Similarly, neuropeptides and hormones (gut peptides) that are released from enteroendocrine (EE) cells in response to other luminal factors, such as nutrients, toxins or antigens, can act both in an endocrine fashion, reaching targets in the brain (area postrema, dorsal vagal complex and hypothalamus), and through receptor activation on spinal and vagal afferents, in a paracrine fashion. Enterochromaffin (EC) cells signal to both IPANs and vagal afferents.
b) Encoding of multiple luminal signals by enteroendocrine (EE) cells. Different classes of enteroendocrine (EE) cells are interspersed between gut epithelial cells throughout the gastrointestinal tract. Upon luminal stimulation (or upon activation by postganglionic sympathetic or vagal nerves), these cells can release up to 20 different gut peptides from their basolateral (and possibly luminal) surface. Released peptides can activate closely adjacent vagal afferent nerve terminals in a paracrine fashion, or when released into the circulation they can exert an endocrine effect, signalling to various sites in the brain and other parts of the gastrointestinal tract. Different types of receptors have been identified on the luminal side of enteroendocrine (EE) cells, including G protein-coupled taste receptors (GPCRs) for sweet and bitter tastants, GPCRs that are responsive to fatty acids and toll-like receptors (TLRs). The intestinal taste receptors that are shown are coupled to a specific Gα protein subunit, gustducin (Gαgust), and receptor-induced increases in intracellular calcium result in peptide release from the basolateral membrane.
Abbreviations: [Ca2+]i = intracellular calcium concentration; DAG = diacylglycerol; GI peptide = gastrointestinal peptide; GPR40 = G protein-coupled receptor 40; InsP3 = Inositol-1,4,5-trisphosphate;. PIP2 = aquaporin PIP2 member; PKC = protein kinase C; PLCβ2 = phospholipase Cβ; T1R = taste receptor type 1 member; TRPM5 = transient receptor potential cation channel subfamily M member 5 (specifically linked to taste receptor signalling); VSCC = voltage-sensitive Ca2+ channel.
[Source 4 ]Butterflies in stomach causes
The Brain-Gut axis describes a network of neurons that connect the human enteric nervous system (ENS) with the central nervous system (CNS) 5. The enteric nervous system consists of a series of neurons that exist within the gastrointestinal tract, and which govern visceral sensation and perception, as well as the secretion of various chemicals into our gut. Comparatively, the central nervous system consists of a wide range of neural networks and fibers that interact within higher cortical regions to govern our thoughts, feelings and emotions, as well as basic physiological processes such as those involved in homeostasis.
A major scientific breakthrough in understanding the interaction of the nervous system with the digestive system occurred with the discovery of the so-called enteric nervous system (ENS) in the middle of the nineteenth century 4. Even though it is now considered the third branch of the autonomic nervous system (ANS), the enteric nervous system has been referred to as the ‘second brain’, based on its size, complexity and similarity — in neurotransmitters and signalling molecules — with the brain 6. Even before the discovery of the enteric nervous system, the importance of interactions between the brain and the digestive system in health and disease has been recognized for many centuries, and has been studied by prominent psychologists, psychiatrists and physiologists during the later part of the nineteenth and earlier part of the twentienth centuries 7. Both top-down modulation of gastrointestinal function by stress and emotions 8, and bottom-up signalling from visceral afferents to the brain in abdominal pain syndromes, as well as possible emotion regulation 9 have been reported by early investigators. This topic has received increased attention during the past two decades, largely owing to a series of independent but converging scientific discoveries from various fields of research, including enteric neuroscience 10, neuroimaging 11, intestinal microbiology and host microbial interactions 12, and most recently, microbial gut–brain signalling 13.
The brain communicates to the internal organs (viscera), including the gastrointestinal tract, through multiple parallel pathways, including the two branches of the autonomic nervous system (autonomic nervous system), the hypothalamic–pituitary–adrenal (HPA) axis and the sympatho–adrenal axis (modulating the gut-associated lymphoid tissue), and descending monoaminergic pathways (modulating gain of spinal reflexes and dorsal horn excitability) 4. Two key subcortical structures that generate these outputs are the hypothalamus and the amygdala. They receive inputs from a network of cortical regions (the medial prefrontal cortex network) 14, including subregions of the medial prefrontal cortex and anterior cingulate cortex. Whereas ventral anterior cingulate cortex regions (for example, subgenual cingulate cortex and Brodmann area 25) project primarily to the medullary vagal complex, pregenual anterior cingulate cortex regions (for example, Brodmann area 24) project to the periaqueductal grey 15. The medial prefrontal cortex network receives input from a lateral prefrontal cortex and orbitofrontal cortex network that provides integrated multisensory information about the representation of complex homeostatic body states, including those related to gut homeostasis, food intake and visceral pain. Outputs from subregions of the medial network, amygdala and hypothalamus are integrated into distinct motor patterns within the mesencephalic periaqueductal grey 16. The most caudal component of the central autonomic networks is represented by pontine and medullary nuclei, including the serotonergic raphe nuclei, the locus coeruleus complex (including Barrington’s nucleus) and the dorsal vagal complex. This system of parallel outflows from cortico–limbic–pontine networks, which is engaged by distinct homeostatic states, has been referred to as the emotional motor system and consists of integrated motor autonomic, neuroendocrine and pain modulatory components 17. The medial component of the emotional motor system (including the midline raphe nuclei) is thought to have a role primarily in the tonic modulation through serotonergic, noradrenergic and opioidergic descending spinal pathways of the gain of various spinal reflexes involved in the regulation of gastrointestinal functions, as well as in the modulation of dorsal horn neuronal excitability, to regulate pain sensitivity. An example of the gut-related engagement of the medial component of the emotional motor system is the observation that food consumption inhibits pain-related behaviors in the rat, an analgesia mechanism related to the engagement of descending serotonergic pain modulation pathways 18. Descending opioid-dependent pain modulation pathways are also engaged by other behavioural and motivational states, including vigilance and fear 19. By contrast, the lateral system (including subregions of the periaqueductal grey, the central nucleus of the amygdala, bed nucleus of the striae terminalis, lateral hypothalamus and pontine locus coeruleus complex) may play a part in executing distinct regional motor patterns of the viscera mediated by the engagement of function-specific subsets of sympathetic and parasympathetic pathways.
Autonomic nervous system output can be triggered reflexively by ascending interoceptive signals from the gut, by descending cognitive or emotional influences or in response to external or internal demands. For example, top-down modulation can override local (for example, enteric nervous system-based) reflex function in the context of threats to body homeostasis (haemorrhagic shock), severe environmental stressors 20 or during strong emotions such as fear, anger and sadness 21.
Outputs from medullary pontine nuclei project to function-specific sympathetic preganglionics located in the intermediolateral column of the spinal cord, and projections to the medullary vagal complex and the pontine Barrington’s nucleus modulate function-specific vagal and sacral parasympathetic outflows, respectively 22. Preganglionic parasympathetic neurons are viscerotopically organized in the dorsal motor nucleus of the vagus and are modulated by a variety of hormonal and humoral substances 23.
Effects on the gut
The sympathetic innervation of the gastrointestinal tract and its role in the modulation of gastrointestinal function has been extensively reviewed 10. It has been divided into subclasses of postganglionic vasoconstrictor neurons, secretion inhibiting neurons and motility inhibiting neurons. The overall effect of sympathetic outflow to the gut is inhibitory, slowing gastrointestinal transit and secretion. This inhibitory effect is largely accomplished by inhibitory modulation of cholinergic transmission and by a stimulatory effect on smooth muscle in sphincteric regions. Another subset of sympathetic postganglionics is involved in mucosal immune modulation 24 and possibly in the modulation of interactions between the micro-flora and the mucosa 25. Although the best experimental evidence for such communication between the sympathetic nervous system, lymphocytes and macrophages comes from the spleen 26, evidence for sympathetically mediated immune modulation in the gut has been shown both in Peyer’s patches and in the non-follicular mucosa in close proximity to different classes of immune cells, including dendritic cells, mast cells and B lymphocytes 25. In addition, evidence of a noradrenaline-mediated reduction in the expression of toll-like receptors (TLRs) by intestinal epithelial cells has been reported 27. Finally, noradrenaline-mediated modulation of microbial virulence has been reported for a number of pathogens31, even though the mechanisms by which intraluminal bacteria are exposed to noradrenaline that is released from sympathetic nerve terminals is unclear.
The parasympathetic innervation of the gastrointestinal tract has been studied intensively 28. It is comprised of the vagal and sacral parasympathetic divisions, which innervate foregut and hindgut structures, respectively. Function-specific vagal motor neurons provide input to the stomach, small intestine and proximal portion of the colon. Excitatory vagal input occurs to ganglia within the ENS to mediate vago–vagal motor reflexes and the cephalic phase of gastric acid secretion, to gastrin- and somatostatin-containing enteroendocrine cells and histamine releasing enterochromaffin cells, and to enterochromaffin cells to mediate 5-hydroxytryptamine (5-HT) release 29. While vago–vagal reflexes are the primary neural mechanism to regulate gastric function, such extra intestinal reflexes play a lesser part (compared to intrinsic reflexes) in the modulation of intestinal function. Vagal modulation of macrophage activation through nicotinic acetylcholine receptors has been reported as part of a vago–vagal anti-inflammatory reflex 30.
Top-down engagement of subsets of function-specific postganglionic sympathetic and parasympathetic neurons are likely to mediate reported emotion-related patterns of regional changes in motor, secretory and possibly immune activity in the gastrointestinal tract 31, which may be viewed as analogous to distinct emotion-related facial expressions and body postures (mediated by the somatic branch of the emotional motor system). These emotion-related changes in peripheral target cells will directly or indirectly (for example, through changes in smooth muscle activity) influence the interoceptive feedback to the brain, possibly contributing to the characteristic prolonged duration of many emotional states, which can outlast the initiating event by hours. Prolonged alterations in ANS output to the gut is likely to induce changes in peripheral target cells — such as downregulation of adrenergic receptors on immune cells 32, reduced expression of toll-like receptors 27 on epithelial cells or changes in primary afferent neurons 33 — changing the gain of gut to brain signalling chronically and possibly resulting in the remodelling of brain regions that receive this enhanced input 34. Such lasting changes in brain–gut signalling may be associated with tonic autonomic nervous system dysfunction, which is in turn associated with altered emotional states such as anxiety disorders or depression.
The link between the brain and the gut is thought to play a major role in a group of disorders known as the functional gastrointestinal disorders. These disorders include a variety of gastrointestinal symptoms that have no single unifying patho-physiological cause, and which are associated with various psychological symptoms including anxiety, low mood, and heightened visceral sensation.
Arguably the most common functional gastrointestinal disorder is Irritable Bowel Syndrome (IBS) 35. IBS is a disorder involving chronic abdominal pain and discomfort, and altered defecation habits including diarrhea and constipation. Patients with IBS often have a heightened sensitivity to visceral sensations and are more likely to perceive stomach pain than those without the disorder. Further, the role of psychological processes such as stress and anxiety is such that these symptoms tend to exacerbate symptoms of IBS. Patients with this disorder are often immensely frustrated at the prospect of being diagnosed with a disorder that has no specific physical cause.
The Link between Stress, Anxiety, and Gut Sensation
Returning back to the example of butterflies in the stomach before giving a presentation — let us explore what is happening at the biological and psychological levels here. In response to stress, the brain releases the stress hormone known as cortisol; the release of which is a hallmark of sympathetic nervous system activation, which characterizes our bodies “fight-flight-freeze” response. In response to the release of cortisol, activity within our gut mucosa changes, which corresponds with alterations in the normal speed of digestion once the sympathetic nervous system is activated. These changes in gut mucosa activity make us more vulnerable to experiencing pain and discomfort, and this heightened sensitivity normally recedes once the initial psychological stressor disappears and the body’s parasympathetic relaxation response kicks in.
For individuals who experience stress and anxiety at a chronic and clinical level, however, the sympathetic nervous system is constantly active and thus the gut undergoes various neuro-endocrine changes which make the gut mucosa more reactive to stress, and thus more sensitive to the transmission of pain signals to the brain in response to normal visceral sensation. This in part explains why functional gastrointestinal disorders such as Irritable Bowel Syndrome (IBS) are so commonly co-morbid with psychological disorders such as generalized anxiety disorder and major depressive disorder 36.
Remediation with antidepressants and cognitive behavioral therapy
Due to the interplay between psychological and physiological factors in brain-gut disorders such as IBS, researchers have aimed to treat these disorders with therapies that are typically used for mental health concerns. Indeed, cognitive-behavioral therapy; which is a common and evidence-based psychological therapy, is effective in reducing symptom distress and symptom severity in patients with IBS and other functional gastrointestinal disorders. This corresponds with the relative efficacy afforded by antidepressants such as the selective serotonin reuptake inhibitors. Thus changes in psychological activity in patients with IBS and associated disorders appear to correspond with changes in gut activity, and the heightened sensations that are associated with this activity.
References- Neurotic butterflies in my stomach: the role of anxiety, anxiety sensitivity and depression in functional gastrointestinal disorders. J Psychosom Res. 1999 Sep;47(3):233-40. https://doi.org/10.1016/S0022-3999(99)00032-X
- Sailer, Christian, Wasner, Susanne. Differential Diagnosis Pocket. Hermosa Beach, CA: Borm Bruckmeir Publishing LLC, 2002:77 ISBN 1591032016
- Kahan, Scott, Smith, Ellen G. In A Page: Signs and Symptoms. Malden, Massachusetts: Blackwell Publishing, 2004:68 ISBN 140510368X
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. Published 2011 Jul 13. doi:10.1038/nrn3071 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845678
- Mayer, E. A., & Tillisch, K. (2011). The brain-gut axis in abdominal pain syndromes. Annual review of medicine, 62.
- Gershon MD. The Second Brain. Harper Collins; New York: 1998.
- Mayer EA, Brunnhuber S. Gastrointestinal disorders. Handb Clin Neurol. 2012;106:607-31. doi: 10.1016/B978-0-444-52002-9.00036-X. https://doi.org/10.1016/B978-0-444-52002-9.00036-X
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431.
- James W. What is an emotion? Mind. 1884;9:188–205.
- Furness JB. The Enteric Nervous System. Blackwell; Oxford: 2006. A comprehensive overview of all aspects of the enteric nervous system.
- Mayer EA, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596.
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Rev Immunol. 2009;9:313–323.
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16.
- Ongur D, Price JL. The organization of networks within the orbital and medical prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219.
- Vogt BA. In: The Cerebral Cortex. Jones EG, Peters A, editors. Plenum Press; New York: 1985. pp. 89–149.
- Bandler R, Keay KA. In: The Emotional Motor System. Progress in Brain Research. Holstege G, Bandler R, Saper CB, editors. Elsevier; Amsterdam: 1996. pp. 285–300.
- Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869.
- Mason P. From descending pain modulation to obesity via the medullary raphe. Pain. 2010;152:S20–S24.
- Fields H. State-dependent opioid control of pain. Nature Rev Neurosci. 2004;5:565–575.
- Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088.
- Welgan P, Meshkinpour H, Ma L. Role of anger in antral motor activity in irritable bowel syndrome. Dig Dis Sci. 2000;45:248–251.
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunction. Trends in Pharmacol Sci. 1999;20:253–260.
- Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13.
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:585–638.
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2010;343:23–32. A comprehensive overview of evidence for interactions between peripheral stress mediators and bidirectional interactions of the mucosa and intestinal microbiota.
- Hori T, Katafuchi T, Take S, Shimizu N, Niijima A. The autonomic nervous system as a communication channel between the brain and the immune system. Neuroimmunomodulation. 1995;2:203–215.
- Gopal R, Birdsell D, Monroy FP. Regulation of toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 2008;30:563–576.
- Powley TL, et al. In: Brain–Gut Interactions. Tache Y, Wingate D, editors. CRC Press; Boston: 1991. pp. 73–82.
- Stephens RL, Tache Y. Intracisternal injection of a TRH analogue stimulates gastric luminal serotonin release in rats. Am J Physiol. 1989;256:G377–G383.
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499.
- Welgan P, Meshkinpour H, Beeler P. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology. 1988;94:1150–1156.
- Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann NY Acad Sci. 2002;966:290–303.
- Khasar SG, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. The first evidence that psychosocial stressors can modulate the phenotype of afferent neurons, providing the possible mechanisms for stress induced hyperalgesia.
- Seminowicz DA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57.
- Lee, S., Wu, J., Ma, Y. L., Tsang, A., GUO, W. J., & Sung, J. (2009). Irritable bowel syndrome is strongly associated with generalized anxiety disorder: a community study. Alimentary pharmacology & therapeutics,30(6), 643-651.
- Drossman, D. A. (2006). The functional gastrointestinal disorders and the Rome III process. gastroenterology, 130(5), 1377-1390.