What is cholecystokinin
Cholecystokinin also known as CCK or CCK-PZ, is a peptide hormone of about 33 amino acids, secreted by the enteroendocrine cells in the duodenum (the first portion of the small intestine) and also found in the central nervous system, specifically in the hippocampus, cerebral cortex, and striatum 1. Cholecystokinin receptors are present in the nucleus of tractus solitarius and area postrema of the lower portion of the brain stem 2. Cholecystokinin (CCK) is found throughout the small intestine but is located predominantly in the mucosal I cells of the duodenum and jejunum. In the ileum and colon, it is localized in nerve endings, and it is widely distributed throughout the peripheral and central nervous systems. Cholecystokinin causes gallbladder contraction leading to the release of bile and release of pancreatic exocrine (or digestive) enzymes for the digestion of fat and protein and affects other gastrointestinal functions. Cholecystokinin may also act as an appetite suppressant and has been studied for weight management regimens. Normally, cholecystokinin is an endogenous hormone but is available commercially for diagnostic processes and replacement in pancreatic insufficiency in the octapeptide form. Cholecystokinin is one of the first gastrointestinal hormones discovered, identified more than 90 years ago due to its ability to stimulate gallbladder contraction in 1928. Soon after, cholecystokinin was recognized to be identical to the factor responsible for stimulating pancreatic exocrine secretion in 1943. Cholecystokinin hormone has also been shown to have positive effects on enteric smooth muscle contraction and on nerve activity at multiple locations in the peripheral and central nervous system 3. In addition to its roles in promoting smooth muscle cell contraction/exocrine cell secretion, cholecystokinin promotes cell growth, energy production, gene expression and protein synthesis, processes that have profound for drug development. Cholecystokinin drug has also been investigated for possible antipsychotic properties, owing to its effect on cholecystokinin receptors in the brain. Recent studies have suggested that cholecystokinin also plays a major role in inducing drug tolerance to opioids such as morphine and heroin, and is partially implicated in experiences of pain hypersensitivity during opioid withdrawal.
The specific affinity of membrane receptors on target cells determines the action of cholecystokinin and gastrin. CCK-1 and 2 are part of the class 1 G-protein-coupled receptor family. It is made up of seven transmembrane domains connected by intracellular and extracellular loops with an extracellular N-terminal and intracellular C-terminal tails. They divide these receptors into subtypes based on their affinity to cholecystokinin or gastrin. The CCK-1 receptor has an affinity 500-times higher for cholecystokinin than gastrin. The CCK2 receptor has the same affinity for cholecystokinin as it does for gastrin. CCK-1 receptor also has a very high affinity for sulfated cholecystokinin than non-sulfated cholecystokinin while CCK-2 receptor cannot differentiate between the 2. One of the extracellular loops of CCK-2 receptor contains 5 amino acids paramount to gastrin sensitivity. Loss of His207 from CCK-2 receptor leads to a loss of cholecystokinin binding. His207 is also found in the CCK-1 receptor binding site, and an exchange in this region for another amino acid and the Asp region of cholecystokinin leads to a loss or gain of affinity. Therefore, the binding sites of CCK-1 and CCK-2 receptors share some homologous regions and the Asp of cholecystokinin further demarcates the binding site of the CCK-2 receptor 1.
Where is cholecystokinin produced?
Levels of cholecystokinin-producing neurons in the brain are low at birth but steadily increase into adulthood. Low levels of cholecystokinin are found in the thyroid C cells, adrenal medulla, bronchial mucosa, pituitary corticotrophs, and spermatogenic cells. CCK-1 and 2 are part of class 1 G-protein-coupled receptor family. CCK1R are found in the gallbladder smooth muscles, chief and D cells of gastric mucosa, pancreatic acinar cells, and selected areas of central and peripheral nervous systems while CCK2R/GR are identified in the stomach (in the parietal, chief, and ECL cell of gastric mucosa), human pancreas and central nervous system (CNS) 4.
In peripheral neurons such as those found in the intestinal mucosa, they express cholecystokinin in I-cells. Food intake, pituitary adenylate cyclase-activating polypeptide (PACAP), and glucocorticoids play a role in regulating cholecystokinin expression. Estrogen, dopamine, and injury situations activate cholecystokinin-mRNA in neuronal cells. Activation of transcription factors of these signaling pathways is unknown. Receptors in the CNS are G protein-coupled; CCK-2 receptor subtype (formerly known as CCK-B) are found mainly in the brain, and CCK-1 receptor subtype (formerly known as CCK-A) found mainly peripherally, for example, in the pancreas. Adenylyl cyclase is activated inducing a rise in intracellular cyclic adenosine 3′, 5′-monophosphate (cAMP) and the activation of protein kinase A (PKA). They associate this rise in cAMP with cholecystokinin-receptor-mediated pathway with high concentrations of cholecystokinin. cAMP-dependent protein kinase activity is cholecystokinin concentration dependent. PKA activity peaks rapidly and maintains a high level of activity at high doses of cholecystokinin 5. Cholecystokinin receptors found on pancreatic acinar cells have two types of affinity to cholecystokinin which elicits different responses. At low concentrations, it stimulates zymogen secretion while at high concentrations cholecystokinin inhibits stimulation and secretion of intracellular zymogen proteolysis 5. Binding sites of CCK-1 and CCK-2 receptors have different affinities for various cholecystokinin neuropeptide fragments. CCK-A binding has a higher affinity for sulfated, intermediate, neuropeptide CCK-8 than CCK-4; therefore, most of its action is peripheral. CCK-2R has a high affinity for all cholecystokinin fragments with a higher affinity for CCK-4, alluding to the fact that most of cholecystokinin action is on the brain. Stimulation of neuronal pathways containing cholecystokinin can amount to a panic-like reaction in humans. Panic attacks could arise from the activation of cholecystokinin. cholecystokinin antagonists can provide anxiolytic properties via their action on CCK-2 receptors 6.
What is the function of cholecystokinin
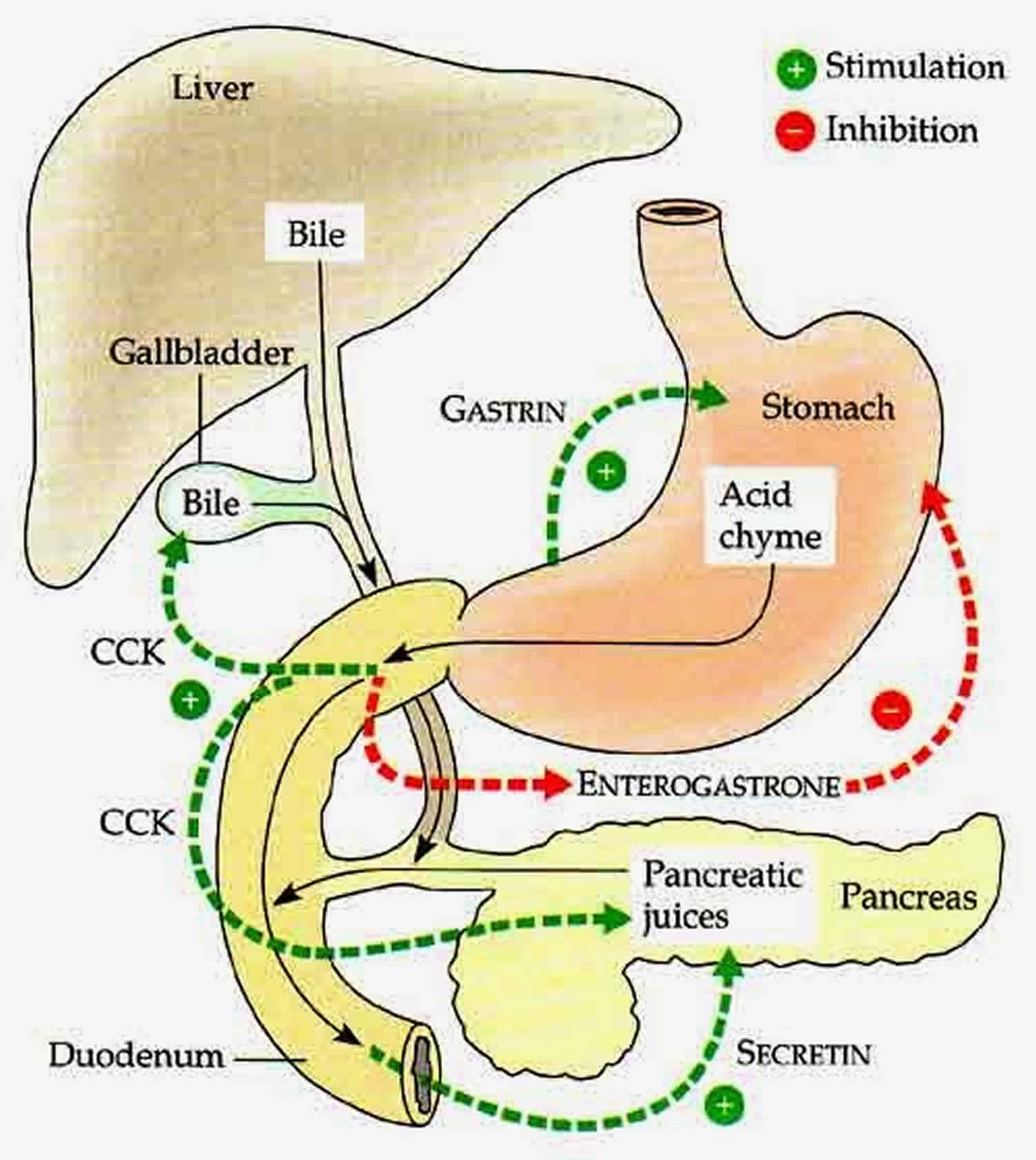
Cholecystokinin makes the gallbladder contract (squeeze together). Cholecystokinin also makes the pancreas produce enzymes, which are some of the juices needed for the digestion of food. In addition, cholecystokinin increases the movements or contractions of the stomach and intestines.
In the Intestine
- Cholecystokinin mediates digestion by regulating the release of pancreatic exocrine enzymes which plays a role in the digestion of fats, proteins, and carbohydrates
- Cholecystokinin causes contraction and relaxation of the gallbladder via the sphincter of Oddi in response to food; cholecystokinin regulates the release of bile acid to aid in further fat digestion in the small intestine
- Cholecystokinin regulates overall gastrointestinal movement, in other words, gut motility 7
- Cholecystokinin regulates gastric emptying: It inhibits gastric emptying to regulate the flow of chyme into the duodenum 8
- Cholecystokinin inhibits gastric acid secretion after a meal by regulating gastrin production via somatostatin 9
- Cholecystokinin enhances the release of leptin which inhibits basal gastric H+ secretion after a meal. In the intestine, it promotes further absorption of proteins 10.
- Cholecystokinin stimulates of cell growth 10.
- Cholecystokinin stimulates energy production
- Cholecystokinin stimulates gene expression
- Cholecystokinin stimulates protein synthesis
In the Brain
- Cholecystokinin regulates feeding behavior: Leptin acts on the brain to inhibit food intake resulting in satiety 10.
- Cholecystokinin manages anxiety
- Cholecystokinin regulates pain perception
- Cholecystokinin regulates memory
Fatty acids and proteins stimulate the release of cholecystokinin via a direct action on the I-cells. GPR40 is a G-protein-coupled receptor expressed on I-cells that responds to long-chain fatty acids. Discharge of vagal efferent neurons is stimulated by the action of cholecystokinin on CCK-1 receptors and increase intracellular calcium. These neurons are found in both the stomach and small intestines, and cholecystokinin initially activates the afferent fibers in the small intestine via a paracrine mechanism. This inhibits the excitatory vagal efferent pathway to the distal stomach. Gastric vagal afferents are stimulated in response to the hormonal effect coupled with the inhibitory vagal efferent pathway to the proximal stomach 11. Due to the mechanism mentioned above, cholecystokinin can inhibit gastric emptying by relaxing the proximal portion of the stomach, which increases tension in the pyloric sphincter. At high levels of cholecystokinin can increase the effect of how fast gastric emptying occurs, and it does this by increasing the excitatory effect it has on both the small and large intestine, which leads to movement in the bowels or by improving the tension of the pyloric sphincter 12. Therefore, the reflex control of gastric emptying is regulated by cholecystokinin action on vasovagal reflexes and the hormonal activation of a variety of pathways that are coupled to vagal efferent pathways controlling gastric motility.
Gallbladder dysfunction testing
Gallbladder dysfunction is defined as an abnormally low gallbladder ejection fraction 13. Gallbladder disease can manifest as bladder dyskinesia, chronic acalculous cholecystitis, biliary dyskinesia, and functional gallbladder disorder, among others. Cholecystokinin scintigraphy (CCK-HIDA) assesses gallbladder ejection fraction and is used to test patients presenting with chronic upper abdominal pain together with a normal upper abdominal ultrasonography 14. Tc-99m-labeled HIDA collects in the gallbladder after it is absorbed by the liver and excreted by the biliary system. Cholecystokinin is injected to stimulate gallbladder contraction to calculate the gallbladder ejection fraction 13.
Role of cholecystokinin
Obesity blunts the effect of cholecystokinin, which means there is insensitivity of vagal afferent neurons to cholecystokinin. This reduced expression of cholecystokinin accounts for reduced effect on satiety and the fact that most obese people always complain about feeling hungry. Consumption of high-fat diets with diminished expression of the CCK-1 receptor increases the levels of ghrelin in plasma. This increases food intake, and it does this by suppressing the expression of satiety peptide cocaine and amphetamine-regulated transcript (CART) in vagal afferent neurons. cholecystokinin is also involved in metabolic regulation and lipid absorption. Linking inactivation of the cholecystokinin signaling pathway to reduced weight gain. Inactivation increases energy expenditure and lowers energy extraction 15.
Cholecystokinin acts via the vasovagal pathway and is activated peripherally via gastric wall distension. Doctors use intragastric balloons in clinical practice to treat weight loss by mimicking this pathway. By distending the stomach it activates the vagal nerve and the nucleus of tractus solitarius and the paraventricular nucleus, leading to a centrally mediated feeling of satiety 5. These devices physically reduce food intake by obstructing the outlet, delaying gastric emptying and physically reducing the capacity of the stomach. Pancreatic peptide (PP) secretion is impaired due to decreased gastric emptying which leads to reduced gut wall interactions with nutrients such as fat and protein which elicit a pancreatic peptide response. Pancreatic peptide (PP) secretion is biphasic, and food and secretion of cholecystokinin control the second phase 15.
Cholecystokinin plays a minor role in the release of incretin as compared to glucagon-like peptide (GLP-1) from islet cells. Decreased size of islet cells and mass of beta-cells correlates with upregulation of cholecystokinin expression and increased sensitivity to cholecystokinin for insulin release in obesity. It, therefore, can mediate compensatory mechanisms within the islets of Langerhans 15. Fat and energy restricted meals stimulate cholecystokinin and pancreatic peptide secretion and together with delayed gastric emptying secondary to the intragastric balloon can cause extensive weight loss and improved glucose homeostasis 5. Presence of cholecystokinin in various regions of the midbrain suggests it plays a role in behavioral processes such and anxiety. Panic disorder is described as the feeling of unprovoked fear and an overwhelming feeling of anxiety. People with panic disorder or those that experience panic attacks present to the emergency department feeling a sense of impending doom, chest pain, abdominal pain, sometimes even shortness of breath. Scientists know that cholecystokinin is expressed in the nucleus of tractus solitarius and area postrema. They associate these areas with nociception and patients with Parkinson disease are usually sensitive with bodily sensations. Noradrenaline and serotonin (5-HT) containing nuclei found in the brainstem interact with these areas as well implicating then to the pathogenesis of Parkinson disease 7.
Cancers of the gastrointestinal tract including medullary thyroid cancer, small cell lung cancer all express gastrin and CCK-2 receptors. Gastrin and CCK2R/gastrin receptor (GR) play a role in regulating cellular proliferation, loss of cell-cell adhesion, differentiation and morphology and the enhanced motility/invasion of epithelial cells. The activation of the CCK2R/GR via the intracellular signaling pathway can lead to carcinogenesis. These receptors, therefore, play a crucial role in starting the events leading up to preneoplastic lesions and cancer development 16.
References- Beinfeld MC. An introduction to neuronal cholecystokinin. Peptides. 2001 Aug;22(8):1197-200.
- Okonkwo O, Adeyinka A. Biochemistry, Cholecystokinin (CCK) [Updated 2019 Jan 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534204
- Ma J, Dankulich-Nagrudny L, Lowe G. Cholecystokinin: an excitatory modulator of mitral/tufted cells in the mouse olfactory bulb. PLoS ONE. 2013;8(5):e64170
- Beinfeld MC. An introduction to neuronal cholecystokinin. Peptides. 2001 Aug;22(8):1197-200
- Marino CR, Leach SD, Schaefer JF, Miller LJ, Gorelick FS. Characterization of cAMP-dependent protein kinase activation by CCK in rat pancreas. FEBS Lett. 1993 Jan 18;316(1):48-52.
- van Megen HJ, den Boer JA, Westenberg HG. On the significance of cholecystokinin receptors in panic disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1994 Dec;18(8):1235-46.
- Cao SG, Wu H, Cai ZZ. Dose-dependent effect of ghrelin on gastric emptying in rats and the related mechanism of action. Kaohsiung J. Med. Sci. 2016 Mar;32(3):113-7.
- Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J. Nutr. 1994 Aug;124(8 Suppl):1334S-1339S.
- Burckhardt B, Delco F, Ensinck JW, Meier R, Bauerfeind P, Aufderhaar U, Ketterer S, Gyr K, Beglinger C. Cholecystokinin is a physiological regulator of gastric acid secretion in man. Eur. J. Clin. Invest. 1994 Jun;24(6):370-6.
- Konturek JW, Konturek SJ, Kwiecień N, Bielański W, Pawlik T, Rembiasz K, Domschke W. Leptin in the control of gastric secretion and gut hormones in humans infected with Helicobacter pylori. Scand. J. Gastroenterol. 2001 Nov;36(11):1148-54
- Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012 Feb;19(1):8-12.
- Cao SG, Wu H, Cai ZZ. Dose-dependent effect of ghrelin on gastric emptying in rats and the related mechanism of action. Kaohsiung J. Med. Sci. 2016 Mar;32(3):113-7
- Richmond BK, DiBaise J, Ziessman H. Utilization of cholecystokinin cholescintigraphy in clinical practice. J. Am. Coll. Surg. 2013 Aug;217(2):317-23.
- Watson A, Better N, Kalff V, Nottle P, Scelwyn M, Kelly MJ. Cholecystokinin (CCK)-HIDA scintigraphy in patients with suspected gall-bladder dysfunction. Australas Radiol. 1994 Feb;38(1):30-3.
- Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J. Nutr. 1994 Aug;124(8 Suppl):1334S-1339S
- Rai R, Chandra V, Tewari M, Kumar M, Shukla HS. Cholecystokinin and gastrin receptors targeting in gastrointestinal cancer. Surg Oncol. 2012 Dec;21(4):281-92.