Chorioamnionitis
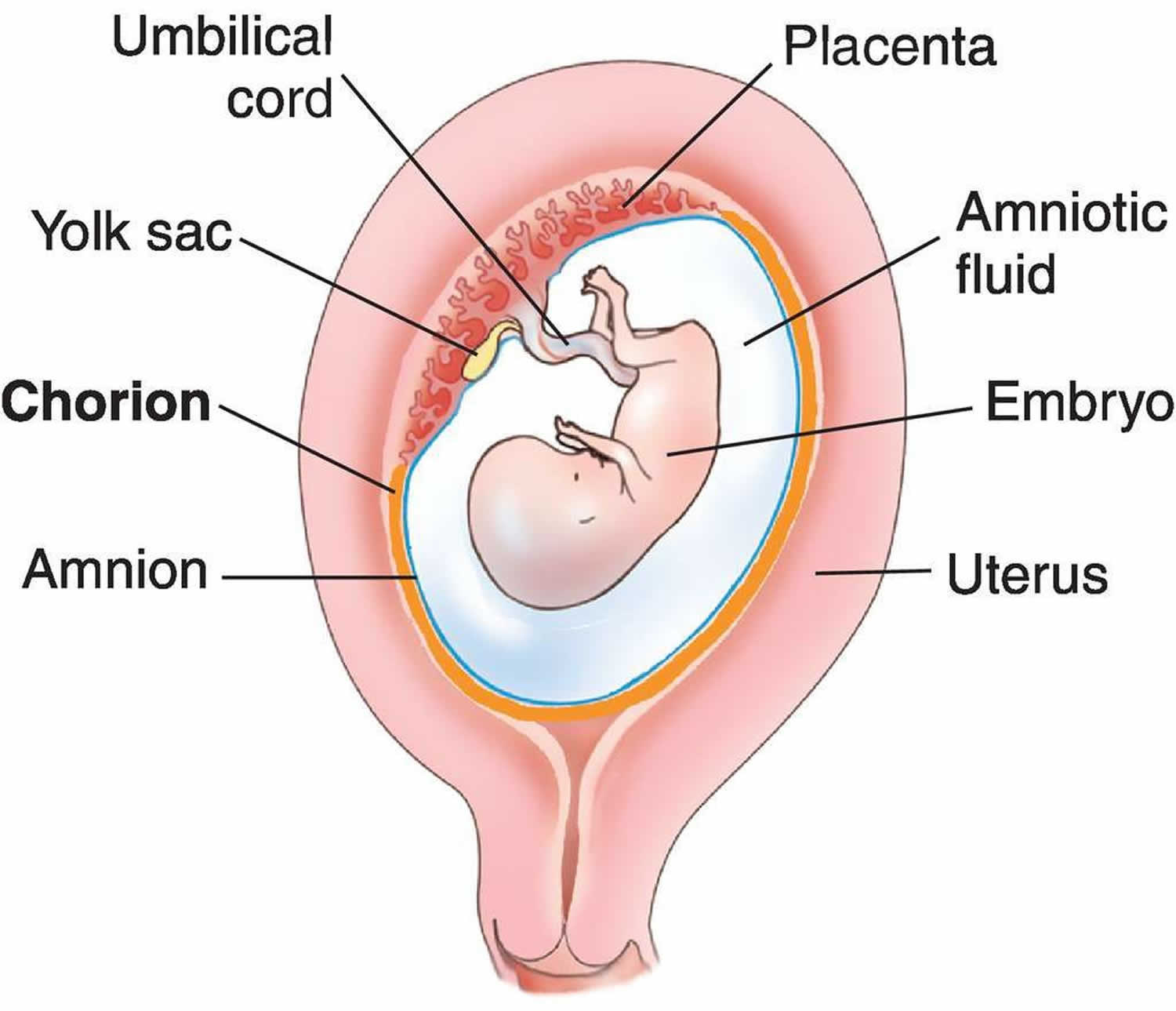
Chorioamnionitis is an intraamniotic inflammation or infection of the amniotic fluid, placenta, fetus, fetal membranes (amnion and chorion membranes) that surround the amniotic fluid and decidua, which can occur before labor, during labor, or after delivery 1. Recently, some authors have suggested changing the name of this condition to “intraamniotic infection and inflammation” to more accurately reflect the full spectrum of the disease process 2. Chorioamnionitis is a complication of pregnancy and can be acute, subacute, or chronic. In general, the clinical presentation of chorioamnionitis is defined as acute chorioamnionitis 3. Subacute chorioamnionitis is associated with chronic lung disease in the infant 4. Chronic chorioamnionitis is associated with retinopathy of prematurity, very low birth weight, and impaired brain development in the premature infant. Chronic chorioamnionitis is common 5. This terminology refers to histologic chorioamnionitis. Histologic chorioamnionitis at term is rarely infectious. In histologic chorioamnionitis, symptoms may be absent, and the placenta or cultures may not show evidence of chorioamnionitis 3.
The National Institute of Child Health and Human Development workshop recommended using the term “triple I” to address the heterogeneity of chorioamnionitis 2. The term “triple I” refers to intrauterine infection or inflammation or both, and it is defined by strict diagnostic criteria; however, this terminology has not been universally accepted 6. It is important to differentiate between clinical and histologic chorioamnionitis; the latter tend to be “silent” and present only with preterm labor or preterm premature rupture of membranes (PPROM). The risk of neonatal sepsis is increased when chorioamnionitis is diagnosed in the laboring mother; however, the risk is much lower than anticipated based on historical figures when widespread use of intrapartum antibiotics was not a common practice 7.
Chorioamnionitis often is polymicrobial in origin, commonly involves aerobic and anaerobic bacteria, and frequently originates from the vaginal flora 8. It predominantly occurs by ascending bacterial invasion from the lower genital tract to the typically sterile amniotic cavity. Chorioamnionitis also can occur, although rarely, after invasive procedures (eg, amniocentesis or chorionic villus sampling) or by a hematogenous route secondary to maternal systemic infection (eg, Listeria monocytogenes). Estimates suggest that approximately 2–5% of term deliveries are complicated by a clinically apparent chorioamnionitis 9. More recent data suggest that the relative risk for chorioamnionitis and neonatal infection may increase after 40 completed weeks of gestation 10. Chorioamnionitis may be identified postdelivery or postmortem on a pathologic review of the placenta and cord.
Most commonly, chorioamnionitis is associated with preterm labor, prolonged rupture of membranes, prolonged labor, tobacco use, nulliparous pregnancy, meconium stained fluid, multiple vaginal exams post rupture of membranes, and in women with known bacterial or viral infections 3. However, it can occur at term and in women without prior infections. Left untreated, chorioamnionitis can lead to morbidity and mortality for the mother and neonate. Neonatal morbidity and mortality increase in severity and occurrence with earlier gestations. Antibiotic therapy has been shown to reduce the incidence and severity of the infection in both the mother and neonate. However, antibiotics do not eradicate the infection in all cases.
Chorioamnionitis occurs in about 4% of deliveries at term but occurs more frequently in preterm deliveries and premature rupture of membranes. In evaluating women with symptoms of chorioamnionitis, studies show a strong correlation between histologic chorioamnionitis and the key clinical symptoms of fever, uterine tenderness, meconium aspiration syndrome, and foul-smelling vaginal discharge 11. Histologic chorioamnionitis with vasculitis is associated with a higher incidence of premature rupture of membranes and preterm delivery 12.
In deliveries between 21 and 24 weeks gestation, chorioamnionitis can be found in more than 94% of the placentas on evaluation 13. Term deliveries of mothers with chorioamnionitis are associated with failure to progress. Chorioamnionitis in preterm labor is likely to end in preterm delivery. Studies show that inflammation of the placenta or chorioamnionitis can be found in approximately 8% to 50% of preterm deliveries 14. In the term pregnancy, chorioamnionitis is most likely associated with labor and a history of prolonged ruptured membranes.
Chorioamnionitis can be associated with acute neonatal morbidity, including neonatal pneumonia, meningitis, sepsis, and death 15. The use of intrapartum antibiotic treatment given either in response to maternal group B streptococcal colonization or in response to evolving signs of chorioamnionitis during labor has been associated with a nearly 10-fold decrease in group B streptococcal-specific neonatal sepsis 16. Decreases in non-group B streptococcal neonatal infections also have been noted 17. The protective effect of maternal intrapartum antibiotic administration has been demonstrated in recent multivariate risk models of individual infant risk of neonatal sepsis 10.
Chorioamnionitis can be associated with long-term complications for the infant, such as bronchopulmonary dysplasia and cerebral palsy 18, potentially due to the effect of inflammation alone. A recent meta-analysis of 15 studies found a significantly higher relative risk of cerebral palsy among primarily premature infants exposed to either histologic chorioamnionitis (odds ratio 1.8) or clinical chorioamnionitis (odds ratio 2.4) 19. It is nonetheless important to acknowledge that the overall absolute risk of cerebral palsy remains quite low (approximately 2 per 1,000 live births) 20.
Maternal morbidity from chorioamnionitis also can be significant, and may include dysfunctional labor requiring increased intervention, postpartum uterine atony with hemorrhage, endometritis, peritonitis, sepsis, adult respiratory distress syndrome and, rarely, death 21.
Figure 1. Chorion placenta anatomy
Chorioamnionitis causes
Chorioamnionitis is an ascending infection, originating in the lower genitourinary tract and migrating to the amniotic cavity. The infection usually originates from the cervical and vaginal area. Vertical transmission has been documented in bacterial and viral infections transmitted to the fetus 22. The medical literature defines chorioamnionitis as an inflammatory and infectious process. Inflammation in utero is linked to preterm birth, brain abnormalities, and retinopathy 12. Infection can be due to a bacterial, fungal, or viral agent. Bacterial agents in chorioamnionitis can vary depending on the geographic location and population. Common bacterial agents found in chorioamnionitis include group B streptococcus, Mycoplasma pneumoniae 22, Ureaplasma, Gardnerella vaginalis, Escherichia coli, and Bacteroides. Candida species are identified as risk factors for chorioamnionitis, leading to preterm birth and adverse fetal outcomes 23. In adolescents with sexually transmitted infections, studies show that trichomoniasis is a risk factor for the development of chorioamnionitis. Although chorioamnionitis is a risk factor for vertical transmission in pregnancy, the incidence of chorioamnionitis in HIV-positive versus HIV-negative women is not significantly different during labor. In one study of 298 women with similar risk factors and demographics, both groups of women had a high incidence of chorioamnionitis. The higher incidence for each group was strongly associated with the number of vaginal exams during labor 24.
Chorioamnionitis symptoms
The characteristic clinical signs and symptoms of chorioamnionitis include the following:
- Maternal fever (intrapartum temperature >100.4°F or >38.0°C) 25.
- Other observed signs include the following 2:
- Baseline fetal tachycardia (>160 beats per min for 10 min or longer, excluding accelerations, decelerations, and periods of marked variability)
- Maternal leukocytosis (total blood leukocyte count >15,000 cells/μL) in the absence of corticosteroids
- Definite purulent fluid from the cervical os
Other nonspecific signs such as maternal tachycardia and uterine tenderness are deemphasized by a report from a workshop conducted by the National Institute of Child Health and Human Development 2.
Chorioamnionitis complications
Neonatal complications of chorioamnionitis include premature birth, cerebral palsy, retinopathy of prematurity, neurologic abnormalities, respiratory distress syndrome, bronchopulmonary dysplasia in premature infants, neonatal sepsis, and neonatal death. Neonatal sepsis is suspected as a complication of chorioamnionitis; however, in more than 99% of cases, cultures are negative. Perinatal listeriosis is associated with high morbidity. Current antibiotic regimens may not cover listeriosis in chorioamnionitis.
Maternal complications of chorioamnionitis include severe pelvic infections, subcutaneous wound infections, preterm delivery, postpartum hemorrhage, operative delivery, and maternal sepsis.
Chorioamnionitis is associated with vertical transmission of HIV in pregnancy 26.
Chorioamnionitis diagnosis
The diagnosis of clinical chorioamnionitis in pregnancy is commonly made based on clinical findings of fever plus fetal tachycardia, maternal leukocytosis, or purulent fluid coming from the cervical os. Additionally, the pregnant woman with chorioamnionitis may appear ill, even toxic, and she may exhibit hypotension, diaphoresis, and/or cool or clammy skin. However, especially when dealing with histologic chorioamnionitis, maternal clinical signs or symptoms of infection may be absent (silent chorioamnionitis) 27.
Furthermore, clinical signs and symptoms of chorioamnionitis are not always associated with placental evidence of inflammation 28. This is particularly true if maternal fever is the sole criterion for the diagnosis.
Examination for suspected sepsis in the neonate of a mother with chorioamnionitis often yields nonspecific and subtle findings, which may include the following:
- Behavioral abnormalities (eg, lethargy, hypotonia, weak cry, poor suck)
- Pulmonary: Tachypnea, respiratory distress, cyanosis, pulmonary hemorrhage, and/or apnea
- Cardiovascular: Tachycardia, hypotension, prolonged capillary refill time, cool and clammy skin, pale or mottled appearance, and/or oliguria
- Gastrointestinal: Abdominal distention, vomiting, diarrhea, and/or bloody stools
- Central nervous system: Thermal regulatory abnormalities, behavioral abnormalities, apnea, and/or seizures
- Hematologic and/or hepatic: Pallor, petechiae or purpura, and overt bleeding
Laboratory tests
During the intrapartum period, the diagnosis of chorioamnionitis is usually based on clinical criteria, particularly for pregnancies at term.
Laboratory studies for asymptomatic pregnant mothers who present with premature labor or prelabor rupture of membrane (PROM) include the following:
- Examination of amniotic fluid
- Maternal blood studies
- Maternal urine studies
- Maternal group B streptococcal screening test
Testing in febrile pregnant women with suspected chorioamnionitis may include the following:
- White blood cell (WBC) counts
- C-reactive protein (CRP) levels
- Alpha1-proteinase inhibitor (A1PI) complex measurement
- Serum interleukin-6 (IL-6) or ferritin levels
Studies to evaluate amniotic fluid and urogenital secretions may include the following:
- Bacterial cultures
- Leukocyte count
- Gram staining
- pH
- Glucose concentration
- Leukocyte esterase activity 29
- Endotoxin, lactoferrin, and/or cytokine levels (especially IL-6)
- Polymerase chain reaction (PCR) for specific microorganisms
- Fetal fibronectin, insulinlike growth factor binding protein-1 (IGFBP-1), and sialidase levels
- Proteomic profiling 30
The criterion standard for diagnosing early-onset bacteremia, pneumonia, or meningitis in neonates is the growth of bacteria in an appropriate specimen (ie, blood, tracheal secretions, cerebrospinal fluid). Screening tests for neonatal sepsis include white blood cell profiles and C-reactive protein (CRP) determinations.
Other tests that may be used to diagnose early-onset neonatal sepsis include the following:
Imaging studies
Before the fetus is viable, vaginal ultrasonography can be used to identify women with a shortened cervical canal. A shortened cervical canal is associated with a higher risk of preterm delivery 33.
Ultrasonography may also be used to ascertain fetal well-being, utilizing the biophysical profile.
Procedures
Procedures that may be used to evaluate suspected chorioamnionitis or neonatal early-onset sepsis include the following:
- Needle aspiration and analysis of amniotic fluid, with ultrasonographic guidance: Can confirm the diagnosis of acute chorioamnionitis
- Gross/microscopic examination of placenta, fetal membranes, umbilical cord 34
- Complete blood cell (CBC) count and inflammatory biomarkers, blood culture, and chest x-ray
- Controversial: Lumbar puncture of neonates
Chorioamnionitis treatment
Therapy for the mother and/or neonate with chorioamnionitis includes early delivery, supportive care, and antibiotic administration.
Pharmacotherapy
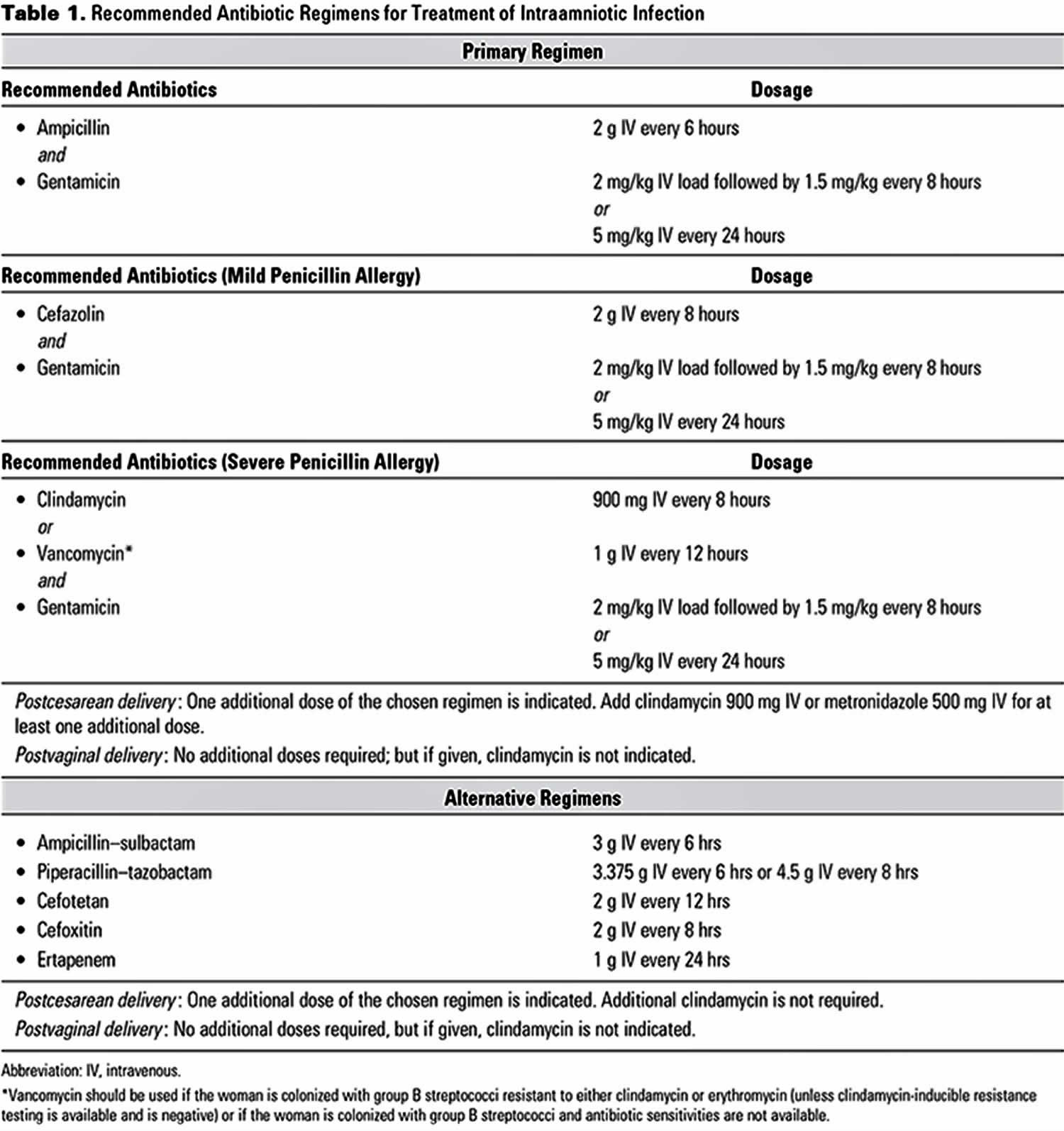
Antibiotic agents used in the treatment of chorioamnionitis include the following:
- Ampicillin and gentamicin
- Clindamycin or metronidazole when endometritis is suspected (postdelivery)
- Vancomycin for penicillin-allergic patients
- Alternatives: Monotherapy with ampicillin-sulbactam, ticarcillin-clavulanate, cefoxitin, cefotetan, or piperacillin-tazobactam
- Penicillin G: Used exclusively for group B streptococcus intrapartum prophylaxis; if intraamniotic infection is suspected, broaden the antibiotic coverage.
The most common antibiotics used are ampicillin and gentamicin. Alternative antibiotics include clindamycin, cefazolin, and vancomycin in women allergic to penicillin. After delivery, the current recommendation is to administer one additional dose with a cesarean section but no additional antibiotics for vaginal deliveries. Additional broad-spectrum antibiotics may be required, depending on the clinical status 35.
Nonpharmacotherapy
Supportive care of the septic neonate may include the following:
- Warmth, monitoring of vital signs
- Preparedness to perform a full resuscitation, including intubation, providing positive-pressure ventilation
- Treatment of hypovolemia, shock, and respiratory and/or metabolic acidosis
- Surfactant replacement therapy
- Glucose homeostasis
- Assessment and treatment of thrombocytopenia and coagulopathy, if present
Surgical option
Cesarean section may be indicated to expedite the delivery.
Although surgical intervention in the newborn is infrequently required in early-onset bacterial infections of the neonate, conditions that may require such intervention include the following:
- Epidural or brain abscess
- Subcutaneous abscesses
- Infections localized to the pleural space
- Certain intraabdominal infections (especially if intestinal perforation is present)
- Bone or joint infections
Chorioamnionitis prognosis
Chorioamnionitis is a risk factor for both maternal and neonatal complications. Endometritis can occur in up to one-third of women treated for chorioamnionitis who undergo a cesarean section. The rate of endometritis is the same in vaginal deliveries and cesarean deliveries following chorioamnionitis. Recent studies show that management with postpartum antibiotics does not decrease the risk of endometritis following chorioamnionitis 36.
The majority of women with chorioamnionitis will recover and not require further antibiotics after delivery.
Maternal complications
Acute chorioamnionitis may result in labor abnormalities (dysfunctional labor) that increase the risk for cesarean delivery, uterine atony, and postpartum bleeding, as well as the need for blood transfusion 2. These complications are likely to occur more often when the amniotic fluid is infected with invasive organisms (eg, E coli and group B Streptococcus) as compared with low-virulence organisms (eg, Ureaplasma urealyticum) 37. Chorioamnionitis may also lead to the development of other infectious complications, including endometritis, localized pelvic infections requiring drainage, septic pelvic thrombophlebitis, and intraabdominal infections 38. More serious sequelae such as sepsis, coagulopathy, and adult respiratory distress syndrome are rare, especially when treatment with broad-spectrum antibiotics is initiated. Additionally, chorioamnionitis may initiate uteroplacental bleeding or a placental abruption 39. The risk of intrauterine infection is increased in placenta previa and may manifest with vaginal bleeding 40.
Neonatal complications
The most serious risks of neonatal exposure to chorioamnionitis are preterm delivery 41 and early-onset neonatal infections (especially sepsis and pneumonia). Other adverse outcomes include perinatal death, asphyxia, intraventricular hemorrhage, cerebral white matter damage, and long-term disability (including cerebral palsy), as well as other morbidities related to preterm birth 42. The outcome of neonatal infections depends on the causative organism, the nature of the infection, the time of infection onset to time of administration of appropriate therapy, the symptoms at time of birth, and the gestational age of the infant. Prematurity and birth defects are confounding factors that must be considered when a prognosis is offered to parents or caregivers of an infected newborn. Outcomes may not be evident during the neonatal period, and long-term follow-up care is indicated in these infected neonates.
Neonatal mortality and morbidity
In a study that evaluated the whole US population and linked infant birth and death certificate files for the year 2008, the neonatal mortality rate for infants exposed to chorioamnionitis was 1.40 per 1000 live births versus 0.81 per 1000 live births for infants without chorioamnionitis, with an odds ratio of 1.72 43. The odds ratio for neonatal death for infants with chorioamnionitis exposure who received antibiotics versus those who did not was 0.69 43. In another study of infants born at 23-32 weeks’ gestation with evidence of intrauterine infection and inflammation, the neonatal death rate was 9.9%-11.1% 44.
Preterm infants born to mothers with chorioamnionitis have unfavorable short-term (meningitis and intraventricular hemorrhage and periventricular leukomalacia) and long-term (cerebral palsy and neurodevelopmental impairment) neurologic outcomes 45. Cerebral palsy 46 and cognitive impairment without cerebral palsy 47 have been linked to exposure to maternal chorioamnionitis. In particular, funisitis and the fetal inflammatory response syndrome have been associated with white matter brain injury or periventricular leukomalacia that is linked to activation of cytokine networks 48. Interleukin (IL)-1beta, IL-6, IL-8, IL-17, IL-18, and tumor necrosis factor (TNF)-alpha are among the cytokines identified as agents related to the fetal inflammatory response syndrome that results in brain injury 49. However, more recent systematic reviews suggest that the evidence for a causal or associative role of chorioamnionitis in cerebral palsy is weak 50 and that improvements in neonatal intensive care may have attenuated the impact of chorioamnionitis on brain health outcomes 51.
The relationship of chorioamnionitis and neonatal cardiopulmonary morbidity is conflicting. Different studies have evaluated the risk of respiratory distress syndrome, bronchopulmonary dysplasia and childhood asthma after fetal exposure to chorioamnionitis. Although some studies showed chorioamnionitis to be associated with lower risk of respiratory distress syndrome 52 other studies found an increased risk of respiratory distress syndrome 53 or no association after adjusting to other variables 45. Similar conflicting data exist for the link of chorioamnionitis and bronchopulmonary dysplasia; however, a 2017 French national prospective, population-based, cohort study that included 2513 live-born singletons delivered at 24-31 weeks of gestation and 1731 placentas concluded that histologic chorioamnionitis is not associated with bronchopulmonary dysplasia 54.
Chorioamnionitis caused by Ureaplasma has been studied extensively 55 (including in animal models) and has been linked to congenital pneumonia, prolonged mechanical ventilation, and cytokine release in the neonatal lungs with subsequent development of bronchopulmonary dysplasia 56. However, studies that looked at antibiotic therapy with erythromycin to reduce the incidence bronchopulmonary dysplasia when the neonatal lungs are colonized or infected with Ureaplasma have been disappointing. More recent studies with azithromycin are encouraging 57.
The link between fetal exposure to chorioamnionitis and the future development of childhood asthma was implied by a systematic review but there was much variation in the included studies with regard to the type of maternal infection, age of the children, and methods of exposure ascertainment that made the conclusion less certain 58. Lastly, with regard to the association between chorioamnionitis and patent ductus arteriosus, two meta-analyses reached opposing conclusions about the association 59.
References- Intrapartum Management of Intraamniotic Infection. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Intrapartum-Management-of-Intraamniotic-Infection
- Higgins RD, Saade G, Polin RA, et al, for the Chorioamnionitis Workshop Participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016 Mar. 127(3):426-36.
- Fowler JR, Simon LV. Chorioamnionitis. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532251
- Ohyama M, Itani Y, Yamanaka M, Goto A, Kato K, Ijiri R, Tanaka Y. Re-evaluation of chorioamnionitis and funisitis with a special reference to subacute chorioamnionitis. Hum. Pathol. 2002 Feb;33(2):183-90.
- Bennet L, Dhillon S, Lear CA, van den Heuij L, King V, Dean JM, Wassink G, Davidson JO, Gunn AJ. Chronic inflammation and impaired development of the preterm brain. J. Reprod. Immunol. 2018 Feb;125:45-55.
- Barth WH Jr. Lost in translation: the changing language of our specialty. Obstet Gynecol. 2016 Mar. 127(3):423-5.
- Benitz WE, Wynn JL, Polin RA. Reappraisal of guidelines for management of neonates with suspected early-onset sepsis. J Pediatr. 2015 Apr. 166(4):1070-4.
- Sperling RS, Newton E, Gibbs RS. Intraamniotic infection in low-birth-weight infants. J Infect Dis 1988;157:113–7.
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52.
- Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns >/= 34 weeks’ gestation. Pediatrics 2014;133:30–6.
- Suzuki S. Association between clinical chorioamnionitis and histological funisitis at term. J Neonatal Perinatal Med. 2019;12(1):37-40.
- Palmsten K, Nelson KK, Laurent LC, Park S, Chambers CD, Parast MM. Subclinical and clinical chorioamnionitis, fetal vasculitis, and risk for preterm birth: A cohort study. Placenta. 2018 Jul;67:54-60.
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015 Oct;213(4 Suppl):S29-52.
- Conti N, Torricelli M, Voltolini C, Vannuccini S, Clifton VL, Bloise E, Petraglia F. Term histologic chorioamnionitis: a heterogeneous condition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015 May;188:34-8.
- Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol 1993;36:795–808.
- ABCs Report: Group A Streptococcus, 2014. Active Bacterial Core Surveillance (ABCs): Emerging Infections Program Network. https://www.cdc.gov/abcs/reports-findings/survreports/gas14.html
- Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016;138:e20162013.
- Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol 2016;33:1076–8.
- Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol 2010;116:387–92.
- Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis [published erratum appears in Dev Med Child Neurol 2016;58:316]. Dev Med Child Neurol 2013;55:509–19.
- Rouse DJ, Landon M, Leveno KJ, Leindecker S, Varner MW, Caritis SN, et al. The Maternal-Fetal Medicine Units Cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol 2004;191:211–6.
- Huber BM, Meyer Sauteur PM, Unger WWJ, Hasters P, Eugster MR, Brandt S, Bloemberg GV, Natalucci G, Berger C. Vertical Transmission of Mycoplasma pneumoniae Infection. Neonatology. 2018;114(4):332-336.
- Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida Chorioamnionitis Leads to Preterm Birth and Adverse Fetal-Neonatal Outcome. Infect Dis Obstet Gynecol. 2017;2017:9060138.
- Newman T, Cafardi JM, Warshak CR. Human immunodeficiency virus-associated pulmonary arterial hypertension diagnosed postpartum. Obstet Gynecol. 2015 Jan;125(1):193-5.
- Snyder M, Crawford P, Jamieson B, Neher JO. Clinical inquiries. What treatment approach to intrapartum maternal fever has the best fetal outcomes?. J Fam Pract. 2007 May. 56(5):401-2.
- Ocheke AN, Agaba PA, Imade GE, Silas OA, Ajetunmobi OI, Echejoh G, Ekere C, Sendht A, Bitrus J, Agaba EI, Sagay AS. Chorioamnionitis in pregnancy: a comparative study of HIV-positive and HIV-negative parturients. Int J STD AIDS. 2016 Mar;27(4):296-304.
- Horvath B, Lakatos F, Toth C, Bodecs T, Bodis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med. 2014 Jul. 42(4):441-7.
- Apantaku O, Mulik V. Maternal intra-partum fever. J Obstet Gynaecol. 2007 Jan. 27(1):12-5.
- Hsu CD, Meaddough E, Hong SF, Aversa K, Lu LC, Copel JA. Elevated amniotic fluid nitric oxide metabolites and interleukin-6 in intra-amniotic infection. J Soc Gynecol Investig. 1998 Jan-Feb. 5(1):21-4.
- Buhimschi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007 Jan. 4(1):e18.
- Joram N, Boscher C, Denizot S. Umbilical cord blood procalcitonin and C reactive protein concentrations as markers for early diagnosis of very early onset neonatal infection. Arch Dis Child Fetal Neonatal Ed. 2006 Jan. 91(1):F65-6.
- Hedegaard SS, Wisborg K, Hvas AM. Diagnostic utility of biomarkers for neonatal sepsis–a systematic review. Infect Dis (Lond). 2015 Mar. 47(3):117-24.
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007 Oct. 30(5):706-14.
- Reilly SD, Faye-Petersen OM. Chorioamnionitis and funisitis. NeoReviews. Sept 2008. 9(9):e411-7.
- Committee on Obstetric Practice. Committee Opinion No. 712: Intrapartum Management of Intraamniotic Infection. Obstet Gynecol. 2017 Aug;130(2):e95-e101.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment Utility of Postpartum Antibiotics in Chorioamnionitis Study. Am J Perinatol. 2016 Jul;33(8):732-7.
- Silver RK, Gibbs RS, Castillo M. Effect of amniotic fluid bacteria on the course of labor in nulliparous women at term. Obstet Gynecol. 1986 Nov. 68(5):587-92.
- Rouse DJ, Landon M, Leveno KJ, et al, for the National Institute of Child Health And Human Development, Maternal-Fetal Medicine Units Network. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004 Jul. 191(1):211-6.
- Nath CA, Ananth CV, Smulian JC, Shen-Schwarz S, Kaminsky L. Histologic evidence of inflammation and risk of placental abruption. Am J Obstet Gynecol. 2007 Sep. 197(3):319.e1-6.
- Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010 May. 38(3):275-9.
- Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol. 2014 May. 210(5):450.e1-10.
- Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016. 29(9):1525-9.
- Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J Perinatol. 2014 Aug. 34(8):611-5.
- Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol. 2006 Oct. 195(4):1020-4.
- Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009 Apr. 200(4):372.e1-6.
- Neufeld MD, Frigon C, Graham AS, Mueller BA. Maternal infection and risk of cerebral palsy in term and preterm infants. J Perinatol. 2005 Feb. 25(2):108-13.
- Versland LB, Sommerfelt K, Elgen I. Maternal signs of chorioamnionitis: persistent cognitive impairment in low-birthweight children. Acta Paediatr. 2006 Feb. 95(2):231-5.
- Lu HY, Zhang Q, Wang QX, Lu JY. Contribution of histologic chorioamnionitis and fetal inflammatory response syndrome to increased risk of brain injury in infants with preterm premature rupture of membranes. Pediatr Neurol. 2016 Aug. 61:94-98.e1.
- Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014 Jan. 32(1):56-67.
- Shi Z, Ma L, Luo K, et al. Chorioamnionitis in the development of cerebral palsy: a meta-analysis and systematic review. Pediatrics. 2017 Jun. 139(6).
- Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014 Mar. 41(1):83-103.
- Liu Z, Tang Z, Li J, Yang Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr Neonatol. 2014 Feb. 55(1):35-40.
- Jones MH, Corso AL, Tepper RS, et al. Chorioamnionitis and subsequent lung function in preterm infants. PLoS One. 2013. 8(12):e81193.
- Torchin H, Lorthe E, Goffinet F, et al. Histologic chorioamnionitis and bronchopulmonary dysplasia in preterm infants: the epidemiologic study on low gestational ages 2 cohort. J Pediatr. 2017 Aug. 187:98-104.e3.
- Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol. 2013 Apr. 37(2):94-101.
- Viscardi RM, Kallapur SG. Role of ureaplasma respiratory tract colonization in bronchopulmonary dysplasia pathogenesis: current concepts and update. Clin Perinatol. 2015 Dec. 42(4):719-38.
- Smith C, Egunsola O, Choonara I, Kotecha S, Jacqz-Aigrain E, Sammons H. Use and safety of azithromycin in neonates: a systematic review. BMJ Open. 2015 Dec 9. 5(12):e008194.
- Zhu T, Zhang L, Qu Y, Mu D. Meta-analysis of antenatal infection and risk of asthma and eczema. Medicine (Baltimore). 2016 Aug. 95(35):e4671.
- Behbodi E, Villamor-Martinez E, Degraeuwe PL, Villamor E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Sci Rep. 2016 Nov 28. 6:37967.