Clove oil
Clove oil also known as Caryophylii floris aetheroleum, is the essential oil extracted by steam distillation from the dried flower buds of clove Syzygium aromaticum (L.) or Eugenia caryophyllata L. (Myrtaceae), that is used as a topical application to relieve pain and to promote healing and also finds use in the fragrance and flavoring industries 1. Clove essential oil is known for its to its antimicrobial, antioxidant, antifungal and antiviral activity 2. In addition, clove essential oil possesses antiinflammatory, cytotoxic, insect repellent and anaesthetic properties. Clove essential oil is a complex mixture of chemical substances, the main components being phenylpropanoids such as carvacrol, thymol, eugenol and cinnamaldehyde. The eugenol content noted from 63.8 to 84.8% 3 and its strong antibacterial activity make the clove oil a good candidate for the anti-biofilm agent 4. According to the US Food and Drug Administration (FDA) clove essential oil was approved generally recognized as safe (GRAS) to use as a food additive and in dentistry 5; however, it is lethal when taken via an oral dose of 3.75 g/kg body weight 6. The traditional use of clove oil for dental emergencies, mainly for symptomatic temporary relief of toothache due to a dental cavity and inflammation in the mouth and throat. The relief of toothache by clove essential oil is only a provisional measure. Dental attention should be sought as soon as possible. In this regard, clove oil has been reported to be used in preparation of certain toothpastes and mouth washes 7.
Owing to its antibacterial, antioxidant, anti-inflammatory, and analgesic properties, clove oil is one of the ideal essential oils for the healing of wounds. Clove essential oil fabricated as nanofibers with various polymers show strong antibacterial properties, especially against Staphylococcus aureus, Escherichia coli, Pseudomonas fluorescens, and Bacillus subtilis 8. The wound-healing effect of clove oil in nanoemulsion form was studied, and it was confirmed that the oil in nanoemulsion has a marked wound-healing capacity and reduced the incidence of inflammatory cells at the site of a wound when compared to pure clove oil and enhanced cell viability 9. Poly(ε-Caprolactone)/Gelatin nanofibers loaded with clove oil showed good antibacterial as well as marked healing properties 8. Clove oil-based nanofibrous mats showed good antifungal properties when used for candida-associated denture stomatitis prevention and treatment 10. Topical application of 1% clove oil cream demonstrated a significant beneficial effect when applied to patients suffering from chronic anal fissure 11.
Clove oil becomes yellowish and is chemically unstable in air; moisture, light and temperature during storage cause highly volatile losses its activity 12.
Clove (Caryophylli flos) is traditionally used as spice such as for gingerbread flavoring. Many spice blends, including curry contain powdered cloves, most herb liqueurs and bitter liqueurs contain clove macerates 13. Clove has been traditionally used in dyspeptic complaints, flatulence and diarrhea as a decoction 13.
Table 1. Clove oil main chemical compounds and concentrations
| Compound | Concentration (%) |
| Eugenol | 86.99 ± 0.09 |
| α- Copaene | 00.07 ± 0.01 |
| β-Caryophyllene | 08.76 ± 0.04 |
| Cadina-1(6),4-diene | 00.04 ± 0.01 |
| α-Humulene | 01.91 ± 0.03 |
| ɣ-Muurolene | 00.02 ± 0.01 |
| β-Selinene | 00.02 ± 0.01 |
| α-Selinene | 00.05 ± 0.02 |
| β-Farnesene | 00.06 ± 0.01 |
| (Z)-Calamenene | 00.11 ± 0.02 |
| δ-Cadinene | 00.27 ± 0.03 |
| Cadina-1,4-diene | 00.03 ± 0.01 |
| Humulene epoxide | 00.11 ± 0.01 |
| Total | 98.44 |
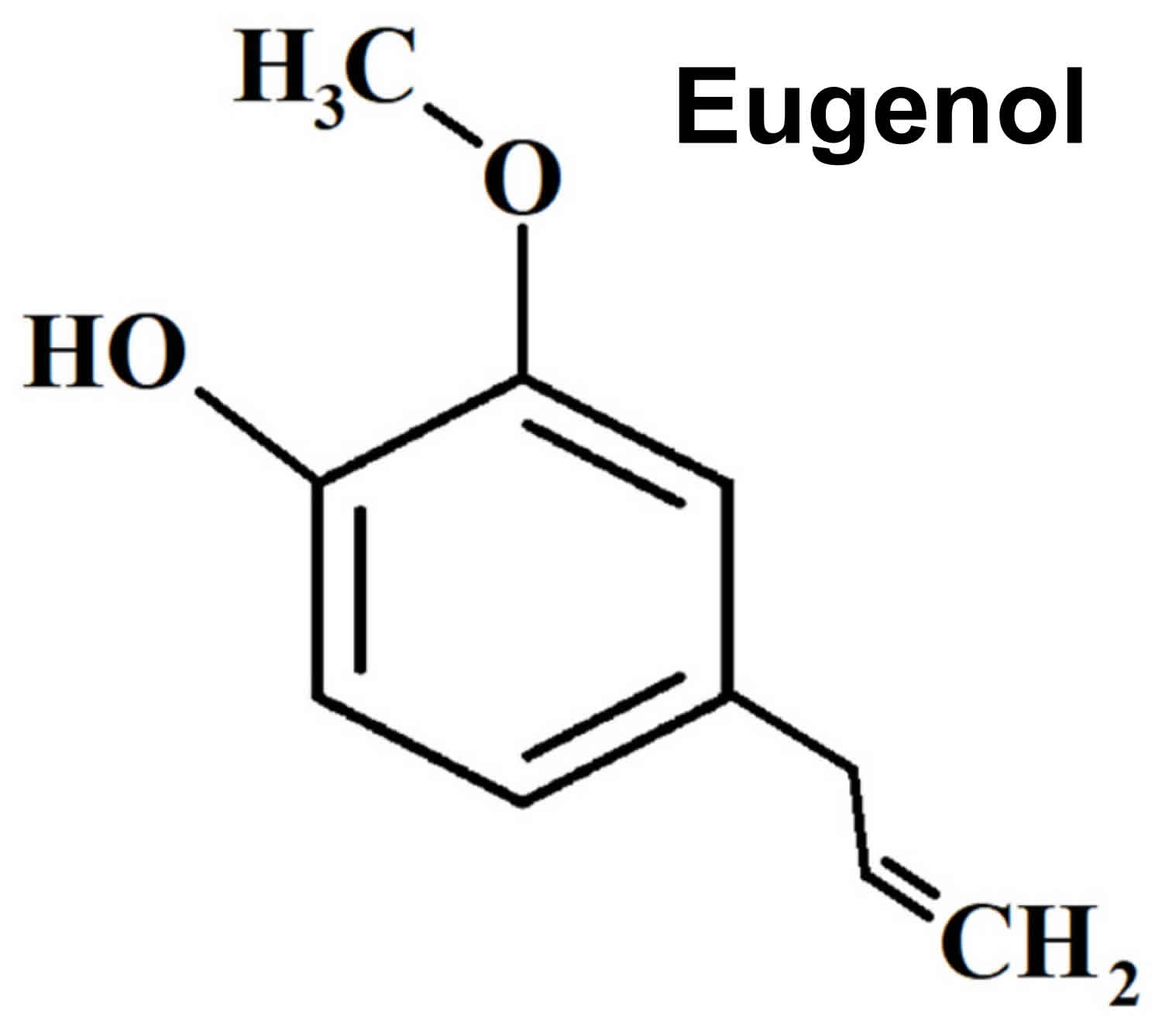
Figure 1. Eugenol chemical structure
Clove oil benefits
Clove oil expresses a strong antimicrobial activity 14. Clove oil’s minimum inhibitory concentrations (MICs) against bacteria Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa were noted in the range of 0.2–0.625 mg/mL 15. Minimum inhibitory concentrations (MICs) are defined as the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation, and minimum bactericidal concentrations (MBCs) as the lowest concentration of antimicrobial that will prevent the growth of an organism after subculture on to antibiotic-free media, usually reported as mg/L or mcg/mL 16. Clove oil antibacterial effect is assigned to its main compound eugenol interactions with bacterial cell membranes and disruption of DNA synthesis 15. The antifungal activity of the clove oil was proved against a variety of yeast and molds: Candida albicans, Candida parapsilosis, Candida krusei, Alternaria sp., Aspergillus niger, Aspergillus fumigatus, Penicillum sp., Microsporum canis, Microsporum gypseum, Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum 17. The antifungal activity of clove oil is attributed to a decrease of ergosterol synthesis, the component of the fungal cell wall 18. Clove oil at the concentrations in the range of 2.5–10.0% was also effective against food contaminants Bacillus cereus, Bacillus subtilis, Staphylococcus sp., E. coli, Aspergillus sp., Penicillium sp., and Rhizopus sp. 17. Clove oil action against food borne pathogens Listeria monocytogenes, S. aureus, E. coli O157:H7, and Salmonella sp. was also confirmed 19. The vivid antibiofilm effect of clove oil was observed against E. coli O157:H7 20, Pseudomonas aeruginosa, and Aeromonas hydrophila 21. The clove oil even in its sub-minimum inhibitory concentrations substantially inhibited the bacterial biofilm formation and reduced virulence factors of the pathogens. Interestingly, solid liposomes of clove oil applied on raw vegetables exhibited anti-biofilm activity against E. coli O157:H7 without any adverse effects on sensory quality of food 22.
In the recent past, studies have demonstrated that clove is antimutagenic 23, antioxidant, antithrombotic, antiparasitic and anti-inflammatory 24. Studies in the arthritic rat model have shown that eugenol exhibits anti-inflammatory and anti-arthritic activities 25. Feng and Lipton 26 have demonstrated that eugenol significantly reduced fever when given to rabbits made febrile by the injection of interleukin 1 (IL-1). These findings provide support for some of the traditionally accepted medicinal uses of clove oil.
Antibacterial effects
The majority of publications on pharmacological effects of clove, clove essential oil and eugenol deal with the antimicrobial effects. Only some selected references are cited below.
Eugenol (1 mg/ml) showed pronounced antibacterial properties against Gram-positive as well as against Gram-negative microorganisms comparable with 500 μg/ml neomycin 27. Growth inhibition was even more pronounced for Candida species in comparison with nystatin (5000 U/ml).
0.4% clove oil in 63% sugar syrup inactivated after 2–7 minutes Candida albicans, Clostridium perfringens, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus 28. The effect was not affected by addition of serum. Added sugar is not needed for an effect; however, it stabilizes the dispersion of the essential oil 28.
The effect of 0–300 ppm clove oil on the growth and synthesis of aflatoxins from Aspergillus parasiticus was studied in submerged culture 29. 0–250 ppm led to a slower growth, but had no influence on the weight of the mycelium after 21 days. 300 ppm completely inhibited the growth. The production of aflatoxins was dose-dependently delayed 29.
Clove oil was superior to rosemary oil when tested against several Gram-positive and Gram-negative bacteria as well as against two fungi. A synergistic effect was observed against Candida albicans, an antagonism for Aspergillus niger 30.
In an investigation of various aromatic waters, clove oil–water had no sustainable growth-inhibiting effect on Pseudomonas. Clove oil-water exhibited in the serial dilution test and agar diffusion test only a weak antimicrobial effect. The minimum inhibitory concentration (MIC) in the serial dilution test was in the case of Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa for each 1:20, for Mycobacterium phlei 1:640 and for Staphylococcus aureus 1:160 or 1:320 31. The minimal fungicidal dilution was 1:320 in case of Aspergillus niger, for Penicillium chrysogenum 1:40 and for Mucor, Rhizopus and Candida albicans for each 1:20. The results in the agar diffusion test differ in part from that in the serial dilution test 31.
Ali et al 32 found that eugenol inhibits the growth of 30 Helicobacter pylori strains tested, at a concentration of 2μ/ml after 9 and 12 hour of incubation. A lower pH-value increased the activity. The bacteria did not develop any resistance even after 10 passages grown at sub-inhibitory concentrations. The authors conclude that eugenol may prevent the growth of Helicobacter pylori 32.
Dorman and Deans 33 tested the antibacterial activity of clove essential oil in 25 bacteria. The results suggest that the essential oil is equally effective against both Gram-positive and Gram-negative microorganisms. A different sensitivity of the bacteria tested was observed.
Saini et al 34 investigated the effect of orally administered clove essential oil on respiratory tract infections with Klebsiella pneumoniae in rats. The daily oral supplementation was 0.5 ml of a 1% w/v solution. The comparison of short term (15 days) and long term (30 days) treatment resulted in a significantly lower bacterial load in the lungs of mice fed clove oil for 30 days. The authors stated also a significant decrease of bacterial colonization already after 15 days 34.
Khan et al 35 studied the influence of clove oil and of eugenol on quorum sensing (QS = mechanism by which bacterial populations coordinate the expression according to the density of the local population) regulated functions in bacteria. The production of violacein by Chromobacterium violaceum is QS-controlled. Clove oil reduces at sub-MICs this production up to 78% compared to control. The swarming motility in Pseudomonas aeruginosa which is also QS-controlled was reduced up to 78%. Eugenol was not responsible for these effects 35.
The antibacterial activity of eugenol may be due to an interaction of eugenol with the bacterial cell membrane 36. The membrane is disrupted and macromolecules of the membrane are deformed.
Antifungal effect
The antifungal activity of clove essential oil against Aspergillus section Flavi was evaluated in sterile maize grain 37. The effect of clove essential oil added to maize grains on growth rate, lag phase and aflatoxin B1 (AFB1) accumulation of Aspergillus section Flavi were evaluated at different water activity conditions (a measure for water content; 0.982; 0.955; and 0.90). The essential oil had an inhibitory effect on Aspergillus section Flavi growth rate; the efficacy depended mainly on the water activity and concentration. Clove essential oil showed a considerable inhibitory effect on the AFB1 accumulation. When the water activity was 0.982, the AFB1 inhibition percentage for all aflatoxigenic strains exceeded 98% at all clove essential oil concentrations 37.
The antifungal effect of clove oil was tested against several dermatophytes using the agar diffusion method 38. Hyphal growth was completely inhibited in Trichophyton mentagrophytes, T. rubrum and Microsporum gypseum in concentrations of 0.2 mg clove oil per ml. Eugenol was found to be the most effective compound against Trichophyton mentagrophytes and Microsporum canis 38.
The composition and antifungal activity of clove essential oil were tested by Pinto et al 39. MICs, determined according to Clinical and Laboratory Standards Institute protocols, and minimum fungicidal concentration were used to evaluate the antifungal activity of the clove oil and its main component eugenol, against Candida, Aspergillus clinical isolates (e.g. American Type Culture Collection strains). The essential oil and eugenol showed inhibitory activity against all the tested strains. Propidium iodide rapidly penetrated the majority of the yeast cells when the cells were treated with concentrations just over the MICs. Therefore the fungicidal effect may result from extensive lesions of the cell membrane. Clove oil and eugenol also caused a considerable reduction in the quantity of ergosterol, a specific fungal cell membrane component. Germ tube formation by Candida albicans was completely or almost completely inhibited by the essential oil and eugenol concentrations below the MIC values. The authors conclude that the results indicate that clove oil and eugenol have considerable antifungal activity against clinically relevant fungi, including fluconazole-resistant strains 39.
Eugenol significantly reduced the number of colony forming units (CFUs) sampled from the oral cavity of immunosuppressed rats treated for 8 days 40. Eugenol was used in a concentration of 24 mM (= double MIC) in agar solution. Nystatin was used as a poisitive control in a concentration of 58 μM (= tenfold MIC). Eugenol and nystatin gave similar results. Only few zones were occupied by hyphae with eugenol, while under nystatin hyphae were found in the folds of the tongue mucosa 40.
There was a significant reduction of colony counts in a prophylactic approach and a treatment approach in cases of vaginal candidiasis in an immunosuppressed rat model. The rats received 10 mg/kg/day eugenol via an intravaginal route 41. Lee et al 42 evaluated the antifungal effect of eugenol against skin lesions in guinea pigs infected with Microsporum gypseum. Eugenol was adjusted to 10% concentration with a base of vaseline petroleum jelly and was applied topically to the skin lesions daily for 3 weeks. Eugenol was clinically active.
For eugenol no significant antiviral activity against herpes simplex virus type 1 was found in vitro using the plaque reduction assay 43.
Antiprotozoal effects
Clove oil inactivated in vitro Trichomonas vaginalis in a dose- and time-depending manner 13. After addition of 4, 2, 1, 0.5 and 0.25 mg/ml to the culture medium no surviving Trichomonads were detectable after an incubation period of 5 min to 8 hours. Concentrations of 0.01 and 0.05 mg/ml were not effective. With eugenol comparable effects were achieved. With 4–0.05 mg/ml of the reference substance metronidazol the effect was achieved after 30 min to 2 hours 13.
The IC50 is the concentration of drug required for 50% inhibition.Treatment of epimastigotes of Trypanosoma cruzi with different concentrations of clove essential oil resulted in a dose-dependent growth inhibition with IC50/24 hours of about 99.5 μg/ml; IC50/24 hours values obtained after treatment of bloodstream trypomastigotes were about 57.5 μg/ml 44. The values obtained for epimastigotes treated with eugenol were 246 μg/ml, while treatment of bloodstream trypomastigotes resulted in IC50/24 hours values of 76 μg/ml for eugenol 44.
Antiparasitic effects
In a study by Eamsobhana et al 45 commercially produced essential oils of 13 plant species and ethanol (control) were tested for repellent activity against host-seeking larvae of chigger mite (Leptotrombidium imphalum). Dilutions of each essential oil were prepared in absolute ethanol. Clove essential oil exhibited 100% repellent activity at 5% concentration.
Analgesic effects
Patch-clamp experiments showed that eugenol reversibly activates calcium ion channels and chloride ion channels in dorsal root ganglion cells from rats 46. The applied eugenol concentrations ranged from 0.125 to 1 mmol/l. These effects may be responsible for the analgesic activity 46. Sodium and calcium channels act as targets for eugenol for its analgesic effect. Eugenol inhibits ATP-induced P2X currents in trigeminal ganglion neurons, which contributes to the analgesic effect 47.
Intrathecal treatment of mice with eugenol (12.5 to 50 μg) for 24 hours, dose-dependently inhibited the formalin-induced nociceptive response 48. Capsazepine shifted the dose-response curves in parallel to the right. Eugenol may exert its antinociceptive effect via the capsaicin receptor located on sensory terminals in the spinal cord. These results indicate that eugenol acts as a capsaicin-like substance 48.
Anti-inflammatory effects
Eugenol inhibited the nitric oxide (NO) production in a dose-dependent manner in RAW264.7 cells treated with 1 μg/ml lipopolysaccharide for 24 hours 49. Isoeugenol was more effective. LPS-dependent expression of COX-2 was also inhibited by isoeugenol and less effectively by eugenol 49.
Sedative effect
Wagner and Sprinkmeyer 13 investigated the sedative effect of clove essential oil. Mice received 1 to 100 mg/kg orally. The motility in the photocell cage was compared with the results of the day before (without treatment). The authors observed a non dose-dependent reduction of motility 13.
Spasmolytic effect
A saturated aqueous solution of clove oil was active in vitro on isolated organs against various spasmogens: rat/duodenum/acetylcholine: 20 to 40% inhibition; rat/duodenum/barium chloride: 40 to 60% inhibition; guinea-pig/ileum/histamine: >60% inhibition; rabbit/jejunum/nicotine: >60% inhibition 13. No further details on the methodology are available 13. Clove oil antagonized in vitro the carbachol-induced spasm of guinea pig trachea muscles and the electrically stimulated contraction of longitudinal muscles of guinea pig ileum. Half maximal effective concentration (EC50) refers to the concentration of the drug at a stable state inducing half of the maximum effect. The EC50 was 3.8 mg/ml (trachea; isoprenaline EC50 = 3.9 nmol/l) or 6.8 mg/ml (ileum; papaverine EC50 = 3.7 μmol/l) 50. Eugenol relaxes the rabbit thoracic aorta while suppressing the Ca2+ sensitivity and both the uptake and extrusion mechanisms for Ca2+ 51.
Anticancer effects
Mice received 20 mg of isolated sesquiterpenes once every 2 days. The sesquiterpenes ß-caryophyllene, ß-caryophyllene oxide, alpha-humulene, alpha-humulene epoxide and eugenol induced the detoxifying enzyme glutathione S-transferase in the mouse liver and intestine 52. Eugenol showed chemopreventive effects. Eugenol, but not its isomer isoeugenol, was found to be a potent inhibitor of melanoma cell proliferation. It inhibits the growth of melanoma cells in culture (50% inhibition by 0.5 μM). Eugenol causes significant tumour growth delay, decrease of tumor size and prevents tumor metastasis in mice (125 mg/kg) 53.
Effect on coagulation
Clove oil inhibited in vitro the platelet aggregation which was induced by arachidonic acid, epinephrine and collagen 54. The formation of thromboxane B2 induced by arachidonic acid was inhibited in intact and in lysed platelet preparations. The effect, which exceeds the in vitro effect of acetylsalicylic acid, might be attributed to eugenol and eugenyl acetate. The combination of these compounds inhibits the platelet aggregation in a superadditive manner 54.
The IC50 is the concentration of drug required for 50% inhibition. The IC50 of eugenol (3.0 x 10-7 M) and isoeugenol (7.2 x 10-7 M) were comparable with indomethacin (2.2 x 10-7 M) on platelet aggregation 27.
Eugenol and isoeugenol inhibit the arachidonic acid (1 x 10-4 g/ml) induced platelet aggregation, the IC50 values were 4.5 x 10-8 g/ml and 1 x 10-7 g/ml respectively 55.
Effect on prostaglandin synthesis
The addition of 37 μM clove oil to in vitro preparations from sheep seminal vesicles inhibits (based on average molecular weight of 200) the prostaglandin synthesis from [1-14C] arachidonic acid by 84.1% compared to a control without the essential oil 56. The IC50 of eugenol was 11 μM; the IC50 of indomethacin was 1.2 μM 56. Eugenol and its derivatives are inhibitors of LOX-5 and COX-2 57.
Clove oil uses
Clove oil has been traditionally used externally or locally for the temporary relief of toothache and the treatment of minor infections of the mouth and skin, dressing of minor wounds, sore throats and coughs associated with the common cold, muscle ache (myalgia), rheumatic complaints, insect bites, flatulent colic or nausea 58. Clove oil can be used on adults, the elderly and children over 2 years old. The German commission E (a scientific advisory board of the Federal Institute for Drugs and Medical Devices similar to the FDA) proposes the use of the clove essential oil for treatment of inflammations of the oral and pharyngeal mucosa and in dentistry for topical anaesthesia 59. Clove oil or eugenol alone is widely used in dentistry mixed with zinc oxide as temporary filling material 13. Undiluted clove essential oil or solutions in a strength of minimum 50% or gels in a strength of 20% is authorized products in the UK for the temporary relief of toothache due to dental cavity 60. Repeat administration after 20 minutes, then every 2 hours thereafter if necessary. However, the relief of toothache by clove essential oil is only a provisional measure. Dental attention should be sought as soon as possible. Topical application of 1% clove oil cream demonstrated a significant beneficial effect when applied to patients suffering from chronic anal fissure 11.
- DO NOT use Clove oil in teething.
- DO NOT use Clove oil on surrounding gums.
Clove oil mouth washes
Clove oil mouth washes corresponding to 1–5% essential oil is used for the inflammations of the oral and pharyngeal mucosa 59.
Traditional use in children and adolescents
- Oromucosal use:
Clove oil dosage
Clove oil for dental use
Undiluted clove essential oil or solutions in a strength of minimum 50% or gels in a strength of 20%. According to the electronic medicines compendium of UK medicinal products 62: A small piece of cotton wool should be soaked in the undiluted clove oil or in a diluted solution; semisolid dosage forms should be placed on a cotton bud. Cotton bud or cotton wool should be accurately directed to the decayed part of the tooth as required. Avoid contact with gums. Repeat administration after 20 minutes, then every 2 hours if necessary. Repeated use may cause gum damage. Not to be used for more than 1 week. The relief of toothache by clove essential oil is only a temporary measure. See your dentist as soon as possible. The use in children and adolescents under 18 years of age is not recommended due to lack of adequate data.
Use of eugenol in dentistry: Eugenol can be part of temporary pulp fillings in dentistry. Eugenol is mixed with zinc oxide, giving a paste which hardens quickly when coming into contact with saliva.
Clove oil for mouthwashes
Mouthwashes corresponding to 1–5% clove essential oil. Apply several times daily. The use in children and adolescents under 18 years of age is not recommended due to lack of adequate data.
Clove oil side effects
Clove oil can have side effects, although these don’t affect everyone. Side effects are 62:
- Temporary skin irritation.
- Dermatitis (skin rash, itching, flaking).
- Swelling of the lips.
- Blistering and swelling in the mouth.
- You may also become sensitive to clove oil.
- If you notice these or any other side effect notincluded above, stop use and tell your dentist or pharmacist. They will tell you what to do.
Clove essential oil acts in high concentrations as local irritant, allergic reactions may also be possible 60. However, when applied in diluted form, no reports on severe adverse events are published. When applied correctly in the proposed routes of administration, clove essential oil can be considered as clinically safe 60.
LD50 assessment
Lethal dose 50 (LD50) is the amount of an ingested substance that kills 50 percent of a test sample. It is expressed in mg/kg, or milligrams of substance per kilogram of body weight. The median intraperitoneal LD50 of clove oil was 161.9 mg/kg 63. Mice treated with up to 50 mg/kg clove oil and observed for 96 hours did not show abnormal symptoms, consistent with no decrease in food intake (data not shown). Furthermore, no deaths were observed. These data indicate that the toxicity of the clove oil is limited. Injection of doses ≥90 mg/kg body weight produced various observable effects, including in motor activity and respiration, within 45 minutes. Mice showed ataxia (unsteady gait), sedation and decreased motor activity, piloerection, tremor, ptosis and 25% mortality. Also, a dose-dependent mortality was observed after increasing the doses: 292 mg/kg clove oil was 100% lethal 63.
Skin and mucosal irritations
In concentrated form, clove oil may be irritating to mucosal tissues 46. In contrast to this, Anton et al 64 report that there is no skin irritation (undiluted clove oil) on hairless mice. Under occlusion the undiluted clove oil was moderately irritating in rabbits.
Allergic effects
In patients sensitized to Peru balsam, a hexane extract of clove, caused, in concentrations higher than 0.12% in petrolatum, local reactions. In a concentration of 1% in petrolatum, in two of four patients, a moderate reaction was observed, in the other two, an intense reaction occurred (large, infiltrated, dark spots with numerous vesicles) 65. In a skin test with clove powder (on filter paper moistened with water), out of 78 patients with allergy against Peru balsam, 36 reacted positive. In a control group of 156 probands lacking Peru balsam allergy, nobody responded positively 66 . A 22 years old patient with eczema on the hands reacted to a oral stress test 2 times 100 mg clove powder in gelatine capsules) with blisters on palms and fingers 66. Clove cigarettes have been reported to cause acute respiratory problems in humans that rapidly progress to hemorrhagic pulmonary edema or pneumonia 13.
In a patch test study a 10% ethanol extract of clove was investigated among other herbal preparations used in the Traditional Chinese Medicine. Out of 30 patients 8 reacted positively to clove extract 67.
Clove oil, 20% incorporated in petrolatum, produced redness in 2 of 25 healthy subjects 68. In concentrations of 2% and 0.2% in petrolatum, no reactions were observed 68.
When trying to administer clove essential oil onto an aching tooth, a 24 year old woman disposed accidentally the oil on the upper lip and cheek 69. Although she tried to remove the essential oil, a sensation of burning and inflammation occurred, which disappeared within a few hours. Subsequently, local anaesthesia and reduced sweat production in the affected areas were observed. The medical examination after 11 months revealed a dry, slightly erythematous skin with reduced pressure sensitivity. During the following 9 months the situation remained unchanged 69.
Root canal fillings with eugenol cement
When eugenol cement is applied near the pulpa (intact dentin layer) no toxicity is observed 60. However, when applied directly to the exposed pulp, pulp necrosis and inflammation appeared 70.
A root canal filling with eugenol cement resulted in a patient with a generalized urticaria (hives or red itchy welts) 71. In the skin test, the patient responded positively to Peru balsam and cloves. A distributed oral provocation test with 0.1 to 0.5 ml of eugenol, in water, resulted in urticaria which persisted for several weeks 71.
Pregnancy and breastfeeding
Safety during pregnancy and lactation has not been established. In the absence of sufficient data, the use during pregnancy and lactation is not recommended.
Clove oil contraindications
The medicinal use of clove essential oil should be contraindicated in cases of hypersensitivity to clove essential oil or Eugenol as well hypersensitivity to Peru balsam 13.
Serious events
A 17-year-old male high-school student died of rapidly progressive inflammatory lung disease that developed hours after smoking a clove cigarette 60. The student was recovering from a lower respiratory infection at the time. The California Department of Health Service and Centers for Disease Control (CDC) collected 110 cases of clove cigarette toxicity by 1984, two of which were fatal 46. During 1984 and 1985, the Centers for Disease Control received 11 case reports of clove cigarette smoking-associated acute respiratory system injury in adolescents and young adults; two deaths were also reported. The reported respiratory adverse effects included hemoptysis, bronchospasm, hemorrhagic and nonhemorrhagic pulmonary oedema, pleural effusion, respiratory insufficiency, respiratory infection and aspiration of foreign material 46.
Clove oil overdose
No case of clove oil overdose from oromucosal use or dental use has been reported. After oral administration of 5-10 ml of clove oil in children below 2 years of age, life threatening conditions were observed. Overdose may lead to central nervous system depression, urinary abnormalities, anion gap acidosis, deterioration of liver function, coma, seizure and low blood glucose levels. Treatment should be supportive and symptomatic; there have been reports in the literature that N-acetylcysteine has been successfully used as an antidote 72.
Case reports
A 7-month-old boy received 1 teaspoon of clove oil 73. The dose corresponds to about 500 mg/kg eugenol. On admission to hospital, an attenuation of the central nervous system (awake, but without a direct response to environment), leukocytosis, proteinuria and ketonuria were observed 73. The also observed metabolic acidosis was attributed to the already existing diarrhea. 3 hours after ingestion, gastric lavage was performed with the addition of activated carbon. An endoscopy the next morning showed no evidence of mucosal damage in the stomach or esophagus. After 48 hours acidosis and leukocytosis were no longer detectable. The patient recovered completely and was released from hospital after 4 days 73.
A 2 year old boy drank 5 to 10 ml of clove oil 74. After 1 hour only mild drowsiness was observed. Within the next 3 hours, a drastic deterioration occurred with deep coma and severe acidosis. 8.5 hours after the ingestion, generalized cramps occurred which were treated with diazepam. The patient had an unrecordable blood glucose level which was treated with intravenous dextrose. 24 hours after ingestion, the patient was unconscious 74. A severely impaired liver function and disseminated intravascular coagulopathy (therapy with plasma, heparin, antithrombin III, protein C, factor VII) was observed. The liver function deteriorated further in the following days. During the 5th day, the patient awoke and on day 6, he was fully conscious. From this time point, the symptoms gradually disappeared, the patient fully recovered 74.
A very similar case – ingestion of about 10 ml of clove oil by a 2 year old boy resulting in convulsions, unconsciousness and severe coagulation – is described by Brown et al 75. The patient was treated with heparin and fresh frozen plasma, and, following specific hemostasis assays, with appropriate coagulation factor and inhibitor concentrates 75.
A 3 month old girl developed a fulminant hepatic failure after ingestion of less than 8 ml of clove oil (exact amount not documented) 76. She was successfully treated with N-acetylcystein infusion and recovered completely 76.
A similar case is reported by Janes et al 77: a 15 month old boy developed a fulminant hepatic failure after ingestion of 10 ml of clove oil. After 24 hour, the ALT level was in excess of 13,000 U/l, with blood urea and creatinine of 11.8 mmol and 134 μmol/l respectively. The hepatic impairment resolved after intravenous administration of N-acetylcysteine so that 6 h later, the ALT level was approximately 10,000 U/l. The liver function and clinical status improved over the next 4 days 77.
Clove oil drug interactions
The antiplatelet effect of clove oil may increase the risk of bleeding if taken with these medications. Clove may result in a false increase in phenytoin levels 46. However, the routes of administration of clove oil for oromucosal and dental use; the duration of use is limited. Therefore these mentioned theoretical drug interactions are not relevant for the traditional use of clove oil for the short term treatment of toothache or as an antiseptic mouthwash 60.
References- Chaieb, K., Hajlaoui, H., Zmantar, T., Kahla-Nakbi, A.B., Rouabhia, M., Mahdouani, K. and Bakhrouf, A. (2007), The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother. Res., 21: 501-506. https://doi.org/10.1002/ptr.2124
- Radünz M, da Trindade MLM, Camargo TM, Radünz AL, Borges CD, Gandra EA, Helbig E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019 Mar 15;276:180-186. doi: 10.1016/j.foodchem.2018.09.173
- Nowak K., Ogonowski J., Jaworska M., Grzesik K. Clove oil—Properties and applications. Chemik. 2012;66:145–152.
- Bevilacqua A., Corbo M.R., Sinigaglia M. Use of essential oils to inhibit Alicyclobacillus acidoterrestris: A short overview of the literature. Front. Microbiol. 2011;2:195. doi: 10.3389/fmicb.2011.00195
- Sellamuthu R. Encyclopedia of Toxicology. Reference Module in Biomedical Sciences. 3rd ed. Elsevier; Amsterdam, The Netherlands: 2014. Eugenol; pp. 539–541.
- Hameed M, Rasul A, Waqas MK, Saadullah M, Aslam N, Abbas G, Latif S, Afzal H, Inam S, Akhtar Shah P. Formulation and Evaluation of a Clove Oil-Encapsulated Nanofiber Formulation for Effective Wound-Healing. Molecules. 2021; 26(9):2491. https://doi.org/10.3390/molecules26092491
- Milind P, Khanna D. Clove: a champion spice. IJRAP. 2011;2:47–54.
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and antibacterial properties of peppermint essential oil loaded poly (ε-caprolactone)(PCL) electrospun fiber mats for wound healing. Front. Bioeng. Biotechnol. 2019, 7, 346.
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cellsnanomed. Biotechnol. 2017, 45, 591–597.
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fabrication and evaluation of nanostructured herbal oil/hydroxypropyl-β-cyclodextrin/polyvinylpyrrolidone mats for denture stomatitis prevention and treatment. AAPS Pharmscitech 2016, 17, 1441–1449.
- Elwakeel, H.A., Moneim, H.A., Farid, M. and Gohar, A.A. (2007), Clove oil cream: a new effective treatment for chronic anal fissure. Colorectal Disease, 9: 549-552. https://doi.org/10.1111/j.1463-1318.2006.01185.x
- Jun-xia, X.; Hai-yan, Y.; Jian, Y. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011, 125, 1267–1272.
- Blaschek W, Ebel S, Hackenthal E, Holzgrabe U, Keller K, Reichling J, Schulz V. HagerROM 2008: Hagers Handbuch der Drogen und Arzneistoffe. Wissenschaftl. VerlagsgesmbH, Stuttgart 2008
- Kunicka-Styczyńska, A., Tyfa, A., Laskowski, D., Plucińska, A., Rajkowska, K., & Kowal, K. (2020). Clove Oil (Syzygium aromaticum L.) Activity against Alicyclobacillus acidoterrestris Biofilm on Technical Surfaces. Molecules (Basel, Switzerland), 25(15), 3334. https://doi.org/10.3390/molecules25153334
- Xu J.G., Liu T., Hu Q.P., Cao X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. 2016;21:1194. doi: 10.3390/molecules21091194
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001 Jul;48 Suppl 1:5-16. doi: 10.1093/jac/48.suppl_1.5. Erratum in: J Antimicrob Chemother 2002 Jun;49(6):1049.
- Gupta C., Garg A., Uniyal R., Gupta S. Comparison of antimicrobial activities of clove oil and its extract on some food borne microbes. Internet J. Microbiol. 2009;7:1–6.
- Pinto E., Vale-Silva L., Cavaleiro C., Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009;58:1454–1462. doi: 10.1099/jmm.0.010538-0
- Hoque M.M., Bari M.L., Juneja V.K., Kawamoto S. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria, and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Rep. National Food Res. Inst. 2008;72:9–21.
- Kim Y.-G., Lee J.-H., Gwon G., Kim S.-I., Park J.G., Lee J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016;6:36377. doi: 10.1038/srep36377
- Husain F.M., Ahmad I., Asif M., Tahseen Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013;38:835–844. doi: 10.1007/s12038-013-9385-9
- Cui H., Zhang C., Li C., Lin L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT. 2020;117:108656. doi: 10.1016/j.lwt.2019.108656
- Kouidhi B, Zmantar T, Bakhrouf A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann Microbiol. 2010;60:599–604.
- Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
- Sharma JN, Srivastava KC, Gan EK. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology. 1994 Nov;49(5):314-8. doi: 10.1159/000139248
- Feng J, Lipton JM. Eugenol: antipyretic activity in rabbits. Neuropharmacology. 1987 Dec;26(12):1775-8. doi: 10.1016/0028-3908(87)90131-6
- Laekeman GM, Van Hoof L, Haemers A, Vanden Berghe DA, Herman AG, Vlietinck AJ. Eugenol a valuable compound for in vitro experimental research and worthwhile for further in vivo investigation. Phytother Res 1990, 4: 90-96.
- Briozzo J, Nunez L, Chirife J, Herszage L, D’Aquino M. Antimicrobial activity of clove oil dispersed in a concentrated sugar solution. J Appl Bacteriol 1989, 66: 69-75.
- Bullerman LB, Lieu FY, Seier SA. Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol. J Food Sci 1977, 42: 1107-1109.
- Fu YJ, Chen LY, Shi XG, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 2007, 21: 989-994.
- Yousef RT, Tawil GG. Antimicrobial activity of volatile oils. Pharmazie 1980, 35: 698-701.
- Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrobials 2005, 4:20.
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000, 88: 308-316.
- Saini A, Sharma S, Chhibber S. Induction of resistance to respiratory tract infection with Klebsiella pneumoniae in mice fed on a diet supplemented with tulsi (Ocimum sanctum) and clove (Syzygium aromaticum) oils. J Microbiol Immunol Infect 2009, 42: 107-113.
- Khan MSA, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol 2009, 49: 354-360.
- Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol 2010, 130: 107-115.
- Bluma RV, Etcheverry MG. Application of essential oils in maize grain: Impact on Aspergillus section Flavi growth parameters and aflatoxin accumulation. Food Microbiol 2008, 25: 324-334.
- Park MJ, Gwak KS, Yang I, Choi WS, Jo HJ, Chang JW, Jeung EB, Choi IG. Antifungal activities of the essential oils in Syzygium aromaticum and Leptospermum petersonii and their constituents against various dermatohytes. J Microbiol 2007, 45: 460-465.
- Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009, 58: 1454-62.
- Chami N, Chami F, Bennis S, Truillas J, Remmal A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Diseases 2004, 8: 217-226.
- Chami F, Chami N, Bennis S, Truillas J, Remmal A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J Antimicrob Chemother2004a, 54: 909-914.
- Lee SJ, Han JI, Lee GS, Park MJ, Choi IG, Na KJ, Jeung EB. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol Pharm Bull 2007, 30: 184-188.
- Astani A, Reichling J, Schnitzler P. Screening for antiviral activities of isolated compounds from essential oil. Evid Based Complement Alternat Med 2009.
- Santoro GF, Cardoso MG, Guimaràes LGL, Mendonça LZ, Soares MJ. Trypanosoma cruzi: Activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp Parasitol 2007, 116: 283-290.
- Eamsobhana P, Yoolek A, Kongkaew W, Lerdthusnee K, Khlaimanee N, Parsartvit A, Malainuai N, Yong HS. Laboratory evaluation of aromatic essential oils from thirteen plant species as candidate repellents against Leptotrombidium chiggers (Acari: Trombiculidae), the vector of scrub typhus. Exp Appl Acarol2009, 47: 257-262.
- Gruenwald J, Brendler T, Jaenicke C, LaGow B, editors. PDR for Herbal Medicines. 3rd ed. Thomson PDR, Montvale 2004
- Li HY, Lee BK, Kim JS, Jung SJ, Oh SB. Eugenol inhibits ATP-induced P2X currents in trigeminal ganglion neurons. Korean J Physiol Pharmacol 2008, 12: 315-321.
- Ohkubo T, Shibata M. The selective capsaicin antagonist capsazepine abolished the antinociceptive action of eugenol and guaiacol. J Dent Res 1997, 76: 848-851.
- Li W, Tsubouchi R, Qiao S, Haneda M, Murakami K, Yoshino M. Inhibitory action of Eugenol compounds on the production of nitric oxide in RAW264.7 macrophages. Biomed Res 2006, 27: 69-74.
- Reiter M, Brandt W. Relaxant effects on tracheal and ileal smooth muscles of the guinea pig. Arzneim Forsch 1985, 35: 408-414.
- Nishijima H, Uchida R, Kameyama K, Kawakami N, Ohkubo T, Kitamura K. Mechanisms mediating the vasorelaxing action of eugenol, a pungent oil, on rabbit arterial tissue. Jpn J Pharmacol 1999, 79: 327-334.
- Zheng GQ, Kenney PM, Lam LKT. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J Nat Prod 1992, 55: 999-1003.
- Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem 2005, 280: 5812-5819.
- Srivastava KC. Antiplatelet principles from a food spice clove (Syzygium aromaticum L). Prostagland Leukotr Essent Fatty Acids 1993, 48:363-372; erratum 1993, 49: 885.
- Rasheed A, Laekeman G, Totte J, Vlietinck AJ, Herman AG. Eugenol and prostaglandin biosynthesis. New England J Med 1984, 310: 50-51.
- Wagner H, Wierer M, Bauer R. In vitro Inhibition of prostaglandin biosynthesis by essential oils and phenolic compounds. Planta Med 1986, 52: 184-187.
- Hübner J. Komplementäre Onkologie: Supportive Maßnahmen und evidenzbasierte Empfehlungen. Schattauer GmbH, Stuttgart 2008
- Dingermann T, Hiller K, Schneider G, Zündorf I. Arzneidrogen. 5th ed. Elsevier, München 2004
- Blumenthal M, Busse WR, Goldberg A, Gruenwald J, et al., editors. The Complete German Commission E Monographs. American Botanical Council, Austin Texas 1998, 221
- Assessment report on Syzygium aromaticum (L.) Merill et L.M. Perry, flos and Syzygium aromaticum (L.) Merill et L.M. Perry, floris aetheroleum. European Medicines Agency Committee on Herbal Medicinal Products (HMPC) 13 September 2011. EMA/HMPC/534946/2010. https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-syzygium-aromaticum-l-merill-et-lm-perry-flos-syzygium-aromaticum-l-merill_en.pdf
- Dorsch W, Loew D, Meyer-Buchtela E, Schilcher H. Kinderdosierungen von Phytopharmaka. 3rd ed. Kooperation Phytopharmaka, Bonn 2002
- Clove Oil BP. https://www.medicines.org.uk/emc/files/pil.4817.pdf
- Taher, Y. A., Samud, A. M., El-Taher, F. E., ben-Hussin, G., Elmezogi, J. S., Al-Mehdawi, B. F., & Salem, H. A. (2015). Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. The Libyan journal of medicine, 10, 28685. https://doi.org/10.3402/ljm.v10.28685
- Anton R, Patri F, Silano V. Plants in cosmetics. Vol 2. Council of Europe Publishing, Strasbourg 2001
- Bouhlal K, Meynadier J, Peyron JL, Meynadier J. The cutaneous effects of the common concretes and absolutes used in the perfume industry. J Essent Oil Res 1989, 1: 169-195.
- Niinimäki A. Delayed-type allergy to spices. Contact Dermatitis 1984, 11: 34-40
- List of references supporting the assessment of Syzygium aromaticum (L.) Merill et L.M. Perry, flos and Syzygium aromaticum (L.) Merill et L.M. Perry, floris aetheroleum EMA/HMPC/534948/2010 Page 3/8Chen HH, Sun CC, Tseng MP, Hsu CJ. A patch test study of 27 crude drugs commonly used in Chinese topical medicaments. Contact Dermatitis 2003, 49: 8-14
- Opdyke DL. Monographs on fragrance raw materials. Food Cosmet Toxicol 1975, 13: 761-763
- Isaacs G. Permanent local anaesthesia and anhidrosis after clove oil spillage. Lancet 1983, 1(8329): 882
- Reichl FX, Mohr K, Hein L, Hickel R. Taschenatlas der Pharmakologie und Toxikologie für Zahnmediziner. Georg Thieme Verlag, Stuttgart 2007
- Grade AC, Martens BPM. Chronic urticaria due to dental eugenol. Dermatologica 1989, 178: 217-22
- Community herbal monograph on Syzygium aromaticum(L.) Merill et L. M. Perry, floris aetheroleum. European Medicines Agency Committee on Herbal Medicinal Products (HMPC) 13 September 2011. https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-syzygium-aromaticum-l-merill-et-l-m-perry-floris-aetheroleum_en.pdf
- Lane BW, Ellenhorn MJ, Hulbert TV, McCarron M. Clove oil ingestion in an infant. Hum Exp Toxicol1991, 10: 291-294.
- Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child 1993, 69: 392-393.
- Brown SA, Biggerstaff J, Savidge GF. Disseminated intravascular coagulation and hepatocellular necrosis due to clove oil. Blood Coagul Fibrinol 1992, 3: 665-668.
- Eisen JS, Koren G, Juurlink DN, Ng VL. N-acetylcysteine fort he treatment of clove oil-induced fulminant hepatic failure. J Toxicol Clon Toxicol 2004, 42: 89-92.
- Janes SE, Price CS, Thomas D. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur J Pediatr 2005, 164: 520-522.