Collateral circulation
Collateral circulation is a network of alternate circulation around a blocked artery or vein via another path, such as nearby minor vessels. Collateral circulation is present in most tissues and provides protection against ischemic injury caused by ischemic stroke, coronary atherosclerosis, peripheral artery disease, and other conditions and diseases 1. Collateral circulation may occur via preexisting vascular redundancy (analogous to engineered redundancy), as in the circle of Willis in the brain, or it may occur via new branches formed between adjacent blood vessels (neovascularization), as in the eye after a retinal embolism. Its formation may be provoked by pathological conditions such as high vascular resistance or ischemia. Unfortunately, this protection falls far short in a large fraction of individuals because of differences in the number or diameter of these vessels present before or their ability to undergo adaptive growth after the onset of occlusive disease.
Collateral circulation are naturally occurring artery-to-artery or arteriole-to-arteriole anastomoses present in healthy tissues that increase their anatomic diameter, that is, outwardly remodel, in obstructive disease. Because they cross-connect 2 feed arteries or the crowns of adjacent arterial trees, respectively, blood flow along their length comes from opposite directions in the healthy tissue at baseline 2. This results in a to-and-fro flow that prevents hemostatic thrombosis, resulting in little or no net flow at a point generally near the midpoint of the collateral. Thus, collaterals reside in a unique hemodynamic environment of low and oscillatory shear stress. Collaterals should not be confused with arteriovenous anastomoses (shunt vessels), which have long been called collaterals in some clinical areas (eg, lung) 3.
Two types of collaterals are distinguished:
- Collateral Arteries: These are artery-to-artery anastomoses that tend to be present in similar locations among human and other mammalian species. They usually carry explicit names in human, for example, superior ulnar collateral artery, genicular artery and other anastomotic arteries around elbows, knees, and other articulations; palmar and plantar arch collaterals, ileolumbar-superior epigastric communicating artery, bronchial-to-pulmonary vein arteries, other collateral arteries in the abdomen and thorax; and anterior and posterior communicating arteries/collaterals of the circle of Willis. Compared with microvascular collaterals (defined below), collateral arteries in healthy young adults generally exhibit minimal or no tortuosity, undergo considerably less anatomic lumen enlargement on a percentage basis (remodeling) in response to a chronic increase in shear stress in obstructive disease 4 and form during embryogenesis by a different process.
- Microvascular Collaterals: These are arteriole-to-arteriole anastomoses that cross- connect a small fraction (generally <0.05%) of arterioles in the crowns of adjacent arterial trees. They are on average <100 μm in diameter in most healthy species, including in human and are present in most but not all tissues (eg, absent in the retinal circulation and noncapsular kidney except in rare circumstances) 5. Examples include pial (leptomenigeal) collaterals of the brain and spinal cord, coronary collaterals, collaterals in skeletal muscle and skin. Depending on species and tissue, several collaterals may have artery-size calibers (>150 μm; the diameter generally used to distinguish arterioles from arteries) 6, for example, in the healthy heart 7 and between the crowns of the superior and inferior epigastric and other thoracoabdominal artery trees. Characteristics of microvascular collaterals include significant tortuosity even in young adults, outward remodeling of their lumen diameters generally by 5- to 10-fold in humans with occlusive disease 8 and, at least in mouse strains, large genetic background–dependent variability in their number, diameter, and remodeling 9. Because many collateral arteries carry explicit names (as given above), the use of term collaterals alone will usually imply the population of microvascular collaterals in a given tissue.
Collateral arteries and microvascular collaterals usually have ≥1 side branches. Thus, besides presumably helping to optimize regional metabolic control of oxygen delivery in healthy tissues, collaterals also serve as scaffolds for delivery of blood flow to parenchymal tissue within the collateral zone (watershed) between adjacent artery trees 9.
The above collateral vessels are further distinguished from other types of arterial anastomoses that are collateral-like but are not denoted as collaterals per se. There are two types:
- Intratree Anastomoses: These are arteriole-to-arteriole anastomoses that, where present, connect adjacent branches within a given tree. They are present in many tissues, for example, in heart 7, skeletal muscle 10, intestinal mesentery,8 and the cerebral cortex of many species (eg, rat16 but not mouse) 11. Like collaterals, they can have opposing flow at their midpoint 6. They also serve the same endogenous bypass function of collaterals if an adjacent branch becomes obstructed 12. However, because they protect much less tissue, do not interconnect separate arterial trees, are much shorter, and have little or no tortuosity, they are denoted with a unique term.
- Arcade Arteries: These are arteries that often take an arching course and may or may not, depending on the individual, anastomose with another artery, for example, gastric and omental arcade arteries, marginal mesenteric arteries, pancreaticoduodenal arcade, gastroepiploic artery, internal thoracic artery, collateral intercostal artery, vasa vasorum of the aorta, and arteries supplying long axial structures (eg, bile duct, trachea, spinal cord) 13.
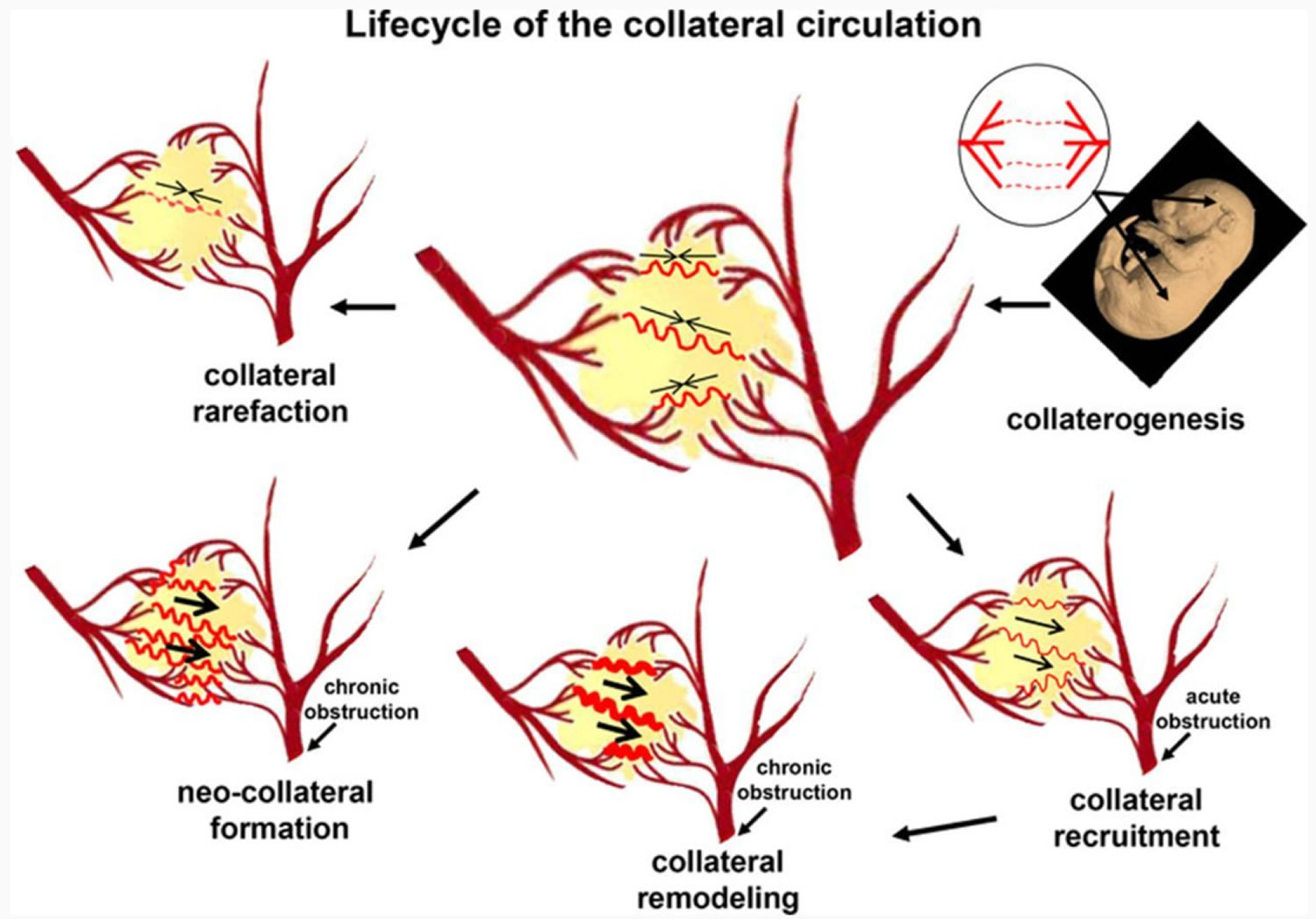
Footnote: Major aspects of the vascular biology of the collateral circulation. Embryonic formation of native collaterals (collaterogenesis), which is depicted in the top right as several collaterals forming between 2 trees, begins at ≈E14 in mouse and determines collateral extent in the adult (middle diagram with several collaterals shown connecting 2 arterial trees). Acute obstruction induces flow across the collateral network (recruitment), followed by remodeling, and, potentially, formation of additional collaterals in chronic obstructive disease (neocollateral formation). Loss of native collaterals (rarefaction) can be caused by aging and other risk factors.
[Source 1 ]Coronary collateral circulation
Anastomotic channels, known as collateral vessels, connect a territory supplied by one epicardial coronary artery with that supplied by another 14. Collateral arteries therefore provide an alternative source of blood supply to myocardium that has been jeopardized by occlusive coronary artery disease, and they can help to preserve myocardial function in the setting of coronary artery disease 15.
Coronary collateral circulation is a network of tiny blood vessels, and under normal conditions, not open. When the coronary arteries narrow to the point that blood flow to the heart muscle is limited (coronary artery disease), collateral vessels may enlarge and become active. This allows blood to flow around the blocked artery to another artery nearby or to the same artery past the blockage, protecting the heart tissue from injury. While their growth is often thought to be initiated by ischemia, collateral arteries are also present in individuals who do not have coronary disease 16. Obviously, other factors seem to play a more important role.
Although collateral blood flow after epicardial coronary occlusion may be sufficient in some patients to meet myocardial needs at rest, the prevalent view is that collateral circulation is generally not sufficient to meet myocardial demands during exercise 17 and may not prevent myocardial ischemia during coronary occlusion. To prevent myocardial ischemia during acute vessel occlusion, a flow of 20% to 25% is generally regarded as sufficient to provide the blood supply needed at rest 18. One in four patients without coronary artery disease has sufficient collaterals as compared with one in three patients with coronary artery disease 19. The reasons for this are not fully understood, but genetic factors are likely to play a role 20.
Coronary collateral circulation confers a protective blood supply to the myocardium jeopardized by ischemia. During embryological development, an extensive overlay of inter coronary anastomoses derives from endothelial precursor cells which, in response to local signals, migrate and differentiate into new vessels in a process termed vasculogenesis. With progressive luminal encroachment of atheromatous plaque, collateral vasculature is remodeled to reduce overall resistance to the epicardial coronary flow which entails a decrease in the number of conduits and an increase in the caliber from 10–200 μm to 100–800 μm 21. Following total or subtotal occlusion of an epicardial artery, arteriogenesis (recruitment of preexisting collateral channels) occurs conjointly with angiogenesis (sprouting of new vessels) to salvage the ischemic myocardium by shunting the blood from nonjeopardized territories. The mechanism responsible for modulating the processes of coronary vasculogenesis, arteriogenesis, and angiogenesis remains elusive. In the setting of myocardial infarction, the presence of well-developed collateralization is associated with a reduced infarct size 22, mitigated QT prolongation 23, lessened post-infarct ventricular dilatation 24 and decreased mortality 25. However, further prospective studies are warranted to determine whether therapeutic promotion of coronary collateral growth translates into favorable cardiovascular outcomes.
Table 1. Clinical factors that can influence collateral circulation in the heart
| Factor (reference) | Remarks |
|---|---|
| Degree of coronary stenosis | Strongest predictor, confirmed in several studies |
| Proximal lesion location | |
| Longer duration of symptoms | |
| Longer duration of lesion occlusion | In patients with chronic total occlusions |
| Heart rate (lower) | Only in patients without coronary artery disease |
Figure 1. Coronary collateral circulation
Footnote: Schematic drawing of the coronary artery circulation with (A) and without (B) interarterial anastomoses between the right coronary artery and the occluded left anterior descending artery (LAD; occluded beyond the third diagonal branch). The gray area indicates the area at risk for myocardial infarction in case of the left anterior descending artery occlusion and in the absence of collaterals (corresponding to the infarct size in the example on the right side).
[Source 29 ]Assessing the collateral circulation of the heart
Except for the situation with a known chronic total coronary occlusion, there is currently no technique to quantify the collateral circulation non-invasively in human. The easiest strategy is the visual assessment of collateral arteries by coronary angiography. This can be performed in a semiquantitative manner as described by Rentrop et al. 30. The Rentrop method involves balloon occlusion of the contralateral coronary artery, which is rarely performed. Collateral vessels from patent to occluded are classified ranging from grade 0 (no visible filling of any collateral channel), grade 1 (filling of the side branches of the occluded artery, with no dye reaching the epicardial segment), grade 2 (partial filling of the epicardial vessel), and grade 3 (complete filling of the epicardial vessel by collaterals) 31.
Instead, most clinicians and investigators apply the Rentrop score without occluding the contralateral vessels. However, a patent contralateral coronary artery increases the back pressure in this collateral-receiving territory, which underestimates the degree of collateralization. This visual method has several other limitations: it is not a very objective measure, and it is influenced by blood pressure and the force of contrast injection as well as the duration of filming.
The currently most accurate assessment method measures the so-called collateral flow index (CFI). Two methods are available: one is based on Doppler velocity measurements, which is limited by frequent artifacts. The second one is more accurate and based on pressure measurement. For the Doppler approach, the collateralization of a certain coronary artery can be measured by placement of a Doppler sensor tipped guide wire. Then, the antegrade flow through the coronary artery needs to be blocked with an angioplasty balloon. The flow velocity measured with the Doppler sensor distal to the occluded vessel derives from collaterals. Then, the vessel is angioplastied so that there is no remaining lesion and the flow velocity measured again, which represents the flow through the normal vessel. The collateral flow velocity is then compared to the flow velocity through the open coronary artery and indicates the percentage of normal blood flow that can be preserved via the collateral circulation in case of an abrupt vessel occlusion 32.
Pressure-based collateral flow index (CFI)
The pressure index of the distal pressure during vessel occlusion divided by the systemic blood pressure both subtracted by central venous pressure measures a pressure-derived collateral flow index (CFI). The central venous pressure has to be taken into account as a back pressure 33. Another, simpler, cheaper and very accurate way to measure collateral function is an intracoronary electrocardiogram (ECG) 18. Studies have defined a threshold of ST-segment elevation ≥0.1 mV during a 1 to 2 minute vessel occlusion with an angioplasty balloon to define insufficient collateralization. In addition, if the patient develops chest pain during balloon occlusion of the vessel, this can be regarded as a sign of insufficient collateral function.
All three methods, Rentrop score, collateral flow index (CFI) and intracranial ECG, predict clinical outcomes and are therefore useful 34. For research purposes, the CFI is clearly superior because it is a continuous value while the Rentrop score is an ordinal and the ECG a dichotomous variable. The CFI is therefore more informative and increases the statistical power.
Other methods have been described, such as ‘wash-out collaterometry’ whereby the time to contrast dye clearance distal to a balloon occluded artery is measured. The washout is quicker the better the vessel is collateralized 35. However, in contrast to the above-mentioned methods, none of these have shown a predictive value in clinical practice.
Clinical significance of coronary collateral circulation
The clinical relevance has been disputed repeatedly since the anastomoses are often incapable of restoring flow to normal levels 36. In fact, the presence of collaterals was sometimes even assumed to signify a worsening prognosis 37.
In the setting of an acute infarction, the relevance of coronary collaterals has been shown in preserving myocardial function 38, limiting infarct size 39, and positively influence post-infarct remodeling 40. Increased collateral flow was also associated with less need for intra-aortic balloon pumping post-percutaneous coronary intervention (PCI) and better myocardial blush grade 41. The presence of collaterals also appears to reduce mortality in patients, primarily due to a lower frequency of cardiogenic shock 42. Such observations support the view that collateral flow is a modifying factor, capable of alleviating the deleterious effects of atherosclerosis on cardiovascular morbidity and mortality.
To date, 12 studies have investigated the effect of collaterals on mortality. The first of these studies was published in 1971 in the New England Journal of Medicine 43. Only three of these trials demonstrated a clear benefit for collaterals. This inconsistency did not actually help to resolve the dispute 33. The inconsistency is partially explainable by the method of collateral assessment used in most of the studies; collaterals were ‘qualified’ visually during the coronary angiography 44. This represents a rather crude approach. Intracoronary flow or pressure-based methods (collateral flow index) using a pressure or Doppler sensor tipped guide wire are more accurate 19. The relevance of the collateral circulation in case of a chronic total occlusion of a coronary artery with normal left ventricular function is fairly obvious. There are even extreme examples of patients with left main artery occlusion or three-vessel occlusion with only mild symptoms 45. Beside this anecdotal evidence, a pooled analysis of the above mentioned 12 studies (including 6,529 patients) clearly showed that, overall, well developed collaterals are associated with a reduced mortality 46. On average, the mortality was reduced by about 35%.
Even though collaterals seem to have a protective effect balancing all the available evidence, they have been found to be associated with a higher risk for restenosis. A meta-analysis of 7 studies recruiting a total of 1,425 patients showed that patients with good collateralization have a 40% higher risk of restenosis as compared with patients with poor collateralization 47. However, whether this association is causal or whether collaterals just present a risk marker is unclear. It could be an indication for the function of collaterals that prevent sufficient flow through the stented vessel. Potentially, collaterals would have been able to supply the subtended myocardium alone in these situations, rendering stenting unnecessary. Regardless, collaterals seem to be a useful and easily available marker on an individual patient level for the clinical decision-making process. In patients with better developed collaterals, cardiologists should try to reduce the risk of restenosis by using drug-eluting instead of bare-metal stents, or by prescribing cilostazol 48.
Multiple strategies to enhance collateral function have been tested (Table 2). The important role of shear stress and of monocytes have both been used as targets for the therapeutic induction of collaterals. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) are growth factors that increase monocyte numbers and they have both shown to improve collateral function 49. Their mechanism of action is probably via their effect on the number but also on the gene expression profile of monocytes, a further mechanism is the release of endothelial progenitor cells from the bone marrow 49. Another therapeutic option is to increase shear stress via external counterpulsation 50 or via physical exercise 51; both strategies have demonstrated an effect on collateral function. External counterpulsation (ECP) can be regarded as a simulation of physical exercise in that it increases shear forces on endothelial cells. It has repeatedly been shown to reduce symptoms in patients with angina pectoris but the mechanism of action has remained unknown for years. The first controlled trial in a group of patients with coronary artery disease undergoing a 30-h program of high-pressure external counterpulsation (300 mmHg) and in a group undergoing sham external counterpulsation (ECP) at 80 mmHg inflation pressure has demonstrated a relevant improvement of the collateral function (CFI) between baseline and follow-up at 4 weeks 50.
Another promising means to increase collateral artery growth is heart rate reduction using ivabradine. Bradycardia is known to be associated with better collateralization (Table 2), probably because, due to prolongation of the diastole, the lower heart rate increases the endothelial shear stress. Experimental studies indicated a benefit of ivabradine on collateral growth 52. A clinical study to test this concept in human is currently underway (clinicaltrials.gov identifier NCT01039389).
Table 2. Factors that have been tested to improve coronary collateral circulation
| Method (references) | Positive effecta | Application | Remarks |
|---|---|---|---|
| Exercise 53 | Yes | No randomized data, increase in CFI. | |
| External counterpulsation 50 | Yes | One randomized controlled trial (RCT), observational studies, increase in CFI, improvement of angina symptoms | |
| GM-CSF 54 | Yes | Intracoronary, subcutaneous | Two small RCTs (n = 21, n = 12). Stopped early because of potential plaque destabilization. Increase in CFI. |
| G-CSF 55 | Yes | Subcutaneous | One small randomized trial (n = 52). Increase in CFI. |
| Dipyridamole 56 | Yes | Very small trial (n = 30); angiographic collateral assessment, which is not very accurate | |
| VEGF 57 | No | Intracoronary | No difference in angina symptoms or exercise tolerance |
| FGF4 (adenovirus) 58 | No | Intracoronary | No change in exercise tolerance |
Footnote: a) Based on a random effects meta-analysis model if more than one study.
Abbreviations: CFI = collateral flow index; FGF4 = fibroblast growth factor 4; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; VEGF = vascular endothelial growth factor.
Summary
Coronary collateral arteries serve as conduits that bridge severe stenosis or connect a territory supplied by one epicardial artery with that supplied by another. They can be recruited if required. While coronary collaterals provide substantial blood flow to the resting heart, they are often insufficient during increased myocardial oxygen demand (for example, exercise). Collateral arteries can reduce infarct size, the risk for post-infarct complications and they can also reduce mortality. Besides known triggers of tangential shear stress and the presence of bone-marrow-derived mononuclear cells for collateral growth, first clinical proof-of-concept trials have demonstrated that collateral growth can be promoted therapeutically by physical exercise, external counterpulsation and certain growth factors and cytokines (granulocyte colony-stimulating factor [G-CSF] and granulocyte-macrophage colony-stimulating factor [GM-CSF]).
Collateral circulation brain
Collateral circulation of the brain refers to the auxiliary vascular structures that compensate cerebral blood flow when ‘normal’ blood flow is impaired or restricted due to severe stenosis or occlusion of the principal supplying arteries or other focal or systemic situations 59. Cerebral collateral circulation is usually divided into primary, secondary and tertiary collaterals. Primary collaterals refer to the arterial segments of the circle of Willis; secondary collaterals include the ophthalmic artery and leptomeningeal arteries, as well as other anastomoses between the distal, small-caliber arteries; and tertiary collaterals refer to newly developed microvessels through angiogenesis at the periphery of ischemic regions 60.
The arterial anatomy of the collateral circulation includes extracranial sources of cerebral blood flow (Figure 2) and intracranial routes of ancillary perfusion (Figure 3) that are commonly divided into primary or secondary collateral pathways. Primary collaterals include the arterial segments of the circle of Willis, whereas the ophthalmic artery and leptomeningeal vessels constitute secondary collaterals. Interhemispheric blood flow across the anterior communicating artery and reversal of flow in the proximal anterior cerebral artery provide collateral support in the anterior portion of the circle of Willis. The posterior communicating arteries may supply collateral blood flow in either direction between the anterior and posterior circulations. Additional interhemispheric collaterals include the proximal posterior cerebral arteries at the posterior aspect of the circle of Willis. Considerable variability exists in the anatomy of the circle of Willis, with frequent asymmetry and an ideal configuration in only a minority of cases. Anatomic studies note absence of the anterior communicating artery in 1% of subjects, absence or hypoplasia of the proximal anterior cerebral artery in 10%, and absence or hypoplasia of either posterior communicating artery in 30% 61. Reversal of blood flow within the ophthalmic artery may provide secondary collateral support. Anastomoses between distal segments of the major cerebral arteries also contribute ancillary collateral blood flow. The number and size of these anastomotic vessels are greatest between anterior and middle cerebral arteries, with smaller and fewer connections between middle and posterior cerebral arteries and even less prominent terminal anastomoses between posterior and anterior cerebral arteries. Distal branches of the major cerebellar arteries similarly provide collateral links across the vertebral and basilar segments of the posterior circulation. Leptomeningeal and dural arteriolar anastomoses with cortical vessels further enhance the collateral circulation. Other collateral routes are less commonly encountered in acute stroke, such as the tectal plexus joining supratentorial branches of the posterior cerebral artery with infratentorial branches of the superior cerebellar artery; the orbital plexus linking the ophthalmic artery with facial, middle meningeal, maxillary, and ethmoidal arteries; and the rete mirabile caroticum connecting internal and external carotid arteries. The course and anatomic characteristics of collaterals may vary extensively, with atypical collaterals such as anterior choroidal supply from the posterior circulation induced by pathophysiological conditions 62. Moyamoya syndrome represents the ultimate example of excessive collateralization over a chronic time course, recruiting a wide range of leptomeningeal and deep parenchymal vessels. The deep parenchymal arteries of the basal ganglia are normally poorly developed. Collateral vessels are formed during the prenatal period, although pathophysiological conditions may cause secondary changes. The collateral ability of a vessel is ultimately determined by luminal caliber 63.
Venous collaterals augment drainage of cerebral blood flow when principal routes are occluded or venous hypertension ensues. The anatomy of venous collateral circulation is highly variable, allowing diversion of blood through numerous routes when exiting the brain (Figure 4).
Figure 2. Extracranial arterial collateral circulation
Footnote: Extracranial arterial collateral circulation. Shown are anastomoses from the facial (a), maxillary (b), and middle meningeal (c) arteries to the ophthalmic artery and dural arteriolar anastomoses from the middle meningeal artery (d) and occipital artery through the mastoid foramen (e) and parietal foramen (f).
[Source 64 ]Figure 3. Intracranial arterial collateral circulation
Footnote: Intracranial arterial collateral circulation in lateral (A) and frontal (B) views. Shown are posterior communicating artery (a); leptomeningeal anastomoses between anterior and middle cerebral arteries (b) and between posterior and middle cerebral arteries (c); tectal plexus between posterior cerebral and superior cerebellar arteries (d); anastomoses of distal cerebellar arteries (e); and anterior communicating artery (f).
[Source 64 ]Figure 4. Venous collateral circulation
Footnote: Venous collateral circulation. Shown are pterygoid plexus (a), deep middle cerebral vein (b), inferior petrosal sinus and basilar plexus (c), superior petrosal sinus (d), anastomotic vein of Trolard (e), anastomotic vein of Labbé (f), condyloid emissary vein (g), mastoid emissary vein (h), parietal emissary vein (i), and occipital emissary vein (j).
[Source 64 ]Collateral circulation brain pathophysiology
The process of collateral recruitment depends on the caliber and patency of primary pathways that may rapidly compensate for decreased blood flow and the adequacy of secondary collateral routes. Primary collaterals provide immediate diversion of cerebral blood flow to ischemic regions through existing anastomoses. Secondary collaterals such as leptomeningeal anastomoses may be anatomically present, although enhanced capacity of these alternative routes for cerebral blood flow likely requires time to develop. Although the specific pathophysiological factors leading to the development of collaterals are uncertain, diminished blood pressure in downstream vessels is considered a critical variable 65. The opening of collaterals likely depends on several compensatory hemodynamic, metabolic, and neural mechanisms. Angiogenesis may stimulate collateral growth at the periphery of an ischemic region 66. Focal cerebral ischemia may lead to the secretion of angiogenic peptides with some potential for collateral formation, although these vessels may be designed for removal of necrotic debris rather than augmentation of cerebral blood flow 67. Experimental data on middle cerebral artery occlusion in rats demonstrates the temporal dependence of collateral development 68. Clinical observations further emphasize the pace of cerebral ischemia as a critical variable, with collateral capacity improving over time 69. The influence of comorbidities and other clinical variables on the development of intracranial collaterals in humans is unknown, as no prospective studies have been conducted. Hypertension decelerates the development of collaterals in rats, and the anastomoses are significantly narrower, with diminished collateral capacity 68. Extrapolation from rats to humans is limited, however, by anatomic and likely pathophysiological differences 70.
The incipient development of collaterals does not guarantee their persistence. Hemodynamic fluctuations may influence the endurance of collaterals, possibly threatening cerebral blood flow. Similarly, distal fragmentation of a thrombus within the parent vessel may occlude distal branches supplying retrograde collateral flow from cortical arteries. The efficacy of collateral vessels likely depends on age, duration of ischemia, and associated comorbidities.
Chronic hypoperfusion due to arterial flow restrictions such as extracranial carotid stenosis or intracranial stenotic disease promotes collateral development, although the relationship of these collaterals with cerebral blood flow and clinical symptomatology remains unclear. Secondary collateral pathways that require time to develop are presumed to be recruited once primary collaterals at the circle of Willis have failed. Although longitudinal studies have not chronicled this sequence of collateral failure, the presence of secondary collateral pathways is considered a marker of impaired cerebral hemodynamics. Increasing severity of carotid stenosis has been correlated with a greater extent of collateralization. Attempts to correlate various collateral patterns with hemodynamic and metabolic parameters have yielded conflicting results across several studies 71. Some of these discrepancies may result from employing variable methodology, including MR spectroscopy, CO2 reactivity with transcranial Doppler ultrasonography, and positron emission tomography. Inadequate angiographic assessment of all potential collateral routes may also account for these conflicting results. The clinical manifestations of carotid occlusive disease likely depend on multiple variables including time course, degree of luminal stenosis, and status of the collateral circulation ultimately effecting changes in cerebral perfusion pressure. The definition of a “hemodynamically significant” carotid stenosis must therefore account for the status of collaterals 72.
The collateral circulation is also a critical determinant of cerebral perfusion pressure in acute cerebral ischemia. The hemodynamic effects of the collateral circulation may be important in maintaining perfusion to penumbral regions, but these collateral vessels may also facilitate clearance of fragmented thrombus from more proximal locations 73. Deep parenchymal collaterals within the striatum may be less effective, allowing undissolved thrombus to be retained for longer periods of time. These factors may be involved in the development of large subcortical infarcts with cortical sparing of the basal ganglia in middle cerebral artery occlusion and limited thalamic infarction in posterior cerebral artery occlusion. Studies of regional cerebral blood flow with various modalities have demonstrated decreased regional cerebral blood flow in cortical areas peripheral to subcortical infarcts. Although metabolic factors and diaschisis may account for these findings, diminished regional cerebral blood flow may simply be the result of marginal collateral blood flow 74.
Assessing the collateral circulation of the brain
Numerous techniques, including xenon-enhanced CT, single-photon emission CT, positron emission tomography, CT perfusion, and MR perfusion, assess cerebral blood flow and thereby infer the status of collaterals. These diagnostic modalities provide information regarding the amount of blood flow to specific regions of the brain, although the arterial source of sustained perfusion may not be evident when the parent vessel is occluded. Prolonged transit times of arterial blood flow may be indicative of collateral blood supply on perfusion studies. Relatively subtle findings such as vascular enhancement on conventional neuroimaging studies, including CT and MRI, may also be representative of collateral blood flow 75. Vascular enhancement may persist for several weeks after the onset of ischemia. Vascular hyperintensities on fluid-attenuated inversion recovery (FLAIR) MRI sequences may be another relatively subtle manifestation of collateral flow (Figure 5). Although such indirect evidence of collaterals may be apparent with multiple imaging techniques, only limited information regarding collaterals can be accrued.
Direct visualization of collaterals is limited to angiographic methods including transcranial Doppler ultrasonography, CT angiography (CTA), MR angiography (MRA), and conventional angiography. Technical aspects of each of these diagnostic modalities confer specific advantages as well as limitations. Conventional angiography is considered the gold standard, although objective evaluation of collaterals is rarely performed. Variation in contrast volume and pressure during injection may distort the appearance of distal vessels. Angiographic scales incorporating aspects of collateral blood supply are considerably subjective, with inconsistent use across studies 76. Incomplete information regarding collaterals is obtained unless multivessel injections are performed. Noninvasive techniques have limited resolution, precluding evaluation of leptomeningeal and other secondary collateral pathways. transcranial Doppler ultrasonography is used primarily for evaluation of collateral routes at the circle of Willis, although inadequacy of transtemporal bone windows frequently limits evaluation. Transcranial color-coded duplex ultrasonography identifies vessels on color-coded B-mode images 63, which may be improved with contrast administration 77. Cerebral vasomotor reactivity testing with transcranial Doppler ultrasonography may provide information on autoregulation and collateral status, employing serial evaluation of blood flow in response to a vasodilatory stimulus, such as CO2 inhalation, acetazolamide injection, or apnea 78. These vasodilatory stimuli have somewhat different hemodynamic effects, conferring relative advantages and disadvantages of each approach 79. Transcranial Doppler ultrasonography vasomotor reactivity testing with CO2 has been correlated with stroke risk in carotid stenosis and the need for shunting during carotid endarterectomy 80. Impaired vasomotor reactivity has also been correlated with the extent of collateralization 81. Transcranial Doppler ultrasonography performance and interpretation, however, are subject to considerable variability, and validation of vasomotor reactivity testing has been suboptimal 82. CT angiography source images may contain valuable information regarding collaterals, but systematic review of these raw images has met limited success 83. Postprocessing of CT angiography data may be more informative, but use of these images is far less practical. Collateral assessment with MR angiography (MRA) is generally limited to proximal arterial segments at the circle of Willis. MRA velocity encoding during acquisition allows for flow-sensitive images in 3 orthogonal planes; however, these images are constrained by anatomic resolution and are therefore only useful in proximal segments as well 84.
Inconsistency in the results of studies focused on collaterals may be due in part to variability in the diagnostic evaluation. Although both approaches provide information regarding collaterals, perfusion techniques cannot be directly compared with angiographic modalities. The specific advantages and limitations of each modality must be considered, and the timing of studies is crucial as collaterals evolve with time from the incipient ischemic event. The contribution of all potential collateral routes must be considered, and objective scales, rather than the presence or absence of specific arterial segments, must be employed to adequately formulate conclusions regarding the role of collaterals.
Figure 5. Collateral blood flow MRI
Footnote: Collateral blood flow distal to an occlusion of the middle cerebral artery manifest as vascular enhancement (A, arrow) and FLAIR vascular hyperintensity (B, arrow).
Collateral circulation of the brain prognostic implications
The status of collaterals in acute stroke may have several prognostic implications. Numerous studies have shown that residual perfusion in an ischemic region of the brain is an important determinant of clinical recovery and hemorrhagic transformation 85. Although the extent of collateral blood supply affects residual perfusion in ischemic territory, the exact relationship of these variables and the relative impact of all collateral vessels are unclear. Early clinical improvement during the first 48 hours of ischemia may be linked to the presence of collaterals 86. Systematic angiographic evaluation of collaterals before thrombolysis suggests an increased mortality in the absence of significant collateralization 87. The presence of leptomeningeal collaterals is also predictive of improved long-term clinical outcome in patients treated with and without thrombolysis for middle cerebral artery occlusion 88. Reperfusion of ischemic regions by collaterals may improve blood flow and minimize the extent of infarction, but collateral flow may also promote hemorrhagic transformation.
The presence of collaterals on conventional angiography has been associated with a lower risk of hemispheric stroke and transient cerebral ischemia in patients with carotid stenosis 89. This finding promotes the use of angiography for prognostication and risk stratification in carotid endarterectomy candidates. Studies of carotid artery occlusion have suggested that individuals with impaired collaterals suffer from an increased incidence of stroke 90. Border zone infarcts have been associated with absence of anterior communicating artery flow 91 and hypoplasia or absence of the posterior communicating artery 92. Others have suggested that cerebral metabolism and hemodynamics may be normal as long as one of the primary collateral pathways is present, although complete absence of the primary collateral pathways has pathophysiological consequences 93. Hypertension may impair collateral development in the setting of carotid occlusion and therefore increase stroke risk 94.
Summary
The collateral circulation constitutes an important aspect of cerebrovascular disease that remains largely unappreciated. Diagnostic modalities for the detection and characterization of collaterals require further refinement. As multivessel conventional angiography is impractical for all subjects, the development of noninvasive approaches that combine angiographic information with perfusion data would promote our understanding of the collateral circulation considerably. Objective scales for assessment of collateral perfusion need to be practical, with proven reliability and validity. Clarification of the relationship between collaterals and perfusion mismatch may advance development of an image-based thrombolytic window for acute stroke. Further understanding of collaterals may also explain differences in clinical outcome, enable risk stratification for individual subjects, and expand treatment options for acute stroke and chronic cerebrovascular disorders.
References- A Brief Etymology of the Collateral Circulation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:1854–1859 https://doi.org/10.1161/ATVBAHA.114.303929
- Toriumi H, Tatarishvili J, Tomita M, Tomita Y, Unekawa M, Suzuki N. Dually supplied T-junctions in arteriolo-arteriolar anastomosis in mice: key to local hemodynamic homeostasis in normal and ischemic states?Stroke. 2009; 40:3378–3383.
- Porres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins.Radiographics. 2013; 33:999–1022.
- Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age.Stroke. 2012; 43:3052–3062.
- Shoja MM, Tubbs RS, Loukas M, Shokouhi G, Ghabili K, Agutter PS. The sub-peritoneal arterial plexus of Sir William Turner.Ann Anat. 2010; 192:194–198.
- Zweifach BW. Microcirculation.Annu Rev Physiol. 1973; 35:117–150.
- van Horssen P, Siebes M, Spaan JA, Hoefer IE, van den Wijngaard JP. Innate collateral segments are predominantly present in the subendocardium without preferential connectivity within the left ventricular wall.J Physiol. 2014; 592(Pt 5):1047–1060.
- van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research.J Am Coll Cardiol. 2009; 55:17–25.
- Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke.J Cereb Blood Flow Metab. 2010; 30:923–934.
- Eriksson E, Germann G, Mathur A. Microcirculation in muscle.Ann Plast Surg. 1986; 17:13–16.
- Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation.J Mol Cell Cardiol. 2010; 49:251–259.
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke.Stroke. 2001; 32:2179–2184.
- Uflacker RAtlas of Vascular Anatomy. An Angiographic Approach, 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.
- Interarterial coronary anastomoses. Occurrence in normal hearts and in certain pathologic conditions. PITT B. Circulation. 1959 Nov; 20():816-22.
- The human coronary collateral circulation. Seiler C. Eur J Clin Invest. 2010 May; 40(5):465-76.
- Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40:465–476. doi: 10.1111/j.1365-2362.2010.02282.x.
- Bache RJ, Schwartz JS. Myocardial blood flow during exercise after gradual coronary occlusion in the dog. Am J Physiol. 1983;245:H131–H138.
- de Marchi SF, Streuli S, Haefeli P, Gloekler S, Traupe T, Warncke C, Rimoldi SF, Stortecky S, Steck H, Seiler C. Determinants of prognostically relevant intracoronary electrocardiogram st-segment shift during coronary balloon occlusion. Am J Cardiol. 2012;110:1234–1239. doi: 10.1016/j.amjcard.2012.06.023
- Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–2220. doi: 10.1161/01.CIR.0000066321.03474.DA
- Meier P, Antonov J, Zbinden R, Kuhn A, Zbinden S, Gloekler S, Delorenzi M, Jaggi R, Seiler C. Non-invasive gene-expression-based detection of well-developed collateral function in individuals with and without coronary artery disease. Heart. 2009;95:900–908. doi: 10.1136/hrt.2008.14538
- ARTERIAL ANASTOMOSES IN THE CORONARY CIRCULATION. I. ANATOMICAL FEATURES IN NORMAL AND DISEASED HEARTS DEMONSTRATED BY STEREOARTERIOGRAPHY. Scott Med J. 1963 Nov;8:420-34. https://doi.org/10.1177/003693306300801102
- Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation. 1991 Mar;83(3):739-46. https://doi.org/10.1161/01.CIR.83.3.739
- Pascal Meier, Steffen Gloekler, Stefano F. de Marchi, Rainer Zbinden, Etienne Delacrétaz, Christian Seiler, An indicator of sudden cardiac death during brief coronary occlusion: electrocardiogram QT time and the role of collaterals, European Heart Journal, Volume 31, Issue 10, May 2010, Pages 1197–1204, https://doi.org/10.1093/eurheartj/ehp576
- Collateral channels that develop after an acute myocardial infarction prevent subsequent left ventricular dilation. J Am Coll Cardiol. 1996 Apr;27(5):1133-9. https://doi.org/10.1016/0735-1097(95)00596-X
- Pascal Meier, Harry Hemingway, Alexandra J. Lansky, Guido Knapp, Bertram Pitt, Christian Seiler, The impact of the coronary collateral circulation on mortality: a meta-analysis, European Heart Journal, Volume 33, Issue 5, March 2012, Pages 614–621, https://doi.org/10.1093/eurheartj/ehr308
- de Marchi SF, Gloekler S, Meier P, Traupe T, Steck H, Cook S, Vogel R, Seiler C. Determinants of preformed collateral vessels in the human heart without coronary artery disease. Cardiology. 2011;118:198–206. doi: 10.1159/000328648
- Piek JJ, van Liebergen RA, Koch KT, Peters RJ, David GK. Clinical, angiographic and hemodynamic predictors of recruitable collateral flow assessed during balloon angioplasty coronary occlusion. J Am Coll Cardiol. 1997;29:275–282. doi: 10.1016/S0735-1097(96)00499-8
- Werner GS, Ferrari M, Betge S, Gastmann O, Richartz BM, Figulla HR. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation. 2001;104:2784–2790. doi: 10.1161/hc4801.100352
- The collateral circulation of the heart. BMC Med. 2013; 11: 143. doi: 10.1186/1741-7015-11-143 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3689049
- Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/S0735-1097(85)80380-6
- Seiler C. Collateral Circulation of the Heart. London, UK: Springer-Verlag; 2009.
- Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–1279. doi: 10.1016/S0735-1097(98)00384-2
- Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40:465–476. doi: 10.1111/j.1365-2362.2010.02282.x
- Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33:614–621. doi: 10.1093/eurheartj/ehr308
- Seiler C, Billinger M, Fleisch M, Meier B. Washout collaterometry: a new method of assessing collaterals using angiographic contrast clearance during coronary occlusion. Heart. 2001;86:540–546. doi: 10.1136/heart.86.5.540
- Meier P, Seiler C. The coronary collateral circulation-clinical relevances and therapeutic options. Heart. in press.
- Schaper W. Collateral vessels reduce mortality. Eur Heart J. 2012;33:564–566. doi: 10.1093/eurheartj/ehr385
- Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–476. doi: 10.1161/01.CIR.74.3.469
- Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, Bolli R. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation. 1991;83:739–746. doi: 10.1161/01.CIR.83.3.739
- Kodama K, Kusuoka H, Sakai A, Adachi T, Hasegawa S, Ueda Y, Mishima M, Hori M, Kamada T, Inoue M, Hirayama A. Collateral channels that develop after an acute myocardial infarction prevent subsequent left ventricular dilation. J Am Coll Cardiol. 1996;27:1133–1139. doi: 10.1016/0735-1097(95)00596-X
- Elsman P, Hof AW v ’t, de Boer MJ, Hoorntje JC, Suryapranata H, Dambrink JH, Zijlstra F. Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J. 2004;25:854–858. doi: 10.1016/j.ehj.2004.03.005
- Perez-Castellano N, Garcia EJ, Abeytua M, Soriano J, Serrano JA, Elizaga J, Botas J, Lopez-Sendon JL, Delcan JL. Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am Coll Cardiol. 1998;31:512–518.
- Helfant RH, Vokonas PS, Gorlin R. Functional importance of the human coronary collateral circulation. N Engl J Med. 1971;284:1277–1281. doi: 10.1056/NEJM197106102842301
- Meier P. The sword of Damocles: an illustrative example of the life-saving effect of the collateral circulation. J Invasive Cardiol. 2011;23:E47–48.
- Saraon T, Chadow HL, Castillo R. The power of collateral circulation: a case of asymptomatic chronic total occlusion of the left main coronary artery. J Invasive Cardiol. 2012;24:E196–198.
- Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2011;33:614–621.
- Meier P, Indermuehle A, Pitt B, Traupe T, de Marchi SF, Crake T, Knapp G, Lansky AJ, Seiler C. Coronary collaterals and risk for restenosis after percutaneous coronary interventions: a meta-analysis. BMC Med. 2012;10:62. doi: 10.1186/1741-7015-10-62
- Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho KI, Kim BH, Park YH, Je HG, Jeong YH, Kim WJ, Lee JY, Lee SW. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology. 2012;122:133–143. doi: 10.1159/000339238
- Meier P, Gloekler S, Oezdemir B, Indermuehle A, Traupe T, Vogel R, de Marchi S, Seiler C. G-CSF induced arteriogenesis in humans: molecular insights into a randomized controlled trial. Curr Vasc Pharmacol. 2013;11:38–46. doi: 10.2174/157016113804547674
- Gloekler S, Meier P, de Marchi SF, Rutz T, Traupe T, Rimoldi SF, Wustmann K, Steck H, Cook S, Vogel R, Togni M, Seiler C. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart. 2010;96:202–207. doi: 10.1136/hrt.2009.184507
- Zbinden R, Zbinden S, Meier P, Hutter D, Billinger M, Wahl A, Schmid JP, Windecker S, Meier B, Seiler C. Coronary collateral flow in response to endurance exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:250–257. doi: 10.1097/HJR.0b013e3280565dee
- Schirmer SH, Degen A, Baumhäkel M, Custodis F, Schuh L, Kohlhaas M, Friedrich E, Bahlmann F, Kappl R, Maack C, Böhm M, Laufs U. Heart-rate reduction by If-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur Heart J. 2012;33:1223–1231. doi: 10.1093/eurheartj/ehr255
- Heaps CL, Parker JL. Effects of exercise training on coronary collateralization and control of collateral resistance. J Appl Physiol. 2011;111:587–598. doi: 10.1152/japplphysiol.00338.2011
- Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642. doi: 10.1016/j.jacc.2005.01.068
- Meier P, Gloekler S, de Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation. 2009;120:1355–1363. doi: 10.1161/CIRCULATIONAHA.109.866269
- Belardinelli R, Belardinelli L, Shryock JC. Effects of dipyridamole on coronary collateralization and myocardial perfusion in patients with ischaemic cardiomyopathy. Eur Heart J. 2001;22:1205–1213. doi: 10.1053/euhj.2000.2446
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. VIVA Investigators: The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.CIR.0000061911.47710.8A
- Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, West A, Rade JJ, Marrott P, Hammond HK, Engler RL. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595
- Collateral circulation. Liebeskind DS. Stroke. 2003 Sep; 34(9):2279-84. https://doi.org/10.1161/01.STR.0000086465.41263.06
- Chinese consensus statement on the evaluation and intervention of collateral circulation for ischemic stroke. Liu LP, Xu AD, Wong LK, Wang DZ, Wang YJ, Expert consensus group of the evaluation & intervention of collateral circulation for ischemic stroke. CNS Neurosci Ther. 2014 Mar; 20(3):202-8.
- Lippert H, Pabst R. Arterial Variations in Man. Munich, Germany: JF Bergmann Verlag; 1985:92–93.
- Takahashi S, Tobita M, Takahashi A, Sakamoto K. Retrograde filling of the anterior choroidal artery: vertebral angiographic sign of obstruction in the carotid system. Neuroradiology. 1992; 34: 504–507.
- Hoksbergen AW, Fulesdi B, Legemate DA, Csiba L. Collateral configuration of the circle of Willis: transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke. 2000; 31: 1346–1351.
- Collateral circulation. Stroke. 2003 Sep;34(9):2279-84. Epub 2003 Jul 24. https://doi.org/10.1161/01.STR.0000086465.41263.06
- Meyer JS, Denny-Brown D. The cerebral collateral circulation, I: factors influencing collateral blood flow. Neurology. 1957; 7: 447–458.
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001; 32: 2179–2184.
- Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001; 21: 1223–1231.
- Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991; 28: 183–189.
- Toyoda K, Minematsu K, Yamaguchi T. Long-term changes in cerebral blood flow according to different types of ischemic stroke. J Neurol Sci. 1994; 121: 222–228.
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995; 66: 149–173.
- Derdeyn CP, Shaibani A, Moran CJ, Cross DT III, Grubb RL Jr, Powers WJ. Lack of correlation between pattern of collateralization and misery perfusion in patients with carotid occlusion. Stroke. 1999; 30: 1025–1032.
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991; 29: 231–240.
- Wang CX, Todd KG, Yang Y, Gordon T, Shuaib A. Patency of cerebral microvessels after focal embolic stroke in the rat. J Cereb Blood Flow Metab. 2001; 21: 413–421.
- Bartolini A, Gasparetto B, Furlan M, Roncallo F, Sullo L, Trivelli G, Primavera A. Functional circulation images by angio-CT in the assessment of small deep cerebral infarctions. Comput Med Imaging Graph. 1995; 19: 313–323.
- Essig M, von Kummer R, Egelhof T, Winter R, Sartor K. Vascular MR contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol. 1996; 17: 887–894.
- Roberts HC, Dillon WP, Furlan AJ, Wechsler LR, Rowley HA, Fischbein NJ, Higashida RT, Kase C, Schulz GA, Lu Y, Firszt CM. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002; 33: 1557–1565.
- Koga M, Kimura K, Minematsu K, Yamaguchi T. Relationship between findings of conventional and contrast-enhanced transcranial color-coded real-time sonography and angiography in patients with basilar artery occlusion. AJNR Am J Neuroradiol. 2002; 23: 568–571.
- Gur AY, Bornstein NM. TCD and the Diamox test for testing vasomotor reactivity: clinical significance. Neurol Neurochir Pol. 2001; 35 (suppl 3): 51–56.
- Kazumata K, Tanaka N, Ishikawa T, Kuroda S, Houkin K, Mitsumori K. Dissociation of vasoreactivity to acetazolamide and hypercapnia: comparative study in patients with chronic occlusive major cerebral artery disease. Stroke. 1996; 27: 2052–2058.
- Blaser T, Hofmann K, Buerger T, Effenberger O, Wallesch CW, Goertler M. Risk of stroke, transient ischemic attack, and vessel occlusion before endarterectomy in patients with symptomatic severe carotid stenosis. Stroke. 2002; 33: 1057–1062.
- Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis. 2002; 14: 22–26.
- Pindzola RR, Balzer JR, Nemoto EM, Goldstein S, Yonas H. Cerebrovascular reserve in patients with carotid occlusive disease assessed by stable xenon-enhanced CT cerebral blood flow and transcranial Doppler. Stroke. 2001; 32: 1811–1817.
- Grond M, Rudolf J, Schneweis S, Terstegge K, Sobesky J, Kracht L, Neveling M, Heiss WD. Feasibility of source images of computed tomographic angiography to detect the extent of ischemia in hyperacute stroke. Cerebrovasc Dis. 2002; 13: 251–256.
- Patrick JT, Fritz JV, Adamo JM, Dandonna P. Phase-contrast magnetic resonance angiography for the determination of cerebrovascular reserve. J Neuroimaging. 1996; 6: 137–143.
- Lee KH, Cho SJ, Byun HS, Na DG, Choi NC, Lee SJ, Jin IS, Lee TG, Chung CS. Triphasic perfusion computed tomography in acute middle cerebral artery stroke: a correlation with angiographic findings. Arch Neurol. 2000; 57: 990–99
- Toni D, Fiorelli M, Bastianello S, Falcou A, Sette G, Ceschin V, Sacchetti ML, Argentino C. Acute ischemic strokes improving during the first 48 hours of onset: predictability, outcome, and possible mechanisms: a comparison with early deteriorating strokes. Stroke. 1997; 28: 10–14.
- Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery. 2002; 50: 1405–1414;comment 1414–1415.
- Kucinski T, Koch C, Eckert B, Becker V, Kromer H, Heesen C, Grzyska U, Freitag HJ, Rother J, Zeumer H. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003; 45: 11–18.
- Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ, for the North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke. 2000; 31: 128–132.
- Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, Caltagirone C, Silvestrini M. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001; 32: 1552–155
- Miralles M, Dolz JL, Cotillas J, Aldoma J, Santiso MA, Gimenez A, Capdevila A, Cairols MA. The role of the circle of Willis in carotid occlusion: assessment with phase contrast MR angiography and transcranial duplex. Eur J Vasc Endovasc Surg. 1995; 10: 424–430.
- Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994; 330: 1565–1570.
- van Everdingen KJ, Visser GH, Klijn CJ, Kappelle LJ, van der Grond J. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol. 1998; 44: 167–176.
- Hedera P, Bujdakova J, Traubner P, Pancak J. Stroke risk factors and development of collateral flow in carotid occlusive disease. Acta Neurol Scand. 1998; 98: 182–186.