Left ventricular outflow tract obstruction
Left ventricular outflow tract obstruction or LVOT obstruction refers to any functional or anatomic obstruction of flow out of the left ventricle into the ascending aorta. Left ventricular outflow tract obstruction can occur at the valvular, subvalvular, or supravalvular level 1. Left ventricular outflow tract obstructions involve stenotic lesions starting in the anatomic left ventricular outflow tract and stretching to the descending portion of the aortic arch. In general, there is an obstruction to forward flow which increases afterload, and if untreated, can result in hypertrophy, dilatation, and eventual failure of the left ventricle. In the United States, most cases of left ventricular outflow tract obstruction are congenital in individuals younger than 50 years of age 2. All patients with left ventricular outflow tract obstruction at a high risk for developing infective endocarditis and prophylaxis should be instituted 2.
Left ventricular outflow tract obstruction causes
Subaortic stenosis
Subaortic stenosis is narrowing at the level of the aortic valve. It may be due to a discrete ridge or fibrous ring encircling the left ventricular outflow tract. This fibrous membrane may extend onto the aortic valve cusps and make contact with the ventricular side of the anterior mitral leaflet. The obstruction may be focal or more diffuse, resulting in a tunnel leading out of the left ventricle. The discrete form is most common. Complex subaortic stenosis can also be seen which leads to abnormal adherence to the anterior leaflet of the mitral valve to the septum and the presence of endocardial tissue in the left ventricular outflow tract. These type of obstructions are commonly seen in patients with ventral septal defects (VSDs).
Subaortic stenosis signs and symptoms
If a gradient is present, a systolic ejection murmur is heard along the lower left sternal border, which peaks later with the severity of Subaortic stenosis. The clinical course of Subaortic stenosis is progressive, with increasing obstruction and progression of aortic regurgitation in more than 80% of untreated patients.
Patients may present with one of three symptoms associated with severe valvular aortic stenosis: angina, heart failure, or syncope.
Subaortic stenosis diagnosis
The electrocardiogram is usually normal. However, with isolated forms of LVOT obstruction, left ventricular hypertrophy and left axis deviation may be present.
Transthoracic echocardiography will often demonstrate a focal or diffuse narrowing of the left ventricular outflow tract. Membranes located adjacent to the aortic valve or extending to the anterior leaflet of the mitral valve are more likely to result in a progressive obstruction as well as more likely to cause aortic valve damage with aortic regurgitation. A continuous-wave Doppler peak gradient of greater than or equal to 50 mmHg is considered severe and indicates a poor prognosis if left untreated.
Subaortic stenosis treatment
Surgical resection is the intervention of choice and is done via a transaortic approach. Surgical intervention is a consideration in patients with lower gradients (peak pressure gradient less than 50 mmHg) if there is left ventricular systolic dysfunction, moderate to severe aortic regurgitation, or a ventral septal defect. Usually, the approach has been to intervene when the mean gradient across the left ventricular outflow tract is more than 30mmHg to avoid further aortic valve damage. Patients who develop symptoms with exertion and patients planning to become pregnant should be considered for Subaortic stenosis resection if the gradient is greater to or equal 30 mmHg. Surgery involves fibromyectomy with concomitant aortic valve repair if significant aortic regurgitation is present. Subaortic stenosis can recur and requires reoperation in up to 20% of cases 3.
Supravalvular aortic stenosis
Supravalvar aortic stenosis is the rarest lesion of the LVOT obstructions. There are three anatomic types: hourglass type, membranous type and diffuse hypoplasia of the ascending aorta. The most common is the hourglass type with thickening and disorganization of elastin fibers within the aortic media producing a constricting annular ridge at the margin of the sinuses of Valsalva. This leads to reduced elasticity and increased shear stress within the ascending aorta, which incites smooth muscle hypertrophy and increased collagen deposition. The coronary arteries are near the site of outflow obstruction which causes them to be subjected to elevated systolic pressures leading to dilatation, tortuosity, and accelerated atherosclerosis.
supravalvular aortic stenosis occurs in an autosomally inherited form and in a rare sporadic form. In all three types, the underlying cause has been identified as a mutation of the elastin gene on chromosome 7.
Supravalvar aortic stenosis signs and symptoms
Supravalvular aortic stenosis is associated with Williams syndrome which is a multisystem disorder with features including failure to thrive, intellectual impairment, elfin facies, gastrointestinal problems, and urinary tract abnormalities.
Physical findings of isolated supravalvular aortic stenosis are similar to aortic valves stenosis. A systolic murmur is heard which projects to the jugular notch. A hallmark feature of supravalvular aortic stenosis is that systolic pressure in the right arm is usually higher than the left arm. The patient may experience symptoms of angina, dyspnea, and syncope.
Supravalvar aortic stenosis diagnosis
Electrocardiogram reveals left ventricular hypertrophy when an obstruction is severe. Right ventricular hypertrophy can also be seen if pulmonary arteries are narrowed.
Transthoracic echocardiography may reveal dilated sinuses of Valsalva, and the ascending aorta and arch are usually small or of normal size. The diameter of the aortic annulus is greater than the sinotubular junction.
Angiography is usually done to assess the gradient across the LVOT and to assess the coronary arteries.
Supravalvar aortic stenosis treatment
Surgical intervention is recommended in symptomatic patients or those with a mean pressure gradient of more than or equal to 50 mmHg. Obstruction can be relieved by an excision of a focal stenosis with end-to-end anastomosis of the ascending aorta, patch enlargement of the sinotubular junction, or aortoplasty. Prognosis is good following surgical intervention 4.
Bicuspid aortic valve
Bicuspid aortic valve is one of the most common congenital cardiovascular malformations, present in about 1% to 2% of the population and more common in males. bicuspid aortic valve can be inherited, and family clusters have been documented. In those cases, inheritance patterns are usually autosomal dominant with variable penetrance. A mutation in the NOTCH1 gene has also been described.
Bicuspid aortic valves arise from abnormal vasculogenesis and cusp formation, resulting in the formation of 1 smaller cusp and one larger cusp. More commonly, the right and left coronary cusps are fused. bicuspid aortic valve is usually progressive, and most valves function normally until later in life. The abnormal valve formation leads to increased leaflet stress, more turbulent flow and restricted motion which leads to accelerated valve changes including scarring, calcification, aortic stenosis, and regurgitation. bicuspid aortic valve is associated with dilation of the ascending aorta and increased risk of aortic dissection.
Bicuspid aortic valve signs and symptoms
The most common complication of bicuspid aortic valve is aortic stenosis. Therefore, clinical features are similar. Patients may present with a midsystolic murmur that increases in harshness and peaks later as the degree of stenosis worsens. Concomitant aortic regurgitation results in an early diastolic murmur. When aortic stenosis is present, patients may complain of chest pain, dyspnea or have episodes of syncope.
Bicuspid aortic valve diagnosis
Transthoracic echocardiography may show irregularities in the cusp of the aortic valve along with evidence of progressive thickening and calcification leading to a stenotic valve. Patients with bicuspid aortic valve make up more than 50% of cases with clinically significant aortic stenosis.
Bicuspid aortic valve treatment
There are no effective medical therapies to prevent valve deterioration or aortic root dilatation in patients with bicuspid aortic valve. Surgical repair is indicated for patients with severe stenosis who are symptomatic or have decreased left the ventricular function. Asymptomatic patients who desire to become pregnant or want to increase their exercise tolerance may also be considered for surgery. Severe aortic regurgitation that is associated with symptoms, severe aortic root enlargement, or left ventricular dysfunction should be surgically corrected. For patients who will undergo valve replacement, concurrent aortic root replacement is recommended if the aortic diameter is greater to or equal to 4.5 cm. In those without aortic valve disease, aortic root replacement is recommended when the aortic diameter exceeds 5.5 cm and may be considered in patients with an aortic diameter of 5.0 cm if there is a positive family history or history of rapid progression. Balloon valvuloplasty may decrease the gradient and improve symptoms in those without a calcified valve 5.
Coarctation of the aorta
Coarctation of the aorta is an aorta narrowing, located at the insertion of the ductus arteriosus just distal to the left subclavian. More diffuse forms of the disease may involve the arch or isthmus. The exact pathogenesis which causes this narrowing is unknown, although multiple theories have been described. The stenotic lesion causes left ventricular outflow tract obstruction resulting in increased systolic pressure in the left ventricle and proximal aorta. Left ventricular hypertrophy and collateral blood flow are mechanisms to try and circumvent this obstruction.
Coarctation of the aorta signs and symptoms
Adults may initially present with systemic arterial hypertension. A patient with systemic arterial hypertension should have upper and lower extremity arterial blood pressures measured. Radial and femoral pulses should also be checked. With coarctation of the aorta, upper limb hypertension is usually present, and there is a systolic pressure differential of at least 10 mmHg between the upper and lower extremities. A delay or decrease in amplitude of the femoral pulse may also be present. A systolic or continuous murmur may be heard on auscultation in the interscapular region or throughout the chest wall from intercostal collateral arteries.
Coarctation of the aorta diagnosis
An electrocardiogram may show left ventricular hypertrophy. For more complicated lesions, right ventricular hypertrophy may also be present.
Characteristic rib notching is often present on chest x-ray or CT due to extensive collateral formation bypassing the area of coarctation. A figure “three” sign may also be seen on chest x-ray due to pre-stenotic and post-stenotic dilatation.
Coarctation of the aorta is best seen on echocardiography via the suprasternal notch view. When performed, this may demonstrate a posterior shelf, well-expanded isthmus, and transverse aortic arch. A high-velocity jet will likely be seen through the coarctation site.
MRI and angiogram may also be performed before or at the time of intervention if indicated.
Coarctation of the aorta treatment
Surgical repair usually relieves the obstruction with low mortality rates. Stent implantation decreases the risk of aneurysm formation and has good long-term outcomes in both native and recurrent coarctation. For discrete stenoses, balloon angioplasty can be used as a primary intervention, but it is not the best option for long-segment or tortuous forms of coarctation 6.
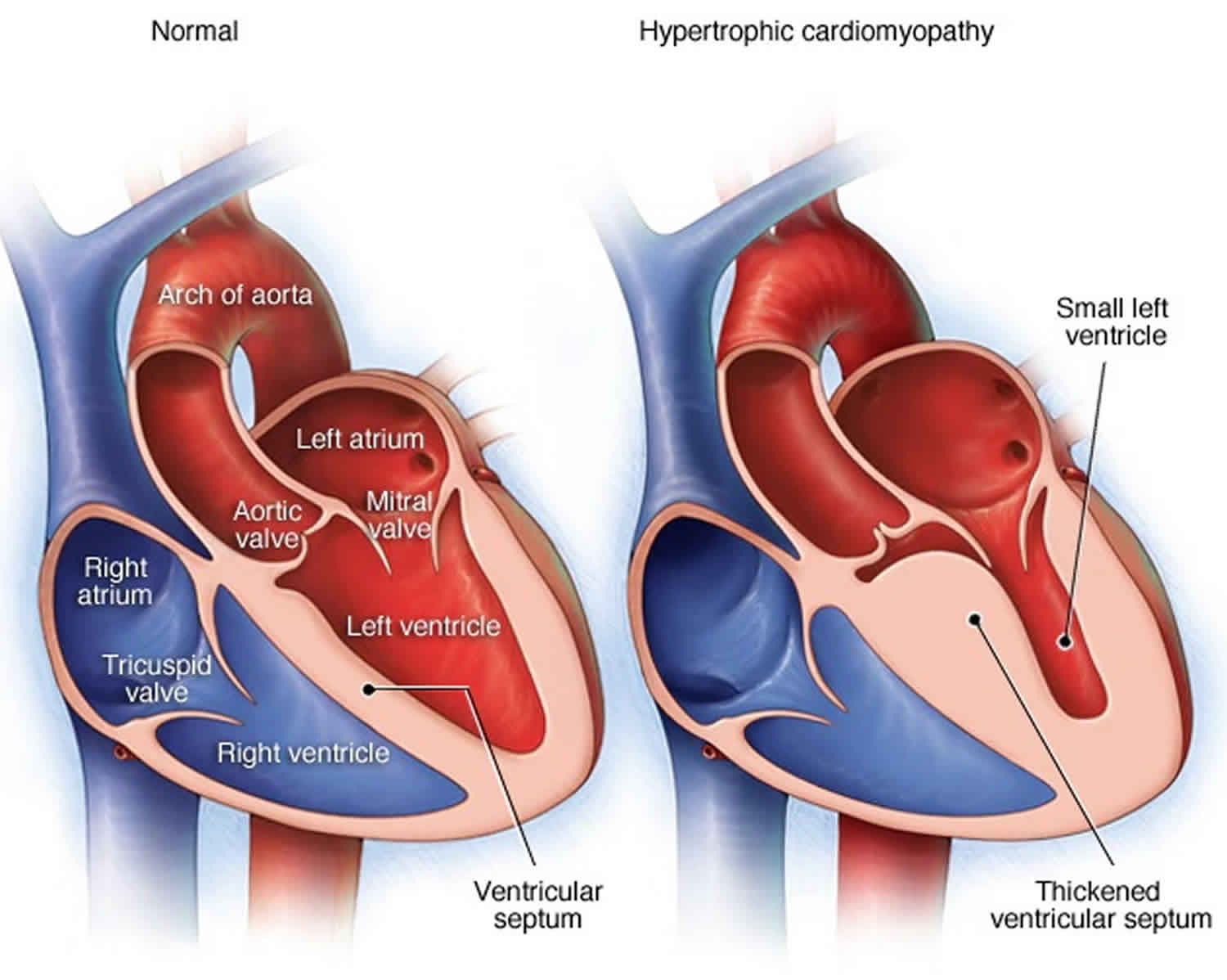
Hypertrophic obstructive cardiomyopathy
Hypertrophic obstructive cardiomyopathy is characterized by disorganized myocytes which lead to hypertrophy of the left ventricle, usually the septum. However, hypertrophy can be extensive involving the left ventricle free wall as well. Hypertrophic obstructive cardiomyopathy is an autosomal dominant disease with equal prevalence in males and females.
About two-thirds of patients have the obstructive form. In most patients, LV outflow obstruction occurs via systolic anterior motion (SAM) of the mitral valve in which the elongated leaflets contact the septum in mid-systole due to the high-velocity flow of blood directly on the leaflets. The narrowed diameter of the LVOT due to increased septal wall thickness contributes to this obstruction. This leads to increased intraventricular pressures that over time can lead to left ventricular dysfunction.
Hypertrophic obstructive cardiomyopathy signs and symptoms
Symptoms may include chest pain, dyspnea, exertional fatigue, dizziness, palpitation and other symptoms of heart failure. Patients may experience near-syncope or syncope due to outflow obstruction or arrhythmia. A systolic ejection murmur may be heard at the lower left sternal border and apex that varies with the subaortic gradient
Hypertrophic obstructive cardiomyopathy diagnosis
Most common findings on ECG are left ventricular hypertrophy, T wave inversions, left atrial enlargement, deep and narrow Q waves and diminished R waves in the precordial leads.
Transthoracic echocardiography usually reveals a thickened septum.
Hypertrophic obstructive cardiomyopathy treatment
Medical treatment with beta blockers, calcium channel blockers, and disopyramide has been used for symptomatic patients. Surgical myectomy is the preferred option for patients with severe symptoms refractory to medical therapy or with LVOT gradient greater than 50 mmHg. Percutaneous alcohol septal ablation is an alternative to myectomy in selected patients. An implantable cardiac device is effectively used for primary prevention of lethal ventricular tachyarrhythmias 2.
Right ventricular outflow tract obstruction
Right ventricular outflow tract obstruction or RVOT obstruction and/or pulmonary artery obstruction are present in some form in 25 % of all congenital heart defects. The right ventricular outflow tract obstruction includes stenosis or narrowing of the pulmonary valve, the tissue above the valve (supravalvar obstruction) and below it (subvalvar obstruction). Congenital subvalvar and supravalvar right ventricular outflow tract stenosis usually occurs with other congenital heart defects such as ventricular septal defect (VSD) or Tetralogy of Fallot. The timing and type of surgery will vary from patient to patient depending upon the severity of the obstruction and the associated congenital defect.
If right ventricular outflow tract obstruction is present and the ventricular septum is intact, usually the right ventricle will adapt better given that no right to left shunting is present. This absence of shunting may result in sufficient pulmonary blood flow to allow the patient to remain asymptomatic longer.
Usually therapy is directed to increase pulmonary blood flow and decompressing the right ventricle (RV) and it will depend on the severity and location of the defect. According to the anatomic features of the pulmonary valve stenosis, the physician may use balloon dilatation (a minimally invasive transvenous procedure) to dilate the obstruction during cardiac catheterization, but if the pulmonary artery is the structure involved, stenting (insertion of a small tube) within the narrowed region can be done following balloon angioplasty. Standard treatment of right ventricular outflow tract or pulmonary artery obstruction involves open chest surgery.
Right ventricular outflow tract obstruction usually occurs in newborn. However, the age at presentation depends on the severity of the obstruction with many patients with mild obstruction presenting at adolescence or adulthood. Isolated pulmonic valvular stenosis with intact ventricular septum is the second most common congenital cardiac defect.
Right ventricular outflow tract obstruction represents 8-12% of all congenital heart defects in children 7 and 15% of all congenital heart defects in adults 8. Isolated pulmonic valvular stenosis with intact ventricular septum is the second most common congenital cardiac defect.
Right ventricular outflow tract obstruction secondary to rheumatic fever is rare and it seldom causes serious pulmonic valvular deformity. It usually may occur in association with the pulmonary hypertension that occurs at high altitudes. The prevalence of rheumatic disease in developed nations is steadily declining. Developing countries, in contrast, have higher rates of rheumatic fever and subsequent mitral stenosis with a prevalence of more than 10 cases per 1,000 in India and 4-10 cases per 1,000 in China, Russia, Africa and Australia 9.
Right ventricular outflow tract obstruction causes
Right ventricular outflow tract obstruction common causes
Congenital
- Tetralogy of fallot
- Noonan syndrome
- Leopard syndrome
- William’s syndrome
- Alagille syndrome
- Congenital rubella syndrome
Acquired
- Carcinoid syndrome
- Rheumatic fever
- Homograft dysfunction 10
- Pulmonary hypertension
Pulmonary valve stenosis
Pulmonary valve stenosis accounts for 8% of all congenital heart disease and worldwide the prevalence of pulmonary valve stenosis is 1 per 2000 births 11. The pulmonic valve stenosis is classified into 3 different subtypes based on the location of the stenosis. Isolated valvular stenosis is the most common sub-type, with dome shaped morphology and dysplastic valves. Patients with mild stenosis usually have a beningn course and do not progress, patients with moderate to severe stenosis manifest symptoms of dyspnea, chest pain, fatigue and syncope. If left untreated patients progress to right heart failure. 2D Echo is the standard diagnostic test to identify the location and to assess the severity of the stenosis. Symptomatic patients undergo valvulotomy or balloon valvuloplasty based on the morphology of the affected valves. Timely intervention in patients with valvular stenosis has good outcomes and excellent prognosis. Guidelines for evaluation, approach and treatment are well-defined.
Pulmonary valve stenosis classification
Based on the anatomic location
Pulmonic stenosis is classified into valvular, subvalvular (infundibular) and supravalvular based on the location of the stenosis in relation to the pulmonary valve. Valvular stenosis is most common of the three sub-types.
- Sub-valvular stenosis: It can be infudibular or sub-infundibular. Infundibular stenosis is a feature of tetralogy of Fallot. Sub-infundibular pulmonic stenosis is known as ‘double chambered right ventricle’ dividing the right ventricle into a high pressure inlet and a low pressure outlet causing a progressive right ventricular outflow tract obstruction 12.
- Valvular stenosis: It is the most common cause of pulmonic stenosis. The valves are usually dome shaped or dysplastic affecting the movement of the cusps. It can be isolated or associated with other congenital heart diseases such as atrial septal defect, Ebstein’s anomaly, double outlet right ventricle, and transposition of the great arteries.
- Supravalvular stenosis: The obstruction is usually in the common pulmonary trunk or in the bifurcation or the pulmonary branches. It is commonly associated with other congenital syndromes such as Williams–Beuren, Noonan, Allagile syndrome, DiGeorge, and Leopard syndrome.
Based on the severity of the stenosis
Severity of pulmonary stenosis is classified based on the estimated peak velocity and peak resting gradient calculated using modified Bernoulli equation. It is classified into 13:
- Mild: Peak velocity less than 3m/s and peak gradient is less than 36 mm Hg.
- Moderate: Peak velocity is 3 to 4m/s and peak gradient is 36 to 64mm Hg.
- Severe: Peak velocity is greater than 4m/s and peak gradient is greater than 64mm Hg.
According to 2014 American Heart Association and American CC Guideline for the Management of Patients With Valvular Heart Disease, stages of severe pulmonic stenosis are defined as follows 14:
Pulmonary valve stenosis causes
Pulmonary valve stenosis is due to a structural changes resulting from thickening and fusion of the pulmonary valve. The valve pathology can be congenital or acquired. The following is the list of causes:
Congenital causes
These account for 95% of the cases with pulmonic stenosis which include isolated pulmonic valve pathologies and its associations with other congenital heart diseases 15.
Associated with congenital heart disease
- Tetralogy of Fallot 16
- Double outlet right ventricle
- Univentricular atrio-ventricular connection
- Atrioventricular canal defect
- Bicuspid pulmonary valve 17
- Quadricuspid pulmonary valve: Benign and an incidental finding 18
- Isolated pulmonic stenosis 19
- Acommissural pulmonary valves: Valve has a prominent systolic doming of the cusps and an eccentric orifice 20.
- Dysplastic pulmonary valves: Thickened and deformed cusps with no commissural fusion 21. It is a common finding associated with Noonan syndrome 21.
- Unicommissural pulmonary valve
- Bicuspid valve with fused commissures
Acquired causes
These are less frequent and account for less than 5% of the cases
- Carcinoid Syndrome: most common acquired cause 22
- Post infectious: Infective endocarditis
- Calcification of the pulmonary valve 23
- Rheumatic heart disease 24
- Ross procedure 25
- Functional pulmonic stenosis: Primary cardiac tumors obstructing the right ventricular outflow tract such as leiomyosarcoma 26
Risk factors for pulmonic valve stenosis
Common risk factors in the development of congenital heart disease apply for pulmonic stenosis and include 27:
- Maternal gestational diabetes mellitus
- Consanguineous marriage 28
- Phenylketonuria
- Febrile illness
- Vitamin A use
- Marijuana use
- Exposure to organic solvents
Pulmonary valve stenosis symptoms
The severity of symptoms and age of symptom onset depends on the severity of the pulmonic stenosis. Clinical presentations vary as follows:
- Critical pulmonary stenosis:It presents in first few hours to days of life with cyanosis. It is a condition with a very small or pin-hole orifice in the pulmonary valve which can be diagnosed prenatally. These patients have an intact interventricular septum, poorly complaint hypoplastic right ventricle and areductus dependent. Cyanosis in these patients is due to the right to left shunting at the level of the foramen ovale 29.
- Mild Pulmonic Stenosis: Patients with mild stenosis are asymptomatic and are diagnosed by routine examination with an ejection systolic murmur.
- Moderate Pulmonic Stenosis: Patients present with exertional dyspnea and fatigue.
- Severe Pulmonic Stenosis: Patients present with exertional dyspnea, chest pain and syncope.
- Untreated patients develop features of right ventricular failure which include:
- Exercise intolerance
- Fatigue
- Shortness of breath
- Swelling of the feet or ankles
- Abdominal discomfort
- Patients with subinfundibular/infundibular pulmonic stenosis can be asymptomatic or they may present with angina, dyspnea, dizziness, or syncope.
- Patients with supravalvular pulmonic stenosis may be asymptomatic or have symptoms of dyspnea and fatigue on exertion.
The common physical examination findings include:
- Patients with isolated pulmonary stenosis usually appear normal. In patients diagnosed with syndromes associated with pulmonic stenosis syndrome specific physical examination findings are demonstrated.
- Cardiac examination findings are dependent on the degree of the pulmonary stenosis, the pathology of the valve and associated cardiac lesions. The common findings include as follows:
- In mild stenosis findings include normal jugular venous pulse, absent right ventricle lift, ejection click in the pulmonary area which decreases with inspiration, ejection systolic murmur in the pulmonary area heard in the ending of mid systole increasing in intensity during inspiration 30.
- In severe stenosis findings include:
- Elevated JVP with a prominent “a” wave
- Right ventricular heave
- Louder and longer ejection murmur in the left parasternal area in second and third intercostal space
- Ejection click is softer and absent with increasing severity
- Wide split S2 with reduced or absent P2 component
- Right sided S4 can be audible
Pulmonary valve stenosis diagnosis
Gold standard diagnosis of pulmonic stenosis and assessment of severity is done by 2D echocardiography 31. Cardiac MRI is very useful to study the anatomy of the right ventricular outflow tract, pulmonary artery and to locate the exact level of stenosis 32.
Cardiac catheterization is useful to measure the pressure gradients directly, but its not performed on a regular basis as echo is a reliable and non-invasive test to measure the pressure gradients 33.
Pulmonary valve stenosis treatment
Medical therapy
There is no specific medical therapy for the treatment of pulmonic stenosis. However, patients diagnosed with right heart failure diuretics are recommended to decrease the fluid overload.
Surgery
Indications for surgical intervention
Surgical correction is recommended based on the peak gradient and other associated clinical features 34:
- Surgery is advised regardless of the symptoms if the doppler derived peak instantaneous gradient greater than 64 mm Hg (peak velocity >4 m/s).
- In patients with doppler derived peak instantaneous gradient less than 64 mm Hg (peak velocity>4 m/s), surgery is advised if any of the following is present:
- Symptomatic patient
- Decreased right ventricular function
- Double chambered right ventricle
- Arrhythmia
- Right to left shunting via the VSD or ASD
- Asymptomatic patients with a systolic RV pressure greater than 80 mm Hg (tricuspid regurgitation jet velocity >4.3 m/s).
Pulmonary subvalvular stenosis
There is hypertrophy of the infundibular muscle causing obstruction. The isolated form is rare, more commonly develops in response to an underlying VSD.
Pulmonary subvalvular stenosis signs and symptoms
Similar to valvar pulmonic stenosis. No ejection click in contrast to valvar pulmonic stenosis. No poststenotic dilation of the pulmonary artery in contrast to valvar pulmonic stenosis.
Pulmonary supravalvular stenosis
Supra valvular pulmonary artery stenosis also called peripheral pulmonary stenosis, is defined as stenosis of the pulmonary artery above the level of the valve area. May have one or many stenoses of the pulmonary artery or its branches.
Pulmonary supravalvular stenosis infrequently occurs as an isolated lesion, more commonly occurs as part of Tetralogy of Fallot.
Pulmonary atresia
Pulmonary atresia is a congenital malformation of the pulmonary valve in which the valve orifice fails to develop. Atresia means “no opening”. The valve is completely closed thereby obstructing the outflow of blood from the heart to the lungs.
Oxygen-poor blood travels directly from the right atrium to the left side of the heart through a shunt (patent foramen ovale or any other type of atrial septal defect). This oxygen-poor blood is then pumped through the aorta to the rest of the body, making fingers, toes, and lips appear blue or cyanotic.
Babies with this type of cyanotic congenital heart disease survive only for the first few days of life while the normal fetal shunts (patent ductus arteriousus, patent foramen ovale) between left and right circulations remain patent. Without an operation in that period to open the pulmonary valve or to make a shunt between the aorta and the pulmonary arteries, the condition is fatal.
The type of surgery recommended depends on the size of the right ventricle and the pulmonary artery. If they are normal in size and the right ventricle is able to pump blood, open heart surgery can be performed to make blood flow through the heart in a normal pattern. If the right ventricle is small and unable to act as a pump, doctors may perform an operation called the Fontan procedure. In this procedure, the right atrium is connected directly to the pulmonary artery. Many children with Pulmonary Atresia will go on to lead ‘normal’ lives.
Right ventricular outflow tract obstruction symptoms
Symptoms of right ventricular outflow tract obstruction are infrequent (25% of patients) and include dyspnea, fatigue, syncope, cough and pedal edema.
Symptoms develop in only approximately 25% of patients because progression of the disease is infrequent.
- Chest pain
- Syncope
- Congestive heart failure
- Dyspnea
- Fatigue
- Pedal edema
- Cough
- Cough with pink frothy sputum
- Cardiomegaly
With a gradient of > 75 mm Hg symptoms include fatigability, dyspnea on exertion, angina, syncope and central cyanosis if there is a right-to-left shunt through a patent foramen ovale (PFO).
Right ventricular outflow tract obstruction diagnosis
Gold standard diagnosis of right ventricular outflow tract obstructions and assessment of severity is done by 2D echocardiography 31. Cardiac MRI is very useful to study the anatomy of the right ventricular outflow tract, pulmonary artery and to locate the exact level of stenosis 32.
Cardiac catheterization is useful to measure the pressure gradients directly, but its not performed on a regular basis as echo is a reliable and non-invasive test to measure the pressure gradients 33.
Right ventricular outflow tract obstruction treatment
Surgery
- In patients with severe stenoses, there is impaired exercise tolerance and changes in the RV myocardium.
- Surgery is indicated in patients with fatigability, DOE, cyanosis or CHF.
- Surgery is recommended in the absence of symptoms if the gradient is greater than 75 mm Hg, and is also preferred if the gradient is as low as 50 to 60 mm Hg.
- For those with gradients less than 50 mm Hg, then follow-up is recommended.
- Long-term results of pulmonary valvuloplasty are not yet available, but short term results appear to be excellent.
- Restenosis is likely is the residual gradient is greater than 30 mm Hg.
- 79% of these patients have residual pulmonic regurgitation.
- Following these procedures there is a soft residual systolic murmur, a diastolic murmur of pulmonic insufficiency, and some regression of the ECG criteria of right ventricular hyperthrophy.
According to 2008 American College of Cardiology and the American Heart Association guidelines 35, following are the indications for balloon valvotomy in pulmonary stenosis:
- “Balloon valvotomy is recommended in adolescent and young adult patients with pulmonic stenosis who have exertional dyspnea, angina, syncope, or presyncope and an RV–to–pulmonary artery peak-to-peak gradient greater than 30 mm Hg at catheterization.”
- “Balloon valvotomy is recommended in asymptomatic adolescent and young adult patients with pulmonic stenosis and RV–to–pulmonary artery peak-to-peak gradient greater than 40 mm Hg at catheterization.”
- “Balloon valvotomy is not recommended in asymptomatic adolescent and young adult patients with pulmonic stenosis and RV–to–pulmonary artery peak-to-peak gradient less than 30 mm Hg at catheterization.”
- “Balloon valvotomy may be reasonable in asymptomatic adolescent and young adult patients with pulmonic stenosis and an RV–to–pulmonary artery peak-to-peak gradient 30 to 39 mm Hg at catheterization.”
- Vilcant V, Hai O. Left Ventricular Outflow Tract Obstruction. [Updated 2019 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470446
- Jain P, Patel PA, Fabbro M. Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction: Expecting the Unexpected. J. Cardiothorac. Vasc. Anesth. 2018 Feb;32(1):467-477.
- Costa MACD, Wippich AC. Correction of Left Ventricular Outflow Tract Obstruction Caused by Anomalous Papillary Muscle and Subaortic Membrane. Braz J Cardiovasc Surg. 2018 Nov-Dec;33(6):634-637.
- Carr K, Aldoss O, Thattaliyath B, Bansal M. Balloon angioplasty for supravalvular aortic stenosis as an early complication following arterial switch operation. Ann Pediatr Cardiol. 2018 Sep-Dec;11(3):315-317.
- Liu T, Xie M, Lv Q, Li Y, Fang L, Zhang L, Deng W, Wang J. Bicuspid Aortic Valve: An Update in Morphology, Genetics, Biomarker, Complications, Imaging Diagnosis and Treatment. Front Physiol. 2018;9:1921.
- Doshi AR, Chikkabyrappa S. Coarctation of Aorta in Children. Cureus. 2018 Dec 05;10(12):e3690.
- Nadas A. Pulmonary stenosis. In: Fyler DC, ed. Nadas’ Pediatric Cardiology. Hanley & Belfus;1992:459-470.
- Johnson LW, Grossman W, Dalen JE, Dexter L (1972). “Pulmonic stenosis in the adult. Long-term follow-up results”. N Engl J Med. 287 (23): 1159–63. doi:10.1056/NEJM197212072872301
- Seckeler MD, Hoke TR (2011). “The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease”. Clin Epidemiol. 3: 67–84. doi:10.2147/CLEP.S12977
- Interventional catheterization in adult congenital heart disease. Circulation. 2007 Mar 27;115(12):1622-33. https://doi.org/10.1161/CIRCULATIONAHA.105.592428
- Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Nov 15;58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. https://doi.org/10.1016/j.jacc.2011.08.025
- Double-chambered right ventricle. European Heart Journal, Volume 16, Issue 5, May 1995, Pages 682–686, https://doi.org/10.1093/oxfordjournals.eurheartj.a060973
- Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009 Jan;22(1):1-23; quiz 101-2. doi: 10.1016/j.echo.2008.11.029. https://www.onlinejase.com/article/S0894-7317(08)00776-1/fulltext
- 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jun 10;63(22):2438-88. doi: 10.1016/j.jacc.2014.02.537. Epub 2014 Mar 3. https://doi.org/10.1016/j.jacc.2014.02.537
- Altrichter PM, Olson LJ, Edwards WD, Puga FJ, Danielson GK (1989). “Surgical pathology of the pulmonary valve: a study of 116 cases spanning 15 years”. Mayo Clin Proc. 64 (11): 1352–60.
- Greenberg SB, Crisci KL, Koenig P, Robinson B, Anisman P, Russo P (1997). “Magnetic resonance imaging compared with echocardiography in the evaluation of pulmonary artery abnormalities in children with tetralogy of Fallot following palliative and corrective surgery”. Pediatr Radiol. 27 (12): 932–5. doi:10.1007/s002470050275
- Jashari R, Van Hoeck B, Goffin Y, Vanderkelen A (2009). “The incidence of congenital bicuspid or bileaflet and quadricuspid or quadrileaflet arterial valves in 3,861 donor hearts in the European Homograft Bank”. J Heart Valve Dis. 18 (3): 337–44.
- Fernández-Armenta J, Villagómez D, Fernández-Vivancos C, Vázquez R, Pastor L (2009). “Quadricuspid pulmonary valve identified by transthoracic echocardiography”. Echocardiography. 26 (3): 288–90. doi:10.1111/j.1540-8175.2008.00798.x
- Gikonyo BM, Lucas RV, Edwards JE (1987). “Anatomic features of congenital pulmonary valvar stenosis”. Pediatr Cardiol. 8 (2): 109–16. doi:10.1007/BF02079465
- Jonas SN, Kligerman SJ, Burke AP, Frazier AA, White CS (2016). “Pulmonary Valve Anatomy and Abnormalities: A Pictorial Essay of Radiography, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI)”.
- Koretzky ED, Moller JH, Korns ME, Schwartz CJ, Edwards JE (1969). “Congenital pulmonary stenosis resulting from dysplasia of valve”. Circulation. 40 (1): 43–53.
- Paredes A, Valdebenito M, Gabrielli L, Castro P, Zalaquett R (2014). “[Tricuspid and pulmonary valve involvement in carcinoid syndrome. Report of two cases]”. Rev Med Chil. 142 (5): 662–6. doi:10.4067/S0034-98872014000500017
- Gabriele OF, Scatliff JH (1970). “Pulmonary valve calcification”. Am Heart J. 80 (3): 299–302.
- Vela JE, Contreras R, Sosa FR (1969). “Rheumatic pulmonary valve disease”. Am J Cardiol. 23 (1): 12–8.
- Raanani E, Yau TM, David TE, Dellgren G, Sonnenberg BD, Omran A (2000). “Risk factors for late pulmonary homograft stenosis after the Ross procedure”. Ann Thorac Surg. 70 (6): 1953–7.
- Vakilian F, Shabestari MM, Poorzand H, Teshnizi MA, Allahyari A, Memar B (2016). “Primary Pulmonary Valve Leiomyosarcoma in a 35-Year-Old Woman”. Tex Heart Inst J. 43 (1): 84–7. doi:10.14503/THIJ-14-4748
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ; et al. (2011). “Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis”. J Am Coll Cardiol. 58 (21): 2241–7.
- Naderi S (1979). “Congenital abnormalities in newborns of consanguineous and nonconsanguineous parents”. Obstet Gynecol. 53 (2): 195–9.
- Capuruço CA, Vercosa NC, Lopes RM (2016). “EP08.08: Critical pulmonary valve stenosis in dichorionic and diamniotic twins: prenatal diagnosis, pregnancy outcomes and postnatal development”. Ultrasound Obstet Gynecol. 48 Suppl 1: 297–8. doi:10.1002/uog.16888
- Hultgren HN, Reeve R, Cohn K, McLeod R (1969). “The ejection click of valvular pulmonic stenosis”. Circulation. 40 (5): 631–40.
- Kim DH, Park SJ, Jung JW, Kim NK, Choi JY (2013). “The Comparison between the Echocardiographic Data to the Cardiac Catheterization Data on the Diagnosis, Treatment, and Follow-Up in Patients Diagnosed as Pulmonary Valve Stenosis”. J Cardiovasc Ultrasound. 21 (1): 18–22. doi:10.4250/jcu.2013.21.1.18
- Rajiah P, Nazarian J, Vogelius E, Gilkeson RC (2014). “CT and MRI of pulmonary valvular abnormalities”. Clin Radiol. 69 (6): 630–8. doi:10.1016/j.crad.2014.01.019
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA; et al. (2008). “ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease)”. Circulation. 118 (23): 2395–451. doi:10.1161/CIRCULATIONAHA.108.190811
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA; et al. (2008). “ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease)”. Circulation. 118 (23): e714–833. doi:10.1161/CIRCULATIONAHA.108.190690
- 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 Sep 23;52(13):e1-142. doi: 10.1016/j.jacc.2008.05.007. https://doi.org/10.1016/j.jacc.2008.05.007