Coronaviruses (CoV) are a large family of common viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV), Severe Acute Respiratory Syndrome (SARS-CoV) and Coronavirus Disease 2019 (COVID-19) 1. Middle East respiratory syndrome coronavirus (MERS-CoV) was first identified in humans in 2012 2. In 2019, a new coronavirus now known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak that originated in China was identified as the cause of a disease called the Coronavirus Disease 2019 (COVID-19). In March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic. COVID-19 (Coronavirus Disease 2019) is an infectious disease caused by an enveloped single-stranded RNA novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 3.
Coronaviruses are named for the crown-like spikes on the surface of the virus. Some coronaviruses only affect animals called enzootic infections in birds and mammals, but in the last few decades, have shown to be capable of infecting humans as well (zoonotic infections, meaning they are transmitted between animals and people). Most people get infected with human coronaviruses at some time in their life. This usually causes mild to moderate upper-respiratory infections, like the common cold. But they can also cause more severe illnesses such as bronchitis and pneumonia.
There are several different types of human coronaviruses, including the Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viruses. The outbreak of severe acute respiratory syndrome (SARS) in 2003 and, more recently, Middle-East respiratory syndrome (MERS) has demonstrated the lethality of coronaviruses when they cross the species barrier and infect humans 4. Detailed investigations found that SARS-coronavirus was transmitted from civet cats to humans and MERS-coronavirus from dromedary camels to humans 5. MERS-coronavirus and SARS-coronavirus have been known to frequently cause severe symptoms. MERS symptoms usually include fever, cough, and shortness of breath which often progress to pneumonia 6. About 3 or 4 out of every 10 patients reported with MERS have died. MERS cases continue to occur, primarily in the Arabian Peninsula 6. SARS symptoms often included fever, chills, and body aches which usually progressed to pneumonia 6. No human cases of SARS have been reported anywhere in the world since 2004 6.
Several known coronaviruses are circulating in animals that have not yet infected humans. A renewed interest in coronaviral research has led to the discovery of several novel human coronaviruses and since then much progress has been made in understanding the coronavirus life cycle. The coronavirus envelope (E) protein is a small, integral membrane protein involved in several aspects of the virus’ life cycle, such as assembly, budding, envelope formation, and pathogenesis. Recent studies have expanded on its structural motifs and topology, its functions as an ion-channelling viroporin, and its interactions with both other coronavirus proteins and host cell proteins.
Common human coronaviruses, including types 229E, NL63, OC43, and HKU1, usually cause mild to moderate upper-respiratory tract illnesses, like the common cold. Most people get infected with these coronaviruses at some point in their lives. These illnesses usually only last for a short amount of time. Symptoms may include 6:
- runny nose
- headache
- cough
- sore throat
- fever
- a general feeling of being unwell
Human coronaviruses can sometimes cause lower-respiratory tract illnesses, such as pneumonia or bronchitis. This is more common in people with cardiopulmonary disease, people with weakened immune systems, infants, and older adults.
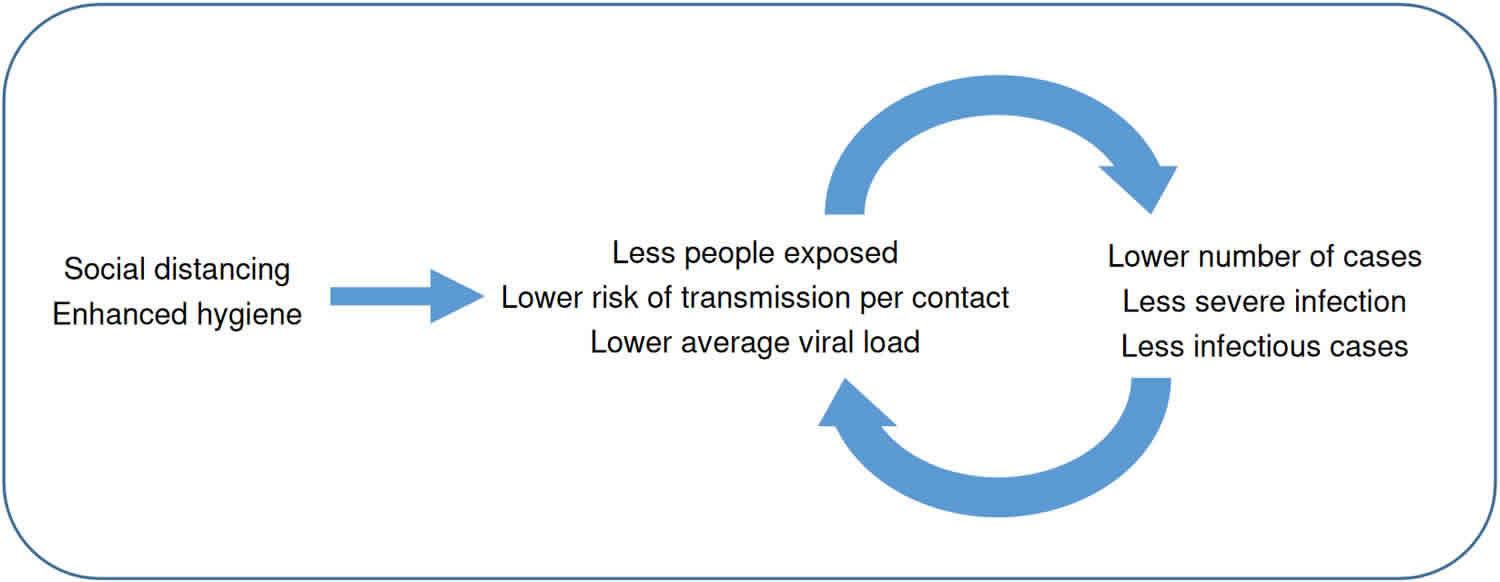
Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
There are no specific treatments for coronavirus infections. Most people will get better on their own. However, you can relieve your symptoms by:
- Taking over-the-counter medicines for pain, fever, and cough. However, do not give aspirin to children. And do not give cough medicine to children under four.
- Using a room humidifier or taking a hot shower to help ease a sore throat and cough
- Getting plenty of rest
- Drinking fluids
Coronavirus is most likely to spread from person to person, when you come into close contact with one another. You can all help stop the spread by keeping your distance.
If you are sick, stay away from others – that is the most important thing you can do.
You should also practise good hand and sneeze/cough hygiene:
- wash your hands frequently with soap and water, before and after eating, and after going to the toilet
- cover your cough and sneeze, dispose of tissues, and use alcohol-based hand sanitizer, and
- if unwell, avoid contact with others (stay more than 1.5 meters from people).
As well as these, you can start a range of social distancing and low cost hygiene actions now. These simple, common sense actions help reduce risk to you and to others. They will help to slow the spread of disease in the community – and you can use them every day – in your home, workplace, school and while out in public.
If you are worried about your symptoms, contact your health care provider.
Look for emergency warning signs* for COVID-19. If someone is showing any of these signs, seek emergency medical care immediately:
- Trouble breathing
- Persistent pain or pressure in the chest
- New confusion
- Inability to wake or stay awake
- Bluish lips or face
*This list is not all possible symptoms. Please call your medical provider for any other symptoms that are severe or concerning to you.
Because some of the symptoms of flu (influenza) and COVID-19 are similar, it may be hard to tell the difference between them based on symptoms alone, and testing may be needed to help confirm a diagnosis.
Call your local emergency services number or call ahead to your local emergency facility: Notify the operator that you are seeking care for someone who has or may have COVID-19.
Virus mutations are common, so it’s not surprising that COVID-19 (SARS-CoV-2) variants have been detected. Multiple COVID-19 (SARS-CoV-2) variants are circulating globally. Several new variants emerged in the fall of 2020, most notably:
- In the United Kingdom (UK), a new variant of COVID-19 (SARS-CoV-2) (known as 20I/501Y.V1, VOC 202012/01 or B.1.1.7) emerged with a large number of mutations. This variant has since been detected in numerous countries around the world, including the United States (US). In January 2021, scientists from UK reported evidence 7 that suggests the B.1.1.7 variant may be associated with an increased risk of death compared with other variants. More studies are needed to confirm this finding. This variant was reported in the US at the end of December 2020.
- In South Africa, another variant of COVID-19 (SARS-CoV-2) (known as 20H/501Y.V2 or B.1.351) emerged independently of B.1.1.7. This variant shares some mutations with B.1.1.7. Cases attributed to this variant have been detected in multiple countries outside of South Africa. This variant was reported in the US at the end of January 2021.
- In Brazil, a variant of COVID-19 (SARS-CoV-2) (known as P.1) emerged that was first was identified in four travelers from Brazil, who were tested during routine screening at Haneda airport outside Tokyo, Japan. This variant has 17 unique mutations, including three in the receptor binding domain of the spike protein. This variant was detected in the US at the end of January 2021.
Scientists are working to learn more about these variants to better understand how easily they might be transmitted and the effectiveness of currently authorized vaccines against them. New information about the virologic, epidemiologic, and clinical characteristics of these variants is rapidly emerging.
B.1.1.7 lineage (a.k.a. 20I/501Y.V1 Variant of Concern (VOC) 202012/01)
- This variant has a mutation in the receptor binding domain (RBD) of the spike protein at position 501, where the amino acid asparagine (N) has been replaced with tyrosine (Y). The shorthand for this mutation is N501Y. This variant also has several other mutations, including:
- 69/70 deletion: occurred spontaneously many times and likely leads to a conformational change in the spike protein
- P681H: near the S1/S2 furin cleavage site, a site with high variability in coronaviruses. This mutation has also emerged spontaneously multiple times.
- This variant is estimated to have first emerged in the UK during September 2020.
- Since December 20, 2020, several countries have reported cases of the B.1.1.7 lineage, including the United States.
- This variant is associated with increased transmissibility (i.e., more efficient and rapid transmission).
- In January 2021, scientists from UK reported evidence 7 that suggests the B.1.1.7 variant may be associated with an increased risk of death compared with other variants.
- B.1.1.7 (United Kingdom) is 50-70% more efficient in spreading from person to person.
- Early reports 8, 9, 10 found no evidence to suggest that the variant has any impact on the severity of disease or vaccine efficacy for the two vaccines authorized for use in the U.S.
B.1.351 lineage (a.k.a. 20H/501Y.V2)
- This variant has multiple mutations in the spike protein, including K417N, E484K, N501Y. Unlike the B.1.1.7 lineage detected in the UK, this variant does not contain the deletion at 69/70.
- This variant was first identified in Nelson Mandela Bay, South Africa, in samples dating back to the beginning of October 2020, and cases have since been detected outside of South Africa, including the United States
- The variant also was identified in Zambia in late December 2020, at which time it appeared to be the predominant variant in the country.
- Individuals infected with B1.351 (South Africa) strain showed higher viral loads which may increase transmissibility.
- Currently there is no evidence to suggest that this variant has any impact on disease severity.
- There is some evidence to indicate that one of the spike protein mutations, E484K, may affect neutralization by some polyclonal and monoclonal antibodies 11.
- Early studies with several COVID-19 vaccines resulted lower neutralizing antibody titers against this variant. However, titers were still well within the expected range to be effective.
P.1 lineage (a.k.a. 20J/501Y.V3)
- The P.1 variant is a branch off the B.1.1.28 lineage that was first reported by the National Institute of Infectious Diseases in Japan in four travelers from Brazil, sampled during routine screening at Haneda airport outside Tokyo.
- The P.1 lineage contains three mutations in the spike protein receptor binding domain: K417T, E484K, and N501Y.
- There is evidence to suggest that some of the mutations in the P.1 variant may affect its transmissibility and antigenic profile, which may affect the ability of antibodies generated through a previous natural infection or through vaccination to recognize and neutralize the virus.
- A recent study reported on a cluster of cases in Manaus, the largest city in the Amazon region, in which the P.1 variant was identified in 42% of the specimens sequenced from late December 11.
- In this region, it is estimated that approximately 75% of the population had been infected with SARS-CoV2 as of October 2020. However, since mid-December the region has observed a surge in cases. The emergence of this variant raises concerns of a potential increase in transmissibility or propensity for SARS-CoV-2 re-infection of individuals.
- This variant was identified in the United States at the end of January 2021.
Coronavirus disease 2019 (COVID-19) also called the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can make anyone seriously ill. But for some people, the risk is higher.
There are 2 levels of higher risk:
- People at high risk (clinically extremely vulnerable)
- People at moderate risk (clinically vulnerable)
People at high risk (clinically extremely vulnerable)
You may be at high risk from coronavirus (COVID-19) if you:
- have had an organ transplant
- are having chemotherapy or antibody treatment for cancer, including immunotherapy
- are having an intense course of radiotherapy (radical radiotherapy) for lung cancer
- are having targeted cancer treatments that can affect the immune system (such as protein kinase inhibitors or PARP inhibitors)
- have blood or bone marrow cancer (such as leukemia, lymphoma or myeloma)
- have had a bone marrow or stem cell transplant in the past 6 months, or are still taking immunosuppressant medicine
- have been told by a doctor you have a severe lung condition (such as cystic fibrosis, severe asthma or severe chronic obstructive pulmonary disease [COPD])
- have a condition that means you have a very high risk of getting infections (such as severe combined immunodeficiency [SCID] or sickle cell)
- are taking medicine that makes you much more likely to get infections (such as high doses of steroids or immunosuppressant medicine)
- have a serious heart condition and are pregnant
- have a problem with your spleen or your spleen has been removed (splenectomy)
- are an adult with Down’s syndrome
- are an adult who is having dialysis or has severe (stage 5) long-term kidney disease
- have been classed as clinically extremely vulnerable, based on clinical judgement and an assessment of your needs
People at moderate risk (clinically vulnerable)
People at moderate risk from coronavirus (COVID-19) include people who:
- are 70 or older
- have a lung condition that’s not severe (such as asthma, COPD, emphysema or bronchitis)
- have heart disease (such as heart failure)
- have diabetes
- have chronic kidney disease
- have liver disease (such as hepatitis)
- have a condition affecting the brain or nerves (such as Parkinson’s disease, motor neurone disease, multiple sclerosis or cerebral palsy)
- have a condition that means they have a high risk of getting infections
- are taking medicine that can affect the immune system (such as low doses of steroids)
- are very obese (a BMI of 40 or above)
- are pregnant
If you’re at high risk (clinically extremely vulnerable) from coronavirus (COVID-19), there are things you can do to help keep yourself safe.
DO
- try to stay at least 2 meters (3 steps) away from anyone you do not live with (or anyone not in your support bubble)
- wash your hands with soap and water often – do this for at least 20 seconds
- use hand sanitizer gel if soap and water are not available
- wash your hands as soon as you get home
- wear something that covers your nose and mouth in places where it’s hard to stay away from other people, such as on public transport, in shops and in hospitals
Work
- You should work from home if possible. Your employer should support you to do this.
- If you cannot work from home and you’re concerned about having to go to work, talk to your employer. Employers should make sure suitable arrangements are in place so you can go to work.
Shopping
To reduce your risk from coronavirus (COVID-19), you may want to:
- do your shopping online
- ask family or friends to collect shopping for you
- avoid busy times if you go shopping.
Pregnancy and COVID-19
There’s no evidence that pregnant women are more likely to get seriously ill from coronavirus (COVID-19). But pregnant women have been included in the list of people at moderate risk (clinically vulnerable) as a precaution. This is because pregnant women can sometimes be more at risk from viruses like flu. It’s not clear if this happens with COVID-19. But because it’s a new virus, it’s safer to include pregnant women in the moderate-risk group.
It may be possible for you to pass COVID-19 to your baby before they are born. But when this has happened, the babies have got better.
There’s no evidence COVID-19 causes miscarriage or affects how your baby develops in pregnancy.
Call your doctor (obstetrician) or maternity team immediately if:
- your baby is moving less than usual
- you cannot feel your baby moving
- there is a change to your baby’s usual pattern of movements
- you have any bleeding from your vagina
- you’re feeling very anxious or worried
- you have a headache that does not go away
- you get shortness of breath when resting or lying down
Do not wait until the next day – call immediately, even if it’s the middle of the night.
What to do if you’re pregnant
If you’re pregnant, it’s important you:
- wash your hands regularly
- stay at home as much as possible and follow the advice on social distancing, such as staying at least 2 meters (3 steps) away from other people
- stay away from anyone who has symptoms of COVID-19
You still need to go to all of your pregnancy (antenatal) scans and appointments unless you’re told not to.
You may find that:
- some midwife appointments are online, by phone or by video call
- you may be asked to wear a mask or gown when you’re in a hospital or clinic
- some appointments may be cancelled or rescheduled – if an appointment is cancelled, it will be rescheduled, or you’ll be able to rebook it
This is to help keep everyone safe and stop the spread of COVID-19.
If you’re unsure if you can bring your partner to your appointment, ask your midwife or maternity team.
Is hydroxychloroquine effective for COVID-19?
Coronavirus disease 2019 (COVID-19) is caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus type 2), the cause of the global pandemic of respiratory illness. In laboratory tests and cell culture systems, both hydroxychloroquine and chloroquine have been shown to have a spectrum of antiviral activity that is believed to be due to interference with viral binding to glycoprotein cell receptors or inhibition of endosomal pH regulation, which inhibits fusion between SARS-CoV-2 and the host cell membrane 12. Hydroxychloroquine can prevent the SARS-CoV and SARS-CoV-2 viruses from attaching to and entering cells. Chloroquine inhibits glycosylation of the cellular angiotensin-converting enzyme 2 (ACE 2) receptor, which may interfere with the binding of SARS-CoV to the cell receptor 13. In vitro studies (test tube studies) have suggested that both chloroquine and hydroxychloroquine may block the transport of SARS-CoV-2 from early endosomes to endolysosomes, possibly preventing the release of the viral genome 13. Both chloroquine and hydroxychloroquine also have immunomodulatory effects, which have been hypothesized to be another potential mechanism of action for the treatment of COVID-19.
Azithromycin has antiviral and anti-inflammatory properties. When used in combination with hydroxychloroquine, it has been shown to have a synergistic effect on SARS-CoV-2 in vitro and in molecular modeling studies 14, 15. However, despite demonstrating antiviral activity in some in vitro systems, neither hydroxychloroquine plus azithromycin nor hydroxychloroquine alone reduced upper or lower respiratory tract viral loads or demonstrated clinical efficacy in a rhesus macaque model 16.
In face of the growing burden of severe illness posed by COVID-19, chloroquine and hydroxychloroquine were proposed as possibly effective in preventing or ameliorating the course and prevent mortality 17, 18, 19. However, multiple human clinical studies have provided data that hydroxychloroquine (Plaquenil) does not provide a medical benefit for hospitalized patients with COVID-19 20, 21, 22.
Use of hydroxychloroquine is controversial, and has been politicized in the U.S. by various groups. Mixed studies have reported both a positive and negative effect, and data may not be robust or reliable: it can include data from study reviews, nonrandomized groups, retrospective research, observational data or from a statistically small sample size of patients. Research for COVID-19 is often quick to be published in non-peer reviewed, preprint online services due to the urgency of the pandemic. However, in general, preprint data should not be used to guide clinical practice. In addition, some hydroxychloroquine studies have been retracted due to lack of confidence in the data, including a Lancet study 23 and one from the New England Journal of Medicine 24.
Hydroxychloroquine, chloroquine, and azithromycin are not approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 25. Furthermore, in June 15, 2020, the FDA revoked the emergency use authorization (EUA) of oral hydroxychloroquine and chloroquine phosphate for the treatment of COVID-19 26. An emergency use authorization (EUA) can allow quicker access to critical medical products when there are no approved alternative options.
- Based on an evaluation of the scientific data to date, the FDA concluded that chloroquine and hydroxychloroquine are not likely to be effective in the treatment of COVID-19 for the authorized uses in the EUA.
- In addition, the risk for serious side effects with hydroxychloroquine and chloroquine phosphate are a concern. This includes the possibility of adverse cardiovascular (heart) events such as an abnormal heart rhythm which could be fatal.
- Additional worldwide studies are still ongoing to assess the use of these agents for the treatment or prevention or COVID-19, including early-stage outpatient and use with supplements such as zinc or vitamin D or with azithromycin. However, the FDA states hydroxychloroquine should not be used outside of clinical trials in the U.S.
The World Health Organization (WHO) and the U.S. National Institutes of Health (NIH) have also stopped studies evaluating hydroxychloroquine for the treatment of COVID-19 due to a lack of benefit. Current NIH and US treatment guidelines do not recommend use of hydroxychloroquine and chloroquine phosphate for COVID-19 treatment outside of clinical studies.
Although earlier studies suggested that hydroxychloroquine could inhibit the SARs-CoV-2 virus and was more potent than chloroquine, recent studies do not support the use of hydroxychloroquine or chloroquine phosphate. The FDA stated on June 15, 2020 that the suggested dosing regimens for chloroquine and hydroxychloroquine are unlikely to kill or inhibit the virus that causes COVID-19 26.
Do studies show hydroxychloroquine is not effective for COVID-19?
Yes, multiple studies provide data that hydroxychloroquine is ineffective in the treatment of SARS-CoV-2, the virus that causes COVID-19 disease.
Hospitalized patients
In a large, randomized, controlled, open-label study evaluating a number of potential treatments for patients hospitalized with COVID-19 in the United Kingdom, the RECOVERY Trial from the University of Oxford 27. The RECOVERY study is being conducted by researchers at the University of Oxford in the UK (the hydroxychloroquine arm is now halted) 28. Hydroxychloroquine did not decrease 28-day mortality when compared to the usual standard of care 29. Patients who were randomized to receive hydroxychloroquine had a longer median hospital stay than those who received the standard of care. In addition, among patients who were not on invasive mechanical ventilation at the time of randomization, those who received hydroxychloroquine were more likely to subsequently require intubation or die during hospitalization than those who received the standard of care 29.
- In the RECOVERY Trial, investigators reported that there was no beneficial effect or reduction of death in hospitalized patients with COVID-19 receiving hydroxychloroquine 28.
- In this study, 1561 patients received hydroxychloroquine and were compared to 3155 patients receiving standard care only. No difference was found in the primary endpoint, which was the incidence of death at 28 days (26.8% hydroxychloroquine vs. 25% usual care).
- In addition, hydroxychloroquine treatment was associated with an increased length of stay in the hospital and increased need for invasive mechanical ventilation.
- Based on this data, investigators stopped enrollment in the RECOVERY hydroxychloroquine arm on June 5th, 2020 28.
The results from several additional large randomized controlled trials have been published; these trials have failed to show a benefit for hydroxychloroquine with or without azithromycin or azithromycin alone in hospitalized adults with COVID-19. In the Solidarity trial 30, an international randomized controlled platform trial that enrolled hospitalized patients with COVID-19, the hydroxychloroquine arm was halted for futility. There was no difference in in-hospital mortality between patients in the hydroxychloroquine arm and those in the control arm 30. Similarly, PETAL 31, a randomized, placebo-controlled, blinded study, was stopped early for futility. In this study, there was no difference in the median scores on the COVID Outcomes Scale between patients who received hydroxychloroquine and those who received placebo 31. Data from two additional randomized studies of hospitalized patients with COVID-19 did not support using hydroxychloroquine plus azithromycin over hydroxychloroquine alone 32, 33. In RECOVERY, azithromycin alone (without hydroxychloroquine) did not improve survival or other clinical outcomes when compared to the usual standard of care 27.
In addition to these randomized trials, data from large retrospective observational studies do not consistently show evidence of a benefit for hydroxychloroquine with or without azithromycin in hospitalized patients with COVID-19 34, 35, 36.
In a multicenter, randomized, open-label, controlled trial published in July 2020 by Cavalcanti and colleagues in the New England Journal of Medicine 37, hydroxychloroquine use was studied in patients who were hospitalized with mild-to-moderate COVID-19.
- Patients received hydroxychloroquine (400 mg twice daily for 7 days), hydroxychloroquine with azithromycin (hydroxychloroquine 400 mg twice daily + azithromycin 500 mg once daily for 7 days), or standard care only.
- The clinical status of these patients at day 15 was not improved as compared with the patients receiving only standard care.
- In addition, researchers noted that prolonged QT intervals (which may lead to abnormal heart rates and death) and elevated liver enzymes were higher in patients receiving hydroxychloroquine, either with or without azithromycin.
A retrospective, observational study conducted from March to early May of 2020 36 did report a positive effect with hydroxychloroquine on hospitalized patient mortality, used alone and with azithromycin when compared to no treatment. The study authors note a limitation to their analysis was the retrospective, non-randomized, non-blinded study design 36.
- Researchers looked at 2,541 patients, with a median total hospitalization time of 6 days.
- Mortality, by treatment, was 20.1% for hydroxychloroquine + azithromycin, 13.5% for hydroxychloroquine alone, 22.4% for azithromycin alone, and 26.4% for neither drug. The primary cause of death was respiratory failure in 88% of patients.
- Adjunct therapy with corticosteroids (methylprednisolone and/or prednisone) and anti-IL-6 tocilizumab was provided in 68% and 4.5% of patients, respectively.
- Factors such as greater glucocorticoid use in the hydroxychloroquine groups and the nonrandomized study design suggested this data may be flawed and that prospective, randomized controlled studies were needed to validate these results.
Given the lack of a benefit seen in the randomized clinical trials, the COVID-19 Treatment Guidelines Panel (the Panel) recommends against using hydroxychloroquine or chloroquine and/or azithromycin to treat COVID-19 in hospitalized patients 38.
Nonhospitalized patients
Several randomized trials have not shown a clinical benefit for hydroxychloroquine in nonhospitalized patients with early, asymptomatic, or mild COVID-19 39, 40. In an open-label trial, Mitja et al. 40 randomized 307 nonhospitalized people who were recently confirmed to have COVID-19 to receive hydroxychloroquine or no antiviral treatment. Patients in the hydroxychloroquine arm received hydroxychloroquine 800 mg on Day 1 followed by 400 mg daily for an additional 6 days. The authors reported no difference in the mean reduction in SARS-CoV-2 RNA at Day 3 or the time to clinical improvement between the two arms 40. In another trial 41, treating patients who had asymptomatic or mild COVID-19 with hydroxychloroquine with or without azithromycin did not result in greater rates of virologic clearance (as measured by a negative polymerase chain reaction [PCR] result on Day 6).
A randomized, double-blind, placebo-controlled trial from Skipper and colleagues published in the Annals of Internal Medicine in July 2020 39 was conducted in 423 outpatients (not in the hospital) with early COVID-19.
- Patients received oral hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 more days) or a placebo (inactive treatment).
- Researchers found that over a 14 day period a change in symptom severity and the percent of patients with ongoing symptoms did not differ significantly between groups, signaling no effect from the hydroxychloroquine treatment.
- However, side effects were significantly greater in the group receiving hydroxychloroquine compared to placebo (43% hydroxychloroquine versus 22% placebo). Rates of hospitalizations and deaths did not differ significantly.
An open-label, prospective, randomized trial compared oral azithromycin 500 mg once daily for 3 days plus standard of care to standard of care alone in nonhospitalized, high-risk, older adults who had laboratory-confirmed or suspected COVID-19 42. No differences were observed between the arms in the primary endpoints of time to first self-reported recovery and hospitalization or death due to COVID-19 42. These findings remained consistent in an analysis that was restricted to participants with positive SARS-CoV-2 PCR results. The study was ultimately halted due to futility 42. Similarly, in a preliminary report from ATOMIC-2, adding oral azithromycin 500 mg once daily to standard of care for 14 days did not reduce the risk of hospitalization or death among 292 participants with mild to moderate COVID-19 43.
While ongoing clinical trials are still evaluating the use of chloroquine, hydroxychloroquine, and azithromycin in outpatients, the existing data suggest that it is unlikely that clinical benefits will be identified for these agents. The COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of chloroquine or hydroxychloroquine and/or azithromycin for the treatment of COVID-19 in nonhospitalized patients 38.
Hydroxychloroquine study for prevention after exposure to COVID-19
A randomized, double-blind, placebo-controlled study published online in the New England Journal of Medicine in June 2020 44 looked at prevention of COVID-19 after exposure to the virus (post-exposure prophylaxis or PEP).
- Researchers evaluated over 800 people in the U.S. and Canada who had been exposed to COVID-19. The primary outcome was the incidence of either laboratory-confirmed COVID-19 or illness compatible with the virus within 14 days.
- Hydroxychloroquine was given as 800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days. Patients started treatment within 4 days after exposure, defined as being in close contact with a COVID-19 patient for more than 10 minutes without protection.
- Results showed that hydroxychloroquine did not prevent COVID-19 when compared to a placebo (used as post-exposure prophylaxis). The incidence of COVID-19 did not differ significantly between those who took hydroxychloroquine (11.8%) and those who took placebo (14.3%).
- Side effects were more common in the hydroxychloroquine group (40.1% compared to 16.8% with placebo), but were not reported as serious. Common adverse events included nausea, loose stools, and stomach pain.
- Limitations in this study 44 were many, and included inability to confirm self-reported COVID-19 exposure, adherence to study drug, starting drug up to 4 days after reported exposure to the virus, lack of survey completion, and enrollment of a lower-risk population.
Can ivermectin be used for preventing and treating COVID‐19?
Ivermectin is an anti-parasite medication with activity against several parasitic nematodes (parasitic worms) and scabies and is the treatment of choice for onchocerciasis (also called “river blindness” because the blood-feeding flies that transmit the ultimately blinding disease inhabit lush, fertile land alongside the rivers in which they breed). Ivermectin has a broad spectrum of activity against several nematodes (Ascaris, Trichuris, Ancylostoma), cestodes (Taenia) and trematodes (Fasciola, Schistosoma). Ivermectin has particularly potent activity against onchocerciasis (river blindness) and lymphatic filariasis, which are important endemic diseases in Africa and South America. In tropical Africa, Onchocerca parasites (Onchocerca volvulus) are transmitted primarily by Dipteran blackflies of the Simulium damnosum complex, members of the Simulium neavei group also being vectors. Some game animals, notably elands and buffalo, are possible reservoir hosts. In the Western hemisphere, varieties of Simulium species bite humans and may transmit parasites. Ivermectin is a lipophilic drug that belongs to the avermectin class (mostly avermectin H2B1a with some avermectin H2B1b) of macrocyclic lactone compounds 45. Ivermectin acts as an endectocide (i.e., kills both endoparasites and ectoparasites). Ivermectin tablet (Stromectol) is approved by the FDA for use in humans to treat intestinal strongyloidiasis (Strongyloides stercoralis) and onchocerciasis (Onchocerca volvulus). Ivermectin cream 1% (Soolantra) is also FDA approved for rosacea.
Ivermectin is currently being investigated as a treatment for coronavirus SARS-CoV-2, which is the virus that causes COVID-19. The FDA has not approved ivermectin for use in treating or preventing COVID-19 in humans. The World Health Organization (WHO) recommend not to use ivermectin in patients with COVID-19, except in clinical trials.
In cell culture systems, ivermectin has activity against several viruses including the novel coronavirus known as COVID‐19 (Severe Acute Respiratory Syndrome coronavirus-type 2 [SARS-CoV-2]) virus, the cause of the global pandemic of respiratory illness 46. In the face of growing burden of severe illness posed by COVID-19, drugs with antiviral activity against SARS-CoV-2 in vitro (test tube studies) were often tried (repurposed) to improve the course and prevent death 47, 48, 49. A plausible and well‐characterized antiviral mechanism of ivermectin has been proposed to be the inhibition of nuclear translocation of viral proteins, facilitated by mammalian host importin also known as karyopherin α/β‐1 heterodimerization 48. Based on this mechanism, ivermectin binds to the importin alpha (armadillo repeat) domain causing thermal stability and a conformational change in alpha‐helicity that prevents binding to importin beta‐1 50.
Ivermectin was evaluated in several open label trials (14 studies with 1678 participants investigating ivermectin compared to no treatment, placebo, or standard of care) with suggestive evidence of benefit, but in more carefully designed, larger trials ivermectin in doses of 20 to 14 mg daily for 3 to 5 days had little or no effect in either preventing infection or ameliorating its outcome 51.
Ivermectin compared to placebo or standard of care for treating people in hospital with COVID‐19 (inpatient COVID‐19 treatment)
- Scientists are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality and clinical worsening up to day 28 assessed as need for invasive mechanical ventilation (IMV) or need for supplemental oxygen (0 participants required supplemental oxygen; 1 study, 45 participants; very low‐certainty evidence), adverse events within 28 days and viral clearance at day seven 52. Ivermectin may have little or no effect compared to placebo or standard of care on clinical improvement up to 28 days and duration of hospitalization. No study reported quality of life up to 28 days 52.
Ivermectin compared to placebo or standard of care for outpatient COVID‐19 treatment
- Scientists are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality up to 28 days and clinical worsening up to 14 days assessed as need for invasive mechanical ventilation or non‐invasive mechanical ventilation or high flow oxygen requirement. Researchers are uncertain whether ivermectin compared to placebo reduces or increases viral clearance at seven days. Ivermectin may have little or no effect compared to placebo or standard of care on the number of participants with symptoms resolved up to 14 days and adverse events within 28 days. None of the studies reporting duration of symptoms were eligible for primary analysis. No study reported hospital admission or quality of life up to 14 days 52.
Ivermectin compared to no treatment for prevention of COVID‐19 infection
- Researchers found one study. Mortality up to 28 days was the only outcome eligible for primary analysis. The researchers are uncertain whether ivermectin reduces or increases mortality (death rate) compared to no treatment. The study reported results for development of COVID‐19 symptoms and adverse events up to 14 days that were included in a secondary analysis due to high risk of bias. No study reported COVID‐19 infection, hospital admission, and quality of life up to 14 days.
Based on the current very low‐ to low‐certainty evidence, scientists are uncertain about the effectiveness and safety of ivermectin used to treat or prevent COVID‐19 52. The completed studies are small and few are considered high quality. Several studies are underway that may produce clearer answers in review updates. Overall, the reliable evidence available does not support the use ivermectin for treatment or prevention of COVID‐19 outside of well‐designed randomized trials.
Evaluation of ivermectin is continuing in 31 ongoing studies, and we will update this post with their results when they become available.
ICON Clinical Study Results
- Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study 53, 54
- The ICON study, which was a multihospital retrospective cohort study, involved 280 patients, 173 patients were treated with at least one oral dose of 200 μg/kg ivermectin and 107 patients had no ivermectin treatment. Patients could also be treated with hydroxychloroquine, azithromycin, or both.
- The results from this study showed that:
- Overall mortality was significantly lower for the ivermectin group (15%) compared to the usual treatment group (25.2%) using figures from the unmatched cohort.
- Mortality for the subgroup of patients who had severe pulmonary involvement was lower in the ivermectin treatment group (38.8%) compared to the usual treatment group (80.7%) from the unmatched cohort.
- There was no significant difference between the two treatment groups regarding successful extubation rates of mechanically ventilated patients. Successful extubation is when the patient has had the breathing tube that has been used for mechanical ventilation removed and they can successfully breath on their own.
- Length of hospital stay was not significantly different between the ivermectin treatment group and the regular treatment group.
- For secondary analysis of the results propensity score matching was performed, which is when the researcher matches a person in the treatment group to a person in the non-treatment group who has similar characteristics. This reduces the effects of confounding and selection bias.
- For the propensity matched cohort figures the mortality was significantly lower in the ivermectin treatment group compared to the regular treatment group (13.3% vs 24.5%).
- The study noted that “Interpretation of these findings are tempered by the limitations of the retrospective design and the possibility of confounding” and that “Further studies in appropriately designed randomized trials are recommended before any conclusions can be made.”
- There has been feedback in regard to this study querying some of the important variables and how they may have affected the study outcome.
IVERCOR-COVID-19 trial
- Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial 55
- The IVERCOR-COVID-19 trial was a randomized, double-blind, placebo controlled trial involving 501 patients. The aim of this trial was to determine whether ivermectin treatment could prevent hospitalization of individuals with early COVID-19. One group was treated with ivermectin plus standard treatment and the other group was treated with placebo with standard treatment. Patients were not allowed to take hydroxychloroquine, chloroquine or antiviral drugs.
- The results of this trial were published on 2 July 2021 showed that:
- The percentage of patients that needed hospitalization was 5.6% in the ivermectin treatment group and 8.4% in the placebo group, but the difference between the two groups was not statistically significantly
- There was no statistically significant difference in median time from patient enrollment to hospitalization between the treatment groups. The ivermectin treatment group median time to hospitalization was 3.5 days and placebo group 3 days.
- There was no statistically significant difference in mean time from study enrolment until a patient was put on invasive mechanical ventilatory support (MVS). The ivermectin treatment group was 5.25 days compared to the placebo group which was 10 days.
- The limitations in this study was noted as a:
- Low percentage of hospitalized patients
- The mean dose of ivermectin was 192.37 μg/kg/day which was below the dose proposed as probably being effective.
PRINCIPLE TRIAL
- Platform Randomised Trial of Treatments in the Community for Epidemic and Pandemic Illnesses (PRINCIPLE) 56.
- The PRINCIPLE trial is a current trial investigating treatments for COVID-19 patients in the community who are more at risk of serious illness. The study is investigating a number of different treatments including ivermectin.
- Participants in the ivermectin part of the trial will be:
- Between the age 18 and 64 years old
- With a underlying health condition or shortness of breath due to COVID-19
- Must join trial within first 14 days of covid-19 symptoms or a positive test
- Will be assigned to receive 3 day course of oral ivermectin treatment
- They will be compared to a group of patients receiving standard NHS COVID-19 care.
The ivermectin part of this trial started in June 2021 and results will be published when available.
Here’s what you need to know about Ivermectin
- Ivermectin is not FDA approved to treat COVID-19 patients. Ivermectin should not be used in patients for COVID-19, unless it is part of a clinical trial.
- With known antiviral properties, ivermectin has been shown to reduce SARS-CoV-2 replication in laboratory studies 56. Small pilot studies show that early administration with ivermectin can reduce viral load and the duration of symptoms in some patients with mild COVID-19. Even though ivermectin is used routinely in some countries to treat COVID-19, there is little evidence from large-scale randomised controlled trials to demonstrate that it can speed up recovery from the illness or reduce hospital admission 56.
- Currently there is not enough high quality evidence supporting the use of ivermectin for COVID-19 treatment 57. Clinical trials assessing ivermectin tablets for the prevention or treatment of COVID-19 in people are ongoing.
- More randomized clinical trials with a higher certainty of evidence are needed for ivermectin in the treatment COVID-19.
- Ivermectin tablets are FDA approved medicine for some types of intestinal worms and ivermectin cream is FDA approved for rosacea treatment.
- Both oral and topical ivermectin have a good safety profile at standard dosing levels.
The WHO Therapeutics and COVID-19 living guideline is the World Health Organization’s (WHO) most up-to-date recommendations of treatments of COVID-19.
- Their recommendation is that ivermectin should not used in patients with COVID-19 unless it is in a research setting, as part of a clinical trial 58.
- This recommendation is the result of a systematic review of randomised clinical trials that used ivermectin for COVID-19. After analysing the information from these studies, they believe that there is a high degree of uncertainty on whether ivermectin is helpful or harmful in treating COVID-19.
- The uncertainty in some of the ivermectin trials for COVID-19 is due to:
- serious risk of bias
- serious risk of imprecision.
There are far fewer randomized controlled trials for ivermectin compared to other COVID treatments. The randomized controlled trials included multiple small trials, that had fewer patients enrolled and had fewer events recorded.
More randomized controlled trials are needed with higher quality of evidence to determine if ivermectin is successful at treating COVID-19.
Is vitamin C effective against COVID-19?
Vitamin C also known as ascorbic acid, is currently making headlines in the fight against COVID-19. Interest in the use of vitamin C supplements to treat COVID-19 comes from research showing that taking 200 mg/day or more vitamin C supplements on a regular basis helps reduce the duration of the common cold and the severity of its symptoms 59. Vitamin C supplements also appear to reduce the risk of developing a cold in people exposed to extreme physical stress—including marathon runners, skiers, and soldiers in subarctic areas 60. In addition, vitamin C supplementation might benefit people with pneumonia who have low vitamin C levels 61, as well as people with viral infections, including Epstein-Barr and herpes zoster 62. Vitamin C’s antioxidant action might also help reduce oxidative stress during infections 59. People with low vitamin C status might benefit more from vitamin C supplementation than those who already obtain sufficient vitamin C 63.
A few observational studies have examined the effects of vitamin C supplementation on mortality rates in patients with COVID-19 and have had mixed findings 64. For example, a retrospective chart review of 102 patients (median age 63 years) with COVID-19 who were receiving intensive care included 73 patients who received vitamin C plus zinc (doses not specified); the other patients did not receive these supplements 65. Vitamin C and zinc supplementation did not affect mortality. Another retrospective chart review included 152 patients with COVID-19 (median age 68 years) who were on mechanical ventilation 66. The 79 patients who received vitamin C supplements (doses not specified) had a significantly lower mortality rate than those who did not receive vitamin C supplements. In addition, self-reported use of vitamin C supplements (dose not reported) more than three times per week for at least 3 months among 372,720 U.K. residents aged 16 to 90 years, 45,757 individuals in the United States, and 27,373 individuals in Sweden was not associated with higher or lower risk of SARS-CoV-2 infection 67.
The COVID A to Z trial compared the effects of daily supplementation with 8,000 mg ascorbic acid, 50 mg zinc (as zinc gluconate), or both for 10 days with standard of care in 214 adults (mean age 45.2 years) with COVID-19 who were not hospitalized 68. None of the supplements shortened symptom duration.
Table 1. Average daily recommended amount of vitamin C
| Life Stage | Recommended Amount |
| Birth to 6 months | 40 mg |
| Infants 7–12 months | 50 mg |
| Children 1–3 years | 15 mg |
| Children 4–8 years | 25 mg |
| Children 9–13 years | 45 mg |
| Teens 14–18 years (boys) | 75 mg |
| Teens 14–18 years (girls) | 65 mg |
| Adults (men) | 90 mg |
| Adults (women) | 75 mg |
| Pregnant teens | 80 mg |
| Pregnant women | 85 mg |
| Breastfeeding teens | 115 mg |
| Breastfeeding women | 120 mg |
Footnote: If you smoke, add 35 mg to the above values to calculate your total daily recommended amount.
Table 2. Food sources of Vitamin C
| Food | Milligrams (mg) per serving | Percent (%) DV* |
| Red pepper, sweet, raw, ½ cup | 95 | 106 |
| Orange juice, ¾ cup | 93 | 103 |
| Orange, 1 medium | 70 | 78 |
| Grapefruit juice, ¾ cup | 70 | 78 |
| Kiwifruit, 1 medium | 64 | 71 |
| Green pepper, sweet, raw, ½ cup | 60 | 67 |
| Broccoli, cooked, ½ cup | 51 | 57 |
| Strawberries, fresh, sliced, ½ cup | 49 | 54 |
| Brussels sprouts, cooked, ½ cup | 48 | 53 |
| Grapefruit, ½ medium | 39 | 43 |
| Broccoli, raw, ½ cup | 39 | 43 |
| Tomato juice, ¾ cup | 33 | 37 |
| Cantaloupe, ½ cup | 29 | 32 |
| Cabbage, cooked, ½ cup | 28 | 31 |
| Cauliflower, raw, ½ cup | 26 | 29 |
| Potato, baked, 1 medium | 17 | 19 |
| Tomato, raw, 1 medium | 17 | 19 |
| Spinach, cooked, ½ cup | 9 | 10 |
| Green peas, frozen, cooked, ½ cup | 8 | 9 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin C is 90 mg for adults and children age 4 years and older [13]. FDA does not require food labels to list vitamin C content unless vitamin C has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 69 ]Is intravenous vitamin C effective against COVID-19?
Studies have also examined the effects of vitamin C administered intravenously. Intravenous (IV) vitamin C is NOT a cure for COVID-19. However, there are reports say that intravenous (IV) vitamin C may help people suffering from COVID-19. Many researchers recommend studying vitamin C as an adjuvant therapy for COVID-19, including its possible ability to reduce inflammation and vascular injury in these patients 70, 71, 59.
Intravenous (IV) vitamin C is a solution of vitamin C is delivered directly into the bloodstream through a vein, typically in the arm. A physician’s order is required to receive this treatment. It is important to get IV vitamin C only in a clinic or other medical setting. IV vitamin C goes straight into the bloodstream. Because it skips the stomach and intestines, vitamin C in the blood rises to very high levels and very quickly.
Intravenous administration of vitamin C can produce plasma concentrations that are much higher than those produced by oral doses 72. The FDA classifies intravenous forms of vitamin C as drugs; only oral forms can be classified as dietary supplements.
Recently, IV vitamin C clinical trials began in China and Italy. Doctors in hard-hit areas of the United States are now reporting using it. Furthermore, IV vitamin C appears in some COVID-19 critical care guidelines, like at the Eastern Virginia Medical School.
According to some case reports from China, for example, high-dose intravenous vitamin C (10–20 g per day administered over 8 to 10 hours) increased the oxygenation index in 50 patients with moderate to severe COVID-19; all patients eventually recovered 73. In a pilot trial in China, 56 patients with COVID-19 (mean age 66.7 years) in ICU received either intravenous vitamin C (12 g twice daily) or placebo for 7 days or until ICU discharge or death 74. Vitamin C administration did not affect 28-day mortality rates. In another trial of 60 patients with severe COVID-19 infection (mean age 58 to 61 years) and receiving oral lopinavir/ritonavir and hydroxychloroquine, 30 patients were also given intravenous vitamin C (1.5 g four times daily) for 5 days 75. Vitamin C administration did not affect mortality, length of ICU stay, or oxygen saturation at discharge.
The National Institutes of Health COVID-19 Treatment Guidelines Panel notes that in patients who do not have COVID-19, intravenous vitamin C alone or in combination with other nutrients and medications improves some but not all outcomes in critically ill patients with sepsis, acute respiratory distress syndrome, or pneumonia 76. However, the National Institutes of Health COVID-19 Treatment Guidelines Panel concludes that data are insufficient to support a recommendation for or against the use of vitamin C to treat COVID-19 76.
Several other clinical trials are examining whether vitamin C (administered intravenously or as a dietary supplement) in combination with other dietary supplement ingredients, medications, or both helps prevent or treat COVID-19. For example, one trial in Italy is investigating intravenous administration of 10 g ascorbic acid in addition to conventional therapy in about 500 children and adults who are hospitalized with COVID-19 pneumonia 77. Another trial is evaluating whether daily supplementation with 1,000 mg ascorbic acid plus 10 mg melatonin for 14 days affects the symptoms and outcomes of COVID-19 in about 150 adults aged 50 years and older who are not hospitalized 78.
There are several theories about how IV vitamin C may help treat COVID-19:
- Very high vitamin C levels may create free radicals that destroy viruses and bacteria. Our body’s cells have defenses against these free radicals, but viruses do not.
- Another possibility is IV vitamin C renews the body’s antioxidant protection. Serious infections can use up our body’s vitamin C and other antioxidants very quickly.
Vitamin C is a vital part of the immune system, but there is no reason to think intravenous vitamin C can boost your immune system beyond normal, healthy status. Daily vitamin C from food or supplements is enough to support healthy immunity. The amount of vitamin C you need each day depends on your age. Average daily recommended amounts for different ages are listed below in milligrams (mg) (Table 1). Fruits and vegetables are the best sources of vitamin C (see Table 2) 79. Citrus fruits, tomatoes and tomato juice, and potatoes are major contributors of vitamin C to the American diet 80. Other good food sources include red and green peppers, kiwifruit, broccoli, strawberries, Brussels sprouts, and cantaloupe (see Table 2) 80. Although vitamin C is not naturally present in grains, it is added to some fortified breakfast cereals. The vitamin C content of food may be reduced by prolonged storage and by cooking because ascorbic acid is water soluble and is destroyed by heat 81. Steaming or microwaving may lessen cooking losses. Fortunately, many of the best food sources of vitamin C, such as fruits and vegetables, are usually consumed raw. Consuming five varied servings of fruits and vegetables a day can provide more than 200 mg of vitamin C.
Vitamin C has low toxicity and is not believed to cause serious adverse effects at high intakes 82. The most common complaints are diarrhea, nausea, abdominal cramps, and other gastrointestinal disturbances due to the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract 83. High vitamin C doses might also cause falsely high or low readings on some blood glucose meters that are used to monitor glucose levels in people with diabetes 84. In people with hemochromatosis, high doses of vitamin C could exacerbate iron overload and damage body tissues 83.
What are COVID-19 variants?
Multiple genetic variants of SARS-CoV-2, the virus that causes COVID-19 have been documented in the United States and globally during this pandemic. Viruses constantly change through mutation and become more diverse. Some variations allow the virus to spread more easily or make it resistant to treatments or vaccines. Those variants must be monitored more carefully. Scientists monitor these changes, including changes to the spikes on the surface of the COVID-19 virus. If you think about a virus like a tree growing and branching out; each branch on the tree is slightly different than the others. By comparing the branches, scientists can label them according to the differences. These small differences, or variants, have been studied and identified since the beginning of the pandemic.
Viral mutations and variants in the United States are routinely monitored through sequence-based surveillance, laboratory studies, and epidemiological investigations. By carefully studying COVID-19 viruses, scientists can learn how changes to SARS-CoV-2 virus might affect how it spreads and how sick people will get from it.
US government interagency group developed a Variant Classification scheme that defines three classes of SARS-CoV-2 variants:
- Variant of Interest: A variant with specific genetic markers that have been associated with changes to receptor binding, reduced neutralization by antibodies generated against previous infection or vaccination, reduced efficacy of treatments, potential diagnostic impact, or predicted increase in transmissibility or disease severity.
- Current variants of interest in the United States that are being monitored and characterized are:
- B.1.427
- B.1.429
- B.1.525
- B.1.526
- B.1.617.1
- B.1.617.3
- P.2
- Current variants of interest in the United States that are being monitored and characterized are:
- Variant of Concern: A variant for which there is evidence of an increase in transmissibility, more severe disease (e.g., increased hospitalizations or deaths), significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures.
- Current variants of concern in the United States that are being closely monitored and characterized by federal agencies are:
- B.1.1.7
- B.1.351
- B.1.617.2
- P.1
- Current variants of concern in the United States that are being closely monitored and characterized by federal agencies are:
- Variant of High Consequence: A variant of high consequence has clear evidence that prevention measures or medical countermeasures have significantly reduced effectiveness relative to previously circulating variants. A variant of high consequence would require notification to WHO under the International Health Regulations, reporting to CDC, an announcement of strategies to prevent or contain transmission, and recommendations to update treatments and vaccines.
- Currently there are no SARS-CoV-2 variants that rise to the level of high consequence.
COVID-19 variants in the United States
Scientists are monitoring multiple COVID-19 variants; currently there are four notable variants in the United States:
- B.1.1.7 (Alpha): This variant was first detected in the United States in December 2020. It was initially detected in the United Kingdom.
- B.1.351 (Beta): This variant was first detected in the United States at the end of January 2021. It was initially detected in South Africa in December 2020.
- P.1 (Gamma): This variant was first detected in the United States in January 2021. P.1 was initially identified in travelers from Brazil, who were tested during routine screening at an airport in Japan, in early January.
- B.1.617.2 (Delta): This variant was first detected in the United States in March 2021. It was initially identified in India in December 2020.
These variants seem to spread more easily and quickly than other variants, which may lead to more cases of COVID-19. An increase in the number of cases will put more strain on healthcare resources, lead to more hospitalizations, and potentially more deaths. Based on current data, variant B.1.1.7 is the most common variant across the country 85. So far, studies suggest that the current authorized vaccines work on the circulating variants. Scientists will continue to study these and other variants.
COVID-19 Delta Variant
The CDC report on the Delta variant (B.1.617.2 variant which was initially identified in India in December 2020) is causing more infections and spreads faster than early forms of SARS-CoV-2 86:
- The Delta variant is more contagious: The Delta variant is highly contagious, more than 2x as contagious as previous variants.
- Vaccines continue to reduce a person’s risk of contracting the virus that cause COVID-19, including the Delta variant. The COVID-19 vaccines authorized in the United States are highly effective at preventing severe disease and death, including against the Delta variant. But they are not 100% effective and some fully vaccinated people will become infected (called a breakthrough infection) and experience illness. For such people, the vaccine still provides them strong protection against serious illness and death.
- Some data suggest the Delta variant might cause more severe illness than previous strains in unvaccinated persons. In two different studies from Canada and Scotland, patients infected with the Delta variant were more likely to be hospitalized than patients infected with Alpha or the original virus strains.
- Unvaccinated people remain the greatest concern: Although breakthrough infections happen much less often than infections in unvaccinated people, individuals infected with the Delta variant, including fully vaccinated people with symptomatic breakthrough infections, can transmit it to others. CDC is continuing to assess data on whether fully vaccinated people with asymptomatic breakthrough infections can transmit. However, the greatest risk of transmission is among unvaccinated people who are much more likely to contract, and therefore transmit the virus.
- Fully vaccinated people with Delta variant breakthrough infections can spread the virus to others. However, vaccinated people appear to be infectious for a shorter period: Previous variants typically produced less virus in the body of infected fully vaccinated people (breakthrough infections) than in unvaccinated people. In contrast, the Delta variant seems to produce the same high amount of virus in both unvaccinated and fully vaccinated people. However, like other variants, the amount of virus produced by Delta breakthrough infections in fully vaccinated people also goes down faster than infections in unvaccinated people. This means fully vaccinated people are likely infectious for less time than unvaccinated people.
A CDC presentation on the Delta variant was leaked to the press (https://www.documentcloud.org/documents/21026654-57c98604-3b54-44f0-8b44-b148d8f75165). The key points from that presentation are as follows:
- The risk of infection among vaccinated persons compared to unvaccinated is 21 vs. 177/100,000, for hospitalization is 0.1 vs. 2.52/100,000, and for mortality is 0.04 vs. 0.96/100,000.
- Vaccine effectiveness for the Delta variant is 87% to 90% overall. However, for immunocompromised persons, the effectiveness of vaccines is lower at 59% to 80%. There is also a lower effectiveness in older people; for example, in long-term care facilities, the effectiveness is 70% to 75%.
- The Delta variant has an R0 (effective reproductive number or average number of persons infected per case) of 5 to 10, compared with 1.5 to 3 for the ancestral strain of SARS-CoV-2. This is comparable to chickenpox in infectiousness.
- Also, the Delta variant results in a much higher viral load, and it is detectable longer (18 vs. 13 days median). The risk of reinfection with Delta variant is higher than with Alpha variant, but only if the initial infection was more than six months ago. Breakthrough cases of Delta variant have about 10´ the viral load of other strains and are likely as transmissible as cases in unvaccinated persons.
There is mounting evidence from studies in Canada, Singapore, and Scotland that the Delta variant causes more severe disease. For example, a study in Canada found higher risk for hospitalization (Adjusted Odds Ratio, 1.5), ICU admission (AOR, 1.89), and death (AOR, 1.51) 87. Given the greater likelihood of breakthrough infection cases and high viral load in those cases, masking for all people is required to reduce transmission.
Coronaviruses are named for the crown-like spikes on their surface. There are four main sub-groupings of coronaviruses, known as alpha, beta, gamma, and delta 88.
Human coronaviruses were first identified in the mid-1960s. The seven coronaviruses that can infect people are 88:
Common human coronaviruses
- 229E (alpha coronavirus)
- NL63 (alpha coronavirus)
- OC43 (beta coronavirus)
- HKU1 (beta coronavirus)
Other human coronaviruses
- MERS-coronavirus (the beta coronavirus that causes Middle East Respiratory Syndrome, or MERS)
- SARS-coronavirus (the beta coronavirus that causes severe acute respiratory syndrome, or SARS)
- 2019 Novel Coronavirus (COVID-19)
People around the world commonly get infected with human coronaviruses 229E, NL63, OC43, and HKU1 88.
Sometimes coronaviruses that infect animals can evolve and make people sick and become a new human coronavirus. Three recent examples of this are 2019 Novel Coronavirus (COVID-19), SARS-coronavirus, and MERS-coronavirus.
On January 9, 2020, the World Health Organization (WHO) reported that a novel (new) coronavirus (COVID-19) was identified by Chinese authorities, which has resulted in hundreds of confirmed cases in China, including cases outside Wuhan City, with additional cases being identified in a growing number of countries internationally. The 2019 Novel Coronavirus (COVID-19) is a virus identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China. Early on, many of the patients in the outbreak in Wuhan, China reportedly had some link to a large seafood and animal market, suggesting animal-to-person spread. However, a growing number of patients reportedly have not had exposure to animal markets, suggesting person-to-person spread is occurring. At this time, it’s unclear how easily or sustainably this virus is spreading between people. The first two case in the United States was announced on January 21 and 24, 2020, both in travelers returning from Wuhan 89.
The situation with regard to 2019 Novel Coronavirus (COVID-19) is still unclear. While severe illness, including illness resulting in a number of deaths has been reported in China, other patients have had milder illness and been discharged.
There are ongoing investigations to learn more. This is a rapidly evolving situation and information will be updated as it becomes available.
It was estimated that approximately two thirds of COVID-19 cases exported from China from 1 to 13 January 2020 have gone undetected globally 90. Most of these exported cases will be mild and may only be detected after several hundred cases have accumulated and severe or fatal cases are recognized 5 to 8 weeks later, as has likely occurred in the recent COVID-19 outbreaks in Iran, South Korea, Italy, and Seattle in the USA 91.
The spread of novel coronavirus COVID-19 transmission globally has been very rapid. The basic reproduction number is estimated at between 2 to 3 92. The mode of transmission is thought to be droplet and contact infection, although opportunistic or close range airborne infection may be involved 93.
The transmission dynamics of the early cases of COVID-19 were significantly different to those during the SARS epidemic. In particular the proportion of cases from healthcare settings was low and the proportion with no known risk exposures was high 93. Another significant factor is that viral loads in nasopharyngeal and respiratory secretions are highest soon after symptom onset in COVID-19 cases 94 compared with a peak of around 10 days in SARS cases (6), making transmission before entering health care facilities more likely.
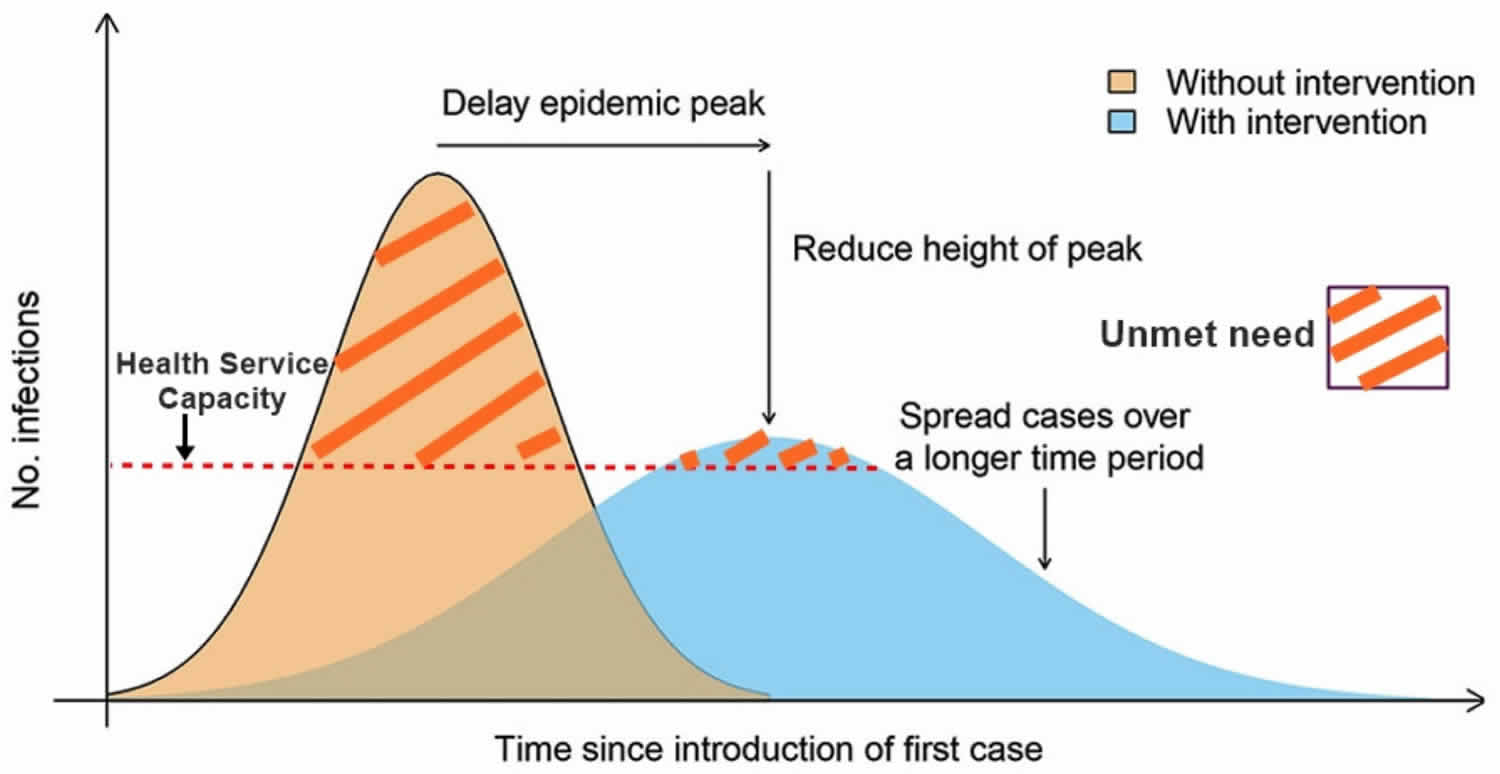
Even though the understanding of transmission dynamics is at an early stage, they do suggest that the step-wise introduction of stringent measures will be necessary to control this epidemic and highlights the importance of early community control.
Limited information is available to characterize the spectrum of clinical illness associated with COVID-19. No vaccine or specific treatment for COVID-19 infection is available; care is supportive.
Of 138 hospitalized patients with 2019 novel coronavirus infected pneumonia, the median age was 56 years and 75 (54.3%) were men 95. Hospital-associated transmission was suspected as the presumed mechanism of infection for affected health professionals (40 [29%]) and hospitalized patients (17 [12.3%]). Common symptoms included fever (136 [98.6%]), fatigue (96 [69.6%]), and dry cough (82 [59.4%]). Lymphopenia (lymphocyte count, 0.8 × 109/L) occurred in 97 patients (70.3%), prolonged prothrombin time (13.0 seconds) in 80 patients (58%), and elevated lactate dehydrogenase (261 U/L) in 55 patients (39.9%). Chest computed tomographic scans showed bilateral patchy shadows or ground glass opacity in the lungs of all patients. Most patients received antiviral therapy (oseltamivir, 124 [89.9%]), and many received antibacterial therapy (moxifloxacin, 89 [64.4%]; ceftriaxone, 34 [24.6%]; azithromycin, 25 [18.1%]) and glucocorticoid therapy (62 [44.9%]). Thirty-six patients (26.1%) were transferred to the intensive care unit (ICU) because of complications, including acute respiratory distress syndrome (22 [61.1%]), arrhythmia (16 [44.4%]), and shock (11 [30.6%]). The median time from first symptom to dyspnea was 5.0 days, to hospital admission was 7.0 days, and to ARDS was 8.0 days. Patients treated in the ICU (n = 36), compared with patients not treated in the ICU (n = 102), were older (median age, 66 years vs 51 years), were more likely to have underlying comorbidities (26 [72.2%] vs 38 [37.3%]), and were more likely to have dyspnea (23 [63.9%] vs 20 [19.6%]), and anorexia (24 [66.7%] vs 31 [30.4%]). Of the 36 cases in the ICU, 4 (11.1%) received high-flow oxygen therapy, 15 (41.7%) received noninvasive ventilation, and 17 (47.2%) received invasive ventilation (4 were switched to extracorporeal membrane oxygenation). As of February 3, 47 patients (34.1%) were discharged and 6 died (overall mortality, 4.3%), but the remaining patients are still hospitalized. Among those discharged alive (n = 47), the median hospital stay was 10 days 95.
Is it safe to travel to Wuhan, China or other countries where COVID-19 cases have occurred?
The CDC has issued at a Level 3 Travel Health Notice (https://wwwnc.cdc.gov/travel/notices/warning/novel-coronavirus-wuhan-china) recommending people avoid all nonessential travel to Wuhan, China. The CDC has also issued a Level 1 Travel Health Notice (https://wwwnc.cdc.gov/travel/notices/watch/novel-coronavirus-china) for the rest of China: Practice Usual Precautions. The notice advises travelers to other parts of China to protect their health by avoiding contact with sick people, avoiding animals (alive or dead) and animal markets, and washing their hands often. The situation is evolving. These notices will be updated as more information becomes available.
What if I recently traveled to Wuhan, China and got sick?
If you were in Wuhan and feel sick with fever, cough, or difficulty breathing, within 14 days after you left Wuhan, you should:
- Seek medical care right away. Before you go to a doctor’s office or emergency room, call ahead and tell them about your recent travel and your symptoms.
- Avoid contact with others.
- Not travel while sick.
- Cover your mouth and nose with a tissue or your sleeve (not your hands) when coughing or sneezing.
- Wash hands often with soap and water for at least 20 seconds to avoid spreading the virus to others. Use an alcohol-based hand sanitizer if soap and water are not available.
The CDC does have additional specific guidance for travelers available online (https://wwwnc.cdc.gov/travel/destinations/traveler/none/china#travel-notices).
Is COVID-19 the same as the MERS-CoV or SARS virus?
No. Coronaviruses are a large family of viruses, some causing illness in people and others that circulate among animals, including camels, cats and bats. The recently emerged 2019-Novel Coronavirus is not the same as the coronavirus that causes Middle East Respiratory Syndrome (MERS) or the coronavirus that causes Severe Acute Respiratory Syndrome (SARS) in 2003. There are ongoing investigations to learn more. This is a rapidly evolving situation and information will be updated as it becomes available.
Should I be concerned about pets or other animals and COVID-19?
While this virus seems to have emerged from an animal source, it is now spreading from person-to-person. The CDC recommends that people traveling to China avoid animals both live and dead, but there is no reason to think that any animals or pets in the United States might be a source of infection with this new coronavirus.
What about animals or animal products imported from China?
The CDC does not have any evidence to suggest that animals or animal products imported from China pose a risk for spreading COVID-19 in the United States. This is a rapidly evolving situation and information will be updated as it becomes available. The United States Department of Agriculture regulatesexternal icon the importation of animals and animal products, and CDC regulates the importation of animals and animal products capable of spreading human disease.
How does the COVID-19 spreads?
Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS and SARS. Many of the patients in the pneumonia outbreak caused by COVID-19 in Wuhan, China had some link to a large seafood and live animal market, suggesting animal-to-person spread. However, a growing number of patients reportedly have not had exposure to animal markets, indicating person-to-person spread is occurring.
When person-to-person spread has occurred with MERS and SARS, it is thought to have happened via respiratory droplets produced when an infected person coughs or sneezes, similar to how influenza and other respiratory pathogens spread. Spread of SARS and MERS between people has generally occurred between close contacts.
It’s important to note that how easily a virus spreads person-to-person can vary. Some viruses are highly contagious (like measles), while other viruses are less so. It’s not clear yet how easily 2019-Novel Coronavirus spreads from person-to-person. It’s important to know this in order to better understand the risk associated with this virus.
There is much more to learn about the transmissibility, severity, and other features associated with COVID-19 and investigations are ongoing.
Person-to-person spread
The virus is thought to spread mainly from person-to-person.
- Between people who are in close contact with one another (within about 6 feet)
- Via respiratory droplets produced when an infected person coughs or sneezes.
- These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.
Spread from contact with infected surfaces or objects
It may be possible that a person can get COVID-19 by touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes, but this is not thought to be the main way the virus spreads.
When does COVID-19 spread happen?
- People are thought to be most contagious when they are most symptomatic (the sickest).
- Some spread might be possible before people show symptoms; there have been reports of this with this new coronavirus, but this is not thought to be the main way the virus spreads.
How efficiently does the COVID-19 spread?
How easily a 2019-Novel Coronavirus spreads from person-to-person can vary. Some viruses are highly contagious (like measles), while other viruses are less so. Another factor is whether the spread continues over multiple generations of people (if spread is sustained). The virus that causes 2019-Novel Coronavirus seems to be spreading easily and sustainably in Hubei province and other parts of China. In the United States, spread from person-to-person has occurred only among a few close contacts and has not spread any further to date.
There is currently no vaccine to prevent COVID-19 infection. The best way to prevent infection is to avoid being exposed to this virus. Right now, COVID-19 has not been found to be spreading in the United States, so there are no additional precautions recommended for the general public to take. However, as a reminder, CDC always recommends everyday preventive actions to help prevent the spread of respiratory viruses, including:
- Wash your hands often with soap and water for at least 20 seconds. If soap and water are not available, use an alcohol-based hand sanitizer.
- Avoid touching your eyes, nose, and mouth with unwashed hands.
- Avoid close contact with people who are sick.
- Stay home when you are sick.
- Cover your cough or sneeze with a tissue, then throw the tissue in the trash.
- Clean and disinfect frequently touched objects and surfaces.
- Follow CDC’s recommendations for using a facemask.
- CDC does not recommend that people who are well wear a facemask to protect themselves from respiratory diseases, including COVID-19.
- Facemasks should be used by people who show symptoms of COVID-19 to help prevent the spread of the disease to others. The use of facemasks is also crucial for health workers and people who are taking care of someone in close settings (at home or in a health care facility).
These are every day habits that can help prevent the spread of several viruses.
Enhanced hygiene and social distancing measures may reduce both numbers of cases and severity of cases through several mechanisms.
The basic reproductive number (R0) is the average number of secondary cases of an infectious disease that arise from cases in a totally susceptible population, and reflects the epidemic potential of a pathogen 96. R0 (R nought) is a function of the number of contacts an infectious person has, the risk of transmission per contact, and the duration of infectiousness.
Enhanced hygiene and social distancing measures may reduce both numbers of cases and severity of cases through several mechanisms.
Social distancing mostly acts on the first factor, by reducing the number of contacts each person makes. Hygiene measures mostly act on the second factor, as they reduce the risk of transmission if a contact occurs. There are epidemiological observations from the outbreak in China that might indicate the effectiveness of pre-emptive implementation of the measures in the community. The WHO-China Joint Mission on COVID-19 determined that widespread community transmission and outbreaks occurred in Wuhan prior to the implementation of comprehensive control measures 93. However in other parts of China, community transmission has been limited and most transmission has occurred in families. For example, among 344 clusters involving 1308 cases (out of a total 1836 cases reported) in Guangdong Province and Sichuan Province, 78%-85% have occurred in families 93. This is likely due to the intense quarantine and social distancing measures implemented in areas outside Hubei prior to the establishment of widespread community transmission.
Community wide interventions may decrease the average viral exposure dose encountered in the community. People exposed to a higher viral dose (inoculum) are more likely to become infected and suffer more severe disease. Animal models for other coronavirus infections demonstrate that increased viral inocula lead to more severe disease and higher viral loads in the lungs and other organs/fluids 97. The Amoy Gardens SARS outbreak in 2003 provided evidence that cases with presumed higher exposure to the index case had higher nasopharyngeal viral loads and more severe illness 98. Modelling of the 2009 influenza pandemic also supported a hypothesis that severe illness was due to a higher infectious dose of the virus mediated by the number of simultaneous infectious contacts 99. Viral loads in severe MERS cases were higher than those in a mild group and the patients in the severe group had more prolonged viral shedding in respiratory secretions, beyond 21 days after the onset of symptoms, whereas viral RNA was no longer detected by 21 days in the mild group 100.
Therefore, it is proposed that early measures that lower the number of contacts, the likelihood of transmission, and average viral infective dose in an area of new transmission could have a multiplier effect leading to less cases, and less severe cases who are less infectious. This early reduction of the R0 (R nought) would result in fewer cases overall and have a significant negative multiplier effect on the overall impact of the epidemic, including the number of deaths (see Figure 1 below). The higher case fatality rate in Wuhan, compared with other provinces in China may partially relate to health-care resource availability and shortages in the face of overwhelming community transmission, as well as greater severity of disease due to higher infection doses 101. These interventions will be particularly important for people over 60 years of age and those with underlying medical conditions.
Steps to help prevent the spread of COVID-19 if you are sick
If you are sick with COVID-19 or suspect you are infected with the virus that causes 2019-Novel Coronavirus, follow the steps below to help prevent the disease from spreading to people in your home and community.
Stay home except to get medical care
You should restrict activities outside your home, except for getting medical care. Do not go to work, school, or public areas. Avoid using public transportation, ride-sharing, or taxis.
Separate yourself from other people and animals in your home
- People: As much as possible, you should stay in a specific room and away from other people in your home. Also, you should use a separate bathroom, if available.
- Animals: You should restrict contact with pets and other animals while you are sick with COVID-19, just like you would around other people. Although there have not been reports of pets or other animals becoming sick with COVID-19, it is still recommended that people sick with COVID-19 limit contact with animals until more information is known about the virus. When possible, have another member of your household care for your animals while you are sick. If you are sick with COVID-19, avoid contact with your pet, including petting, snuggling, being kissed or licked, and sharing food. If you must care for your pet or be around animals while you are sick, wash your hands before and after you interact with pets and wear a facemask.
Call ahead before visiting your doctor
If you have a medical appointment, call the healthcare provider and tell them that you have or may have COVID-19. This will help the healthcare provider’s office take steps to keep other people from getting infected or exposed.
Wear a facemask
You should wear a facemask when you are around other people (e.g., sharing a room or vehicle) or pets and before you enter a healthcare provider’s office. If you are not able to wear a facemask (for example, because it causes trouble breathing), then people who live with you should not stay in the same room with you, or they should wear a facemask if they enter your room.
Cover your coughs and sneezes
Cover your mouth and nose with a tissue when you cough or sneeze. Throw used tissues in a lined trash can; immediately wash your hands with soap and water for at least 20 seconds or clean your hands with an alcohol-based hand sanitizer that contains 60 to 95% alcohol, covering all surfaces of your hands and rubbing them together until they feel dry. Soap and water should be used preferentially if hands are visibly dirty.
Clean your hands often
Wash your hands often with soap and water for at least 20 seconds or clean your hands with an alcohol-based hand sanitizer that contains 60 to 95% alcohol, covering all surfaces of your hands and rubbing them together until they feel dry. Soap and water should be used preferentially if hands are visibly dirty. Avoid touching your eyes, nose, and mouth with unwashed hands.
Avoid sharing personal household items
You should not share dishes, drinking glasses, cups, eating utensils, towels, or bedding with other people or pets in your home. After using these items, they should be washed thoroughly with soap and water.
Clean all “high-touch” surfaces everyday
High touch surfaces include counters, tabletops, doorknobs, bathroom fixtures, toilets, phones, keyboards, tablets, and bedside tables. Also, clean any surfaces that may have blood, stool, or body fluids on them. Use a household cleaning spray or wipe, according to the label instructions. Labels contain instructions for safe and effective use of the cleaning product including precautions you should take when applying the product, such as wearing gloves and making sure you have good ventilation during use of the product.
Monitor your symptoms
Seek prompt medical attention if your illness is worsening (e.g., difficulty breathing). Before seeking care, call your healthcare provider and tell them that you have, or are being evaluated for, 2019-Novel Coronavirus. Put on a facemask before you enter the facility. These steps will help the healthcare provider’s office to keep other people in the office or waiting room from getting infected or exposed. Ask your healthcare provider to call the local or state health department. Persons who are placed under active monitoring or facilitated self-monitoring should follow instructions provided by their local health department or occupational health professionals, as appropriate.
If you have a medical emergency and need to call your local emergency services number, notify the dispatch personnel that you have, or are being evaluated for 2019-Novel Coronavirus. If possible, put on a facemask before emergency medical services arrive.
Discontinuing home isolation
Patients with confirmed 2019-Novel Coronavirus should remain under home isolation precautions until the risk of secondary transmission to others is thought to be low. The decision to discontinue home isolation precautions should be made on a case-by-case basis, in consultation with healthcare providers and state and local health departments.
Social distancing includes ways to stop or slow the spread of infectious diseases. It means less contact between you and other people. Social distancing is important because COVID-19 is most likely to spread from person-to-person through 102:
- direct close contact with a person while they are infectious or in the 24 hours before their symptoms appeared
- close contact with a person with a confirmed infection who coughs or sneezes, or
- touching objects or surfaces (such as door handles or tables) contaminated from a cough or sneeze from a person with a confirmed infection, and then touching your mouth or face.
So, the more space between you and others, the harder it is for the virus to spread.
Social distancing at home
- Households
- To reduce the spread of germs:
- Practise good hand and sneeze/cough hygiene
- Avoid handshaking and kissing
- Regularly disinfect high touch surfaces, such as tables, kitchen benches and doorknobs
- Increase ventilation in the home by opening windows or adjusting air conditioning
- Visit shops sparingly and buy more goods and services online
- Consider whether outings and travel, both individual and family, are sensible and necessary
- To reduce the spread of germs:
- Households where people are ill (in addition to the measures above)
- Care for the sick person in a single room if possible
- Keep the number of carers to a minimum
- Keep the door to the sick person’s room closed and, if possible, a window open
- Both the sick person and the people caring for them should wear a surgical mask when they are in the same room
- Protect other vulnerable family members, such as people over 65 years or people with a chronic illness, including, if practicable, finding alternative accommodation
- Social distancing in the workplace
- To reduce the spread of germs in the workplace:
- Stay at home if you are sick
- Stop handshaking as a greeting
- Hold meetings via video conferencing or phone call
- Defer large meetings
- Hold essential meetings outside in the open air if possible
- Promote good hand and sneeze/cough hygiene and provide hand sanitizers for all staff and workers
- Take lunch at your desk or outside rather than in the lunch room
- Clean and disinfect high touch surfaces regularly
- Consider opening windows and adjusting air conditioning for more ventilation
- Limit food handling and sharing of food in the workplace
- Reconsider non-essential business travel
- Promote strictest hygiene among food preparation (canteen) staff and their close contacts
- Consider if large gatherings can be rescheduled, staggered or cancelled
- To reduce the spread of germs in the workplace:
- Social distancing in schools
- To reduce the spread of germs in schools:
- If your child is sick, do not send them to school (or childcare)
- Sanitize hands when entering school and at regular intervals
- Defer activities that lead to mixing between classes and years
- Avoid queuing and consider cancelling school assemblies
- Promote a regular handwashing schedule
- Clean and disinfect high touch surfaces regularly
- Conduct lessons outdoors where possible
- Consider opening windows and adjusting conditioning for more ventilation
- Promote strictest hygiene among food preparation (canteen) staff and their close contacts
- To reduce the spread of germs in schools:
- Social distancing in public
- To reduce the spread of germs:
- Sanitize your hands wherever possible, including entering and leaving buildings
- Use tap and pay rather than handling money
- Try and travel at quiet times and try to avoid crowds
- Public transport workers and taxi drivers should open vehicle windows where possible, and regularly clean and disinfect high touch surfaces
- To reduce the spread of germs:
Things to consider when organizing public gatherings
Events where a large number of people are in one place can increase the risk of transmission of viruses. If you are organizing a gathering, consider whether you can postpone, reduce size or frequency or cancel the event. If you decide to go ahead, you should assess the risks and reconsider any aspect that may increase risk of transmission.
How to slow the transmission of COVID-19
Pre-emptive interventions is a way to slow the transmission of COVID-19 and limit the impact on health services, particularly hospitals and intensive care units, to ensure access to high level care when needed 102. The concept of pre-emptive deployment is based upon the following assumptions which
require further exploration and are elaborated upon below:
- Community wide COVID-19 transmission may be occurring undetected or may only be recognized after containment is no longer feasible.
- Interventions implemented after community wide transmission is detected will be less effective.
- Reducing the force of COVID-19 infection, particularly early, will delay the epidemic peak, blunt the epidemic peak, spread cases over a longer time, and help to limit the potential for critical
care services to be overwhelmed, which may be lifesaving 103. - Enhanced hygiene and social distancing interventions should:
- a. Decrease the total number of cases per week but extend duration of the epidemic
- b. Decrease the severity of cases through reducing viral inocula.
Figure 1 below illustrates the concept of limiting the peak in COVID-19 cases so that health services are less likely to be overwhelmed (red dashed line) and there is less unmet health service need.
Unmet need may include inability to admit patients to a hospital or to provide hospitalized patients in critical condition access to intensive care. Interventions to reduce COVID-19 infection lead to longer, but less peaked, epidemics. A slower evolution in the COVID-19 epidemic also allows time for health care staff to provide better care, for recovery of infected health care workers, for learning and adapting to the evolving situation by administrators, and for vaccines and treatments to be developed. Although the concept have not been validated for COVID-19 epidemics it is sufficiently validated in simulations for influenza that it would appear a reasonable assumption in response to this emergent disease 104.
Figure 1. Intended impact of enhanced hygiene and social distancing measures on the COVID-19 pandemic
[Source 103 ]Risk Factors for severe illness in adults with COVID-19
Clinical factors
- Body mass index (BMI) ≥ 30 kg/m²
- Dyspnea, increased respiratory rate, decreased oxygen saturation
- Male sex
- Older age
- Among adults 65 to 84 years of age who are diagnosed with COVID-19, 31% to 59% are hospitalized, 11% to 31% are admitted to the intensive care unit (ICU), and 4% to 11% die. Among adults 85 years and older who are diagnosed with COVID-19, 31% to 70% are hospitalized, 10% to 27% are admitted to the ICU, and 10% to 27% die 105.
Comorbidities
- Blood disorders (e.g., sickle cell disease, patient taking blood thinners)
- Chronic kidney disease (CKD)
- Chronic liver disease
- Chronic lung disease (e.g., chronic obstructive pulmonary disease [COPD], asthma)
- Current or recent (within two weeks) pregnancy
- Endocrine disorders (e.g., diabetes mellitus)
- Heart disease (e.g., congestive heart failure, coronary artery disease, hypertension)
- Immunosuppression (e.g., autoimmune disease, malignancy, HIV infection)
- Neurologic or neurodevelopmental conditions
Laboratory findings
- Elevated C-reactive protein, lactate dehydrogenase, alanine transaminase, aspartate transaminase, or d-dimer level
- High white blood cell count (leukocytosis)
- Low albumin level
- Lower-than-normal number of lymphocytes in the blood, also called lymphopenia, lymphocytopenia or lymphocytic leukopenia
- Higher neutrophil count (a type of white blood cell) in the blood than the normal (also called neutrophilia)
COVID-19 breakthrough infection
COVID-19 breakthrough infection is when fully vaccinated people become infected with COVID-19 (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) 106. COVID-19 vaccines are effective at preventing most infections. However, like most vaccines, they are not 100% effective. Because vaccines are not 100% effective, as the number of people who are fully vaccinated goes up, the number of breakthrough infections will also increase.
Fully vaccinated people with a breakthrough infection are less likely to develop serious illness than those who are unvaccinated and get COVID-19 106. This means they are much less likely to be hospitalized or die than people who are not vaccinated. Therefore, the CDC recommends people get a COVID-19 vaccine as soon as they can to protect themselves from severe disease and death. However, people who get vaccine breakthrough infections can be contagious.
COVID-19 infection symptoms
People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness. Symptoms may appear in as few as 2 days or as long as 14 days after exposure to the virus. People with these symptoms may have COVID-19:
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue
- Muscle or body aches
- Headache
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
- Diarrhea
This list does not include all possible symptoms. Health authorities will continue to update this list as they learn more about COVID-19.
Anyone can have mild to severe symptoms. Older adults and people who have severe underlying medical conditions like heart or lung disease or diabetes seem to be at higher risk for developing more serious complications from COVID-19 illness.
Criteria to Guide Evaluation of Patients Under Investigation for COVID-19
- Patients in the United States who meet the following criteria should be evaluated as a Patients Under Investigation in association with the outbreak of 2019-Novel Coronavirus in Wuhan City, China.
- The criteria are intended to serve as guidance for evaluation. Patients should be evaluated and discussed with public health departments on a case-by-case basis if their clinical presentation or exposure history is equivocal (e.g., uncertain travel or exposure).
| Clinical Features | & | Epidemiologic Risk |
|---|---|---|
| Fever1 and symptoms of lower respiratory illness (e.g., cough, difficulty breathing) | and | In the last 14 days before symptom onset, a history of travel from Wuhan City, China.– or – In the last 14 days before symptom onset, close contact2 with a person who is under investigation for COVID-19 while that person was ill. |
| Fever1 or symptoms of lower respiratory illness (e.g., cough, difficulty breathing) | and | In the last 14 days, close contact2 with an ill laboratory-confirmed COVID-19 patient. |
Footnote:
1) Fever may not be present in some patients, such as those who are very young, elderly, immunosuppressed, or taking certain fever-lowering medications. Clinical judgment should be used to guide testing of patients in such situations.
2) Close contact is defined as—
- a) being within approximately 6 feet (2 meters), or within the room or care area, of a novel coronavirus case for a prolonged period of time while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection); close contact can include caring for, living with, visiting, or sharing a health care waiting area or room with a novel coronavirus case.– or –
- b) having direct contact with infectious secretions of a novel coronavirus case (e.g., being coughed on) while not wearing recommended personal protective equipment.
See CDC’s Interim Healthcare Infection Prevention and Control Recommendations for Patients Under Investigation for 2019 Novel Coronavirus (https://www.cdc.gov/coronavirus/2019-ncov/infection-control.html).
Data to inform the definition of close contact are limited. Considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk) and the clinical symptoms of the person with novel coronavirus (e.g., coughing likely increases exposure risk as does exposure to a severely ill patient). Special consideration should be given to those exposed in health care settings.
How do you test a person for COVID-19?
If you develop symptoms of coronavirus disease 2019 (COVID-19) or you’ve been exposed to the COVID-19 virus, contact your doctor. Also let your doctor know if you’ve had close contact with anyone who has been diagnosed with COVID-19.
Factors used to decide whether to test you for the virus that causes COVID-19 may differ depending on where you live. Depending on your location, you may need to be screened by your clinic to determine if testing is appropriate and available.
In the U.S., your doctor will determine whether to conduct tests for the virus that causes COVID-19 based on your signs and symptoms, as well as whether you have had close contact with someone diagnosed with COVID-19. Your doctor may also consider testing if you are at higher risk of serious illness or you are going to have a medical procedure.
To test for the COVID-19 virus, a health care provider takes a sample from the nose (nasopharyngeal swab) or throat (throat swab). The samples are then sent to a lab for testing. If you’re coughing up sputum, that may be sent for testing. The U.S. Food & Drug Administration (FDA) has authorized at-home tests for the COVID-19 virus. These are available only with a doctor’s prescription.
COVID-19 tests are available at no cost nationwide at health centers and select pharmacies. Contact your health care provider or your state or local public health department for more information.
- Find a health center near you exit disclaimer icon for available COVID-19 screening and testing (https://findahealthcenter.hrsa.gov)
- Community-Based Testing Sites for COVID-19: https://www.hhs.gov/coronavirus/community-based-testing-sites/index.html
COVID-19 infection treatment
Currently, only one medication has been approved to treat COVID-19. No cure is available for COVID-19. Antibiotics aren’t effective against viral infections such as COVID-19. Researchers are testing a variety of possible treatments.
The FDA has approved the antiviral drug remdesivir (Veklury) to treat COVID-19 in hospitalized adults and children who are age 12 and older in the hospital. The FDA has granted an emergency use authorization for the rheumatoid arthritis drug baricitinib (Olumiant) to treat COVID-19 in some cases. Baricitinib is a pill that seems to work against COVID-19 by reducing inflammation and having antiviral activity. The FDA states baricitinib may be used in combination with remdesivir in people who are hospitalized with COVID-19 who are on mechanical ventilators or need supplemental oxygen.
Three monoclonal antibody medications have received Emergency Use Authorization (EUA) from the FDA. Monoclonal antibodies are proteins created in a lab that can help the immune system fight off viruses. One medication is called bamlanivimab, and the second medication is a combination of two antibodies called casirivimab and imdevimab. Both drugs are used to treat mild to moderate COVID-19 in people who have a higher risk of developing serious illness due to COVID-19. Treatment consists of a single intravenous infusion given in an outpatient setting. To be most effective, these medications need to be given soon after COVID-19 symptoms start and prior to hospitalization.
The U.S. National Institutes of Health recently recommended the corticosteroid dexamethasone for people hospitalized with severe COVID-19 who require supplemental oxygen or mechanical ventilation. Other corticosteroids, such as prednisone, methylprednisolone or hydrocortisone, may be used if dexamethasone isn’t available.
The FDA has also granted emergency use authorization (EUA) for convalescent plasma therapy to treat COVID-19. Convalescent plasma is blood donated by people who’ve recovered from COVID-19. It’s used to treat people who are ill with COVID-19 in the hospital.
Supportive care is aimed at relieving symptoms and may include:
- Pain relievers (ibuprofen or acetaminophen)
- Cough syrup or medication
- Rest
- Fluid intake
There is no evidence that ibuprofen or other nonsteroidal anti-inflammatory drugs (NSAIDs) need to be avoided.
If you have mild symptoms, your doctor may recommend that you recover at home. He or she may give you special instructions to monitor your symptoms and to avoid spreading the illness to others. You’ll likely be asked to isolate yourself as much as possible from family and pets while you’re sick, wear a mask when you’re around people and pets, and use a separate bedroom and bathroom.
Your doctor will likely recommend that you stay in home isolation for a period of time except to get medical care. Your doctor will likely follow up with you regularly. Follow guidelines from your doctor and local health department about when you can end home isolation.
If you’re very ill, you may need to be treated in the hospital.
COVID-19 Monoclonal Antibody Therapies
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome encodes four major structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as nonstructural and accessory proteins 107. The spike protein is further divided into two subunits, S1 and S2, that mediate host cell attachment and invasion. Through its receptor-binding domain (RBD), S1 spike protein attaches to angiotensin-converting enzyme 2 (ACE2) on the host cell; this initiates a conformational change in S2 that results in virus-host cell membrane fusion and viral entry 108. Monoclonal antibodies that target the spike protein have been shown to have a clinical benefit in treating COVID-19 infection. Preliminary data suggest that monoclonal antibodies may play a role in preventing COVID-19 infection in household contacts of infected patients 109 and during skilled nursing and assisted living facility outbreaks 110.
In the United States, there are currently three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of mild to moderate COVID-19 in nonhospitalized patients with laboratory-confirmed SARS-CoV-2 infection who are at high risk for progressing to severe disease and/or hospitalization. The issuance of an EUA does not constitute FDA approval. These products are:
- Bamlanivimab plus etesevimab: These are neutralizing monoclonal antibodies that bind to different but overlapping epitopes in the spike protein RBD of SARS-CoV-2.
- The distribution of bamlanivimab plus etesevimab was paused on June 25, 2021, because both the Gamma (P.1) and Beta (B.1.351) variants of concern that are currently circulating in the United States have reduced susceptibility to bamlanivimab and etesevimab 111.
- Casirivimab plus imdevimab: These are recombinant human monoclonal antibodies that bind to nonoverlapping epitopes of the spike protein receptor-binding domain (RBD) of SARS-CoV-2.
- Sotrovimab: This monoclonal antibody was originally identified in 2003 from a SARS-CoV survivor. It targets an epitope in the receptor-binding domain (RBD) of the spike protein that is conserved between SARS-CoV and SARS-CoV-2.
The FDA also updated the Emergency Use Authorization (EUA) for casirivimab plus imdevimab as post-exposure prophylaxis for certain individuals who are at high risk of acquiring SARS-CoV-2 infection and, if infected, are at high risk of progressing to serious illness 112.
In laboratory studies, SARS-CoV-2 variants that contain certain substitutions in the spike protein cause a marked reduction in susceptibility to bamlanivimab and may have reduced sensitivity to etesevimab and casirivimab. The L452R substitution found in the B.1.427 and B.1.429 lineages has been shown to cause a significant reduction in susceptibility to bamlanivimab and a modest decrease in susceptibility to the combination of bamlanivimab and etesevimab, although the clinical implications of this modest decrease are not known. The E484K substitution found in the B.1.351, P.1, and B.1.526 lineages also results in a marked reduction in susceptibility to bamlanivimab, as well as the combination of bamlanivimab and etesevimab. Laboratory studies also suggest that the K417N and K417T substitutions, which are present in the B.1.351 and P.1 variants, respectively, along with the E484K mutation, reduces virus susceptibility to casirivimab, although the combination of casirivimab and imdevimab appears to retain activity. There is no reported reduction in susceptibility of variants to sotrovimab.
Recommendations
The strength of the evidence for using anti-SARS-CoV-2 monoclonal antibodies varies depending on the factors that place patients at high risk for progression to severe COVID-19 and/or hospitalization. The COVID-19 Treatment Guidelines Panel (the Panel) recommendations for using anti-SARS-CoV-2 monoclonal antibodies according to the updated EUA criteria are based on preliminary results from the clinical trials that evaluated these products. The details on the study designs, methods, and follow-up periods for these trials are currently limited. When peer-reviewed data from the Phase 3 trials become publicly available, the Panel will review the results and update the recommendations if necessary.
- The COVID-19 Treatment Guidelines Panel (the Panel) recommends using one of the following anti-SARS-CoV-2 monoclonal antibodies, listed in alphabetical order, to treat nonhospitalized patients with mild to moderate COVID-19 who are at high risk of clinical progression:
- Casirivimab plus imdevimab; or
- Sotrovimab 500 mg intravenous (IV) infusion
- When using casirivimab plus imdevimab, the Panel recommends:
- Casirivimab 600 mg plus imdevimab 600 mg IV infusion
- If IV infusions are not feasible or would cause a delay in treatment, casirivimab 600 mg plus imdevimab 600 mg administered by four subcutaneous injections (2.5 mL per injection) can be used as an alternative.
- When using monoclonal antibodies, treatment should be started as soon as possible after the patient receives a positive result on a SARS-CoV-2 antigen or nucleic acid amplification test (NAAT) and within 10 days of symptom onset.
- At this time, the Panel recommends against the use of bamlanivimab plus etesevimab because the Gamma (P.1) and Beta (B.1.351) variants of concern, which have reduced susceptibility to both agents, are circulating in the United States.
- The use of anti-SARS-CoV-2 monoclonal antibodies should be considered for patients with mild to moderate COVID-19 who are hospitalized for a reason other than COVID-19 if they otherwise meet the EUA criteria for outpatient treatment.
- Anti-SARS-CoV-2 monoclonal antibodies are not currently authorized for use in patients who are hospitalized with severe COVID-19; however, they may be available through expanded access programs for patients who have not developed an antibody response or who are not expected to mount an effective immune response to SARS-CoV-2 infection.
The recommendations for treatment are based on the following criteria from the FDA EUAs.
Medical conditions or other factors that were represented in clinical trials that evaluated anti-SARS-CoV-2 Monoclonal Antibodies:
- Aged ≥65 years
- Obesity (BMI >30)
- Diabetes
- Cardiovascular disease (including congenital heart disease) or hypertension
- Chronic lung diseases (e.g., chronic obstructive pulmonary disease, moderate-to-severe asthma, interstitial lung disease, cystic fibrosis, pulmonary hypertension)
Other conditions or factors that had limited representation in clinical trials but are considered Risk Factors for Progression to Severe COVID-19 by the Centers for Disease Control and Prevention:
- An immunocompromising condition or immunosuppressive treatment. Many experts strongly recommend therapy for patients with these conditions, despite their limited representation in clinical trials.
- Being overweight (BMI 25–30) as the sole risk factor
- Chronic kidney disease
- Pregnancy
- Sickle cell disease
- Neurodevelopmental disorders (e.g., cerebral palsy) or other conditions that confer medical complexity (e.g., genetic or metabolic syndromes and severe congenital anomalies)
- Medical-related technological dependence (e.g., tracheostomy, gastrostomy, or positive pressure ventilation that is not related to COVID-19)
It is important to note that the likelihood of developing severe COVID-19 increases when a person has multiple high-risk conditions or comorbidities 113. Other factors (e.g., race or ethnicity) or medical conditions may also place individual patients at high risk for progression to severe COVID-19. The current EUAs state that the use of anti-SARS-CoV-2 monoclonal antibodies may be considered for many of these other patients.
COVID-19 prognosis
International data suggest that 85% of people with COVID-19 have only mild illness, whereas 14% have severe disease requiring hospitalization, including 5% of adults and 2% of children who require admission to an intensive care unit 114. Children tend to have a better prognosis than adults (Arnaout R, et al., unpublished data, 2020) 115.
The overall mortality rate from COVID-19 has been estimated to be 0.66% to 0.9%, although estimates are difficult because of the number of undiagnosed cases 116. Observed rates vary considerably (2.3% to 7.2%) depending on location and test availability. As of July 21, 2020, the Johns Hopkins Center for Health Security reported a U.S. case fatality rate of 3.7%.2 Case fatality rates increase with age 117.
Predictors of more severe disease or death include clinical factors, comorbidities, and laboratory findings 118. Common risk factors in hospitalized patients include 119:
- Cardiovascular disease
- Chronic kidney disease
- Chronic liver disease
- Chronic obstructive pulmonary disease (COPD)
- Diabetes mellitus
- Hypercholesterolemia
- Hypertension
- Malignancy
- Obesity (BMI >30)
- Obstructive sleep apnea (OSA)
In pregnant women, COVID-19 may increase the risk of complications such as preterm birth and preterm premature rupture of membranes. The risk of vertical transmission and risks to newborns are not well understood 120.
Acute complications of COVID-19 include pneumonia, acute respiratory distress syndrome, stroke, and arterial and venous thrombosis. Hospitalized patients may have debility 121 and mental health effects 122. Factors that may increase the risk of these complications include:
- A diet with inadequate fruits and vegetables 123
- Body mass index (BMI) greater than 40 kg/m² 124
- Sedentary lifestyle
- Smoking
The long-term health effects of COVID-19 are under investigation.
Approximately 35% of people with COVID-19 have not returned to their previous level of health 14 to 21 days after diagnosis. These “long haulers” have a syndrome referred to as long COVID 125.
Middle East Respiratory Syndrome
Middle East Respiratory Syndrome (MERS) is a severe respiratory illness that mainly involves the upper respiratory tract. It causes fever, coughing, and shortness of breath. About 30% of people who have gotten this illness have died. Some people only have mild symptoms.
Middle East Respiratory Syndrome (MERS), was first identified in a patient with lower respiratory disease by a physician in Saudi Arabia, and subsequently, the Middle East Respiratory Syndrome coronavirus (MERS-CoV) was identified and characterized from this patient’s sputum sample by researchers in Saudi Arabia and at Erasmus Medical Center in the Netherlands 126. MERS was first reported in Saudi Arabia in 2012 and then spread to many countries. Most cases were spread from people who traveled to the Middle Eastern countries. Since then (to December 2018), 2266 laboratory-confirmed MERS-coronavirus cases and 804 deaths across 27 countries (35.5% crude case fatality rate) have been reported to the World Health Organization (WHO) 127, with the majority of cases (1888) and deaths (730) reported from Saudi Arabia (World Health Organization 2018a, World Health Organization/EMRO 2018). Cases continue to occur (World Health Organization/EMRO 2018). To date, there have only been 2 cases of MERS in the United States. They were in people traveling to the United States from Saudi Arabia and diagnosed in 2014. The Middle East Respiratory Syndrome coronavirus poses a very low risk to people in the United States.
The most common symptoms of MERS disease have included fever, chills, and cough. Less common symptoms include coughing up blood, diarrhea, and vomiting. However, these symptoms can also occur in a number of other conditions.
All people with MERS had an abnormal chest x-ray.
Right now, there is no vaccine for MERS and no specific treatment. Supportive care is given.
How MERS Spreads?
No one knows exactly where the MERS coronavirus comes from. MERS coronavirus has also been found in camels and in one bat. While it is believed to come from animals, that is still unclear. Scientists need more information to understand the interactions between humans and camels that are important for MERS coronavirus transmission.
MERS-coronavirus, like other coronaviruses, likely spreads from an infected person’s respiratory secretions, such as through coughing. However, scientists still don’t fully understand the precise ways that it spreads.
MERS coronavirus can spread between people in close contact. MERS-coronavirus has spread from ill people to others through close contact, such as caring for or living with an infected person. Infected people have spread MERS-coronavirus to others in healthcare settings, such as hospitals. Researchers studying MERS have not seen any ongoing spreading of MERS-coronavirus in the community.
All reported cases have been linked to countries in and near the Arabian Peninsula. Most infected people either lived in the Arabian Peninsula or recently traveled from the Arabian Peninsula before they became ill. A few people have gotten MERS after having close contact with an infected person who had recently traveled from the Arabian Peninsula. The largest known outbreak of MERS outside the Arabian Peninsula occurred in the Republic of Korea in 2015 and was associated with a traveler returning from the Arabian Peninsula.
Public health agencies continue to investigate clusters of cases in several countries to better understand how MERS-coronavirus spreads from person to person.
The incubation period of MERS coronavirus is not precisely known. This is the amount of time between when a person is exposed to the virus and when symptoms occur. In the first cases, the average incubation period seemed to be 5 days, but there are cases that occurred up to 14 days after exposure.
MERS prevention
If you plan to travel to one of the countries where MERS is present, the Centers for Disease Control Prevention (CDC) advises taking the following steps to prevent illness.
- Wash your hands often with soap and water for 20 seconds. Help young children do the same. If soap and water are not available, use an alcohol-based hand sanitizer.
- Cover your nose and mouth with a tissue when you cough or sneeze then throw the tissue in the trash.
- Avoid touching your eyes, nose, and mouth with unwashed hands.
- Avoid close contact, such as kissing, sharing cups, or sharing eating utensils, with sick people.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs.
- If you come in contact with animals, such as camels, wash your hands thoroughly afterward. It has been reported that some camels carry the MERS virus.
People who may be at increased risk for MERS
Recent travelers from the Arabian Peninsula
If you develop a fever* and symptoms of respiratory illness, such as cough or shortness of breath, within 14 days after traveling from countries in or near the Arabian Peninsula**, you should call ahead to a healthcare provider and mention your recent travel.
Close contacts of an ill traveler from the Arabian Peninsula
If you have had close contact*** with someone within 14 days after they traveled from a country in or near the Arabian Peninsula**, and the traveler has/had fever* and symptoms of respiratory illness, such as cough or shortness of breath, you should monitor your health for 14 days, starting from the day you were last exposed to the ill person.
If you develop fever* and symptoms of respiratory illness, such as cough or shortness of breath, you should call ahead to a healthcare provider and mention your recent contact with the traveler.
Close contacts of a confirmed case of MERS
If you have had close contact*** with someone who has a confirmed MERS-coronavirus infection, you should contact a healthcare provider for an evaluation. Your healthcare provider may request laboratory testing and outline additional recommendations, depending on the findings of your evaluation and whether you have symptoms. You most likely will be asked to monitor your health for 14 days, starting from the day you were last exposed to the ill person. Watch for these symptoms:
- Fever*. Take your temperature twice a day.
- Coughing
- Shortness of breath
- Other early symptoms to watch for are chills, body aches, sore throat, headache, diarrhea, nausea/vomiting, and runny nose.
If you develop symptoms, call ahead to your healthcare provider as soon as possible and tell them about your possible exposure to MERS-coronavirus so the office can take steps to keep other people from getting infected. Ask your healthcare provider to call the local or state health department.
Healthcare personnel not using recommended infection-control precautions
Healthcare personnel should adhere to recommended infection control measures, including standard, contact, and airborne precautions, while managing symptomatic close contacts, patients under investigation, and patients who have probable or confirmed MERS-coronavirus infections. They should also use recommended infection control precautions when collecting specimens.
Healthcare personnel who had close contact*** with a confirmed case of MERS while the case was ill, if not using recommended infection control precautions (e.g., appropriate use of personal protective equipment), are at increased risk of developing MERS-coronavirus infection. These individuals should be evaluated and monitored by a healthcare professional with a higher index of suspicion.
People with exposure to camels
Direct contact with camels is a risk factor for human infection with MERS-coronavirus.
The World Health Organization has posted a general precaution for anyone visiting farms, markets, barns, or other places where animals are present. Travelers should practice general hygiene measures, including regular handwashing before and after touching animals, and avoiding contact with sick animals. Travelers should also avoid consumption of raw or undercooked animal products.
The World Health Organization considers certain groups to be at high risk for severe MERS. These groups include people with diabetes, kidney failure, or chronic lung disease, and people who have weakened immune systems. The World Health Organization recommends that these groups take additional precautions:
- Avoid contact with camels
- Do not drink raw camel milk or raw camel urine
- Do not eat undercooked meat, particularly camel meat
*Fever may not be present in some patients, such as those who are very young, elderly, immunosuppressed, or taking certain medications. Clinical judgement should be used to guide testing of patients in such situations.
**Countries considered in and near the Arabian Peninsula include: Bahrain; Iraq; Iran; Israel, the West Bank, and Gaza; Jordan; Kuwait; Lebanon; Oman; Qatar; Saudi Arabia; Syria; the United Arab Emirates (UAE); and Yemen.
***Close contact is defined as a) being within approximately 6 feet (2 meters), or within the room or care area, of a confirmed MERS case for a prolonged period of time (such as caring for, living with, visiting, or sharing a healthcare waiting area or room with, a confirmed MERS case) while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection); or b) having direct contact with infectious secretions of a confirmed MERS case (e.g., being coughed on) while not wearing recommended personal protective equipment. See CDC’s Interim Infection Prevention and Control Recommendations for Hospitalized Patients with MERS (https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html). Data to inform the definition of close contact are limited; considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk) and the clinical symptoms of the person with MERS (e.g., coughing likely increases exposure risk). Special consideration should be given to those exposed in healthcare settings. For detailed information regarding healthcare personnel (HCP) please review CDC’s Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Middle East Respiratory Syndrome coronavirus Exposure (https://www.cdc.gov/coronavirus/mers/hcp/monitoring-movement-guidance.html). Transient interactions, such as walking by a person with MERS, are not thought to constitute an exposure; however, final determination should be made in consultation with public health authorities.
MERS symptoms
Some infected people had mild symptoms (such as cold-like symptoms) or no symptoms at all. The symptoms of MERS start to appear about 5 or 6 days after a person is exposed, but can range from 2 to 14 days.
Most people confirmed to have MERS-coronavirus infection have had severe respiratory illness with symptoms of:
- fever
- cough
- shortness of breath
Some people also had diarrhea and nausea/vomiting. For many people with MERS, more severe complications followed, such as pneumonia and kidney failure. About 3 or 4 out of every 10 people reported with MERS have died. Most of the people who died had a pre-existing medical condition that weakened their immune system, or an underlying medical condition that hadn’t yet been discovered. Medical conditions sometimes weaken people’s immune systems and make them more likely to get sick or have severe illness.
Pre-existing conditions among people who got MERS have included:
- diabetes
- cancer
- chronic lung disease
- chronic heart disease
- chronic kidney disease
MERS treatment
There is no specific antiviral treatment recommended for MERS-coronavirus infection. Individuals with MERS often receive medical care to help relieve symptoms. For severe cases, current treatment includes care to support vital organ functions.
Severe Acute Respiratory Syndrome (SARS) is a viral respiratory illness caused by SARS-associated coronavirus (SARS-CoV). Severe acute respiratory syndrome (SARS) is a serious form of pneumonia. Infection with the SARS coronavirus causes acute respiratory distress (severe breathing difficulty) and sometimes death. SARS was first reported in Asia in February 2003. Over the next few months, the illness spread to more than two dozen countries in North America, South America, Europe, and Asia before the SARS global outbreak of 2003 was contained. Since 2004, there have not been any known cases of SARS reported anywhere in the world 128.
SARS causes
SARS is caused by a member of the coronavirus family of viruses (the same family that can cause the common cold). It is believed the 2003 Severe Acute Respiratory Syndrome (SARS) epidemic started when the virus spread from small mammals in China.
When someone with SARS coughs or sneezes, infected droplets spray into the air. You can catch the SARS virus if you breathe in or touch these particles. The SARS virus may live on hands, tissues, and other surfaces for up to several hours in these droplets. The virus may be able to live for months or years when the temperature is below freezing.
While the spread of droplets through close contact caused most of the early SARS cases, SARS might also spread by hands and other objects the droplets has touched. Airborne transmission is a real possibility in some cases. Live virus has even been found in the stool of people with SARS, where it has been shown to live for up to 4 days.
With other coronaviruses, becoming infected and then getting sick again (reinfection) is common. This may also be the case with SARS.
Symptoms usually occur about 2 to 10 days after coming in contact with the virus. In some cases, SARS started sooner or later after first contact. People with active symptoms of illness are contagious. But it is not known for how long a person may be contagious before or after symptoms appear.
SARS prevention
Reducing your contact with people who have SARS lowers your risk for the disease. Avoid travel to places where there is an uncontrolled SARS outbreak. When possible, avoid direct contact with people who have SARS until at least 10 days after their fever and other symptoms are gone.
Hand hygiene is the most important part of SARS prevention. Wash your hands or clean them with an alcohol-based instant hand sanitizer.
Cover your mouth and nose when you sneeze or cough. Droplets that are released when a person sneezes or coughs are infectious.
DO NOT share food, drink, or utensils.
Clean commonly touched surfaces with an EPA-approved disinfectant.
Masks and goggles may be useful for preventing the spread of the disease. You may use gloves when handling items that may have touched infected droplets.
SARS symptoms
The main symptoms of SARS are:
- Cough
- Difficulty breathing
- Fever of 100.4°F (38.0°C) or higher
- Other breathing symptoms
The most common symptoms are:
- Chills and shaking
- Cough, usually starts 2 to 3 days after other symptoms
- Headache
- Muscle aches
- Tiredness
Less common symptoms include:
- Cough that produces phlegm (sputum)
- Diarrhea
- Dizziness
- Nausea and vomiting
In some people, the lung symptoms get worse during the second week of illness, even after the fever has stopped.
SARS complications
SARS complications may include:
- Respiratory failure
- Liver failure
- Heart failure
- Kidney problems
SARS diagnosis
Your health care provider may hear abnormal lung sounds while listening to your chest with a stethoscope. In most people with SARS, a chest x-ray or chest CT show pneumonia, which is typical with SARS.
Tests used to diagnose SARS might include:
- Arterial blood tests
- Blood clotting tests
- Blood chemistry tests
- Chest x-ray or chest CT scan
- Complete blood count (CBC)
Tests used to quickly identify the virus that causes SARS include:
- Antibody tests for SARS
- Direct isolation of the SARS virus
- Rapid polymerase chain reaction (PCR) test for SARS virus
All current tests have some limitations. They may not be able to easily identify a SARS case during the first week of the illness, when it is most important.
SARS treatment
People who are thought to have SARS should be checked right away by a provider. If they are suspected of having SARS, they should be kept isolated in the hospital.
Treatment may include:
- Antibiotics to treat bacteria that cause pneumonia (until bacterial pneumonia is ruled out or if there is bacterial pneumonia in addition to SARS)
- Antiviral medicines (although how well they work for SARS is unknown)
- High doses of steroids to reduce swelling in the lungs (it is not known how well they work)
- Oxygen, breathing support (mechanical ventilation), or chest therapy
In some serious cases, the liquid part of blood from people who have already recovered from SARS has been given as a treatment.
There is no strong evidence that these treatments work well. There is evidence that the antiviral medicine, ribavirin, does not work.
SARS prognosis
In the 2003 outbreak, the death rate from SARS was 9% to 12% of those diagnosed. In people over age 65, the death rate was higher than 50%. The illness was milder in younger people.
In the older population, many more people became sick enough to need breathing assistance. And even more people had to go to hospital intensive care units.
Public health policies have been effective at controlling outbreaks. Many nations have stopped the epidemic in their own countries. All countries must continue to be careful to keep this disease under control. Viruses in the coronavirus family are known for their ability to change (mutate) in order to spread among humans.
Human coronaviruses usually spread from an infected person to others through:
- The air by coughing and sneezing
- Close personal contact, such as touching or shaking hands
- Touching an object or surface with the virus on it, then touching your mouth, nose, or eyes before washing your hands
- Rarely, feces (poop)
Right now, there aren’t any vaccines to prevent human coronavirus infections. But you may able to reduce your risk of getting or spreading an infection by:
- Washing hands often with soap and water for at least 20 seconds
- Avoiding touching your face, nose, or mouth with unwashed hands
- Avoiding close contact with people who are sick
- Cleaning and disinfecting surfaces that you frequently touch
- Covering coughs and sneezes with a tissue. Then throw away the tissue and wash your hands.
- Staying home when sick
The symptoms depend on the type of coronavirus and how serious the infection is. If you have a mild to moderate upper-respiratory infection such as the common cold, your symptoms may include:
- Runny nose
- Headache
- Cough
- Sore throat
- Fever
- Not feeling well overall
Some coronaviruses can cause severe symptoms. The infections may turn into bronchitis and pneumonia, which cause symptoms such as:
- Fever, which may be quite high if you have pneumonia
- Cough with mucus
- Shortness of breath
- Chest pain or tightness when you breathe and cough
Severe infections are more common in people with heart or lung diseases, people with weakened immune systems, infants, and older adults.
To make a coronavirus infection diagnosis, your health care provider will:
- Take your medical history, including asking about your symptoms
- Do a physical exam
- May do blood tests
- May do lab tests of sputum, a sample from a throat swab, or other respiratory specimens
Your healthcare provider may order laboratory tests on respiratory specimens and serum (part of your blood) to detect human coronaviruses. Laboratory testing is more likely to be used if you have severe disease or are suspected of having MERS.
If you are experiencing symptoms, you should tell your healthcare provider about any recent travel or contact with animals. Most MERS-coronavirus infections have been reported from countries in the Arabian Peninsula. Therefore reporting a travel history or contact with camels or camel products is very important when trying to diagnose MERS.
Most state laboratories are approved to test for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) using CDC’s real-time reverse transcription–PCR (rRT-PCR) assay.
The U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization on June 5, 2013, to authorize use of CDC’s 2012 real-time reverse transcription–PCR assay to test for MERS-coronavirus in clinical respiratory, serum, and stool specimens 129. CDC has distributed this assay to qualified laboratories in the United States and around the world.
There are no specific treatments for coronavirus infections. Most people will get better on their own. However, you can relieve your symptoms by
- Taking over-the-counter medicines for pain, fever, and cough. However, do not give aspirin to children. And do not give cough medicine to children under four.
- Using a room humidifier or taking a hot shower to help ease a sore throat and cough
- Getting plenty of rest
- Drinking fluids
If you are worried about your symptoms, contact your health care provider.
References- Coronavirus. https://www.who.int/health-topics/coronavirus
- Dawson P, Malik MR, Parvez F, Morse SS. What Have We Learned About Middle East Respiratory Syndrome Coronavirus Emergence in Humans? A Systematic Literature Review. Vector Borne Zoonotic Dis. 2019;19(3):174–192. doi:10.1089/vbz.2017.2191 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6396572
- COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. Published 2019 May 27. doi:10.1186/s12985-019-1182-0 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6537279
- SARS and MERS: recent insights into emerging coronaviruses. de Wit E, van Doremalen N, Falzarano D, Munster VJ. Nat Rev Microbiol. 2016 Aug; 14(8):523-34.
- Coronavirus Symptoms and Diagnosis. https://www.cdc.gov/coronavirus/about/symptoms.html
- https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961037/NERVTAG_note_on_B.1.1.7_severity_for_SAGE_77__1_.pdf
- mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. Kai Wu, Anne P. Werner, Juan I. Moliva, Matthew Koch, Angela Choi, Guillaume B. E. Stewart-Jones, Hamilton Bennett, Seyhan Boyoglu-Barnum, Wei Shi, Barney S. Graham, Andrea Carfi, Kizzmekia S. Corbett, Robert A. Seder, Darin K. Edwards bioRxiv 2021.01.25.427948; doi: https://doi.org/10.1101/2021.01.25.427948
- Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Allison J. Greaney, Andrea N. Loes, Katharine H.D. Crawford, Tyler N. Starr, Keara D. Malone, Helen Y. Chu, Jesse D. Bloom bioRxiv 2020.12.31.425021; doi: https://doi.org/10.1101/2020.12.31.425021
- Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020;9:e61312 doi: 10.7554/eLife.61312
- Spike E484K mutation in the first SARS-CoV-2 reinfection case confirmed in Brazil, 2020. https://virological.org/t/spike-e484k-mutation-in-the-first-sars-cov-2-reinfection-case-confirmed-in-brazil-2020/584
- Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., Shi, Z., Hu, Z., Zhong, W., & Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research, 30(3), 269–271. https://doi.org/10.1038/s41422-020-0282-0
- Vincent, M. J., Bergeron, E., Benjannet, S., Erickson, B. R., Rollin, P. E., Ksiazek, T. G., Seidah, N. G., & Nichol, S. T. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology journal, 2, 69. https://doi.org/10.1186/1743-422X-2-69
- Fantini, J., Chahinian, H., & Yahi, N. (2020). Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. International journal of antimicrobial agents, 56(2), 106020. https://doi.org/10.1016/j.ijantimicag.2020.106020
- Andreani, J., Le Bideau, M., Duflot, I., Jardot, P., Rolland, C., Boxberger, M., Wurtz, N., Rolain, J. M., Colson, P., La Scola, B., & Raoult, D. (2020). In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microbial pathogenesis, 145, 104228. https://doi.org/10.1016/j.micpath.2020.104228
- Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, Naninck T, Pizzorno A, Lemaitre J, Gonçalves A, Kahlaoui N, Terrier O, Fang RHT, Enouf V, Dereuddre-Bosquet N, Brisebarre A, Touret F, Chapon C, Hoen B, Lina B, Calatrava MR, van der Werf S, de Lamballerie X, Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020 Sep;585(7826):584-587. doi: 10.1038/s41586-020-2558-4
- Infante, M., Ricordi, C., Alejandro, R., Caprio, M., & Fabbri, A. (2021). Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert review of anti-infective therapy, 19(1), 5–16. https://doi.org/10.1080/14787210.2020.1799785
- Frie K., Gbinigie K. The Centre for Evidence-Based Medicine; 2020. Chloroquine and Hydroxychloroquine: Current Evidence for Their Effectiveness in Treating COVID-19.
- Spinelli F.R., Ceccarelli F., Di Franco M., Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann. Rheum. Dis. 2020;79:666–667.
- Singh, B., Ryan, H., Kredo, T., Chaplin, M., & Fletcher, T. (2021). Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. The Cochrane database of systematic reviews, 2(2), CD013587. https://doi.org/10.1002/14651858.CD013587.pub2
- Joshi, G., Thakur, S., Mayank, & Poduri, R. (2021). Exploring insights of hydroxychloroquine, a controversial drug in Covid-19: An update. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 151, 112106. https://doi.org/10.1016/j.fct.2021.112106
- Abd-Elsalam, S., Esmail, E. S., Khalaf, M., Abdo, E. F., Medhat, M. A., Abd El Ghafar, M. S., Ahmed, O. A., Soliman, S., Serangawy, G. N., & Alboraie, M. (2020). Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study. The American journal of tropical medicine and hygiene, 103(4), 1635–1639. https://doi.org/10.4269/ajtmh.20-0873
- Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Published:May 22, 2020 https://doi.org/10.1016/S0140-6736(20)31180-6
- Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med 2020; 382:e102 DOI: 10.1056/NEJMoa2007621 https://www.nejm.org/doi/full/10.1056/NEJMoa2007621
- Chloroquine or Hydroxychloroquine and/or Azithromycin. Last Updated: July 8, 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/chloroquine-or-hydroxychloroquine-and-or-azithromycin
- https://www.fda.gov/media/138945/download
- Recovery Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605-612. https://doi.org/10.1016/S0140-6736(21)00149-5
- https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf
- RECOVERY Collaborative Group, Horby, P., Mafham, M., Linsell, L., Bell, J. L., Staplin, N., Emberson, J. R., Wiselka, M., Ustianowski, A., Elmahi, E., Prudon, B., Whitehouse, T., Felton, T., Williams, J., Faccenda, J., Underwood, J., Baillie, J. K., Chappell, L. C., Faust, S. N., Jaki, T., … Landray, M. J. (2020). Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. The New England journal of medicine, 383(21), 2030–2040. https://doi.org/10.1056/NEJMoa2022926
- WHO Solidarity Trial Consortium, Pan, H., Peto, R., Henao-Restrepo, A. M., Preziosi, M. P., Sathiyamoorthy, V., Abdool Karim, Q., Alejandria, M. M., Hernández García, C., Kieny, M. P., Malekzadeh, R., Murthy, S., Reddy, K. S., Roses Periago, M., Abi Hanna, P., Ader, F., Al-Bader, A. M., Alhasawi, A., Allum, E., Alotaibi, A., … Swaminathan, S. (2021). Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. The New England journal of medicine, 384(6), 497–511. https://doi.org/10.1056/NEJMoa2023184
- Self WH, Semler MW, Leither LM, et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(21):2165–2176. doi:10.1001/jama.2020.22240 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7653542
- Furtado, R., Berwanger, O., Fonseca, H. A., Corrêa, T. D., Ferraz, L. R., Lapa, M. G., Zampieri, F. G., Veiga, V. C., Azevedo, L., Rosa, R. G., Lopes, R. D., Avezum, A., Manoel, A., Piza, F., Martins, P. A., Lisboa, T. C., Pereira, A. J., Olivato, G. B., Dantas, V., Milan, E. P., … COALITION COVID-19 Brazil II Investigators (2020). Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet (London, England), 396(10256), 959–967. https://doi.org/10.1016/S0140-6736(20)31862-6
- Cavalcanti, A. B., Zampieri, F. G., Rosa, R. G., Azevedo, L., Veiga, V. C., Avezum, A., Damiani, L. P., Marcadenti, A., Kawano-Dourado, L., Lisboa, T., Junqueira, D., de Barros E Silva, P., Tramujas, L., Abreu-Silva, E. O., Laranjeira, L. N., Soares, A. T., Echenique, L. S., Pereira, A. J., Freitas, F., Gebara, O., … Coalition Covid-19 Brazil I Investigators (2020). Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. The New England journal of medicine, 383(21), 2041–2052. https://doi.org/10.1056/NEJMoa2019014
- Rosenberg, E. S., Dufort, E. M., Udo, T., Wilberschied, L. A., Kumar, J., Tesoriero, J., Weinberg, P., Kirkwood, J., Muse, A., DeHovitz, J., Blog, D. S., Hutton, B., Holtgrave, D. R., & Zucker, H. A. (2020). Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA, 323(24), 2493–2502. https://doi.org/10.1001/jama.2020.8630
- Geleris, J., Sun, Y., Platt, J., Zucker, J., Baldwin, M., Hripcsak, G., Labella, A., Manson, D. K., Kubin, C., Barr, R. G., Sobieszczyk, M. E., & Schluger, N. W. (2020). Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. The New England journal of medicine, 382(25), 2411–2418. https://doi.org/10.1056/NEJMoa2012410
- Arshad, S., Kilgore, P., Chaudhry, Z. S., Jacobsen, G., Wang, D. D., Huitsing, K., Brar, I., Alangaden, G. J., Ramesh, M. S., McKinnon, J. E., O’Neill, W., Zervos, M., & Henry Ford COVID-19 Task Force (2020). Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 97, 396–403. https://doi.org/10.1016/j.ijid.2020.06.099
- Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020; 383:2041-2052 DOI: 10.1056/NEJMoa2019014 https://www.nejm.org/doi/full/10.1056/NEJMoa2019014
- Chloroquine or Hydroxychloroquine and/or Azithromycin. Last Updated: July 8, 2021 https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/chloroquine-or-hydroxychloroquine-and-or-azithromycin
- Skipper, C. P., Pastick, K. A., Engen, N. W., Bangdiwala, A. S., Abassi, M., Lofgren, S. M., Williams, D. A., Okafor, E. C., Pullen, M. F., Nicol, M. R., Nascene, A. A., Hullsiek, K. H., Cheng, M. P., Luke, D., Lother, S. A., MacKenzie, L. J., Drobot, G., Kelly, L. E., Schwartz, I. S., Zarychanski, R., … Boulware, D. R. (2020). Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Annals of internal medicine, 173(8), 623–631. https://doi.org/10.7326/M20-4207
- Mitjà, O., Corbacho-Monné, M., Ubals, M., Tebe, C., Peñafiel, J., Tobias, A., Ballana, E., Alemany, A., Riera-Martí, N., Pérez, C. A., Suñer, C., Laporte, P., Admella, P., Mitjà, J., Clua, M., Bertran, L., Sarquella, M., Gavilán, S., Ara, J., Argimon, J. M., … BCN PEP-CoV-2 RESEARCH GROUP (2020). Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaa1009. Advance online publication. https://doi.org/10.1093/cid/ciaa1009
- Omrani AS, Pathan SA, Thomas SA, et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe COVID-19. EClinicalMedicine. 2020. Published online 2020 Nov 20. doi: 10.1016/j.eclinm.2020.100645 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7678437
- PRINCIPLE Trial Collaborative Group (2021). Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet (London, England), 397(10279), 1063–1074. https://doi.org/10.1016/S0140-6736(21)00461-X
- A randomised clinical trial of azithromycin versus standard care in ambulatory COVID-19 – the ATOMIC2 trial. Timothy SC Hinks, Lucy Cureton, Ruth Knight, Ariel Wang, Jennifer L Cane, Vicki S Barber, Joanna Black, Susan J Dutton, James Melhorn, Maisha Jabeen, Phil Moss, Rajendar Garlapati, Tanya Baron, Graham Johnson, Fleur Cantle, David Clarke, Samer Elkhodair, Jonathan Underwood, Daniel Lasserson, Ian D Pavord, Sophie Morgan, Duncan Richards. medRxiv 2021.04.21.21255807; doi: https://doi.org/10.1101/2021.04.21.21255807
- Boulware D, Pullen M, Bangdiwala A, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med 2020; 383:517-525 DOI: 10.1056/NEJMoa2016638 https://www.nejm.org/doi/full/10.1056/nejmoa2016638
- Dreyer SM, Morin KJ, Vaughan JA. Differential susceptibilities of Anopheles albimanus and Anopheles stephensi mosquitoes to ivermectin. Malaria Journal. 2018;17:148. doi:10.1186/s12936-018-2296-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5883420/
- Kinobe, R. T., & Owens, L. (2021). A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundamental & clinical pharmacology, 35(2), 260–276. https://doi.org/10.1111/fcp.12644
- Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID‐19 complementary regimen. J. Antibiot. (Tokyo). 2020;73:593‐602. 10.1038/s41429-020-0336-z
- Jans DA, Wagstaff KM. Ivermectin as a broad‐spectrum host‐directed antiviral: the real deal? Cells. 2020;9(9):2100‐10.3390/cells9092100
- Formiga FR, Leblanc R, Reboucas JDS et al. Ivermectin: an award‐winning drug with expected antiviral activity against COVID‐19. J. Control Release. 2020; 329: 758–761. 10.1016/j.jconrel.2020.10.009
- Lundberg L, Pinkham C, Baer A et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 2013;100:662‐672. 10.1016/j.antiviral.2013.10.004
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Ivermectin. [Updated 2021 Apr 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548921
- Popp, M., Stegemann, M., Metzendorf, M. I., Gould, S., Kranke, P., Meybohm, P., Skoetz, N., & Weibel, S. (2021). Ivermectin for preventing and treating COVID-19. The Cochrane database of systematic reviews, 7(7), CD015017. https://doi.org/10.1002/14651858.CD015017.pub2
- Rajter, J. C., Sherman, M. S., Fatteh, N., Vogel, F., Sacks, J., & Rajter, J. J. (2021). Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study. Chest, 159(1), 85–92. https://doi.org/10.1016/j.chest.2020.10.009
- Ortega-Guillén, E., Meneses, G., & Coila, E. (2021). Remarks About Retrospective Analysis of Ivermectin Effectiveness on Coronavirus Disease 2019 (ICON Study). Chest, 159(5), 2110–2111. https://doi.org/10.1016/j.chest.2020.10.088
- Vallejos, J., Zoni, R., Bangher, M. et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis 21, 635 (2021). https://doi.org/10.1186/s12879-021-06348-5
- Ivermectin to be investigated in adults aged 18+ as a possible treatment for COVID-19 in the PRINCIPLE trial. https://www.principletrial.org/news/ivermectin-to-be-investigated-as-a-possible-treatment-for-covid-19-in-oxford2019s-principle-trial
- Drug treatments for covid-19: living systematic review and network meta-analysis BMJ 2021; 373 :n967 doi:10.1136/bmj.n967
- Rochwerg B, Agarwal A, Siemieniuk R A, Agoritsas T, Lamontagne F, Askie L et al. A living WHO guideline on drugs for covid-19 BMJ 2020; 370 :m3379 doi:10.1136/bmj.m3379
- Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD000980. doi: 10.1002/14651858.CD000980.pub4
- Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013 Aug 8;(8):CD005532. doi: 10.1002/14651858.CD005532.pub3
- Johnston CS. Vitamin C. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition 11th ed. Cambridge, MA: Elsevier; 2020:155-69.
- Hemilä H. Vitamin C and Infections. Nutrients. 2017 Mar 29;9(4):339. doi: 10.3390/nu9040339
- Milani GP, Macchi M, Guz-Mark A. Vitamin C in the Treatment of COVID-19. Nutrients. 2021 Apr 1;13(4):1172. doi: 10.3390/nu13041172
- Capone S, Abramyan S, Ross B, Rosenberg J, Zeibeq J, Vasudevan V, Samad R, Gerolemou L, Pinelis E, Gasperino J, Orsini J. Characterization of Critically Ill COVID-19 Patients at a Brooklyn Safety-Net Hospital. Cureus. 2020 Aug 17;12(8):e9809. doi: 10.7759/cureus.9809
- Krishnan S, Patel K, Desai R, Sule A, Paik P, Miller A, Barclay A, Cassella A, Lucaj J, Royster Y, Hakim J, Ahmed Z, Ghoddoussi F. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020 Dec;67:110005. doi: 10.1016/j.jclinane.2020.110005
- Louca P, Murray B, Klaser K, Graham MS, Mazidi M, Leeming ER, et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr Prev Health 2021.
- Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il’Giovine ZJ, Mehra R, McWilliams C, Nissen SE, Desai MY. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open. 2021 Feb 1;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369
- Vitamin C. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional
- Patterson T, Isales CM, Fulzele S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021 Feb 1;12(1):14-26. doi: 10.14336/AD.2020.0918
- Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020 May 19;12(5):1466. doi: 10.3390/nu12051466
- Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006 Mar 28;174(7):937-42. doi: 10.1503/cmaj.050346
- Cheng RZ. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020 Mar;5:100028. doi: 10.1016/j.medidd.2020.100028
- Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H, Peng Z. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021 Jan 9;11(1):5. doi: 10.1186/s13613-020-00792-3
- Jamali Moghadam Siahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani M, Sekhavati Moghadam E, Abbasian L, Dehghan Manshadi SA, Salehi M, Hasannezhad M, Ghaderkhani S, Meidani M, Salahshour F, Jafari F, Manafi N, Ghiasvand F. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021 Feb 11;26(1):20. doi: 10.1186/s40001-021-00490-1
- COVID-19 Treatment Guidelines. Vitamin C. https://www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-c
- Use of Ascorbic Acid in Patients With COVID 19. https://clinicaltrials.gov/ct2/show/NCT04323514
- The Effect of Melatonin and Vitamin C on COVID-19. https://clinicaltrials.gov/ct2/show/NCT04530539
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001 Sep;108(3):E55. doi: 10.1542/peds.108.3.e55
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000.
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002 Mar-Apr;5(2):66-74. doi: 10.1046/j.1523-5408.2002.00005.x
- Cho J, Ahn S, Yim J, Cheon Y, Jeong SH, Lee SG, Kim JH. Influence of Vitamin C and Maltose on the Accuracy of Three Models of Glucose Meters. Ann Lab Med. 2016 May;36(3):271-4. doi: 10.3343/alm.2016.36.3.271
- COVID Data Tracker. Variant Proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- Delta Variant: What We Know About the Science. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
- Progressive Increase in Virulence of Novel SARS-CoV-2 Variants in Ontario, Canada. David N. Fisman, Ashleigh R. Tuite. medRxiv 2021.07.05.21260050; doi: https://doi.org/10.1101/2021.07.05.21260050
- Human Coronavirus Types. https://www.cdc.gov/coronavirus/types.html
- First Travel-related Case of 2019 Novel Coronavirus Detected in United States. https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html
- Report 6: Relative sensitivity of international surveillance. Imperial College London COVID-19 Response Team https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-international-surveillance-21-02-2020.pdf
- MacIntyre, C.R., 2020. Global spread of COVID-19 and pandemic potential. Global Biosecurity, 1(3), p.None. DOI: http://doi.org/10.31646/gbio.55
- Liu, Y., Gayle, A. A., Wilder-Smith, A. & Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of Travel Medicine. 2020. doi:10.1093/jtm/taaa021
- Q&A on coronaviruses (COVID-19). https://www.who.int/news-room/q-a-detail/q-a-coronaviruses
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Feb 19 https://www.nejm.org/doi/pdf/10.1056/NEJMc2001737
- Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. Published online February 7, 2020. doi:10.1001/jama.2020.1585
- Fine, P. Introduction: the basics – infections, transmission and models. In: Vynnycky E & White R.An Introduction to Infectious Disease Modelling. 2010. UK: Oxford University Press.
- Douglas, M. G., Kocher, J. F., Scobey, T., Baric, R. S. & Cockrell, A. S. Adaptive evolution influences the infectious dose of MERS-CoV necessary to achieve severe respiratory disease. Virology 2018 517, 98–107.
- Chu C-M, Cheng VCC, Hung IFN, Chan K-S, Tang BSF, Tsang THF, et al. Viral load distribution in SARS outbreak. Emerg Infect Dis 2005 Dec http://dx.doi.org/10.3201/eid1112.040949
- Paulo AC, Correia‐Neves M, Dominguos T et al. Influenza infectious dose may explain the high mortality of the second and third wave of 1918–19 influenza pandemic. PLoS ONE 2010; 5: e11655.. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0011655#s2
- Oh MD, Park WB, Choe PG, Choi SJ, Kim JI, Chae J, Park SS, Kim EC, Oh HS, Kim EJ, Nam EY, Na SH, Kim DK, Lee SM, Song KH, Bang JH, Kim ES, Kim HB, Park SW, Kim NJ. Viral load kinetics of MERS coronavirus infection. N Engl J Med 2016 375:1303–1305. DOI: 10.1056/NEJMc1511695
- Yunpeng Ji,Zhongren Ma, Maikel P Peppelenbosch, Qiuwei Pan. Potential association between COVID-19 mortality and health-care resource availability. 2020 DOI: https://doi.org/10.1016/S2214-109X(20)30068-1
- Pre-emptive low cost social distancing and enhanced hygiene implemented before local COVID-19 transmission could decrease the number and severity of cases. https://www.mja.com.au/system/files/2020-03/FINAL%20Dalton%20preprint%20mja20.00300.pdf
- Fong MW, Gao H, Wong JY, Xiao J, Shiu EYC, Ryu S, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—social distancing measures. Emerg Infect Dis. 2020 May. https://doi.org/10.3201/eid2605.190995
- Kelso, J.K., Milne, G.J. & Kelly, H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 2009 9, 117. https://doi.org/10.1186/1471-2458-9-117
- Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;(8):CD013705.
- The Possibility of COVID-19 after Vaccination: Breakthrough Infections. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html
- Anti-SARS-CoV-2 Monoclonal Antibodies. https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
- Jiang, S., Hillyer, C., & Du, L. (2020). Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends in immunology, 41(5), 355–359. https://doi.org/10.1016/j.it.2020.03.007
- O’Brien, M. P., Forleo-Neto, E., Musser, B. J., Isa, F., Chan, K. C., Sarkar, N., Bar, K. J., Barnabas, R. V., Barouch, D. H., Cohen, M. S., Hurt, C. B., Burwen, D. R., Marovich, M. A., Hou, P., Heirman, I., Davis, J. D., Turner, K. C., Ramesh, D., Mahmood, A., Hooper, A. T., … Weinreich, D. M. (2021). Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention. medRxiv : the preprint server for health sciences, 2021.06.14.21258567. https://doi.org/10.1101/2021.06.14.21258567
- Cohen MS, Nirula A, Mulligan MJ, et al. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA. 2021;326(1):46–55. doi:10.1001/jama.2021.8828
- Pause in the Distribution of bamlanivimab/etesevimab. https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab-etesevimab/Pages/bamlanivimab-etesevimab-distribution-pause.aspx
- FACT SHEET FOR HEALTH CARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF REGEN-COVTM (casirivimab and imdevimab). https://www.fda.gov/media/145611/download
- Zhang, Y., Luo, W., Li, Q., Wang, X., Chen, J., Song, Q., Tu, H., Ren, R., Li, C., Li, D., Zhao, J., McGoogan, J. M., Shan, D., Li, B., Zhang, J., Dong, Y., Jin, Y., Mao, S., Qian, M., Lv, C., … Feng, Z. (2021). Risk Factors for Death Among the First 80 543 COVID-19 Cases in China: Relationships Between Age, Underlying Disease, Case Severity, and Region. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciab493. Advance online publication. https://doi.org/10.1093/cid/ciab493
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242.
- Liguoro I, Pilotto C, Bonanni M, et al. SARS-CoV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179(7):1029–1046.
- Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis [published correction appears in Lancet Infect Dis. 2020;20(6):e116]. Lancet Infect Dis. 2020;20(6):669–677.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776.
- https://www.cdc.gov/coronavirus/2019-ncov/index.html
- Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal [published correction appears in BMJ. 2020;369:m2204]. BMJ. 2020;369:m1328.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320.
- Hashem MD, Nallagangula A, Nalamalapu S, et al. Patient outcomes after critical illness: a systematic review of qualitative studies following hospital discharge. Crit Care. 2016;20(1):345.
- Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121–1129.
- Outpatient Management of COVID-19: Rapid Evidence Review. Am Fam Physician. 2020 Oct 15;102(8):478-486. https://www.aafp.org/afp/2020/1015/p478.html
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
- Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026.
- Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. N Engl J Med. 2012 Nov 8; 367(19):1814-20.
- Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/en/
- Severe Acute Respiratory Syndrome (SARS). https://www.cdc.gov/sars/index.html
- Emergency Use Authorizations. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations