What is Diindolylmethane
Diindolylmethane also called DIM or 3,3′-diindolylmethane, is a bioactive compound found in cruciferous vegetables like broccoli, brussels sprouts, cauliflower, cabbage, kale, bok choy, radishes, turnips, and kohlrabi, that is being studied in the treatment of prostate cancer and in the prevention of cervical cancer and breast cancer 1, 2. Thomson and colleagues 3 previously reported, from a secondary analysis, a 52% reduction in breast cancer recurrence among women consuming more cruciferous vegetables, they hypothesized to be attributed to higher exposure to indole-3-carbinol (I3C) and the related dimer, DIM diindolylmethane 4.
Diindolylmethane has been shown to regulate immune responses and exhibit a broad range of biological activities in several disease models, including various cancers 5 and inflammatory diseases such as multiple sclerosis 6, colitis 7 and arthritis 8. Moreover, diindolylmethane has shown potential effects against infections with enteric viruses 9 and Staphylococcus aureus 10. The immunomodulatory properties of diindolylmethane are associated with its ability to regulate multiple receptors 11 and signaling pathways, such as JNK, p38, NF-ĸB, AP-1, and FAK 12, suggesting that the anti-inflammatory properties of diindolylmethane are associated with the regulation of T-cell differentiation and inhibition of Th1/Th17 cells 13.
Cruciferous vegetable consumption may confer a protective effect against cancer, possibly attributable to their glucosinolates 14. Glucobrassicin is a predominant glucosinolate 15, is converted upon chewing of cruciferous vegetables into indole-3-carbinol (I3C), which then undergoes acid condensation in the stomach, predominantly to DIM (3,3′-diindolylmethane) a compound with anti-cancer effects 16.
DIM is readily detected in the livers and feces of rodents fed indole-3-carbinol (I3C), whereas the parent I3C compound has not been detected in tissues of these rodents 17. Thus, the natural effects of I3C (indole-3-carbinol) are attributable to diindolylmethane (DIM), which show evidence of anti-cancer activities in vivo (animal) and in vitro (test tube) by reducing the growth of prostate, colon, and breast cancer cells 18. I3C and its derivatives also suppress cell propagation and induce apoptosis in colon cancer cells 19, as well as in other types of cancer cells including prostate cancer 20, breast cancer 18, bladder cancer 21, pancreatic cancer 22 and liver cancer 23. However, a number of studies have found that I3C actually promoted or enhanced the development of cancer when administered chronically after the carcinogen 24. The cancer promoting effects of I3C is first reported in a trout model of liver cancer 24. However, I3C also has been found to promote cancer of the thyroid, liver, colon, and uterus in rats 25. Although the long-term effects of I3C supplementation on cancer risk in humans are not known, but the contradictory results of animal studies have led some to caution against the widespread use of I3C and DIM supplements in humans until their potential risks and benefits are better understood 25.
Reed et al. 26 in his extensive article of pharmacokinetic studies reported that women received oral doses of 400, 600, 800, 1,000, and 1,200 mg I3C (indole-3-carbinol) and these serial plasma samples were analyzed by high-performance liquid chromatography-mass spectrometry method for the detection and quantitation of the I3C and diindolylmethane (DIM). I3C itself is not detectable in plasma 26. The only detectable I3C-derived product is DIM. High initial value, plasma DIM for all subjects, decreased to near or below the limit of quantitation within the 12 hour sampling period 26. Physiologically based pharmacokinetic model is developed using plasma and tissue (brain, heart, liver, kidneys, and lungs) concentration data for DIM to compare the pharmacokinetic properties and biodistribution of pure crystalline and a novel formulation (BioResponse-DIM) of DIM after oral administration to mice 27. I3C is hard to develop as a drug because it is highly unstable and can transform into many other derivatives such as DIM, 5,6,11,12,17,18-hexahydrocyclonona[1,2-b:4,5-b′:7,8-b′′] triindole (CTr), indolo [3,2-b] carbazole (ICZ), N-methoxyindole-3-carbinol (NI3C), two tetramers, one linear (LTET) and one cyclic (CTET), and 1-(3-hydroxymethyl) indolylmethane (HI-IM). Anderton et al. 17 reported oral doses of I3C to female CD-1 mice, and studied the disposition of I3C and its acid condensation products diindolylmethane (DIM), ICZ, LTr(1), and HI-IM in blood, liver, kidney, lung, heart, and brain. I3C was rapidly absorbed, distributed, and eliminated from plasma and tissues, falling below the limit of detection by 1 hour. The major acid condensation product of I3C, diindolylmethane (DIM), has proven very stable in acidic conditions during prolonged exposure to high temperature and humidity 28. Another report BioResponse-DIM has proven very stable and it is not converted into other forms 28. The tissue distribution of I3C has been determined in mice by radio-labelled I3C 29. Both I3C and DIM have been detected in kidneys, lungs, heart, liver, plasma, and brain samples of mice treated with 250 mg/kg of I3C as early as 15 mins after administration 29. These results suggest that I3C is rapidly absorbed and spread to a number of well-perfused tissues, where it is transformed to diindolylmethane (DIM) to perform its anticancer actions 29. Based on these outcomes researchers suggested that one could exploit further development of diindolylmethane (DIM) as a potential therapeutic agent 2.
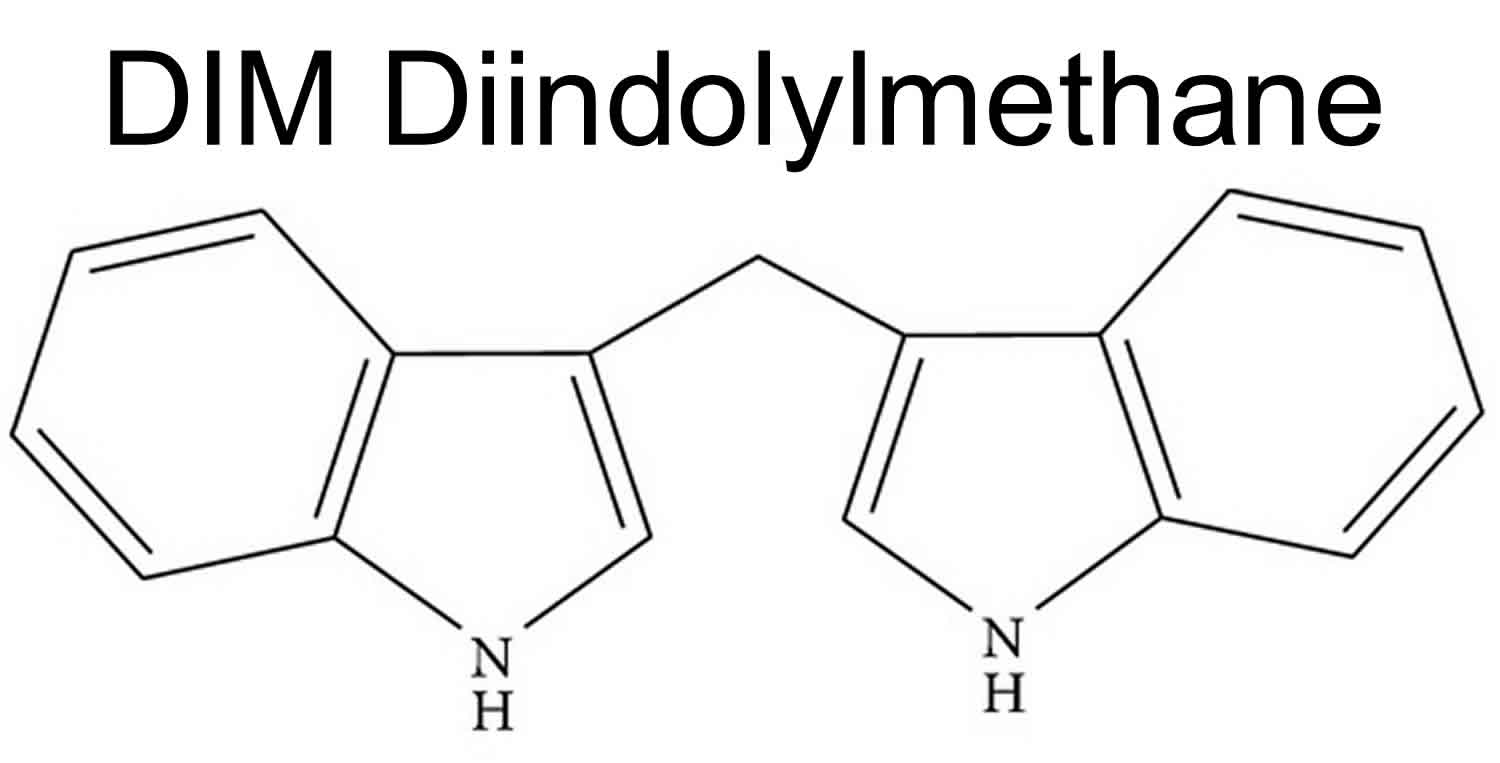
Figure 1. Diindolylmethane (DIM)
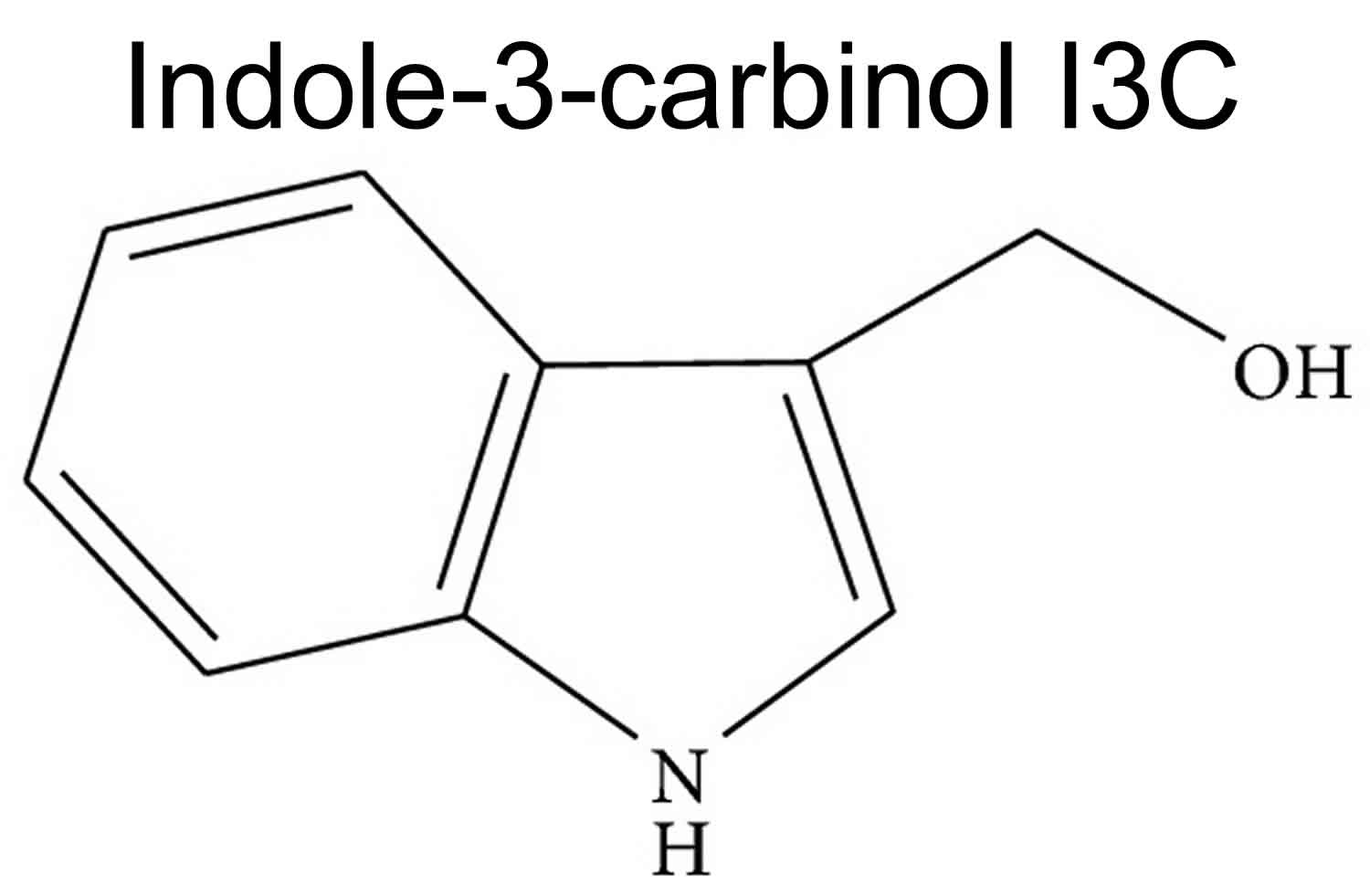
Figure 2. Indole-3-carbinol (I3C)
[Source 2 ]Diindolylmethane benefits
For more than 25 years, the interdependence between nutrition and the development and progression of cancer have been recognized 30. The key challenge is to identify the specific components responsible for contributing to this relationship 30. In the United States, cancers of the lungs, colon, rectum, breast, and prostate account for almost half of the total cancer incidence 30. Cohen et al. 31 examined the association of fruit and vegetable intake and prostate cancer risk among newly diagnosed men residing in the Seattle, WA, area. With each 10 g of cruciferous vegetables consumed per day, one could expect an 8% decrease in risk for colorectal cancer.
Several recent case-control studies in the US, Sweden, and China found that measures of cruciferous vegetable intake are significantly lower in women diagnosed with breast cancer than in cancer-free control groups 32. High intake of cruciferous vegetables has been associated with lower risk of lung and colorectal cancer in some epidemiological studies, but there is evidence that genetic polymorphisms may influence the effectiveness of cruciferous vegetables on human cancer risk 33. Epidemiological studies show that consumption of large quantities of fruits and vegetables, particularly cruciferous vegetables, is associated with a reduced occurrence of cancer 33.
Studies in various experimental models have shown that indole-3-carbinol (I3C) can alter the metabolism of carcinogens and provide protection against chemically induced carcinogenesis 34. When administered before or at the same time as the carcinogen, oral I3C has been originated to inhibit the spreading out of cancer in a variety of animal models and tissues, including cancers of breast cancer 18, colon cancer 19, stomach cancer 34, lung cancer 29 and liver cancer 24. However, a number of studies have found that I3C actually promoted or enhanced the development of cancer when administered chronically after the carcinogen 24.
The cancer promoting effects of I3C is first reported in a trout model of liver cancer 24. However, I3C also has been found to promote cancer of the thyroid, liver, colon, and uterus in rats 25. Although the long-term effects of I3C supplementation on cancer risk in humans are not known, but the contradictory results of animal studies have led some to caution against the widespread use of I3C and DIM supplements in humans until their potential risks and benefits are better understood 25.
Other uses of diindolylmethane supplements, such as weight loss and acne 35 treatment, have not been studied in humans.
Anticancer effects
DIM inhibits proliferation of human breast cancer cells at concentrations achievable through oral supplementation with I3C (10–50 μM) 36. Recently a report by Fan et al. 37 demonstrated that DIM protects cancer cells and normal epithelial cells against reactive oxygen species (ROS) in a breast cancer type 1 susceptibility protein (BRCA1-)dependent manner. Moreover I3C inhibits DMBA initiated and TPA promoted mouse skin tumor formation 38. I3C also exhibits inhibitory and preventive effects on prostate tumors in mice 20. Other investigators reported that this activity of I3C is associated with its action as a nonspecific inducer of powerful cytochrome enzymes responsible for “Phase I” detoxification metabolism, the efficiency of I3C to inhibit prostate tumor growth 34. The anticancer effect of I3C is well proven including the reduction of cervical intraepithelial neoplasia (CIN) and its progression to cervical cancer 39. It is perceived that the diminished phosphatase and tensin homolog protein (PTEN) expression is observed during the progression from low-grade to high-grade cervical dysplasia in humans and in a mouse model for cervical cancer, the K14HPV16 transgenic mice promoted with estrogen 39. PTEN could impede tumor progression by inhibiting proliferation and by increasing tumor cell apoptosis 39. This is supported by the previous findings that I3C decreased proliferating cell nuclear antigen- (PCNA-)positive cells and increased TdT-mediated dUTP nick-end labeling- (TUNEL-)positive cells in abnormal cervical epithelium of HPV16 mice 40.
Diindolylmethane estrogen blocker
Breast cancer survivors frequently self-prescribe bioactive dietary supplements with the intention to obtain survival benefit 41. Of these, DIM or diindolylmethane (3,3′-diindolylmethane), a stable in vivo metabolite of indole-3-carbinol (I3C) found in cruciferous vegetables 42, is among the most well-studied 43. Thomson et al. 44 previously reported, from a secondary analysis, a 52% reduction in breast cancer recurrence among women consuming more cruciferous vegetables, hypothesized to be attributed to higher exposure to indole-3-carbinol (I3C) and the related dimer, diindolylmethane 45.
Accumulating evidence favors several anti-tumor actions of diindolylmethane, including favorable changes in estrogen metabolism toward 2-hydroxylation of estrogen metabolism 46; increasing the ratio of 2-hydroxyestrone (2-OHE1) (anti-tumorigenic) to 16α-hydroxyestrone (16α-OHE1) (pro-tumorigenic) estrogens 47, a shift associated with lower breast cancer risk 48. Support of anti-estrogenic effect of diindolylmethane are evident in thyroid disease 49, cell culture studies demonstrating diminished estrogen receptor alpha (ERα) levels 50 and activation of estrogen receptor beta (ERβ) target genes 51.
This randomized, controlled trial of diindolylmethane supplementation 46 provided as BioResponse-DIM, found that diindolylmethane modulates estrogen metabolism through increased 2-hydroxylation of estrogen metabolism and, ultimately, increasing the ratio of this “favorable” metabolite to the “punitive” 16α-hydroxylated metabolite confirming the results in a pilot clinical trial using one third the diindolylmethane dose in breast cancer survivors not on tamoxifen. The present results support prior observations in 3 patients taking tamoxifen where the 2-hydroxyestrone (2-OHE1)/16α-hydroxyestrone (16α-OHE1) ratio increased 126–229% with a daily dose of 100 mg diindolylmethane from BioResponse-DIM for 30 days 52. Mechanistically, 2-hydroxyestrone (2-OHE1) (anti-tumorigenic) has been shown to bind ERα with high affinity, but lacks the ability to induce transcriptional activity resulting in the reported anti-estrogenic/anti-growth effects 53. Higher urinary 2-hydroxyestrone/16α-hydroxyestrone ratio has been associated with a lower risk of breast cancer in most 54, but not all studies 55. While a higher 2-hydroxyestrone/16α-hydroxyestrone ratio has been hypothesized as being anti-tumorigenic, direct evidence that manipulating 2-hydroxyestrone/16α-hydroxyestrone ratio prevents breast cancer or improves outcomes are lacking 56.
In this study 56, diindolylmethane did not affect serum estrogen levels, but increased sex hormone-binding globulin (SHBG), exposures which have been extensively studied in relation to breast cancer risk. In an analysis of worldwide data, the Endogenous Hormones and Breast Cancer Collaborative Group 57 confirmed that breast cancer risk increased with higher total Estradiol (E2), free E2, non-SHBG-bound E2, Estrone (E1), E1 sulfate, and testosterone, despite some contrary findings 58. As in this study, low levels and the high variations in measures may limit interpretation. Sex hormone-binding globulin (SHBG) has been inversely associated with breast cancer risk 59 with recent evidence suggesting a strong inverse association with breast cancer specific mortality 58. As noted by Duggan et al 60, sex hormone-binding globulin has been shown to induce apoptosis and inhibit growth of breast cancer cells via a receptor-mediated binding, suggesting that the effects of diindolylmethane on sex hormone-binding globulin may be beneficial to breast cancer outcomes independent of serum hormone or tamoxifen metabolite levels.
In this study 56, diindolylmethane lowered plasma levels of all three major Phase I tamoxifen metabolites. The clinical significance of lowered levels of tamoxifen metabolites remains unclear. Endoxifen and 4-OH tamoxifen, which exhibit higher activity for estrogen receptor at the tissue level, have been postulated as the active agents in tamoxifen therapy. While early work in the field failed to demonstrate a direct relationship between tamoxifen dose and tissue Ki67 levels as a biomarker of effects on proliferation, subsequent work suggested that low circulating endoxifen levels may limit the efficacy of tamoxifen. For example, in breast cancer survivors on tamoxifen circulating endoxifen levels > 5.6 ng/ml (upper four quintiles) were associated with a marginally significant lower risk of recurrence (26%) 61. Despite study limitations, this evidence has promoted inclusion of the 5.6 ng/ml endoxifen cut-point as a putative therapeutic threshold of tamoxifen 61. Lower doses of tamoxifen, including 1 and 5 mg daily, have demonstrated similar antitumor effects to therapeutic dosing and challenge the validity of the endoxifen cut-point for determining efficacy 62. Furthermore, diindolylmethane did not result in unfavorable change in breast density or sex hormone-binding globulin (SHBG), exposures which have been associated with tamoxifen efficacy 63.
Apoptosis
Apoptosis or programmed cell death is a highly regulated process that involves activation of a series of molecular events, leading to cell death that is characterized by cellular morphological change, chromatin condensation, and apoptotic bodies which are associated with DNA cleavage into ladders 64. The nuclear factor kappa B (NF-κB) signaling plays critical roles in regulating cell proliferation, survival, tumor invasion, metastasis, drug resistance, and stress response 65. Banerjee et al 66 confirmed that NF-κB activity is significantly upregulated by docetaxel, gemcitabine or oxaliplatin treatment and that the NF-κB inducing activity of these agents was completely abrogated in cells pretreated with DIM. They found that diindolylmethane or the formulated BR-DIM treatment, could restrict its nuclear localization and inactivate NF-κB DNA-binding activity in prostate 67, breast 68 and pancreatic cancer cells 66, resulting in the inhibition of transcriptional downregulation of several NF-κB downstream genes causing inhibition of cell growth and inducing apoptotic cell death. Collectively, these results clearly suggest that diindolylmethane pretreatment, which inactivates NF-κB activity, along with other cellular effects of DIM, may contribute to enhanced cell growth inhibition and apoptosis with suboptimal doses of cytotoxic chemotherapeutic agents with minimal side effects.
I3C trigger the stress-induced MAP-kinases p38 and C-jun N-terminal kinase (JNK) in prostate cancer cells and to inhibit constitutively active STAT3, a transcription factor, in pancreatic cancer cells 69. Irrespective of the cell type I3C suppressed NF-κB activation induced by various agents 69. NF-κB inhibition correlated with suppression of inhibitor of kappa B kinase (IKK) and IκBα phosphorylation, ubiquitination, degradation with p65 phosphorylation, nuclear translocation, and acetylation 70. I3C also downregulated NF-κB α regulated reporter gene transcription and gene products involved in cell proliferation, antiapoptosis, and invasion 70. This led to the potentiation of apoptosis induced by cytokines and chemotherapeutic agents 70. Collectively, the concerted effects on those proapoptotic components underlie the ability of I3C or diindolylmethane to induce mitochondrial dependent apoptosis in tumor cells 70.
Regulation of redox status
Reactive oxygen species (ROS) including hydrogen peroxide (H2O2) can cause different combinations of apoptosis, necrosis, and autophagy in a cell line dependent and stimulus-dependent manner 71. The capacity of I3C to form adducts with electrophiles or free radicals appears too autonomous on their chemical reactivity hence the scavenging ability of I3C is compatible with the adduct formation 72. Arnao et al. 72 investigate the ability of I3C to trap a metastable synthetic-free radical and inhibition of carcinogenesis. This induction may be produced by I3C itself and/or I3C derived polymerization products such as diindolylmethane and others 73.
According to Benabadji et al. 74, they reported that diindolylmethane and 6-methoxy-diindolylmethane in DPPH model, their IC50, were 50% and 40% smaller than that of vitamin E, due to their hydrogen-donating ability with the presence of two N–H groups as an H-donating group necessary to react with free radical and slightly less potent than the standard phenolic antioxidant BHA in β-carotene model with IC50 4% and 9% smaller for diindolylmethane and 6-methoxy-diindolylmethane.
Cell cycle arrest
Cell cycle arrest is defined as the halt of the cell cycle. I3C is reported to inhibit cyclin dependent kinase 2 (CDK2) kinase activities in MCF-7 cells through selective alterations in cyclin E composition, size distribution, and subcellular localization of the CDK2 protein complex 75. Cell-cycle arrest involves the upregulation of the CDK inhibitors p21 WAF1 and p27 KIP1 and the concurrent downregulation of cyclin D1, cyclin E, and CDKs 2, 4, and 6 attributable to the effect of I3C and diindolylmethane on regulating SP1-promoter binding activity [19]. Inhibition of CDK4/6 cyclin D1 and CDK2 cyclin E activities led to decreased Rb phosphorylations, which cause the Rb protein to bind to the E2F transcription factor 76. This E2F sequestration blocks the transcription of S phase genes resulting in G1 arrest; also the involvement of p53 and cell cycle arrest in the I3C-mediated effect has been studied in various cancer cells 77.
Treatment of I3C betters the expression of p53 (Ser 15) and CDKIs such as p21 and p27, while cyclin D1 expression is suppressed and cyclin E is not altered 78. Collectively, the data for the cell cycle analysis, the Plk-1 assay, and the western blot of p53 and CDKIs explain that I3C augments the expression of p53 and CDKIs and that I3C induced cell cycle arrest at G0/G1 in A549 cells 78. In addition, cotreatment with I3C and wortmannin prevents both phosphorylation of p53 at Ser 15 and p21 expression. Hence it is clear that A549 cell arrest by I3C is involved in the PI3 K and p53 signal pathways 78.
Angiogenesis
Angiogenesis is the physiological process through which new blood vessels form from preexisting vessels. This is distinct from vasculogenesis, which is the de novo formation of endothelial cells from mesoderm cell precursors 79. Dysregulated angiogenesis that consists of the unbalanced production of pro- and antiangiogenic factors is linked to a number of pathological situations 80. For example, the overexpression of angiogenic factors, including vascular endothelial growth factor (VEGF), IL-6, and matrix metalloproteinases (MMP-9) is closely associated with the development of cancers and metastasis [21]. The effect of I3C on LPS-activated macrophage-induced tube formation and its associated factors in endothelial EAhy926 cells are investigated 80. LPS significantly enhanced the capillary-like structure of endothelial cells cocultured with macrophages, but no such effect was observed in single-cultured ECs 80. I3C, on the other hand, suppressed such enhancement in concert with decreased secretions of VEGF, NO, IL-6, and MMPs 80. The results obtained from cultivating endothelial cells with conditioned medium collected from macrophages suggested that both endothelial cells and macrophages were inactivated by I3C 80.
Anti-inflammatory effect
The effect of diindolylmethane on inflammatory responses and its molecular mechanisms of diindolylmethane have been examined using lipopolysaccharide (LPS) stimulated RAW264.7 murine macrophages 81. Diindolylmethane inhibits LPS-induced increases in protein levels of inducible nitric oxide synthase (iNOS), which are accompanied by decreased iNOS mRNA levels and transcriptional activity 81. In addition, diindolylmethane suppresses LPS-induced NF-κB transcriptional and DNA-binding activity, translocation of p65 (RelA) to the nucleus, and degradation of inhibitor of kappa B alpha (IκBα) 81. Also the results of RT-PCR analysis exposed that LPS augmented the steady-state levels of proinflammatory cytokines such as TNF-α, IL-1β, PLA2, and interleukin-6 (IL-6) transcripts, which are substantially suppressed by diindolylmethane treatment 81. Diindolylmethane pretreatment significantly repressed LPS-induced phosphorylation of SAPK/JNK, whereas the phosphorylation of other MAPK family proteins (p38 or ERK-1/2) is unaltered by diindolylmethane pretreatment 82. Consequently, the use of certain anti-inflammatory agents, such as indole-3-carbinol (I3C) and 3,3’-diindolylmethane (DIM), could be beneficial for the treatment of autoimmune diseases 6.
Detoxification
Detoxification is the physiological or medicinal removal of toxic substances from living organisms 83. Both the Phase I and Phase II detoxification centers in the liver and the intestinal epithelial cells can be accelerated by some minor exogenous agent like I3C 83. Many researchers indicate that the ability of cruciferous vegetables to motivate Phase I and Phase II detoxification, particularly their I3C content, is a primary factor in which these nutrients are related to reduce cancer risk in humans 84. Animals are exposed to or injected with carcinogens; the animals receiving the cruciferous vegetables or the I3C in their food supply have a significantly lower tumor incidence than the animals fed the same diet, but without cruciferous vegetables or I3C fortification 84.
Diindolylmethane dosage
Due to limited research, proper dosages for DIM or diindolylmethane are unknown. In human research, doses typically range from 108–900 mg per day — though these studies were only related to treatments for cancer and prostate enlargement 85, 86.
However, a study in 24 healthy people 87 found that although diindolylmethane doses of up to 200 mg were well tolerated and didn’t cause side effects, one person experienced nausea, headache, and vomiting after taking a 300-mg dose, suggesting that higher doses may be associated with adverse side effects. Therefore, it’s best to talk to your healthcare provider to obtain personalized dosage recommendations based on your intended use.
Diindolylmethane side effects
Due to a lack of research in humans, little is known about the long-term safety and side effects of diindolylmethane supplements. Current human research doesn’t show diindolylmethane supplements to be toxic or have serious side effects. The most common side effects include darkening of the urine, an increase in bowel movements, headaches, and gas 85. Less common side effects include nausea, vomiting, diarrhea, and skin rash 88.
As diindolylmethane supplements interact with estrogen levels, they may affect people with hormone-sensitive cancers or who are on hormone therapies. Such individuals should steer clear of diindolylmethane supplements unless under the supervision of a medical professional. No matter your medical history, it’s important to consult your healthcare provider before taking diindolylmethane supplement.
Diindolylmethane summary
Diindolylmethane is a compound found in cruciferous (Brassica) vegetables. These vegetables include cabbage, cauliflower, Brussels sprouts, kale, turnips, and broccoli 89. Limited studies suggest that diindolylmethane supplements may help reduce prostate enlargement, prostatic intraepithelial neoplasia (PIN) 86 and protect against certain cancers. However, diindolylmethane’s effectiveness for other hormone-related conditions hasn’t been widely studied.
DIM supplements have not been shown to cause serious side effects, though more safety research is needed. People on hormone therapies or with certain hormone-related cancers should avoid diindolylmethane supplement.
References- Ciska E, Verkerk R, Honke J. Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3′-diindolylmethane in fermented cabbage. J Agric Food Chem. 2009 Mar 25;57(6):2334-8. doi: 10.1021/jf803477w
- Maruthanila, V. L., Poornima, J., & Mirunalini, S. (2014). Attenuation of Carcinogenesis and the Mechanism Underlying by the Influence of Indole-3-carbinol and Its Metabolite 3,3′-Diindolylmethane: A Therapeutic Marvel. Advances in pharmacological sciences, 2014, 832161. https://doi.org/10.1155/2014/832161
- Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, Pierce JP. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women’s Healthy Eating and Living Study. Breast Cancer Res Treat. 2011 Jan;125(2):519-27. doi: 10.1007/s10549-010-1014-9
- Fujioka N, Ainslie-Waldman CE, Upadhyaya P, Carmella SG, Fritz VA, Rohwer C, Fan Y, Rauch D, Le C, Hatsukami DK, Hecht SS. Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev. 2014 Feb;23(2):282-7. doi: 10.1158/1055-9965.EPI-13-0645
- Kim SM. Cellular and molecular mechanisms of 3,3’-diindolylmethane in gastrointestinal cancer. Int. J. Mol. Sci. 2016; 17: 1155 10.3390/ijms17071155
- Rouse M, Rao R, Nagarkatti M, Nagarkatti P.S. 3,3’-Diindolylmethane ameliorates experimental autoimmune encephalomyelitis by promoting cell cycle arrest and apoptosis in activated T cells through microRNA signaling pathways. J. Pharmacol. Exp. Ther. 2014; 350: 341–352. 10.1124/jpet.114.214742
- Jeon EJ, Davaatseren M, Hwang JT, Park JH, Hur HJ, Lee AS, et al. Effect of oral administration of 3,3’-diindolylmethane on dextran sodium sulfate-induced acute colitis in mice. J. Agric. Food Chem. 2016; 64: 7702–7709. 10.1021/acs.jafc.6b02604
- Dong L, Xia S, Gao F, Zhang D, Chen J, Zhang J. 3,3’-Diindolylmethane attenuates experimental arthritis and osteoclastogenesis. Biochem. Pharmacol. 2010; 79: 715–721. 10.1016/j.bcp.2009.10.010
- Xue L, Pestka JJ, Li M, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane stimulates murine immune function in vitro and in vivo. J. Nutr. Biochem. 2008; 19: 336–344. 10.1016/j.jnutbio.2007.05.004
- Elliott DM, Nagarkatti M, Nagarkatti PS. 3,3’-Diindolylmethane ameliorates Staphylococcal enterotoxin B-induced acute lung injury through alteration in the expression of microRNA that target apoptosis and cell-cycle arrest in activated cells. J. Pharmacol. Exp. Ther. 2016; 357: 177–187. 10.1124/jpet.115.226563
- Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3’-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003; 278: 21136–21145. 10.1074/jbc.M300588200
- Li WX, Chen LP, Sun MY, Li JT, Liu HZ, Zhu W. 3’3-diindolylmethane inhibits migration, invasion and metastasis of hepatocellular carcinoma by suppressing FAK signaling. Oncotarget. 2015; 6: 23776–23792. 10.18632/oncotarget.4196
- Fernandez-Colorado, C. P., Cammayo, P., Flores, R. A., Nguyen, B. T., Kim, W. H., Kim, S., Lillehoj, H. S., & Min, W. (2020). Anti-inflammatory activity of diindolylmethane alleviates Riemerella anatipestifer infection in ducks. PloS one, 15(11), e0242198. https://doi.org/10.1371/journal.pone.0242198
- Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010 Aug;36(5):377-83. doi: 10.1016/j.ctrv.2010.01.002
- Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J Agric Food Chem. 2000;48(7):2862–7.
- International Agency for Research on Cancer. IARC handbooks of cancer prevention. Vol. 9. Lyon: IARC Press; 2004. Cruciferous vegetables, isothiocyanates and indoles.
- Anderton MJ, Manson MM, Verschoyle RD, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clinical Cancer Research. 2004;10(15):5233–5241.
- Rahman KMW, Sarkar FH. Inhibition of nuclear translocation of nuclear factor-κB contributes to 3,3′-Diindolylmethane-induced apoptosis in breast cancer cells. Cancer Research. 2005;65(1):364–371.
- Chintharlapalli S, Smith R, III, Samudio I, Zhang W, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor γ-mediated growth inhibition, transactivation, and differeatiation markers in colon cancer cells. Cancer Research. 2004;64(17):5994–6001.
- Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Indole-3-carbinol and 3,3′-diindolylmethane induce apoptosis in human prostate cancer cells. Food and Chemical Toxicology. 2003;41(6):745–752.
- Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. Inhibition of bladder tumor growth by 1,1-bis(3′-indolyl)-1-(p- substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Research. 2006;66(1):412–418.
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-Diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27(4):717–728.
- Gong Y, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane is a novel topoisomerase IIα catalytic inhibitor that induces S-phase retardation and mitotic delay in human hepatoma HepG2 cells. Molecular Pharmacology. 2006;69(4):1320–1327.
- Hendrich S, Bjeldanes LF. Effects of dietary cabbage, Brussels sprouts, Illicium verum, Schizandra chinensis and alfalfa on the benzo[a]pyrene metabolic system in mouse liver. Food and Chemical Toxicology. 1983;21(4):479–486.
- Lee BM, Park KK. Beneficial and adverse effects of chemopreventive agents. Mutat Res. 2003 Feb-Mar;523-524:265-78. doi: 10.1016/s0027-5107(02)00342-1
- Reed GA, Arneson DW, Putnam WC, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiology Biomarkers and Prevention. 2006;15(12):2477–2481.
- Anderton MJ, Manson MM, Verschoyle R, et al. Physiological modeling of formulated and crystalline 3,3′ -diindolylmethane pharmacokinetics following oral administration in mice. Drug Metabolism and Disposition. 2004;32(6):632–638.
- Banerjee S, Kong D, Wang Z, Bao B, Hillman GG, Sarkar FH. Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): from bench to clinic. Mutation Research. 2011;728(1-2):47–66.
- Kassie F, Anderson LB, Scherber R, et al. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Research. 2007;67(13):6502–6511.
- Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer. 2001;41(1-2):17-28. doi: 10.1080/01635581.2001.9680607
- Jennifer H. Cohen, Alan R. Kristal, Janet L. Stanford, Fruit and Vegetable Intakes and Prostate Cancer Risk, JNCI: Journal of the National Cancer Institute, Volume 92, Issue 1, 5 January 2000, Pages 61–68, https://doi.org/10.1093/jnci/92.1.61
- Christine B. Ambrosone, Susan E. McCann, Jo L. Freudenheim, James R. Marshall, Yueshang Zhang, Peter G. Shields, Breast Cancer Risk in Premenopausal Women Is Inversely Associated with Consumption of Broccoli, a Source of Isothiocyanates, but Is Not Modified by GST Genotype, The Journal of Nutrition, Volume 134, Issue 5, May 2004, Pages 1134–1138, https://doi.org/10.1093/jn/134.5.1134
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007 Mar;55(3):224-36. doi: 10.1016/j.phrs.2007.01.009
- Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Research. 1978;38(5):1410–1413.
- Melnik B. (2012). Dietary intervention in acne: Attenuation of increased mTORC1 signaling promoted by Western diet. Dermato-endocrinology, 4(1), 20–32. https://doi.org/10.4161/derm.19828
- Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19(9):1631–1639.
- Fan S, Meng Q, Saha T, Sarkar FH, Rosen EM. Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Research. 2009;69(15):6083–6091.
- Srivastava B, Shukla Y. Antitumour promoting activity of indole-3-carbinol in mouse skin carcinogenesis. Cancer Letters. 1998;134(1):91–95.
- Chen D-Z, Qi M, Auborn KJ, Carter TH. Indole-3-carbinol and diindolylmethane induce apoptosis of human cervical cancer cells and in murine HPV16-transgenic preneoplastic cervical epithelium. The Journal of Nutrition. 2001;131(12):3294–3302.
- Davidson B, Goldberg I, Kopolovic J. Angiogenesis in uterine cervical intraepithelial neoplasia and squamous cell carcinoma: An immunohistochemical study. International Journal of Gynecological Pathology. 1997;16(4):335–338.
- Greenlee H, Kwan ML, Ergas IJ, Strizich G, Roh JM, Wilson AT, Lee M, Sherman KJ, Ambrosone CB, Hershman DL, Neugut AI, Kushi LH. Changes in vitamin and mineral supplement use after breast cancer diagnosis in the Pathways Study: a prospective cohort study. BMC Cancer. 2014 May 29;14:382. doi: 10.1186/1471-2407-14-382
- Ciska E, Verkerk R, Honke J. Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3′-diindolylmethane in fermented cabbage. J Agric Food Chem. 2009;57(6):2334–2338. doi: 10.1021/jf803477w
- Thomson CA, Ho E, Strom MB. Chemopreventive properties of 3,3′-diindolylmethane in breast cancer: evidence from experimental and human studies. Nutr Rev. 2016 Jul;74(7):432-43. doi: 10.1093/nutrit/nuw010
- Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, Pierce JP. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women’s Healthy Eating and Living Study. Breast Cancer Res Treat. 2011;125(2):519–527. doi: 10.1007/s10549-010-1014-9
- Fujioka, N., Ainslie-Waldman, C. E., Upadhyaya, P., Carmella, S. G., Fritz, V. A., Rohwer, C., Fan, Y., Rauch, D., Le, C., Hatsukami, D. K., & Hecht, S. S. (2014). Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 23(2), 282–287. https://doi.org/10.1158/1055-9965.EPI-13-0645
- Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161–167. doi: 10.1207/s15327914nc5002_5
- Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7(2):112–129.
- Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids. 2015 Jul;99(Pt A):67-75. doi: 10.1016/j.steroids.2015.02.015
- Rajoria S, Suriano R, Parmar PS, Wilson YL, Megwalu U, Moscatello A, Bradlow HL, Sepkovic DW, Geliebter J, Schantz SP, Tiwari RK. 3,3′-diindolylmethane modulatesestrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21(3):299–304. doi: 10.1089/thy.2010.0245
- Okino ST, Pookot D, Basak S, Dahiya R. Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Phila). 2009 Mar;2(3):251-6. doi: 10.1158/1940-6207.CAPR-08-0146
- Vivar OI, Saunier EF, Leitman DC, Firestone GL, Bjeldanes LF. Selective activation of estrogen receptor-beta target genes by 3,3′-diindolylmethane. Endocrinology. 2010 Apr;151(4):1662-7. doi: 10.1210/en.2009-1028
- Bradlow HL. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo. 2008 Jul-Aug;22(4):441-5.
- Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrinol. 1989 Feb;61(2):239-46. doi: 10.1016/0303-7207(89)90135-4
- Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013 Apr 22;15(2):R34. doi: 10.1186/bcr3416
- Giske Ursin, Stephanie London, Frank Z. Stanczyk, Elisabet Gentzschein, Annlia Paganini-Hill, Ronald K. Ross, Malcolm C. Pike, Urinary 2-Hydroxyestrone/16α-Hydroxyestrone Ratio and Risk of Breast Cancer in Postmenopausal Women, JNCI: Journal of the National Cancer Institute, Volume 91, Issue 12, 16 June 1999, Pages 1067–1072, https://doi.org/10.1093/jnci/91.12.1067
- Thomson, C. A., Chow, H., Wertheim, B. C., Roe, D. J., Stopeck, A., Maskarinec, G., Altbach, M., Chalasani, P., Huang, C., Strom, M. B., Galons, J. P., & Thompson, P. A. (2017). A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast cancer research and treatment, 165(1), 97–107. https://doi.org/10.1007/s10549-017-4292-7
- The Endogenous Hormones and Breast Cancer Collaborative Group, Endogenous Sex Hormones and Breast Cancer in Postmenopausal Women: Reanalysis of Nine Prospective Studies, JNCI: Journal of the National Cancer Institute, Volume 94, Issue 8, 17 April 2002, Pages 606–616, https://doi.org/10.1093/jnci/94.8.606
- Duggan C, Stanczyk F, Campbell K, Neuhouser ML, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard R, McTiernan A. Associations of sex steroid hormones with mortality in women with breast cancer. Breast Cancer Res Treat. 2016 Feb;155(3):559-67. doi: 10.1007/s10549-016-3704-4
- He XY, Liao YD, Yu S, Zhang Y, Wang R. Sex hormone binding globulin and risk of breast cancer in postmenopausal women: a meta-analysis of prospective studies. Horm Metab Res. 2015 Jun;47(7):485-90. doi: 10.1055/s-0034-1395606
- Kahn SM, Li YH, Hryb DJ, Nakhla AM, Romas NA, Cheong J, Rosner W. Sex hormone-binding globulin influences gene expression of LNCaP and MCF-7 cells in response to androgen and estrogen treatment. AdvExp Med Biol. 2008;617:557–564. doi: 10.1007/978-0-387-69080-3_57
- Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011 May;89(5):718-25. doi: 10.1038/clpt.2011.32
- Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S, Gulisano M, Johansson H, Galimberti V, Cassano E, Moroni SM, Formelli F, Lien EA, Pelosi G, Johnson KA, Bonanni B. Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009 Aug 10;27(23):3749-56. doi: 10.1200/JCO.2008.19.3797
- Johansson H, Bonanni B, Gandini S, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, MacisD, Puccio A, Sandri MT, Gulisano M, Formelli F, Decensi A. Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose tamoxifen and fenretinide. Breast Cancer Res Treat. 2013;142(3):569–578. doi: 10.1007/s10549-013-2768-7
- Chen Y-C, Lin-Shiau S-Y, Lin J-K. Involvement of p53 and HSP70 proteins in attenuation of UVC-induced apoptosis by thermal stress in hepatocellular carcinoma cells. Photochemistry and Photobiology. 1999;70(1):78–86.
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Reviews, Immunology Cancer Cell. 2005;5(10):749–759.
- Banerjee S, Wang Z, Kong D, Sarkar FH. 3,3′-diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Research. 2009;69(13):5592–5600.
- Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by Akt and NF-kappaB pathways in prostate cancer cells. Frontiers in Bioscience. 2005;10(1):236–243.
- Rahman KMW, Banerjee S, Ali S, et al. 3,3′-diindolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Research. 2009;69(10):4468–4475.
- Lian JP, Word B, Taylor S, Hammons GJ, Lyn-Cook BD. Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention effects of indole-3-carbinol (I3C) and genistein. Anticancer Research. 2004;24(1):133–137.
- Takada Y, Andreeff M, Aggarwal BB. Indole-3-carbinol suppresses NF-κB and IκBα kinase activation, causing inhibition of expression of NF-κB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005;106(2):641–649.
- Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death and Differentiation. 2008;15(1):171–182.
- Arnao MB, Sanchez-Bravo J, Acosta M. Indole-3-carbinol as a scavenger of free radicals. Biochemistry and Molecular Biology International. 1996;39(6):1125–1134.
- Sun S, Han J, Ralph WM, et al. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro . Cell Stress and Chaperones. 2004;9:76–87.
- Benabadji SH, Wen R, Zheng J-B, Dong X-C, Yuan S-G. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacologica Sinica. 2004;25(5):666–671.
- Garcia HH, Brar GA, Nguyen DHH, Bjeldanes LF, Firestone GL. Indole-3-carbinol (I3C) inhibits cyclin-dependent kinase-2 function in human breast cancer cells by regulating the size distribution, associated cyclin E forms, and subcellular localization of the CDK2 protein complex. The Journal of Biological Chemistry. 2005;280(10):8756–8764.
- Safe S, Papineni S, Chintharlapalli S. Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Letters. 2008;269(2):326–338.
- Chen C-Y, Hsu Y-L, Tsai Y-C, Kuo P-L. Kotomolide A arrests cell cycle progression and induces apoptosis through the induction of ATM/p53 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Food and Chemical Toxicology. 2008;46(7):2476–2484.
- Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. The Journal of Nutrition. 2003;133(7):2448S–2455S.
- Risau W, Flamme I. Vasculogenesis. Annual Review of Cell and Developmental Biology. 1995;11:73–91.
- Kunimasa K, Kobayashi T, Kaji K, Ohta T. Antiangiogenic effects of indole-3-carbinol and 3,3′-diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube-forming HUVEC. The Journal of Nutrition. 2010;140(1):1–6.
- Cho HJ, Seon MR, Lee YM, et al. 3,3′-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. The Journal of Nutrition. 2008;138(1):17–23.
- Karin M. Inflammation-activated protein kinases as targets for drug development. Proceedings of the American Thoracic Society. 2005;2(4):386–390.
- Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. Journal of the National Cancer Institute. 1975;54(4):985–988.
- McDanell R, McLean AEM, Hanley AB. Differential induction of mixed-function oxidase (MFO) activity in rat liver and intestine by diets containing processed cabbage: correlation with cabbage levels of glucosinolates and glucosinolate hydrolysis products. Food and Chemical Toxicology. 1987;25(5):363–368.
- Thomson CA, Chow HHS, Wertheim BC, Roe DJ, Stopeck A, Maskarinec G, Altbach M, Chalasani P, Huang C, Strom MB, Galons JP, Thompson PA. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res Treat. 2017 Aug;165(1):97-107. doi: 10.1007/s10549-017-4292-7
- Paltsev, M., Kiselev, V., Drukh, V., Muyzhnek, E., Kuznetsov, I., Andrianova, E., & Baranovskiy, P. (2016). First results of the double-blind randomized placebo-controlled multicenter clinical trial of DIM-based therapy designed as personalized approach to reverse prostatic intraepithelial neoplasia (PIN). The EPMA journal, 7(1), 5. https://doi.org/10.1186/s13167-016-0057-3
- Reed, G. A., Sunega, J. M., Sullivan, D. K., Gray, J. C., Mayo, M. S., Crowell, J. A., & Hurwitz, A. (2008). Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 17(10), 2619–2624. https://doi.org/10.1158/1055-9965.EPI-08-0520
- Castañon A, Tristram A, Mesher D, Powell N, Beer H, Ashman S, Rieck G, Fielder H, Fiander A, Sasieni P. Effect of diindolylmethane supplementation on low-grade cervical cytological abnormalities: double-blind, randomised, controlled trial. Br J Cancer. 2012 Jan 3;106(1):45-52. doi: 10.1038/bjc.2011.496
- Cho HJ, Seon MR, Lee YM, Kim J, Kim JK, Kim SG, et al. 3,3’-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J. Nutr. 2008; 138: 17–23. 10.1093/jn/138.1.17