What is Huperzine A
Huperzine A is a dietary supplement derived from the Chinese club moss plant Huperzia serrata also known as Lycopodium serratum Thunb. Huperzine A is a sesquiterpene alkaloid and acts as a powerful and reversible inhibitor of acetylcholinesterase (AChE) — a type of medication that works by improving the levels of neurotransmitters in the brain 1. Acetylcholinesterase (AChE) specifically hydrolyzes acetylcholine (ACh) into choline and acetic acid to avoid over-stimulation in post-synaptic nerves 2. Huperzine A may increase the levels of acetylcholine (ACh) and other neurotransmitters in the brain and has been studied as a possible treatment for the symptoms of Alzheimer’s disease. Acetylcholine (ACh) is a neurotransmitter found predominantly in the human brain and has an important role in arousal, attention, memory and motivation 3. Alzheimer’s disease patients have lower acetylcholine (ACh) levels due to age related degeneration of their cholinergic system and/or brain injuries 2. The cholinergic hypothesis of Alzheimer’s disease suggests a strategy to treat neurodegeneration is to restore acetylcholine deficiency 3. Therefore, acetylcholinesterase (AChE) inhibitors are served as cognition enhancing agents to treat patients with mild to moderate Alzheimer’s disease, including tacrine (Cognex), donepezil (Aricept), rivastigmine (Exelon) and physostigmine (Mestinon) 2.
Small early studies suggest that Huperzine A might improve memory and protect nerve cells, which could slow the cognitive decline associated with Alzheimer’s disease. Huperzine A might slightly improve memory and cognition for patients with vascular dementia and Alzheimer’s disease; however, the small size of the studies prevents firm conclusions. Very low- to low-quality evidence from up to 7 randomized controlled trials containing 648 participants found clinically meaningful improvements in cognition and activities of daily living in people living with mild to moderate Alzheimer’s disease offered Huperzine A versus placebo or no intervention 4. However, a recent systematic review found that the quality of the evidence of Huperzine A’s effectiveness was low 5. Moderate-quality evidence from 1 randomized controlled trial containing 210 participants could not detect clinically meaningful differences in behavioral and psychological symptoms between people living with mild to moderate Alzheimer’s disease offered Huperzine A versus placebo or usual care 4.
Huperzine A has been used in China for centuries for the treatment of contusions, swellings, fever, blood disorders, pain and schizophrenia 6, 7, 1. Huperzine A has been authorized for treating Alzheimer’s disease and benign memory deficits since 1994 in China 8. Memory tests that are used to measure the progression of Alzheimer’s disease are applied in clinical trials to assess the performance of a treatment 9; clinical trials performed in China used these memory tests (e.g. mini-mental state evaluation, memory quotient and Alzheimer’s disease assessment scale-cognitive section) to evaluate the efficacy of Huperzine A in the treatment of patients suffering from age related memory dysfunction or dementia 6. Huperzine A reportedly led to significant cognitive enhancement in these patients and as a result, the China Food and Drug Administration (CFDA) approved huperzine A (0.2 mg; twice daily) to treat the cognitive symptoms of Alzheimer’s disease 9. However, the lack of randomization in these clinical trials and evidence-based literature on the safety and efficacy have prevented Huperzine A medical approval outside of China 10. Huperzine A drug is available as a nutraceutical in the US 11. In the US, “nutraceuticals” are largely unregulated, as they exist in the same category as dietary supplements and food additives by the US Food and Drug Administration (FDA), under the authority of the Federal Food, Drug, and Cosmetic Act.
Compared with the cholinesterase inhibitors tacrine (Cognex), donepezil (Aricept) and rivastigmine (Exelon), Huperzine A has better penetration through the blood-brain barrier (BBB), higher oral bioavailability, and longer duration of AChE inhibitory action 12. A recent small randomized controlled trial performed in China directly compared Donepezil (N = 21) and Huperzine A (N = 21) in people with vascular dementia 13. It concluded that both drugs were equally efficacious and well-tolerated. Recent data have shown that Huperzine A may have neuroprotective effects that go beyond the inhibition of acetylcholinesterase (AChE). These effects include modification of beta-amyloid peptide processing, reduction of oxidative stress, neuronal protection against apoptosis, and regulation of the expression and secretion of nerve growth factor and nerve growth factor signalling 14.
Up until now, there’s a lack of long-term safety data on Huperzine A with most clinical studies having lasted three months or less and many participants in the clinical trials had side effects, including nausea and vomiting. More studies are needed to determine possible benefits and long-term risks of huperzine A. Huperzine A appears safe for short-term use, but evidence for long-term safety is lacking. Consult your doctor before starting any dietary supplement, including Huperzine A. The Alzheimer’s Association recommends not taking Huperzine A, especially if you’re taking a prescribed cholinesterase inhibitor, such as donepezil (Aricept), rivastigmine (Exelon) or galantamine (Razadyne). Taking both could increase your risk of serious side effects.
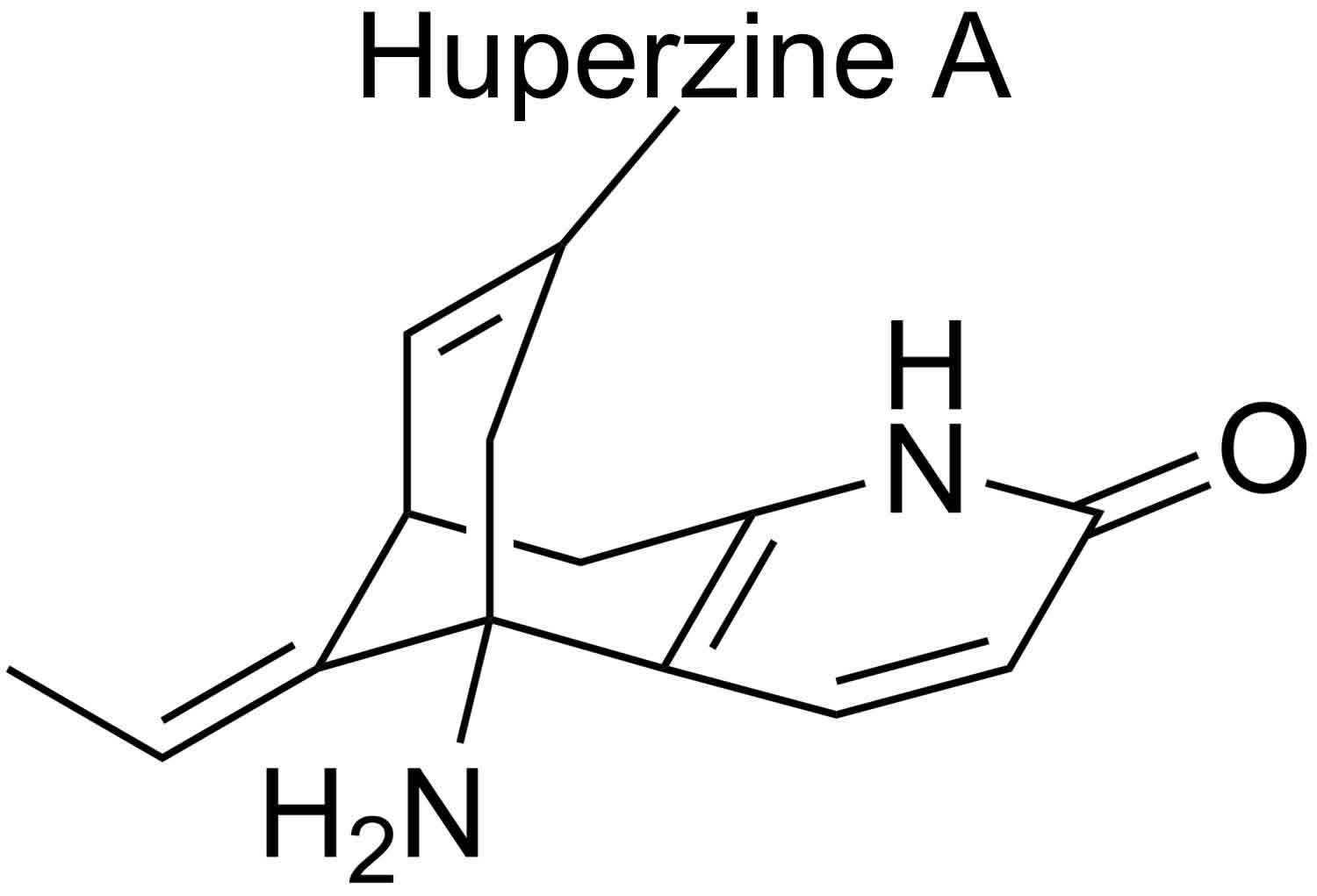
Figure 1. Huperzine A
Footnote: Huperzine A is an alkaloid comprising an unusual bicyclo[3.3.1] ring system fused with an ethylidene group and a 2-pyridone moiety.
[Source 15 ]Huperzine A benefits
The cholinesterases are a family of enzymes present in the central nervous system (brain and spinal cord) that break down choline-based esters 2. Two types of cholinesterases have been characterized; acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) 2. Acetylcholinesterase (AChE) specifically hydrolyzes acetylcholine (ACh) into choline and acetic acid to avoid over-stimulation in post-synaptic nerves; butyrylcholinesterase (BuChE) (also known as pseudocholinesterase) is a nonspecific cholinesterase that breaks down different choline-based esters 2. Acetylcholine (ACh) is a neurotransmitter found predominantly in the human brain and has an important role in arousal, attention, memory and motivation 3. Alzheimer’s disease patients have lower acetylcholine (ACh) levels due to age related degeneration of their cholinergic system and/or brain injuries 2. The cholinergic hypothesis of Alzheimer’s disease suggests a strategy to treat neurodegeneration is to restore acetylcholine deficiency 3. Therefore, acetylcholinesterase (AChE) inhibitors are served as cognition enhancing agents to treat patients with mild to moderate Alzheimer’s disease, including tacrine (Cognex), donepezil (Aricept), rivastigmine (Exelon) and physostigmine (Mestinon) 2.
The inhibitory activity of Huperzine A has been examined against both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) in vitro (test tubes) 16. The inhibitory activity on acetylcholinesterase (AChE) induced by huperzine A (IC50 = 0.082 µM) was slightly more potent than tacrine (a nonselective cholinesterase inhibitor; IC50 = 0.093 µM), but ~8-fold weaker than the drug donepezil (a selective acetylcholinesterase (AChE) inhibitor; IC50 = 0.010 µM) 16. The inhibitory activity on butyrylcholinesterase (BuChE) induced by huperzine A (IC50 = 74.43 µM) was ~15 and ~1000-fold less potent than donepezil (IC50 = 5.01 µM) and tacrine (IC50 = 0.074 µM) respectively 16. These findings indicated that huperzine A displays high selectivity for acetylcholinesterase (AChE) over butyrylcholinesterase (BuChE) 6. Oral administration of huperzine A to rats led to significant inhibition of acetylcholinesterase (AChE) (16% inhibition at 1 µmol/kg), ~15 and 140-fold more potent than donepezil (9% inhibition at 8 µmol/kg) and tacrine (7% inhibition at 60 µmol/kg), respectively 16. However, upon intracerebroventricular (ICV) injection, the anti-acetylcholinesterase (AChE) activity of huperzine A (21% inhibition at 0.066 µmol/kg) was ~3-fold less potent than donepezil (35% inhibition at 0.038 µmol/kg) but ~2-fold stronger than tacrine (11% inhibition at 0.068 µmol/kg), a pattern similar to the in vitro results 16. The different administration routes clearly affect the bioavailability of huperzine A, with the intracerebroventricular route facilitating access to the brain 6. In a subsequent in vivo (animal) experiment, the level of acetylcholine (ACh) in the whole rat brain upon huperzine A administration was also measured 16. Huperzine A displayed the most prolonged increase in acetylcholine level when compared with donepezil and tacrine, lasting for at least 6 hours after administration 16. Moreover, the activity of choline acetyltransferase and the level of choline did not change, indicating that the increase of acetylcholine (ACh) level was not a result of an increase in acetylcholine (ACh) synthesis 17. A clear inverse relationship was observed between acetylcholinesterase (AChE) activity and acetylcholine level, further confirming that the increase was mediated through the acetylcholinesterase (AChE) inhibition induced by huperzine A 16. Given that the lack of acetylcholine (ACh) in the brain is a common symptom in Alzheimer’s disease patients, the high binding specificity to acetylcholinesterase (AChE) of huperzine A suggests therapeutic potential 6.
The effect of huperzine A on glutamate-induced neuron toxicity was investigated by Ved and co-workers 18. Neurons derived from rat embryonic forebrain were treated for 45 min with either 100 µM glutamate, 100 nM huperzine A or a mixture of 100 nM huperzine A and 100 µM glutamate. Neuronal cell death caused by glutamate-induced toxicity was found to be ~55% upon treatment with glutamate alone, which was ~50% greater than the group treated with huperzine A alone. Treatment with both huperzine A and glutamate resulted in a ~30% neuron death, suggesting that huperzine A had partially suppressed the glutamate-induced toxicity in neurons 6. In the same study, the effect of huperzine A on calcium mobilization was also investigated 18. Neurons derived from embryonic forebrain were exposed to 10 µM of either glutamate or Bay-K8644 (a potent calcium channel agonist), followed by huperzine A (100 nM) and the level of evation was measured. Upon huperzine A treatment, the glutamate-induced calcium mobilization was reduced from 811 to 668 nM, but a minimal effect was observed in the neurons that were exposed to Bay-K8644 (calcium elevation: 505 nM vs 521 nM).116 These results inferred that huperzine A acts on glutamate receptors to exert neuroprotective properties on glutamate-induced toxicity 6.
Studies examining the effect of huperzine A on the N-methyl-D-aspartate (NMDA) receptor, an ionotropic glutamate receptor that controls synaptic plasticity and memory function, have also been performed 19. Huperzine A (100 µM) did not inhibit the binding of [3H]-glutamate (an agonist that binds to the N-methyl-D-aspartate (NMDA) agonist site), [3H]-selfotel (an antagonist that binds to the N-methyl-D-aspartate (NMDA) agonist site), [3H]-dichlorokynurenic acid (an antagonist that binds to the N-methyl-D-aspartate (NMDA) glycine regulatory site) or [3H]-ifenprodil (an antagonist that binds to the N-methyl-D-aspartate (NMDA) polyamine regulatory site) 19; however, both [3H]-dizocilpine and [3H]-thienylcyclohexylpiperidine (non-competitive antagonists that bind to the N-methyl-D-aspartate (NMDA) ion channel) were displaced by huperzine A at Ki = 5.6 and 9.5 µM respectively, indicating that huperzine A interacts with N-methyl-D-aspartate (NMDA) receptor by binding to the ion channel via a non-competitive inhibition mechanism 19. The N-methyl-D-aspartate (NMDA)-induced neuronal toxicity was prevented as the survival rate of cell culture increased from 35% to 85% upon pre-treatment with huperzine A.117 Thus, huperzine A is a potent non-competitive N-methyl-D-aspartate (NMDA) ion channel antagonist and exerts neuroprotective properties by blocking NMDA-induced toxicity in neuronal cells 19.
The effect of huperzine A on cognitive enhancement has been examined in several animal models (e.g. rats, chicks and monkeys) 6. Tang and co-workers 20 demonstrated that both working and reference memory deficits induced by scopolamine (a mAChR antagonist commonly used to induce cognitive deficits) were significantly improved upon huperzine A administration. The improvements were more pronounced on working memory than on reference memory 20. Upon oral administration to aged rats, huperzine A was ~3.5 and 9-fold more potent at ameliorating memory impairments than donepezil and tacrine respectively, in agreement with the results reported by Tang and co-workers 16. In a subsequent study, the cognitive enhancement induced by huperzine A was investigated on memory deficits induced by both scopolamine and GABA in chicks 21. The results revealed that huperzine A was able to reverse the memory disruptions caused by scopolamine and GABA, suggesting huperzine A improved memory formation processes through both acetylcholinesterase (AChE) inhibition and GABA receptor antagonism 17. Cai and co-workers 22 also established that huperzine A (0.01 mg/kg; intramuscularly) significantly improved the spatial working memory impairments induced by catecholamine depleting agent reserpine (0.1 mg/kg; intramuscular) in monkeys. The results revealed an inverted U-shaped dose–response pattern, inferring that huperzine A putatively improves the working memory deficits through an adrenergic mechanism 22.
A large number of preclinical studies and clinical trials had shown the potential effect of Huperzine A in treating acetylcholine‐deficit dementia including Alzheimer’s disease and protect the brain through multiple pathways 23, but it is not yet clear whether these benefits translate to humans. No studies have tested whether Huperzine A can prevent dementia or cognitive decline. Huperzine A supplements are often promoted as nootropic (i.e., promoting cognitive function) but a quasi-randomized double-blind study of 84 healthy individuals in the military reported that huperzine A did not improve cognitive function 24. Another randomized double-blind clinical trial in China reported that 50 microgram (ug) of Huperzine A twice per day for four weeks improved memory in junior high students with subjective memory problems 25. However, the trial was small, and it is not clear whether the effect was large enough to be noticed in daily life 25.
Dementia
Many small clinical trials have tested Huperzine A as a treatment for Alzheimer’s disease. These were reviewed in four meta-analyses 26, 27, 28, 29. Another two trials tested Huperzine A for vascular dementia 30, 31. While most trials saw clinically meaningful improvements, the trials were small, short, and at a high risk of bias that casts some doubt on the conclusions 32. Because huperzine A is thought to work in a similar manner to most Alzheimer’s drugs, including Aricept (donepezil), it is unclear whether it would have added benefit on patients who already take these drugs. Indeed, the addition of huperzine A may raise the risk of side effects.
Huperzine A dosage
Huperzine A is available as a dietary supplement and is often marketed as a nootropic, a substance that may enhance memory. A variety of manufacturers offer Huperzine A oral supplements in doses of 0.05 to 0.2 mg. Clinical trials in dementia patients (Alzheimer disease) have used Huperzine A doses between 0.2 to 0.4 mg/day 33. A Phase 2 clinical trial reported that 0.4 mg twice per day, but not 0.2 mg twice per day, showed some benefit in patients with Alzheimer’s disease 34. Huperzine A is also found in several multi-supplement formulations.
Huperzine A side effects
Serious side effects have not been reported in the small clinical trials completed for huperzine A. To date, few side effects have been observed in Huperzine A clinical trials conducted in China and those side effects most frequently observed (headaches, mild dizziness, blurred vision, abdominal distension, and nausea) were transient and were either resolved by stopping the medication or improved with symptomatic treatment 35. Cholinergic adverse reactions have been noted, including hyperactivity, nasal obstruction, nausea, vomiting, diarrhea, insomnia, anxiety, dizziness, thirst, and constipation. One trial reported abnormalities in electrocardiogram (ECG) patterns (cardiac ischemia and arrhythmia). Since all trials lasted 36 weeks or less, long-term safety is unknown. One study did find that huperzine A might lower heart rate and worsen some epilepsy symptoms 36.
Drug interactions
Although the Drugs Interactions Checker lists no harmful interactions between Huperzine A and prescription medications, not every medication has been evaluated. Since huperzine A and common Alzheimer’s disease drugs such as Aricept behave similarly, it may exacerbate the side effects of these drugs. It may also reduce the effectiveness of other drugs with anti-cholinergic effects such as some anti-histamines and anti-depressants.
Toxicity
The Lethal dose 50 (LD50) of huperzine A lies between 2 and 4 mg/kg in female rats and >4 mg/kg in male rats 9. LD50 (Lethal dose 50) is the amount of an ingested substance that kills 50 percent of a test sample. It is expressed in mg/kg or milligrams of substance per kilogram of body weight. Huperzine A is less toxic and induces less adverse effects than classic acetylcholinesterase (AChE) inhibitors that are currently used to treat Alzheimer’s disease (e.g. donepezil and tacrine) 17.
Symptoms of acute Huperzine A toxicity are similar to those of other cholinergic inhibitors and include muscular tremor, drooling, tears, increased bronchial secretions, and incontinence. No mutagenicity or teratogenicity were found in rodent studies.
References- Zangara A. The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer’s disease. Pharmacol Biochem Behav. 2003 Jun;75(3):675-86. doi: 10.1016/s0091-3057(03)00111-4
- Cummings JL. Cholinesterase inhibitors: A new class of psychotropic compounds. Am J Psychiatry. 2000 Jan;157(1):4-15. doi: 10.1176/ajp.157.1.4
- Lamy P.P. The role of cholinesterase inhibitors in Alzheimer’s disease. CNS Drugs. 1994;1(2):146–165.
- National Institute for Health and Care Excellence (UK). Dementia: Assessment, management and support for people living with dementia and their carers. London: National Institute for Health and Care Excellence (UK); 2018 Jun. (NICE Guideline, No. 97.) 13, Non-pharmacological interventions for people living with dementia. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536485
- Laver K, Dyer S, Whitehead C, Clemson L, Crotty M. Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews. BMJ Open. 2016 Apr 27;6(4):e010767. doi: 10.1136/bmjopen-2015-010767. Erratum in: BMJ Open. 2017 Jun 7;7(5):e010767corr1
- Yang H., Ma Y., Wang X., Zhu D. Huperzine A: A mini-review of biological characteristics, natural sources, synthetic origins, and future prospects. Russ J Org Chem. 2020;56(1):148–157.
- Ha G.T., Wong R.K., Zhang Y. Huperzine A as potential treatment of Alzheimer’s disease: An assessment on chemistry, pharmacology, and clinical studies. Chem Biodivers. 2011;8(7):1189–1204.
- Han Yan, et al. Review on clinical application of Huperzine A in China. New Drugs Clin Rem. 2006;25(9):682–8.
- Ha GT, Wong RK, Zhang Y. Huperzine a as potential treatment of Alzheimer’s disease: an assessment on chemistry, pharmacology, and clinical studies. Chem Biodivers. 2011 Jul;8(7):1189-204. doi: 10.1002/cbdv.201000269
- Bai D. Development of huperzine A and B for treatment of Alzheimer’s disease. Pure Appl Chem. 2007;79(4):469–479.
- Little JT, Walsh S, Aisen PS. An update on huperzine A as a treatment for Alzheimer’s disease. Expert Opin Investig Drugs. 2008 Feb;17(2):209-15. doi: 10.1517/13543784.17.2.209
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006 Jan;27(1):1-26. doi: 10.1111/j.1745-7254.2006.00255.x
- Wang WL, Jiang SF, Xu WM, Sheng JQ, Gong Q, Gao J, et al. Research on Donepezil and Huperzine A’s curative effect on vascular dementia. Hebei Medicine. 2010;16(10):1163–6.
- Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci. 2006 Dec;27(12):619-25. doi: 10.1016/j.tips.2006.10.004
- Liu J.-S., Zhu Y.-L., Yu C.-M. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem. 1986;64(4):837–839.
- Tang X., De Sarno P., Sugaya K., Giacobini E. Effect of huperzine A, a new cholinesterase inhibitor, on the central cholinergic system of the Rat. J Neurosci Res. 1989;24(2):276–285.
- Zhu D.-Y., Tan C.-H., Li Y.-M. Wiley; 2006. Medicinal chemistry of bioactive natural products; pp. 143–182. Chapter 4-The Overview of Studies on Huperzine A: A Natural Drug for the Treatment of Alzheimer’s Disease.
- Ved H.S., Koenig M.L., Dave J.R., Doctor B.P. Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. NeuroReport. 1997;8(4):963–967.
- Gordon RK, Nigam SV, Weitz JA, Dave JR, Doctor BP, Ved HS. The NMDA receptor ion channel: a site for binding of Huperzine A. J Appl Toxicol. 2001 Dec;21 Suppl 1:S47-51. doi: 10.1002/jat.805
- Wang T., Tang X.C. Reversal of scopolamine-induced deficits in radial maze performance by (−)-Huperzine A: comparison with E2020 and tacrine. Eur J Pharmacol. 1998;349(2–3):137–142.
- Gao Y., Tang X., Guan L., Kuang P. Huperzine A reverses scopolamine-and muscimol-induced memory deficits in chick. Acta Pharmacol Sin. 2000;21(12):1169–1173.
- Ou L.Y., Tang X.C., Cai J.X. Effect of Huperzine A on working memory in reserpine-or yohimbine-treated monkeys. Eur J Pharmacol. 2001;433(2–3):151–156.
- Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Pérez B, Clos MV, Badia A. Huprine X and huperzine A improve cognition and regulate some neurochemical processes related with Alzheimer’s disease in triple transgenic mice (3xTg-AD). Neurodegener Dis. 2013;11(3):129-40. doi: 10.1159/000336427
- Morasch KC, Aaron CL, Moon JE, Gordon RK. Physiological and neurobehavioral effects of cholinesterase inhibition in healthy adults. Physiol Behav. 2015 Jan;138:165-72. doi: 10.1016/j.physbeh.2014.09.010
- Sun QQ, Xu SS, Pan JL, Guo HM, Cao WQ. Huperzine-A capsules enhance memory and learning performance in 34 pairs of matched adolescent students. Zhongguo Yao Li Xue Bao. 1999 Jul;20(7):601-3.
- Li J, Wu HM, Zhou RL, Liu GJ, Dong BR. Huperzine A for Alzheimer’s disease. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD005592. https://doi.org/10.1002/14651858.CD005592.pub2
- Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer’s disease: an updated meta-analysis. J Neural Transm (Vienna). 2009 Apr;116(4):457-65. doi: 10.1007/s00702-009-0189-x
- Xing, S. H., Zhu, C. X., Zhang, R., & An, L. (2014). Huperzine a in the treatment of Alzheimer’s disease and vascular dementia: a meta-analysis. Evidence-based complementary and alternative medicine : eCAM, 2014, 363985. https://doi.org/10.1155/2014/363985
- Yang G, Wang Y, Tian J, Liu JP. Huperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013 Sep 23;8(9):e74916. doi: 10.1371/journal.pone.0074916
- Xu ZQ, Liang XM, Juan-Wu, Zhang YF, Zhu CX, Jiang XJ. Treatment with Huperzine A improves cognition in vascular dementia patients. Cell Biochem Biophys. 2012 Jan;62(1):55-8. doi: 10.1007/s12013-011-9258-5
- Hao, Z., Liu, M., Liu, Z., & Lv, D. (2009). Huperzine A for vascular dementia. The Cochrane database of systematic reviews, (2), CD007365. https://doi.org/10.1002/14651858.CD007365.pub2
- Yang, G., Wang, Y., Tian, J., & Liu, J. P. (2013). Huperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. PloS one, 8(9), e74916. https://doi.org/10.1371/journal.pone.0074916
- Li J, Wu HM, Zhou RL, Liu GJ, Dong BR. Huperzine A for Alzheimer’s disease. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD005592. https://doi.org/10.1002/14651858.CD005592.pub2
- Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012 Mar;2(3):a006395. doi: 10.1101/cshperspect.a006395
- Zhong ZG, Liang KZ. Clinical observation of huperzine A in the treatment of nineteen patients with vascular dementia. Journal of Hainan Medical College. 2004;10(4):251–2.
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 2015 Mar;111:85-141. doi: 10.1016/j.eplepsyres.2015.01.001