What is Pterostilbene
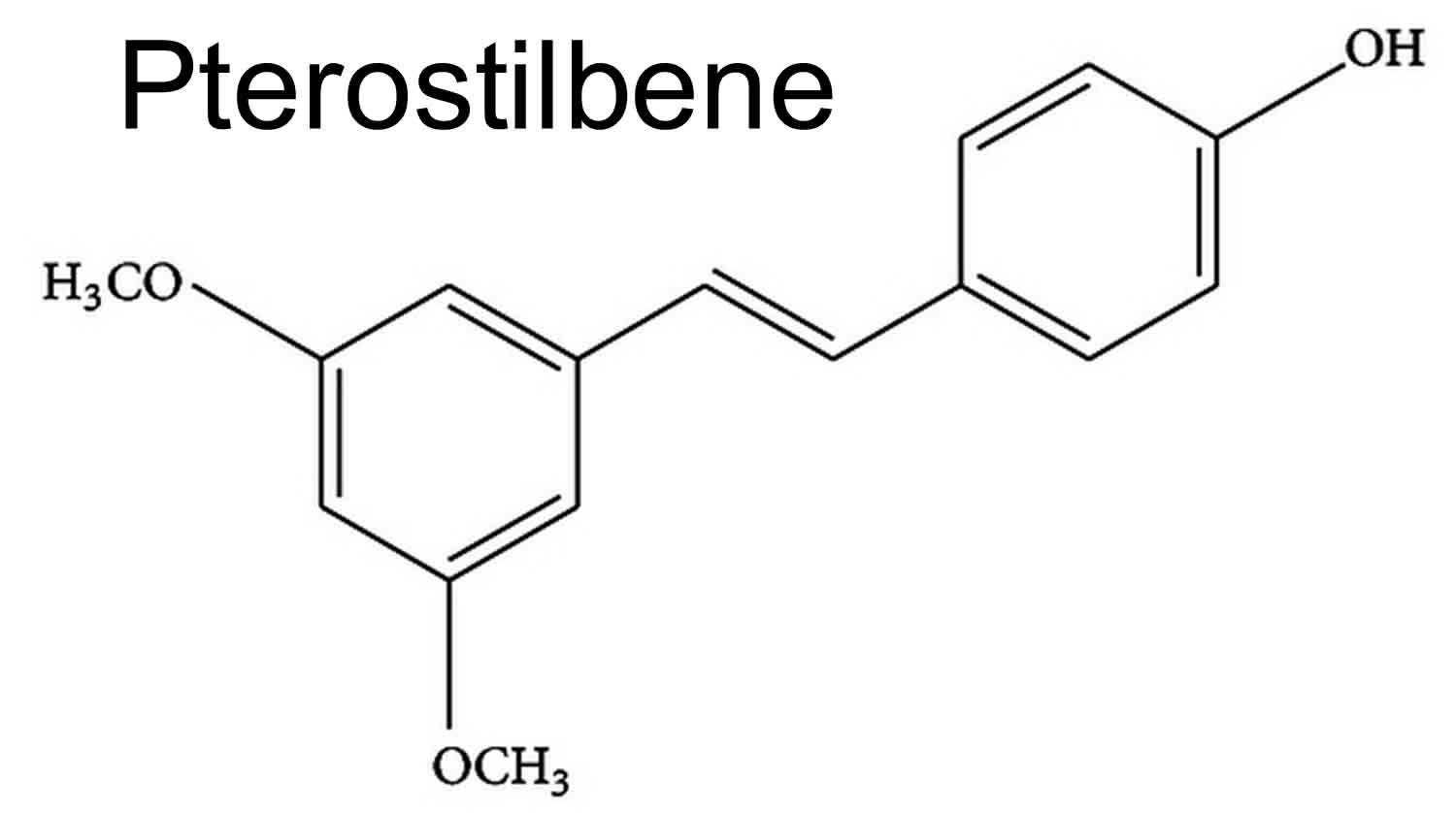
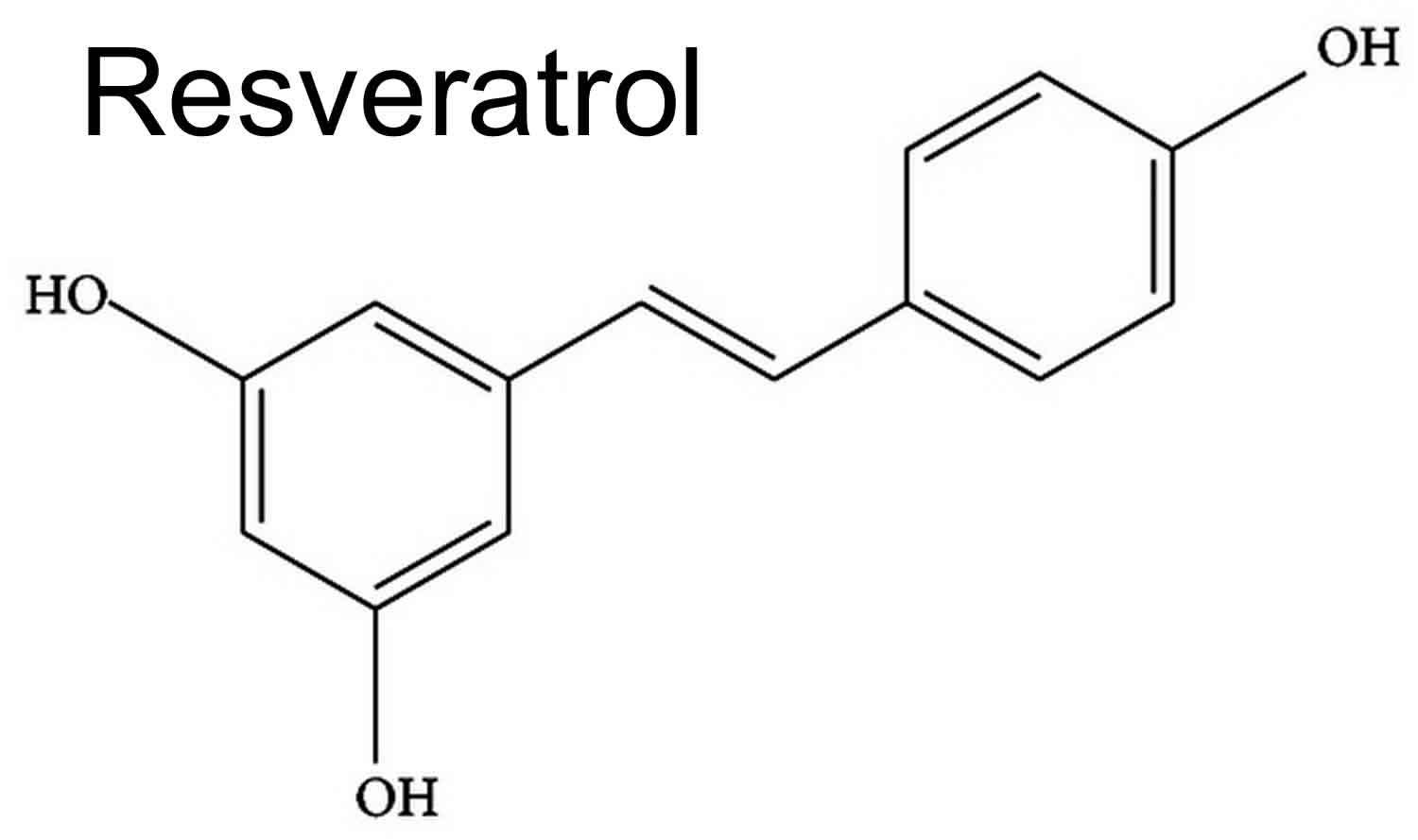
Pterostilbene also known as trans-3,5-dimethoxy-4-hydroxystilbene and is biologically classified as a phytoalexin, is a naturally occurring anti-fungal agent naturally created by plants like blueberries, grapes and heartwood of red sandalwood 1. Pterostilbene is involved (as are other natural polyphenols) in plant defense against different stressful conditions, i.e., UV radiation, aggression by pathogens, low soil fertility, high/low temperatures, severe drought, or grazing pressure 2. Pterostilbene is a natural 3,5-dimethoxy analog of resveratrol that has antioxidant and antifungal activities, but pterostilbene is a much stronger antifungal agent (>10 times) than resveratrol 3. The antioxidant activity of pterostilbene has been implicated in cancer chemoprophylaxis, modulation of neurological disease, anti-inflammation, attenuation of vascular disease, and amelioration of diabetes 4. Similar to other stilbene compounds, pterostilbene has two more methoxy groups on the A-benzene ring than Resveratrol, indicating its higher liposolubility that may lead to the increased permeability of the cell membrane, which increases oral absorption and bioavailability (see Figures 1 and 2) 5. In many cases, pterostilbene showed significantly higher bioactivity than Resveratrol 6. In animal studies, pterostilbene was shown to have 80% bioavailability compared to 20% for resveratrol making it potentially advantageous as a therapeutic agent 5. Pterostilbene can easily pass through the blood-brain barrier because of its low molecular weight and good liposolubility 7. Due to pterostilbene’s increased lipophilic and oral absorption, it is expected to be more effective than resveratrol but its effects in humans are largely unknown and it was reported to increase LDL (low-density lipoprotein or “bad” cholesterol). While more bioavailable than resveratrol, very little is known about pterostilbene’s safety or efficacy or whether it could either help or harm. Pterostilbene is also a major phenolic compound of Darakchaava, an herbal preparation used in traditional Indian Ayuvedic medicine. There is very little clinical data exist on pterostilbene used as a supplement.
Some of the concerns around resveratrol also apply to pterostilbene. The effects are difficult to predict because the compounds and their metabolites may affect many biological pathways 8 yet those pathways can be difficult to identify because the compounds are pan-assay interference compounds, leading to many false-positive hits in vitro assays 9.
Substantial evidence suggests that pterostilbene may have numerous preventive and therapeutic properties in a vast range of human diseases that include neurological, cardiovascular, metabolic, and hematologic disorders. Further benefits of pterostilbene have been reported in preclinical trials, in which pterostilbene was shown to be a potent anticancer agent in several cancers 10. Pterostilbene is characterized by low molecular weight (Figure 1) and good liposolubility, allowing it to easily cross the blood-brain barrier 11.
A resveratrol O-methyltransferase, which catalyzes the synthesis of pterostilbene from resveratrol, was identified in grapevine leaves where it is induced by different types of stress 12. The amount of daily pterostilbene consumption varies according to dietary fruit intake and it has been estimated that pterostilbene content per blueberry varies from 99 ng to 520 ng/gram depending on the type of berry ingested (approx. 10–15 mg/kg of fresh weight) 13. Experiments showed that pterostilbene can be rapidly absorbed and is widely distributed in the body. Pterostilbene has suitable metabolic stability and bioavailability 14, indicating its suitable pharmacokinetic characteristics. In addition, most available data in human and animal models show that pterostilbene has no significant toxic effects 15. Therefore, pterostilbene is a potential natural small-molecular medicine with a good development prospect.
In addition to being extracted from natural products, pterostilbene can also be synthesized by biological and chemical methods 7. Shi et al. 16 studied a new process. The yield of the process was approximately 78%, the reaction conditions were mild, the market supply of raw materials was sufficient, and no special low temperature or pressure device was used. The whole process did not need column separation or distillation, and the product was purified only by recrystallization, which was suitable for industrial production.
Figure 1. Pterostilbene
Figure 2. Resveratrol
Pterostilbene benefits
Interest in pterostilbene has been renewed in recent years, and studies have found that pterostilbene possesses an array of pharmacological properties, including neuroprotective 17, chemopreventive, anti-inflammatory 18, antidiabetic 19, lipid-lowering 20 and antiatherosclerotic 1 effects. The multiple benefits of pterostilbene in the treatment and prevention of human disease have been attributed to its anti-oxidant 21, anti-inflammatory 18 and anti-cancer 22 properties leading to improved function of normal cells and inhibition of malignant cells 23. Only two clinical trials have reported relevant results for Pterostilbene. In hypercholesterolemic patients, it was reported to have both benefits and harm on cardiovascular risk factors. Specifically, it increased LDL (low-density lipoprotein or “bad” cholesterol), but decreased blood pressure 24. In another short-term cross-over trial, a combination of 5 supplements decreased some blood biomarkers of cardiovascular disease and oxidative stress in the elderly 25.
Pterostilbene and cancer
Pterostilbene has garnered increasing interest from cancer researchers over the last decade as a natural molecule with anticancer properties that has been shown to be safe for human use. Published reports that have examined the anticancer effects of Pterostilbene under in vitro (test tube) (Table 1) and in vivo (animal) (Table 2) conditions. In vitro, Pterostilbene inhibited the proliferation of a variety of tumor cells, including stomach, lung, liver, oral cavity, pancreas, lymph, colon, prostate, breast, melanoma, leukemia, and myeloma tumor cells. In vivo, pterostilbene inhibited tumor occurrence and metastasis and showed almost no toxicity 26. Manlio et al. 27 found that pterostilbene had a stronger ability to induce the apoptosis of chronic myeloid leukemia cells than resveratrol. Resveratrol has almost no effect on inducing apoptosis of drug-resistant cancer cell lines, while pterostilbene can induce apoptosis of drug-resistant cancer cell lines, and there was no significant difference between the AC50 of drug-resistant cancer cell lines and the AC50 of sensitive cancer cell lines, indicating that pterostilbene has a strong apoptosis-inducing effect on drug-resistant cancer cell lines. Pterostilbene was less toxic on normal hemopoietic stem cells than on leukemia cells 27. Cultured human glioblastoma cell lines U87MG and GBM8401, human promyelocytic acute leukemia cell line HL-60, gastric cancer AGS cells, colon cancer COLO205 cells, colorectal cancer HT-29 cells, and hepatocellular carcinoma HepG2 cells were reduced in a time- and concentration-dependent manner, with IC50 values of 1.42, 2.99, 46.7, 50.7, 71.2, and 82.8 μM respectively 28.
Sun et al. 29 determined for the first time that pinostilbene was the main pterostilbene metabolite in the colon of pterostilbene-fed mice. The inhibitory effect of pinostilbene on the growth of human colon cancer cells was similar to that of pterostilbene and these effects were related to the regulation of a variety of key proteins involved in cell cycle arrest and apoptosis. The bioactivities of pinostilbene in the colon and its significance in the prevention of colon cancer by oral food containing pterostilbene need to be studied to further clarify the mechanism of pterostilbene’s inhibition of the growth of colon cancer cells.
There are also some novel anti-tumor effects such as autophagy or apoptosis-independent pathways of pterostilbene. Ko et al. 30 studied the effect of pterostilbene on human oral cancer cells and found that pterostilbene can induce autophagy of oral cancer cells by activating JNK1/2, inhibiting Akt, ERK1/2 and p38, which is expected to be a new drug for the treatment of oral cancer. Wang et al. 31 depleted PolyFN suspended Lewis lung cancer cells by silencing endogenous FN expression or pterostilbene, and observed whether pterostilbene could inhibit the metastasis of lung cancer cells. It was found that pterostilbene inhibited PolyFN assembly and lung metastasis on Lewis lung cancer suspension cells regulated by AKT/ERK. Its preventive and therapeutic effects on lung metastasis of lung cancer cells include apoptosis-independent manner 31.
The signal pathways related to pterostilbene’s anti-cancer mechanism are summarized in Table 3.
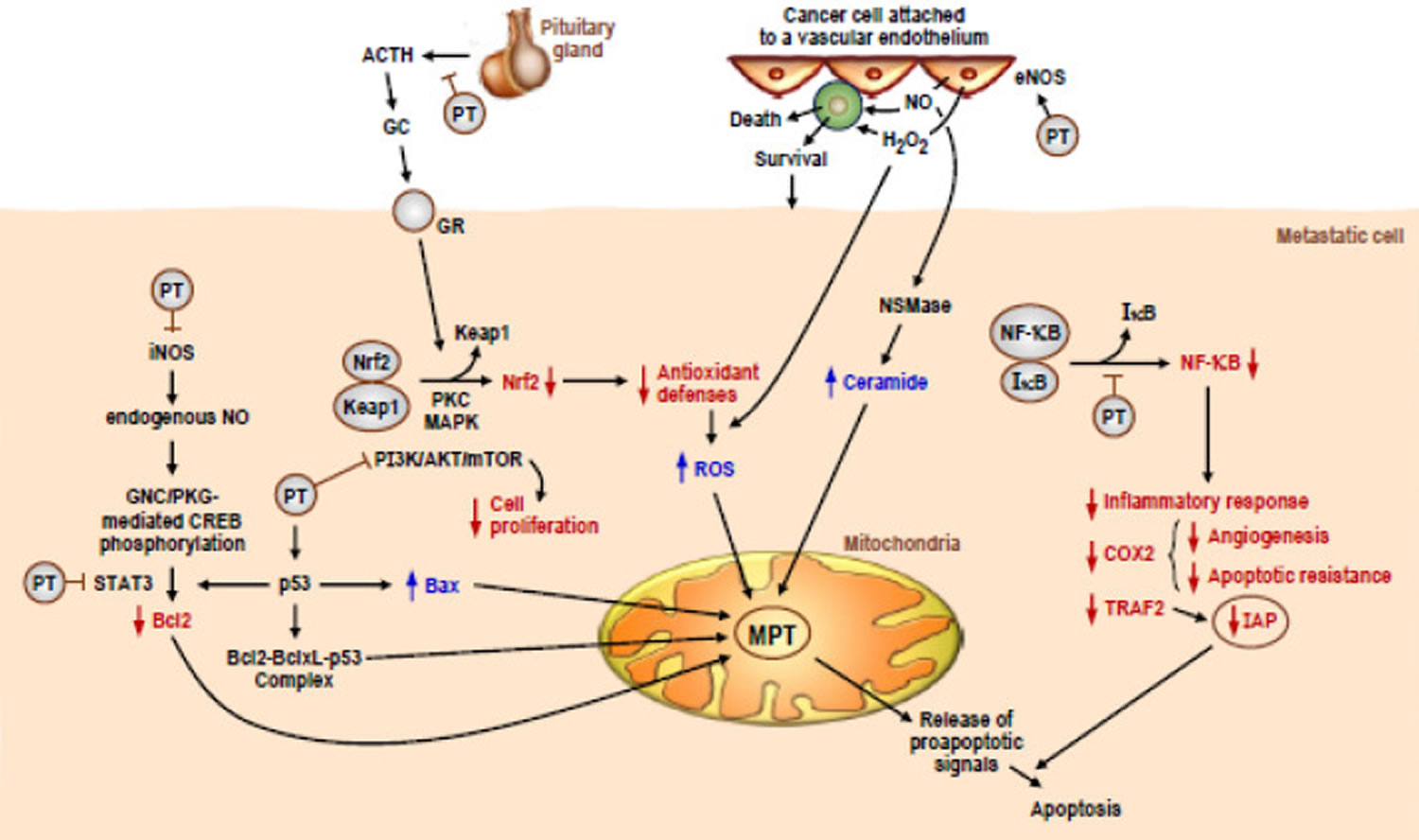
While published accounts of the mechanism of action of Pterostilbene against cancer cells can sometimes look contradictory, it is clear that Pterostilbene possesses several different mechanisms of anticancer action in its repertoire. Figure 3 depicts several distinct anticancer activities of Pterostilbene. Specifically, Pterostilbene has been shown to downregulate inducible nitric oxide synthase (iNOS) in both skin 32 and colon cancer 33 models, which leads to the increased apoptosis of the cancer cells. Along the same downstream pathway, Pterostilbene has been shown to inhibit signal transducer and activator of transcription 3 (STAT3) in lung 34, ovarian 35, pancreatic 36 and endometrial cancer 37. Pterostilbene can also function through inhibiting the AKT/mTOR pathway in pancreatic 38 and breast cancers 39. Furthermore, Pterostilbene can exhibit anticancer activity through the inhibition of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (NF-κB) signaling in colon 33 and skin cancer 32. Finally, Figure 3 shows an anticancer activity of Pterostilbene that only works in vivo, when Pterostilbene indirectly downregulates Nrf2 through the inhibition of glucocorticoid secretion from the pituitary gland, which decreases antioxidant defenses of metastatic melanoma cells and leads to apoptosis 40. The Benlloch et al. 40 paper demonstrates the importance of using in vivo experimental models to examine the anticancer activity of Pterostilbene as Pterostilbene has indirect as well as direct effects on inducing cancer cell death 41.
Pterostilbene stands out (as compared to almost all other natural polyphenols) in its ability to cross the blood–brain barrier, which may confer the potential advantage of being useful in the therapy of tumors of the central nervous system, such as glioblastoma multiforme. Nevertheless, reaching effective concentrations in tumors, under in vivo conditions, and for a sufficient time to exert its antineoplastic actions, is a fundamental unsolved problem 41. A key problem directly related to this is Pterostilbene’s short half-life and low bioavailability under in vivo conditions. In fact, the most common discrepancy between experimental and clinical observations is the use of nonphysiologically relevant concentrations of the stilbene in mechanistic studies. Thus, it remains highly controversial how applicable underlying mechanisms are with bioavailable concentrations and biological half-life. In fact, the assumption that Pterostilbene-induced anticancer effects derive from its direct interaction with the tumor cells frequently ignores the limitations involving in vivo concentrations and time of exposure 41. This reasoning leads to some obvious needs for future developments, i.e., (a) improvement of pharmaceutical formulations and delivery systems to increase bioavailability, (b) Pterostilbene has chemo- and radiosensitizing effects which make it suitable as adjuvant in oncotherapy, (c) its very low systemic toxicity is a clear advantage for its combination with conventional/targeted anticancer treatments, (d) the combination of Pterostilbene with other polyphenols may show synergic/additive effects. However, we still need more studies to show that these technological advances impact on improvements in the in vivo anti-cancer efficacy of the stilbene. Furthermore, advances in experimental models still must be applied to humans. At present, there is only one clinical trial (phase 2) running investigating the effect of the combination of Pterostilbene and megestrol acetate in treating patients with endometrial cancer 42. In this regard, experimental evidences suggest the potential of combining Pterostilbene with, prooxidant and/or anti-inflammatory strategies, anti-Bcl-2 therapies, HSP70 inhibitors, or PI3K/AKT/mTOR inhibitors.
Figure 3. Pterostilbene-induced cancer cell death potential molecular mechanisms involved
Footnotes: The multiple molecular interactions and signaling mechanisms are based on or deduced from data obtained in metastatic melanoma cells under in vivo conditions. Pterostilbene (PT) is encircled, and its interactions (inhibitions or activations are indicated by a T line or an arrow, respectively).
Abbreviations: iNOS = inducible nitric oxide synthase; NO = nitric oxide; GNC = guanylate cyclase; CREB = CAMP responsive element binding protein; STAT3 = signal transducer and activator of transcription 3; Bcl2 = B-cell lymphoma 2; BclxL = B-cell lymphoma-extra large; p53 = tumor protein p53; Bax = Bcl2-assciated X protein; ACTH = adrenocorticotropic hormone; GC = glucocorticoid; GR = glucocorticoid receptor; Nrf2 = nuclear factor erythroid 2-related factor 2; Keap1 = Kelch-like ECH-associated protein 1; PKC = protein kinase C; MAPK = mitogen-activated protein kinase; PI3/AKT/mTOR = phosphoinositide 3 kinase/protein kinase B/mechanistic target of rapamycin; eNOS = endothelial nitric oxide synthase; ROS = reactive oxygen species; NSMase = neutral sphingomyelinase; MPT = mitochondrial permeability transition; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells; IkB = nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; COX2 = cyclooxygenase 2; TRAF2 = TNF receptor-associated factor 2; IAP = inhibitors of apoptosis proteins.
[Source 41 ]Table 1. Pterostilbene and cancer cells under in vitro (test tube) conditions: effects and proposed mechanisms
| Cancer Type | Concentration(s) Analyzed | Time of Incubation (hours) | Anticancer Effect | Proposed Mechanism | Reference |
| Lung | Pterostilbene (10 μM) + Osimertinib (0.02 μM) | 24 | Synergistic anticancer effect against two EGFR-mutation positive non-small cell lung cancer cells | The combination reversed osimertinib-induced STAT3 activation and suppressed src activation | 34 |
| Cervical | Pterostilbene (20 and 40 μM) | 48 | Inhibition of growth and metastatic ability of both adherent and stem-like cancer cells | Induction of reactive oxygen species (ROS)-induced apoptosis and inhibition of MMP 2/9 expression | 43 |
| Pancreatic | Pterostilbene (50 and 75 μM) | 72 | Induced cell cycle arrest and apoptosis in Gemcitabine-resistant cancer cells | Inhibitions of multidrug resistance protein (MDR1) expression via reduction in Akt signaling | 38 |
| Ovarian | Pterostilbene (18.5 to 300 μM) +/− Cisplatin (3.125 to 50 μM) | 48 | Induction of cell cycle arrest and apoptosis against several ovarian cancer cell lines and synergy with cisplatin | Downregulation of JAK/STAT3 pathway | 35 |
| Oral | Pterostilbene (50 and 75 μM) | 24 or 48 | Induction of apoptosis of cisplatin-resistant oral cancer cells | Activation of intrinsic apoptosis cascade and downregulation of MDR1 | 44 |
| Breast | Pterostilbene (2.5 to 10 μM) | 24 | Upregulation of apoptotic pathways in two mutant-p53 cell lines | Induction of pro-apoptotic Bax protein and caspase-3 activity. Decreased mutant p53 protein | 45 |
| Breast | Pterostilbene (10 and 20 μM) + Tamoxifen (5 μM) | 24 | Pterostilbene + Tamoxifen showed an additive inhibitory effect on breast cancer cells | Increased apoptosis | 46 |
| Gastrointestinal | Pterostilbene (10 and 100 μM) | 48 | Pterostilbene showed dose-dependent inhibition of cell proliferation in three gastrointestinal cancer cell lines | Increase in mitochondrial membrane potential, ROS and lipid peroxide | 47 |
| Prostate | Pterostilbene (10 to 100 μM) | 48 | Pterostilbene showed dose-dependent inhibition of cellular proliferation in three prostate cancer cell lines | Activation of AMPK | 48 |
| Pancreatic | Pterostilbene (10 to 100 μM) | 72 | Pterostilbene is cytotoxic against two pancreatic cancer cell lines. | Inhibition of cell proliferation and/or cell death, mitochondrial membrane depolarization and activation of caspases. | 49 |
| Melanoma, colon, breast, and lung | Pterostilbene (10 to 50 μM) | 72 | Pterostilbene demonstrates differential toxicity to various cancer cell lines | Pterostilbene is more efficacious in melanoma and lung cancer cells that have low HSP70 expression than in high HSP70 colon and breast cancer cells | 50 |
Table 2. Pterostilbene and cancer in vivo (animal) studies: effects and proposed mechanisms
| Cancer Type | Concentration(s) Analyzed | Administration | Anticancer Effect | Proposed Mechanism | Reference |
| Cervical | Pterostilbene (1 mM) | Intralesional injection daily for 5 days | Pterostilbene inhibits tumor development in HPV E6-positive cervical cancer mouse model | Decrease in tumor size due to increase in apoptosis, and downregulation of E6 and VEGF tumor protein levels | 51 |
| Breast | Pterostilbene (40 μg/kg) + Vitamin E (42 IU/kg or 99 IU/kg) | Pterostilbene oral 3 times per week Vit E in diet | Pterostilbene and vit E inhibited breast tumor growth and invasion in mouse xenograft model | Inhibition of Akt and downregulation of cell cycle proteins | 39 |
| Breast | Pterostilbene (56 mg/kg every 4 days for 3 weeks) | Oral gavage | Pterostilbene induces apoptosis and inhibits tumor growth of ER- Breast cancer xenograft model | Inhibition of ER-a36 (a variant of full-length Estrogen receptor) resulting in inhibition of Akt signaling | 52 |
| Prostate | Pterostilbene (50 mg/kg) | Intraperitoneal Injections daily (5 days/week) for 39 days | Pterostilbene reduced tumor growth in mouse xenograft model | Downregulation of miR-17-5p and miR-106-5p expression in both tumors and circulation | 53 |
| Breast | Pterostilbene (10 mg/kg) | Intraperitoneal injections 3 times a week | Pterostilbene suppressed tumor growth and metastasis in xenograft mouse model | Reduction in src signaling and inhibition of EMT | 54 |
| Pancreatic | Pterostilbene (100 μg/kg, 500 μg/kg or 1 mg/kg) | Oral gavage | Pterostilbene inhibited tumor growth rates | Increases Mn SOD antioxidant activity; inhibits STAT3 activity | 36 |
| Melanoma | Pterostilbene (30 mg/kg) every 48 h for 5 weeks | Intravenous | Pterostilbene decreased tumor growth in mouse xenograft model | Downregulated adrenocorticotropin hormone (ACTH) resulting in decrease Nrf2-mediated antioxidant defenses | 55 |
| Lymphoma | Pterostilbene (30 mg/kg every 2 days for 20 days) | Intravenous | Pterostilbene inhibited tumor growth in diffuse large B-cell lymphoma xenograft mouse model | Cytotoxic effect due to reduction in mitochondrial membrane potential and increase in apoptosis and ROS levels | 56 |
| Breast | Pterostilbene (0.1% w/w in diet) | Oral | Pterostilbene suppressed tumor growth in triple-negative breast cancer xenograft mouse model | Inhibition of Akt activationand upregulation of Bax | 57 |
| Prostate | Pterostilbene (50 mg/kg/day) | Intraperitoneal | Pterostilbene inhibited tumor growth and metastasis in prostate cancer xenografts | Reduction in metastasis-associated protein 1 (MTA1) and increased apoptosis | 58 |
| Endometrial | Pterostilbene (30 mg/kg/day) + Megestrol acetate (10 mg/kg/day) | Oral gavage | Pterostilbene synergizes with megestrol acetate for reduction of tumor growth in xenografts | Suppression of STAT3 activation as well as decreased ER expression | 37 |
| Biliary | Pterostilbene (30 and 60 mg/kg every 2 days For 3 weeks) | Intraperitoneal | Pterostilbene inhibited tumor growth in xenograft mouse model | Inhibited cell progression and induced autophagy | 59 |
| Multiple Myeloma | Pterostilbene (50 mg/kg/day For 2 weeks) | Intraperitoneal | Pterostilbene reduced tumor volume in mouse xenografts | Inhibited cell progression. Induction of apoptosis through increased ROS generation and activation of ERK1/2 and of JNK signaling | 60 |
| Colon | Pterostilbene (40 ppm diet for 45 weeks) | Oral | Pterostilbene reduced azoxymethane-induced colon tumor multiplicity | Inhibits cell proliferation via reduced PCNA expression and reduced beta-catenin and cyclin D1. Reduction of inflammatory markers | 61 |
| Colorectal | Pterostilbene (20 mg/kg/day) + quercetin (20 mg/kg/day) | Intravenous | Pterostilbene + QUER inhibited tumor growth by 51% in xenografts | Increase in SOD2 expression and decrease in Bcl-2 expression | 62 |
| Liver | Pterostilbene (100 and 200 mg/kg/day) | Intraperitoneal | Pterostilbene dose-dependently inhibited hepatocellular carcinoma tumor growth in mouse model | Increase in p53 expression and ROS generation and activation of apoptosis | 63 |
| Skin | Pterostilbene (1-2 μmol) | Topical | Pterostilbene prevented UV-B induced skin cancer in mouse model | Maintenance of skin antioxidant defenses including Nrf2 activation | 64 |
| Skin | Pterostilbene (1 and 5 μmol) | Topical | Pterostilbene suppressed 12-O-tetradecanoylphorbol-13-acetate-induced skin cancer in mouse model | Downregulation of iNOS and COX-2 | 32 |
| Glioblastoma Multiforme | Pterostilbene (2 mg/kg, three times a week) | Intraperitoneal | Pterostilbene suppressed tumorigenesis in glioma stem cell mouse xenograft | Inhibition of GRP78 | 65 |
| Colon | Pterostilbene (50 and 250 ppm in diet, 24 weeks) | Oral | Pterostilbene prevents azoxymethane-induced colon tumorigenesis. | Reduction of NF-κB activation, as well as iNOS and COX-2 expression Activation of Nrf2 signaling | 33 |
| Melanoma | Pterostilbene (20 mg/kg/day) + QUER (20 mg/kg/day) | Intravenous | Pterostilbene + QUER shown to inhibit metastasis of melanoma in xenografts | Inhibition of Bcl-2 | 66 |
Table 3. Signaling pathway of the anti-tumor effect of pterostilbene
| Signaling Pathway | Model | Pterostilbene Dose | Reference |
| EGFR, Akt/mTOR, Stat3, ERK1/2, and NFκB pathways | urethane-caused lung tumor | 250 mg/kg | 67 |
| microRNA 448 circuit | MDA-MB-231 cells were cocultured with M2 TAM and were subcutaneously injected into the left flank of NOD/SCID mice | 5 mg/kg | 68 |
| JAK/STAT3 signaling pathway | Breast cancer cell lines (MDA-231 and ZR-751) | 75 μM | 69 |
| Src/Fak signaling pathway | MDA-MB-231-bearing NOD/SCID mice | 10 mg/kg | 70 |
| Rac1/WAVE/Arp2/3 pathway | MDA-MB-231 cells | 10 μM | 71 |
| β-catenin/p65 downstream signaling pathway | F344 rats were given two AOM injections subcutaneously | 0.004% in the diet for 45 weeks | 72 |
| ATM/CHK/p53 pathway | non-small cell lung cancer cell (A549) | 21 μM | 73 |
| GRP78 signaling pathway | human glioblastoma cell lines GBM8401 and U87MG | 2.99, 1.42 μM | 28 |
| p53/SOD2/ROS pathway | HepG2 cells | 100 μM | 74 |
| miR-663b/BCL2L14 signaling pathway | HTB-111 and Ishikawa cells | 71.64 nM, 74.34 µM | 75 |
| JAK2/STAT3 signaling pathway | human osteosarcoma cell line, SOSP-9607 | 1.81 µM | 76 |
| AKT/mTOR/p70S6K and ERK1/2 pathways | T24 human bladder cancer cell | 66.58 ± 1.84 µM | 77 |
| Fas/FasL pathway | human AGS gastric carcinoma cells (CCRC 60102) | 50.7 μM | 78 |
| ERS signaling pathway | Human EC109 and TE1 esophageal cancer cells | 150 μM | 79 |
| RAGE/PI3K/Akt signaling pathway | MIA PaCa-2 and MIA PaCa-2GEMR cells (GEM-resistant cells) | 41.8, 42.0 µM | 80 |
| signal transducer and activator of transcription 3 signaling pathway | HeLa, CaSki, and SiHa cervical cancer adherent cells | 32.67, 14.83, 34.17 µM | 81 |
Breast cancer
The anticancer effects of Pterostilbene were first examined against two breast cancer cell lines (MCF7 and ZR-751) in combination with tamoxifen 46. These cells were treated with Pterostilbene (10 and 20 μM) for 24 h prior to Tamoxifen (5 μM) being added and assayed for cell viability and apoptosis. This combination demonstrated the inhibition of cell proliferation and increased apoptosis in both cell lines. In addition, Pterostilbene + Tamoxifen was shown to be additive in the reduction of cell viability in the ZR-751 cell line, but not the MCF7 cell line 46. This finding is consistent with a subsequent study showing MCF7 to be resistant to Pterostilbene anticancer activities due to high levels of HSP70 expression 50. Pterostilbene was then examined against the mutant p53-breast cancer cell lines MDA-MB-231 and T-47D, and found to decrease mutant p53 protein expression while increasing the expression of the pro-apoptotic Bax protein 45.
Pterostilbene has been assessed in several mouse models of breast cancer as well. Pterostilbene was shown to induce apoptosis in ER-α66 negative cells both in vitro and in a mouse xenograft model through the inhibition of Erk and Akt activation 52. Specifically, oral Pterostilbene (56 mg/kg) every four days for 3 weeks induced apoptosis and inhibited tumor growth. Interestingly, the authors show that knockdown of ER-α36 (a variant of ER-α66 present in this cell line) desensitized the cancer cells to Pterostilbene 52.
Pterostilbene was then examined for activity against the invasive ability of three breast cancer cell lines MCF7, Hs578t and MDA-MB-231 and found to inhibit the migratory potential of MDA-MB-231 and Hs578t cells 54. Once again MCF7 cells proved resistant to Pterostilbene treatment potentially due to high HSP70 expression 50. Importantly, Pterostilbene was shown to suppress tumor growth and metastasis in an MDA-MB-231 xenograft mouse model through reduction in src expression 54.
Pterostilbene was then shown to inhibit tumor growth and invasion in combination with Vitamin E in a breast cancer xenograft model through inhibition of Akt and down regulation of cell cycle proteins 39. In this study Pterostilbene (40 μg/kg) was given orally three times per week while Vitamin E (42 and 99 IU/kg) were given in the diet daily.
After demonstrating that Pterostilbene exhibited the greatest dose-dependent antiproliferative activity against triple-negative MDA-MB-468 cells of the three different subtypes of breast cancer test in vitro, oral administration of Pterostilbene was examined in an MDA-MB-468 xenograft mouse model 82. Pterostilbene (0.1% w/w in diet) suppressed tumor growth in this mouse model 82. Investigation into the potential mechanism of Pterostilbene antitumor activity using the MDA-MB-468 cell line revealed an inhibition of Akt and an upregulation of Bax protein 82.
Cervical cancer
Efficacy of Pterostilbene (20 and 40 μM) was examined in cervical cancer in both HeLa adherent and stem-like cells 43. Pterostilbene was found to inhibit growth, viability and migration of HeLa adherent cells through cell cycle arrest via induction of p53 coupled with reduction of cyclin E1 and cyclin B1 expression. Pterostilbene also induced apoptosis through ROS-induced activation of caspase-3 and caspase-9 as well as downregulation of the antiapoptotic proteins Bcl-2 and Bcl-xL and the inhibition of MMP-2 and MMP-9 expression 43. Furthermore, this was the first study to look the possible effect of Pterostilbene on cervical cancer stem cell-like cells (CSCs). The authors report that Pterostilbene suppressed tumor-sphere forming ability and migration of CSCs through reducing stemness supporting transcription factors (Sox2, Oct4 and Nanog) as well as decreasing the activation of STAT3 43.
A separate study examined the effect of Pterostilbene on HPV-E6 positive cervical cancer in vitro and in vivo 51. Pterostilbene was shown to be cytotoxic to E6-TC1 cells though decreasing the expression of E6 oncoprotein in vitro. A mouse xenograft of this cell line was then used to evaluate Pterostilbene (1 mM) by intralesional injection daily for 5 days. In the model Pterostilbene significantly reduced the size of the tumors by an average of 72% when compared to controls. Furthermore, immunohistochemistry of the Pterostilbene-treated tumors depicted in increase in apoptosis through caspase-3 activation and reduced E6 expression as compared with control tumors 51.
Colon cancer
The effects of Pterostilbene in colon cancer were first assessed in 2008. A combination of Pterostilbene with another polyphenol, quercetin was shown to inhibit the growth of HT-29 colorectal cancer cells by about 56% via an increase in SOD2 expression and a decrease in Bcl-2 expression 62. Intravenous administration of this combination, Pterostilbene and quercetin (each at 20 mg/kg/day), suppressed tumor growth by approximately 51% in HT-29 xenografts 62.
Another lab examined the role of Pterostilbene alone in treating an azoxymethane-induced colon cancer rat model 61. Pterostilbene was continuously administered orally via the diet at a concentration of 40 ppm for 45 weeks and resulted in reduced tumor multiplicity and downregulated the expression of proliferating cell nuclear antigen (PCNA), β-catenin and cyclin D1 61.
An independent study of Pterostilbene in the azoxymethane-induced colon cancer model (in mice this time) once again showed that Pterostilbene can inhibit azoxymethane-induced colon tumorigenesis 33. This study presented evidence that Pterostilbene is working through reduction of NF-κB activation, decreasing the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2) and aldose reductase as well as through activation of Nrf-2 33.
Endometrial cancer
Another investigation demonstrated that Pterostilbene as a single treatment led to an increased cleavage of an apoptotic marker, caspase 3, and a decreased expression of cell survival proteins, Bcl-2 and Bcl-xL, in endometrial cells, similar to the pro-apoptotic effects by Pterostilbene reported for other cancer cell lines 37. In addition, Pterostilbene inhibited the expression of the cell cycle regulators, such as cyclin D1, cyclin B1 and CDK4. These activities were further enhanced when Pterostilbene was combined with megestrol acetate, a hormonal treatment in endometrial cancer, resulting in a synergistic antiproliferative effect in endometrial cancer cells. Investigation into molecular mechanisms leading to this synergy revealed that the combination more effectively suppressed the activation of the ERK1/2 pathway and STAT3, as well as ER expression. The combination of Pterostilbene (30 mg/kg/day) and megestrol acetate (10 mg/kg/day) by oral gavage also significantly reduced tumor growth in a xenograft endometrial cancer mouse model, as demonstrated by reduction in tumor weight and volume 37.
An ongoing phase II randomized controlled, neoadjuvant trial for patients with endometrial cancer, currently investigates the in vivo effect of the combination of Pterostilbene and megestrol acetate 42. This is an open-label phase II randomized controlled trial of neoadjuvant therapy during the preoperative window period with megestrol acetate ± Pterostilbene in patients with stage I endometrial cancer. The objective is to evaluate the in vivo antiproliferative impact of Pterostilbene in endometrial cancer, by the comparison of the Ki-67 proliferation index in post-treatment hysterectomy samples with pre-treatment endometrial samples. Other potentially predictive molecular and/or clinicopathologic biomarkers will be explored.
Ovarian cancer
The anti-tumor activity of Pterostilbene in human ovarian cancer was demonstrated in vitro. Pterostilbene inhibited cell viability by suppressing cell cycle progression and inducing apoptosis. Additionally, it potentiated the anti-proliferative effects of cisplatin, a first line agent for ovarian cancer treatment. Pterostilbene reduced proliferation and migration in ovarian cancer cells by the inhibition of the STAT3 pathway 35. Pterostilbene was found to inhibit STAT3 activation in OVCAR-8 and Caov-3 cells. STAT3 is constitutively activated in ovarian cancer and the inhibition of STAT3 pathway effectively suppresses ovarian cancer growth and progression. Pterostilbene also caused the decreased expression of STAT3 target proteins, including anti-apoptotic proteins, such as Mcl-1 and Bcl-2 and cell cycle protein, such as cyclin D1. As a result, Pterostilbene treatment leads to the induction of apoptosis and inhibition of cell cycle progression.
The effect of Pterostilbene on cell cycle progression appeared to be concentration dependent in both OVCAR-8 and Caov-3 cells. While a low concentration of Pterostilbene (25 μM) led to an increase in cells in the S-phase, a higher concentration of Pterostilbene (50–150 μM) caused an increase in cells in the G0/G1 phase. Consistent with these results, one of the critical proteins controlling cell cycle progression, cyclin D1, was remarkably downregulated by Pterostilbene in a dose-dependent manner in both OVCAR-8 and Caov-3 cells. Therefore, it is possible that Pterostilbene induced cell cycle arrest through the cyclin–CDK checkpoint.
Prostate cancer
Initially, Pterostilbene was examined in two prostate cancer cell lines for it anticancer activity, the p53 wild type LNCaP cells and the p53 null PC3 cell line 48. This study demonstrated that Pterostilbene exhibited a dose-dependent inhibition of cell proliferation regardless of p53 status through activation of AMPK. However, Pterostilbene only induced apoptosis in the p53 null PC3 cells 48. Another lab subsequently examined the ability of Pterostilbene to regulate p53 activity in a prostate cancer xenograft model 58. Intraperitoneal injection of Pterostilbene (50 mg/kg/day) was reported to be effective in this model resulting in significantly inhibited tumor growth, progression, local invasion and metastasis. This reduction was reflective of decreased metastasis-associated protein 1 (MTA1) expression and subsequent decrease in p53 inactivation. In addition, the knockdown of metastasis-associated protein 1 (MTA1) further sensitized tumors to Pterostilbene treatment 58.
This same lab reported on the ability of Pterostilbene to regulate another tumor suppressor, PTEN, in prostate cancer pathology 53. Building on a previous publication that elucidated the overexpression of the oncogenic microRNA-17 (miR-17) family, which downregulates PTEN in prostate cancer 83, this study focused on Pterostilbene anticancer activity in a xenograft model of prostate cancer 53. The authors show that I.P injection of Pterostilbene (50 mg/kg) daily for 5 days/week for 39 days reduced tumor growth through the downregulation of miR-17-5p and miR-106-5p which rescued PTEN expression 53.
Pancreatic cancer
The anticancer activities of Pterostilbene in pancreatic cancer were first assessed in two cell lines, MIA PaCa and PANC-1 49. Treatment of these cells with Pterostilbene (10 to 100 μM) showed a decrease in cell proliferation in a concentration- and time-dependent manner through the induction of cell cycle arrest, mitochondrial membrane depolarization and activation of caspases 49. Another lab recently reported a similar finding that the treatment of Pterostilbene (50 and 75 μM) inhibited cell proliferation of two pancreatic cell lines, MIA PaCa-2 and the gemcitabine-resistant MIA PaCa-2GEMR 38. Pterostilbene was reported to induce cell cycle arrest in S-phase, increase apoptosis and inhibit the expression of the multidrug resistance protein (MDR1) in both cell lines. The reduction in MDR1 expression was due to decreased Akt activation 38.
Microarray analysis was performed on MIA PaCa-2 cells that were treated with Pterostilbene (50 μM) to ascertain differential gene regulation of pancreatic cancer cells by Pterostilbene 36. This genetic analysis revealed that Pterostilbene upregulated the pro-apoptotic genes including DDIT-3, growth differentiation factor 15 and SOD2. Pterostilbene was then administered orally at one of three doses (100 μg/kg/day, 500 μg/kg/day or 1 mg/kg/day) to a MIA PaCa-2 xenograft mouse model. All doses of Pterostilbene were found to inhibit tumor growth and tumor histology confirmed that all Pterostilbene-treated tumors showed prominent central necrosis encompassing 60–80% of the tissue 36.
Skin cancer
The anticancer activity of Pterostilbene against skin cancer was first reported in 2005. The intravenous administration of Pterostilbene (20 mg/kg/day) in combination with quercetin (20 mg/kg/day) was shown to be effective in a B16-F10 melanoma metastasis mouse model 66. Specifically, Pterostilbene + quercetin inhibited 73% of liver metastasis of B16-F10 cells. The observed antimetastatic activity was reported to be due to the inhibition of vascular adhesion molecule 1 expression in the hepatic sinusoidal epithelium thereby decreasing B16-F10 cell adhesion to the endothelium as well as down regulation of Bcl-2 in the metastatic cells 66. Similarly, Pterostilbene (10 to 50 μM) alone was shown to inhibit the cell proliferation and induce apoptosis of A375 melanoma cells, which have low HSP70 expression, in vitro 50.
Pterostilbene prevented 7,12-dimethylbenz[a]anthracene (DMBA) + 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin tumor formation via the downregulation of iNOS and COX-2 expression in mouse skin 32. In this model, Pterostilbene (1 or 5 μmol) or vehicle was applied topically 30 min prior to TPA application twice weekly for 20 weeks. After 20 weeks the control mice had an average of 38 skin tumors/mouse while the Pterostilbene treated animals had significantly fewer tumors. The Pterostilbene (1μmol) group had an average of 50% fewer tumors while the Pterostilbene (5 μmol) group had a decrease in tumor number of about 63% 32.
Similar results were observed in a UVB-induced skin cancer mouse model. The topical administration of Pterostilbene prevented chronic UVB (180 mJ/cm², three times a week for 6 months)-induced carcinogenesis (90% of Pterostilbene-treated mice did not develop skin carcinomas, whereas a large number of tumors were observed in all controls). In these experiments, 1–2 μmol Pterostilbene/cm² of skin were administered 20 min before each UVB irradiation 64. The authors report that the anticancer activity of Pterostilbene was attributed to Nrf2-dependent antioxidant response through the robust maintenance of glutathione levels as well as catalase, superoxide and GSH peroxidase activities 64.
The Nrf2 signaling pathway was examined further in mouse xenograft models of three melanoma cell lines, A2058, MeWo and MelJuso 40. Interestingly, Pterostilbene (15 μM) was not cytotoxic to any of these three cell lines in vitro, while i.v. Pterostilbene (30 mg/kg) was efficacious against xenograft mouse models of these same three melanoma cell lines. Specifically, i.v. Pterostilbene administered every 48 h for 5 weeks caused the significant inhibition of tumor growth of 70% in A2058, 65% in MeWo and 49% in MelJuso xenografted mice 40. The mechanism of action proposed shows Pterostilbene reduces circulating levels of adrenocorticotropin hormone (ACTH) resulting in a decrease in Nrf2-mediated antioxidant defenses in the melanoma cells. This mechanism does explain why Pterostilbene was not effective against melanoma cells in culture and underscores the importance of testing potential anticancer agents under in vivo conditions.
Lung cancer
The most robust study of the anticancer activity of Pterostilbene against lung cancer to date examined Pterostilbene in combination with Osimertinib against five EGFR-mutation positive non-small cell lung cancer (NSCLC) cell lines. Pterostilbene plus Osimertinib demonstrated synergistic effects on cell proliferation for all five cell lines tested 34. Intriguingly, while Pterostilbene alone did not have an effect on Src phosphorylation in these cells, Pterostilbene did reverse the Osimertinib-induced STAT3, YAP1 and CUB domain containing protein (CDCP1) phosphorylation when treated in combination. If these results are replicated in vivo, the addition of Pterostilbene could greatly improve the current monotherapy of Osimertinib in NSCLC since Pterostilbene abrogates the known Osimertinib-activated resistance pathways 34. In addition to this study, Pterostilbene (10 to 50 μM) has been shown to effectively inhibit the growth of A549 lung cancer cells in vitro since this cell line has low HSP70 expression 50.
Liver cancer
Pterostilbene (100 and 200 mg/kg/day) via i.p. injection was shown to inhibit hepatocellular carcinoma tumor growth in a chemically induced liver cancer mouse model 63. In fact, a dose-dependent suppression of tumor growth was observed as there were both fewer tumors per mouse as well as reduced size of the tumors. The proposed mechanism for Pterostilbene anticancer activity in liver cancer was assessed using HepG2 cells in vitro as well as in tumors and was attributed to Pterostilbene-induced ROS generation through increased p53 expression which, in turn, decreased SOD2 expression 63.
Blood cancers
Two robust studies have looked at the anticancer effects of Pterostilbene in hematological cancers. The first examined Pterostilbene against six diffuse large B-cell lymphoma cell lines as well as i.v. Pterostilbene (30 mg/kg) every two days for 20 days in a large B-cell lymphoma xenograft mouse model 56. Pterostilbene (12.5–100 μM) exhibited a dose-dependent inhibition of cell viability in vitro through the reduction in mitochondrial membrane potential, increased ROS generation and increased apoptosis via caspase activation. Similarly, Pterostilbene administered i.v. significantly inhibited large B-cell lymphoma tumor growth in mice 56. The second study tested the potential anticancer activity of Pterostilbene against multiple myeloma (MM) cell lines in a xenograft mouse model. Specifically, Pterostilbene (10-50 μM) demonstrated a dose-dependent reduction in cell proliferation on four multiple myeloma cell lines 60. The observed cytotoxic effect was due to increased caspase activation specifically in multiple myeloma cells and not in normal peripheral blood mononuclear cells. Pterostilbene also increased ROS generation and reduced mitochondrial membrane potential. Additionally, i.p. injection of Pterostilbene (50 mg/kg) daily for two weeks inhibited MM tumor growth in a mouse xenograft model 60. All of these findings echo the anticancer activity of Pterostilbene in large B-cell lymphoma 56.
In addition, it has been proposed the mechanism of action to be ERK1/2 and JNK activation 60. This seems counterintuitive on its own as ERK1/2 activation has been shown to stimulate Nrf2 nuclear localization and induce antioxidant defenses 84, however, since tumor growth was clearly reduced after Pterostilbene treatment, it is entirely possible that Pterostilbene inhibits AKT in MM and thereby inhibits Nrf2 activation as evidenced through enhanced ROS generation. Although AKT activation was not assessed in this study, AKT inhibition by Pterostilbene has been shown in pancreatic 38 and breast cancer 39. Furthermore, the inhibition of mTOR (though the inhibition of AKT) has been proposed as a potential adjunct therapy in multiple myeloma 85. The authors of this manuscript opine that the use of an inhibitor of mTOR complex 2, via the inhibition of AKT, in combination with an inhibitor of mTOR complex 1 in multiple myeloma would be useful for the anti-angiogenic management of patients 85. Thus, a potential clinical study is warranted using Pterostilbene as an mTOR complex 1 inhibitor with a TBD mTOR complex 2 inhibitor. 5.12. Other Cancers (Oral, Gastrointestinal, Biliary, Glioblastoma Multiforme)
The anticancer activity of Pterostilbene was examined in a cisplatin-resistant human oral cancer CAR cells in vitro. Pterostilbene (50 μM and 75 μM) treatment of these cells reduced cell viability though the induction of apoptotic caspase activation and the downregulation of MDR1 through reduced Akt activation 86. Pterostilbene was also shown to inhibit the cell proliferation of three gastrointestinal cancer cell lines in vitro 47. Once again, Pterostilbene (10 and 100 μM) treatment was found to increase mitochondrial membrane potential and increase ROS generation, leading to increased apoptosis 47.
Instead of inducing apoptosis in human cholangiocarcinoma, a cancer of the biliary tract, Pterostilbene (15–120 μM) treatment in vitro of two cholangiocarcinoma cell lines showed dose-dependent cytotoxic effects through autophagy 59. This study also demonstrated that Pterostilbene (30 mg/kg and 60 mg/kg) administered i.p. every two days for three weeks inhibited cholangiocarcinoma tumor growth in a mouse xenograft model 59.
Another lab targeting glioblastoma multiforme examined Pterostilbene anticancer activity against glioma stem cells since they have been shown to contribute to the tumorigenesis, recurrence and resistance to treatment 65. They showed that Pterostilbene suppressed self-renewal and irradiation resistant properties of glioma stem cells and this activity was associated with miR-205 increased expression. This microRNA downregulates GRP78, a protein that is highly expressed in glioma stem cells and contributes to treatment resistance. Pterostilbene also suppressed tumorigenesis in glioma stem cell xenografted mice 65.
Antioxidation activity
The anti-oxidation effect of pterostilbene is the basis for its use in the treatment of numerous diseases. In vitro, nuclear transcription factor (Nrf2) is the “main regulator” of cytoprotective and antioxidant genes. In the STZ-induced diabetic model, Nrf2 activation and its downstream target gene expression were observed during pterostilbene treatment, the oxidative damage on the pancreatic tissue was reduced 87. As an effective activator of Nrf2, pterostilbene protected against arsenic-induced cytotoxicity and apoptosis of human keratinocytes 88. In a high glucose environment, the proliferation of human retinal endothelial cells was enhanced, the expressions of TNF-α and IL-1β increased, the level of NF-κB protein was significantly up-regulated, the production of reactive oxygen species (ROS) significantly increased, and the activity of superoxide dismutase (SOD) significantly decreased. Compared with the high glucose group, pterostilbene significantly inhibited the excessive proliferation of human retinal endothelial cells, decreased TNF-α and IL-1β levels, inhibited the expression of NF-κB protein, decreased the production of ROS, and increased SOD activity. Pterostilbene may inhibit the excessive proliferation of human retinal endothelial cells through antioxidation, thereby delaying the progression of diabetic retinopathy 89. In vivo, rats were administered 50 ppm pterostilbene daily for 6 weeks. By activating the Nrf2 signal pathway, the expressions of HO-1 and glutathione reductase were significantly increased, and antioxidation ensued. Cellular defense mechanisms, such as the activities of antioxidant enzymes (SOD, catalase, and glutathione peroxidase), protected cells from active free radicals and other oxidants. The activities of SOD, catalase, glutathione peroxidase, and glutathione transferase and glutathione content in the liver and kidney of diabetic rats were significantly lower in the treatment group than in the normal control group. After treatment with 40 mg/kg pterostilbene for 6 weeks, the abovementioned activities significantly improved 21. These results indicate that pterostilbene is a promising antioxidant.
Anti-inflammation activity
Anti-inflammatory effect is another important research field in the study of pterostilbene’s bioactivities.
In vitro experiments showed that pterostilbene could significantly reduce the expression of pro-inflammatory mediators TNF-α, IL-1β, IL-6, matrix metallopeptidase 2 (MMP2), and matrix metallopeptidase 9 (MMP9) in hypertonic cultured human corneal epithelial cells and had a protective effect on human corneal inflammation induced by hyperosmotic stress 90. Pterostilbene significantly reduced inflammatory reaction by reducing the production of cytokines sICAM1, IL-8, MCP-1, and sE-selectin and by inhibiting the adhesion of U937 monocytes to human umbilical vein endothelial cells. Furthermore, pterostilbene decreased the expression of ERS-related proteins eIF2a, ICAM1, MMP9, and GRP78 under endoplasmic reticulum stress, which effectively reduced the inflammation of vascular endothelial cells 22. Pterostilbene (IC50, 22.4 μmol/L) was more effective as an anti-inflammatory compound than resveratrol (IC50, 43.8 μmol/L) in inhibiting the occurrence of colon cancer in HT-29 human adenocarcinoma cell line 11.
In vivo, pterostilbene showed its potential anti-inflammatory effect by inhibiting the lipopolysaccharide-induced expressions of IL-6 and TNF-α mRNA in rat hippocampus. The decrease of inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) in streptozotocin (STZ)-induced diabetic mice proved that treatment with pterostilbene can significantly improve inflammatory response 91. In addition, pterostilbene may become a new therapeutic intervention to reverse vascular inflammation by inhibiting the metabolic activity of intestinal microflora and reducing the metabolism of carnitine into trimethylamine-N-oxide 92. What is more, targeting to NLRP3 is a novel anti-inflammation effect of pterostilbene. Liu et al. 93 analyzed the inflammasome activity of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) by Western blot, and found that pterostilbene treatment decreased the activation of NLRP3 inflammasome, suggesting that pterostilbene may reduce early brain injury after subarachnoid hemorrhage by inhibiting NLRP3 inflammatory bodies. These results suggest that pterostilbene has potential as an anti-inflammatory agent.
Lipid-lowering activity
Investigations conducted to evaluate the hypolipidemic activity of pterostilbene against streptozotocin (STZ)-nicotinamide-induced diabetic rats showed it to be effective in ameliorating dyslipidemia, which is thought to play a significant role in the increased cardiovascular mortality seen in diabetics 94. Oral administration of high-dose pterostilbene (40 mg/kg body weight) for six weeks significantly reduced serum VLDL and LDL (low-density lipoprotein or “bad” cholesterol) cholesterol and increased serum HDL (high-density lipoprotein or “good” cholesterol) cholesterol. Triglycerides, phospholipids, free fatty acids, and total cholesterol were reduced 94. Pterostilbene also increased antioxidant activity in diabetic rats, who demonstrated normalization of lipid peroxidation with pterostilbene treatment 95.
A study by Rimando et al. 20 stated that pterostilbene is an agonist of peroxisome proliferator-activated receptor; it had the same activity as the fibrate antihyperlipidemic drugs used in clinic. Resveratrol is not an activator of peroxisome proliferator-activated receptor. Compared with resveratrol, pterostilbene may be a more effective hypolipidemic compound, making it a possible choice for the treatment of dyslipidemia 20.
Pterostilbene exerts its lipid-lowering activity by reducing the amount of white adipose tissue and increasing the amount of brown adipose tissue. Koji et al. 96 found that pterostilbene inhibits the accumulation of white adipose tissue by enhancing energy metabolism and by partially inhibiting adipogenesis in obese Otsuka Long–Evans Tokushima fatty rats (Table 4). Pterostilbene can also induce the browning of white adipose tissue. The analysis of inguinal white adipose tissue showed that the tendency of browning and the transcription of several marker genes (CIDA, EBF2, PGC1α, PPARγ, Sirt1, and Tbx1) increased significantly 97. Aguirre et al. 98 studied brown adipose tissue. By analyzing the effects of two doses of pterostilbene (15 and 30 mg/kg/d) on several indexes of thermogenic ability in a hereditary obesity model, researchers found that pterostilbene could improve the thermogenic and oxidative capacities of brown adipose tissue in obese rats. In addition, the degreasing mechanism of pterostilbene was determined. Pterostilbene decreased fatty acid availability and promoted the synthesis of triglyceride, the assembly of very low density lipoprotein, and the oxidation of fatty acids 99.
Table 4. Lipid-lowering activity of pterostilbene
| Model | Pterostilbene Dose | Reference |
| H4IIEC3 cells | 100 µM | 20 |
| Obese Otsuka Long–Evans Tokushima fatty rats | 0.5% diet for 4 weeks | 96 |
| 3T3-L1 mature adipocytes | 5 µM | 97 |
| Mice fed an obesogenic high-fat diet | 352 µmol/kg/d for 30 weeks | 97 |
| Genetic obesity Zucker (fa/fa) rats | 15, 30 mg/kg body weight/day for 6 weeks | 99 |
| Wistar rats fed an obesogenic diet | 15, 30 mg/kg body weight/day for 6 weeks | 100 |
| 3T3-F442A preadipocytes | 1–10 μM | 101 |
| 3T3-L1 preadipocytes | 5–40 μM | 102 |
| Genetic obesity Zucker (fa/fa) rats | 15 mg/kg body weight/day for 6 weeks | 103 |
Pterostilbene and weight loss
Under overfeeding conditions, pterostilbene can reduce the weight of subcutaneous adipose tissue and prevent the decrease of triglyceride 9 caused by tumorigenic feeding, thereby reducing the absorption of glycerol and promoting the accumulation of triglycerides 100. Gómez-Zorita et al. 104 studied the effects of pterostilbene on rats fed an obesogenic diet (Table 4). The decrease of lipogenesis in adipose tissue and the increase of fatty acid oxidation in liver contributed to the anti-obesity effect of pterostilbene. If you compare this result with that of resveratrol, then you would observe that pterostilbene is more effective than resveratrol at a dose of 15 mg/kg/d. Gómez-Zorita et al. 101 also observed that pterostilbene had an anti-adipogenic effect on preadipocytes and quickly inhibited the incorporation of glucose into the lipids of mature adipocytes, this anti-lipid effect can occur in vivo. Studies by Chin et al. 102 showed that pterostilbene had an anti-adipogenic effect on preadipocytes, differentiated adipocytes, and mature adipocytes. Pterostilbene can also change the composition of intestinal microflora. The weight loss and cholesterol-lowering effect induced by pterostilbene in Zucker rats may be related to the enrichment of the Verrucomicrobia phylum 103. Pterostilbene can potentially be used to treat and prevent obesity due to its lipid-lowering activity.
Neuroprotective activity
The primary rationale for Pterostilbene’s neuroprotective activity comes from evidence on resveratrol, which is believed to have similar but less potent effects than Pterostilbene. However, the effects of resveratrol in a clinical trial on dementia patients was mixed with some biomarkers suggesting benefit but others suggesting harm. For Pterostilbene specifically, in a mouse model of accelerated aging (SAMP8), Pterostilbene, but not resveratrol, improved cognitive function, with measured effects on markers of cellular stress, inflammation, Alzheimer’s-like pathology, and increased PPAR alpha activity. Oddly, neither Pterostilbene nor resveratrol activated SIRT1 105. Another study in aged rats reported benefit to cognitive aging and specifically working memory 106. Other than that, some in vitro studies suggest relevant mechanisms of action like on inflammation and oxidative stress but no effects have yet been confirmed in humans 8.
Pterostilbene attenuated glutamate-induced oxidative stress damage of HT22 cells in mouse hippocampal neurons through the Nrf2 signal pathway 107 and attenuated the central nervous system damage induced by high glucose via the activation of nuclear factor erythroid 2-related factor 2 108. Pterostilbene preconditioning can improve tissue and functional damage through oxidative stress, programmed cell death, and inflammation mediated by HO-1, thereby preventing neonatal brain injury caused by hypoxic-ischemic encephalopathy 109. Pterostilbene showed estrogenic activity in its neuroprotection effect by up-regulating the expression of anti-apoptotic Bcl-2 and activating MAPK/ERK and PI3K/AKT signal pathways. ER-α was the main receptor involved in the neuroprotective effect of pterostilbene 110. Therefore, pterostilbene may be a putative neuroprotective substance for nerve injury treatment and prevention.
Cerebral ischemia
Pterostilbene plays an important role in the occurrence and development of cerebral ischemia. Zhou et al. 87 found that after oral administration, pterostilbene improved motor function in a time- and concentration-dependent manner before and after ischemia. It reduced the infarct volume and weakened the damage to the blood-brain barrier after ischemia reperfusion in the mouse model of middle cerebral artery occlusion. The best dose was 10 mg/kg, which was administered within 1 h after ischemia reperfusion. This protective effect may be at least partly related to the inhibition of oxidative stress and apoptosis of neurons in the penumbra of the cortex. Another article showed the same conclusion by studying this model; however, the mechanism was pterostilbene’s reduction of astrocyte-mediated inflammation and oxidative damage after ischemia reperfusion by inhibiting NF-κB phosphorylation and nuclear translocation 111.
Pterostilbene was used to treat mouse brain in a common carotid artery occlusion model in vivo and HT22 neuronal cells in vitro. Pterostilbene reduced mitochondrial oxidative damage induced by cerebral ischemia-reperfusion by activating heme oxygenase-1 (HO-1) signal pathway 112. Longxue Tongluo capsule was widely used to treat ischemic stroke. Jing et al. quantified the multi-components in the plasma or tissue of rats after oral administration of Longxue Tongluo capsule and clarified the kinetic characteristics of the main phenolic derivatives. Among the 11 analytes, the concentration of pterostilbene in the brain was the highest (141.4 ± 25.48 ng/g) 113. These findings indicated that pterostilbene may be effective for alleviating neurological and histological abnormalities after cerebral ischemia reperfusion.
Alzheimer’s disease
Pterostilbene can be used to treat Alzheimer’s disease. Chang et al. 17 found that pterostilbene was a more effective cognitive and cellular stress regulator than resveratrol in mouse susceptibility No. 8 (SAMP8) Alzheimer’s disease model of accelerated aging at the same and achievable dietary dose of 120 mg/kg. This action may be due to the increase of liposolubility caused by the substitution of hydroxyl groups in pterostilbene, which increased the expression of peroxisome proliferator-activated receptor α 17. Pterostilbene can alleviate lipopolysaccharide-induced learning and memory impairment. The underlying mechanism may be related to its inhibition of microglial activation, which resulted in the obvious decrease of lipopolysaccharide-induced production of NO, TNF-α, and IL-6 in N9 microglial cells and the protection of neuronal damage. Thus, pterostilbene improved lipopolysaccharide-induced learning and memory impairment by suppressing doublecortin expression and increasing neuronal nuclear antigen expression 114. Moreover, pterostilbene inhibited Aβ apoptosis through PI3K/Akt signal pathway [74] through its antioxidant activity in the brain and further through the inhibition of acetylcholinesterase activity; thus, it had anti-Alzheimer’s disease activity 115. These results suggest that pterostilbene may be involved in the dietary intervention for Alzheimer’s disease prevention and may be used as an adjuvant treatment of Alzheimer’s disease.
Anti-anxiety effect
Pterostilbene showed anti-anxiety effect by down-regulating the phosphorylation level of extracellular regulated kinase in the hippocampus of mice 116. Pterostilbene had a similar antidepressant effect on chronic unexpectedly stressed rats and its mechanism may involve the promotion of hippocampal neurogenesis; its molecular mechanism may be related to the activation of BDNF/ERK/CREB neurotrophic signal pathway 117.
Antidiabetic activity
Administration of pterostilbene significantly reduced pathological changes seen in liver and kidney of diabetic rats. Pterostilbene given at 40 mg/kg significantly decreased plasma glucose and increased insulin levels in normal and diabetic rats. Pterostilbene administration also resulted in a significant reduction of glycosylated hemoglobin 118.
Pterostilbene was found in vitro to be a PPAR-a agonist, which can lower both plasma cholesterol and glucose. Feeding hypercholesterolemic hamsters a diet containing 25 ppm pterostilbene resulted in a 29-percent lower plasma LDL cholesterol, seven-percent higher HDL cholesterol, 14-percent lower glucose and a lower LDL/HDL ratio, compared to controls 119. Intraperitoneal administration of pterostilbene in rats significantly lowered blood glucose, an effect comparable to that of metformin 120.
Pterostilbene exerts its hypoglycemic activity by stimulating the increase of glycolysis and the decrease of gluconeogenesis. In STZ-nicotinamide-induced diabetic male albino Wistar rats, the concentrations of blood glucose and glycosylated hemoglobin were significantly decreased after oral administration of pterostilbene (10, 20, and 40 mg/kg) for 2, 4, and 6 weeks. After treatment, the expressions of hexokinase increased significantly, whereas the expressions of glucose-6-phosphatase, and fructose-1,6-bisphosphatase, decreased significantly. The expression of glucose-6-phosphatase and fructose-1-line 6-diphosphatase regulated glucose homeostasis 121.
Pterostilbene exerted its hypoglycemic activity by inhibiting the apoptosis of islet β cells. It increased the expression of anti-apoptotic protein Bcl-2 and down-regulated the expression of pro-apoptotic Bax and caspase-3, thereby inhibiting the apoptosis of islet β cells in diabetic rats 122. A recent study suggested that the anti-diabetic mechanism of pterostilbene may be related to the activation of PPARγ and PI3K/AKT signaling pathways in adipose tissue 123. These results suggest that pterostilbene may be a candidate for the treatment of diabetes in the future.
Nuclear transcription factor Nrf2 is the “main regulator” of cytoprotective and antioxidant genes. Bhakkiyalakshmi et al. 19 evaluated the effect of pterostilbene on islet β cytotoxicity in INS-1E rats induced by STZ. The cells were pretreated with pterostilbene in a time- and concentration-dependent manner (0–48 h and 0–16 μM, respectively) and then treated with 10 mM STZ for 1 h. Pterostilbene pretreatment (0–8 μM) for 48 h significantly protected islet β-cell from damage induced by STZ in a time-dependent manner. The survival rate of pretreated cells at concentrations of 4 and 8 μM increased by 67 ± 3.4% and 72 ± 2.7%, respectively. The activation of Nrf2 and its downstream target gene expression was observed during pterostilbene treatment, which played a protective role in islet β cells [12]. This finding showed that pterostilbene can exert its hypoglycemic activity by protecting the islet β cells. Elango et al. injected several small doses of STZ (50 mg/kg) intraperitoneally into 6-week-old male albino Swiss mice for 5 days consecutively. After 1 week, moderate diabetic mice with glycosuria and hyperglycemia (with blood glucose concentrations of 200 and 300 mg/d) were injected intraperitoneally with pterostilbene (5 mg/kg), resveratrol (10 mg/kg), and glibenclamide (600 μg/kg body weight) for 5 weeks. The mice with hyperglycemia were intraperitoneally injected with pterostilbene (10 min), resveratrol (10 min), and glibenclamide (600 μg/kg body weight) for 5 weeks. Compared with resveratrol, a known Nrf2 activator, pterostilbene administration significantly improved the level of serum insulin in diabetic mice. The ability to reduce the level of fasting blood glucose in diabetic mice may be due to the increased insulin secretion of residual β-cells, which is equivalent to the activity of the antidiabetic drug glibenclamide. Compared with diabetic mice, those treated with pterostilbene showed restored activity of downstream targets of Nrf2; pterostilbene further limited the production of free radicals 124. Pterostilbene plays a hypoglycemic role by stimulating insulin secretion of the residual islet β cells.
These are the effects of pterostilbene on type 2 diabetic rats induced by STZ-nicotinamide. Gómez-Zorita et al. 125 studied the effect of pterostilbene on insulin resistance associated with obesity feeding. Wistar rats were randomly divided into three experimental groups with nine rats in each group, which were fed with a commercial obesogenic diet that was high in sucrose (20.0%) and fat (22.5%). The latter two groups were added to the diet according to the guaranteed amount of 15 mg/kg body weight/day or 30 mg/kg body weight/day. The duration of the experiment was 6 weeks. The results showed that pterostilbene improved the serum glucose control of insulin-resistant rats induced by obese diet. The mechanism may be the increase of liver glucokinase activity and skeletal muscle glucose uptake. Compared with their previously reported results on the effects of resveratrol, the dose of 15 mg/kg body weight/day was not as effective as pterostilbene in reducing serum glucose levels 125.
Antifungal activity
Studies on antifungal activity showed that 160 μg/mL of resveratrol and 60 μg/mL of pterostilbene completely inhibited the germination of conidia of Botrytis cinerea 126. In addition, pterostilbene had a strong bacteriostatic effect on Fusobacterium nucleatum, a key periodontal pathogen, and the minimum inhibitory concentration was more than 60 times lower than that of resveratrol; pterostilbene’s antibacterial activity mechanism involved inducing the leakage of cell contents, which resulted in the loss of bacterial cell vitality 127. Pterostilbene is expected to be a candidate for adjuvant treatment of periodontitis.
Pterostilbene shows potent antiviral activity against hepatitis C virus (HCV) at 10 μM, with no associated toxicity 128. Pterostilbene inhibited infectious particle assembly and secretion and caused a reduction of intracellular infectivity. Pterostilbene is also an agonist of the peroxisome proliferator-activated receptor α (PPAR-α) that is known to be required for hepatitis C virus RNA replication 129.
Resveratrol and pterostilbene completely blocked HIV-1 infection in resting CD4 T cells in the reverse transcription step at low molar concentrations. Resveratrol alone did not inhibit HIV-1 infection in activated T cells, but it co-inhibited reverse transcription with nucleoside reverse transcriptase inhibitors in these cells 130. In the transformed fibroblast cell line (293T), 30 μM resveratrol had no anti-HIV-1 effect, whereas 10 μM pterostilbene showed 50% inhibitory effect 131. Thus, resveratrol and pterostilbene are promising adjuvants in anti-HIV pre-exposure prophylaxis formulations.
Pterostilbene dosage
Pterostilbene is available as a supplement from numerous companies. Data extrapolation from animal studies suggest a dose of Pterostilbene 50-100 mg twice daily. The only clinical trial to-date used 50 to 175 mg twice daily. The supplement Elysium includes Pterostilbene and nicotinamide riboside based on the theory that the increase in NAD+ caused by nicotinamide riboside will lead to much stronger SIRT1 activation by Pterostilbene. However, to date, no studies have been published that either confirm or counter this hypothesis for a synergistic effect.
Pterostilbene side effects
Pterostilbene is not known to be toxic or cause adverse effects in humans. In mice fed trans-pterostilbene for 28 days at doses up to 3000 mg/kg body weight per day, equivalent to 500 times the estimated mean human intake (25 mg/day), no significant toxic effects or adverse biochemical parameters were noted, compared to controls 132.
Pterostilbene has been tested in one Chromadex-sponsored clinical trial in patients with hypercholesterolemia at 50 or 125 mg oral twice daily for 6-8 weeks. No adverse reactions or side effects were reported. The treatment did increase LDL levels 133. Pterostilbene was also used in a combination therapy in young adults with no safety concerns raised 134. In multiple myeloma patients, a trial on a related compound (a bioavailable formation of resveratrol) was terminated early due to safety concerns of kidney damage 135. High doses of Drakshasava, an herbal Ayurvedic medicine that contains pterostilbene, is reported online to cause diarrhea and gastrointestinal discomfort.
Toxicity
In Swiss mice fed with a pterostilbene enriched diet at doses of 0, 30, 300, and 3000 mg/kg body weight per day, the red blood cell number and the hematocrit increased (approx. 25%) compared to control mice, whereas biochemical parameters were not significantly affected 136. Histopathology, hematology, clinical chemistry, and urinary balance studies found no alterations induced by pterostilbene as compared to controls 136, thus concluding that orally administered pterostilbene, even at the highest dose administered, was nontoxic. More recent studies suggest a no-observed-adverse-effect-level (NOAEL) of 200 mg of 3’-hydroxy-pterostilbene (a natural pterostilbene analog)/kg body weight per day in rats after oral administration 137.
At present, there are 13 registered clinical trials where pterostilbene has been used in humans, alone or in combination with other compounds. Based on the available data, orally administered pterostilbene appears safe at a dose of 125 mg twice daily 138. Safety concerns forced a previous trial to stop, where the safety and activity of SRT501 (a formulation of resveratrol claimed to increase its in vivo bioavailability), alone or in combination with bortezomib, were being evaluated in patients with multiple myeloma 139. In this trial, where 5g of SRT501/day was administered orally for 20 consecutive days, kidney damage developed in some patients, thus raising the question of whether high doses (>1 g/day), administered chronically could pose toxicity problems 139.
Intravenous administration has been also evaluated in nude mice 62. Pterostilbene was found to be pharmacologically safe (no organ-specific or systemic toxicity, i.e., tissue histopathologic examination and regular hematology and clinical chemistry data) even when administered i.v. (intravenuously) (dissolved in DMSO:ethanol [2:1]) at a dose of 30 mg/kg per day × 23 days 62 or at a dose of 40 mg/kg every 48h × 5 weeks 40. The i.v. administration has never been tried in humans. However, the availability of a hydrosoluble disodium salt of pterostilbene phosphate may facilitate its use in cancer 41.
References- Kosuru R, Rai U, Prakash S, Singh A, Singh S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur J Pharmacol. 2016 Oct 15;789:229-243. doi: 10.1016/j.ejphar.2016.07.046
- Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. Engl. 2011;50:586–621. doi: 10.1002/anie.201000044
- Estrela JM, Ortega A, Mena S, Rodriguez ML, Asensi M. Pterostilbene: Biomedical applications. Crit Rev Clin Lab Sci. 2013 May-Jun;50(3):65-78. doi: 10.3109/10408363.2013.805182
- McCormack, D., & McFadden, D. (2013). A review of pterostilbene antioxidant activity and disease modification. Oxidative medicine and cellular longevity, 2013, 575482. https://doi.org/10.1155/2013/575482
- Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011 Sep;68(3):593-601. doi: 10.1007/s00280-010-1525-4
- Tsai H.Y., Ho C.T., Chen Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017;25:134–147. doi: 10.1016/j.jfda.2016.07.004
- Liu, Y., You, Y., Lu, J., Chen, X., & Yang, Z. (2020). Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules (Basel, Switzerland), 25(21), 5166. https://doi.org/10.3390/molecules25215166
- Poulose SM, Thangthaeng N, Miller MG, Shukitt-Hale B. Effects of pterostilbene and resveratrol on brain and behavior. Neurochem Int. 2015 Oct;89:227-33. doi: 10.1016/j.neuint.2015.07.017
- Dahlin JL, Walters MA. The essential roles of chemistry in high-throughput screening triage. Future Med Chem. 2014 Jul;6(11):1265-90. doi: 10.4155/fmc.14.60
- McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012 Apr;173(2):e53-61. doi: 10.1016/j.jss.2011.09.054
- Deng L., Li Y., Zhang X., Chen B., Deng Y., Li Y. UPLC-MS method for quantification of pterostilbene and its application to comparative study of bioavailability and tissue distribution in normal and Lewis lung carcinoma bearing mice. J. Pharm. Biomed. Anal. 2015;114:200–207. doi: 10.1016/j.jpba.2015.04.045
- Schmidlin L., Poutaraud A., Claudel P., Mestre P., Prado E., Santos-Rosa M., Wiedemann-Merdinoglu S., Karst F., Merdinoglu D., Hugueney P. A Stress-Inducible Resveratrol O-Methyltransferase Involved in the Biosynthesis of Pterostilbene in Grapevine. Plant Physiol. 2008;148:1630–1639. doi: 10.1104/pp.108.126003
- Rodríguez-Bonilla P, López-Nicolás JM, Méndez-Cazorla L. Development of a reversed phase high performance liquid chromatography method based on the use of cyclodextrins as mobile phase additives to determine pterostilbene in blueberries. Journal of Chromatography B. 2011;879(15-16):1091–1197.
- Jeandet P., Sobarzo-Sánchez E., Silva A., Clément C., Nabavi S., Battino M., Rasekhian M., Belwal T., Habtemariam S., Koffas M., et al. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020;39:107461. doi: 10.1016/j.biotechadv.2019.107461
- Riche D.M., McEwen C.L., Riche K.D., Sherman J.J., Wofford M.R., Deschamp D., Griswold M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013;2013:463595. doi: 10.1155/2013/463595
- Yubai S., Cheng L., Shaohua M. A new process for synthesis of trans stilbene compound pterostilbene. Chin. J. Pharm. 2016;47:4. (In Chinese).
- Chang J., Rimando A., Pallas M., Camins A., Porquet D., Reeves J., Shukitt-Hale B., Smith M.A., Joseph J.A., Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging. 2012;33:2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015
- Liu J., Fan C., Yu L., Yang Y., Jiang S., Ma Z., Hu W., Li T., Yang Z., Tian T., et al. Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells. Cytokine. 2016;77:88–97. doi: 10.1016/j.cyto.2015.11.006
- Bhakkiyalakshmi E., Shalini D., Sekar T.V., Rajaguru P., Paulmurugan R., Ramkumar K.M. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 2014;171:1747–1757. doi: 10.1111/bph.12577
- Rimando A.M., Nagmani R., Feller D.R., Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J. Agric. Food Chem. 2005;53:3403–3407. doi: 10.1021/jf0580364
- Amarnath Satheesh M., Pari L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006;58:1483–1490. doi: 10.1211/jpp.58.11.0009
- Ma Z., Zhang X., Xu L., Liu D., Di S., Li W., Zhang J., Zhang H., Li X., Han J., et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019;145:104265. doi: 10.1016/j.phrs.2019.104265
- Remsberg CM, Yáñez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytotherapy Research. 2008;22(2):169–179.
- Riche DM, Riche KD, Blackshear CT, McEwen CL, Sherman JJ, Wofford MR, Griswold ME. Pterostilbene on metabolic parameters: a randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med. 2014;2014:459165. doi: 10.1155/2014/459165
- Qureshi AA, Khan DA, Mahjabeen W, Papasian CJ, Qureshi N. Nutritional Supplement-5 with a Combination of Proteasome Inhibitors (Resveratrol, Quercetin, δ-Tocotrienol) Modulate Age-Associated Biomarkers and Cardiovascular Lipid Parameters in Human Subjects. J Clin Exp Cardiolog. 2013 Mar 2;4(3):238. doi: 10.4172/2155-9880.1000238
- Daniel M., Tollefsbol T.O. Pterostilbene down-regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. J. Cell Biochem. 2018;119:3326–3337. doi: 10.1002/jcb.26495
- Tolomeo M., Grimaudo S., Di Cristina A., Roberti M., Pizzirani D., Meli M., Dusonchet L., Gebbia N., Abbadessa V., Crosta L., et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005;37:1709–1726. doi: 10.1016/j.biocel.2005.03.004
- Huynh T.T., Lin C.M., Lee W.H., Wu A.T., Lin Y.K., Lin Y.F., Yeh C.T., Wang L.S. Pterostilbene suppressed irradiation-resistant glioma stem cells by modulating GRP78/miR-205 axis. J. Nutr. Biochem. 2015;26:466–475. doi: 10.1016/j.jnutbio.2014.11.015
- Sun Y., Wu X., Cai X., Song M., Zheng J., Pan C., Qiu P., Zhang L., Zhou S., Tang Z., et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016;60:1924–1932. doi: 10.1002/mnfr.201500989
- Ko C., Lin C., Chen M., Yang S., Chiou H., Hsieh M. Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol. 2015;51:593–601. doi: 10.1016/j.oraloncology.2015.03.007
- Wang Y., Lin J., Cheng L., Chang W., Kao Y., Chang M., Wang B., Cheng H. Pterostilbene prevents AKT-ERK axis-mediated polymerization of surface fibronectin on suspended lung cancer cells independently of apoptosis and suppresses metastasis. J. Hematol. Oncol. 2017;10:72. doi: 10.1186/s13045-017-0441-z
- Tsai M.-L., Lai C.-S., Chang Y.-H., Chen W.-J., Ho C.-T., Pan M.-H. Pterostilbene, a Natural Analogue of Resveratrol, Potently Inhibits 7,12-Dimethylbenz[a]Anthracene (DMBA)/12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Skin Carcinogenesis. Food Funct. 2012;3:1185–1194. doi: 10.1039/c2fo30105a
- Chiou Y.-S., Tsai M.-L., Nagabhushanam K., Wang Y.-J., Wu C.-H., Ho C.-T., Pan M.-H. Pterostilbene Is More Potent than Resveratrol in Preventing Azoxymethane (AOM)-Induced Colon Tumorigenesis via Activation of the NF-E2-Related Factor 2 (Nrf2)-Mediated Antioxidant Signaling Pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103
- Bracht J.W.P., Karachaliou N., Berenguer J., Pedraz-Valdunciel C., Filipska M., Codony-Servat C., Codony-Servat J., Rosell R. Osimertinib and Pterostilbene in EGFR-Mutation-Positive Non-Small Cell Lung Cancer (NSCLC) Int. J. Biol. Sci. 2019;15:2607–2614. doi: 10.7150/ijbs.32889
- Wen W., Lowe G., Roberts C.M., Finlay J., Han E.S., Glackin C.A., Dellinger T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. Int. J. Mol. Sci. 2018;19:1983. doi: 10.3390/ijms19071983
- McCormack D.E., Mannal P., McDonald D., Tighe S., Hanson J., McFadden D. Genomic Analysis of Pterostilbene Predicts Its Antiproliferative Effects against Pancreatic Cancer in Vitro and in Vivo. J. Gastrointest. Surg. 2012;16:1136–1143. doi: 10.1007/s11605-012-1869-7
- Wen W., Lowe G., Roberts C.M., Finlay J., Han E.S., Glackin C.A., Dellinger T.H. Pterostilbene, a Natural Phenolic Compound, Synergizes the Antineoplastic Effects of Megestrol Acetate in Endometrial Cancer. Sci. Rep. 2017;7:12754. doi: 10.1038/s41598-017-12922-2
- Hsu Y.-H., Chen S.-Y., Wang S.-Y., Lin J.-A., Yen G.-C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules. 2020;10:709. doi: 10.3390/biom10050709
- Tam K.-W., Ho C.-T., Tu S.-H., Lee W.-J., Huang C.-S., Chen C.-S., Wu C.-H., Lee C.-H., Ho Y.-S. α-Tocopherol Succinate Enhances Pterostilbene Anti-Tumor Activity in Human Breast Cancer Cells in Vivo and in Vitro. Oncotarget. 2018;9:4593–4606. doi: 10.18632/oncotarget.23390
- Benlloch M., Obrador E., Valles S.L., Rodriguez M.L., Sirerol J.A., Alcácer J., Pellicer J.A., Salvador R., Cerdá C., Sáez G.T., et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016;24:974–990. doi: 10.1089/ars.2015.6437
- Obrador, E., Salvador-Palmer, R., Jihad-Jebbar, A., López-Blanch, R., Dellinger, T. H., Dellinger, R. W., & Estrela, J. M. (2021). Pterostilbene in Cancer Therapy. Antioxidants (Basel, Switzerland), 10(3), 492. https://doi.org/10.3390/antiox10030492
- Megestrol Acetate With or Without Pterostilbene in Treating Patients With Endometrial Cancer Undergoing Hysterectomy. https://clinicaltrials.gov/ct2/show/NCT03671811
- Shin H.J., Han J.M., Choi Y.S., Jung H.J. Pterostilbene Suppresses Both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules. 2020;25:228. doi: 10.3390/molecules25010228
- Chang H.-P., Lu C.-C., Chiang J.-H., Tsai F.-J., Juan Y.-N., Tsao J.-W., Chiu H.-Y., Yang J.-S. Pterostilbene Modulates the Suppression of Multidrug Resistance Protein 1 and Triggers Autophagic and Apoptotic Mechanisms in Cisplatin-Resistant Human Oral Cancer CAR Cells via AKT Signaling. Int. J. Oncol. 2018;52:1504–1514.
- Elsherbini A.M., Sheweita S.A., Sultan A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr. Cancer. 2020:1–9. doi: 10.1080/01635581.2020.1817513
- Mannal P., McDonald D., McFadden D. Pterostilbene and Tamoxifen Show an Additive Effect against Breast Cancer in Vitro. Am. J. Surg. 2010;200:577–580. doi: 10.1016/j.amjsurg.2010.07.022
- Mori S., Kishi S., Honoki K., Fujiwara-Tani R., Moriguchi T., Sasaki T., Fujii K., Tsukamoto S., Fujii H., Kido A., et al. Anti-Stem Cell Property of Pterostilbene in Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2020;21:9347. doi: 10.3390/ijms21249347
- Lin V.C.-H., Tsai Y.-C., Lin J.-N., Fan L.-L., Pan M.-H., Ho C.-T., Wu J.-Y., Way T.-D. Activation of AMPK by Pterostilbene Suppresses Lipogenesis and Cell-Cycle Progression in P53 Positive and Negative Human Prostate Cancer Cells. J. Agric. Food Chem. 2012;60:6399–6407. doi: 10.1021/jf301499e
- Mannal P.W., Alosi J.A., Schneider J.G., McDonald D.E., McFadden D.W. Pterostilbene Inhibits Pancreatic Cancer in Vitro. J. Gastrointest. Surg. 2010;14:873–879. doi: 10.1007/s11605-010-1164-4
- Mena S., Rodríguez M.L., Ponsoda X., Estrela J.M., Jäättela M., Ortega A.L. Pterostilbene-Induced Tumor Cytotoxicity: A Lysosomal Membrane Permeabilization-Dependent Mechanism. PLoS ONE. 2012;7:e44524. doi: 10.1371/journal.pone.0044524
- Chatterjee K., Mukherjee S., Vanmanen J., Banerjee P., Fata J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in Vitro and in Vivo Analysis. Front. Oncol. 2019;9:352. doi: 10.3389/fonc.2019.00352
- Pan C., Hu Y., Li J., Wang Z., Huang J., Zhang S., Ding L. Estrogen Receptor-A36 Is Involved in Pterostilbene-Induced Apoptosis and Anti-Proliferation in in Vitro and in Vivo Breast Cancer. PLoS ONE. 2014;9:e104459. doi: 10.1371/journal.pone.0104459
- Dhar S., Kumar A., Rimando A.M., Zhang X., Levenson A.S. Resveratrol and Pterostilbene Epigenetically Restore PTEN Expression by Targeting OncomiRs of the MiR-17 Family in Prostate Cancer. Oncotarget. 2015;6:27214–27226. doi: 10.18632/oncotarget.4877
- Su C.-M., Lee W.-H., Wu A.T.H., Lin Y.-K., Wang L.-S., Wu C.-H., Yeh C.-T. Pterostilbene Inhibits Triple-Negative Breast Cancer Metastasis via Inducing MicroRNA-205 Expression and Negatively Modulates Epithelial-to-Mesenchymal Transition. J. Nutr. Biochem. 2015;26:675–685. doi: 10.1016/j.jnutbio.2015.01.005
- Benlloch M, Obrador E, Valles SL, Rodriguez ML, Sirerol JA, Alcácer J, Pellicer JA, Salvador R, Cerdá C, Sáez GT, Estrela JM. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid Redox Signal. 2016 Jun 10;24(17):974-90. doi: 10.1089/ars.2015.6437
- Kong Y., Chen G., Xu Z., Yang G., Li B., Wu X., Xiao W., Xie B., Hu L., Sun X., et al. Pterostilbene Induces Apoptosis and Cell Cycle Arrest in Diffuse Large B-Cell Lymphoma Cells. Sci. Rep. 2016;6:37417. doi: 10.1038/srep37417
- Wakimoto R., Ono M., Takeshima M., Higuchi T., Nakano S. Differential Anticancer Activity of Pterostilbene against Three Subtypes of Human Breast Cancer Cells. Anticancer Res. 2017;37:6153–6159
- Li K., Dias S.J., Rimando A.M., Dhar S., Mizuno C.S., Penman A.D., Lewin J.R., Levenson A.S. Pterostilbene Acts through Metastasis-Associated Protein 1 to Inhibit Tumor Growth, Progression and Metastasis in Prostate Cancer. PLoS ONE. 2013;8:e57542
- Wang D., Guo H., Yang H., Wang D., Gao P., Wei W. Pterostilbene, An Active Constituent of Blueberries, Suppresses Proliferation Potential of Human Cholangiocarcinoma via Enhancing the Autophagic Flux. Front. Pharmacol. 2019;10:1238. doi: 10.3389/fphar.2019.01238
- Xie B., Xu Z., Hu L., Chen G., Wei R., Yang G., Li B., Chang G., Sun X., Wu H., et al. Pterostilbene Inhibits Human Multiple Myeloma Cells via ERK1/2 and JNK Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2016;17:1927. doi: 10.3390/ijms17111927
- Paul S., DeCastro A.J., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Zhou R., Rimando A.M., Suh N. Dietary Intake of Pterostilbene, a Constituent of Blueberries, Inhibits the Beta-Catenin/P65 Downstream Signaling Pathway and Colon Carcinogenesis in Rats. Carcinogenesis. 2010;31:1272–1278. doi: 10.1093/carcin/bgq004
- Priego S., Feddi F., Ferrer P., Mena S., Benlloch M., Ortega A., Carretero J., Obrador E., Asensi M., Estrela J.M. Natural Polyphenols Facilitate Elimination of HT-29 Colorectal Cancer Xenografts by Chemoradiotherapy: A Bcl-2- and Superoxide Dismutase 2-Dependent Mechanism. Mol. Cancer Ther. 2008;7:3330–3342. doi: 10.1158/1535-7163.MCT-08-0363
- Guo L., Tan K., Wang H., Zhang X. Pterostilbene Inhibits Hepatocellular Carcinoma through P53/SOD2/ROS-Mediated Mitochondrial Apoptosis. Oncol. Rep. 2016;36:3233–3240. doi: 10.3892/or.2016.5151
- Sirerol J.A., Feddi F., Mena S., Rodriguez M.L., Sirera P., Aupí M., Pérez S., Asensi M., Ortega A., Estrela J.M. Topical Treatment with Pterostilbene, a Natural Phytoalexin, Effectively Protects Hairless Mice against UVB Radiation-Induced Skin Damage and Carcinogenesis. Free Radic. Biol. Med. 2015;85:1–11. doi: 10.1016/j.freeradbiomed.2015.03.027
- Huynh T.-T., Lin C.-M., Lee W.-H., Wu A.T.H., Lin Y.-K., Lin Y.-F., Yeh C.-T., Wang L.-S. Pterostilbene Suppressed Irradiation-Resistant Glioma Stem Cells by Modulating GRP78/MiR-205 Axis. J. Nutr. Biochem. 2015;26:466–475. doi: 10.1016/j.jnutbio.2014.11.015
- Ferrer P., Asensi M., Segarra R., Ortega A., Benlloch M., Obrador E., Varea M.T., Asensio G., Jordá L., Estrela J.M. Association between Pterostilbene and Quercetin Inhibits Metastatic Activity of B16 Melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337
- Chen R.J., Tsai S.J., Ho C.T., Pan M.H., Ho Y.S., Wu C.H., Wang Y.J. Chemopreventive effects of pterostilbene on urethane-induced lung carcinogenesis in mice via the inhibition of EGFR-mediated pathways and the induction of apoptosis and autophagy. J. Agric. Food Chem. 2012;60:11533–11541. doi: 10.1021/jf302778a
- Mak K.K., Wu A.T., Lee W.H., Chang T.C., Chiou J.F., Wang L.S., Wu C.H., Huang C.Y., Shieh Y.S., Chao T.Y., et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013;57:1123–1134. doi: 10.1002/mnfr.201200549
- McCormack D., Schneider J., McDonald D., McFadden D. The antiproliferative effects of pterostilbene on breast cancer in vitro are via inhibition of constitutive and leptin-induced Janus kinase/signal transducer and activator of transcription activation. Am. J. Surg. 2011;202:541–544. doi: 10.1016/j.amjsurg.2011.06.020
- Su C.-M., Lee W.-H., Wu A.T.H., Lin Y.-K., Wang L.-S., Wu C.-H., Yeh C.-T. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J. Nutr. Biochem. 2015;26:675–685. doi: 10.1016/j.jnutbio.2015.01.005
- Ko H.S., Kim J.S., Cho S.M., Lee H.J., Ahn K.S., Kim S.H., Lee E.O. Urokinase-type plasminogen activator expression and Rac1/WAVE-2/Arp2/3 pathway are blocked by pterostilbene to suppress cell migration and invasion in MDA-MB-231 cells. Bioorg. Med. Chem. Lett. 2014;24:1176–1179. doi: 10.1016/j.bmcl.2013.12.115
- Paul S., DeCastro A.J., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Zhou R., Rimando A.M., Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the -catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis. 2010;31:1272–1278. doi: 10.1093/carcin/bgq004
- Lee H., Kim Y., Jeong J.H., Ryu J.H., Kim W.Y. ATM/CHK/p53 Pathway Dependent Chemopreventive and Therapeutic Activity on Lung Cancer by Pterostilbene. PLoS ONE. 2016;11:e0162335. doi: 10.1371/journal.pone.0162335
- Guo L., Tan K., Wang H., Zhang X. Pterostilbene inhibits hepatocellular carcinoma through p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol. Rep. 2016;36:3233–3240. doi: 10.3892/or.2016.5151
- Wang Y.L., Shen Y., Xu J.P., Han K., Zhou Y., Yang S., Yin J.Y., Min D.L., Hu H.Y. Pterostilbene suppresses human endometrial cancer cells in vitro by down-regulating miR-663b. Acta Pharmacol. Sin. 2017;38:1394–1400. doi: 10.1038/aps.2017.60
- Liu Y., Wang L., Wu Y., Lv C., Li X., Cao X., Yang M., Feng D., Luo Z. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology. 2013;304:120–131. doi: 10.1016/j.tox.2012.12.018
- Chen R.J., Ho C.T., Wang Y.J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010;54:1819–1832. doi: 10.1002/mnfr.201000067
- Pan M.H., Chang Y.H., Badmaev V., Nagabhushanam K., Ho C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007;55:7777–7785. doi: 10.1021/jf071520h
- Feng Y., Yang Y., Fan C., Di S., Hu W., Jiang S., Li T., Ma Z., Chao D., Feng X., et al. Pterostilbene Inhibits the Growth of Human Esophageal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Cell Physiol. Biochem. 2016;38:1226–1244. doi: 10.1159/000443071
- Hsu Y.H., Chen S.Y., Wang S.Y., Lin J.A., Yen G.C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules. 2020;10:709. doi: 10.3390/biom10050709
- Shin H.J., Han J.M., Choi Y.S., Jung H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules. 2020;25:228. doi: 10.3390/molecules25010228
- Wakimoto R., Ono M., Takeshima M., Higuchi T., Nakano S. Differential Anticancer Activity of Pterostilbene against Three Subtypes of Human Breast Cancer Cells. Anticancer Res. 2017;37:6153–6159.
- Kumar A., Rimando A.M., Levenson A.S. Resveratrol and Pterostilbene as a MicroRNA-Mediated Chemopreventive and Therapeutic Strategy in Prostate Cancer. Ann. N. Y. Acad. Sci. 2017;1403:15–26. doi: 10.1111/nyas.13372
- Zipper L.M., Mulcahy R.T. Erk Activation Is Required for Nrf2 Nuclear Localization during Pyrrolidine Dithiocarbamate Induction of Glutamate Cysteine Ligase Modulatory Gene Expression in HepG2 Cells. Toxicol. Sci. 2003;73:124–134. doi: 10.1093/toxsci/kfg083
- Lamanuzzi A., Saltarella I., Desantis V., Frassanito M.A., Leone P., Racanelli V., Nico B., Ribatti D., Ditonno P., Prete M., et al. Inhibition of MTOR Complex 2 Restrains Tumor Angiogenesis in Multiple Myeloma. Oncotarget. 2018;9:20563–20577. doi: 10.18632/oncotarget.25003
- Chang H.-P., Lu C.-C., Chiang J.-H., Tsai F.-J., Juan Y.-N., Tsao J.-W., Chiu H.-Y., Yang J.-S. Pterostilbene Modulates the Suppression of Multidrug Resistance Protein 1 and Triggers Autophagic and Apoptotic Mechanisms in Cisplatin-Resistant Human Oral Cancer CAR Cells via AKT Signaling. Int. J. Oncol. 2018;52:1504–1514
- Zhou Y., Zhang X.M., Ma A., Zhang Y.L., Chen Y.Y., Zhou H., Li W.J., Jin X. Orally administrated pterostilbene attenuates acute cerebral ischemia-reperfusion injury in a dose- and time-dependent manner in mice. Pharmacol. Biochem. Behav. 2015;135:199–209. doi: 10.1016/j.pbb.2015.06.009
- Zhou J., Ci X., Ma X., Yu Q., Cui Y., Zhen Y., Li S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019;10:497. doi: 10.3389/fphar.2019.00497
- Shen H., Rong H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Exp. Pathol. 2015;8:12589–12594.
- Li J., Ruzhi D., Hua X., Zhang L., Lu F., Coursey T.G., Pflugfelder S.C., Li D.Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016;6:19408. doi: 10.1038/srep19408
- Li Y.R., Li S., Lin C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44:69–82. doi: 10.1002/biof.1400
- Koh Y.C., Li S., Chen P.Y., Wu J.C., Kalyanam N., Ho C.T., Pan M.H. Prevention of Vascular Inflammation by Pterostilbene via Trimethylamine-N-Oxide Reduction and Mechanism of Microbiota Regulation. Mol. Nutr. Food Res. 2019;63:e1900514. doi: 10.1002/mnfr.201900514
- Liu H., Zhao L., Yue L., Wang B., Li X., Guo H., Ma Y., Yao C., Gao L., Deng J., et al. Pterostilbene Attenuates Early Brain Injury Following Subarachnoid Hemorrhage via Inhibition of the NLRP3 Inflammasome and Nox2-Related Oxidative Stress. Mol. Neurobiol. 2017;54:5928–5940. doi: 10.1007/s12035-016-0108-8
- Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J Appli Biomed 2008;6:31-37.
- Satheesh MA, Pari L. The antioxidant role of pterostilbene in streptozotocin-nicotin-amide-induced type 2 diabetes mellitus in Wistar rats. J Pharm Pharmacol2006;58:1483-1490.
- Nagao K., Jinnouchi T., Kai S., Yanagita T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J. Nutr. Biochem. 2017;43:151–155. doi: 10.1016/j.jnutbio.2017.02.009
- La Spina M., Galletta E., Azzolini M., Gomez Zorita S., Parrasia S., Salvalaio M., Salmaso A., Biasutto L. Browning Effects of a Chronic Pterostilbene Supplementation in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2019;20:5377. doi: 10.3390/ijms20215377
- Aguirre L., Milton-Laskibar I., Hijona E., Bujanda L., Rimando A.M., Portillo M.P. Effects of pterostilbene in brown adipose tissue from obese rats. J. Physiol. Biochem. 2016;73:457–464. doi: 10.1007/s13105-017-0556-2
- Aguirre L., Palacios-Ortega S., Fernandez-Quintela A., Hijona E., Bujanda L., Portillo M.P. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients. 2019;11:961.
- Gomez-Zorita S., Trepiana J., Fernandez-Quintela A., Gonzalez M., Portillo M.P. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. Int. J. Mol. Sci. 2018;19:2654. doi: 10.3390/ijms19092654
- Gomez-Zorita S., Belles C., Briot A., Fernandez-Quintela A., Portillo M.P., Carpene C. Pterostilbene Inhibits Lipogenic Activity similar to Resveratrol or Caffeine but Differently Modulates Lipolysis in Adipocytes. Phytother. Res. 2017;31:1273–1282. doi: 10.1002/ptr.5852
- Hsu C.L., Lin Y.J., Ho C.T., Yen G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012;3:49–57. doi: 10.1039/C1FO10209E
- Etxeberria U., Hijona E., Aguirre L., Milagro F.I., Bujanda L., Rimando A.M., Martinez J.A., Portillo M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017;61:61. doi: 10.1002/mnfr.201500906
- Gómez-Zorita S., Fernández-Quintela A., Lasa A., Aguirre L., Rimando A., Portillo M. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J. Agric. Food Chem. 2014;62:8371–8378. doi: 10.1021/jf501318b
- Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012 Sep;33(9):2062-71. doi: 10.1016/j.neurobiolaging.2011.08.015
- Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B. Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J Agric Food Chem. 2008 Nov 26;56(22):10544-51. doi: 10.1021/jf802279h
- Wang B., Liu H., Yue L., Li X., Zhao L., Yang X., Wang X., Yang Y., Qu Y. Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res. 2016;1643:70–79. doi: 10.1016/j.brainres.2016.04.048
- Yang Y., Fan C., Wang B., Ma Z., Wang D., Gong B., Di S., Jiang S., Li Y., Li T., et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:827–837. doi: 10.1016/j.bbadis.2017.01.005
- Li D., Song T., Yang L., Wang X., Yang C., Jiang Y. Neuroprotective actions of pterostilbene on hypoxic-ischemic brain damage in neonatal rats through upregulation of heme oxygenase-1. Int. J. Dev. Neurosci. 2016;54:22–31. doi: 10.1016/j.ijdevneu.2016.08.005
- Song Z., Han S., Pan X., Gong Y., Wang M. Pterostilbene mediates neuroprotection against oxidative toxicity via oestrogen receptor alpha signalling pathways. J. Pharm. Pharmacol. 2015;67:720–730. doi: 10.1111/jphp.12360
- Liu H., Wu X., Luo J., Wang X., Guo H., Feng D., Zhao L., Bai H., Song M., Liu X., et al. Pterostilbene Attenuates Astrocytic Inflammation and Neuronal Oxidative Injury After Ischemia-Reperfusion by Inhibiting NF-kappaB Phosphorylation. Front. Immunol. 2019;10:2408. doi: 10.3389/fimmu.2019.02408
- Yang Y., Wang J., Li Y., Fan C., Jiang S., Zhao L., Di S., Xin Z., Wang B., Wu G., et al. HO-1 Signaling Activation by Pterostilbene Treatment Attenuates Mitochondrial Oxidative Damage Induced by Cerebral Ischemia Reperfusion Injury. Mol. Neurobiol. 2016;53:2339–2353. doi: 10.1007/s12035-015-9194-2
- Sun J., Huo H., Song Y., Zheng J., Zhao Y., Huang W., Wang Y., Zhu J., Tu P., Li J. Method development and application for multi-component quantification in rats after oral administration of Longxuetongluo Capsule by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2018;156:252–262. doi: 10.1016/j.jpba.2018.04.030
- Hou Y., Xie G., Miao F., Ding L., Mou Y., Wang L., Su G., Chen G., Yang J., Wu C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog Neuropsychopharmacol. Biol. Psychiatry. 2014;54:92–102. doi: 10.1016/j.pnpbp.2014.03.015
- Naik B., Nirwane A., Majumdar A. Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn. Neurodyn. 2017;11:35–49. doi: 10.1007/s11571-016-9413-1
- Al Rahim M., Rimando A.M., Silistreli K., El-Alfy A.T. Anxiolytic action of pterostilbene: Involvement of hippocampal ERK phosphorylation. Planta Med. 2013;79:723–730. doi: 10.1055/s-0032-1328553
- Yang L., Ran Y., Quan Z., Wang R., Yang Q., Jia Q., Zhang H., Li Y., Peng Y., Liang J., et al. Pterostilbene, an active component of the dragon’s blood extract, acts as an antidepressant in adult rats. Psychopharmacology. 2019;236:1323–1333. doi: 10.1007/s00213-018-5138-7
- Pari L, Satheesh MA. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci2006;79:641-645.
- Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholes-terolemic hamsters. J Agric Food Chem2005;53:3403-3407.
- Manickam M, Ramanathan M, Jahromi MA, et al. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod 1997;60:609-610.
- Pari L., Satheesh M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006;79:641–645. doi: 10.1016/j.lfs.2006.02.036
- Paul S., Rimando A.M., Lee H.J., Ji Y., Reddy B.S., Suh N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. (Phila.) 2009;2:650–657. doi: 10.1158/1940-6207.CAPR-08-0224
- Sun H., Liu X., Long S.R., Teng W., Ge H., Wang Y., Yu S., Xue Y., Zhang Y., Li X., et al. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur. J. Pharmacol. 2019;859:172526. doi: 10.1016/j.ejphar.2019.172526
- Elango B., Dornadula S., Paulmurugan R., Ramkumar K.M. Pterostilbene Ameliorates Streptozotocin-Induced Diabetes through Enhancing Antioxidant Signaling Pathways Mediated by Nrf2. Chem. Res. Toxicol. 2016;29:47–57. doi: 10.1021/acs.chemrestox.5b00378
- Gómez-Zorita S., Fernández-Quintela A., Aguirre L., Macarulla M., Rimando A., Portillo M. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015;6:1968–1976. doi: 10.1039/C5FO00151J
- Paul B., Masih I., Deopujari J., Charpentier C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J. Ethnopharmacol. 1999;68:71–76. doi: 10.1016/S0378-8741(99)00044-6
- Lim Y.R.I., Preshaw P.M., Lim L.P., Ong M.M.A., Lin H.S., Tan K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020;10:9072. doi: 10.1038/s41598-020-66031-8
- Gastaminza P, Whitten-Bauer C, Chisari FV. Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. Proc Natl Acad Sci U S A 2010;107:291-296.
- Rakic B, Sagan SM, Noestheden M, et al. Peroxisome proliferator-activated receptor alpha antagonism inhibits hepatitis C virus replication. Chem Biol 2006;13:23-30.
- Chan C.N., Trinite B., Levy D.N. Potent Inhibition of HIV-1 Replication in Resting CD4 T Cells by Resveratrol and Pterostilbene. Antimicrob. Agents Chemother. 2017;61:1–10. doi: 10.1128/AAC.00408-17
- Pflieger A., Waffo Teguo P., Papastamoulis Y., Chaignepain S., Subra F., Munir S., Delelis O., Lesbats P., Calmels C., Andreola M.L., et al. Natural stilbenoids isolated from grapevine exhibiting inhibitory effects against HIV-1 integrase and eukaryote MOS1 transposase in vitro activities. PLoS ONE. 2013;8:e81184. doi: 10.1371/journal.pone.0081184
- Ruiz MJ, Fernández M, Picó Y, et al. Dietary administration of high doses of pterostil-bene and quercetin to mice is not toxic. J Agric Food Chem 2009;57:3180-3186.
- Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, Griswold M. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol. 2013;2013:463595. doi: 10.1155/2013/463595
- Joy, J. M., Vogel, R. M., Moon, J. R., Falcone, P. H., Mosman, M. M., & Kim, M. P. (2016). Twelve weeks supplementation with an extended-release caffeine and ATP-enhancing supplement may improve body composition without affecting hematology in resistance-trained men. Journal of the International Society of Sports Nutrition, 13, 25. https://doi.org/10.1186/s12970-016-0136-9
- GlaxoSmithKline Halts All Further Development Of Resveratrol Drug SRT501. https://myelomabeacon.org/news/2010/11/30/glaxosmithkline-halts-all-further-development-of-resveratrol-drug-srt501
- Ruiz M.J., Fernández M., Picó Y., Mañes J., Asensi M., Carda C., Asensio G., Estrela J.M. Dietary Administration of High Doses of Pterostilbene and Quercetin to Mice Is Not Toxic. J. Agric. Food Chem. 2009;57:3180–3186. doi: 10.1021/jf803579e
- Majeed M., Bani S., Natarajan S., Pandey A., Naveed S. Evaluation of 90 Day Repeated Dose Oral Toxicity and Reproductive/Developmental Toxicity of 3’-Hydroxypterostilbene in Experimental Animals. PLoS ONE. 2017;12:e0172770. doi: 10.1371/journal.pone.0172770
- Riche D.M., McEwen C.L., Riche K.D., Sherman J.J., Wofford M.R., Deschamp D., Griswold M. Analysis of Safety from a Human Clinical Trial with Pterostilbene. J. Toxicol. 2013;2013:463595. doi: 10.1155/2013/463595
- A Clinical Study to Assess the Safety and Activity of SRT501 Alone or in Combination With Bortezomib in Patients With Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT00920556